Abstract
Background
Digital health is transforming healthcare delivery.
Objective
To compare the current digital health landscape in select groups of cardiac electrophysiology (EP) professionals prior to and during the COVID-19 era.
Methods
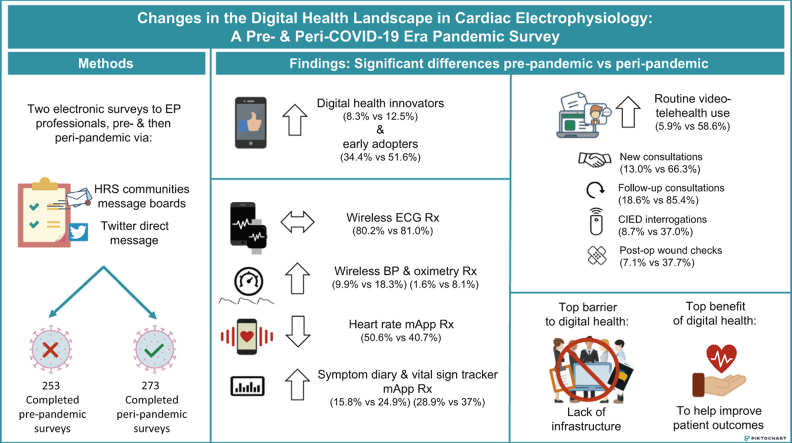
Two online surveys were emailed to 4 Heart Rhythm Society communities and tweeted out to Twitter EP, 1 before and 1 during the pandemic. Categorical variables were analyzed using the χ2 test and reported as absolute numbers and percentages.
Results
There were 253 pre-pandemic (S1) and 273 follow-up surveys (S2) completed. The majority of respondents to both surveys were male, aged <55 years (70.6% vs 75.1%), university-affiliated (52.6% vs 55%), and physicians (83.3% vs 87.9%). Between S1 and S2, routine use of video-telehealth increased (5.9% vs 58.6%; P < .001) for all types of consultations (P < .001 for all). Wireless electrocardiogram prescribing was prevalent and similar (80.2% vs 81.0%), whereas wireless blood pressure monitoring (9.9% vs 18.3%) and wireless oximetry (1.6% vs 8.1%; P = .006 for both) prescribing both increased. For smartphone mobile applications (mApps), prescriptions for heart rate mApps decreased (50.6% vs 40.7%; P = .022), while vital sign (28.9% vs 37%; P = .04) and symptom trackers (15.8% vs 24.9%; P = .01) prescribing increased. A majority in both surveys (84.6% vs 75.5%) reported no workplace infrastructure or support for digital health with concerns for lack of parity in reimbursement.
Conclusion
Our results show an increase in adoption of digital health by EP during the COVID-19 pandemic. Concerns regarding a lack of supportive infrastructure persisted. Development of professional society guidelines on optimal clinical workflow, infrastructure, and reimbursement may help advance and sustain digital health integration in EP.
Keywords: Digital health, Mobile applications, Telehealth, Wearables
Graphical abstract
Introduction
Growing interest in improving healthcare efficiency, personalization, and precision has resulted in exponential growth of digital health technologies over the past decade. The worldwide digital health market, currently valued at an estimated $100 million U.S. dollars, is projected to increase to nearly $400 million by the year 2025.1 Surveys of U.S. adults have shown that nearly 80% used at least 1 type of digital health tool, with many willing to share health data with their clinicians in hopes of living longer and healthier lives.2 According to a study of 1360 U.S. physicians conducted by the American Medical Association, interest in digital heath and its adoption into medical practice increased between the years 2016 and 2019.3 Almost 90% saw at least some advantages for digital health tools, with an increase in the number of physicians using digital tools as well as the number of digital tools used.
Cardiac electrophysiology (EP) has been a leader in the digital health space. Transtelephonic remote monitoring of cardiac implantable electronic devices (CIEDs) was introduced in the 1970s, and remote monitoring technology has continued to evolve with miniaturization of the monitoring device itself and incorporation of wireless transmission and cloud-based data storage technology. Owing to studies demonstrating that earlier detection of clinically actionable and device-related events results in improved healthcare efficiency and outcomes, the Heart Rhythm Society has designated CIED remote monitoring a class I indication, making it the standard of care for all CIED patients.4 In addition, the field of EP has pioneered and revolutionized ambulatory electrocardiogram (ECG) monitoring and mobile cardiac telemetry.5
While EP has been successful in pushing digital health forward with CIED remote monitoring and ambulatory ECG, less is known about other aspects of digital health, including the use of video-telehealth, digital health tools, and mobile applications. In November of 2019, prior to the COVID-19 pandemic, we administered a survey to EP professionals to assess adoption of these digital technologies. Since then, societal statements have recommended the use of these technologies to provide EP care during the pandemic,6 but how the use of digital health in EP has been impacted by the pandemic and these new guidelines has not been characterized. We took the opportunity to assess the impact of the COVID-19 pandemic on the landscape of digital health in EP by readministering a second survey in July 2020 to assess trends in digital health adoption by EP professionals prior to and during the pandemic era.
