Abstract
Background
A smartphone-enabled device has been developed that provides a single-lead electrocardiogram using a portable monitor. The increase in direct-to-consumer medical devices may lead to health disparities affecting members of socially disadvantaged populations.
Objective
Here we provide a single center’s experience in the use of this device in a pediatric cardiology clinic using a loan-based program. We also compare it to retrospective data from patients who received a traditional nonlooping event monitor.
Methods
Forty AliveCor Kardia monitor devices were purchased with grant support from the South Carolina TeleHealth Alliance. The devices were provided between June 2018 and August 2019 to patients presenting to the pediatric cardiology clinic who would have otherwise received a nonlooping event monitor. A retrospective chart review was performed for all patients who were given a MicroER nonlooping event monitor between May and December of 2017.
Results
Over a 15-month period, 65 patients were given the smartphone device. A total of 692 tracings were recorded by patients with 9 abnormal recordings. Of the devices expected to be returned, 35 devices have been returned to clinic (54%). Over an 8-month period, 61 patients received the traditional event monitors, accounting for a total of 142 transmissions with 3 abnormal transmissions.
Conclusion
Our results reveal adequate use of the device with reliable tracings and show more frequent utilization of the smartphone-enabled device. Utilization of these devices in a loan-based program may improve access to care with improved methods to ensure return of the devices.
Keywords: Ambulatory monitoring, Health equity, Pediatric cardiology, Smartphone-enabled device
Key Findings.
-
•
A smartphone-enabled monitor can be used successfully to evaluate for arrhythmias in a pediatric cardiology clinic as a replacement for a more traditional event monitor.
-
•
The use of a smartphone-enabled device resulted in a statistically significant increase in transmission frequency when compared to a more traditional event monitor.
-
•
The smartphone-enabled device was well accepted by families, based on survey responses.
-
•
A loan-based program can be used with a smartphone-enabled monitor in a pediatric cardiology clinic but requires improved methods for device return.
Introduction
With the advancement of technology in medicine there has been an increase in the introduction of direct-to-consumer medical devices. Many devices have focused on providing consumers with data regarding their cardiovascular health, including heart rate variability and single-lead electrocardiography (ECG).1 These technologies have been advanced by the introduction of wireless technologies for communication and networking (Wi-Fi, Bluetooth) as well as advances in novel materials used for ECG sensors.2 Smartphone ownership has also increased rapidly, with recent studies showing that 95% of citizens under the age of 34 years in the United States currently own a smartphone device.3 Integrating new technologies into the current health environment is essential to creating sustainable healthcare systems that provide adequate care that includes underserved pediatric populations.4
The AliveCor Kardia heart monitor (AliveCor, San Francisco, CA) is a small, direct-to-consumer wireless ECG monitor that pairs with a smartphone via an app. It provides a 30-second single-lead ECG comparable to lead 1 on a standard ECG by having the patient place a finger from each hand on the electrodes or place the device directly on the chest. The device has been studied as a method to evaluate palpitations in competitive athletes during play as a means to allow them to return to play faster.5 The Kardia monitor has also been shown to accurately measure heart rate, rhythm, and interval lengths when compared to 12-lead ECG.6,7 Previous studies have also evaluated the use of the Kardia monitor in pediatric patients with previously diagnosed arrhythmias and have shown that the monitor provides adequate tracings to identify tachyarrhythmias in pediatric patients.8,9 The introduction of direct-to-consumer medical devices and their rapid development and distribution may allow healthcare providers to access more patient data and can aid in the diagnosis and management of disease. However, they also risk exacerbating the already present inequities in healthcare for those who cannot afford these devices.
In this study we evaluate a loan-based program to provide the smartphone-enabled device to families during routine management of patients presenting to our outpatient clinics with concerns for arrhythmia. We also will compare the device use to a more traditional nonlooping event monitor in a similar patient population.
