Abstract
Uncontrolled inflammation following COVID-19 infection is an important characteristic of the most seriously ill patients. The present study aims to describe the clusters of inflammation in COVID-19 and to analyze their prognostic role. This is a retrospective observational study including 15,691 patients with a high degree of inflammation. They were included in the Spanish SEMI-COVID-19 registry from March 1, 2020 to May 1, 2021. The primary outcome was in-hospital mortality. Hierarchical cluster analysis identified 7 clusters. C1 is characterized by lymphopenia, C2 by elevated ferritin, and C3 by elevated LDH. C4 is characterized by lymphopenia plus elevated CRP and LDH and frequently also ferritin. C5 is defined by elevated CRP, and C6 by elevated ferritin and D-dimer, and frequently also elevated CRP and LDH. Finally, C7 is characterized by an elevated D-dimer. The clusters with the highest in-hospital mortality were C4, C6, and C7 (17.4% vs. 18% vs. 15.6% vs. 36.8% vs. 17.5% vs. 39.3% vs. 26.4%). Inflammation clusters were found as independent factors for in-hospital mortality. In detail and, having cluster C1 as reference, the model revealed a worse prognosis for all other clusters: C2 (OR = 1.30, p = 0.001), C3 (OR = 1.14, p = 0.178), C4 (OR = 2.28, p < 0.001), C5 (OR = 1.07, p = 0.479), C6 (OR = 2.29, p < 0.001), and C7 (OR = 1.28, p = 0.001). We identified 7 groups based on the presence of lymphopenia, elevated CRP, LDH, ferritin, and D-dimer at the time of hospital admission for COVID-19. Clusters C4 (lymphopenia + LDH + CRP), C6 (ferritin + D-dimer), and C7 (D-dimer) had the worst prognosis in terms of in-hospital mortality.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11739-021-02924-4.
Keywords: Coronavirus, COVID-19, Cluster analysis, Inflammation, Prognosis, Mortality
Introduction
As of May 2021, COVID-19 has infected more than 170 million people worldwide and caused the death of more than 3.5 million people [1]. Death is largely due to the inflammatory escalation or host cytokine storm secondary to SARS-CoV-2 infection [2]. This inflammatory response has clearly analytically identifiable components [3]. Clinically, most individual patients showed the changes of lymphocyte counts, D-dimer, interleukin-6 (IL-6), C-reactive protein (CRP), ferritin, LDH, etc. Why some patients become more inflamed than others is not known for certain but it is possible that there is a genetic background that facilitates this. Among those patients who present with this exaggerated inflammatory response, different degrees of inflammation have been described that predict the short-term future of these patients [4]. However, this inflammation is far from being homogeneous in all patients. From these studies, we know that the more parameters of analytical inflammation the worse the prognosis. The next step is to find out if some parameters have more prognostic value than others. In fact, in clinical practice, we see how some patients start their analytical inflammation in one way and others in another. This suggests different pathways and perhaps also different prognoses.
The present study aims to describe the different clusters or differentiated groups of inflammation in COVID-19 and to analyze their prognostic role.
Materials and methods
Study design, patient selection, and data collection
The present study is a retrospective observational study of consecutive patients included in the Spanish SEMI-COVID-19 Registry, created by the Spanish Society of Internal Medicine (SEMI). This is a multicenter, nationwide registry with over 150 hospitals registered so far. From March 1, 2020 to May 1, 2021, 21,962 hospitalized patients were included in the Registry. The characteristics of this registry have been detailed in previous reports [5]. In brief, all included patients were diagnosed by polymerase chain reaction (PCR) test or rapid antigenic test for SARS-CoV-2 taken from a nasopharyngeal sample, sputum, or bronchoalveolar lavage. The collection of data from each patient in terms of sociodemographic data, comorbidities, laboratory data, treatments, and outcomes was verified by the principal investigator of each center through the review of clinical records. All participating centers in the register received confirmation from the relevant Ethics Committees, including Bellvitge University Hospital (PR 128/20). Informed consent was obtained from the subjects.
The inclusion criteria were all patients in the registry with a community (non-nosocomial) SARS-CoV-2 infection and belonging to the high-risk category of inflammation according to our previous report and based on the lab test on admission [4]. This category of high risk is based on the decrease in the lymphocyte count 101.5 mg/L, lactate dehydrogenase (LDH) > 394U/L, ferritin > 1359.9mcg/L, and D-dimer > 1580 ng/mL.
The treatments received were in accordance with the medical guidelines available at the time of the pandemic [6–11]. In the absence of clinical evidence of any of the treatments at the initial time of the pandemic, their use was allowed off-label.
Outcomes definition
The primary outcome of the study was in-hospital mortality. Secondary outcomes were the requirement of high-flow nasal cannula (HFNC), non-invasive mechanical ventilation (NIMV), invasive mechanical ventilation (IMV), or ICU admission.
Statistical analysis
Multiple imputations of missing data were performed. Categorical variables were expressed as absolute numbers and percentages. Continuous variables are expressed as mean plus standard deviation (SD) in the case of parametric distribution or median [IQR] in the case of non-parametric distribution. Differences between groups were assessed using the Chi-square test for categorical variables and ANOVA or Kruskal–Wallis test as appropriate for continuous variables. p values < 0.05 indicated statistical significance.
The cluster analysis was performed by ascendant hierarchical clustering on the 5 laboratory variables previously selected using Ward’s minimum variance method with Euclidean squared distance [12]. Results are graphically depicted by a dendrogram. The number of clusters was estimated by the kmeans method. The cluster analysis model was included in a binary logistic regression, taking in-hospital mortality as the dependent variable. We introduced in the multivariate model those variables with a p value < 0.10 in the univariate model. In-hospital mortality between clusters was depicted by the Kaplan–Meier curves with their logarithmic range test (event: death; censored data: hospital discharge).
Statistical analysis was performed by IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY, USA: IBM Corp.
Results
General baseline data between groups
From March 1, 2020 to May 1, 2021, 20,641 patients admitted for non-nosocomial COVID-19 were included in the SEMI-COVID-19 registry. Of these, 15,691 fell into the high-risk category for inflammation according to the laboratory tests at admission and were therefore included in the present study (Figure S1).
