Abstract
Context: Spasticity is one of the most common secondary impairment after spinal cord injury (SCI). It can lead to an increase in the level of disability. The functional electrical stimulation cycling (FES-cycling) promotes recovery in patients with SCI. No systematic review has been published examining the influence of FES-cycling on the spasticity of lower extremities post-SCI.
Objective: This review aimed to investigate the effects of the FES-cycling on the lower extremities spasticity in patients with SCI.
Methods: PubMed, Scopus, PEDro, REHABDATA, Web of Science, and MEDLINE were searched until December 2019. The methodological quality was assessed using the Physiotherapy Evidence Database (PEDro) scale.
Results: Ten studies were met the inclusion criteria. Two were randomized clinical trials, cohort study (n = 2), and pilot study (n=6). The scores on the PEDro scale ranged from one to nine, with a median score of three. The results showed evidence for the beneficial effects of FES-cycling on the spasticity of lower extremities in individuals with SCI.
Conclusion: The FES-cycling intervention may reduce the lower extremities spasticity in patients with various injury levels of SCI. It is not a suitable intervention for medically unstable patients or with contraindication for lower extremities movement. Further randomized controlled trials with a large sample size strongly warranted to confirm our findings.
Keywords: Spinal cord injury, Electrical stimulation, Spasticity, Bicycling, Rehabilitation, Muscle spasm
Introduction
Spinal cord injury (SCI) is a leading cause of disability worldwide.1 Traumatic SCI most commonly occurs in persons aged between 16 and 20 years.2 Spasticity occurs in the upper motor neuron injury conditions (above T12/L1).3 Approximately 70% of patients post-SCI exhibit spasticity causing functional disability.4,5 It characterized by increases in muscle tone, hyperreflexia, clonus sign, and muscle spasms,6,7 which can cause functional impairments, contractures, pain, and ulcers that are capable of reducing the individuals’ quality of life (QoL).4,8
Medications such as Botulinum toxin are commonly used for reducing spasticity in patients with SCI.9 However, the common side effects for these agents include muscle weakness, malaise, and painful sensations at the injection site.9 As well, oral anti-spastic medications such as Baclofen can cause muscle weakness and may disturb the functional activities in patients with SCI.10 Despite their widespread use, there is insufficient evidence to guarantee pharmacological medications for reducing the spasticity.11 In the last decade, many physiotherapy modalities were prescribed for performing the physical exercises in patients with SCI.12,13 However, they have disadvantages such as time-consuming and high cost. Recently, numerous treatment technological devices have been used as a rehabilitation training methods. Among these is the functional electrical stimulation (FES) cycling.
The FES-cycling is an exercise modality that allows the quadriplegic and paraplegic patients to exercise of their paretic or paralyzed lower extremities muscles.14–17 It increases muscle mass and blood flow, enhances muscle oxidative capacity, and reduces spasticity in the paralyzed lower extremities.18 Numerous studies demonstrated the beneficial effects of the FES-cycling on the long-term cardiopulmonary and metabolic dysfunctions, as well on the functional recovery in individuals with SCI.19–23
The stimulation of the motor axons using external electrical stimuli may alter the nerve conduction by adjusting the synaptic organization in the central nervous system.24,25 Also, the reciprocal movements (i.e. cycling) may lead to central activation via reciprocal sensory feedback of lower extremities.20 In this context, the FES-cycling may have a positive therapeutic effect in reducing the lower extremities’ spasticity. To date, there were no systematic reviews have been published examining the impact of FES-cycling on the spasticity post-SCI. Therefore, this study aimed to investigate the effects of FES-cycling on the spasticity of lower extremities in patients with SCI.
Methods
Searching strategy
The search was conducted in the following databases: PubMed, Scopus, PEDro, REHABDATA, the web of science, and MEDLINE from inception until December 2019. The key search terms were (functional electrical stimulation cycling OR FES cycling OR FES OR cycling OR electrical stimulation OR functional electrical OR electrical) AND (spinal cord injury OR spinal cord injuries OR spinal cord OR spine cord OR traumatic spinal cord injury) AND (spasticity OR motor impairments OR physical OR impairment OR movement OR spasm OR muscle spasms). The present systematic review follows to all guidelines of Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement.26
Study selection
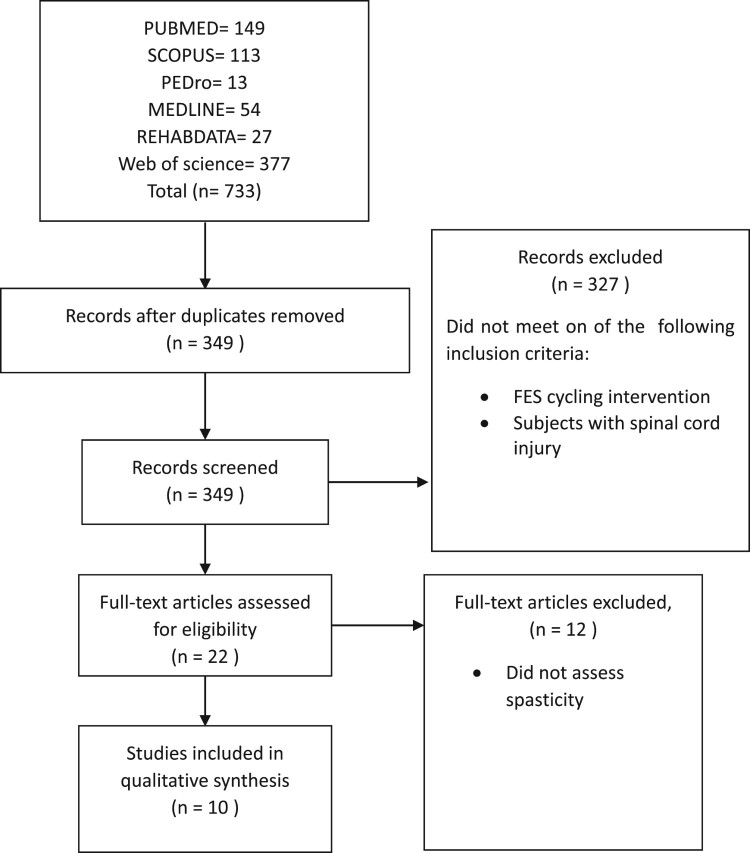
Studies were included in this systematic review if they (a) used functional electrical stimulation cycling intervention, (b) conducted human models, (c) published in the English language, (d) assessed individuals with SCI, and (e) examined lower extremities spasticity. Studies were excluded if they (a) assessed individuals with no confirmed diagnosis for SCI, (b) used animal models, (c) used motorized FES-cycling, (d) used the medications as the main intervention, and (e) included individuals complain from other neurological disorders (e.g. traumatic brain injury, stroke). Two reviewers independently performed the initial analysis of articles selection by analyzing the titles and the abstracts. Wherever necessary, the entire text of the articles was studied, and all effort was assumed to avoid subjective bias.27 Any disagreement between the reviewers discussed between them. The process of study selection was presented in Figure 1.
Figure 1.
Summary of literature review process.
