Abstract
Calcium (Ca2+) and manganese (Mn2+) are essential elements for plants and have similar ionic radii and binding coordination. They are assigned specific functions within organelles, but share many transport mechanisms to cross organellar membranes. Despite their points of interaction, those elements are usually investigated and reviewed separately. This review takes them out of this isolation. It highlights our current mechanistic understanding and points to open questions of their functions, their transport, and their interplay in the endoplasmic reticulum (ER), vesicular compartments (Golgi apparatus, trans-Golgi network, pre-vacuolar compartment), vacuoles, chloroplasts, mitochondria, and peroxisomes. Complex processes demanding these cations, such as Mn2+-dependent glycosylation or systemic Ca2+ signaling, are covered in some detail if they have not been reviewed recently or if recent findings add to current models. The function of Ca2+ as signaling agent released from organelles into the cytosol and within the organelles themselves is a recurrent theme of this review, again keeping the interference by Mn2+ in mind. The involvement of organellar channels [e.g. glutamate receptor-likes (GLR), cyclic nucleotide-gated channels (CNGC), mitochondrial conductivity units (MCU), and two-pore channel1 (TPC1)], transporters (e.g. natural resistance-associated macrophage proteins (NRAMP), Ca2+ exchangers (CAX), metal tolerance proteins (MTP), and bivalent cation transporters (BICAT)], and pumps [autoinhibited Ca2+-ATPases (ACA) and ER Ca2+-ATPases (ECA)] in the import and export of organellar Ca2+ and Mn2+ is scrutinized, whereby current controversial issues are pointed out. Mechanisms in animals and yeast are taken into account where they may provide a blueprint for processes in plants, in particular, with respect to tunable molecular mechanisms of Ca2+ versus Mn2+ selectivity.
Calcium and manganese play essential roles in organellar compartments, yet they share many transport mechanisms, implying synergistic, and antagonistic interactions of both cations.
Introduction
Compartmentation enables the eukaryotic cell to simultaneously carry out processes with different physicochemical requirements, such as pH, redox potential, or ion concentrations. In consequence, mineral elements fulfill specific functions in different compartments. Cellular compartments may also serve as stores for essential elements and places to safely sequester toxic compounds, with the plant vacuole representing the prime example. In any case, transport proteins are required to load and unload the compartments and thus maintain this elemental homeostasis. This review covers our current understanding of the compartmentation of two cations, calcium (Ca2+) and manganese (Mn2+), which are usually investigated and reviewed in isolation of each other. It aims to show that a more integrative view of both elements is overdue, since classical, as well as more recent work, points to an interaction of those two cations, owing to their similar coordination geometry and ionic radii. For instance, induction of Ca2+ deficiency symptoms and diminished Ca2+ translocation by excess Mn2+ in bean (Phaseolus vulgaris) plants has already been observed in the 1970s (Horst and Marschner, 1978). More recently, an interference of Mn2+ with Ca2+ translocation was confirmed for Arabidopsis and other species (Blamey et al., 2015; Lesková et al., 2017).
In plants, two primary functions for Ca2+ are most evident (Peiter, 2014). The bivalent cation provides structural stability by bridging carboxyl groups of galacturonans in pectin and by binding to phospholipids at cell membranes. Second, Ca2+ has been established as one of the universal second messengers, involved in responses to nearly every environmental cue and in a large number of developmental processes. Thereby, temporally and spatially defined elevations of free Ca2+, triggered by the perception of a stimulus, are sensed and “decoded” by an array of Ca2+-binding proteins, that either bind to or modify downstream proteins, for example, by phosphorylation. Plant Ca2+ signaling has been the subject of numerous recent reviews that highlight different aspects (Dodd et al., 2010; Costa et al., 2018; Kudla et al., 2018; Thor, 2019; Tian et al., 2020; Pirayesh et al., 2021). Here, we concentrate on the roles of Ca2+ in an organellar context, and in particular on its interaction with Mn2+.
An interference of Mn2+ in Ca2+ signaling is highly likely on the levels of both generation and perception of Ca2+ signals. Transport proteins of many families discriminate poorly between the two cations. Where known, this is highlighted for individual proteins throughout this review, and potential mechanisms of selectivity are addressed. However, for many transport processes, this interaction has not been studied yet. In animals, Ca2+ channels of the Ca2+ release-activated Ca2+ channel (CRAC)-, IP3 receptor (IP3R)-, and voltage-gated Ca2+ channel (VGCC)-type also conduct Mn2+ (Kamer et al., 2018). Mn2+ permeability is largely uncharted for Ca2+ channels in plants, albeit external Mn2+ affects Ca2+ influx into the cytosol (McAinsh et al., 1995; Wymer et al., 1997), and thus potentially the generation of Ca2+ signals. Regarding Ca2+ signal decoding, Mn2+ binds with high affinity to Ca2+-sensing proteins, for example, of the Calcineurin B-like (CBL) family (Sánchez-Barrena et al., 2005); the physiological effect of this competition again is unknown. Its binding to calmodulin locks the protein in a closed conformation and may, therefore, negatively regulate Ca2+ signaling (Senguen and Grabarek, 2012).
Our current view of Mn2+ in plants has been the subject of a number of comprehensive reviews (Pittman, 2005; Socha and Guerinot, 2014; Shao et al., 2017; Andresen et al., 2018; Alejandro et al., 2020). The role of Mn2+ considered as most important in plants is that as a redox-active constituent of the oxygen (O2)-evolving complex (OEC) in photosystem II (PSII), where Mn2+ and Ca2+ form a Mn4CaO cluster to catalyze the splitting of water (H2O). The chloroplast that harbors this machinery, hence, needs to accumulate large amounts of Mn2+, in parallel with dynamically modulating free Ca2+ as regulatory element, as discussed below. Further roles of Mn2+ are distributed amongst different compartments, such as reactive O2 scavenging in the mitochondria by Mn2+-Superoxide Dismutase (SOD), phytohormone deconjugation in the endoplasmic reticulum (ER), and glycosylation in the Golgi apparatus (Andresen et al., 2018; Alejandro et al., 2020). In yeast lacking the Golgi-localized Ca2+/Mn2+ transporter Gcr1-dependent translation factor1 (GDT1), high-Ca2+ media cause glycosylation defects which could be rescued by the addition of Mn2+ (Dulary et al., 2018), again indicating a competition of Mn2+ and Ca2+. In addition to activating enzymes, the idea of a regulatory function of Mn2+ in metabolism has recently been put forward (Bloom, 2019). Intriguingly, an immunity signaling mechanism based on organellar Mn2+ release operates in humans (Wang et al., 2018).
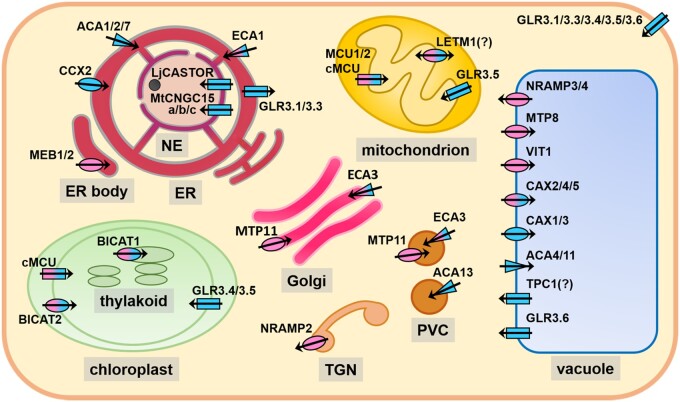
Cellular functions and interactions of Ca2+ and Mn2+ in organelles depend on their loading and unloading by an array of transport proteins from different families, which are displayed in Figure 1 and listed in Table 1. An up-to-date overview of those permeating Ca2+ is provided by the review of Demidchik et al. (2018); Ca2+ transport in the model eukaryote yeast is covered in Lange and Peiter (2020). Proteins transporting Mn2+ have recently been treated by Alejandro et al. (2020) and fungal mechanisms by Reddi et al. (2009). In plant organelles, primary active transport of Ca2+ is mediated by P2A- and P2B-type ATPases, called ER Ca2+-ATPases (ECAs) and Autoinhibited Ca2+-ATPases (ACAs), respectively. The former also transport Mn2+, and the latter are feedback-regulated by a Ca2+/calmodulin-binding autoinhibitory domain. Those pumps have recently been reviewed by Bossi et al. (2020). A large number of secondary active transporters, belonging to different families, contribute to organellar Ca2+ and Mn2+ homeostasis. The Ca2+ exchanger (CAX) family, reviewed by Pittman and Hirschi (2016), contains both Ca2+-specific transporters and those transporting Ca2+ and Mn2+; both are energized by H+ antiport (Waight et al., 2013). The bivalent cation transporter (BICAT) family, with its chloroplast-localized members BICAT1 and BICAT2, has been identified only recently as a family of Ca2+/Mn2+ transport proteins with a yet unresolved transport mechanism (Thines et al., 2020). The inconsistent nomenclature of these transporters is explained in the chloroplast section of this review. The founding member of this family is GDT1 in yeast (Demaegd et al., 2014). Ca2+ and Mn2+ transport have been shown directly for GDT1 (Colinet et al., 2016; Thines et al., 2018), and also for the human homolog Transmembrane protein165 (TMEM165; Stribny et al., 2020). As the mutation of these transporters causes both Ca2+- and Mn2+-related phenotypes in yeast and in plants (Thines et al., 2020), they are an ideal case to study Ca2+/Mn2+ interactions, as discussed later.
Figure 1.
Transport proteins for Ca2+ and Mn2+ discussed in this review. Pumps (triangles), transporters (ellipses), and channels (rectangles) that were experimentally shown to permeate Ca2+ (blue), Mn2+ (magenta), or both (blue/magenta) are displayed in a hypothetical plant cell containing the organelles discussed in this review. Only Arabidopsis proteins are shown, except for LjCASTOR and MtCNGC15s, for which a function of the Arabidopsis homologs as Ca2+ channels has not been examined yet. Characterized orthologs of Arabidopsis proteins described in the text are listed in Table 1. Note that the absence of an experimentally confirmed substrate, for example, Mn2+ in the case of Ca2+ channels (GLR, CNGC, and TPC1) does not exclude its permeation. Conductance of Mn2+ by (c)MCUs is inferred from their mammalian homologs. The permeation of Ca2+ by NRAMPs can be excluded on a structural basis, as discussed in the text. ER, endoplasmic reticulum; NE, nuclear envelope; PVC, pre-vacuolar compartment; TGN, trans-Golgi network.
Table 1.
Organellar transport proteins for Ca2+ and Mn2+ discussed in this review.
MtCNGC15a,b,ca
| Protein | Type | Substrates | References |
|---|---|---|---|
| ER, NE | |||
| AtECA1 | P2A ATPase | Ca2+, Mn2+ | Liang et al., 1997; Wu et al., 2002; Shkolnik et al., 2018 |
| MtMCA8 | P2A ATPase | Ca2+, Mn2+ (?)e | Capoen et al., 2011 |
| AtACA1, AtACA4, AtACA7 | P2B ATPase | Ca2+ | Harper et al., 1998; Rahmati Ishka et al., 2021 |
| AtCCX2 | Transporter | Ca2+ (?) | Corso et al., 2018 |
| AtMEB1, AtMEB2 | Transporter | Mn2+, Fe2+ | Yamada et al., 2013 |
| LjCASTOR | Channel | K+, Ca2+ | Charpentier et al., 2008; Kim et al., 2019 |
| MtCNGC15abc | Channel | Ca2+ | Charpentier et al., 2016 |
| AtCNGC15 | Channel | Ca2+ (?) | Leitao et al., 2019 |
| AtGLR3.1, AtGLR3.3a | Channel | Ca2+ | Nguyen et al., 2018 |
|
Golgi Apparatus, PVC, TGN | |||
| AtECA3 | P2A ATPase | Ca2+, Mn2+ | Li et al., 2008; Mills et al., 2008 |
| AtMTP11 | Transporter | Mn2+ | Delhaize et al., 2007; Peiter et al., 2007a |
| OsMTP11 | Transporter | Mn2+ | Farthing et al., 2017; Ma et al., 2018 |
| AtNRAMP2 | Transporter | Mn2+ | Alejandro et al., 2017; Gao et al., 2018 |
|
Vacuole |
|||
| AtACA4, AtACA11 | P2B ATPase | Ca2+ | Geisler et al., 2000; Lee et al., 2007; Hilleary et al., 2020 |
| AtCAX1, AtCAX3 | Transporter | Ca2+ | Hirschi et al., 1996; Conn et al., 2011; Punshon et al., 2012 |
| AtCAX2 | Transporter | Ca2+, Mn2+, Cd2+ | Hirschi et al., 2000; Schaaf et al., 2002; Pittman et al., 2004 |
| AtCAX4 | Transporter | Ca2+, Mn2+, Cd2+ | Cheng et al., 2002; Mei et al., 2009 |
| AtCAX5 | Transporter | Ca2+, Mn2+ | Edmond et al., 2009 |
| OsCAX1a | Transporter | Ca2+, Mn2+ | Kamiya et al., 2005; 2006 |
| VCAX1 | Transporter | Ca2+ | Ueoka-Nakanishi et al., 2000 |
| VvCAX3 | Transporter | Ca2+, Mn2+ | Martins et al., 2017 |
| AtMTP8 | Transporter | Mn2+, Fe2+ | Eroglu et al., 2016, 2017; Chu et al., 2017 |
| OsMTP8.1, OsMTP8.2 | Transporter | Mn2+ | Chen et al., 2013; Takemoto et al., 2017 |
| ShMTP8 | Transporter | Mn2+ | Delhaize et al., 2003 |
| AtNRAMP3, AtNRAMP4 | Transporter | Mn2+, Fe2+, Cd2+ | Thomine et al., 2003; Lanquar et al., 2010 |
| TcNRAMP3, TcNRAMP4 | Transporter | Mn2+, Fe2+, Cd2+ | Oomen et al., 2009 |
| AtVIT1 | Transporter | Mn2+, Fe2+ | Kim et al., 2006 |
| OsVIT1, OsVIT2 | Transporter | Mn2+, Fe2+, Zn2+ | Zhang et al., 2012b |
| TaVIT1 | Transporter | Mn2+, Fe2+ | Connorton et al., 2017 |
| AtZIP1 | Transporter | Mn2+, Zn2+ | Milner et al., 2013 |
| AtGLR3.6a | Channel | Ca2+ | Nguyen et al., 2018 |
| AtTPC1 | Channel | Ca2+, K+, Mg2+ | Hedrich et al., 1986; Peiter et al., 2005 |
|
Chloroplast | |||
| AtBICAT1b | Transporter | Ca2+, Mn2+ | Schneider et al., 2016; Wang et al., 2016; Frank et al., 2019 |
| AtBICAT2c | Transporter | Ca2+, Mn2+ | Eisenhut et al., 2018; Zhang et al., 2018; Frank et al., 2019 |
| AtGLR3.4a | Channel | Ca2+ (?) | Teardo et al., 2011 |
| AtGLR3.5a | Channel | Ca2+ (?) | Teardo et al., 2015 |
| AtcMCUd | Channel | Ca2+, Mn2+ (?) | Teardo et al., 2019 |
|
Mitochondrion | |||
| AtGLR3.5a | Channel | Ca2+ (?) | Teardo et al., 2015 |
| AtMCU1d | Channel | Ca2+, Mn2+ (?) | Teardo et al., 2017; Selles et al., 2018 |
| AtMCU2d | Channel | Ca2+, Mn2+ (?) | Selles et al., 2018 |
| AtcMCUd | Channel | Ca2+, Mn2+ (?) | Teardo et al., 2019 |
Note that the absence of an experimentally confirmed substrate, for example, Mn2+ in the case of Ca2+ channels, does not exclude its permeation.
In other studies, localization and function of AtGLR3.1, 3.3, 3.4, 3.5, and 3.6 in the plasma membrane have been demonstrated. For details, see text.
BICAT1 has also been named PAM71 and CCHA1.
BICAT2 has also been named PAM71-HL and CMT1.
Conductance of Mn2+ by (c)MCUs is inferred from their mammalian homologs.
(?), Substrates inferred from homologous proteins and not experimentally determined.
Members of the cation diffusion facilitator (CDF) family, called metal tolerance proteins (MTPs) in plants, mediate the H+-driven active export of metals, including Mn2+, out of the cytosol into organelles (Montanini et al., 2007; Gustin et al., 2011; Ricachenevsky et al., 2013). Transport of Mn2+, besides Fe2+, into the vacuolar organelle is also mediated by transporters of the vacuolar iron transporter (VIT) family (Kim et al., 2006). Natural resistance-associated macrophage proteins (NRAMP), in turn, import metals, including Mn2+, from the apoplast or from organelles into the cytosol (Nevo and Nelson, 2006). The ZRT-, IRT-like protein (ZIP) family also harbors proteins that transport Mn2+ across organellar membranes (Milner et al., 2013). For many Mn2+ transporters, Ca2+ transport has not been examined yet, and cannot be excluded. An exception may be NRAMP proteins, for which a coordination of Ca2+ was excluded on a structural basis (Ehrnstorfer et al., 2014). Within the metal-binding site, the sulfur of a conserved methionine selects against transport of Ca2+, while allowing the permeation of transition metals, including Mn2+ (Bozzi et al., 2016). All plant NRAMPs except NRAMP5 contains this selectivity motif.
The release of Ca2+, and potentially also Mn2+, from organelles along their electrochemical gradient is mediated by channel proteins. Although pharmacological analyses have frequently pointed to a contribution of organellar Ca2+ release to the generation of Ca2+ signals, the respective Ca2+ stores and the identity of the channels have remained mostly undefined (e.g. Thor and Peiter, 2014). Beginning with the identification of the vacuolar two-pore channel1 (TPC1; Peiter et al., 2005), a limited number of Ca2+-permeable channels in organellar membranes, which belong to the families of cyclic nucleotide-gated channels (CNGCs), glutamate receptor-likes (GLRs), mitochondrial conductivity units (MCUs), and possibly annexins have been uncovered (Demidchik et al., 2018). This review will indicate the relevance of those families in a physiological context.
Ca2+ and Mn2+ in the ER and the nuclear envelope
Functions of Ca2+
Within the ER of eukaryotic cells, the Ca2+-binding protein calreticulin is an important molecular chaperone with diverse functions (Jia et al., 2009). Unlike its animal counterpart, plant calreticulin is glycosylated. As a molecular chaperone, it mediates folding of glycoproteins and determines growth, development, and stress responses. It has been shown in animals that calreticulin modulates Ca2+ homeostasis due to its high capacity for Ca2+-binding. In animal cells, the ER indeed represents the main intracellular Ca2+ store for the generation of cytosolic Ca2+ signals. Inverse changes of [Ca2+]cyt and [Ca2+]ER have been directly demonstrated by simultaneous imaging of reporters targeted to both compartments (Palmer et al., 2004). Due to its reticulate structure, the ER is predestined to mediate localized Ca2+ fluxes, which are a prerequisite for spatio-temporally specific [Ca2+]cyt signals. Polarization of Ca2+ fluxes across ER membranes allows some animal cell types to employ the ER as intracellular “Ca2+ tunnel” (Petersen et al., 2017), a mechanism that has not been demonstrated in plants for Ca2+ yet, but, intriguingly, for Zn2+ (Sinclair et al., 2018).
In plants, due to the overwhelming size of the vacuole, the role of the ER in Ca2+ signaling has been taken little into consideration in the past. However, this picture is currently changing. Using an ER-targeted Ca2+ reporter, distinct changes in [Ca2+]ER upon stimulation have been revealed (Bonza et al., 2013). Unlike in animals, [Ca2+]ER in plants increased upon [Ca2+]cyt transients, which implies a role of the ER as Ca2+ buffer rather than Ca2+ source (Bonza et al., 2013). It has recently become apparent that [Ca2+]ER homeostasis determines NaCl-induced [Ca2+]cyt signals and plant sensitivity to salt stress (Corso et al., 2018). Furthermore, the phloem ER has been identified as Ca2+ store mediating a long-distance [Ca2+]cyt wave in response to H2O potential gradients, hence determining the root growth toward H2O (Shkolnik et al., 2018). Specifically in some leguminous plants, Ca2+ release from the phloem ER plays a further role in the swelling of forisomes, proteins that expand upon Ca2+ binding and in turn block sieve tubes in response to disturbances (Furch et al., 2009).
The ER is continuous with the nuclear envelope (NE), which engulfs the nucleus. The steady-state [Ca2+] in the nucleus is similar to that in the cytosol and increases transiently or repetitively upon perception of environmental and developmental stimuli (Mazars et al., 2009), including pathogen-associated molecular patterns (PAMPs; Lecourieux et al., 2005) or microbial-derived symbiotic molecules, such as Nod factors that initiate symbiosis with rhizobial bacteria (Sieberer et al., 2009). The topic of nuclear Ca2+ signaling has recently been covered in an excellent review (Charpentier, 2018). Nuclear Ca2+ transients often follow cytosolic ones, but nuclei have also been demonstrated to be autonomous in the generation of Ca2+ signals, what requires Ca2+ release from the NE. Nuclear Ca2+ signals, whose kinetics differ with different stimuli, can be decoded by Ca2+-regulated protein kinases, such as CCaMK in the initiation of symbioses (Singh and Parniske, 2012). Besides their central position in symbiotic signaling, nuclear Ca2+ signatures also regulate root development (Leitao et al., 2019). As discussed below, Ca2+-permeable channels and transporters that generate those Ca2+ signals have been identified.
Functions of Mn2+
In the ER, Mn2+ is required for phytohormone balance. Auxin amidohydrolases are Mn2+-dependent enzymes that participate in the activation of auxin by hydrolysis of IAA-Ala, -Leu, and -Phe into biologically active IAA (LeClere et al., 2002). In Arabidopsis, the auxin amidohydrolase family consists of seven members, of which ILR1, ILL1, ILL2, and IAR3 are located in the ER (Sanchez Carranza et al., 2016). ilr1iar3ill2 mutant seeds and seedlings have reduced levels of free IAA and accumulate more IAA conjugates, indicating that amidohydrolases contribute free IAA to the auxin pool during germination (Rampey et al., 2004). Interestingly, IAR3 is also involved in the hydrolysis of jasmonic acid (JA)-Ile, which is the bioactive form of the phytohormone jasmonate (Widemann et al., 2013). Thus, the ER might also represent a site of signaling crosstalk between auxin and jasmonate pathways dependent on Mn2+.
Another role of Mn2+ in the ER lies in purine degradation (i.e. the ureide pathway), which can be divided into two phases. In peroxisomes, the pyrimidine ring of uric acid is cleaved to produce (S)-allantoin. In the second phase, (S)-allantoin is converted into glyoxylate in a stepwise manner by four different enzymes located in the ER (Werner and Witte, 2011). Three of these enzymes, that is, allantoate amidohydrolase (Werner et al., 2008), (S)-ureidoglycine aminohydrolase (Shin et al., 2012), and (S)-ureidoglycolate amidohydrolase (Shin et al., 2014), require Mn2+ as cofactor. Thus, the last part of uric acid degradation to ammonia and glyoxylate is a Mn2+-dependent pathway in plants.
Import of Ca2+ and Mn2+
Active import of Ca2+ into the plant ER is likely mediated by P2A- and P2B-type ATPases, of which ECA1 (Liang et al., 1997) and ACA1, 2, and 7 (Harper et al., 1998; Rahmati Ishka et al., 2021) have been localized to this compartment.
ACA1, 2, and 7 are functionally redundant (Rahmati Ishka et al., 2021). Loss of the three genes caused a defect in pollen transmission and aberrances in immunity, such as salicylic acid-dependent lesions in leaves, a phenotype also found in plants devoid of vacuolar ACAs (see below). As both, fertilization and immunity are regulated by [Ca2+]cyt, the phenotypes indicate a role of the pumps in Ca2+ sequestration by the ER. This notion is supported by markedly increased [Ca2+]cyt responses in the mutants to blue light and to the PAMP flg22.
Knockout mutants for ECA1 are sensitive to low-Ca2+ media (Wu et al., 2002). A role of this pump in cytosolic Ca2+ signaling has recently been shown in Arabidopsis, where its activity shapes systemic Ca2+ signals triggered by a H2O potential gradient (Shkolnik et al., 2018). ECA1 physically interacts with MIZ1 and is inhibited by this protein (Shkolnik et al., 2018). A homolog of ECA1 has been described in rice, where it is upregulated by drought (Huda et al., 2013). This may point to a similar role in H2O tracking as it has been shown in Arabidopsis. In Medicago truncatula, an ER- and NE-localized ECA is essential for the generation of nuclear Ca2+ oscillations upon Nod factor perception, and it determines symbiotic establishment (Capoen et al., 2011). Sequestration of Ca2+ by ATPases is thus crucial in diverse Ca2+ signaling scenarios.
ECA1 is the only transport protein described so far that moves Mn2+ into the plant ER (Liang et al., 1997; Johnson et al., 2009). ECA2 and ECA4 contain an ER retention motif, but have not been functionally characterized yet (Bossi et al., 2020). Arabidopsis eca1 mutants show growth inhibition by high Mn2+, indicating that ECA1 is important for tolerance to toxic levels of Mn (Wu et al., 2002). The absence of a phenotype under standard growth conditions suggests the existence of other mechanisms responsible for Mn2+ transport into the ER, with ECA2 and ECA4 being suitable candidates. Since ECA pumps are a major factor in Ca2+ and Mn2+ homeostasis, yet unknown mechanisms must exist to discriminate between both ions.
Apart from P-type ATPases, an ER-localized transporter of the Ca2+/cation exchanger family, CCX2, contributes to Ca2+ homeostasis in ER and cytosol, thereby modulating abiotic stress responses (Corso et al., 2018). Free Ca2+ in the ER is increased and concentrations in cytosol are decreased upon NaCl treatment if CCX2 is absent, which implies that CCX2 either transports Ca2+ itself or regulates a Ca2+ transport protein.
Specific ER subcompartments, named ER bodies, contain two Mn2+/Fe2+ transporters, membrane protein of ER body 1 (MEB1) and MEB2, that are distantly related to the vacuolar transporter VIT1 (Yamada et al., 2013). ER bodies have been found only in Brassicales (Matsushima et al., 2003), where they are constitutively present in roots and induced by wounding or jasmonate treatment in epidermal cells of mature leaves. It has been proposed that this organellar structure functions in metal sequestration and in the defense against herbivores by accumulating EE-type myrosinases (Yamada et al., 2011). Since Mn2+ may stimulate myrosinase activity (Ohtsuru and Kawatani, 1979), the loading of Mn2+ into ER bodies is likely required for their function.
