Abstract
Rhinoviruses are the most common cause of the common cold, but they can cause more severe illnesses in people with underlying lung disorders such as asthma, chronic obstructive pulmonary disease, or cystic fibrosis. Epidemiologic studies with sensitive detection methods such as PCR have identified rhinovirus infection as a major source of asthma exacerbations in both children and adults, especially during the spring and fall. Since rhinoviruses cause little tissue destruction, it is presumed that the immune response to the infection may play an important role in the pathogenesis of rhinovirus-induced exacerbations of asthma. This review examines the epidemiologic association between rhinovirus infections and exacerbations of asthma and outlines current information on immune responses to rhinovirus infection and potential connections between antiviral responses and preexisting allergic inflammation. Finally, current and future strategies for treating rhinovirus infections and virus-induced exacerbations of asthma are discussed.
Human rhinoviruses (RV) cause approximately half of all common colds each year and account for the majority of respiratory illnesses during the spring and fall. In addition to contracting rhinitis, sinusitis, and middle-ear dysfunction, certain populations are at risk for developing lower respiratory complications such as wheezing, small-airway obstruction, bronchitis, and pneumonia during RV infections. These high-risk populations include infants, the elderly, and people with conditions associated with preexisting airway inflammation, such as asthma, cystic fibrosis, and tobacco smoking. In this review, we focus on the association between RV infections and exacerbations of asthma and examine both potential mechanisms linking these events and treatments designed to interrupt these processes.
EPIDEMIOLOGY OF RHINOVIRUS INFECTIONS AND ASTHMA
In children or adults with existing asthma, respiratory viruses frequently trigger exacerbations of asthma (19, 81, 102, 104). Initial studies designed to establish this relationship identified respiratory pathogens in association with wheezing episodes by culture or detection of increasing titers of virus-specific antibody (28, 104), however, these are relatively insensitive diagnostic techniques. Consequently, the development of reverse transcription-PCR assays, which are much more sensitive for detecting viruses than are standard cell culture techniques (9, 45, 82), provides a tool to more precisely define the effects of respiratory viruses on asthma. Using PCR along with standard viral diagnostic tests, Johnston et al. determined that 80 to 85% of school-aged children with wheezing episodes tested positive for a virus and that the virus most commonly detected was RV (81). Furthermore, about half of the exacerbations in adults with asthma are associated with RV infection (102). Moreover, virus-induced asthma may be severe: seasonal patterns of upper respiratory virus prevalence correlate closely with hospital admissions for asthma, especially in children (80). Furthermore, RV and other respiratory viruses are frequently detected in children hospitalized for asthma (82). Together, these studies indicate that viral infections, and particularly respiratory illnesses from RV, are the most common cause of asthma exacerbations in children and also contribute substantially to the asthma morbidity in adults.
In addition to epidemiologic studies linking respiratory viral infections to exacerbations of asthma, there is clinical evidence to implicate respiratory allergies as a risk factor for developing lower airway symptoms during infections with RV. For example, Duff et al. evaluated risk factors for wheezing in infants and children who presented to a hospital emergency department (31). Wheezing children older than 2 years were more likely to have respiratory allergies (odds ratio [OR] = 4.5) or a confirmed viral infection (OR = 3.7) than were children without wheezing. Children with the greatest risk (OR = 10.8) for wheezing were those who had both respiratory allergies and a viral infection. These findings indicated that viral infections and respiratory allergies may have synergistic inflammatory effects on lower-airway physiology that greatly increase the likelihood of wheezing.
Together, these studies indicate that infections with RV and other respiratory viruses frequently exacerbate of asthma and suggest that there may be specific interactions between viral infections and the variations in airway biology produced by respiratory allergies or asthma. Since bronchospasm during asthma is a result of airway inflammation and hyperresponsiveness, the effects of viral infections on these conditions are of special interest. In this review, we explore potential mechanisms by which respiratory viruses affect lower-airway function and/or inflammation to produce small-airway obstruction, wheezing, and asthma.
RHINOVIRUS INFECTIONS AND AIRWAY HYPERRESPONSIVENESS
One of the cardinal features of asthma is airway hyperresponsiveness, which is defined as the increased sensitivity of the small airways to bronchoconstriction in response to inhaled substances, such as histamine or methacholine. It is therefore of great interest that viral respiratory infections can transiently increase airway responsiveness in humans and in animals. Increased airway responsiveness usually begins early during the acute viral infection and can be observed in response to inhalation of histamine, methacholine, citric acid, or allergen (14, 33, 43, 86, 87, 89, 120, 127). The use of an experimentally induced infection of volunteers with RV or influenza viruses has enabled longitudinal examination of lung physiology before, during, and after infections. Cheung et al. (24) inoculated 14 subjects with mild asthma with either RV16 (type 16 rhinovirus) or placebo and found that airway responsiveness transiently increased during the acute infection, and returned to baseline levels by 1 week after the inoculation. In addition to increasing the sensitivity of the airway, RV16 infection increased the maximal response to inhaled methacholine, and, in contrast to changes in airway responsiveness, the maximal responses remained elevated for up to 15 days after the acute infection. Thus, viral infections can enhance both the reactivity of the lower airway and the magnitude of bronchoconstriction in response to inhaled contractile substances in asthma, and the latter effect can persist for weeks after the acute infection.
Some studies of normal and allergic volunteers have not found changes in lower-airway physiology after experimental infection (4, 63, 116, 117, 122). The reasons for the variability of lower-airway effects after experimental inoculation are not established but may be related to differences in host factors, inoculation technique, timing of the histamine challenge, viral inocula, or severity of the induced colds. It is also possible that there are inherent differences in the pathogenicity of different RV serotypes, since lower-airway changes have generally been observed with RV16, but less often with RV39 or RV2. Alternately, since picornaviruses mutate rapidly, viruses used in human inoculation studies may have been attenuated by being grown in tissue culture. It should be noted, however, that a large number of RV serotypes can affect lower-airway physiology during natural infections: more than 30 serotypes have been cultured from patients with concurrent acute asthma symptoms and upper respiratory infection (28). This indicates that although some serotypes may indeed be more likely to induce wheezing in asthma, this property is not confined to a small subset of highly pathogenic serotypes. Finally, the selection criteria for study subjects could bias the outcome of experimental RV infections in that, for ethical reasons, asthmatic children or individuals with a history of severe or frequent virus-induced exacerbations of asthma may be excluded from such studies.
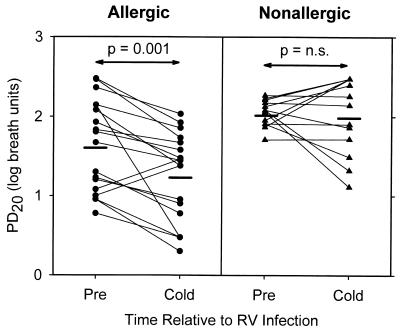
There is evidence that allergy and asthma can influence the effect of respiratory viral infection on airway responsiveness. Experimental infection with RV16 induces greater changes in airway responsiveness in volunteers with respiratory allergy (14, 50) or mild allergic asthma (43) than in normal control subjects (Fig. 1). In addition, RV-induced increases in airway responsiveness were greater in subjects with lower baseline pulmonary function (forced expiratory volume in 1 [FEV1]), suggesting that virus-induced changes in airway responsiveness can be influenced by baseline lung function (50).
FIG. 1.
Effect of allergy on RV16-induced changes in lower-airway histamine responsiveness. Subjects were grouped according to the presence or absence of allergy, and histamine PD20 was plotted precold (pre), and 2 days following RV16 inoculation (cold). Heavy horizontal lines represent group mean data. n.s., not significant. Reprinted from reference 50 with permission of the publisher.
When considered together, these studies suggest that factors related to both the host and the virus determine the effects of respiratory viral infections on airway responsiveness. Despite the limitations of experimental techniques for inducing viral infections, these studies have provided evidence that factors such as allergy and baseline FEV1 can influence the changes in lower-airway physiology caused by RV infection and may contribute to the increased lower-airway effects of RV infection in subjects with asthma.
MECHANISMS OF VIRUS-INDUCED AIRWAY OBSTRUCTION AND ASTHMA
Effects of Virus on the Lower Airway: Study Designs
Studies to determine the mechanisms by which viruses cause lower-airway dysfunction are hampered by the difficulty in sampling lower-airway cells and secretions and safety concerns in performing bronchoscopy in patients with acute virus-induced airway obstruction. Several experimental approaches have been used to deal with these constraints, including the use of animal models and sampling of upper-airway cells and secretions. Animal models have provided many insights into potential mechanisms linking viral infections and lower airway effects, but there are species-specific differences that can limit the interpretation of these data. For example, guinea pigs develop an accentuated eosinophilic response to viral infection (131) compared to most other species. Furthermore, there is no animal model for RV infection: major-group RV do not bind to mouse intercellular cell adhesion molecule type 1 (ICAM-1), and although minor-group RV bind to mouse epithelial cells, replication occurs only in the presence of systemic immunosuppression.
The upper airway provides a readily accessible model to study interactions between viral infections, allergic inflammation, and airway dysfunction. It is reasonable to assume that allergen- or virus-induced epithelial-cell inflammatory responses are similar in the upper and lower airways. Analysis of virus-induced effects on upper-airway cells and secretions can therefore provide clues about lower-airway pathologic findings, and in some studies, changes in upper respiratory cells or mediators have correlated with virus-induced lower-airway dysfunction (Table 1). These studies suggest either that virus-induced changes in the upper airway cause lower-airway dysfunction or, more probably, that sampling of upper-airway events provides an approximation of changes that occur in the lower airway.
TABLE 1.
