ABSTRACT
Background
Systemic oppression, particularly towards sexual minorities, continues to be deeply rooted in the bedrock of many societies globally. Experiences with minority stressors (e.g. discrimination, hate-crimes, internalized homonegativity, rejection sensitivity, and microaggressions or everyday indignities) have been consistently linked to adverse mental health outcomes. Elucidating the neural adaptations associated with minority stress exposure will be critical for furthering our understanding of how sexual minorities become disproportionately affected by mental health burdens.
Methods
Following PRISMA-guidelines, we systematically reviewed published neuroimaging studies that compared neural dynamics among sexual minority and heterosexual populations, aggregating information pertaining to any measurement of minority stress and relevant clinical phenomena.
Results
Only 1 of 13 studies eligible for inclusion examined minority stress directly, where all other studies focused on investigating the neurobiological basis of sexual orientation. In our narrative synthesis, we highlight important themes that suggest minority stress exposure may be associated with decreased activation and functional connectivity within the default-mode network (related to the sense-of-self and social cognition), and summarize preliminary evidence related to aberrant neural dynamics within the salience network (involved in threat detection and fear processing) and the central executive network (involved in executive functioning and emotion regulation). Importantly, this parallels neural adaptations commonly observed among individuals with posttraumatic stress disorder (PTSD) in the aftermath of trauma and supports the inclusion of insidious forms of trauma related to minority stress within models of PTSD.
Conclusions
Taken together, minority stress may have several shared neuropsychological pathways with PTSD and stress-related disorders. Here, we outline a detailed research agenda that provides an overview of literature linking sexual minority stress to PTSD and insidious trauma, moral affect (including shame and guilt), and mental health risk/resiliency, in addition to racial, ethnic, and gender related minority stress. Finally, we propose a novel minority mosaic framework designed to inform future directions of minority stress neuroimaging research from an intersectional lens.
KEYWORDS: Minority stress, sexual minorities, neurobiology, neuroimaging, stress, intrinsic connectivity networks, PTSD
HIGHLIGHTS
Minority stress exposure may be associated with alterations within intrinsic connectivity networks.
There currently exists a limited number of neuroimaging studies directly investigating the neural correlates of minority stress.
Here, we propose a novel minority mosaic framework.
Short abstract
Antecedentes: La opresión sistémica, en particular hacia las minorías sexuales, sigue estando profundamente arraigada en los cimientos de muchas sociedades a nivel mundial. Las experiencias con los factores de estrés de las minorías (por ejemplo, la discriminación, los delitos de odio, la homonegatividad interiorizada, la sensibilidad al rechazo y las microagresiones o humillaciones cotidianas) se han relacionado sistemáticamente con resultados adversos para la salud mental. La elucidación de las adaptaciones neuronales asociadas con la exposición al estrés de las minorías será fundamental para avanzar en nuestra comprensión de cómo las minorías sexuales se ven afectadas de manera desproporcionada por las cargas de salud mental.
Métodos: Siguiendo las directrices PRISMA, revisamos sistemáticamente los estudios de neuroimagen publicados que comparaban la dinámica neural entre las poblaciones de minorías sexuales y heterosexuales, agregando la información relativa a cualquier medición del estrés de minorías y los fenómenos clínicos relevantes.
Resultados: Sólo 1 de los 13 estudios elegibles para su inclusión examinó directamente el estrés de las minorías, mientras que todos los demás estudios se centraron en investigar las bases neurobiológicas de la orientación sexual. En nuestra síntesis narrativa, destacamos temas importantes que sugieren que la exposición al estrés de las minorías puede estar asociada con la disminución de la activación y la conectividad funcional dentro de la red del modo por defecto (relacionada con el sentido del yo y la cognición social), y resumimos la evidencia preliminar relacionada con la dinámica neuronal aberrante dentro de la red de saliencia (involucrada en la detección de amenazas y el procesamiento del miedo) y la red ejecutiva central (involucrada en el funcionamiento ejecutivo y la regulación de las emociones). Es importante destacar que esto es paralelo a las adaptaciones neuronales comúnmente observadas entre los individuos con trastorno de estrés postraumático (TEPT) después del trauma y apoya la inclusión de formas insidiosas de trauma relacionadas con el estrés de las minorías dentro de los modelos de TEPT.
Conclusiones: En conjunto, el estrés de las minorías puede tener varias vías neuropsicológicas compartidas con el TEPT y los trastornos relacionados con el estrés. Aquí, esbozamos una agenda de investigación detallada que proporciona una visión general de la literatura que vincula el estrés de las minorías sexuales con el TEPT y el trauma insidioso, el afecto moral (incluyendo la vergüenza y la culpa), y el riesgo/resiliencia de la salud mental, además del estrés de las minorías relacionadas con la raza, la etnia y el género. Por último, proponemos un marco de mosaico de minorías novedoso diseñado para informar sobre las futuras direcciones de la investigación de neuroimagen del estrés de las minorías desde una perspectiva interseccional.
