Abstract
Context/Objective: Wheelchair users with spinal cord injury (SCI) have a high risk of developing shoulder pain, caused by rotator cuff disease. Platelet-rich plasma (PRP) is a potential treatment after conservative treatments fail and prior to surgical intervention; however, it has not been tested in wheelchair users who have recalcitrant shoulder pain associated with rotator cuff disease. The objective of this pilot project was to test the safety and potential treatment effect of an ultrasound-guided PRP injection for shoulder pain in the aforementioned population.
Design: Prospective, quasi-experimental.
Setting: Clinical research center.
Participants: Six wheelchair users with SCI (3 paraplegia, 3 tetraplegia) who had chronic shoulder pain due to rotator cuff disease (presence of anterior shoulder pain, positive physical examination tests for rotator cuff disease, and tendinopathy demonstrated by ultrasound) and failed at least six months of conservative treatment.
Interventions: Ultrasound-guided PRP injection into pathological shoulder tendons, targeting the supraspinatus. Subjects were provided a standardized stretching and strengthening program and were followed for 4, 8, 12, and 24 weeks post-intervention with outcomes collected at each time-point.
Outcome Measures: Wheelchair User's Shoulder Pain Index (WUSPI); pain Numerical Rating Scale (NRS); physical and ultrasound examinations for supraspinatus tendinopathy; 5-point patient global impression of change (PGIC).
Results: WUSPI (69.9%, P < 0.001), NRS (49.6%, P < 0.01), and physical exam scores (35.7%, P < 0.01) decreased 24 weeks after treatment. Participants reported overall improvement in their status as a result of the treatment. No adverse events were noted, and no changes in ultrasound markers for tendinopathy were observed.
Conclusion: A single, ultrasound-guided PRP injection into the supraspinatus tendon, followed by a stretching and strengthening exercise program, was safe and provided improvements in shoulder pain outcome measures in this sample for 24 weeks. Lack of blinding, short-term follow-up, and a suitable control group warrant a larger randomized controlled trial.
Trial Registration: NCT01355549
Keywords: Platelet-rich plasma, Spinal cord injury, Rotator cuff injuries, Treatment outcome, Rehabilitation
Introduction
Shoulder pain is common in individuals with spinal cord injury (SCI), with a prevalence ranging between 30% and 73%.1,2 Nichols et al. were the first to coin the term, “wheelchair user's shoulder”, when they reported that individuals with SCI who used wheelchairs were more likely to develop pain around the shoulders than any age group in the general population.3 Shoulder pain can lead to substantial disability, resulting in decreased functional independence.4 Although there are a number of conditions that can cause shoulder pain in wheelchair using individuals with SCI, rotator cuff disease is most common.1
Shoulder pain in wheelchair users with SCI often resolves with non-operative treatments such as rest, pharmacologic agents (e.g. nonsteroidal anti-inflammatory drugs, corticosteroid injections), modalities (e.g. heat, ice, ultrasound), exercises, equipment modification, and education.2,5 Once these conventional treatments fail, surgery may be indicated to restore function, reduce pain, and prevent further damage to the rotator cuff structures.6 In people with SCI, several groups have evaluated post-surgery outcomes.6–10 Many reported favorable outcomes with regard to improvements in pain and function.7,9,10 However, post-operative management included several months of power wheelchair use, assistance with weight-bearing activities of daily living (e.g. transfers), and in some cases hospitalization.7,9–11 A re-tear rate of 33% was observed in one study despite these post-operative precautions.10 Participants in two of the studies reported high satisfaction with post-surgical outcomes, indicating surgery may be a viable option after all others fail.6,10 Considering the costs associated with a surgical procedure and the functional limitations during the post-operative period, conservative treatment options that delay or prevent the need for surgery are preferable.
