Abstract
Background
Six million Americans suffer from atrial fibrillation (AF), a heart rhythm abnormality that significantly increases the risk of stroke. AF is responsible for 15% of ischemic strokes, which lead to permanent disability in 60% of cases and death in up to 20%. Anticoagulation (AC) is the mainstay for stroke prevention in patients with AF. Despite guidelines recommending AC for patients, up to half of eligible patients are not on AC. Clinical decision support tools in the electronic health record (EHR) can help bridge the disparity in AC prescription for patients with AF.
Objective
To enhance and assess the effectiveness of our previous rule-based alert on AC initiation and persistence in a diverse patient population from UMass-Memorial Medical Center and University of Florida at Jacksonville.
Methods/Results
Using the EHR, we will track AC initiation and persistence. We will interview both patients and providers to determine a measure of satisfaction with AC management. We will track digital crumbs to better understand the alert’s mechanism of effect and further add enhancements. These enhancements will be used to refine the alert and aid in developing an implementation toolkit to facilitate use of the alert at other health systems.
Conclusion
If the number of AC starts, the likelihood of persisting on AC, and the frequency alert use are found to be higher among intervention vs control providers, we believe such findings will confirm our hypothesis on the effectiveness of our alert.
Keywords: Anticoagulation, Atrial fibrillation, Clinical decision support, Clinical trials, Electronic medical records
Key Findings.
-
•
Anticoagulation (AC) is the mainstay for stroke prevention in patients with atrial fibrillation (AF), but there is a significant gap in AC use in eligible patients.
-
•
Many barriers prevent AC initiation and persistence. Clinical decision support (CDS) tools can help improve AC initiation and persistence.
-
•
We describe our protocol for a prospective randomized controlled trial for the assessment and evaluation of an enhanced CDS tool—a rule-based alert—that will support providers in making decisions about AC. The tool includes a prescription order set and educational resources for both providers and patients.
Background
In the United States, 6 million people suffer from atrial fibrillation (AF), with 12 million projected to have AF by 2050.1, 2, 3 AF causes 15% of ischemic strokes, which result in permanent disability in 60% of cases and death in up to 20%.4 Anticoagulation (AC) is the gold standard treatment for stroke prevention. However, only 60% of eligible patients are on AC despite professional society guidelines recommending AC for patients.5 This low adherence to recommendations results from both lack of AC initiation when indicated and premature discontinuation of AC. This disparity is more prominent in minority populations, in which AC use is lower and stroke rates are higher.6,7 Beliefs about AC effectiveness, health literacy, lack of trust in physicians and AC, and inaccurate risk perception of AF contribute to the diminished use in minority populations.8, 9, 10, 11
Several barriers prevent initiation and persistence of AC. A common barrier is patient refusal.12 Many providers report a lack of comfort with the newer direct oral anticoagulants (DOACs). AC often is prematurely discontinued after isolated setbacks such as falls or isolated bleeding episodes despite expert guidelines indicating that these events are not absolute contraindications, compared to active interlobar bleeding, which is an absolute contradiction.13,14
A clinical decision support (CDS) tool can help surmount these barriers and therefore would be beneficial in reducing the known disparities in AC use and outcomes and improve AC initiation and persistence.15, 16, 17 In our previous work, we explored the role of provider e-mail messaging, in-basket messaging, and educational outreach and its effects on rates of AC prescription. We found that it was possible to reach providers and saw an improvement in patient comfort with AC prescription, but no significant increase in AC prescription. The data gathered from exit interviews with providers demonstrated the need for a more structured CDS, with contraindications and dosing criteria for DOACs.12,13
To meet this need, we designed a rule-based alert in the electronic health record (EHR) as an exemplary CDS tool that only fires or becomes active when the target conditions are met.18 We launched the prototype of the alert to “fire” when a provider has an appointment with a patient with AF who is not on AC. The alert was tied to a novel smart order set (also known as a smart set) to help providers prescribe AC, order laboratory tests, provide links to educational material and resources for peer support to patients, and make specialty referrals. Through a quasi-experimental study, we found that although responsiveness to our alert was high, there was no increase in AC prescription.19
In this article, we discuss a protocol for a prospective randomized controlled trial for the implementation of our existing novel AC CDS tool within the EHR to examine its effect on AC prescription behavior at two safety net health care systems in the Eastern and Southeastern United States. In contrast to our previous study, we will add several enhancements to the alert and test those enhancements in a cluster randomized controlled trial. In addition, through provider interviews and detailed study of “digital crumbs” (ie, data trails left by providers), we will assess engagement with the alert. In executing these multiple aims, we are guided by the RE-AIM implementation science framework, which encompasses the following principles: Reach into the target population (which we evaluate through examination at two separate sites, including minority subsets); Effectiveness of the intervention (our controlled trial); Adoption by target setting (vis-à-vis responsiveness to alert); Implementation concerns (digital crumbs and refinement of alert); and Maintenance of the intervention effects (including persistence of AC use) in individuals and settings over time.