Methods
Two electronic surveys were created using Zoho Surveys (Zoho, Ltd, Pleasanton, CA). The target audience for both surveys was cardiac EP professionals, including cardiac EP physicians, nurse practitioners, physician assistants, nurses, technicians, scientists, and pharmacists. The surveys were sent via 2 avenues. A message containing an explanation of the voluntary study as well as a link to the electronic survey was posted broadly to the following Heart Rhythm Society communities’ message boards: Member open forum, Allied Professionals community of practice, Early-Career community of practice, and the Women in EP community of practice. The same message containing the survey link was also sent via Twitter to cardiac EP professionals and was retweeted organically by the Twitter EP community using the #EPeeps hashtag and tagging @EPeeps_Bot. A tweet with the survey link was also sent out in Spanish by the Latin American Heart Rhythm Society and in Portuguese by the Sociedade Brasileira de Arritmias Cardíacas for amplification to their followers. Each respondent could only complete the survey once.
Both surveys remained active for 1 week. The first survey (S1) was conducted between November 29, 2019, and December 6, 2019 (prior to the COVID-19 pandemic) and consisted of 17 multiple choice questions. For some questions more than 1 response could be chosen. Demographic questions included gender, age, primary occupation, and practice setting. Other questions pertained to details of digital health adoption, including use of video-telehealth, digital health tools, smartphone mobile applications (mApps, defined as smartphone-based interactive applications solely using the smartphone and not requiring additional tools or using a wearable device) and prescriptions, perceived benefits and barriers to digital health, and self-characterization of digital health adoption. For the latter, 5 choices were provided with the following definitions. Innovators were defined as risk-takers who have the highest risk tolerance, early adopters were opinion leaders with moderate risk tolerance, early majority adopters were those who need evidence and prioritize utility or practicality, late adopters were skeptics who adapt out of necessity, and very late adopters were traditionalists or resistant to change.7 The second survey (S2) was conducted between July 6, 2020, and July 13, 2020, approximately 6 months after the first COVID-19 case was reported in the United States.8 The same questions from the first survey were included in the second survey with the addition of 5 questions to assess respondents’ perceptions of the effect of the pandemic on their adoption of digital health. Categorical variables are reported as absolute numbers and percentages and responses to S1 and S2 were compared using the χ2 test, under the assumption that the responses to both surveys were predominantly independent of each other. A 2-sided P value <.05 was used to denote statistical significance. The research reported in this study adhered to the Helsinki Declaration as revised in 2013.
Results
Baseline demographics
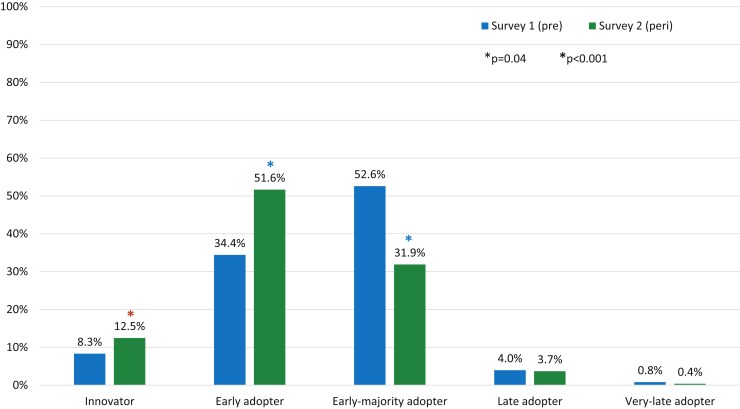
The total number of respondents increased between S1 and S2 (253 vs 273), as did the number of male respondents (61.7% vs 72.5%, P = .008). Other baseline demographics of the respondents did not change significantly between the 2 surveys. The majority of respondents to both surveys were between the ages of 35 and 44 years, were physicians, and practiced in a university or academic center (Table 1). Respondents identifying as digital health innovators and early adopters significantly increased between S1 and S2 (8.3% vs 12.5%, P = .04, and 34.4% vs 51.6%, P < .001), while early majority adopters significantly decreased (52.6% vs 31.9%, P < .001) (Figure 1).
Table 1.