Autologous blood injections are a promising treatment for muscle injury and tendinopathy.12 It has been hypothesized that growth factors and cytokines carried in a person's own blood act as mediators to induce healing. Platelets, small cell fragments normally involved in blood clotting, have been shown to contain growth factors with healing properties that may enhance the reparative response after tendon injury.13 Platelet-rich plasma (PRP) is a treatment in which platelets are isolated from a sample of a person's own blood using simple cell-separating systems such as centrifugation. The resulting product is a highly concentrated sample of platelets that can be injected into an injury site to promote healing. In vitro studies have observed enhanced collagen formation and cell proliferation,14, 15 and increased synthesis of vascular endothelial growth factor (VEGF).13
Research studies reporting the use of PRP for chronic rotator cuff tendinopathy have primarily been in able-bodied, non-SCI populations. A multicenter, retrospective review reported moderate improvement to complete resolution of symptoms after PRP injections in the rotator cuff in 81% of participants.16 However, a recent systematic review and meta-analysis demonstrated equivocal results with respect to improvements in pain compared to the control group.17 In the rotator cuff, PRP has been compared to corticosteroid injections,18 dry-needling,19 and standardized exercise.20 In a study by Rha et al.,19 able-bodied patients who received PRP showed greater reduction in pain compared to dry-needling after six months. Symptoms improved after PRP compared to corticosteroids in a study by Shams et al.,18 yet the improvements appeared to dissipate after six months. Kesikburun et al.20 observed no improvements in participants who received PRP and a standardized exercise program compared to the control group that received only the exercise program. The differences in outcomes may be due to the post-treatment protocol. The study with less-favorable outcomes prescribed a strict, 6-week regimen starting with passive supervised range of motion exercises followed by home-based strengthening and stretching exercises. The others either provided a less structured regimen19 or did not allow physiotherapy in any capacity.18
The only investigation completed in the SCI population found that a PRP injection into the biceps tendon resulted in some pain reduction.21 However, the supraspinatus is most often the primary source of rotator cuff pathology in this population22,23 and thus should be the primary target of any therapy designed to reduce pain following a diagnosis of tendinopathy. The objective of this exploratory pilot study was to test the safety and treatment effect of a PRP injection under ultrasound guidance into the supraspinatus tendon in people with SCI who have recalcitrant shoulder pain caused by rotator cuff disease. Positive findings may help guide treatment for these ailments and lead to larger randomized control trials. It was hypothesized that a PRP injection, followed by a standardized stretching and strengthening protocol, would prove to be a safe and result in less pain intensity up to six months post-treatment. Pain intensity was measured using a pain numerical rating scale (NRS), the Wheelchair User's Shoulder Pain Index (WUSPI),24 and eight physical examination tests for rotator cuff disease. The treatment would be considered safe if no adverse events related to the procedure were reported. It was further hypothesized that ultrasound signs for supraspinatus pathology would lessen in severity, assessed using the Ultrasound Shoulder Pathology Rating Scale (USPRS).23
Methods
Subjects
Inclusion criteria included: (1) between 18 and 60 years of age, inclusive; (2) SCI with neurological level of injury between C6 and L5, inclusive, that occurred at least 12 months prior to the Screening Visit; (3) use of a manual or power wheelchair as primary means of mobility (> 40 h/week); (4) presence of chronic shoulder pain due to rotator cuff disease in spite of at least six months of conservative treatment; (5) presence of average shoulder pain of 5 or above on an 11-point numerical rating scale (NRS; 0, no pain; 10, maximum pain imaginable) during the week leading up to the Screening Visit; and (6) ability and willingness to comply with the protocol. Rotator cuff disease was defined as pain over the anterolateral shoulder with direct palpation and pain at the shoulder with provocative tests for rotator cuff disease, and was confirmed after identifying tendinopathic changes on ultrasound imaging. Provocative tests included palpation of the supraspinatus tendon over the greater tuberosity, supraspinatus (“empty can”, Jobe's) test,25 painful arc,26 and Hawkins–Kennedy test,27 and Neer's test.28 Ultrasound signs include edema, tendon thickening, and hypoechoic areas within the tendon consistent with the absence of tendon tissue (e.g. a tear).29
Subjects were excluded from the study if they: (1) reported prior PRP treatment in the same shoulder; (2) reported a history of systemic disorders such as diabetes or rheumatoid arthritis; (3) had contra-indications to the procedure such as infection, coagulopathy, or were currently taking anti-coagulants; (4) had a glucocorticoid injection to the affected shoulder within the past four weeks; (5) were pregnant; or (6) had any medical condition, including psychiatric illness, that would interfere with the interpretation of the study results or the conduct of the study.
Treatment procedure
Approximately 60 mL of blood was drawn from the uninvolved arm into a syringe containing an anti-coagulant. The blood was processed using a PRP preparation system (Harvest® SmartPReP®, Somerset, NJ, USA) according to instructions. Approximately 6mL of PRP was obtained from each patient. An 8.4% sodium bicarbonate solution was added into the PRP to buffer to physiologic pH. Between 3 and 5 mL were peppered into the affected tendon of the participant under direct ultrasound guidance using sterile technique. The supraspinatus was targeted in all participants, with one participant receiving an additional injection into the biceps tendon (Table 1). A 5–13 MHz linear probe was used for all injections and ultrasound measures (12L-RS probe; GE Logiq e, GE Healthcare, Chicago, Illinois, USA).