Methods
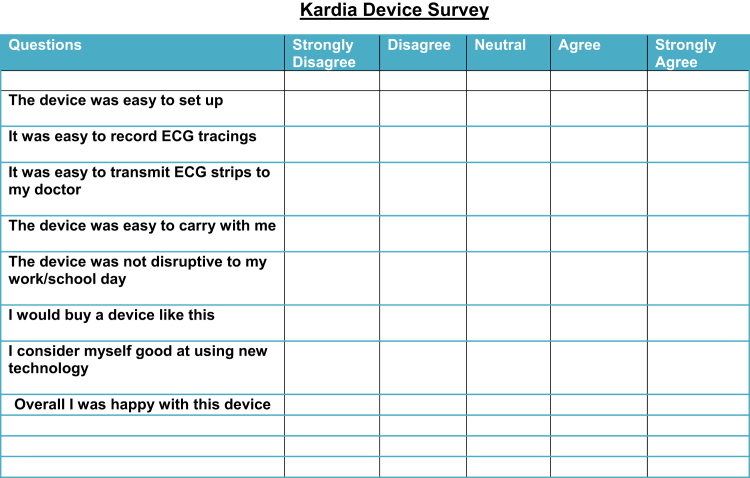
In order to evaluate the use of the novel smartphone-enabled device, we conducted a retrospective single-center case-control study. The Institutional Review Board at the Medical University of South Carolina (MUSC) approved this retrospective study. Forty Kardia Monitor devices were purchased with grant support from the South Carolina TeleHealth Alliance. Beginning in June 2018, we provided the Kardia tele-ECG devices on a loan basis to patients at no charge in our outpatient pediatric cardiology clinics. The device was set up in the clinic and the patients were instructed on its use and assisted in the download of the smartphone app as well as set-up of an online account. A postmarked envelope with postage was provided to allow the family to return the device and a survey after completing a prespecified interval. The survey (Figure 1) included questions on the ease of setting up the device as well as its use. Patient selection was based on clinician preference and was used in place of more traditional event monitors. Patient transmissions were automatically uploaded to AliveCor’s secure, encrypted servers and available for review on AliveCor’s website. All patients given the Kardia monitor between June 2018 and August 2019 were included in the retrospective review. All transmissions were reviewed by 1 of 2 board-certified pediatric electrophysiologists. Data regarding patient demographics, past medical history, presenting complaint, and results of transmissions was collected. A retrospective chart review was performed between May and December 2017 prior to the use of the Kardia monitor. The time period selected for the traditional event monitor was prior to the use of the Kardia monitor in order to obtain a representative sample of controls without confounding the results owing to selection bias. All patients who received the MicroER event monitor (Instromedix, San Diego, CA) within the study period were included. Data regarding patient demographics, past medical history, presenting complaint, and results of transmissions were collected from the electronic medical record. Demographic and clinical indication data were presented as frequencies for categorical variables. Transmission frequency data were presented as median with 25% and 75% interquartile range (IQR). Descriptive and χ2 analyses were performed where appropriate. The significance level was set at an alpha level of P ≤ .05. A Mann-Whitney test was performed for continuous variables.
Figure 1.
Survey provided to families who received Kardia monitor (AliveCor, San Francisco, CA).
Results
A total of 126 patients were included in the study. Of these, 65 were given the smartphone-enabled device between June 2018 and August 2019. Patients ranged between 3 months and 49 years of age (average 15 years of age); 48% were male and 89.2% white (Table 1). Of the patients given the smartphone-enabled device, 12 (18.5%) had a history of structural heart disease, with 4 of those 12 having a previously diagnosed arrhythmia. An additional 15 (23.1%) patients without structural heart disease also had a previously diagnosed arrhythmia. The remaining 38 patients (58.5%) had no history of structural heart disease or arrhythmia. Indication for the device included a history of palpitations, chest pain, or syncope (Table 1).
Table 1.
Demographics and indication of study population and control group
| Characteristic | Kardia monitor | MicroER | P value |
|---|---|---|---|
| Male/female | 31/34 | 25/36 | .57 |
| Race (%) | .27 | ||
| White | 58 (89.2%) | 48 (78.7%) | |
| Black | 6 (9.2%) | 11 (18%) | |
| Other | 1 (1.5%) | 2 (3%) | |
| Ethnicity (%) | |||
| Non-Hispanic | 62 (95.4%) | 57 (93.4%) | .63 |
| Hispanic | 3 (4.6%) | 4 (6.6%) | |
| Mean age (SD) | 15 (9) | 13 (8) | .15 |
| Indication (%) | |||
| Palpitations | 44 (67.7%) | 52 (85.2%) | .004 |
| Arrhythmia monitoring | 17 (26.2%) | 2 (3.3%) | |
| Chest pain | 3 (4.6%) | 5 (8.2%) | |
| Syncope | 1 (1.5%) | 2 (3.3%) |
SD = standard deviation.
