Abstract
Background
Atrial fibrillation (AF) is the most common cardiac arrhythmia in the adult population. Herein, is a systematic review with meta-analysis to determine the impact of AF/atrial flutter (AFL) on mortality, as well as individual complications in patients hospitalized with the coronavirus disease 2019 (COVID-19).
Methods
A systematic search of the SCOPUS, Medline, Web of Science, CINAHL and Cochrane databases was performed. The a priori primary outcome of interest was in-hospital mortality. A random-effects model was used to pool study results.
Results
Nineteen studies which included 33,296 patients were involved in this meta-analysis. In-hospital mortality for AF/AFL vs. no-AF/AFL groups varied and amounted to 32.8% vs. 14.2%, respectively (risk ratio [RR]: 2.18; 95% confidence interval [CI]: 1.79–2.65; p < 0.001). In-hospital mortality in new onset AF/AFL compared to no-AFAFL was 22.0% vs. 18.8% (RR: 1.86; 95% CI: 1.54–2.24; p < 0.001). Intensive care unit (ICU) admission was required for 17.7% of patients with AF/AFL compared to 10.8% for patients without AF/AFL (RR: 1.94; 95% CI: 1.04–3.62; p = 0.04).
Conclusions
The present study reveals that AF/AFL is associated with increased in-hospital mortality and worse outcomes in patients with COVID-19 and may be used as a negative prognostic factor in these patients. Patients with AF/AFL are at higher risk of hospitalization in ICU. The presence of AF/AFL in individuals with COVID-19 is associated with higher risk of complications, such as bleeding, acute kidney injury and heart failure. AF/AFL may be associated with unfavorable outcomes due to the hemodynamic compromise of cardiac function itself or hyperinflammatory state typical of these conditions.
Keywords: atrial fibrillation, atrial flutter, new onset atrial fibrillation, COVID-19, outcome, systematic review, meta-analysis
Introduction
Cardiovascular diseases (CVD) are known to affect the prognosis of patients hospitalized with coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) [1, 2]. It has been demonstrated that patients with pre-existing comorbidities, e.g., hypertension, coronary artery disease (CAD) or congestive heart failure are more likely to suffer from the severe course of COVID-19 [3, 4], more often require admission to the intensive care unit (ICU) [4–6], use mechanical ventilation [3, 7] and have higher mortality [3, 7, 8], compared to patients without CVD. This is a sign of increased vulnerability towards the virus and subsequent disease.
Atrial fibrillation (AF) is the most common cardiac arrhythmia in the adult population. According to European Society of Cardiology, global prevalence of AF oscillates between 2% and 4% and is expected to further increase due to longevity, including an expanding group of people with long-lasting underlying CVD [9]. The incidence of atrial flutter (AFL) in individuals without recent predisposing events and preexisting comorbidities is estimated to reach 1.7% [10]. Pathophysiological mechanisms responsible for those arrhythmias include i.e.: structural and electrical atrial remodeling through fibrosis, hypertrophy, inflammation or oxidative stress [9, 11, 12]. Acute inflammation in the course of COVID-19 may alter atrial electrophysiology and structural substrates, therefore playing a major role in the development of these conditions in patients with COVID-19 [13]. Due to the well-established links between inflammation and AF, the association between COVID-19 and AF constitutes an interesting and thus far, unexplored subject.
Outcome analysis of patients hospitalized with COVID-19 provides valuable data that can generate new hypotheses regarding the pathophysiology of AF and AFL, help to identify patients at a higher risk for adverse outcomes and improve patient management within hospital wards. Previously published literature on the outcomes of COVID-19 patients with AF/AFL consists mainly of retrospective studies, rarely single-center prospective ones and very often provides conflicting results. This problem has previously been addressed in the “discussion forum” section of European Heart Journal [14, 15] or in review articles [16].
However, to reach solid conclusions regarding the association between AF/AFL and outcomes of patients with COVID-19, a systematic analysis of available data is indispensable. The available research is insufficient, with one meta-analysis published in January 2021, providing data about the influence of AF on outcomes of patients with COVID-19 [17]. However, it only evaluated the mortality outcomes, without considering other complications, and it has been limited to the European and United States populations. Furthermore, due to the constant changes in our understanding of COVID-19, development of new treatment protocols and pandemic dynamics itself, it is essential to provide updated, high-quality data regarding the association between COVID-19 and CVD. A systematic review with meta-analysis was performed herein, to determine the impact of AF/AFL on mortality, as well as individual complications in patients hospitalized with COVID-19.
Methods
The current systematic review and meta-analysis was complied with the widely recognized Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Suppl. Table S2) [18]. Due to the study design, neither an institutional review board approval nor patient informed consent were required.
Search strategy
An extensive search was conducted of the relevant data using the SCOPUS, Medline, Web of Science, CINAHL and the Cochrane Central Register for Controlled Trials from these databases inception through to October 10th, 2021. The search was performed using the following terms: “atrial fibrillation” OR “AF” OR “atrial flutter” OR “AFL” AND “COVID-19” OR “coronavirus disease 2019” OR “SARS-CoV-2”. Two of the reviewers (M.P. and A.G.) independently selected candidates for the study, and conflicts were resolved through discussion with a third reviewer (L.S.).
Studies comparing adult COVID-19 patients more than 18 years old with and without AF/AFL were systematically searched as noted. All randomized controlled trials (RCTs) and observational studies were included in this review. Case reports, case series, and conference abstracts were excluded.
Data extraction
Two reviewers (L.S. and M.P.) independently assessed each article to determine whether they met the inclusion criteria. In cases of suspected data discrepancies, the relevant author was contacted directly, moreover, care was taken to avoid including data from duplicate publications.
Primary and secondary outcomes
The a priori primary outcome of interest was in-hospital mortality. Secondary outcomes were: occurrence of adverse events, in-hospital cardiovascular death or hospital- or ICU-length of stay.
