Abstract
Alzheimer’s disease (AD) is a progressive and fatal neurodegenerative disease. The strongest genetic risk factor for sporadic AD is carriage of the ε4 allele of the Apolipoprotein E (APOE) gene. Strategies to slow the progression of AD, including dietary interventions, may be modified by the pathogenic effect of this polymorphism. Our objective in this review was to determine the extent and quality of the literature investigating how dietary factors and interventions interact with the APOE ε4 genotype to impact cognitive decline in AD. To that end, we performed a systematic scoping review of published English-language articles involving human subjects. We found evidence suggesting that adherence to a Mediterranean diet may reduce cognitive decline among APOE ε4 carriers, whereas ketogenic agents appear to be ineffective. Diets high in saturated fats may be particularly harmful for APOE ε4 carriers. We identified several topics, including the use of ω-3 fatty acid and antioxidant supplements, for which additional high level evidence is needed.
Keywords: Cognition, Apolipoprotein E, Alzheimer’s disease
Introduction
Sporadic Alzheimer’s disease (AD) is a progressive neurodegenerative disease and the leading cause of dementia. AD is characterized by memory loss, impaired language, executive dysfunction, mood and other behavioral changes. These cognitive losses lead to functional impairment, dependence on others for care, and ultimately death. AD affects 5.8 million people in the United States, including 32% of individuals older than age 85 (1). Both environmental and genetic factors contribute to AD risk. The strongest genetic risk factor for the development of AD and the age of onset is the Apolipoprotein E (APOE) genotype (2–4). APOE is the major lipid-carrying protein synthesized in the central nervous system (CNS), and has a role in systemic and brain lipid metabolism (5). APOE is found in three isoforms in humans that are differentiated by only one or two single nucleotide polymorphisms: ε2, ε 3, and ε4. APOE ε3 is the most common allele, whereas APOE ε4 confers increased risk for AD and is found in 13.7% of the population. APOE ε2 is protective against AD and is found in 8.4% of the population (6). APOE ε4 occurs more frequently in equatorial populations and risk attributed to the gene may be affected by ethnicity, although there may be confounding sociocultural factors (7–9). APOE ε4/ε4 has been proposed to be a “thrifty” genotype, given its higher incidence in populations with sporadic food availability (10). APOE ε4 reduces brain glucose metabolism as measured by FDG-PET (11, 12) and reduce levels of expression of glucose transporters in neurons (13). This suggests that APOE isoform can affect metabolism in the CNS, a possible contributing mechanism to neurodegenerative disease.
Testing for APOE ε4 in clinical settings is discouraged due to limited impact that the results have on treatment recommendations and prognosis (14). However, education, counseling, and careful in-person disclosure of APOE ε4 status to cognitively intact individuals in a clinical research setting does not result in psychological harm (15, 16). The literature is inconsistent on whether providing this information can motivate APOE ε4 positive patients to improve their lifestyle choices that might reduce AD risk; one study found no significant impact of learning APOE ε4 genotype on diet or exercise habits (16), whereas another found that after disclosure APOE ε4 carriers consume a healthier diet that improved hyperlipidemia (17). In spite of the consensus that routine testing for APOE ε4 is premature, direct-to-consumer genetic testing for APOE ε4 has become widely accessible and inexpensive, and interest in preventative strategies for cognitive decline in APOE ε4 carriers has grown (18, 19).
In this scoping review, we sought to determine—in a systematic manner—which dietary factors and interventions are of utility for individuals carrying an APOE ε4 genotype. Dietary interventions to prevent dementia in APOE ε4 carriers have been previously reviewed (5), but not with a systematic approach. Scoping reviews use systematic, reproducible methods, but unlike meta-analysis they do not address a narrow or quantitative question, rather they aim to describe the literature relevant to broad, complex, or under-studied topics. The scoping review format was used here because our question was exploratory, and scoping reviews allow for a wider range of study types to be included.
Methods
Review strategy
This review was conducted in two parts. First, we performed a scoping systematic review to identify interventions conferring risk or protection for cognitive decline that have been examined for interactions with APOE ε4 genotype. A scoping review uses transparent methods to identify and analyze the literature relevant to a topic, with the goal of presenting a descriptive overview of a potentially large and diverse body of literature (20). Compared to a quantitative systematic review, which extracts data and thoroughly assesses the quality of each study, scoping reviews address broader research questions, including a variety of methods and study types, and can be used to determine the extent of the current literature, summarize findings and trends, and identify deficiencies in existing scientific knowledge. The PRISMA ScR checklist was followed throughout the search and manuscript preparation (21) (Figure 1). Our scoping review was followed by targeted searches of the topics identified focusing on diet.
Figure 1.
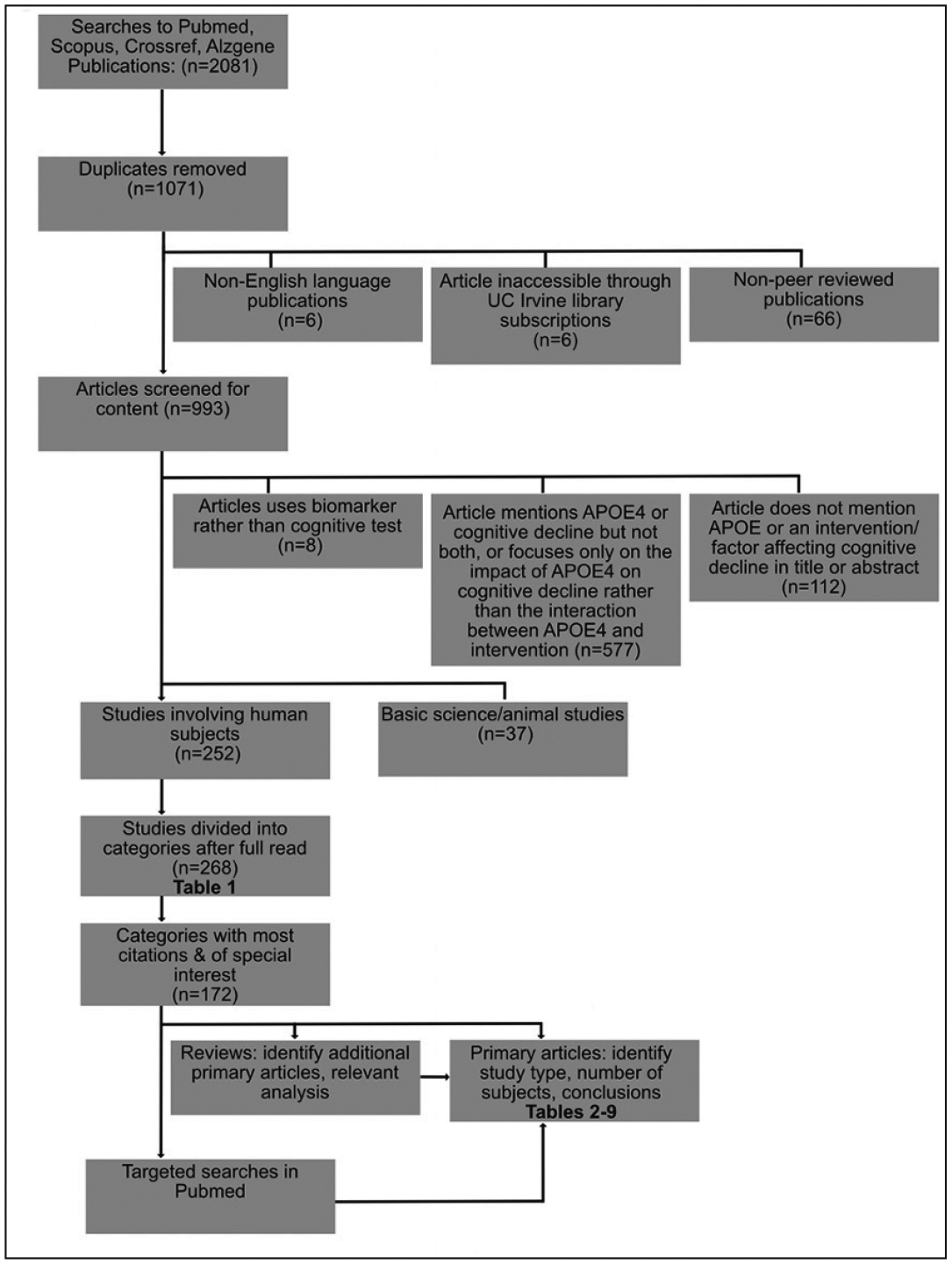
Flow chart depicting search strategy and inclusion criteria
Scoping review search
Sources included in the scoping review included all English-language peer-reviewed articles from any publication year, primary or review, discussing how APOE ε4 impacts the effect of an intervention or risk factor on cognitive decline in human subjects. This search was performed on April 9th 2020.
The following databases were searched: pubmed, scopus, crossref, and alzgene. Databases were searched with the following terms: “APOE4 prevent cognitive decline”, “APOE4 intervention cognitive decline”, “APOE4 therapy cognitive decline”, “APOE4 treatment cognitive decline”, and “APOE4 interaction cognitive decline”. Alzgene was searched for “APOE2/3/4”. The total number of discovered titles was 2081. After duplicates were removed, 1071 unique titles remained. These abstracts were screened by two authors (GF or NG) to determine eligibility according to the following exclusion criteria: not English language, not a peer-reviewed article, not accessible through UC Irvine services or library subscriptions. Only 6 out of 1071 articles could not be accessed. Nine hundred ninety-three articles met the criteria and the full text was screened for content according to the following exclusion criteria: article does not mention APOE ε4, cognitive decline, or the interaction between APOE ε4 and a dietary factor, does not use a cognitive test or dementia conversion as an outcome, or article focuses on basic science or animal studies rather than clinical/human subject studies. Two hundred fifty-two articles met all inclusion criteria; these articles were divided into categories according to the APOE4-interacting factor examined in each study. These categories were coded as pertaining to dietary, behavioral, unmodifiable, or medical interventions by GF and NG.