Methodological quality
Two reviewers assessed the methodological quality of the selected studies using the Physiotherapy Evidence Database scale (PEDro). It provides an overview of the internal and external validity of the studies.28 Four items of the PEDro scale have been validated while the other items have face validity.26 Also, acceptable inter-rater reliability has been verified.28,29
Data extraction and analysis
Upon selection process for review, the following data were extracted separately: (a) author and date of publication, (b) study design and participant characteristics (c) session details, (d) FES-cycling application, (e) experimental group design, and (f) control group design. The study characteristics were presented in Table 1.
Table 1. Study characteristics.
| Author, year | Participants | Sessions | FES-cycling application | Experimental group design | Control group design |
|---|---|---|---|---|---|
| Krause et al., 200835 | Study design: Randomized crossover trial Sample size: 5 Sex (M/F): 3/2 Mean age: 46.6 ASIA: A Level of injury, number/ Range: T /(T3–T7) Duration of injury Mean (Range): 6.2 years (3–9 years) |
Sessions (n): 10 Session duration: 60–100 min Frequency: – Time period: – |
Type: Microstim 8 (Krauth & Timmermann, Germany). Pulse: Biphasic rectangular pulses. Pulse width: 500 ms Frequency: 20 Hz Amplitude: 0 to 99mA Electrodes: 12 (Flextrodes, 5*9 rectangle) Target muscles: quadriceps, hamstrings and glutei muscles. RPM: – |
FES cycling The participants legs were moved by the FES applied to the leg while fastened to the ergometer. |
Passive cycling The participants legs were moved only by the engine of ergometer with same frequency and with the same period of time, FES was not applied. First 5 sessions participants underwent FES cycling (experimental phase) then 5 sessions of passive cycling (control phase). Other participants performed same treatment protocol but in reverse order (crossover design). |
| Kuhn et al., 201430 | Study design: Clinical cohort study Sample size: 30 Sex (M/F): 30/0 Mean age: 44 ASIA: A=10, B=3,C=15, D=2 Level of injury, number/ Range: C=13, T=11, L=6/(C4–L4) Duration of injury Mean (Range): 8.4 months (0–112 months) |
Sessions (n): 8 Session duration: 20 min Frequency: 2–3 times weekly Time period: 4 weeks |
Type: Computer-controlled leg cycle (RECK-Medizintechnik GmbH &Co. KG, Betzenweiler, Germany) Pulse: Biphasic rectangular pulses Pulse width: 250 ms Frequency: 30 Hz Amplitude: 10–130 mA Electrodes: 6 (Self-adhesive 5*9 cm) Target muscles: hamstring, quadriceps, and the gluteal muscles. RPM: 15–55 |
FES cycling: The range of the pedalling cadence was set between 15–55 rpm. When could not reached minimum cadence actively, the pedalling was achieved passively by motor power. When cadence exceed 55 rpm, the resistance was adjusted manually (3 W). On days without FES cycling, the participants received physiotherapy and occupational therapy for 45 min. The physiotherapy intervention focused on proprioceptive neuromuscular facilitation (PNF) techniques, whereas occupational therapy interventions were focused on activities of daily life. |
– |
| Mazzoleni et al., 201732 | Study design: Pilot Sample size: 7 Sex (M/F): 5/2 Mean age: 45.3 ASIA: A Level of injury, number/ Range: T/(T4–T12) Duration of injury Mean (Range): NA |
Sessions (n): 40 Session duration: – Frequency: 3 times weekly Time period: – |
Type: FES cycling system (Pegaso, Biotech Srl, Italy). Pulse: Square biphasic alternated pulses Pulse width: 500 ms Frequency of 50 Hz Amplitude: 35-75 mA (Quadriceps), 25-50 mA (Biceps femoris) Electrodes: – Target muscles: quadriceps and biceps femoris muscles. RPM: – |
The patients received 20 sessions of FES cycling, an integrated cycle-ergometer system based on an electronic control, followed by 20 training sessions based on an over-ground robotic exoskeleton (Ekso GT, Ekso Bionics,USA). | – |
| Mazzoleni et al., 201331 | Study design: Pilot Sample size: 5 Sex (M/F): 4/1 Mean age: 43 ASIA: A=1, B=2, C=2 Level of injury, number/ Range: C=2, T=3/(C7–T12) Duration of injury Mean (Range): NA |
Sessions (n): 20 Session duration: 15 min, 5 min were incrementally added, till to 30 min Frequency: 3 times weekly Time period: 7 weeks |
Type: FES cycling system (Pegaso, Biotech Srl, Italy). Pulse: Balanced biphasic pulse; timing: 50–500 μs Pulse width: – Frequency: – Amplitude: 140 mA Electrodes: 6 Target muscles: quadriceps, femoral biceps and gluteus muscles. RPM: – |
Each session was included the following four phases: (1)Warm-up (90 sec, maximum speed: 40 cycles/min); (2) Preparation (2 min, maximum stimulation: 30%); (3) Active phase (30 min, target speed: 30 cycles/min, resistance: 5 Nm); (4) De-fatigue (20 sec, speed kept by motor: 20 cycles/min). In addition to FES cycling, rehabilitation intervention using exercises to increase control movement of the head, arm and trunk. |
– |
| Ralston et al., 201336 | Study design: Randomized crossover trial Sample size: 14 Sex (M/F): 11/3 Mean age: 25 ASIA: A=13, B=1 Level of injury, number/ Range: C=8, T=6/(C4–T10) Duration of injury Mean (Range): 118 days (64–135 days) |
Sessions (n): 20 Session duration: 30-45 min Frequency: 4 times weekly Time period: 5 weeks |
Type: Microstim 8 (Krauth & Timmermann, Germany). Pulse: Biphasic rectangular pulses. Pulse width: 350 ms Frequency 33 Hz Amplitude: 140 mA Electrodes: – Target muscles: quadriceps, hamstrings, and gluteal muscles. RPM: – |
First group received FES cycling + usual rehabilitation. Usual rehabilitation included physiotherapy and occupational therapy for 2 weeks. Second group received usual rehabilitation only. Next 3 weeks the first group received usual rehabilitation only. Second group received FES cycling + usual rehabilitation. | – |
| Reichenfelser et al., 201334 | Study design: Pilot Sample size: 9 Sex (M/F): NA Mean age: NA ASIA: Incomplete (B,C, and/ or D) Level of injury, number/ Range: Paraplegia (T2–L5) Duration of injury Mean (Range): NA |
Sessions (n): 7–14 Session duration: 30 min Frequency: 3 times weekly Time period: 2 months |
Type: tricycle (AnthroTech, Eckental, Germany) Pulse: Rectangular biphasic pulses Pulse width: 600 ms Frequency of 50 Hz Amplitude 20 mA Electrodes: 10 Target muscles: quadriceps, hamstring, and gluteus muscles RPM: 10,20,30,40,50, and 60 |