Table 1. Participant demographic information and treatment notes for platelet-rich plasma injection procedure.
| Subject | Age (yrs) | DOI (yrs) | Race/Ethnicity | Injury level | Ultrasound indicators | PRP volume, treatment locations |
|---|---|---|---|---|---|---|
| 1* | 51 | 13 | W, NH | C6 | Clear PT tear | 3 mL, Bursal and articular side of partial thickness tear |
| 2 | 48 | 20 | W, H | T12 | Mild tendinosis | 5 mL, Subacromial space |
| 3† | 60 | 45 | W, H | T12 | Possible FT tear | 4 mL, Tendon, long axis |
| 4 | 58 | 29 | W, NH | C7 | Clear FT tear | 3 mL, Tendon, unspecified |
| 5† | 62 | 22 | W, NH | C5 | Clear FT tear | 4 mL, Articular sided tear |
| 6 | 48 | 31 | W, NH | T6 | Clear PT tear | 4 mL, Areas of tendinopathy and partial tear |
Notes. W = White. H/NH = Hispanic/Non-Hispanic. PT = partial thickness. FT = full thickness. DOI = duration of injury. All participants were male.
* Biceps tendon injection (2 mL) in addition to supraspinatus.
† Supraspinatus intratendinous calcifications needled in addition to PRP injection.
Participants were instructed to rest the arm during the first 24 h following PRP treatment. This included limiting upper-limb weight-bearing activities (e.g. transfers, manual WC propulsion, etc.) whenever possible, and avoiding strenuous upper-limb activities (e.g. driving, prolonged wheeling, repeated overhead reaching, etc.). Subjects who drove were recommended to have a “helper” drive them home following the treatment. A 4-day course of tramadol or oxycodone/acetaminophen was prescribed for immediate post-injection pain, alternatively, participants could take 1–2 acetaminophen 500 mg tablets up to four times daily. Use of aspirin, NSAIDs, and corticosteroids was prohibited. During the first week, ADL could be increased as tolerated. Four weeks after the procedure (i.e. after evaluation at 4-Week Follow-up Visit), participants were allowed to proceed with normal sporting or recreational activities as tolerated.
Participants began a standardized stretching protocol after the 24-hour resting period, which transitioned to a formal strengthening program one month after PRP injection. Both stretching and strengthening protocols were adapted from a randomized controlled trial conducted by Mulroy et al.30 The stretching protocol consisted of maneuvers that targeted the anterior and posterior shoulder joint structures, as well as the upper trapezius. Strengthening exercises included scapular retraction using an elastic band, weighted shoulder abduction in the scapular plane, and weighted shoulder external rotation. Participants were instructed to continue the stretching and strengthening programs for the duration of the study.
Pain questionnaires
Participants completed questionnaires about functional and global pain intensity, including the WUSPI24 and an 11-point NRS. The WUSPI is a well-validated and reliable tool to measure shoulder pain in people with SCI during functional activities over the past week.31 The metric utilizes 10 centimeter visual analog scales across fifteen activities, with total scores ranging from 0 (no pain) to 150 (maximum pain). The NRS is a commonly-used metric that inquires about average shoulder pain over the past week using a 0 (“no pain”) to 10 (“worst pain imaginable”) scale. It is recommended for use in SCI pain research32 and clinical trials in pain as a primary outcome measure.33 After the treatment, participants were asked how much their overall status has changed using a 7-item Likert scale with 1 indicating “very much improved” and 7 indicating “very much worse.”34
Physical examination
A modified version of the Physical Examination of the Shoulder Scale (PESS)23 was included as a clinically-relevant and objective measure of shoulder pain. Five tests were chosen from the original PESS that were specific to the supraspinatus tendon: palpation over the greater tuberosity, the supraspinatus (“empty can” or Jobe's) test,25 painful arc test,26 and Hawkins-Kennedy27 and Neer's (impingement) signs.28 Other examinations were eliminated from the analysis because the supraspinatus was targeted for injection and inclusion of the other exam scores would likely introduce error. Each maneuver was given a numerical score of 0 (absent), 1 (equivocal), and 2 (present)23 Scores were summed for a total between 0 and 10.