Targeted review search
Six categories pertaining to diet were selected for targeted review, based on number of citations and overlap with other included categories (for example, fish, Mediterranean diet, and ω-3 fatty acids) (Table 1). The following sources were used to develop the list of publications included in targeted reviews: first, the citations found in the scoping review were reviewed in full. Second, any additional relevant citations referenced in the publications identified in the scoping review were included. Finally, targeted searches were performed for some topics at reviewers’ discretion, in order to ensure full coverage and that relevant citations were not missed. These searches were performed only in Pubmed, and included the following keywords: “‘APOE’ and ‘saturated fat’ and ‘cognitive’”, “‘APOE’ and ‘dietary cholesterol’ and ‘cognitive’“, “‘APOE’ and ‘Mediterranean diet’ and «cognitive’”, “‘ketosis APOE’, ‘Ketogenic APOE’, “‘APOE’. The same criteria was used for determining eligibility in these searches as listed above for scoping review results. These searches were performed between April 11th and August 1st of 2020.
Table 1.
Scoping review search results pertaining to diet
| APOE genotype Interacting Factor | Number of citations (initial search) | Primary articles (initial search) | Primary articles (including additional searches) |
|---|---|---|---|
| Antioxidants and Ginkgo biloba | 10 | 10 | 17 |
| ω-3 fatty acids | 17 | 7 | 12 |
| Fish consumption | 3 | 3 | 3 |
| Mediterranean diet | 2 | 2 | 6 |
| Dietary cholesterol and saturated fat | 9 | 3 | 6 |
| Ketogenic diet/ketogenic agents | 3 | 0 | 6 |
| Homotaurine | 3 | Not reviewed | |
| B vitamins | 2 | Not reviewed | |
| Anserine | 1 | Not reviewed | |
| Carnitine | 1 | Not reviewed | |
| Kynurenine | 1 | Not reviewed |
Data charting process
For each study identified in the targeted review, the following variables were charted: whether the study was observational or a randomized controlled trial, number of subjects included, average age of subjects, patient diagnosis, cognitive test used, qualitative record of whether the intervention/risk factor examined had an effect on cognitive decline, and whether that effect was modified by the presence of APOE ε4. Some studies, with longitudinal, cohort, cross-sectional observational designs included individuals various diagnoses, in these instances “various” or “various longitudinal” was indicated in the diagnosis column. Some studies did not list all cognitive tests used or listed an extensive battery of tests used to produce a cognitive Z score; in these cases, “testing battery” was indicated in the cognitive test column.
Results
Overall search results
Our search returned a total of 252 articles that examined the role of APOE genotype and another factor on cognitive decline in a clinical context. These articles were broken into categories (Figure 1). All articles were assigned to at least one category, and some articles were used in multiple categories, thus the total of 268 listed in the table is higher than the total search results. Forty-four topics were identified, with ten pertaining diet (Table 1). Six diet-related categories were reviewed in the following results sections (Tables 2–6).
Table 2.
Studies investigating risk of low antioxidant levels or benefit of antioxidant supplementation for cognitive decline among APOE4 carriers
| Study | Study Type | Number of Patients | Avg Age | Patient Diagnosis | Antioxidant | Test/Measurement Used | Risk Observed in Primary Outcome | Risk in APOE Subgroup Analysis |
|---|---|---|---|---|---|---|---|---|
| Kharrazi 2008 | Obs Case-control | n=182 | 73.2, 75 | Various | Total antioxidant status | MMSE, NINCDS/ ADRDA | Yes | APOE4 > non-carriers |
| Dursun 2016 | Obs Case-control | n=196 | 57, 61, 73, 74, 76 | Various (Ctrl, MCI, AD groups) | Vitamin D | CDR; MMSE | No | No interaction |
| Bunce 2004 | Obs Cross-sectional | n=167 | 82.81 | Various | Vitamin B12, folate | Free recall of semantically unrelated words; Free and cued recall of organizable words | Yes | APOE4 > non-carriers |
| Huang 2018 | Obs Cross-sectional | n=1754 | 65.31 | Cognitively healthy | α-tocopherol/retinol | MoCA | Yes | Independent of genotype, more prevalent in APOE4 |
| Miller, 2016 | Longitudinal cohort | n=382 | 75.5 | Various | 25-OHD | CDR, Uniform Data Set Neuropsychological Battery, DSM-III, Spanish and English Neuropsychological Assessment Scales |
Yes | Controlled |
| Study | Study Type | Number of Patients | Avg Age | Patient Diagnosis | Antioxidant | Test/Measurement Used | Significant Benefit Observed in Primary Outcome | Benefit in APOE Subgroup Analysis |
| Nishimaki 2018 | RCT | n=73 | 73.97, 74.45 | MCI | H2 infused water | ADAS-cog | No | APOE4 |
| Snitz 2009 | RCT | n=3069 | 79 | Dementia-free and MCI | Ginkgo biloba | MMMSE, ADAS-Cog, neuropsychology battery, TICS | No | No Interaction |
| Petersen, 2005 | RCT | n=769 | 72.9 | aMCI | Vitamin E | MMSE, ADAS-cog, CDR, ADCS Mild Cognitive Impairment Activities of Daily Living Scale, Global Deterioration Scale, neuropsychological battery | No | No |
| Aisen, 2009 | RCT | n=409 | 76.3 | Mild to moderate AD | Folate, vitamin B6, vitamin B12 | ADAScog, MMSE, CDR-SOB, ADCSADL, NPI, QOL-AD | No | No |
| Yasuno 2012 | CT | n=663 | 72.7, 73 | Dementia-free | ω-3 fatty acids, lycopene, Ginkgo biloba | Set- dependent activity; CCR; category fluency test; WAIS-R | Yes | APOE4 > non-carriers |
| Engelhart 2002 | Prospective cohort | n=5395 | 67.7 | Dementia-free | Beta carotene, flavonoids, vitamin C, vitamin E | DSM-III-R; NINCDS-ADRDA criteria | Yes (vitamin C and vitamin E only) | No interaction |
| Maddock 2015 | Prospective cohort | n=4848 | ~50 | Various | High vitamin D | Immediate and delayed word recall | Yes | Beneficial for E4 homozygotes |
| Dai 2008 | Prospective cohort | n=1836 | 71.6, 71.7, 72.1 | Dementia-free | Vitamin C, vitamin E, beta carotene | CASI; CERAD; DSMIV; NINCDS-ADRDA | Yes (drinking fruit and vegetable juices) | Non-significant: APOE4 > non-carriers |
| Noguchi-Shinohara 2018 | Prospective longitudinal 1 | n=349 | 72.2 | Dementia-free | Vitamin C (women) | MMSE; CDR; DSMIII-R | Yes | APOE4 > non-carriers |
| Vitamin E (men) | Yes | APOE4 < non-carriers | ||||||
| Goodwill 2018 | Prospective longitudinal | n=252 | 59.8 | Various | High vitamin D | CERAD; CVLT-II, verbal fluency; TMT-B | Yes | No Interaction |
| Morris 2002 | Prospective longitudinal | n=815 | 73.3 | Dementia-free | Vitamin E from food | Clinical evaluation | Yes (vitamin E from food only) | Only non-carriers |
Abbreviations: Obs; observational study, MMSE; mini-mental status examination, MoCA; Montreal Cognitive Assessment, CDR; Clinical Dementia Rating, DSM-III; Diagnostic and Statistical Manual of Mental Disorders, AD; Alzheimer’s disease, MCI; mild cognitive impairment, aMCI; amnestic subtype of mild cognitive impairment, SPMSQ; Short Portable mental Status Questionnaire, DSM; Diagnostic and Statistical Manual of Mental Disorders, ADAS-Cog; Alzheimer’s Disease Assessment Scale-Cognitive Subscale, CCR; category cued recall, WAISR; Wechsler Adult Intelligence Scale-Revised, CERAD; Consortium to Establish a Registry for Alzheimer’s Disease, CVLT; California Verbal Learning Test, TMT; Trail Making Test, CASI; Cognitive Abilities Screening Instrument, MMMSE; modified mini mental status examination, TICS; Telephone Interview for Cognitive Status.
Table 6.
Studies investigating benefit of ketosis for cognitive decline among APOE4 carriers
| Study | Study Type | Number of Patients | Avg Age | Patient Diagnosis | Test/Measurement Used | Ketogenic intervention | Significant Benefit Observed in Primary Outcome | Benefit in APOE Subgroup Analysis |
|---|---|---|---|---|---|---|---|---|
| Morrill 2019 | CS | n=1 | 71 | Mild AD | MoCA | Diet | Yes | NA |
| Stoykovich 2019 | CS | n=1 | 68 | Mild AD | MoCA | Diet | Yes | NA |
| Brown 2018 | CS | n=1 | 38 | MCI | MoCA | Diet | Yes | NA |
| Reger 2004 | RCT | n=20 | 74.7 | Probable AD or aMCI | ADAS-Cog, MMSE, SCWIT | Ketogenic agent | Yes | No |
| Henderson 2009 | RCT | n=152 | 76 | Mild to moderate AD | ADAS-Cog, MMSE, ADCS-CGIC | Ketogenic agent | Yes | No |
| Henderson 2020 | RCT | n=413 | 76 | Mild to moderate AD | ADAS-Cog11 ADCS-CGIC | Ketogenic agent | No | No |
Abbreviations: CS; Case study, RCT; Randomized Controlled Trial, MCI; Mild Cognitive Impairment, AD; Alzheimer’s Disease, MoCA; Montreal Cognitive Assessment, ADAS-Cog; Alzheimer’s Disease Assessment Scale-Cognitive Subscale, MMSE; mini-mental status examination, SCWIT; Stroop Colour Word Interference Task, ADCS-CGIC; Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change.
