We identified olaparib 100 mg b.i.d. (intermittent) with gemcitabine 600 mg/m2 as a tolerated dose combination, which could be considered for future evaluation. Given the encouraging response observed in patients with a BRCAm in previous olaparib trials, further investigation of clinical benefit in this patient subset compared with chemotherapy alone is warranted.
Keywords: olaparib, pancreatic cancer, gemcitabine
Abstract
Background
Olaparib (Lynparza™) is an oral poly(adenosine diphosphate [ADP]-ribose) polymerase inhibitor that induces synthetic lethality in cancers with homologous recombination defects.
Patients and methods
In this phase I, dose-escalation trial, patients with advanced solid tumours received olaparib (50–200 mg capsules b.i.d.) continuously or intermittently (days 1–14, per 28-day cycle) plus gemcitabine [i.v. 600–800 mg/m2; days 1, 8, 15, and 22 (cycle 1), days 1, 8, and 15 (subsequent cycles)] to establish the maximum tolerated dose. A separate dose-escalation phase evaluated olaparib in tablet formulation (100 mg o.d./b.i.d.; days 1–14) plus gemcitabine (600 mg/m2). In an expansion phase, patients with genetically unselected locally advanced or metastatic pancreatic cancer were randomised 2 : 1 to the tolerated olaparib capsule combination dose or gemcitabine alone (1000 mg/m2).
Results
Sixty-six patients were treated [dose-escalation phase, n = 44 (tablet cohort, n = 12); dose-expansion phase, n = 22 (olaparib plus gemcitabine, n = 15; gemcitabine alone, n = 7)]. In the dose-escalation phase, four patients (6%) experienced dose-limiting toxicities (raised alanine aminotransferase, n = 2; neutropenia, n = 1; febrile neutropenia, n = 1). Grade ≥3 adverse events were reported in 38/47 patients (81%) treated with olaparib capsules plus gemcitabine; most common were haematological toxicities (55%). Tolerated combinations were olaparib 100 mg b.i.d. capsule (intermittently, days 1–14) plus gemcitabine 600 mg/m2 and olaparib 100 mg o.d. tablet (intermittently, days 1–14) plus gemcitabine 600 mg/m2. There were no differences in efficacy observed during the dose-expansion phase.
Conclusions
Olaparib 100 mg b.i.d. (intermittent dosing; capsules) plus gemcitabine 600 mg/m2 is tolerated in advanced solid tumour patients, with no unmanageable/unexpected toxicities. Continuous dosing of olaparib or combination with gemcitabine at doses >600 mg/m2 was not considered to have an acceptable tolerability profile for further study.
ClinicalTrials.gov
introduction
In tumours with deficiencies in homologous recombination (HR), poly(adenosine diphosphate [ADP]-ribose) polymerase inhibition (PARPi) is thought to lead to the accumulation of single-strand breaks in DNA, which are converted into double-strand breaks (DSBs) during replication. DSBs cannot be accurately repaired and result in cell death, a concept known as synthetic lethality [1]. BRCA2 defects and other HR repair deficiencies have been reported in a small but significant proportion (5%–7%) of patients with pancreatic cancers [2].
Olaparib (Lynparza™) is an oral PARP inhibitor that induces lethality in tumours with HR deficiencies, such as BRCA mutations (BRCAm) [3]. Monotherapy with olaparib has yielded significant clinical benefit in patients with BRCAm [3, 4]. Preclinical studies have shown that PARPi potentiates the effects of DNA-damaging agents [5]. In mice bearing human pancreatic tumour xenografts, PARPi with gemcitabine significantly reduced tumour weight and increased survival [6]. Olaparib in combination with gemcitabine may increase clinical benefit in pancreatic cancer when compared with gemcitabine monotherapy.