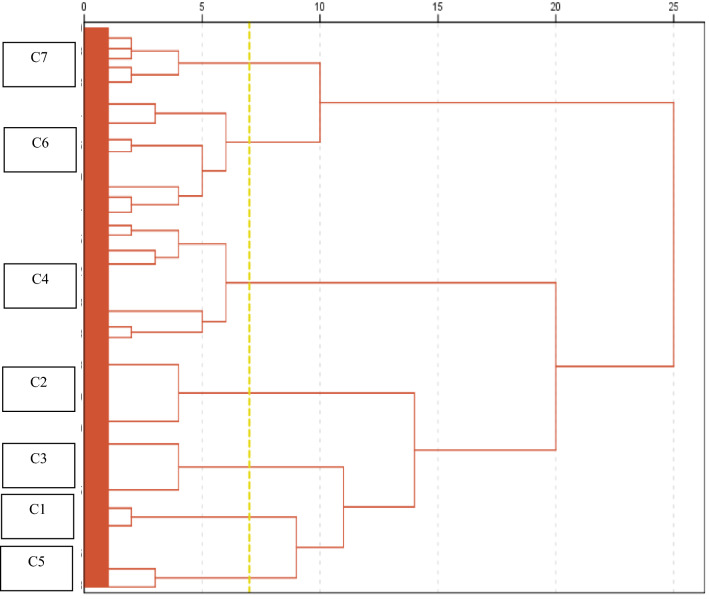
Hierarchical cluster analysis identified 7 clearly differentiated clusters (C1–C7) (Fig. 1). The baseline characteristics of the 7 clusters are shown in Table 1. It is noteworthy that C1 patients attend the hospital earlier from the onset. C2 and C3 patients have a lower Charlson index. C4 patients are older and predominantly male and have more comorbidity (mainly hypertension and dyslipidemia). C5 patients have more comorbidity (mainly hypertension, dyslipidemia and diabetes mellitus). Patients in groups C6 and C7 are older and have more dependency and comorbidity (mainly hypertension, dyslipidemia and diabetes mellitus).
Fig. 1.
Dendrogram. Clusters based on analytical inflammatory parameters upon admission. Cluster C1 is characterized by the presence of lymphopenia, cluster C2 by elevated ferritin, and C3 by elevated LDH. Cluster C4 is characterized by lymphopenia plus elevated CRP and LDH and frequently also elevated ferritin. Cluster C5 is defined by elevated CRP, and cluster C6 by elevated ferritin and D-dimer and frequently also elevated CRP and LDH. Finally, cluster C7 is characterized by elevated D-dimer
Table 1.
Patient characteristics between clusters of inflammation
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | p value | |
|---|---|---|---|---|---|---|---|---|
| N | 2348 | 3574 | 1712 | 1014 | 1920 | 1598 | 3525 | |
| Age, median [IQR] |
71.1 [59.2–80.6] |
66 [54.6–76.5] |
67.3 [54.7–77.7] |
71.6 [60.5–80.3] |
68.4 [56.6–78.2] |
74.6 [62–83.5] |
76.1 [63.4–85.1] |
< 0.001 |
| Gender (males), i (%) | 1412 (60.1) | 2469 (69.1) | 896 (52.3) | 734 (72.4) | 1099 (57.2) | 1039 (65) | 1830 (51.9) | < 0.001 |
|
Race, n (%) Caucasian Black Hispanic Asian Others |
2171 (92.5) 9 (0.4) 140 (6) 6 (0.3) 22 (0.9) |
3171 (88.7) 24 (0.7) 318 (8.9) 20 (0.6) 41 (1.1) |
1537 (89.7) 7 (0.4) 138 (8.1) 14 (0.8) 16 (0.9) |
932 (91.9) 3 (0.3) 66 (6.5) 5 (0.5) 8 (0.8) |
1648 (85.8) 9 (0.5) 223 (11.6) 13 (0.7) 27 (1.4) |
1458 (91.2) 8 (0.5) 105 (6.6) 5 (0.3) 22 (1.4) |
3242 (92) 17 (0.5) 217 (6.2) 9 (0.3) 40 (1.1) |
< 0.001 |
|
Days from onset to admission, median [IQR] |
6 [3–9] | 7 [4–10] | 7 [4–9] | 7 [4–9] | 7 [4–10] | 6 [3–9] | 6 [3–9] | < 0.001 |
| BMI, median [IQR] | 28 [25–32] | 29 [25–32] | 28 [26–32] | 29 [26–33] | 29 [26–33] | 29 [25–84] | 28 [25–32] | < 0.001 |
|
Smoking behavior, n (%) Never smoker Former smoker Current smoker |
1604 (68.3) 658 (28) 86 (3.7) |
2412 (67.5) 970 (27.1) 192 (5.4) |
1190 (69.5) 442 (25.8) 80 (4.7) |
663 (65.4) 302 (29.8) 49 (4.8) |
1347 (70.2) 492 (25.6) 81 (4.2) |
1046 (65.5) 452 (28.3) 100 (6.3) |
2466 (70) 878 (24.9) 181 (5.1) |
< 0.001 |
|
Degree of dependency, n (%) None or mild Moderate Severe |
1958 (83.4) 224 (9.5) 166 (7.1) |
3,156 (88.3) 243 (6.8) 175 (4.9) |
1503 (87.8) 130 (7.6) 79 (4.6) |
873 (86.1) 90 (8.9) 51 (5) |
1648 (85.8) 142 (7.4) 130 (6.8) |
1211 (75.8) 206 (12.9) 181 (11.3) |
2558 (72.6) 535 (15.2) 432 (12.3) |
< 0.001 |
| Arterial hypertension, n (%) | 1244 (53) | 1732 (48.5) | 831 (48.5) | 597 (58.9) | 1038 (54.1) | 902 (56.4) | 2086 (59.2) | < 0.001 |
| Dyslipidemia, n (%) | 932 (39.7) | 1332 (37.3) | 662 (38.7) | 447 (44.1) | 813 (42.3) | 672 (42.1) | 1500 (42.6) | < 0.001 |
| Diabetes mellitus, n (%) | 50 (22.1) | 609 (17) | 287 (16.8) | 209 (20.6) | 480 (25) | 403 (25.2) | 852 (24.2) | < 0.001 |
| Atrial fibrillation, n (%) | 349 (14.9) | 319 (8.9) | 182 (10.6) | 147 (14.5) | 174 (9.1) | 180 (11.3) | 407 (11.5) | < 0.001 |
| Ischemic cardiopathy, n (%) | 227 (9.7) | 224 (6.3) | 123 (7.2) | 80 (7.9) | 148 (7.7) | 153 (9.6) | 332 (9.4) | < 0.001 |
| Cerebrovascular disease, n (%) | 182 (7.8) | 216 (6) | 113 (6.6) | 90 (8.9) | 124 (6.5) | 134 (8.4) | 326 (9.2) | < 0.001 |
| Peripheral arterial disease, n (%) | 95 (4) | 116 (3.2) | 50 (2.9) | 49 (4.8) | 75 (3.9) | 113 (7.1) | 212 (6) | < 0.001 |
| Dementia, n (%) | 221 (9.4) | 234 (6.5) | 126 (7.4) | 68 (6.7) | 150 (7.8) | 236 (14.8) | 596 (16.9) | < 0.001 |
| Chronic heart failure, n (%) | 199 (8.5) | 159 (4.4) | 110 (6.4) | 77 (7.6) | 97 (5.1) | 138 (8.6) | 351 (10) | < 0.001 |
| Chronic liver disease, n (%) | 88 (3.7) | 115 (3.2) | 60 (3.5) | 39 (3.8) | 50 (2.6) | 81 (5.1) | 132 (3.7) | 0.008 |
| Severe chronic renal failure, n (%) | 163 (6.9) | 179 (5) | 82 (4.8) | 60 (5.9) | 84 (4.4) | 153 (9.6) | 320 (9.1) | < 0.001 |
| Cancer, n (%) | 280 (11.9) | 296 (8.3) | 150 (8.8) | 93 (9.2) | 142 (7.4) | 215 (13.5) | 419 (11.9) | < 0.001 |
| COPD, n (%) | 197 (8.4) | 225 (6.3) | 97 (5.7) | 71 (7) | 119 (6.2) | 122 (7.6) | 290 (8.2) | < 0.001 |
| Asthma, n (%) | 162 (6.9) | 224 (6.3) | 127 (7.4) | 52 (5.1) | 148 (7.7) | 90 (5.6) | 210 (6) | 0.022 |
| OSAS, n (%) | 138 (5.9) | 226 (6.3) | 100 (5.8) | 68 (6.7) | 127 (6.6) | 86 (5.4) | 216 (6.1) | 0.722 |
| Charlson index median [IQR] | 1 [0–2] | 0 [0–1] | 0 [0–2] | 1 [0–2] | 1 [0–2] | 1 [0–2] | 1 [0–2] | < 0.001 |
BMI body mass index, IQR interquartile range, COPD chronic obstructive pulmonary disease, OSAS obstructive sleep apnea syndrome, Severe chronic renal failure: Creatinine > 300 mg/dl or dyalisis
Symptoms at the time of hospital admission are listed in Table S1. In particular, group C1 presents more frequently with a headache. C2 and C3 present more frequently with headaches, arthromyalgia, sore throat, cough, diarrhea, anosmia, and ageusia. C4 patients present more frequently with cough, tachypnea, and less diarrhea. Cluster C5 most frequently presents with headache, arthromyalgia, cough, anosmia, and ageusia. Clusters C6 and C7 present less frequently with fever and cough.