Methods
Population
Providers
We will include all ambulatory care providers, including physicians, physician assistants, and nurse practitioners, who opt in to contribute patients to our analysis set. For patients being seen by residents, we will assign them to the attending physicians supervising the residents.
Patients
We will include patients with AF, age ≥18 years, and elevated CHA2DS2-VASc (≥2 for men or ≥3 for women; equivalent to a combined stroke and embolism risk ≥2.9% per year) seeing a cardiology provider or primary care physician. More specifically, we will use the International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnostic codes associated with active problems in the problem list in the electronic chart of each patient to identify AF and calculate the CHA2DS2-VASc score following the example of our prior work. Inclusion criteria are given in Figure 1, and characteristics of the baseline patient population are listed in Table 1. Our previous work demonstrated high specificity for electronic capture of AF (98%) and CHA2DS2-VASc score ≥2 (100%).13
Figure 1.
Summary of the study population.
Table 1.
Key patient characteristics at study sites
| Characteristic | University of Massachusetts |
University of Florida |
|---|---|---|
| Frequency (% of 1571) | Frequency (% of 1061) | |
| Age (y) | ||
| 75+ | 993 (63.2) | 443 (41.8) |
| 65–74 | 388 (24.7) | 209 (29.1) |
| <65 | 190 (12.1) | 209 (29.1) |
| Gender | ||
| Female | 750 (47.7) | 517 (48.7) |
| Male | 821 (52.3) | 544 (51.3) |
| Race | ||
| Nonwhite | 101 (6.4) | 301 (28.4)∗ |
| White | 1468 (93.4) | 724 (68.2)∗ |
| Missing | 2 (0.1) | 26 (3.4)∗ |
| Hispanic ethnicity† | ||
| Hispanic | 53 (3.4) | 30 (2.8)† |
| Non-Hispanic | 1506 (95.9) | 1031 (97.2)† |
| CHA2D2-VASc score | ||
| 2–3 | 448 (28.5) | 369 (24.9) |
| 4–5 | 692 (44.1) | 394 (37.0) |
| 6+ | 431 (27.4) | 298 (28.0) |
| Setting | ||
| Academic | 1116 (71.0) | 281 (26.6) |
| Community/private | 455 (29.0) | 779 (73.4) |
Projected values based on system-level data.
Thirteen patients are missing ethnicity information at the University of Massachusetts.
Setting
We will be testing the alert at UMass-Memorial Medical Center (UMass) and University of Florida at Jacksonville (UFL), which both utilize Epic Systems (Verona, WI) EHR. Combined, UMass and UFL provide care to more than 1.5 million Americans.
Procedures
Recruitment
After identifying a list of all cardiology providers and primary care physicians, we will send them an e-mail with both information and a request for them to join the study. We will send this e-mail through REDCap, our data collection platform. Our e-mail will also include a link to a survey that allows providers to opt out. This follows our prior SUPPORT-AF (Supporting Use of Anticoagulants Through Provider Profiling of Oral Anticoagulant Therapy for Atrial Fibrillation) II protocol.13 We will track information on providers (specialty, years since graduation, and credentials) to determine whether there is any significant selection bias.
Randomization
We will randomize providers (excluding those who opt out) in a 1:1 ratio to either control or intervention using randomly permuted blocks. Patient allocation to either arm will be dictated by the allocation of the provider conducting the first visit and will be stratified by provider type (cardiologist or primary care physician) and clinical site (UMass or UFL).
Contamination
To avoid contamination, we will configure the alert not to fire in any control patient (ie, any patient whose index visit was with a control provider) subsequently seeing an intervention provider. In intervention patients, the alert will continue to fire until the patients start AC or the provider selects a permanent reason for not initiating AC.