Ultrasound examination
A physician visualized the soft-tissue structures in the subjects’ treated shoulders, using ultrasound, at three time points: before the treatment and 12 and 24 weeks post-treatment. The USPRS was used to apply a numerical score to seven different static and dynamic ultrasonographic tests that assess shoulder pathology. Static tests included biceps and supraspinatus tendinopathy, glenohumeral joint effusion, bursal thickness, and irregularity of the greater tuberosity cortical surface. Dynamic tests assessed degrees of supraspinatus and subscapularis impingement. A score of zero implies no observable pathology. Complete details of each test and its grading system have been published previously.23,35 Of particular interest for this study was the 6-point supraspinatus tendinopathy subscale, which applies a number to the various signs of tendinopathy. Lower scores indicate less severe pathology and higher scores indicated greater degrees of pathology, with the highest score being a clear full-thickness tear.23
Statistical analysis
All analyses were performed using SPSS v21 (IBM, Inc., Armonk, New York, USA). An alpha of 0.05 was considered significant. Descriptives were calculated for demographic variables and outcome measures. Mixed effects models were built to test changes in outcomes (WUSPI, NRS, PESS, USPRS) over time. Random intercepts were built into the models to account for between-subjects heterogeneity. The independent variable was time since treatment (4, 8, 12, and 24 weeks). Measurements at each post-treatment time point were compared to baseline; Bonferroni corrections were applied to each pairwise comparison.
Results
Subjects
Six male participants were enrolled and completed the study (three with tetraplegia, three with paraplegia). The average age was 54.5 ± 6.3 years with an average injury duration of 26.7 ± 11.1 years. All participants reported bilateral shoulder pain during wheelchair use. Subject ages, injury durations, races/ethnicities, and injury characteristics are presented in Table 1. Ultrasound signs for supraspinatus tendinopathy are detailed in Table 1
PRP was injected directly into the supraspinatus tendons of five participants (one into the subacromial space), and one participants’ biceps tendon (Table 1). Calcifications present in two participants supraspinatus tendons were needled at the time of the procedure (Table 1).
Pain and imaging outcomes
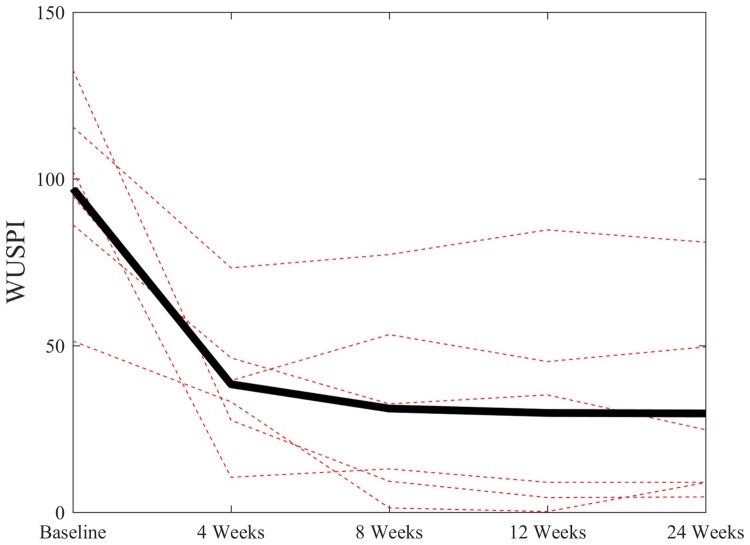
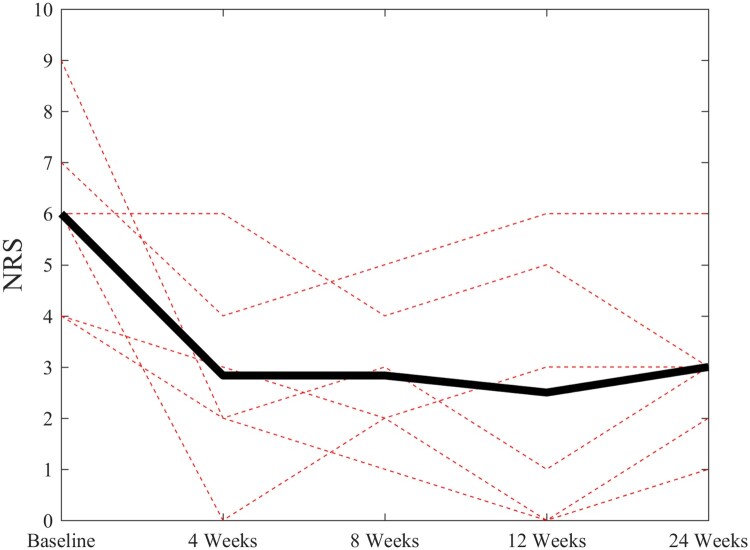
The PRP treatment resulted in marked decreases in all three pain outcomes after 4, 8, 12, and 24 weeks: NRS (P < 0.01), WUSPI (P < 0.001), and physical examination findings (P < 0.01). After six months, averages of 69.9% and 49.6% decreases were observed in WUSPI and pain NRS scores (Table 2). Subject-specific and mean changes in WUSPI and NRS scores are presented in Figures 1 and 2, respectively. The average decrease in modified PESS scores was 35.7% (Table 2). Frequencies of positive signs between baseline and six months post-treatment for each physical examination test are presented in Table 3. No changes in ultrasound markers for supraspinatus were found after 24 weeks (Table 2). Two participants reported their status as minimally improved as a result of the intervention, while three reported much improved and one reported very much improved.