Laboratory tests between groups
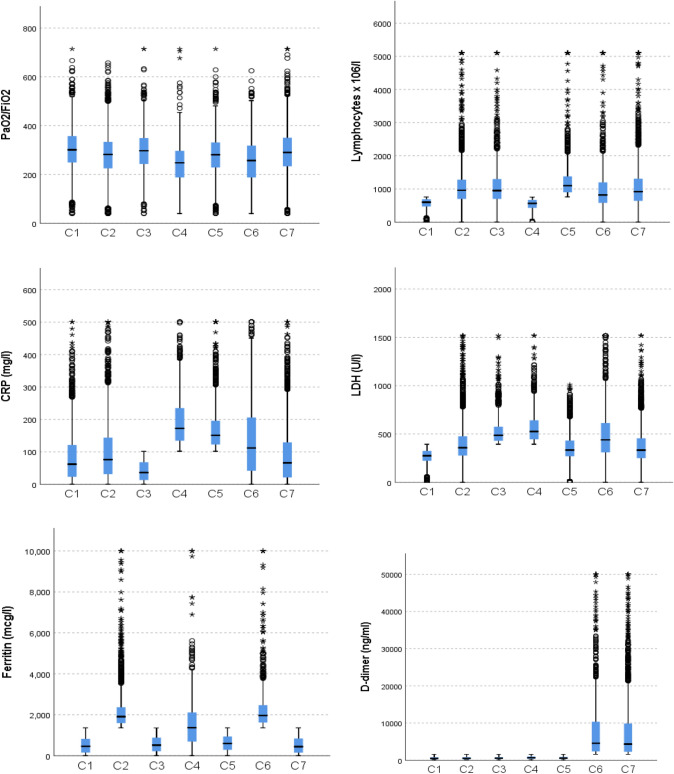
Table S2 shows the inflammatory analytical parameters presented by each of the clusters. According to the most relevant analytical feature in each group, we can define cluster C1 by the presence of lymphopenia, cluster C2 by elevated ferritin, and C3 by elevated LDH. Cluster C4 is characterized by lymphopenia plus elevated CRP and LDH and often also elevated ferritin. Cluster C5 is defined by elevated CRP, and cluster C6 by elevated ferritin and D-dimer and often also elevated CRP and LDH. Finally, group C7 is characterized by elevated D-dimer. Figure 2 shows the distribution of each of the inflammatory parameters in each cluster.
Fig. 2.
Distribution of the parameters of inflammation between clusters. Mann–Whitney U test (p < 0.001 for all parameters). Each box defines the median and the 25th and 75th percentile. The whiskers define the dispersion, the circles the outliers and the asterisks the extreme values
It should be noted that cluster C4 is the cluster with the lowest PaO2/FiO2 on admission. This fact possibly shows a greater inflammatory component and greater evolution to ARDS.
Treatments during admission
Table S3 shows the treatments received during admission. Logically, the clusters with a greater analytical inflammatory component (C4) received corticosteroids and tocilizumab more frequently. On the other hand, clusters with higher D-dimer elevation (C6 and C7) more frequently received high doses of LMWH.
Outcomes between groups
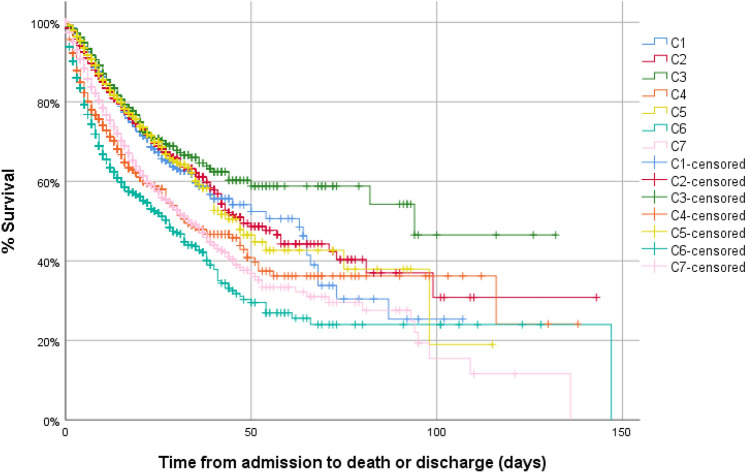
The clusters with the highest in-hospital mortality were C4, C6, and C7 (36.8% vs. 39.3% vs. 26.4%) (Table 2; Fig. 3). It is also noteworthy that the C4 cluster with a higher analytical inflammatory component is the group with the highest requirements for HFNC, NIMV, IMV, and ICU admission. On the other hand, the C6 and C7 clusters with greater prominence of D-dimer have high mortality but not so high requirements for HFNC, NIMV, IMV, and ICU admission.
Table 2.
Outcomes between clusters of inflammation
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | p value | |
|---|---|---|---|---|---|---|---|---|
| Primary outcome n (%) | ||||||||
| In-hospital mortality | 408 (17.4) | 644 (18) | 267 (15.6) | 373 (36.8) | 336 (17.5) | 628 (39.3) | 932 (26.4) | < 0.001 |
| Secondary outcomes n (%) | ||||||||
| HFNC | 242 (10.3) | 386 (10.8) | 142 (8.3) | 212 (20.9) | 230 (12) | 205 (12.8) | 292 (8.3) | < 0.001 |
| NIMV | 165 (7) | 252 (7.1) | 93 (5.4) | 138 (13.6) | 118 (6.1) | 125 (7.8) | 181 (5.1) | < 0.001 |
| IMV | 142 (6) | 344 (9.6) | 136 (7.9) | 203 (20) | 174 (9.1) | 190 (11.9) | 215 (6.1) | < 0.001 |
| ICU admission | 213 (9.1) | 429 (12) | 172 (10) | 249 (24.6) | 229 (11.9) | 222 (13.9) | 277 (7.9) | < 0.001 |
HFNC High-flow nasal cannula, NIMV non-invasive mechanical ventilation, IMV invasive mechanical ventilation, ICU intensive care unit
Fig. 3.