Risk of bias assessment
Risk Of Bias In Non-randomized Studies was used — the Interventions (ROBINS-I) tool was utilized to assess the quality of the studies’ design and extent of potential bias [19]. The ROBINS-I tool examines five bias domains: (1) bias due to confounding; (2) bias due to selection of participants; (3) bias in classification of interventions; (4) bias due to deviations from intended interventions; (5) bias due to missing data; (6) bias in measurement of outcomes; (7) bias in selection of the reported result. The overall ROBINS-I judgment at domain and study level was attributed according to the criteria specified in the ROBVIS tool [20].
Statistical analysis
The Cochrane Statistical Package Review Manager ver. 5.4 (Cochrane Collaboration, London, United Kingdom) was used for data synthesis and analysis. For dichotomous data, odds ratios (ORs) or risk ratios (RRs) as the effect measure were used with 95% confidence intervals (CIs), and for continuous data, standard mean differences (SMDs) with 95% CI were used. In cases where the continuous outcome was reported in a study as median, range, and interquartile range, means and standard deviations were estimated using the formula described by Hozo et al. [21]. Heterogeneity was quantified in each analysis by the tau-squared and I2 statistics. Values of I2 > 50% and > 75% were considered to indicate moderate and significant heterogeneity among studies, respectively. A random-effects model was used to pool study results independently of the p-value for heterogeneity or I2 [22].
Results
Characteristics of studies included in the meta-analysis
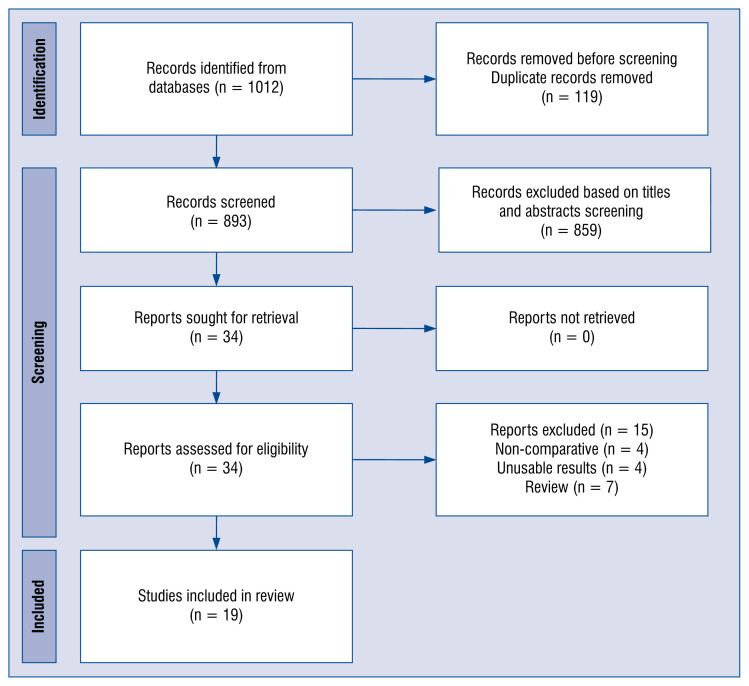
A total of 1,012 articles were identified from the Medline (PubMed), Embase, Cochrane library, and the manual search as described above. Ultimately, 19 studies [23–41] published from 2020 to 2021 which included 33,296 patients in the meta-analysis (Fig. 1). The details of the selected trials are summarized in Table 1.
Figure 1.
Flow diagram showing stages of the database search and study selection as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Table 1.
Characteirsitcs of included trials.
| Study | Country | Study design | Study group | No. of patients | Age, mean ± SD | Sex, male | Diabetes | Hypertension | CAD | LVEF, mean ± SD |
|---|---|---|---|---|---|---|---|---|---|---|
| Abdulrahman et al. 2021 | Bahrain | Retrospective study | With AF | 30 | 70.0 ± 11.8 | 30 (100%) | 14 (46.7%) | 17 (56.7%) | 10 (33.3%) | 48.6 ± 11.7 |
| Without AF | 462 | 52.3 ± 15.7 | 462 (100%) | 166 (35.9%) | 181 (39.2%) | 49 (10.6%) | 51.2 ± 12.3 | |||
| Denegri et al. 2021 | Italy | Retrospective study | With AF | 30 | 78.5 ± 12.6 | 18 (60.0%) | 10 (33.3%) | 26 (86.7%) | 14 (46.7%) | NS |
| Without AF | 171 | 66.8 ± 14.4 | 111 (64.9%) | 27 (15.9%) | 88 (51.2%) | 21 (12.4%) | NS | |||
| Ergün et al. 2021 | Turkey | Retrospective study | With AF | 37 | 78.4 ± 3.6 | 29 (78.4%) | 14 (37.8% | 31 (83.8%) | 11 (29.7%) | NS |
| Without AF | 211 | 69.5 ± 3 | 147 (69.7%) | 77 (36.5%) | 144 (68.2%) | 54 (25.6%) | NS | |||
| García-Granja et al. 2021 | Spain | Retrospective study | With AF | 54 | 81.6 ± 8.7 | 35 (64.8%) | 13 (24.1%) | 40 (74.1%) | NS | 58.1 ± 11.7 |
| Without AF | 463 | 66.5 ± 14.9 | 255 (55.1%) | 78 (16.4%) | 219 (47.3%) | NS | 62.6 ± 6.9 | |||
| Harrison et al. 2020 | UK | Retrospective study | With AF | 6,589 | 73.6 ± 10.9 | 3,587 (54.4%) | 2692 (40.9%) | 5101 (77.4%) | 3038 (46.1%) | NS |
| Without AF | 6,589 | 73.3 ± 10.9 | 3,611 (54.8%) | 2746 (41.7%) | 5223 (79.3%) | 3062 (46.5%) | NS | |||
| Iacopino et al. 2020 | Italy | Retrospective study | With AF | 12 | 75.9 ± 8.8 | 10 (83.3%) | 5 (41.7%) | 12 (100.0%) | NS | 52.5 ± 5.8 |