Olaparib monotherapy (capsule formulation) is generally well tolerated at doses up to 400 mg twice daily (b.i.d.) using a continuous schedule [3]. However, olaparib combined with chemotherapeutic agents has been associated with increased haematological toxicity [7]. This phase I study was designed to determine the safety, tolerability, and maximum tolerated dose (MTD) of olaparib combined with gemcitabine in patients with advanced solid tumours. Safety and efficacy of the MTD of olaparib (capsules) and gemcitabine in chemotherapy-naïve patients with locally advanced and metastatic pancreatic cancer was evaluated in a randomised dose-expansion phase.
methods
patients
Eligible patients were aged ≥18 years with: clinical or radiological evidence of disease; Eastern Cooperative Oncology Group performance status ≤2; life expectancy ≥12 weeks; adequate bone marrow, hepatic, and renal function. For dose escalation with the capsule formulation, patients had a histologically or cytologically confirmed malignant solid tumour refractory to standard therapy or for which gemcitabine was a potential treatment option. In the tablet cohort of the dose-escalation phase and the dose-expansion phase (capsule), eligible patients had histologically or cytologically confirmed adenocarcinoma of the pancreas with locally advanced or metastatic disease, irrespective of genetic status. For the capsule dose-escalation phase, prior chemotherapy (including gemcitabine) was not allowed within 4 weeks before the start of study treatment; patients who had received >2 courses of prior chemotherapy were excluded from the study. For the tablet dose-escalation phase and the dose-expansion (capsule) phase, no prior chemotherapy was allowed. All patients provided written informed consent.
study design
Phase I, open-label, multicentre study comprising two phases: dose-escalation phase to establish the MTD of continuous or intermittent olaparib dosing (capsule) in combination with gemcitabine; dose-expansion phase, where the selected combination dose (olaparib capsule plus gemcitabine) was compared with gemcitabine alone to further establish safety and efficacy in patients with locally advanced or metastatic pancreatic cancer. A separate dose-escalation evaluation assessed olaparib tablet formulation in combination with gemcitabine to determine a tablet dose for future use (supplementary Appendix, available at Annals of Oncology online). The tablet formulation delivers the therapeutic dose in fewer, smaller dose units versus the capsule, reducing the number of pills required. All patients received 28-day treatment cycles of olaparib in combination with gemcitabine, or gemcitabine alone. Patients received treatment as long as it was tolerable without disease progression. The institutional review boards/independent ethics committees of all investigational sites approved the protocol. The study was carried out in accordance with the Declaration of Helsinki, Good Clinical Practice, and the AstraZeneca policy on Bioethics [8].
dose-escalation phase
Olaparib capsules were administered orally b.i.d. on days 1–28 (intermittent cohort days 1–14) per cycle. Three patients were recruited to a cohort; if no dose-limiting toxicities (DLTs) occurred, recruitment to the next dose commenced (supplementary Appendix, available at Annals of Oncology online). In the continuous schedule, olaparib (capsule formulation) was increased from 50 mg b.i.d. to at least 200 mg b.i.d. over three cohorts, together with 800 mg/m2 of gemcitabine (unless a DLT occurred). MTD was defined as the dose level below that which caused DLTs in ≥2 patients in a cohort.
In the continuous dosing schedule, olaparib was administered for 1 week (on days −7 to −5 and day −4) before the first cycle of chemotherapy to allow samples to be taken for assessment of olaparib and gemcitabine pharmacokinetics (PK).
Patients in the tablet-escalation phase received olaparib once daily on days 1–14.
dose-expansion phase
The MTD determined the olaparib/gemcitabine dose combination used in the expansion phase. Patients in the combination arm received intermittent olaparib (capsule) b.i.d. for all cycles. In the dose-expansion phase, gemcitabine was administered on days 1, 8, 15 (all cycles), and day 22 in cycle 1. The dose-expansion phase was randomised in an unblinded 2 : 1 ratio (combination : gemcitabine alone). The stratification for randomisation was based on disease stage and locally advanced or metastatic disease.
study end points and assessments
The primary objective was to determine the safety and tolerability, and establish the MTD, of olaparib in combination with gemcitabine. Safety was monitored throughout the study and assessed according to Common Terminology Criteria for Adverse Events v3, biochemical laboratory tests, vital signs, and physical examination. Secondary objectives were: identification of DLTs of the combination olaparib/gemcitabine; preliminary assessment of antitumour activity [objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and percentage change in tumour size] in patients with locally advanced/metastatic pancreatic cancer; and determination of the plasma PK profile for olaparib and gemcitabine alone and in combination. Tumour size was determined by CT/MRI scans at baseline and following every two cycles. Tumour response was assessed by the investigator according to Response Evaluation Criteria in Solid Tumours (RECIST) v1.0.