Antioxidants
Oxidative damage may increase risk for cognitive decline (22), and antioxidant supplementation may counteract these effects in cognitively impaired individuals (23, 24). Several studies have shown greater oxidative damage in brain tissue in APOE ε4 carriers (25, 26), possibly due to decreased antioxidant activity (26). We identified five observational studies assessing the risk associated with low antioxidant levels and twelve studies investigating the benefits of antioxidant supplementation (Table 2), including four RCTs and one non-randomized clinical trial. All of these studies investigated the interaction between APOE ε4 and antioxidants in affecting cognitive decline.
Four retrospective studies (two case-control, two cross sectional) investigating potential harmful effects of low antioxidant levels were identified. One case-control study showed that lower levels of total antioxidant, lower activity of the antioxidant enzymes catalase and glutathione peroxidase, and higher levels of (Cu-Zn) superoxide dismutase, a part of an enzymatic antioxidant defense system, were found in AD participants compared to controls, a pattern that was also observed in APOE ε4 carriers with AD compared to non-APOE ε4 carriers with AD and controls, suggesting that APOE ε4 carriers are at greater risk with low antioxidant levels (27). Another case-control study found that LOAD patients who were APOE ε4 non-carriers showed pronounced 25OHD (vitamin D) deficiency, but the same was not true of APOE ε4 carriers (28). A cross-sectional study found that low retinol (vitamin A) and high α-tocopherol (vitamin E)/retinol ratio was associated with greater risk, with both APOE ε2 and APOE ε4 carriers showing greater α-tocopherol/retinol compared to APOE ε3 (29). Another cross-sectional study showed that only in cases of low cognitive support (defined as the most demanding cognitive tasks, such as allowing only for a short encoding time for episodic memory tasks) there was a significant association between vitamin B12 levels and APOE ε4 with respect to free recall (30). A similar association was found between folate and APOE ε4, however it was not significant (30). Thus, the majority of studies suggest that low antioxidant levels, with the exception of vitamin D, pose a greater risk of cognitive decline for APOE ε4 carriers.
Of the twelve studies exploring the possible benefits of antioxidant supplementation, five were trials. A RCT of one year supplementation with H2-infused water in MCI patients found no significant difference in ADAS-cog score between the H2-group and the control, however a secondary analysis showed a significant improvement in two tasks among APOE ε4 carriers including APOE ε4 carriers with MCI (31). Snitz et al. conducted a RCT in which 1524 participants were administered 120 mg of ginkgo biloba or placebo twice a day. No benefit was observed in either cognitively normal or MCI participants (32). A secondary subgroup analysis performed no genotype interaction (32). A RCT from Petersen et al. in which 769 MCI participants were given 2000 IU of vitamin E, 10 mg of donepezil, or placebo daily over a three year period found that neither vitamin E nor donepezil had significant effect on progression from MCI to AD. Secondary analysis showed no effect among APOE ε4 carriers for vitamin E versus placebo, however, donepezil appeared to decrease progression to AD among ε4 carriers (Petersen, 2005). Aisen et al. randomized 409 participants with mild to moderate AD to antioxidant supplements (5 mg folate, 25 mg vitamin B6, and 1 mg vitamin B12) or placebo for 18 months. The primary analysis found no difference in the rate of change of ADAS-cog between treatment groups; secondary analyses showed no difference among APOE genotypes (Aisen, 2009). An open-label uncontrolled clinical trial by Yasuno et al. investigated the effects of supplementation with a combination of antioxidants in dementia-free participants over three years. They found that cognitive function was improved, with this effect being stronger in APOE ε4 carriers compared to non carriers (33).
Eight observational studies investigating the potential benefits of antioxidant supplementation were identified in addition to the controlled trials. Each of the seven prospective studies found at least a partial benefit of antioxidant supplementation. A cohort study found that greater supplementation of vitamin C and E were associated with a decreased risk of AD, and that this association was independent of APOE genotype (34). Another study found that vitamin D levels greater than 25nmol/L in midlife were associated with greater performance on tasks involving executive function later in life; secondary analysis showed no significant differences between APOE genotypes (35). Two studies found that antioxidant intake from foods was associated with reduced risk for AD (36, 37). In subgroup analyses, Morris et al. found that vitamin E intake through food was beneficial for APOE ε4 non-carriers only (36). Dai et al. found that fruit and vegetable juices high in polyphenols delayed the onset of AD in all APOE genotypes, though the association was stronger in APOE ε4 carriers compared to non-carriers. Maddock et al. found that both high and low levels of vitamin D (with ranges consisting of <25, 25–49, 50–74, and ≥75 nmol/l) were associated with decreased memory function after adjustment for number of APOE ε4 alleles, but that high levels were beneficial only for APOE ε4 homozygotes (38). A study by Noguchi-Shinohara et al. found that high levels of vitamin C were associated with reduced risk of cognitive decline in APOE ε4 women while higher levels of vitamin E were associated with reduced risk of decline in men who are non-carriers (39). A single retrospective study found that beta-carotene supplementation may be protective against cognitive decline for APOE ε4 carriers (40).
In spite of the majority of observational studies pointing towards antioxidant benefit for APOE ε4 carriers, individual antioxidants had mixed results. Studies investigating beta carotene (34, 40–42), vitamin C39, vitamin E34, Ginkgo biloba (33), and vitamin D35, indicated a benefit while others proved to be ineffective with no APOE ε4 interaction (32, 36, 39). The benefit of Ginkgo biloba seems dubious, as the only study showing that it may benefit APOE ε4 carriers used it in combination with ω-3 fatty acids and lycopene (33), and a high quality randomized controlled trial (RCT) showed no benefit (32).
Vitamin D in particular has contradictory results in three observational studies. One study showed benefit of higher levels (35) especially in APOE ε4 carriers. Another study showed that high levels were only beneficial for APOE ε4 homozygotes and were detrimental for APOE ε4 heterozygotes and non-carriers (38). A third small study showed that low levels of vitamin D were not associated with MMSE or age of onset in APOE ε4 carriers or non-carriers (28). Another showed that vitamin D deficiency was associated with accelerated decline in episodic memory and executive function after controlling for APOE ε4 (43). Previous work indicates that APOE ε4 carriers and APOE ε4 mouse models have higher levels of vitamin D, suggesting that APOE modulates vitamin D status (44). Further study is warranted to clarify how APOE modulates vitamin D levels and what effect this has on cognition.
In summary, the literature on antioxidant supplementation has produced mixed results, but several studies suggest that the APOE ε4 allele is associated with decreased levels of antioxidants and that antioxidant supplementation may be associated with decreased rate of cognitive decline, especially for APOE ε4 carriers. The benefit of individual antioxidants for APOE ε4 carriers requires further investigation.
ω-3 fatty acid supplementation
APOE ε4 carriers on average have lower plasma levels of DHA than non-carriers (45). We identified three RCTs, one trial without a randomized control group, and eight observational studies that investigated APOE genotype interaction with ω-3 fatty acid supplementation or higher levels of ω-3 fatty acids with an outcome of cognitive function (Table 3).
Table 3.