| Without AF | 18 | 74.7 ± 10.2 | 10 (55.6%) | 6 (33.3%) | 14 (77.8%) | NS | 55 ± 2.9 | |||
| Ip et al. 2021 | USA | Multicenter retrospective cohort study | With AF | 60 | 74 | NS | NS | NS | NS | NS |
| Without AF | 111 | 63 | NS | NS | NS | NS | NS | |||
| Kelesoglu et al. 2021 | Turkey | Single-center prospectively study | With AF | 33 | 72.42 ± 6.1 | 16 (48.5%) | 7 (21.2%) | 22 (66.6%) | NS | NS |
| Without AF | 625 | 53.78 ± 13.8 | 356 (57.0%) | 112 (17.9%) | 188 (30.0%) | NS | NS | |||
| Kelesoglu et al. 2021 (B) | Turkey | Single-center prospectively study | With AF | 41 | 61.8 ± 6.1 | 20 (48.8%) | 9 (22.0%) | 19 (46.3%) | 6 (14.6%) | 62.8 ± 1.4 |
| Without AF | 741 | 55.5 ± 3.3 | 418 (56.4%) | 121 (16.3%) | 226 (30.5%) | 107 (14.4%) | 63.3 ± 1.2 | |||
| Lee et al. 2021 | Republic of Korea | Retrospective study | With AF | 130 | 71.9 ± 13.7 | 70 (53.9%) | 46 (35.4%) | 98 (75.4%) | 39 (30.0%) | NS |
| Without AF | 7032 | 47.3 ± 18.5 | 2799 (39.1%) | 962 (13.7%) | 1511 (21.5%) | 232 (3.3%) | NS | |||
| Mountantonakis et al. 2021 | USA | Retrospective study | With AF | 1238 | 73.1 ± 13.5 | 777 (62.8%) | 547 (44.2%) | 925 (74.7%) | 584 (47.2%) | NS |
| Without AF | 1238 | 73.6 ± 13.3 | 774 (62.5%) | 548 (44.3%) | 940 (75.9%) | 593 (47.9%) | NS | |||
| Musikantow et al. 2021 | USA | Retrospective study | With AF | 375 | 76.8 ± 2.8 | 225 (60.0%) | 125 (33.3%) | 208 (55.5%) | 90 (24.0%) | NS |
| Without AF | 3595 | 65 ± 3.7 | 2063(57.4%) | 851 (23.7%) | 1159 (32.2%) | 320 (9.0%) | NS | |||
| Pardo Sanz et al. 2021 | Spain | Retrospective study | With AF | 12 | 75.9 ± 9.6 | 8 (66.7%) | 3 (25.5%) | 9 (75.0%) | NS | NS |
| Without AF | 148 | 64.9 ± 16.3 | 88 (59.5%) | 22 (14.9%) | 66 (44.6%) | NS | NS | |||
| Peltzer et al. 2020 | USA | Retrospective study | With AF | 166 | 74.5 ± 13.0 | 120 (72.3%) | 50 (30.1%) | 114 (68.7%) | 45 (27.1%) | 59.4 ± 2.9 |
| Without AF | 887 | 60.1 ± 17.0 | 536 (60.4%) | 263 (29.7%) | 454 (51.2%) | 112 (12.6%) | 58.3 ± 2.8 | |||
| Rubini-Costa et al. 2021 | Spain | Retrospective study | With AF | 151 | 74.6 ± 11.0 | 82 (54.3%) | 46 (30.5%) | 115 (76.2%) | NS | NS |
| Without AF | 151 | 75.1 ± 12.0 | 51 (53.6%) | 46 (30.5%) | 105 (69.5%) | NS | NS | |||
| Russo et al. 2021 | Italy | Retrospective multicenter study | With AF | 71 | 73.69 ± 9.9 | 46 (64.8%) | 22 (31.0%) | 57 (80.3%) | 21 (29.6%) | NS |
| Without AF | 343 | 65.54 ± 15.48 | 207 (60.3%) | 84 (24.6%) | 206 (60.2%) | 45 (13.2%) | NS | |||
| Slipczuk et al. 2021 | USA | Retrospective study | With AF | 16 | 65.7 ± 4.1 | 12 (75.0%) | 9 (56.3%) | 14 (87.5%) | 8 (50.0%) | NS |
| Without AF | 363 | 67.5 ± 3 | 171 (47.1%) | 218 (60.1%) | 254 (70.0%) | 143 (39.4%) | NS | |||
| Spinoni et al. 2021 | Italy | Retrospective study | With AF | 134 | NS | NS | NS | NS | NS | NS |
| Without AF | 503 | NS | NS | NS | NS | NS | NS | |||
| Urbarri et al. 2021 | Spain | Retrospective study | With AF | 233 | 79.7 ± 9.7 | 134 (57.5%) | 69 (29.6%) | 189 (81.1%) | NS | NS |
| Without AF | 233 | 79.1 ± 11.5 | 134 (57.5%) | 69 (29.6%) | 189 (81.1%) | NS | NS |
AF — atrial fibrilation; CAD — coronary artery disease; LVEF — left ventricular ejection fraction; NS — not specified; SD — standard deviation; UK — United Kingdom; USA — United States of America
Male gender in the AF/AFL and no-AF/AFL groups varied and amounted to 56.6% vs. 52.4% (OR: 1.23; 95% CI: 1.05–1.44; I2: 64%; p = 0.01). Mean age of patients in AF/AFL group was 73.8 ± 11.2 years compared to 61.8 ± 17.5 years for no AF/AFL group (SMD: 0.90; 95% CI: 0.39–1.41; I2: 99%; p < 0.001). Detailed characteristics of patient comorbidities are presented in the Supplementary Table S1.