Once an appropriate dose of gemcitabine/olaparib combination therapy had been determined, patients with advanced pancreatic cancer were enrolled in the expansion phase. Retrospective BRCA1/2 testing for dose-expansion patients was optional.
statistical analysis
A maximum of 70 patients were to be enrolled in the overall study to determine a tolerable dose of olaparib tablet/capsule formulation in combination with gemcitabine. ORR was compared using Fisher's exact test with difference in ORR [95% confidence interval (CI)] calculated using the Wilson score-based method. PFS and OS were compared using a Cox proportional hazards model with a factor for treatment group [hazard ratio (HR) and 95% CI]. Descriptive statistics were used to describe change in tumour size and safety variables.
results
patients
In total, 46 patients were enrolled in the dose-escalation phase; 44 received treatment (intent-to-treat population) (Figure 1) . In the dose-expansion phase, 23 patients were enrolled; 22 received study medication (Figure 1). Demographics and baseline characteristics were generally well balanced (Table 1). The dose-escalation strategy is outlined in Table 2. The intended study population for the dose-expansion phase was patients with pancreatic cancer who had not received prior chemotherapy; however, two patients with BCRA-positive disease who had prior adjuvant chemotherapy for breast cancer were randomised to combination treatment.
Figure 1.
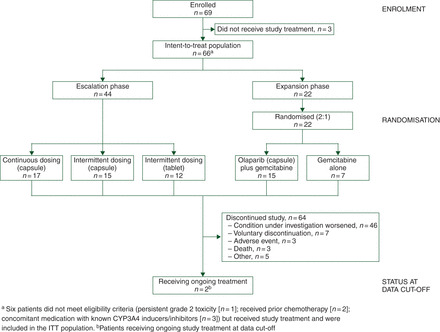
Study design.
Table 1.
Patient characteristics and baseline demographics
| Characteristic | Dose-escalation phasea |
Dose-expansion phaseb |
|
|---|---|---|---|
| Olaparib plus gemcitabine (N = 44) | Olaparib plus gemcitabine (N = 15) | Gemcitabine alone (N = 7) | |
| Median (range) age, years | 59 (29–79) | 65 (47–79) | 66 (44–73) |
| Sex, n (%) | |||
| Female | 19 (43) | 7 (47) | 4 (57) |
| ECOG status, n (%) | |||
| 0 | 25 (57) | 5 (33) | 4 (57) |
| 1 | 18 (41) | 9 (60) | 3 (43) |
| 2 | 1 (2) | 1 (7) | 0 |
| Primary tumour site, n (%) | |||
| Pancreatic adenocarcinoma | 30 (68) | 15 (100) | 7 (100) |
| Malignant melanoma | 4 (9) | 0 | 0 |
| Otherc | 10 (23) | 0 | 0 |
| Disease characteristics, n (%) | |||
| First-line pancreaticd | 28 (64) | 15 (100) | 7 (100) |
| Locally advanced | – | 2 (13) | 2 (29) |
| Metastatic | – | 13 (87) | 5 (71) |
| Refractory solid tumoure | 13 (30) | 0 | 0 |
| BRCA mutation status | |||
| BRCA 1 mutation | – | 2 (13) | 0 |
| BRCA 2 mutation | – | 1 (7) | 0 |
| No mutation detected | – | 3 (20) | 3 (43) |
| Unknown | 44 (100) | 9 (60) | 4 (57) |
| Prior chemotherapy, n (%) | 13 (30) | 2 (13)f | 0 |
| Prior immune/hormonal therapy, n (%) | 2 (5) | 0 | 0 |
| Prior hormonal therapy | 2 (5) | 1 (7) | 0 |
| Prior radiation therapy | 9 (20) | 2 (13) | 0 |
| Median (range) duration of disease, months | 2 (0–131) | 1 (0–4) | 1 (1–2) |
aDose-escalation phase: olaparib capsules (50–200 mg b.i.d.; intermittent dosing days 1‒14 or continuous dosing days 1‒28) plus gemcitabine 600/800 mg/m2 or olaparib tablets (100 mg o.d. days 1‒14) plus gemcitabine 600 mg/m2.
bDose-expansion phase: olaparib capsules plus gemcitabine: olaparib 100 mg b.i.d. (14 days) and gemcitabine 600 mg/m2; gemcitabine alone: 1000 mg/m2. In both arms, gemcitabine was administered on days 1, 8, 15, and 22 of cycle 1 and on days 1, 8, and 15 of subsequent cycles.
cOther primary sites included: gallbladder and extrahepatic bile duct (n = 3); ovary (n = 2); breast (n = 2); oesophagus (n = 1); uterus (n = 1); primary tumour site unknown (n = 1).
dLocally advanced or metastatic pancreatic cancer information was not collected for the dose-escalation phase of the study.
eIncludes patients with pancreatic cancer who received prior therapy.
fPatients had prior adjuvant chemotherapy for breast cancer in the remote past.