PALABRAS CLAVE: Estrés de minorías, minorías sexuales, neurobiología, neuroimagen, estrés, redes de conectividad intrínseca, TEPT
Short abstract
背景: 系统性压迫, 尤其是对性少数群体的压迫, 持续深深植根于全球许多社会的基石。少数群体应激源 (例如歧视, 犯罪仇恨, 内化性恐同, 排斥敏感性以及微攻击或日常侮辱) 的经历一直与不良的心理健康结果有关。阐明与少数群体应激暴露相关的神经适应对于进一步了解性少数群体如何不成比例地受到心理健康负担影响至关重要。
方法: 按照 PRISMA 指南, 我们系统综述了比较了性少数和异性恋群体的神经动力学, 汇总了与少数群体应激和相关临床现象的一切测量相关信息的已发表神经影像学研究。
结果: 符合纳入条件的 13 项研究中只有 1 项直接考查了少数群体应激, 而所有其他研究都侧重于考查性取向的神经生物学基础。在我们的叙述性综合中, 我们强调了一些重要主题, 表明少数应激暴露可能与默认模式网络 (与自我意识和社会认知相关) 内激活和功能连接减少有关, 并总结了突显网络 (参与威胁检测和恐惧处理) 和中央执行网络 (参与执行功能和情绪调节) 中神经动力学异常的相关初步证据。重要的是, 这与通常在创伤后应激障碍 (PTSD) 个体中观察到的神经适应相似, 并支持在 PTSD 模型中纳入少数应激相关的潜在形式创伤。
结论: 总体来说, 少数群体的应激可能与 PTSD 和应激相关疾病有几个共同的神经心理通路。在这里, 我们概述了一个将性少数应激与 PTSD 和潜在创伤, 道德影响 (包括羞耻和内疚) 和心理健康风险/韧性, 以及种族, 民族和少数派应激相关性别联系起来的详细研究计划。最后, 我们提出了一种新颖的旨在从交叉镜头中启发少数群体应激神经影像研究未来方向的少数群体马赛克框架。
关键词: 少数群体应激, 性少数, 神经生物学, 神经影像学, 应激, 内在连接网络, PTSD
1. Introduction
It has been well documented that individuals identifying as sexual minorities, including gay, lesbian, bisexual and other non-heterosexual people, are disproportionately affected by mental health burdens across their lifetime (Dürrbaum & Sattler, 2020; Hatzenbuehler, 2009; Pitoňák, 2017; Plöderl & Tremblay, 2015; Russell & Fish, 2016; Sattler, Zeyen, & Christiansen, 2017). Recent studies indicate elevated rates of comorbid posttraumatic stress disorder (PTSD), mood disorders, anxiety disorders, substance use disorders, body image disturbances, eating disorders, and suicide ideation and attempts among both sexual minority adolescents and adults (Dürrbaum & Sattler, 2020; Pachankis, 2015; Pitoňák, 2017; Plöderl & Tremblay, 2015; Russell & Fish, 2016; Wawrzyniak & Sabbag, 2018). Of importance, experiences of sexual orientation minority stressors intersect with other racial, ethnic, gender and social identities (Purdie-Vaughns & Eibach, 2008), where Black, Indigenous and People of Colour (BIPOC) who also identify as a sexual minority face unique challenges and experiences of discrimination due to their multidimensional minority status (Balsam, Molina, Beadnell, Simoni, & Walters, 2011; Berger & Sarnyai, 2015; Dale & Safren, 2019; Hatzenbuehler, 2009).
Minority stress theory (Meyer, 2003) is the most prominent theoretical framework utilized to explain these mental health disparities and posits that sexual minorities experience distinct, uncontrollable, and chronic stressors related to their stigmatized identities (Berg, Munthe-Kaas, & Ross, 2016; Burton, Marshal, Chisolm, Sucato, & Friedman, 2013; Feinstein, 2020; Frost, 2017; Hatzenbuehler, 2017; Meyer, 2016; Pachankis, 2015; Pachankis, Mahon, Jackson, Fetzner, & Bränström, 2020). Specifically, several types of minority stressors have been identified along a distal–proximal continuum, where distal minority stressors are defined as objective events (e.g. discrimination, violence, hate crimes, and microaggressions), while conversely, proximal minority stressors are defined as subjective processes that involve individual perceptions, appraisals, and emotions (e.g. internalized homonegativity, rejection-related cognitions, and sexual orientation concealment; Meyer, 2003). Together, these experiences disproportionately compromise the mental health of sexual minorities, and lead to significant adverse changes in emotions, cognitions, and behaviour (Dürrbaum & Sattler, 2020; Newcomb & Mustanski, 2010; Russell & Fish, 2016) as described in detail below. Indeed, sexual identity minority stressors including victimization, rejection-related emotions and cognitions, as well as internalized homonegativity, have been found to mediate the association between one’s sexual identity and aforementioned mental health burdens (Sattler et al., 2017). Critically, minority stress theory suggests that systemic societal oppression as well as interpersonal experiences of discrimination and stigmatization from an early age compromises the health of sexual minorities through several shared neuropsychological pathways of PTSD and stress-related disorders, including altered stress reactivity, avoidance, hypervigilance, disrupted attachment, social cognition/interpersonal difficulties, emotion dysregulation, and alterations in the sense-of-self (Fenster, Lebois, Ressler, & Suh, 2018; Hatzenbuehler, 2009; Pachankis, 2015; Robinson & Rubin, 2016; Yehuda et al., 2015).