Training session included: Spasticity test routine Warm-up (5 min) Isokinetic training (5 min) Power output test Constant torque training (5 min) Isokinetic training (5 min) Spasticity test routine |
– |
| Reichenfelser et al., 201233 | Study design: Pilot Sample size: 23 Sex (M/F): 20/3 Mean age: 40 ASIA: B=4, C=10, D=9 Level of injury, number/ Range: C=9, T=9, L=5/(C4–L1) Duration of injury Mean (Range): 9 months (1–29 months) |
Sessions (n): 18 Session duration: 30 min Frequency: 3 times weekly Time period: 2 months |
Type: tricycle (AnthroTech, Eckental, Germany) Pulse: Rectangular biphasic pulses Pulse width: 600 ms Frequency: 50 Hz Amplitude: 20 mA Electrodes: (Axelgaard CF5090, 2 * 9 cm, Fallbrook, California). Target muscles: quadriceps, hamstrings and gluteus muscles. RPM: 10,20,30,40,50, and 60 |
Training session included: Spasticity test routine (3 min) Warm up (5 min) Isokinetic training (5 min) Active power output test Constant torque training (5 min) Isokinetic training (5 min) Spasticity test routine (3 min) |
– |
| Sadowsky et al., 201319 | Study design: Retrospective cohort study Sample size: 45 Sex (M/F): 38/7 Mean age: 36 ASIA: A=31, B=9, C=5 Level of injury, number/ Range: Quadriplegia (C1–T1) = 28, Paraplegia (T2–L5) = 17 Duration of injury Mean (Range): 23 patients = 6 months-5 years, 22 patients = more than 5 years |
Sessions (n): 36 Session duration: 45–60 min Frequency: 3 times weekly Time period: 30 months |
Type: ERGYS2 (Therapeutic Alliances Inc., Fairborn, OH, USA) Pulse: – Pulse width: 500 ms Frequency: 100 Hz Amplitude: 140 mA Electrodes: – Target muscles: quadriceps, gluteal, and hamstring muscles. RPM: 50 |
Participants underwent FES cycling with bilateral reciprocal leg cycling, typically at 50 RPM | Standard care: received range of motion and stretching. |
| Szecsi and Schiller 200914 | Study design: Pilot Sample size:13 Sex (M/F):9/4 Mean age: 39.9 ASIA: A Level of injury, number/ Range: C7–T12 Duration of injury Mean (Range): 10.1 years (–) |
Sessions (n): 3 Session duration: 20 min Frequency: – Time period: 2 weeks |
Type: – Parameters: LFRP (rectangular, biphasic, charged balanced pulses with a frequency of 20 Hz, maximum pulse amplitude of 127 mA, and constant pulse width of 500 μs). And MFAC (4 KHz sinusoidal modulated with 50 Hz on-off rectangles and duty cycle of 1:1). Electrodes: 4 (auto-adhesive gel electrodes 4.5 × 9.5 cm,Hamburg, Germany) Target muscles: quadriceps, gluteal, and hamstring muscles. RPM: 35–55 |
Each participant underwent 3 different experimental sessions: (1) isometric torque generation using LFRP and MFAC stimulation; (2) ergometer using LFRP stimulation; (3) ergometer using MFAC stimulation. The order of the sessions 1, 2, and 3 was randomized; each session was performed on a different day |
– |
| Yaşar et al., 201523 | Study design: Pilot Sample size: 10 (1 non traumatic SCI) Sex (M/F): 6/4 Mean age: 37.5 ASIA: C=1, D=9 Level of injury, number/ Range: C=5, T=5/(C4–T12) Duration of injury Mean (Range): 27.4 months (24–33 months) |
Sessions (n): 48 Session duration: 60 min Frequency: 3 times weekly Time period: 16 weeks |
Type: (RT 300-SLSA; Restorative Therapies, Baltimore, MD, USA). Pulse: – Pulse width, 250 ms Frequency: 20 Hz Amplitude: 10–140 mA. Electrodes: 6 (3 * 4 cm2 adhesive surface electrodes) Target muscles: quadriceps, hamstrings and gluteal muscles. RPM: 40–50 |
Participants underwent FES cycling. The participants were warned not to make any voluntary muscle contraction during cycling and the lower extremities were passively moved. The range of pedalling cadence was 40–50 rpm | – |
Notes: C, cervical; T, thoracic; L, lumber; ASIA, American spinal cord association; SCI, spinal cord injury; NA, not available; M, male; F, female; LFRP, low frequency rectangular pulse; MFAC, middle-frequency alternating current; RPM, rotations per minute.
Table 2 displays the outcome measures of the included studies. The following data were documented: (a) author and date of publication, (b) outcome measures and assessed muscles, (c) assessment time, (d) experimental group (Mean ± SD), (e) control group (Mean ± SD), and (f) the results. The data were not pooled for meta-analysis because of the heterogeneity and the inability to contact the authors of selected studies.
Table 2. Outcome measures and results.
| Author, year | Outcome measure/ tested muscles | Assessment time | Experimental group (mean ± SD) | Control group (mean + SD) | Results |
|---|---|---|---|---|---|
| Krause et al., 200835 | MAS /quadriceps muscle | At baseline and post treatment | AVG of Left leg Pre 2.75 ± 0.5 Post1.5 ± 0.3 AVG of right leg Pre 2.80 ± 0.07 Post 1.35.03 |
AVG of Left leg Pre 2.75 ± 0.6 Post 1.83 ± 0.6 AVG of right leg Pre 2.62 ± 0.4 Post 1.81 ± 0.2 |
There was significant in spasticity reduction in active session with FES (P < 0.001) and passive movement (P < 0.05) sessions. |
| Pendulum/ quadriceps muscle. | At baseline and post treatment | Relaxation index Active session with FES Post-Pre: 0.41 ± 0.18 Passive movement session Post-Pre:0.09 ± 0.14 Peak velocity Active session with FES Post-Pre:149 ± 0.70 Passive movement session Post-Pre:2 ± 61 |
– | The relaxation index and peak velocity increased on average after the active session with FES and passive movement session. but The increase after the passive movement session was smaller. | |
| Kuhn et al., 201430 | MAS / hip (abduction, adduction) knee joint (extension, flexion), and the foot (dorsal extension or plantar flexion). | At baseline and post treatment | Hip abduction Reduction of (70%) P = 0.002 Hip adduction Reduction of (98.1%) P = 0.016 Knee flexion Reduction of (66.8%) (P = 0.003) Knee extension Reduction of (76.6%) (P < 0.001) Dorsiflexion Reduction of (67.8%) (P = 0.001) |
– | There was significant reduction in spasticity of lower extremities |
| Mazzoleni et al., 201732 | MAS/ hip, knee and ankle flexors and extensors. | Baseline (Pre) After the FES-cycling training (Post1) After the overground robotic exoskeleton gait training (Post 2) |
Pre 7.14 ± 5.36 Post1 4.28 ± 3.68 Post2 3.57 ± 4.04 |
– | There was significant reduction in spasticity of lower extremities between T0-T1 and T0-T2. |
| NRS-spasticity/hip, knee and ankle flexors and extensors. | Baseline (Pre) After the FES-cycling training (Post1) After the overground robotic exoskeleton gait training (Post 2) |
