Abstract
Objective
Previous longitudinal studies have found that cognitive function affected oral health, and vice versa. However, research is lacking on the reciprocal relationships between cognitive function and edentulism simultaneously, especially in developing countries. The present study aimed to examine the reciprocal relationship between cognitive function and edentulism among middle-aged and older adults in China.
Methods
Data were derived from the 2011 and 2015 waves of the China Health and Retirement Longitudinal Study. The sample included 14,038 respondents aged 45 or older. A two-wave cross-lagged analysis was adopted to test the hypothesized model.
Results
Among respondents aged 45–59, baseline cognitive function was associated with subsequent edentulism [b = −0.017, standard deviation (SD) = 0.006, P < 0.01]. In contrast, baseline edentulism was not significantly associated with poorer cognitive function at the follow-up wave (b = −0.744, SD = 0.383, P > 0.05). However, among respondents aged 60 or older, baseline cognitive function was associated with subsequent edentulism (b = −0.017, SD = 0.005, P < 0.01), and baseline edentulism was also associated with follow-up lower levels of cognitive function (b = −0.419, SD = 0.143, P < 0.01).
Conclusions
These findings demonstrated the reciprocal relationships of cognitive function and edentulism. However, such relationships varied across age groups. This study demonstrates the importance of developing programs and services to promote both cognitive and oral health, especially for those in older age.
Keywords: cognitive function, edentulism, reciprocal relationship, China, older people
Key points
There is a lack of research on the bidirectional relationship between edentulism and cognitive function in China.
Baseline edentulism was associated with subsequent poorer cognitive function in older adults.
Baseline poor cognitive function was associated with subsequent edentulism among both middle-aged and older adults.
Having good oral health may be beneficial for maintaining cognitive health.
Preventive strategies should be promoted to enhance oral health conditions among adult populations in China.
Introduction
Cognitive function is a critical dimension of healthy aging in developing and developed countries [1, 2]. Empirical evidence reveals negative consequences of cognitive impairment and dementia, including a significant risk of mortality and a decline in mental health and quality of life among older adults and their family caregivers [3, 4]. Furthermore, recent reviews have summarized cognition’s effects on oral health in later life [5, 6]. Individuals with cognitive impairment tend to be dependent in their functional health and often need assistance from caregivers for daily oral care [6]. Relevant studies have indicated that individuals with cognitive impairment and dementia are relatively more likely to have poor oral hygiene, develop dental caries and suffer from periodontal disease [7–9]. Although edentulism is generally related to chronic and severe inflammation and periodontal disease, the findings on the association between cognitive function and edentulism have been mixed [5, 6].
Oral health is a worldwide public health issue for older populations [10]. Poor oral health is a well-known determinant of poor nutritional intake and carries a high risk of cardiovascular disease, pain and reduced quality of life [5, 6, 11]. Further, it is considered a potential risk factor for reduced cognitive function [5, 12, 13]. Systemic inflammatory markers are evident in older adults with poor oral health and dementia, a relationship that could be a biological explanation for the association between oral health and cognition [6]. However, the effects of oral health on cognition are less clear, especially in terms of edentulism and cognition. Whereas some studies have identified statistically significant associations between the two factors [14–16], others have generated non-significant results [17, 18].
In summary, the evidence solidly supports the effects of cognitive function and dementia on oral health, but oral health’s effects on cognition are inconclusive [5, 6]. Particularly, evidence is mixed regarding edentulism’s effects on cognition and cognition’s effects on edentulism [5, 6]. Furthermore, the relevant studies were conducted primarily in developed countries, with only a limited number in developing countries, which differ significantly from developed regions in terms of socioeconomic development, health care and insurance systems, culture, nutritional intake and health education and practices [2, 16]. Finally, studies on the reciprocal relationship between edentulism and cognitive function are lacking. Only one longitudinal study, in England, investigated the bidirectional relationship between oral health and cognitive function, and it found a positive relationship [19]. However, China and England have different healthcare systems [15, 19, 20], and their dietary patterns and oral health status among older populations differ [15, 19]. Furthermore, edentulism is a relatively severe issue among older adults in China (see Table 1). Because cognitive function tends to differentially impact various oral health indicators [6], whether the reciprocal relationship between cognitive function and edentulism is significant in the Chinese context remains uncertain. Finally, the prevalence of people with both cognitive impairment and edentulism increases with age [15, 21], making the bidirectional relationship more salient in older age and necessitating separate testing in middle-aged adults and older adults.