RV-induced immune responses and association with clinical observationsa
| Immune response | Locationb | Associated clinical observationsb | Reference(s) |
|---|---|---|---|
| Upper airway | |||
| ↑ Kinins | NS | ↑ Symptom scores | 98 |
| ↑ Neutrophils or myeloperoxidase | NS | ↑ Symptom scores | 91, 98, 125 |
| ↓ Lymphocytes | Blood | ↑ Symptom scores | 90 |
| ↑ Lymphocytes | NS | ↑ Symptom scores | 91 |
| ↑ RV-specific T-cell proliferation | Blood | ↑ Nasal mucus | 75 |
| ↑ Albumin | NS | ↑ Symptom scores | 98 |
| ↑ IL-1 | NS | ↑ Symptom scores | 105 |
| ↑ IL-2 (ex vivo) | Blood | ↓ Nasal mucus, ↓ duration of viral shedding | 75 |
| ↑ IL-6 | NS | ↑ Symptom scores | 139 |
| ↑ IL-8 | NS | ↑ Symptom scores | 58, 130 |
| Lower airway | |||
| ↑ Eosinophil cationic protein | Sputum | ↑ Airway responsiveness | 57 |
| ↓ Lymphocytes | Blood | ↑ Airway responsiveness, MFEV1 | 24, 43 |
| ↑ Neutrophils | NS | ↑ Airway responsiveness | 58 |
| ↑ IL-8 | NS | ↑ Airway responsiveness | 58 |
| ↑ IL-11 | NS | Wheezing | 32 |
Modified from reference 39 with permission of the publisher.
Abbreviations: NS, nasal secretions; MFEV1, maximum fall in forced expiratory volume, during a methacholine challenge.
Despite these similarities, differences in the upper- and lower-airway environment (i.e., neutrophils are found in upper airway secretions, while macrophages predominate in bronchial secretions) could produce site-specific differences in virus-induced immune responses. It is therefore important to validate observations in the upper airway with studies of lower-airway cells and secretions. Studies involving bronchoscopy have generally been limited to include patients with allergic rhinitis or mild asthma because of safety concerns. Recently, studies have been conducted to evaluate changes in sputum biology during respiratory viral infections (35, 37), and this technique provides a relatively noninvasive means of examining lower-airway inflammatory changes during viral infections. The ability to repeatedly sample lower-airway cells during a respiratory viral infection is likely to provide additional insights into mechanisms of pathogenesis.
Site of Infection during Virus-Induced Wheezing
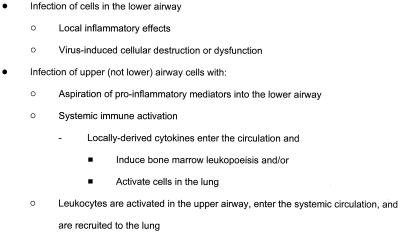
There is little doubt that infections with respiratory viruses such as influenza virus, respiratory syncytial virus, and parainfluenza virus can extend to lower-airway tissues, thereby inducing tissue inflammation and lower-airway obstruction. It remains to be established, however, whether RV infections extend into the lower airway during exacerbations of asthma and whether this is a mechanism of increased asthma severity. If RV infection does involve the lower airway, this suggests that viral replication in the lower airway could trigger a local inflammatory response and directly enhance preexisting airway inflammation. Alternatively, RV infection could be confined to the upper airway in asthma, and symptoms could be increased through remote mechanisms (Fig. 2).
FIG. 2.
Potential mechanisms for lower-airway dysfunction caused by RV infections.
The former scenario seems more likely, however, based on several studies that support the concept that RV is a lower-airway pathogen. For example, some (73, 84, 96) but not all (26, 70) epidemiologic studies have linked RV to lower-airway syndromes such as bronchitis, bronchiolitis, and pneumonia. There are also reports of fatal RV pneumonia in children, including a case in which RV was recovered from lung tissue at autopsy (88). In addition, RV is frequently detected in the nasal secretions of elderly individuals with lower respiratory tract symptoms (101). Obtaining lower-airway secretions to culture RV has remained a problem, since samples obtained via sputum induction or bronchoscopy may be contaminated with material from the upper airway (65). Given this limitation, several studies have attempted to detect RV in lower-airway samples. Horn et al. performed viral cultures on samples from children with wheezy bronchitis and found that RV was obtained about twice as often from the sputum than from samples from the nose or throat (74). Furthermore, in experimentally- infected volunteers, RV has been cultured from lower-airway brushings (64) and RV16 RNA has been detected by reverse transcription-PCR in bronchial lavage cells (53). Furthermore, there are preliminary data to indicate that RV RNA can be detected in bronchial biopsy tissue by in situ hybridization (15). These data suggest that, at least under some conditions, RV infections extend into the lower airway and that replication of RV in bronchial epithelial cells and the induction of lower-airway inflammation could contribute to the pathogenesis of virus-induced exacerbations of asthma.
Effects of Viral Infections on Neural Regulation of the Airway
Although neural mechanisms of virus-induced bronchoconstriction and airway obstruction are of great interest, they are particularly difficult to study in humans, because definitive experiments often require the disruption of neural tissue. Consequently, much of our understanding has been gained through the use of animal models infected with respiratory viruses other than RV. Viral infections could potentially cause bronchoconstriction and increased airway responsiveness by enhancing parasympathetic bronchoconstrictive responses, by stimulating reflex bronchospasm or neuropeptide release from sensory C fibers, or by interfering with the function of nonadrenergic, noncholinergic neurons, which produce the potent bronchodilator nitric oxide. Each of these mechanisms has been explored, and they the subject of recent reviews (12, 39, 41). Further advances in the understanding of virus effects on neural control of the airways await the development of new specific antagonists of neural pathways or the development of new experimental techniques. In addition, there is relatively little information about the interface between airway inflammation and neural dysregulation, and more research is needed to define these pathways.
Effects of Rhinovirus on Cellular Inflammation
Although the precise mechanisms by which RV cause symptoms are unknown, there is increasing evidence that the immune response induced by the virus plays a major role in symptom pathogenesis. For example, RV infections provoke symptoms of asthma but do not cause extensive epithelial-cell destruction (133). Second, several investigators have found that levels of mediators (kinins) or cytokines (interleukin-1 [IL-1], IL-8, and IL-11) correlate with the severity of respiratory symptoms during respiratory infections (Table 1). Whether these factors are participating in symptom pathogenesis or are markers of inflammatory cell activation has not yet been established. Third, there is evidence to support the role of the immune response to viruses in symptom pathogenesis in animal models of viral infection. For example, virus-induced airway hyperresponsiveness can be passively transferred in guinea pigs by passive infusion of bronchial alveolar lavage cells from infected animals (42), and morbidity and mortality of viral infections in mice is increased after passive transfer of certain virus-specific T cells (2, 3, 23).
These data indicate that the immune response to viral infections, although important in the clearance of viral infections, can also contribute to pathologic changes in the airway. The immune responses to viruses and allergens are complex, and both involve multiple airway cells, cytokines, and mediators. Since cytokines are major regulators of inflammation associated with respiratory allergies and asthma, it seems likely that virus-induced cytokines secreted by lower-airway cells could augment existing airway inflammation and consequently adversely affect lung physiology. In addition, several cells are of particular interest in the context of virus-induced exacerbations of asthma.
Epithelial cells.
The respiratory epithelial cell is the host cell for RV replication, and the degree of RV replication in the epithelial cell strongly influences the severity of respiratory symptoms associated with RV colds. Studies in which shielded catheters were used to map patterns of RV infection in vivo have demonstrated a patchy pattern of infection throughout the nasal cavities and nasopharynx (134). This concept has been confirmed at the cellular level: analysis of infected tissue by in situ hybridization has shown that only a subset of epithelial cells are infected (7, 8, 13). The susceptibility of epithelial cells to infection with RV may be determined by the density of viral receptors on the cell membrane. Specialized nonciliated cells in the lymphoepithelium of adenoidal tissue express large amounts of ICAM-1, the receptor used by over 90% of RV serotypes (134a). This high expression of ICAM-1 may make these cells especially susceptible to RV infection (8). It is possible, but as yet unproven, that increased ICAM-1 expression in the lower airway renders patients with asthma, or other disorders characterized by chronic airway inflammation, more susceptible to RV infections of the lower airways.
Peak viral titers in nasal secretions correlate with the maximum severity of cold symptoms (50, 54), and during asthma exacerbations, lower-respiratory tract symptoms are worse in patients with severe upper-respiratory tract symptoms (58, 94, 95). Moreover, the epithelial cell, once regarded as a passive host for respiratory virus infection, is now recognized as playing an active and important role in airway immune responses through secretion of a broad array of cytokines and mediators with chemotactic and inflammatory effects. Studies of epithelial cells or cell lines indicate that in vitro inoculation with RV induces the secretion of IL-1β, IL-6, IL-8, IL-11, tumor necrosis factor alpha (TNF-α), RANTES, and granulocyte-macrophage colony-stimulating factor (29, 32, 46, 79, 119, 121, 124, 138). Several of these cytokines have profound effects on inflammatory cells that can potentiate asthma. For example, RANTES is a chemoattractant for eosinophils and memory T cells, and IL-8 is a chemoattractant for neutrophils and activated eosinophils: recruitment of these cell types could enhance airway inflammation and increase airway obstruction (83, 136). Furthermore, granulocyte-macrophage colony-stimulating factor increases eosinophil survival in vitro and is a cofactor for eosinophil superoxide production and degranulation (92, 99, 115). IL-11 is secreted in very large amounts after epithelial cells are infected with RV or RSV in vitro and may have direct effects on bronchial hyperresponsiveness (32, 123).
