Abstract
Objective: Acupuncture combined with moxibustion (AM) therapy has been applied to treat spinal cord injury (SCI), but the underlying mechanism is unclear. The present study aimed to confirm the effect and mechanism of AM treatment on the recovery of SCI.
Design: Male Sprague–Dawley rats were used to establish the SCI model by impact method. SCI rat models were subjected to AM treatment at Dazhui (GV14) and Jiaji points (T7-T12), Yaoyangguan (GV3), Zusanli (ST36) and Ciliao (BL32).
Outcome measures: Motor function and cell apoptosis in rats after SCI. The mRNA and protein expression levels of Shh and Gli-1 were determined by real-time quantitative polymerase chain reaction, western blot and immunohistochemistry.
Results: After AM treatment, the hindlimb motor function of SCI rats was significantly increased than the SCI group at 7, 9, 11, 14 days (P < 0.05). AM treatment 7 d and 14 d significantly preserved the nissl-stained positive neurons and significantly decreased number of apoptotic cells, compared to that of SCI 7 and 14 d groups (P < 0.05). AM treatment improved the mRNA protein levels of Shh and Gli-1 after 7 and 14 days treatment compared to the SCI group (P < 0.05).
Conclusion: AM could improve the expression of Shh and Gli-1 in injured spinal cord of rats. That could be part of underlying mechanisms of AM treatment including recover motor function and preserve the neuron cells and alleviate the apoptosis of nerve cells in rats after SCI.
Keywords: Acupuncture, Moxibustion, Spinal cord injury, Shh, Gli-1
Introduction
Spinal cord injury (SCI) results in the loss of voluntary sensory and motor function, leads to severe disability. The loss of nerve function is mainly caused by two pathological factors, the directly mechanical damage on the spinal cord and the secondary injury caused by pathophysiological changes after SCI.1 At present, there still is no effective treatment for the injured spinal cord. In the adult central nervous system (CNS) the regeneration of neurons is limited and it is hard to regenerate after severe injury. Due to the limitation of nerve regeneration after injury, it is difficult to achieve complete recovery from SCI. Previous work revealed that the regeneration of nerve cells depends on their surrounding microenvironment.2 The surrounding environment is critical for the regeneration progress of CNS and various signal pathways could regulate the nerve growth and regeneration. Those secondary injuries caused by SCI, such as hemorrhage, ischemia, inflammation and apoptosis break the balance of microenvironment, which could not support the regeneration of CNS. Johansson et al. found that various signal pathways can be observed in microenvironment where neural stem cells congregate in embryo, those signal pathways might participate the proliferation and differentiation of neural stem cells.3
The Hedgehog (Hh) signaling pathway regulates an enormous variety of developmental events in embryo.4 The vertebrate Hh family is represented by at least three members: Desert Hedgehog (Dhh), Indian Hedgehog (Ihh) and Sonic Hedgehog (Shh). As one of three Hh signaling pathway members, Shh is the most extensively characterized homolog, and has been identified within a variety of species. Shh is expressed in the notochord, the floor plate of the neural tube in the human embryo.5 Shh ligand interacts with the transmembrane receptor Patched-1 (Ptc1) or Patched-2 (Ptc2) then relieves the signal transducer smoothened (Smo), which activating the zinc finger protein glioma-associated oncogene homolog-1 (Gli-1) and leads to Gli-1 protein entering the nucleus and acting as a transcriptional activator for downstream genes.6 Recent studies revealed that Shh signaling pathway could be abnormally activated in various tissues derived from endoderm, which lead to the continuous damage repair and regeneration of cells in tissue, in some cases inducing tumor formation ultimately.7,8 The Shh/Gli-1 signaling pathway has been recognized as key mediator of many fundamental processes in embryonic development, including the growth, patterning, and morphogenesis of CNS.9 Previous research has revealed that the Shh pathway could contribute to nerve regeneration and may ultimately promote SCI recovery.10
Acupuncture combined with moxibustion (AM) therapy has been used to treat SCI clinically, but the underlying mechanism is unclear. Previous studies have indicated electroacupuncture or moxibustion could promote the cell proliferation and be beneficial for the recovery of SCI.11,12 However, the relationship between Shh/Gli-1 signaling pathway and AM has been less studied in SCI. Therefore, this study was conducted to investigate the effect and mechanism of AM on the recovery of SCI.
Methods
Animals
Age-matched male Sprague–Dawley rats weighing 150–200 g were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Rats were housed in a standard polypropylene cage containing sterile bedding under a controlled condition of temperature, humidity, and light (12 h light/dark cycles) and were allowed free access to food and water ad libitum. This study was carried out in accordance with the recommendations of the NIH Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Use Committee at Jiangxi University of Traditional Chinese Medicine.
Spinal cord injury model
After being anesthetized with ketamine (100 mg/kg body weight) combined with xylazine (25 mg/kg body weight), the rats were fixed on the operating table in a prone position. The skin and muscle of the spinal cord area (T8-T12 vertebral body) were incised and the spinous process and neural plate were exposed. The T10 spinous process and neural plate were removed by micro-rongeur forceps to expose the spinal cord. The spinal cord was set at the bottom of a stainless steel impact rod (diameter, 1.8 mm). A 10 g weight was released 8 cm vertically away from impact rod, the impact rod hit into spinal cord for about 1.5 mm. Then the incision was sutured. A successful SCI model was defined as a spinal cord with hemorrhage and edema, followed by contractions of the lower extremity, acutely swung tail, and flaccid paralysis. Rats of sham surgery group only removed spinous process and neural plate without spinal cord damaged. All SCI rats received assisted urination the bladder was gently pressed three times daily until the rat recovered the spontaneous urination.
