ABSTRACT
Long-tailed macaques (Macaca fascicularis) are known to harbour a variety of infectious pathogens, including zoonotic species. Long-tailed macaques and humans coexist in Thailand, which creates potential for interspecies pathogen transmission. This study was conducted to assess the presence of B virus, Mycobacterium spp., simian foamy virus (SFV), hepatitis B virus (HBV), and Plasmodium spp. in 649 free-living Thai long-tailed macaques through polymerase-chain reaction. DNA of SFV (56.5%), HBV (0.3%), and Plasmodium spp. (2.2%) was detected in these macaques, whereas DNA of B virus and Mycobacterium spp. was absent. SFV infection in long-tailed macaques is broadly distributed in Thailand and is correlated with age. The HBV sequences in this study were similar to HBV sequences from orangutans. Plasmodium spp. DNA was identified as P. inui. Collectively, our results indicate that macaques can carry zoonotic pathogens, which have a public health impact. Surveillance and awareness of pathogen transmission between monkeys and humans are important.
KEYWORDS: Hepatitis B virus, long-tailed macaque, Macaca fascicularis, Plasmodium inui, simian foamy virus
1. Introduction
The most infectious diseases are zoonoses, which can be transmitted from animals to humans [1]. Zoonotic diseases are caused by viruses, bacteria, fungi, parasites, and prions [1] and pose a serious threat to public health and economy [2]. Wildlife is a major source of zoonotic disease [2]. Non-human primates (NHPs) are a reservoir host of several infectious diseases [2–4]. Of the 25 important zoonotic diseases in humans, 5 originate from NHPs [5]. NHPs can spread pathogens to humans via body fluids, contaminated food or water, and insect vectors. NHPs can carry several pathogens that lead to human infections, such as B virus, Mycobacterium spp., simian foamy virus (SFV), hepatitis B virus (HBV), and Plasmodium spp. The seropositivity rate for the B virus in adult captive macaques is high and without clinical signs [6]. B virus infections in humans are usually transmitted by macaques. Although the incidence of B virus infection in humans is low, the mortality is high (>70%) [6]. Mycobacterium spp. is another pathogen that poses a serious problem for public health worldwide [7]. Macaques are the most susceptible species to Mycobacterium infections [7]. SFV is a retrovirus that is found in both Old and New World NHP [8], especially macaques [9]. Approximately 1–5% of the humans in close contact with NHPs were found to be infected with SFV [10]. HBV is mainly reported in Old World NHPs, including the long-tailed macaques [11,12]. This virus is a global health problem with a high prevalence in humans [13]. Plasmodium spp. cause vector-borne diseases and their occurrence in NHPs results in morbidity and mortality in humans [14]. Three simian malaria species (P. cynomolgi, P. knowlesi, and P. inui) are natural parasites in Southeast Asian macaques that can infect humans [14–16].
Thailand supports 15 native NHP species [17], with a larger population of long-tailed macaques (Macaca fascicularis) [18,19]. Long-tailed macaque habitats are located throughout Thailand [18,19]. Most habitats are shared with human communities due to macaque overcrowding, the adaptation of macaque behaviour, the sizes of human communities, and the nature of human activities [18,19]. Conflicts between these NHP and humans are common [18,19]. Such contact creates opportunities for cross-transmission of pathogens between macaques and humans. This study This study was carried out to investigate B virus, Mycobacterium spp., SFV, HBV, and Plasmodium spp. infection in free-living long-tailed macaques inhabiting urban areas in Thailand.
2. Materials and methods
2.1. Ethical statement
The protocol of this study was approved by the Animal Care and Use Committee, Faculty of the Veterinary Science, Mahidol University (FVS-MU-IACUC) (Ethical approval No. MUVS-2020-02-05).
2.2. Sample collection and DNA extraction
Accessing sample size, Slovin’s formula was used to calculate the number of long-tail macaques in Thailand for this study. A total of 649 free-living, long-tailed macaques were captured from different locations in 26 urban areas in 5 regions in Thailand (Figure 1). Sites and samples were distributed throughout the country, including eight sites in the Central (n = 206), four sites in the Eastern (n = 100), five sites in the Western (n = 125), five sites in the Southern (n = 118), and four sites in Northeastern (n = 100) regions (Table 1). Blood and oropharyngeal swabs were obtained from 649 anesthetized animals under tiletamine HCl-zolazepam (3 mg/kg) -xylazine (0.5 mg/kg). After the animals fully recovered, returning them to their homeland. Animals were classified as adults and sub-adults as judged by body size, genital organs, teeth, and muscular development. Samples were collected between March and September 2019. DNA was extracted from blood and swab samples using DNeasy Blood & Tissue Kit (QIAGEN, Germany), following the manufacturer’s instructions. Animals consisted of 446 males (147 sub-adults, 299 adults) and 203 females (81 sub-adults, 122 adults) (Table 1). Macaques were judged healthy by physical examination.