Results of the meta-analysis
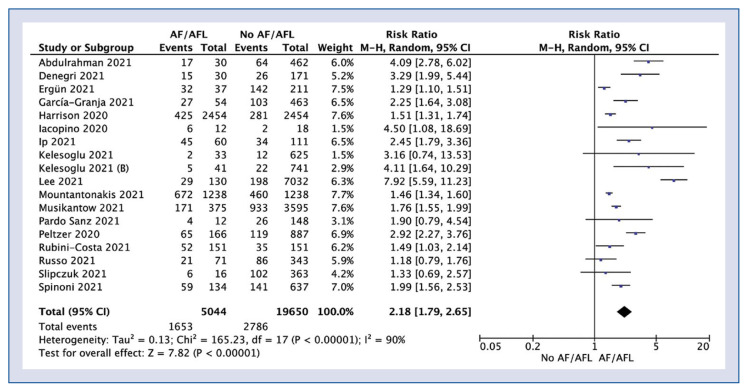
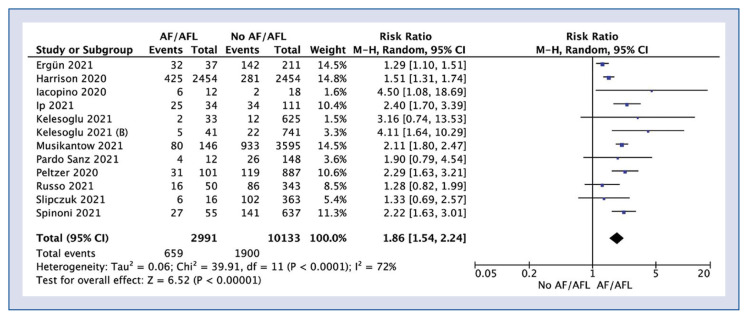
In-hospital mortality was reported in 18 studies and was 32.8% for AF/AFL group compared to 14.2% (RR: 2.18; 95% CI: 1.79–2.65; I2: 90%; p < 0.001; Fig. 2). Sub-analysis showed that in-hospital mortality in new onset AF/AFL compared to the non-AF/AFL group amounted to 22.0% vs. 18.8% (RR: 1.86; 95% CI: 1.54–2.24; I2: 72%; p < 0.001; Fig. 3).
Figure 2.
Forest plot of in-hospital mortality in atrial fibrillation/atrial flutter (AF/AFL) and no-AF/AFL groups. The center of each square represents the weighted risk ratios for individual trials, and the corresponding horizontal line stands for a 95% confidence interval (CI). The diamonds represent pooled results.
Figure 3.
Forest plot of in-hospital mortality in new onset atrial fibrillation/atrial flutter (AF/AFL) no-AF/AFL groups. The center of each square represents the weighted risk ratios for individual trials, and the corresponding horizontal line stands for a 95% confidence interval (CI). The diamonds represent pooled results.
In-hospital cardiovascular death was reported in 1 study [40] and was 10.4% vs. 5.2% respectively for patients with and without AF/AFL (RR: 2.02; 95% CI: 1.11–3.66; p = 0.02). Uribarri et al. [41] also showed 60-day mortality which was 43.3% vs. 30.9%, respectively (RR: 1.40; 95% CI: 1.10–1.79; p = 0.02).
Intensive care unit admission was required for 17.7% of patients with AF/AFL compared to 10.8% for patients without AF/AFL (RR: 1.94; 95% CI: 1.04–3.62; I2: 72%; p = 0.04).
Mechanical ventilation was reported in 6 studies and was 14.4% vs. 5.2% for patients with and without AF/AFL (RR: 1.76; 95% CI: 0.92–3.36; I2: 89%; p = 0.09).
A pooled analysis of the observed adverse events is presented in Table 2. Patients with AF/AFL had a higher risk of bleeding events (9.1% vs. 3.2%; RR: 3.50; 95% CI: 1.55–7.91; I2: 47%; p = 0.003), heart failure (HF) (23.1% vs. 18.2%; RR: 1.39; 95% CI: 1.01–1.91; I2: 35%; p = 0.04) as well as higher risk of acute kidney injury (AKI) (41.9% vs. 40.1%; RR: 1.31; 95% CI: 1.10–1.57; I2: 0%; p = 0.003), compared to patients without AF/AFL.
Table 2.
Polled analysis of adverse events among included trials.
| Adverse event | No. of studies | Events/participants | Events | Heterogeneity between trials | P-value for differences across groups | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| AF/AFL | No AF/AFL | RR | 95% CI | P-value | I2 statistic | |||
| Embolic events | 3 | 253/2699 (9.4%) | 181/2835 (6.4%) | 2.81 | 0.75–10.51 | 0.001 | 86% | 0.12 |
| APE | 2 | 3/91 (3.3%) | 14/674 (2.1%) | 1.80 | 0.10–32.61 | 0.07 | 70% | 0.69 |
| Stroke | 2 | 7/429 (1.6%) | 34/4058 (0.8%) | 1.95 | 0.87–4.37 | 0.75 | 0% | 0.11 |
| Bleeding events | 4 | 41/450 (9.1%) | 32/995 (3.2%) | 3.50 | 1.55–7.91 | 0.13 | 47% | 0.003 |
| Acute MI | 2 | 0/91 (0.0%) | 11/674 (1.6%) | 0.77 | 0.07–8.87 | 0.25 | 25% | 0.84 |
| Heart failure | 3 | 75/324 (23.1%) | 165/907 (18.2%) | 1.39 | 1.01–1.91 | 0.22 | 35% | 0.04 |
| Myocarditis | 2 | 0/66 (0.0%) | 1/481 (0.2%) | 2.81 | 0.12–68.19 | NA | NA | 0.53 |
| Ventricular arrhythmia | 1 | 1/54 (1.9%) | 0/463 (0.0%) | 25.31 | 1.04–613.72 | NA | NA | 0.05 |
| CPR | 1 | 1/37 (2.7%) | 8/211 (3.8%) | 0.71 | 0.09–5.53 | NA | NA | 0.75 |
| Acute kidney injury | 2 | 113/270 (41.9%) | 178/444 (40.1%) | 1.31 | 1.10–1.57 | 0.66 | 0% | 0.003 |
| RRT | 1 | 14/37 (37.8%) | 52/211 (24.6%) | 1.54 | 0.95–2.47 | NA | NA | 0.08 |
AF — atrial fibrillation; AFL — atrial flutter; APE — acute pulmonary embolism; CI — confidence interval; CPR — cardiopulmonary resuscitation; MI — myocardial infarction; NA — not applicable; RR — risk ratio; RRT — renal replacement therapy
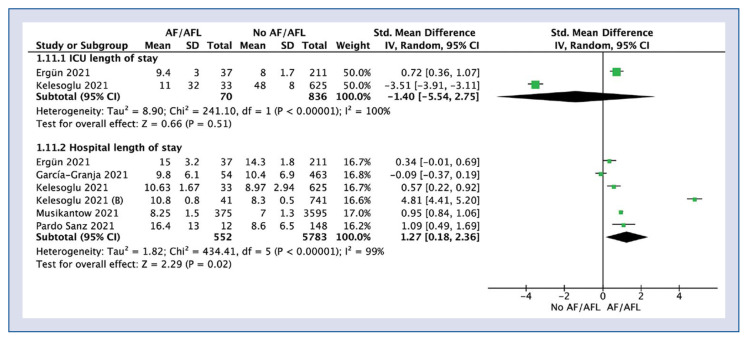
Length of stay in ICU was reported in 2 studies and was 10.2 ± 21.9 days for AF/AFL group compared to 37.9 ± 18.7 days for no AF/AFL group (SMD: −1.40; 95% CI: −5.54 to 2.75; I2: 100%; p = 0.51; Fig. 4).