Treatments and groups
Acupuncture (AP), moxibustion (MX) and acupuncture combined with moxibustion (AM) therapy were performed on the SCI rats. Dazhui (GV14) and Jiaji points (T7-T12), Yaoyangguan (GV3), Zusanli (ST36), Ciliao (BL32) of both sides were selected. Acupuncture stainless 0.18-mm-diameter needles were inserted approximately 5 mm deep into those acupoints. Meanwhile Dazhui (GV14) Yaoyangguan (GV3) and Zusanli (ST36) were treated by moxibustion (Made by Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang, China). AP was acupuncture alone without MX, MX was moxibustion alone without AP. Each treatment was performed for 30 min once per day. The SCI rats were randomly divided into nine groups: 7 days or 14 days model control group (SCI, n = 12/each group), 7 days or 14 days AP treatment group (SCI + AP, n = 12/each group), 7 days or 14 days MX treatment group (SCI + MX, n = 12/each group), 7 days or 14 days AM treatment group (SCI + AM, n = 12/each group) and sham surgery rats were set as sham group (Sham, n = 12). Rats of treatment groups were received treatments 24 h after SCI for 7 days or 14 days.
Measurement of rat behavior
The hindlimb motor function of SCI rats was assessed at 1, 3, 5, 7, 9, 11 and 14 days after SCI according to BBB score. Rats were placed on the open flat space. Then motor functions were observed for 5 min and scored by two experienced individuals who were not involved in treatment. The BBB scores were recorded as the average values of two independent BBB scores by different persons.
Histology and Nissl staining
Spinal cord tissues were fixed in 4% paraformaldehyde for 24 h and then transferred to ethanol for dehydration. Embedded in paraffin and sliced into sections. For Nissl staining, sections were deparaffinized in xylene and rehydrated with ethanol and double distilled water, then staining in 1% Cresyl violet for 10 min. Finally dehydrated in ethanol and mounted with Permount (Thermo Fisher Scientific). The slides were observed and imaged using an optical microscope. Five randomly selected fields were measured for each section.
TUNEL assay
Apoptosis was assessed by TUNEL staining. The sections were firstly deparaffinized and rehydrated, and then were incubated with proteinase K for 20 min at 37°C. After washing three times with TBS buffer, the sections were incubated with a 20 μL labeling buffer containing 1 μL DIG-d-UTP and 1 μL TdT Enzyme for 2 h at 37°C in the dark. After washing the slides were incubated with 50 μL blocking buffer for 30 min. The specimen was covered in Streptavidin-HRP for 30 min and washed in TBS three times. The slides were visualized by diaminobenzidine (DAB) (AR1022, Boster Biological Technology, Wuhan, China) substrate and observed by microscope. Brown cells were considered positive and were counted as apoptotic cells, the apoptotic index was calculated as a ratio of apoptotic cells and total cells in each field.
Immunohistochemistry
The SABC Immunohistochemistry Kit (SA1055, Boster Biological Technology, Wuhan, China) was used in this experiment. The sections of spinal cord tissues were firstly deparaffinized in xylene and rehydrated with gradient ethanol. After incubation with 3% H2O2 for 10 min, the sections were heated and cooled in citrate buffer for 30 min to retrieve antigen. After incubated with blocking buffer for 30 min at 37°C, three randomly selected sections in each group were incubated with the rabbit polyclonal anti-Gli-1 antibody (1:200) (Abcam, Cambridge, UK) or mouse monoclonal anti-Shh antibody (1:100) (Santa Cruz Biotechnology, Santa Cruz, USA) at 4°C overnight. The sections were incubated with biotinylated rabbit anti-goat/ rabbit anti-mouse IgG (Boster Biological Technology, Wuhan, China) for 20 min at 37°C. After washing with PBS, sections were incubated with SABC reagent for 20 min at 37°C, visualized with DAB (AR1022, Boster Biological Technology, Wuhan, China) for 5–10 min, dyed with hematoxylin and mounted. The slides were finally observed and imaged under a light microscope. Five randomly selected fields were measured for each section. The positive rate was calculated as a ratio of positive cells and total cells in each field.
RT-qPCR
RT-qPCR was performed using SYBR Green system. Total RNA was isolated from the spinal cords of rats in each group using TRIzol solution (Invitrogen, Carlsbad, CA, USA). The mRNA expression levels of Shh and Gli-1 were measured using a RT-qPCR system with SYBR Green (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was amplified by PCR using primers for each target gene. RT-qPCR conditions are as follows: 94°C for 5 min, followed by 40 cycles of 95°C for 15 s, 60°C for 45 s and 72°C for 30 s. The amplification efficiency was compared between the target and reference control GAPDH (glyceraldehyde 3-phosphate dehydrogenase) using the 2−ΔΔCt method. Shh forward primer: 5′-AAA AGC TGA CCC CTT TAG CC-3′ and reverse primer: 5′-GAT GTC GGG GTT GTA ATT GC-3′; Gli forward primer: 5′-CAG CTC AAA GCT CAG CTC CT-3′ and reverse primer: 5′-CTT GGG GCT CTG ATA TGG AA-3′; GAPDH forward primer: 5′- CCG GTG CTG AGT ATG TCG TG-3′ and reverse primer: 5′- CCT TTT GGC TCC ACC CTT C-3′.
Western blot analysis
Spinal cord tissues obtained at 7and 14 days in each group were mixed in lysis buffer to collect total protein. Protein concentration was determined by bicinchoninic acid (BCA). The protein samples were mixed with SDS loading buffer and boiled at 94°C for 10 min. Equal amounts of proteins were separated by SDS-PAGE, and then the resolved proteins were transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). After blocking by 5% skimmed milk, the membranes were incubated with primary antibodies overnight at 4°C. Antibodies used for western blot including the rabbit polyclonal anti-Gli-1 antibody (1:1000, Abcam, Cambridge, UK), mouse monoclonal anti-Shh antibody (1:500, Santa Cruz Biotechnology, Santa Cruz, USA), anti-beta-Actin antibody (1:1000, Santa Cruz Biotechnology, Santa Cruz, USA), goat anti-rabbit IgG-HRP (1:1000, Boster Biological Technology, Wuhan, China) and goat anti-mouse IgG-HRP (1:1000, Boster Biological Technology, Wuhan, China). Subsequently, the membranes were incubated with secondary antibody at room temperature for 2 h. The membranes were immersed in an enhanced chemiluminescence reagent (ECL, Thermo Fisher Scientific, Waltham, USA). The gray values of bands were quantified using Image J software (Fujifilm, Tokyo, Japan). The relative expression of protein was calculated based on the gray value ratio of target to loading control.