Baseline demographics
| S1 | S2 | P value | |
|---|---|---|---|
| Total n | 253 | 273 | |
| Gender, n (%) | |||
| Male | 156 (61.7) | 198 (72.5) | .008 |
| Female | 97 (38.3) | 75 (27.5) | |
| Age (years), n (%) | |||
| 25–34 | 11 (4.4) | 16 (5.9) | .432 |
| 35–44 | 118 (46.6) | 119 (43.6) | .482 |
| 45–54 | 61 (24.1) | 86 (31.5) | .059 |
| 55–64 | 50 (19.8) | 42 (15.4) | .187 |
| 65–74 | 12 (4.7) | 10 (3.7) | .536 |
| >75 | 1 (0.4) | 0 | n/a |
| Occupation, n (%) | |||
| Physician | 216 (85.4) | 240 (87.9) | .392 |
| Nurse practitioner / physician assistant | 24 (9.5) | 23 (8.4) | .670 |
| Registered nurse / licensed vocational nurse | 7 (2.8) | 2 (0.7) | .072 |
| Technician | 2 (0.8) | 4 (1.5) | .467 |
| Researcher | 4 (1.5) | 3(1.1) | .630 |
| Pharmacist | 0 | 1 (0.4) | n/a |
| Other | 0 | 0 | n/a |
| Practice setting, n (%) | |||
| University/academic | 133 (52.6) | 150 (54.9) | .585 |
| Private practice | 68 (26.9) | 86 (31.5) | .244 |
| Health Maintenance Organization | 2 (0.8) | 3 (1.1) | .716 |
| Public/county facility | 10 (3.9) | 7 (2.6) | .368 |
| Government | 17 (6.7) | 9 (3.3) | .070 |
| Other | 23 (9.1) | 18 (6.6) | .286 |
S1 = pre-pandemic survey; S2 = peri-pandemic survey.
Figure 1.
Comparison of self-characterization of digital health adoption, Survey 1 (pre-pandemic) vs survey 2 (peri-pandemic). Categories based on Rogers.7 ∗ = statistically significant as listed in figure legend.
Video-telehealth use
Two-hundred forty-two respondents (88.6%) reported that video-telehealth use had increased compared to before the pandemic. Routine and occasional users of video-telehealth significantly increased between S1 and S2 (5.9% vs 58.6% and 13% vs 31.9%, respectively; P < .001 for both) (Table 2). The use of video-telehealth for all types of consultations (new and follow-up consultations, CIED interrogations, and postoperative wound check) all significantly increased (P < .001 for all) (Table 2). The percentage of respondents who did not use video-telehealth dropped substantially (74.3% vs 8.8%, respectively; P < .001) between the 2 surveys.
Table 2.
Comparison of video-telehealth use, overall and by consultation type, pre-pandemic vs peri-pandemic
| S1 | S2 | P value | |
|---|---|---|---|
| Video-telehealth use, n (%) | |||
| Routinely | 15 (5.9) | 160 (58.6) | <.001 |
| Occasionally | 33 (13) | 87 (31.9) | <.001 |
| Never, but would like to | 149 (58.9) | 18 (6.6) | <.001 |
| Never, would rather not | 51 (20.12) | 5 (1.8) | <.001 |
| Characteristics of video-telehealth use, n (%) | |||
| New consultation | 33 (13) | 181 (66.3) | <.001 |
| Follow-up consultation | 47(18.6) | 233 (85.4) | <.001 |
| CIED interrogation | 22 (8.7) | 101 (37) | <.001 |
| Wound-check only | 18 (7.1) | 103 (37.7) | <.001 |
| Other | 6 (2.4) | 14 (5.1) | <.001 |
| I do not use video-telehealth | 188 (74.3) | 24 (8.8) | <.001 |
CIED = cardiac implantable electronic device; S1 = pre-pandemic survey; S2 = peri-pandemic survey.
Video-telehealth use overall and for all types of consultations increased significantly in S2.
Use of digital health tools and mobile applications
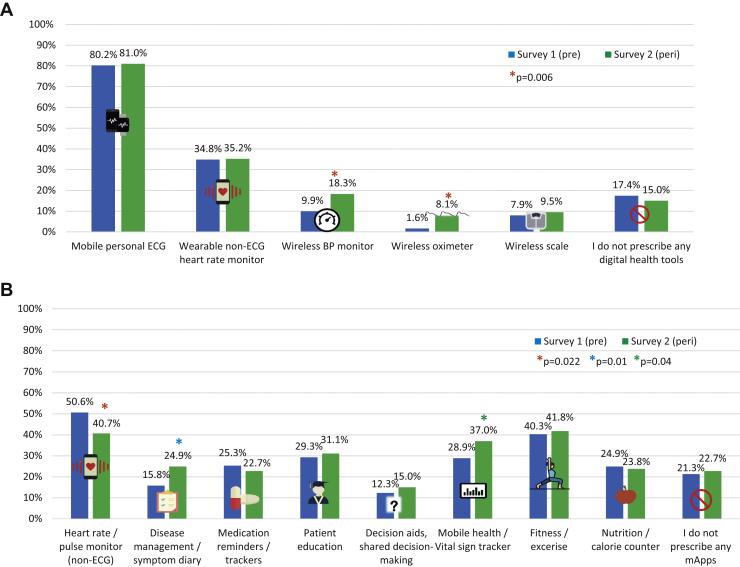
A majority of respondents to both surveys recommended or prescribed mobile or personal ECG and this number was similar between S1 and S2 (80.2% vs 81.0%). Similarly, wearable non-ECG heart rate monitor and wireless scale prescribing did not change, while wireless blood pressure monitor and wireless oximeter prescribing both significantly increased (9.9% vs 18.3% and 1.6% vs 8.1%, respectively, P = .006 for both) (Table 3, Figure 2A). With respect to smartphone mApps, a significantly lower percentage recommended non-ECG heart rate and pulse monitors to their patients (50.6% vs 40.7%, P = .022), whereas a significantly larger percentage recommended disease management and vital sign trackers (28.9% vs 37%, P = .04, and 15.8% vs 24.9%, P = .01, respectively). Prescribing of other mApps did not change (Table 3, Figure 2B).
