Abstract
Background
The Apple Watch Series 4 (AW) can detect atrial fibrillation and perform a single-lead electrocardiogram (ECG), but the clinical accuracy of AW ECG waveforms compared to lead 1 of a 12-lead ECG is unclear.
Objective
The purpose of this study was to assess the accuracy of interval measurements on AW ECG tracings in comparison to lead 1 on a 12-lead ECG.
Methods
We obtained ECGs at a university hospital of healthy volunteers age >18 years. ECG waveforms were measured with calipers to the nearest 0.25 mm. When possible, 3 consecutive waveforms in lead 1 were measured. Waveform properties, including intervals, were recorded. Concordance correlation coefficients and Bland-Altman plots were used to assess level of agreement between devices.
Results
Twelve-lead (n = 113) and AW (n = 129) ECG waveforms from 43 volunteers (mean age 31 years; 65% female) were analyzed. Sinus rhythm interpretation between devices was 100% concordant. No arrhythmias were recorded. Mean difference (d) for heart rate was 1.16 ± 4.33 bpm (r = 0.94); 3.83 ± 113.54 ms for RR interval (r = 0.79); 5.43 ± 17 ms for PR interval (r = 0.83); –6.89 ± 14.81 ms for QRS interval (r = 0.65); –11.27 ± 22.9 ms for QT interval (r = 0.79); and –11.67 ± 27 ms for QTc interval (r = 0.57). There was moderate (d <40 ms) to strong (d <20 ms or < 5 bpm) agreement between devices represented by Bland-Altman plots.
Conclusion
The AW produces accurate ECGs in healthy adults with moderate to strong agreement of basic ECG intervals.
Keywords: Apple, Apple watch, Atrial fibrillation, ECG interval: ECG monitoring, Kardia, Lead 1, Photoplethysmography, Smartwatch, Wearable
Key Findings.
-
•
The Apple Watch produces a high-quality electrocardiogram (ECG), and in our cohort, waveform interval measurements seem to have moderate (<40 ms) to strong (<20 ms) reliability and validity compared to the corresponding lead 1 of a 12-lead ECG.
-
•
Automated heart rate measurement between the Apple Watch and 12-lead ECG has strong correlation.
-
•
Given high-quality ECG tracings, the Apple Watch may have the potential to help identify first-degree atrioventricular (AV) blocks, second-degree AV blocks, narrow-complex tachycardias, wide-complex tachycardias, and segment elevations or depressions in lead 1.
-
•
Given our data and analysis, Apple Watch likely has the potential to be introduced as a new tool in the outpatient setting, and mobile health care application developers should explore utilizing our findings to help create future applications.
Introduction
Sensor technology, coupled with complex algorithms designed through neural networks,1 has made its way into wearable consumer electronic devices. With advancements in computing and battery technology, these devices have become exponentially miniaturized with the ability to measure health metrics and medical data in the ambulatory setting.
In September 2018, Apple Inc. (Cupertino, CA) released the Apple Watch Series 4 (AW). This wearable smartwatch contains built-in software and hardware to perform a single-lead electrocardiogram (ECG) and detect atrial fibrillation. AW utilizes green light–based photoplethysmography (PPG), which uses light beams and sensors to detect changes in blood volume in the microvasculature.2 Data are plotted on a tachogram to estimate heart rate. A single-lead ECG can be recorded by using electrodes located on the back of the watch (in direct contact with the ipsilateral dorsal wrist) and on the crown of the watch (in direct contact with the contralateral index finger). Once a single-lead ECG is recorded, a 30-second rhythm strip corresponding to lead 1 on a 12-lead ECG is presented on a paired iPhone (Apple) within the “Health” application. This rhythm strip is presented on standard ECG paper and can be printed to allow for manual caliper measurements similar to a routine 12-lead ECG. Using a proprietary algorithm, AW is able to analyze data from the PPG sensor and the single-lead ECG to classify rhythms under 5 categories: sinus rhythm, atrial fibrillation, low heart rate, high heart rate, or inconclusive.