There are data to suggest that there may be quantitative differences in virus-induced cytokine secretion related to wheezing illnesses and asthma. Einarsson et al. analyzed nasal secretions from patients with either upper respiratory symptoms or wheezing associated with a viral respiratory infection (32), and IL-11 levels in nasal secretions were significantly increased in the patients with wheezing. IL-11 is of particular interest in light of two recent murine studies in which IL-11 administration to normal mice caused airway hyperresponsiveness (32) and targeted airway expression of IL-11 in transgenic mice caused airway inflammation and hyperresponsiveness (123).
In addition, Grünberg and colleagues analyzed IL-8 levels in the nasal secretions of volunteers with allergic asthma after experimental inoculation with either RV16 or a placebo (58). IL-8 levels were increased in the nasal secretions of subjects who were inoculated with RV16 but not in those given the placebo. Furthermore, the quantity of IL-8 detected 2 days after inoculation significantly correlated with cold and asthma symptom scores and with increases in airway hyperresponsiveness. Together with the findings of Einarsson et al. (32), these data suggest that greater amounts of cytokines are generated in respiratory viral infections that are associated with adverse effects on the lower airway.
Viral infections damage respiratory epithelium to a variable extent and could reduce the synthesis of enzymes that have anti-inflammatory effects. For example, airway epithelial cells synthesize histamine methyltransferase and neutral endopeptidase, the enzymes that metabolize histamine and tachykinins (e.g., substance P), respectively (100). Damage to epithelial cells could lead to decreased production of these molecules and thus enhance airway inflammation or obstruction during viral infections.
In addition, airway epithelial cells express both constitutive and inducible forms of nitric oxide synthase and may be an important source of nitric oxide. Exhaled nitric oxide (NO) is increased during asthma and is increased in some (25, 85) but not all (37) studies of upper respiratory infections; it could have complex effects on airway physiology. On the one hand, NO is a potent vasodilator, which might increase airway wall edema and thereby increase nasal or bronchial airway obstruction. In contrast, the effects of NO that could be beneficial include inhibition of viral (including RV) replication (112) and bronchodilation through relaxation of bronchial smooth muscle. Findings in volunteers with asthma suggest that the overall effect of NO is positive, since greater NO production during experimentally induced RV infections correlated with a smaller increase in airway responsiveness (25). Similar trends were noted in a study of guinea pigs inoculated with parainfluenza type III virus (40). In these experiments, virus-induced increases in airway responsiveness were prevented by treatment with an aerosol containing l-arginine, a nitric oxide precursor.
Endothelial cells.
Endothelial cells are likely to contribute to airway dysfunction during respiratory illnesses through at least two pathways. First, endothelial cells could have a major impact on airway inflammation and respiratory symptoms through their role in recruiting leukocytes to the airway during RV infections. Second, RV infections quickly cause transudation of plasma proteins from the vascular tissue of the nasal mucosa, leading to increased nasal secretions and congestion (77). Peak levels of plasma proteins such as albumin and immunoglobulin G (IgG) coincide with the time of maximal cold symptoms (77) and increases in bronchial responsiveness (24), suggesting that similar processes occur in the lower airway. Activation of kinins has been advanced as a possible mechanism for cold-induced increased endothelial-cell permeability (98, 106); however, clinical trials have so far failed to confirm this hypothesis. For example, the bradykinin antagonist NPC 567 did not improve cold symptoms (72), and oral prednisone therapy decreased kinin levels in nasal secretions without reducing cold symptoms (59). Thus, increased vascular permeability contributes to cold symptoms and may also produce lower-airway obstruction and increased symptoms of asthma, but the mechanisms by which viral infections increase vascular permeability are as yet unclear.
Granulocytes.
Granulocyte recruitment and activation are likely to contribute to airway dysfunction during RV infections. Neutrophils are the main cells recruited to the airway during the acute stages of a cold (34, 91, 128, 133), and this is likely to be in response to chemotactic factors such as IL-8 and leukotriene B4 (6, 17, 38, 58, 125, 137). Respiratory viruses can activate neutrophil inflammatory functions, as indicated by enhancement of superoxide responses, chemotaxis, and adhesion (20, 108).
Evidence that neutrophils may participate in the pathogenesis of virus-induced asthma exacerbations is derived from several human studies. Neutrophils comprise the majority of nonepithelial cells in sputum during acute exacerbations of asthma, of which approximately 50% are associated with symptoms of the common cold (35). Furthermore, in subjects with atopic asthma experimentally infected with RV16, peripheral-blood neutrophil counts correlated with cold and asthma symptom scores and with cold-induced changes in airway hyperresponsiveness (58). These data are consistent with the hypothesis that neutrophils contribute to the pathogenesis of respiratory symptoms induced by RV infection, and this could occur through the generation of superoxide or the release of cytokines. One potential mechanism to explain the association between neutrophils and increased airway symptoms is that neutrophil proteases are potent secretagogues for airway submucosal glands (114) and that increased mucus production could further contribute to airway obstruction during viral infections of the lower airway.
Although the neutrophil is the predominant cell found in nasal secretions during acute viral infections, eosinophil granular proteins have also been detected in the nasal secretions of children with wheezing illnesses caused by RV or respiratory syncytial virus (47, 71). In addition, increases in sputum eosinophilic cationic protein (ECP) during the acute phase of experimentally induced RV infection correlated with increased airway responsiveness in a group of adult asthmatics after experimental inoculation with RV16 (57). Experiments conducted in vitro indicate that RV do not activate eosinophils directly (66), and so it is likely that eosinophil activation is secondary to the activity of virus-induced mediators or cytokines secreted by T cells, epithelial cells, or other airway cells (55). Together, these studies provide evidence that viral infections can trigger increased recruitment and activation of eosinophils, and they suggest that eosinophils contribute to virus-induced airway hyperresponsiveness. Although these data are intriguing, additional information is needed to clarify the contribution of eosinophil recruitment and activation to the pathogenesis of respiratory symptoms in virus-induced exacerbations of asthma.
Macrophages.
Airway macrophages are likely to be involved in antiviral immune responses because they express ICAM-1 (the receptor for major-group RV), bind RV in vitro, and secrete cytokines that have antiviral (alpha interferon [IFN-α]) and/or proinflammatory (IL-1 and TNF-α) effects (52, 67). Nasal secretions of RV-infected volunteers or children with naturally acquired upper respiratory infections contain IL-1 (103, 105), which can cause systemic symptoms such as fever and/or malaise that are commonly associated with RV colds in children (27). In addition, airway macrophages incubated with RV in vitro secrete TNF-α (52); this cytokine can increase the expression of ICAM-1 and other adhesion molecules on a number of different cell types (111), and its presence has been closely associated with wheezing illnesses in infancy (11) and the development of the late-phase allergic reaction and asthma (5, 56). TNF-α also increases the susceptibility of an epithelial cell line (BEAS2B) to become infected with major-group RV, probably by increasing the expression of ICAM-1 receptors (121). Thus, macrophages have the capacity to contribute to early antiviral immune responses but also could increase airway inflammation, leading to increased respiratory symptoms.
T lymphocytes.
T-cell responses may be of particular importance because of their central role in orchestrating immune responses to both allergens and viruses, through the regulation of effector cells that are virucidal and/or cause airway inflammation. Respiratory viral infections induce specific and nonspecific T-cell activation, and there is evidence linking these T-cell responses to asthma disease severity during viral infections (49). RV infection is usually accompanied by peripheral-blood lymphopenia, and the degree of lymphopenia correlates with severity of cold symptoms (90) and changes in airway responsiveness (24, 43). Moreover, lymphocyte numbers are increased in the nasal secretions and lower-airway epithelium during acute RV infection, coincident with increases in airway responsiveness (43, 91).
Natural killer cells, CD4+ T cells, and CD8+ T cells all participate in antiviral immune responses. Cytotoxic CD8+ T cells are a major source of IFN-γ during viral infections, and they exert potent antiviral activity by lysing virus-infected cells. CD4+ T cells have less cytotoxic potential but can direct immune responses through the secretion of cytokines. Studies of mice have shown that synthesis of IFN-γ, IL-4, IL-5, and IL-10 is increased in the lungs during respiratory viral infection (16, 113), indicating that viral infections induce a broad array of cytokines. The balance of Th1- and Th2-like responses is likely to be important in coordinating both cytotoxic and antibody responses to viral infection, which provide antiviral activity and immunity to reinfection, respectively.
Virus-specific T-cell responses are generally not detectable in the airway or blood until 7 to 10 days after inoculation with virus. This time delay raises questions about whether virus-specific T cells contribute to virus-induced asthma symptoms, which often begin during the first few days of the respiratory infection. With this in mind, there are three possible scenarios to describe T-cell involvement in the pathogenesis of virus-induced exacerbations of asthma: (i) T cells help to clear viral infections but do not contribute to asthma symptoms; (ii) virus-specific T-cell responses contribute to asthma symptoms, but only during the latter stages of viral infections; or (iii) viral infections rapidly activate T cells, which contribute to airway inflammation and symptoms during the entire illness.