Table 3.
Prescribing of digital health tools and smartphone mobile applications (mApps), pre-pandemic vs peri-pandemic
| S1 | S2 | P value | |
|---|---|---|---|
| Digital health tools, n (%) | |||
| Mobile personal ECG (smartwatch & non-smartwatch) | 203 (80.2) | 221 (81) | .834 |
| Wearable non-ECG heart rate monitor | 88 (34.8) | 96 (35.2) | .927 |
| Wireless blood pressure monitor | 25 (9.9) | 50 (18.3) | .006 |
| Wireless oximeter | 4 (1.6) | 22 (8.1) | .006 |
| Wireless scale | 20 (7.9) | 26 (9.5) | .511 |
| I do not prescribe/recommend any digital health tools | 44 (17.4) | 41 (15) | .46 |
| Smartphone mApps, n (%) | |||
| Heart rate / pulse monitor (non-ECG) | 128 (50.6) | 111 (40.7) | .022 |
| Disease management / symptom diary | 40 (15.8) | 68 (24.9) | .01 |
| Medication reminders / trackers | 64 (25.3) | 62 (22.7) | .487 |
| Patient education | 74 (29.3) | 85 (31.1) | .638 |
| Decision aids, shared decision-making | 31 (12.3) | 41 (15) | .357 |
| Mobile health / vital sign tracker | 73 (28.9) | 101 (37) | .04 |
| Fitness / exercise | 102 (40.3) | 114 (41.8) | .737 |
| Nutrition / calorie counter | 63 (24.9) | 65 (23.8) | .771 |
| I do not recommend any mApps | 54 (21.3) | 62 (22.7) | .066 |
ECG = electrocardiogram; mApps = mobile applications; S1 = pre-pandemic survey; S2 = peri-pandemic survey.
Prescribing wireless blood pressure monitor and wireless oximetry as well as mApps for disease management and vital sign trackers all increased significantly, while heart rate monitoring mApp prescribing decreased significantly in S2.
Figure 2.
Comparison of digital health tool (A) and smartphone mobile application (mApp) (B) prescribing, survey 1 (pre-pandemic) vs survey 2 (peri-pandemic). ∗ = statistically significant as listed in figure legend. BP = blood pressure; ECG = electrocardiogram; Peri = peri-pandemic; Pre = pre-pandemic.
Estimated use of wearable and smart devices by patients, and frequency patients were seen with smart device–detected arrhythmias
Estimations of patient usage of digital wearables and smart devices did not change significantly between S1 and S2 (P = .852 for trend). The number of patients seen with an arrhythmia diagnosed by a wearable or a smart device did not change significantly either (P = .966 for trend).