Figure 1.
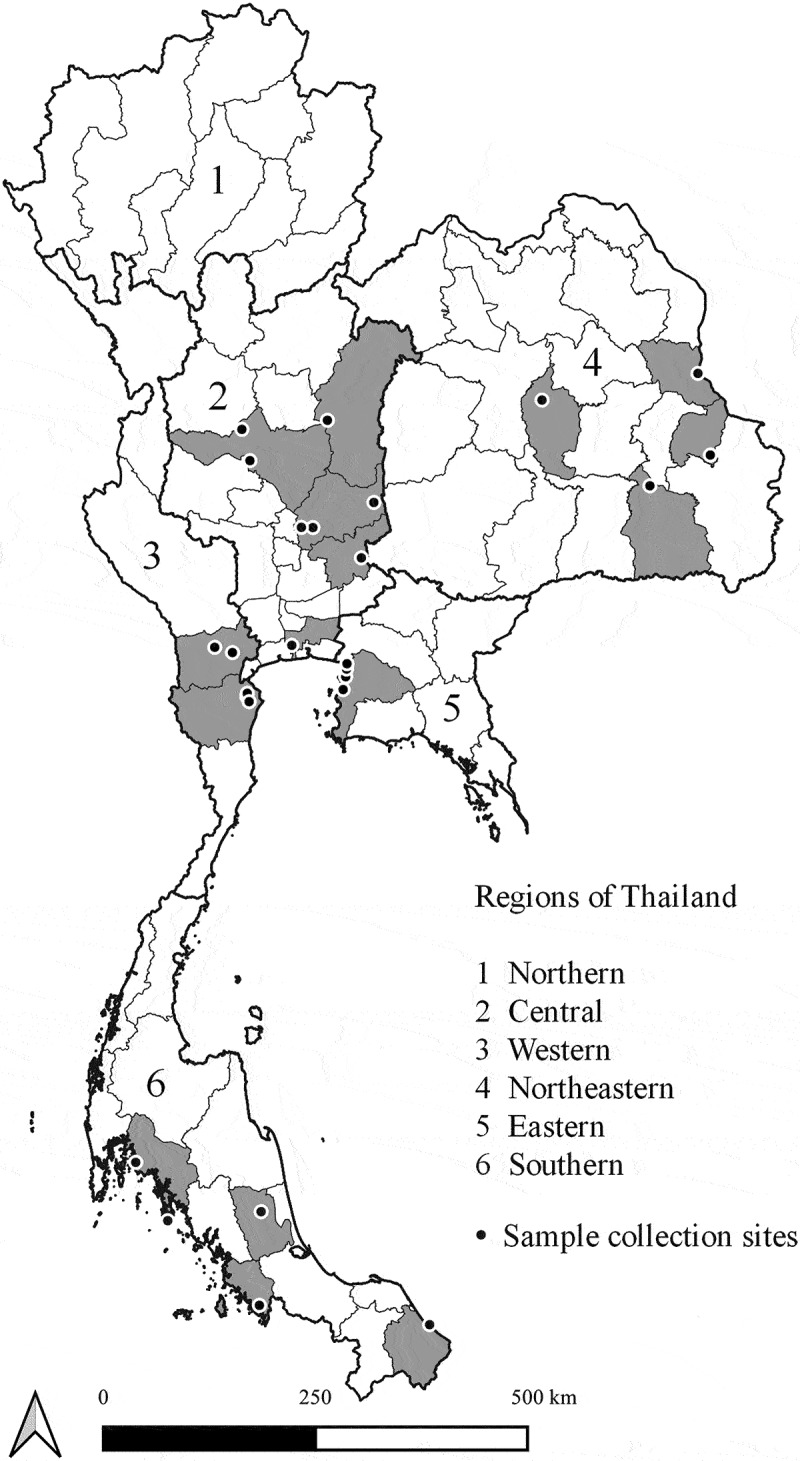
Location of collection sites of long-tailed macaques in Thailand. The map was created using QGIS version 3.8.3-Zanzibar, a free and open source geographic information system.
Table 1.