In a multicenter study sponsored by Apple, the AW demonstrated 98.3% sensitivity and 99.6% specificity in detecting atrial fibrillation3 and was subsequently granted Food and Drug Administration (FDA) approval to detect atrial fibrillation due to its high fidelity, reliability, and performance. However, no formal published studies have been performed comparing the accuracy and correlation of basic intervals derived from AW single-lead ECG and the corresponding lead 1 from a 12-lead ECG. This is important because such studies will provide validation for patients and health care providers that this technology is a reliable, decentralized health monitoring tool to document, track, and help guide interventions in patients with suspected arrhythmias.
The primary objective of this study was to compare the accuracy and correlation of the intervals and waveforms derived from the single-lead AW ECG with lead 1 of a standard 12-lead ECG by performing manual interval measurements and waveform analysis in a healthy adult population.
Methods
This prospective study received expedited approval from the University of Texas at Austin (UT) and Seton Healthcare institutional review boards. This research adhered to human research guidelines consistent with the Helsinki Declaration as revised in 2013. ECGs were performed on healthy adults at Dell Seton Medical Center’s cardiovascular laboratory. A Philips PageWriter TC70 ECG machine (Philips USA, Andover, MA), provided by the hospital, was used for 12-lead ECGs. One AW (Figure 1) and 1 iPhone 8 was funded by an internal grant through University of Texas at Austin residency programs. All data were collected through the default Apple Health application on iOS 12.3.1 and watchOS 5.2. All ECGs were standard calibration with a paper speed of 25 mm/s.
Figure 1.
Apple Watch Series 4 used to obtain single-lead electrocardiograms (ECGs) that represent lead 1 on a standard 12-lead ECG.
Health care workers and students were recruited using internal UT and Seton Health e-mail lists. Inclusion criteria included all adults age >18 years who were able to provide written consent. Individuals with any self-reported cardiac history or newly found ECG abnormalities requiring urgent cardiology evaluations were excluded.
Written consent was obtained from all participants before any ECGs were performed or data were recorded. Each participant’s age, gender, weight, height, and ethnicity were recorded. The AW ECG and standard 12-lead ECG were performed consecutively, with patients given instructions to lie as still as possible without shifting positions for the entire duration of both recordings. Due to signal interference, the AW ECG and 12-lead ECG could not be performed simultaneously. The 12-lead ECG was always obtained first, followed by disconnection of the lead wires and immediate placement of the AW on the patient’s left wrist to record the single-lead AW ECG. Time between both ECGs per participant typically was <60 seconds. Any abnormal ECG was immediately evaluated by a board-certified cardiologist. All AW ECGs were automatically saved in the “Health” application on a paired iPhone and subsequently printed and de-identified for offline analysis. ECG collection and analysis occurred over a period of 4 months.
Printed and de-identified AW ECGs and corresponding 12-lead ECGs were independently analyzed by 3 internal medicine residents. When possible, 3 consecutive waveforms in lead 1 were manually measured with calipers to the nearest 0.25 mm. On the AW ECG, the first triplet of waveforms with a discernible P, QRS, and T wave was chosen for analysis. On the 12-lead ECG, all discernible waveforms (up to 3) in lead 1 were chosen for analysis. Automated heart rate, automated heart rhythm, manual heart rhythm, and caliper-measured intervals (RR, PR, QRS, ST, QT) were recorded using lead 1 of both ECGs. QTc was calculated using the Bazett formula by inputting both caliper-measured RR intervals and automated heart rate–derived RR intervals.
Statistical analysis
Summary data are presented as frequency with percentage. Descriptive statistics are presented as mean with range. Bland-Altman analysis using absolute mean differences and concordance correlation coefficients were used to assess the level of agreement between devices. Based on the absolute difference of the means (d) of up to 3 consecutive waveform interval measurements per device, moderate agreement was defined as d <40 ms, and strong agreement was defined as d <20 ms or heart rate <5 bpm.