Experiments with mice indicate that the majority of T cells attracted to the lungs during an acute viral infection are not virus specific (30), and there is evidence that experimentally induced RV infection produces both antigen-dependent and antigen-independent T-cell activation (75). These observations suggest that viral infections can activate a broad range of T cells and raise the possibility that this effect contributes to airway inflammation and dysfunction. Several mechanisms for the rapid activation of T cells have been proposed. For example, T-cell responses induced by either influenza virus or RV can be cross-reactive among different viral serotypes (51, 109, 132). Since both of these viruses cause recurrent infections with different serotypes, part of the T-cell response could be a memory response, which would be expected to have faster kinetics and potentially be of a greater magnitude than a primary T-cell response. In addition, the large number of cytokines and mediators generated during a viral infection could activate lung T cells in an antigen-independent manner through “bystander” effects. In support of this hypothesis, in vitro incubation of RV with peripheral blood mononuclear cells causes activation of 25 to 50% of T cells, as indicated by expression of the early activation marker CD69 and production of IFN-γ mRNA and protein (55). This effect is mediated by soluble factors secreted by RV-activated monocytes and does not require antigen presentation to the T cells. Antigen-independent T-cell activation can also be observed in mice during a systemic viral infection (lymphocytic choriomeningitis virus) (126) and in vitro by incubating T cells with high concentrations of RANTES, a chemokine induced by respiratory viruses (10). Together, these studies indicate that RV infections could cause nonspecific T-cell recruitment and activation early during the course of infection and suggest that these effects could significantly augment airway inflammation and thus contribute to respiratory symptoms.
Interactions between Allergic Inflammation and Viral Infections
To test the hypothesis that viral infections enhance preexisting airway inflammation due to allergy, several studies have been conducted to measure lower-airway responses to allergen in subjects with allergic rhinitis before, during, and after experimental inoculation with RV (21, 22, 48, 50, 89). RV infections increased airway responsiveness to histamine, methacholine, and allergen and increased the probability of developing a late asthmatic response after whole-lung antigen inhalation (89). The effects of RV on airway responsiveness were most pronounced in subjects who developed a late allergic response after allergen inhalation (22). Furthermore, subjects who developed a late allergic response to allergen after RV infection also had a greater increase in histamine levels in plasma after the inhaled allergen challenge (22).
In studies involving bronchoscopy, RV infection caused an increased release of histamine into the lower airways after segmental allergen challenge and augmented the recruitment of both total leukocytes and eosinophils into the airway 48 h after allergen challenge (21). These effects were noted only in allergic individuals, indicating that RV infection specifically enhanced allergen-induced responses in the airway. In a separate study of subjects with asthma experimentally infected with RV16, immunohistochemistry of bronchial biopsies revealed that both T cells and eosinophils are recruited to the lower airway during the acute infection (43). Asthmatic subjects differed from normal controls in having persistent increases in the numbers of lower-airway eosinophils when they were reevaluated 4 weeks after inoculation. Thus, these data show that RV infection can intensify both the immediate and late responses to allergen challenge and that this is accompanied by increasing mast cell or basophil mediator release and the recruitment of eosinophils to the lower airway.
Anecdotal reports suggest that the development of allergic symptoms in young children is sometimes preceded by a viral respiratory infection (44), prompting speculation that common-cold viruses might participate in the initiation of respiratory allergies. Recently, a mechanism for the stimulation of IgE synthesis by viral infections was been proposed. Infection of a B-cell line with either RV14 or RV16 caused activation of the double-stranded RNA-dependent protein kinase and an increase in Ig isotype switching to IgE (107). Furthermore, RV39 inoculation was found to increase total IgE levels but not levels of allergen-specific IgE in a group of experimentally infected volunteers (118). Additional studies are needed to determine whether this effect is reproducible and is of clinical relevance.
SUMMARY AND THERAPEUTIC IMPLICATIONS
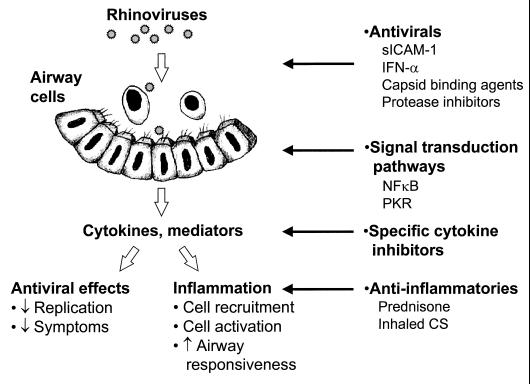
In older children and adults, common-cold viruses, such as RV, that produce relatively mild symptoms in most individuals can trigger severe episodes of lower-airway dysfunction in the presence of asthma. There is now evidence that the immune response to respiratory viral infections, although critical to clear virus from the airway, also contributes to airway obstruction and respiratory symptoms. The mechanisms by which these changes occur are not fully established but appear to be associated with the ability of respiratory viruses to induce the production of proinflammatory cytokines and mediators (Fig. 3). This inflammatory cascade, together with direct effects of viral infections on the airway mucosa, activates neural mechanisms to enhance airway hyperresponsiveness and airway obstruction. In the context of asthma, the immune response to respiratory viruses can enhance preexisting airway inflammation, which leads to clinical exacerbations.
FIG. 3.
Mechanisms of RV-induced exacerbations of asthma, and therapeutic implications. See the text for details. Abbreviations: sICAM-1, soluble ICAM-1; PKR, double-stranded-RNA-activated protein kinase; CS, corticosteroids.
This model suggests several opportunities, and a number of challenges, for the design of therapeutic interventions for virus-induced lower airway dysfunction (Fig. 3). Given the large number of RV serotypes, vaccination is not a viable option. However, several classes of anti-RV medications have been developed and are now in various stages of clinical trials. For RV infection, prophylactic use of topical IFN-α, soluble ICAM-1, or capsid binding agents that prevent viral binding or uncoating can prevent infections (67, 69, 76, 110, 129). The clinical utility of these medications is limited, however, since they are generally ineffective if their use is delayed until after the onset of symptoms (1). In addition, IFN-α administered to patients with chronic respiratory diseases (including asthma) after close contact with people with upper-respiratory symptoms does not prevent cold-related lower-respiratory symptoms (135). With the success of protease inhibitors for the treatment of human immunodeficiency virus infections, there is renewed interest in finding specific inhibitors of RV proteases, and several compounds have shown promising results in vitro (93). Finding a broad-spectrum anti-RV agent that is affordable and safe enough to be used for cold prophylaxis remains a major challenge.
Peak upper-respiratory symptoms typically coincide with peak viral titers in nasal secretion, and by this time it is probably too late to modify the disease course with antiviral medications. Instead, therapy at this stage is aimed at either reducing virus-induced immune responses or providing symptomatic relief. Recent well-powered clinical studies have demonstrated that certain common-cold symptoms are amenable to treatment. For example, first-generation antihistamines such as clemastine and brompheniramine reduce sneezing and rhinorrhea, although these medications may produce side effects such as drowsiness (60, 61). In addition, topical vasoconstrictors and anticholinergic agents can temporarily relieve nasal congestion and rhinorrhea, respectively (37, 68). Whether the statistically significant yet clinically incomplete symptom relief provided by these medications justifies the cost and side effects associated with therapy continues to be debated.
A number of studies have evaluated the effects of dietary supplements such as vitamin C or zinc on common-cold symptoms. Zinc lozenges reduced cold symptoms in some but not all placebo-controlled clinical trials, but their side effects such as bad taste and nausea make it difficult to conduct a truly blinded trial (62, 78, 97). Vitamin C therapy does not prevent colds from occurring, although some studies have found that it shortens the duration or severity of colds (reviewed in reference 28).
For virus-induced exacerbations of asthma, the most effective treatment now available is to prescribe a short “burst” of oral corticosteroid early during the course of a viral respiratory infection (18). Despite their proven efficacy in lessening the lower-airway consequences of viral respiratory infections in patients with asthma, systemic corticosteroids have little effect on common-cold symptoms (36, 59), indicating that the mechanisms that cause upper- and lower-respiratory symptoms associated with RV infection may be distinct. The use of systemic corticosteroids may be associated with side effects, and it is clearly desirable to develop treatments that inhibit the proinflammatory effects of viral infections while avoiding immunosuppression and corticosteroid-induced morbidity.
RV infections induce a host of immunologic changes in the blood and airways, and some of these responses have been correlated with cold symptoms, changes in airway responsiveness, or lower-airway symptoms (Table 1). It remains to be determined which of these factors participate in the pathogenesis of respiratory symptoms and which are merely indicators of an ongoing immune response. In any case, since many of these cells, cytokines, and mediators have overlapping inflammatory effects, it seems unlikely that therapeutic approaches aimed at inhibiting any one factor will reverse the effects of RV on airway function. Intracellular mechanisms by which viruses activate cytokine genes are now being defined, with the hope that viruses may trigger a common signaling pathway(s) that leads to the activation of multiple proinflammatory genes. For example, NF-κB activation appears to be essential for RV-induced synthesis of the proinflammatory cytokines IL-6 and IL-8 (138, 139), and this would seem to be a reasonable target for future strategies to decrease virus-induced inflammation.
Finally, basic questions about the pathogenesis of RV infections in patients with asthma remain unanswered. The specific factors related to asthma that are responsible for the increased lower-airway effects of RV infection have yet to be determined. Are antiviral immune responses impaired in the presence of atopy or asthma? Alternatively, are the more severe clinical manifestations of RV infections in asthma a result of the different types of cells or, perhaps, the heightened activation state of the cells in the asthmatic airway? With the answers to these questions will come a better appreciation of how airway inflammation is regulated in asthma and how best to treat this process when it is augmented by viral infection.
ACKNOWLEDGMENTS
We thank Elizabeth Grimm for the artwork in Fig. 3.
This study was supported by NIH grants AI26609, AI34891, AI40685, and HL44098.