Perceived barriers and benefits to digital health
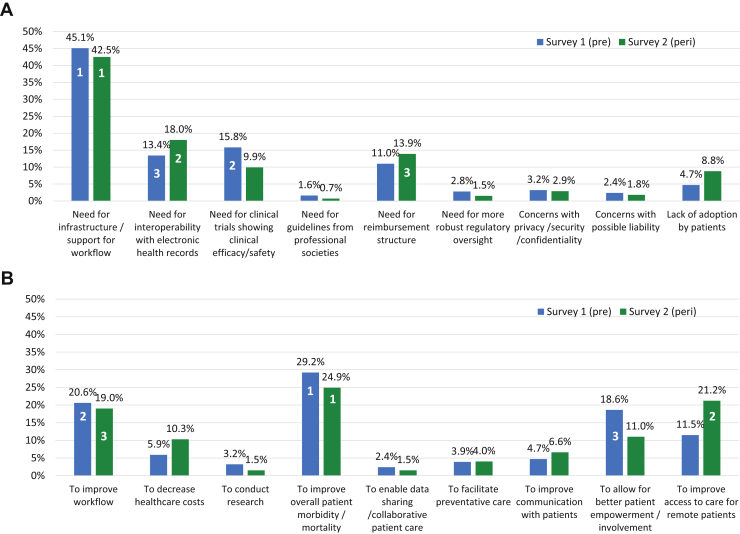
A majority of respondents in both surveys reported lack of workplace infrastructure or support for digital health. However, this lack of infrastructure decreased significantly between S1 and S2 (214 [84.6%] vs 206 [75.5%], P = .009). In S1, the top 3 perceived barriers to the adoption of digital health were, in order of importance, the need for workplace digital health infrastructure/support, the need for clinical trials showing efficacy and/or safety, and the need for interoperability with electronic medical records (EMR). In S2, the top perceived barrier remained the same as S1, while the second became the need for interoperability with EMR, and the third was need for reimbursement structure (Figure 3A). In S1, the top 3 perceived benefits of digital health were to improve overall patient morbidity or mortality, to improve workflow, and for better patient empowerment/involvement in healthcare. In S2, the top perceived benefit remained the same as in S1, while the second became to improve access to care for remote patients, and third was to improve workflow (Figure 3B).
Figure 3.
Comparison of perceived barriers (A) and benefits (B) to digital health, survey 1 (pre-pandemic) vs survey 2 (peri-pandemic). Lower number signifies greater importance. Peri = peri-pandemic; Pre = pre-pandemic.
Perceptions by respondents on the impact of the pandemic on digital health
In the second survey, we included additional questions regarding the perception of how the pandemic impacted adoption of digital health technologies. Two-hundred forty-two (88.6%) responded that their use of video-telehealth had increased compared to prior to the pandemic; 193 (70.7%) responded that they increased their regular use of digital tools, wearables, and smart devices; and 113 (41.4%) reported that their mApp prescribing increased as well. One hundred and one (61.5%) felt that there was no change in the number of patients being seen for smart device–detected arrhythmias. With regard to infrastructure to support digital technologies, 158 (57.9%) stated that there was improvement, and 93 (34.1%) reported minimal or no change in infrastructure.
Discussion
In our current survey comprising over 500 responses from EP professionals, we found a significantly increased overall adoption of video-telehealth for all forms of EP consultations, as well as continued incorporation of digital health tools and mobile applications—particularly wireless ECG, wireless blood pressure, and wireless oximetry. Lack of infrastructure, EMR interoperability, and reimbursement concerns were identified as top barriers, while improvement in patient outcomes, workflow, and access to care were identified as potential benefits to digital health.
Prior to the COVID-19 pandemic, only 6% of EP professionals reported routinely using video-telehealth, with 60% interested in eventually incorporating it into their practice. With the pandemic, the routine use of video-telehealth reported on this survey was nearly 10-fold higher. This growth in routine use of video-telehealth was more robust than that seen between the years 2016 and 2019 (14% to 28%) as assessed in the 2019 AMA digital health survey.3 The EP practitioners surveyed reported high integration of video-telehealth into all aspects of their practices, including new and follow-up consultations, CIED interrogations, and wound checks. This increase in adoption is particularly important, as some studies are beginning to suggest that video-telehealth consultations improve patient access to specialty care, decrease hospital admissions or emergency room visits, and possibly improve outcomes.9,10 Some patients and clinicians who have used virtual video visits find them to be equivalent to in-person visits, without a perceived differential in the quality of care.11,12 In a study from the Cleveland Clinic cardiac EP group, 60% of patients and nearly 70% of clinicians preferred to continue with virtual telehealth visits for further follow-up care.13 Telehealth visits for EP care, as compared to in-person visits, may also reduce subsequent hospital encounters or emergency room visits.14 However, those who used telehealth in this latter study were younger and had fewer comorbidities as compared to those who had in-person visits, and this selection bias may have led to higher hospital encounters. In addition, data regarding hard outcomes are lacking, and several randomized trials of telehealth use in heart failure patients failed to show a difference in outcomes, including readmissions and all-cause mortality.15, 16, 17
Incorporation of digital health tools was found to be highly prevalent even prior to the pandemic. Specifically, mobile smartwatch and non-smartwatch ECG devices were prescribed to patients by 80% of respondents to both surveys. Use of these devices may help in the care of cardiac arrhythmia patients and may soon prove to improve health outcomes. In the iHEART study, use of a mobile ECG device paired with a smartphone increased the detection of recurrent atrial arrhythmias after atrial fibrillation ablation, which strongly predicted later arrhythmia recurrence with a trend toward lower hospitalization and emergency room visits.18 The randomized Heartline study (ClinicalTrials.gov identifier: NCT04276441) is currently underway to determine if use of a 1-lead ECG–capable smartwatch can not only increase the diagnosis of atrial fibrillation but also improve major cardiovascular outcomes of stroke, myocardial infarction, and all-cause mortality.