Kaplan–Meier curves of in-hospital mortality between clusters of inflammation. C1: 17.4%, C2: 18%, C3: 15.6%, C4: 36.8%, C5: 17.5%, C6: 39.3%, C7: 26.4%; p < 0.001
Risk factors for in-hospital mortality
Table S4 shows the risk factors for in-hospital mortality. These included age (OR = 1.07, p < 0.001), female sex (OR = 0.71, p < 0.001), Black race (OR = 0.19, p = 0.032), higher BMI (OR = 1.02, p < 0.001), time from disease onset to admission (OR = 0.96, p < 0.001), higher degree of dependency (OR = 1.39, p < 0.001 for moderate and OR = 1.66, p < 0.001 for severe), ischemic heart disease (OR = 1.32, p < 0.001), higher Charlson index (OR = 1.14, p < 0.001), lower PaO2/FiO2 (OR = 0.99, p < 0.001), tachypnea on admission (OR = 2.72, p < 0.001), and inflammation clusters. In detail and, having cluster C1 as reference, the model revealed a worse prognosis for all other clusters: C2 (OR = 1.30, p = 0.001), C3 (OR = 1.14, p = 0.178), C4 (OR = 2.28, p < 0.001), C5 (OR = 1.07, p = 0.479), C6 (OR = 2.29, p < 0.001), and C7 (OR = 1.28, p = 0.001). The drugs received during admission (steroids, remdesivir, and tocilizumab) were included in the uni- and multivariate models due to their clinical importance and statistical influence on the rest of the variables but, despite reaching statistical significance in the multivariate model, their results must be interpreted with caution since this is not the type of study to assess their efficacy. Asthma was found to be a protective factor for in-hospital death.
Discussion
In the present study, we demonstrate the presence of 7 clearly differentiated inflammation clusters. It is clear that COVID-19 elicits an exaggerated inflammatory response but it is also clear that patients do not all become inflamed in the same way. Recently, our group had highlighted risk categories based on the degree of analytical inflammation in patients with COVID-19 at the time of hospital admission [4]. We know from that research the additive importance of such analytical inflammatory criteria. We now also know from the present study that it is important not only how many criteria the patient meets but also which ones. And this differentiation in the type of inflammation can be predicted at the time of admission. Afterward, the cascade of inflammation will continue, but the onset of inflammation already translates into differential characteristics and a very important predictive power when it comes to directing our strategies as clinicians.
The fact that the clusters with the worst prognosis C4, C6, and C7 are also those that occur in the older population suggests that age alone is not only a risk factor, but also that this is a population that is more inflamed. Whether this is due to a failure in the autoregulation of inflammatory mechanisms inherent to age should be answered with further studies. They are also clusters with higher associated comorbidity (especially C4) and one would think that those associated diseases provide an inflammatory basis that could influence. However, the C5 cluster also has considerably associated comorbidity and yet does not have as much inflammation at entry.
Inflammation clusters add to the list of factors associated with increased in-hospital mortality in COVID-19 [13–15]. All the clusters have a worse prognosis compared to cluster C1, which we could consider the reference cluster. However, we should focus our efforts on detecting the C4, C6, and C7 clusters first, as they are really the ones with the worst prognosis. The C4 cluster presents a greater inflammatory component in the form of lymphopenia, elevated CRP and LDH, and very frequently, also elevated ferritin. It is a cluster that will evolve to acute respiratory distress syndrome (ARDS) and will require more HFNC, NIMV, IMV, and ICU admission. On the contrary, cluster C7 is a cluster with a prominence of D-dimer. It also has high mortality but clearly requires less HFNC, NIMV, IMV, and ICU admission. This could translate into higher mortality from thromboembolic disease and less progression to ARDS. Finally, cluster C6 is a mixture of clusters C4 and C7, i.e., a cluster with a prominence of D-dimer but also of ferritin and, very frequently, CRP, LDH, and lymphopenia.
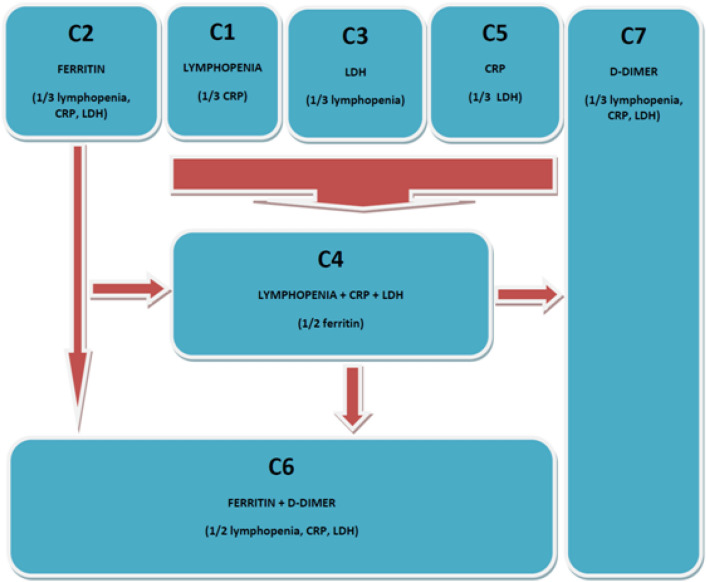
Whether these 7 clusters respond to different pathways of inflammation and perhaps respond to genetic differences that condition a different host response to the same external aggression of SARS-CoV-2 is something that we cannot answer with the present study. As we have discussed previously, the inflammation cascade in patients with COVID-19 does not occur equally in all patients. In Fig. 4, we propose a logical sequence in the inflammation of these patients. It is based on the analytical characteristics of each of them and the additive possibilities. It also has a pathophysiological logic behind it as the initiation of the inflammatory cascade in COVID-19 is defined by macrophage activation. This includes elevation of LDH, IL6, CRP, ferritin, and cytopenias. Why some patients elevate some parameters earlier and other patients elevate others is unknown. After this first step, vascular endothelial damage occurs and there appears the elevation of D-dimer.
Fig. 4.
Proposed algorithm for analytic inflammatory pathways in patients with COVID-19. It is based on the analytical characteristics of each of them and the additive possibilities. The initiation of the inflammatory cascade in COVID-19 is defined by macrophage activation. This includes elevation of LDH, IL6, CRP, ferritin, and cytopenias. After this first step, vascular endothelial damage occurs and there appears the elevation of D-dimer. Cluster C7 suggests that there is a group of patients who present endothelial damage with subsequent micro- or macrothrombosis without going through a previous step of major inflammation
From such a model, there are several comments to consider. First, there are 3 clusters that should sound the alarm when detected, such as clusters C4, C6, and C7. They translate more advanced stages of the disease and early action should be taken to block such escalation. On the other hand, cluster C2 does not represent such an advanced stage of inflammation but possibly the transition to cluster C6 is just around the corner in these patients (compared to clusters C1, C3, and C5 where we would have more room for maneuver). Thus, ferritin and D-dimer should be the key indicators of increased risk in patients hospitalized for COVID-19. Finally, cluster C7 suggests that there is a group of patients who present endothelial damage with subsequent micro- or macrothrombosis without going through a previous step of major inflammation. This is a puzzling fact that should make us rethink the doses of LMWH in patients with little inflammation.