Studies investigating interaction between APOE4 and omega 3 fatty acids or fish consumption in cognitive decline
| Study | Study type | Number of patients | Avg Age | Diagnosis | Form of ω-3 fatty acid or fish consumption | Cognitive test | Benefit observed in primary outcome | Benefit in APOE subgroup analysis |
|---|---|---|---|---|---|---|---|---|
| Stonehouse 2013 | RCT | n=176 | 33 | Cognitively healthy | DHA supplementation | Computerized mental performance assessment system | Yes | Greater effect in APOE4 males |
| Quinn 2010 | RCT | n=295 | 76 | AD | DHA supplementation | ADAS-Cog, CDR sum of boxes | No | Non-carriers |
| van de Rest 2008 | RCT | n=302 | 70 | Cognitively healthy | EPA-DHA supplementation | 5-test battery | No | APOE4-attention |
| Yasuno, 2012 | Trial | n=663 | 72.7, 73 | Dementia-free | ω-3 fatty acid supplementation | Set- dependent activity; CCR; category fluency test; WAIS-R | Yes | APOE4>non-carriers |
| Ronnemaa 2012 | Prospective cohort | n=2009 | 50 | Longitudinal (various) | PUFA levels | Demenita diagnosis | No | No interaction |
| Samieri 2011 | Prospective cohort | n=1228 | 74 | Non-institutionalized | EPA and DHA plasma levels | Test battery including MMSE | No | APOE4 |
| Kroger 2009 | Prospective cohort | n=633 | 81 | Dementia-free | DHA levels, Mercury levels | MMSE, dementia diagnosis | No* | No interaction |
| Whalley 2008 | Prospective longitudinal** | n=120 | 64 | Dementia-free | Erythrocyte membrane ω-3 fatty acid levels | MMSE, RPM, AVLT, UCOT, DSST | Yes | Non-carriers |
| Kivipelto 2008 | Longitudinal retrospective | n=1449 | 57 | Longitudinal (various) | PUFA consumption from spreads | DWRT, DSST/WAIS, WFT | Yes | APOE4>non-carriers |
| Laitinen 2006 | Longitudinal retrospective | n=1449 | 50 | Longitudinal (various) | PUFA consumption from spreads | MMSE, dementia diagnosis | Yes | APOE4 |
| Beydoun 2007 | Prospective cohort | n=2251 | 57 | Longitudinal (various) | Plasma ω-3 fatty acid levels | DWRT, DSST, WFT | Yes | No interaction |
| Laurin 2003 | Cross sectional and prospective | n=65 | 76 | Cross sectional (various) and prospective (unimpaired at start of study) | ω-3 fatty acid levels | MMMSE, dementia diagnosis | No*** | No**** |
| Huang 2005 | Prospective Cohort | n=2233 | 71 | Cognitively healthy | Fatty fish 2x/week | MMSE, TICS, IQcode, dementia diagnosis |
Yes | Non-carriers |
| Barberger-Gateau 2007 | Prospective cohort | n=8085 | >65 | Non-demented | Weekly fish, ω-3 fatty acid | Dementia diagnosis | Yes | Non-carriers |
| Daiello 2015 | Retrospective Cohort | n=819 | 75 | Cognitively healthy, MCI, AD | Fish oil supplements | ADAS-Cog, MMSE | Yes | Cognitively healthy non-carriers |
| van de Rest, 2016 | Longitudinal retrospective | n=915 | 81 | Longitudinal (various) | Fish in diet, fish oil supplements | 21-test battery | Yes | APOE4 |
| Samieri 2018 | Retrospective cohort meta-analysis | n=23,688 | 74 | Various | Fish consumption | In person interviews, telephone battery | Yes | No interaction |
| Danthiir 2014 | Cross-sectional | n=390 | 73 | Cognitively healthy | Current and childhood fish consumption | Cognitive test battery | No | No interaction |
Abbreviations: Obs; observational study, RCT; randomized controlled trial, AD; Alzheimer’s disease, MMSE; mini-mental status examination, CCR; category cued recall, WAISR; Wechsler Adult Intelligence Scale-Revised, WAIS; Wechsler Adult Intelligence Scale, DSST; Digit Symbol Substitution Test, WFT; Word Fluency Test, MMMSE; modified mini mental status examination; RPM; Raven’s Progressive Matrices, AVLT; Auditory-Verbal Learning Test, UCOT; Universal Cognitive Aptitude Test, TICS; Telephone Interview for Cognitive Status, IQcode; Informant Questionnaire on Cognitive Decline in the Elderly, ADAS-Cog; Alzheimer’s Disease Assessment Scale-Cognitive Subscale, CDR; Clinical Dementia Rating.
However, dementia risk reduction was observed in individuals in the highest quartile of mercury concentration and also had high DHA, suggesting high fish consumption.
Cognition was assessed both at age 11, 64, 66, 68.
In prospective analysis, higher levels of EPA, DHA, omega 3 and PUFAs were found in patients with dementia or cognitive impairment.
APOE4 carriers with dementia had lower levels of n-6 and total PUFAs than controls. Non-carriers with dementia had higher levels of DHA. Subgroup analysis performed only in the cross-sectional analysis, not prospective.
The evidence from the RCTs that ω-3 fatty acid supplementation would help prevent cognitive decline in APOE ε4 carriers was weak. A study investigating cognitive performance in healthy, cognitively young (18–45 years old) patients found significant benefit of DHA supplementation for episodic and working memory overall. No interaction between APOE ε4 genotype and treatment was found. Although a treatment × APOE ε4 × sex interaction was found, multiplicity is a concern in the interpretation of these statistical comparisons. While male APOE ε4 carriers and non-carriers had improvement with supplementation, the effect size was greater in male APOE ε4 carriers. Similarly, attention was significantly improved in male APOE ε4 carriers whereas it was not improved in male non-carriers45. A RCT of DHA supplementation in older individuals (average 76 years old) with mild/moderate AD revealed no benefit on the primary cognitive outcomes, but a pre-planned exploratory analysis found that APOE ε4 non-carriers had a significantly lower decline in cognition with DHA, whereas there was no effect in APOE ε4 carriers (47). Another randomized controlled trial of docosohexanoic acid (DHA) and eicosapentaenoic acid (EPA) supplementation was conducted in 2008 in cognitively healthy adults over 65 years old. The primary outcome was a neuropsychological test battery, which revealed no significant difference between EPA-DHA supplement and control. A pre-planned secondary analysis of a five-test battery revealed that APOE ε4 carriers improved in attention after EPA-DHA supplementation (48), although none of the other cognitive domains were significantly improved and therefore the conclusions that can be drawn are limited.
One clinical trial did not include a randomized controlled group, but instead used patients with no treatment from the same community dwelling as controls, a study design subject to bias (33). The study reported that there was significant benefit overall after 3 years of ω-3 fatty acid supplementation as well as lycopene and ginko biloba. In subgroup analysis, there was a positive effect for both APOE carriers and APOE ε4 non-carriers, but the effect size was greater for APOE ε4 carriers.
Among the clinical trials of ω-3 fatty acid supplementation, three were limited to patients who were cognitively healthy and one was limited to patients who were diagnosed with AD. Of the trials examining patients who were cognitively healthy, two found a greater positive impact on cognitive decline for APOE ε4 carriers upon subgroup analysis (33, 45) and one found benefit only in APOE ε4 carriers (48) in cognitive testing batteries. In the RCT limited to cognitively impaired patients, a positive effect was found for non-carriers only on subgroup analysis of cognitive testing (47).
In addition to RCTs assessing the efficacy of DHA supplements, we identified seven observational studies (one of which was associated with two publications) that assessed poly-unsaturated fatty acid (PUFA) consumption through surveys or plasma levels of ω-3 fatty acids. One of these studies included a prospective portion and a cross-sectional portion, but only the cross-sectional portion was used for secondary analysis of groups separated by APOE genotype (49). Out of four prospective cohort studies, higher ω-3 fatty acid levels were associated with reduced cognitive decline in the primary analysis in two studies (50, 51). There was no significant effect in the primary analysis in four studies (49, 52–54). Secondary subgroup analyses of APOE ε4 carriers and non-carriers were performed in five of these prospective studies; three studies found no interaction between APOE and ω-3 fatty acid levels (50, 52, 54) in the effect on cognitive decline, one found a benefit only for non-carriers (51), and one found benefit only for carriers (53). One retrospective study with two associated publications showed that moderate consumption of PUFA from spreads (butter, margarine) reduced the risk of dementia in the primary analysis; in subgroup analysis this effect was observed only in APOE ε4 carriers (55, 56). One small cross-sectional study found no benefit of higher ω-3 fatty acid levels and no difference in secondary analysis of APOE genotypes (57).
Two observational studies were limited to cognitively healthy patients only; one of these found benefit in cognitive testing for non-carriers only in subgroup analysis (51) and the other found no positive effect and no APOE genotype interaction (52).
Overall, the literature we identified on the efficacy of ω-3 fatty acid supplementation for preventing cognitive decline was mixed. While six studies showed some benefit for APOE ε4 carriers, four studies showed either no benefit and no genotype interaction or benefit only for APOE ε4 non-carriers. Some of this inconsistency may be explained by defects in ω-3 fatty acid absorption and trafficking caused by APOE ε4 (58, 59). A study in rodents has found that long-term, high-dose DHA supplementation in APOE ε4 mice can prevent cognitive decline (60), Suggesting that especially high doses of ω-3 fatty acids may be required for APOE ε4 carriers to derive cognitive benefit.
Fish consumption
A major dietary source of ω-3 fatty acid is fish consumption. We identified two prospective, three retrospective, and one cross-sectional observational study examining the effect of fish consumption on cognitive decline (Table 3). Both of the prospective studies were conducted in patients who were cognitively healthy at the start of the study and measured fish consumption using surveys. Both observed reduced cognitive decline with higher fish consumption in the primary analysis, but in secondary analysis found that only APOE ε4 non-carriers benefited (61, 62).
Retrospective multivariate meta-analysis of five cohort studies revealed that higher fish intake was associated with slower decline in both cognition and memory, and found no interaction with APOE genotype (63). Of the studies we identified, one retrospective study showed that fish oil supplements were associated with less frequent occurrence of dementia but only in APOE ε4 non-carriers (64), and one small cross-sectional study showed no association of current or childhood fish consumption with better performance on cognitive testing overall or for either genotype (in fact, higher current fish consumption predicted worse performance on cognitive speed tests) (65). One retrospective study found no benefit overall, but in secondary analysis found that fish in the diet, but not fish oil supplements, improved cognitive testing in APOE ε4 carriers only (66). Overall, the evidence suggests that fish consumption may be beneficial for preventing cognitive decline in the general population, but less effective in APOE ε4 carriers. These results are consistent with findings showing that fish consumption has a more positive association with plasma EPA and DHA levels in APOE ε4 non-carriers than in APOE ε4 carriers (67).