REFERENCES
- 1.al-Nakib W, Tyrrell D A. Drugs against rhinoviruses. J Antimicrob Chemother. 1992;30:115–117. doi: 10.1093/jac/30.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alwan W H, Kozlowska W J, Openshaw P J. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alwan W H, Record F M, Openshaw P J M. CD4+ T-cells clear virus but augment disease in mice infected with respiratory syncytial virus - comparison with the effects of CD8+ T-cells. Clin Exp Immunol. 1992;88:527–536. doi: 10.1111/j.1365-2249.1992.tb06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelini B, Van Deusen B S, Doyle W J, Seroky J, Cohen S, Skoner D P. Lower airway responses to rhinovirus-Hanks in healthy subjects with and without allergy. J Allergy Clin Immunol. 1997;99:618–619. doi: 10.1016/s0091-6749(97)70022-8. [DOI] [PubMed] [Google Scholar]
- 5.Anticevich S Z, Hughes J M, Black J L, Armour C L. Induction of human airway hyperresponsiveness by tumour necrosis factor-alpha. Eur J Pharmacol. 1995;284:221–225. doi: 10.1016/0014-2999(95)00463-u. [DOI] [PubMed] [Google Scholar]
- 6.Arnold R, Humbert B, Werchau H, Gallati H, Konig W. Interleukin-8, interleukin-6, and soluble tumour necrosis factor receptor type I release from a human pulmonary epithelial cell line (A549) exposed to respiratory syncytial virus. Immunology. 1994;82:126–133. [PMC free article] [PubMed] [Google Scholar]
- 7.Arruda E, Boyle T R, Winther B, Pevear D C, Gwaltney J M, Hayden F G. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J Infect Dis. 1995;171:1329–1333. doi: 10.1093/infdis/171.5.1329. [DOI] [PubMed] [Google Scholar]
- 8.Arruda E, Mifflia T E, Gwaltney J M Jr, Winther B, Hayden F G. Localization of rhinovirus replication in vitro with in situ hybridization. J Med Virol. 1991;54:634–638. doi: 10.1002/jmv.1890340107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arruda E, Pitkaranta A, Witek T J J, Doyle C A, Hayden F G. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997;35:2864–2868. doi: 10.1128/jcm.35.11.2864-2868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacon K B, Premack B A, Gardner P, Schall T J. Activation of dual T cell signalling pathways by the chemokine RANTES. Science. 1995;269:1727–1730. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 11.Balfour-Lynn I M, Valman H B, Wellings R, Webster A D B, Taylor G W, Silverman M. Tumour necrosis factor-alpha and leukotriene E4 production in wheezy infants. Clin Exp Allergy. 1994;24:121–126. doi: 10.1111/j.1365-2222.1994.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 12.Bardin P G, Johnston S L, Pattemore P K. Viruses as precipitants of asthma symptoms. II. Physiology and mechanisms. Clin Exp Allergy. 1992;22:809–822. doi: 10.1111/j.1365-2222.1992.tb02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bardin P G, Johnston S L, Sanderson G, Robinson B S, Pickett M A, Fraenkel D J, Holgate S T. Detection of rhinovirus infection of the nasal mucosa by oligonucleotide in situ hybridization. Am J Respir Cell Mol Biol. 1994;10:207–213. doi: 10.1165/ajrcmb.10.2.8110476. [DOI] [PubMed] [Google Scholar]
- 14.Bardin P G, Sanderson G, Robinson B S, Holgate S T, Tyrrell D A J. Experimental rhinovirus infection in volunteers. Eur Respir J. 1996;9:2250–2255. doi: 10.1183/09031936.96.09112250. [DOI] [PubMed] [Google Scholar]
- 15.Bates P J, Bardin P G, Fraenkel D J, Sanderson G, Holgate S T, Johnston S L. Localisation of rhinovirus in the bronchial epithelium of experimentally-infected human volunteers. Am J Respir Crit Care Med. 1998;157:A25. . (Abstract.) [Google Scholar]
- 16.Baumgarth N, Brown L, Jackson D, Kelso A. Novel features of the respiratory tract T-cell response to influenza virus infection: lung T cells increase expression of gamma interferon mRNA in vivo and maintain high levels of mRNA expression for interleukin-5 (IL-5) and IL-10. J Virol. 1994;68:7575–7581. doi: 10.1128/jvi.68.11.7575-7581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker S, Quay J, Soukup J. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J Immunol. 1991;147:4307–4312. [PubMed] [Google Scholar]
- 18.Brunette M G, Lands L, Thibodeau L P. Childhood asthma: prevention of attacks with short-term corticosteroid treatment of upper respiratory tract infections. Pediatrics. 1988;81:624–629. [PubMed] [Google Scholar]
- 19.Busse W W, Gern J E. Viruses in asthma. J Allergy Clin Immunol. 1997;100:147–150. doi: 10.1016/s0091-6749(97)70216-1. [DOI] [PubMed] [Google Scholar]
- 20.Busse W W, Vrtis R F, Steiner R, Dick E C. In vitro incubation with influenza virus primes human polymorphonuclear leukocyte generation of superoxide. Am J Respir Cell Mol Biol. 1991;4:347–354. doi: 10.1165/ajrcmb/4.4.347. [DOI] [PubMed] [Google Scholar]
- 21.Calhoun W J, Dick E C, Schwartz L B, Busse W W. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94:2200–2208. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calhoun W J, Swenson C A, Dick E C, Schwartz L B, Lemanske R F, Jr, Busse W W. Experimental rhinovirus 16 infection potentiates histamine release after antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1991;144:1267–1273. doi: 10.1164/ajrccm/144.6.1267. [DOI] [PubMed] [Google Scholar]
- 23.Cannon M J, Openshaw P J M, Askonas B A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung D, Dick E C, Timmers M C, De Klerk E P A, Spaan W J M, Sterk P J. Rhinovirus inhalation causes long-lasting excessive airway narrowing in response to methacholine in asthmatic subjects in vivo. Am J Respir Crit Care Med. 1995;152:1490–1496. doi: 10.1164/ajrccm.152.5.7582282. [DOI] [PubMed] [Google Scholar]
- 25.de Gouw H W F M, Grünberg K, Schot R, Kroes A C M, Dick E C, Sterk P J. Relationship between exhaled nitric oxide and airway hyperresponsiveness following experimental rhinovirus infection in asthmatic subjects. Eur Respir J. 1998;11:126–132. doi: 10.1183/09031936.98.11010126. [DOI] [PubMed] [Google Scholar]
- 26.Denny F W, Clyde W A., Jr Acute lower respiratory tract infections in nonhospitalized children. J Pediatr. 1986;108:635–646. doi: 10.1016/s0022-3476(86)81034-4. [DOI] [PubMed] [Google Scholar]
- 27.Dick E C, Blumer C R, Evans A S. Epidemiology of infections with rhinoviruses type 43 and 5 in a group of University of Wisconsin student families. Am J Epidemiol. 1967;86:386–400. doi: 10.1093/oxfordjournals.aje.a120749. [DOI] [PubMed] [Google Scholar]
- 28.Dick E C, Inhorn S L. Rhinoviruses. In: Feigin R D, Cherry J D, editors. Textbook of pediatric infectious diseases. 3rd ed. Philadelphia, Pa: The W. B. Saunders Co.; 1992. pp. 1507–1532. [Google Scholar]
- 29.Dicosmo B F, Geba G P, Picarella D, Elias J A, Rankin J A, Stripp B R, Whitsett J A, Flavell R A. Airway epithelial cell expression of interleukin-6 in transgenic mice—uncoupling of airway inflammation and bronchial hyperreactivity. J Clin Invest. 1994;94:2028–2035. doi: 10.1172/JCI117556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doherty P C, Hou S, Tripp R A. CD8+ T-cell memory to viruses. Curr Opin Immunol. 1994;6:545–552. doi: 10.1016/0952-7915(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 31.Duff A L, Pomeranz E S, Gelber L E, Price H W, Farris H, Hayden F G, Platts-Mills T A E, Heymann P W. Risk factors for acute wheezing in infants in infants and children: viruses, passive smoke, and IgE antibodies to inhalant allergens. Pediatrics. 1993;92:535–540. [PubMed] [Google Scholar]
- 32.Einarsson O, Geba G P, Zhu Z, Landry M, Elias J A. Interleukin-11: stimulation in vivo and in vitro by respiratory viruses and induction of airways hyperresponsiveness. J Clin Invest. 1996;97:915–924. doi: 10.1172/JCI118514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Empey D W, Laitinen L A, Jacobs L, Gold W M, Nadel J A. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis. 1976;113:131–139. doi: 10.1164/arrd.1976.113.2.131. [DOI] [PubMed] [Google Scholar]
- 34.Everard M L, Swarbrick A, Wrightham M, Mcintyre J, Dunkley C, James P D, Sewell H F, Milner A D. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child. 1994;71:428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahy J V, Kim K W, Liu J, Boushey H A. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95:843–852. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 36.Farr B M, Gwaltney J M J, Hendley J O, Hayden F G, Naclerio R M, McBride T, Doyle W J, Sorrentino J V, Riker D K, Proud D. A randomized controlled trial of glucocorticoid prophylaxis against experimental rhinovirus infection. J Infect Dis. 1990;162:1173–1177. doi: 10.1093/infdis/162.5.1173. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson E A, Eccles R. Changes in nasal nitric oxide concentration associated with symptoms of common cold and treatment with a topical nasal decongestant. Acta Oto-Laryngol. 1997;117:614–617. doi: 10.3109/00016489709113447. [DOI] [PubMed] [Google Scholar]