Statistical analysis
All data are presented as mean ± SD. Statistical analysis was performed with SPSS 21.0 software (IBM, Armonk, USA). Data were analyzed by a one-way or two-way analysis of variance (ANOVA) test followed by a post hoc Student–Newman–Keuls (SNK) test or Dunnett’s post hoc test to compare the difference between groups. A value of P < 0.05 is considered statistically significant.
Results
AM improved the motor function of SCI rats
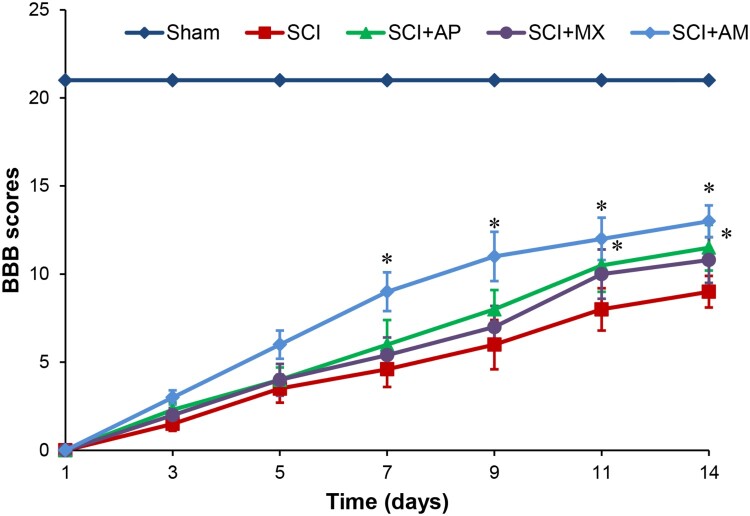
BBB scores were used to evaluate the motor function of hindlimbs in each group. As shown in Fig. 1, BBB scores of SCI rats were significantly reduced than that of the sham control rats (P < 0.05). After AM treatment, the hindlimb motor function of SCI rats were improved, BBB scores were significantly increased than SCI group at 7, 9, 11, 14 days (P < 0.05). However, in AP and MX treatment groups, the BBB scores were only significantly raised at 11 and 14 days (P < 0.05). AM treatment group BBB scores were higher than the both of AP and MX alone treatment groups at 7, 9, 11, 14 days (P < 0.05). These results indicated that AM treatment gradually recovered the hindlimb motor function in SCI rats, and AM had a better effect than AP or MX alone at early recovery of SCI, providing experiment evidence supporting the treatment effect of AM under SCI.
Figure 1.
BBB scores indicated AM improved the motor function of SCI rats. Motor function assessment of rat hindlimbs at 1, 3, 5, 7, 9, 11 and 14 days after SCI surgery according to BBB scores. Data are expressed as the mean ± SD (n = 12). * Compared to that from SCI group, P < 0.05. BBB: Basso, Beattie, and Bresnahan; SCI: spinal cord injury; AP: acupuncture; MX: moxibustion; AM: acupuncture combined with moxibustion
AM alleviated the apoptosis of neural cells after SCI
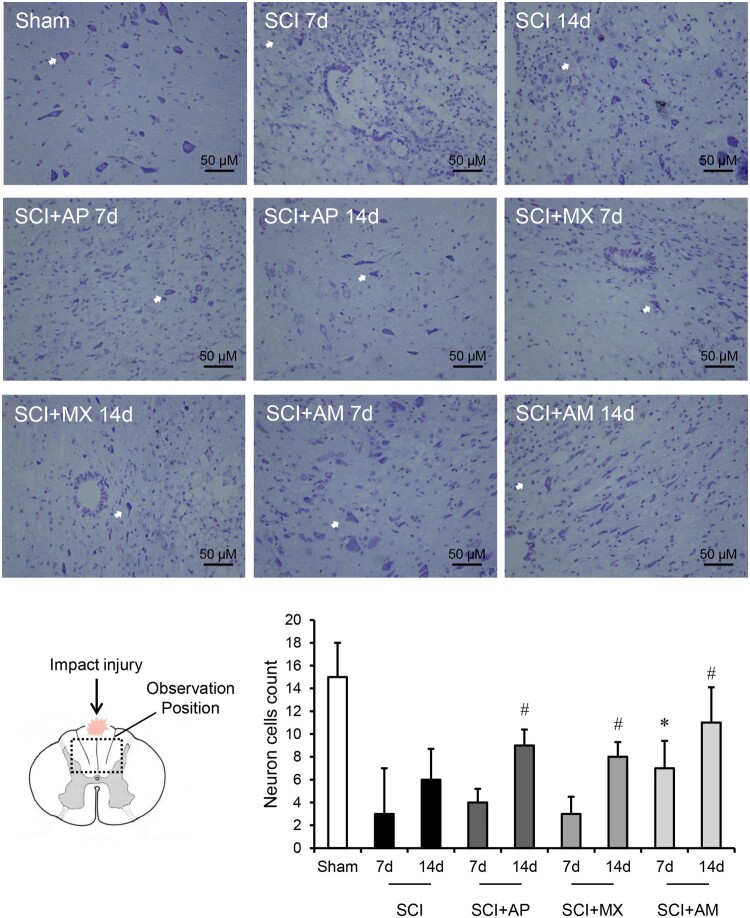
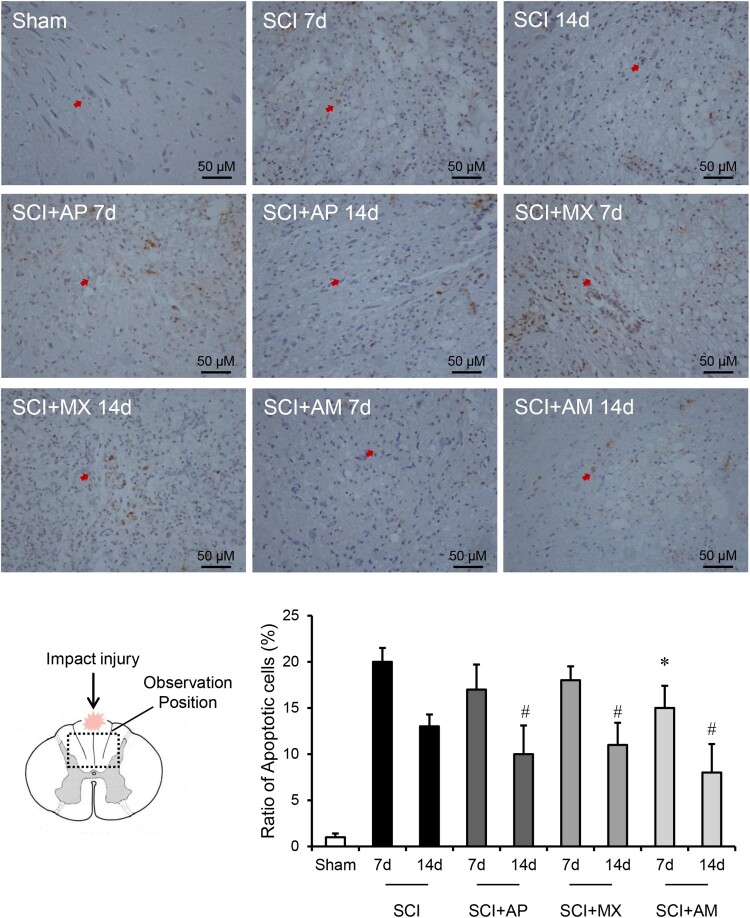
Nissl stain was used to evaluate the numbers of neurons and the apoptosis after SCI was detected by TUNEL. As shown in Fig. 2, the nissl-stained neuron cells were reduced in SCI 7 d and 14 d groups compared to the sham group (P < 0.05). AM treatment 7 d and 14 d significantly preserved the nissl-stained positive neurons, compared to that of SCI 7 d and 14 d groups (P < 0.05). AP and MX alone treatments had no significant change at 7 days compared to