Abstract
Streptococcus pneumoniae is a problematic infectious agent, whose seriousness to human health has been underscored by the recent rise in the frequency of isolation of multidrug-resistant strains. Pneumococcal pneumonia in the elderly is common and often fatal. Young children in the developing world are at significant risk for fatal pneumococcal respiratory disease, while in the developed world otitis media in children results in substantial economic costs. Immunocompromised patients are extremely susceptible to pneumococcal infection. With 90 different capsular types thus far described, the diversity of pneumococci contributes to the challenges of preventing and treating S. pneumoniae infections. The current capsular polysaccharide vaccine is not recommended for use in children younger than 2 years and is not fully effective in the elderly. Therefore, innovative vaccine strategies to protect against this agent are needed. Given the immunogenic nature of S. pneumoniae proteins, these molecules are being investigated as potential vaccine candidates. Pneumococcal surface protein A (PspA) has been evaluated for its ability to elicit protection against S. pneumoniae infection in mouse models of systemic and local disease. This review focuses on immune system responsiveness to PspA and the ability of PspA to elicit cross-protection against heterologous strains. These parameters will be critical to the design of broadly protective pneumococcal vaccines.
Streptococcus pneumoniae is a major cause of morbidity and mortality worldwide. In the United States, there are over 100,000 cases of bacteremic pneumococcal pneumonia each year, primarily in the elderly, with at least 40,000 resultant deaths (34a). In the developed world, fatal pneumococcal respiratory infections are most frequently seen in the elderly and are rare in the very young. In contrast, in the developing world, pneumococcal respiratory infections are a major cause of death in children younger than 5 years. In children, the pneumococcus is a major cause of otitis media, while pneumococcal meningitis occurs in both children and the elderly. Pneumococcal sepsis also occurs with high frequency in those infected with the human immunodeficiency virus (47).
Since S. pneumoniae was found to be sensitive to penicillin, pneumococcal disease was not considered to be a problem after penicillin came into routine use for the treatment of pneumonia patients. Deaths in elderly individuals with pneumonia who were treated with penicillin were assumed to be due to viral infections. Through the efforts of Robert Austrian and others, it was demonstrated that despite the availability of penicillin therapy, pneumococcal pneumonia caused more deaths in this country than almost any other infectious disease (6, 7, 35, 52). From a comparison of mortality data from the pre- and postantibiotic eras, Austrian and Gold demonstrated that patients who die during the first few days after being diagnosed with bacteremic pneumonia are generally not protected by treatment with antibiotics. However, patients who would have died at later times after the diagnosis can frequently be saved by antibiotic treatment. The early deaths apparently occurred because of the very rapid progress of the disease in some of the infected individuals. Austrian and Gold reasoned that the best way to protect these individuals was to develop a vaccine that would prevent disease progression and the associated fatal infections (6). Their studies and those of others led to the development of the 14-valent and subsequently the 23-valent capsular polysaccharide vaccines for the prevention of infection in adults (99). The need for pneumococcal vaccines has been further emphasized by more recent studies demonstrating a recent rapid increase in both the prevalence and level of resistance of multiple-antibiotic-resistant pneumococci (4, 33).
PNEUMOCOCCAL VACCINES
Ideally, a vaccine that could protect against pneumococcal pneumonia would also protect against pneumococcal meningitis, otitis media, and bacteremia in young children. Since pneumococci are generally acquired from carriers and since nasopharyngeal carriage generally precedes infection, much of the recent thinking regarding the development of a pneumococcal vaccine is that the optimal vaccines would be those that protected against carriage as well as invasive disease.
The original decision to develop a capsular polysaccharide vaccine was based on a large body of research originating in the laboratories of Avery and others, which indicated that the outer surface of the pneumococcus was covered with a polysaccharide capsule. Antibodies generated against the capsular polysaccharide are typically highly protective against lethal infection (9, 110). The vaccine design was complicated, however, by the fact that protection elicited by the capsule is type specific and there are at least 90 different capsular types of S. pneumoniae (64). Taking advantage of the fact that certain capsular types are more commonly associated with human disease than are others, a vaccine composed of polysaccharides of the 14 (and later the 23) most common types was constructed (99). This vaccine was highly effective in young adults working in gold mines in South Africa, whose high rate of pneumococcal infection was caused largely by their working and living environments (5). However, the vaccine is only about 60% effective in preventing fatal pneumococcal bacteremia in the elderly (110). Unfortunately, the vaccine is unable to elicit adequate antibody responses to most of the capsular polysaccharides in children younger than 2 years (40). A recent survey of urgently needed vaccines in the developing and developed world places an improved pneumococcal vaccine among the top three vaccine priorities of industrialized countries (39).
Polysaccharide-Protein Conjugate Vaccines
Polysaccharide-protein conjugates are being developed as a potential solution to the less than adequate immunogenicity of the multivalent capsular vaccine in children and the elderly. This approach is based on early work with animals, which showed that polysaccharides could be rendered more immunogenic by conjugating them to proteins (8, 10, 56). This process endows the conjugated polysaccharide with certain properties of a T-cell-dependent antigen, allowing it to be antigenic in infants (68, 114). Pneumococcal conjugates have been prepared by coupling capsular polysaccharides to several carriers including tetanus toxoid, diphtheria toxoid, CRM197 (a nontoxic mutant of diphtheria toxin), pneumolysin, and meningococcal outer membrane proteins (112). Of these strategies, conjugates to tetanus toxoid and diphtheria toxoid have progressed the farthest in human trials. In general, conjugates of pneumococcal polysaccharides and proteins have been found to be more immunogenic in children than have the soluble polysaccharides (70, 131). Whether they will be more efficacious in adults than the soluble polysaccharides and whether they will elicit protection against carriage is still under study (112).
The basic approach of using polysaccharide-protein conjugates has proven highly successful with the Haemophilus influenzae type b polysaccharide vaccine in children, where only a single conjugate is necessary (118). However, for S. pneumoniae, it will be important that the vaccine contain conjugates of as many of the polysaccharide types as possible. Since each conjugate requires unique conjugation substrates and reaction conditions, individual conjugates must be separately constructed. Due to the amount of conjugated protein required to elicit immunity to a single polysaccharide, the number of different conjugates included in a single vaccine will of necessity be limited. In addition, one must be mindful that there is some variation in the most common pneumococcal serotypes in different parts of the world. This could be addressed by further increasing the number of polysaccharides in the vaccine or by making different mixtures of polysaccharides and conjugates for different regions of the world.
Immunogenic Considerations for Conjugate Vaccines
The finding that very young children failed to respond to the pneumococcal polysaccharide vaccine led to the discovery that human infants and infant young of other vertebrate species exhibit late maturation of their antipolysaccharide immune responsiveness (in comparison to their responsiveness to protein antigens). Children do not become adequately responsive to polysaccharides until after 2 years of age. In mice, anti-polysaccharide responsiveness does not occur until after 2 weeks of age (40, 57, 71, 93).
Since the ability of infants to make antibodies to polysaccharides would undoubtedly aid in their resistance to encapsulated bacteria, the absence of antipolysaccharide responsiveness in infants raises the possibility that there is some selective immunologic or developmental disadvantage in making such responses (46, 63). Hayrinen and colleagues suggest that tolerance induced by host tissue polysialic acid and bacterial polysaccharide cross-reactivities may play a role in the poor immunogenicity of meningococcal capsular polysaccharides (63). Polysialyated glycoproteins have been found in human embryonal brain (63) and newborn rat kidney, heart, and muscle but not in the corresponding adult tissues (49). This suggests that the absence of responsiveness to this and other polysaccharide antigens in children may prevent the production of antibodies reactive with developing tissues. Based on these data, Finne and coworkers have raised caution regarding meningococcal group B vaccine development for children (49, 50). Whether similar concerns should be raised about other conjugate vaccines in infants is a matter of speculation.
Another possible problem with early vaccination of mice with polysaccharides may come from deleterious modulation of the murine antibody response (46, 129, 130). This effect is well demonstrated by the response to phosphocholine (PC). In the mouse, the antibody response to PC has served as a model for the effects of antibody specificity on neonatal immunization after mice reach maturity. PC is present in both F-antigen and C-polysaccharide (29, 32) and is an immunodominant determinant of these molecules in the mouse. C-polysaccharide is the name given to pneumococcal cell wall teichoic acid, which is released by the activity of the pneumococcal autolysin. F-antigen is a lipoteichoic acid of the pneumococcus which possesses a teichoic acid which is essentially identical to the cell wall teichoic acid (128). When mice are immunized with killed pneumococci, their immune response is dominated by antibodies to PC (84, 134); these antibodies are quite protective against subsequent S. pneumoniae infection (17, 24, 72, 90, 117). The majority of BALB/c antibodies to PC are of the T15 idiotype and are encoded by germ line light- and heavy-chain genes (98). Although BALB/c mice make antibodies to PC of other idiotypes, only the T15 idiotype antibodies are highly protective against pneumococcal infection (19, 20). In this context, “idiotype” refers to the tertiary structure of the complementary determining regions of antibodies. Although other tests can be used, the expression of different idiotypes is generally determined by the use of antisera or monoclonal antibodies (MAb) specific for the antigen binding site of the antibody in question (22).
In mice, the adult repertoire of antibodies is strongly affected by idiotype interactions during fetal and early neonatal development (46). To test the effect of neonatal exposure to C-polysaccharide, mice were injected with heat-killed, protease-treated pneumococci 2 days after birth and then immunized with the same killed vaccine as 7-week-old adolescents (129). It was observed that pretreatment with killed pneumococci resulted in an anti-PC response of 7-week-old mice that was largely deficient in antibodies of the T15 idiotype. In contrast, nonimmunized control mice were able to mount a primarily T15 response when immunized with killed pneumococci at 7 weeks of age. When sera from these mice were tested by passive injection into naive CBA/N mice, the sera from immunized, normal mice were protective against pneumococcal infection whereas the sera from mice also injected neonatally with killed pneumococci were not (129).
In subsequent studies, 2-day-old mice were injected with F-antigen, C-polysaccharide, or saline only. Strong suppression of the adult T15 response was observed when the mice were immunized with isolated C-polysaccharide but not F-antigen (Table 1). The protective capacity of these sera was evaluated by passive immunization of CBA/N mice with serum containing 3 μg of antibody to PC followed by challenge with capsular type 3 strain WU2. As expected from its low T15 content, the antibody to PC from mice that had received C-polysaccharide at 2 days of age was relatively nonprotective, whereas antibodies to PC elicited in mice treated with F-antigen or saline at 2 days caused significant extension of life (Table 1).
TABLE 1.
Modulation of the expressed adult repertoire of BALB/c mouse antibodies to PC by injection of C-polysaccharide or F-antigen 2 days after birth
| Antigen injected 2 days after birtha | Immune response of mice to heat-killed pneumococcal vaccine at 7 weeks of agebc
|
Median time (h) to death afforded by passive protection with 3 μg of PC antibodybd | ||
|---|---|---|---|---|
| Heat-killed pneumococcal immunogen | Geometric mean concn of anti-PC antibody (μg/ml) (SE factor)e | Geometric mean % T15 antibody response (SE factor)e | ||
| Saline | Yes | 63 (1.1) | 72 (1.2) | 166 (P < 0.05)g |
| F-antigen | Yes | 135 (1.3) | 20 (1.5) | 161 (P = 0.05) |
| C-polysaccharide | Yes | 224 (1.1) | <2 | 53 |
| None | No | <5 | 36f | |
Doses of C-polysaccharide and F-antigen were adjusted to contain the same amounts of antibody-detectable PC.