- 38.Fiedler M A, Wernke-Dollries K, Stark J M. Respiratory syncytial virus increases IL-8 gene expression and protein release in A549 cells. Am J Physiol. 1995;269:L865–L872. doi: 10.1152/ajplung.1995.269.6.L865. [DOI] [PubMed] [Google Scholar]
- 39.Folkerts G, Busse W W, Nijkamp F P, Sorkness R, Gern J E. State of the art: virus-induced airway hyperresponsiveness and asthma. Am J Respir Crit Care Med. 1998;157:1708–1720. doi: 10.1164/ajrccm.157.6.9707163. [DOI] [PubMed] [Google Scholar]
- 40.Folkerts G, Linde van der H J, Nijkamp F P. Virus-induced airway hyperresponsiveness in guinea pigs is related to a deficiency in nitric oxide. J Clin Invest. 1995;95:26–30. doi: 10.1172/JCI117649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Folkerts G, Nijkamp F P. Virus-induced airway hyperresponsiveness: Role of inflammatory cells and mediators. Am J Resp Crit Care Med. 1995;151:1666–1674. doi: 10.1164/ajrccm.151.5.7735631. [DOI] [PubMed] [Google Scholar]
- 42.Folkerts G, Verheyen A, Janssen M, Nijkamp F P. Virus-induced airway hyperresponsiveness in the guinea pig can be transferred by bronchoalveolar cells. J Allergy Clin Immunol. 1992;90:364–72. doi: 10.1016/s0091-6749(05)80016-8. [DOI] [PubMed] [Google Scholar]
- 43.Fraenkel D J, Bardin P G, Sanderson G, Lampe F, Johnston S L, Holgate S T. Lower airway inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1995;151:879–886. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 44.Frick W E, German D, Mills J. Development of allergy in children. I. Association with virus infection. J Allergy Clin Immunol. 1979;63:228–241. doi: 10.1016/0091-6749(79)90106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gama R E, Horsnell P R, Hughes P J, North C, Bruce C B, al-Nakib W, Stanway G. Amplification of rhinovirus specific nucleic acids from clinical samples using the polymerase chain reaction. J Med Virol. 1989;28:73–77. doi: 10.1002/jmv.1890280204. [DOI] [PubMed] [Google Scholar]
- 46.Garafalo R, Mei F, Espejo R, Ye H, Haeherde H, Baron S, Ogra P, Reyes V E. Respiratory syncytial virus infection of human respiratory epithelial cells up-regulates class I MHC expression through induction of IFN-β and IL-1α. J Immunol. 1996;157:2506–2513. [PubMed] [Google Scholar]
- 47.Garofalo R, Kimpen J L L, Welliver R C, Ogra P L. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr. 1992;120:28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- 48.Gern J E, Busse W W. The effects of rhinovirus infections on allergic airway responses. Am J Respir Crit Care Med. 1995;152:S40–S45. doi: 10.1164/ajrccm/152.4_Pt_2.S40. [DOI] [PubMed] [Google Scholar]
- 49.Gern J E, Busse W W. Role of T cells in virus-induced asthma. In: Liggett S B, Meyers D A, editors. The genetics of asthma. New York, N.Y: Marcel Dekker, Inc.; 1996. pp. 39–66. [Google Scholar]
- 50.Gern J E, Calhoun W J, Swenson C, Shen G, Busse W W. Rhinovirus infection preferentially increases lower airway responsiveness in allergic subjects. Am J Respir Crit Care Med. 1997;155:1872–1876. doi: 10.1164/ajrccm.155.6.9196088. [DOI] [PubMed] [Google Scholar]
- 51.Gern J E, Dick E C, Kelly E A B, Vrtis R, Klein B. Rhinovirus-specific T cells recognize both shared and serotype-restricted viral epitopes. J Infect Dis. 1997;175:1108–1114. doi: 10.1086/516449. [DOI] [PubMed] [Google Scholar]
- 52.Gern J E, Dick E C, Lee W M, Murray S, Meyer K, Handzel Z T, Busse W W. Rhinovirus enters but does not replicate inside monocytes and airway macrophages. J Immunol. 1996;156:621–627. [PubMed] [Google Scholar]
- 53.Gern J E, Galagan D M, Jarjour N N, Dick E C, Busse W W. Detection of rhinovirus RNA in lower airway cells during experimentally-induced infection. Am J Respir Crit Care Med. 1997;155:1159–1161. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 54.Gern J E, Sukow K A, Swenson C, Dick E C, Busse W W. Correlation of nasal cytokines and symptoms during experimental rhinovirus infection. J Allergy Clin Immunol. 1997;99:S130. . (Abstract.) [Google Scholar]
- 55.Gern J E, Vrtis R, Kelly E A B, Dick E C, Busse W W. Rhinovirus produces nonspecific activation of lymphocytes through a monocyte-dependent mechanism. J Immunol. 1996;157:1605–1612. [PubMed] [Google Scholar]
- 56.Gosset P, Tsicopoulos A, Wallaert B, Vannimenus C, Joseph M, Tonnel A B, Capron A. Increased secretion of tumor necrosis factor α and interleukin-6 by alveolar macrophages consecutive to the development of the late asthmatic reaction. J Allergy Clin Immunol. 1991;88:561–571. doi: 10.1016/0091-6749(91)90149-i. [DOI] [PubMed] [Google Scholar]
- 57.Grünberg K, Smits H H, Timmers M C, de Klerk E P A, Dolhain R J, Dick E C, Hiemstra P S, Sterk P J. Experimental rhinovirus 16 infection. Effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am J Respir Crit Care Med. 1997;156:609–616. doi: 10.1164/ajrccm.156.2.9610079. [DOI] [PubMed] [Google Scholar]
- 58.Grünberg K, Timmers M C, Smits H H, De Klerk E P A, Dick E C, Spaan W J M, Hiemstra P S, Sterk P J. Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin-8 in nasal lavage in asthmatic subjects in vivo. Clin Exp Allergy. 1997;27:36–45. doi: 10.1111/j.1365-2222.1997.tb00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gustafson L M, Proud D, Hendley J O, Hayden F G, Gwaltney J M. Oral prednisone therapy in experimental rhinovirus infections. J Allergy Clin Immunol. 1996;97:1009–1014. doi: 10.1016/s0091-6749(96)80077-7. [DOI] [PubMed] [Google Scholar]
- 60.Gwaltney J M, Jr, Druce H M. Efficacy of brompheniramine maleate for the treatment of rhinovirus colds. Clin Infect Dis. 1997;25:1188–1194. doi: 10.1086/516105. [DOI] [PubMed] [Google Scholar]
- 61.Gwaltney J M, Jr, Park J, Paul R A, Edelman D A, O’Connor R R, Turner R B. Randomized controlled trial of clemastine fumarate for treatment of experimental rhinovirus colds. Clin Infect Dis. 1996;22:656–662. doi: 10.1093/clinids/22.4.656. [DOI] [PubMed] [Google Scholar]
- 62.Gwaltney J M., Jr Where’s the bias? Ann Intern Med. 1998;128:75. doi: 10.7326/0003-4819-128-1-199801010-00025. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 63.Halperin S A, Eggleston P A, Beasley P, Suratt P, Hendley J O, Gröschel D H, Gwaltney J M., Jr Exacerbations of asthma in adults during experimental rhinovirus infection. Am Rev Respir Dis. 1985;132:976–980. doi: 10.1164/arrd.1985.132.5.976. [DOI] [PubMed] [Google Scholar]
- 64.Halperin S A, Eggleston P A, Hendley J O, Suratt P M, Gröschel D H, Gwaltney J M Jr. Pathogenesis of lower respiratory tract symptoms in experimental rhinovirus infection. Am Rev Respir Dis. 1983;128:806–810. doi: 10.1164/arrd.1983.128.5.806. [DOI] [PubMed] [Google Scholar]
- 65.Halperin S A, Suratt P M, Gwaltney J M, Jr, Gröschel D H, Hendley J O, Eggleston P A. Bacterial cultures of the lower respiratory tract in normal volunteers with and without experimental rhinovirus infection using a plugged double catheter system. Am Rev Respir Dis. 1982;125:678–680. doi: 10.1164/arrd.1982.125.6.678. [DOI] [PubMed] [Google Scholar]
- 66.Handzel Z T, Busse W W, Sedgwick J B, Vrtis R, Lee W M, Kelly E A B, Gern J E. Eosinophils bind rhinovirus and activate virus-specific T cells. J Immunol. 1998;160:1279–1284. [PubMed] [Google Scholar]
- 67.Hayden F G, Albrecht J K, Kaiser D L, Gwaltney J M Jr. Prevention of natural colds by contact prophylaxis with intranasal alpha2-interferon. N Engl J Med. 1986;314:71–75. doi: 10.1056/NEJM198601093140202. [DOI] [PubMed] [Google Scholar]
- 68.Hayden F G, Diamond L, Wood P B, Korts D C, Wecker M T. Effectiveness and safety of intranasal ipratropium bromide in common colds—a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1996;125:89–89. doi: 10.7326/0003-4819-125-2-199607150-00002. [DOI] [PubMed] [Google Scholar]
- 69.Hayden F G, Hipskind G J, Woerner D H, Eisen G F, Janssens M, Janssen P A J, Andries K. Intranasal pirodavir (R77,975) treatment of rhinovirus colds. Antimicrob Agents Chemother. 1995;39:290–294. doi: 10.1128/aac.39.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henderson F W, Clyde W A, Jr, Collier A M, Denny F W, Senior R J, Sheaffer C I, Conley W G, Christian R M. The etiologic and epidemiologic spectrum of bronchiolitis in pediatric practice. J Pediatr. 1979;95:183–190. doi: 10.1016/s0022-3476(79)80647-2. [DOI] [PubMed] [Google Scholar]