Release of Ca2+ and Mn2+
Our molecular understanding of Ca2+ release from the ER only partially explains biochemical findings. In animals, it is facilitated by two classes of ligand-gated channels: Ryanodine receptors (RyRs) activated by cyclic adenosine diphosphate ribose (cADPR), and IP3Rs. Genomes of plants do not encode these proteins. Yet, cADPR activates Ca2+ release from ER membrane vesicles (Navazio et al., 2001), and this molecule has been assigned a role in the circadian clock (Dodd et al., 2007), in abscisic acid signaling (Sánchez et al., 2004), and in NO-dependent responses (Abdul-Awal et al., 2016), all involving an increase of [Ca2+]cyt. Further complexity is added by a likely interference of cADPR synthesis and poly-ADP(ribosyl)ation due to their common precursor, NAD+ (Rissel and Peiter, 2019). The source of cADPR in plants has been completely obscure until recently, when the biosynthesis of a cADPR variant, v-cADPR, by the TIR domain of nucleotide-binding leucine-rich repeat (NLR) immune receptors was shown (Wan et al., 2019). NLR-mediated synthesis of v-cADPR promotes cell death. It is, however, still unknown if the enzymatic activity of the TIR domain is also responsible for cADPR synthesis in the circumstances mentioned above.
Besides cADPR, the NAD+-based nucleotide NAADP was shown to release Ca2+ from plant ER-derived vesicles (Navazio et al., 2000). In animals, NAADP activates TPCs resident in endolysosomal membranes (Calcraft et al., 2009). However, as discussed below, plant TPC proteins reside in the vacuolar membrane, and they are not activated by NAADP. In addition to ligand-gated Ca2+ conductances, voltage-gated Ca2+ channels have been detected by lipid bilayer electrophysiology in reconstituted plant ER membranes (Klüsener et al., 1995; Klüsener and Weiler, 1999). At present, the molecular mechanisms and physiological relevance of all those Ca2+ release pathways are unclear. However, the early biochemical and electrophysiological observations might be associated with channel proteins that have been localized to the ER.
Two channel proteins of the GLR family, GLR3.1 and GLR3.3, have recently been assigned a localization to ER-like structures of xylem contact cells and phloem sieve elements, respectively (Nguyen et al., 2018). In these tissues, those channels are essential for the propagation of electropotential waves and systemic Ca2+ signals triggered by wounding and herbivory (Farmer et al., 2020). For GLR3.3, Ca2+ permeability has been shown by expression in Cos-7 cells (Wudick et al., 2018) and HEK293T cells (Shao et al., 2020). The apparent localization of the channels to endomembranes cannot explain the defects of mutants for those genes, namely their inability to elicit systemic plasma membrane potential changes and [Ca2+]cyt responses to apoplastic glutamate (Mousavi et al., 2013; Toyota et al., 2018). Furthermore, GLR3.1 functions, together with GLR3.5, as L-Met-activated Ca2+ channel in the plasma membrane of stomatal guard cells (Kong et al., 2016). Also, GLR3.3 has been described before to mediate Ca2+ entry into root cells, plasma membrane depolarization, and macroscopic currents upon stimulation with various amino acids, glutathione, or 1-aminocyclopropane-1-carboxylic acid (Qi et al., 2006; Mou et al., 2020). Accordingly, the ligand-binding domain of GLR3.3, the structure of which has recently been solved, binds glutamate and other amino acids in the micromolar range (Alfieri et al., 2020). GLR3.3 also localizes to the plasma membrane in pollen sperm cells, where its exit from the ER is regulated by CORNICHON proteins (Wudick et al., 2018). This mechanism might also control its localization in the vascular system. There, besides the reported ER localization, a low number of channels may be present in the plasma membrane that mediate the observed chemo-electrical responses (Grenzi et al., 2020). It is unclear at present if and under which circumstances GLR3.1 and 3.3 mediate Ca2+ release from the ER.
The generation of the above-mentioned oscillatory nuclear Ca2+ signals that initiate symbiotic interactions with rhizobial bacteria or mycorrhizal fungi relies on Ca2+ release from the NE, which is continuous with the ER (Zipfel and Oldroyd, 2017). Three CNGC15 paralogs have been identified as Ca2+ channels that mediate those fluxes (Charpentier et al., 2016). These channels physically interact with and are regulated by another cation channel, DMI1 in M. truncatula, or CASTOR/POLLUX in Lotus japonicus, the absence of which completely abolishes the nuclear Ca2+ spiking. The localization of DMI1 preferably in the inner nuclear membrane supports its role in nuclear Ca2+ signal generation (Capoen et al., 2011). Recently, the generation of nuclear Ca2+ signals in Arabidopsis, which does not enter symbiotic interactions, was demonstrated to also involve orthologs of DMI1, and possibly CNGC15 (Leitao et al., 2019). Medicago DMI1 modulates Ca2+ signals upon expression in yeast (Peiter et al., 2007b), and, based on lipid bilayer analyses, DMI1 and CASTOR/POLLUX are believed to primarily conduct K+ and to regulate Ca2+ oscillations by altering the NE’s membrane potential (Charpentier et al., 2008; Venkateshwaran et al., 2012). However, this view has recently been challenged by Kim et al. (2019), who reported Ca2+ permeability and Ca2+ activation of CASTOR, which would render it a Ca2+-induced Ca2+ release (CICR) channel. A large gating ring structure of the protein contains Ca2+-binding sites that biphasically stimulate or inhibit channel activity (Kim et al., 2019).
The Mn2+ permeability of ER-localized Ca2+ channels has not been analyzed yet, but is likely of relevance, given the fact that ECA1 accumulates Mn2+ into this organelle. Specific mechanisms of Mn2+ release from the ER in plants are also still unknown. There is neither evidence in humans nor in yeast of transporters mediating Mn2+ efflux from ER, although in yeast Mn2+ release from the ER to the Golgi via vesicular trafficking has been proposed (Garcia-Rodriguez et al., 2015).
Ca2+ and Mn2+ in the Golgi, TGN, and PVC
Functions of Ca2+
The luminal homeostasis of ions, including Ca2+ and Mn2+, next to the pH determines Golgi functions (Kellokumpu, 2019). However, our functional understanding of Ca2+ in the plant’s Golgi apparatus and other vesicular compartments is still scant. In the Golgi, post-translational modifications like glycosylation often require bivalent cations as cofactors. Albeit this is mostly Mn2+, some enzymes, such as ER-type α-mannosidase I (MNS3), employ Ca2+ or Mn2+ for their catalytic activity (Liebminger et al., 2009). In tobacco (Nicotiana tabacum), Ca2+ serves as cofactor for a Golgi-localized calreticulin, likely required for correct protein folding similarly to ER-located calreticulin (Nardi et al., 2006).
In animal cells, a [Ca2+] gradient exists along the compartments of the secretory pathway that is likely of functional relevance (Suzuki et al., 2016). Whereas the basal free Ca2+ in the ER is around 700 µM, free Ca2+ in the Golgi is lower, decreasing from 150–300 µM in the cis-Golgi to 50–100 µM in the trans-Golgi (Aulestia et al., 2015). Similarly, there is a pH gradient along the compartments of the animal secretory pathway (Kellokumpu, 2019). A gradient of decreasing pH is also observed along the plant secretory pathway (Shen et al., 2013), whereby an increase from Golgi to pre-vacuolar compartment (PVC) and late PVC has been observed (Martinière et al., 2013). In contrast, this has not been assessed for Ca2+ yet. Intriguingly, the single available report on [Ca2+] in the plant Golgi showed a concentration of merely 0.7 µM (Ordenes et al., 2012). This indicates that [Ca2+]Golgi homeostasis in plants may differ substantially from that in animals. However, determination of absolute [Ca2+] in the secretory pathway is challenging. Albeit precise targeting to subcompartments is pursuable by genetically encoded Ca2+ indicators (GECIs), a formidable challenge lies in the pH dependence of most of those fluorescence-based reporters (Suzuki et al., 2016). Because of this, the luminescent reporter aequorin is still a suitable tool to report Ca2+ in acid compartments due to its independence of pH (Brini, 2008).
Free Ca2+ in the plant Golgi is responsive to various stimuli. In response to cold shock, mechanical stimulation, or hyperosmotic stress, Ordenes et al. (2012) monitored an increase in [Ca2+]Golgi that was delayed compared to the [Ca2+]cyt rises. This led to the conclusion that Ca2+ is moved into the Golgi apparatus to restore the basal [Ca2+]cyt. Apart from that, an increase in [Ca2+]Golgi induced by environmental stimuli may regulate vesicular trafficking, which is required to cope with stresses, for example, salt stress (Leshem et al., 2006).
Secretory proteins, molecules, and lipids traverse through the secretory pathway including the Golgi apparatus, the trans-Golgi network (TGN), and the PVC. In plants, vacuolar sorting receptors (VSRs) are involved in cargo transport to the vacuole (Miao et al., 2006). VSRs contain epidermal growth factor (EGF)-like repeats which play a role in Ca2+ interaction, and the binding of vacuolar sorting determinants (VSDs) to VSRs occurs in a Ca2+-dependent manner (Suen et al., 2010; Robinson and Pimpl, 2014). Consequently, Künzl et al. (2016) concluded that VSDs bind to VSRs in ER and Golgi at neutral pH and high [Ca2+], whereas VSRs release VSDs in the TGN or in PVCs under mildly acidic pH and low [Ca2+]. In addition, in animals, the sorting of secretory proteins depends on the import of Ca2+ into the TGN by the secretory pathway Ca2+-ATPase1 (SPCA1) in a sphingomyelin-dependent manner (Deng et al., 2018).
Besides the potential regulatory activity of Ca2+ within organelles of the secretory pathway, its release will cause local [Ca2+]cyt elevations that may be sensed by secretory pathway-localized proteins. For instance, the auxin derivate 2,4-D induced a decrease in [Ca2+]Golgi (Ordenes et al., 2012). Several Ca2+-sensing proteins associated with the secretory pathway have been identified. For instance, AGD12 and EHB1, located to the TGN, contain a cytosolic Ca2+-binding C2 domain (Dümmer et al., 2016). AGD12 is an activator of ARF GTPases that mediate vesicular transport and membrane docking (Nielsen et al., 2008). Mutants lacking either of those proteins are affected in gravitropism, which is regulated by auxin (Dümmer et al., 2016) and [Ca2+]cyt-dependent (Monshausen et al., 2011; Shih et al., 2015). A decrease in [Ca2+]Golgi might thus lead to local [Ca2+]cyt elevations around the Golgi that trigger AGD12 and EHB1. Interestingly, EHB1 has recently been described to inhibit the Fe2+ (and Mn2+) uptake transporter IRT1 in a Ca2+-dependent way, albeit in that study, EHB1 was localized to the plasma membrane (Khan et al., 2019).
In Arabidopsis, two calmodulin-like proteins, CML4 and CML5, with yet unknown function are localized to organelles of the secretory pathway (Ruge et al., 2016). Their CaM domain faces the cytosol and will thus sense [Ca2+]cyt elevations around those compartments. Finally, synaptotagmins (SYTs), which contain two cytosolic C2 domains, are also predestined targets of such local [Ca2+]cyt hotspots. In animals, SYTs regulate membrane trafficking and fusion in a Ca2+-dependent manner (Südhof, 2013). Out of the five SYTs in Arabidopsis, SYT2 has been localized to the Golgi apparatus and suggested to be required for unconventional secretion (Zhang et al., 2011) as well as for conventional exocytosis in pollen tubes, whereby it is additionally localized at the plasma membrane (Wang et al., 2015).
Functions of Mn2+
As cofactor of glycosyltransferases (GTs), Mn2+ plays a pivotal role in glycosylation reactions in the Golgi apparatus (Nagashima et al., 2018). GTs are classified into three groups based on their 3D folds, GT-A, GT-B, and GT-C (Moremen and Haltiwanger, 2019). Most of the GT-A fold enzymes contain conserved DxD (Asp-[any amino acid]-Asp) motifs which interact with the phosphate group of the nuclear sugar donor through the coordination of a divalent cation, which is typically Mn2+ (Breton et al., 2006). Although no DxD motif exists in GT-B fold GTs, some of them require a divalent cation for their optimal activity (Breton et al., 2006). For instance, Mn2+ strongly enhances the catalytic activity of a fucosyltransferase by increasing its affinity to receptors, even though the divalent cation is not absolutely essential for its enzymatic activity (Palma et al., 2004).
In all eukaryotes, GTs modify proteins in a complex and coordinated way (Nguema-Ona et al., 2014; Schoberer and Strasser, 2018). Thereby, N-linked protein glycosylation is initiated in the ER. The intermediate N-glycans are transmitted to the cis-Golgi and processed further by MNS3 to remove a mannose residue, followed by maturation while progressing through the Golgi stack from cis- to trans-face. This is accomplished by Golgi α-mannosidase I (MNS1 and MNS2), N-acetylglucosaminyltransferase I (GNTI), Golgi α-mannosidase II (GMII), N-acetylglucosaminyltransferase II (GNTII), α-1,3-fucosyltransferase (FUT11 and FUT12), β-1,2-xylosyltransferase (XYLT), β-1,3-galactosyltransferase (GALT1), and α-1,4-fucosyltransferase (FUT13; Nagashima et al., 2018). Among them, some have been shown to coordinate Mn2+ for their catalytic activity (Figure 2A), and mutants of them show abnormal growth phenotypes.
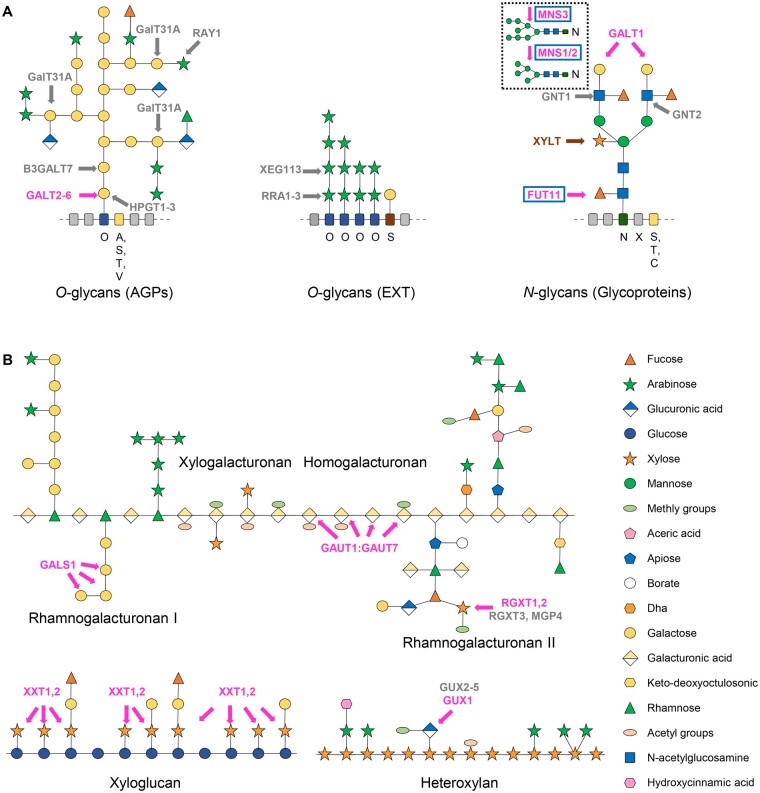
Figure 2.
Mn2+ and Ca2+ dependence of glycosylation in the Golgi. Enzymes are either marked in magenta for those experimentally confirmed to require Mn2+ or in gray for those predicted to be Mn2+-dependent. Enzymes that are also activated by Ca2+ are framed in blue. An enzyme that does not absolutely depend on Mn2+ is marked in brown. A, Schematic representation of enzymes involved in synthesis of O-glycans attached to plant AGPs and EXTs, and specific complex-type N-glycans attached to plant glycoproteins. The glycan models presented are modified from Nguema-Ona et al. (2014) and Showalter and Basu (2016). Among those core enzymes of deglycosylation (insert) and glycosylation, MNS1, MNS2, MNS3 (Liebminger et al., 2009), and FUT11 (Both et al., 2011) are activated by Ca2+ or Mn2+. XYLT (Pagny et al., 2003), GALT1 (Strasser et al., 2007), and GALT2 to 6 (Basu et al., 2015a, 2015b) are reported to coordinate Mn2+ for their catalytic activity. Activity of XYLT is stimulated or inhibited by Mn2+ (Bencúr et al., 2005). Based on the Uniprot database (www.uniprot.org), some further Arabidopsis GTs involved in N- or O-glycosylation are predicted to contain a DxD motif or to bind Mn2+ by sequence similarity, that is, β-1,2-N-acetylglucosaminyltransferase (GNT1 and 2), β-1,2-arabinosyltransferase (RRA1,2,3 and XEG113), hydroxyproline-O-galactosyltransferase (HPGT1,2,3), β-1,3-galactosyltransferase (B3GALT7), β-1,6-galactosyltransferase (GALT31A), and β-arabinofuranosyltransferase (RAY1). B, Schematic representation of enzymes involved in the synthesis of matrix sugars of the plant cell wall. The structures of matrix sugars are modified from Burton et al. (2010). Enzymes shown to require Mn2+ are galactan synthase 1 (AtGALS1) that catalyzes the addition of galactose from UDP-α-D-Gal to β-1,4-galactan chains of rhamnogalacturonan I and the transfer of an arabinopyranose from UDP-β-L-Arap to galactan chains (Ebert et al., 2018; Laursen et al., 2018); a polygalacturonate (1,4)-α-D-galacturonosyltransferase complex (GAUT1:GAUT7) that catalyzes the transfer of galacturonic acid onto homogalacturonan (Amos et al., 2018); (1,3)-α-D-xylosyltransferases (AtRGXT1 and 2) that synthesize rhamnogalacturonan-II (RG II; Egelund et al., 2006; Petersen et al., 2009); xyloglucan xylosyltransferases (XXT1 and 2) that are involved in xyloglucan biosynthesis (Culbertson et al., 2016; 2018); and GUX1 that adds GlcA to xylan (Rennie et al., 2012). In addition, based on the Uniprot database (www.uniprot.org), further Arabidopsis GTs involved in matrix sugar biosynthesis are predicted to contain a DxD motif or to bind Mn2+ by sequence similarity, that is, rhamnogalacturonan α-1,3-D-xylosyltransferase (MGP4 and RGXT3) for RGII biosynthesis, and UDP-GlcA:xylan glucuronyltransferase (GUX2,3,4,5) for heteroxylan biosynthesis.
Extensins (EXTs) and arabinogalactan proteins (AGPs) are two major O-glycosylated protein components of the plant cell wall. They belong to the hydroxyproline-rich glycoprotein superfamily and account for around 10% of cell wall dry weight. AtGALT2 to 6 are Golgi-localized Hyp-O-galactosyltransferases, responsible for transferring galactose to hydroxyproline residues of AGPs, and require Mn2+ for their optimal activity (Figure 2A). Mutants show aberrant root growth, as well as seed mucilage and seed set phenotypes (Basu et al., 2015a, 2015b). Based on the Uniprot database (www.uniprot.org), some further Arabidopsis GTs involved in O-glycosylation are predicted to contain a DxD motif or to bind Mn2+ by sequence similarity (Figure 2A).
Unlike protein glycosylation, the synthesis of matrix polysaccharides (pectin and hemicellulose) takes place exclusively in the Golgi. Some of the Arabidopsis GTs involved in this process have been described to work in a Mn2+-dependent manner (Figure 2B). There are eight plant glycogenin-like starch initiation proteins (PGSIPs) in Arabidopsis, of which PGSIP1 to 5 were later annotated as glucuronic acid substitution of xylan1 (GUX1) to 5, respectively. Albeit initially believed to be involved in starch synthesis, GUX1 encodes a glucuronosyltransferase mediating the addition of GlcA to xylan and requires Mn2+ for its catalytic activity (Rennie et al., 2012). Another member of this protein family, PGSIP6/IPUT1/MOCA1, is a showcase example for a Mn2+-dependent glycosylation process playing multiple crucial roles in signaling and development. Using the same sugar donor as GUX1, PGSIP6 catalyzes the transfer of a GlcA residue to glycosyl inositol phosphorylceramide (GIPC) sphingolipids and hence was renamed to inositol phosphorylceramide glucuronosyltransferase1 (IPUT1; Rennie et al., 2014). GIPCs are abundant in the plasma membrane, where they make up about a quarter of the total lipids, and also reside in tonoplast and ER membranes. Homozygous iput1 T-DNA insertional loss-of-function mutants are lethal (Rennie et al., 2014), but expression of IPUT1 under a pollen-specific promoter in an iput1 knockout line allowed to generate pollen-specifically rescued homozygous iput1 mutants (Tartaglio et al., 2017). These mutants contain fewer GIPCs and a severely altered sphingolipidome, and showed severe dwarfism, compromised pollen tube guidance, and constitutive activation of salicylic acid-mediated defense pathways, indicating important roles of GIPC sphingolipid glycosylation.
Based on its homology to GUX1, PGSIP6/IPUT1/MOCA1 is bound to be Mn2+-dependent, which has yet to be confirmed experimentally. In this respect, it is interesting to note that Bian et al. (2018) identified a mutant of this gene in a screen for hypersensitivity to low Mn2+ supply. This mutant contains a nonsynonymous point mutation which, however, is not close to the Mn2+-binding site of the protein, but which may cause a partial loss in enzymatic activity, leading to the Mn2+-dependent growth defect. It is unclear if the mutant protein requires a higher [Mn2+]Golgi to function, or if Mn2+ may even have a regulatory role.
Finally, the most recent characterization of the PGSIP6/IPUT1/MOCA1 protein combines this role of Mn2+ with Ca2+ signaling. Intriguingly, a mutant called monocation-induced [Ca2+]i increases1 (moca1) that carries a four-amino acid-deletion in PGSIP6/IPUT1/MOCA1, was identified in a screen for aberrant [Ca2+]cyt signals in response to salt (Na+) stress (Jiang et al., 2019). Transient [Ca2+]cyt elevation is an early and essential response to salt stress (Dodd et al., 2010). However, the Na+ sensor in plants has been unknown. Due to the abolished Na+-triggered [Ca2+]cyt signal in the moca1 mutant, GIPCs synthesized by the PGSIP6/IPUT1/MOCA1 protein are believed to fulfill this role by coordinating Na+ to gate Ca2+ influx channels (Jiang et al., 2019). Taken together, this implies that Na+ sensing, Ca2+ signal generation, and hence salt tolerance, depend on a correct Mn2+ supply to the Golgi. Furthermore, based on the multitude of reported phenotypes in mutants of PGSIP6/IPUT1/MOCA1, it remains to be confirmed whether its enzymatic product indeed functions as Na+ sensor, or whether its role is more indirect.
The requirement of bivalent cations by GTs is likely to be more complex as generally anticipated, as the concentration of the cation is an important factor, and in some cases, cations can act in an inhibitory way. For instance, the activity of GALT5 is enhanced by Mn2+ but inhibited by Ca2+ (Basu et al., 2015b). Besides, the activity of XylT, which does not absolutely depend on metal ion cofactors, is enhanced by 1 mM Mn2+, while higher Mn2+ concentrations are inhibitory (Bencúr et al., 2005). Cation concentrations and interactions in the Golgi thus appear to modulate, or even regulate GT enzymes. Considering the specific targeting of enzymes to distinct Golgi subcompartments as well as the different cation requirements of GTs, a concerted regulation of the concentration and ratio of Ca2+ and Mn2+ in the Golgi apparatus is likely to be crucial. However, our understanding of this matter in plants is very rudimentary. In animal cells, the Ca2+/Mn2+ transporter TMEM165 is important for glycosylation, and some Ca2+/Mn2+-ATPases are also potentially involved in Ca2+ and Mn2+ supply of Golgi-localized GTs, as discussed below. In yeast, the Ca2+/Mn2+-ATPase PMR1 and the Ca2+/Mn2+ transporter GDT1 are required for protein glycosylation (Dürr et al., 1998; Colinet et al., 2016). In contrast, no cation transport protein has been associated with the maintenance of glycosylation reactions in plants.
There is evidence that the Golgi and TGN take in a central position in subcellular Mn2+ allocation. In yeast, SMF2, an NRAMP transporter, is assumed to be crucial for releasing Mn2+ from vesicular compartments (trans-Golgi and late endosomes) to the cytosol (Garcia-Rodriguez et al., 2015), whereby Mn2+ can subsequently be transferred to the mitochondria by unknown transporters and to the Golgi by PMR1. Accordingly, a Δsmf2 mutant exhibits reduced SOD2 activity and Mn2+ accumulation in mitochondria, and defective glycosylation of secreted invertase (Luk and Culotta, 2001). In Arabidopsis, the TGN-localized NRAMP2 is believed to be functionally epistatic to NRAMP3 and NRAMP4, two vacuolar Mn2+ and Fe2+ transporters, involved in the redistribution of Mn2+ to vacuoles and chloroplasts under Mn2+ deficiency (Alejandro et al., 2017). Mutants for NRAMP2 show reduced photosynthesis and Mn2+ concentrations in chloroplasts and vacuoles. Based on a model put forward by Krieger-Liszkay and Thomine (2018), Mn2+ released from TGN and vacuole to cytosol can be subsequently translocated to the chloroplast stroma by BICAT2 and further imported into the thylakoid lumen by BICAT1 to supply the H2O-splitting complex and maintain photosynthetic efficiency under Mn2+ deficiency. This implies that vesicular compartments are essential for inter-organellar Mn2+ distribution.
Studies in the animal field suggest a crucial role of the Golgi in Mn2+ storage and detoxification. By combining synchrotron X-ray fluorescence (SXRF) nanoprobe with particle-induced X-ray emission (PIXE) microanalysis and backscattering spectrometry, Carmona et al. (2010) observed an accumulation of Mn2+ within the Golgi of PC12 dopaminergic cells at physiological concentrations, and a further increase of the Golgi-allocated fraction when cells were exposed to 100 µM MnCl2, a subcytotoxic level. Upon exposure to toxic concentrations, Mn2+ was also detected in cytoplasm and nucleus. Based on monitoring the subcellular distribution of Mn2+ in living HEK293T cells by a fluorescent Mn2+ sensor and by nano-SXRF imaging, Das et al. (2019) obtained evidence that the Golgi, besides its function as a Mn2+ storage organelle under physiological conditions, has an additional trafficking role under subcytotoxic Mn2+ conditions. No direct measurement of compartmental Mn2+concentrations has been conducted in plants yet. However, a crucial role of the Golgi in Mn2+ detoxification has been inferred from the Mn2+-hypersensitive phenotype of the Golgi-localized Mn2+ transporter MTP11 (Peiter et al., 2007a). It would be highly interesting to develop genetically encoded Mn2+ indicators to monitor the subcellular Mn2+ distribution under different levels of Mn2+ supply. In analogy to GECIs, specificity to Mn2+ may be conferred by a Mn2+-specific binding domain, for example from the cyanobacterial Mn2+ sensor MntS (Yamaguchi et al., 2002). In combination with a more complete inventory of Golgi-localized Mn2+ transport proteins, this would help to elucidate poorly understood plant Golgi functions, such as different Mn2+ requirements of complex glycosylation processes, inter-organellar Mn2+ transit, Mn2+ storage and detoxification, and, in particular, Mn2+–Ca2+ interactions.