Results
Demographics
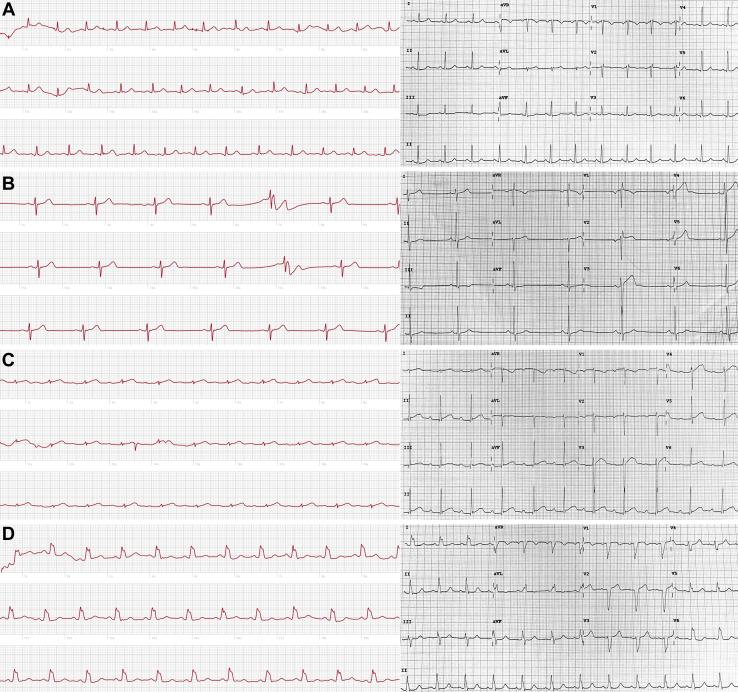
Forty-three healthy volunteers were recruited between April 2019 and June 2019. Demographics are summarized in Table 1. All ECGs (86/86 [100%]) were of diagnostic quality on first attempt (Figure 2). With regard to our goal of analyzing intervals on three lead 1 waveforms per device per volunteer, 113 of 129 12-lead ECG waveforms (87.5%) and 129 of 129 AW ECG waveforms (100%) were analyzed. Three full waveforms could not be analyzed (Figure 2B) in 16 of 129 lead 1 tracings (12.5%) on 12-lead ECGs due to the inability to make lead 1 the default rhythm strip on the 12-lead ECG machine.
Table 1.
Demographics of enrolled participants (N = 43)
| Sex∗ | |
| Male | 15 (35) |
| Female | 28 (65) |
| Age (y) | 31 ± 8.46 |
| Age distribution (y) | |
| 18–29 | 23 (53.5) |
| 30–39 | 15 (34.9) |
| ≥40 | 5 (11.6) |
| Race or ethnicity∗ | |
| White | 24 (55.9) |
| Hispanic | 2 (4.6) |
| Black | 2 (4.6) |
| Asian | 8 (18.6) |
| Middle Eastern | 7 (16.3) |
Values are given as n (%) or mean ± SD.
Sex and race or ethnic group were reported by the participants.
Figure 2.
Apple Watch Series 4 single-lead electrocardiograms (left) and 12-lead electrocardiograms (right) show similar waveform and rhythm properties, such as normal sinus rhythm (NSR) without abnormality (A), sinus bradycardia (B), NSR with first-degree atrioventricular block (C), and NSR with widened QRS (D).