Interestingly, mobile blood pressure and oximetry prescribing increased significantly. This could be explained by 2 factors. With the “stay-at-home” orders, telehealth was adopted at an exponential rate, both in cardiac EP and in medicine as a whole, in an effort to continue to provide safe healthcare. The increase in mobile blood pressure and oximetry monitor prescribing may have simply been a reaction to this pivot to telehealth, allowing clinicians the ability to still obtain vital signs for their virtual patient visits. Secondly, mobile ECG devices can be costlier than mobile blood pressure or oximetry monitoring devices or may not be readily available or affordable to a worldwide population. Mobile blood pressure and oximetry monitors often include photoplethysmography tracing that produces a heart rate reading and can also be used as a basic surrogate for heart rhythm. In some instances, cardiac EP professionals may have had to choose the more affordable and available monitoring tool for their patients, leading to the mild increase in prescribing patterns.
Heart rate or pulse detection mApps were prescribed by 40% to 50% of participants in our study. Although ECG or heart rate detection smartwatches may appear to be ubiquitous, a multitude of photoplethysmography mApps solely using a smartphone camera to detect heart rate are more readily available and more affordable to a majority of the public. They are also easily used, requiring little instruction without the need for extra equipment outside of the smartphone itself, and have been shown to strongly correlate with gold-standard ECG heart rates.19 Despite this, heart rate detection mApp prescriptions decreased with the pandemic, while mApps for vital signs and symptom tracking increased. As previously mentioned, our survey showed an increase in mobile blood pressure and oximetry prescribing and because these devices also often read heart rate, stand-alone heart rate monitoring mApps may have been less necessary. With the exception of fitness mApps, prescriptions for other mApps were otherwise relatively evenly distributed in both surveys, which may speak to a perceived lack of usefulness or benefit of other mApps for cardiac EP and their patients.
With the pandemic, our findings showed that providing access to care for those in remote areas became the second most important perceived benefit of telehealth. Telehealth may be effective in helping to improve clinical outcomes in older adults, providing an avenue for healthcare in patients with mobility or distance and travel issues.20 Access to care and continued care have become an increasingly important priorities during the pandemic—especially with limitations of various modes of public transportation and the need to socially distance. As a solution, virtual care and digital health have been adopted at an exponential rate. Stanford University primary care converted from in-person to over 75% virtual care and 20,000 video visits within 2 months of the pandemic, and the Veterans Affairs Healthcare System saw a near 12-fold increase in primary care virtual visits between March and April 2020 alone.21,22 However, the rapid and necessary adoption of virtual care during the pandemic has further highlighted healthcare inequities, and barriers to access continue despite the digital health surge. In the United States, 30% of adults with household incomes less than $30,000 do not own a smartphone and nearly 50% do not have broadband or a computer, making virtual care an impossibility.23 Globally, less than 50% of developing countries have access to the internet.24 Active initiatives to improve telehealth access are beginning. The U.S. federal government recently passed the Coronavirus Aid, Relief, and Economic Security (CARES) Act, which will increase funding from $8.7 million to $29 million for telehealth technologies used in rural and medically underserved areas.25 While these are steps in the right direction, solutions must still be sought for populations such as those with cognitive impairments or language barriers, who continue to face increased challenges with virtual care.21 In addition, visual inspection over video-telehealth without in-person physical examination for many health conditions is simply inadequate and could lead to the semblance of stability while hiding critical illness.26 Overall, despite perceived and real advantages to digital health and telehealth, it must be remembered that these technologies are not replacements for face-to-face care. Rather, all of these forms of care are complementary, with each enhancing the other.