Import of Ca2+ and Mn2+
In animals and fungi, the import of Ca2+ and Mn2+ into the Golgi apparatus is mediated by pumps and transporters, whose cooperative function may ensure optimal supply. The P2A-type ATPases SERCA2 and SPCA1 and 2 are localized to the Golgi apparatus of animal cells with specific distribution across the cisternae (Wong et al., 2013; Pizzo et al., 2011). In addition, SPCA1 is localized to the TGN, where it was shown to determine vesicular transport of secretory proteins (Deng et al., 2018). Yeast owns a sole P2A pump, PMR1. Both PMR1 and SPCA1 have structural features that determine Ca2+ versus Mn2+ selectivity. SPCA1 contains an N-terminal Ca2+-binding EF-hand motif, which increases the relative turnover rate of Ca2+ (Chen et al., 2019). A similar mechanism to modulate substrate specificity is present in PMR1 (Wei et al., 1999), in addition to other factors based on sequence and structure that determine selectivity (Mandal et al., 2000, 2003). These Golgi-localized ATPases thus appear to be equipped with mechanisms to specifically modulate Mn2+ and Ca2+ transport, which may be important to provide the optimal cation concentrations for the processes described above. Apart from supplying the Golgi for glycosylation reactions, PMR1 is a Mn2+ tolerance factor by clearing cytosolic Mn2+, which is then secreted by exocytosis (Dürr et al., 1998).
Besides ATP-driven pumps, members of a recently identified transporter family, CaCA2, determine Ca2+ and Mn2+ homeostasis in the Golgi: TMEM165 in humans and GDT1 in yeast. Initially believed to be Ca2+/H+ antiporters that regulate Ca2+ and pH homeostasis (Demaegd et al., 2013), they were subsequently shown to transport also Mn2+ (Stribny et al., 2020). This renders them crucial for Golgi-localized Mn2+-dependent glycosylation processes (Potelle et al., 2016; Foulquier and Legrand, 2020). Interestingly, TMEM165 and GDT1 themselves are sensitive to Mn2+ and degraded upon elevated levels of cytosolic Mn2+ (Potelle et al., 2017; Dulary et al., 2018). An abolition of SPCA1-mediated Mn2+ loading of the Golgi thus entails the shut-down of the alternative TMEM165 pathway to supply the Golgi with Mn2+. This astonishing interaction might serve to protect the Golgi from Mn2+ overload that might interfere with Ca2+-dependent processes. The interaction of Ca2+ and Mn2+ transport by TMEM165 and GDT1 is not completely understood yet, and it was even suggested that the proteins may mediate a Ca2+/Mn2+ antiport (Dulary et al., 2018).
As compared to the Ca2+ and Mn2+ supply of animal and yeast Golgi, information on plants is even more rudimentary. One P2A-type ATPase, ECA3, is localized in the plant secretory pathway, and suggested to function as Ca2+/Mn2+ pump as shown in yeast complementation experiments. Two studies localized it to the Golgi and the PVC (Li et al., 2008; Mills et al., 2008). An involvement in Ca2+ and Mn2+ homeostasis is supported by phenotypes of eca3 mutants, which are hypersensitive to low Ca2+ or high Mn2+ supply. The protein was thus suggested to detoxify Mn2+ via loading it into vesicles followed by exocytosis, and to ensure Ca2+-dependent processes in the Golgi (Li et al., 2008). Potential regulation of this pump by Ca2+ or Mn2+ has yet to be studied.
Similar to ECA3, MTP11, a member of the CDF family, was localized to the Golgi (Peiter et al., 2007a) and the PVC (Delhaize et al., 2007). Its capacity to transport Mn2+ by Mn2+/H+ antiport was shown by direct transport assays (Delhaize et al., 2007; Peiter et al., 2007a). mtp11 knockout mutants are hypersensitive and MTP11 overexpressors hypertolerant to Mn2+ toxicity. Since knockout mutants accumulated more Mn2+, Peiter et al. (2007a) suggested a mechanism of Mn2+ detoxification by MTP11-mediated Mn2+ loading of vesicles followed by exocytosis. As an equivalent mechanism has been suggested for ECA3, it ought to be analyzed if both proteins operate in the same pathway or even interact. Interestingly, mtp11 mutants were hypertolerant to low Mn2+ supply, indicating that the supply of critical Mn2+-dependent processes was improved.
Orthologs of Arabidopsis MTP11 from poplar (PtdMTP11.1/2) and rice (OsMTP11) also complemented a Mn2+-sensitive yeast strain deleted in PMR1, and OsMTP11 rescued the Mn2+ sensitivity of an Arabidopsis mtp11 mutant, suggesting the functional conservation of this protein in different species (Peiter et al., 2007a; Farthing et al., 2017; Ma et al., 2018). This is supported by a growth inhibition of Osmtp11 knockdown mutants under Mn2+ toxicity, and an increased Mn2+ tolerance of OsMTP11 overexpressor lines (Ma et al., 2018). In a parallel study, higher Mn2+ sensitivity of Osmtp11 knockout lines was only apparent in the absence of OsMTP8.1, a vacuolar Mn2+ transporter (Tsunemitsu et al., 2018). As for Arabidopsis MTP11, localization of OsMTP11 is discrepant in different studies. It was found in the trans-Golgi (Farthing et al., 2017; Tsunemitsu et al., 2018) and the TGN, with a partial localization to the plasma membrane in N. benthamiana epidermal cells upon exposure to high extracellular Mn2+ concentration (Ma et al., 2018). Across all studies, the localization of all Golgi-bound Mn2+ transporters appears to vary and likely to depend on experimental conditions and/or cell types. A systemic analysis of this aspect is highly warranted.
Unlike in animals and fungi, a Ca2+ transporter accompanying the pump is not known yet. Also, the transport proteins identified so far have been associated with Mn2+ detoxification, whereas the Mn2+ supply of the complex Golgi-localized glycosylation processes is completely obscure. A role of ECA3 in glycosylation remains to be elucidated, and further transport proteins to support this function remain to be identified, whereby homologs of GDT1/TMEM165 represent promising candidates.
Release of Ca2+ and Mn2+
In animal cells, the release of Ca2+ from the Golgi is mediated by RyRs and IP3Rs (Kellokumpu, 2019). In plants, there is no evidence of channels that take on this function. Also, [Ca2+]Golgi in Arabidopsis is thought to be substantially lower than that in animals (Ordenes et al., 2012). It remains possible that this concentration is maintained by vesicular trafficking alone, but studies are required that monitor Ca2+ fluxes across vesicular membranes.
While a mechanism of Ca2+ release awaits discovery, a transporter that mediates the release of Mn2+ from the secretory pathway has recently been uncovered (Alejandro et al., 2017; Gao et al., 2018). The Arabidopsis NRAMP2 protein localizes to the TGN and rescues the low Mn2+-sensitive phenotype of the Δsmf2 yeast mutant, which is devoid of the yeast NRAMP transporter SMF2 that mediates intracellular Mn2+ distribution in yeast. Growth of nramp2 mutants was strikingly sensitive to Mn2+ deficiency (Alejandro et al., 2017; Gao et al., 2018), and in one study, expression of NRAMP2 was induced in both shoots and roots under this stress (Alejandro et al., 2017). Defects in Mn2+ supply of chloroplasts and vacuoles suggest that NRAMP2 redistributes Mn2+ to these organelles, as discussed above (Alejandro et al., 2017). Moreover, NRAMP2 has been suggested to promote the reutilization of root-Mn2+, as Mn2+ concentrations were found to be higher in roots and lower in young leaves under Mn2+ starvation (Gao et al., 2018). However, since in another study, nramp2 shoots accumulated more Mn2+ than those of the wild-type (Alejandro et al., 2017), this aspect demands further scrutiny.
Ca2+ and Mn2+ in the vacuole
Functions of Ca2+
Due to its tremendous size, the central vacuole represents the quantitatively most important organelle for storage and detoxification of metabolites and ions, including Ca2+ and Mn2+. Free Ca2+ in the vacuole is in the micro to millimolar range, but this parameter has been little studied. Owing to the steep electrochemical gradient of Ca2+ from the vacuole to the cytosol, the opening of cation channels in the tonoplast can rapidly increase [Ca2+]cyt and thus contribute to cytosolic Ca2+ signaling (Peiter, 2011). However, while extracellular Ca2+ chelators make it relatively straightforward to discriminate between the apoplast and intracellular stores as source of [Ca2+]cyt signals, it is less trivial to pinpoint the vacuole as intracellular source. The identification of the vacuolar channel protein TPC1, discussed below, eventually allowed to assign the vacuole a role in the generation of systemic Ca2+ signals in response to salt stress (Choi et al., 2014), herbivory and wounding (Kiep et al., 2015), and aphid feeding (Vincent et al., 2017).
Besides contributing to cytosolic Ca2+ signaling, luminal Ca2+ regulates vacuolar proteins either by direct binding or in an indirect way. The former is exemplified by TPC1 that has an inhibitory Ca2+-binding site on the luminal side, as discussed below (Dadacz-Narloch et al., 2011). Indirect regulation by luminal Ca2+ is assumed for a Na+ transporter that interacts with and is regulated by a vacuolar Calmodulin-like protein (Yamaguchi et al., 2005). However, the physiological relevance of this mechanism is unclear.
Release of vacuolar Ca2+ will first lead to a local increase in [Ca2+]cyt at the tonoplast. This may be perceived again either directly or indirectly. Vacuolar channels, for example, TPC1 (Peiter et al., 2005), two-pore potassium 1 (TPK1; Gobert et al., 2007), and TPK3 (Höhner et al., 2019; Jaslan et al., 2019) are activated by Ca2+-binding EF-hand domains. Indirectly, autoinhibited Ca2+-ATPases in the tonoplast are activated by binding Ca2+-calmodulin (see below). CBL proteins together with CBL-interacting protein kinases (CIPKs) form modules to transduce Ca2+ signal information into phosphorylation events. In Arabidopsis, four CBLs (CBL2, 3, 6, and 10) localize to the tonoplast (Batistic et al., 2010) and may thus specifically sense Ca2+ released from the vacuole.
Functions of Mn2+
Vacuoles play an important role in both detoxification and storage of Mn2+ in plant cells. Under Mn2+ excess, the sequestration of Mn2+ in vacuoles is a tolerance mechanism to avoid toxic effects in the cytosol. This mechanism is very obvious in Mn2+-(hyper)accumulating species that store vacuolar Mn2+ mainly in complex with carboxylates, such as malate, citrate, or oxalate (Dou et al., 2009; Fernando et al., 2010; Blamey et al., 2015).
Vacuolar Mn2+ sequestration is also activated under nutritional circumstances that lead to increased Mn2+ influx. Phosphate-efficient plants, such as lupins, mobilize phosphate by releasing large amounts of carboxylates into the rhizosphere (Lambers et al., 2015). This leads to the concurrent mobilization of Mn2+ that is massively taken up and deposited in leaf vacuoles (Peiter et al., 2000; Blamey et al., 2015). The transporter mediating this sequestration is unknown.
Iron deficiency is another nutritional imbalance that leads to critical Mn2+ influx. In Fe2+-limited dicotyledonous plants, Fe2+ is acquired by the poorly selective transporter IRT1. Mn2+ taken up by IRT1 has the potential to inhibit Fe(III) reduction mediated by ferric chelate reductase (FRO2), hence triggering iron chlorosis (Eroglu et al., 2016). In Arabidopsis, this problem is circumvented by sequestration of Mn2+ in root vacuoles by the vacuolar transporter MTP8 (Eroglu et al., 2016). Consequently, mtp8 mutants are prone to iron chlorosis upon iron limitation in the presence of Mn2+. Vacuolar Mn2+ accumulation by MTP8 also protects imbibing seeds from Mn2+ toxicity (Eroglu et al., 2017). In cold temperatures, seeds can undergo numerous wet-and-dry cycles that mobilize soil Mn2+ by alternating reductive and oxidative conditions. This Mn2+ load is critical if not sequestered in the vacuole (Eroglu et al., 2017).
Apart from detoxification, vacuolar Mn2+ serves as storage pool. Under Mn2+-limited conditions, vacuolar Mn2+ can thus be re-distributed to chloroplasts, which requires NRAMP3 and 4 (Lanquar et al., 2010; Alejandro et al., 2017). In the embryo of developing Arabidopsis seeds, Mn2+ is allocated specifically in the subepidermal cell layer on the abaxial side of the cotyledons and in the cortex of the hypocotyl, whereby MTP8 mediates the import into protein storage vacuoles of those cells (Chu et al., 2017; Eroglu et al., 2017; Figure 3). The physiological importance of this vacuolar Mn2+ storage pattern is reflected in poor germination of mtp8 mutant seeds derived from Mn2+-limited mother plants (Eroglu et al., 2017). In the absence of MTP8, Mn2+ is allocated to the prevascular bundles by VIT1 (Figure 3).
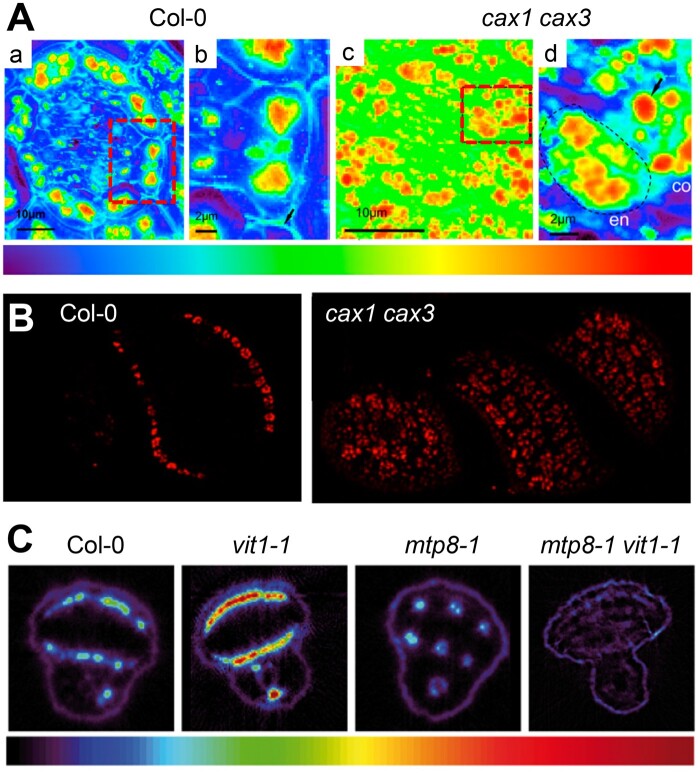
Figure 3.
Synchrotron µXRF analyses visualize the allocation of Ca2+ and Mn2+ in Arabidopsis seeds by vacuolar transporters. A, High-resolution SXRF maps of Ca2+ distribution in endodermal layers of the hypocotyl (a and c) and single endodermal cells (b and d; positions indicated by rectangles in a and c, respectively) in seeds from Col-0 and cax1cax3 plants. Colors indicate normalized fluorescence (logarithmic scale). Figure taken from Punshon et al. (2012), modified. B, High-resolution SXRF maps of Mn distribution in whole seeds of Col-0 and cax1cax3 plants. Figure taken from Punshon et al. (2012), modified. C, Distribution of Mn in intact seeds from Col-0, vit1-1, mtp8-1, and mtp8-1vit1-1 plants determined by µSXRF tomography. The color scale ranges from 0 to 1,100 µg g−1 Mn. Figure taken from Eroglu et al. (2017), modified.
Import of Ca2+ and Mn2+
Ca2+ and Mn2+ are loaded into the vacuole by secondary active transporters, which employ the proton motive force (pmf) across the tonoplast, and by ACAs, of which ACA11 resides in the membrane of lytic vacuoles (Lee et al., 2007) and ACA4 in smaller vacuolar vesicles of Arabidopsis (Geisler et al., 2000). Knockout of both pumps causes the formation of lesions due to hypersensitive response, which is dependent on salicylic acid signaling (Boursiac et al., 2010). This phenotype has recently been related to an aberrant [Ca2+]cyt regulation in aca4aca11 plants. In the double mutant, basal [Ca2+]cyt as well as flg22-induced Ca2+ signals in leaves were markedly increased (Hilleary et al., 2020). Both lesion formation and flg22-triggered Ca2+ signals were suppressed at elevated temperatures, which confirms the role of those tonoplast Ca2+ pumps as negative regulators of PAMP-triggered Ca2+ signals and innate immunity. In a separate study, the [Ca2+]cyt response of root tips to external ATP was only minimally higher in aca4aca11 as compared to the wild-type (Behera et al., 2018). The different outcomes point to a stimulus- or tissue-specific role of vacuolar ACAs. Transport of Mn2+ by those proteins has not been reported yet.
The molecular basis of Ca2+/H+ antiport across the tonoplast was uncovered with the identification of Arabidopsis CAX1 and CAX2 (Hirschi et al., 1996), which are regulated by an N-terminal autoinhibitory domain (Pittman et al., 2002). It has later become apparent that antiporters of the CAX family vary in substrate spectrum. CAX1 and CAX3 are selective for Ca2+, whereas CAX2 and CAX5 transport both Ca2+ and Mn2+ (Schaaf et al., 2002; Pittman and Hirschi, 2016).
Various physiological circumstances in which CAX proteins determine allocation and functions of both elements have been described. Overexpression of Arabidopsis CAX1 in tobacco increased Ca2+ accumulation while causing symptoms of Ca2+ deficiency and defects in stress responses involving Ca2+ signaling (Hirschi, 1999). In contrast, CAX1 knockout causes no strong growth phenotype, likely due to an ectopic upregulation of CAX3 in the mutants (Cheng et al., 2003). Hence, double knockout plants for both genes are severely perturbed in Ca2+ distribution and ionome, triggering a host of physiological aberrations (Cheng et al., 2005; Conn et al., 2011). In dicots, including Arabidopsis, most Ca2+ is accumulated in vacuoles of mesophyll cells (Storey and Leigh, 2004), in which CAX1 is most highly expressed. The cax1cax3 mutant accumulates less Ca2+ in mesophyll vacuoles and contains higher Ca2+ concentrations in the apoplast. This leads to a reduced cell wall extensibility going along with transcriptional alterations of genes involved in cell wall modifications, and to defects in stomatal opening that again causes decreased CO2 assimilation (Conn et al., 2011). In contrast to dicots, in graminaceous plants, CAX1 is mainly expressed in the epidermis (Conn and Gilliham, 2010), which again corresponds to the preferential accumulation of Ca2+ in this tissue (Karley et al., 2000).
The absence of CAX1 alone and in combination with CAX3 has a strong effect on the ionome, that is, the plant’s mineral element composition. Notably, Mn concentrations in shoots are reduced in cax1, while they are increased in cax1cax3, whereas Ca2+ and Mg2+ are decreased in the double mutant (Cheng et al., 2005). In addition, the deletion of CAX transporters reduces vacuolar H+-ATPase activity, which exerts numerous secondary effects.
CAX1 has been identified as a negative factor for adaptation to serpentine soils, which have a very high Mg2+:Ca2+ ratio, besides often high concentrations of heavy metals and a lack of nitrogen, phosphorus, and potassium (Bradshaw, 2005). Serpentine conditions are lethal to wild-type Arabidopsis, whereas cax1 mutants are resistant to very high Mg2+ concentrations and high Mg2+:Ca2+ ratio, what has been explained by an increased cytosolic Ca2+ availability caused by the decreased sequestration (Bradshaw, 2005; Cheng et al., 2005).
CAX1 and CAX3 are both expressed during seed development, and SXRF analyses showed that their deletion disrupted the allocation of Ca2+ to organelles of the embryo (Punshon et al., 2012; Figure 3). Accordingly, deletion of CAXs negatively affects seed germination (Connorton et al., 2012). Distribution of Mn2+ is also disturbed in cax1cax3 seeds (Punshon et al., 2012) although both transporters are selective for Ca2+ (Figure 3).
CAX1 and CAX3 are also co-expressed in guard cells and during stress responses, and both proteins physically interact, forming a transporter with altered kinetics (Zhao et al., 2009; Hocking et al., 2017). The functional relevance of CAX heteromers in stomatal regulation and in stress responses has been inferred from mutant analyses (Hocking et al., 2017).
CAX2 appears not to play a major role in Ca2+ homeostasis, but mediates vacuolar Mn2+ sequestration (Pittman et al., 2004). A three-amino acid region has been identified as instrumental for the Mn2+ specificity of this transporter (Shigaki et al., 2003). However, albeit its overexpression in tobacco caused an increased Mn2+ tolerance (Hirschi et al., 2000), sensitivity to Mn2+ was initially reported to be unaffected (Pittman et al., 2004), but later shown to be increased (Connorton et al., 2012) in a cax2 knockout mutant. This discrepancy indicates that this transporter is not generally important for Mn2+ detoxification. CAX5 also transports Ca2+ and Mn2+, but had a lower transport velocity for both elements than its close relative CAX2 and also differs in expression pattern, with only CAX5 being upregulated by elevated levels of Mn2+ (Edmond et al., 2009). Further work is required to examine a potential functional redundancy or co-operation of CAX2 and CAX5.
The vacuolar Ca2+ transporter CAX4 is transcriptionally upregulated by Mn2+ stress and Ca2+ depletion, and a cax4 knockout mutant is Mn2+-sensitive (Cheng et al., 2002; Mei et al., 2009), making it likely that CAX4 is involved in the tolerance to Mn2+ toxicity. In this respect, the resistance of cax1 mutants to Mn2+ toxicity and their altered Mn2+ accumulation may be the result of an induction of CAX4 expression (Cheng et al., 2003). In addition, auxin responses of roots were altered in the cax4 mutant (Mei et al., 2009). Mechanistic explanation of this phenotype may lie in the involvement of Ca2+ homeostasis in auxin signaling (Dindas et al., 2018) or in the role of Mn2+ in auxin conjugation (see above). Auxin responses were also affected in cax1 and cax3 mutants, causing a defective regulation of stomatal guard cells (Cho et al., 2012). This was explained by an increased apoplastic pH in the mutants, leading to a decrease in polar auxin transport. It remains to be shown if the auxin-related phenotypes of cax1/3 and cax4 are mechanistically related.
Orthologs of Arabidopsis CAXs have been described to reside in the vacuole of other species, such as VCAX1 in mung bean (Ueoka-Nakanishi et al., 2000) or VvCAX3 in grapevine (Martins et al., 2017). Albeit closely related to Arabidopsis CAX1/3, the latter also appears to transport Mn2+. Upon heterologous expression in yeast, OsCAX1a/b/c, 3, and 4 from rice confer Ca2+ tolerance, and OsCAX1a, 3, and 4 also Mn2+ tolerance, indicating that their substrate spectrum does not closely reflect that of their closest homologs in Arabidopsis (Kamiya et al., 2005; Yamada et al., 2014). OsCAX1a is expressed most highly and further induced by high Ca2+ levels (Kamiya et al., 2006), which suggests that it plays a crucial role in Ca2+ homeostasis similar to CAX1, its closest homolog in Arabidopsis. However, further studies using multiple mutants are required to dissect the relevance of CAXs in Mn2+ and Ca2+ homeostasis of rice.
Examination of homologs of Arabidopsis CAXs in other species revealed a phylogenetic diversity in their regulation. HvCAX2 of barley is apparently not regulated by an N-terminal domain, whereas in LeCAX2 of tomato the N-terminus only affects Mn2+ transport (Edmond et al., 2009). The structural basis as well as physiological consequences of those differences are still unknown.
To date, transporters of the CDF family have been described to mediate most physiological roles of vacuolar Mn2+ sequestration. As described above, in Arabidopsis, expression of MTP8 is highly induced by Fe deficiency, but also by high Mn2+ levels (Eroglu et al., 2016), and this transporter additionally builds vacuolar Mn2+ pools in specific embryo tissues (Chu et al., 2017; Eroglu et al., 2017). Similar to Arabidopsis, the rice transporters OsMTP8.1 and OsMTP8.2 mediate Mn2+ tolerance through sequestration of Mn2+ into vacuoles (Chen et al., 2013; Takemoto et al., 2017). The founding member of the Mn2+-transporting CDF subfamily, Stylosanthes hamata MTP8, transports Mn2+ upon expression in yeast. Its overexpression in Arabidopsis conferred Mn2+ tolerance (Delhaize et al., 2003), but its function in S. hamata has yet to be confirmed.
Proteins of the VIT family, such as Arabidopsis VIT1, have also been described as vacuolar Mn2+ transporters, albeit the primary function of VIT1 is to move Fe2+ into vacuoles of developing embryos (Kim et al., 2006). Similarly, OsVIT1 and OsVIT2 are vacuolar Mn2+ transporters, but their likely main function in rice is to mediate the vacuolar sequestration of Fe2+ and Zn2+ in flag leaves for future transport to the seeds (Zhang et al., 2012b). In wheat, ectopic expression of the ortholog TaVIT2 in the endosperm caused a higher accumulation of Mn2+ and Fe2+, which was suggested as biofortification strategy (Connorton et al., 2017). In general, Mn2+ appears to be a secondary substrate of VIT proteins.
Release of Ca2+ and Mn2+
Our mechanistic understanding of vacuolar Ca2+ release is still rudimentary. In fungi, this is mediated by proteins of the transient receptor potential (TRP) family (Lange et al., 2016), which are absent in plants. Flux analyses and patch-clamp studies on vacuolar membranes of various plant species have demonstrated the presence of Ca2+ conductances activated by IP3 or cADPR (see Peiter, 2011 for review). In animals, these ligands activate IP3Rs and RyRs, respectively, as discussed above for the ER and the Golgi. As plants lack those protein families (Edel et al., 2017), the mode of action of those molecules is still unclear. Their effectiveness on isolated vacuolar membranes suggests a direct action on channels or associated proteins, which is supported by the binding of IP3 to the vacuolar membrane with high-affinity (Brosnan and Sanders, 1993). However, later it was shown that IP6 is a far more effective agonist of Ca2+ release, and that IP3 is rapidly converted to IP6 (Lemtiri-Chlieh et al., 2003). We are still awaiting the breakthrough that solves the enigma of IPx-mediated Ca2+ signaling in plants.