Twelve mice per group were used in immunization and passive-protection studies.
Adult mouse immunizations were as described previously (129).
CBA/N mice were challenged with 350 CFU of capsular type 3 S. pneumoniae WU2. Passive-protection studies were conducted with the sera described in the first four columns..
The SE factor is the number by which the geometric mean may be multiplied and divided to calculate the upper and lower bounds of standard error.
For passive protection, a volume of normal mouse serum was injected comparable to that containing 3 μg of anti-PC in immune mice pretreated with saline.
P values in comparisons with no antigen.
When adult mice were immunized with the same two preparations, F-antigen elicited the strongest anti-PC responses and C-polysaccharide appeared to be tolerogenic at high doses (Table 2). The greater immunogenicity of F-antigen than of C-polysaccharide has been known since the 1940s (55) and is presumed to be due to the lipid content of F-antigen. Taken together, these results demonstrate that neonatal exposure of mice to polysaccharide antigens can lead to idiotypic modulation and nonprotective immune responses. Interestingly, the strongest effect on the adult antibody repertoire was mediated by the form of the antigen that was least immunogenic. If this is found to be true for human infants, rigorous removal of nonconjugated tolerogenic polysaccharide fragments might minimize any immunomodulatory effects of polysaccharide-protein vaccines.
TABLE 2.
Antigenicity of F-antigen and C-polysaccharide
| Dose of antigen (μg) | Amt of anti-PC antibody (μg/ml)a elicited by:
|
|
|---|---|---|
| F-antigen | C-polysaccharide | |
| 125 | 26 | 2 |
| 6.3 | 20 | 11 |
| 0.3 | 41 | 16 |
Data for pools of serum from groups of five mice.
In spite of these concerns, it must be pointed out that millions of infants have been immunized at 3 months of age with the H. influenzae group B polysaccharide-protein conjugate vaccines and there have been no reports of deleterious immunologic or developmental effects. For a pneumococcal vaccine, however, five or more different conjugates would be required, possibly increasing the risk of deleterious consequences, especially if they are dependent on idiotype network interactions that may interfere with B-cell differentiation (46, 130).
Pneumococcal Protein Vaccines
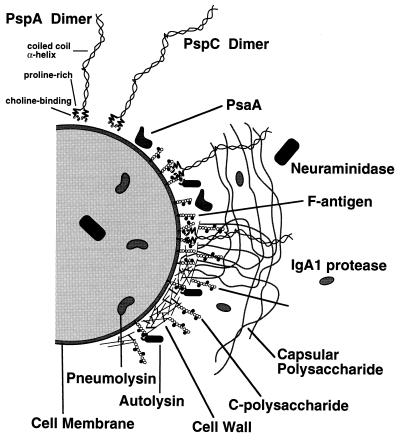
In addition to the capsule, a number of protein antigens are either exposed on the surface or released from the pneumococcus (Fig. 1). Several laboratories have examined the possibility that antigens other than capsular polysaccharides elicit protection against pneumococcal infection (113). One approach has been to immunize animals with known toxins and surface proteins to see if they elicit immunity against infection. Using this approach, it has been shown that antibodies to pneumolysin, autolysin (pneumococcal amidase), and neuraminidase are protective (77, 78). Of these, pneumolysin and autolysin are the most effective at eliciting protection against sepsis. Antibodies to autolysin appear to exert their effect in part because they prevent the autolysin-dependent release of pneumolysin. Recent studies, however, indicate that strains with a mutation in autolysin may be less virulent than strains with a mutation in pneumolysin (34). If this is the case, autolysin may play other roles in virulence, such as the release of virulence factors in addition to pneumolysin.
FIG. 1.
Hypothetical representation of the pneumococcal surface depicting several noncapsular antigens shown to elicit protective immune responses in mice. C-polysaccharide (teichoic acid) attached to the cell wall is thought to be similar in structure to F-antigen (lipoteichoic acid), except that the latter contains lipids allowing it to insert in the cell membrane. Neuraminidase has been depicted both in the cytoplasm and beyond the capsule, since it is thought to be secreted by pneumococci. Although its role in virulence is mediated at a distance from pneumococci, pneumolysin is depicted in the cytoplasm of the cell shown here, since its release is dependent on the autolytic activity of autolysin. For PspA, an effort has been made to draw its extension from the surface to scale with respect to the thickness of the cell wall and capsule. It has been hypothesized that the lysines of the PspA α-helix interact with the capsular polysaccharides to stabilize the coverage of the surface by the capsule. This hypothetical function is depicted by showing individual capsular polysaccharide strands interacting with more than one PspA molecule. Since the location of PsaA with respect to other cell surface structures is unknown, its depiction here is completely hypothetical. Reprinted from reference 21 with permission of the publisher.
Based on in vitro studies, a number of biologic functions have been ascribed to pneumolysin, including complement fixation (97), Fc binding (91), and damage to epithelial cells (48, 105). It has also been shown that pneumolysin plays a role in ocular infections (65, 69) and pneumococcal infections initiated in mice by the intranasal, intratracheal, and parenteral routes (1, 14, 34, 77, 96, 104). It is clear that in the presence of pneumolysin, capsular type 2 strains are able to grow exponentially in mice and cause fatal disease, whereas in the absence of pneumolysin, pneumococci increase in number only until they reach 106 CFU/ml of blood. From that point on, the mouse is largely able to control their numbers to about 106 CFU/ml, with death being delayed for several days and at times prevented altogether. Although the mechanism by which pneumolysin acts is unclear, it appears to regulate the host inflammatory response. In the absence of pneumolysin, the inflammation that develops is highly effective and can even protect against subsequent infections with pneumolysin-producing strains. In the presence of pneumolysin, the elicited inflammatory response is not effective in curtailing the S. pneumoniae infection (12).
Another approach used for identifying protection-eliciting pneumococcal proteins has been to produce MAb to proteins by immunizing with whole nonencapsulated pneumococci or with pneumococcal extracts. Protective MAb were then used to identify the eliciting proteins and to aid in the cloning of their structural genes. This approach was used originally to identify pneumococcal surface protein A (PspA) (84) and, more recently, a 37-kDa protein, PsaA (106, 126). PspA immunization can protect against pneumococcal sepsis following either intravenous (i.v.) or intraperitoneal (i.p.) pneumococcal challenge doses of >105 times the 50% lethal dose (LD50) of pneumococci (18, 23). As in the case of pneumolysin, immunization with PspA has been found to elicit protection against strains of more than one capsular type (1, 125). PsaA was named “pneumococcal surface adhesin A” based on the similarity of its predicted amino acid sequence to those of adhesins of certain oral streptococci (107). PsaA has been shown to elicit protection against fatal infection in mice with a single capsular type 3 strain (120). PspC and several other choline binding proteins have been identified through the homology of their genes to the structural genes for autolysin (21, 103, 111).
In the development of protein-based vaccines, it seems likely that the optimal vaccine might contain more than one protein antigen. In this regard, it is important to note that many of the proteins, such as PsaA, pneumolysin, and PspA, might be found to play even more important roles in protection against carriage, lung infection, or invasion than they do against sepsis. Until the studies of their roles in virulence are completed, the full value of these proteins as immunogens will not be known.
PNEUMOCOCCAL SURFACE PROTEIN A (PSPA)
Identification of PspA by Using MAb
PspA was first identified by using MAb produced from lymph node cells of CBA/N mice immunized with heat-killed nonencapsulated R36A pneumococci (84). Nonencapsulated pneumococci were used as the immunogen to maximize the chance of eliciting antibodies to noncapsular antigens. CBA/N mice express an X-linked immunodeficiency (XID) trait which prevents them from making immune responses to most polysaccharide antigens, including the PC determinants of C-polysaccharide and F-antigen (2, 24, 93). The failure of these mice to make antibody responses to PC was especially relevant to this study, since PC is a major immunologic determinant when mice are immunized with killed nonencapsulated pneumococci (36).
Growing hybrids were screened for antibodies against heat-killed R36A S. pneumoniae. In later fusions, mice were immunized with whole encapsulated pneumococci or cell wall extracts of pneumococci. Although XID mice were used, about 60% of the MAb reactive with R36A were specific for the PC epitope (84). The observation that antibody responses to polysaccharides are seen more readily in MAb than in serum antibody of XID mice has also been reported by others (109). In almost every case, MAb elicited by immunization with whole pneumococci or pneumococcal cell wall extracts that were nonreactive with PC were found to react with proteins (81, 84). The only exceptions observed in MAb from numerous independent fusions were a single MAb reactive with the type 5 capsule that was generated by immunization with a heat-killed capsular type 5 pneumococcus (27) and a MAb reactive with a non-PC component of pneumococcal teichoic acids (88, 135). All but one of the anti-protein MAb reacted with the same protein. This protein was designated PspA (27, 85). The only other protein we identified by this approach was PspB, which was serologically variable like PspA. Unlike PspA MAb, the MAb to PspB was not protective against pneumococcal infection (82). Once it became available, recombinant PspA was used to elicit additional MAb (83). PspA is now identified by over 12 MAb. Each of these has been shown to react with immunologically distinct epitopes and recognizes different but overlapping subsets of pneumococcal strains. This finding was the first evidence for the complex serological diversity and cross-reactivity of PspA (43, 83, 84).
Because R36A is a nonencapsulated pneumococcus, the protective capacity of the MAb was evaluated by challenge with capsular type 3 pneumococcal strain WU2. It was observed that five of nine MAb tested were protective against fatal WU2 infection (83) and that the four MAb which did not protect against this strain reacted poorly with WU2 PspA (83).
Role of PspA in Virulence
To determine if PspA is required for full virulence of pneumococci, insertion duplication mutagenesis (80, 123) was used to disrupt a gene required for PspA expression. Mutants of nonencapsulated strain Rx1 were derived by transformation with a library of restriction-digested pneumococcal DNA fragments ligated to pVA891. This plasmid carries an erythromycin resistance gene and lacks a functional origin of replication in S. pneumoniae (80). Thus, erythromycin-resistant colonies contain plasmid DNA integrated into the chromosome by homologous recombination. If the DNA fragment is internal to a gene, that gene will be interrupted by the integrated plasmid and a truncated gene product will be produced. By screening colony blots of erythromycin-resistant pneumococci with a PspA-specific MAb, transformants which had lost the ability to produce detectable PspA were identified (89).
From one of these mutants, WG44.1, we isolated pKSD300, a derivative of pVA891 that contained a 583-bp fragment of pneumococcal DNA. By transforming pKSD300 back into Rx1, it was possible to eliminate the expression of surface PspA in 100% of transformants (89). By adapting the transformation procedure for use with encapsulated pneumococci (145), it was possible to use pKSD300 to produce similar mutations in the virulent strain D39 (89). Later studies demonstrated that the D39 and Rx1 mutants prepared by transformation with pKSD300 actually produced a PspA fragment comprising the N-terminal 245 amino acids of the protein. However, these fragments were not surface attached and were secreted into the medium (144). Similar mutations were prepared in encapsulated type 3 S. pneumoniae WU2. The reduced virulence of the D39 PspA− and WU2 PspA− mutants was manifest as increased LD50s and more rapid clearance of PspA− pneumococci from the blood of infected mice (27, 89). Originally it was also reported that PspA− mutants were also prepared from a type 5 strain (27). Later it was discovered that, for reasons that are still unclear, these particular mutants had also lost their ability to make capsule. Thus, the lack of virulence in the PspA− type 5 strain cannot be attributed solely to a loss of PspA. In the D39 and WU2 mutants, however, capsule was present in quantities essentially identical to those in the parent strains (27).