Table 2. Changes in pain outcome measures after four, eight, twelve, and twenty-four weeks post-treatment.
| Baseline | Week 4 | Week 8 | Week 12 | Week 24 | |
|---|---|---|---|---|---|
| WUSPIa | 97.2 (27.8) | 38.4 (21.0) | 31.1 (29.4) | 29.8 (32.4) | 29.6 (30.1) |
| NRSb | 6.0 (1.9) | 2.86 (2.0) | 2.83 (1.5) | 2.5 (2.6) | 3.0 (1.7) |
| PESS-STc | 9.0 (1.3) | 2.4 (1.0) | 4.0 (3.2) | 3.0 (2.7) | 5.17 (2.9) |
| USPRS-STe | 3.2 (1.7) | NA | NA | 3.3 (1.5) | 3.2 (1.3) |
Notes. WUSPI = Wheelchair User's Shoulder Pain Index. NRS = Numerical Rating Scale. PESS-ST = Physical Examination of the Shoulder Scale supraspinatus tendon subscale. USPRS-ST = Ultrasound Shoulder Pathology Rating Scale supraspinatus tendon subscale.
aP < 0.001; significantly different from baseline at all points (P < 0.001).
bP < 0.01; significantly different from baseline at all points (P < 0.01).
cP < 0.01; significantly different from baseline at all points (P < 0.05).
eP > 0.05.
Figure 1.
Subject-specific changes (dotted lines) and mean changes (solid black line) in Wheelchair User's Shoulder Pain Index (WUSPI) scores over the 24-week follow-up period.
Figure 2.
Subject-specific changes (dotted lines) and mean changes (solid black line) in pain Numerical Rating Scale (NRS) scores over the 24-week follow-up period.
Table 3. Numbers and percentages of participants with positive physical examination findings at baseline and at 24 weeks after treatment with Platelet-Rich Plasma.
| Test | Baseline | Week 24 |
|---|---|---|
| GT Tenderness | 5 (83.3%) | 4 (66.7%) |
| Empty Can | 5 (83.3%) | 3 (50.0%) |
| Painful Arc | 5 (83.3%) | 3 (50.0%) |
| Neer's Impingement | 4 (66.7%) | 2 (33.3%) |
| Hawkin's Impingement | 5 (83.3%) | 3 (50.0%) |
Notes. GT = greater tuberosity.
Discussion
Shoulder pain is common in the SCI population and is most likely caused by tendinopathy of the rotator cuff structures due to overuse from activities of daily living.1 In this way, people with SCI are unique in their near-constant exposure to upper limb loading, and thus the inability of their tendons to heal after such repeated exposure. PRP was thought to be a useful treatment in this population because of its innate healing properties, which may help to offset the deleterious effects of chronic wheelchair use without the need for surgery. This was the first investigation to present outcomes of a PRP injection in the degenerative rotator cuffs of a sample of wheelchair users with SCI. Overall, the intervention reduced global and activity-specific pain intensity after six months without any changes in ultrasound signs for supraspinatus tendinopathy. No adverse reactions to the treatment were reported.
The approximate 50% and 70% reductions in NRS and WUSPI scores, respectively, after 24 weeks were similar to other groups who reported functional and general pain outcomes in the able-bodied population.19,20 Only one group has reported on the efficacy and safety of PRP for shoulder pain in the SCI population, reporting overall positive outcomes.21 It is difficult to compare their findings due to their targeting of the biceps tendon and a shorter follow-up period of eight weeks. Additionally, although their participants had a history of chronic shoulder pain for at least six months, there was no indication as to whether they had failed conservative treatment prior to enrolling in study. Similar to the present investigation, Ibrahim, et al. described a reduction in visual analog pain scores for the injected arm after eight weeks with no change in ultrasound markers for pathology.21 In contrast, however, marked decreases in physical examination findings for supraspinatus pathology were observed in the current investigation. One study that followed able-bodied participants for a year showed a continued decrease in pain measures between six and twelve months.20 It is possible the same pattern would have been observed in this sample, yet this was not tested. Future investigations should continue to follow individuals for at least one year to determine when pain levels begin to reverse toward baseline.