Table 2.
Treatment regimens and study recruitment
| Cohort | Olaparib dose and formulation | Olaparib schedule | Gemcitabine dose | Patients (N) | Tolerability | Cohort decision |
|---|---|---|---|---|---|---|
| 1 | 50 mg b.i.d. capsule | Continuous | 800 mg/m2 | 10 | One DLT (grade 3 increased ALT) in cycle 1 | No DLTs in initial four patients; cohort expanded to seven patients |
| Following DLT, cohort expanded to 10 patients | ||||||
| No further DLTs; dose escalated | ||||||
| 2 | 100 mg b.i.d. capsule | Continuous | 800 mg/m2 | 8a | Two DLTs (grade 3 increased ALT and grade 3 neutropenia) in cycle 1 | Study protocol amended to allow intermittent olaparib dosing |
| 3 | 100 mg b.i.d. capsule | Intermittent | 800 mg/m2 | 5 | No DLTs in cycle 1 | AEs leading to frequent dose delays of olaparib or gemcitabine led to other dosing regimens being explored |
| 4 | 100 mg b.i.d. capsule | Intermittent | 600 mg/m2 | 4 | One DLT (grade 3 febrile neutropenia) in cycle 1 | Cohort considered tolerable; dose escalated |
| Dose level subsequently selected for the randomised expansion phase | ||||||
| 5 | 200 mg b.i.d. capsule | Intermittent | 600 mg/m2 | 6 | No DLTs in cycle 1 | Dose level not considered suitable for expansion phase due to dose delays and reductions |
| 6 | 100 mg o.d. tablet | Intermittent | 600 mg/m2 | 13a | No DLTs in cycle 1 | This olaparib tablet cohort was opened in parallel with the randomisation phase of the olaparib capsule. No DLTs in initial six patients; cohort expanded to 13 patients to better understand safety |
aOne patient discontinued before receiving combination treatment so was excluded from the ITT and safety populations.
tolerability
tolerable dosing combination
Four patients (60%) in the dose-escalation phase experienced DLTs (continuous cohorts, n = 3; intermittent cohorts, n = 1; Table 2). All DLTs resolved following gemcitabine dose modifications. Continuous dosing of olaparib capsules with a gemcitabine dose >600 mg/m2 was considered non-tolerated following two DLTs [grade 3 increased alanine aminotransferase (ALT) and grade 3 neutropenia and persistent fatigue] in patients receiving continuous olaparib 100 mg b.i.d. capsules and gemcitabine 800 mg/m2 (Table 2). Intermittent olaparib dosing was explored. Olaparib 100 mg b.i.d. (capsule: days 1–14) in combination with gemcitabine 600 mg/m2 was considered to have acceptable tolerability and selected for the expansion phase. A dose schedule of 200 mg b.i.d. intermittent olaparib (capsule) with gemcitabine 600 mg/m2 was not considered tolerable for the expansion phase because of dose delays/reductions to both treatments, mainly relating to haematological adverse events (AEs).
adverse events
The predominant treatment-emergent AEs (TEAEs) encountered in olaparib capsule cohorts are shown in Table 3. Overall, 38/47 (81%) patients treated with olaparib capsule and gemcitabine reported grade ≥3 TEAEs. The most common were haematological toxicities, which occurred in 26/47 (55%) patients.
Table 3.