Despite some perceived improvements during the pandemic, lack of infrastructure was still cited as the most common major barrier to adoption of digital health, with lack of healthcare record interoperability ranking second. Major medical societies, including the European Society of Cardiology and the American College of Cardiology, as well as the Heart Rhythm Society, have created digital health working groups in an effort to better help incorporate digital health into day-to-day care of cardiovascular disease patients. Creation of society-endorsed optimal clinical workflows and infrastructure guidelines will be key to increasing the adoption of digital health not just by cardiac EPs but by other clinicians. Notably with the pandemic, the need for a digital health / telehealth reimbursement structure became the third most important barrier, as payment parity with in-person visits was found to be lacking. While governing agencies have revised reimbursement rules for telehealth to be more encompassing and are requiring payment parity during the pandemic, whether this will remain in effect after the pandemic subsides remains to be seen.27 Lastly, while the need for clinical trials showing efficacy and/or safety was the second most important barrier to the adoption of digital health pre-pandemic, this did not rank into the top 3 barriers as the pandemic progressed. Clinicians on the whole, as well as our own survey respondents, may have felt that the need for, and benefits of, decreasing potential exposure of both patients and clinicians to SARS-CoV-2 through digital health outweighed risks from the current lack of trials to establish digital health outcomes or safety. Increased adoption may also reflect mandated use of digital health by employers or institutions owing to the pandemic.28 Importantly, clinical trials in this space should be conducted to better understand the role, safety, and outcomes of continued routine use of digital health and telehealth in patient care outside of pandemic times. In this vein, a recent “think tank” consisting of stakeholders from cardiology academia, industry, professional organizations as well as government and regulatory agencies was convened to help lay the groundwork to address issues surrounding delivery of digital healthcare and conducting digital health research.29
Limitations
This study has several limitations. First, the number of responses to our survey was small, despite distribution to a large audience. This did not allow for more definitive conclusions to be drawn based on analysis of subgroups of respondents (ie, digital health adopter type, practice setting, etc); future trials with larger sample sizes with these objectives specified a priori may be needed to better understand survey results. Second, our methods used to compare the survey responses assumed that responses were independent, and because the survey was anonymous we do not know whether, or to what degree, there might have been overlap between the respondents. Third, this was an electronic survey with respondents who were mainly <55 years of age, of undisclosed race/ethnicity, practicing in an academic center. Respondents who are more technologically advantaged (ie, having better access to digital or mobile devices and/or internet access) may be overrepresented, and thus the opinions and adoption of digital and telehealth may not be representative of all EP practitioners. In addition, questions did not geolocate respondents, and results may be less applicable to areas where digital technology, internet access, and infrastructure may be less readily available. Lastly, this survey was conducted during a time when the COVID-19 pandemic had subsided in some parts of the world but was still active or surging in other parts. Some institutions are already reporting a substantial decrease of telehealth usage in areas where hospitals have been allowed to reopen for face-to-face visits (personal communication). Sustainability of this rapid digital health adoption should be evaluated post pandemic, when rules and regulations may return to the former nonpandemic status.
Conclusion
This survey, composed of over 500 responses from cardiac EP professionals comparing digital health adoption before and with the current COVID-19 global pandemic, shows a rapid adoption of digital health and, more specifically, video-telehealth by EP practitioners. There was enthusiasm for continued uptake of all forms of digital health within the practices of respondents, including video-telehealth, digital health tools (particularly mobile ECG), and mobile applications, with hopes of improving current workflow, patient outcomes, and healthcare accessibility. Creation of society-sponsored digital health guidelines, especially for supporting infrastructure and workflow, as well as institution of fair and equitable telehealth reimbursement structures, will be crucial to furthering the adoption and sustainability of digital health by cardiac EP.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Conflict of Interest: Dr Han receives speaking honoraria from Abbott and Medtronic. Dr Al-Khatib receives research, speaking, and consultancy fees from Medtronic, as well as research and speaking fees from Abbott. Dr Albert has no conflicts to disclose.
References
- 1.Valuates Reports Digital Health Market Size to Reach USD 385.8 Billion by 2025 [Internet]. PR Newswire. 2020 [cited 2020 May 15] https://www.prnewswire.com/news-releases/digital-health-market-size-to-reach-usd-385-8-billion-by-2025--valuates-reports-301046093.html Available from.
- 2.Day S., Seninger C., Kothari M., et al. Stanford Center for Digital Health / Rock Health; 2019. Digital Consumer Adoption Report 2019; pp. 1–30. [Google Scholar]
- 3.AMA Digital Health Research . American Medical Association; 2020. Physicians’ motivations and requirements for adopting digital health adoption and attitudinal shifts from 2016 to 2019; pp. 1–37. [Google Scholar]