Participants were given a standardized stretching and strengthening program to complete after the PRP treatment. It is entirely possible that the observed pain reductions were due to the exercise and stretching program. The largest comparative study of PRP to physical therapy in able-bodied individuals showed no differences between the two interventions after 12 months.36 In wheelchair users with SCI, Mulroy et al. conducted a 12-week home-based exercise, stretching, and movement optimization intervention.30 In the 26 individuals who received the intervention, the reductions in WUSPI scores were virtually no different than those in the present study who received the PRP injection (70.9% versus 70.0%, respectively).30 Interestingly, Numerical Pain Rating Scale reductions were greater in their study (72.5% versus 49.6%). It is possible that differences in sample characteristics were at least partially responsible for these observations. Participants in the study by Mulroy et al. were on average younger with shorter injury durations, lesser WUSPI scores, and fewer physical examination signs for shoulder pathology than the current sample.30 Considering the improvements noted in this sample, there is a need for further investigation with a larger sample size, randomization, and a control group. Future studies could also include pain measurements of the untreated, contralateral limb for comparison.
Individuals with SCI often present signs of soft-tissue pathology in multiple locations within the shoulder; for example, the biceps tendon, supraspinatus tendon, subacromial bursa, or glenohumeral joint. It is important to note that different approaches were taken depending on the participants and their specific pathology. For example, supraspinatus tendon calcifications were needled in two participants and an additional injection was performed in one participant's biceps tendon. The introduction of this treatment heterogeneity introduces some error in that these additional procedures may have influenced the effect of the intervention on outcomes. However, it is important to consider this heterogeneity during treatment to optimize outcomes.
Limitations
This was a pilot study; thus, the primary limitations were the small sample size, lack of a control group and randomization, and a relatively short follow up period of 24 weeks. Recruiting a larger sample and conducting a randomized, controlled, double-blinded study would help to show efficacy of the treatment. Although PRP volume and location of the injection were recorded, additional data should be collected on the count and concentration of platelets in the PRP product. These variables would provide a more complete understanding of the treatment effects, and perhaps be used to predict a more successful outcome. The sample only consisted of White (Hispanic and non-Hispanic) males. Including a higher percentage of females and people of other races in future studies would improve generalizability to the SCI population.
Conclusions
A PRP injection under ultrasound guidance, followed by a shoulder stretching and strengthening program, reduced functional and general shoulder pain intensity for up to 24 weeks. No adverse events related to the procedure were reported. This pilot study showed that PRP is a safe and potentially efficacious treatment for recalcitrant shoulder pain caused by rotator cuff disease in wheelchair users with SCI. A larger, more definitive randomized controlled trial is warranted. Inclusion of quantitative outcome measures, such as MRI T2 mapping or quantitative ultrasound, may yield additional insight into evidence of tendon healing after PRP treatment.
Disclosures
This data was presented at the 2018 Academy of Spinal Cord Injury Professionals Annual Meeting in New Orleans, Louisiana, USA. A subset of these data were also presented at the 2019 Meeting of the American Spinal Injury Association in Honolulu, Hawaii, USA.
Disclaimer statements
Contributors None.
Conflicts of interest None.
Funding Statement
This study was supported by a grant from the Kessler Foundation, the Derfner Foundation, and the National Institute on Disability, Independent Living, and Rehabilitation Research [grant number 90SI5011]--NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health & Human Services (HHS). The contents of this report do not necessarily represent the policy of NIDILRR, ACL, or HHS, and you should not assume endorsement by the Federal Government.