Table 1 .
Social demographics of older respondents (n = 14,038; unweighted)
| Age 45–59 (N = 8,005) | Age 60+ (N = 6,033) | |||
|---|---|---|---|---|
| N (%) | Mean (SD) | N (%) | Mean (SD) | |
| Age | 52.3 (4.4) | 67.6 (6.4) | ||
| Gender | ||||
| Female | 4,250 (53.1) | 3,045 (50.5) | ||
| Male | 3,755 (46.9) | 2,988 (49.5) | ||
| Marital status | ||||
| Married | 7,565 (94.5) | 4,803 (79.6) | ||
| Other marital status | 440 (5.5) | 1,230 (20.4) | ||
| Education | ||||
| Primary school education or lower | 4,567 (57.1) | 4,951 (82.1) | ||
| Secondary school education or higher | 3,438 (42.9) | 1,082 (17.9) | ||
| Household assets (RMB) | 3613.0 (5883.9) | 2512.6 (8796.6) | ||
| Agricultural HRS | 6,641 (83.0) | 4,781 (79.2) | ||
| Good/very good self-rated health | 20,798 (26.0) | 1,169 (19.3) | ||
| No ADL disabilities | 7,038 (87.9) | 4,585 (76.0) | ||
| Number of chronic diseases | 1.3 (1.3) | 1.7 (1.5) | ||
| Had health insurance | 7,375 (92.1) | 5,552 (92.0) | ||
| Had dental care in 2015 | 1,462 (18.3) | 1,016 (16.8) | ||
| Was edentulous in 2011 | 216 (2.7) | 947 (15.7) | ||
| Was edentulous in 2015 | 617 (7.7) | 1780 (29.5) | ||
| Cognitive function in 2011 | 3.8 (1.7) | 3.6 (1.8) | ||
| Cognitive function in 2015 | 3.1 (1.7) | 2.5 (1.8) | ||
Notes: ADL, activities of daily living; HRS, household registration status; 100 RMB = 14.32USD.
Because the global population is aging, cognitive health and oral health are likely to play increasingly salient roles in healthy aging and overall well-being in later life [22]. Such issues are particularly important in China: In 2010, the prevalence of dementia among China’s older populations ranged from 4 to 7%, accounting for roughly 9.19 million people [4], and that figure is estimated to increase to 30 million by 2050 [4]. Oral disease is also common in China, and unmet needs regarding dental treatment and lack of oral health education among older adults are major urgent issues [5, 15]. Therefore, this study aimed to test the reciprocal relationship between cognitive function and edentulism among middle-aged and older adults in the Chinese context.
Methods
Context
We conducted a secondary data analysis based on the China Health and Retirement Longitudinal Study (CHARLS), a longitudinal panel study that was conducted between 2011 and 2015.
Data collection
In CHARLS, multistage cluster sampling was applied to recruit a nationally representative sample of community-dwelling adults aged 45 and older in China, and details of its sampling strategies can be found elsewhere [23]. Face-to-face computer-assisted personal interviews were conducted with respondents from 28 provinces in 2011 and 2012, and the interviewer used a computer or other electronic device to record the interviewees’ answers. A total of 17,708 respondents completed the baseline survey between 2011 and 2012, with a response rate of 80.5%. In the 2013 and 2015 waves, 18,612 and 21,097 respondents participated, respectively. For those who could not answer the questionnaire by themselves, family members were invited as a proxy to help them answer certain factual questions. At baseline, 8.7% of the questionnaires were assisted by proxies.
The respondents in this study were at baseline aged 45 or older and had an agricultural or non-agricultural household registration status, and all participated in the 2011 and 2015 waves of the survey. A total of 17,076 respondents participated in the baseline survey and 14,038 of the original respondents participated in the 2015 wave.
Measurement
Cognitive function was assessed by episodic memory, a 10-item Telephone Interview of Cognitive status (TICS) [24] and figure drawing. Respondents were asked to recall 10 Chinese nouns after interviewers read the nouns to them (i.e. immediate recall). Four minutes later, they were again asked to recall the 10 nouns (i.e. delayed recall). Average scores of immediate and delayed recall were calculated (range 0–10). The 10 items of TICS included the season of the year, the date (day, week, month and year) and serial subtraction of 7 from 100 (up to five times). In addition, the interviewer showed a picture of two overlapping pentagons to the respondents and asked them to complete a similar figure (0 = failed attempt; 1 = successful completion). Scores of the three tests were summed to represent each person’s overall cognitive function, with answers ranging from 0 to 21 (a measurement that has been used previously with CHARLS data [25, 26]).