There have been several important extensions of minority stress theory that have significantly advanced our understanding of how oppression compromises the mental health of sexual minorities. Hatzenbuehler (2009) proposed a Psychological Mediation Framework (PMF) that identifies several general psychological processes that may mediate the association between minority stress and psychopathology. Importantly, this model highlights that stigma-related stressors trigger alterations in emotion regulation (e.g. ruminative tendencies), social/interpersonal dynamics (e.g. isolation), and cognitive processes (e.g. negative self-schemas) that then serve to mediate the association between experiencing minority stress and mental health outcomes. Most recently, Feinstein (2020) introduced the Rejection Sensitivity Model as a framework for furthering the mechanistic understanding of minority stress. Here, rejection-related cognitions include not only the expectation of rejection, but also anticipatory emotions/behaviours related to the threat of rejection (Feinstein, 2020). As such, this model emphasizes the role of perception in stigma-related experiences, anticipatory emotions (e.g. anger, anxiety), proximal minority stressors, and their dynamic relation to mental health (Feinstein, 2020). Rejection-related cognitions have been linked to negative mental health outcomes including depression, social anxiety, generalized anxiety, and PTSD, and have also been shown to mediate associations between discrimination and mental health burdens (Cohen, Rodriguez-Seijas, Feinstein, Taylor, & Newman, 2016; Dyar, Feinstein, Eaton, & London, 2018; Feinstein, 2020; Feinstein, Goldfried, & Davila, 2012; Sattler et al., 2017). Importantly, it has also been hypothesized that rejection-related emotions and cognitions may serve as an adaptive mechanism by which individuals identifying as sexual minorities learn to avoid unsafe situations, particularly important for youth who have less control over their environment as compared to adults (Feinstein, 2020).
Pachankis and colleagues (2015) have suggested several mechanistic pathways by which minority stress may transdiagnostically mediate psychosocial health outcomes among sexual minorities, particularly sexual minority men. Here, hypothesized mechanisms linking minority stress with psychosocial syndemics include disruptions within negative valence systems (i.e. avoidance, hypervigilance, loss), social functioning (i.e. disrupted attachment, low agency, social submission, poor social communication, low self-knowledge), and positive valence systems (i.e. approach motivation, reward learning, habit; Pachankis, 2015). With respect to negative valence systems more specifically, chronic and uncontrollable experiences with discrimination, harassment, ambiguously stigmatizing social encounters, and sexual orientation concealment may predispose gay and bisexual men towards hypervigilance (Pachankis, 2007, 2015). Indeed, hypervigilance in the form of expectations of gay-related rejection has been shown to be associated with depression, social anxiety, substance use, sexual compulsivity, and intimate partner violence among gay and bisexual men (Carvalho, Lewis, Derlega, Winstead, & Viggiano, 2011; Feinstein, 2020; Pachankis, 2015). Moreover, symptoms of avoidance may also manifest via emotional numbing, substance abuse, and forms of impulsive behaviour enacted to escape painful affective experiences (Pachankis, 2015). Critically, cognitive avoidance has been shown to mediate the relationship between minority stress experiences, such as discrimination, and depression and anxiety among sexual minority individuals (Hatzenbuehler, 2009). In relation to social functioning pathways, several minority stressors have been shown to be associated with disrupted attachment among gay and bisexual men (Pachankis, 2015). For example, both avoidant and anxious attachment are associated with sexual orientation concealment, internalized homophobia, and lack of maternal support for one’s sexual orientation (Mohr & Fassinger, 2003; Sherry, 2007). In the context of stigmatizing social climates, sexual minorities may develop poor emotion regulation strategies, including an inability to trust one’s emotions as valid sources of wants, needs, or desires, and to adaptively act on these emotions (Pachankis, 2015). Emerging evidence suggests that sexual minority individuals might exhibit greater difficulties with emotion regulation, and have attenuated emotional self-knowledge than heterosexuals, where indeed this has been shown to mediate the relationship between minority stress and depression and anxiety (Hatzenbuehler, McLaughlin, & Nolen-Hoeksema, 2008). Finally, with respect to positive valence systems, strong evidence exists that sexual minority stress disrupts stress pathways that result in developmental alterations within motivational and seeking systems from an early age (Pachankis, 2015). This includes, for example, increased impulsivity in the face of stress which is associated with substance use, other addictive behaviours, and eating disturbances (Pachankis, 2015).