Alert enhancements
We will enhance our previously developed noninterruptive rule-based alert. Specifically, we will include an actionable in-workflow decision support tool customized to each override reason in order to provide evidence about timing or starting AC in the setting of a “fall” or “bleeding event.”20
We will include links to high-quality patient educational materials generated by the American Heart Association titled, “Answers by Heart,” and from a previously funded trial that includes information about individual prescriptions.21 In addition, we will include a link to StopAfib.org, a website that provides information about patients coping with or contemplating starting AC. This resource can be added to the patient’s take-home instructions or after-visit summary.
Our alert will include links to 2 shared decision-making tools. The first tool is the free mobile app AFib2gether developed by Pfizer Inc., which improves shared decision-making between patients with AF on AC and cardiology providers.22 The second tool is part of a CardioSmart initiative, which is a patient engagement program designed to support patient and clinician partnership and includes “A Decision Aid for Afib Stroke Prevention for Patients with Atrial Fibrillation.”23
Lastly, we will add a comment feature that allows providers to leave suggestions on how the alert, associated smart set, appearance of alert, overall user experience, and linkages can be optimized for the best experience for providers. These comments will help us to make iterative improvements to the alert after it is launched. It is important to note that we do not anticipate making significant changes that may require recalculating sample sizes; however, we will perform sensitivity analyses to account for this.
Measuring digital crumbs with AC initiation and persistence
We will record in patient charts several digitals crumbs where an alert fired. First, we will download the user action log records and Clarity orders table from the Epic databases. Then we will search patient charts for the following items: alert-specific actions, alert-specific orders, other orders, and access log actions. Access log actions refer to actions taken when a health care provider accesses different parts of the patient’s chart, including provider notes, medication lists, and laboratory test results.
Provider and patient satisfaction interviews
Provider interviews
We will interview a representative sample of at least 20 providers for whom the alert fired (the alert must have fired 5 times for cardiologists and 3 times for primary care physicians) with questions from domains listed in Table 2. From both UMass and UFL, we will interview physicians who utilized the alert and physicians who did not. For providers participating in interviews, a stipend of $100 will be provided for their knowledge and expertise, which will be used to improve the alert. Domains were mapped from the COM-B model (Capability, Opportunity, Motivation–Behavior).
Table 2.
Provider interview domains
| Alert-specific domains |
| Usability of alert |
| Acceptability of alert |
| Attitude toward educational material and communication tools |
| Impact on workflow |
| Appropriateness of timing |
| Recommendations for research team |
| General AC management domains |
| Barriers to AC management |
| Confidence initiating AC |
| Experience talking to patients after setback (fall, bleed) |
AC = anticoagulation.
Patient interviews
We will interview a representative sample of at least 25 patients (and at least 10 minorities) who had discussions about AC after the alert fired. First, we decide which patients to interview by using chart reviews to ensure AC discussion took place. Second, we will confirm by telephone calls with those patients whether AC discussions took place. Finally, we will obtain consent from patients, ask questions in domains listed in Table 3, and audio record interviews with subsequent transcription. Domains were mapped from the COM-B model.
Table 3.
Patient interview domains
| Perception of personal stroke risk |
| Knowledge of AC |
| Trust in provider |
| Choice (AC vs not AC) offered by provider |
| Provider encouraged patient participation in decision-making |
| Provider discussed risks and benefits of AC |
AC = anticoagulation.
Primary outcomes
Initiation
In a 12-month time frame after initiating the enhanced alert, we will record AC starts using each patient’s first encounter. In our sensitivity analysis, we will allow for subsequent patient encounters to contribute additional opportunities for AC starts. We will count an AC start if an anticoagulant is added to a patient’s medication list or if the patient has an international normalized ratio ≥1.5 in the 2 months following the index encounter. Our previous work determined that the accuracy of our AC status definition was excellent, with 99% sensitivity and 90% specificity.13 We estimate that 2632 eligible patients will have a visit with approximately 400 cardiology providers or primary care physicians. With this number of patients, an AC initiation percentage of 14% for control patients found in our previous work, a power of 80%, and 2-sided alpha of 0.05, the detectable between-group absolute percentage difference is 4.0%.