Of all the treatments tested in COVID-19, it is the immunosuppressants/immunomodulators (corticosteroids and tocilizumab) that have demonstrated the greatest effectiveness to date [16–25]. They are especially indicated in those patients with analytical parameters of inflammation such as the patients included in the present study. However, it is plausible to think that their effectiveness is not the same in all inflammation clusters. We believe that perhaps in C4 (with a greater inflammatory component) and even C6 they could clearly demonstrate their benefit but perhaps in C7, with a greater role of D-dimer, they might not be so useful.
In conclusion, the present study identifies 7 inflammation groups based on the presence of lymphopenia, elevated CRP, LDH, ferritin, and D-dimer at the time of hospital admission for COVID-19. Clusters C4 (lymphopenia + LDH + CRP), C6 (ferritin + D-dimer), and C7 (D-dimer) were the worst prognostic clusters in terms of in-hospital mortality.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We gratefully acknowledge all the investigators who participate in the SEMI-COVID-19 Registry. We also thank the SEMI-COVID-19 Registry Coordinating Center for their quality control data, logistic and administrative support.
Appendix
List of the SEMI-COVID-19 Network members
Coordinator of the SEMI-COVID-19 Registry: José Manuel Casas Rojo.
SEMI-COVID-19 Scientific Committee Members: José Manuel Casas Rojo, José Manuel Ramos Rincón, Carlos Lumbreras Bermejo, Jesús Millán Núñez-Cortés, Juan Miguel Antón Santos, Ricardo Gómez Huelgas.
Members of the SEMI-COVID-19 Group
H. Univ. de Bellvitge. L'Hospitalet de Llobregat (Barcelona)
Xavier Corbella, Narcís A. Homs, Abelardo Montero, Jose María Mora-Luján, Francesc Formiga, Joan Albà-Albalate, Manuel Rubio-Rivas.
H. U. 12 de Octubre. Madrid
Paloma Agudo de Blas, Coral Arévalo Cañas, Blanca Ayuso, José Bascuñana Morejón, Samara Campos Escudero, María Carnevali Frías, Santiago Cossio Tejido, Borja de Miguel Campo, Carmen Díaz Pedroche, Raquel Diaz Simon, Ana García Reyne, Laura Ibarra Veganzones, Lucia Jorge Huerta, Antonio Lalueza Blanco, Jaime Laureiro Gonzalo, Jaime Lora-Tamayo, Carlos Lumbreras Bermejo, Guillermo Maestro de la Calle, Rodrigo Miranda Godoy, Barbara Otero Perpiña, Diana Paredes Ruiz, Marcos Sánchez Fernández, Javier Tejada Montes.
H. Costa del Sol. Marbella (Málaga)
Victoria Augustín Bandera, Javier García Alegría, Nicolás Jiménez-García, Jairo Luque del Pino, María Dolores Martín Escalante, Francisco Navarro Romero, Victoria Nuñez Rodriguez, Julián Olalla Sierra.
H. U. Gregorio Marañon. Madrid
Laura Abarca Casas, Álvaro Alejandre de Oña, Rubén Alonso Beato, Leyre Alonso Gonzalo, Jaime Alonso Muñoz, Crhistian Mario Amodeo Oblitas, Cristina Ausín García, Marta Bacete Cebrián, Jesús Baltasar Corral, Maria Barrientos Guerrero, Alejandro D. Bendala Estrada, María Calderón Moreno, Paula Carrascosa Fernández, Raquel Carrillo, Sabela Castañeda Pérez, Eva Cervilla Muñoz, Agustín Diego Chacón Moreno, Maria Carmen Cuenca Carvajal, Sergio de Santos, Andrés Enríquez Gómez, Eduardo Fernández Carracedo, María Mercedes Ferreiro-Mazón Jenaro, Francisco Galeano Valle, Alejandra Garcia, Irene Garcia Fernandez-Bravo, María Eugenia García Leoni, María Gómez Antúnez, Candela González San Narciso, Anthony Alexander Gurjian, Lorena Jiménez Ibáñez, Cristina Lavilla Olleros, Cristina Llamazares Mendo, Sara Luis García, Víctor Mato Jimeno, Clara Millán Nohales, Jesús Millán Núñez-Cortés, Sergio Moragón Ledesma, Antonio Muiño Míguez, Cecilia Muñoz Delgado, Lucía Ordieres Ortega, Susana Pardo Sánchez, Alejandro Parra Virto, María Teresa Pérez Sanz, Blanca Pinilla Llorente, Sandra Piqueras Ruiz, Guillermo Soria Fernández-Llamazares, María Toledano Macías, Neera Toledo Samaniego, Ana Torres do Rego, Maria Victoria Villalba Garcia, Gracia Villarreal, María Zurita Etayo.
H. de Cabueñes. Gijón (Asturias)
Ana María Álvarez Suárez, Carlos Delgado Vergés, Rosa Fernandez-Madera Martínez, Eva Mª Fonseca Aizpuru, Alejandro Gómez Carrasco, Cristina Helguera Amezua, Juan Francisco López Caleya, Diego López Martínez, María del Mar Martínez López, Aleida Martínez Zapico, Carmen Olabuenaga Iscar, Lucía Pérez Casado, María Luisa Taboada Martínez, Lara María Tamargo Chamorro.
H. Reg. Univ. de Málaga
Mª Mar Ayala-Gutiérrez, Rosa Bernal López, José Bueno Fonseca, Verónica Andrea Buonaiuto, Luis Francisco Caballero Martínez, Lidia Cobos Palacios, Clara Costo Muriel, Francis de Windt, Ana Teresa Fernandez-Truchaud Christophel, Paula García Ocaña, Ricardo Gómez Huelgas, Javier Gorospe García, José Antonio Hurtado Oliver, Sergio Jansen-Chaparro, Maria Dolores López-Carmona, Pablo López Quirantes, Almudena López Sampalo, Elizabeth Lorenzo-Hernández, Juan José Mancebo Sevilla, Jesica Martín Carmona, Luis Miguel Pérez-Belmonte, Iván Pérez de Pedro, Araceli Pineda-Cantero, Carlos Romero Gómez, Michele Ricci, Jaime Sanz Cánovas.