SCI group, but AP and MX both preserved the neurons in 14 days (P < 0.05). As shown in Fig. 3, the percentage of TUNEL positive cells was calculated, apoptosis was obvious increased after SCI (P < 0.05). SCI + AM 7 d and 14 d groups exhibited a significantly decreased number of apoptotic cells than in the SCI 7 d and 14 d groups (P < 0.05). AP and MX alone treatments could protect the apoptosis at 14 days but not 7 days.
Figure 2.
AM preserved the nissl-stained neuron cells after SCI. Spinal cord tissues were prepared and assessed by nissl-staining at 7 and 14 days after SCI. Nissl-stained neurons were pointed by white arrows in the field of vision. The count number of positive cells in the field of vision was divided by the number of fields. Data are expressed as the mean ± SD (n = 4). * Compared to that from SCI 7d group, P < 0.05; # Compared to that from SCI 7d group, P < 0.05. SCI: spinal cord injury; AP: acupuncture; MX: moxibustion; AM: acupuncture combined with moxibustion
Figure 3.
AM alleviated the apoptosis of neural cells after SCI. Spinal cord tissues were prepared and assessed by TUNEL at 7 and 14 days after SCI. The brown positive cells were apoptotic cells and pointed by red arrows. Ratio of apoptotic cells to total cells was counted. Data are expressed as the mean ± SD (n = 4). * Compared to that from SCI 7d group, P < 0.05; # Compared to that from SCI 7d group, P < 0.05. SCI: spinal cord injury; AP: acupuncture; MX: moxibustion; AM: acupuncture combined with moxibustion
AM increased the expression of Shh and Gli-1 in spinal cord after SCI
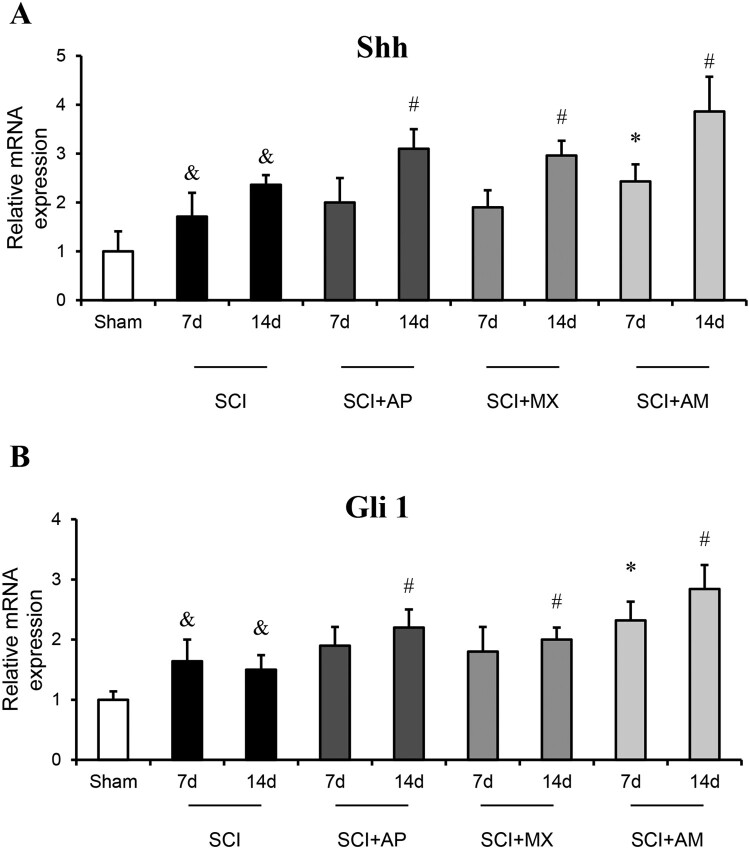
To investigate the AM treatment effect on the Shh/Gli1 signaling pathway, mRNA expression of Shh and Gli-1 were detected by qRT-PCR and protein levels were evaluated by western blot and immunohistochemistry in spinal cord tissues. As shown in Fig. 4, mRNA expression of Shh and Gli-1 were significantly increased at 7 and 14 days after SCI compared to the sham group (P < 0.05). After AM treatment, the mRNA levels of Shh and Gli-1 were higher in SCI + AM 7 d group than that in SCI 7d group (P < 0.05), Shh and Gli-1mRNA levels of SCI + AM 14 d group were also increased than that of SCI 14d group (P < 0.05). In AP and MX alone treatment groups, the mRNA levels of Shh and Gli-1were significantly raised at 14 days rather than 7 days.