Treatment-emergent adverse events reported in >50% (any grade) or ≥15% (grade ≥3) of patients in any group, by MedDRA preferred term
| Adverse event, N (%) | Dose-escalation phase |
Dose-expansion phase |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial dose-level olaparib capsule, mg b.i.d. (days)/gemcitabine, mg/m2 |
Olaparib tablet, mg o.d. (days)/gemcitabine, mg/m² |
Olaparib capsule, mg b.i.d. (days)/gemcitabine, mg/m2 |
Gemcitabine alone, mg/m2 |
|||||||||||||
| 50 (28)/800 (N = 10) |
100 (14)/600 (N = 4) |
100 (14)/800 (N = 5) |
100 (28)/800 (N = 7) |
200 (14)/600 (N = 6) |
100 (14)/600 (N = 12) |
100 (14)/600 (N = 15) |
1000 (N = 7) |
|||||||||
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Nausea | 9 (90) | 0 | 2 (50) | 0 | 4 (80) | 0 | 3 (43) | 0 | 3 (50) | 0 | 11 (92) | 1 (8) | 9 (60) | 1 (7) | 6 (86) | 0 |
| Fatigue | 8 (80) | 0 | 4 (100) | 0 | 3 (60) | 0 | 3 (43) | 0 | 4 (67) | 0 | 9 (75) | 1 (8) | 9 (60) | 1 (7) | 3 (43) | 1 (14) |
| Anaemia | 7 (70) | 0 | 3 (75) | 1 (25) | 1 (20) | 0 | 6 (86) | 2 (29) | 4 (67) | 0 | 1 (8) | 0 | 4 (27) | 1 (7) | 2 (29) | 1 (14) |
| Vomiting | 4 (40) | 0 | 1 (25) | 0 | 2 (40) | 0 | 3 (43) | 1 (14) | 1 (17) | 0 | 5 (42) | 0 | 1 (7) | 1 (7) | 1 (14) | 0 |
| Deep vein thrombosis | 4 (40) | 3 (30) | 0 | 0 | 0 | 0 | 2 (29) | 2 (29) | 1 (17) | 1 (17) | 0 | 0 | 3 (20) | 2 (13) | 2 (29) | 2 (29) |
| Neutropenia | 3 (30) | 3 (30) | 1 (25) | 1 (25) | 3 (60) | 3 (60) | 6 (86) | 6 (86) | 3 (50) | 2 (33) | 4 (33) | 4 (33) | 5 (33) | 5 (33) | 3 (43) | 3 (43) |
| Abdominal pain | 3 (30) | 0 | 1 (25) | 1 (25) | 0 | 0 | 1 (14) | 0 | 3 (50) | 0 | 5 (42) | 2 (17) | 2 (13) | 0 | 1 (14) | 1 (14) |
| Constipation | 3 (30) | 0 | 1 (25) | 0 | 1 (20) | 0 | 0 | 0 | 2 (33) | 0 | 3 (25) | 0 | 10 (67) | 0 | 1 (14) | 0 |
| Rash | 3 (30) | 0 | 0 | 0 | 1 (20) | 0 | 4 (57) | 0 | 1 (17) | 0 | 4 (33) | 0 | 2 (13) | 0 | 1 (14) | 0 |
| Thrombocytopenia | 2 (20) | 0 | 3 (75) | 1 (25) | 3 (60) | 2 (40) | 4 (57) | 2 (29) | 6 (100) | 3 (50) | 4 (33) | 0 | 8 (53) | 4 (27) | 3 (43) | 1 (14) |
| ALT increased | 2 (20) | 2 (20) | 0 | 0 | 1 (20) | 0 | 4 (57) | 3 (43) | 1 (17) | 0 | 1 (8) | 1 (8) | 0 | 0 | 2 (29) | 0 |
| Pyrexia | 1 (10) | 0 | 0 | 0 | 0 | 0 | 4 (57) | 0 | 0 | 0 | 1 (8) | 0 | 7 (47) | 0 | 2 (29) | 0 |
| Anorexia | 4 (40) | 0 | 2 (50) | 0 | 1 (20) | 0 | 3 (43) | 0 | 3 (50) | 0 | 5 (42) | 1 (8) | 2 (13) | 0 | 1 (14) | 0 |
| Dyspnoea | 1 (10) | 0 | 0 | 0 | 1 (20) | 0 | 3 (43) | 2 (29) | 2 (33) | 0 | 3 (25) | 0 | 6 (40) | 1 (7) | 0 | 0 |
| Peripheral oedema | 1 (10) | 0 | 3 (75) | 0 | 0 | 0 | 2 (29) | 0 | 2 (33) | 0 | 1 (8) | 0 | 8 (53) | 1 (7) | 2 (29) | 0 |
| Pulmonary embolism | 2 (20) | 2 (20) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (13) | 2 (13) | 0 | 0 |
| Bacteraemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (29) | 2 (29) |
Patients with multiple events in a category were counted only once in that category. Includes events occurring post-treatment during the 30-day follow-up.