Pre 3.71 ± 2.14 Post1 3.14 ± 2.54 Post2 2.42 ± 2.22 |
– | There was significant reduction in spasticity of lower extremities between T0-T2. | |
| Mazzoleni et al., 201331 | MAS/ hip, knee and ankle flexors and extensors. | Baseline (Pre) mid-treatment (Post1), that is after 10 sessions, at the end of the treatment (Post2), that is after 20 sessions | Pre 2.60 ± 0.89 Post1 2.40 ± 0.55 Post2 2.20 ± 0.84 |
– | There was no significant difference in spasticity of lower extremities after end of treatment |
| Ralston et al., 201336 | Ashworth Scale/ quadriceps, hamstrings, plantarflexor, and hip adductor muscles. | At baseline and post treatment | FES cycling+ usual rehabilitation Pre 5.6 ± 4.6 Post 2.8 ± 2.3 Usual rehabilitation+ FES cycling Pre 6.1 ± 5.7 Post 5.1 ± 4.6 |
– | There was no significant difference in spasticity of lower extremities after end of treatment |
| PRISM/quadriceps, hamstrings, plantarflexor, and hip adductor muscles. | At baseline and post treatment | FES cycling+ usual rehabilitation Pre 24 ± 11 Post 22 ± 9 Usual rehabilitation+ FES cycling Pre 23 ± 10 Post 26 ± 20 |
– | There was no significant difference in spasticity of lower extremities after end of treatment | |
| Reichenfelser et al., 201334 | MAS/ hip and knee joints. | At baseline and post treatment | average MAS of 1.5 that dropped to 1.2 after the FES training | – | The spasticity in both legs decreased after FES-cycling intervention |
| Reichenfelser et al., 201233 | MAS/hip and knee joints | At baseline and post treatment | Hip joints: Mean Modified Ashworth Scale (MAS) value >1, averaged mean MAS = 2.0, SD 0.4 Knee joints: Mean MAS value < 1, averaged mean MAS = 0.2, SD 0.2 |
– | There was decreasee in spasticity of lower extremities after FES-cycling |
| Sadowsky et al., 201319 | Measured quantitatively | At baseline and post treatment | The lower level of spasticity observed in the FES group is unlikely due to higher doses of anti-spasticity medication as the mean dose of the anti-spasticity medication baclofen was significantly lower (P = 0.02) in the FES group (20.2 mg, SD = 29.6) than in the controls (56.0 mg, SD = 59.2) | – | There was significant reduction in spasticity in FES-cycling group more than control group |
| Szecsi and Schiller 200914 | MAS/quadriceps, hamstring | At baseline and post treatment | MFAC P = 0.001 LFRP P = 0.001 |
– | There was decrease in spasticity after MFAC and LTRP without significant differences |
| Yaşar et al., 201523 | MAS/ quadriceps, hamstring | Baseline (Pre0) 3 months after the baseline measurement. (Post1), 6 months after the baseline measurement (Post2) |
Rectus femoris: Pre 2.1 ± 0.3 Post1 0.6 ± 0.5 Post2 0.9 ± 0.7 Hamstring: Pre 1.7 ± 0.4 Post1 0.4 ± 0.5 Post2 0.7 ± 0.4 |
– | There was significant decrease in spasticity of both rectus femoris and hamstring muscles at 3 and 6 months compared with baseline |
Notes: AVG, average; NRS, Numerical Rating Scale; MAS, Modified Ashworth Scale; PRISM, Patient-Reported Impact of Spasticity Measure; FES, functional electrical stimulation; MFAC, middle-frequency alternating current; LFRP, low frequency rectangular pulse; PRISMA, Patient-Reported Impact of Spasticity Measure.
Results
Study selection
An electronic search of PubMed (yielding 149 articles), SCOPUS (113), PEDro (13), REHABDATA (27), MEDLINE (54), and Web of Science (377) produced a total of 733 articles. After removing duplicates, 349 articles were reviewed. Out of those, 327 articles were excluded because they did not match our inclusion criteria. Twenty-two articles were subjected to more detailed analysis. Twelve articles were eliminated because they did not assess the spasticity in the lower extremities. A total of ten articles were identified for the inclusion criteria in this systematic review. The process of the study selection was presented in Figure 1.
Methodological quality
The PEDro scale was used to evaluate the risk of bias. Of ten studies, two studies were randomized crossover trials,35,36 cohort studies (n = 2),19,30 and pilot studies (n = 6).14,23,31–34 The score on the PEDro scale ranged from one to nine, with a median of three. Overall, one study met nine criteria,36 seven criteria (n = 1),35 four criteria (n = 2),14,19 three criteria (n = 2),32,33 two criteria (n = 3),23,30,31 and one criterion (n = 1)34 for low risk of bias. Table 3 displays the methodological quality scores for the included studies.
Table 3. Methodological quality scores.
| Question number on PEDro scale | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Score |
| Krause et al., 200835 | Y | Y | N | Y | N | N | Y | Y | Y | N | Y | 7 |
| Kuhn et al., 201430 | Y | N | N | N | N | N | N | Y | N | N | N | 2 |
| Mazzoleni et al., 201732 | Y | N | N | N | N | N | N | Y | Y | N | N | 3 |
| Mazzoleni et al., 201331 | N | N | N | N | N | N | N | Y | Y | N | N | 2 |
| Ralston et al., 201336 | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 9 |
| Reichenfelser et al., 201334 | N | N | N | N | N | N | N | N | N | N | Y | 1 |
| Reichenfelser et al., 201233 | N | N | N | N | N | N | N | Y | Y | N | Y | 3 |
| Sadowsky et al., 201319 | Y | N | N | N | N | N | N | Y | Y | N | Y | 4 |
| Szecsi and Schiller, 200914 | N | N | N | N | N | N | N | Y | Y | Y | N | 4 |
| Yaşar et al., 201523 | N | N | N | N | N | N | N | Y | N | N | Y | 2 |
| Median Score = 3 | ||||||||||||
Notes: Scores: 1, Eligibility criteria were specified; 2, Subjects were randomly allocated to groups or to a treatment order; 3, Allocation was concealed; 4, The groups were similar at baseline;5, There was blinding of all subjects; 6 There was blinding of all therapists; 7, There was blinding of all assessors; 8, Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups 9, Intention to treat analysis was performed or all subjects received the treatment or control condition as allocated; 10, The results of between-group statistical comparisons are reported for at least one key outcome; 11, The study provides both point measures and measures of variability for at least one key outcome. Yes, low risk of bias; No, high risk of bias.
Participant characteristics
PICOS approach (Patients, Intervention, Control, Outcomes, and Subjects) was used.37 A total of 161 patients with SCI 16.15% of whom were females. The mean age for all participants was 36.45 years old.