Figure 4.
Forest plot of length of hospital stay in atrial fibrillation/atrial flutter (AF/AFL) and no-AF/AFL groups. The center of each square represents the weighted standard mean differences for individual trials, and the corresponding horizontal line stands for a 95% confidence interval (CI). The diamonds represent pooled results; ICU — intensive care unit.
Hospital length of stay in AF/AFL and no AF/AFL amounted to 9.4 ± 3.7 vs. 8.0 ± 3.1 days, respectively (SMD: 1.27; 95% CI: 0.18–2.36; I2: 99%; p = 0.02).
Discussion
General considerations and study population
Cardiovascular diseases, such as hypertension, have already been shown to worsen the prognosis of COVID-19 patients, both in terms of morbidity (increased risk of developing severe disease, need for hospitalization within ICU) and mortality [4, 8]. However, so far, no consensus has been reached regarding the impact of AF on the outcome of patients with COVID-19.
Atrial fibrillation and flutter are arrhythmias occurring mostly in the elderly, with hypertensive heart disease and coronary heart disease being the most frequently observed underlying disorders. According to previous studies, at least one risk factor, most often hypertension, is present among COVID-19 patients developing AF [42, 43]. However, there are also reports regarding new-onset AF emerging without any pre-existing illness [42, 44, 45]. The most prevalent comorbidities in patients with AF/AFL in this study included hypertension, chronic obstructive pulmonary disease (COPD) and CAD. Furthermore, the groups with AF/AFL tend to be of older age compared to groups without AF/AFL, with the difference in the mean age reaching as high as 24.6 years in one study [32].
Mortality in new-onset vs. pre-existing AF/AFL
Based on our findings, mortality was 2.18-fold higher in COVID-19 patients with pre-existing AF/AFL, compared to the non-AF/AFL group. This increase is much higher, compared to the previous meta-analysis, where the mortality was only 1.13-fold higher in patients with AF [17]. The reason for this difference, may be the inclusion of different populations in the present study, since the previous meta-analysis took into consideration only studies conducted in Europe and the United States. The magnitude of the increased risk in patients with pre-existing AF hospitalized due to COVID-19, remains to be established.
Due to a high prevalence of broad spectrum of comorbidities in patients with AF/AFL (e.g., hypertension, COPD, CAD) the distinction needs to be made between the impact of AF/AFL and the impact of other chronic diseases on in-hospital mortality. To date, in spite of the burden of comorbidities in patients with AF/AFL, many studies included in this meta-analysis confirmed that AF/AFL is an independent negative prognostic factor in patient with COVID-19 [23, 24, 29, 32, 33, 36, 37, 40, 41]. To confirm this finding, AF was associated with higher in-hospital mortality mainly in patients with a low or intermediate CHA2DS2-VASc score [15], suggesting that the existence of AF/AFL is not only the cumulative measure of risk due to other chronic diseases, but a novel prognostic factor. Hence, AF/AFL are potentially useful in clinical routine to identify patients at a higher risk of death, suggesting a need for a closer monitoring and a more intensive therapy.
Interestingly, the current study demonstrated that COVID-19 patients with new-onset AF/AFL had a 1.8-fold higher risk of mortality, compared to patients without AF/AFL, whereas patients with pre-existing AF had 2.18-fold higher risk of mortality, as compared to patients without AF/AFL. This suggests that especially pre-existing AF/AFL exerts its negative effects in the course of COVID-19. The higher risk of mortality in the group with long-lasting AF/AFL can also be an indicator of a long-term hemodynamic compromise, caused by the persistent effect of arrhythmia on the effectivity of atrial systole, which may produce higher susceptibility for adverse outcomes.
Mid-term mortality
One study provided additional data on 60-day mortality, which indicated a slightly lower increase in mortality between the AF/AFL group, compared to the non-AF/AFL group (RR: 1.40) [41]. This may suggest that the highest burden of AF/AFL falls on the period of first hospitalization, indicating the special need of intensive care and monitoring in the acute phase.
Reasons for increased mortality in AF/AFL
There are several theories explaining the association of AF/AFL with worse outcomes in COVID-19 that are strongly related to the molecular mechanisms underlying the electrical instability of atrial arrhythmias, hyperinflammatory state and mechanical stress on the cardiomyocytes.