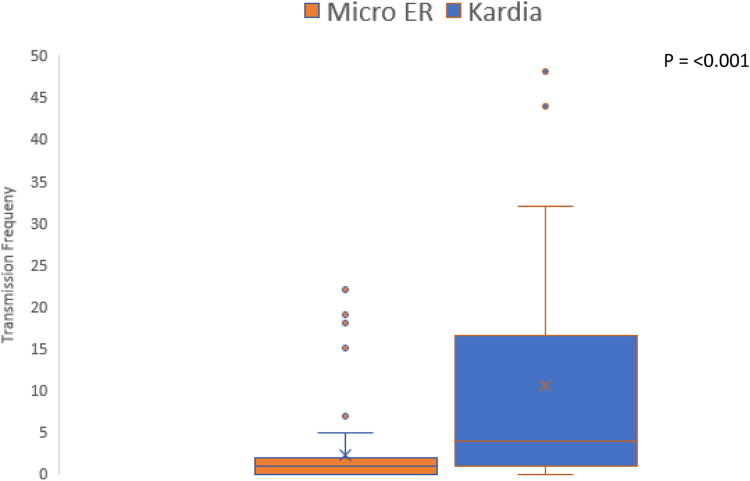
A total of 692 transmissions were received from the smartphone-enabled devices. Of the 692 submitted transmissions, 688 (99.4%) were deemed to be of adequate quality, with 683 (98.7%) normal and 9 (1.3%) abnormal transmissions, all with supraventricular tachycardia (SVT). Among the 9 abnormal transmissions, 3 transmissions were in 2 patients without a history of arrhythmia or congenital heart disease that presented for palpitations. The remaining 6 abnormal transmissions were in 3 patients, 2 with known history of SVT and 1 with congenital heart disease without a history of arrhythmia. Infrequent atrial and ventricular ectopy as well as 1 patient with paced rhythm were included in the normal transmissions. Of the submitted transmissions, 57 (8.2%) also included voice recordings associated with symptoms. The number of transmissions per patient ranged between 0 and 94 transmissions, with a median of 4 and IQR of 1–16 (Figure 2). Fourteen (21.5%) patients submitted no transmissions.
Figure 2.
Number of transmissions in those given traditional event monitor MicroER (Instromedix, San Diego, CA) compared to those given smartphone-enabled device Kardia monitor (AliveCor, San Francisco, CA). Median number of transmissions in MicroER group, with median of 1 and interquartile range (IQR) of 0–2, compared to Kardia group, with median of 4 and IQR of 1–16, showing higher transmission frequency in Kardia group (P < .001).
The comparison group included 61 patients who were provided the traditional event monitor. Patient ranged between 1 month and 57 years of age (average 13 years of age); 41% were male and 78.7% white (Table 1). Three (4.9%) patients had a history of structural heart disease and 11 (18%) patients without structural heart disease had a previously diagnosed arrhythmia. The remaining 47 patients (77%) had no history of structural heart disease or arrhythmia. The most common indication was palpitations (Table 1).
A total of 142 transmissions were sent. Of the 142 transmissions, all but 1 was interpretable. Three transmissions (2.1%) were abnormal and showed SVT. Two were from patients with no history of arrhythmia or congenital heart disease and 1 was from a patient with history of SVT. The number of transmissions per patient ranged between 0 and 22 transmissions, with a median of 1 and IQR of 0–2 (Figure 2). Twenty-four (39.3%) patients submitted no transmissions.
There was no statistically significant difference in sex, age, race, or ethnicity between the 2 groups (Table 1). There was a significantly higher prevalence of arrhythmia monitoring in the smartphone-enabled device group when compared to the traditional event monitor. Those who received the smartphone-enabled device transmitted tracings significantly more frequently than those who received the traditional event monitor, P < .001.
Of the patients who performed the survey (38%), all either agreed or strongly agreed that the smartphone-enabled device was easy to set up and use and were overall happy with the device. No respondents strongly disagreed or disagreed with any survey questions (Figure 1).