H. U. La Paz. Madrid
Jorge Álvarez Troncoso, Francisco Arnalich Fernández, Francisco Blanco Quintana, Carmen Busca Arenzana, Sergio Carrasco Molina, Aranzazu Castellano Candalija, Germán Daroca Bengoa, Alejandro de Gea Grela, Alicia de Lorenzo Hernández, Alejandro Díez Vidal, Carmen Fernández Capitán, Maria Francisca García Iglesias, Borja González Muñoz, Carmen Rosario Herrero Gil, Juan María Herrero Martínez, Víctor Hontañón, Maria Jesús Jaras Hernández, Carlos Lahoz, Cristina Marcelo Calvo, Juan Carlos Martín Gutiérrez, Monica Martinez Prieto, Elena Martínez Robles, Araceli Menéndez Saldaña, Alberto Moreno Fernández, Jose Maria Mostaza Prieto, Ana Noblejas Mozo, Carlos Manuel Oñoro López, Esmeralda Palmier Peláez, Marina Palomar Pampyn, Maria Angustias Quesada Simón, Juan Carlos Ramos Ramos, Luis Ramos Ruperto, Aquilino Sánchez Purificación, Teresa Sancho Bueso, Raquel Sorriguieta Torre, Clara Itziar Soto Abanedes, Yeray Untoria Tabares, Marta Varas Mayoral, Julia Vásquez Manau.
H. Royo Villanova. Zaragoza
Nicolás Alcalá Rivera, Anxela Crestelo Vieitez, Esther del Corral Beamonte, Jesús Díez Manglano, Isabel Fiteni Mera, Maria del Mar Garcia Andreu, Martin Gericó Aseguinolaza, Cristina Gallego Lezaun, Claudia Josa Laorden, Raul Martínez Murgui, Marta Teresa Matía Sanz.
H. Clínico de Santiago de Compostela (A Coruña)
Maria del Carmen Beceiro Abad, Maria Aurora Freire Romero, Sonia Molinos Castro, Emilio Manuel Paez Guillan, María Pazo Nuñez, Paula Maria Pesqueira Fontan.
H. Clínico San Carlos. Madrid
Inés Armenteros Yeguas, Javier Azaña Gómez, Julia Barrado Cuchillo, Irene Burruezo López, Noemí Cabello Clotet, Alberto E. Calvo Elías, Elpidio Calvo Manuel, Carmen María Cano de Luque, Cynthia Chocron Benbunan, Laura Dans Vilan, Claudia Dorta Hernández, Ester Emilia Dubon Peralta, Vicente Estrada Pérez, Santiago Fernandez-Castelao, Marcos Oliver Fragiel Saavedra, José Luis García Klepzig, Maria del Rosario Iguarán Bermúdez, Esther Jaén Ferrer, Alejandro Maceín Rodríguez, Alejandro Marcelles de Pedro, Rubén Ángel Martín Sánchez, Manuel Méndez Bailón, Sara Miguel Álvarez, Maria José Nuñez Orantos, Carolina Olmos Mata, Eva Orviz García, David Oteo Mata, Cristina Outon González, Juncal Perez-Somarriba, Pablo Pérez Mateos, Maria Esther Ramos Muñoz, Xabier Rivas Regaira, Laura Mª Rodríguez Gallardo, Iñigo Sagastagoitia Fornie, Alejandro Salinas Botrán, Miguel Suárez Robles, Maddalena Elena Urbano, Andrea María Vellisca González, Miguel Villar Martínez.
H. Universitario Dr. Peset. Valencia
Juan Alberto Aguilera Ayllón, Arturo Artero, María del Mar Carmona Martín, María José Fabiá Valls, Maria de Mar Fernández Garcés, Ana Belén Gómez Belda, Ian López Cruz, Manuel Madrazo López, Elisabeth Mateo Sanchis, Jaume Micó Gandia, Laura Piles Roger, Adela Maria Pina Belmonte, Alba Viana García.
H. U. Puerta de Hierro. Madrid
María Álvarez Bello, Ane Andrés Eisenhofer, Ana Arias Milla, Isolina Baños Pérez, Laura Benítez Gutiérrez, Javier Bilbao Garay, Silvia Blanco Alonso, Jorge Calderón Parra, Alejandro Callejas Díaz, José María Camino Salvador, Mª Cruz Carreño Hernández, Valentín Cuervas-Mons Martínez, Sara de la Fuente Moral, Miguel del Pino Jimenez, Alberto Díaz de Santiago, Itziar Diego Yagüe, Ignacio Donate Velasco, Ana María Duca, Pedro Durán del Campo, Gabriela Escudero López, Esther Expósito Palomo, Ana Fernández Cruz, Esther Fiz Benito, Andrea Fraile López, Amy Galán Gómez, Sonia García Prieto, Claudia García Rodríguez-Maimón, Miguel Ángel García Viejo, Javier Gómez Irusta, Edith Vanessa Gutiérrez Abreu, Isabel Gutiérrez Martín, Ángela Gutiérrez Rojas, Andrea Gutiérrez Villanueva, Jesús Herráiz Jiménez, Pedro Laguna del Estal, Mª Carmen Máinez Sáiz, Cristina Martín Martín, María Martínez Urbistondo, Fernando Martínez Vera, Susana Mellor Pita, Patricia Mills Sánchez, Esther Montero Hernández, Alberto Mora Vargas, Cristina Moreno López, Alfonso Ángel-Moreno Maroto, Victor Moreno-Torres, Concha, Ignacio Morrás De La Torre, Elena Múñez Rubio, Rosa Muñoz de Benito, Ana Muñoz Gómez, Alejandro Muñoz Serrano, Jose María Palau Fayós, Lina Marcela Parra Ramírez, Ilduara Pintos Pascual, Arturo José Ramos Martín-Vegue, Antonio Ramos Martínez, Isabel Redondo Cánovas del Castillo, Alberto Roldán Montaud, Lucía Romero Imaz, Yolanda Romero Pizarro, Enrique Sánchez Chica, David Sánchez Órtiz, Mónica Sánchez Santiuste, Patricia Serrano de la Fuente, Pablo Tutor de Ureta, Ángela Valencia Alijo, Mercedes Valentín-Pastrana Aguilar, Juan Antonio Vargas Núñez, Jose Manuel Vázquez Comendador, Gema Vázquez Contreras, Carmen Vizoso Gálvez.
H. U. Reina Sofía. Córdoba
Antonio Pablo Arenas de Larriva, Pilar Calero Espinal, Javier Delgado Lista, Francisco Fuentes-Jiménez, María del Carmen Guerrero Martínez, María Jesús Gómez Vázquez, Jose Jiménez Torres, Laura Limia Pérez, José López-Miranda, Laura Martín Piedra, Marta Millán Orge, Javier Pascual Vinagre, Pablo Pérez-Martinez, María Elena Revelles Vílchez, Angela Rodrigo Martínez, Juan Luis Romero Cabrera, José David Torres-Peña.
C. H. U. de Badajoz
Rafael Aragon Lara, Inmaculada Cimadevilla Fernandez, Juan Carlos Cira García, Gema Maria García García, Julia Gonzalez Granados, Beatriz Guerrero Sánchez, Francisco Javier Monreal Periáñez, Maria Josefa Pascual Perez.
H. Moisès Broggi. Sant Joan Despí (Barcelona)
Judit Aranda Lobo, Lucía Feria Casanovas, Jose Loureiro Amigo, Miguel Martín Fernández, Isabel Oriol Bermúdez, Melani Pestaña Fernández, Nicolas Rhyman, Nuria Vázquez Piqueras.