Concerning completeness of injury, three studies included patients with complete SCI,14,32,35 incomplete SCI (n = 3),23,33,34 and mixed (Complete & incomplete) SCI (n = 4).19,30,31,36 In terms of the injury level, three studies included paraplegic SCI patients,32,34,35 while the remaining studies included mixed (paraplegic & tetraplegic) patients.19,14,23,30,31,33,36 In terms of the injury severity, three studies included SCI patients with level A on the American Spinal cord Injury Association (ASIA) scale,14,32,35 (A,B, and C) (n = 2),19,31 (B,C, and D) (n = 2),33,34 (A and B) (n = 1),36 and (A,B,C, and D) (n = 1).30 Finally, in terms of the injury duration, one study included patients with acute SCI (less than 6 months),36 chronic (more than 6 months) (n = 4),19,14,23,35 and mixed (acute & chronic) (n = 2),30,33 the duration of injury was not reported in the remaining studies.31,32,34 Participant characteristics were presented in Table 1.
Study design
In the study by Krause et al.35, the interventional protocols include an active session (FES-cycling) and the passive movements (cycling) protocol. The participants in the experimental and control phase were seated on the ergometer. The participants underwent the FES-cycling for five sessions, followed by five sessions of passive cycling. Other participants performed the same treatment protocol in the reverse order (crossover design). In the active session, the lower extremities were moved by the FES-cycling. A total of 12 electrodes were placed over the proximal and distal fourth of quadriceps, hamstring, and gluteal muscles on both sides. Biphasic rectangular pulses were used (pulse widths 500 ms, frequency 20 Hz, and currents 0–99 mA). In the passive session, the lower extremities were moved by the ergometer engine without applying FES. In total, all participants received ten 60-100-minute sessions.35
In the study by Kuhn et al.30, the computer-controlled FES-cycling was used for four weeks. The rotations per minute (RPMs) ranged from 15 to 55 RPMs. Six self-adhesive electrodes were placed over the quadriceps, hamstring, and gluteal muscles on both sides. The biphasic rectangular pulses were applied (pulse widths 250 ms, frequency 30 Hz, and currents 10–130 mA). In case of the minimum cadence could not be reached actively (by stimulation), the pedalling was achieved passively by motor power without FES stimulation. In total, all participants received eight 20-minute sessions. On days without FES-cycling, the participants received physiotherapy and occupational therapy treatment for 45 min. The physiotherapy treatment interventions focused on the proprioceptive neuromuscular facilitation (PNF) techniques. While occupational therapy treatment interventions focused on the activities of daily life (ADLs).30
In the study by Mazzoleni et al.31, all participants underwent FES-cycling training. Six electrodes were used to stimulate quadriceps, biceps femoris, and gluteus muscles on both lower extremities. A balanced biphasic pulse was used (impulse 50-500μs and amplitude 140 mA). In addition to FES-cycling training, the rehabilitation-training intervention (coordination exercises) was included to increase the control movement of the head, upper extremities, and trunk. The first session was used to familiarize the participants. The second session duration was 15 min, 5 min were added incrementally from the third session to session number 20th, till to 30 min. Each session composed from four phases: first (warm-up) (90 s, maximum speed: 40 cycles/min), second (preparation) (2 min, maximum stimulation: 30%), third (active phase) (30 min, target speed: 30 cycles/min, resistance: 5 Nm), and fourth (de-fatigue) (20 se, speed kept by motor: 20 cycles/min).31
Moreover, in the study by Mazzoleni et al.32, all participants underwent 20 sessions of FES-cycling (3 sessions per week), followed by 20 sessions of over-ground robotic exoskeleton training (3 sessions per week). In terms of the FES-cycling, square biphasic alternated pulses were used (frequency 50 Hz and impulse 500 μs). The quadriceps (amplitude 35-75 mA) and biceps femoris (amplitude 25–50 mA) muscles were stimulated.32
Furthermore, in the study by Ralston et al.36, the first group underwent FES-cycling plus usual rehabilitation. In terms of FES-cycling, biphasic rectangular pulses were used (frequency 33 Hz, pulse width 350 ms, and amplitude 140 mA). The quadriceps, biceps femoris, and gluteus muscles were stimulated on both sides. The usual rehabilitation intervention included physiotherapy and occupational therapy interventions. The second group received the usual rehabilitation intervention only. Next three weeks, the reverse order was applied (crossover design). In total, all participants received 20 of the 30-45-minute sessions, four sessions per week for five weeks.36
In the study by Reichenfelser et al.,33 the phases of training were: first, the spasticity test. It is a 3-minute test where the lower extremities are passively propelled at six isokinetic cadences. Second, the warm-up. It includes 5 min of active pedalling (30 RPMs). The amplitude (20 mA) was applied to the quadriceps, biceps femoris, and gluteus muscles on both sides. Third, isokinetic training, the participants pedal actively for 5 min, and stimulation was added. The rectangular biphasic pulses were applied (frequency 50 Hz, amplitude 39 mA, and a pulse duration of 600 μs). Fourth, the active power output test, it performed without stimulation (30 rpm). Fifth, the participants performed five minutes of the training with constant motor torque and FES. The motor resistance set at ± 1000 mA. Sixth, the isokinetic training repeated for five minutes. Seventh, the spasticity test repeated for three minutes. The RPMs increased gradually with time (10, 20, 30, 40, 50, and 60 rpm). In total, all participants underwent 18 training of 30-minute sessions, three sessions per week for two months.33
In the study by Reichenfelser et al.,34 all participants underwent a 5-minute warm-up followed by isokinetic training (10, 20, 30, 40, 50, and 60 RPMs) for five minutes. The participants propel actively with supporting electrical stimulation. Subsequently, the participants pedal as strong as possible without stimulation. Next, the motor resistance is set at ± 1000 mA to enable participants a smooth pedaling. Finally, isokinetic training is repeated for five minutes. The rectangular biphasic pulses were applied (amplitude 20 mA, frequency 50 Hz, and pulse duration of 600μs). The quadriceps, biceps femoris, and gluteus muscles on both sides were stimulated. The participants received in total 7–14 sessions of 30- minute sessions, three sessions per week for two months.34
In the study by Sadowsky et al.,19 the participants in the experimental group performed the FES-cycling with bilateral reciprocal leg cycling (50 RPMs, amplitude 140 mA, pulse width 500 μs, and the frequency 100 Hz). Electrodes were placed on the quadriceps, biceps femoris, and gluteus muscles on both sides. The control group performed standard care including the range of motion (ROM) and stretching exercises. The participants in both groups received 36 of 45-60-minute sessions, three sessions per week for three months.19
Additionally, in the study by Szecsi and Schiller14, each participant underwent three different experimental sessions. Four electrodes were placed over the proximal and distal fourth of the quadriceps, hamstring, and gluteal muscles on both sides. First session, low-frequency rectangular pulse (LFRP) (frequency 20 Hz, amplitude 127 mA, and constant pulse width 500 μs) and middle-frequency alternating current (MFAC) (current 4 kHz with 50 Hz on–off rectangles, and duty cycle of 1:1) stimulation were applied. Second, the ergometer using LFRP stimulation was applied. And third, the ergometer using MFAC stimulation (35–55 RPMs) was applied. The order of sessions was randomized and was performed on different days. Each participant received three different 20-minute sessions over two weeks.14
Finally, in the study by Yaşar et al.,23 all participants underwent FES-cycling intervention (40-50 RPMs, pulse width, 250 μs, frequency 20 Hz, and amplitude 10–140 mA). Six electrodes were applied over the quadriceps, hamstrings, and gluteal muscles on both sides. All participants received 48 of 60-minute sessions, three sessions per week over 16 weeks.23
Outcome measures
The selected studies measured the lower extremities spasticity using the Modified Ashworth Scale (MAS).14,23,30–35 The MAS designed to evaluates the muscle tone and to assess the level of spasticity.38 Additionally, other spasticity tests were applied; Pendulum test,35 Numerical Rating Scale (NRS-spasticity),32 Patient-Reported Impact of Spasticity Measure (PRISM),36 and Ashworth scales.36 As well as, quantitive spasticity test.19 It is done by measured resistance torque by passively moving the joints through ROM at multiple speeds 30–180 degrees/sec for the knee joints, and f 120 degrees/second for the ankle joints.19 The selected studies measured the spasticity of hips extensors,14,23,31–35 flexors,14,23,31–34 abductors,30 and adductors30. Knees extensors30–34 and flexors30–34. As well as, ankles extensors30–32 and flexors.30–32
Effect of FES-cycling on spasticity
The selected studies demonstrated a significant reduction in the MAS23,30,32,33,34 and NRS-spsticity32 scores (reduce spasticity) after FES-cycling post-treatment,23,30,32-34 and at three23 and six months23 follow-ups (P < 0.05). One study reported a reduction in the MAS scores and changes in Pendulum scores (reduce spasticity) following FES-cycling experimental and passive cycling control conditions (P < 0.05).35 Another study by Szecsi and Schiller14 demonstrated a reduction in the MAS scores after MFAC and LTRP conditions (P < 0.05). The study by Sadowsky et al.19 reported a significant reduction in the spasticity in the experimental FES-cycling group (P = 0.02) than in the standard care control group, however, with no significant difference between groups (P > 0.05). The remaining studies showed no significant changes in the MAS,31 Ashworth,36 and PRISM36 scores following FES-cycling (P > 0.05).