Figure 4.
AM increased the mRNA expression of Shh and Gli-1 in spinal cord of SCI rats. (A) The mRNA expression of Shh in spinal cord tissues of the sham group, SCI, SCI + AP, SCI + MX and SCI + AM groups at 7 and 14 days after SCI surgery, determined by qRT-PCR. (B) The mRNA expression of Gli-1 in spinal cord tissues of the sham group, SCI, SCI + AP, SCI + MX and SCI + AM groups at 7 and 14 days after SCI surgery, determined by qRT-PCR. Data are expressed as the mean ± SD (n = 4). & Compared to that from Sham group, P < 0.05; * Compared to that from SCI 7d group, P < 0.05; # Compared to that from SCI 7d group, P < 0.05. SCI: spinal cord injury; AP: acupuncture; MX: moxibustion; AM: acupuncture combined with moxibustion
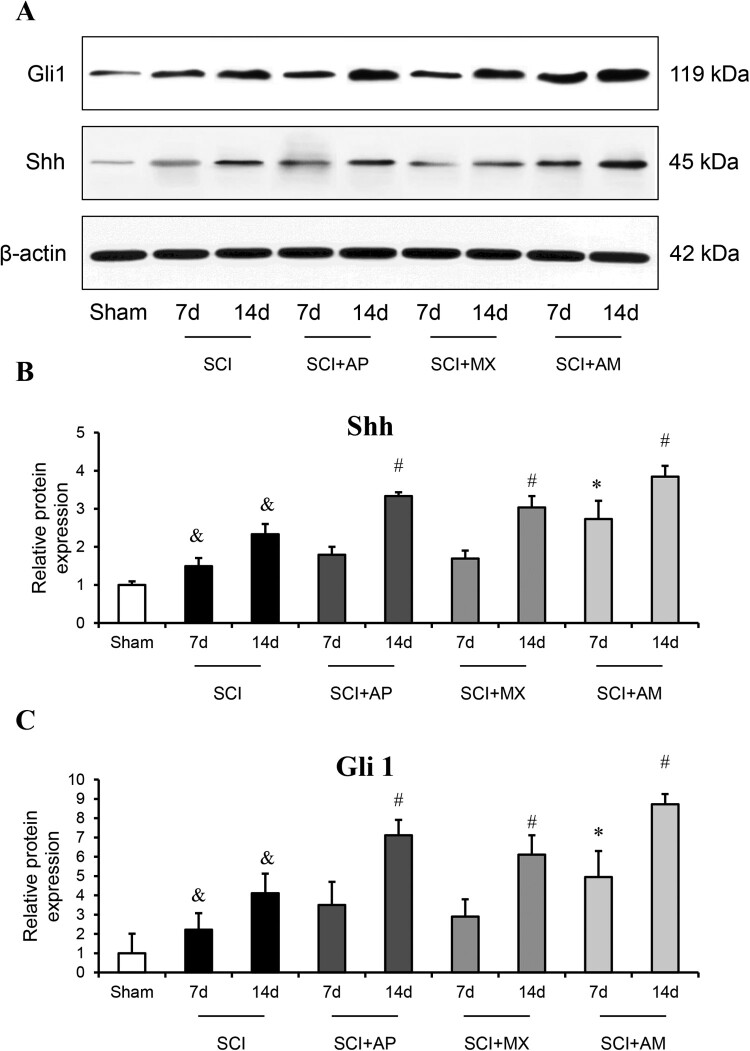
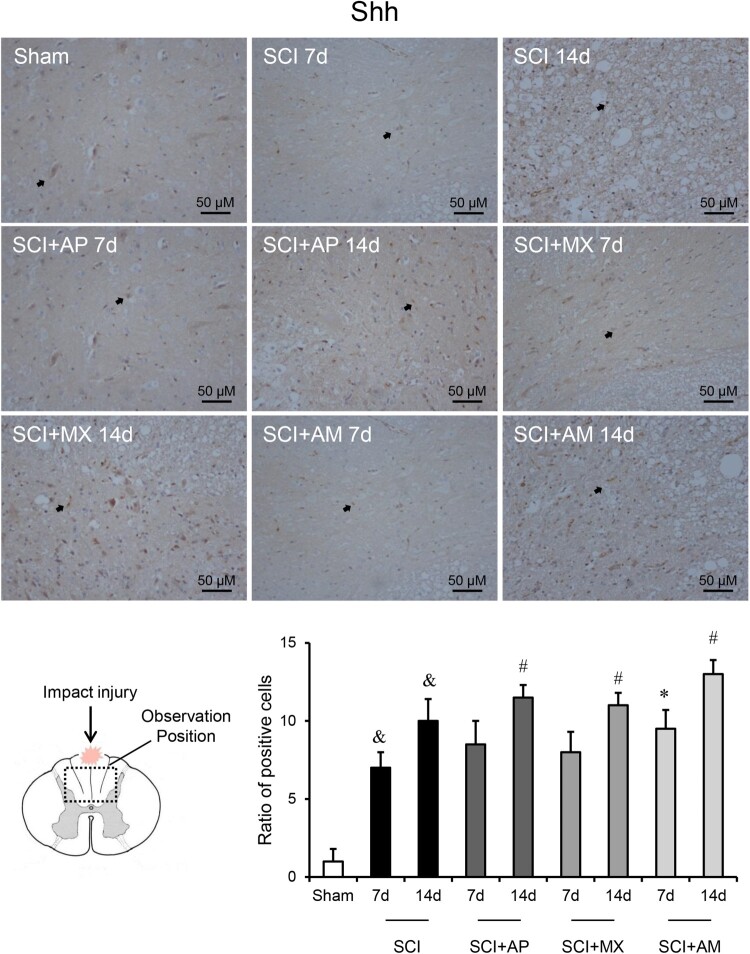
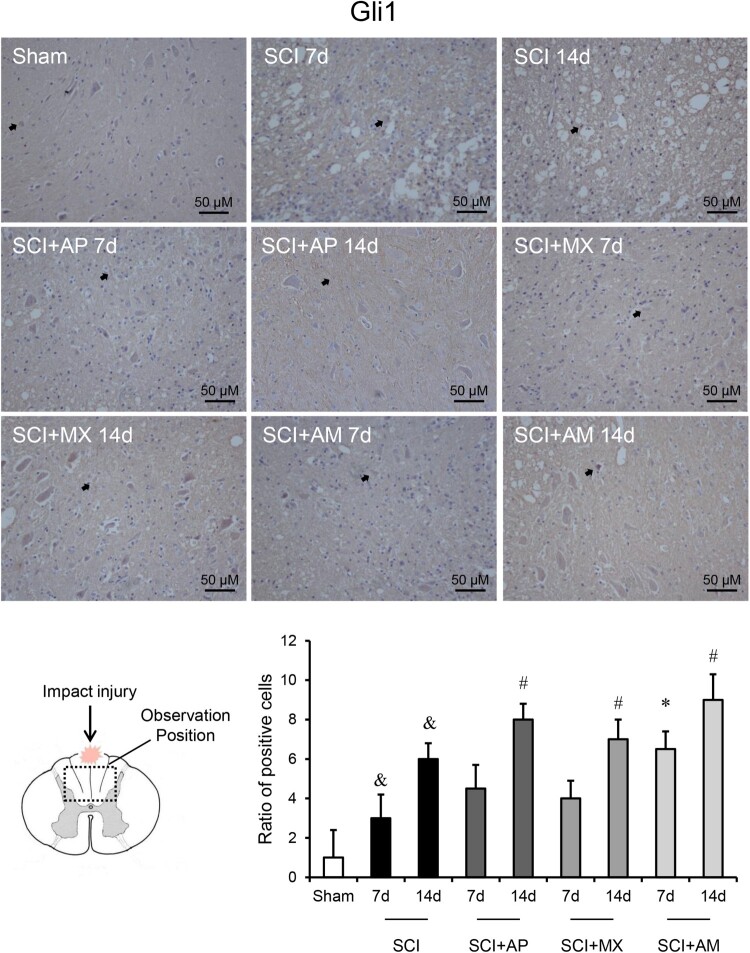
As shown in Fig. 5, the protein levels of Shh and Gli-1 exhibited the similar results to mRNA changes. Shh and Gli-1 protein in the spinal cord were significantly up-regulated at 7 and 14 days SCI compared to that of the sham group (P < 0.05). AM improved the protein levels of Shh and Gli-1 after 7 and 14 days treatment compared to the SCI group (P < 0.05). In AP and MX alone treatment groups, the protein levels of Shh and Gli-1were significantly raised at 14 days rather than 7 days. As shown in Figs. 6 and 7, Immunohistochemistry detected the Shh and Gli-1 protein among the sham, SCI, SCI + AP, SCI + MX and SCI + AM groups. The results were consistent with western blot results; Shh and Gli-1 positive staining of SCI 7d and 14d groups were increased than that of the sham group, AP and MX increased the positive staining cells at 14 days rather than 7 days compare to SCI groups. AM obviously increased the Shh and Gli-1 than that of SCI group at 7 and 14 days after SCI.