Mediterranean diet
Mediterranean diet traditionally includes olive oil, legumes, unrefined cereal, fruit, vegetables, fish, moderate consumption of dairy and wine, and low consumption of meat, and has been found to reduce risk of AD (68). Six studies, including four RCTs, were identified that included analyses of APOE genotype interaction with the effect of Mediterranean diet on cognitive decline (Table 4). All of the studies showed benefit overall in preventing cognitive decline in the primary analysis. Three RCTs showed no APOE genotype interaction (69–71). One RCT that included Mediterranean diet as part of a multidomain intervention included only older patients at high risk, with cardiovascular risk factors and average or below average cognition, showed that cognitive outcomes improved significantly only for APOE ε4 carriers in secondary within group analysis (72). Both prospective cohort studies (73, 74) found a benefit of Mediterranean diet intervention overall; one showed benefit only for APOE ε4 carriers (73) on sub-analysis whereas the other showed no interaction (74). Among studies limited to cognitively healthy participants, one observational study (73) found benefit only for APOE ε4 carriers, one observational and one RCT found general benefit with no APOE interaction (71, 74).
Table 4.
Studies investigating APOE4 and Mediterranean diet in cognitive decline
| Study | Study type | Number of patients | Avg Age | Diagnosis | Cognitive test | Benefit observed in primary outcome | Benefit in APOE subgroup analysis |
|---|---|---|---|---|---|---|---|
| Solomon 2018 | RCT | n=1175 | 69 | At-risk older individuals | Neuropsychological Test Battery | NA | APOE4** |
| Martinez-Lapiscina 2014 | RCT | n=522 | 67 | Various* | MMSE, CDT | Yes | No interaction |
| Martinez-Lapiscina 2013 | RCT | n=268 | 67 | Various* | Test battery including MMSE | Yes | No interaction |
| Valls-Pedret 2015 | RCT | n=447 | 66 | Cognitively healthy | 8-test battery including MMSE | Yes | No interaction |
| Keenan 2020 | Obs Prospective cohort | n=7756 | 70 | Dementia-free | Test battery including MMMSE | Yes | No interaction |
| Gardener 2015 | Obs Prospective cohort | n=527 | 69 | Cognitively heathy | Test battery | Yes | APOE4 |
Abbreviations: Obs; observational study, RCT; randomized controlled trial, MCI; mild cognitive impairment, MMSE; mini-mental status examination, CDT; Clock Drawing Test.
Inclusion criteria stated that participants must have a cardiovascular risk factor but no previous cardiovascular incidents and no other chronic conditions. Average subject MMSE was 27–28 suggesting subjects were generally cognitively healthy.
Within group analysis (intervention vs no intervention); no significant interaction of randomization group × time × APOE.
In conclusion, all the studies we reviewed showed either cognitive improvement or reduction in cognitive decline with Mediterranean diet among APOE ε4 carriers, and one study showed benefit exclusively in these individuals.
Dietary saturated fat and cholesterol
APOE is a lipid transport protein and it has long been observed that APOE isoforms differentially affect the body’s response to a diet rich in saturated fats (75). We identified three observational studies (two prospective cohort and one cross-sectional) that investigated the effect of dietary cholesterol on cognition and interaction with APOE genotype (Table 5). All of these studies were conducted in middle-aged subjects (average age 53, 59, 57.7). Two studies performed in dementia-free patients found that amount of egg and dietary cholesterol consumption was not associated with cognitive decline, regardless of APOE genotype (76, 77). One cross-sectional study including patients at risk for dementia (first-degree blood relatives of AD patients) found that intake of cholesterol was significantly higher in subjects with altered cognitive performance as measured by a neurocognitive battery, but there was no difference between APOE ε4 carriers and non-carriers (78).
Table 5.
Studies investigating risk of dietary cholesterol or saturated fats for cognitive decline among APOE4 carriers
| Study | Study Type | Number of Patients | Avg Age | Patient Diagnosis | Type of dietary fat | Test/Measurement Used | Risk observed in primary outcome | Risk in APOE subgroup analysis |
|---|---|---|---|---|---|---|---|---|
| Ylilauri 2017 | Obs Prospective cohort | n=2497 | 53 | Dementia-free | Cholesterol | Dementia diagnosis, MMSE, TMT, VFT, SRT, VRT | No | No |
| An 2019 | Obs Prospective cohort | n=2514 | 59 | No neuropsychiatric problems | Cholesterol | MoCA, SDMT, AVLT, LMT, DSF, WMS-RC | No | No |
| Salerno Kennedy 2007 | Obs Cross-sectional | n=20 | 57.7 | Various | Cholesterol | MMSE, PWLT, LDCT SCWT | Yes | No interaction |
| Laitinen 2006, Eskelinen 2008 | Obs Prospective cohort | n=1449 | 50.4 | Unspecified | Saturated fat | MMSE, dementia diagnosis | Yes | APOE4 only |
| Luchsinger 2002 | Obs Prospective cohort | n=980 | 75 | Cognitively healthy | Saturated fat | Dementia diagnosis | Yes | APOE4 only |
| Hanson 2015 | RCT | n=20 | 70 | MCI, normal cognition | Saturated fat | Neurocognitive battery | Yes | APOE4 carriers without impairment perform worse, APOE4 with impairment perform better |
Abbreviations: Obs; observational study, MMSE; mini-mental status examination, TMT; Trail Making Test, VFT; Verbal fluency test, SRT; Simple Reaction Time, VRT; Visual Reaction Time, MoCA; Montreal Cognitive Assessment, SDMT; Symbol Digit Modalities Test, AVLT; Auditory-Verbal Learning Test, LMT; Letter Memory Test, DSF; Digit Span Forwards, WMS-RC; Wechsler Memory Scale-Revised in China, PWLT; Picture Word Learning Test, LDCT; Letter Digit Coding Test, SCWT; Stroop Colour Word Test.
Two well-powered observational studies (one of which produced two publications) with over 900 participants each showed that consumption of saturated fat increased risk of cognitive decline, and on subgroup analysis this risk was found to be more profound in APOE ε4 carriers (55, 79, 80) (Table 5). One study was limited to cognitively healthy older adults (80), and the other was a longitudinal study examining cognition; at the start of the study middle-aged subjects were cognitively intact, with some subjects becoming cognitively impaired at the follow-up time point 21 years later (55). One pilot RCT was identified involving cognitive testing following a single 800 calorie high fat (50%) meal. In a sample of 19 cognitively healthy and 27 MCI patients, following a high calorie, high fat meal, APOE ε4 non-carriers with no pre-existing cognitive decline performed worse than their baseline, whereas APOE ε4 carriers and APOE ε4 non-carriers with cognitive impairment performed better than baseline (81).
Ketogenic diet
While the available evidence may suggest overall that a long-term diet rich in saturated fat is detrimental to cognitive health of APOE ε4 carriers, there have been several reviews (82–85) and primary articles (86–92) on the efficacy of the ketogenic diet or ketogenic supplements for preventing cognitive decline in APOE ε4 carriers and non-carriers. The ketogenic diet is rich in fats, with very low carbohydrates. A study on the feasibility of the ketogenic diet for patients with dementia found that although there was a high dropout rate in the patients with advanced dementia in part due to caregiver burden, milder patients less frequently dropped out, achieved ketosis and showed improved cognitive performance by an average of 4.1 points on the ADAS-cog (93).
Three case studies were identified that each investigated a single APOE ε4 carrier patient with mild cognitive impairment or AD (86–88) who showed cognitive benefit after being on the ketogenic diet (Table 6). To our knowledge, no properly powered observational or RCTs have investigated the efficacy of the ketogenic diet specifically for APOE ε4 carriers to date.
Four RCTs have examined the efficacy of ketogenic compounds or supplements for improving cognition in patients with mild cognitive impairment and dementia. One found no significant benefit for cognition in APOE ε4 carriers or non-carriers using the caprylic triglyceride formulation AC-1204 (92). Another study by the same group using a slightly different formulation, AC-1202, found a significant benefit only for APOE ε4 non-carriers (89, 90). Finally, one study found that a single supplement of medium chain triglycerides acutely increased β-hydroxybutyrate levels in blood and enhanced cognitive performance only for APOE ε4 non-carriers (91). Ketogenic supplements appear to have no benefit for APOE ε4 carriers in well-powered studies.
Discussion
We performed a systematic search of the literature on dietary factors that may interact with the APOE ε4 genotype to have an impact on cognitive decline. We reviewed six dietary factors, including three with multiple RCTs. Mediterranean diet appeared to be effective for reducing cognitive decline in individuals of all APOE genotypes, and fish consumption showed consistent benefit across several well-powered observational studies, whereas ω-3 fatty acid supplementation was associated with mixed results and weak evidence of benefit. Ketogenic agents were not beneficial for APOE ε4 carriers. Several high-quality randomized studies investigating antioxidants and Ginkgo biloba showed no evidence of effectiveness. There were no randomized studies but some well-designed and powered observational studies investigating dietary saturated fat; this evidence indicated that consuming a diet high in saturated fat is detrimental for APOE ε4 carriers. Limited studies showed weak and mixed results for the effectiveness of low dietary cholesterol in preventing cognitive decline in APOE ε4 carriers.
Our results are largely in agreement with the Lancet’s 2020 report on dementia prevention, intervention, and care for the general population, which recommends a Mediterranean diet but suggests that there is not yet sufficient evidence to recommend dietary supplements for the prevention of cognitive decline (94).