In line with this, a recent systematic review has documented substantial evidence to support the relationship between minority stress and biological outcomes among sexual minorities (Flentje, Heck, Brennan, & Meyer, 2020), including alterations in stress hormones, immune response, hypothalamic–pituitary–adrenocortical (HPA) axis functioning, and physical health. Critically, however, neural correlates of minority stress (e.g. alterations in functional brain networks) were beyond the scope of this review. Moreover, in addition to minority stressors on the individual level (e.g. discrimination), structural stigma (i.e. the societal-level conditions, cultural norms, and institutional policies that constrain the opportunities, resources, and wellbeing of the stigmatized) (Hatzenbuehler & Link, 2014) has been shown to alter stress reactivity within the HPA axis among sexual minorities (Hatzenbuehler & McLaughlin, 2014). Specifically, LGB young adults who were raised in highly stigmatizing environments as adolescents evidenced a blunted cortisol response following social stress, compared to those from low-stigma environments (Hatzenbuehler & McLaughlin, 2014). The authors of this study suggest that the stress of growing up in environments that target sexual minorities for social exclusion may indeed exert biological effects that are similar to traumatic life experiences (Hatzenbuehler & McLaughlin, 2014). In relation, individuals with PTSD and other forms of severe trauma have also been shown to display basal hypocortisolism (Yehuda et al., 2015). Miller and colleagues have posited several factors that may result in diminished HPA axis reactivity, including chronic stressors, stress that is severe and persistent, and stress that results in feelings of shame (Miller, Chen, & Zhou, 2007). Notably, these factors (chronicity, severity, persistence of stress, and feelings of shame) are core components of minority stress among sexual minorities (Hatzenbuehler & McLaughlin, 2014; Meyer, 2003). Interestingly, higher levels of minority stress have also been shown to be related to differential expression of genes that are functionally related to inflammation and immune functioning (Flentje et al., 2018).
Collectively, these findings suggest that experiences of minority stress not only alter multiple emotional and psychological processes, but also have a profound effect on the stress response among sexual minorities. However, in contrast to a sizable body of evidence on biological correlates of minority stress (e.g. altered immune or HPA response, Flentje et al., 2020), little is known about the neural correlates of minority stress. This is a critical limitation of the current evidence base given that several neural networks (e.g. the default-mode, salience, and central executive networks) have been shown to display aberrant function and architecture following chronic stress and trauma exposure and are transdiagnostically implicated in psychopathogenesis, including the development of PTSD, depression, and anxiety (Lanius, Terpou, & Mckinnon, 2020; Lanius, Frewen, Tursich, Jetly, & Mckinnon, 2015; Menon, 2011; Nicholson et al., 2020). Thus, it seems plausible that minority stressors might also lead to alterations within these neural networks, thereby contributing to the disproportionally higher rates of psychopathology (e.g. PTSD, depression, anxiety, substance use, and suicidality) observed among sexual minority populations. Importantly, the triple network model of psychopathology by Menon (2011) highlights that aberrant organization and functioning within the default-mode (DMN), salience (SN), and central executive (CEN) networks are prominent neurobiological features of several major psychiatric and neurological disorders. These networks are involved in autobiographical memory, self-referential processing, and social cognition (DMN), interoception, threat, and fear processing (SN), and executive functioning (CEN), respectively (Menon, 2011). Indeed, this supports the notion of developing links between core features of symptoms rather than syndromes, among psychological conditions. This is in line with the Pachankis (2015) transdiagnostic minority stress model for treating mental health conditions among gay men (i.e. depression, anxiety, substance use) that focuses on syndemic pathways which are assumed to be altered by minority stress. Here, elucidating minority stress neural pathways will be critical for furthering our understanding of how sexual minorities become disproportionately affected by mental health burdens. Of importance, this will have significant implications for transdiagnostic interventions targeting these stress pathways (Burton, Wang, & Pachankis, 2019).
The aim of the current study is to systematically review the neural correlates of minority stress among sexual minority populations in order to better understand the neurobiological basis of this stress pathway. Our primary objective is to define how exposure to minority stress may be associated with altered neural dynamics within the brain. We will achieve this by systematically reviewing neuroimaging studies that compare directly neural dynamics among sexual minority and heterosexual groups, aggregating information pertaining to any measurement of minority stress (e.g. internalized homonegativity, rejection sensitivity, microaggressions, discrimination and violence) and any relevant clinical phenomena.
2. Methods
2.1. Protocol and registration
The current systematic review was conducted in line with PRISMA-guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009), and was submitted to PROSPERO on 14 May 2020 (CRD42020179705; date of registration: July, 5 2020) before completion of formal screening of the search results against the prespecified eligibility criteria. Following standard procedures, ethics board approval and informed consent were not obtained for this systematic review.