Discussion
Concern for primary arrhythmia is a common cause for referral to pediatric cardiology, with studies showing an incidence of 24.4 per 100,000 live births.10 SVT is the most common arrhythmia found in the pediatric population, with the majority secondary to the presence of an accessory pathway.11 Multiple studies have been performed to evaluate the effectiveness of ambulatory ECG monitoring in the pediatric population. In 1 single-center study with 495 pediatric patients, 48% of patients with a transtelephonic ECG monitor had a useful diagnosis, with the remainder of patients failing to submit a single eligible recording.12
The diagnostic yield of an ambulatory ECG monitor is dependent on both the ability to detect abnormal rhythms and the ability to exclude arrhythmia in the presence of symptoms for which the patient was referred. Our study population had a significantly better diagnostic yield, with only 21.5% of patients not submitting a transmission compared to 39.3% of patients who were given the traditional event monitor. This may be related to selection bias, as patients were not randomly selected to receive the monitor over other more traditional forms of ambulatory ECG monitoring and, as such, may be more likely to submit transmissions. The increased transmission rate may also be related to the fact that the smartphone-enabled device is easier to carry and is linked to the smartphone, which is likely always carried with the patient. There were a total of 5 patients with SVT in the study group and 3 patients in the traditional event monitor group. The increased transmission frequency in the Kardia group, with the majority being normal transmissions, resulted in a decreased percentage of abnormal results (1.15%) when compared to the traditional event monitor group (2%). While an increased transmission frequency may aid in the diagnostic yield of ambulatory monitors, it could also result in larger volumes of data that physicians and care teams must evaluate.
Previous studies in adults have shown that the Kardia monitor can be used effectively to evaluate patients presenting with palpitations in the outpatient setting.13,14 A study performed in pediatric patients presenting with palpitations showed a greater diagnostic arrhythmia yield using the Kardia monitor than a standard event recorder.15 Our data show similar results, with over half of patients presenting with palpitations in both study groups and nearly 75% of those with palpitations submitting recordings in the smartphone-enabled device group vs 62% in the traditional event monitor group.
At a cost of $89.00, the AliveCor Kardia monitor is available as a direct-to-consumer medical device. A more recent device has also been released by AliveCor that allows for a 6-lead ECG recording at a cost of $149.00. These devices are significantly less expensive than many more conventional forms of ambulatory ECG devices and could allow for decreased healthcare-related costs for many common presentations in the pediatric cardiology outpatient visit. Other forms of ambulatory monitoring also require costly analysis systems, while the Kardia monitor review system is $15.00 per patient for the month. The Kardia monitor does not require frequent maintenance and has a reported battery life of 12 months. During our study we have not had any Kardia monitors that have required maintenance or battery replacement. A case study by the York Health Economics Consortium16 to evaluate cost effectiveness of the use of the Kardia monitor in the diagnosis of atrial fibrillation in adults found a cost savings of £968 per patient per year from a National Health System perspective.
However, direct-to-consumer medical devices create an environment that may allow for health disparities affecting members of socially disadvantaged populations who do not have the same access to these devices. Our approach hoped to provide a framework that can allow for improved access to such devices that are deemed clinically beneficial to these populations. The devices used in this study were purchased using grant funding and provided to patients as a loan with instruction to return the devices after use. This method allows for the reuse of a small batch of devices with multiple patients at a small initial cost. The subsequent reuse of the devices also decreases the cost associated with outpatient rhythm monitoring and can provide significant cost savings for this patient population.
Given the need for device reuse, device return is an important aspect of the loan-based program, and this study attempted to evaluate postmarked envelopes as a means of device return. Of the 65 patients included in the study 35 (54%) returned the device. All patients also received 1 reminder call and an e-mail to have them return the device after their period of monitoring was complete. A return rate of 54% would not be financially sustainable, and this reveals one of the major barriers to successful implementation of this program in a general pediatric cardiology clinic. A successful loan-based program would need improved methods to assist in the return of the device or methods to offset the cost of lost devices. One method may be the scheduling of a follow-up appointment after completion of the study period to discuss the results of the monitor as well as return the device. This can create an incentive for the patient to return to the clinic to receive his or her results and improve device return. Charging the families for devices that are not returned may also improve device return and would also offset the financial burden of a poor device return rate. This method is commonly used for other event monitors that also face similar problems with device return. Patients who lose the MicroER event monitor are charged $120 by the company, a fee that serves to improve device return and offset the cost of lost devices.
The survey results show that the device was well accepted by the patients and their families, with all respondents stating that they agreed or strongly agreed that the smartphone-enabled device was easy to set up and use and were overall happy with the device.
Conclusion
This study is the first to evaluate the use of a smartphone-enabled device in a loan-based program as a replacement of more commonly used ambulatory rhythm monitoring devices in a pediatric cardiology clinic setting. Our results show that the device provides tracings of adequate diagnostic quality and can be used successfully in the pediatric cardiology clinic setting for the evaluation of arrhythmias. Our data also shows improved diagnostic yield of the smartphone-enabled device when compared to a more standard nonlooping event recorder, with a statistically significantly increased number of transmissions in the smartphone-enabled device group. Our results showed that inadequate device return is one of the primary barriers to successful implementation of a loan-based program and that strategies to mitigate poor return are required to implement such a program in the outpatient setting.