Figure 5.
Western blot analysis showed that AM increased the protein levels of Shh and Gli-1 in spinal cord of SCI rats. (A) Representative western blot bands of Shh and Gli-1 in sham, SCI, SCI + AP, SCI + MX and SCI + AM groups. (B) The protein level of Shh in spinal cord tissues of the sham group, SCI, SCI + AP, SCI + MX and SCI + AM groups at 7 and 14 days after SCI surgery. (C) The protein level of Gli-1 in spinal cord tissues of the sham group, SCI, SCI + AP, SCI + MX and SCI + AM groups at 7 and 14 days after SCI surgery. Data are expressed as the mean ± SD (n = 4). & Compared to that from Sham group, P < 0.05; * Compared to that from SCI 7d group, P < 0.05; # Compared to that from SCI 7d group, P < 0.05. SCI: spinal cord injury; AP: acupuncture; MX: moxibustion; AM: acupuncture combined with moxibustion
Figure 6.
Immunohistochemistry indicated that AM increased the protein levels of Shh in spinal cord. Spinal cord tissues were prepared and Shh protein was assessed by immunohistochemistry. The brown positive cells were pointed by black arrows. Representative positive staining of Shh protein in sham, SCI, SCI + AP, SCI + MX and SCI + AM groups at 7 and 14 days after SCI surgery. Ratio of positive cells to total cells was counted. Data are expressed as the mean ± SD (n = 4). & Compared to that from Sham group, P < 0.05; * Compared to that from SCI 7d group, P < 0.05; # Compared to that from SCI 7d group, P < 0.05. SCI: spinal cord injury; AP: acupuncture; MX: moxibustion; AM: acupuncture combined with moxibustion
Figure 7.