Interval analysis
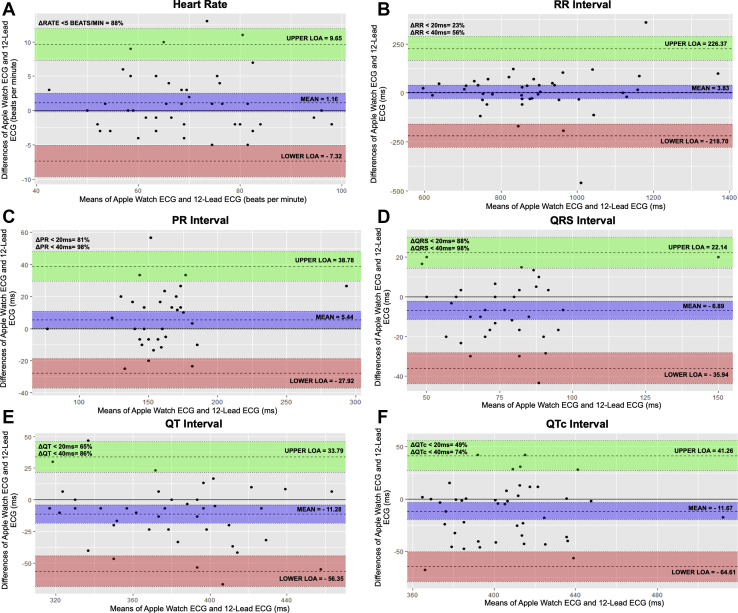
Absolute mean difference for automated heart rate was 1.16 ± 4.33 bpm (r = 0.94); 3.83 ± 113 ms for RR interval (r = 0.79); 5.43 ± 17 ms for PR interval (r = 0.83); –6.89 ± 14.8 ms for QRS interval (r = 0.65); –4.19 ± 21.4 ms for ST interval (r = 0.80); –11.2 ± 22.9 ms for QT interval (r = 0.79); and –11.6 ± 27 ms for QTc interval (r = 0.57) using Bland-Altman analysis. Moderate agreement existed in 216 of 257 total intervals (84%): 24 of 43 RR (55.8%); 42 of 42 PR (100%); 42 of 43 QRS (97.6%); 39 of 43 ST (90.6%); 37 of 43 QT (86%); and 32 of 43 QTc (74.4%) measurements. Strong agreement was present in 38 of 43 (88%) heart rate measurements and 162 of 257 total intervals (63%): 10 of 43 RR (23.2%); 35 of 42 PR (83.3%); 38 of 43 QRS (88.3%); 30 of 43 ST (69.8%); 28 of 43 QT (65.1%); and 21 of 43 QTc (48.8%) measurements (Figure 3). Three of 43 participants (7%) unintentionally began to stand before starting the AW ECG, which caused a clear fluctuation in automated heart rate measurement.
Figure 3.
Bland-Altman plots show varying levels of agreement (LOA) between heart rate (A), RR interval (B), PR interval (C), QRS interval (D), QT interval (E), and QTc interval (F) in lead 1 of the Apple Watch Series 4 electrocardiogram (ECG) and lead 1 of 12-lead ECG. Heart rate measurements were derived from each device’s rate measurement algorithm and presented in beats per minute. All interval absolute mean differences are measured in milliseconds. Upper LOA and lower LOA represent +1.96 and –1.96 standard deviations, respectively.
AW QTc, calculated using caliper-measured QT and RR intervals, demonstrated at least moderate agreement (d = –11.6 ms; standard deviation of d = 27 ms) with 12-lead ECG using Bland-Altman analysis (Figure 3F). AW QTc also calculated using caliper-measured QT intervals and automated heart rate–derived RR intervals yielded essentially identical results as the previous method (d = –12.2 ms; standard deviation of d = 26.3 ms). Two methods to calculate QTc were used to help validate our approach of using means of 3 consecutively measured RR intervals vs deriving RR intervals from the automated heart rate produced by the AW algorithm. The RR interval had a clinically important standard deviation of absolute mean difference of 113 ms, which helps explain why the AW QTc interval achieved strong agreement with 12-lead ECG in <50% of participants. Outlier data points (Figure 3B) are mainly secondary to a combination of irregular sinus arrhythmia and a single measurable RR interval on the 12-lead ECG. Interestingly, when participants with irregular sinus arrhythmia were removed from our data set, mean difference for the RR interval corrected to 6.5 ± 70.5 ms, but mean difference for QTc remained relatively unchanged.