H. U. Río Hortega. Valladolid
Irene Arroyo Jiménez, Marina Cazorla González, Marta Cobos-Siles, Luis Corral-Gudino, Pablo Cubero-Morais, María González Fernández, José Pablo Miramontes González, Marina Prieto Dehesa, Pablo Sanz Espinosa.
H. U. S. Juan de Alicante (Alicante)
Marisa Asensio Tomás, David Balaz, David Bonet Tur, Ruth Cañizares Navarro, Paloma Chazarra Pérez, Jesús Corbacho Redondo, Eliana Damonte White, María Escamilla Espínola, Leticia Espinosa Del Barrio, Pedro Jesús Esteve Atiénzar, Carles García Cervera, David Francisco García Núñez, Francisco Garrido Navarro, Vicente Giner Galvañ, Angie Gómez Uranga, Javier Guzmán Martínez, Isidro Hernández Isasi, Lourdes Lajara Villar, Verónica Martínez Sempere, Juan Manuel Núñez Cruz, Sergio Palacios Fernández, Juan Jorge Peris García, Rafael Piñol Pleguezuelos, Andrea Riaño Pérez, José Miguel Seguí Ripoll, Azucena Sempere Mira, Philip Wikman-Jorgensen.
H. Nuestra Señora del Prado. Talavera de la Reina (Toledo)
Sonia Casallo Blanco, Jeffrey Oskar Magallanes Gamboa, Cristina Salazar Mosteiro, Andrea Silva Asiain.
H. de Pozoblanco (Córdoba)
José Nicolás Alcalá Pedrajas, Antonia Márquez García, Inés Vargas.
H. G. U. de Elda (Alicante)
Carmen Cortés Saavedra, Jennifer Fernández Gómez, Borja González López, María Soledad Hernández Garrido, Ana Isabel López Amorós, Santiago López Gil, Maria de los Reyes Pascual Pérez, Nuria Ramírez Perea, Andrea Torregrosa García.
H. U. Infanta Cristina. Parla (Madrid)
Juan Miguel Antón Santos, Ana Belén Barbero Barrera, Blanca Beamonte Vela, Coralia Bueno Muiño, Charo Burón Fernández, Ruth Calderón Hernáiz, Irene Casado López, José Manuel Casas Rojo, Andrés Cortés Troncoso, Pilar Cubo Romano, Francesco Deodati, Alejandro Estrada Santiago, Gonzalo García Casasola Sánchez, Elena García Guijarro, Francisco Javier García Sánchez, Pilar García de la Torre, Mayte de Guzmán García-Monge, Davide Luordo, María Mateos González, José A. Melero Bermejo, Cruz Pastor Valverde, José Luis Pérez Quero, Fernando Roque Rojas, Lorea Roteta García, Elena Sierra Gonzalo, Francisco Javier Teigell Muñoz, Juan Vicente de la Sota, Javier Villanueva Martínez.
H. Santa Marina. Bilbao
María Areses Manrique, Ainara Coduras Erdozain, Ane Labirua-Iturburu Ruiz.
H. San Pedro. Logroño (La Rioja)
Diana Alegre González, Irene Ariño Pérez de Zabalza, Sergio Arnedo Hernández, Jorge Collado Sáenz, Beatriz Dendariena, Marta Gómez del Mazo, Iratxe Martínez de Narvajas Urra, Sara Martínez Hernández, Estela Menendez Fernández, Jose Luís Peña Somovilla, Elisa Rabadán Pejenaute.
H. U. Son Llàtzer. Palma de Mallorca
Andrés de la Peña Fernández, Almudena Hernández Milián.
C. H. U. Ourense
Raquel Fernández González, Amara Gonzalez Noya, Carlos Hernández Ceron, Isabel Izuzquiza Avanzini, Ana Latorre Diez, Pablo López Mato, Ana María Lorenzo Vizcaya, Daniel Peña Benítez, Milagros María Peña Zemsch, Lucía Pérez Expósito, Marta Pose Bar, Lara Rey González, Laura Rodrigo Lara.
H. U. La Fe. Valencia
Dafne Cabañero, María Calabuig Ballester, Pascual Císcar Fernández, Ricardo Gil Sánchez, Marta Jiménez Escrig, Cristina Marín Amela, Laura Parra Gómez, Carlos Puig Navarro, José Antonio Todolí Parra.
H. de Mataró. Barcelona
Raquel Aranega González, Ramon Boixeda, Javier Fernández Fernández, Carlos Lopera Mármol, Marta Parra Navarro, Ainhoa Rex Guzmán, Aleix Serrallonga Fustier.
H. de Sagunto (Valencia)
Enrique Rodilla Sala, Jose María Pascual Izuel, Zineb Karroud Zamrani.
H. Alto Guadalquivir. Andújar (Jaén)
Begoña Cortés Rodríguez.
C. H. U. de Ferrol (A Coruña)
Hortensia Alvarez Diaz, Tamara Dalama Lopez, Estefania Martul Pego, Carmen Mella Pérez, Ana Pazos Ferro, Sabela Sánchez Trigo, Dolores Suarez Sambade, Maria Trigas Ferrin, Maria del Carmen Vázquez Friol, Laura Vilariño Maneiro.
H. Infanta Margarita. Cabra (Córdoba)
María Esther Guisado Espartero, Lorena Montero Rivas, Maria de la Sierra Navas Alcántara, Raimundo Tirado-Miranda.
H. Público de Monforte de Lemos (Lugo)
José López Castro, Manuel Lorenzo López Reboiro, Cristina Sardiña González.
H. U. Virgen del Rocío. Sevilla
Reyes Aparicio Santos, Máximo Bernabeu-Wittel, Santiago Rodríguez Suárez, María Nieto, Luis Giménez Miranda, Rosa María Gámez Mancera, Fátima Espinosa Torre, Carlos Hernandez Quiles, Concepción Conde Guzmán, Juan Delgado de la Cuesta, Jara Eloisa Ternero Vega, María del Carmen López Ríos, Pablo Díaz Jiménez, Bosco Baron Franco, Carlos Jiménez de Juan, Sonia Gutiérrez Rivero, Julia Lanseros Tenllado, Verónica Alfaro Lara, Aurora González Estrada.
H. Marina Baixa. Villajoyosa (Alicante)
Javier Ena, José Enrique Gómez Segado.
C. A. U. de Salamanca
Gloria María Alonso Claudio, Víctor Barreales Rodríguez, Cristina Carbonell Muñoz, Adela Carpio Pérez, María Victoria Coral Orbes, Daniel Encinas Sánchez, Sandra Inés Revuelta, Miguel Marcos Martín, José Ignacio Martín González, José Ángel Martín Oterino, Leticia Moralejo Alonso, Sonia Peña Balbuena, María Luisa Pérez García, Ana Ramon Prados, Beatriz Rodríguez-Alonso, Ángela Romero Alegría, Maria Sanchez Ledesma, Rosa Juana Tejera Pérez.
H. General Defensa. Zaragoza
Anyuli Gracia Gutiérrez, Leticia Esther Royo Trallero.
H. de Palamós (Girona)
Ana Alberich Conesa, Mari Cruz Almendros Rivas, Miquel Hortos Alsina, José Marchena Romero, Anabel Martin-Urda Diez-Canseco.