The number of collected long-tailed macaque samples within different geographical regions
| Thailand Region | Site | Male |
Female |
||||
|---|---|---|---|---|---|---|---|
| Sub-adult | Adult | Total | Sub-adult | Adult | Total | ||
| Central | 8 | 35 | 104 | 139 | 28 | 39 | 67 |
| Eastern | 4 | 36 | 42 | 78 | 12 | 10 | 22 |
| Western | 5 | 29 | 77 | 106 | 7 | 12 | 19 |
| Southern | 5 | 33 | 45 | 78 | 25 | 15 | 40 |
| Northeastern | 4 | 14 | 31 | 45 | 9 | 46 | 55 |
| Total | 147 | 299 | 446 | 81 | 122 | 203 | |
2.3. Molecular detection of pathogens
All macaques were screened for several pathogens, including B virus, Mycobacterium spp., SFV, HBV, and Plasmodium spp., by polymerase-chain reaction (PCR). DNA from oropharyngeal swabs was investigated in the presence of SFV, B virus, and Mycobacterium spp. while the extracted DNA from blood collected into EDTA-containing tubes was examined for HBV and Plasmodium spp.
The status of B virus samples was assessed with the glycoprotein G (G) gene by real-time PCR [20]. The identification of Mycobacterium spp. was carried out through a multiplex PCR with three primer sets [21]. These primers target insertion sequences 6110 (IS6110), 32-kD alpha protein (32-kDa), and MTP40 species-specific protein (mtp40) genes, which are specific for the M. tuberculosis complex, Mycobacterium spp., and M. tuberculosis, respectively [21]. To detect SFV, Nested PCR was used to amplify the polymerase (pol) gene of the proviral DNA [22]. HBV was screened using a duplex real-time PCR based on the surface (S) and core (C) genes [23]. Positive HBV samples were identified with nested PCR to amplify S as previously documented [24]. For Plasmodium spp., a real-time PCR was conducted [25]. Macaques positive for Plasmodium infection were screened for four human malaria species (P. falciparum, P. vivax, P. ovale, and P. malariae) and five simian malaria species (P. knowlesi, P. cynomolgi, P. coatneyi, P. inui, and P. fieldi) using nested PCR [26,27]. Species in the genus Plasmodium were identified in the first round of PCR, and individual species were identified in the second round. Both real-time and nested PCRs target the 18S small subunit ribosomal RNA (18S rRNA) genes. Specific primer sets and PCR conditions for this study were previously described [20–27] (Table 2).
Table 2.
Sequences of primers and probes for pathogens detection in this study
| Primer/Probe | Sequences (5’-3’) | Annealing Temperature (°C) | Product size (bp) | Reference |
|---|---|---|---|---|
| B virus detection | ||||
| gG_BV323F | TGGCCTACTACCGCGTGG | 60 | 123 | [20] |
| gG_BV446R | TGGTACGTGTGGGAGTAGCG | |||
| gG_BV403P | FAM-CCGCCCTCTCCGAGCACGTG-TAMRA | |||
| Mycobacterium spp. detection | ||||
| M. tuberculosis complex | ||||
| IS5-F | CGGAGACGGTGCGTAAG | 70 | 984 | [21] |
| IS6-R | GATGGACCGCCAGGGCTTGC | |||
| Mycobacterium spp. | ||||
| MT1 | TTCCTGACCAGCGAGCTGCCG | 70 | 506 | |
| MT2 | CCCCAGTACTCCCAGCTGTGC | |||
| M. tuberculosis | ||||
| PT1 | CGGCAACGCGCCGTCGGTGG | 70 | 396 | |
| PT2 | CCCCCCACGGCACCGCCGGG | |||
| SFV detection | ||||
| SFV_pol_F1 | GTGGNAAGGTGGAAAGGA AAA ATAGTGANA | 45 | 227 | [22] |
| SFV-pol_R1 | NTANAGANNNNCNAATTTCCTGTAAAAGAGA | |||
| SFV_pol_F2 | NGTNGGNNGNCCTNCNAAGTGGTATGA | 47 | 153 | |
| SFV_pol_R2 | NAANTCAAGTGTATCNNNNTTTGCAAANGG | |||
| HBV detection | ||||
| HBV-C-F | TTCCGGAAACTACTGTTGTTAGAC | 55 | 125 | [23] |
| HBV-C-R | ATTGAGATTCCCGAGATTGAGA | |||
| HBV-C-P | FAM-CCCTAGAAGAAGAACTCCCTCGCCTC-BHQ1 | |||
| HBV-S-F | GATGTGTCTGCGGCGTTTTA | 55 | 91 | |
| HBV-S-R | GCAACATACCTTGATAGTCCAGAAGAA | |||
| HBV-S-P | Cy5-CCTCTICATCCTGCTGCTATGCCTCA-BHQ2 | |||
| HBV confirmation | ||||
| HBV-S1 | CATCAGGAYTCCTAGGACCCCT | 55 | 238 | [24] |