The slow vacuolar (SV) channel, encoded by TPC1, has emerged as one of the regulators of [Ca2+]cyt. Identified electrophysiologically in the 1980s (Hedrich et al., 1986), it represents the longest-known and most extensively scrutinized ion channel in plants (Hedrich and Marten, 2011; Hedrich et al., 2018; Pottosin and Dobrovinskaya, 2018). Its molecular identity was revealed 20 years later (Peiter et al., 2005). Studies of SV currents have unearthed a plethora of factors that regulate this channel in vitro, including membrane potential, redox potential, phosphorylation, pH, Ca2+, Mg2+, polyamines, and 14-3-3 proteins (Peiter, 2011). These factors do not act independently on the channel, and some have opposite effects on either side of the membrane, which adds further complexity. Albeit tempting, an amalgamation of these studies is difficult as they have been conducted on different species and tissues and with different experimental set-ups.
The TPC1 protein, which functions as dimer, contains two Shaker-like structures, each containing six transmembrane spans and a pore domain, fused by a cytosolic linker. The structure of Arabidopsis TPC1 has been solved in independent labs with high resolution (Guo et al., 2016; Kintzer and Stroud, 2016), which allows to elucidate the molecular basis of its electrophysiological characteristics (Kintzer and Stroud, 2018). In animals, TPC channels are the molecular basis of NAADP-activated Ca2+ release from lysosomes and endosomes (see Jin et al., 2020 for review), while other studies found them to be Na+-selective and activated by the phosphoinositide PI(3,5)P2 (Wang et al., 2012). Intriguingly, recent research indicated that, depending on the ligand, animal TPC channels show different selectivities toward Ca2+ or Na+ (reviewed in Gerndt et al., 2020). Arabidopsis TPC1 is neither sensitive to NAADP nor to PI(3,5)P2 (Boccaccio et al., 2014).
It is now commonly accepted that TPC1 resides exclusively in the vacuolar membrane of mono- and dicotyledonous plants (Peiter et al., 2005; Larisch et al., 2012; Dadacz-Narloch et al., 2013). It conducts mono- and divalent cations, including Ca2+. Nevertheless, it is still controversially debated if TPC1 is able to function as a bona fide Ca2+ release channel (Hedrich et al., 2018). It has been discovered early that TPC1 currents are activated by increases in [Ca2+]cyt (Hedrich and Neher, 1987), which is owing to the presence of two EF-hands in the cytosolic linker, of which only EF2 appears to be involved in Ca2+ activation (Schulze et al., 2011). This feature would allow TPC1 to function as CICR channel (Ward and Schroeder, 1994; Bewell et al., 1999). Patch-clamp measurements of ion currents combined with [Ca2+] determination by a fluorescent reporter directly demonstrated the dependence of Ca2+ fluxes across the vacuolar membrane on TPC1 (Gradogna et al., 2009; Carpaneto and Gradogna, 2018). However, unphysiologically positive voltages required to activate TPC1 and its inhibition by luminal Ca2+ have raised doubts about its role in Ca2+ release (Rienmüller et al., 2010). The structural basis of this inhibition by luminal Ca2+ lies in a Ca2+-binding domain on the luminal side (Dadacz-Narloch et al., 2011). Defunctionalization of this domain in the fou2 mutant results in a hyperactive TPC1 that leads to constitutive JA signaling (Bonaventure et al., 2007; Beyhl et al., 2009), which is thought to be caused by an altered tonoplast potential (Lenglet et al., 2017).
It was recently shown that TPC1, together with TPK1 and TPK3, is a prerequisite for vacuolar excitability (Jaslan et al., 2019). Mutant analyses have also pointed to roles of TPC1 in stomatal regulation and seed germination (Peiter et al., 2005). Two studies have shown that TPC1 is a target of selection on serpentine soils, which have a very low Ca2+ versus Mg2+ availability (Turner et al., 2010; Arnold et al., 2016). Arabidopsis arenosa and A. lyrata populations adapted to those extreme conditions have a severely altered ionome with a very high Ca2+/Mg2+ ratio, which coincides with alterations in several genes associated with ion transport and homeostasis (Arnold et al., 2016). These comprise a non-synonymous mutation in the pore of TPC1, which may provide a mechanistic basis of the altered Ca2+ and Mg2+ handling or of changes in Ca2+ signaling in serpentine-adapted plants.
Attempts to assign a role in Ca2+ signal generation to this channel had initially failed (Ranf et al., 2008). This changed when the propagation of systemic [Ca2+]cyt signals evoked by salt stress, wounding, and aphid feeding was demonstrated to require TPC1. In a tpc1 mutant, a NaCl-triggered Ca2+ wave and local-systemic Ca2+ signals caused by aphids are dampened (Choi et al., 2014; Vincent et al., 2017), while a wounding-induced Ca2+ wave is even abolished (Kiep et al., 2015). A recent study suggested that [Ca2+]cyt homeostasis in systemic signaling is related to the TPC1/TPK1/TPK3-dependent excitability of the vacuolar membrane (Dindas et al., 2021). In this model, which deserves further scrutiny, TPC1 as part of a Ca2+-homeostat plays an indirect role in [Ca2+]cyt control by determining H+/Ca2+ antiport across the vacuolar membrane. The specific involvement of TPC1 in systemic [Ca2+]cyt signal generation provokes the question of which factors regulate its activity in vivo.
Recently, a member of the GLR family, GLR3.6, was suggested to be localized to vacuolar membranes in xylem contact cells (Nguyen et al., 2018). Mutation of this channel together with that of GLR3.3 (see above) attenuated or abolished wounding-induced membrane potential changes and Ca2+ waves (Mousavi et al., 2013; Vincent et al., 2017; Nguyen et al., 2018; Shao et al., 2020). Ca2+ permeability of GLR3.6 has been demonstrated by expression in HEK293T cells (Shao et al., 2020). Similar to GLR3.3 and 3.1, most evidence points to a function of GLR3.6 in the plasma membrane (Mou et al., 2020). The subcellular localization of those channels thus requires further scrutiny.
A further potential mechanism of vacuolar Ca2+ release may rely on annexins, that have been localized to the tonoplast besides the plasma membrane (Seals et al., 1994) and shown to permeate Ca2+ in lipid bilayer experiments (Laohavisit et al., 2012). Annexin-mediated Ca2+ currents are activated by reactive oxygen species (ROS), which would allow them to contribute to a ROS wave-driven Ca2+ wave (Richards et al., 2014), and, accordingly, Annexin1 is required for the propagation of systemic Ca2+ signals (Malabarba et al., 2021). The contribution of annexins to the generation of [Ca2+]cyt signals demands further attention.
It has not been studied yet if the potential vacuolar Ca2+ release channels also conduct Mn2+. For TPC1, this is highly expected, since it is permeable to Mg2+ in a hydrated state (Pottosin and Dobrovinskaya, 2018), but this awaits to be studied. Transporters that move vacuolar Mn2+ into the cytosol have been identified. ZIP1 of the ZRT family is a transporter localized in root vacuoles and likely involved in remobilizing Mn2+, but its function in cellular Mn2+ homeostasis is still unknown (Milner et al., 2013). Proteins of the NRAMP family, Arabidopsis NRAMP3 and 4, as well as Thlaspi caerulescens TcNRAMP3 and 4, also remobilize vacuolar Mn2+ (Thomine et al., 2003; Oomen et al., 2009). Mutation of NRAMP3 and 4 renders the plant hypersensitive to Mn2+ deficiency, which is associated with a decrease in chloroplastic Mn2+ and reduced photosynthetic activity (Lanquar et al., 2010). This indicates an important role of these vacuolar Mn2+ efflux transporters for Mn2+ loading into the chloroplasts. A mechanistic explanation of the distribution of released vacuolar Mn2+ remains to be established, and possibly involves vesicular trafficking, as discussed above.
Ca2+ and Mn2+ in the chloroplast
Functions of Ca2+
The chloroplast is the site of photosynthesis and many biosynthetic pathways, and it accumulates high concentrations of Ca2+ and Mn2+ (Finazzi et al., 2015). Both cations play important roles in chloroplast structure and functions. One Ca and four Mn atoms are present in the Mn4CaO5 cluster in the OEC of PSII, which oxidizes H2O to provide electrons needed in the photosynthetic electron transport chain and protons to generate ATP (Umena et al., 2011). While Mn catalyzes the oxidation of H2O, the Ca2+ modulates the redox potential of the OEC to allow Mn to transfer electrons away from the O2 (Tsui et al., 2013). A redox-active tyrosine is oxidized and reduced in a proton-coupled electron transfer reaction, which is dependent on Ca2+ (Guo et al., 2018). Furthermore, Ca2+ is capable of binding to PsbO, an extrinsic protein of the PSII complex (Murray and Barber, 2006).
Ca2+ regulates many proteins involved in photosynthetic reactions, organelle division, and the chloroplastic protein import machinery (Rocha and Vothknecht, 2012; Stael et al., 2012; Hochmal et al., 2015). The ion may be an important regulator of several key enzymes of the photosynthetic light-independent reaction, the Calvin–Benson–Bassham cycle, including fructose 1,6-bisphosphatase and sedoheptulose 1,7-bisphosphatase (Charles and Halliwell, 1980; Kreimer et al., 1988). Furthermore, the import of nuclear-encoded proteins via the translocon at the inner envelope membrane of chloroplasts (TIC) complexes may be under the regulation of Ca2+. Calmodulin directly binds to the stromal side of TIC32, whereas Ca2+ itself has a strong effect on TIC110 channel activity in vitro (Chigri et al., 2006; Balsera et al., 2009). An important target of Ca2+ in the chloroplast is the Ca2+-sensing receptor (CAS), a thylakoid membrane-associated Ca2+-binding protein that has been identified as a central regulator of cellular Ca2+ homeostasis and signaling. CAS modulates chloroplast stromal Ca2+ signals induced by darkness, flg22, and heat, and, surprisingly, it governs external-Ca2+-induced Ca2+ transients in the cytosol (Nomura et al., 2008; Weinl et al., 2008). The protein regulates photosynthetic efficiency, modulates MAPK signaling, and participates in plant stress responses, where it plays a role in stomatal regulation and innate immunity (Vainonen et al., 2008; Weinl et al., 2008; Nomura et al., 2012). The mechanistic basis of CAS action is poorly understood. Finally, the vacuolar Ca2+-activated K+ channel TPK3 (see above), has also been described to localize to thylakoid membranes and to be vital for the generation of the pmf (Carraretto et al., 2013). However, both localization of TPK3 in chloroplasts and its involvement in photosynthetic processes have recently been questioned (Höhner et al., 2019). The reason for the diametrically opposed findings in those studies remains to be determined.
The regulatory role of Ca2+ in the chloroplast implies dynamic fluctuations of free Ca2+ in its subcompartments. Changes in [Ca2+]stroma with different kinetics are indeed induced by numerous biotic (flg22, chitin, cryptogein, and oligogalacturonides) and abiotic (osmotic, heat, cold, oxidative, and salt) stresses as well as environmental stimuli (light-to-dark transition; Navazio et al., 2020). [Ca2+]stroma signals in response to stress occur in chloroplasts and nongreen plastids, whereas the response to light-dark transition is chloroplast-specific, suggesting a potential role of the thylakoids in its generation (Sello et al., 2016). Imaging of individual chloroplasts revealed the occurrence of [Ca2+]stroma spiking to blue pulsed light, in addition to the previously described sustained transient (Loro et al., 2016). The [Ca2+]stroma transient at the onset of darkness is hypothesized to initiate an alteration of metabolic processes, as recently shown for ppGpp synthesis (Ono et al., 2020). It is not accompanied by a decrease in [Ca2+]cyt, which suggests that it is generated by Ca2+ release from an intrachloroplastic store (Sai and Johnson, 2002). This store might be the thylakoid lumen, in which free Ca2+ is 3–5 times higher than in the stroma (Sello et al., 2018). However, [Ca2+]thylakoid does not decrease reciprocally to the transient rise in [Ca2+]stroma, but rather shows a steady increase (Sello et al., 2018). Alternatively, Ca2+ may be released from negatively charged thylakoid membranes or Ca2+-binding proteins, to which it can be reversibly bound.
Intriguingly, the dark-induced [Ca2+]stroma transient has recently been demonstrated to be accompanied by a transient increase in [Ca2+]cyt. Both the [Ca2+]cyt and the [Ca2+]stroma response are gated by the circadian clock and reach maximum levels at anticipated dusk (Ruiz et al., 2020). These findings suggest that the circadian clock determines chloroplast function by gating [Ca2+]stroma signals. It furthermore opens the question if both [Ca2+] elevations are mechanistically linked, for example, by dark-induced Ca2+ release from the chloroplast.
Recently, elevated temperatures ˃30°C were shown to cause a [Ca2+]stroma elevation dependent on absolute temperature, rather than the rate of heating (Lenzoni and Knight, 2019). This Ca2+ response involves the CAS protein. It is specific to the chloroplast and is thereby clearly distinguished from a Ca2+ signal elicited by stronger heat, which occurs in the cytosol by influx of apoplastic Ca2+ (Finka et al., 2012).
Taken together, Ca2+ dynamics in the chloroplast are initiated by different stimuli. They likely have many regulatory roles in the organelle and influence the entire cellular signaling network. However, our mechanistic understanding of the physiological consequences of plastidial Ca2+ signals is still very incomplete.
Functions of Mn2+
As the catalyst in the OEC of PSII, Mn2+ takes center stage in photosynthesis. Therefore, Mn2+ deficiency strongly affects this process, leading to reduced Mn2+ binding to PSII, which causes the destabilization of its super and subcomplexes (Schmidt et al., 2016; 2020). PsbP is one of the extrinsic proteins and has two Mn2+-binding sites, suggesting PsbP may function as a Mn2+ carrier that facilitates the access of Mn2+ ions to the OEC (Cao et al., 2015). Moreover, the phosphatase TAP38/PPH1, which specifically targets light-harvesting complex II, contains a Mn2+ (or Mg2+) binuclear center, which unravels a crucial step in state transitions between PSI and PSII (Wei et al., 2015).
In addition to photosynthesis, Mn2+ is required by enzymes in biosynthetic pathways. This includes imidazoleglycerol-phosphate dehydratase involved in histidine biosynthesis (Bisson et al., 2015), as well as 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase, which is the first enzyme of the Shikimate pathway for the production of chorismate, the precursor of aromatic amino acids and secondary metabolites (Entus et al., 2002). Finally, the catalytic activity of glutamine synthetase is regulated by Mn2+ and Mg2+ (Eisenberg et al., 2000).
Several other enzymes, including phytoene synthetase, mevalonic kinase, and ent-kaurene synthetase in the isoprenoid biosynthetic pathway that produces building blocks of carotenoids, chlorophyll, and gibberellins, require Mn2+ as a cofactor (Dogbo et al., 1988; Rohdich et al., 2006). The decrease in the production of pigments and gibberellins under Mn2+ deficiency has been assigned to this function (Wilkinson and Ohki, 1988), what should be followed-up. Intriguingly, it is hypothesized that Mn2+ and Mg2+ function as toggle switch for some chloroplastic enzymes, including Rubisco, malic enzyme, and phosphoglycolate phosphatase, to improve the energy balance between photorespiration and other metabolic processes (Bloom and Lancaster, 2018).
Import and allocation of Ca2+ and Mn2+
Three types of membranes (an inner and outer envelope and a thylakoid membrane) divide the chloroplast into three distinct internal compartments, consisting of the intermembrane space between the two chloroplast envelope membranes, the stroma, and the thylakoid lumen. To transport ions and metabolites through these three membranes, the chloroplast harbors transport proteins in each of them (Carraretto et al., 2016; Schmidt et al., 2020).
Early biochemical studies demonstrated that Ca2+ uptake from the cytosol into the chloroplast is a light-dependent process (e.g. Muto et al., 1982), and that Ca2+ is imported into the thylakoid lumen by Ca2+/H+ antiport (Ettinger et al., 1999). Some candidate proteins localized to the chloroplast of Arabidopsis were proposed to mediate Ca2+ flux across the chloroplast inner envelope. This includes a heavy-metal ATPase (Moreno et al., 2008) and the GLR channels GLR3.4 and GLR3.5 (Teardo et al., 2011; 2015), with additional biochemical evidence supporting GLR activity in chloroplast inner envelope of spinach (Teardo et al., 2010). However, none of these were demonstrated to alter [Ca2+] or mediate Ca2+ transport in intact chloroplasts, and GLR3.4 and GLR3.5 have also been reported to be localized in the plasma membrane (Meyerhoff et al., 2005; Teardo et al., 2011; Kong et al., 2016).
This situation has changed recently with the identification of chloroplast-targeted homologs of yeast GDT1 that contributes to Ca2+/Mn2+ homeostasis in the Golgi of yeast, as described above. These transporters were described independently by several groups as representing long-sought Mn2+ and Ca2+ pathways across chloroplast membranes and tagged with an array of nonsystematic names that may lead to confusion. Owing to its phenotype, a thylakoid-localized transporter was first described as photosynthesis-affected mutant71 (PAM71), with a second member named PAM71-homolog (PAM71-HL; Schneider et al., 2016). For its presumed transport function and localization, this protein was also named Chloroplast-localized Ca2+/H+ Antiporter 1 (CCHA1; Wang et al., 2016). Later, PAM-71-HL was renamed chloroplast Mn2+ transporter 1 (CMT1) based on one of its substrates and its localization (Eisenhut et al., 2018; Zhang et al., 2018). This situation was unsatisfactory because proteins of this family are neither all likely to be localized to chloroplasts, nor are they selective transporters for Ca2+ or Mn2+. In our analysis of those proteins, we, therefore, renamed them bivalent cation transporter1 (BICAT1) and BICAT2, based on the substrate spectrum of all known eukaryotic homologs (Frank et al., 2019). This nomenclature has found wide acceptance in the community (Stael, 2019; Navazio et al., 2020; Ruiz et al., 2020; Schmidt et al., 2020; Pirayesh et al., 2021).
BICAT1 is localized to the thylakoid, while BICAT2 resides in the inner envelope of the chloroplast (Schneider et al., 2016; Wang et al., 2016; Eisenhut et al., 2018; Zhang et al., 2018; Frank et al., 2019). The complementation of yeast mutants defective in Mn2+ and Ca2+ transport indicated the permeation of both cations by those proteins. Interestingly, two studies employing different mutants indicated that BICAT2 mediates Mn2+ influx into or Mn2+ efflux from the cytosol (Eisenhut et al., 2018; Zhang et al., 2018). Transport of Ca2+ by BICAT1 and BICAT2 was confirmed by transport assays employing Escherichia coli or yeast vesicles (Frank et al., 2019). In agreement with an activity in Ca2+ transport, mutants devoid of BICAT1 or BICAT2 were defective in 45Ca2+ uptake by illuminated isolated thylakoids and chloroplasts, respectively (Frank et al., 2019). Expectedly, the impeded Ca2+ movement impinges on Ca2+ homeostasis in the stroma, as detected by a stroma-targeted aequorin reporter: the [Ca2+]stroma transient evoked by light-dark transition was almost completely abolished in bicat2 mutants, but increased in bicat1 mutants (Frank et al., 2019). Based on the presumed Ca2+ fluxes that contribute to this Ca2+ signal, it is conceivable that BICAT2 imports cytosolic Ca2+ during the light phase. Imported Ca2+ may be bound, as described above, and released to the stroma upon darkness. Stromal Ca2+ may subsequently be translocated by BICAT1 into thylakoids or released into the cytosol. This sequence is supported by the Ca2+ dynamics in cytosol and lumen (Sello et al., 2018; Ruiz et al., 2020) in combination with the effects of BICAT knockout (Frank et al., 2019). The kinetics of dark-induced [Ca2+]stroma changes in a bicat1bicat2 double mutant are indistinguishable from those of bicat2 single mutants, as expected from a model where bicat2 acts upstream of bicat1 (Frank et al., 2019). The regulation of chloroplast Ca2+ homeostasis by BICAT proteins is bound to affect the many Ca2+-regulated processes described above, what remains to be studied. This is, however, complicated by the other essential function of those transporters, the transport of Mn2+ into the chloroplast and the lumen.
Mutants of bicat2 are defective in Mn2+ accumulation in chloroplasts, and Mn2+ partitioning is altered in bicat1 (Schneider et al., 2016; Eisenhut et al., 2018; Zhang et al., 2018). Consequently, Mn2+ binding to PSII complexes is reduced and the mutants are defective in PS II activity, which is most severe in bicat2. In bicat1, but not in bicat2, PSII activity was restored by supplementation of Mn2+ (Schneider et al., 2016; Eisenhut et al., 2018).
Phenotypically, bicat2 mutants display severe defects in growth, chloroplast morphology, and photosynthetic activity, while bicat1 mutants are less affected, and the bicat1bicat2 phenotype is similar to that of bicat2. This highlights again the dominant role of BICAT2 in chloroplast structure and functions (Schneider et al., 2016; Eisenhut et al., 2018; Zhang et al., 2018; Frank et al., 2019). An ortholog of BICAT1 in Zea mays ssp. mexicana L., ZmmCCHA1, was recently described to be functionally comparable to its Arabidopsis counterpart, which indicates the conservation of these mechanisms in monocots (Wang et al., 2020).
Further investigations are required to unravel the transport mode and selectivity of chloroplast BICATs that determine their dual function. Their parallel interference in Ca2+ and Mn2+ homeostasis necessitates discriminative mechanisms and complicates the analysis of the role of those transporters in planta.
With the recent description of a chloroplast-localized mitochondrial Ca2+ uniporter (cMCU), a Ca2+ channel localized in the chloroplast inner envelope as well as in root mitochondria was identified (Teardo et al., 2019). By mediating the flux of Ca2+ into the chloroplast, it contributes to the elevation of [Ca2+]stroma in response to oxidative and osmotic stress. In accordance with a role in osmotic (drought) stress responses, signaling events downstream of the Ca2+ signal were altered, and mutants were drought-tolerant (Teardo et al., 2019). This breakthrough provokes a host of new questions. Why does it only contribute to [Ca2+]stroma elevations to some specific signals? What is its functional cooperation with BICAT2 in the same membrane? Owing to different affinities, they may operate at different ion concentrations (Frank et al., 2019; Stael, 2019). Finally, since mammalian MCU proteins are permeable to Mn2+ (Kamer et al., 2018), as discussed for mitochondria below, a further role of cMCU may lie in Mn2+ supply or release of chloroplasts, what remains to be studied.
Ca2+ and Mn2+ in the mitochondrion
Functions of Ca2+
Compared to chloroplasts, the role of Ca2+ in plant mitochondria has been less studied. Excellent reviews provide an overview of the subject (Stael et al., 2012; Wagner et al., 2016; Costa et al., 2018; Pirayesh et al., 2021). In animals, free mitochondrial Ca2+, [Ca2+]mito, is a central regulator of aerobic metabolism (Szabadkai and Duchen, 2008). This regulatory function is based on the parallel activation of Ca2+-dependent enzymes of the tricarboxylic acid (TCA) cycle, that is, pyruvate dehydrogenase (PDH), NAD+-isocitrate dehydrogenase (NAD-ICDH), and 2-oxoglutarate dehydrogenase (OGDH; McCormack et al., 1990). Regulation by Ca2+ is pursued by direct binding (NAD-ICDH, OGDH) or Ca2+-dependent dephosphorylation (PDH; Giacomello et al., 2007). The ATP synthase is also Ca2+-activated. Collectively, this regulates the production of NAD(P)H+ and ATP synthesis. Such a role of [Ca2+]mito is less clear in plants.
A particular role of [Ca2+]mito in animals is to trigger apoptotic cell death. Thereby the mitochondrial permeability transition pore, a membrane-spanning multi-protein complex, that likely originates from the F-ATP synthase, is activated by mitochondrial Ca2+ overaccumulation, causing membrane rupture and the release of apoptotic signals, such as cytochrome c (Giacomello et al., 2007; Carraro et al., 2019). This sequence of events is also found in plant mitochondria (Arpagaus et al., 2002; De Col et al., 2018), indicating a high degree of conservation.
A regulation by [Ca2+]mito is to be expected for mitochondrial proteins that carry a Ca2+-binding EF hand. In plants, this includes NAD(H)-dependent glutamate dehydrogenase2 (GDH2), but not GDH1 (Turano et al., 1997). Both isoforms form homo- and hetero-hexamers in all combinations, hence assembling seven different isozymes with different total numbers of EF-hands. This may produce a spectrum of Ca2+ dependencies, which has indeed been observed in vitro in older studies (Loulakakis and Roubelakis-Angelakis, 1990). In plants, GDH operates primarily in the catabolic direction to release NH4+ and 2-oxoglutarate from glutamate. Intriguingly, Ca2+ appears to stimulate GDH only in the opposite, aminating direction, required to detoxify high amounts of NH3, which may occur in mitochondria during photorespiration or uptake of NH4+ from the soil (Tercé-Laforgue et al., 2004). [Ca2+]mito may thus play a decisive role in GDH activation and fine-tuning, what remains to be demonstrated in vivo.
There are still numerous loose ends regarding the action of Ca2+ in plant mitochondria. Pharmacological studies indicated that the import of nuclear-encoded proteins into plant mitochondria across the inner membrane is Ca2+- and CaM-regulated (Kuhn et al., 2009). As yeast mitochondria are unaffected by the pharmacological treatments, this trait is suspected to be plant-specific, whereby the mechanism of action is unclear. Possibly related to this, a calmodulin-like protein, CML30, has been localized to mitochondria, but its role has not been revealed yet (Chigri et al., 2012).
The concentration of free Ca2+ in plant mitochondria is about twice than that in the cytosol and responsive to stimuli (Logan and Knight, 2003). The dynamics of [Ca2+]mito, that regulate the mitochondrial Ca2+-dependent processes, are linked to those in the cytosol. Generally, [Ca2+]mito follows [Ca2+]cyt elevations, but with specific kinetics (Loro et al., 2012). Mitochondria may thus act as sinks of cytosolic Ca2+ and in turn shape the spatiotemporal signature of cytosolic Ca2+ signals. This property has been demonstrated in animal cells (e.g. in cardiomyocytes, Drago et al., 2012), whereas in plants it remains to be shown.