Edentulism was assessed by a single question: The respondents were asked whether they had lost all of their teeth, and their responses were assessed by a binary variable (0 = no, 1 = yes).
Covariates of age, gender, marital status, education and household registration status were recoded as dichotomous variables (0 = 45–59, 1 = 60 and older; 0 = female, 1 = male; 0 = other marital status, 1 = married; 0 = primary school education or lower, 1 = secondary school education or higher; 0 = agricultural, 1 = non-agricultural). The log value of household assets was calculated. The respondents were also asked whether they had difficulties in conducting a range of activities of daily living [27], whether they had ever smoked in the past year (0 = no or had quit smoking during the year, 1 = still smoking) and whether they had physician-diagnosed diabetes (0 = no; 1 = yes). Self-rated health was measured by a 5-point Likert scale (1 = very poor, 3 = fair, 5 = very good). Finally, respondents in the 2015 wave were asked whether they had seen a dentist for dental care (not available at baseline; 0 = no, 1 = yes).
Statistical analyses
We used a cross-lagged analytic approach based on a structural equation modelling framework to test the proposed model. The estimator was a diagonally weighted least square mean and variance (WLSMV) that was specifically developed for ordinal variables [28, 29]. Fit indexes examined the model fit, including the chi-square test, the Tucker–Lewis index (TLI), the comparative fit index (CFI), the root mean square error of approximation (RMSEA) and the weighted root mean square residual (WRMR) [30]. Mplus 7 was used.
We conducted cross-lagged analyses for two age groups (45–59 and 60 or older). Cognitive function at the follow-up wave was regressed on edentulism at the baseline, whereas edentulism at the time of the follow-up wave was regressed on cognitive function simultaneously. Baseline sociodemographic characteristics, socioeconomic status, health conditions and smoking status were controlled for in the final model. The WLSMV with pairwise deletion was used to handle missingness [29]. Finally, weights were used in the analysis.
Ethics
Peking University’s Ethical Review Committee approved the study protocol.
Results
Characteristics of the sample
The respondents’ characteristics at baseline are presented in Table 1. They were predominantly married (88.1%) and had rural household registration status (81.4%), and they ranged in age between 45 and 101 years [M = 58.9, standard deviation (SD) = 9.3]. More than half of the respondents were female (52.0%). Approximately 42.9% of the middle-aged respondents and only 17.9% of the older respondents had completed a secondary school education or above. Older respondents reported higher levels of limitations in activities of daily living than middle-aged participants did (24.0 versus 12.1%, respectively) and more chronic diseases (1.7 versus 1.3). More than 90% of the respondents had health insurance at baseline.
Two-wave cross-lagged model
We built a two-wave cross-lagged model to test the association between cognitive function and edentulism. The results of fit indexes indicated a good model fit for each of the two age groups (for those aged 45–59: χ2[5] = 10.603, P = 0.0599, RMSEA = 0.012 [0.000–0.022], CFI = 0.997, TLI = 0.970 and WRMR = 0.439; and for those aged 60 or older: χ2[17] = 27.498, P = 0.0512, RMSEA = 0.010 [0.000–0.017], CFI = 0.993, TLI = 0.978 and WRMR = 0.670).
Among the middle-aged respondents, the path between 2011 and 2015 for cognitive function (b = 0.467, SD = 0.012, P < 0.001) and that for edentulism (b = 2.876, SD = 0.243, P < 0.001) were both statistically significant. Furthermore, the associations between cognitive function and edentulism were significant in both waves (2011: b = −0.027, SD = 0.008, P < 0.01; 2015: b = −0.262, SD = 0.083, P < 0.01). Finally, the results showed that poor cognitive function in 2011 was a significant predictor of edentulism in 2015 (b = −0.017, SD = 0.006, P < 0.01). Interestingly, however, edentulism in 2011 was not a significant predictor of poor cognitive function in 2015 (b = −0.744, SD = 0.383, P > 0.05).