| HBV-S5 | GAGGCATAGCAGCAGGATGMAGAGG | |||
| HBV-S3 | CGTGTTACAGGCGGKGTKTTTCTTGT | 55 | 206 | |
| HBV-S6 | ATGATAAAACGCCGCAGACACATC3 | |||
| Plasmodium spp. detection | ||||
| Plasmo-F | GCTCTTTCTTGATTTCTTGGATG | 60 | 99 | [25] |
| Plasmo-R | AGCAGGTTAAGATCTCGTTCG | |||
| Plasmo-P | FAM-ATGGCCGTTTTTAGTTCGTG-TAMRA | |||
| Human and simian malaria species identification | ||||
| Plasmodium spp. | ||||
| rPLU5 | CCTGTTGTTGCCTTAAACTTC | 55 | 1,100 | [26] |
| rPLU6 | TTAAAATTGTTGCAGTTAAAACG | |||
| P. falciparum | ||||
| rFAL1 | TTAAACTGGTTTGGGAAAACCAAATATATT | 55 | 205 | |
| rFAL2 | ACACAATGAACTCAATCATGACTACCCGTC | |||
| P. vivax | ||||
| rVIV1 | CGCTTCTAGCTTAATCCACATAACTGATAC | 55 | 120 | |
| rVIV2 | ACTTCCAAGCCGAAGCAAAGAAAGTCCTTA | |||
| P. ovale | ||||
| rOVA1 | ATCTCTTTTGCTATTTTTTAGTATTGGAGA | 55 | 800 | |
| rOVA2 | GGAAAAGGACACATTAATTGTATCCTAGTG | |||
| P. malariae | ||||
| rMAL1 | ATAACATAGTTGTACGTTAAGAATAACCGC | 55 | 144 | |
| rMAL2 | AAAATTCCCATGCATAAAAAATTATACAAA | |||
| P. knowlesi | ||||
| Pmk8 | GTTAGCGAGAGCCACAAAAAAGCGAAT | 60 | 153 | [27] |
| Pmk9r | ACTCAAAGTAACAAAATCTTCCGT | |||
| P. cynomolgi | ||||
| CY2F | GATTTGCTAAATTGCGGTCG | 61 | 137 | |
| CY4R | CGGTATGATAAGCCAGGGAAGT | |||
| P. coatneyi | ||||
| PctF1 | CGCTTTTAGCTTAAATCCACATAACAGAC | 61 | 504 | |
| PctR1 | GAGTCCTAACCCCGAAGGGAAAGG | |||
| P. inui | ||||
| PinF2 | CGTATCGACTTTGTGGCATTTTTCTAC | 61 | 479 | |
| INAR3 | GCAATCTAAGAGTTTTAACTCCTC | |||
| P. fieldi | ||||
| PfldF1 | GGTCTTTTTTTTGCTTCGGTAATTA | 62 | 421 | |
| PfldR2 | AGGCACTGAAGGAAGCAATCTAAGAGTTTC | |||
2.4. Phylogenetic analysis
S amplicons of HBV are sequenced and used for phylogenetic tree construction. The tree was generated by bootstrap analysis with 1,000 replicates. Phylogenetic relationships of various HBV strains were constructed using the ClustalW alignment and the maximum likelihood with the MEGA 7 software [28]. Distances were evaluated with the Kimura two-parameter model assuming a Gamma distribution. The reference sequences for HBV for comparison were obtained from GenBank NCBI (http://www.ncbi.nlm.nih.gov/genbank).
2.5. Nucleotide sequence accession numbers
Partial S gene sequences of HBV detected from the Central (MW2536/62) and the Northeastern (MW2227/62) regions were deposited on GenBank with accession no. OL711942 and OL711943, respectively.
2.6. Statistical analysis
Statistical relationships between positive results and status data, including sex and age of free-living NHPs, were analysed using Pearson chi-square tests using SPSS 26 for Windows (SPSS Inc., USA). Statistical significance was indicated at a P-value of <0.05.
3. Results
A total of 649 long-tailed macaques native to Thailand were screened for B virus, Mycobacterium spp., SFV, HBV, and Plasmodium spp. using specific PCR-based methods. No macaque samples were positive for B virus or Mycobacterium spp. DNA from SFV, HBV, and Plasmodium spp. was recovered (Table 3). SFV was encountered in over half of the oropharyngeal swabs (56.5%, 367/649) and was present at every sampling site. SFV was identified in 56.5% (252/446) of male and 56.7% (115/203) of female macaques. Further, SFV-positive males included 63.2% (189/299) of adults and 42.9% (63/147) of sub-adults. Similarly, 61.5% (75/122) and 49.4% (40/81) of positive females were adults and sub-adults, respectively. Association analysis showed no significant difference between the positive results for SFV and sex (P = 0.972), but a significant relationship was observed between SFV and age (P < 0.001). Furthermore, the number of SFV-positive cases in the female group was not significantly associated with age (P = 0.089) but showed a significant relationship with age in the male group (P < 0.001).