Functions of Mn2+
An important function of Mn2+ in mitochondria is as part of the active site of MnSOD, which removes superoxide anion radicals generated as a side-product of energy metabolism (Andresen et al., 2018). Decrease in MnSOD activity inhibits the TCA flux and affects the mitochondrial redox balance, causing a decrease in plant growth (Morgan et al., 2008). Although mitochondrial MnSOD function is reduced by Mn deficiency in Chlamydomonas (Allen et al., 2007), MnSOD activity in plants, as Arabidopsis, is unaffected under low Mn2+ supply (Lanquar et al., 2010; Alejandro et al., 2017). This indicates that organelles are prioritized differently in both organisms.
Arginase is another Mn2+-containing metalloenzyme in plant mitochondria, whose function is to hydrolyze arginine to ornithine and urea, which is recycled by urease to ammonia (Cao et al., 2010). Arabidopsis has two mitochondrial arginases, ARGAH1 and ARGAH2 (Flores et al., 2008), of which ARGAH2 was also localized in chloroplasts (Patel et al., 2017). A coordinated action of arginase and urease remobilizes nitrogen to meet the metabolic demands of developing seedlings (Winter et al., 2015). Single and double mutants show an increased nitric oxide (NO) accumulation, going along with altered auxin responses in roots (Flores et al., 2008) and increased abiotic stress resistance (Shi et al., 2013). NO production in plant mitochondria may be mediated by NO synthase (NOS)-like enzymes that convert arginine into NO and citrulline (Winter et al., 2015), besides a nitrate-dependent pathway (Planchet et al., 2005). As arginine is also a precursor of polyamines, arginase mutants have increased levels of putrescine and spermine (Shi et al., 2013). Polyamines may also be precursors of NO biosynthesis (Tun et al., 2006). Hence, Mn2+-containing arginases play distinctive roles in the crosstalk between arginine metabolism and the accumulation of polyamines and NO, resulting in a range of responses to abiotic stresses.
In animal cells, the main enzymes capable of generating reducing power (i.e. NADH) in mitochondria are the NAD-dependent malic enzymes (NAD-MEs), which catalyze the oxidative decarboxylation of malate to yield pyruvate and CO2, and the NAD-ICDHs that catalyze the oxidative decarboxylation of isocitrate to 2-oxoglutarate. Both require Mn2+ or Mg2+ for the catalysis (Yang et al., 2002; Ma et al., 2017). Intriguingly, NAD-ICDH is also activated by Ca2+, as mentioned above. Arabidopsis contains two genes encoding catalytic ICDH subunits and three genes encoding regulatory subunits (Lemaitre et al., 2007), as well as two NAD-ME proteins that form homodimers or a heterodimer with distinct functional characteristics (Tronconi et al., 2010). Their activity has been analyzed in vitro in the presence of Mn2+ (Lemaitre et al., 2007; Tronconi et al., 2010) but the importance of this metal and an interference with Ca2+ in the regulation of their activity in vivo requires further investigation.
Import of Ca2+ and Mn2+
Due to the high membrane potential of around −180 mV across the inner mitochondrial membrane (IMM), which is due to the activity of the electron transport chain, Ca2+ would accumulate to 100–200 mM in the mitochondrial matrix if it was in Nernstian equilibrium. This would of course be detrimental. In mammalian mitochondria, Ca2+ accumulates to only 1–10 µM in the matrix, implying the operation of regulated influx pathways and efflux mechanisms (Szabadkai and Duchen, 2008). The outer mitochondrial membrane (OMM) is mostly regarded as unselectively permeable, not restricting Ca2+ import (Wagner et al., 2016). Conversely, Ca2+ influx across the inner membrane of animal mitochondria is mediated by a Ca2+-selective channel, the MCU (Kirichok et al., 2004). The channel protein responsible for those Ca2+ currents contains only two transmembrane domains and a short pore loop (Baughman et al., 2011; De Stefani et al., 2011). Its structure has been solved, revealing the basis of its high ion selectivity (Baradaran et al., 2018). Notably, its absence abrogates Ca2+ uptake by animal mitochondria, whereas there is not yet a clear in vivo demonstration that MCU is the principal mechanism of mitochondrial Ca2+ accumulation in plants, as discussed below. The animal MCU core forms a complex with a number of interacting proteins essential for its proper function: the splice variant MCUb, essential MCU regulator (EMRE), MCUR1, MICU1, and MICU2 (Wagner et al., 2016). In principle, MCU is similarly permeable to Ca2+ and Mn2+, like most other Ca2+ channel proteins, but in this case, the enigma, how the homeostasis of both ions can be separated, has been solved. Selectivity for Ca2+ is conferred by MICU1, which possesses Ca2+-binding EF-hands (Kamer et al., 2018). At low [Ca2+]cyt, MICU1 inhibits MCU (Perocchi et al., 2010). This inhibition is relieved by Ca2+ binding at increased [Ca2+]cyt. Mn2+ is also bound by MICU1 with high affinity, but is unable to exert the Ca2+-specific structural changes that activate MCU (Kamer et al., 2018). In consequence, Mn2+ can only permeate MCU upon elevated [Ca2+]cyt, but not upon elevated [Mn2+]cyt, which safeguards the mitochondria from Mn2+ overload. Accordingly, mutation of MCU renders cells more resistant, and mutation of MICU1 more sensitive to Mn2+ (Kamer et al., 2018).
Besides facilitated Ca2+ influx by MCU, animal mitochondria are able to accumulate Ca2+ with high affinity. A protein localized to the mitochondrial inner membrane, leucine zipper EF-hand-containing transmembrane protein 1 (LETM1), has been identified to mediate this uptake of Ca2+ in exchange of H+ at [Ca2+]cyt < 1 µM (Jiang et al., 2009). As described below, its activity is reversible, the direction being determined by the pH and [Ca2+] gradients. Albeit LETM1 was initially described as K+/H+ antiporter, reconstitution in proteoliposomes unequivocally demonstrated an electroneutral Ca2+/2H+ antiport activity of this protein (Tsai et al., 2014). Aberrances in K+ homeostasis in letm1 mutant mitochondria may be explained by the stimulation of Ca2+-activated K+ channels at higher [Ca2+]mito (Jiang et al., 2009). Competition assays further demonstrated that Mn2+ is also transported with micromolar affinity, pointing to an additional role of LETM1 in Mn2+ homeostasis and Ca2+/Mn2+ interaction.
Largely based on findings in animals, Ca2+ transport mechanisms in plant mitochondria are being unveiled (Carraretto et al., 2016; Wagner et al., 2016). Based on genome information, the MCU complex can serve as blueprint for a mitochondrial Ca2+ import mechanism in plants, albeit some of its proteins, like EMRE, are not present in plant genomes. In contrast, MCU itself has fanned out: the Arabidopsis genome harbors six genes encoding MCU homologs. As described above, at least one member of the family, cMCU, is localized in the chloroplast as well as in root mitochondria. A member with mitochondrial localization, MCU1, shows Ca2+ channel activity upon reconstitution in planar lipid bilayers (Teardo et al., 2017). It is inhibited by Gadolinium and Ruthenium Red, which resembles its animal counterpart, and it is downregulated by MICU. Knockout mutants for this gene have a reduced root length and altered mitochondrial morphology, but only slight changes in mitochondrial Ca2+ dynamics, which implies the activity of redundant Ca2+ influx pathways. This needs to be solved by multiple knockout mutants. For Arabidopsis MCU1 and MCU2, mitochondrial Ca2+ influx has also been shown by yeast complementation (Selles et al., 2018), and MCU2 has been functionally expressed in HEK293 cells (Tsai et al., 2016). Both proteins are present in pollen mitochondria, and pollen germination of mcu2 is impaired, indicating the importance of [Ca2+]mito in this process, which requires further clarification.
The only homolog of MICU in Arabidopsis binds Ca2+ through three EF-hands, like its animal counterparts (Wagner et al., 2015). Responses of [Ca2+]mito to ATP and auxin were elevated in micu knockout mutants, which is explained by the function of Arabidopsis MICU as a negative regulator of mitochondrial Ca2+ influx (Wagner et al., 2015). Its knockout alters mitochondrial ultrastructure and causes changes in the respiratory machinery, that, however, do not provoke defects in whole-plant development. It is tempting to speculate that one role of MICU in plants lies in the discrimination of Ca2+ and Mn2+, like that of its mammalian counterpart. This also provokes the question to which extent plant MCU complexes contribute to mitochondrial Mn2+ uptake.
Apart from MCU complexes, a member of the GLR family, GLR3.5, has been localized to the IMM in Arabidopsis (Teardo et al., 2015). This channel is dually targeted, whereby two splice variants encode proteins that are directed either to chloroplasts or to mitochondria. Mitochondrial ultrastructure is altered and mitochondrial Ca2+ uptake is slightly reduced in a knockout mutant. It remains to be demonstrated whether GLR3.5 functions as a Ca2+ channel or whether it indirectly regulates Ca2+ fluxes mediated by other channels. In addition, GLR3.5 has also been shown to localize to the plasma membrane and to co-operate with GLR3.1 in guard cells (Kong et al., 2016).
A potential pathway for sequential Mn2+ uptake into the matrix of animal mitochondria consists of two poorly selective Fe2+ transporters, divalent metal transporter 1 (DMT1; synon. NRAMP2) and mitoferrin 1 (Mfrn1). There is evidence that DMT1 transports Mn2+ from the cytosol across the OMM to the intermembrane space (Wolff et al., 2018). This is intriguing because DMT1 has been described before as an endosomal transporter that translocates metals into the cytosol, and because the OMM is considered to be unselectively permeable to solutes. A homolog of DMT1 in Arabidopsis, NRAMP2, functions as Mn2+ transporter in the TGN/EE compartment, as described above. There is no evidence of its localization in mitochondria, and mitochondrial MnSOD is not altered in nramp2 mutants (Alejandro et al., 2017). Mn2+ flux across the IMM into the mitochondrial matrix of animals is mediated by Mfrn1, which belongs to the mitochondrial carrier family (Christenson et al., 2018). Interestingly, in Arabidopsis, two homologs to Mfrn1, mitochondrial iron transporter1 (MIT1) and MIT2, were recently described as essential mitochondrial Fe2+ transporters (Jain et al., 2019). These are very promising candidates for a metal-selective high-affinity Mn2+ influx pathway alongside a low-affinity influx by MCUs.
Release of Ca2+ and Mn2+
In mammals, Ca2+ release mechanisms counteract the voltage-driven passive accumulation of Ca2+ in the mitochondrial matrix mediated by MCU. In excitable cells, matrix Ca2+ is released by the Na+/Ca2+ exchanger NCLX (Luongo et al., 2017). Knockout mice for this gene displayed mitochondrial Ca2+ overload causing superoxide generation, necrosis and, eventually, cardiac failure, which demonstrates that mitochondrial Ca2+ efflux is essential for mitochondrial function and cell survival.
Another mechanism to release Ca2+ from the mitochondrial matrix is based on LETM1, which mediates bidirectional mitochondrial Ca2+/2H+ antiport, as described above (Jiang et al., 2009). An absence of LETM1 therefore not only results in reduced Ca2+ uptake by mitochondria at low [Ca2+]cyt, but also in a Ca2+ overload of mitochondria that accumulate Ca2+ by MCU activity. As LETM1 is essential to maintain [Ca2+]mito homeostasis, knockout of LETM1 is embryo-lethal in mice and causes severe metabolic and neurological defects in humans (Jiang et al., 2013). Owing to its affinity for Mn2+, LETM1 may also release this cation when operating in reverse direction, what remains to be studied.
Two homologs of LETM1 have been described in Arabidopsis (Zhang et al., 2012a). The double knockout mutation of these genes is lethal; developing seeds are aborted. In hemizygous plants, the abundance of mitochondrial proteins is affected and growth is reduced. These phenotypes suggest a conservation of LETM1 function in the homeostasis of [Ca2+]mito and possibly [Mn2+]mito of plants, what remains to be investigated.
Ca2+ and Mn2+ in the peroxisome
Functions of Ca2+
Peroxisomes play numerous roles in metabolism and development, which have been the subject of a recent review (Pan et al., 2020). Ca2+ appears to function in intra-peroxisomal signaling, since they contain Ca2+ sensor proteins which regulate diverse metabolic processes. These include Ca2+-dependent protein kinases, such as GhCPK33, which regulates JA biosynthesis in cotton (Hu et al., 2018), and PiCDPK2, that regulates pollen tube growth in petunia (Guo et al., 2013), as well as the CaM-like protein CML3, which mediates dimerization of the DEG15 protease in Arabidopsis (Dolze et al., 2013). A peroxisomal catalase, Cat3, which degrades H2O2 into H2O and O2, is also activated by Ca2+/CaM (Yang and Poovaiah, 2002). The identity of the regulatory CaM is unknown, with CML3 being a candidate. This relationship is supported by an increase of guard cell peroxisomal H2O2 scavenging activity upon increased [Ca2+]peroxi (Costa et al., 2010). The peroxisomal import and activity of arginine-dependent NO synthase (NOS) also require Ca2+/CaM (Corpas and Barroso, 2014). However, the identity of the NOS, as well as the mechanisms of its regulation by Ca2+/CaM, is unknown.
Functions of Mn2+
Peroxisomes are important in the detoxification of free radicals (Pan et al., 2020). For some plant species, such as pea, watermelon, cucumber, castor bean, and carnation, a MnSOD has been shown to be present in plant peroxisomes (Corpas et al., 2017).
Import and release of Ca2+ and Mn2+
The presence of Ca2+ signal-decoding machinery, as well as a Mn2+-dependent SOD, implies the operation of transport mechanisms for those cations in the peroxisomal membrane. This is supported by peroxisomal [Ca2+] measurements showing that [Ca2+]peroxi dynamics follow those of [Ca2+]cyt upon stimulation (Costa et al., 2010).
No membrane proteins that mediate those fluxes are known to date. However, plant peroxisomes contain a protein of the MVP17/PMP22 family, members of which from mammals and yeast form a poorly selective channel with a wide pore, which is permeable to ions and metabolites (see Charton et al., 2019 for review). Albeit channel activity of a plant PMP22 has yet to be demonstrated, this protein is a likely basis for Ca2+ and Mn2+ fluxes across the peroxisomal membrane.
Conclusions and outlook
Ca2+ and Mn2+ govern a plethora of processes in organellar compartments of plants, the most prominent of which were covered in this review. Ca2+ acts as signaling agent within organelles, like in the cytosol, and Mn2+ may interfere with this function owing to its ability to (unproductively?) occupy Ca2+-binding domains. Both cations also cooperate, as in the OEC. Such functions and interactions of Ca2+ and Mn2+ are determined by their free concentrations in the different compartments, which might be examined by genetically encoded indicators, that need yet to be developed for Mn2+. This issue may also be approached by direct subcellular imaging of Ca2+ and Mn2+, for example, by synchrotron radiation, but such techniques with subcellular resolution are not widely available, extraordinarily complex, and unable to show dynamic changes. Both approaches need to be pushed forward (see Advances and Outstanding Questions).
Membrane transport proteins are the backbone of cellular cation (re-)distribution, and in the last 5 years, a number of new players have shown up on the pitch. Notably, routes for Ca2+ and Mn2+ into chloroplasts and mitochondria are now known, albeit not fully understood, and further candidates are lining up to be tested. The plant ER/NE has turned out as a hub of Ca2+ signaling in different circumstances, and channel proteins in this compartment have been found: The identification of CNGCs in the NE solved the long-standing question how nuclear Ca2+ signals are generated in symbiotic signaling, which has very recently taken another turn with the characterization of CASTOR as Ca2+ channel. The finding that GLRs essential for systemic Ca2+ signaling may also reside in ER and vacuole, besides their operation in the plasma membrane, demands a scrutiny of their roles in those endomembranes. In this respect, the function and regulation of the most-studied plant channel, TPC1, is also far from clear. Some major gaps in the array of organellar transporters still need to be filled. For example, in the plant Golgi, no transporters associated with Mn2+ import for glycosylation and no Ca2+ release channels are known; peroxisomal Ca2+ and Mn2+ transport is a black box.
Mechanisms on protein, organellar, and cellular levels ought to be in place that define the distribution and ratio of Ca2+ and Mn2+. Here, tunable discrimination mechanisms have become apparent from nonplant work in the form of EF-hands, either in accessory subunits (as in animal MCU-MICU) or in the transport protein itself (as in yeast PMR1 or human SPCA1). Conversely, for the chloroplastic BICAT proteins that supply the organelle with Mn2+ and shape its Ca2+ signals, mechanisms of tell Mn2+ from Ca2+ are unknown, and even the mode of transport of this family is debated. The search for interactors, transport assays on proteoliposomes, and eventually the solving of a protein structure will lead toward a better understanding.
Finally, the way Ca2+ and Mn2+ are allocated to organelles is far from clear. How do organelles get prioritized? Are there hard-wired logics? To test the simplest assumption that substrate allocation is determined by different affinities of the transport proteins, their kinetics need to be determined, which is not trivial. However, a more sophisticated coordination and communication between compartments and subcompartments seem inevitable for an efficient utilization of the cations and the optimum supply of target proteins. An impressive example of coordinated ion concentrations is the animal secretory pathway with its subcompartmental [Ca2+] gradient. Yet there is some evidence for specific supply chains in plants. Mn2+ delivery to chloroplasts, for example, is believed to require sequential Mn2+ release from the TGN and the vacuole by NRAMP2 and NRAMP3/4, respectively. This bears some similarity to yeast, where the NRAMP2 homolog SMF2 supplies the mitochondria by endosomal Mn2+ release.
What guarantees that these intraorganellar fluxes reach the correct recipient? Besides spatial proximity, membrane contact sites, at which Ca2+ or Mn2+ are passed from one organelle to the next, are likely to play a major role that has yet to be unveiled in plants. Examples from yeast and animals are in support of this, for instance between ER and mitochondria, where Ca2+ transport proteins of different organelles even physically interact.
After completing the toolbox of Ca2+ and Mn2+ transport, the regulation of those proteins and their interaction within and between organelles needs to be resolved to reach an understanding of Ca2+ and Mn2+ handling by the plant cell. Sneaking a peek at animals and yeast has proven fruitful in the past and will yield more insights in the future.
ADVANCES
The molecular basis of many Ca2+ and Mn2+ transport activities across organellar membranes has been revealed. In particular, transporters for Ca2+ and Mn2+, and a Ca2+ channel, have recently been identified in the chloroplast.
The contribution of organelles, in particular the ER and the vacuole, to cytosolic Ca2+ signaling in plants has become evident.
Transporters that detoxify Mn2+ have been identified in the Golgi and the vacuole.
A role of the TGN in cellular Mn2+ distribution has become apparent.
OUTSTANDING QUESTIONS
What is the identity of the missing transport proteins, e.g. to release Ca2+ from the Golgi, or to move Ca2+ and Mn2+ across peroxisomal membranes? Which transport proteins supply the Golgi and ER with Ca2+ and Mn2+ for glycosylation processes?
What are the (sub)organellar concentrations of total and free Ca2+ and Mn2+? Is there a gradient of Ca2+ along the secretory pathway, as there is in animals?
How are Mn2+ and Ca2+ homeostasis controlled individually if transport proteins are unspecific?
What is the contribution of different organelles to specific Ca2+ signals?
How is the vacuolar cation channel TPC1 integrated in systemic Ca2+ signaling?
Does Mn2+ interfere with Ca2+-dependent signaling processes, and does it have a signaling function on its own?
How are Ca2+ and Mn2+ allocated to different organelles, especially under limiting conditions?
Acknowledgments
We thank Bastian Meier and Stefanie Höller for critically reading the manuscript and Julia Rödiger for support in preparing the figures.
Funding
This work was supported by the European Regional Development Fund within the State Research Focus Program “Molecular Biosciences as a Motor for a Knowledge-Based Economy” (ZS/2016/06/79740 to E.P.), by the European Social Fund within the AGRIPOLY graduate school (ZS/2016/08/80644 to E.P.), by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) within the Research Training Group GRK2498 (400681449, project 06 to E.P.), and by a China Scholarship Council Ph.D. scholarship to J.H.
Conflict of interest statement. None declared.
E.P. conceived the article. J.H., N.R., M.T.T.H., S.A., and E.P. wrote the article. E.P. agrees to serve as the author responsible for contact and ensures communication.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Author (https://academic.oup.com/plphys/pages/general-instructions) is: Edgar Peiter (edgar.peiter@landw.uni-halle.de).