References
- 1.Dyson-Hudson TA, Kirshblum SC.. Shoulder pain in chronic spinal cord injury, Part I: epidemiology, etiology, and pathomechanics. J Spinal Cord Med 2004;27(1):4–17. doi: 10.1080/10790268.2004.11753724 [DOI] [PubMed] [Google Scholar]
- 2.Paralyzed veterans of America consortium for spinal cord Medicine . Preservation of upper limb function following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med. 2005;28(5):434-70. doi: 10.1080/10790268.2005.11753844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols PJ, Norman PA, Ennis JR.. Wheelchair user’s shoulder? shoulder pain in patients with spinal cord lesions. Scand J Rehabil Med 1979;11(1):29–32. [PubMed] [Google Scholar]
- 4.Dalyan M, Cardenas DD, Gerard B.. Upper extremity pain after spinal cord injury. Spinal Cord 1999;37(3):191–5. doi: 10.1038/sj.sc.3100802 [DOI] [PubMed] [Google Scholar]
- 5.Van Straaten MG, Cloud BA, Zhao KD, Fortune E, Morrow MMB.. Maintaining shoulder Health after spinal cord injury: a guide to understanding treatments for shoulder pain. Arch Phys Med Rehabil 2017;98(5):1061–3. doi: 10.1016/j.apmr.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fattal C, Coulet B, Gelis A, Rouays-Mabit H, Verollet C, Mauri C, et al. . Rotator cuff surgery in persons with spinal cord injury: relevance of a multidisciplinary approach. J Shoulder Elbow Surg 2014;23(9):1263–71. doi: 10.1016/j.jse.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 7.Jung HJ, Sim GB, Jeon IH, Kekatpure AL, Sun JH, Chun JM.. Reconstruction of rotator cuff tears in wheelchair-bound paraplegic patients. J Shoulder Elbow Surg 2015;24(4):601–5. doi: 10.1016/j.jse.2014.09.028 [DOI] [PubMed] [Google Scholar]
- 8.Goldstein B, Young J, Escobedo EM.. Rotator cuff repairs in individuals with paraplegia. Am J Phys Med Rehabil 1997;76(4):316–22. doi: 10.1097/00002060-199707000-00011 [DOI] [PubMed] [Google Scholar]
- 9.Popowitz RL, Zvijac JE, Uribe JW, Hechtman KS, Schurhoff MR, Green JB.. Rotator cuff repair in spinal cord injury patients. J Shoulder Elbow Surg 2003;12(4):327–32. doi: 10.1016/S1058-2746(03)00035-1 [DOI] [PubMed] [Google Scholar]
- 10.Kerr J, Borbas P, Meyer DC, Gerber C, Buitrago Tellez C, Wieser K.. Arthroscopic rotator cuff repair in the weight-bearing shoulder. J Shoulder Elbow Surg 2015;24(12):1894–9. doi: 10.1016/j.jse.2015.05.051 [DOI] [PubMed] [Google Scholar]
- 11.Robinson MD, Hussey RW, Ha CY.. Surgical decompression of impingement in the weightbearing shoulder. Arch Phys Med Rehabil 1993;74(3):324–7. [PubMed] [Google Scholar]
- 12.Taylor DW, Petrera M, Hendry M, Theodoropoulos JS.. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sports Med 2011;21(4):344–52. doi: 10.1097/JSM.0b013e31821d0f65 [DOI] [PubMed] [Google Scholar]
- 13.Eppley BL, Woodell JE, Higgins J.. Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plastic Reconstruct Surg 2004;114(6):1502–8. doi: 10.1097/01.PRS.0000138251.07040.51 [DOI] [PubMed] [Google Scholar]
- 14.Anitua E, Sanchez M, Nurden AT, Zalduendo M, de la Fuente M, Orive G, et al. . Autologous fibrin matrices: A potential source of biological mediators that modulate tendon cell activities. J Biomed Mat Res Part A 2006;77(2):285–93. doi: 10.1002/jbm.a.30585 [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Qiu Y, Triffitt J, Carr A, Xia Z, Sabokbar A.. Proliferation and differentiation of human tenocytes in response to platelet rich plasma: An in vitro and in vivo study. J Orthop Res 2012;30(6):982–90. doi: 10.1002/jor.22016 [DOI] [PubMed] [Google Scholar]
- 16.Mautner K, Colberg RE, Malanga G, Borg-Stein JP, Harmon KG, Dharamsi AS, et al. . Outcomes after ultrasound-guided platelet-rich plasma injections for chronic tendinopathy: A multicenter, retrospective review. PM R 2013;5(3):169–75. doi: 10.1016/j.pmrj.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 17.Miller LE, Parrish WR, Roides B, Bhattacharyya S.. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: Systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Ex Med 2017;3(1):e000237. doi: 10.1136/bmjsem-2017-000237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shams A, El-Sayed M, Gamal O, Ewes W.. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Europ J Orthop Surg Traumatol 2016;26(8):837–42. doi: 10.1007/s00590-016-1826-3 [DOI] [PubMed] [Google Scholar]