Among the older respondents, the relationships between 2011 and 2015 for cognition (b = 0.520, SD = 0.023, P < 0.001) and edentulism (b = 1.984, SD = 0.112, P < 0.001) were statistically significant, and the relationships between cognition and edentulism were also significant at both the 2011 and 2015 waves (2011: b = −0.099, SD = 0.024, P < 0.001; 2015: b = −0.194, SD = 0.061, P < 0.01). Finally, poor cognitive function in 2011 was a significant predictor of edentulism in 2015 (b = −0.017, SD = 0.005, P < 0.01), and in this group, edentulism in 2011 was also a significant predictor of poor cognitive function in 2015 (b = −0.419, SD = 0.143, P < 0.01; see Figures 1 and 2).
Figure 1 .
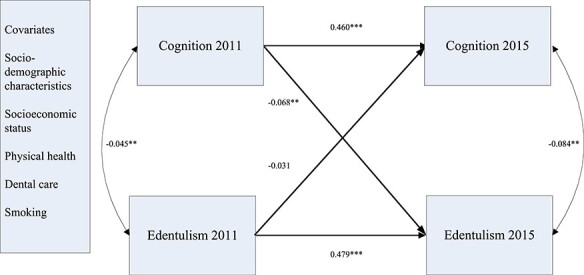
Results of the cross-lag model among middle-aged respondents aged 45–60. Notes: **P < 0.01 (two-tailed); ***P < 0.001 (two-tailed). Standardized coefficients were reported here.
Figure 2 .
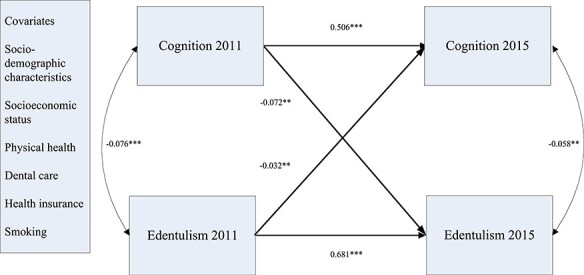
Results of the cross-lag model among older respondents age 60 and older. Notes: **P < 0.01 (two-tailed); ***P < 0.001 (two-tailed). Standardized coefficients were reported here.
Discussion
Findings
Overall, edentulous people aged 60 or older were more likely to have poorer cognitive function over time than were individuals with teeth, even after controlling for edentulism and covariates at baseline. Certain physiological mechanisms could link edentulism to poor cognitive function. A recent study found a key pathogen (Porphyromonas gingivalis) for chronic periodontitis in the brains of Alzheimer’s patients [12]. Tooth loss occurs from the long-term cumulative effects of periodontitis. Other potential explanations include a decline in masticatory function, presence of cardiovascular disease and insufficient nutrition due to edentulism [5, 15]. Thus, our study generated important scientific evidence for early detection of cognitive impairment and dementia. Finally, the non-significant associations in the middle-aged group may have resulted from the fact that this group was still relatively young and might not have experienced a significant decline in cognitive function during the 4-year study period.
Furthermore, although previous findings related to cognitive function and edentulism have been mixed [5, 6, 14–18], our study in the Chinese context found that individuals with relatively poor cognitive function were more likely to experience edentulism. The inconsistency between our results and those of others may have been due to disparities in the age range, race and measurements of cognitive function and oral health of the individuals studied. Many older adults with cognitive impairment or dementia rely on others to help them maintain their oral hygiene, and some may resist assistance with oral hygiene and dental care [2, 5].
Strength and limitations
Using a nationally representative sample, this study was an initial attempt to simultaneously test the effect of edentulism on cognitive function and the effect of cognitive function on edentulism among adults aged 45 and older in China. We examined the reciprocal relationships among middle-aged and older adults separately, and our findings provide new empirical evidence that the association between cognitive function and edentulism becomes more salient as people get older.
This study had the following limitations. First, because we were analysing secondary data, we could only select measures from our data source, the CHARLS questionnaire. Future studies on edentulism and cognition should collect additional information on the progress of oral health status and other related health outcomes, such as tooth loss, periodontal disease, denture use, oral health history, frailty, inflammatory biomarkers and masticatory function. Second, our follow-up period to assess edentulism and cognitive function was 4 years, but the reciprocal relationship might be more evident in studies with longer follow-up periods. Third, the results may be subject to recall bias, because all the information was self-reported.