Adverse effects after FES cycling
In the study by Ralston et al.36, an increase in spasticity and perception of bowel accident were reported. The remaining studies did not report any adverse/side effects or uncomfortable sensations following FES cycling intervention.
Discussion
The purpose of this systematic review was to examine the effectiveness of the FES-cycling on the spasticity of lower extremities post-SCI. We included the low-quality studies because of the paucity of high-quality studies that investigated the influence of FES-cycling on the lower extremities spasticity following SCI. The main findings based on ten studies showed evidence for the positive effects of the FES-cycling on the lower extremities spasticity in patients with SCI. Similar to our findings, numerous studies have proven that the FES-cycling reduced the spasticity and frequency of the muscle spasms in patients with SCI.18,21 The FES-cycling induced neural activity may cause neuroanatomical plasticity and changing the balance of excitation and inhibition within the spinal cord.39 Earlier studies have shown that the electrical stimulation of paralyzed muscles can alter the H reflex parameters; hence, potentially reducing the spasticity.40
Spasticity can lead to secondary changes such as soft-tissue contractures. As well, muscle fiber and tendon properties change.5,41 However, a mild to moderate degree of spasticity may have a positive influence on daily activities.3
Concerning the methodological quality of selected studies, two of the selected studies were superior to the research pyramid being randomized crossover studies. 35,36 These studies were of high methodological quality on the PEDro scale.35,36 The remaining studies were of low methodological quality.14,19,23,30-34 Moreover, all studies had poor results in the blinding of subjects and therapists, leading to potential bias. Additionally, except for Kuhn et al.30 and Sadowsky et al.,19 the sample sizes were small (<25) for remaining studies. The significant differences not allowed to calculate in a small sample size.42
Two studies combined the FES-cycling with the passive cycling (Motor system)35 and with the usual rehabilitation (physiotherapy and occupational therapy).30 The remaining studies used FES-cycling alone during treatment intervention. Except for studies by Mazzoleni et al.31 and Ralston et al.,36 the included studies showed a significant improvement in the spasticity of lower extremities in patients with SCI following FES-cycling intervention. Ralston et al.36 have shown that his findings cannot be interpreted as proof of no treatment effect because this interpretation relies on determining a minimally beneficial treatment effect. As well as, it is not clear what size treatment effect therapists and individuals with SCI would consider adequate to justify the time and cost associated with FES cycling.36 While no explanations were provided by Mazzoleni et al.42, we propose that was due to small sample size (n = 5). As a small sample size, significant differences cannot be calculated.42
In the study by Mazzoleni et al.,32 the participants underwent 20 sessions of the FES-cycling (Phase 1) followed by 20-sessions of the overground exoskeleton training (Phase 2). The outcome results showed a reduction in spasticity after each phase. In this context, it seems that both overground exoskeleton training and FES-cycling reduce spasticity in patients with SCI.
Owing the spontaneous motor recovery occurs within three to nine months after the injury;43,44 hence, may the patients in some of the selected studies30,33,36 improved spontaneously not due to treatment intervention as they included acute SCI patients with injury history less than six months. Moreover, one study included patients on anti-spasticity medication (Baclofen), thereby may the patients had a reduction in spasticity due to medication effect.19
Although the electrical stimulation was applied to the thigh muscles (quadriceps, hamstring, and gluteal muscles), however, some studies reported an improvement in the dorsi-flexors and the planter-flexors of the ankle joints. Rösche et al.45 have shown that the reduction in the ankle muscle spasticity may occur because of rhythmic passive leg movements. Although the muscles stimulated by the electrical current following FES-cycling experience rapid fatigue.46 However, except for the study by Ralston et al.,36 the studies did not notice any adverse or side effects after FES-cycling intervention. Ralston et al. (2013) reported some of the adverse effects, such as an increase in spasticity and bowel accident perception. We propose that occurred because all the participants were with acute SCI, which there is a more probability for developing the symptoms quickly than those in the chronic stage.
Janssen et al.48 suggested that stronger spasticity will develop after FES-cycling than before, because of increased muscle strength. However, in the selected studies, 14,19,23,30,33,34 the RPMs ranged from 10 to 60 RPMs showed a positive effect of the FES-cycling in reducing spasticity. Similar to Glaser et al.47, he showed that the FES-cycling reduced the lower extremities spasticity in the patients with SCI.
Many intervention details were not reported in some of the selected studies such as: frequency of treatment,14,35 treatment time period,32,35 session duration,32 number of electrodes,19,32,33,36 pulse type,19,23 type of FES-cycling system,14 pulse width,31 frequency,31 and RPM.31,32,35,36 So we are unable to identify the treatment effect size and the effective treatment protocol.
In the selected studies,19,30,32,35 patients were excluded if they have the following limitations: (1) severe reduction in the range of motion, (2) heterotopic ossifications, (3) severe spasticity, (4) fractures, (5)metal plants in lower extremities, (6) pressure ulcers, and (7) cardiovascular instability. In this context, we propose that the FES-cycling is not a suitable intervention for medically unstable SCI patients or who with contraindication for lower extremities movement.