Electrical signal noise (Figure 2) of the AW ECG was noted during our analysis. Low-energy positive or negative deflections of noise were noted in 123 of 129 PR segments, 123 of 129 ST segments, 54 of 129 TP intervals, and 18 of 129 T waves. Signal noise did not alter overall rhythm assessment but may have altered interval measurements based on the physical location of the noise on the waveform.
Rhythm analysis
Automated sinus rhythm interpretation between devices was concordant in 43 of 43 participants (100%). No arrhythmia, including atrial fibrillation, was identified by the algorithm of either device. The AW did not incorrectly classify any rhythm as “high heart rate” or inconclusive.” Manual review of ECG rhythms revealed that 2 participants exhibited a sinus arrhythmia that was captured by both devices. The AW classified these rhythms as “sinus rhythm.” Twelve-lead ECG identified 1 tracing as sinus bradycardia (41 bpm), and AW ECG algorithm correctly identified this tracing as “low heart rate” (Figure 2B). AW ECG tracings showed a first-degree atrioventricular (AV) block (Figure 2C) in 2 tracings but only one of two 12-lead ECGs corresponded to this finding. AW ECG showed a wide QRS (Figure 2D) in 1 tracing, and this finding was confirmed to be a left bundle branch block on corresponding 12-lead ECG. Two participants had isolated premature atrial or ventricular contractions noted on AW or 12-lead ECG, but these premature beats were captured on both modalities 0 of 2 times due to serial acquisition of tracings. Zero volunteers were excluded from this study because no urgent cardiology evaluations (determined by a cardiologist after immediate review of any abnormal tracing) were required.
The absolute mean difference and standard deviation of waveform characteristics for age and gender subgroups are listed in Table 2.
Table 2.
Absolute mean difference and standard deviation of waveform characteristics divided by age and gender subgroups between lead 1 of Apple Watch Series 4 and 12-lead ECG
| Heart rate (bpm) | RR interval (ms) | PR interval (ms) | QRS interval (ms) | ST interval (ms) | QT interval (ms) | QTc interval (ms) | |
|---|---|---|---|---|---|---|---|
| Male | |||||||
| Age 18–29 y | 0.57 ± 2.57 | 55.2 ± 149 | 13.1 ± 22.1 | –9.52 ± 20.1 | 2.38 ± 20.5 | –8.33 ± 19.8 | –16.6 ± 34.9 |
| Age 30–39 y | 2.14 ± 5.87 | 25 ± 62.2 | 10.9 ± 15.9 | –12.6 ± 9.01 | –7.14 ± 28.7 | –20 ± 35.4 | –20.5 ± 31.1 |
| Age ≥40 y∗ | 3 | –30 | –5 | 3.33 | –50 | –46.6 | –45.6 |
| All ages | 1.46 ± 4.29 | 35.4 ± 108 | 10.8 ± 18.3 | –10.1 ± 14.9 | –5.55 ± 26.6 | –16.3 ± 28.4 | –20.4 ± 31.4 |
| Female | |||||||
| Age 18-29 y | 2 ± 4.7 | –48.3 ± 138 | 6.14 ± 14.8 | –8.85 ± 13.5 | –2.08 ± 19.2 | –10.8 ± 20.6 | –0.39 ± 22.6 |
| Age 30–39 y | 0.75 ± 3.95 | 35 ± 48.3 | –3.6 ± 14.2 | –2.7 ± 16.5 | –5.41 ± 21.7 | –8.12 ± 21.7 | –19.5 ± 27.1 |
| Age ≥40 y | –2.5 ± 2.64 | 31.6 ± 36.7 | –2 ± 21.4 | 4.58 ± 13 | –5 ± 10 | –0.41 ± 8.43 | –8.12 ± 7.95 |
| All ages | 1 ± 4.42 | –13 ± 114 | 2.4 ± 15.7 | –5.17 ± 14.7 | –3.45 ± 18.5 | –8.57 ± 19.5 | –6.97 ± 23.5 |
Values are given as absolute mean difference ± SD.