Analysis of Nucleotide and Amino Acid Sequences
The first amino acid sequence determined for PspA was that of the N-terminal 45 amino acids of an isolated N-terminal fragment of Rx1 PspA (121). The fragment was produced by an Rx1 strain that had been transformed with pKSD300. The fragment migrated with an apparent molecular mass of 43 kDa in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). In later cloning experiments, the deduced amino acid sequence indicated that the actual size of this PspA protein fragment was 27 kDa and that PspA fragments could have some tertiary structure even in SDS-containing gels (143, 144). The N-terminal sequence of PspA was unblocked. It contained a proline in amino acid position 4, and the remainder of the 45-amino-acid sequence was consistent with that of an α-helical coiled-coil protein (121).
Numerous attempts to clone the pspA gene, using MAb to detect expression in Escherichia coli, were unsuccessful. In retrospect, our failures to clone pspA by this method are probably explained by the fact that expression of full-length PspA greatly retards the growth of E. coli. The complete pspA gene was finally cloned, however, by a circuitous approach. It was known that insertional inactivation, directed by the 583-bp pneumococcal DNA fragment present in pKSD300, resulted in an absence of PspA surface expression (89). It was assumed that the insertion directed by this fragment either interrupted a necessary regulatory gene, interfered with the promotion of the pspA structural gene, or interrupted the pspA structural gene itself. To examine these possibilities, the WG44.1 chromosome containing the insert was cloned, mapped, and sequenced (144). DNA sequencing and subsequent cloning revealed a previously undescribed 2-kb open reading frame which included the 583-bp fragment (143). Guided by the sequence of the open reading frame and knowledge of the restriction enzyme sites that had been identified during the mapping and cloning, the two halves of the pspA gene were reassembled and cloned into E. coli. Even though recombinant PspA was somewhat toxic, full-length PspA was readily detected in the transformed E. coli (143, 144). While these studies were in progress, pspA-containing DNA from strain D39 was successfully cloned into lambda (86). However, again, the slow growth of E. coli producing the intact gene product thwarted attempts to move the functional pspA gene from the lambda clone to a plasmid in E. coli.
Although the sequence of the Rx1 pspA gene predicted an amino acid sequence for a 65-kDa protein, the PspA of strain Rx1 migrated in SDS-PAGE with an apparent molecular mass of 84 kDa (121, 143, 144). It seems likely that the larger than expected apparent size of the PspA in SDS-containing gels is due to tertiary structure rather than to polysaccharide substitutions, since substituted proteins are thought to be rare in bacteria. Moreover, the size of PspA is similar whether it is produced in E. coli or pneumococci (86, 144). Most cloned truncated PspA fragments were also smaller than their apparent size in SDS-PAGE (144).
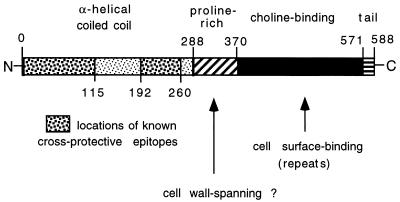
Based on its deduced amino acid sequence, it is possible to divide PspA into four domains (Fig. 1 and Fig. 2). The N-terminal 50% of the molecule has a sequence consistent with the α-helical coiled-coil structure frequently found in surface fibrillar proteins of gram-positive bacteria (143). The surface exposure of the α-helical region is supported by mapping studies in which individual MAb have been localized to epitopes in this region (43, 83). PspA MAb have been shown to bind intact pneumococci in enzyme-linked immunosorbent assay studies with immobilized whole bacteria (84), immunofluorescence microscopy (142), and electron microscopy (58). The surface exposure of PspA was also confirmed by surface labeling of pneumococci with radioactive iodine before immunoprecipitation with PspA MAb (85).
FIG. 2.
Schematic presentation of the domains of PspA delineated from the deduced amino acid sequence of Rx1 pspA. The locations of cross-protective MAb epitopes, determined by mapping studies, are shown. Numbers indicate amino acid residues. The two arrows point to the proline-rich (amino acids 289 to 370) and choline-binding (amino acids 371 to 571) regions, respectively. The functions of these regions are largely unknown but may include mechanistic roles, in addition to those described herein.
There is a proline-rich region, C-terminal to the α-helical sequence, in which the predominant sequence motif contains alternating proline and alanine residues. It is anticipated that this proline-rich region of PspA spans the cell wall of S. pneumoniae, as is the case for proline-rich regions of other surface proteins of gram-positive bacteria (45). C-terminal to the proline-rich region are 10 20-amino-acid repeats followed by a 17-amino-acid nonrepeating tail at the C terminus. The repeat region is composed of hydrophobic and hydrophilic amino acids that form a binding site for choline. The repeat region forms a binding site that attaches PspA to choline residues of F-antigen (also referred to as lipoteichoic acid) in the pneumococcal cell membrane (146). The polysaccharide chains of the F-antigen extend into the cell wall (128), and therefore the attachment of PspA to the F-antigen may occur within the cell wall. Such a scenario would require at least the start of the proline-rich region to reside within the pneumococcal cell wall. If this were the case, the α-helical region might extend at least 43 nm from the pneumococcal cell wall surface (143). Although the bulk of PspA is known to be associated with F-antigen, the possibility cannot be excluded that some small fraction of PspA binds to choline residues in the C-polysaccharide (also known as teichoic acids). Should that be the case, PspA might extend even further from the bacterial surface.
Putative Function of PspA
Although the role of PspA in virulence remains unresolved, it is known that it retards the clearance of pneumococci from the blood (89). It seems likely, therefore, that the major effect of PspA on virulence involves interference with the antiphagocytic properties of pneumococci. In the absence of PspA, pneumococci fix complement more effectively as measured by the bystander complement assay (21). However, this assay does not measure complement deposited on the pneumococcal surface, and thus the significance to pathogenesis requires additional study. With regard to antibody-mediated protection, it is clear that antibody to PspA enhances the clearance of pneumococci from the blood and that prior treatment with cobra venom factor largely eliminates the protective effects of passively administered PspA antibody (26).
A possible virulence mechanism for PspA is suggested by the amino acid composition of the PspA sequence. The α-helical region of Rx1 PspA contains a higher concentration of lysines than that generally found in coiled-coil proteins. The positions of the heptad repeat of coiled-coil proteins exposed to the fluid phase are b, c, e, f, and g. In PspA, 26% of these positions are occupied by lysines, while in other fibrous proteins, only about 12% of the exposed positions contain lysines (38). Even M protein of group A streptococci contains only about 18% lysines in positions b, c, e, f, and g (51). Capsular polysaccharides of almost all pneumococci that are virulent in humans are negatively charged (75). Since the flexible side chains of lysines carry a positive charge, they are ideally suited for interactions with the negatively charged capsular polysaccharide. If such interactions occur, they could serve to stabilize the capsular structure and maximize the ability of capsule to mask the pneumococcal cell wall. Alternatively, the proposed ionic interactions might simply permit PspA to extend into and protrude through the capsule layer.
It should be noted, however, that the overall net charge of the α-helical half of Rx1 PspA is negative at neutral pH. In the α-helical region, there are 61 amino acids (glutamic acid and aspartic acid) with negatively charged side chains and 56 (including 50 lysines) with positively charged side chains. The predicted isoelectric point is approximately 4.9 (143). Thus, even if there are charge interactions between individual lysines and the capsular polysaccharide, the net charge of the surface would remain negative. Any negative charges of the polysaccharide that are neutralized by lysines would be more than compensated for by the negatively charged amino acids of PspA.
Protective Effect of Immunity to PspA
As previously indicated, the first evidence that immunity to PspA might be protective was that PspA-specific MAb protected mice from otherwise fatal sepsis. It was observed that passive protection by the injection of immunoglobulin G (IgG) or IgM MAb to Rx1 PspA protected mice from death following i.v. or i.p. injection with 105 times the LD50 of S. pneumoniae WU2 (18). The passive antibody resulted in clearance of pneumococci from the blood of challenged mice (18, 84) in a complement-dependent manner (26).
The observations that MAb to PspA passively protected mice against experimental pneumococcal sepsis suggested that active immunization with PspA might elicit a protective response. Unfortunately, PspA proved difficult to isolate in pure form because the native molecule aggregates readily (122). Thus, the original analyses of the ability of immunization with PspA to elicit protection were indirect. One approach was to immunize XID mice with heat-killed Rx1 or with heat-killed WG44.1, a PspA− mutant of Rx1 containing an inactivated pspA gene (89). Immunization with rough pneumococci elicits strong antibody responses to PC in immunologically normal mice. Anti-PC antibody of mice, especially that of the T15 idiotype, can be quite protective against pneumococcal infection (17, 24, 134). Thus, immunodeficient XID mice were chosen for this study, since they lack the ability to produce serum antibody to PC (24) but still make antibody responses to proteins. At the time, it was known that S. pneumoniae WG44.1 lacked PspA on its surface but it was not known if the mutation was in the pspA structural gene or a regulatory gene. It was later found that WG44.1 unexpectedly lacks the 5′ half of the pspA gene, including the sequence encoding the pspA promoter region (144). Thus, WG44.1 is completely unable to make PspA or fragments of PspA.
Mice were immunized with heat-killed pneumococci suspended in saline and then challenged i.v. with 25 to 50 LD50 of type 3 strain WU2. The nonencapsulated PspA+ strain Rx1 elicited protection against fatal infection, whereas the PspA− strain WG44.1 did not (89). This finding made it clear that immunization with heat-killed nonencapsulated pneumococci elicited protection in mice only if PspA was present. This study did not rule out the involvement of or the requirement for other pneumococcal molecules as adjuvants or coimmunogens.
Immunity to PspA in the Absence of Other Pneumococcal Antigens
Before the completion of the cloning studies of Yother et al. (143, 144), the pspA structural gene was cloned by using a lambda gt11 library (86). Efforts to resolve the full-length gene from lambda into an expression plasmid in E. coli were unsuccessful due to the toxicity of full-length PspA in E. coli. The lambda clone did, however, provide a means of evaluating the ability of full-length PspA to elicit protection. The pspA gene was expressed during lysogeny, and the PspA produced was recovered as the “lambda lysate” by washing the agar plate with buffer. As a control, lysates were prepared from clones of the lambda library that contained random unidentified (non-PspA encoding) pneumococcal DNA fragments. The lysates were emulsified in complete Freund’s adjuvant and used as immunogens. Mice immunized with the PspA-containing lysates were protected from fatal infection following i.v. pneumococcal infection of CBA/N mice with 3,000 CFU (300 LD50) of capsular type 3 strain WU2. Similar immunization with preparations from non-PspA-producing lambda lysates did not protect mice from pneumococcal infection (86).