2.2. Eligibility criteria
A comprehensive list of eligibility criteria for the current systematic review is reported in Table 1. To be included, empirical studies needed to have the following characteristics: a) published in a peer-reviewed journal in English, with the publication year being >1999, b) utilized structural or functional brain imaging techniques (e.g. MRI, fMRI, DTI, MRS, PET or SPECT), c) included members from a sexual minority group (as categorized by identity, attraction, or behaviour) of any age, including mixed samples of sexual and gender minorities (if separate results for sexual minorities were reported), d) examined direct group comparisons between sexual minority and heterosexual participants or examined neural correlates of minority stress within sexual minority populations. Given our specific research aim and scope of the study, exclusion criteria included: a) studies focusing solely on heterosexual groups or gender minority groups (i.e. discrimination among heterosexuals and/or transgender individuals), b) studies including participants with a current or past history of bipolar or psychotic disorders (as assessed by standard diagnostic measures e.g. ICD-11 or DSM-5) following standard methods for neuroimaging studies investigating trauma-related psychopathology and/or depression (Nicholson et al., 2020; Rive et al., 2013; Young et al., 2017), c) neuroimaging studies among individuals diagnosed with a major medical illness as an effort to limit potentially confounding variables (unless the study examined relevant group comparisons for the current review, e.g. HIV/AIDS studies including analyses with HIV negative sexual minorities as compared to HIV negative heterosexuals), d) studies concerned with paedophilia or sexual arousal studies. No exclusion criteria were applied for measurements of specific minority stressors. Studies including participants with HIV/AIDS diagnoses were excluded given the psychological impact and pervasive societal stigma associated with this diagnosis/illness, which together, may confound neural pathways associated with minority stress (Vanable, Carey, Blair, & Littlewood, 2006; Weinstein & Li, 2016).
Table 1.
Inclusion and exclusion criteria according to population, intervention, controls, outcome, and study type (PICOS)
| Criterion | Inclusion | Exclusion |
|---|---|---|
| Publication and study type | -Empirical, peer-reviewed articles published in English language -Publication year > 1999 -Use of structural or functional brain imaging techniques such as: magnetic resonance imaging (MRI), functional magnetic resonance imaging (fMRI), diffusion tensor imaging (DTI), magnetic resonance spectrometry (MRS), positron emission tomography (PET), single-photon emission computed tomography (SPECT) |
-Any nonempirical works (e.g. reviews) -Studies not published in peer-reviewed journal articles and/or in a language other than English -Studies not including neuroimaging analyses |
| Population | -Members of a sexual minority group (assessed via identity, attraction, behaviour) of any age, including mixed samples of sexual and gender minorities | -Studies focusing exclusively on members of the sexual majority (i.e. heterosexual participants; by identity, behaviour, attraction) -Studies focusing exclusively on gender minority groups -Studies including participants with a current or past history of substance abuse, bipolar or psychotic disorders -Studies conducting neuroimaging among individuals diagnosed with a major medical illness, including HIV/AIDS. Exceptions included if studies examined relevant group comparisons for the current review (e.g. HIV negative sexual minorities as compared to HIV negative heterosexuals) -Studies concerned with paedophilia or sexual arousal |
| Intervention/Exposurea | n/a | n/a |
| Controlsa | n/a | n/a |
| Outcome | -Examined the neural correlates of any minority stressor, including implicit measures (i.e. analyses of sexual minorities in general will implicitly examine minority stress); or - directly examined group differences between sexual minority and heterosexual participants |
n/a |
aNo inclusion/exclusion criteria specified.
2.3. Data sources
We searched the following databases on 15 April 2020 (update: 2 February 2021): PubMed, Scopus, Web of Science (all databases). Databases were selected due to their suitability for conducting systematic reviews (Gusenbauer & Haddaway, 2020), their broad coverage, as well as their thematic fit with the research question of the review. In addition, we conducted forward (Google Scholar) and backward searches of articles eligible for inclusion in the study (30 November 2020).
2.4. Search strategy
We combined two different sets of keywords (AND-operator) relating to (1) sexual orientation/sexual minority status, and (2) brain imaging techniques or neural correlates using free and controlled vocabulary in respective databases. Apart from publication year (>1999), no search limits were set. Given heterogeneous terminology utilized in the field with regard to various concepts and specific forms of minority stress (e.g. internalized homonegativity, sexual orientation concealment, discrimination) and based on a preliminary literature search, we did not include a separate set of search terms related to this construct. This would have likely resulted in the omission of eligible studies, due to i) discrepancies in the operationalization of minority stress between studies, or ii) because the study assessed group differences between sexual minorities and heterosexuals without assessing minority stress directly. Instead, we chose an overinclusive approach and coded for the assessment of minority stress manually among any eligible studies. Due to the nascent stage of the field, studies were still included if they only examined group differences between sexual minority and heterosexual groups using neuroimaging data, thereby allowing us to narratively synthesize important themes. The rationale for this was because these studies unavoidably and indirectly would examine minority stress, due to the chronic and systemic nature of these stressors (i.e. analyses of sexual minorities in general will implicitly examine minority stress). Critically, however, we then coded and report minority stress variables and constructs using an exhaustive approach during data extraction. The full search string for all databases is available within the PROSPERO registration: https://www.crd.york.ac.uk/PROSPEROFILES/179705_STRATEGY_20200514.pdf.