Table 3.
Age and sex distribution of zoonotic pathogens among Thai long-tailed macaques
| Number of samples (%) | Number of positive |
||||
|---|---|---|---|---|---|
| SFV (%) | HBV (%) | Plasmodium spp. (%) | |||
| Sex | Male | 446 (68.7) | 252 (56.5) | 2 (0.4) | 10 (2.2) |
| Female | 203 (31.3) | 115 (56.7) | 0 (0) | 4 (2) | |
| P-value | 0.972 | 0.339 | 0.825 | ||
| Age | Sub-adult | 228 (35.1) | 103 (45.2) | 0 (0) | 5 (2.2) |
| Adult | 421 (64.9) | 264 (62.7) | 2 (0.5) | 9 (2.1) | |
| P-value | < 0.001 | 0.297 | 0.963 | ||
| Total | 649 | 367 (56.5) | 2 (0.3) | 14 (2.2) | |
Out of the 649 long-tailed macaques, 2 (0.3%) were HBV positive by duplex real-time PCR. The positive HBV samples were adult males from Central (n = 1) and Northeastern (n = 1) regions. These animals were co-infected with SFV. Further, these positive samples showed 100% similarity to sequences of HBV found in orangutans from Thailand using a BLASTn search. Positive sequences clustered with the HBV from orangutans in a phylogenetic tree based on the HBV S gene (Figure 2).
Figure 2.
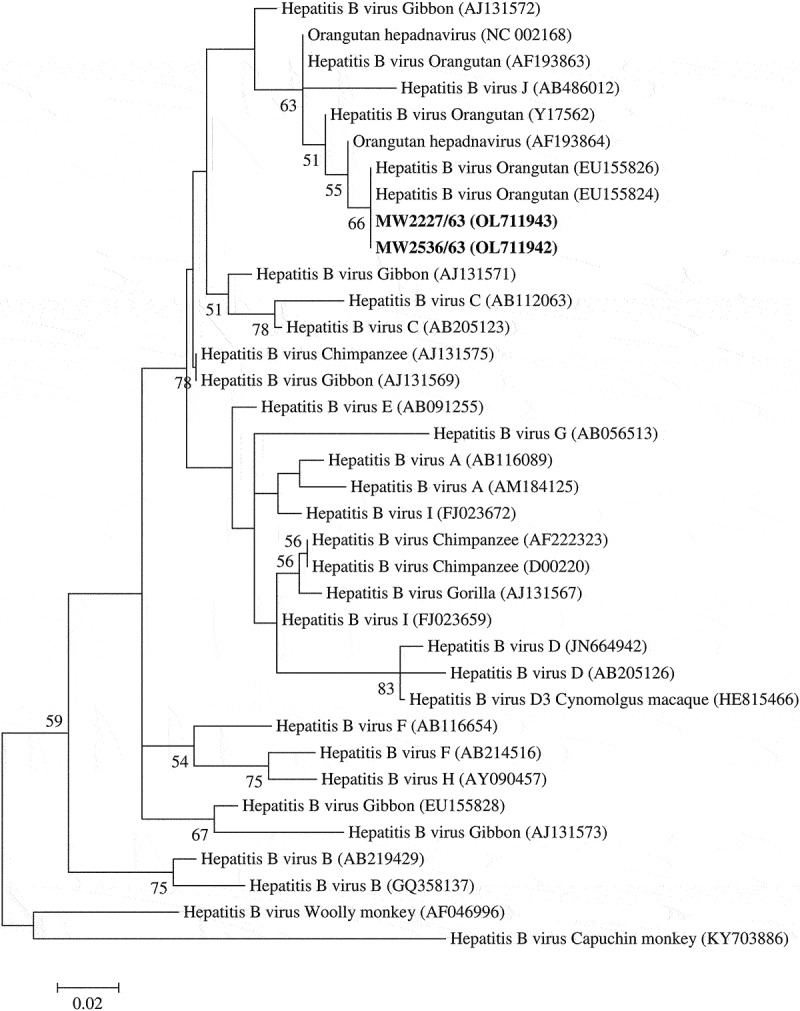
Phylogenetic tree based on S gene of HBV was constructed by the Maximum likelihood method. The bold is the positive samples in this study. The phylogenetic tree illustrated sequence relationships among positive samples, genotypes of human HBV, and sequences of NHP HBV. Bootstrap analysis was calculated with 1,000 replicates. Percentages of bootstrap values were displayed on the nodes of the tree, although values below 50% were excluded.