Immunization with the 27-kDa N-terminal fragment of the α-helical region of Rx1 PspA first demonstrated that an isolated PspA protein could elicit protection. This fragment, composed of amino acids 1 to 245 of PspA, was originally produced in pneumococci by insertion duplication mutagenesis with pKSD300 (143, 144). The 27-kDa PspA protein fragment was isolated by preparative SDS-PAGE from a periplasmic extract of E. coli expressing the cloned product (121). When the 27-kDa PspA fragment was used for immunization, its complete structure had not been determined; due to its electrophoretic mobility, it was referred to as a 43-kDa fragment of Rx1 PspA (121). Immunization of CBA/N (XID) mice with 5 μg of the 245-amino-acid fragment was carried out with a primary subcutaneous injection of 5 μg of material in complete Freund’s adjuvant followed by an i.p. booster dose in phosphate-buffered saline, while control mice were immunized likewise with adjuvant and phosphate-buffered saline but no antigen. Mice immunized with the fragment were protected from subsequent i.v. challenge with 300 CFU (30 LD50) of S. pneumoniae WU2 (121). We subsequently demonstrated that immunization with a fragment containing the N-terminal 243 or 260 amino acids of Rx1 PspA was unsuccessful without adjuvant (23, 139). The antigenicity of the 243-amino-acid fragment was greatly enhanced however, by expressing it as a fusion protein with interleukin-2 (139). More recent studies involving immunization with 1 or 5 μg of a fragment comprising the N-terminal 303 amino acids of Rx1 PspA in the absence of adjuvant have shown it to be immunogenic in mice and able to elicit protection against fatal infection following i.v. challenge with 480 CFU of A66.1, a strain that is significantly more virulent than WU2. The greater immunogenicity of the 303-amino-acid fragment in the absence of adjuvant may be due to its larger size (67).
It seems likely that the major mediator of protection following PspA immunization is antibody rather than some form of cell-mediated immunity. It is clear that immunization with PspA elicits IgG antibody in serum and that passive anti-PspA IgG and IgM MAb can protect against fatal infection (84). Moreover, immune sera from immunized mice (86) or rabbits (124) can passively protect mice from otherwise fatal sepsis with injection doses of 102 to 104 times the LD50 of the challenge pneumococci.
Isolation and Immunogenicity of PspA
Initial attempts to isolate full-length PspA from cell wall extracts of pneumococci were greatly hampered by the fact that native PspA aggregates (122). Isolation of PspA was greatly enhanced after the discovery that the repeat region of PspA was homologous to the choline-binding domain of autolysin (143). For autolysin, binding to the PC epitope of teichoic acid is necessary for activation of its enzymatic activity (108) and is sufficient for attachment of purified autolysin to the cell wall (53). However, elution with high choline concentrations or growth in medium containing ethanolamine rather than choline did not release autolysin for cell surfaces (15, 146), suggesting that autolysin may also have a non-choline-dependent method of attachment to the pneumococcal surface.
For PspA, however, the repeat region was found to be both required and sufficient for binding PspA to the pneumococcal surface by attachment to choline residues of F-antigen. This finding led to several successful approaches to the release of PspA from pneumococci and its subsequent isolation (23, 146). The most direct isolation protocol was to grow pneumococci under normal culture conditions, wash them with buffered saline, and then elute PspA from the bacterial surface with 2% choline chloride. An alternative approach was to grow the organisms in 1.2% choline chloride, a previously known means of blocking the action of autolysin by preventing its binding to the choline of cell wall teichoic acids (29, 54, 127). As for PspA, growth in 1.2% choline chloride results in a significant proportion of PspA being released into the medium (146). Additionally, PspA is released from the surface of pneumococci when they are grown in a defined medium containing ≤0.00001% choline chloride and 0.03% ethanolamine (146). This latter technique also leads to the loss of activity of autolysin because of the absence of choline in teichoic acids (128). Growth in either high concentrations of choline chloride or 0.03% ethanolamine media also inhibits the action of the enzyme(s) responsible for separation of cells during growth (28). As a result, pneumococci grown in these media remain in long chains and do not autolyse.
Each of these techniques releases PspA from the pneumococcal surface, but the PspA is in the presence of other bacterial proteins. Even so, the contribution of PspA to the protection elicited by the fractions could be clearly demonstrated by comparing the resistance of mice immunized with material prepared from PspA+ Rx1 and PspA− WG44.1 strains (23). In every case, the PspA-containing preparations were able to elicit protection in the absence of Freund’s complete adjuvant whereas the preparations lacking PspA were not. It was observed, however, that in the presence of adjuvant, non-PspA components present in the medium of PspA− pneumococci grown in 1.2% choline chloride or 0.03% ethanolamine (in place of choline) medium were able to elicit protection if the spent medium was injected at high concentrations. These results indicated that these preparations contained additional non-PspA protection-eliciting molecules (23). The purer PspA preparations were obtained when pneumococci were washed before elution with 2% choline chloride (146). If similar elutions were made from PspA+ Rx1 and PspA− WG44.1, protection was elicited only by preparations made from the PspA+ pneumococci, even in the presence of adjuvant (23).
The most successful strategy for purification of PspA has been to run PspA-containing extracts over a choline (or choline analog) column and subsequently elute the bound PspA with 2% choline chloride. The spent medium from cultures grown in ethanolamine can be chromatographed directly on the choline-Sepharose column. If PspA is to be isolated on a choline-Sepharose column from the spent medium of pneumococci grown in 1.2% choline chloride or the 2% choline chloride eluate of live pneumococci, prior dialysis to remove excess choline is required. PspA isolated from a choline-Sepharose column is more than 95% pure based on SDS-PAGE with silver staining (23, 146). The isolated material is immunogenic in mice at doses as low as 0.1 μg in the absence of adjuvant. When a mock isolation is conducted with spent medium from a PspA− strain, even a 100-fold-greater volume of the choline-eluted material does not elicit protection. Even in the presence of adjuvant, the mock-isolated material fails to elicit protection. These results verify that the only protection-eliciting molecule isolated by this procedure is PspA (23).
Cross-Reactivity and Cross-Protection
Even though PspA can be divided into numerous serologically distinguishable groups, it is a very cross-reactive protein. Sera from a single rabbit immunized with PspA can recognize PspA in Western blots of all pneumococci (43). Moreover, seven MAb to individual epitopes were each observed to react with 20 to 50% of PspAs and collectively with 95% of PspAs examined (43). The best evidence for cross-reactions between PspAs is the observation that immunization with a single PspA can protect mice against strains with serologically distinguishable PspAs (86, 125). In fact, cross-protection appears to be the rule rather than the exception. This was seen when mice were challenged with a panel of 14 S. pneumoniae strains following immunization with individual PspAs (Table 3) (25). Although certain PspAs elicited better protection against some strains than others, each PspA exhibited cross-protection against most challenge strains. Another major finding is that it is easier to protect against some pneumococcal strains than others. For example, it was difficult to obtain complete protection against capsular type 2 and 4 strains, even when mice were immunized with the homologous PspA. This finding may be partially due to the use of hypersusceptible, immunodeficient CBA/N mice. Preliminary results, however, indicate that protection against death due to capsular type 2 strain D39 and capsular type 4 strain EF3296 can be readily elicited by PspA in immunologically normal BALB/cByJ mice (25).
TABLE 3.
Ability of three PspAs to elicit protection against 14 diverse S. pneumoniae challenge strainsa
| Strain | Pneumococcal challenge
|
% of mice alive 21 days after challenge of mice immunized withb:
|
||||
|---|---|---|---|---|---|---|
| Capsule type | PspA type | D39 | WU2 | BG9739 | Nonec | |
| D39 | 2 | 25 | 38 | 60 | 3 | |
| WU2 | 3 | 1 | 100 | 100 | 100 | 2 |
| A66 | 3 | 13 | 75 | 100 | 80 | 5 |
| EF10197 | 3 | 18 | 100 | 80 | 0 | |
| ATCC 6303 | 3 | 7 | 100 | 0 | ||
| BG9739 | 4 | 26 | 11 | 60 | 13 | 0 |
| EF3296 | 4 | 20 | 25 | 20 | 10 | 0 |
| EF5668 | 4 | 12 | 22 | 25 | 60 | 9 |
| L81905 | 4 | 23 | 10 | 0 | 31 | 0 |
| DBL5 | 5 | 33 | 10 | 14 | 0 | |
| EF6796 | 6A | 1 | 100 | 0 | ||
| DBL6A | 6A | 19 | 67 | 25 | 33 | 4 |
| BG9163 | 6B | 21 | 89 | 20 | ||
| BG7322 | 6B | 24 | 100 | 60 | 25 | 6 |
Immunized and control CBA/N mice (groups of 10 to 50 animals) were challenged with ∼100 LD50 of each strain.
Where no data are shown, no experiment was conducted. The results of the same studies have been previously presented as median days alive after challenge (26). Underlined numbers are statistically different from those in the right-hand column (P < 0.05).
“None” includes unimmunized mice and mice immunized with mock preparations from S. pneumoniae lacking the pspA gene.
Location of Protection-Eliciting Epitopes of PspA
From the first cloning studies, it was apparent that MAb that were able to protect against pneumococcal infection reacted with fragments of PspA containing the majority of the α-helical region of PspA (43, 121, 144). The first evidence that this region could elicit protective immunity was not obtained, as was described above, until an insertionally inactivated mutant PspA, comprising the N-terminal 245 amino acids, was used for immunization (121). The same general region of PspA was shown to elicit protection against diverse strains of S. pneumoniae when mice were immunized with a recombinant Mycobacterium bovis BCG vaccine expressing a fragment containing amino acids 4 to 299 of Rx1 PspA (74). Subsequently, a fragment of PspA comprising only the first 115 amino acids was also shown to elicit protection against pneumococcal infection (23).
By using recombinant fragments of PspA, five MAb protective against the mouse-virulent encapsulated strain WU2 have been localized in two regions of Rx1 PspA (83). One of the five cross-protective MAb reacted with the fragment containing the N-terminal 115 amino acids of PspA. The other four MAb reacted with epitopes located between amino acids 192 and 260. No significant reactivity was observed with any of the cross-protective MAb in the region between 115 and 192 or in the portion of the α-helical region from amino acids 260 to 288 (Fig. 2).
It has also been shown that the PspA fragment of S. pneumoniae Rx1 extending from amino acid 192 to the C terminus (amino acid 588) is highly immunogenic and capable of eliciting statistically significant cross-protection against a diversity of pneumococci with very different PspAs (125). By using Rx1-specific primers for the respective ends of the sequence from amino acids 192 to 588, a number of homologous fragments of diverse PspAs were amplified and cloned (125). Three of these clones were expressed, and the recombinant PspAs were used to immunize mice. In all three cases, cross-protection was elicited against unrelated strains of pneumococci (125). These results indicate that cross-protection can be efficiently elicited by epitopes C terminal to amino acid 192 of PspA. Additionally, an Rx1 pspA fragment, BAR416, which expressed amino acids 192 to 299 was cloned (83). This PspA peptide proved capable of eliciting protection against capsule type 3 strain WU2 (83) and five additional strains expressing diverse PspAs. Moreover, PspA fragments homologous to BAR416 from other pneumococcal strains also elicit protection against infections with S. pneumoniae WU2 (26).