References
- Abdul-Awal SM, Hotta CT, Davey MP, Dodd AN, Smith AG, Webb AAR (2016) NO-mediated [Ca2+]cyt increases depend on ADP-ribosyl cyclase activity in Arabidopsis. Plant Physiol 171:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alejandro S, Cailliatte R, Alcon C, Dirick L, Domergue F, Correia D, Castaings L, Briat J-F, Mari S, Curie C (2017) Intracellular distribution of manganese by the trans-Golgi network transporter NRAMP2 is critical for photosynthesis and cellular redox homeostasis. Plant Cell 29:3068–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alejandro S, Höller S, Meier B, Peiter E (2020) Manganese in plants: from acquisition to subcellular allocation. Front Plant Sci 11:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri A, Doccula FG, Pederzoli R, Grenzi M, Bonza MC, Luoni L, Candeo A, Armada NR, Barbiroli A, Valentini G,. et al. (2020) The structural bases for agonist diversity in an Arabidopsis thaliana glutamate receptor-like channel. Proc Natl Acad Sci USA 117:752–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MD, Kropat J, Tottey S, Del Campo JA, Merchant SS (2007) Manganese deficiency in Chlamydomonas results in loss of photosystem II and MnSOD function, sensitivity to peroxides, and secondary phosphorus and iron deficiency. Plant Physiol 143:263–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos RA, Pattathil S, Yang JY, Atmodjo MA, Urbanowicz BR, Moremen KW, Mohnen D (2018) A two-phase model for the non-processive biosynthesis of homogalacturonan polysaccharides by the GAUT1:GAUT7 complex. J Biol Chem 293:19047–19063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen E, Peiter E, Küpper H (2018) Trace metal metabolism in plants. J Exp Bot 69:909–954 [DOI] [PubMed] [Google Scholar]
- Arnold BJ, Lahner B, DaCosta JM, Weisman CM, Hollister JD, Salt DE, Bomblies K, Yant L (2016) Borrowed alleles and convergence in serpentine adaptation. Proc Natl Acad Sci USA 113:8320–8325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpagaus S, Rawyler A, Braendle R (2002) Occurrence and characteristics of the mitochondrial permeability transition in plants. J Biol Chem 277:1780–1787 [DOI] [PubMed] [Google Scholar]
- Aulestia FJ, Alonso MT, Garcia-Sancho J (2015) Differential calcium handling by the cis and trans regions of the Golgi apparatus. Biochem J 466:455–465 [DOI] [PubMed] [Google Scholar]
- Balsera M, Goetze TA, Kovács-Bogdán E, Schürmann P, Wagner R, Buchanan BB, Soll J, Bölter B (2009) Characterization of Tic110, a channel-forming protein at the inner envelope membrane of chloroplasts, unveils a response to Ca2+ and a stromal regulatory disulfide bridge. J Biol Chem 284:2603–2616 [DOI] [PubMed] [Google Scholar]
- Baradaran R, Wang C, Siliciano AF, Long SB (2018) Cryo-EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature 559:580–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D, Tian L, Wang W, Bobbs S, Herock H, Travers A, Showalter AM (2015a) A small multigene hydroxyproline-O-galactosyltransferase family functions in arabinogalactan-protein glycosylation, growth and development in Arabidopsis. BMC Plant Biol 15:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D, Wang W, Ma S, DeBrosse T, Poirier E, Emch K, Soukup E, Tian L, Showalter AM (2015b) Two hydroxyproline galactosyltransferases, GALT5 and GALT2, function in arabinogalactan-protein glycosylation, growth and development in Arabidopsis. PLoS One 10:e0125624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistic O, Waadt R, Steinhorst L, Held K, Kudla J (2010) CBL-mediated targeting of CIPKs facilitates the decoding of calcium signals emanating from distinct cellular stores. Plant J 61:211–222 [DOI] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL,. et al. (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476:341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera S, Xu Z, Luoni L, Bonza MC, Doccula FG, De Michelis MI, Morris RJ, Schwarzländer M, Costa A (2018) Cellular Ca2+ signals generate defined pH signatures in plants. Plant Cell 30:2704–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencúr P, Steinkellner H, Svoboda B, Mucha J, Strasser R, Kolarich D, Hann S, Köllensperger G, Glössl J, Altmann F, et al. (2005) Arabidopsis thaliana β1,2-xylosyltransferase: an unusual glycosyltransferase with the potential to act at multiple stages of the plant N-glycosylation pathway. Biochem J 388:515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewell MA, Maathuis FJM,, Allen GJ, Sanders D (1999) Calcium-induced calcium release mediated by a voltage-activated cation channel in vacuolar vesicles from red beet. FEBS Lett 458:41–44 [DOI] [PubMed] [Google Scholar]
- Beyhl D, Hörtensteiner S, Martinoia E, Farmer EE, Fromm J, Marten I, Hedrich R (2009) The fou2 mutation in the major vacuolar cation channel TPC1 confers tolerance to inhibitory luminal calcium. Plant J 58:715–723 [DOI] [PubMed] [Google Scholar]
- Bian B, Kageshima S, Yano K, Fujiwara T, Kamiya T (2018) Screening Arabidopsis thaliana mutants for low sensitivity to manganese identifies novel alleles of NRAMP1 and PGSIP6. J Exp Bot 69:1795–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson C, Britton KL, Sedelnikova SE, Rodgers HF, Eadsforth TC, Viner RC, Hawkes TR, Baker PJ, Rice DW (2015) Crystal structures reveal that the reaction mechanism of imidazoleglycerol-phosphate dehydratase is controlled by switching Mn(II) coordination. Structure 23:1236–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamey FPC, Hernandez-Soriano MC, Cheng M, Tang C, Paterson DJ, Lombi E, Wang WH, Scheckel KG, Kopittke PM (2015) Synchrotron-based techniques shed light on mechanisms of plant sensitivity and tolerance to high manganese in the root environment. Plant Physiol 169:2006–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ (2019) Metal regulation of metabolism. Curr Opin Chem Biol 49:33–38 [DOI] [PubMed] [Google Scholar]
- Bloom AJ, Lancaster KM (2018) Manganese binding to Rubisco could drive a photorespiratory pathway that increases the energy efficiency of photosynthesis. Nat Plants 4:414–422 [DOI] [PubMed] [Google Scholar]
- Boccaccio A, Scholz-Starke J, Hamamoto S, Larisch N, Festa M, Gutla PVK, Costa A, Dietrich P, Uozumi N, Carpaneto A (2014) The phosphoinositide PI(3,5)P2 mediates activation of mammalian but not plant TPC proteins: functional expression of endolysosomal channels in yeast and plant cells. Cell Mol Life Sci 71:4275–4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure G, Gfeller A, Proebsting WM, Hörtensteiner S, Chételat A, Martinoia E, Farmer EE (2007) A gain-of-function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. Plant J 49:889–898 [DOI] [PubMed] [Google Scholar]
- Bonza MC, Loro G, Behera S, Wong A, Kudla J, Costa A (2013) Analyses of Ca2+ accumulation and dynamics in the endoplasmic reticulum of Arabidopsis root cells using a genetically encoded cameleon sensor. Plant Physiol 163:1230–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi JG, Kumar K, Barberini ML, Dominguez GD, Guerrero YDR, Marino-Buslje C, Obertello M, Muschietti JP, Estevez JM (2020) The role of P-type IIA and P-type IIB Ca2+-ATPases in plant development and growth. J Exp Bot 71:1239–1248 [DOI] [PubMed] [Google Scholar]
- Both P, Sobczak L, Breton C, Hann S, Nöbauer K, Paschinger K, Kozmon S, Mucha J, Wilson IB (2011) Distantly related plant and nematode core α1,3-fucosyltransferases display similar trends in structure-function relationships. Glycobiology 21:1401–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y, Lee SM, Romanowsky S, Blank R, Sladek C, Chung WS, Harper JF (2010) Disruption of the vacuolar calcium-ATPases in Arabidopsis results in the activation of a salicylic acid-dependent programmed cell death pathway. Plant Physiol 154:1158–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzi AT, Bane LB, Weihofen WA, McCabe AL, Singharoy A, Chipot CJ, Schulten K, Gaudet R (2016) Conserved methionine dictates substrate preference in Nramp-family divalent metal transporters. Proc Natl Acad Sci USA 113:10310–10315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw HD (2005) Mutations in CAX1 produce phenotypes characteristic of plants tolerant to serpentine soils. New Phytol 167:81–88 [DOI] [PubMed] [Google Scholar]
- Breton C, Snajdrová L, Jeanneau C, Koca J, Imberty A (2006) Structures and mechanisms of glycosyltransferases. Glycobiology 16:29R–37R [DOI] [PubMed] [Google Scholar]
- Brini M (2008) Calcium-sensitive photoproteins. Methods 46:160–166 [DOI] [PubMed] [Google Scholar]
- Brosnan JM, Sanders D (1993) Identification and characterization of high-affinity binding sites for inositol trisphosphate in red beet. Plant Cell 5:931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Gidley MJ, Fincher GB (2010) Heterogeneity in the chemistry, structure and function of plant cell walls. Nat Chem Biol 6:724–732 [DOI] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, et al. (2009) NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459:596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao FQ, Werner AK, Dahncke K, Romeis T, Liu LH, Witte CP (2010) Identification and characterization of proteins involved in rice urea and arginine catabolism. Plant Physiol 154:98–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Xie Y, Li M, Pan X, Zhang H, Zhao X, Su X, Cheng T, Chang W (2015) Crystal structure analysis of extrinsic PsbP protein of photosystem II reveals a manganese-induced conformational change. Mol Plant 8:664–666 [DOI] [PubMed] [Google Scholar]
- Capoen W, Sun J, Wysham D, Otegui MS, Venkateshwaran M, Hirsch S, Miwa H, Downie JA, Morris RJ,, Ané JM, et al. (2011) Nuclear membranes control symbiotic calcium signaling of legumes. Proc Natl Acad Sci USA 108:14348–14353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona A, Devès G, Roudeau S, Cloetens P, Bohic S, Ortega R (2010) Manganese accumulates within Golgi apparatus in dopaminergic cells as revealed by synchrotron X-ray fluorescence nanoimaging. ACS Chem Neurosci 1:194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpaneto A, Gradogna A (2018) Modulation of calcium and potassium permeation in plant TPC channels. Biophys Chem 236:1–7 [DOI] [PubMed] [Google Scholar]
- Carraretto L, Formentin E, Teardo E, Checchetto V, Tomizioli M, Morosinotto T, Giacometti GM, Finazzi G, Szabó I (2013) A thylakoid-located two-pore K+ channel controls photosynthetic light utilization in plants. Science 342:114–118 [DOI] [PubMed] [Google Scholar]
- Carraretto L, Teardo E, Checchetto V, Finazzi G, Uozumi N, Szabo I (2016) Ion channels in plant bioenergetic organelles, chloroplasts and mitochondria: from molecular identification to function. Mol Plant 9:371–395 [DOI] [PubMed] [Google Scholar]
- Carraro M, Checchetto V, Szabó I, Bernardi P (2019) F-ATP synthase and the permeability transition pore: fewer doubts, more certainties. FEBS Lett 593:1542–1553 [DOI] [PubMed] [Google Scholar]
- Charles SA, Halliwell B (1980) Action of calcium ions on spinach (Spinacia oleracea) chloroplast fructose bisphosphatase and other enzymes of the calvin cycle. Biochem J 188:775–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier M (2018) Calcium signals in the plant nucleus: origin and function. J Exp Bot 69:4165–4173 [DOI] [PubMed] [Google Scholar]
- Charpentier M, Bredemeier R, Wanner G, Takeda N, Schleiff E, Parniske M (2008) Lotus japonicus CASTOR and POLLUX are ion channels essential for perinuclear calcium spiking in legume root endosymbiosis. Plant Cell 20:3467–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier M, Sun J, Martins TV, Radhakrishnan GV, Findlay K, Soumpourou E, Thouin J, Véry AA, Sanders D, Morris RJ, et al. (2016) Nuclear-localized cyclic nucleotide-gated channels mediate symbiotic calcium oscillations. Science 352:1102–1105 [DOI] [PubMed] [Google Scholar]
- Charton L, Plett A, Linka N (2019) Plant peroxisomal solute transporter proteins. J Integr Plant Biol 61:817–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Smaardijk S, Mattelaer CA, Pamula F, Vandecaetsbeek I, Vanoevelen J, Wuytack F, Lescrinier E, Eggermont J, Vangheluwe P (2019) An N-terminal Ca2+-binding motif regulates the secretory pathway Ca2+/Mn2+-transport ATPase SPCA1. J Biol Chem 294:7878–7891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Fujii Y, Yamaji N, Masuda S, Takemoto Y, Kamiya T, Yusuyin Y, Iwasaki K, Kato S, Maeshima M. et al. (2013) Mn tolerance in rice is mediated by MTP8.1, a member of the cation diffusion facilitator family. J Exp Bot 64:4375–4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Barkla BJ, Shigaki T, Hirschi KD (2003) The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 15:347–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Shigaki T, Hirschi KD (2002) Characterization of CAX4, an Arabidopsis H+/cation antiporter. Plant Physiol 128:1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD (2005) Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 138:2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigri F, Flosdorff S, Pilz S, Kölle E, Dolze E, Gietl C, Vothknecht UC (2012) The Arabidopsis calmodulin-like proteins AtCML30 and AtCML3 are targeted to mitochondria and peroxisomes, respectively. Plant Mol Biol 78:211–222 [DOI] [PubMed] [Google Scholar]
- Chigri F, Hörmann F, Stamp A, Stammers DK, Bölter B, Soll J, Vothknecht UC (2006) Calcium regulation of chloroplast protein translocation is mediated by calmodulin binding to Tic32. Proc Natl Acad Sci USA 103:16051–16056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D, Villiers F, Kroniewicz L, Lee S, Seo YJ, Hirschi KD, Leonhardt N, Kwak JM (2012) Vacuolar CAX1 and CAX3 influence auxin transport in guard cells via regulation of apoplastic pH. Plant Physiol 160:1293–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WG, Toyota M, Kim SH, Hilleary R, Gilroy S (2014) Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci USA 111:6497–6502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson ET, Gallegos AS, Banerjee A (2018) In vitro reconstitution, functional dissection, and mutational analysis of metal ion transport by mitoferrin-1. J Biol Chem 293:3819–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HH, Car S, Socha AL, Hindt MN, Punshon T, Guerinot ML (2017) The Arabidopsis MTP8 transporter determines the localization of manganese and iron in seeds. Sci Rep 7:11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet AS, Sengottaiyan P, Deschamps A, Colsoul ML, Thines L, Demaegd D, Duchene MC, Foulquier F, Hols P, Morsomme P (2016) Yeast Gdt1 is a Golgi-localized calcium transporter required for stress-induced calcium signaling and protein glycosylation. Sci Rep 6:24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn S, Gilliham M (2010) Comparative physiology of elemental distributions in plants. Ann Bot 105:1081–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn SJ, Gilliham M,, Athman A, Schreiber AW, Baumann U, Moller I, Cheng NH, Stancombe MA, Hirschi KD, Webb AAR, et al. (2011) Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 23:240–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connorton JM, Jones ER, Rodriguez-Ramiro I, Fairweather-Tait S, Uauy C, Balk J (2017) Wheat vacuolar iron transporter TaVIT2 transports Fe and Mn and is effective for biofortification. Plant Physiol 174:2434–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connorton JM, Webster RE, Cheng N, Pittman JK (2012) Knockout of multiple Arabidopsis cation/H+ exchangers suggests isoform-specific roles in metal stress response, germination and seed mineral nutrition. PLoS One 7:e47455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ,, Barroso JB (2014) Peroxisomal plant nitric oxide synthase (NOS) protein is imported by peroxisomal targeting signal type 2 (PTS2) in a process that depends on the cytosolic receptor PEX7 and calmodulin. FEBS Lett 588:2049–2054 [DOI] [PubMed] [Google Scholar]
- Corpas FJ,, Barroso JB, Palma JM, Rodriguez-Ruiz M (2017) Plant peroxisomes: a nitro-oxidative cocktail. Redox Biol 11:535–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso M, Doccula FG, de Melo JRF, Costa A, Verbruggen N (2018) Endoplasmic reticulum-localized CCX2 is required for osmotolerance by regulating ER and cytosolic Ca2+ dynamics in Arabidopsis. Proc Natl Acad Sci USA 115:3966–3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Drago I, Behera S, Zottini M, Pizzo P, Schroeder JI, Pozzan T, Lo Schiavo F (2010) H2O2 in plant peroxisomes: an in vivo analysis uncovers a Ca2+-dependent scavenging system. Plant J 62:760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Navazio L, Szabo I (2018) The contribution of organelles to plant intracellular calcium signalling. J Exp Bot 69:4175–4193 [DOI] [PubMed] [Google Scholar]
- Culbertson AT, Ehrlich JJ, Choe JY, Honzatko RB, Zabotina OA (2018) Structure of xyloglucan xylosyltransferase 1 reveals simple steric rules that define biological patterns of xyloglucan polymers. Proc Natl Acad Sci USA 115:6064–6069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson AT, Tietze AA,, Tietze D, Chou YH, Smith AL, Young ZT, Zabotina OA (2016) A homology model of Xyloglucan Xylosyltransferase 2 reveals critical amino acids involved in substrate binding. Glycobiology 26:961–972 [DOI] [PubMed] [Google Scholar]
- Dadacz-Narloch B, Beyhl D, Larisch C, López-Sanjurjo EJ, Reski R, Kuchitsu K, Müller TD, Becker D, Schönknecht G, Hedrich R (2011) A novel calcium binding site in the slow vacuolar cation channel TPC1 senses luminal calcium levels. Plant Cell 23:2696–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadacz-Narloch B, Kimura S, Kurusu T, Farmer EE, Becker D, Kuchitsu K, Hedrich R (2013) On the cellular site of two-pore channel TPC1 action in the Poaceae. New Phytol 200:663–674 [DOI] [PubMed] [Google Scholar]
- Das S, Carmona A, Khatua K, Porcaro F, Somogyi A, Ortega R, Datta A (2019) Manganese mapping using a fluorescent Mn2+ sensor and nanosynchrotron X-ray fluorescence reveals the role of the Golgi apparatus as a manganese storage site. Inorg Chem 58:13724–13732 [DOI] [PubMed] [Google Scholar]
- De Col V, Petrussa E, Casolo V, Braidot E, Lippe G, Filippi A, Peresson C, Patui S, Bertolini A, Giorgio V, et al. (2018) Properties of the permeability transition of pea stem mitochondria. Front Physiol 9:1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476:336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Gruber BD, Pittman JK, White RG, Leung H, Miao Y, Jiang L, Ryan PR, Richardson AE (2007) A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. Plant J 51:198–210 [DOI] [PubMed] [Google Scholar]
- Delhaize E, Kataoka T, Hebb DM, White RG, Ryan PR (2003) Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell 15:1131–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaegd D, Colinet A-S, Deschamps A, Morsomme P (2014) Molecular evolution of a novel family of putative calcium transporters. PLoS One 9:e100851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaegd D, Foulquier F, Colinet AS, Gremillon L, Legrand D, Mariot P, Peiter E, Van Schaftingen E, Matthijs G, Morsomme P (2013) Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells. Proc Natl Acad Sci USA 110:6859–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Shabala S, Isayenkov S, Cuin TA, Pottosin I (2018) Calcium transport across plant membranes: mechanisms and functions. New Phytol 220:49–69 [DOI] [PubMed] [Google Scholar]
- Deng Y, Pakdel M, Blank B, Sundberg EL, Burd CG, von Blume J (2018) Activity of the SPCA1 calcium pump couples sphingomyelin synthesis to sorting of secretory proteins in the trans-Golgi network. Dev Cell 47:464–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindas J, Dreyer I, Huang S, Hedrich R, Roelfsema MRG (2021) A voltage-dependent Ca2+-homeostat operates in the plant vacuolar membrane. New Phytol doi: 10.1111/nph.17272. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Dindas J, Scherzer S, Roelfsema MRG, von Meyer K, Müller HM, Al-Rasheid KAS, Palme K, Dietrich P, Becker D, Bennett MJ, Hedrich R (2018) AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling. Nat Commun 9:1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Gardner MJ, Hotta CT, Hubbard KE, Dalchau N, Love J, Assie JM, Robertson FC, Jakobsen MK, Goncalves J, et al. (2007) The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science 318:1789–1792 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D (2010) The language of calcium signaling. Annu Rev Plant Biol 61:593–620 [DOI] [PubMed] [Google Scholar]
- Dogbo O, Laferrière A, D’Harlingue A, Camara B (1988) Carotenoid biosynthesis: isolation and characterization of a bifunctional enzyme catalyzing the synthesis of phytoene. Proc Natl Acad Sci USA 85:7054–7058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolze E, Chigri F, Höwing T, Hierl G, Isono E, Vothknecht UC, Gietl C (2013) Calmodulin-like protein AtCML3 mediates dimerization of peroxisomal processing protease AtDEG15 and contributes to normal peroxisome metabolism. Plant Mol Biol 83:607–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou CM, Fu XP, Chen XC, Shi JY, Chen YX (2009) Accumulation and detoxification of manganese in hyperaccumulator Phytolacca americana. Plant Biol 11:664–670 [DOI] [PubMed] [Google Scholar]
- Drago I, De Stefani D, Rizzuto R, Pozzan T (2012) Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc Natl Acad Sci USA 109:12986–12991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulary E, Yu SY, Houdo M, de Bettignies G, Decool V, Potelle S, Duvet S, Krzewinski-Recchi MA, Garat A, Matthijs G, et al. (2018) Investigating the function of Gdt1p in yeast Golgi glycosylation. Biochim Biophys Act 1862:394–402 [DOI] [PubMed] [Google Scholar]
- Dümmer M, Michalski C, Essen L-O, Rath M, Galland P, Forreiter C (2016) EHB1 and AGD12, two calcium-dependent proteins affect gravitropism antagonistically in Arabidopsis thaliana. J Plant Physiol 206:114–124 [DOI] [PubMed] [Google Scholar]
- Dürr G, Strayle J, Plemper R, Elbs S,, Klee SK, Catty P, Wolf DH, Rudolph HK (1998) The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Biol Cell 9:1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Birdseye D, Liwanag AJM, Laursen T, Rennie EA, Guo XY, Catena M, Rautengarten C, Stonebloom SH, Gluza P, et al. (2018) The three members of the Arabidopsis glycosyltransferase family 92 are functional β-1,4-galactan synthases. Plant Cell Physiol 59:2624–2636 [DOI] [PubMed] [Google Scholar]
- Edel KH, Marchadier E, Brownlee C, Kudla J, Hetherington AM (2017) The evolution of calcium-based signalling in plants. Curr Biol 27:R667–R679 [DOI] [PubMed] [Google Scholar]
- Edmond C, Shigaki T, Ewert S, Nelson MD, Connorton JM, Chalova V, Noordally Z, Pittman JK (2009) Comparative analysis of CAX2-like cation transporters indicates functional and regulatory diversity. Biochem J 418:145–154 [DOI] [PubMed] [Google Scholar]
- Egelund J, Petersen BL, Motawia MS, Damager I, Faik A, Olsen CE, Ishii T, Clausen H, Ulvskov P, Geshi N (2006) Arabidopsis thaliana RGXT1 and RGXT2 encode Golgi-localized (1,3)-α-D-xylosyltransferases involved in the synthesis of pectic rhamnogalacturonan-II. Plant Cell 18:2593–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnstorfer IA, Geertsma ER, Pardon E, Steyaert J, Dutzler R (2014) Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport. Nat Struct Mol Biol 21:990–996 [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Gill HS, Pfluegl GMU, Rotstein SH (2000) Structure-function relationships of glutamine synthetases. Biochim Biophys Act 1477:122–145 [DOI] [PubMed] [Google Scholar]
- Eisenhut M, Hoecker N, Schmidt SB, Basgaran RM, Flachbart S, Jahns P, Eser T, Geimer S, Husted S, Webers APM, et al. (2018) The plastid envelope CHLOROPLAST MANGANESE TRANSPORTER1 is essential for manganese homeostasis in Arabidopsis. Mol Plant 11:955–969 [DOI] [PubMed] [Google Scholar]
- Entus R, Poling M, Herrmann KM (2002) Redox regulation of Arabidopsis 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase. Plant Physiol 129:1866–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu S, Giehl RFH, Meier B, Takahashi M, Terada Y, Ignatyev K, Andresen E, Küpper H, Peiter E, von Wirén N (2017) Metal Tolerance Protein 8 mediates manganese homeostasis and iron re-allocation during seed development and germination. Plant Physiol 174:1633–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu S, Meier B, von Wirén N, Peiter E (2016) The vacuolar manganese transporter MTP8 determines tolerance to iron deficiency-induced chlorosis in Arabidopsis. Plant Physiol 170:1030–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger WF, Clear AM, Fanning KJ, Peck ML (1999) Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiol 119:1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Gao YQ, Lenzoni G, Wolfender JL, Wu Q (2020) Wound- and mechanostimulated electrical signals control hormone responses. New Phytol 227:1037–1050 [DOI] [PubMed] [Google Scholar]
- Farthing EC, Menguer PK, Fett JP, Williams LE (2017) OsMTP11 is localised at the Golgi and contributes to Mn tolerance. Sci Rep 7:15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando DR, Mizuno T, Woodrow IE, Baker AJM, Collins RN (2010) Characterization of foliar manganese (Mn) in Mn (hyper)accumulators using X-ray absorption spectroscopy. New Phytol 188:1014–1027 [DOI] [PubMed] [Google Scholar]
- Finazzi G, Petroutsos D, Tomizioli M, Flori S, Sautron E, Villanova V, Rolland N, Seigneurin-Berny D (2015) Ions channels/transporters and chloroplast regulation. Cell Calcium 58:86–97 [DOI] [PubMed] [Google Scholar]
- Finka A, Cuendet AFH, Maathuis FJM, Saidi Y, Goloubinoff P (2012) Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24:3333–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores T, Todd CD, Tovar-Mendez A, Dhanoa PK, Correa-Aragunde N, Hoyos ME, Brownfield DM, Mullen RT, Lamattina L, Polacco JC (2008) Arginase-negative mutants of Arabidopsis exhibit increased nitric oxide signaling in root development. Plant Physiol 147:1936–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulquier F, Legrand D (2020) Biometals and glycosylation in humans: Congenital disorders of glycosylation shed lights into the crucial role of Golgi manganese homeostasis. Biochim Biophys Act 1864:129674. [DOI] [PubMed] [Google Scholar]
- Frank J, Happeck R, Meier B, Hoang MTT, Stribny J, Hause G, Ding H, Morsomme P, Baginsky S, Peiter E (2019) Chloroplast-localized BICAT proteins shape stromal calcium signals and are required for efficient photosynthesis. New Phytol 221:866–880 [DOI] [PubMed] [Google Scholar]
- Furch ACU, van Bel AJE, Fricker MD, Felle HH, Fuchs M, Hafke JB (2009) Sieve element Ca2+ channels as relay stations between remote stimuli and sieve tube occlusion in Vicia faba. Plant Cell 21:2118–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Xie W, Yang C, Xu J, Li J, Wang H, Chen X, Huang CF (2018) NRAMP2, a trans-Golgi network-localized manganese transporter, is required for Arabidopsis root growth under manganese deficiency. New Phytol 217:179–193 [DOI] [PubMed] [Google Scholar]
- Garcia-Rodriguez N, Manzano-Lopez J, Munoz-Bravo M, Fernandez-Garcia E, Muniz M, Wellinger RE (2015) Manganese redistribution by calcium-stimulated vesicle trafficking bypasses the need for P-type ATPase function. J Biol Chem 290:9335–9347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Frangne N, Gomès E, Martinoia E, Palmgren MG (2000) The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol 124:1814–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerndt S, Krogsaeter E, Patel S, Bracher F, Grimm C (2020) Discovery of lipophilic two-pore channel agonists. FEBS J 287:5284–5293 [DOI] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Pizzo P, Pozzan T (2007) Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ 14:1267–1274 [DOI] [PubMed] [Google Scholar]