Persistence
By review of medical records, we will track the number of days that patients stay on AC for 1 year after start of AC. We will allow a buffer period up to 1 month for patients who go off AC because of events such as bleeding or surgical procedures, as long as they resume AC before the end of 12-month follow up. Sample size for persistence will depend on AC initiation. For initiation percentage of 14% in control-provider patients and 18% in intervention-provider patients, using 15% censoring in each group, 80% power, and 2-sided alpha = 0.05, we can detect a hazard ratio of 0.74 for the intervention-control difference in AC discontinuation.
Secondary outcomes (stroke and major bleeding)
Stroke/transient ischemic attack/systemic embolism
We will utilize ICD-10 codes to identify the incidence of new stroke, transient ischemic attack, and systemic embolism (Appendix A). We will count stroke only if it is the principal diagnosis for a patient, as secondary diagnosis codes can be related to history of stroke. This follows recent published methodology.24 Using chart reviews, we will verify the presence of stroke, transient ischemic attack, and systolic embolism.
Bleeding
We will include major hemorrhage and clinically relevant nonmajor bleeding, following an example reported in the literature.24 We will use ICD-10 codes to identify bleeding events (Appendix A) and then will verify accuracy using manual chart reviews.
Independent variable
Patient and provider assignment to intervention vs control will be the independent variable.
Covariates
We will select patient-specific, provider-specific, and other covariates, as detailed in Table 4. These covariates will include comorbidities based on ICD-10 codes as well as those comorbidities that form the CHA2DS2-VASc score. In our previous work, we found 100% specificity, that is, no instances in which our algorithm misidentified a patient as having a CHA2DS2-VASc score of 2+ when it was really <2.13
Table 4.
Patient and provider variables with source
| Type | Variable | Source within EHR |
|---|---|---|
| Primary outcomes | Adherence (AC start)—yes/no by 1 of 2 criteria
|
Medication records with active status∗, including outside pharmacy records through Surescripts health information network Laboratory records |
| Secondary outcomes | Stroke/TIA/systemic embolism Bleeding—major + clinically relevant |
ICD codes† and then verification by chart review |
| Independent | Intervention vs control | Encounter records |
| Covariates | Patient factors | |
|
ICD codes | |
|
ICD codes | |
Demographics
|
Demographic records | |
Risk score
|
Demographic records for age, ICD codes | |
Comorbidities‡
|
ICD codes + laboratory test results | |
| Provider factors | ||
| Age | Credentialing office records | |
| ||
| ||
|
Scheduling database in EHR |
AC = anticoagulation; AF = atrial fibrillation; CHA2DS2-VASc = stroke risk score consisting of congestive heart failure, hypertension, age 75+, diabetes, stroke, vascular disease, age 65 to 75, sex; EHR = electronic health record; ICD = International Classification of Disease; INR = international normalized ratio; TIA= transient ischemic attack.
Active status includes any script for which status is not discontinued.
ICD codes detailed in Appendix A.
Those not included in CHA2DS2-VASc score.
Analysis
Primary
Initiation
The hypothesis we are testing is that the number of AC starts in patients seen by intervention providers will be higher than that in patients seen by control providers. Initial analyses will estimate a generalized linear mixed model for AC start (yes/no) at the patient’s first visit with intervention vs control provider as the independent variable, adjusting for covariates and including a random effect for the provider to account for possible within-provider clustering.25 As a sensitivity analysis, we will include all of a patient’s visits in the 12-month interval and leverage Markov (transition) modeling, which will integrate predictors of starting AC among those who had not done so on a prior visit as well as predictors of discontinuing AC among those on AC at the prior visit. These Markov-based analyses will adjust for randomization assignment of the provider at each visit in addition to randomization assignment of the first-visit provider.