While APOE ε4 carriers are at increased risk for cognitive decline, they are also at increased risk for cardiovascular disease, and the effect of dietary factors on both of these disease processes must be considered. Consumption of dietary saturated fat has a greater effect on APOE ε4 carriers’ plasma lipid levels, which may compound their high risk of heart disease due to high plasma LDL at baseline (95, 96). Likewise, while ω-3 fatty acids may lower plasma LDL levels in APOE ε4 non-carriers, LDL levels increase with ω-3 fatty acid levels in APOE ε4 carriers (97–99). Thus, a possible source of caution in recommending ω-3 fatty acid supplementation could be additional cardiovascular risk factors. High serum LDL is also correlated with cerebral amyloidosis (100), possibly contributing to Alzheimer’s disease pathology. Therefore, the risk of a diet resulting in high LDL for APOE ε4 carriers is compounded by both possible cognitive decline and heart disease. In contrast, consumption of eggs and cholesterol are not particularly detrimental for cognition in APOE ε4 carriers. This is consistent with reports that reducing dietary saturated fat may be more effective than dietary cholesterol for attenuating hyperlipidemia (101).
The ketogenic diet has recently become popular as a weight loss strategy and has been investigated in several case studies for effect on cognitive decline. Strong evidence indicates that ketogenic supplements do not prevent cognitive decline in APOE ε4 carriers with mild cognitive impairment. It is possible that ketogenic diet may be more effective than ketogenic agents at promoting ketosis; this may explain why case studies of dietary interventions show benefit for APOE ε4 carriers whereas RCTs of ketogenic agents do not. However, case studies are a low level of evidence and this diet must be investigated further before it can be recommended to APOE ε4 carriers. It should be noted that in contrast to many other dietary interventions, studies identified testing the efficacy of ketogenic agents and ketogenic diet were all limited to patients with MCI/mild AD, and further investigation in pre-symptomatic patients may reveal different results.
Gaps in the current knowledge of how dietary interventions differentially affect people of different APOE genotypes remain. Randomized controlled trials assessing the effectiveness of diets low in saturated fat or cholesterol would be helpful to determine the effectiveness of these interventions. The literature on APOE genotype interaction with supplemental homotaurine, B vitamins, Anserine, Carnitine, or Kynurenine is nascent; future prospective RCTs could help to determine their effectiveness. Although the body of literature on ω-3 fatty acids in APOE ε4 is robust, it would benefit from studies weighing the cost of increased plasma LDL in APOE ε4 carriers against the benefit for cognitive decline. Possibly due to ω-3 fatty acid trafficking deficiencies, APOE ε4 carriers require higher doses of ω-3 fatty acid supplementation to raise CSF levels (102, 103); future trials should confirm whether high doses over a long time course can consistently reduce cognitive decline in among APOE ε4 carriers.
Limitations
This scoping review was limited in several ways. The initial scoping review search was very broad and included reviews and primary articles. The results of the targeted reviews were also heterogeneous, with a wide variety of study designs, levels of evidence, diagnostic populations, statistical methods, and measurements of cognitive function. Quantitative meta-analysis was not performed in this review, results were qualitative and coded either beneficial, harmful, or no effect on cognition with greater effect or less effect in APOE ε4 carriers. A future quantitative systematic review would be beneficial in several of the topics identified. Additionally, while some animal and basic science studies were referenced for context, our search criteria focused on human subjects research. More thorough systematic review of basic science and animal studies may provide insight into mechanisms of the effects observed in humans and provide pre-clinical assessment of efficacy for new therapies. Assessment of study quality and bias was limited in this review, as the main objective was to describe the range/scope of literature addressing APOE genotype interactions with cognitive decline interventions, rather than critical appraisal of the quality of each individual study.
Conclusions
The available evidence suggests that a Mediterranean diet is potentially beneficial for avoiding cognitive decline in APOE ε4 carriers. We hope that this scoping overview of the current state of the literature will help better inform APOE ε4 carriers and their clinicians of the breadth and strength of the current literature on dietary factors intended to prevent cognitive decline in this at-risk population. While there is still much research to be done, there is hope that these simple and relatively safe lifestyle modifications could impact the personal and public impact of cognitive decline.
Acknowledgements:
This project was funded by NIH as follows: 1F30AG060704-01A1 (to G.M.F.), T32GM008620 (to G.M.F.), T32AG000096 (to G.M.F.). Funding to G.M.F. from the Lorna Carlin Fellowship.
Footnotes
Statement of competing interests: The authors have no financial or non-financial competing interests to report.
Ethical standards: This study adhered to the PRISMA guidelines for scoping reviews. The authors have no conflicts of interest to disclose.
References
- 1.2019 ALZHEIMER’S DISEASE FACTS AND FIGURES Includes a Special Report on Alzheimer’s Detection in the Primary Care Setting: Connecting Patients and Physicians.
- 2.Corder EH et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science (80-.) 1993;261, 921–923; 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 3.Cosentino S et al. APOE ε4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology 2008;70, 1842–1849; 10.1212/01.wnl.0000304038.37421.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertram L, McQueen MB, Mullin K, Blacker D & Tanzi RE Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat. Genet 2007;39, 17–23; 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 5.Yassine HN & Finch CE APOE Alleles and Diet in Brain Aging and Alzheimer’s Disease. Frontiers in Aging Neuroscience 2020;12, 150; 10.3389/fnagi.2020.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrer LA et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis. J. Am. Med. Assoc 1997;278, 1349–1356; 10.1001/jama.278.16.1349. [DOI] [PubMed] [Google Scholar]
- 7.Belloy ME, Napolioni V & Greicius MD A Quarter Century of APOE and Alzheimer’s Disease: Progress to Date and the Path Forward. Neuron 2019;101, 820–838; 10.1016/j.neuron.2019.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egert S, Rimbach G & Huebbe P ApoE genotype: From geographic distribution to function and responsiveness to dietary factors. Proc. Nutr. Soc 2012;71, 410–424; 10.1017/S0029665112000249. [DOI] [PubMed] [Google Scholar]
- 9.Singh PP, Singh M & Mastana SS APOE distribution in world populations with new data from India and the UK. Ann. Hum. Biol 2006;33, 279–308; 10.1080/03014460600594513. [DOI] [PubMed] [Google Scholar]
- 10.Prentice AM, Rayco-Solon P & Moore SE Insights from the developing world: thrifty genotypes and thrifty phenotypes, 2020. doi: 10.1079/PNS2005421 [DOI] [PubMed] [Google Scholar]
- 11.Hoyer S Age-Related Changes in Cerebral Oxidative Metabolism: Implications for Drug Therapy. Drugs Aging 1995;6, 210–218; 10.2165/00002512-199506030-00004. [DOI] [PubMed] [Google Scholar]
- 12.Reiman EM et al. Correlations between apolipoprotein E ε4 gene dose and brain-imaging measurements of regional hypometabolism. Proc. Natl. Acad. Sci. U. S. A 2005;102, 8299–8302; 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu L, Zhang X & Zhao L Human apoe isoforms differentially modulate brain glucose and ketone body metabolism: Implications for Alzheimer’s disease risk reduction and early intervention. J. Neurosci 2018;38, 6665–6681; 10.1523/JNEUROSCI.2262-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michael Arribas-Ayllon. The Ethics of Disclosing Genetic Diagnosis for Alzheimer’s Disease: Do We Need a New Paradigm? - PubMed. Br Med Bull 2011;100, 7–21; 10.1093/bmb/ldr023. [DOI] [PubMed] [Google Scholar]
- 15.Grill JD Disclosing Risk Factors to Individuals Without Cognitive Impairment. Pract. Neurol. Mag 2019;63, 63–66. [Google Scholar]
- 16.Green RC et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N. Engl. J. Med 2009;361, 245–254; 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hietaranta-Luoma H-L et al. A Long-Term Follow-Up Study on Disclosing Genetic Risk Information (APOE) to Promote Healthy Lifestyles in Finland. Lifestyle Genomics 2018;11, 147–154; 10.1159/000500199. [DOI] [PubMed] [Google Scholar]