Funding Statement
This work was supported by a grant received from the South Carolina TeleHealth Alliance and was used to purchase the devices. The South Carolina TeleHealth Alliance was not involved in study design, collection of data, analysis, or writing the report. Conflict of Interest: The authors have no conflicts to disclose.
Acknowledgment
The research reported in this paper adhered to the Helsinki Declaration as revised in 2013.
References
- 1.Li K.H.C., White F.A., Tipoe T., et al. The current state of mobile phone apps for monitoring heart rate, heart rate variability, and atrial fibrillation: narrative review. JMIR Mhealth Uhealth. 2019;7 doi: 10.2196/11606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guzik P., Malik M. ECG by mobile technologies. J Electrocardiol. 2016;49:894–901. doi: 10.1016/j.jelectrocard.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Taylor K., Silver L. Pew Research Center; Washington, DC: 2019. Smartphone ownership is growing rapidly around the world, but not always equally. [Google Scholar]
- 4.Singh A., Wilkinson S., Braganza S. Smartphones and pediatric apps to mobilize the medical home. J Pediatr. 2014;165:606–610. doi: 10.1016/j.jpeds.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 5.Peritz D.C., Howard A., Ciocca M., Chung E.H. Smartphone ECG aids real time diagnosis of palpitations in the competitive college athlete. J Electrocardiol. 2015;48:896–899. doi: 10.1016/j.jelectrocard.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Gropler M.R.F., Dalal A.S., Van Hare G.F., Silva J.N.A. Can smartphone wireless ECGs be used to accurately assess ECG intervals in pediatrics? A comparison of mobile health monitoring to standard 12-lead ECG. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haberman Z.C., Jahn R.T., Bose R., et al. Wireless smartphone ECG enables large-scale screening in diverse populations. J Cardiovasc Electrophysiol. 2015;26:520–526. doi: 10.1111/jce.12634. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen H.H., Van Hare G.F., Rudokas M., Bowman T., Silva J.N. SPEAR Trial: Smartphone Pediatric ElectrocARdiogram Trial. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferdman D.J., Liberman L., Silver E.S. A smartphone application to diagnose the mechanism of pediatric supraventricular tachycardia. Pediatr Cardiol. 2015;36:1452–1457. doi: 10.1007/s00246-015-1185-6. [DOI] [PubMed] [Google Scholar]
- 10.Turner C.J., Wren C. The epidemiology of arrhythmia in infants: a population-based study. J Paediatr Child Health. 2013;49:278–281. doi: 10.1111/jpc.12155. [DOI] [PubMed] [Google Scholar]
- 11.Ko J.K., Deal B.J., Strasburger J.F., Benson D.W., Jr. Supraventricular tachycardia mechanisms and their age distribution in pediatric patients. Am J Cardiol. 1992;69:1028–1032. doi: 10.1016/0002-9149(92)90858-v. [DOI] [PubMed] [Google Scholar]
- 12.Saarel E.V., Stefanelli C.B., Fischbach P.S., Serwer G.A., Rosenthal A., Dick M., 2nd Transtelephonic electrocardiographic monitors for evaluation of children and adolescents with suspected arrhythmias. Pediatrics. 2004;113:248–251. doi: 10.1542/peds.113.2.248. [DOI] [PubMed] [Google Scholar]
- 13.Newham W.G., Tayebjee M.H. Excellent symptom rhythm correlation in patients with palpitations using a novel Smartphone based event recorder. J Atr Fibrillation. 2017;10:1514. doi: 10.4022/jafib.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reed M.J., Grubb N.R., Lang C.C., et al. Multi-centre randomised controlled trial of a smart phone-based event recorder alongside standard care versus standard care for patients presenting to the Emergency Department with palpitations and pre-syncope - the IPED (Investigation of Palpitations in the ED) study: study protocol for a randomised controlled trial. Trials. 2018;19:711. doi: 10.1186/s13063-018-3098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macinnes M., Martin N., Fulton H., McLeod K.A. Comparison of a smartphone-based ECG recording system with a standard cardiac event monitor in the investigation of palpitations in children. Arch Dis Child. 2019;104:43–47. doi: 10.1136/archdischild-2018-314901. [DOI] [PubMed] [Google Scholar]
- 16.York Health Economics Consortium; York, United Kingdom: 2018. Economic impact evaluation case study: AliveCor Kardia Mobile. [Google Scholar]