Immunity to Pneumococcal Carriage Elicited by PspA
The human reservoir of pneumococci is maintained by asymptomatic nasopharyngeal carriage. Strains acquired by adults and children are thought to be generally obtained from carriers, and carriage is known to precede infection (59). Therefore, a vaccine that could block carriage would be able to protect not only the immunized individual but also individuals who have not been immunized or who are unable to respond effectively because of immunodeficiency or age. This principle has been well established for the case of type b H. influenzae, where immunization with protein conjugates of the type b polysaccharide have virtually eliminated both carriage and disease (118).
We have developed a mouse model of pneumococcal carriage whereby carriage can be achieved with strains of at least five capsule types, including type 6B and 4 strains, which are highly virulent in mice when injected parenterally (141). By using this model, protection against nasopharyngeal carriage can be obtained by immunization with full-length PspA or tetanus toxoid conjugates of capsular type 6B polysaccharide in the presence of cholera toxin B subunit as an adjuvant (140). Studies with other pneumococcal surface molecules such as PsaA (120) and PspC (21), a molecule very similar in structure to PspA, are needed to ensure that an optimal vaccine is developed to protect against carriage.
Existence of a PspA-Related Protein, PspC
In virtually all pneumococci, there are two chromosomal loci homologous to the Rx1 pspA gene (87). Both of these loci, pspA and pspC (originally called the pspA-like locus), have homology to regions of the pspA/Rx1 gene encoding the α-helical, proline-rich, and repeat regions of Rx1 PspA (21, 87, 115). Evidence now exists that most strains produce products from both genes (31, 42). The pspC gene has also been discovered by others and has been designated spsA and cbpA (62, 103).
Potential Use of PspA as a Human Vaccine
It has been known since 1992, from the unpublished work of Marilyn Crain, that the sera of virtually all adult humans and many children contain antibody to PspA as detected by Western blotting (41). The results of quantitative studies of human response to natural exposure and active immunization with PspA are not yet available. If vaccination with PspA is found to elicit an even higher antibody response in humans than does natural exposure and if the elicited antibodies are found to be efficacious against pneumococcal infections, PspA could be used as a vaccine in one of several ways. If it is found to be highly efficacious against all pneumococcal infections, it might be used by itself as a 3- to 7-valent PspA vaccine. It could also be used as a carrier for pneumococcal polysaccharides, provided that the conjugation did not significantly impair the protection elicited by PspA itself. Alternatively, it could be used as a nonconjugated protein in combination with a limited number of protein-polysaccharide conjugates or with other pneumococcal proteins such as pneumolysin or PsaA, which might elicit protection that is complementary to that elicited by PspA.
DIVERSITY IN PSPA AND PNEUMOCOCCI
With regard to vaccine development, antigenic variation may be an important consideration. While it would be much easier to prepare a vaccine by using an invariant molecule, the existence of variability in a protein suggests that there is immunity to the molecule in the normal host that exerts selective pressure for variation. Thus, the protein molecules that elicit the best immunity may be those which, like capsular polysaccharides of pneumococci and M-proteins of group A streptococci, exhibit considerable serologic variation within the population. If variable proteins are used as vaccines, however, it becomes essential to understand the extent of this variation and the degree of cross-protection elicited by different variants of each protein.
Understanding the variability of pneumococci themselves is important because it is becoming clear that not all pneumococci cause equivalent infections or use identical infection strategies. This is quite apparent for animal infections (13, 16, 141) but is also seen with regard to capsular type differences for human infections and carriage in adults and children.
Variability of PspAs and pspA Genes
PspA is one of the more variable gene products of pneumococci. By using MAb to PspA, it has been possible to define more than 40 different serotypes (43, 95). By examining the apparent molecular weight of the PspAs, it is evident that differences can frequently be observed and that most individual isolates have unique combinations of PspA serotype and PspA molecular weight (43, 138). As expected, the pspA gene is also highly variable at the level of DNA sequence (66). pspA genes from more than 50% of independent isolates analyzed have been amplified by PCR. Examination of more than 70 amplified pspA genes revealed 17 different restriction fragment length polymorphism (RFLP) patterns (30, 116). Closely related but unique amino acid sequences have been identified in the 5′ half of each of over 25 different pspA genes (66).
The variability in chromosomal DNA containing pspA and pspC sequences can also be detected by Southern blot analysis of restriction fragments with full-length pspA or pspA oligonucleotide probes (87, 111, 115). The full-length pspA probe detects both the pspA and the pspC loci in virtually all strains, even at high stringency. An oligonucleotide probe from within the α-helical region of pspA hybridizes with DNA from a minority of strains at high stringency. These findings demonstrated that for both genes, the 5′ half is more variable than the 3′ half. As the stringency is decreased, the probe for the α-helical region recognizes one or both of the pspA-homologous sequences in most strains (115).
Other genes exhibiting variability among pneumococci are IgA1 protease and genes for certain penicillin binding proteins of penicillin-resistant isolates (60, 61, 79). However, the genes for pneumococcal neuraminidase, pneumolysin, and PsaA of all pneumococci, as well as those for penicillin binding proteins of penicillin-sensitive strains, appear to be relatively invariant. In Southern blots, probes for the neuraminidase gene (116) and the pneumolysin gene (11) generally hybridize with single bands of similar size in the pneumococcal strains analyzed. Moreover, pneumolysin genes have been sequenced from several pneumococci and the deduced amino acid sequences are >99% identical (92, 133).
Variability of Pneumococci
The first demonstration that pneumococci are genetically quite diverse, even within capsular types, came from studies of the serologic and molecular weight diversity of PspA (43, 136–138). The fact that individual isolates of S. pneumoniae of the same capsular type generally differed either in PspA serotype or in the apparent molecular weight of their PspA suggested that a large number of different pneumococcal clones of each capsular type exists (43, 137). This conclusion was confirmed at the molecular level by RFLP analysis of the Rx1 pspA gene (87), as well as RFLP analysis of PCR-amplified pspA genes from many pneumococcal strains (115). Since PspA (and, by analogy, probably PspC) is a virulence factor, it could even be argued that differences in these genes may be greater than those at other loci.
Although this is probably true, it turns out that pneumococci are quite diverse even within capsular type and even when the analysis is not based on the diversity of PspA or PspC. This diversity has been shown in part by the use of multilocus enzyme electrophoresis (95, 119). More recently, restriction enzyme-digested DNAs of independent pneumococcal isolates have been shown to generally exhibit different restriction patterns by Southern analysis with the IS1167 insertion sequence (116). The IS1167 element, first identified in Morrison’s laboratory (147), has been found by us to be present in copy numbers ranging from 3 to 12 in virtually all pneumococci (100, 101). This considerable diversity of pneumococci is also apparent from other techniques including BOX- and repetitive extragenic palindromic (REP)-PCR (73, 102) and pulsed-field gel RFLP patterns of genomic DNA (76, 132).
The great diversity of pneumococci appears to have accrued over an extended period, since changes have not been observed with repeated isolates from patients or from outbreaks (43, 87, 94, 95, 138). One particularly interesting group of strains is that containing D39 and its descendants, including R36A and Rx1. These strains have been separated by more than 130 years of laboratory and animal passage, yet they all have identical pspA genes and gene products by all analyses described above, including identical IS1167 patterns (43, 87, 100, 138, 147). PspA was monitored in sets of pneumococcal strains of the same capsular types recovered from individual patients over a period of 1 year or more. In each case, all had identical PspAs by serologic and molecular weight determinations (136, 138). Examination of strains from two outbreaks showed no variation in IS1167 pattern or PspA type or pspA chromosomal RFLP pattern within either outbreak (101).
Panmictic Nature of Pneumococci
It would be anticipated that mutational mechanisms undoubtedly account for much of the variability seen in pneumococci. However, the observation that the same PspA epitopes occur in different combinations on PspAs from different strains suggests that there have been numerous crossovers within the pspA locus (43, 137). Moreover, the observation that different PspA types and different PspA epitopes appear to be found in strains of many, if not all, different capsular types suggests that genetic exchange within pneumococci has been important in the development of present-day pneumococcal genotypes (43, 137). Additional evidence for this exchange comes from studies showing that genes for penicillin resistance and genes for capsular type have been exchanged to different genetic backgrounds in certain highly penicillin-resistant strains (37, 44). More recent studies using variability at the IgA protease locus and multienzyme typing have concluded that pneumococci are panmictic; that is, the diversity in their genotypes created by genetic exchange is substantial compared to the diversity created by mutation alone (79).
CONCLUSIONS
Human disease resulting from S. pneumoniae infection has been and continues to be associated with a significant degree of morbidity and mortality and with a significant economic burden. Improved pneumococcal vaccines are badly needed to help combat this threat. Although major efforts are being directed at the development of polysaccharide-protein conjugate vaccines, the immunogenic nature of pneumococcal proteins also makes them prime targets for new vaccine strategies. Although several pneumococcal proteins are capable of eliciting protective immunity in mice, PspA is unique in that it can elicit antibodies that protect mice against inocula at least 100-fold greater than the LD50. PspA enhances the virulence of pneumococci and is able to elicit protective immunity against both pneumococcal sepsis and nasopharyngeal carriage. Although PspA is serologically variable, it is sufficiently cross-reactive that immunization with a single PspA elicits protection against diverse strains of S. pneumoniae. The design of the pneumococcal vaccine must take into consideration the extreme variability of pneumococci. Due to the cross-protective nature of PspA, it is anticipated that an appropriately chosen mixture of different PspAs or PspA fragments (possibly in combination with other pneumococcal proteins) might be able to elicit broad protection against pneumococcal infection.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI-07051, AI-21548, HL-51646, AI-33205, and AI-01200 and by World Health Organization grant V23/181/47.
REFERENCES
- 1.Alexander J E, Lock R A, Peeters C C A M, Poolman J T, Andrew P W, Mitchell T J, Hansman D, Paton J C. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect Immun. 1994;62:5683–5688. doi: 10.1128/iai.62.12.5683-5688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amsbaugh D F, Hansen C T, Prescott B, Stashak P W, Barthold D R, Baker P J. Genetic control of the antibody response to type III pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972;136:931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reference deleted.