ECG = electrocardiogram.
Only 1 male age ≥40 years participated in the study.
Discussion
Wearable technology with built-in direct-to-consumer health sensors is rapidly growing with widespread adoption in the general population, independent of physician guidance and recommendations. As a result, many patients have access to and will use these types of decentralized technologies with the hope of further facilitating and improving their health care. Clinical research to help guide clinical decisions for patients with a wide range of medical arrhythmias, with the exception of atrial fibrillation,4 is lacking with the AW.
Given this use and the need to correlate and validate these technologies, particularly as they relate to single-lead ECG recordings, we pursued this project. Because AW ECG technology is partly dependent on Apple’s proprietary algorithm, we chose waveform intervals as a means to compare the 2 devices in order to find any strengths or weaknesses of the raw waveform tracing. An answer we sought to define for the clinician is whether it would be possible to make any clinical interpretations without being dependent on Apple’s built-in algorithm. Our data likely are clinically relevant, and any agreements or disagreements between the 2 devices should, with caution and reference to future studies, be an indicator of the performance of the AW ECG. This is the first study to directly compare the accuracy and correlation of basic ECG intervals from lead 1 derived from a 12-lead ECG and the single-lead ECG obtained from the AW.
Similar to other watch-based ECG recording devices, we have now shown that AW can accurately measure heart rate and interval lengths in healthy adults without a known cardiac history. With regard to rhythm assessment, agreement was present in all AW algorithm-interpreted rhythms compared to 12-lead ECG interpreted rhythms. All participants in sinus rhythm were correctly classified as sinus rhythm by AW, and our study did not specifically enroll participants with known abnormal rhythms. One patient found to have sinus bradycardia on 12-lead ECG was classified as “low heart rate” by AW ECG. There was no false-positive identification of atrial fibrillation or “high heart rate” based on AW detection algorithms in our small cohort. There were no inconclusive recordings. A cardiologist was immediately available to review any abnormal ECG finding, and all of the reviewed tracings were included in our study given no need for urgent cardiology evaluation. In addition, when comparing basic ECG intervals between the AW and lead 1 from 12-lead ECGs, strong correlation existed in PR and QRS intervals and moderate correlation existed in RR, QT, and QTc intervals based on Bland-Altman analysis. We chose <20 ms as the cutoff point for strong correlation and <40 ms as moderate correlation based on previous studies.5
No previous studies have validated interval agreement with AW; however, the AliveCor (Mountain View, CA) KardiaMobile (KM) seems to be the most similar device in the wearables field with published clinical research. In a cohort of athletes, healthy adults, and cardiology clinic patients, Haberman et al6 reported QTc absolute mean difference as 33 ± 44 ms with KM. In a separate study of pediatric patients, Gropler et al5 reported QTc absolute mean difference as 15.6 ± 12.7 ms with KM. In comparison, absolute mean difference of QTc measured in our cohort was –11.6 ± 27 ms, and this measurement, along with other interval measurements, is comparable to those measured with KM in the studies by Haberman et al and Groplier et al. As noted earlier, our definition of strong agreement <20 ms is similar to that chosen by previous studies, and most tracings (63%) achieved strong agreement between AW and 12-lead ECG.