Limitations
This systematic review has several limitations: First, the included studies published in English. Thus, the studies published in other languages were not selected. Second, it included low-quality research types due to the paucity of studies published about this issue. Third, because of the heterogeneity of the included studies, the meta-analysis was not conducted.
Owing to the paucity of high-quality studies concerning the effect of FES-cycling on the lower extremities spasticity in individuals with SCI, further studies are certainly warranted. It is recommended that the quality of further studies is improved by conducting randomized controlled trials and using greater sample sizes. Future studies are also needed to define the most effective FES-cycling training parameters comparing with other rehabilitation interventions and to exploring multiple FES-cycling protocols with different stimulation. Due to insufficient evidence, we are unable to identify if there is a significant difference between acute and chronic SCI following FES-cycling intervention. Future studies should include long-term follow-ups to determine how long the reduction in the spasticity might last and to identify which SCI population most likely benefit from the intervention (i.e. Complete vs Incomplete, Chronic vs Acute).
Conclusion
In conclusion, the quantity but not quality of the published studies into the effects of FES-cycling on spasticity of the lower extremities in those with SCI is relatively good for an emerging modality. Promising results provide insights for the positive effects of FES-cycling in reducing the lower extremities spasticity in patients with various injury levels of SCI. It considers suitable intervention for medically stable SCI patients with an indication for lower extremities movement. Further randomized controlled trials with large sample sizes strongly needed to confirm our findings and to verify our hypothesis.
Disclaimer statements
Contributors None.
Funding The authors have no source of funding or any potential conflicts of interest to disclose
Conflict of interest The authors have no conflict of interests to declare.
References
- 1.Braddom R. Physical Medicine and rehabilitation. 3rd ed. Philadelphia, PA: Saunders Elsevier; 2007. [Google Scholar]
- 2.Sadowsky C, Volshteyn O, Schultz L, McDonald J.. Spinal cord injury. Disabil Rehabil 2002;24:680–7. doi: 10.1080/09638280110110640 [DOI] [PubMed] [Google Scholar]
- 3.Burchiel K, Hsu F K.. Pain and spasticity after spinal cord injury. Spine 2001;26(Supplement):S146–S160. doi: 10.1097/00007632-200112151-00024 [DOI] [PubMed] [Google Scholar]
- 4.Gorgey A, Chiodo A, Zemper E, Hornyak J, Rodriguez G, Gater D.. Relationship of spasticity to soft tissue body composition and the metabolic profile in persons with chronic motor complete spinal cord injury. J Spinal Cord Med 2010;33(1):6–15. doi: 10.1080/10790268.2010.11689669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rekand T, Hagen E, Grønning M.. Spastisitet etter ryggmargsskade. Tidsskrift for Den norske legeforening 2012;132(8):970–3. doi: 10.4045/tidsskr.10.0872 [DOI] [PubMed] [Google Scholar]
- 6.Sheean G. The pathophysiology of spasticity. Eur J Neurol 2002;9(s1):3–9. doi: 10.1046/j.1468-1331.2002.0090s1003.x [DOI] [PubMed] [Google Scholar]
- 7.Rabchevsky A, Kitzman P.. Latest Approaches for the treatment of spasticity and autonomic dysreflexia in chronic spinal cord injury. Neurotherapeutics 2011;8(2):274–82. doi: 10.1007/s13311-011-0025-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westgren N, Levi R.. Quality of life and traumatic spinal cord injury. Arch Phys Med Rehabil 1998;79(11):1433–9. doi: 10.1016/S0003-9993(98)90240-4 [DOI] [PubMed] [Google Scholar]
- 9.Elbasiouny S, Moroz D, Bakr M, Mushahwar V.. Management of spasticity after spinal cord injury: current techniques and future directions. Neurorehabil Neural Repair 2009;24(1):23–33. doi: 10.1177/1545968309343213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirshblum S. Treatment alternatives for spinal cord injury related spasticity. J Spinal Cord Med 1999;22(3):199–217. doi: 10.1080/10790268.1999.11719570 [DOI] [PubMed] [Google Scholar]
- 11.Taricco M, Adone R, Pagliacci C, Telaro E.. Pharmacological interventions for spasticity following spinal cord injury. Cochrane Database Syst Rev 2000;(2):CD001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alashram A, Annino G, Mercuri N.. Rhythmic auditory stimulation in gait rehabilitation for traumatic brain and spinal cord injury. J Clin Neurosci 2019;69:287–8. doi: 10.1016/j.jocn.2019.08.080 [DOI] [PubMed] [Google Scholar]
- 13.Alashram A, Padua E, Annino G.. Effects of whole-body vibration on motor impairments in patients with neurological disorders. Am J Phys Med Rehabil 2019;98(12):1084–98. doi: 10.1097/PHM.0000000000001252 [DOI] [PubMed] [Google Scholar]
- 14.Szecsi J, Schiller M.. FES-propelled cycling of SCI subjects with highly spastic leg musculature. NeuroRehabilitation 2009;24(3):243–53. doi: 10.3233/NRE-2009-0475 [DOI] [PubMed] [Google Scholar]
- 15.Petrofsky J, Philhillips C, Heaton H, Glaser R.. Bicycle ergometer for paralyzed muscle. J Clin Eng 1984;9(1):13–20. doi: 10.1097/00004669-198401000-00003 [DOI] [Google Scholar]
- 16.Edlich R, Wilder R, Wind T.. Functional electrical stimulation cycle ergometer exercise for spinal cord injured patients. J Long Term Eff Med Implants 2002;12(3):14. [PubMed] [Google Scholar]
- 17.Jacobs P, Nash M.. Exercise recommendations for individuals with spinal cord injury. Sports Med 2004;34(11):727–51. doi: 10.2165/00007256-200434110-00003 [DOI] [PubMed] [Google Scholar]
- 18.Raymond J, Crameri R.. Electrical stimulation for individuals with spinal cord injury. Am J Med Sports 2001;22(8):209–22. [Google Scholar]
- 19.Sadowsky C, Hammond E, Strohl A, Commean P, Eby S, Damiano D, et al. Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J Spinal Cord Med 2013;36(6):623–31. doi: 10.1179/2045772313Y.0000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donaldson N, Perkins T, Fitzwater R, Wood D, Middleton F.. FES cycling may promote recovery of leg function after incomplete spinal cord injury. Spinal Cord 2000;38(11):680–2. doi: 10.1038/sj.sc.3101072 [DOI] [PubMed] [Google Scholar]
- 21.Davis G, Hamzaid N, Fornusek C.. Cardiorespiratory, metabolic, and biomechanical responses during functional electrical stimulation leg exercise: health and fitness benefits. Artif Organs 2008;32(8):625–9. doi: 10.1111/j.1525-1594.2008.00622.x [DOI] [PubMed] [Google Scholar]