Recommendations for practice
Because we found that increasing edentulism occurred in association with a decline in cognitive function among both middle-aged and older respondents, adequate access to oral healthcare should be ensured for those with cognitive impairment at each life stage. Moreover, because edentulism predicted poor cognitive function among the older respondents, dental care services should be included in long-term care systems for individuals with cognitive impairment and especially in individualized oral healthcare strategies in later stages of dementia [10]. Furthermore, daily care (whether self or assisted) is important for oral health. Training for maintaining and evaluating hygiene and health should be provided to caregivers of individuals with cognitive impairments. Finally, community oral health education programs should strongly promote oral health examinations and dental services in China. The Chinese medical insurance system should also be expanded to cover the cost of dental care among middle-aged and older populations.
Conclusions
Poor cognitive function was associated with an increased risk of edentulism among both middle-aged and older adults, whereas edentulism was associated with decreasing cognitive function only among older adults. A better understanding of these reciprocal relationships not only plays an important role in the early detection of cognitive impairment and dementia but also has major clinical implications for improving both oral health and cognitive function in older populations. Future longitudinal studies should examine the roles of oral health, the delivery of oral health services at different stages of cognitive impairment and dementia and the underlying mechanisms linking oral health and cognitive function.
Acknowledgements:
We thank the CHARLS research team and field team for collecting the data and making the data publically accessible.
Contributor Information
Nan Lu, Department of Social Work and Social Policy, School of Sociology and Population Studies, Renmin University of China, Beijing, China.
Bei Wu, NYU Aging Incubator, Rory Meyers College of Nursing, New York University, New York, NY, USA.
Yaolin Pei, NYU Aging Incubator, Rory Meyers College of Nursing, New York University, New York, NY, USA.
Declaration of Conflict of Interest:
None.
Declaration of Funding:
This study was supported by a Key Project of National Social Science Foundation of China (Grant No. 19ASH018). This analysis is based on the 2011 and 2015 waves of the China Health and Retirement Longitudinal Study (CHARLS).
References
- 1. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet 2011; 377: 1019–31. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 2. Wu B, Plassman BL, Liang J, Wei L. Cognitive function and dental care utilization among community-dwelling older adults. Am J Public Health 2007; 97: 2216–21. doi: 10.2105/AJPH.2007.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ju YJ, Nam CM, Lee SG, Park S, Hahm M, Park EC. Evaluation of the South Korean national long-term care insurance-funded cognitive function training programme for older people with mild dementia. Age Ageing 2019; 48: 636–42. doi: 10.1093/ageing/afz067. [DOI] [PubMed] [Google Scholar]
- 4. Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015. The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends, Alzheimer’s Disease International. London, 2015. [Google Scholar]
- 5. Wu B, Fillenbaum GG, Plassman BL, Guo L. Association between oral health and cognitive status: a systematic review. J Am Geriatr Soc 2016; 64: 739–51. doi: 10.1111/jgs.14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daly B, Thompsell A, Sharpling Jet al. Evidence summary: the relationship between oral health and dementia. Br Dent J 2017; 223: 846–53. doi: 10.1038/sj.bdj.2017.992. [DOI] [PubMed] [Google Scholar]
- 7. Hatipoglu MG, Kabay SC, Güven G. The clinical evaluation of the oral status in Alzheimer-type dementia patients. Gerodontology 2011; 28: 302–6. doi: 10.1111/j.1741-2358.2010.00401.x. [DOI] [PubMed] [Google Scholar]
- 8. Gil-Montoya JA, Sanchez-Lara I, Carnero-Pardo Cet al. Is periodontitis a risk factor for cognitive impairment and dementia? A case-control study. J Periodontol 2015; 86: 244–53. doi: 10.1902/jop.2014.140340. [DOI] [PubMed] [Google Scholar]
- 9. Chu CH, Ng A, Chau AMH, Lo ECM. Oral health status of elderly Chinese with dementia in Hong Kong. Oral Health Prev Dent 2015; 13: 51–7. doi: 10.3290/j.ohpd.a32343PubMed PMID: 25019106. [DOI] [PubMed] [Google Scholar]