Firstly, AF is associated with increased levels of angiotensin-converting enzyme 2 (ACE-2), the enzyme localized on the surface of coronary endothelial cells, cardiomyocytes and cardiac fibroblasts. In AF, levels of ACE-2 correlate strongly with the remodeling of the left atrium and play a pathophysiologic role by creating the substrate for arrhythmia [41, 46]. At the same time, ACE-2 is a receptor for SARS-CoV-2, allowing for viral entry. Higher levels of ACE-2 are associated with higher susceptibility for infections with SARS-CoV-2 and developing COVID-19 by allowing for a higher viral load within cells [46]. AF may be associated with higher levels of ACE-2, responsible for unfavorable outcomes. On the other hand, ACE-2 may also play a direct role in the cardiac involvement in the course of COVID-19. ACE-2 receptors are present on the cardiomyocytes as well pericytes in the vessels of microvasculature of the heart. Pericytes envelope the endothelial cells of microcirculation, providing vascular integrity [47–49]. It has been speculated that SARS-CoV-2 may interact with ACE-2 receptors on pericytes and lead to vascular leakage and consequent myocardial edema [11]. The edema, in turn, through increased interstitial hydrostatic pressure may lead to aberrations in ion channels conductance, predisposing patients with cardiac complications to AF. Consequently, a new-onset AF may be a manifestation of AKI.
Secondly, an inflammatory state underlies and predicts the onset of AF in humans [41]. An increasing body of evidence demonstrates the role of inflammatory markers (e.g., interleukin-6 and tumor necrosis factor alpha) [50–52], as well as inflammatory infiltrates within the myocardium in pathophysiology of AF [12, 53, 54]. Conversely, persistent AF itself favors remodeling by promoting inflammation, perpetuating the aberrations on the level of electrical conductance and producing the so-called ‘AF begets AF’ phenomenon. Consequently, individuals with long-lasting AF may be at higher risk of developing a hyperinflammatory reaction in the course of COVID-19 but also in patients undergoing COVID-19, developing a hyperinflammatory state, and are more likely to suffer from new-onset AF.
Thirdly, some studies suggest that AF may be the consequence of pulmonary vascular dysfunction (PVD), a condition frequently underlying acute respiratory distress syndrome. In this scenario, PVD is characterized by enhanced inflammatory signaling, remodeling and thrombosis within the microvasculature of the lungs, exerts mechanical stress on the right atrium and consequently on structural and electrical changes in cardiomyocytes. This creates a substrate for arrhythmias, especially AF. Studies conducted before the pandemic supported this hypothesis, showing a higher prevalence of AF in patients with pulmonary hypertension and tachycardia, both of which frequently occur in the course of COVID-19 [55]. In this regard, a new-onset AF would be a condition reflecting the occurrence of PVD.
Elucidating the exact cause or mechanism of death in individuals remains a challenge. This study demonstrated that in-hospital cardiovascular death occurred 2 times more often in the AF/AFL group compared to the non-AF/AFL group. These results are, however, based solely on one retrospective study, hence, does not allow drawing general conclusions. Therefore, we suggest that future studies should make the distinction between cardiovascular death, and non-cardiovascular causes. Also, due to the significance of inflammation in the pathophysiology of AF/AFL, we recommend measuring inflammatory parameters and correlating them with adverse outcomes.
ICU admission
The present study has demonstrated that ICU admission was required more frequently in the AF/AFL group compared to the non-AF/AFL group, although no significant difference in the ICU length of stay was observed. Further analysis of 2 studies [25, 30] provided insights regarding the mechanisms in which AF/AFL may contribute to worse outcomes in the examined group of patients. In the study of Ergün et al. [25], conducted with a group of ICU-patients, the laboratory findings showed an evident increase in the markers of cardiac injury (e.g., high sensitive troponin I; B-type natriuretic peptide) in the group with new-onset atrial fibrillation (NOAF) compared to the group without NOAF. Interestingly, no such difference was visible for C-reactive protein, white blood cell count, lymphocytes or neutrophils. In another study by Uribarri et al. [41] patients with AF have a significantly higher incidence of HF, but lower incidence of respiratory insufficiency, high flow nasal cannula, both with noninvasive and invasive mechanical ventilation.
This suggests that AF/AFL may exert its detrimental effects (reflected by the necessity of therapy in ICU) through myocardial injury rather than only passively reflecting the lung pathology and hyperinflammatory state. These findings may be relevant for the therapy of COVID-19 patients, as the scenario where AF/AFL causes worse outcomes directly through cardiac injury that requires different specific therapy administered by a team with extensive cardiological knowledge, as compared to the scenario, where AF/AFL merely accompanies severe disease where the stress is put on anesthesiologic therapy.
Individual complications
The complications occur significantly more frequently in COVID-19 patients with AF/AFL, compared to those without AF/AFL including bleeding events, AKI and HF.
Bleeding complications occurred 3.5 times more often in the AF/AFL group compared to the non-AF/AFL group, based on 4 studies [26, 35, 37, 41]. Interestingly, 1 study reported that in patients with AF, a percentage of those treated with appropriate doses of anticoagulants was low (57%) [41]. The remaining individuals were either treated with a prophylactic dose only (25.7%) or did not receive any anticoagulant treatment (17.3%). In spite of that, the incidence of relevant bleeding complications in the AF group was more than 4 times higher compared to the non-AF group (OR: 4.03). The study by Rubini-Costa et al. [37] demonstrated no statistical association between any anticoagulant medication and the risk of major bleeding. Consequently, anticoagulants seem to be not the main factor responsible for bleeding and further research is warranted to investigate the pathophysiology behind bleeding complications.
The present study found that AKI is 1.31 higher in the patients with AF/AFL, as compared with patients without AF/AFL. In the study by Ergün et al. [25] in the NOAF group, compared with non-NOAF group, the incidence of secondary bacterial infections was higher (75.7% vs. 51.7%) and comparable to the frequency of AKI, suggesting that there may be links between these 2 phenomena and NOAF [25]. In fact, AF was described as the most common arrhythmia in patients with sepsis [56] and the one associated with increased mortality in this group [57], which demonstrates its links with the acute inflammatory state.