- 19.Rha DW, Park GY, Kim YK, Kim MT, Lee SC.. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: A randomized controlled trial. Clin Rehabil 2013;27(2):113–22. doi: 10.1177/0269215512448388 [DOI] [PubMed] [Google Scholar]
- 20.Kesikburun S, Tan AK, Yilmaz B, Yasar E, Yazicioglu K.. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: A randomized controlled trial with 1-year follow-up. Am J Sports Med 2013;41(11):2609–16. doi: 10.1177/0363546513496542 [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim VM, Groah SL, Libin A, Ljungberg IH.. Use of platelet rich plasma for the treatment of bicipital tendinopathy in spinal cord injury: A pilot study. Top Spinal Cord Inj Rehabil 2012;18(1):77–8. doi: 10.1310/sci1801-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogaboom NS, Worobey LA, Boninger ML.. Transfer technique is associated with shoulder pain and pathology in people with spinal cord injury: A cross-sectional investigation. Arch Phys Med Rehabil 2016;97(10):1770–6. doi: 10.1016/j.apmr.2016.03.026 [DOI] [PubMed] [Google Scholar]
- 23.Brose SW, Boninger ML, Fullerton B, McCann T, Collinger JL, Impink BG, et al. . Shoulder ultrasound abnormalities, physical examination findings, and pain in manual wheelchair users with spinal cord injury. Arch Phys Med Rehabil 2008;89(11):2086–93. doi: 10.1016/j.apmr.2008.05.015 [DOI] [PubMed] [Google Scholar]
- 24.Curtis KA, Roach KE, Applegate EB, Amar T, Benbow CS, Genecco TD, et al. . Development of the wheelchair user’s shoulder pain Index (WUSPI). Paraplegia 1995;33(5):290–3. [DOI] [PubMed] [Google Scholar]
- 25.Jobe FW, Moynes DR.. Delineation of diagnostic criteria and a rehabilitation program for rotator cuff injuries. Am J Sports Med 1982;10(6):336–9. doi: 10.1177/036354658201000602 [DOI] [PubMed] [Google Scholar]
- 26.Brown JT. Early assessment of supraspinatus tears: Procaine infiltration as a guide to treatment. J Bone Joint Surg (Brit) 1949;31b(3):423–5. doi: 10.1302/0301-620X.31B3.423 [DOI] [PubMed] [Google Scholar]
- 27.Hawkins RJ, Kennedy JC.. Impingement syndrome in athletes. Am J Sports Med 1980;8(3):151–8. doi: 10.1177/036354658000800302 [DOI] [PubMed] [Google Scholar]
- 28.Neer CS, 2nd. Impingement lesions. Clin Orthop Rel Res. 1983;173:70–7. doi: 10.1097/00003086-198303000-00010 [DOI] [PubMed] [Google Scholar]
- 29.Allen GM. Shoulder ultrasound imaging-integrating anatomy, biomechanics and disease processes. Eur J Radiol 2008;68(1):137–46. doi: 10.1016/j.ejrad.2008.02.024 [DOI] [PubMed] [Google Scholar]
- 30.Mulroy SJ, Thompson L, Kemp B, Hatchett PP, Newsam CJ, Lupold DG, et al. . Strengthening and optimal movements for painful shoulders (STOMPS) in chronic spinal cord injury: A randomized controlled trial. Phys Ther 2011;91(3):305–24. doi: 10.2522/ptj.20100182 [DOI] [PubMed] [Google Scholar]
- 31.Curtis KA, Roach KE, Applegate EB, Amar T, Benbow CS, Genecco TD, et al. . Reliability and validity of the wheelchair user’s shoulder pain Index (WUSPI). Paraplegia 1995;33(10):595–601. [DOI] [PubMed] [Google Scholar]
- 32.Bryce TN, Budh CN, Cardenas DD, Dijkers M, Felix ER, Finnerup NB, et al. . Pain after spinal cord injury: An evidence-based review for clinical practice and research. Report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures Meeting. J Spinal Cord Med 2007;30(5):421–40. doi: 10.1080/10790268.2007.11753405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. . Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113(1-2):9–19. doi: 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 34.Farrar JT, Young JP, Jr., LaMoreaux L, Werth JL, Poole RM.. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–58. doi: 10.1016/S0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- 35.Collinger JL, Fullerton B, Impink BG, Koontz AM, Boninger ML.. Validation of grayscale-based quantitative ultrasound in manual wheelchair users: Relationship to established clinical measures of shoulder pathology. Am J Phys Med Rehabil 2010;89(5):390–400. doi: 10.1097/PHM.0b013e3181d8a238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ilhanli I, Guder N, Gul M.. Platelet-rich plasma treatment with physical therapy in chronic partial supraspinatus tears. Iran Red Crescent Med J 2015;17(9):e23732. doi: 10.5812/ircmj.23732 [DOI] [PMC free article] [PubMed] [Google Scholar]