- 4.Appelbaum P C. World-wide development of antibiotic resistance in pneumococci. Eur J Clin Microbiol. 1987;6:367–377. doi: 10.1007/BF02013089. [DOI] [PubMed] [Google Scholar]
- 5.Austrian R, Douglas R M, Shiffman G, et al. Prevention of pneumococcal pneumonia by vaccination. Trans Assoc Am Physicians. 1976;89:184–194. [PubMed] [Google Scholar]
- 6.Austrian R, Gold J. Pneumococcal bacteremia with special reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60:759–776. doi: 10.7326/0003-4819-60-5-759. [DOI] [PubMed] [Google Scholar]
- 7.Austrian R A. Pneumococcal infections. In: Germanier R, editor. Bacterial vaccines. New York, N.Y: Academic Press, Inc.; 1984. pp. 257–288. [Google Scholar]
- 8.Avery O T, Goebel W F. Chemo-immunological studies on conjugated carbohydrate-proteins. II. Immunological specificity of synthetic sugar-protein antigens. J Exp Med. 1929;50:533–550. doi: 10.1084/jem.50.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avery O T, Goebel W F. Chemo-immunological studies of the soluble specific substance of pneumococcus. I. The isolation and properties of the acetyl polysaccharide of pneumococcus type 1. J Exp Med. 1933;58:731–755. doi: 10.1084/jem.58.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avery O T, Goebel W F, Babers F H. Chemo-immunological studies on conjugated carbohydrate-proteins. VII. Immunological specificity of antigens prepared by combining α- and β-glucosides of glucose with proteins. J Exp Med. 1932;55:769–780. doi: 10.1084/jem.55.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banks S D, Lee C J. Abstracts of the 91st General Meeting of the American Society for Microbiology 1991. Washington, D.C: American Society for Microbiology; 1991. Analysis of pneumolysin in Streptococcus pneumoniae group 19 strains; p. 97. [Google Scholar]
- 12.Benton K A, Everson M P, Briles D E. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect Immun. 1995;63:448–455. doi: 10.1128/iai.63.2.448-455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benton K A, Paton J C, Briles D E. Differences in virulence of mice among Streptococcus pneumoniae strains of capsular types 2, 3, 4, 5, and 6 are not attributable to differences in pneumolysin production. Infect Immun. 1997;65:1237–1244. doi: 10.1128/iai.65.4.1237-1244.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry A M, Yother J, Briles D E, Hansman D, Paton J C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989;57:2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breise T, Hackenbeck R. Interactions of pneumococcal amidase with lipoteichoic acid and choline. Eur J Biochem. 1985;146:417–427. doi: 10.1111/j.1432-1033.1985.tb08668.x. [DOI] [PubMed] [Google Scholar]
- 16.Briles D E, Crain M J, Gray B M, Forman C, Yother J. A strong association between capsular type and mouse virulence among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briles D E, Forman C, Crain M. Mouse antibody to phosphocholine can protect mice from infection with mouse-virulent human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:1957–1962. doi: 10.1128/iai.60.5.1957-1962.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briles D E, Forman C, Horowitz J C, Volanakis J E, Benjamin W H, Jr, McDaniel L S, Eldridge J, Brooks J. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect Immun. 1989;57:1457–1464. doi: 10.1128/iai.57.5.1457-1464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briles D E, Forman C, Hudak S, Claflin J L. Anti-PC antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briles D E, Forman C, Hudak S, Claflin J L. The effects of idiotype on the ability of IgG1 antiphosphorylcholine antibodies to protect mice from fatal infection with Streptococcus pneumoniae. Eur J Immunol. 1984;14:1027–1030. doi: 10.1002/eji.1830141112. [DOI] [PubMed] [Google Scholar]
- 21.Briles D E, Hollingshead S K, Swiatlo E, Brooks-Walter A, Szalai A, Virolainen A, McDaniel L S, Benton K A, White P, Prellner K, et al. PspA and PspC: their potential for use as pneumococcal vaccines. Microb Drug Resist. 1997;3:401–408. doi: 10.1089/mdr.1997.3.401. [DOI] [PubMed] [Google Scholar]
- 22.Briles D E, Kearney J F. Idiotype antibodies. Methods Enzymol. 1985;116:174–189. doi: 10.1016/s0076-6879(85)16012-x. [DOI] [PubMed] [Google Scholar]
- 23.Briles D E, King J D, Gray M A, McDaniel L S, Swiatlo E, Benton K A. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine. 1996;14:858–867. doi: 10.1016/0264-410x(96)82948-3. [DOI] [PubMed] [Google Scholar]
- 24.Briles D E, Nahm M, Schroer K, Davie J, Baker P, Kearney J, Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briles, D. E., J. S. Sheffield, C. S. Forman, J. D. King, L. S. McDaniel, S. K. Hollingshead, W. H. J. Benjamine, and B. L. Mathews. Unpublished data.
- 26.Briles D E, Tart R C, Wu H-Y, Ralph B A, Russell M W, McDaniel L S. Systemic and mucosal protective immunity to pneumococcal surface protein A. Ann N Y Acad Sci. 1996;797:118–126. doi: 10.1111/j.1749-6632.1996.tb52954.x. [DOI] [PubMed] [Google Scholar]
- 27.Briles D E, Yother J, McDaniel L S. Role of pneumococcal surface protein A in the virulence of Streptococcus pneumoniae. Rev Infect Dis. 1988;10:S372–S374. doi: 10.1093/cid/10.supplement_2.s372. [DOI] [PubMed] [Google Scholar]
- 28.Briles E B, Tomasz A. Radiographic evidence for equatorial wall growth in a gram-positive bacterium: segregation of choline-3H-labeled teichoic acid. J Cell Biol. 1970;47:786–790. doi: 10.1083/jcb.47.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briles E B, Tomasz A. Pneumococcal forssman antigen. A choline-containing lipoteichoic acid. J Biol Chem. 1973;248:6394–6397. [PubMed] [Google Scholar]
- 30.Brooks-Walter, A., L. S. McDaniel, S. K. Hollingshead, and D. E. Briles. Unpublished data.
- 31.Brooks-Walter, A., R. C. Tart, D. E. Briles, and S. K. Hollingshead. Unpublished data.
- 32.Brundish D E, Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem J. 1968;110:573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler J C, Hofmann J, Cetron M S, Elliott J A, Facklam R R, Breiman R F. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention’s pneumococcal sentinel surveillance system. J Infect Dis. 1996;174:986–993. doi: 10.1093/infdis/174.5.986. [DOI] [PubMed] [Google Scholar]
- 34.Canvin J R, Marvin A P, Sivakumaran M, Paton J C, Boulonois G J, Andrew P W, Mitchell T J. The role of pneumolysin and autolysin in the pathology of pneumoniae and septicemia in mice infected with a type 2 pneumococcus. J Infect Dis. 1995;172:119–123. doi: 10.1093/infdis/172.1.119. [DOI] [PubMed] [Google Scholar]
- 34a.Centers for Disease Control. Pneumococcal polysaccharide vaccine, recommendations of the immunization practices. Morbid Mortal Weekly Rep. 1989;38:64–68. [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Pneumonia and influenza death rates—United States 1979–1994. Morbid Mortal Weekly Rep. 1995;44:535–537. [PubMed] [Google Scholar]
- 36.Claflin J L, Lieberman R, Davie J M. Clonal nature of the immune response to phosphocholine. II. Idiotypic specificity and binding characteristics of anti-phosphocholine antibodies. J Immunol. 1974;112:1747–1756. [PubMed] [Google Scholar]
- 37.Coffey T J, Dowson C G, Daniels M, Zhou J, Martin C, Spratt B G, Musser J M. Horizontal transfer of multiple penicillin-binding protein genes, and capsule biosynthetic genes, in natural populations of Streptococcus pneumoniae. Mol Microbiol. 1991;5:2255–2260. doi: 10.1111/j.1365-2958.1991.tb02155.x. [DOI] [PubMed] [Google Scholar]
- 38.Cohen C, Parry D A D. α-Helical coiled coils and bundles: how to design an α-helical protein. Proteins Struct Funct Genet. 1990;7:1–15. doi: 10.1002/prot.340070102. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J. Bumps on the vaccine road. Science. 1994;265:1371–1373. doi: 10.1126/science.8073271. [DOI] [PubMed] [Google Scholar]
- 40.Cowan M J, Ammann A J, Wara D W, Howie V M, Schultz L, Doyle N, Kaplan M. Pneumococcal polysaccharide immunization in infants and children. Pediatrics. 1978;62:721–727. [PubMed] [Google Scholar]
- 41.Crain, M. J., and D. E. Briles. Unpublished data.
- 42.Crain M J, Turner J S, Robinson D A, Coffey T J, Brooks-Walter A, McDaniel L S, Briles D E. Evidence for the simultaneous expression of two PspAs by a clone of capsular serotype 6B Streptococcus pneumoniae. Microb Pathog. 1996;21:265–275. doi: 10.1006/mpat.1996.0060. [DOI] [PubMed] [Google Scholar]
- 43.Crain M J, Waltman II W D, Turner J S, Yother J, Talkington D E, McDaniel L M, Gray B M, Briles D E. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990;58:3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dowson C G, Hutchinson A, Brannigan J A, George R C, Hansman D, Liñares J, Tomasz A, Maynard J, Spratt B G. Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1989;86:8842–8846. doi: 10.1073/pnas.86.22.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dramsi S, Dehoux P, Cossart P. Common features of gram-positive bacterial proteins involved in cell recognition. Mol Microbiol. 1993;9:1119–1122. doi: 10.1111/j.1365-2958.1993.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 46.Elliott M, Kearney J F. Idiotype regulation of development of the B-cell repertoire. Ann N Y Acad Sci. 1992;651:336–345. doi: 10.1111/j.1749-6632.1992.tb24633.x. [DOI] [PubMed] [Google Scholar]
- 47.Farley J J, King J C, Nair P, Hines S E, Tressler R L, Vink P E. Invasive pneumococcal disease among infected and uninfected children of mothers with immunodeficiency virus infection. J Pediatr. 1994;124:853–858. doi: 10.1016/s0022-3476(05)83170-1. [DOI] [PubMed] [Google Scholar]
- 48.Feldman C, Mitchell T J, Andrew P W, Boulnois G J, Raad R, Todd H C, Cole P J, Wilson R. The effect of Streptococcus pneumoniae on human respiratory epithelium in vitro. Microb Pathog. 1990;9:275–284. doi: 10.1016/0882-4010(90)90016-j. [DOI] [PubMed] [Google Scholar]
- 49.Finne J, Bitter-Suermann D, Goridis C, Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J Immunol. 1987;138:4402–4407. [PubMed] [Google Scholar]
- 50.Finne J, Leinonen M, Makela P H. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;ii:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 51.Fischetti V A, Parry D A D, Trus B L, Hollingshead S K, Scott J R, Manjula B N. Conformational characteristics of the complete sequence of group A streptococcal M6 protein. Proteins. 1988;3:60–69. doi: 10.1002/prot.340030106. [DOI] [PubMed] [Google Scholar]
- 52.Gillespie S H. Aspects of pneumococcal infection including bacterial virulence, host response and vaccination. J Med Microbiol. 1989;28:237–248. doi: 10.1099/00222615-28-4-237. [DOI] [PubMed] [Google Scholar]
- 53.Giudicelli S, Tomasz A. Attachment of pneumococcal autolysin to wall teichoic acids, an essential step in enzymatic wall degradation. J Bacteriol. 1984;158:1188–1190. doi: 10.1128/jb.158.3.1188-1190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giudicelli S, Tomasz A. Inhibition of the in vitro and in vivo activity of the pneumococcal autolytic enzyme—by choline and phosphorylcholine. In: Nombela C, editor. Microbial cell wall synthesis and autolysis. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1984. pp. 207–212. [Google Scholar]
- 55.Goebel W F, Adams M H. The immunological properties of the heterophile antigen and somatic polysaccharide of pneumococcus. J Exp Med. 1943;77:435–449. doi: 10.1084/jem.77.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goebel W F, Babers F H, Avery O T. Chemo-immunological studies on conjugated carbohydrate-proteins. VIII. The influence of the acetyl group on the specificity of hexoside-protein antigens. J Exp Med. 1934;60:85–94. doi: 10.1084/jem.60.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gotschlich E C, Goldschneider I, Lepow M L, Gold R. The immune response to bacterial polysaccharides in man. In: Haber E, Krause R M, editors. Antibodies in human diagnosis and therapy. New York, N.Y: Raven Press; 1977. pp. 391–402. [Google Scholar]
- 58.Gray B M. Pneumococcal infection in an era of multiple antibiotic resistance. Adv Pediatr Infect Dis. 1995;11:55–100. [PubMed] [Google Scholar]
- 59.Gray B M, Converse III G M, Dillon H C. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142:923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 60.Hakenbeck R, Briese T, Chalkley L, Ellerbrok H, Kalliokoski R, Latorre C, Leinonen M, Martin C. Antigenic variation of penicillin-binding proteins from penicillin-resistant clinical strains of Streptococcus pneumoniae. J Infect Dis. 1991;164:313–319. doi: 10.1093/infdis/164.2.313. [DOI] [PubMed] [Google Scholar]
- 61.Hakenbeck R, Briese T, Chalkley L, Ellerbrok H, Kalliokoski R, Latorre C, Leinonen M, Martin C. Variability of penicillin-binding proteins from penicillin-sensitive Streptococcus pneumoniae. J Infect Dis. 1991;164:307–312. doi: 10.1093/infdis/164.2.307. [DOI] [PubMed] [Google Scholar]
- 62.Hammerschmidt S, Talay S R, Brandtzaeg P, Chhatwal G S. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol. 1997;25:1113–1124. doi: 10.1046/j.1365-2958.1997.5391899.x. [DOI] [PubMed] [Google Scholar]
- 63.Hayrinen J, Jennings H, Raff H V, Rougon G, Hanai N, Gerardy-Schahn R, Finne J. Antibodies to polysialic acid and its N-propyl derivative: binding properties and interaction with human embryonal brain glycopeptides. J Infect Dis. 1995;171:1481–1490. doi: 10.1093/infdis/171.6.1481. [DOI] [PubMed] [Google Scholar]
- 64.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995;33:2759–2762. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hobden J A, Hagenah M, O’Callaghan R J, Hill J M. Confirmation of the role of pneumolysin in occular infections with Streptococcus pneumoniae. Investig Ophthalmol Visual Sci. 1992;33:1422. [Google Scholar]
- 66.Hollingshead, S. K., R. S. Becker, and D. E. Briles. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 67.Hollingshead, S. K., and D. E. Briles. Unpublished data.