Persistence
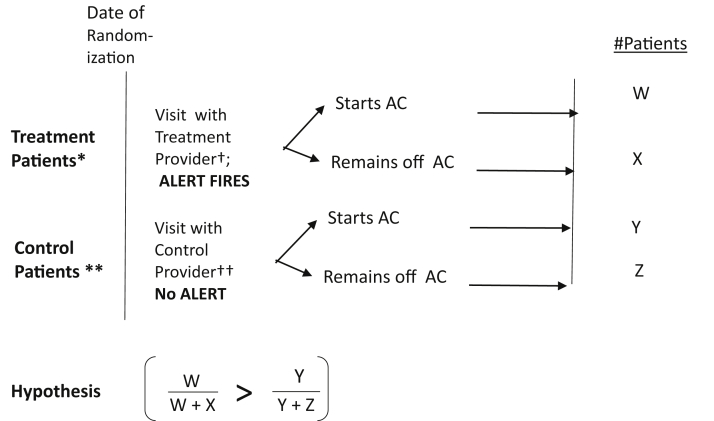
Here the hypothesis we are testing is that the likelihood of persisting on AC will be higher in patients of intervention providers than in patients of control providers. To test this hypothesis, we will model duration of initial AC use among initiators using Kaplan-Meier plots and Cox proportional hazards modeling as a function of the patient’s allocation. Duration will be censored at the end of a patient’s data collection due to death, transfer from our study sites, or end of the 12-month interval. If we observe a non-negligible fraction of patients starting, stopping, and restarting AC, we will use frailty modeling to accommodate within-patient dependence from modeling recurrent events.26 Secondary analyses will use generalized linear mixed modeling to examine a “compound” outcome of starting AC and persisting with no stops. All analyses will be performed using SAS Version 9.4 (SAS Institute, Cary, NC; Figure 2).27
Figure 2.
Schematic of experimental design and hypothesis. ∗Treatment patients are patients with atrial fibrillation (AF) + elevated CHA2DS2-VASc score (≥2 for men or ≥3 for women) + not on anticoagulation (AC) before the first appointment with a treatment provider in 2019 for the pre-launch era OR the first appointment with a treatment provider in 2020 for the post-launch era. ∗∗Control patients are patients with AF + elevated CHA2DS2-VASc score (≥2 for men or ≥3 for women) + not on AC before the appointment with a control provider in 2019 for the pre-launch era OR the first appointment with a control provider in 2020 for post-launch era. These patients did not see a treatment provider in the pre- or post-launch era, respectively. †Treatment providers comprise cardiology providers and primary care providers at study sites (including physicians, nurse practitioners, and physician assistants). ††Control providers comprise all other providers (including physicians, nurse practitioners, and physician assistants). CHA2DS2-VASc = stroke risk score consisting of congestive heart failure, hypertension, age 75+, diabetes, stroke, vascular disease, age 65 to 75, sex.
Association of digital crumbs with AC initiation and persistence
We will calculate the frequencies of each of the crumbs in the patients who started AC compared to those who did not. Our hypothesis is that providers of patients starting AC would more frequently take alert-specific actions and alert-specific orders as well as review medication lists and cardiac test results. To assess associations between digital crumbs and outcomes, we will estimate a series of generalized linear mixed models for AC start (yes/no) and a parallel model for the compound outcome (AC start with persistence), with an independent variable for each model being the presence of a different digital crumb (yes/no), adjusting for the covariates and a random effect for provider and patient. We also will re-examine the measured associations in the subset of minority patients.
Provider and patient interviews
Our investigator team will develop the coding scheme for provider interviews using an iterative process of independently reading 2–3 transcripts, generating and discussing codes associated with certain themes and subthemes, applying codes to 2–3 additional transcripts, and meeting to discuss and modify codes. The first investigator will then code all the transcripts by categorizing relevant statements in the transcripts into one or more codes. The second investigator will review the coded statements. They will resolve disagreements on the codes through discussion.
Secondary
We will calculate the frequency of stroke and bleeding outcomes and examine for differences/trends in differences between the intervention and control groups.
Conclusion
We developed a protocol to test the impact of our modified rule-based alert on AC adherence and persistence in a diverse cohort of patients from UMass and UFL. Using digital crumbs, we have a method to identify the alert’s mechanism of effect. Both patients and providers will be interviewed, thus providing a measure for satisfaction with AC management from alerted providers and the rule-based alert, respectively.
Previous studies demonstrated the difficulty in reducing the treatment gap in AC use. Ashburner et al28 did not find e-mail notifications containing educational material and primary care guidelines for AC management to be beneficial in improving AC prescriptions. SUPPORT-AF I and SUPPORT-AF II studies showed that messages with provider profiles and in-basket messages delivered days or up to 1 week before an encounter with an eligible patient were less desirable than an alert delivered at the time of the ambulatory visit and within the workflow of a provider.29 Our alert meets these criteria and represents a low-cost, nonintrusive solution to improving AC initiation and persistence.