ALT, alanine aminotransferase; MedDRA, Medical Dictionary for Regulatory Activities (version 10.0).
Twenty-nine (44%) patients reported serious AEs (SAEs) during the study. The most common were dyspnoea (n = 4; 14%), abdominal pain (n = 3; 10%), vomiting (n = 3; 10%), and deep vein thrombosis (n = 3; 10%). No SAEs were attributed to olaparib alone; six patients had SAEs that were considered to have a causal relationship with combined olaparib/gemcitabine (primarily infections). There were two deaths which were considered by the investigator to be related to both olaparib and gemcitabine [n = 1 pancreatic adenocarcinoma, 50 mg b.i.d. (continuous) capsule/800 mg/m2 cohort, death due to neutropenic sepsis, died 13 days after last dose of study medication; n = 1 metastatic pancreatic cancer, 100 mg b.i.d. (intermittent) capsule/600 mg/m2 cohort, death due to bacterial peritonitis and renal failure, died 16 days after last dose of study medication]. Three additional AEs led to discontinuation of study treatment: dyspnoea [n = 1, 100 mg b.i.d. (continuous) capsule/800 mg/m2]; grade 3 pneumonitis (bronchoscopy findings consistent with gemcitabine-induced pneumonitis); and grade 2 non-cardiac chest pain [n = 1 each, olaparib 100 mg b.i.d. (tablet, intermittent)/600 mg/m2 cohort].
dosing modifications
In total, 33/47 (70%) patients treated with olaparib capsules and gemcitabine experienced TEAEs that led to dose interruptions; these were predominantly haematological toxicities (thrombocytopenia: 38%, n = 18; neutropenia: 34%, n = 16). There were no dose reductions of olaparib and relatively few dose delays or omissions [delays generally ≤7 days' duration; longer duration delays occurred in the highest dose cohort of olaparib capsule 200 mg b.i.d. (intermittent)/600 mg/m2]. Most patients experienced modifications in gemcitabine dosing (reductions, 65%; omissions, 79%; delays, 52% of patients). TEAEs were the primary reason for gemcitabine dose omissions and reductions (generally neutropenia, thrombocytopenia, or abnormal liver function tests).
antitumour activity
overall response rate
In the dose-escalation phase, ORR was 10% (n = 4/41). In the dose-expansion phase, ORR for the combination was 27% [95% CI 10.9–52.0 (n = 4/15)] compared with 14% [95% CI 2.6–51.3 (n = 1/7)] with gemcitabine alone; the difference was not significant. The sample size in the expansion cohort only included 22 patients with a 2 : 1 randomisation. Details of patients demonstrating an ORR are in supplementary Table S1, available at Annals of Oncology online.
other secondary end points
In the dose-expansion phase, there were no differences in PFS or OS. Best percentage change in relative tumour size from baseline is shown in Figure 2.
Figure 2.
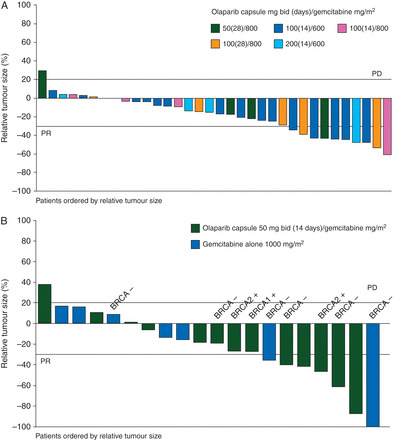
Best percentage change in relative tumour size from baseline for (A) dose-escalation patients* and (B) dose-expansion patients: ITT population. Asterisk denotes that BRCA mutation status was not tested for patients in the dose-escalation phase and was not tested for the majority of patients in the dose-expansion phase; BRCA mutation status for those patients tested is indicated on the dose-expansion phase figure arm.