Plasmodium spp. DNA was identified in 14 of 649 (2.2%) blood samples from 2 sites of Central and 2 sites of Southern areas. Of the 14 Plasmodium-positive animals, 9 were co-infected with SFV. Plasmodium was detected in both males (n = 10) and females (n = 4). Seven and three positive males were adults and sub-adults, respectively. The same number of positive female adults and sub-adults was observed. All Plasmodium-positive samples were identified as P. inui. Positive rates were not statistically significantly different for sex (P = 0.825) or age (P = 0.963).
4. Discussion
Most habitats of long-tailed macaques in Thailand overlap with human communities [18,19]. This overlap creates an opportunity for cross-transmission of pathogens between macaques and humans. Thus, long-tailed macaques from colonies near communities or tourist attractions were examined in the present study.
None of the 649 oropharyngeal swabs collected in this study were positive for the B virus using real-time PCR. B virus DNA was previously identified among long-tailed macaques in Malaysia (39.3%) [29], Japanese macaques in Japan (10%) [30], and rhesus macaques in the USA (2.5%) [31]. Currently, shedding of the B virus has not been reported in Thai macaques. These pathogens are typically found in macaques, but the shedding frequency remains unclear [6]. The shedding frequency of the B virus may not be high [6].
All macaques in this study were negative for Mycobacterium spp. infection. This pathogen can be found in both captive and free-ranging NHPs [32,33]. The most susceptible species are the Old World monkeys, especially rhesus macaque [7,34]; the long-tailed macaque is less susceptible [34]. Our finding contrasts with an earlier study that showed 50% (5/10) incidence in Thai long-tailed macaques [32]. This disparity might be attributed to the health status of macaques because long-tailed macaques included in the previous study had a history of respiratory symptoms [32].
Proviral DNA from SFV in oropharyngeal swabs (56.5%) was amplified and found to be highly prevalent, consistent with the previous information on SFV prevalence in NHPs [8,10,35], particularly in Asian macaques [4,9,36,37]. Our results support a high incidence of SFV antibodies in free-range long-tailed macaques in Thailand (81%) [9]. Altogether, SFV appears to be commonly circulated among long-tailed free-living macaques in Thailand. Such a result is consistent with Gibraltar [38], i.e. SFV prevalence depends on age but does not correlate with sex. In more detail, the increase in SFV prevalence depends on age in the male group (P < 0.001) but is not associated with the female group (P = 0.089). This finding demonstrated a result of social behaviours in which adult males have more aggression, fighting, and biting than sub-adult males and females. This behaviour is a route to transmission of SFVs.
Of the 649 blood samples from long-tailed macaques, 2 (0.3%) were positive for HBV with duplex real-time PCR. This HBV DNA positivity was lower than the previously reported incidence in sera and livers from Mauritius long-tailed macaques (25.8% and 42%, respectively) [11]. In Thailand, HBV DNA was found in 18.81% (19/101) of sera samples from captive gibbons [39]. Conversely, HBV infection in Thai long-tailed macaques has not been previously reported. The lower prevalence in our study might reflect the geographical distribution or type of sampled populations. Acute and chronic HBV infections in humans may present as asymptomatic or with severe signs [40]. None of the HBV-positive macaques in this study showed clinical signs. A previous study reported that NHPs with HBV infection can be asymptomatic carriers [12]. Nucleotide sequence and phylogeny analysis based on the S gene were used to identify HBV in this study. Positive sequences were similar to HBV sequences from captive orangutans in Thailand. In the phylogram, macaque sequences clustered with HBV of captive orangutans in Thailand. Long-tailed macaques and orangutans may be infected with the same HBV strains. The present finding contrast with the previous report that genotype D of HBV was identified in Mauritius long-tailed macaque [11].
Regarding Plasmodium spp. detection, P. inui was identified in all Plasmodium-positive samples, which accounted for 2.2% of all macaques examined in this study. Plasmodium spp., especially P. inui, is normally found in long-tailed macaques [27,41–43]. Positivity for P. inui in wild long-tailed macaques from the Southern region in Thailand was 5.05% (5/99) [43] and 3.59% (7/195) [42]. No correlation between infection and sex or age was observed in the study population. Similar Plasmodium infection incidence was reported in wild macaques in Malaysia [44]. P. inui has been reported to cause infection in humans naturally and experimentally [14–16]. Indeed, natural infection in humans is associated with no clinical signs and a very low number of parasites in blood [15].