Immunohistochemistry indicated that AM increased the protein levels of Gli-1 in spinal cord. Spinal cord tissues were prepared and Gli-1 protein was assessed by immunohistochemistry. The brown positive cells were pointed by black arrows. Representative positive staining of Gli-1 protein in sham, SCI, SCI + AP, SCI + MX and SCI + AM groups at 7 and 14 days after SCI surgery. Ratio of positive cells to total cells was counted. Data are expressed as the mean ± SD (n = 4). & Compared to that from Sham group, P < 0.05; * Compared to that from SCI 7d group, P < 0.05; # Compared to that from SCI 7d group, P < 0.05. SCI: spinal cord injury; AP: acupuncture; MX: moxibustion; AM: acupuncture combined with moxibustion
Discussion
Acupuncture and moxibustion have been widely used in clinical practice as an alternative complementary medicine. According to the traditional Chinese medicine theory of Zang-fu organs and meridians, the pathological change of SCI could be explained by “Governor Vessel” injury.12 “Governor Vessel” is a circulation meridian in the back that position is coincides with spinal cord. Therefore we chose the acupoints located in “Governor Vessel” such as Dazhui (GV14) and Jiaji points (T7-T12) and Yaoyangguan (GV3) to promote the recovery of meridian function. Zusanli (ST36) and Ciliao (BL32) could promote the blood circulation and muscle function recovery. Acupuncture is the use of needle stab into skin to stimulate the acupoints, while moxibustion is the use of infrared light and heat generated by combustion of folium artemisiae argyi to stimulate the acupoints. Acupuncture and moxibustion can enhance the stimulation effect of each other. Generally, acupuncture and moxibustion are used together in clinic to obtain better curative effect.
The current study demonstrates that AM treatment improved hindlimb motor function of SCI rats. According to the BBB scores results, AM significantly increased the BBB scores at 7, 9, 11, 14 days after SCI. However, AP and MX treatment alone were only significantly improved motor function at 11 and 14 days. AM had better treatment effect than AP or MX alone from 7 to 14 days after SCI, which indicated that the combination of AP and MX promote the recovery of SCI in the perspective of behaviors. Nissl-staining resulted revealed that AM preserved the nissl-stained neuron cells which were significantly reduced after SCI. Meanwhile TUNEL staining showed that AM also alleviated the apoptosis after SCI. And the combination of AP and MX was better than alone. Those results indicated the protective effect of AM to the impaired nerve cells could participate in the recovery of SCI.
The Hh pathway has been shown to be activated in nerve damage, and may serve as a potential approach for treating neurodegenerative diseases and dysfunction such as SCI.13 In terms of nerve injury and regeneration, the Shh signaling pathway has a protective role in pathological injury after SCI.14 Studies showed that SCI caused the differentiation of nearby progenitors to scar-producing astrocytes 15 inhibiting the elongation and myelination of axons, finally prevented the re-establish the neural circuitry required for regeneration.16 However the expression of Shh reduced that progress after SCI, those researchers considered Shh signaling pathway as a potential therapy for enhancing regeneration following spinal cord injury.17 Shh is produced in reactive astrocytes after the cerebral cortex injury and participated in regulating the remyelination of injured axons after brain injury.18 Gli-1 is one of three homologs of cytoplasmic zinc finger protein. Shh releases the associated signaling transducer Smo, resulting in upregulation and nuclear translocation of Gli transcription factors.19 Gli is translocated to the nucleus leading to transcriptional activation of Hh target genes, including Gli itself and Patched (Ptc), and take part in the process of promoting regeneration of Hh pathway.
In our study the expression of Shh and Gli-1were up-regulated at both gene and protein levels after SCI. Results revealed that compared to the sham group, Shh and Gli-1 were significantly increased at 7 and 14 days after SCI, and the immunohistochemistry detect coincided with those results. Shh/Gli-1 signaling pathway is activated after SCI as a protective reaction for the injury. Study showed that Shh protein was increased in the hippocampus after brain ischemia and participated promoted in the proliferation of neural progenitor cells (NPCs) which might participate the injury remodeling.20 The elevated Shh and Gli-1 might contribute to preserve the neuron cells and alleviated the apoptosis after SCI. AM treatment improved the mRNA and protein levels of Shh and Gli-1 in injured spinal cord after 7 and 14 days treatment than the SCI groups. The promotion effect of AM at the expression of Shh and Gli-1 in injured spinal cord could accelerate the proliferation of NPCs and the regeneration of axons. AP of MX alone only significantly increased the levels of Shh and Gli-1 at the 14 days, which indicated the combination of AP and MX could improve the expression of Shh and Gli-1 at early stage of SCI treatment and had a better effect than use alone.
The underlying mechanisms of acupuncture and moxibustion have not been thoroughly studied. Various studies have proved the effect of acupuncture in SCI in terms of different mechanisms. Electroacupuncture could inhibit the expression of PTEN and p53, which possibly promote the recovery of mouse SCI motor functions.12 The p53 has been proved that exist the cross-talk with Shh pathway, inhibiting the p53 facilitates the activation of Shh pathway.21 Acupuncture could reduce apoptosis of granulosa cells in rats via up-regulating PI3K/Akt signaling pathway.22 The PI3K/Akt pathway has been considered to mediate Shh pathway in rat astrocytes under oxidative stress.23 Previous study showed that a Shh agonist, purmorphamine, promotes recovery of neurological function and inhibits neuronal apoptosis in intracerebral hemorrhage rat models, and Baihui-penetrating-Qubin acupuncture could produce a series of effects similar to that Shh agonist.24 That study is coincide with our results that AM treatment promotes the recovery of motor function and inhibits apoptosis. Shh/Gli-1 signaling pathway has cross-talk with various critical signaling pathways and molecules, such as the p53 or PI3K/Akt. Acupuncture or electroacupuncture might mediate Shh/Gli-1 signaling pathway by acting those pathways and molecules. Nevertheless, the direct effect of acupuncture and moxbustion on the Shh/Gli-1 signaling pathway required more completely researches in the future.
Conclusion
Acupuncture and moxibustion could improve the expression of Shh and Gli-1 in injured spinal cord. That could be part of underlying mechanisms of AM treatment including recover motor function and preserve the neuron cells and alleviate the apoptosis of nerve cells after SCI. And the combination of AP and MX had a better effect than use alone. This study provides evidence to support the clinical application of AM in SCI and augment our understanding for treatment effects of AM in the SCI and repair. Meanwhile, this study has limitations and further studies are required to thoroughly interpret specific mechanisms of AM in the Shh/Gli-1 signaling pathway in SCI treatment.
Disclaimer statements
Contributors XWH designed the study. LLQD, SFH, PZ, FFZ, TPL, QZ, YY, FH, PX, CKW performed the experiment. LLQD and SFH analyzed the data. LLQD and SFH wrote the manuscript.