In our cohort, absolute mean difference >20 ms was most common in the RR interval, followed by QTc and QT intervals. All participants completed an AW ECG within 60 seconds of a 12-lead ECG. Three participants began to stand before beginning AW ECG, thus altering heart rate and possibly interval measurements, but the etiology of these higher interval absolute mean differences remains multifactorial. Of note, these participants were immediately instructed to lie down, and their AW ECG tracings still were completed <60 seconds after their 12-lead ECG. Neither age nor gender seem to clearly explain these differences. One noted observation, during blinded analysis, of a wide RR interval standard deviation was that 2 participants presented in an irregular sinus arrhythmia. Analysis of these two 12-lead ECGs revealed that the true RR interval on lead 2 rhythm strips was much different (by 300 to 500 ms) than what was presented in the 1 measurable RR interval present in the lead 1 tracing. In addition, based on direct observation, AW is sensitive to motion artifacts (eg, muscle contractions, movement) and seems to have low-energy electrical signal noise present in its tracings, which could have contributed to measurement error. Although strict measurement rules and observations were taught and seemingly standardized for the 3 physicians analyzing all ECGs, interuser variability may have contributed to elevated absolute mean differences. Based on our data and visual inspection of waveforms, this signal noise does not alter our opinion that the AW ECG produces high-quality waveform tracings. It is important to note that lead 1 QTc measurements offer limited clinical applicability, as leads 2 and V5 are classically used to measure this interval for clinical use. A possible application of a lead 1 QTc interval includes trending the change in interval length. Further studies are needed before AW can be independently used to make clinical decisions based on RR, QT, or QTc intervals.
PR and QRS intervals exhibited strong agreement. Thus, AW may be useful in identifying first-degree AV blocks, second-degree AV blocks, narrow-complex tachycardias, and wide-complex tachycardias. Given that AW has frequent low-energy electrical signal noise in its tracings, certain narrow-complex tachycardias and third-degree AV blocks, among other arrhythmias, may be more difficult to diagnose. Based on our visual inspection of all AW waveforms, segment elevations or depressions are also likely interpretable even in the presence of some waveform signal noise. These segment changes require future investigations as our study did not specifically focus on them. The ability of wearable devices to display these waveforms has already been demonstrated with the KM.7
All AW ECGs were performed once per participant and recorded diagnostic-quality data. This reliability may soon open the door for providers to remotely analyze heart rate, heart rhythm, or intervals and provide screening of at-risk patients for cardiac abnormalities such as atrial fibrillation.8 Apple has already shown, with FDA approval, that the AW is able to detect atrial fibrillation with high sensitivity and specificity. Because our study did not focus specifically on atrial fibrillation, further independent studies9 are needed to identify the role of AW in the realm of screening of atrial fibrillation at a population level. As noted in the Results section, our study had no patients with atrial fibrillation during the time of recording.
AW can passively monitor heart rate and rhythm using an algorithm paired with a PPG sensor, which can alert a person of abnormal heart rate activity, with similar accuracy as a 12-lead ECG or telemetry monitor.10 The ease of use of AW allows a person to perform a 1-lead ECG in 30 seconds. The ECG is presented immediately on a paired iPhone, and a share button is visible on the first screen when the ECG notification is selected from the iPhone’s lock screen. If a linked notification is not provided on the paired iPhone’s lock screen, the ECG file can be accessed and shared from the paired iPhone’s “Health” application. Given that diagnostic-quality data are available instantly for those prompted by the AW algorithm or those experiencing specific symptoms, time-sensitive events can be captured and shared with medical providers for clinical interpretation, and true arrhythmias likely can be diagnosed earlier and treated appropriately.11 Nevertheless, it is important to note that those who own wearable devices have been recognized to be younger or more affluent, and identifying the utility of introducing wearable devices into the general population should be of greater interest.12
The cardiovascular data analyzed in this study support the accuracy and validity of the AW, and in specific outpatient clinical scenarios the AW can be incorporated as a tool for use by the clinician given the device’s high-quality tracings. The decentralization of health care technology with tools such as the AW will continue to push physicians and patients toward a truly “patient-centered” ecosystem. This technology will provide relevant diagnostic data to help make clinical care more efficient and potentially more convenient to patients. However, further independent studies are needed to ensure that tools such as the AW can be used safely and effectively in clinical care.