Conclusions
The present study showed that AF/AFL is associated with increased in-hospital mortality and worse outcomes in patients with COVID-19 and may be used as a negative prognostic factor in these patients. Patients with AF/AFL are at higher risk of hospitalization in ICU. The presence of AF/AFL in individuals with COVID-19 is associated with higher risk of complications, such as bleeding, AKI and HF. AF/AFL may be associated with unfavorable outcomes due to the hemodynamic compromise of cardiac function itself or hyperinflammatory state typical of these conditions.
Supplementary Information
Acknowledgments
The study was supported by the Polish Society of Disaster Medicine.
Footnotes
This paper was guest edited by Prof. Togay Evrin
Conflict of interest: None declared
References
- 1.Dzieciatkowski T, Szarpak L, Filipiak KJ, et al. COVID-19 challenge for modern medicine. Cardiol J. 2020;27(2):175–183. doi: 10.5603/CJ.a2020.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruetzler K, Szarpak L, Filipiak K, et al. The COVID-19 pandemic — a view of the current state of the problem. Dis Emerg Med J. 2020 doi: 10.5603/demj.a2020.0015.. [DOI] [Google Scholar]
- 3.Bhatt AS, Jering KS, Vaduganathan M, et al. Clinical outcomes in patients with heart failure hospitalized with COVID-19. JACC Heart Fail. 2021;9(1):65–73. doi: 10.1016/j.jchf.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Bo, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu Bo, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan JWM, Ng CK, Chan YH, et al. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58(8):686–689. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72, 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 9.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 10.Granada J, Uribe W, Chyou PH, et al. Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol. 2000;36(7):2242–2246. doi: 10.1016/s0735-1097(00)00982-7. [DOI] [PubMed] [Google Scholar]
- 11.Stone E, Kiat H, McLachlan CS. Atrial fibrillation in COVID-19: A review of possible mechanisms. FASEB J. 2020;34(9):11347–11354. doi: 10.1096/fj.202001613. [DOI] [PubMed] [Google Scholar]
- 12.Hu YF, Chen YJ, Lin YJ, et al. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12(4):230–243. doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 13.Szarpak Ł, Nowak B, Kosior D, et al. Cytokines as predictors of COVID-19 severity: evidence from a meta-analysis. Pol Arch Intern Med. 2021;131(1):98–99. doi: 10.20452/pamw.15685. [DOI] [PubMed] [Google Scholar]
- 14.Inciardi RM, Adamo M, Lupi L, et al. Atrial fibrillation in the COVID-19 era: simple bystander or marker of increased risk? Eur Heart J. 2020;41(32):3094. doi: 10.1093/eurheartj/ehaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchis-Gomar F, Perez-Quilis C, Lavie CJ. Should atrial fibrillation be considered a cardiovascular risk factor for a worse prognosis in COVID-19 patients? Eur Heart J. 2020;41(32):3092–3093. doi: 10.1093/eurheartj/ehaa509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gawałko M, Kapłon-Cieślicka A, Hohl M, et al. COVID-19 associated atrial fibrillation: Incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc. 2020;30:100631. doi: 10.1016/j.ijcha.2020.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, Liang X, Xu J, et al. Meta-analysis of atrial fibrillation in patients with COVID-19. Am J Cardiol. 2021;144:152–156. doi: 10.1016/j.amjcard.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Sterne JAc, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 21.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ades AE, Lu G, Higgins JPT. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646–654. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 23.Abdulrahman A, Hussain T, Nawaz S, et al. Is atrial fibrillation a risk factor for worse outcomes in severe COVID-19 patients: a single center retrospective cohort. J Saudi Heart Assoc. 2021;33(2):160–168. doi: 10.37616/2212-5043.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denegri A, Morelli M, Pezzuto G, et al. Atrial fibrillation is related to higher mortality in COVID-19/SARS-CoV-2 pneumonia infection. Cardiol J. 2021 doi: 10.5603/CJ.a2021.0102. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ergün B, Ergan B, Sözmen MK, et al. New-onset atrial fibrillation in critically ill patients with coronavirus disease 2019 (COVID-19) J Arrhythm. 2021 doi: 10.1002/joa3.12619. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Granja PE, Veras C, Aparisi Á, et al. Atrial fibrillation in patients with SARS-CoV-2 infection. Med Clin (Barc) 2021;157(2):58–63. doi: 10.1016/j.medcli.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison SL, Fazio-Eynullayeva E, Lane DA, et al. Atrial fibrillation and the risk of 30-day incident thromboembolic events, and mortality in adults ≥ 50 years with COVID-19. J Arrhythm. 2021;37(1):231–237. doi: 10.1002/joa3.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iacopino S, Placentino F, Colella J, et al. New-Onset cardiac arrhythmias during COVID-19 hospitalization. Circ Arrhythm Electrophysiol. 2020;13(11):e009040. doi: 10.1161/CIRCEP.120.009040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ip RJ, Ali A, Baloch ZQ, et al. Atrial fibrillation as a predictor of mortality in high risk COVID-19 patients: a multicentre study of 171 patients. Heart Lung Circ. 2021;30(8):1151–1156. doi: 10.1016/j.hlc.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelesoglu S, Yilmaz Y, Ozkan E, et al. New onset atrial fibrilation and risk faktors in COVID-19. J Electrocardiol. 2021;65:76–81. doi: 10.1016/j.jelectrocard.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelesoglu S, Yilmaz Y, Ozkan E, et al. Usefulness of C-reactive protein/albumin ratio as a predictor of new-onset atrial fibrillation in SARS-COV-2. Biomark Med. 2021;15(13):1167–1175. doi: 10.2217/bmm-2020-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JiH, Hwang YMi, Cho Y, et al. Prognostic impact of atrial fibrillation in patients with severe acute respiratory syndrome coronavirus 2 infection. Medicine (Baltimore) 2021;100(33):e26993. doi: 10.1097/MD.0000000000026993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mountantonakis SE, Saleh M, Fishbein J, et al. Atrial fibrillation is an independent predictor for in-hospital mortality in patients admitted with SARS-CoV-2 infection. Heart Rhythm. 