The limitations of this study are that nucleotide sequencing and phylogenetic tree construction of SFV and P. inui were not performed because SFV and P. inui are generally found in Thai macaques [9,42,43,45] as well as natural infection with SFV and P. inui in humans is still very rare (1–5% for SFV and 2.82% for P. inui) and asymptomatic [10,15]. The public health burden of such pathogens needs to be further investigated in the future
5. Conclusion
In conclusion, SFV, HBV, and Plasmodium spp., were found in wild macaques, indicating that these NHPs may serve as a reservoir for zoonotic pathogens in Thailand. Awareness and surveillance of transmission of such pathogens between monkeys and humans is critical. The findings in this study provide information to government agencies for risky communication on zoonotic diseases in communities that share an interface with free-living macaques.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Lloyd-Smith JO, George D, Pepin KM, et al. Epidemic dynamics at the human-animal interface. Science. 2009;326(5958):1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kruse H, Kirkemo AM, Handeland K.. Wildlife as source of zoonotic infections. Emerg Infect Dis. 2004;10(12):2067–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nakayima J, Hayashida K, Nakao R, et al. Detection and characterization of zoonotic pathogens of free-ranging non-human primates from Zambia. Parasit Vectors. 2014;7(1):490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jones-Engel L, Engel GA, Heidrich J, et al. Temple monkeys and health implications of commensalism, Kathmandu, Nepal. Emerg Infect Dis. 2006;12(6):900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007;447(7142):279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huff JL, Barry PA. B-virus (Cercopithecine herpesvirus 1) infection in humans and macaques: potential for zoonotic disease. Emerg Infect Dis. 2003;9(2):246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ghodbane R, Drancourt M. Non-human sources of Mycobacterium tuberculosis. Tuberculosis (Edinb). 2013;93(6):589–595. [DOI] [PubMed] [Google Scholar]
- [8].Meiering CD, Linial ML. Historical perspective of foamy virus epidemiology and infection. Clin Microbiol Rev. 2001;14(1):165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jones-Engel L, Steinkraus KA, Murray SM, et al. Sensitive assays for simian foamy viruses reveal a high prevalence of infection in commensal, free-ranging Asian monkeys. J Virol. 2007;81(14):7330–7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Murray SM, Linial ML. Foamy virus infection in primates. J Med Primatol. 2006;35(4–5):225–235. [DOI] [PubMed] [Google Scholar]
- [11].Dupinay T, Gheit T, Roques P, et al. Discovery of naturally occurring transmissible chronic hepatitis B virus infection among Macaca fascicularis from Mauritius Island. Hepatology. 2013;58(5):1610–1620. [DOI] [PubMed] [Google Scholar]
- [12].Sa-Nguanmoo P, Rianthavorn P, Amornsawadwattana S, et al. Hepatitis B virus infection in non-human primates. Acta Virol. 2009;53(2):73–82. [DOI] [PubMed] [Google Scholar]
- [13].Liaw Y-F, Chu C-M. Hepatitis B virus infection. Lancet. 2009;373(9663):582–592. [DOI] [PubMed] [Google Scholar]
- [14].Baird JK. Malaria zoonoses. Travel Med Infect Dis. 2009;7(5):269–277. [DOI] [PubMed] [Google Scholar]
- [15].Liew JWK, Bukhari FDM, Jeyaprakasam NK, et al. Natural Plasmodium inui infections in humans and Anopheles cracens Mosquito, Malaysia. Emerg Infect Dis. 2021;27(10):2700–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Coatney GR, Chin W, Contacos PG, et al. Plasmodium inui, a quartan-type malaria parasite of old world monkeys transmissible to man. J Parasitol. 1966;52(4):660–663. [PubMed] [Google Scholar]
- [17].Parr JWK. A guide to the large mammals of Thailand. Bangkok: Sarakadee Press; 2003. [Google Scholar]
- [18].Aggimarangsee N. Survey of semi-tame colonies of macaques in Thailand. Nat Hist Bull Siam Soc. 1992;40(2):103–166. [Google Scholar]
- [19].Malaivijitnond S, Hamada Y. Current situation and status of long-tailed macaques (Macaca fascicularis) in Thailand. Nat Hist J Chulalongkorn Univ. 2008;8:185–204. [Google Scholar]
- [20].Perelygina L, Patrusheva I, Manes N, et al. Quantitative real-time PCR for detection of monkey B virus (Cercopithecine herpesvirus 1) in clinical samples. J Virol Methods. 2003;109(2):245–251. [DOI] [PubMed] [Google Scholar]
- [21].Del Portillo P, Thomas MC, Martínez E, et al. Multiprimer PCR system for differential identification of mycobacteria in clinical samples. J Clin Microbiol. 1996;34(2):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Heneine W, Switzer WM, Sandstrom P, et al. Identification of a human population infected with simian foamy viruses. Nat Med. 1998;4(4):403–407. [DOI] [PubMed] [Google Scholar]