- 4.Slotwiner D., Varma N., Akar J., et al. HRS Expert Consensus Statement on remote monitoring for cardiac electronic implantable devices. Heart Rhythm. 2015;12:E69–E100. doi: 10.1016/j.hrthm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Barrett P.M., Komatireddy R., Haaser S., et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127 doi: 10.1016/j.amjmed.2013.10.003. 95.e11–95.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varma N., Marrouche N.F., Aguinaga L., et al. HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic. Heart Rhythm. 2020;17:E255–E268. doi: 10.1016/j.hrthm.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers E. 1st edition. Free Press of Glencoe; New York: 1962. Diffusion of Innovations [Internet]https://www.worldcat.org/oclc/254636 Available from. [Google Scholar]
- 8.Holshue M.L., DeBolt C., Lindquist S., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Totten A., Hansen R., Wagner J., et al. Agency for Healthcare Research and Quality; Rockville, MD: 2019. Telehealth for Acute and Chronic Care Consultations. Comparative Effectiveness Review No. 216. (Prepared by Pacific Northwest Evidence-based Practice Center under Contract No. 290-2015-00009-I.) AHRQ Publication No. 19-EHC012-EF. [PubMed] [Google Scholar]
- 10.Srivastava A., Do J.M., Sales V.L., Ly S., Joseph J. Impact of patient-centred home telehealth programme on outcomes in heart failure. J Telemed Telecare. 2019;25:425–430. doi: 10.1177/1357633X18775852. [DOI] [PubMed] [Google Scholar]
- 11.Donelan K., Barreto E.A., Sossong S., et al. Patient and clinician experiences with telehealth for patient follow-up care. Am J Manag Care. 2019;25:40–44. [PubMed] [Google Scholar]
- 12.Slightam C., Gregory A.J., Hu J., et al. Patient perceptions of video visits using veterans affairs telehealth tablets: survey study. J Med Internet Res. 2020;22:1–17. doi: 10.2196/15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu P., Hilow H., Patel D., et al. Use of virtual visits for the care of the arrhythmia patient. Heart Rhythm. 2020;17:1179–1783. doi: 10.1016/j.hrthm.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Wosik J., Clowse M., Overton R., et al. Impact of the COVID-19 pandemic on patterns of outpatient cardiovascular care. Am Heart J. 2021;231:1–5. doi: 10.1016/j.ahj.2020.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhry S.I., Mattera J.A., Curtis J.P., et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koehler F., Winkler S., Schieber M., et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: The Telemedical Interventional Monitoring in Heart Failure Study. Circulation. 2011;123:1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 17.Ong M.K., Romano P.S., Edgington S., et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure. JAMA Intern Med. 2016;176:310–318. doi: 10.1001/jamainternmed.2015.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldenthal I., Sciacca R., Riga T., et al. Recurrent atrial fibrillation/flutter detection after ablation or cardioversion using the AliveCor KardiaMobile device: iHEART results. J Cardiovasc Electrophysiol. 2019;30:2220–2228. doi: 10.1111/jce.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avram R., Tison G.H., Aschbacher K., et al. Real-world heart rate norms in the Health eHeart study. npj Digital Medicine. 2019;2 doi: 10.1038/s41746-019-0134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Y., Albright D. The effectiveness of telehealth on self-management for older adults with a chronic condition: a comprehensive narrative review of the literature. J Telemed Telecare. 2018;24:392–403. doi: 10.1177/1357633X17706285. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan M., Phadke A.J., Zulman D., et al. Enhancing patient engagement during virtual care: a conceptual model and rapid implementation at an academic medical center [Internet]. NEJM Catalyst Innovative Care Delivery. 2020 [cited 2020 Nov 20] https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0262 Available from.
- 22.Heyworth L., Kirsh S., Zulman D., Ferguson J.M., Kizer K.W. Expanding Access through Virtual Care : The VA’s Early Experience with Covid-19 [Internet]. NEJM Catalyst Innovative Care Delivery. 2020 [cited 2020 Nov 20] https://catalyst.nejm.org/doi/full/10.1056/cat.20.0327 Available from.
- 23.Anderson M., Madhumitha K. Digital divide persists even as lower-income Americans make gains in tech adoption [Internet]. Pew Research. 2019 [cited 2020 Nov 20] https://www.pewresearch.org/fact-tank/2019/05/07/digital-divide-persists-even-as-lower-income-americans-make-gains-in-tech-adoption/ Available from.
- 24.Makri A. Bridging the digital divide in health care. Lancet Digit Health. 2019;1:e204–e205. [Google Scholar]
- 25.CARES Act Provisions for Healthcare and Health IT [Internet]. HIMMS. 2020 [cited 2020 Jul 24] https://www.himss.org/news/cares-act-provisions-healthcare-and-health-it Available from.
- 26.Singh J. Virtual Care: Fad or Here to Stay? [Internet]. Medium.com. 2020 [cited 2020 Nov 20] https://jagsinghmd.medium.com/virtual-care-fad-or-here-to-stay-a2afb2ddea50 Available from.
- 27.Medicare Telemedicine Health Care Provider Fact Sheet [Internet]. 2020 [cited 2020 May 25] https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet Available from.
- 28.Artandi M., Thomas S., Shah N.R., Srinivasan M. Rapid System Transformation to More Than 75% Primary Care Video Visits within Three Weeks at Stanford: Response to Public Safety Crisis during a Pandemic [Internet]. NEJM Catalyst Innovations in Care Delivery 2020 [cited 2020 Nov 6] https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0100 Available from.
- 29.Sharma A., Harrington R.A., McClellan M.B., et al. Using digital health technology to better generate evidence and deliver evidence-based care. J Am Coll Cardiol. 2018;71:2680–2690. doi: 10.1016/j.jacc.2018.03.523. [DOI] [PubMed] [Google Scholar]