H. Comarcal de Blanes (Girona)
Oriol Alonso Gisbert, Mercé Blázquez Llistosella, Pere Comas Casanova, Angels Garcia Flores, Anna Garcia Hinojo, Ana Inés Méndez Martínez, Maria del Carmen Nogales Nieves, Agnés Rivera Austrui, Alberto Zamora Cervantes.
H. do Salnes. Vilagarcía de Arousa (Pontevedra)
Vanesa Alende Castro, Ana María Baz Lomba, Ruth Brea Aparicio, Marta Fernández Morales, Jesús Manuel Fernández Villar, María Teresa López Monteagudo, Cristina Pérez García, Lorena Rodríguez Ferreira, Diana Sande Llovo, Maria Begoña Valle Feijoo.
H. U. HM Montepríncipe
José F. Varona Arche.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare they have no conflict of interest.
Statement of human and animal rights
The study has been approved by the ethics committee of Bellvitge University Hospital.
Informed consent
Informed consent was obtained from the subjects.
Authorship
All authors had access to the data and a role in writing this manuscript.
Footnotes
A complete list of the SEMI-COVID-19 Network members is provided in the Appendix.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Manuel Rubio-Rivas, Email: mrubio@bellvitgehospital.cat.
José María Mora-Luján, Email: jmora@bellvitgehospital.cat.
Francesc Formiga, Email: fformiga@bellvitgehospital.cat.
Miguel Ángel Corrales González, Email: Dr.corralesg@gmail.com.
María del Mar García Andreu, Email: mariadelmargarciaandreu@gmail.com.
Víctor Moreno-Torres, Email: victor.moreno.torres.1988@gmail.com.
Gema María García García, Email: geminway21@hotmail.com.
José N Alcalá Pedrajas, Email: Jnalcala58@hotmail.com.
Ramon Boixeda, Email: Rboixeda@csdm.cat.
Leticia Pérez-Lluna, Email: doctoraperezlluna@gmail.com.
Begoña Cortés-Rodríguez, Email: begocortesrod@gmail.com.
Carmen Mella-Pérez, Email: mellacarmen@gmail.com.
María de la Sierra Navas Alcántara, Email: maria.sierra.navas@gmail.com.
Manuel Lorenzo López Reboiro, Email: manuel.lorenzo.lopez.reboiro@sergas.es.
Verónica Alfaro-Lara, Email: valfarolara@hotmail.com.
Santiago Pérez-Martín, Email: santino_perez@hotmail.com.
José Ángel Martín-Oterino, Email: jmoterino@saludcastillayleon.es.
Anyuli Gracia Gutiérrez, Email: agraciagut@gmail.com.
Anabel Martín-Urda Díez-Canseco, Email: anabelmartinurda10canseco@gmail.com.
Pere Comas Casanova, Email: 23575pcc@comb.cat.
Cristina Pérez García, Email: saraaloe@hotmail.com.
José F Varona, Email: jfvarona@hmhospitales.com.
Ricardo Gómez-Huelgas, Email: ricardogomezhuelgas@hotmail.com.
Juan-Miguel Antón-Santos, Email: jmanton.hugf@salud.madrid.org.
Carlos Lumbreras-Bermejo, Email: clumbrerasb@gmail.com.
References
- 1.https://coronavirus.jhu.edu/map.html
- 2.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong LZ, Shou ZX, Zheng DM, Jin X. The most important biomarker associated with coagulation and inflammation among COVID-19 patients. Mol Cell Biochem. 2021;476:1–9. doi: 10.1007/s11010-021-04122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubio-Rivas M, Corbella X, Formiga F, et al. Risk categories in COVID-19 based on degrees of inflammation. Data on more than 17,000 patients from the Spanish SEMI-COVID-19 registry. J Clin Med. 2021;10:2214. doi: 10.3390/jcm10102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casas-Rojo JM, Antón-Santos JM, Millán-Núñez-Cortés J, et al. Clinical characteristics of patients hospitalized with COVID-19 in Spain: results from the SEMI-COVID-19 Registry. Rev Clin Esp. 2020;220:480–494. doi: 10.1016/j.rce.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gautret P, Cagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capra R, De Rossi N, Mattioli F, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. 2020;76:31–35. doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campochiaro C, Della-Torre E, Cavalli G, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.A Recovery Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2020;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husson F, Josse J, Pagès J (2010) Principal component methods – hierarchical clustering – partitional clustering: why would we need to choose for visualizing data? Technical Report. http://math.agrocampus-ouest.fr/infoglueDeliverLive
- 13.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients 434 with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodilla E, López-Carmona MD, Cortes X, et al. Impact of arterial stiffness on all-cause mortality in patients hospitalized with COVID-19 in Spain. Hypertension. 2021;77:856–867. doi: 10.1161/HYPERTENSIONAHA.120.16563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubio-Rivas M, Corbella X, Mora-Luján JM, et al. Predicting clinical outcome with phenotypic clusters in COVID-19 pneumonia: an analysis of 12,066 hospitalized patients from the Spanish registry SEMI-COVID-19. J Clin Med. 2020;9:3488. doi: 10.3390/jcm9113488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubio-Rivas M, Ronda M, Padulles A, et al. Beneficial effect of corticosteroids in preventing mortality in patients receiving tocilizumab to treat severe COVID-19 illness. Int J Infect Dis. 2020;101:290–297. doi: 10.1016/j.ijid.2020.09.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon AC, Mouncey PR, Al-Beidh F, REMAP-CAP Investigators et al. Interleukin-6 receptor antagonists in critically Ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tleyjeh IM, Kashour Z, Riaz M, Hassett L, Veiga VC, Kashour T. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis-first update. Clin Microbiol Infect. 2021;27:1076. doi: 10.1016/j.cmi.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubio-Rivas M, Mora-Luján JM, Montero A, et al. The use of corticosteroids or tocilizumab in COVID-19 based on inflammatory markers. J Gen Intern Med. 2021;18:1–8. doi: 10.1007/s11606-021-07146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muñoz-Gómez A, Fernández-Cruz A, Lavilla-Olleros C, et al. Real-life impact of glucocorticoid treatment in COVID-19 mortality: a multicenter retrospective study. J Clin Med. 2021;10(20):4678. doi: 10.3390/jcm10204678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balaz D, Wikman-Jorgensen PE, Galvañ VG, et al. Evolution of the use of corticosteroids for the treatment of hospitalised COVID-19 patients in spain between march and november 2020: SEMI-COVID National Registry. J Clin Med. 2021;10(19):4610. doi: 10.3390/jcm10194610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mora-Luján JM, Tuells M, Montero A, et al. High-dose methylprednisolone pulses for 3 days vs. low-dose dexamethasone for 10 days in severe, non-critical COVID-19: a retrospective propensity score matched analysis. J Clin Med. 2021;10(19):4465. doi: 10.3390/jcm10194465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubio-Rivas M, Forero CG, Mora-Luján JM, et al. Beneficial and harmful outcomes of tocilizumab in severe COVID-19: a systematic review and meta-analysis. Pharmacotherapy. 2021;41(11):884–906. doi: 10.1002/phar.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.