- [23].Liu C, Chang L, Jia T, et al. Real-time PCR assays for hepatitis B virus DNA quantification may require two different targets. Virol J. 2017;14(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].MacDonald DM, Holmes EC, Lewis JC, et al. Detection of hepatitis B virus infection in wild-born chimpanzees (Pan troglodytes verus): phylogenetic relationships with human and other primate genotypes. J Virol. 2000;74(9):4253–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kamau E, Tolbert LS, Kortepeter L, et al. Development of a highly sensitive genus-specific quantitative reverse transcriptase real-time PCR assay for detection and quantitation of Plasmodium by amplifying RNA and DNA of the 18S rRNA genes. J Clin Microbiol. 2011;49(8):2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Snounou G, Viriyakosol S, Zhu XP, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61(2):315–320. [DOI] [PubMed] [Google Scholar]
- [27].Akter R, Vythilingam I, Khaw LT, et al. Simian malaria in wild macaques: first report from Hulu Selangor district, Selangor, Malaysia. Malar J. 2015;14(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee M-H, Rostal M, Hughes T, et al. Macacine herpesvirus 1 in long-tailed macaques, Malaysia, 2009–2011. Emerg Infect Dis. 2015;21(7):1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ohsawa K, Black DH, Torii R, et al. Detection of a unique genotype of monkey B virus (Cercopithecine herpesvirus 1) indigenous to native Japanese macaques (Macaca fuscata). Comp Med. 2002;52(6):555–559. [PubMed] [Google Scholar]
- [31].Wisely SM, Sayler KA, Anderson CJ, et al. Macacine herpesvirus 1 antibody prevalence and DNA shedding among invasive rhesus macaques, Silver Springs State Park, Florida, USA. Emerg Infect Dis. 2018;24(2):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wilbur AK, Engel GA, Rompis A, et al. From the mouths of monkeys: detection of Mycobacterium tuberculosis complex DNA from buccal swabs of synanthropic macaques. Am J Primatol. 2012;74(7):676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rosenbaum M, Mendoza P, Ghersi BM, et al. Detection of Mycobacterium tuberculosis complex in new world monkeys in Peru. Ecohealth. 2015;12(2):288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Montali RJ, Mikota SK, Cheng LI. Mycobacterium tuberculosis in zoo and wildlife species. Rev Sci Tech. 2001;20(1):291–303. [DOI] [PubMed] [Google Scholar]
- [35].Stenbak CR, Pinto-Santini DM, Murray SM, et al. Simian foamy viruses: infections in human and nonhuman primate hosts. In: Knauf S, Jones-Engel L, editors. Neglected diseases in monkeys: from the monkey-human interface to one health. Cham: Springer International Publishing; 2020. [Google Scholar]
- [36].Ayouba A, Duval L, Liégeois F, et al. Nonhuman primate retroviruses from Cambodia: high simian foamy virus prevalence, identification of divergent STLV-1 strains and no evidence of SIV infection. Infect Genet Evol. 2013;18:325–334. [DOI] [PubMed] [Google Scholar]
- [37].Huang F, Wang H, Jing S, et al. Simian foamy virus prevalence in Macaca mulatta and zookeepers. AIDS Res Hum Retroviruses. 2012;28(6):591–593. [DOI] [PubMed] [Google Scholar]
- [38].Engel GA, Pizarro M, Shaw E, et al. Unique pattern of enzootic primate viruses in macaques. Emerg Infect Dis. 2008;14(7):1112–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Noppornpanth S, Haagmans BL, Bhattarakosol P, et al. Molecular epidemiology of gibbon hepatitis B virus transmission. J Gen Virol. 2003;84(Pt 1):147–155. [DOI] [PubMed] [Google Scholar]
- [40].Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64(1):51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fooden J. Malaria in macaques. Int J Primatol. 1994;15(4):573–596. [Google Scholar]
- [42].Putaporntip C, Jongwutiwes S, Thongaree S, et al. Ecology of malaria parasites infecting Southeast Asian macaques: evidence from cytochrome b sequences. Mol Ecol. 2010;19(16):3466–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Seethamchai S, Putaporntip C, Malaivijitnond S, et al. Malaria and Hepatocystis species in wild macaques, southern Thailand. Am J Trop Med Hyg. 2008;78(4):646–653. [PubMed] [Google Scholar]
- [44].Amir A, Shahari S, Liew JWK, et al. Natural Plasmodium infection in wild macaques of three states in Peninsular Malaysia. Acta Trop. 2020;211:105596. [DOI] [PubMed] [Google Scholar]
- [45].Fungfuang W, Udom C, Tongthainan D, et al. Malaria parasites in macaques in Thailand: stump-tailed macaques (Macaca arctoides) are new natural hosts for Plasmodium knowlesi, Plasmodium inui, Plasmodium coatneyi and Plasmodium fieldi. Malar J. 2020;19(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]