- 18.Berkowitz CL et al. Clinical Application of APOE in Alzheimer’s Prevention: A Precision Medicine Approach. J. Prev. Alzheimer’s Dis 2018;5, 245–252; 10.14283/jpad.2018.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez Lopez C et al. The Alzheimer’s Prevention Initiative Generation Program: Study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer’s disease. Alzheimer’s and Dementia: Translational Research and Clinical Interventions 2019;5, 216–227; 10.1016/j.trci.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pham MT et al. A scoping review of scoping reviews: Advancing the approach and enhancing the consistency. Res. Synth. Methods 2014;5, 371–385; 10.1002/jrsm.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tricco AC et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med 2018;169, 467; 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 22.Butterfield DA & Halliwell B Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci 2019;20, 148–160; 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dominguez LJ & Barbagallo M Nutritional prevention of cognitive decline and dementia. Acta Biomedica 2018;89, 276–290; 10.23750/abm.v89i2.7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eshkoor SA, Hamid TA, Mun CY & Ng CK Mild cognitive impairment and its management in older people. Clinical Interventions in Aging 2015;10, 687–693; 10.2147/CIA.S73922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramassamy C et al. Oxidative damage and protection by antioxidants in the frontal cortex of Alzheimer’s disease is related to the apolipoprotein E genotype. Free Radic. Biol. Med 1999;27, 544–553; 10.1016/s0891-5849(99)00102-1. [DOI] [PubMed] [Google Scholar]
- 26.Tamaoka A et al. Apolipoprotein E allele-dependent antioxidant activity in brains with Alzheimer’s disease. Neurology 2000;54, 2319–2321; 10.1212/wnl.54.12.2319. [DOI] [PubMed] [Google Scholar]
- 27.Kharrazi H et al. Association between enzymatic and non-enzymatic antioxidant defense mechanism with apolipoprotein E genotypes in Alzheimer disease. Clin. Biochem 2008;41, 932–936; 10.1016/j.clinbiochem.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Dursun E et al. Vitamin D deficiency might pose a greater risk for ApoEε4 non-carrier Alzheimer’s disease patients. Neurol. Sci 2016; 37, 1633–1643; 10.1007/s10072-016-2647-1. [DOI] [PubMed] [Google Scholar]
- 29.Huang X et al. Diminished circulating retinol and elevated α-TOH/retinol ratio predict an increased risk of cognitive decline in aging Chinese adults, especially in subjects with ApoE2 or ApoE4 genotype. Aging (Albany. NY) 2018;10, 4066–4083.; 10.18632/aging.101694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bunce D, Kivipelto M & Wahlin Å Utilization of Cognitive Support in Episodic Free Recall as a Function of Apolipoprotein E and Vitamin B12 or Folate among Adults Aged 75 Years and Older. Neuropsychology 2004;18, 362–370; 10.1037/0894-4105.18.2.362. [DOI] [PubMed] [Google Scholar]
- 31.Nishimaki K et al. Effects of Molecular Hydrogen Assessed by an Animal Model and a Randomized Clinical Study on Mild Cognitive Impairment. Curr. Alzheimer Res 2018;15, 482–492; 10.2174/1567205014666171106145017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snitz BE et al. Ginkgo biloba for preventing cognitive decline in older adults a randomized trial. JAMA - J. Am. Med. Assoc 2009. 302, 2663–2670; 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasuno F et al. Combination of antioxidant supplements improved cognitive function in the elderly. J. Alzheimer’s Dis 2012;32, 895–903; 10.3233/JAD-2012-121225. [DOI] [PubMed] [Google Scholar]
- 34.Engelhart MJ et al. Dietary intake of antioxidants and risk of Alzheimer disease. J. Am. Med. Assoc 2002;287, 3223–3229; 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 35.Goodwill AM et al. Vitamin D status is associated with executive function a decade later: Data from the Women’s Healthy Ageing Project. Maturitas 2018;107, 56–62; 10.1016/j.maturitas.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Morris MC et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. J. Am. Med. Assoc 2002;287, 3230–3237; 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 37.Dai Q, Borenstein AR, Wu Y, Jackson JC & Larson EB Fruit and Vegetable Juices and Alzheimer’s Disease: The Kame Project. Am. J. Med 2006;119, 751–759.; 10.1016/j.amjmed.2006.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maddock J, Cavadino A, Power C & Hyppönen E 25-Hydroxyvitamin D, APOE ε4 genotype and cognitive function: Findings from the 1958 British birth cohort. Eur. J. Clin. Nutr 2015;69, 505–508; 10.1038/ejcn.2014.201. [DOI] [PubMed] [Google Scholar]
- 39.Noguchi-Shinohara M et al. Higher Blood Vitamin C Levels are Associated with Reduction of Apolipoprotein e E4-related Risks of Cognitive Decline in Women: The Nakajima Study. J. Alzheimer’s Dis 2018;63, 1289–1297; 10.3233/JAD-170971. [DOI] [PubMed] [Google Scholar]
- 40.Hu P et al. Association between serum beta-carotene levels and decline of cognitive function in high-functioning older persons with or without apolipoprotein E 4 alleles: MacArthur studies of successful aging. J. Gerontol. A. Biol. Sci. Med. Sci 2006;61, 616–620; 10.1093/gerona/61.6.616. [DOI] [PubMed] [Google Scholar]
- 41.Zheng Y et al. Lysosomal Proteases Are a Determinant of Coronavirus Tropism. J. Virol 2018;92; 10.1128/JVI.01504-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan Changzheng, 1,2,3 Fondell Elinor,2,3 Ascherio Alberto,2,3,4 Okereke Olivia I,3,4,5 Grodstein Francine,3,4 & Hofman Albert, 4,6 and Willett Walter C 2,. Long-Term Intake of Dietary Carotenoids Is Positively Associated with Late-Life Subjective Cognitive Function in a Prospective Study in US Women. The Journal of Nutrition | 10.1093/jn/nxaa087. J. Nutr. 2020; 10.1093/jn/nxaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller JW, Harvey DJ, Beckett LA, Green R, Farias ST, Reed BR, Olichney JM, Mungas DM, DeCarli C (2015) Vitamin D Status and Rates of Cognitive Decline in a Multiethnic Cohort of Older Adults. JAMA Neurol 72:1295; 10.1001/jamaneurol.2015.2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huebbe P et al. APOE ε 4 is associated with higher vitamin D levels in targeted replacement mice and humans. FASEB J 2011; 25, 3262–3270; 10.1096/fj.11-180935. [DOI] [PubMed] [Google Scholar]
- 45.Chouinard-Watkins R et al. Disturbance in uniformly 13C-labelled DHA metabolism in elderly human subjects carrying the apoE Î4 allele. Br. J. Nutr 2013;110, 1751–1759; 10.1017/S0007114513001268 [DOI] [PubMed] [Google Scholar]
- 46.Stonehouse W et al. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am. J. Clin. Nutr 2013;97, 1134–1143; 10.3945/ajcn.112.053371 [DOI] [PubMed] [Google Scholar]
- 47.Quinn JF et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA - J. Am. Med. Assoc 2010;304, 1903–1911; 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van De Rest O et al. Effect of fish oil on cognitive performance in older subjects: A randomized, controlled trial. Neurology 2008;71, 430–438; 10.1212/01.wnl.0000324268.45138.86 [DOI] [PubMed] [Google Scholar]
- 49.L. D, V. R, L. J, D. É & H. BJ Omega-3 fatty acids and risk of cognitive impairment and dementia. J. Alzheimer’s Dis 2003;5, 315–322; 10.3233/jad-2003-5407 [DOI] [PubMed] [Google Scholar]
- 50.Beydoun May A 1, Kaufman Jay S, Satia Jessie A, Rosamond Wayne, F. AR Plasma n-3 Fatty Acids and the Risk of Cognitive Decline in Older Adults: The Atherosclerosis Risk in Communities Study - PubMed. American Journal of Clinical Nutrition (2007). Available at: https://pubmed.ncbi.nlm.nih.gov/17413112/. (Accessed: 7th May 2020); 10.1093/ajcn/85.4.1103 [DOI] [PubMed] [Google Scholar]
- 51.Whalley LJ et al. n-3 Fatty acid erythrocyte membrane content, APOE 4, and cognitive variation: an observational follow-up study in late adulthood 2008;1–3. Am J Clin Nutr 87; 10.1093/ajcn/87.2.449. [DOI] [PubMed] [Google Scholar]
- 52.Kröger E et al. Omega-3 fatty acids and risk of dementia: The Canadian Study of Health and Aging. Am. J. Clin. Nutr 2009;90, 184–192; 10.3945/ajcn.2008.26987. [DOI] [PubMed] [Google Scholar]
- 53.Samieri C et al. Omega-3 fatty acids and cognitive decline: Modulation by ApoEε4 allele and depression. Neurobiol. Aging 2011;32, 2317.e13–2317.e22; 10.1016/j.neurobiolaging.2010.03.02 [DOI] [PubMed] [Google Scholar]
- 54.Rönnemaa E et al. Serum fatty-acid composition and the risk of Alzheimers disease: A longitudinal population-based study. Eur. J. Clin. Nutr 2012;66, 885–890;0; 10.1038/ejcn.2012.63. [DOI] [PubMed] [Google Scholar]
- 55.Laitinen MH et al. Fat intake at midlife and risk of dementia and Alzheimer’s disease: A population-based study. Dement. Geriatr. Cogn. Disord 2006;22, 99–107; 10.1159/000093478 [DOI] [PubMed] [Google Scholar]
- 56.Kivipelto M et al. Apolipoprotein e ε4 magnifies lifestyle risks for dementia: A population-based study. J. Cell. Mol. Med 2008;12, 2762–2771; 10.1111/j.1582-4934.2008.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laurin D, Verreault R, Lindsay J, Dewailly É & Holub BJ Omega-3 fatty acids and risk of cognitive impairment and dementia. J. Alzheimer’s Dis 2003;5, 315–322; 10.3233/jad-2003-5407. [DOI] [PubMed] [Google Scholar]
- 58.Conway V et al. Apolipoprotein E isoforms disrupt long-chain fatty acid distribution in the plasma, the liver and the adipose tissue of mice. Prostaglandins Leukot. Essent. Fat. Acids 2014;91, 261–267; 10.1016/j.plefa.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Nock TG et al. Carriers of an apolipoprotein E epsilon 4 allele are more vulnerable to a dietary deficiency in omega-3 fatty acids and cognitive decline. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids 2017;1862, 1068–1078; 10.1016/j.bbalip.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 60.Chouinard-Watkins R et al. Docosahexaenoic acid prevents cognitive deficits in human apolipoprotein E epsilon 4-targeted replacement mice. Neurobiol. Aging 2017;57, 28–35; 10.1016/j.neurobiolaging.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Huang TL et al. Benefits of fatty fish on dementia risk are stronger for those without APOE ε4. Neurology 2005;65, 1409–1414; 10.1212/01.wnl.0000183148.34197.2e. [DOI] [PubMed] [Google Scholar]