- 68.Insel R A, Anderson P W. Oligosaccharide-protein conjugate vaccines induce and prime for oligoclonal IgG antibody responses to the Haemophilus influenzae b capsular polysaccharide in human infants. J Exp Med. 1986;163:262–269. doi: 10.1084/jem.163.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson M K, Hobden J A, Hagenah M, O’Callaghan R J, Hill J M. The role of pneumolysin in ocular infections with Streptococcus pneumoniae. Curr Eye Res. 1990;11:1107–1114. doi: 10.3109/02713689008997584. [DOI] [PubMed] [Google Scholar]
- 70.Käythy H, Eskola J. New vaccines for the prevention of pneumococcal infections. Emerg Infect Dis. 1996;2:289–298. doi: 10.3201/eid0204.960404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kearney J F. Idiotypic networks. In: Paul W E, editor. Fundamental immunology. New York, N.Y: Raven Press; 1989. pp. 663–676. [Google Scholar]
- 72.Kenny J J, Guelde G, Fisher R T, Longo D L. Induction of phosphocholine-specific antibodies in X-linked immune deficient mice: in vivo protection against a Streptococcus pneumoniae challenge. Int Immunol. 1993;6:561–568. doi: 10.1093/intimm/6.4.561. [DOI] [PubMed] [Google Scholar]
- 73.Koeuth T, Versalovic J, Lupski J R. Differential subsequence conservation of interspersed repetitive Streptococcus pneumoniae Box elements in diverse bacteria. Genome Res. 1995;5:408–418. doi: 10.1101/gr.5.4.408. [DOI] [PubMed] [Google Scholar]
- 74.Langermann S, Palaszynski S R, Burlein J E, Koenig S, Hanson M S, Briles D E, Stover C K. Protective humoral response against pneumococcal infection in mice elicited by recombinant Bacille Calmette-Guérin vaccines expressing PspA. J Exp Med. 1994;180:2277–2286. doi: 10.1084/jem.180.6.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee C-J, Banks S D, Li J P. Virulence, immunity, and vaccine related to Streptococcus pneumoniae. Crit Rev Microbiol. 1991;18:89–114. doi: 10.3109/10408419109113510. [DOI] [PubMed] [Google Scholar]
- 76.Lefever J C, Faucon G, Sicard A M, Gasc A M. DNA fingerprinting of Streptococcus pneumoniae strains by pulse-field gel electrophoresis. Infect Immun. 1993;31:2724–2728. doi: 10.1128/jcm.31.10.2724-2728.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lock R A, Hansman D, Paton J C. Comparative efficacy of autolysin and pneumolysin as immunogens protecting mice against infection by Streptococcus pneumoniae. Microb Pathog. 1992;12:137–143. doi: 10.1016/0882-4010(92)90116-6. [DOI] [PubMed] [Google Scholar]
- 78.Lock R A, Paton J C, Hansman D. Comparative efficacy of pneumococcal neuraminidase and pneumolysin as immunogens protective against Streptococcus pneumoniae. Microb Pathog. 1988;5:461–467. doi: 10.1016/0882-4010(88)90007-1. [DOI] [PubMed] [Google Scholar]
- 79.Lomholt H. Evidence for recombination and an antigenically diverse immunoglobulin A1 protease among strains of Streptococcus pneumoniae. Infect Immun. 1995;63:4238–4243. doi: 10.1128/iai.63.11.4238-4243.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Macrina F L, Evans R P, Tobian J A, Hartley D L, Clewell D B, Jones K R. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene. 1983;25:145–150. doi: 10.1016/0378-1119(83)90176-2. [DOI] [PubMed] [Google Scholar]
- 81.McDaniel L S, Briles D E. Monoclonal antibodies against surface components of Streptococcus pneumoniae. In: Macario A J L, de Macario E C, editors. Monoclonal antibodies against bacteria. Orlando, Fla: Academic Press, Inc.; 1986. pp. 143–164. [Google Scholar]
- 82.McDaniel L S, Briles D E. A pneumococcal surface protein (PspB) that exhibits the same protease sensitivity as streptococcal R antigen. Infect Immun. 1988;56:3001–3003. doi: 10.1128/iai.56.11.3001-3003.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McDaniel L S, Ralph B A, McDaniel D O, Briles D E. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb Pathog. 1994;17:323–337. doi: 10.1006/mpat.1994.1078. [DOI] [PubMed] [Google Scholar]
- 84.McDaniel L S, Scott G, Kearney J F, Briles D E. Monoclonal antibodies against protease sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J Exp Med. 1984;160:386–397. doi: 10.1084/jem.160.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McDaniel L S, Scott G, Widenhofer K, Carroll J M, Briles D E. Analysis of a surface protein of Streptococcus pneumoniae recognized by protective monoclonal antibodies. Microb Pathog. 1986;1:519–531. doi: 10.1016/0882-4010(86)90038-0. [DOI] [PubMed] [Google Scholar]
- 86.McDaniel L S, Sheffield J S, Delucchi P, Briles D E. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect Immun. 1991;59:222–228. doi: 10.1128/iai.59.1.222-228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McDaniel L S, Sheffield J S, Swiatlo E, Yother J, Crain M J, Briles D E. Molecular localization of variable and conserved regions of pspA, and identification of additional pspA homologous sequences in Streptococcus pneumoniae. Microb Pathog. 1992;13:261–269. doi: 10.1016/0882-4010(92)90036-n. [DOI] [PubMed] [Google Scholar]
- 88.McDaniel L S, Waltman II W D, Gray B, Briles D E. A protective monoclonal antibody that reacts with a novel antigen of pneumococcal teichoic acid. Microb Pathog. 1987;3:249–260. doi: 10.1016/0882-4010(87)90058-1. [DOI] [PubMed] [Google Scholar]
- 89.McDaniel L S, Yother J, Vijayakumar M, McGarry L, Guild W R, Briles D E. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA) J Exp Med. 1987;165:381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McNamara M K, Ward R E, Kohler H. Monoclonal idiotype vaccine against Streptococcus pneumoniae infection. Science. 1984;226:1325–1326. doi: 10.1126/science.6505692. [DOI] [PubMed] [Google Scholar]
- 91.Mitchell T J, Andrew P W, Saunders F K, Smith A N, Boulnois G J. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol Microbiol. 1991;5:1883–1888. doi: 10.1111/j.1365-2958.1991.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 92.Mitchell T J, Mendez F, Paton J C, Andrew P W, Boulnois G J. Comparison of pneumolysin genes and proteins from Streptococcus pneumoniae types 1 and 2. Nucleic Acids Res. 1990;18:4010. doi: 10.1093/nar/18.13.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mosier D E, Zitron I M, Mond J, Aftab A, Scher I, Paul W E. Surface immunoglobulin D as a functional receptor for a subclass of B lymphocytes. Immunol Rev. 1977;37:89–104. doi: 10.1111/j.1600-065x.1977.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 94.Munoz R, Coffey T, Daniels M, Dowson C G, Laible G, Casal J, Hakenbeck R, Jacobs M, Musser J M, Spratt B G, Tomasz A. Intercontinental spread of a multi-resistant clone of serotype 23F of Streptococcus pneumoniae. J Infect Dis. 1991;164:302–306. doi: 10.1093/infdis/164.2.302. [DOI] [PubMed] [Google Scholar]
- 95.Munoz R, Musser J M, Crain M, Briles D E, Marton A, Parkinson A J, Sorensen U, Tomasz A. Geographic distribution of penicillin-resistant clones of Streptococcus pneumoniae: characterization by penicillin-binding protein profile, surface protein A typing, and multilocus enzyme analysis. Clin Infect Dis. 1992;15:112–118. doi: 10.1093/clinids/15.1.112. [DOI] [PubMed] [Google Scholar]
- 96.Paton J C, Lock R A, Hansman D C. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect Immun. 1983;40:548–552. doi: 10.1128/iai.40.2.548-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paton J C, Rowan-Kelly B, Ferrante A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun. 1984;43:1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perlmutter R M, Crews S T, Douglas R, Sorensen G, Johnson N, Nivera N, Gearhart P J, Hood L. The generation of diversity in phosphorylcholine-binding antibodies. Adv Immunol. 1984;35:1–37. doi: 10.1016/s0065-2776(08)60572-6. [DOI] [PubMed] [Google Scholar]
- 99.Robbins J B, Austrian R, Lee C-J, Rastogi S C, Schiffman G, Henrichsen J, Makela P H, Broome C V, Facklam R R, Tiesjema R H, Parke J C., Jr Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983;148:1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- 100.Robinson, D. A., S. K. Hollingshead, A. J. Parkinson, D. E. Briles, and M. J. Crain. The IS1167 insertion sequence is a phenotypically informative marker among isolates of serotype 6B Streptococcus pneumoniae. Mol. Evol., in press. [DOI] [PubMed]
- 101.Robinson, D. A., J. S. Turner, R. R. Facklam, A. J. Parkinson, R. F. Breiman, M. Gratten, S. K. Hollingshead, D. E. Briles, and M. J. Crain. Molecular characterization of a globally distributed lineage of serotype 12F Streptococcus pneumoniae causing invasive disease. Submitted for publication. [DOI] [PubMed]
- 102.Rodriguez-Barradas M C, Tharapel R A, Groover J E, Giron K P, Lacke C E, Houston E D, Hammill R J, Steinhoff M C, Musher D M. Colonization by Streptococcus pneumoniae among human immunodeficiency virus-infected adults: prevalence of antibiotic resistance, impact of immunization, and characterization by polymerase chain reaction with box primers of isolates from persistent S. pneumoniae carriers. J Infect Dis. 1997;175:590–597. doi: 10.1093/infdis/175.3.590. [DOI] [PubMed] [Google Scholar]
- 103.Rosenow C, Ryan P, Weiser J N, Johnson S, Fontan P, Ortqvist A, Masure H R. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 104.Rubins J B, Charboneau D, Paton J C, Mitchell T J, Andrew P W. Dual function of pneumolysin in the early pathogenesis of purine pneumococcal pneumonia. J Clin Invest. 1994;95:142–150. doi: 10.1172/JCI117631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rubins J B, Duane P G, Clawson D, Charboneau D, Young J, Niewoehner D E. Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect Immun. 1993;61:1352–1358. doi: 10.1128/iai.61.4.1352-1358.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Russell H, Tharpe J A, Wells D E, White E H, Johnson J. Monoclonal antibody recognizing a species-specific protein from Streptococcus pneumoniae. J Clin Microbiol. 1990;28:2191–2195. doi: 10.1128/jcm.28.10.2191-2195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sampson J S, O’Connor S P, Stinson A R, Tharpe J A, Russell H. Cloning and nucleotide sequence analysis of psaA, the Streptococcus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp. adhesins. Infect Immun. 1994;62:319–324. doi: 10.1128/iai.62.1.319-324.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sanz J M, Lopez R, Garcia J L. Structural requirements of choline derivatives for ‘conversion’ of pneumococcal amidase. FEBS Lett. 1988;232:308–312. doi: 10.1016/0014-5793(88)80759-2. [DOI] [PubMed] [Google Scholar]