antitumour response by BRCAm status
BRCAm status was available for six patients in the combination arm of the expansion cohort and three patients in the gemcitabine arm (Table 1). In the combination arm, two patients had a BRCA1 mutation and one patient had a BRCA2 mutation (all three patients with BRCAm had metastatic unresectable pancreatic cancer; both patients with BRCA1 mutation had previous breast cancer). Three patients each in the combination and gemcitabine alone arm were known to be BRCA wild type. BRCAm status was unknown for the remaining patients. Analysis of response by BRCAm was not possible. PFS responses of 220 and 229 days and OS responses of 332 and 401 days were observed in two patients with pancreatic cancer and a BRCA1 mutation. The patient with a BRCA2 mutation had PFS of 213 days and OS of 540 days. This compares with a median PFS and OS of 163 and 332 days for the combination arm, and 172 and 359 days for the gemcitabine arm, respectively.
discussion
In this study, it was not possible to administer olaparib with the standard dose of gemcitabine 1000 mg/m2. Combination of olaparib 100 mg b.i.d. (capsule formulation; intermittent dosing days 1‒14) with gemcitabine 600 mg/m2 i.v. on days 1, 8, and 15 every 4 weeks was considered to have an acceptable tolerability profile and could be considered for further study. Continuous/intermittent dosing schedules of olaparib, together with gemcitabine dose levels >600 mg/m2, did not have acceptable tolerability. Four patients in the dose-escalation phase experienced DLTs of raised ALT and neutropenia. Neutropenia incidence was highest in olaparib capsule 100-mg b.i.d. groups (continuous/intermittent) combined with gemcitabine 800 mg/m2. High incidences of thrombocytopenia and raised levels of aspartate aminotransferase (AST)/ALT were reported in these groups. These cohorts recruited a higher proportion of refractory solid tumour patients who had received several prior chemotherapy treatments, potentially increasing susceptibility to treatment-induced toxicities. While increases in AST/ALT are not known to be associated with the use of olaparib, transient increases have been reported in a large proportion of patients administered gemcitabine [9]. There were no signals of potential drug-induced liver injury in this study.
Consistent with previous studies of olaparib combined with chemotherapy, most commonly reported AEs included anaemia, neutropenia, and thrombocytopenia [7]. Myelosuppression at rates higher than expected has been reported in studies of PARPi administered in combination with temozolomide [10]. Nab-paclitaxel combined with gemcitabine increased rates of haematological AEs compared with gemcitabine alone in a similar patient population [11]. We also evaluated the olaparib tablet formulation, which allows a smaller number of daily pills. The 100 mg o.d. (intermittent)/600 mg/m2 gemcitabine dose combination was acceptable and could be considered for future evaluation. In the small PK dataset, no consistent effect on olaparib or gemcitabine PK was observed when dosed in combination (supplementary Appendix, available at Annals of Oncology online).
In the dose-expansion phase conducted in patients with locally advanced or metastatic pancreatic cancer, no difference in efficacy end points (ORR, PFS, or OS) was observed between treatment cohorts. The numbers in each group for this comparative assessment were small (olaparib/gemcitabine: n = 15; gemcitabine alone: n = 7), there was sub-optimal dosing of gemcitabine, and this part of the study was conducted in a genetically unselected pancreatic cancer patient population. Due to tolerability, the gemcitabine dose of 600 mg/m2 administered with olaparib was lower than standard doses of gemcitabine monotherapy in this treatment setting. A study of gemcitabine 600 mg/m2 (initial doses) in combination with radiation in unresectable pancreatic cancer reported a significant improvement in OS compared with gemcitabine alone [12]. The combination of intermittent olaparib 100 mg b.i.d. with 600 mg/m2 gemcitabine may provide meaningful activity against pancreatic adenocarcinoma.
Phase II trials have shown that olaparib has favourable single-agent antitumour activity in patients with breast and ovarian cancers who have a BRCAm [4]. Mutations in the BRCA genes are associated with an increased risk of pancreatic cancer [13], and this patient subset may potentially benefit from targeted therapy with PARPi. In our randomised comparative expansion phase, of nine patients for whom BRCAm status was known, three had a BRCAm and were randomised to combination treatment. Although these patients demonstrated good PFS and OS, efficacy comparison with the gemcitabine arm was not possible.