- 71.Heymann P W, Rakes G P, Hogan A D, Ingram J M, Hoover G E, Platts-Mills T A. Assessment of eosinophils, viruses and IgE antibody in wheezing infants and children. Int Arch Allergy Immunol. 1995;107:380–382. doi: 10.1159/000237043. [DOI] [PubMed] [Google Scholar]
- 72.Higgins P G, Barrow G I, Tyrrell D A. A study of the efficacy of the bradykinin antagonist, NPC 567, in rhinovirus infections in human volunteers. Antiviral Res. 1990;14:339–344. doi: 10.1016/0166-3542(90)90052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horn M E C, Brain E, Gregg I. Respiratory viral infections in childhood. A survey in general practice. J Hyg. 1974;157:157–168. doi: 10.1017/s0022172400024220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horn M E C, Reed S E, Taylor P. Role of viruses and bacteria in acute wheezy bronchitis in childhood: a study of sputum. Arch Dis Child. 1979;54:587–592. doi: 10.1136/adc.54.8.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsia J, Goldstein A L, Simon G L, Sztein M, Hayden F G. Peripheral blood mononuclear cell interleukin-2 and interferon-gamma production, cytotoxicity, and antigen-stimulated blastogenesis during experimental rhinovirus infection. J Infect Dis. 1990;162:591–597. doi: 10.1093/infdis/162.3.591. [DOI] [PubMed] [Google Scholar]
- 76.Huguenel E D, Cohn D, Dockum D P, Greve J M, Fournel M A, Hammond L, Irwin R, Mahoney J, Mcclelland A, Muchmore E, Ohlin A C, Scuderi P. Prevention of rhinovirus infection in chimpanzees by soluble intercellular adhesion molecule-1. Am J Respir Crit Care Med. 1997;155:1206–1210. doi: 10.1164/ajrccm.155.4.9105055. [DOI] [PubMed] [Google Scholar]
- 77.Igarashi Y, Skoner D P, Doyle W J, White M V, Fireman P, Kaliner M A. Analysis of nasal secretions during experimental rhinovirus upper respiratory infections. J Allergy Clin Immunol. 1993;92:722–731. doi: 10.1016/0091-6749(93)90016-9. [DOI] [PubMed] [Google Scholar]
- 78.Jackson J L, Peterson C, Lesho E. A meta-analysis of zinc salts lozenges and the common cold. Arch Intern Med. 1997;157:2373–2376. [PubMed] [Google Scholar]
- 79.Johnston S L, Papi A, Bates P J, Mastronarde J G, Monick M M, Hunninghake G W. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J Immunol. 1998;160:6172–6181. [PubMed] [Google Scholar]
- 80.Johnston S L, Pattemore P K, Sanderson G, Smith S, Campbell M J, Josephs L K, Cunningham A, Robinson B S, Myint S H, Ward M E, Tyrrell D A J, Holgate S T. The relationship between upper respiratory infections and hospital admissions for asthma: a time trend analysis. Am J Respir Crit Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 81.Johnston S L, Pattemore P K, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O’Toole S, Myint S H, Tyrrell D A, Holgate S T. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. Br Med J. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnston S L, Xie P, Johnson W. Comparison of standard virology and PCR in diagnosis of rhinovirus and respiratory syncytial virus infections in nasal aspirates from children hospitalized with wheezing illness and bronchiolitis. Am J Respir Crit Care Med. 1996;153:A503. . (Abstract.) [Google Scholar]
- 83.Kameyoshi Y, Dorschner A, Mallet A I, Christophers E, Schroder J M. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992;176:587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kellner G, Popow-Kraupp T, Kundi M, Binder C, Kunz C. Clinical manifestations of respiratory tract infections due to respiratory syncytial virus and rhinoviruses in hospitalized children. Acta Paediatr Scand. 1989;78:390–394. doi: 10.1111/j.1651-2227.1989.tb11098.x. [DOI] [PubMed] [Google Scholar]
- 85.Kharitonov S A, Yates D, Barnes P J. Increased nitric oxide in exhaled air of normal human subjects with upper respiratory tract infections. Eur Respir J. 1995;8:295–297. doi: 10.1183/09031936.95.08020295. [DOI] [PubMed] [Google Scholar]
- 86.Laitinen L A, Elkin R B, Empey D W, Jacobs L, Mills J, Nadel J A. Bronchial hyperresponsiveness in normal subjects during attenuated influenza virus infection. Am Rev Respir Dis. 1991;143:358–361. doi: 10.1164/ajrccm/143.2.358. [DOI] [PubMed] [Google Scholar]
- 87.Laitinen L A, Kava T. Bronchial reactivity following uncomplicated influenza A infection in healthy subjects and in asthmatic patients. Eur J Respir Dis. 1980;10:51–58. [PubMed] [Google Scholar]
- 88.Las Heras J, Swanson V L. Sudden death of an infant with rhinovirus infection complicating bronchial asthma: case report. Pediatr Pathol. 1983;1:319–323. doi: 10.3109/15513818309040669. [DOI] [PubMed] [Google Scholar]
- 89.Lemanske R F, Jr, Dick E C, Swenson C A, Vrtis R F, Busse W W. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989;83:1–10. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levandowski R A, Ou D W, Jackson G G. Acute-phase decrease of T lymphocyte subsets in rhinovirus infection. J Infect Dis. 1986;153:743–748. doi: 10.1093/infdis/153.4.743. [DOI] [PubMed] [Google Scholar]
- 91.Levandowski R A, Weaver C W, Jackson G G. Nasal secretion leukocyte populations determined by flow cytometry during acute rhinovirus infection. J Med Virol. 1988;25:423–432. doi: 10.1002/jmv.1890250406. [DOI] [PubMed] [Google Scholar]
- 92.Lopez A F, Williamson D J, Gamble J R, Begley C G, Harlan J M, Klebanoff S J, Waltersdorph A, Wong G, Clark S C, Vadas M A. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986;78:1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mills J S. Viral protease inhibitors—what next after HIV? Antiviral Chem Chemother. 1996;7:281–293. [Google Scholar]
- 94.Minor T E, Dick E C, Baker J W, Ouellette J J, Cohen M, Reed C E. Rhinovirus and influenza type A infections as precipitants of asthma. Am Rev Respir Dis. 1976;113:149–153. doi: 10.1164/arrd.1976.113.2.149. [DOI] [PubMed] [Google Scholar]
- 95.Minor T E, Dick E C, DeMeo A N, Ouellette J J, Cohen M, Reed C E. Viruses as precipitants of asthmatic attacks in children. JAMA. 1974;227:292–298. [PubMed] [Google Scholar]
- 96.Monto A S, Cavallaro J J. The Tecumseh study of respiratory illness. II. Patterns of occurrence of infection with respiratory pathogens. Am J Epidemiol. 1971;94:280–289. doi: 10.1093/oxfordjournals.aje.a121321. [DOI] [PubMed] [Google Scholar]
- 97.Mossad S B, Macknin M L, Medendorp S V, Mason P. Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebo-controlled study. Ann Intern Med. 1996;125:81–88. doi: 10.7326/0003-4819-125-2-199607150-00001. [DOI] [PubMed] [Google Scholar]
- 98.Naclerio R M, Proud D, Lichtenstein L M, Kagey-Sobotka A, Hendley J O, Sorrentino J, Gwaltney J M. Kinins are generated during experimental rhinovirus colds. J Infect Dis. 1988;157:133–142. doi: 10.1093/infdis/157.1.133. [DOI] [PubMed] [Google Scholar]
- 99.Nagata M, Sedgwick J B, Busse W W. Synergistic activation of eosinophil superoxide anion generation by VCAM-1 and GM-CSF. Involvement of tyrosine kinase and protein kinase C. Int Arch Allergy Immunol. 1997;114:S78–80. doi: 10.1159/000237725. [DOI] [PubMed] [Google Scholar]
- 100.Nakazawa H, Sekizawa K, Morikawa M, Yamauchi K, Satoh M, Maeyama K, Watanabe T, Sasaki H. Viral respiratory infection causes airway hyperresponsiveness and decreases histamine N-methyltransferase activity in guinea pigs. Am J Respir Crit Care Med. 1994;149:1180–1185. doi: 10.1164/ajrccm.149.5.8173757. [DOI] [PubMed] [Google Scholar]
- 101.Nicholson K G, Kent J, Hammersley V, Cancio E. Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. Br Med J. 1996;313:1119–1123. doi: 10.1136/bmj.313.7065.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nicholson K G, Kent J, Ireland D C. Respiratory viruses and exacerbations of asthma in adults. Br Med J. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Noah T L, Henderson F W, Wortman I A, Devlin R B, Handy J, Koren H S, Becker S. Nasal cytokine production in viral acute upper respiratory infection of childhood. J Infect Dis. 1995;171:584–592. doi: 10.1093/infdis/171.3.584. [DOI] [PubMed] [Google Scholar]
- 104.Pattemore P K, Johnston S L, Bardin P G. Viruses as precipitants of asthma symptoms. I. Epidemiology. Clin Exp Allergy. 1992;22:325–336. doi: 10.1111/j.1365-2222.1992.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Proud D, Gwaltney J M Jr, Hendley J O, Dinarello C A, Gillis S, Schleimer R P. Increased levels of interleukin-1 are detected in nasal secretions of volunteers during experimental rhinovirus colds. J Infect Dis. 1994;169:1007–1013. doi: 10.1093/infdis/169.5.1007. [DOI] [PubMed] [Google Scholar]
- 106.Proud D, Naclerio R M, Gwaltney J M, Hendley J O. Kinins are generated in nasal secretions during natural rhinovirus colds. J Infect Dis. 1990;161:120–123. doi: 10.1093/infdis/161.1.120. [DOI] [PubMed] [Google Scholar]