2021;18(4):501–507. doi: 10.1016/j.hrthm.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musikantow DR, Turagam MK, Sartori S, et al. Atrial fibrillation in patients hospitalized with COVID-19: incidence, predictors, outcomes, and comparison to influenza. JACC Clin Electrophysiol. 2021;7(9):1120–1130. doi: 10.1016/j.jacep.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pardo Sanz A, Salido Tahoces L, Ortega Pérez R, et al. New-onset atrial fibrillation during COVID-19 infection predicts poor prognosis. Cardiol J. 2021;28(1):34–40. doi: 10.5603/CJ.a2020.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peltzer B, Manocha KK, Ying X, et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J Cardiovasc Electrophysiol. 2020;31(12):3077–3085. doi: 10.1111/jce.14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubini-Costa R, Bermúdez-Jiménez F, Rivera-López R, et al. Prevalence of bleeding secondary to anticoagulation and mortality in patients with atrial fibrillation admitted with SARS-CoV-2 infection. Med Clin (Barc) 2021 doi: 10.1016/j.medcli.2021.06.015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russo V, Di Maio M, Mottola FF, et al. Clinical characteristics and prognosis of hospitalized COVID-19 patients with incident sustained tachyarrhythmias: A multicenter observational study. Eur J Clin Invest. 2020;50(12):e13387. doi: 10.1111/eci.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slipczuk L, Castagna F, Schonberger A, et al. Incidence of new-onset atrial fibrillation in COVID-19 is associated with increased epicardial adipose tissue. J Interv Card Electrophysiol. 2021 doi: 10.1007/s10840-021-01029-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spinoni EG, Mennuni M, Rognoni A, et al. Contribution of Atrial Fibrillation to In-Hospital Mortality in Patients With COVID-19. Circ Arrhythm Electrophysiol. 2021;1815;14(2):e009375. doi: 10.1161/CIRCEP.120.009375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uribarri A, Núñez-Gil IJ, Aparisi Á, et al. HOPE COVID-19 investigators. Atrial fibrillation in patients with COVID-19. Usefulness of the CHADS-VASc score: an analysis of the international HOPE COVID-19 registry. Rev Esp Cardiol (Engl Ed) 2021;74(7):608–615. doi: 10.1016/j.rec.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taha ME, Alsafi W, Taha M, et al. Coronavirus disease and new-onset atrial fibrillation: two cases. Cureus. 2020;12(5):e8066. doi: 10.7759/cureus.8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sala S, Peretto G, De Luca G, et al. Low prevalence of arrhythmias in clinically stable COVID-19 patients. Pacing Clin Electrophysiol. 2020;43(8):891–893. doi: 10.1111/pace.13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seecheran R, Narayansingh R, Giddings S, et al. Atrial arrhythmias in a patient presenting with coronavirus disease-2019 (COVID-19) infection. J Investig Med High Impact Case Rep. 2020;8:2324709620925571. doi: 10.1177/2324709620925571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kochav SM, Coromilas E, Nalbandian A, et al. Cardiac arrhythmias in COVID-19 infection. Circ Arrhythm Electrophysiol. 2020;13(6):e008719. doi: 10.1161/CIRCEP.120.008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomasoni D, Italia L, Adamo M, et al. COVID-19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail. 2020;22(6):957–966. doi: 10.1002/ejhf.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray IR, Baily JE, Chen WCW, et al. Skeletal and cardiac muscle pericytes: Functions and therapeutic potential. Pharmacol Ther. 2017;171:65–74. doi: 10.1016/j.pharmthera.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avolio E, Madeddu P. Discovering cardiac pericyte biology: From physiopathological mechanisms to potential therapeutic applications in ischemic heart disease. Vascul Pharmacol. 2016;86:53–63. doi: 10.1016/j.vph.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Marcus GM, Whooley MA, Glidden DV, et al. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J. 2008;155(2):303–309. doi: 10.1016/j.ahj.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amdur RL, Mukherjee M, Go A, et al. Interleukin-6 Is a Risk Factor for Atrial Fibrillation in Chronic Kidney Disease: Findings from the CRIC Study. PLoS One. 2016;11(2):e0148189. doi: 10.1371/journal.pone.0148189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren M, Li X, Hao Li, et al. Role of tumor necrosis factor alpha in the pathogenesis of atrial fibrillation: A novel potential therapeutic target? Ann Med. 2015;47(4):316–324. doi: 10.3109/07853890.2015.1042030. [DOI] [PubMed] [Google Scholar]
- 53.Guo Y, Lip GYH, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60(22):2263–2270. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 54.Savelieva I, Kakouros N, Kourliouros A, et al. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part II: secondary prevention. Europace. 2011;13(5):610–625. doi: 10.1093/europace/eur023. [DOI] [PubMed] [Google Scholar]
- 55.Wanamaker B, Cascino T, McLaughlin V, et al. Atrial arrhythmias in pulmonary hypertension: pathogenesis, prognosis and management. Arrhythm Electrophysiol Rev. 2018;7(1):43–48. doi: 10.15420/aer.2018.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shahreyar M, Fahhoum R, Akinseye O, et al. Severe sepsis and cardiac arrhythmias. Ann Transl Med. 2018;6(1):6. doi: 10.21037/atm.2017.12.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christian SA, Schorr C, Ferchau L, et al. Clinical characteristics and outcomes of septic patients with new-onset atrial fibrillation. J Crit Care. 2008;23(4):532–536. doi: 10.1016/j.jcrc.2007.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.