Limitations of the study include small sample sizes in the randomised dose-expansion phase. Furthermore, blinding of investigators to randomised treatment was not possible due to different gemcitabine doses between the randomised arms. Larger studies are needed to explore whether combination therapy that includes olaparib can be used to increase clinical benefits in advanced pancreatic cancer compared with chemotherapy alone. Given the encouraging response observed in previous olaparib trials involving patients with BRCA-mutated cancers, the evaluation of pancreatic cancer patients with BRCAm is important. Results of ongoing olaparib studies (including NCT01078662 and NCT01296763) may provide insight into the clinical relevance of olaparib in this patient population. Data from study NCT01078662 have shown that olaparib monotherapy had benefits in patients with heavily pre-treated BRCA-mutated pancreatic cancer, as well as other tumour types [14].
In conclusion, the tolerability of olaparib capsules 100 mg b.i.d. (days 1–14) with gemcitabine 600 mg/m2 (i.v. days 1, 8, and 15 every 4 weeks) appeared to be acceptable, with no unmanageable or unexpected toxicities, and could be considered for evaluation in future studies. Further evaluation of olaparib in pancreatic adenocarcinoma with a BRCAm should be explored based on the known biology of these tumours and the activity of olaparib in other BRCA-mutated tumours. A phase III, randomised, double-blind, placebo-controlled study of maintenance olaparib monotherapy in patients with germline BRCA-mutated metastatic pancreatic cancer whose disease has not progressed on first-line platinum-based chemotherapy (NCT02184195) is ongoing.
funding
This work was supported by AstraZeneca. No grants were received.
disclosure
MRM is supported by the Oxford National Institute for Health Research (NIHR) Biomedical Research Centre. IC acknowledges National Health Service funding to the NIHR Biomedical Research Centre. EOR and HH have received research funding from AstraZeneca. AF and WB are employees of AstraZeneca and own stock in AstraZeneca. MRM has received funding from Amgen for serving on an advisory board, Roche and GSK for clinical trial conduct and drug development advice, an institution grant from AstraZeneca, institutional study fees and advisory board funding from BMS, institutional study fees and membership to IDSMC from Eisai, and institutional study fees from Merck, Pfizer, Clovis, Abbot (Abbvie), Vertex, Immunocore, Millennium, Astellas, and Novartis. JB, HB, and IC have no conflicts of interest.
Supplementary Material
acknowledgements
We thank Helen Swaisland for her important contribution to the pharmacokinetic design of the study. We also thank Theradex for their assistance with the data analysis. This study was sponsored by AstraZeneca. Medical writing assistance was provided by Claire Routley, PhD, from Mudskipper Business Ltd, funded by AstraZeneca.
references
- 1. Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol 2008; 26: 3785–3790. [DOI] [PubMed] [Google Scholar]
- 2. Goggins M, Schutte M, Lu J, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res 1996; 56: 5360–5364. [PubMed] [Google Scholar]
- 3. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009; 361: 123–134. [DOI] [PubMed] [Google Scholar]
- 4. Ledermann JA, Harter P, Gourley C, et al. Randomized trial of olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis by BRCA mutation status. Lancet Oncol 2014; 15: 852–861. [DOI] [PubMed] [Google Scholar]
- 5. Donawho CK, Luo Y, Luo Y, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res 2007; 13: 2728–2737. [DOI] [PubMed] [Google Scholar]
- 6. Jacob DA, Bahra M, Langrehr JM, et al. Combination therapy of poly (ADP-ribose) polymerase inhibitor 3-aminobenzamide and gemcitabine shows strong antitumor activity in pancreatic cancer cells. J Gastroenterol Hepatol 2007; 22: 738–748. [DOI] [PubMed] [Google Scholar]
- 7. Rajan A, Carter CA, Kelly RJ, et al. A phase I combination study of olaparib with cisplatin and gemcitabine in adults with solid tumors. Clin Cancer Res 2012; 18: 2344–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. AstraZeneca. Global Policy: Bioethics. 2011. http://www.astrazeneca.com/Responsibility/Code-policies-standards/Our-global-policies (1 March 2014, date last accessed). [Google Scholar]
- 9. Gemcitabine Drug Record. U.S. National Library of Medicine. 2013. http://livertox.nlm.nih.gov/Gemcitabine.htm (1 March 2014, date last accessed).
- 10. Plummer R, Jones C, Middleton M, et al. Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res 2008; 14: 7917–7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loehrer PJ, Sr., Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 2011; 29: 4105–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thompson D, Easton DF. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst 2002; 94: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 14. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2014Nov 3 [epub ahead of print], doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