- 107.Rager K J, Langland J O, Jacobs B L, Proud D, Marsh D G, Imani F. Activation of antiviral protein kinase leads to immunoglobulin E class switching in human B cells. J Virol. 1998;72:1171–1176. doi: 10.1128/jvi.72.2.1171-1176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raz M, Robbins R A, Kelling C L, Stine L C, Leikauf G D, Rennard S I, Spurzem J R. Viral infection of bovine bronchial epithelial cells induces increased neutrophil chemotactic activity and neutrophil adhesion. Exp Lung Res. 1993;85:753–760. doi: 10.1042/cs0850753. [DOI] [PubMed] [Google Scholar]
- 109.Rimmelzwaan G F, Osterhaus A D M E. Cytotoxic T lymphocyte memory: role in cross-protective immunity against influenza? Vaccine. 1995;13:703–705. doi: 10.1016/0264-410x(94)00030-q. [DOI] [PubMed] [Google Scholar]
- 110.Rosenwirth B, Kis Z L, Eggers H J. In vivo efficacy of SDZ 35-682, a new picornavirus capsid-binding agent. Antiviral Res. 1995;26:55–64. doi: 10.1016/0166-3542(94)00065-g. [DOI] [PubMed] [Google Scholar]
- 111.Rothlein R, Czajkowski M, O’Neill M M, Marlin S D, Mainolfi E, Merluzzi V J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. J Immunol. 1988;141:1665–1669. [PubMed] [Google Scholar]
- 112.Sanders S P, Siekierski E S, Porter J D, Richards S M, Proud D. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J Virol. 1998;72:934–942. doi: 10.1128/jvi.72.2.934-942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sarawar S R, Doherty P C. Concurrent production of interleukin-2, interleukin-10, and gamma interferon in the regional lymph nodes of mice with influenza pneumonia. J Virol. 1994;68:3112–3119. doi: 10.1128/jvi.68.5.3112-3119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schuster A, Fahy J V, Ueki I, Nadel J A. Cystic fibrosis sputum induces a secretory response from airway gland serous cells that can be prevented by neutrophil protease inhibitors. Eur Respir J. 1995;8:10–14. doi: 10.1183/09031936.95.08010010. [DOI] [PubMed] [Google Scholar]
- 115.Sedgwick J B, Quan S F, Calhoun W J, Busse W W. Effect of interleukin-5 and granulocyte-macrophage colony stimulating factor on in vitro eosinophil function: Comparison with airway eosinophils. J Allergy Clin Immunol. 1995;96:375–385. doi: 10.1016/s0091-6749(95)70057-9. [DOI] [PubMed] [Google Scholar]
- 116.Skoner D P, Doyle W J, Seroky J, Fireman P. Lower airway responses to influenza A virus in healthy allergic and nonallergic subjects. Am J Respir Crit Care Med. 1996;154:661–664. doi: 10.1164/ajrccm.154.3.8810602. [DOI] [PubMed] [Google Scholar]
- 117.Skoner D P, Doyle W J, Seroky J, Vandeusen M A, Fireman P. Lower airway responses to rhinovirus 39 in healthy allergic and nonallergic subjects. Eur Respir J. 1996;9:1402–1406. doi: 10.1183/09031936.96.09071402. [DOI] [PubMed] [Google Scholar]
- 118.Skoner D P, Doyle W J, Tanner E P, Kiss J, Fireman P. Effect of rhinovirus 39 (RV-39) infection on immune and inflammatory parameters in allergic and non-allergic subjects. Clin Exp Allergy. 1995;25:561–567. doi: 10.1111/j.1365-2222.1995.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 119.Stellato C, Beck L A, Gorgone G A, Proud D, Schall T J, Ono S J, Lichtenstein L M, Schleimer R P. Expression of the chemokine RANTES by a human bronchial epithelial cell line. J Immunol. 1995;155:410–418. [PubMed] [Google Scholar]
- 120.Sterk P J. Virus-induced airway hyperresponsiveness in man. Eur Respir J. 1993;6:894–902. [PubMed] [Google Scholar]
- 121.Subauste M C, Jacoby D B, Richards S M, Proud D. Infection of a human respiratory epithelial cell line with rhinovirus - Induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J Clin Invest. 1995;96:549–557. doi: 10.1172/JCI118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Summers Q A, Higgins P G, Barrow I G, Tyrrell D A, Holgate S T. Bronchial reactivity to histamine and bradykinin is unchanged after rhinovirus infection in normal subjects. Eur Respir J. 1992;5:313–317. [PubMed] [Google Scholar]
- 123.Tang W, Geba G P, Zheng T, Ray P, Homer R J, Kuhn C, Flavell R A, Elias J A. Targeted expression of IL-11 in the murine airway causes lymphocytic inflammation, bronchial remodeling, and airways obstruction. J Clin Invest. 1996;98:2845–2853. doi: 10.1172/JCI119113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Terajima M, Yamaya M, Sekizawa K, Okinaga S, Suzuki T, Yamada N, Nakayama K, Ohrui T, Oshima T, Numazaki Y, Sasaki H. Rhinovirus infection of primary cultures of human tracheal epithelium: role of ICAM-1 and IL-1beta. Am J Physiol. 1997;273:L749–L759. doi: 10.1152/ajplung.1997.273.4.L749. [DOI] [PubMed] [Google Scholar]
- 125.Teran L M, Johnston S L, Schroder J M, Church M K, Holgate S T. Role of nasal interleukin-8 in neutrophil recruitment and activation in children with virus-induced asthma. Am J Respir Crit Care Med. 1997;155:1362–1366. doi: 10.1164/ajrccm.155.4.9105080. [DOI] [PubMed] [Google Scholar]
- 126.Tough D F, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 127.Trigg C J, Nicholson K G, Wang J H, Ireland D C, Jordan S, Duddle J M, Hamilton S, Daview R J. Bronchial inflammation and the common cold: a comparison of atopic and non-atopic individuals. Clin Exp Allergy. 1996;26:665–676. doi: 10.1111/j.1365-2222.1996.tb00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Turner R B. The role of neutrophils in the pathogenesis of rhinovirus infections. Pediatr Infect Dis J. 1990;9:832–835. doi: 10.1097/00006454-199011000-00011. [DOI] [PubMed] [Google Scholar]
- 129.Turner R B, Dutko F J, Goldstein N H, Lockwood G, Hayden F G. Efficacy of oral WIN 54954 for prophylaxis of experimental rhinovirus infection. Antimicrob Agents Chemother. 1993;37:297–300. doi: 10.1128/aac.37.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Turner R B, Weingand K W, Yeh C H, Leedy D W. Association between interleukin-8 concentration in nasal secretions and severity of symptoms of experimental rhinovirus colds. Clin Infect Dis. 1998;26:840–846. doi: 10.1086/513922. [DOI] [PubMed] [Google Scholar]
- 131.Van Oosterhout A J M, van Ark I, Folkerts G, van der Linde H J, Savelkoul HF, Verheyen A K, Nijkamp F P. Antibody to interleukin-5 inhibits virus-induced airway hyperresponsiveness to histamine in guinea pigs. Am J Respir Crit Care Med. 1995;151:177–183. doi: 10.1164/ajrccm.151.1.7812550. [DOI] [PubMed] [Google Scholar]
- 132.Wimalasundera S S, Katz D R, Chain B M. Characterization of the T cell response to human rhinovirus in children: implications for understanding the immunopathology of the common cold. J Infect Dis. 1997;176:755–759. doi: 10.1086/514101. [DOI] [PubMed] [Google Scholar]
- 133.Winther, B., B. Farr, R. B. Thoner, J. O. Hendley, N. Mygrind, and J. M. Gwaltney. 1984. Histopathologic examination and enumeration of polymorphonuclear leukocytes in the nasal mucosa during experimental rhinovirus colds. Acta Otolaryngol (Stockh) 413(Suppl.):19–24. [DOI] [PubMed]
- 134.Winther B, Gwaltney J M Jr, Mygind M, Turner R B, Hendley J O. Sites of rhinovirus recovery after point inoculation of the upper airway. JAMA. 1986;256:1763–1767. [PubMed] [Google Scholar]
- 134a.Winther B, Greve J M, Gwaltney Jr J M, Innes D J, Eastham J R, Mcclelland A, Hendley J O. Surface expression of intercellular adhesion molecule 1 on epithelial cells in the human adenoid. J Infect Dis. 1997;176:523–525. doi: 10.1086/517280. [DOI] [PubMed] [Google Scholar]
- 135.Wiselka M J, Nicholson K G, Kent J, Cookson J B, Tyrrell D A J. Prophylactic intranasal α2 interferon and viral exacerbations of chronic respiratory disease. Thorax. 1991;46:706–711. doi: 10.1136/thx.46.10.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang L, Redington A E, Holgate S T. RANTES: A novel mediator of allergic inflammation? Clin Exp Allergy. 1994;24:899–904. doi: 10.1111/j.1365-2222.1994.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 137.Zhou S, Stark J M, Leikauf G D. Leukotriene B4 formation: human neutrophil-airway epithelial cell interactions. J Appl Physiol. 1995;78:1396–1403. doi: 10.1152/jappl.1995.78.4.1396. [DOI] [PubMed] [Google Scholar]
- 138.Zhu Z, Tang W, Gwaltney J M J, Wu Y, Elias J A. Rhinovirus stimulation of interleukin-8 in vivo and in vitro: role of NF-kappaB. Am J Physiol. 1997;273:L814–L824. doi: 10.1152/ajplung.1997.273.4.L814. [DOI] [PubMed] [Google Scholar]
- 139.Zhu Z, Tang W, Wu Y, Einarsson O, Landry M L, Gwaltney J M, Jr, Elias J A. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor kappa B-dependent transcriptional activation. J Clin Invest. 1996;97:421–430. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]