- 22.Fornusek C, Gwinn T, Heard R.. Cardiorespiratory responses during functional electrical stimulation cycling and electrical stimulation isometric exercise. Spinal Cord 2014;52(8):635–9. doi: 10.1038/sc.2014.85 [DOI] [PubMed] [Google Scholar]
- 23.Yaşar E, Yılmaz B, Göktepe S, Kesikburun S.. Erratum: The effect of functional electrical stimulation cycling on late functional improvement in patients with chronic incomplete spinal cord injury. Spinal Cord 2015;53(12):866–900. doi: 10.1038/sc.2015.19 [DOI] [PubMed] [Google Scholar]
- 24.Daly J, Marsolais E, Mendell L, Rymer W, Stefanovska A.. Therapeutic neural effects of electrical stimulation. IEEE Trans Rehabil Eng 1996;4(4):218–30. doi: 10.1109/86.547922 [DOI] [PubMed] [Google Scholar]
- 25.Rushton D. Functional electrical stimulation and rehabilitation – an hypothesis. Med Eng Phys 2003;25(1):75–8. doi: 10.1016/S1350-4533(02)00040-1 [DOI] [PubMed] [Google Scholar]
- 26.Moher D. Assessing the Quality of Reports of Randomised Trials. Alton: Core Research on behalf of NCCHTA; 1999. Systematic Reviews. York: CRD, University of York; 2009. [Google Scholar]
- 27.Pannucci C, Wilkins E.. Identifying and avoiding bias in research. Plast Reconstr Surg 2010;126(2):619–25. doi: 10.1097/PRS.0b013e3181de24bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maher C, Sherrington C, Herbert R, Moseley A, Elkins M.. Reliability of the PEDro scale for Rating quality of randomized controlled trials. Phys Ther 2003;83(8):713–21. doi: 10.1093/ptj/83.8.713 [DOI] [PubMed] [Google Scholar]
- 29.Foley N, Bhogal S, Teasell R, Bureau Y, Speechley M.. Estimates of quality and reliability with the physiotherapy evidence-based Database scale to assess the Methodology of randomized controlled trials of pharmacological and nonpharmacological interventions. Phys Ther 2006;86(6):817–24. doi: 10.1093/ptj/86.6.817 [DOI] [PubMed] [Google Scholar]
- 30.Kuhn D, Leichtfried V, Schobersberger W.. Four weeks of functional electrical stimulated cycling after spinal cord injury. Int J Rehabil Res 2014;37(3):243–50. doi: 10.1097/MRR.0000000000000062 [DOI] [PubMed] [Google Scholar]
- 31.Mazzoleni S, Stampacchia G, Gerini A, Tombini T, Carrozza M.. FES-cycling training in Spinal Cord Injured patients. 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2013:5339–41. [DOI] [PubMed]
- 32.Mazzoleni S, Battini E, Rustici A, Stampacchia G.. An integrated gait rehabilitation training based on functional electrical stimulation cycling and overground robotic exoskeleton in complete spinal cord injury patients: Preliminary results. IEEE Int Conf Rehab Robot 2017: 289–93. [DOI] [PubMed] [Google Scholar]
- 33.Reichenfelser W, Hackl H, Hufgard J, Kastner J, Gstaltner K, Gföhler M.. Monitoring of spasticity and functional ability in individuals with incomplete spinal cord injury with a functional electrical stimulation cycling system. J Rehabil Med 2012;44(5):444–9. doi: 10.2340/16501977-0979 [DOI] [PubMed] [Google Scholar]
- 34.Reichenfelser W H, Hufgard J H, Gstaltner K, Gfoehler M.. Effect of FES cycling training on spasticity in spinal cord Injured subjects. International Journal of Electrical, Computer, Energetic, Electronic and Communication Engineering 2013;7(2):137–40. [Google Scholar]
- 35.Krause P, Szecsi J, Straube A.. Changes in spastic muscle tone increase in patients with spinal cord injury using functional electrical stimulation and passive leg movements. Clin Rehabil 2008;22(7):627–34. doi: 10.1177/0269215507084648 [DOI] [PubMed] [Google Scholar]
- 36.Ralston K, Harvey L, Batty J, Lee B, Ben M, Cusmiani R, et al. Functional electrical stimulation cycling has no clear effect on urine output, lower limb swelling, and spasticity in people with spinal cord injury: a randomised cross-over trial. J Physiother 2013;59(4):237–43. doi: 10.1016/S1836-9553(13)70200-5 [DOI] [PubMed] [Google Scholar]
- 37.Liberati A. The PRISMA statement for Reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med 2009;151(4):65–94. doi: 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 38.Bohannon R, Smith M.. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987;67(2):206–7. doi: 10.1093/ptj/67.2.206 [DOI] [PubMed] [Google Scholar]
- 39.Tillakaratne N, Mouria M, Ziv N, Roy R, Edgerton V, Tobin A.. Increased expression of glutamate decarboxylase (GAD67) in feline lumbar spinal cord after complete thoracic spinal cord transection. J Neurosci Res 2000;60(2):219. doi: [DOI] [PubMed] [Google Scholar]
- 40.Baoping Y, Gomez J, Gonzalez J, Wenwei Y, Ino S.. H-reflex measurement and a simulation model for interpreting the effect of an auxiliary electrical stimulation on FES. 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology. 2010. [DOI] [PubMed]
- 41.Adams M, Hicks A.. Spasticity after spinal cord injury. Spinal Cord 2005;43(10):577–86. doi: 10.1038/sj.sc.3101757 [DOI] [PubMed] [Google Scholar]
- 42.Portney L, Watkins M.. Foundations of Clinical Research: Applications to Practice. 3rd ed. Upper Saddle River, NJ: Prentice Hall; 2009. [Google Scholar]
- 43.Fawcett J, Curt A, Steeves J, Coleman W, Tuszynski M, Lammertse D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2006;45(3):190–205. doi: 10.1038/sj.sc.3102007 [DOI] [PubMed] [Google Scholar]
- 44.Pollard M, Apple D.. Factors associated with improved neurologic outcomes in patients with incomplete tetraplegia. Spine 2003;28(1):33–8. doi: 10.1097/00007632-200301010-00009 [DOI] [PubMed] [Google Scholar]
- 45.Rösche J, Paulus C, Maisch U, Kaspar A, Mauch E, Kornhuber H.. The effects of therapy on spasticity utilizing a motorized exercise-cycle. Spinal Cord 1997;35(3):176–8. doi: 10.1038/sj.sc.3100376 [DOI] [PubMed] [Google Scholar]
- 46.Thrasher A, Graham G, Popovic M.. Reducing muscle fatigue due to functional electrical stimulation using random modulation of stimulation parameters. Artif Organs 2005;29(6):453–8. doi: 10.1111/j.1525-1594.2005.29076.x [DOI] [PubMed] [Google Scholar]
- 47.Glaser RM. Functional neuromuscular stimulation: exercise conditioning of spinal cord injured patients. Int J Sports Med 1994;15(3):142–8. doi: 10.1055/s-2007-1021036 [DOI] [PubMed] [Google Scholar]
- 48.Janssen T, Glaser R, Shuster D.. Clinical efficacy of electrical stimulation exercise training: effects on health, fitness, and function. Spinal Cord Injury Rehab 1998;3(3):33–49. [Google Scholar]