2.5. Review of search results, data extraction and coding
The study selection process is depicted in Figure 1. The first three authors [AN, MS, JW] independently screened the titles and abstracts of 610 records retrieved via the database search against the eligibility criteria (overall agreement between raters: 95%; κFleiss = .86). In the event that eligibility could not be determined based on titles and abstracts alone, the respective full text was assessed [AN, MS, JW, BLS]. In the case of discrepant eligibility ratings between raters, we used the majority rating. Due to the low number of studies eligible for full text assessment (k = 31), every study was assessed and coded independently by the same three authors using a standardized and piloted spreadsheet. Eligibility and coding information was discussed on a case-by-case basis between the first three authors. Discrepancies (descriptive coding errors only) were resolved via discussion and by verification with original reports. The forward-backward-search of the final sample yielded 7 studies eligible for full-text assessment, of which one was included in the final review.
Figure 1.
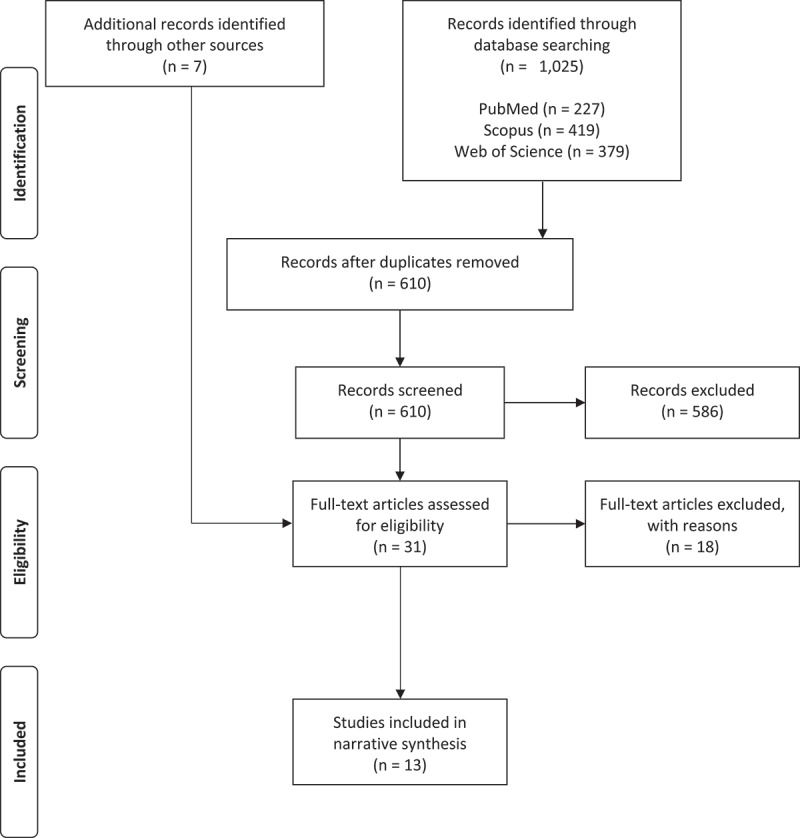
PRISMA Flow Chart depicting study selection process.
The following information was extracted from each study: publication and study information (year of data collection, country of data collection); sample information (sample size, gender composition, sexual orientation composition, age, ethnicity, socioeconomic status, and participant exclusion criteria; see Table 2); assessment of minority stress sensu (Meyer, 2003) and as defined in our introduction (conceptual and measurement level); assessment of clinically relevant variables (conceptual and measurement level); neurobiological information (imaging technique; analysis method; contrasts of interest; neuroimaging results with relevant coordinates [converted to MNI if necessary], and cluster sizes), as well as main study findings (see Table 3). We report relevant characteristics of the studies included and main study findings relevant to this systematic review in Tables 2 and 3, respectively. An exhaustive list of extracted data, including brain region coordinates, can be found in the Supplemental Material section.
Table 2.