Published reports have demonstrated the effectiveness of EHR alerts. Three different studies have shown the ability of rule-based alerts to increase vaccination rates.30, 31, 32 Piazza et al33 investigated the benefit of an alert for hospitalized patients with AF not on AC. They found that patients of providers assigned the alert were much more likely to be prescribed AC in the hospital and subsequently at the time of discharge compared with patients of providers not receiving the alert (23.8% vs 12.9%; P = .003). Our alert distinguishes itself by focusing on outpatients and their continuity providers. The hospital is an important setting to identify potential treatment strategies, but the outpatient setting likely may be the more appropriate place for a patient to discuss the benefits and risks of an intervention such as AC with his/her trusted providers. Our unique alert combines an associated smart order set, referral and laboratory entry options, and embedded logic to facilitate providers in choosing the correct anticoagulant and dose for the patient. With our proposed enhancements—educational materials, peer support resources for patients, shared decision-making tools, and evidence when providers select contraindications—we anticipate promising gains in initiation and persistence of AC.
We have described our protocol for enhancing our unique rule-based alert in the EHR and testing its ability to improve AC initiation and persistence. Previous studies indicate that alerts can be effective. In tracking digital crumbs, we will establish a way to verify the mechanism of effect.
Funding Sources
This work will be supported by the National Institutes of Health (pending R01 HL155343).
Disclosures
Dr Kapoor has received research grant support from Pfizer through its Independent Grants for Learning and Change the Funding Mechanism; and from Bristol-Myers Squibb for Independent Medical Education Grants. More recently, he has received research grant support through a competitive process adjudicated and funded by the alliance, which is formed by both Pfizer and Bristol-Myers Squibb. He also has been awarded a grant by Pfizer to examine conversations between patients and providers. Dr McManus receives sponsored research support from Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer, Biotronik, and Philips Healthcare; has consulted for Bristol-Myers Squibb, FlexCon, Samsung, Philips, and Fitbit; and has equity in Mobile Sense Technologies, LLC. Dr Catanzaro has received sponsored support from Cormatrix, GE, and Abbott Inc. More recently, he has received support from Boston Scientific. Hammad Sadiq, Jay Patel, and Dr Crawford have received research grant support from Bristol-Meyers Squibb in the past 3 years (staff members or co-investigator on grants secured by Dr Kapoor and Dr McManus as previously described). All other authors have nothing to disclose.
Guidelines Statement
Our research will adhere to the relevant CONSORT guidelines per http://www.consortstatement.org/extensions/overview/cluster-trials.
Disclaimer
Given his role as Editor-in-Chief, David McManus had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Dr Hamid Ghanbari.
Appendix
Appendix A.
ICD diagnosis codes for secondary outcomes
| Stroke/TIA/Embolism | Stroke | "43301", "43311", "43321", "43331", "43381", "43401", "43411", "43491", "436", "I6300", "I63019", "I6302", "I63031", "I63039", "I6309", "I6310", "I63131", "I63132", "I63139", "I6319", "I6320", "I63211", "I6322", "I63231", "I63232", "I63239", "I6329", "I6330", "I63311", "I63312", "I63319", "I63322", "I63341", "I63342", "I6339", "I6340", "I63411", "I63412", "I63419", "I63421", "I63422", "I63431", "I63432", "I63441", "I63442", "I63449", "I6349", "I6350", "I63511", "I63512", "I63519", "I63521", "I63522", "I63531", "I63532", "I63541", "I63542", "I63549", "I6359", "I638", "I639", "I6782", "I6789" |
| TIA | "36234", "G450", "G451", "G453", "G458", "G459" | |
| Systemic Embolism | "36231", "44401", "44409", "44421", "44422", "44481", "44489", "4449", "I742", "I743", "I745", "I748", "I749" | |