- 109.Schroer K R, Kim J K, Prescott B, Baker P J. Generation of anti-type III pneumococcal polysaccharide hybridomas from mice with an X-linked B lymphocyte defect. J Exp Med. 1979;150:689–702. doi: 10.1084/jem.150.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shapiro E D, Berg A T, Austrian R, Schroeder D, Parcells V, Margolis A, Adair R K, Clemmens J D. Protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 111.Sheffield J S, Benjamin W H, McDaniel L M. Detection of DNA in Southern blots by chemiluminescence is a sensitive and rapid technique. BioTechniques. 1992;12:836–839. [PubMed] [Google Scholar]
- 112.Shelly M A, Jacoby H, Riley G J, Graves B T, Pichichero M, Treanor J J. Comparison of pneumococcal polysaccharide and CRM197 conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect Immun. 1997;65:242–247. doi: 10.1128/iai.65.1.242-247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siber G R. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 114.Steinhoff M C, Edwards K, Keyserling H, Thoms M L, Johnson C, Madore D, Hogerman D. A randomized comparison of three bivalent Streptococcus pneumoniae glycoprotein conjugate vaccines in young children: effect of polysaccharide size and linkage characteristics. Pediatr Infect Dis J. 1994;13:368–372. doi: 10.1097/00006454-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 115.Swiatlo E, Brooks-Walter A, Briles D E, McDaniel L S. Oligonucleotides identify conserved and variable regions of pspA and pspA-like sequences of Streptococcus pneumoniae. Gene. 1997;188:279–284. doi: 10.1016/s0378-1119(96)00823-2. [DOI] [PubMed] [Google Scholar]
- 116.Swiatlo E, Crain M J, McDaniel L S, Brooks-Walter A, Coffey T J, Spratt B G, Morrison D A, Briles D E. DNA polymorphisms and variant penicillin-binding proteins as evidence that relatively penicillin-resistant pneumococci in western Canada are clonally related. J Infect Dis. 1996;174:884–888. doi: 10.1093/infdis/174.4.884. [DOI] [PubMed] [Google Scholar]
- 117.Szu S C, Clark S, Robbins J B. Protection against pneumococcal infection in mice conferred by phosphocholine antibodies: specificity of the phosphocholine binding in relation to several types. Infect Immun. 1983;39:993–999. doi: 10.1128/iai.39.2.993-999.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Takala A K, Eskola J, Leinonen M, Kaythy H, Nissinen A, Pekkanen E. Reduction of oropharyngeal carriage of Haemophilus influenzae type b (Hib) in children immunized with an Hib conjugate vaccine. J Infect Dis. 1991;164:982–986. doi: 10.1093/infdis/164.5.982. [DOI] [PubMed] [Google Scholar]
- 119.Takala A K, Vuopio-Varkila J, Tarkka E, Leinonen M, Musser J M. Subtyping of common pediatric pneumococcal serotypes from invasive disease and pharyngeal carriage in Finland. J Infect Dis. 1996;173:128–135. doi: 10.1093/infdis/173.1.128. [DOI] [PubMed] [Google Scholar]
- 120.Talkington D F, Brown B G, Tharpe J A, Koening A, Russell H. Protection of mice against fatal pneumococcal challenge by immunization with pneumococcal surface adhesion A (PsaA) Microb Pathog. 1996;21:17–22. doi: 10.1006/mpat.1996.0038. [DOI] [PubMed] [Google Scholar]
- 121.Talkington D F, Crimmins D L, Voellinger D C, Yother J, Briles D E. A 43-kilodalton pneumococcal surface protein, PspA: isolation, protective abilities, and structural analysis of the amino-terminal sequence. Infect Immun. 1991;59:1285–1289. doi: 10.1128/iai.59.4.1285-1289.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Talkington D F, Voellinger D C, McDaniel L S, Briles D E. Analysis of pneumococcal PspA microheterogeneity in SDS polyacrylamide gels and the association of PspA with the cell membrane. Microb Pathog. 1992;13:343–355. doi: 10.1016/0882-4010(92)90078-3. [DOI] [PubMed] [Google Scholar]
- 123.Tao L, LeBlanc D J, Ferretti J J. Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene. 1992;120:105–110. doi: 10.1016/0378-1119(92)90016-i. [DOI] [PubMed] [Google Scholar]
- 124.Tart, R. C., and D. E. Briles. Unpublished data.
- 125.Tart R C, McDaniel L S, Ralph B A, Briles D E. Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J Infect Dis. 1996;173:380–386. doi: 10.1093/infdis/173.2.380. [DOI] [PubMed] [Google Scholar]
- 126.Tharpe J A, Russell H. Purification and seroreactivity of pneumococcal surface adhesin A (PsaA) Clin Diagn Lab Immunol. 1996;3:227–229. doi: 10.1128/cdli.3.2.227-229.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of pneumococcus: chain formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci USA. 1968;59:86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tomasz A. Surface components of Streptococcus pneumoniae. Rev Infect Dis. 1981;3:190–211. doi: 10.1093/clinids/3.2.190. [DOI] [PubMed] [Google Scholar]
- 129.Vakil M, Briles D E, Kearney J F. Antigen-independent selection of T15 idiotype during B-cell ontogeny in mice. Dev Immunol. 1991;1:203–212. doi: 10.1155/1991/45352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vakil M, Kearney J F. Functional relationship between T15 and J558 idiotypes in BALB/c mice. Dev Immunol. 1991;1:213–224. doi: 10.1155/1991/91729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vella P, Marburg S, Staub J, Kniskern P, Miller W, Hagopian A, Tolman R, Rusk C, Chupak L S, Ellis R. Immunogenicity of conjugate vaccines of pneumococcal capsular polysaccharide types 6B, 14, 19F, and 23F with a meningococcal outer membrane protein complex. Infect Immun. 1992;60:4977–4983. doi: 10.1128/iai.60.12.4977-4983.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Viering T P, Fine D P. Genetic analysis of Streptococcus pneumoniae serotypes with the use of DNA fingerprinting. J Infect Dis. 1989;160:76–82. doi: 10.1093/infdis/160.1.76. [DOI] [PubMed] [Google Scholar]
- 133.Walker J A, Allen R L, Falmagne P, Johnson M K, Boulnois G J. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect Immun. 1987;55:1184–1189. doi: 10.1128/iai.55.5.1184-1189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wallick S, Claflin J L, Briles D E. Resistance to Streptococcus pneumoniae is induced by a phosphocholine-protein conjugate. J Immunol. 1983;130:2871–2875. [PubMed] [Google Scholar]
- 135.Waltman W D, II, Gray B, McDaniel L S, Briles D E. Cross-reactive monoclonal antibodies for diagnosis of pneumococcal meningitis. J Clin Microbiol. 1988;26:1635–1640. doi: 10.1128/jcm.26.9.1635-1640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Waltman W D, II, Gray B M, Svanborg C, Facklam R, Briles D E. Epidemiologic studies of group 9 pneumococci in terms of protein type and 9N versus 9V capsular type. J Infect Dis. 1991;163:812–818. doi: 10.1093/infdis/163.4.812. [DOI] [PubMed] [Google Scholar]
- 137.Waltman W D, II, McDaniel L S, Andersson B, Bland L, Gray B M, Svanborg-Eden C, Briles D E. Protein serotyping of Streptococcus pneumoniae based on reactivity to six monoclonal antibodies. Microb Pathog. 1988;5:159–167. doi: 10.1016/0882-4010(88)90018-6. [DOI] [PubMed] [Google Scholar]
- 138.Waltman W D, II, McDaniel L S, Gray B M, Briles D E. Variation in the molecular weight of PspA (pneumococcal surface protein A) among Streptococcus pneumoniae. Microb Pathog. 1990;8:61–69. doi: 10.1016/0882-4010(90)90008-e. [DOI] [PubMed] [Google Scholar]
- 139.Wortham C, Grinberg L, Kaslow D C, Briles D E, McDaniel L S, Lees A, Flora M, Snapper C M, Mond J J. Enhanced protective antibody responses to PspA after intranasal or subcutaneous injections of PspA genetically fused to granulocyte-macrophage colony-stimulating factor or interleukin-2. Infect Immun. 1998;66:1513–1520. doi: 10.1128/iai.66.4.1513-1520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu H-Y, Nahm M, Guo Y, Russell M, Briles D E. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage and infection with Streptococcus pneumoniae. J Infect Dis. 1997;175:893–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 141.Wu H-Y, Virolainen A, Mathews B, King J, Russell M, Briles D E. Establishment of a Streptococcus pneumoniae nasopharyngeal model of pneumococcal carriage in adult mice. Microb Pathog. 1997;23:127–137. doi: 10.1006/mpat.1997.0142. [DOI] [PubMed] [Google Scholar]
- 142.Yother, J. Unpublished data.
- 143.Yother J, Briles D E. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J Bacteriol. 1992;174:601–609. doi: 10.1128/jb.174.2.601-609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yother J, Handsome G L, Briles D E. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J Bacteriol. 1992;174:610–618. doi: 10.1128/jb.174.2.610-618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yother J, McDaniel L S, Briles D E. Transformation of encapsulated Streptococcus pneumoniae. J Bacteriol. 1986;168:1463–1465. doi: 10.1128/jb.168.3.1463-1465.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yother J, White J M. Novel surface attachment mechanism for the Streptococcus pneumoniae protein PspA. J Bacteriol. 1994;176:2976–2985. doi: 10.1128/jb.176.10.2976-2985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhou L, Hui F M, Morrison D A. Characterization of IS1167, a new insertion sequence in Streptococcus pneumoniae. Plasmid. 1995;33:127–138. doi: 10.1006/plas.1995.1014. [DOI] [PubMed] [Google Scholar]