Characteristics of included studies
| |
|
|
|
|
|
|
|
|
Minority stress |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Country |
N sexual minority (M/F) |
Ethnicitysexual minorities |
N heterosexual (M/F) |
Ethnicity heterosexuals |
Age group | Exclusion criteria | Sexual orientation assessment | Concept | Assessment | Findings |
| 1. Abé et al. (2014) | Sweden | 19/0 | 21/21 | Adult | History of psychiatric disorders, head trauma, head injury resulting in loss of consciousness for more than 10 minutes, HIV/AIDS, Hepatitis-C, chronic pain conditions, vision or hearing problems, brain pathology | Kinsey Scale HeM: Range: 0–1 M = 0 (0.5) HoM: Range: 5–6 M = 5.5 (0.5) |
n.a. | ||||
| 2. Burke, Manzouri, & Savic (2017)a | Sweden | 29/30 | 40/40 | Adult | Cisgender group: gender dysphoria, neurological or psychiatric disorders, substance use disorders, family history of psychiatric disorders, ongoing medication, and (previous) use of anabolic steroid and/or hormone supplements | Kinsey Scale HeM: Range: 0–2 M = 0.3 (0.5) HeF: Range: 0–2 M = 0.4 (0.6) HoM Range: 5–6 M = 5.7 (0.5) HoF Range: 4–6 M = 5.5 (0.6) |
n.a. | ||||
| 3. Eckstrand et al. (2019) | USA | 1/7 | 50% White 25% Black/African American 25% Multiracial |
18/20 | 68% White; 24% Black/African American; 8% Multiracial |
Adolescent | Serious medical illness or history of psychiatric disorders/treatment | Attraction (equivalent to National Longitudinal Study on Adolescent Health); grouped into sexual minority or heterosexual | Victimization | Youth Risk Behaviour Survey (YRBS) 2009 (Eaton et al., 2010) | Experiencing victimization was positively associated with interpersonal depressive symptoms, but groups did not differ on rates of victimization |
| 4. Folkierska-Żukowska et al. (2020) | Poland | 46/0 23 classified as gender-conforming; 23 as gender non-conforming in childhood |
100% white | 22/22 | 100% white | Adult | Sell Assessment of Sexual Orientation (Polish Adaptation) Predominantly heterosexual and homosexual groups defined by combination of attraction/behaviour/identity |
n.a. | |||
| 5. Hu et al. (2013)1 | China | 26/0 | 26/0 | Adult | Sexual dysfunction, gender identity disorder, paraphilia, history of major medical illnesses or substance abuse, sexual offences | Kinsey Scale HeM: Range: 0–0 M = 0 HoM: Range: 3–6 M = 4.73 |
n.a. | ||||
| 6. Hu et al. (2014)1 | China | 26/0 | 26/0 | Adult | Sexual dysfunction, gender identity disorder, paraphilia, history of major medical illnesses or substance abuse, sexual offences | Kinsey Scale HeM: Range: 0–0 M = 0 HoM: Range: 3–6 M = 4.73 |
n.a. | ||||
| 7. Kinnunen et al. (2004) | USA | 8/0 | 7/0 | Adult | Physical illness, or psychiatric history | Kinsey Scale + fantasy/attraction/behaviour questionnaire; only exclusively hetero/homosexual (identity, behaviour, attraction) included. Kinsey Scores: HeM: Range: 0–0 M = 0 HoM: Range: 6–6 M = 6 |
n.a. | ||||
| 8. Manzouri & Savic (2018a)2 | Sweden | 30/30 | 40/40 | Adult | History of psychosis, personality disorder, sexual dysfunction, gender dysphoria, hypogonadism, HIV infection, paraphilia, or sexual offences, major or bipolar depression, alcohol or substance abuse, chronic occupational psychosocial stress, major life traumas, chronic fatigue, chronic pain, or systemic disease, head trauma, neurological disease | Kinsey Scale + fantasy/attraction/behaviour interview HeM: Range: 0–1 M = 0.3 (0.5) HeF: Range: 0–1 M = 0.4 (0.5) HoM: Range: 5–6 M = 5.6 (0.5) HoF: Range: 5–6 M = 5.5 (0.5) |
n.a. | ||||
| 9. Manzouri & Savic (2018b)2 | Sweden | 30/0 | 35/38 | Adult | Medical illness, HIV positive, psychosis, personality disorder, sexual dysfunction, gender dysphoria, hypogonadism, paraphilia, sexual offences, major or bipolar depression, alcohol/ substance abuse, chronic occupational psychosocial stress, major life traumas, chronic fatigue, chronic pain, systemic disease, daily medication | Kinsey Scale + fantasy/attraction/behaviour interview Kinsey Scores: HeM/HeF: Range: 0–1 HoM: Range: 5–6 |
n.a. | ||||
| 10. Ponseti et al. (2007) | Germany | 16/15 | 24/25 | Adult | Mentally distressed, history of substance abuse, sexual dysfunction, gender identity disorder, paraphilia, sexual offences | Kinsey Scale HeF/HeM: Range: 0–1 HoF/HoM: Range: 5–6 |
n.a. | ||||
| 11. Savic & Lindström (2008) | Sweden | MRI: 20/20 PET (subsample): 12/12 |
MRI: 25/25 PET (subsample): 13/13 |
Adult | Significant medical illness, HIV positive | Kinsey Scale + fantasy/attraction/behaviour interview Kinsey Scores: HeM/HeF: Range 0–0, M = 0 HoM: Range 6–6 M = 6 HoF Range n.r. M = 5.5 |
n.a. | ||||
| 12. Wang et al. (2020) | China | 53/0 | 47/0 | Adult | History of psychiatric or neurological disorders, such as depression, bipolar disorder, head trauma, and epilepsy, long-term medications or psychoactive substance abuse (including alcohol use and smoking), history of sexual dysfunction, gender identity disorder, or paraphilia which were excluded by the Utrecht Gender Dysphoria Scale (UGS) and the Multiphasic Sex Inventory (MSI). | Kinsey Scale: HeM: Range: 0–0 M = 0 HoM Range: 4–6 M = 5.47 |
n.a. | ||||
| 13.Witelson et al. (2008) | Canada | 12/0 | 100% Caucasian | 10/0 | 100% Caucasian | Adult | History of any neurological or psychiatric disorder; HIV+ | Kinsey Scale HeM: Range: 0–1 HoM: Range: 5–6 |
n.a. | ||
HeM = Heterosexual males; HeF = heterosexual females; HoM = homosexual males; HoF = homosexual females. aResults for cis-gendered participants coded. Studies with matching superscripts constitute partially dependent samples.