- 62.Barberger-Gateau P et al. Dietary patterns and risk of dementia: The Three-City cohort study. Neurology 2007;69, 1921–1930; 10.1212/01.wnl.0000278116.37320.52 [DOI] [PubMed] [Google Scholar]
- 63.Samieri C et al. Original Contribution Fish Intake, Genetic Predisposition to Alzheimer Disease, and Decline in Global Cognition and Memory in 5 Cohorts of Older Persons 187; 10.1093/aje/kwx330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daiello LA, Gongvatana A, Dunsiger S, Cohen RA & Ott BR Association of fish oil supplement use with preservation of brain volume and cognitive function. Alzheimer’s Dement 2015;11, 226–235; 10.1016/j.jalz.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Danthiir V et al. Cognitive Performance in Older Adults Is Inversely Associated with Fish Consumption but Not Erythrocyte Membrane n–3 Fatty Acids. J. Nutr 2014;144, 311–320; 10.3945/jn.113.175695. [DOI] [PubMed] [Google Scholar]
- 66.Van De Rest O et al. APOE e4 and the associations of seafood and long-chain omega-3 fatty acids with cognitive decline. Neurology 2016;86, 2063–2070; 10.1212/WNL.0000000000002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samieri C et al. Relationship between diet and plasma long-chain n-3 PUFAs in older people: Impact of apolipoprotein e genotype. J. Lipid Res 2013;54, 2559–2567; 10.1194/jlr.P036475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh B et al. Association of Mediterranean diet with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. Journal of Alzheimer’s Disease 2014;39, 271–282; 10.3233/JAD-130830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinez-Lapiscina EH et al. Virgin olive oil supplementation and long-term cognition: The Predimed-Navarra randomized, trial. J. Nutr. Heal. Aging 2013;17, 544–552; 10.1007/s12603-013-0027-6. [DOI] [PubMed] [Google Scholar]
- 70.Martínez-Lapiscina EH et al. Genotype patterns at CLU, CR1, PICALM and APOE, cognition and Mediterranean diet: The PREDIMED-NAVARRA trial. Genes Nutr 2014;9; 10.1007/s12263-014-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valls-Pedret C et al. Mediterranean diet and age-related cognitive decline: A randomized clinical trial. JAMA Intern. Med 2015;175, 1094–1103; 10.1001/jamainternmed.2015.1668. [DOI] [PubMed] [Google Scholar]
- 72.Solomon A et al. Effect of the apolipoprotein e genotype on cognitive change during a multidomain lifestyle intervention a subgroup analysis of a randomized clinical trial. JAMA Neurol 2018;75, 462–470; 10.1001/jamaneurol.2017.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gardener SL et al. Dietary patterns and cognitive decline in an Australian study of ageing. Mol. Psychiatry 2015;20, 860–866; [DOI] [PubMed] [Google Scholar]
- 74.Keenan TD et al. Adherence to a Mediterranean diet and cognitive function in the Age-Related Eye Disease Studies 1 & 2. Alzheimer’s Dement 2020. doi: 10.1002/alz.12077; 10.1002/alz.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dreon DM, Fernstrom HA, Miller B & Krauss RM Apolipoprotein E Isoform Phenotype and LDL Subclass Response to a Reduced-Fat Diet. Arterioscler. Thromb. Vasc. Biol 1995;15, 105–111; 10.1161/01.atv.15.1.105. [DOI] [PubMed] [Google Scholar]
- 76.Ylilauri MPT et al. Association of dietary cholesterol and egg intakes with the risk of incident dementia or Alzheimer disease: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr 2017;105, 476–484; 10.3945/ajcn.116.146753. [DOI] [PubMed] [Google Scholar]
- 77.An Y et al. Longitudinal and nonlinear relations of dietary and Serum cholesterol in midlife with cognitive decline: Results from EMCOA study. Mol. Neurodegener 2019;14; 10.1186/s13024-019-0353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salerno-Kennedy R & Cashman KD The relationship between nutrient intake and cognitive performance in people at risk of dementia. Ir. J. Med. Sci 2007;176, 193–198; 10.1007/s11845-007-0036-8. [DOI] [PubMed] [Google Scholar]
- 79.Eskelinen MH et al. Fat intake at midlife and cognitive impairment later in life: A population-based CAIDE study. Int. J. Geriatr. Psychiatry 2008;23, 741–747; 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- 80.Luchsinger JA, Tang MX, Shea S & Mayeux R Caloric intake and the risk of Alzheimer disease. Arch. Neurol 2002;59, 1258–1263; 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- 81.Hanson AJ et al. Differential effects of meal challenges on cognition, metabolism, and biomarkers for apolipoprotein Eβ 4 carriers and adults with mild cognitive impairment. J. Alzheimer’s Dis 2015;48, 205–218; 10.3233/JAD-150273 [DOI] [PubMed] [Google Scholar]
- 82.Cunnane S et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition 2011;27, 3–20; 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharma A, Bemis M & Desilets AR Role of medium chain triglycerides (Axona®) in the treatment of mild to moderate alzheimer’s disease. American Journal of Alzheimer’s Disease and other Dementias 2014;29, 409–414; 10.1177/1533317513518650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Henderson ST Ketosis in Mild Cognitive Impairment and Alzheimer’s Disease. in Diet and Nutrition in Dementia and Cognitive Decline, Elsevier Inc. 2015;447–456 [Google Scholar]
- 85.Broom GM, Shaw IC & Rucklidge JJ The ketogenic diet as a potential treatment and prevention strategy for Alzheimer’s disease. Nutrition 2019;60, 118–121; 10.1016/j.nut.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 86.Morrill SJ & Gibas KJ Ketogenic diet rescues cognition in ApoE4+ patient with mild Alzheimer’s disease: A case study. Diabetes Metab. Syndr. Clin. Res. Rev 2019;13, 1187–1191; 10.1016/j.dsx.2019.01.035 [DOI] [PubMed] [Google Scholar]
- 87.Stoykovich S & Gibas K APOE ε4, the door to insulin-resistant dyslipidemia and brain fog? A case study 2019; 10.1016/j.dadm.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown D & Gibas KJ Metabolic syndrome marks early risk for cognitive decline with APOE4 gene variation: A case study. Diabetes Metab. Syndr. Clin. Res. Rev 12, 2018;823–827; 10.1016/j.dsx.2018.04.030 [DOI] [PubMed] [Google Scholar]
- 89.Henderson ST et al. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Metab 2009;6; 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henderson ST & Poirier J Pharmacogenetic analysis of the effects of polymorphisms in APOE, IDE and IL1B on a ketone body based therapeutic on cognition in mild to moderate Alzheimer’s disease; a randomized, double-blind, placebo-controlled study. BMC Med. Genet 2011;12; 10.1186/1471-2350-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reger MA et al. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging 2004;25, 311–314; 10.1016/S0197-4580(03)00087-3 [DOI] [PubMed] [Google Scholar]
- 92.Henderson ST, Morimoto BH, Cummings JL, Farlow MR & Walker J A Placebo-Controlled, Parallel Group, Randomized Clinical Trial of AC-1204 in Mild-to-Moderate Alzheimer’s Disease. J. Alzheimer’s Dis 2020;1–11; 10.3233/JAD-191302. [DOI] [PubMed] [Google Scholar]
- 93.Taylor MK, Sullivan DK, Mahnken JD, Burns JM & Swerdlow RH Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv 2018;4, 28–36; 10.1016/j.trci.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Livingston G et al. The Lancet Commissions Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396, 413–446; 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rubin J & Berglund L Apolipoprotein E and diets: A case of gene-nutrient interaction? Curr. Opin. Lipidol 2002;13, 25–32; 10.1097/00041433-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Masson Lindsey F, McNeill Geraldine and A. A Genetic variation and the lipid response to dietary intervention: asystematic review. Am J Clin Nutr 2003;77, 1098–111; 10.1093/ajcn/77.5.1098 [DOI] [PubMed] [Google Scholar]
- 97.Anil E The impact of EPA and DHA on blood lipids and lipoprotein metabolism: Influence of apoE genotype. in Proceedings of the Nutrition Society 2007;66, 60–68 (Proc Nutr Soc).; 10.1017/S0029665107005307. [DOI] [PubMed] [Google Scholar]
- 98.Minihane AM et al. ApoE polymorphism and fish oil supplementation in subjects with an atherogenic lipoprotein phenotype. Arterioscler. Thromb. Vasc. Biol 2000;20, 1990–1997; 10.1161/01.atv.20.8.1990. [DOI] [PubMed] [Google Scholar]
- 99.Olano-Martin E et al. Contribution of apolipoprotein E genotype and docosahexaenoic acid to the LDL-cholesterol response to fish oil. Atherosclerosis 2010;209, 104–110; 10.1016/j.atherosclerosis.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 100.Reed B et al. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol 2014;71, 195–200; 10.1001/jamaneurol.2013.5390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ball M, Geekie M, Carter R, Benfield L & Fisher K Effect of dietary cholesterol on plasma cholesterol concentration in subjects following reduced fat, high fibre diet. Br. Med. J. (Clin. Res. Ed) 1987;294, 333–336; 10.1136/bmj.294.6568.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yassine HN et al. Association of docosahexaenoic acid supplementation with Alzheimer disease stage in Apolipoprotein e ε4 carriers: A review. JAMA Neurology 2017;74, 339–347; 10.1001/jamaneurol.2016.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arellanes IC et al. Brain delivery of supplemental docosahexaenoic acid (DHA): A randomized placebo-controlled clinical trial. EBioMedicine 2020;59; 10.1016/j.ebiom.2020.102883 [DOI] [PMC free article] [PubMed] [Google Scholar]