Study limitations
This was a single-center study at a tertiary care university hospital involving healthy participants, 88% of whom were age <40 years, with no known cardiac history. This population does not allow for generalizability of our results to a wider age group having a variety of medical pathologies, and it is important to assess interval measurements during abnormal tracings in future studies. AW and 12-lead ECGs were obtained serially rather than simultaneously, leading to potential variability in our overall rhythm analysis. It is possible that this limitation likely caused our data to have less agreement between devices, but it is important to note that significant waveform interference was present on both devices when both ECGs were obtained simultaneously. AW ECG records a 30-second tracing and 12-lead ECG records a 10-second tracing, so variability in automated and manual measurements could have occurred in rates and intervals (specifically RR and QTc). In addition, only 113 of 129 possible 12-lead ECG waveforms (87.5%) were measurable on lead 1 for analysis. Because lead 1 was not made the default rhythm strip on 12-lead ECGs, having <3 waveforms of 12-lead ECG data likely affected the absolute mean difference of all intervals, especially RR and QTc intervals. Lastly, baseline waveform noise was present in most of the AW ECGs. Even though the 3 resident physicians who analyzed the ECGs were made aware of AW waveform noise, interuser variability of waveform analysis likely contributed to differences in measurement between AW and 12-lead ECGs.
Conclusion
The AW produces accurate 1-lead ECGs in healthy adults with no known cardiac history. Appropriate application of this wearable technology in the outpatient and ambulatory medical setting should be further assessed by clinicians.
Acknowledgments
We thank Clay Cauthen, MD, Peter Monteleone, MD, and Mauricio Hong, MD for assistance with conceptualization and design.
Funding Sources
This work was supported by the SERF grant (ID: 67-APPLE-WATCH) provided through Dell Seton Medical Center at The University of Texas at Austin.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Tison G., Sanchez J., Ballinger B., et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 2018;3:409–416. doi: 10.1001/jamacardio.2018.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isakadze N., Martin S. How useful is the smartwatch ECG? Published online October 31, 2019. Trends Cardiovasc Med. 2019 doi: 10.1016/j.tcm.2019.10.010. S1050-1738(19)30149-5. [DOI] [PubMed] [Google Scholar]
- 3.Apple Using Apple Watch for arrhythmia detection. December 2018. https://www.apple.com/healthcare/docs/site/Apple_Watch_Arrhythmia_Detection.pdf Available at.
- 4.Perez M., Mahaffey K., Hedlin H., et al. Large-Scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;38:1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gropler M., Dalal A., Van Hare G., Silva J., Liu N. Can smartphone wireless ECGs be used to accurately assess ECG intervals in pediatrics? A comparison of mobile health monitoring to standard 12-lead ECG. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haberman Z., Jahn R., Bose R., et al. Wireless smartphone ECG enables large-scale screening in diverse populations. Cardiovasc Electrophysiol. 2015;26:520–526. doi: 10.1111/jce.12634. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen H., Van Hare G., Rudokas M., Bowman T., Silva J., Nguyen H. SPEAR trial: Smartphone Pediatric ElectrocARdiogram Trial. PloS One. 2015 doi: 10.1371/journal.pone.0136256. 10:e0136256–e0136256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halcox P., Wareham P., Cardew B., et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation. 2017;136:1784–1794. doi: 10.1161/CIRCULATIONAHA.117.030583. [DOI] [PubMed] [Google Scholar]
- 9.Seshadri D., Bittel B., Browsky D., et al. Accuracy of the Apple Watch 4 to measure heart rate in patients with atrial fibrillation. IEEE J Transl Eng Health Med. 2020;8:1–4. doi: 10.1109/JTEHM.2019.2950397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seshadri R., Bittel K., Browsky Y., et al. Accuracy of Apple Watch for detection of atrial fibrillation. Circulation. 2020;141:702–703. doi: 10.1161/CIRCULATIONAHA.119.044126. [DOI] [PubMed] [Google Scholar]
- 11.Steinhubl S., Waalen J., Edwards A., et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA. 2018;320:146–155. doi: 10.1001/jama.2018.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strain T., Wijndaele K., Brage S. Physical activity surveillance through smartphone apps and wearable trackers: examining the UK potential for nationally representative sampling. JMIR Mhealth Uhealth. 2019;7 doi: 10.2196/11898. [DOI] [PMC free article] [PubMed] [Google Scholar]