Conflicts of interest Authors have no conflict of interests to declare.
Funding Statement
This project was supported by a grant from the National Natural Science Foundation of China [grant number 81760888 to XWH].
References
- 1.Schwab ME. Repairing the injured spinal cord. Science. 2002;295(5557):1029–31. doi: 10.1126/science.1067840 [DOI] [PubMed] [Google Scholar]
- 2.Cajal SRY, May RM, DeFelipe J, Jones EG, Trumpler M.. Cajal's degeneration and regeneration of the nervous system. ISIS. 1994;85(3):543–4. doi: 10.1086/356955 [DOI] [Google Scholar]
- 3.Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisen J.. Neural stem cells in the adult human brain. Exp Cell Res. 1999;253(2):733–6. doi: 10.1006/excr.1999.4678 [DOI] [PubMed] [Google Scholar]
- 4.Harris PJ, Speranza G, Dansky Ullmann C.. Targeting embryonic signaling pathways in cancer therapy. Expert Opin Ther Targets. 2012;16(1):131–45. doi: 10.1517/14728222.2011.645808 [DOI] [PubMed] [Google Scholar]
- 5.McMahon AP. More surprises in the Hedgehog signaling pathway. Cell. 2000;100(2):185–8. doi: 10.1016/S0092-8674(00)81555-X [DOI] [PubMed] [Google Scholar]
- 6.Patel SS, Tomar S, Sharma D, Mahindroo N, Udayabanu M.. Targeting sonic hedgehog signaling in neurological disorders. Neurosci Biobehav Rev. 2017;74(Pt A):76–97. doi: 10.1016/j.neubiorev.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 7.Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB.. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422(6929):313–17. doi: 10.1038/nature01493 [DOI] [PubMed] [Google Scholar]
- 8.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, et al. . Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431(7009):707–12. doi: 10.1038/nature02962 [DOI] [PubMed] [Google Scholar]
- 9.Ingham PW, McMahon AP.. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–87. doi: 10.1101/gad.938601 [DOI] [PubMed] [Google Scholar]
- 10.Zhang YD, Zhu ZS, Zhang D, Zhang Z, Ma B, Zhao SC, et al. . Lentivirus-mediated silencing of the PTC1 and PTC2 genes promotes recovery from spinal cord injury by activating the Hedgehog signaling pathway in a rat model. Exp Mol Med. 2017;49(12):e412. doi: 10.1038/emm.2017.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu LN, Tian JX, Gao W, Zhu J, Mou FF, Ye XC, et al. . Electroacupuncture and moxibustion promote regeneration of injured sciatic nerve through Schwann cell proliferation and nerve growth factor secretion. Neural Regen Res. 2018;13(3):477–83. doi: 10.4103/1673-5374.228730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei Z, Zhao W, Schachner M.. Electroacupuncture restores locomotor functions after mouse spinal cord injury in correlation with reduction of PTEN and p53 expression. Front Mol Neurosci. 2018;11(411):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams JA. Hedgehog and spinal cord injury. Expert Opin Ther Targets. 2005;9(6):1137–45. doi: 10.1517/14728222.9.6.1137 [DOI] [PubMed] [Google Scholar]
- 14.Kong YL, Wang YF, Zhu ZS, Deng ZW, Chen J, Zhang D, et al. . Silencing of the MEKK2/MEKK3 pathway protects against spinal cord injury via the Hedgehog pathway and the JNK pathway. Mol Ther – Nucleic Acids 2019;17:578–89. doi: 10.1016/j.omtn.2019.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Nishimura S, Yasuda A, Iwai H, Takano M, Kobayashi Y, Nori S, et al. . Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. doi: 10.1186/1756-6606-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones LL, Sajed D, Tuszynski MH.. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003;23(28):9276–88. doi: 10.1523/JNEUROSCI.23-28-09276.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas AM, Palma JL, Shea LD.. Sponge-mediated lentivirus delivery to acute and chronic spinal cord injuries. J Controlled Release. 2015;204:1–10. doi: 10.1016/j.jconrel.2015.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amankulor NM, Hambardzumyan D, Pyonteck SM, Becher OJ, Joyce JA, Holland EC.. Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J Neurosci. 2009;29(33):10299–308. doi: 10.1523/JNEUROSCI.2500-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Imitola J, Rasmussen S, O'Connor KC, Khoury SJ.. Paradoxical dysregulation of the neural stem cell pathway sonic hedgehog-Gli1 in autoimmune encephalomyelitis and multiple sclerosis. Ann Neurol. 2008;64(4):417–27. doi: 10.1002/ana.21457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sims JR, Lee SW, Topalkara K, Qiu J, Xu J, Zhou Z, et al. . Sonic hedgehog regulates ischemia/hypoxia-induced neural progenitor proliferation. Stroke. 2009;40(11):3618–26. doi: 10.1161/STROKEAHA.109.561951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malek R, Matta J, Taylor N, Perry ME, Mendrysa SM.. The p53 inhibitor MDM2 facilitates Sonic Hedgehog-mediated tumorigenesis and influences cerebellar foliation. PLoS One. 2011;6(3):e17884. doi: 10.1371/journal.pone.0017884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Lin S, Zhu M, Li C, Chen S, Pu L, et al. . Acupuncture reduces apoptosis of granulosa cells in rats with premature ovarian failure via restoring the PI3K/Akt signaling pathway. Int J Mol Sci 2019;20(24):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia YP, Dai RL, Li YN, Mao L, Xue YM, He QW, et al. . The protective effect of sonic hedgehog is mediated by the phosphoinositide [corrected] 3-kinase/AKT/Bcl-2 pathway in cultured rat astrocytes under oxidative stress. Neuroscience 2012;209:1–11. doi: 10.1016/j.neuroscience.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, Dai XH, Yu XP, Zou W, Teng W, Sun XW, et al. . Baihui (DU20)-penetrating-Qubin (GB7) acupuncture inhibits apoptosis in the perihemorrhagic penumbra. Neural Regen Res 2018;13(9):1650. doi: 10.4103/1673-5374.237139 [DOI] [PMC free article] [PubMed] [Google Scholar]