- Gobert A, Isayenkov S, Voelker C, Czempinski K, Maathuis FJM (2007) The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc Natl Acad Sci USA 104:10726–10731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradogna A, Scholz-Starke J, Gutla PVK, Carpaneto A (2009) Fluorescence combined with excised patch: measuring calcium currents in plant cation channels. Plant J 58:175–182 [DOI] [PubMed] [Google Scholar]
- Grenzi M, Bonza MC, Alfieri A, Costa A (2020) Structural insights into long-distance signal transduction pathways mediated by plant glutamate receptor-like channels. New Phytol 229: 1261–1267 [DOI] [PubMed] [Google Scholar]
- Guo F, Yoon GM, McCubbin AG (2013) PiSCP1 and PiCDPK2 localize to peroxisomes and are involved in pollen tube growth in Petunia inflata. Plants 2:72–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Zeng W, Chen Q, Lee C, Chen L, Yang Y, Cang C, Ren D, Jiang Y (2016) Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature 531:196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, He J, Barry BA (2018) Calcium, conformational selection, and redox-active tyrosine YZ in the photosynthetic oxygen-evolving cluster. Proc Natl Acad Sci USA 115:5658–5663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin JL, Zanis MJ, Salt DE (2011) Structure and evolution of the plant cation diffusion facilitator family of ion transporters. BMC Evol Biol 11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JF, Hong B, Hwang I, Guo HQ, Stoddard R, Huang J, Palmgren MG, Sze H (1998) A novel calmodulin-regulated Ca2+-ATPase (ACA2) from Arabidopsis with an N-terminal autoinhibitory domain. J Biol Chem 273:1099–1106 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Flügge UI, Fernandez JM (1986) Patch-clamp studies of ion transport in isolated plant vacuoles. FEBS Lett 204:228–232 [Google Scholar]
- Hedrich R, Marten I (2011) TPC1 - SV channels gain shape. Mol Plant 4:428–441 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Mueller TD, Becker D, Marten I (2018) Structure and function of TPC1 vacuole SV channel gains shape. Mol Plant 11:764–775 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Neher E (1987) Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature 329:833–836 [Google Scholar]
- Hilleary R, Paez-Valencia J, Vens C, Toyota M, Palmgren M, Gilroy S (2020) Tonoplast-localized Ca2+ pumps regulate Ca2+ signals during pattern-triggered immunity in Arabidopsis thaliana. Proc Natl Acad Sci USA 117:18849–18857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD (1999) Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell 11:2113–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ (2000) Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased manganese tolerance. Plant Physiol 124:125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD, Zhen R-G, Cunningham KW, Rea PA, Fink GR (1996) CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci USA 93:8782–8786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmal AK, Schulze S, Trompelt K, Hippler M (2015) Calcium-dependent regulation of photosynthesis. Biochim Biophys Act 1847:993–1003 [DOI] [PubMed] [Google Scholar]
- Hocking B, Conn SJ, Manohar M, Xu B, Athman A, Stancombe MA, Webb AR, Hirschi KD, Gilliham M (2017) Heterodimerization of Arabidopsis calcium/proton exchangers contributes to regulation of guard cell dynamics and plant defense responses. J Exp Bot 68:4171–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhner R, Galvis VC, Strand DD, Völkner C, Krämer M, Messer M, Dinc F, Sjuts I, Bölter B, Kramer DM, et al. (2019) Photosynthesis in Arabidopsis is unaffected by the function of the vacuolar K+ channel TPK3. Plant Physiol 180:1322–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ, Marschner H (1978) Effect of excessive manganese supply on uptake and translocation of calcium in bean plants (Phaseolus vulgaris L.). Zeitschr Pflanzenphysiol 87:137–148 [Google Scholar]
- Hu Q, Zhu L, Zhang X, Guan Q, Xiao S, Min L, Zhang X (2018) GhCPK33 negatively regulates defense against Verticillium dahliae by phosphorylating GhOPR3. Plant Physiol 178:876–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda KMK, Yadav S, Banu MSA, Trivedi DK, Tuteja N (2013) Genome-wide analysis of plant-type II Ca2+ATPases gene family from rice and Arabidopsis: potential role in abiotic stresses. Plant Physiol Biochem 65:32–47 [DOI] [PubMed] [Google Scholar]
- Jain A, Dashner ZS, Connolly EL (2019) Mitochondrial iron transporters (MIT1 and MIT2) are essential for iron homeostasis and embryogenesis in Arabidopsis thaliana. Front Plant Sci 10:1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaslan D, Dreyer I, Lu J, O’Malley R, Dindas J, Marten I, Hedrich R (2019) Voltage-dependent gating of SV channel TPC1 confers vacuole excitability. Nat Commun 10:2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia XY, He LH, Jing RL, Li RZ (2009) Calreticulin: conserved protein and diverse functions in plants. Physiol Plant 136:127–138 [DOI] [PubMed] [Google Scholar]
- Jiang D, Zhao L, Clapham DE (2009) Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326:144–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Zhao L, Clish CB, Clapham DE (2013) Letm1, the mitochondrial Ca2+/H+ antiporter, is essential for normal glucose metabolism and alters brain function in Wolf-Hirschhorn syndrome. Proc Natl Acad Sci USA 110:E2249–E2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Zhou X, Tao M, Yuan F, Liu L, Wu F, Wu X, Xiang Y, Niu Y, Liu F, et al. (2019) Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 572:341–346 [DOI] [PubMed] [Google Scholar]
- Jin X, Zhang Y, Alharbi A, Hanbashi A, Alhoshani A, Parrington J (2020) Targeting Two-Pore Channels: current progress and future challenges. Trends Pharmacol Sci 41:582–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NA, Liu FL, Weeks PD, Hentzen AE, Kruse HP, Parker JJ, Laursen M, Nissen P, Costa CJ, Gatto C (2009) A tomato ER-type Ca2+-ATPase, LCA1, has a low thapsigargin-sensitivity and can transport manganese. Arch Biochem Biophys 481:157–168 [DOI] [PubMed] [Google Scholar]
- Kamer KJ, Sancak Y, Fomina Y, Meisel JD, Chaudhuri D, Grabarek Z, Mootha VK (2018) MICU1 imparts the mitochondrial uniporter with the ability to discriminate between Ca2+ and Mn2 +. Proc Natl Acad Sci USA 115:E7960–E7969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T, Akahori T, Ashikari M, Maeshima M (2006) Expression of the vacuolar Ca2+/H+ exchanger, OsCAX1a, in rice: cell and age specificity of expression, and enhancement by Ca2 +. Plant Cell Physiol 47:96–106 [DOI] [PubMed] [Google Scholar]
- Kamiya T, Akahori T, Maeshima M (2005) Expression profile of the genes for rice cation/H+ exchanger family and functional analysis in yeast. Plant Cell Physiol 46:1735–1740 [DOI] [PubMed] [Google Scholar]
- Karley AJ, Leigh RA, Sanders D (2000) Differential ion accumulation and ion fluxes in the mesophyll and epidermis of barley. Plant Physiol 122:835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellokumpu S (2019) Golgi pH, ion and redox homeostasis: how much do they really matter? Front Cell Dev Biol 7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I, Gratz R, Denezhkin P, Schott-Verdugo SN, Angrand K, Genders L, Basgaran RM, Fink-Straube C, Brumbarova T, Gohlke H, et al. (2019) Calcium-promoted interaction between the C2-domain protein EHB1 and metal transporter IRT1 inhibits Arabidopsis iron acquisition. Plant Physiol 180:1564–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiep V, Vadassery J, Lattke J, Maaß J-P, Boland W, Peiter E, Mithöfer A (2015) Systemic cytosolic Ca2+ elevation is activated upon wounding and herbivory in Arabidopsis. New Phytol 207:996–1004 [DOI] [PubMed] [Google Scholar]
- Kim S, Zeng W, Bernard S, Liao J, Venkateshwaran M, Ane JM, Jiang Y (2019) Ca2+-regulated Ca2+ channels with an RCK gating ring control plant symbiotic associations. Nat Commun 10:3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SA, Punshon T, Lanzirotti A, Li LT, Alonso JM, Ecker JR, Kaplan J, Guerinot ML (2006) Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314:1295–1298 [DOI] [PubMed] [Google Scholar]
- Kintzer AF, Stroud RM (2016) Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature 531:258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintzer AF, Stroud RM (2018) On the structure and mechanism of two-pore channels. FEBS J 285:233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427:360–364 [DOI] [PubMed] [Google Scholar]
- Klüsener B, Boheim G, Liß H, Engelberth J, Weiler EW (1995) Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J 14:2708–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüsener B, Weiler EW (1999) A calcium-selective channel from root tip endomembranes of cress. Plant Physiol 119:1399–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Hu HC, Okuma E, Lee Y, Lee HS, Munemasa S, Cho D, Ju C, Pedoeim L, Rodriguez B, et al. (2016) L-Met activates Arabidopsis GLR Ca2+ channels upstream of ROS production and regulates stomatal movement. Cell Rep 17:2553–2561 [DOI] [PubMed] [Google Scholar]
- Kreimer G, Melkonian M, Holtum JAM, Latzko E (1988) Stromal free calcium concentration and light-mediated activation of chloroplast fructose-1,6-bisphosphatase. Plant Physiol 86:423–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Liszkay A, Thomine S (2018) Importing manganese into the chloroplast: many membranes to cross. Mol Plant 11:1109–1111 [DOI] [PubMed] [Google Scholar]
- Kudla J, Becker D, Grill E, Hedrich R, Hippler M, Kummer U, Parniske M, Romeis T, Schumacher K (2018) Advances and current challenges in calcium signaling. New Phytol 218:414–431 [DOI] [PubMed] [Google Scholar]
- Kuhn S, Bussemer J, Chigri F, Vothknecht UC (2009) Calcium depletion and calmodulin inhibition affect the import of nuclear-encoded proteins into plant mitochondria. Plant J 58:694–705 [DOI] [PubMed] [Google Scholar]
- Künzl F, Früholz S, Fäßler F, Li B, Pimpl P (2016) Receptor-mediated sorting of soluble vacuolar proteins ends at the trans-Golgi network/early endosome. Nat Plants 2:16017. [DOI] [PubMed] [Google Scholar]
- Lambers H, Hayes PE, Laliberté E, Oliveira RS, Turner BL (2015) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90 [DOI] [PubMed] [Google Scholar]
- Lange M, Peiter E (2020) Calcium transport proteins in fungi: the phylogenetic diversity of their relevance for growth, virulence, and stress resistance. Front Microbiol 10:3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M, Weihmann F, Schliebner I, Horbach R, Deising HB, Wirsel SGR, Peiter E (2016) The Transient Receptor Potential (TRP) channel family in Colletotrichum graminicola: a molecular and physiological analysis. PLoS One 11:e0158561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquar V, Schnell Ramos M, Lelièvre F, Barbier-Brygoo H, Krieger-Liszkay A, Krämer U, Thomine S (2010) Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol 152:1986–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Shang Z, Rubio L, Cuin TA, Véry AA, Wang A, Mortimer JC, Macpherson N, Coxon KM, Battey NH, et al. (2012) Arabidopsis Annexin1 mediates the radical-activated plasma membrane Ca2+- and K+-permeable conductance in root cells. Plant Cell 24:1522–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larisch N, Schulze C, Galione A, Dietrich P (2012) An N-terminal dileucine motif directs two-pore channels to the tonoplast of plant cells. Traffic 13:1012–1022 [DOI] [PubMed] [Google Scholar]
- Laursen T, Stonebloom SH, Pidatala VR, Birdseye DS, Clausen MH, Mortimer JC, Scheller HV (2018) Bifunctional glycosyltransferases catalyze both extension and termination of pectic galactan oligosaccharides. Plant J 94:340–351 [DOI] [PubMed] [Google Scholar]
- LeClere S, Tellez R, Rampey RA, Matsuda SPT, Bartel B (2002) Characterization of a family of IAA-amino acid conjugate hydrolases from Arabidopsis. J Biol Chem 277:20446–20452 [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Lamotte O, Bourque S, Wendehenne D, Mazars C, Ranjeva R, Pugin A (2005) Proteinaceous and oligosaccharidic elicitors induce different calcium signatures in the nucleus of tobacco cells. Cell Calcium 38:527–538 [DOI] [PubMed] [Google Scholar]
- Lee SM, Kim HS, Han HJ, Moon BC, Kim CY, Harper JF, Chung WS (2007) Identification of a calmodulin-regulated autoinhibited Ca2+-ATPase (ACA11) that is localized to vacuole membranes in Arabidopsis. FEBS Lett. 581:3943–3949 [DOI] [PubMed] [Google Scholar]
- Leitao N, Dangeville P, Carter R, Charpentier M (2019) Nuclear calcium signatures are associated with root development. Nat Commun 10:4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre T, Urbanczyk-Wochniak E, Flesch V, Bismuth E, Fernie AR, Hodges M (2007) NAD-dependent isocitrate dehydrogenase mutants of Arabidopsis suggest the enzyme is not limiting for nitrogen assimilation. Plant Physiol 144:1546–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EAC, Webb AAR, Manison NF, Brownlee C, Skepper JN, Chen J, Prestwich GD, Brearley CA (2003) Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proc Natl Acad Sci USA 100:10091–10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenglet A, Jaslan D, Toyota M, Mueller M, Müller T, Schönknecht G, Marten I, Gilroy S, Hedrich R, Farmer EE (2017) Control of basal jasmonate signalling and defence through modulation of intracellular cation flux capacity. New Phytol 216:1161–1169 [DOI] [PubMed] [Google Scholar]
- Lenzoni G, Knight MR (2019) Increases in absolute temperature stimulate free calcium concentration elevations in the chloroplast. Plant Cell Physiol 60:538–548 [DOI] [PubMed] [Google Scholar]
- Leshem Y, Melamed-Book N, Cagnac O, Ronen G, Nishri Y, Solomon M, Cohen G, Levine A (2006) Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc Natl Acad Sci USA 103:18008–18013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesková A, Giehl RFH, Hartmann A, Fargasová A, von Wirén N (2017) Heavy metals induce iron-deficiency responses at different hierarchic and regulatory levels. Plant Physiol 174:1648–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chanroj S, Wu Z, Romanowsky SM, Harper JF, Sze H (2008) A distinct endosomal Ca2+/Mn2+ pump affects root growth through the secretory process. Plant Physiol 147:1675–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Cunningham KW, Harper JF, Sze H (1997) ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc Natl Acad Sci USA 94:8579–8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebminger E, Hüttner S, Vavra U, Fischl R, Schoberer J, Grass J, Blaukopf C, Seifert GJ, Altmann F, Mach L, et al. (2009) Class I α-mannosidases are required for N-glycan processing and root development in Arabidopsis thaliana. Plant Cell 21:3850–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DC, Knight MR (2003) Mitochondrial and cytosolic calcium dynamics are differentially regulated in plants. Plant Physiol 133:21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loro G, Drago I, Pozzan T, Lo Schiavo F, Zottini M, Costa A (2012) Targeting of Cameleons to various subcellular compartments reveals a strict cytoplasmic/mitochondrial Ca2+ handling relationship in plant cells. Plant J 71:1–13 [DOI] [PubMed] [Google Scholar]
- Loro G, Wagner S, Doccula FG, Behera S, Weinl S, Kudla J, Schwarzländer M, Costa A, Zottini M (2016) Chloroplast-specific in vivo Ca2+ imaging using Yellow Cameleon fluorescent protein sensors reveals organelle-autonomous Ca2+ signatures in the stroma. Plant Physiol 171:2317–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loulakakis CA, Roubelakis-Angelakis KA (1990) Intracellular localization and properties of NADH-glutamate dehydrogenase from Vitis vinifera L.: purification and characterization of the major leaf isoenzyme. J Exp Bot 41:1223–1230 [Google Scholar]
- Luk EEC, Culotta VC (2001) Manganese superoxide dismutase in Saccharomyces cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transporter, Smf2p. J Biol Chem 276:47556–47562 [DOI] [PubMed] [Google Scholar]
- Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, Carpenter AC, Kolmetzky D, Gao E, van Berlo JH, et al. (2017) The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature 545:93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G, Li JY, Li JJ, Li Y, Gu DF, Chen C, Cui J, Chen X, Zhang W (2018) OsMTP11, a trans-Golgi network localized transporter, is involved in manganese tolerance in rice. Plant Sci 274:59–69 [DOI] [PubMed] [Google Scholar]
- Ma T, Peng Y, Huang W, Liu Y, Ding J (2017) The β and γ subunits play distinct functional roles in the α2βγ heterotetramer of human NAD-dependent isocitrate dehydrogenase. Sci Rep 7:41882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malabarba J,, Meents AK, Reichelt M, Scholz SS, Peiter E, Rachowka J, Konopka-Postupolska D, Wilkins KA, Davies JM, et al. (2021) ANNEXIN1 mediates calcium-dependent systemic defense in Arabidopsis plants upon herbivory and wounding. New Phytol doi: 10.1111/nph.17277. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Mandal D, Rulli SJ, Rao R (2003) Packing interactions between transmembrane helices alter ion selectivity of the yeast Golgi Ca2+/Mn2+-ATPase PMR1. J Biol Chem 278:35292–35298 [DOI] [PubMed] [Google Scholar]
- Mandal D, Woolf TB, Rao R (2000) Manganese selectivity of Pmr1, the yeast secretory pathway ion pump, is defined by residue Gln783 in transmembrane segment 6. Residue Asp778 is essential for cation transport. J Biol Chem 275:23933–23938 [DOI] [PubMed] [Google Scholar]
- Martinière A, Bassil E, Jublanc E, Alcon C, Reguera M, Sentenac H, Blumwald E, Paris N (2013) In vivo intracellular pH measurements in tobacco and Arabidopsis reveal an unexpected pH gradient in the endomembrane system. Plant Cell 25:4028–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins V, Carneiro F, Conde C, Sottomayor M, Gerós H (2017) The grapevine VvCAX3 is a cation/H+ exchanger involved in vacuolar Ca2+ homeostasis. Planta 246:1083–1096 [DOI] [PubMed] [Google Scholar]
- Matsushima R, Hayashi Y, Yamada K, Shimada T, Nishimura M, Hara-Nishimura I (2003) The ER body, a novel endoplasmic reticulum-derived structure in Arabidopsis. Plant Cell Physiol 44:661–666 [DOI] [PubMed] [Google Scholar]
- Mazars C, Bourque S, Mithöfer A, Pugin A, Ranjeva R (2009) Calcium homeostasis in plant cell nuclei. New Phytol 181:261–274 [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Webb AAR, Taylor JE, Hetherington AM (1995) Stimulus-induced oscillations in guard cell cytoplasmic free calcium. Plant Cell 7:1207–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack JG, Halestrap AP, Denton RM (1990) Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70:391–425 [DOI] [PubMed] [Google Scholar]
- Mei H, Cheng NH, Zhao J, Park S, Escareno RA, Pittman JK, Hirschi KD (2009) Root development under metal stress in Arabidopsis thaliana requires the H+/cation antiporter CAX4. New Phytol 183:95–105 [DOI] [PubMed] [Google Scholar]
- Meyerhoff O, Müller K, Roelfsema MR, Latz A, Lacombe B, Hedrich R, Dietrich P, Becker D (2005) AtGLR3.4, a glutamate receptor channel-like gene is sensitive to touch and cold. Planta 222:418–427 [DOI] [PubMed] [Google Scholar]
- Miao Y, Yan PK, Kim H, Hwang I, Jiang L (2006) Localization of green fluorescent protein fusions with the seven Arabidopsis vacuolar sorting receptors to prevacuolar compartments in tobacco BY-2 cells. Plant Physiol 142:945–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RF, Doherty ML, López-Marqués RL, Weimar T, Dupree P, Palmgren MG, Pittman JK, Williams LE (2008) ECA3, a Golgi-localized P2A-type ATPase, plays a crucial role in manganese nutrition in Arabidopsis. Plant Physiol 146:116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner MJ, Seamon J, Craft E, Kochian LV (2013) Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J Exp Bot 64:369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Miller ND, Murphy AS, Gilroy S (2011) Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant J 65:309–318 [DOI] [PubMed] [Google Scholar]
- Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M (2007) Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genom 8:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremen KW, Haltiwanger RS (2019) Emerging structural insights into glycosyltransferase-mediated synthesis of glycans. Nat Chem Biol 15:853–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno I, Norambuena L, Maturana D, Toro M, Vergara C, Orellana A, Zurita-Silva A, Ordenes VR (2008) AtHMA1 is a thapsigargin-sensitive Ca2+/heavy metal pump. J Biol Chem 283:9633–9641 [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Lehmann M, Schwarzländer M, Baxter CJ, Sienkiewicz-Porzucek A, Williams TCR, Schauer N, Fernie AR, Fricker MD, Ratcliffe RG, et al. (2008) Decrease in manganese superoxide dismutase leads to reduced root growth and affects tricarboxylic acid cycle flux and mitochondrial redox homeostasis. Plant Physiol 147:101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou W, Kao YT, Michard E, Simon AA, Li D, Wudick MM, Lizzio MA, Feijó JA, Chang C (2020) Ethylene-independent signaling by the ethylene precursor ACC in Arabidopsis ovular pollen tube attraction. Nat Commun 11:4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SAR, Chauvin A, Pascaud F, Kellenberger S, Farmer EE (2013) GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500:422–426 [DOI] [PubMed] [Google Scholar]
- Murray JW, Barber J (2006) Identification of a calcium-binding site in the PsbO protein of photosystem II. Biochemistry 45:4128–4130 [DOI] [PubMed] [Google Scholar]
- Muto S, Izawa S, Miyachi S (1982) Light-induced Ca2+ uptake by intact chloroplasts. FEBS Lett 139:250–254 [Google Scholar]
- Nagashima Y, von Schaewen A, Koiwa H (2018) Function of N-glycosylation in plants. Plant Sci 274:70–79 [DOI] [PubMed] [Google Scholar]
- Nardi MC, Feron R, Navazio L, Mariani P, Pierson E, Wolters-Arts M, Knuiman B, Mariani C, Derksen J (2006) Expression and localization of calreticulin in tobacco anthers and pollen tubes. Planta 223:1263–1271 [DOI] [PubMed] [Google Scholar]
- Navazio L, Bewell MA, Siddiqua A, Dickinson GD, Galione A, Sanders D (2000) Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proc Natl Acad Sci USA 97:8693–8698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Formentin E, Cendron L, Szabò I (2020) Chloroplast calcium signaling in the spotlight. Front Plant Sci 11:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Mariani P, Sanders D (2001) Mobilization of Ca2+ by cyclic ADP-ribose from the endoplasmic reticulum of cauliflower florets. Plant Physiol 125:2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo Y, Nelson N (2006) The NRAMP family of metal-ion transporters. Biochim Biophys Act 1763:609–620 [DOI] [PubMed] [Google Scholar]
- Nguema-Ona E, Vicré-Gibouin M, Gotté M, Plancot B, Lerouge P, Bardor M, Driouich A (2014) Cell wall O-glycoproteins and N-glycoproteins: aspects of biosynthesis and function. Front Plant Sci 5:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CT, Kurenda A, Stolz S, Chételat A, Farmer EE (2018) Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc Natl Acad Sci USA 115:10178–10183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E, Cheung AY, Ueda T (2008) The regulatory RAB and ARF GTPases for vesicular trafficking. Plant Physiol 147:1516–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H, Komori T, Kobori M, Nakahira Y, Shiina T (2008) Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J 53:988–998 [DOI] [PubMed] [Google Scholar]
- Nomura H, Komori T, Uemura S, Kanda Y, Shimotani K, Nakai K, Furuichi T, Takebayashi K, Sugimoto T, Sano S, et al. (2012) Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat Commun 3:926. [DOI] [PubMed] [Google Scholar]
- Ohtsuru M, Kawatani H (1979) Studies on the myrosinase from Wasabia japonica: purification and some properties of Wasabi myrosinase. Agric Biol Chem 43:2249–2255 [Google Scholar]
- Ono S, Suzuki S, Ito D, Tagawa S, Shiina T, Masuda S (2020) Plastidial (p)ppGpp synthesis by the Ca2+-dependent RelA-SpoT homolog regulates the adaptation of chloroplast gene expression to darkness in Arabidopsis. Plant Cell Physiol 61: 2077–2086 [DOI] [PubMed] [Google Scholar]
- Oomen RJFJ, Wu J, Lelièvre F, Blanchet S, Richaud P, Barbier-Brygoo H, Aarts MGM, Thomine S (2009) Functional characterization of NRAMP3 and NRAMP4 from the metal hyperaccumulator Thlaspi caerulescens. New Phytol 181:637–650 [DOI] [PubMed] [Google Scholar]
- Ordenes VR, Moreno I, Maturana D, Norambuena L, Trewavas AJ, Orellana A (2012) In vivo analysis of the calcium signature in the plant Golgi apparatus reveals unique dynamics. Cell Calcium 52:397–404 [DOI] [PubMed] [Google Scholar]
- Pagny S, Bouissonnie F, Sarkar M, Follet-Gueye ML, Driouich A, Schachter H, Faye L, Gomord V (2003) Structural requirements for Arabidopsis β1,2-xylosyltransferase activity and targeting to the Golgi. Plant J 33:189–203 [DOI] [PubMed] [Google Scholar]
- Palma AS, Morais VA, Coelho AV, Costa J (2004) Effect of the manganese ion on human α3/4 fucosyltransferase III activity. Biometals 17:35–43 [DOI] [PubMed] [Google Scholar]
- Palmer AE, Jin C, Reed JC, Tsien RY (2004) Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci USA 101:17404–17409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan R, Liu J, Wang S, Hu J (2020) Peroxisomes: versatile organelles with diverse roles in plants. New Phytol 225:1410–1427 [DOI] [PubMed] [Google Scholar]
- Patel J, Ariyaratne M, Ahmed S, Ge L, Phuntumart V, Kalinoski A, Morris PF (2017) Dual functioning of plant arginases provides a third route for putrescine synthesis. Plant Sci 262:62–73 [DOI] [PubMed] [Google Scholar]
- Peiter E (2011) The plant vacuole: Emitter and receiver of calcium signals. Cell Calcium 50:120–128 [DOI] [PubMed] [Google Scholar]
- Peiter E (2014) Mineral Deficiencies. InKrauss GJ, Nies DH, eds, Ecological Biochemistry. Wiley-VCH, Weinheim, Germany, pp 209–222 [Google Scholar]
- Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434:404–408 [DOI] [PubMed] [Google Scholar]
- Peiter E, Montanini B, Gobert A, Pedas P, Husted S, Maathuis FJM, Blaudez D, Chalot M, Sanders D (2007a) A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. Proc Natl Acad Sci USA 104:8532–8537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiter E, Sun J, Heckmann AB, Venkateshwaran M, Riely BK, Otegui MS, Edwards A, Freshour G, Hahn MG, Cook DR, et al. (2007b) The Medicago truncatula DMI1 protein modulates cytosolic calcium signaling. Plant Physiol 145:192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiter E, Yan F, Schubert S (2000) Are mineral nutrients a critical factor for lime intolerance of lupins? J Plant Nutr 23:617–635 [Google Scholar]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK (2010) MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 467:291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen BL, Egelund J, Damager I, Faber K, Jensen JK, Yang Z, Bennett EP, Scheller HV, Ulvskov P (2009) Assay and heterologous expression in Pichia pastoris of plant cell wall type-II membrane anchored glycosyltransferases. Glycoconjugate J 26:1235–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OH, Courjaret R, Machaca K (2017) Ca2+ tunnelling through the ER lumen as a mechanism for delivering Ca2+ entering via store-operated Ca2+ channels to specific target sites. J Physiol 595:2999–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirayesh N, Giridhar M, Ben Khedher A, Vothknecht UC, Chigri F (2021) Organellar calcium signaling in plants: an update. Biochim Biophys Act 1868:118948. [DOI] [PubMed] [Google Scholar]
- Pittman JK (2005) Managing the manganese: molecular mechanisms of manganese transport and homeostasis. New Phytol 167:733–742 [DOI] [PubMed] [Google Scholar]
- Pittman JK, Hirschi KD (2016) CAX-ing a wide net: Cation/H+ transporters in metal remediation and abiotic stress signalling. Plant Biol 18:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman JK, Shigaki T, Marshall JL, Morris JL, Cheng NH, Hirschi KD (2004) Functional and regulatory analysis of the Arabidopsis thaliana CAX2 cation transporter. Plant Mol Biol 56:959–971 [DOI] [PubMed] [Google Scholar]