| Bleeding Events | Gastrointestinal | "4560", "45620", "53021", "5307", "53082", "53100", "53120", "53121", "53140", "53141", "53160", "53200", "53201", "53220", "53260", "53300", "53340", "53341", "53400", "53440", "53460", "53501", "53511", "53541", "53551", "53561", "53783", "53784", "56202", "56203", "56212", "56213", "56881", "5693", "56985", "5780", "5781", "5789", "I8501", "I8511", "K226", "K228", "K250", "K251", "K254", "K255", "K260", "K261", "K264", "K265", "K270", "K274", "K275", "K280", "K284", "K2900", "K2901", "K2921", "K2951", "K2961", "K2971", "K2981", "K2991", "K5521", "K5731", "K5733", "K625", "K661", "K920", "K921", "K922" |
| Intracranial | "430", "431", "4320", "4321", "4329", "I6010", "I606", "I607", "I608", "I609", "I610", "I611", "I612", "I613", "I614", "I615", "I618", "I619", "I6200", "I6201", "I6202", "I6203", "I629", "I6900", "I69044", I69051", "I69052", "I69054", "I6910", "I69151", "I69154", "I69159", "I69169", "I69191", "I6920", "I69222", "I69251", "I69259", "I69293" | |
| Other Clinically Relevant Non-major Bleeding Events | "4230", "4590", "5967", "59971", "71911", "71915", "71916", "71918", "7827", "7847", "78630", "78639", "M25022", "M25052", "M25061", "M25062", "N029", "R040", "R042", "R0489", "R233", "R310", "R319", "R58" |
References
- 1.Colilla S., Crow A., Petkun W., Singer D.E., Simon T., Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y., Barnes M.E., Bailey K.R., et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol. 2007;49:986–992. doi: 10.1016/j.jacc.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D., Benjamin E.J., Go A.S., et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 4.Gladstone D.J., Bui E., Fang J., et al. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke. 2009;40:235–240. doi: 10.1161/STROKEAHA.108.516344. [DOI] [PubMed] [Google Scholar]
- 5.January C.T., Wann L.S., Alpert J.S., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Essien U., Magnani J., Gellad W., Fine M., Hernandez I. Race, ethnicity, and sex-related differences in anticoagulation in patients newly diagnosed with atrial fibrillation. Abstracts from the American Heart Association's Quality of Care and Outcomes Research (QCOR) 2019 Scientific Sessions. Circ Cardiovasc Qual Outcomes. 2019;12(Suppl 1):A123. [Google Scholar]
- 7.Essien U.R., Holmes D.N., Jackson L.R., 2nd, et al. Association of race/ethnicity with oral anticoagulant use in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II. JAMA Cardiol. 2018;3:1174–1182. doi: 10.1001/jamacardio.2018.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang M.C., Machtinger E.L., Wang F., Schillinger D. Health literacy and anticoagulation-related outcomes among patients taking warfarin. J Gen Intern Med. 2006;21:841–846. doi: 10.1111/j.1525-1497.2006.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lip G.Y., Kamath S., Jafri M., Mohammed A., Bareford D. Ethnic differences in patient perceptions of atrial fibrillation and anticoagulation therapy: the West Birmingham Atrial Fibrillation Project. Stroke. 2002;33:238–242. doi: 10.1161/hs0102.101817. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez F., Hong C., Chang Y., et al. Limited English proficient patients and time spent in therapeutic range in a warfarin anticoagulation clinic. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson F.L., Racine E., Tekieli V., Williams B. Literacy, readability and cultural barriers: critical factors to consider when educating older African Americans about anticoagulation therapy. J Clin Nurs. 2003;12:275–282. doi: 10.1046/j.1365-2702.2003.00711.x. [DOI] [PubMed] [Google Scholar]
- 12.Kapoor A., Amroze A., Golden J., et al. SUPPORT-AF: piloting a multi-faceted, electronic medical record-based intervention to improve prescription of anticoagulation. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapoor A., Amroze A., Vakil F., et al. SUPPORT-AF II: Supporting Use of Anticoagulants Through Provider Profiling of Oral Anticoagulant Therapy for Atrial Fibrillation: a cluster-randomized study of electronic profiling and messaging combined with academic detailing for providers making decisions about anticoagulation in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.119.005871. [DOI] [PubMed] [Google Scholar]
- 14.Michigan Anticoagulation Quality Improvement Initiative Anticoagulation Toolkit. 2017. http://anticoagulationtoolkit.org/ 2017.