- 10. Nihtilä A, Tuuliainen E, Komulainen Ket al. Preventive oral health intervention among old home care clients. Age Ageing 2017; 46: 846–51. doi: 10.1093/ageing/afx020. [DOI] [PubMed] [Google Scholar]
- 11. van de Rijt LJM, Feast AR, Vickerstaff V, Lobbezoo F, Sampson EL. Prevalence and associations of orofacial pain and oral health factors in nursing home residents with and without dementia. Age Ageing 2019. doi: 10.1093/ageing/afz169. [DOI] [PubMed] [Google Scholar]
- 12. Dominy SS, Lynch CC, Ermini Fet al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv 2019; 5. doi: 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luo J, Wu B, Zhao Qet al. Association between tooth loss and cognitive function among 3063 Chinese older adults: a community-based study. PLoS One 2015; 10. doi: 10.1371/journal.pone.0120986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Batty GD, Li Q, Huxley Ret al. Oral disease in relation to future risk of dementia and cognitive decline: prospective cohort study based on the action in diabetes and vascular disease: Preterax and Diamicron modified-release controlled evaluation (ADVANCE) trial. Eur Psychiatry 2013; 28: 49–52. doi: 10.1016/j.eurpsy.2011.07.005PubMed PMID: 21964484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li J, Xu H, Pan W, Wu B. Association between tooth loss and cognitive decline: a 13-year longitudinal study of Chinese older adults. PLoS ONE 2017; 12: e0171404. doi: 10.1371/journal.pone.0171404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stewart R, Sabbah W, Tsakos G, D'Aiuto F, Watt R. Oral health and cognitive function in the third National Health and nutrition examination survey (NHANES III). Psychosom Med 2008; 70: 936–41. doi: 10.1097/PSY.0b013e3181870aec. [DOI] [PubMed] [Google Scholar]
- 17. Paganinihill A, White SC, Atchison KA. Dentition, dental health habits, and dementia: the leisure world cohort study. J Am Geriatr Soc 2012; 60: 1556–63. doi: 10.1111/j.1532-5415.2012.04064.x. [DOI] [PubMed] [Google Scholar]
- 18. Shimazaki Y, Soh I, Saito Tet al. Influence of dentition status on physical disability, mental impairment, and mortality in institutionalized elderly people. J Dent Res 2001; 80: 340–5. doi: 10.1177/00220345010800010801. [DOI] [PubMed] [Google Scholar]
- 19. Kang J, Wu B, Bunce Det al. Bidirectional relations between cognitive function and oral health in ageing persons: a longitudinal cohort study. Age Ageing 2020. [DOI] [PubMed] [Google Scholar]
- 20. Yip W, Hsiao WC. What drove the cycles of Chinese health system reforms. Health Syst Reform 2015; 1: 52–61. doi: 10.4161/23288604.2014.995005. [DOI] [PubMed] [Google Scholar]
- 21. Ding D, Zhao Q, Guo Qet al. Prevalence of mild cognitive impairment in an urban community in China: a cross-sectional analysis of the shanghai aging study. Alzheimers Dement 2015; 11: 300–9. doi: 10.1016/j.jalz.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 22. Gao M, Kuang W, Qiu P, Wang H, Lv X, Yang M. The time trends of cognitive impairment incidence among older Chinese people in the community: based on the CLHLS cohorts from 1998 to 2014. Age Ageing 2017; 46: 787–93. doi: 10.1093/ageing/afx038. [DOI] [PubMed] [Google Scholar]
- 23. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol 2014; 43: 61–8. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Cogn Behav Neurol 1988; 1: 111–8. [Google Scholar]
- 25. Li J, Cacchione PZ, Hodgson NAet al. Afternoon napping and cognition in Chinese older adults: findings from the China Health and Retirement Longitudinal Study baseline assessment. J Am Geriatr Soc 2017; 65: 373–80. doi: 10.1111/jgs.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ge S, Wei Z, Liu Tet al. Alcohol use and cognitive functioning among middle-aged and older adults in China: findings of the China Health and Retirement Longitudinal Study baseline survey. Alcohol Clin Exp Re 2018; 42: 2054–60. doi: 10.1111/acer.13861. [DOI] [PubMed] [Google Scholar]
- 27. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965; 14: 61–5. [PubMed] [Google Scholar]
- 28. Muthén LK, Muthén B. Mplus User’s Guide. 7th edition. Los Angeles: Muthén & Muthén, 2012. [Google Scholar]
- 29. Asparouhov T, Muthén B. Weighted least squares estimation with missing data. 2010.
- 30. Kline RB. Principles and Practice of Structural Equation Modeling. 3rd edition. New York: The Guilford Press, 2011. [Google Scholar]