- Pittman JK, Sreevidya CS, Shigaki T, Ueoka-Nakanishi H, Hirschi KD (2002) Distinct N-terminal regulatory domains of Ca2+/H+ antiporters. Plant Physiol 130:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P,, LissandronV, , CapitanioP, , Pozzan T (2011) Ca2+ signalling in the Golgi apparatus. Cell Calcium 50: 184–192 [DOI] [PubMed] [Google Scholar]
- Planchet E, Gupta KJ, Sonoda M, Kaiser WM (2005) Nitric oxide emission from tobacco leaves and cell suspensions: rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J 41:732–743 [DOI] [PubMed] [Google Scholar]
- Potelle S, Dulary E, Climer L, Duvet S, Morelle W, Vicogne D, Lebredonchel E, Houdou M, Spriet C, Krzewinski-Recchi MA, et al. (2017) Manganese-induced turnover of TMEM165. Biochem J 474:1481–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potelle S, Morelle W, Dulary E, Duvet S, Vicogne D, Spriet C, Krzewinski-Recchi MA, Morsomme P, Jaeken J, Matthijs G, et al. (2016) Glycosylation abnormalities in Gdt1p/TMEM165 deficient cells result from a defect in Golgi manganese homeostasis. Hum Mol Genet 25:1489–1500 [DOI] [PubMed] [Google Scholar]
- Pottosin I, Dobrovinskaya O (2018) Two-pore cation (TPC) channel: not a shorthanded one. Funct Plant Biol 45:83–92 [DOI] [PubMed] [Google Scholar]
- Punshon T, Hirschi K, Yang J, Lanzirotti A, Lai B, Guerinot ML (2012) The role of CAX1 and CAX3 in elemental distribution and abundance in Arabidopsis seed. Plant Physiol 158:352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Stephens NR, Spalding EP (2006) Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol 142:963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmati Ishka M, Brown E, Rosenberg A, Romanowsky S, Davis JA, Choi WG, Harper JF (2021) Arabidopsis Ca2+-ATPases 1, 2, and 7 in the endoplasmic reticulum contribute to growth and pollen fitness. Plant Physiol 185: 1966–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampey RA, LeClere S, Kowalczyk M, Ljung K, Sandberg G, Bartel B (2004) A family of auxin-conjugate hydrolases that contributes to free indole-3-acetic acid levels during Arabidopsis germination. Plant Physiol 135:978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranf S, Wünnenberg P, Lee J, Becker D, Dunkel M, Hedrich R, Scheel D, Dietrich P (2008) Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J 53:287–299 [DOI] [PubMed] [Google Scholar]
- Reddi AR, Jensen LT, Culotta VC (2009) Manganese homeostasis in Saccharomyces cerevisiae. Chem Rev 109:4722–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie EA, Ebert B, Miles GP, Cahoon RE, Christiansen KM, Stonebloom S, Khatab H, Twell D, Petzold CJ, Adams PD, et al. (2014) Identification of a sphingolipid α-glucuronosyltransferase that is essential for pollen function in Arabidopsis. Plant Cell 26:3314–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie EA, Hansen SF, Baidoo EEK, Hadi MZ, Keasling JD, Scheller HV (2012) Three members of the Arabidopsis glycosyltransferase family 8 are xylan glucuronosyltransferases. Plant Physiol 159:1408–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricachenevsky FK, Menguer PK, Sperotto RA, Williams LE, Fett JP (2013) Roles of plant metal tolerance proteins (MTP) in metal storage and potential use in biofortification strategies. Front Plant Sci 4:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SL, Laohavisit A, Mortimer JC, Shabala L, Swarbreck SM, Shabala S, Davies JM (2014) Annexin 1 regulates the H2O2-induced calcium signature in Arabidopsis thaliana roots. Plant J 77:136–145 [DOI] [PubMed] [Google Scholar]
- Rienmüller F, Beyhl D, Lautner S, Fromm J, Al-Rasheid KAS, Ache P, Farmer EE, Marten I, Hedrich R (2010) Guard cell-specific calcium sensitivity of high density and activity SV/TPC1 channels. Plant Cell Physiol 51:1548–1554 [DOI] [PubMed] [Google Scholar]
- Rissel D, Peiter E (2019) Poly(ADP-ribose) polymerases in plants and their human counterparts: parallels and peculiarities. Int J Mol Sci 20:1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Pimpl P (2014) Receptor-mediated transport of vacuolar proteins: a critical analysis and a new model. Protoplasma 251:247–264 [DOI] [PubMed] [Google Scholar]
- Rocha AG, Vothknecht UC (2012) The role of calcium in chloroplasts-an intriguing and unresolved puzzle. Protoplasma 249:957–966 [DOI] [PubMed] [Google Scholar]
- Rohdich F, Lauw S, Kaiser J, Feicht R, Köhler P, Bacher A, Eisenreich W (2006) Isoprenoid biosynthesis in plants - 2C-methyl-D-erythritol-4-phosphate synthase (IspC protein) of Arabidopsis thaliana. FEBS J 273:4446–4458 [DOI] [PubMed] [Google Scholar]
- Ruge H, Flosdorff S, Ebersberger I, Chigri F, Vothknecht UC (2016) The calmodulin-like proteins AtCML4 and AtCML5 are single-pass membrane proteins targeted to the endomembrane system by an N-terminal signal anchor sequence. J Exp Bot 67:3985–3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MCM, Jung HJ, Webb AAR (2020) Circadian gating of dark-induced increases in chloroplast- and cytosolic-free calcium in Arabidopsis. New Phytol 225:1993–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai J, Johnson CH (2002) Dark-stimulated calcium ion fluxes in the chloroplast stroma and cytosol. Plant Cell 14:1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Barrena MJ, Martínez-Ripoll M, Zhu JK, Albert A (2005) The structure of the Arabidopsis thaliana SOS3: molecular mechanism of sensing calcium for salt stress response. J Mol Biol 345:1253–1264 [DOI] [PubMed] [Google Scholar]
- Sánchez JP, Duque P, Chua NH (2004) ABA activates ADPR cyclase and cADPR induces a subset of ABA-responsive genes in Arabidopsis. Plant J 38:381–395 [DOI] [PubMed] [Google Scholar]
- Sanchez Carranza AP, , Singh A, Steinberger K, Panigrahi K, Palme K, Dovzhenko A, Dal Bosco C (2016) Hydrolases of the ILR1-like family of Arabidopsis thaliana modulate auxin response by regulating auxin homeostasis in the endoplasmic reticulum. Scientific Reports 6: 24212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf G, Catoni E, Fitz M, Schwacke R, Schneider A, von Wirén N, Frommer WB (2002) A putative role for the vacuolar calcium/manganese proton antiporter AtCAX2 in heavy metal detoxification. Plant Biol 4:612–618 [Google Scholar]
- Schmidt SB, Eisenhut M, Schneider A (2020) Chloroplast transition metal regulation for efficient photosynthesis. Trends Plant Sci 25:817–828 [DOI] [PubMed] [Google Scholar]
- Schmidt SB, Powikrowska M, Krogholm KS, Naumann-Busch B, Schjoerring JK, Husted S, Jensen PE, Pedas PR (2016) Photosystem II functionality in barley responds dynamically to changes in leaf manganese status. Front Plant Sci 7:1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Steinberger I, Herdean A, Gandini C, Eisenhut M, Kurz S, Morper A, Hoecker N, Ruhle T, Labs M, et al. (2016) The evolutionarily conserved protein PHOTOSYNTHESIS AFFECTED MUTANT71 is required for efficient manganese uptake at the thylakoid membrane in Arabidopsis. Plant Cell 28:892–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoberer J, Strasser R (2018) Plant glyco-biotechnology. Sem Cell Dev Biol 80:133–141 [DOI] [PubMed] [Google Scholar]
- Schulze C, Sticht H, Meyerhoff P, Dietrich P (2011) Differential contribution of EF-hands to the Ca2+-dependent activation in the plant two-pore channel TPC1. Plant J 68:424–432 [DOI] [PubMed] [Google Scholar]
- Seals DF, Parrish ML, Randall SK (1994) A 42-kilodalton annexin-like protein is associated with plant vacuoles. Plant Physiol 106:1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selles B, Michaud C, Xiong T-C, Leblanc O, Ingouff M (2018) Arabidopsis pollen tube germination and growth depend on the mitochondrial calcium uniporter complex. New Phytol 219:58–65 [DOI] [PubMed] [Google Scholar]
- Sello S, Moscatiello R, Mehlmer N, Leonardelli M, Carraretto L, Cortese E, Zanella FG, Baldan B, Szabò I, Vothknecht UC. et al. (2018) Chloroplast Ca2+ fluxes into and across thylakoids revealed by thylakoid-targeted aequorin probes. Plant Physiol 177:38–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sello S, Perotto J, Carraretto L, Szabò I, Vothknecht UC, Navazio L (2016) Dissecting stimulus-specific Ca2+ signals in amyloplasts and chloroplasts of Arabidopsis thaliana cell suspension cultures. J Exp Bot 67:3965–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senguen FT, Grabarek Z (2012) X-ray structures of magnesium and manganese complexes with the N-terminal domain of calmodulin: insights into the mechanism and specificity of metal ion binding to an EF-hand. Biochemistry 51:6182–6194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao JF, Yamaji N, Shen RF, Ma JF (2017) The key to Mn homeostasis in plants: regulation of Mn transporters. Trends Plant Sci 22:215–224 [DOI] [PubMed] [Google Scholar]
- Shao Q, Gao Q, Lhamo D, Zhang H, Luan S (2020) Two glutamate- and pH-regulated Ca2+ channels are required for systemic wound signaling in Arabidopsis. Sci Signal 13:eaba1453. [DOI] [PubMed] [Google Scholar]
- Shen J, Zeng Y, Zhuang X, Sun L, Yao X, Pimpl P, Jiang L (2013) Organelle pH in the Arabidopsis endomembrane system. Mol Plant 6:1419–1437 [DOI] [PubMed] [Google Scholar]
- Shi H, Ye T, Chen F, Cheng Z, Wang Y, Yang P, Zhang Y, Chan Z (2013) Manipulation of arginase expression modulates abiotic stress tolerance in Arabidopsis: effect on arginine metabolism and ROS accumulation. J Exp Bot 64:1367–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigaki T, Pittman JK, Hirschi KD (2003) Manganese specificity determinants in the Arabidopsis metal/H+ antiporter CAX2. J Biol Chem 278:6610–6617 [DOI] [PubMed] [Google Scholar]
- Shih HW, DePew CL, Miller ND, Monshausen GB (2015) The cyclic nucleotide-gated channel CNGC14 regulates root gravitropism in Arabidopsis thaliana. Curr Biol 25:3119–3125 [DOI] [PubMed] [Google Scholar]
- Shin I, Han K, Rhee S (2014) Structural insights into the substrate specificity of (S)-ureidoglycolate amidohydrolase and its comparison with allantoate amidohydrolase. J Mol Biol 426:3028–3040 [DOI] [PubMed] [Google Scholar]
- Shin I, Percudani R, Rhee S (2012) Structural and functional insights into (S)-ureidoglycine aminohydrolase, key enzyme of purine catabolism in Arabidopsis thaliana. J Biol Chem 287:18796–18805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkolnik D, Nuriel R, Bonza MC, Costa A, Fromm H (2018) MIZ1 regulates ECA1 to generate a slow, long-distance phloem-transmitted Ca2+ signal essential for root water tracking in Arabidopsis. Proc Natl Acad Sci USA 115:8031–8036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter AM, Basu D (2016) Extensin and arabinogalactan-protein biosynthesis: glycosyltransferases, research challenges, and biosensors. Front Plant Sci 7:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberer BJ, Chabaud M, Timmers AC, Monin A, Fournier J, Barker DG (2009) A nuclear-targeted cameleon demonstrates intranuclear Ca2+ spiking in Medicago truncatula root hairs in response to rhizobial nodulation factors. Plant Physiol 151:1197–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair SA, Senger T, Talke IN, Cobbett CS, Haydon MJ, Krämer U (2018) Systemic upregulation of MTP2- and HMA2-mediated Zn partitioning to the shoot supplements local Zn deficiency responses. Plant Cell 30:2463–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Parniske M (2012) Activation of calcium- and calmodulin-dependent protein kinase (CCaMK), the central regulator of plant root endosymbiosis. Curr Opin Plant Biol 15:444–453 [DOI] [PubMed] [Google Scholar]
- Socha AL, Guerinot ML (2014) Mn-euvering manganese: the role of transporter gene family members in manganese uptake and mobilization in plants. Front Plant Sci 5:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stael S (2019) Chloroplast calcium signalling quenches a thirst. Nat Plants 5:559–560 [DOI] [PubMed] [Google Scholar]
- Stael S, Wurzinger B, Mair A, Mehlmer N, Vothknecht UC, Teige M (2012) Plant organellar calcium signalling: an emerging field. J Exp Bot 63:1525–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey R, Leigh RA (2004) Processes modulating calcium distribution in citrus leaves. An investigation using x-ray microanalysis with strontium as a tracer. Plant Physiol 136:3838–3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R, Bondili JS, Vavra U, Schoberer J,, Svoboda B, Glössl J, Léonard R, Stadlmann J, Altmann F, Steinkellner H, et al. (2007) A unique β 1,3-galactosyltransferase is indispensable for the biosynthesis of N-glycans containing Lewis a structures in Arabidopsis thaliana. Plant Cell 19:2278–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stribny J, Thines L, Deschamps A, Goffin P, Morsomme P (2020) The human Golgi protein TMEM165 transports calcium and manganese in yeast and bacterial cells. J Biol Chem 295:3865–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (2013) Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 80:675–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen PK, Shen JB, Sun SSM, Jiang L (2010) Expression and characterization of two functional vacuolar sorting receptor (VSR) proteins, BP-80 and AtVSR4 from culture media of transgenic tobacco BY-2 cells. Plant Sci 179:68–76 [Google Scholar]
- Suzuki J, Kanemaru K, Iino M (2016) Genetically encoded fluorescent indicators for organellar calcium imaging. Biophys J 111:1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Duchen MR (2008) Mitochondria: the hub of cellular Ca2+ signaling. Physiology 23:84–94 [DOI] [PubMed] [Google Scholar]
- Takemoto Y, Tsunemitsu Y, Fujii-Kashino M, Mitani-Ueno N, Yamaji N, Ma JF, Kato S, Iwasaki K, Ueno D (2017) The tonoplast-localized transporter MTP8.2 contributes to manganese detoxification in the shoots and roots of Oryza sativa L. Plant Cell Physiol 58:1573–1582 [DOI] [PubMed] [Google Scholar]
- Tartaglio V, Rennie EA, Cahoon R, Wang G, Baidoo E, Mortimer JC, Cahoon EB, Scheller HV (2017) Glycosylation of inositol phosphorylceramide sphingolipids is required for normal growth and reproduction in Arabidopsis. Plant J 89:278–290 [DOI] [PubMed] [Google Scholar]
- Teardo E, Carraretto L, De Bortoli S, Costa A, Behera S, Wagner R, Lo Schiavo F, Formentin E, Szabo I (2015) Alternative splicing-mediated targeting of the Arabidopsis GLUTAMATE RECEPTOR3.5 to mitochondria affects organelle morphology. Plant Physiol 167:216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teardo E, Carraretto L, Moscatiello R, Cortese E, Vicario M, Festa M, Maso L, De Bortoli S, Cali T, Vothknecht UC, et al. (2019) A chloroplast-localized mitochondrial calcium uniporter transduces osmotic stress in Arabidopsis. Nat Plants 5:581–588 [DOI] [PubMed] [Google Scholar]
- Teardo E, Carraretto L, Wagner S, Formentin E, Behera S, De Bortoli S, Larosa V, Fuchs P, Lo Schiavo F, Raffaello A, et al. (2017) Physiological characterization of a plant mitochondrial calcium uniporter in vitro and in vivo. Plant Physiol 173:1355–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teardo E, Formentin E, Segalla A, Giacometti GM, Marin O, Zanetti M, Lo SF, Zoratti M, Szabò I (2011) Dual localization of plant glutamate receptor AtGLR3.4 to plastids and plasmamembrane. Biochim Biophys Act 1807:359–367 [DOI] [PubMed] [Google Scholar]
- Teardo E, Segalla A, Formentin E, Zanetti M, Marin O, Giacometti GM, Lo Schiavo F, Zoratti M, Szabò I (2010) Characterization of a plant glutamate receptor activity. Cell Physiol Biochem 26:253–262 [DOI] [PubMed] [Google Scholar]
- Tercé-Laforgue T, Dubois F, Ferrario-Méry S, de Crecenzo M-AP, Sangwan R, Hirel B (2004) Glutamate dehydrogenase of tobacco is mainly induced in the cytosol of phloem companion cells when ammonia is provided either externally or released during photorespiration. Plant Physiol 136:4308–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines L, Deschamps A, Sengottaiyan P, Savel O, Stribny J, Morsomme P (2018) The yeast protein Gdt1p transports Mn2+ ions and thereby regulates manganese homeostasis in the Golgi. J Biol Chem 293:8048–8055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines L, Stribny J, Morsomme P (2020) From the Uncharacterized Protein Family 0016 to the GDT1 family: molecular insights into a newly-characterized family of cation secondary transporters. Microbial Cell 7:202–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomine S, Lelièvre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H (2003) AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J 34:685–695 [DOI] [PubMed] [Google Scholar]
- Thor K (2019) Calcium - nutrient and messenger. Front Plant Sci 10:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor K, Peiter E (2014) Cytosolic calcium signals elicited by the pathogen-associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature. New Phytol 204:873–881 [DOI] [PubMed] [Google Scholar]
- Tian W, Wang C, Gao Q, Li L, Luan S (2020) Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat Plants 6:750–759 [DOI] [PubMed] [Google Scholar]
- Toyota M, Spencer D, Sawai-Toyota S, Wang J, Zhang T, Koo AJ, Howe GA, Gilroy S (2018) Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361:1112–1115 [DOI] [PubMed] [Google Scholar]
- Tronconi MA, Maurino VG, Andreo CS, Drincovich MF (2010) Three different and tissue-specific NAD-malic enzymes generated by alternative subunit association in Arabidopsis thaliana. J Biol Chem 285:11870–11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MF, Jiang D, Zhao L, Clapham D, Miller C (2014) Functional reconstitution of the mitochondrial Ca2+/H+ antiporter Letm1. J Gen Physiol 143:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MF, Phillips CB, Ranaghan M, Tsai CW, Wu Y, Williams C, Miller C (2016) Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex. eLife 5:e15545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui EY, Tran R, Yano J, Agapie T (2013) Redox-inactive metals modulate the reduction potential in heterometallic manganese-oxido clusters. Nat Chem 5:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemitsu Y, Genga M, Okada T, Yamaji N, Ma JF, Miyazaki A, Kato S, Iwasaki K, Ueno D (2018) A member of cation diffusion facilitator family, MTP11, is required for manganese tolerance and high fertility in rice. Planta 248:231–241 [DOI] [PubMed] [Google Scholar]
- Tun NN, Santa-Catarina C, Begum T, Silveira V, Handro W, Floh EIS, Scherer GFE (2006) Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol 47:346–354 [DOI] [PubMed] [Google Scholar]
- Turano FJ, Thakkar SS, Fang T, Weisemann JM (1997) Characterization and expression of NAD(H)-dependent glutamate dehydrogenase genes in Arabidopsis. Plant Physiol 113:1329–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TL, Bourne EC, Von Wettberg EJ, Hu TT, Nuzhdin SV (2010) Population resequencing reveals local adaptation of Arabidopsis lyrata to serpentine soils. Nat Genet 42:260–263 [DOI] [PubMed] [Google Scholar]
- Ueoka-Nakanishi H, Tsuchiya T, Sasaki M, Nakanishi Y, Cunningham KW, Maeshima M (2000) Functional expression of mung bean Ca2+/H+ antiporter in yeast and its intracellular localization in the hypocotyl and tobacco cells. Eur J Biochem 267:3090–3098 [DOI] [PubMed] [Google Scholar]
- Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 A. Nature 473:55–60 [DOI] [PubMed] [Google Scholar]
- Vainonen JP, Sakuragi Y, Stael S, Tikkanen M, Allahverdiyeva Y, Paakkarinen V, Aro E, Suorsa M, Scheller HV, Vener AV, et al. (2008) Light regulation of CaS, a novel phosphoprotein in the thylakoid membrane of Arabidopsis thaliana. FEBS J 275:1767–1777 [DOI] [PubMed] [Google Scholar]
- Venkateshwaran M, Cosme A, Han L, Banba M, Satyshur KA, Schleiff E, Parniske M, Imaizumi-Anraku H, Ané JM (2012) The recent evolution of a symbiotic ion channel in the legume family altered ion conductance and improved functionality in calcium signaling. Plant Cell 24:2528–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent TR, Avramova M, Canham J, Higgins P, Bilkey N, Mugford ST, Pitino M, Toyota M, Gilroy S, Miller AJ, et al. (2017) Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. Plant Cell 29:1460–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Behera S, De Bortoli S, Logan DC, Fuchs P, Carraretto L, Teardo E, Cendron L, Nietzel T, Füßl M, et al. (2015) The EF-hand Ca2+ binding protein MICU choreographs mitochondrial Ca2+ dynamics in Arabidopsis. Plant Cell 27:3190–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, De Bortoli S, Schwarzländer M, Szabò I (2016) Regulation of mitochondrial calcium in plants versus animals. J Exp Bot 67:3809–3829 [DOI] [PubMed] [Google Scholar]
- Waight AB,, Pedersen BP, Schlessinger A,, Bonomi M, Chau BH, Roe-Zurz Z, Risenmay AJ, Sali A, Stroud RM (2013) Structural basis for alternating access of a eukaryotic calcium/proton exchanger. Nature 499:107–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Essuman K, Anderson RG, Sasaki Y, Monteiro F, Chung EH, Nishimura EO, DiAntonio A, Milbrandt J, Dangl JL, et al. (2019) TIR domains of plant immune receptors are NAD+-cleaving enzymes that promote cell death. Science 365:799–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Guan Y, Lv M, Zhang R, Guo Z, Wei X, Du X, Yang J, Li T, Wan Y, et al. (2018) Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity 48:675–687 [DOI] [PubMed] [Google Scholar]
- Wang C, Ou D, Wang C, Lu X, Du J, Li J, Lai J, Zhang S, Yang C (2020) Functional characterization of a chloroplast-localized Mn2+Ca2+/H+ antiporter, ZmmCCHA1 from Zea mays ssp. mexicana L. Plant Physiol Biochem 155:396–405 [DOI] [PubMed] [Google Scholar]
- Wang C, Xu W, Jin H, Zhang T, Lai J, Zhou X, Zhang S, Liu S, Duan X, Wang H, et al. (2016) A putative chloroplast-localized Ca2+/H+ antiporter CCHA1 is involved in calcium and pH homeostasis and required for PSII function in Arabidopsis. Mol Plant 9:1183–1196 [DOI] [PubMed] [Google Scholar]
- Wang H, Han S, Siao W, Song C, Xiang Y, Wu X, Cheng P, Li HJ, Jasik J, Micieta K, et al. (2015) Arabidopsis synaptotagmin 2 participates in pollen germination and tube growth and is delivered to plasma membrane via conventional secretion. Mol Plant 8:1737–1750 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, et al. (2012) TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell 151:372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Schroeder JI (1994) Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6:669–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Guo J, Li M, Liu Z (2015) Structural mechanism underlying the specific recognition between the Arabidopsis state-transition phosphatase TAP38/PPH1 and phosphorylated light-harvesting complex protein Lhcb1. Plant Cell 27:1113–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Marchi V, Wang R, Rao R (1999) An N-terminal EF hand-like motif modulates ion transport by Pmr1, the yeast Golgi Ca2+/Mn2+-ATPase. Biochemistry 38:14534–14541 [DOI] [PubMed] [Google Scholar]
- Weinl S, Held K, Schlücking K, Steinhorst L, Kuhlgert S, Hippler M, Kudla J (2008) A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytol 179:675–686 [DOI] [PubMed] [Google Scholar]
- Werner AK, Sparkes IA, Romeis T, Witte CP (2008) Identification, biochemical characterization, and subcellular localization of allantoate amidohydrolases from Arabidopsis and soybean. Plant Physiol 146:418–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner AK, Witte CP (2011) The biochemistry of nitrogen mobilization: purine ring catabolism. Trends Plant Sci 16:381–387 [DOI] [PubMed] [Google Scholar]
- Widemann E, Miesch L, Lugan R, Holder E, Heinrich C, Aubert Y, Miesch M, Pinot F, Heitz T (2013) The amidohydrolases IAR3 and ILL6 contribute to jasmonoyl-isoleucine hormone turnover and generate 12-hydroxyjasmonic acid upon wounding in Arabidopsis leaves. J Biol Chem 288:31701–31714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson RE, Ohki K (1988) Influence of manganese deficiency and toxicity on isoprenoid syntheses. Plant Physiol 87:841–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G, Todd CD, Trovato M, Forlani G, Funck D (2015) Physiological implications of arginine metabolism in plants. Front Plant Sci 6:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff NA, Garrick MD, Zhao L, Garrick LM, Ghio AJ, Thévenod F (2018) A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci Rep 8:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AK, Capitanio P, Lissandron V, Bortolozzi M, Pozzan T, Pizzo P (2013) Heterogeneity of Ca2+ handling among and within Golgi compartments. J Mol Cell Biol 5:266–276 [DOI] [PubMed] [Google Scholar]
- Wu Z, Liang F, Hong B, Young JC, Sussman MR, Harper JF, Sze H (2002) An endoplasmic reticulum-bound Ca2+/Mn2+ pump, ECA1, supports plant growth and confers tolerance to Mn2+ stress. Plant Physiol 130:128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wudick MM, Portes MT, Michard E, Rosas-Santiago P, Lizzio MA, Nunes CO, Campos C, Damineli DSC, Carvalho JC, Lima PT, et al. (2018) CORNICHON sorting and regulation of GLR channels underlie pollen tube Ca2+ homeostasis. Science 360:533–536 [DOI] [PubMed] [Google Scholar]
- Wymer CL, Bibikova TN, Gilroy S (1997) Cytoplasmic free calcium distributions during the develpment of root hairs in Arabidopsis thaliana. Plant J 12:427–439 [DOI] [PubMed] [Google Scholar]
- Yamada K, Hara-Nishimura I, Nishimura M (2011) Unique defense strategy by the endoplasmic reticulum body in plants. Plant Cell Physiol 52:2039–2049 [DOI] [PubMed] [Google Scholar]
- Yamada K, Nagano AJ, Nishina M, Hara-Nishimura I, Nishimura M (2013) Identification of two novel endoplasmic reticulum body-specific integral membrane proteins. Plant Physiol 161:108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Theerawitaya C, Cha-um S, Kirdmanee C, Takabe T (2014) Expression and functional analysis of putative vacuolar Ca2+-transporters (CAXs and ACAs) in roots of salt tolerant and sensitive rice cultivars. Protoplasma 251:1067–1075 [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Suzuki L, Yamamoto H, Lyukevich A, Bodrova I, Los DA, Piven I, Zinchenko V, Kanehisa M, Murata N (2002) A two-component Mn2+-sensing system negatively regulates expression of the mntCAB operon in Synechocystis. Plant Cell 14:2901–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Aharon GS, Sottosanto JB, Blumwald E (2005) Vacuolar Na+/H+ antiporter cation selectivity is regulated by calmodulin from within the vacuole in a Ca2+- and pH-dependent manner. Proc Natl Acad Sci USA 102:16107–16112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Poovaiah BW (2002) Hydrogen peroxide homeostasis: activation of plant catalase by calcium/calmodulin. Proc Natl Acad Sci USA 99:4097–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Lanks CW, Tong L (2002) Molecular mechanism for the regulation of human mitochondrial NAD(P)+-dependent malic enzyme by ATP and fumarate. Structure 10:951–960 [DOI] [PubMed] [Google Scholar]
- Zhang B, Carrie C, Ivanova A, Narsai R, Murcha MW, Duncan O, Wang Y, Law SR, Albrecht V, Pogson B, et al. (2012a) LETM proteins play a role in the accumulation of mitochondrially encoded proteins in Arabidopsis thaliana and AtLETM2 displays parent of origin effects. J Biol Chem 287:41757–41773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Zhang C, Liu C, Jing Y, Wang Y, Jin L, Yang L, Fu A, Shi J, Zhao F, et al. (2018) Inner envelope CHLOROPLAST MANGANESE TRANSPORTER 1 supports manganese homeostasis and phototrophic growth in Arabidopsis. Mol Plant 11:943–954 [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang L, Gao B, Fan H, Jin J, Botella MA, Jiang L, Lin J (2011) Golgi apparatus-localized Synaptotagmin 2 is required for unconventional secretion in Arabidopsis. PLoS One 6:e26477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu YH, Yi HY, Gong JM (2012b) Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J 72:400–410 [DOI] [PubMed] [Google Scholar]
- Zhao J, Shigaki T, Mei H, Guo YQ, Cheng NH, Hirschi KD (2009) Interaction between Arabidopsis Ca2+/H+ exchangers CAX1 and CAX3. J Biol Chem 284:4605–4615 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Oldroyd GED (2017) Plant signalling in symbiosis and immunity. Nature 543:328–336 [DOI] [PubMed] [Google Scholar]