- 15.Durieux P., Nizard R., Ravaud P., Mounier N., Lepage E. A clinical decision support system for prevention of venous thromboembolism: effect on physician behavior. JAMA. 2000;283:2816–2821. doi: 10.1001/jama.283.21.2816. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn L., Reeves K., Taylor Y., et al. Planning for action: the impact of an asthma action plan decision support tool integrated into an electronic health record (EHR) at a large health care system. J Am Board Fam Med. 2015;28:382–393. doi: 10.3122/jabfm.2015.03.140248. [DOI] [PubMed] [Google Scholar]
- 17.Lau B.D., Haider A.H., Streiff M.B., et al. Eliminating health care disparities with mandatory clinical decision support: the venous thromboembolism (VTE) example. Med Care. 2015;53:18–24. doi: 10.1097/MLR.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agency for Healthcare Research and Quality Overview of CDS Five Rights. https://digital.ahrq.gov/ahrq-funded-projects/current-health-it-priorities/clinical-decision-support-cds/chapter-1-approaching-clinical-decision/section-2-overview-cds-five-rights
- 19.Sadiq H., Hoque L., Shi Q., et al. SUPPORT-AF III: supporting use of AC through provider prompting about oral anticoagulation therapy for AF. J Thromb Thrombolysis. 2021 Mar 10 doi: 10.1007/s11239-021-02420-8. Epub ahead of print. PMID: 33694097. [DOI] [PubMed] [Google Scholar]
- 20.Michigan Anticoagulation Quality Improvement Initiative Anticoagulation Toolkit. 2017. http://anticoagulationtoolkit.org/ 2017. 2017. Accessed September 21, 2018.
- 21.Gurwitz J.H., Kapoor A., Garber L., et al. Effect of a multifaceted clinical pharmacist intervention on medication safety after hospitalization in persons prescribed high-risk medications: a randomized clinical trial. JAMA Intern Med. 2021;181:610–618. doi: 10.1001/jamainternmed.2020.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapoor A., Andrade A., Hayes A., et al. Usability, perceived usefulness, and shared decision-making features of the AFib 2gether mobile app: protocol for a single-arm intervention study. JMIR Res Protoc. 2021;10 doi: 10.2196/21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American College of Cardiology Shared Decisions. https://www.cardiosmart.org/SDM/Decision-Aids
- 24.Fralick M., Colacci M., Schneeweiss S., Huybrechts K.F., Lin K.J., Gagne J.J. Effectiveness and safety of apixaban compared with rivaroxaban for patients with atrial fibrillation in routine practice: a cohort study. Ann Intern Med. 2020;172:463–473. doi: 10.7326/M19-2522. [DOI] [PubMed] [Google Scholar]
- 25.Molenberghs G., Verbeke G. Springer; New York: 2005. Models for Discrete Longitudinal Data. [Google Scholar]
- 26.Amorim L.D., Cai J. Modelling recurrent events: a tutorial for analysis in epidemiology. Int J Epidemiol. 2015;44:324–333. doi: 10.1093/ije/dyu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding E.Y., Han D., Whitcomb C., et al. Accuracy and usability of a novel algorithm for detection of irregular pulse using a smartwatch among older adults: observational study. JMIR Cardiol. 2019;3 doi: 10.2196/13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashburner J.M., Atlas S.J., Khurshid S., et al. Electronic physician notifications to improve guideline-based anticoagulation in atrial fibrillation: a randomized controlled trial. J Gen Intern Med. 2018;33:2070–2077. doi: 10.1007/s11606-018-4612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapoor A., Amroze A., Vakil F., et al. SUPPORT-AF II: a cluster-randomized study of electronic profiling and messaging combined with academic detailing for providers making decisions about anticoagulation in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.119.005871. [DOI] [PubMed] [Google Scholar]
- 30.Klatt T.E., Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119(2 Pt 1):301–305. doi: 10.1097/AOG.0b013e318242032a. [DOI] [PubMed] [Google Scholar]
- 31.Ledwich L.J., Harrington T.M., Ayoub W.T., Sartorius J.A., Newman E.D. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61:1505–1510. doi: 10.1002/art.24873. [DOI] [PubMed] [Google Scholar]
- 32.Morgan J.L., Baggari S.R., Chung W., Ritch J., McIntire D.D., Sheffield J.S. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333–337. doi: 10.1097/AOG.0000000000000975. [DOI] [PubMed] [Google Scholar]
- 33.Piazza G., Hurwitz S., Galvin C.E., et al. Alert-based computerized decision support for high-risk hospitalized patients with atrial fibrillation not prescribed anticoagulation: a randomized, controlled trial (AF-ALERT) Eur Heart J. 2020;41:1086–1096. doi: 10.1093/eurheartj/ehz385. [DOI] [PubMed] [Google Scholar]