Table 3.
Neuroimaging findings
| Source | Imaging technique | Paradigm/analysis method | Contrast of interest | Hemisphere | Brain area |
|---|---|---|---|---|---|
| 1. Abé et al. (2014) | MRI2 | Cortical thickness | HeM > HoM | R | Inferior temporal gyrus |
| R | Lateral orbitofrontal cortex | ||||
| R | Pars triangularis | ||||
| R | Lingual gyrus | ||||
| R | Cuneus | ||||
| R | Pericalcarine cortex | ||||
| Subcortical volumes | HeM > HoM | L | Thalamus | ||
| R | Thalamus | ||||
| 2. Burke et al. (2017) | MRI/DTI2 | Fractional anisotropy | HoM & HoF vs. HeM & HeF | ns | |
| 3. Eckstrand et al. (2019) | fMRI1 | Brain activation during social reward task (perception of being liked) | Sexual minorities < heterosexuals (positive > neutral feedback) | R | Medial prefrontal cortex |
| L | Anterior insula | ||||
| R | Temporoparietal junction | ||||
| Brain activation during monetary reward | Sexual minorities vs. heterosexuals (reward anticipation > neutral) | ns | |||
| Negative correlation between depression scores and brain activation during social reward task | Sexual minorities < heterosexuals (positive > neutral feedback) | R | Temporoparietal junction | ||
| Positive correlation between depression scores and brain activation during social reward task | Sexual minorities < heterosexuals (positive > neutral feedback) | L | Anterior insula | ||
| 4. Folkierska-Żukowska et al. (2020) | fMRI1 | ROI activation (mental rotation task vs. baseline) | GC HoM vs. GC HeM | ns | |
| GNC HoM < GC HeM | R | Angular gyrus | |||
| R | Middle temporal gyrus | ||||
| L | Middle temporal gyrus | ||||
| 5. Hu et al. (2013) | fMRI1 | Resting state; ReHo | HoM > HeM | L | Rectal gyrus |
| L | Midbrain | ||||
| R | Midbrain | ||||
| L | Temporal lobe | ||||
| R | Extranuclear white matter | ||||
| HoM < HeM | L | Inferior occipital gyrus | |||
| R | Middle occipital gyrus | ||||
| L | Cuneus | ||||
| R | Superior occipital gyrus | ||||
| R | Precuneus | ||||
| Resting state; functional connectivity of left inferior occipital gyrus (seed) | HoM < HeM | L | Middle temporal gyrus | ||
| L | Supramarginal gyrus | ||||
| L | Inferior occipital gyrus | ||||
| R | Cuneus | ||||
| 6. Hu et al., (2014) | fMRI1 | Resting state; fALFF | HoM > HeM | R | Anterior cerebellar lobe |
| R | Middle frontal gyrus | ||||
| L | Inferior parietal gyrusa | ||||
| HoM < HeM | L | Postcentral gyrus | |||
| L | Lingual gyrus | ||||
| R | Pallidum | ||||
| R | Postcentral gyrus | ||||
| R | Superior temporal gyrus | ||||
| L | Cuneus | ||||
| L | Inferior frontal gyrus | ||||
| Resting state; functional connectivity of the left postcentral gyrus (seed) | HoM < HeM | R | Precuneus | ||
| L | ACC | ||||
| L | Cuneus | ||||
| R | Putamen | ||||
| R | Middle temporal gyrus | ||||
| L | Lingual gyrus | ||||
| L | Gyrus rectus | ||||
| Resting state; functional connectivity of the left cuneus (seed) | HoM < HeM, *indicates significant positive correlation with sexual orientation | R | Paracentral lobule | ||
| L | Superior parietal gyrus* | ||||
| L | Postcentral gyrus | ||||
| R | Precentral gyrus | ||||
| L | Middle cingulate cortex | ||||
| L | Inferior parietal gyrus | ||||
| R | Postcentral gyrus | ||||
| 7. Kinnunen et al. (2004) | PET1 | Metabolic changes after administration of 40 mg fluoxetine as compared to placebo | HoM > HeM smaller decrease in glucose metabolism in response to fluoxetine relative to placebo | Hypothalamus | |
| HoM > HeM greater increase in glucose metabolism in response to fluoxetine relative to placebo | Prefrontal association cortex | ||||
| Cingulate cortex | |||||
| HoM < HeM greater increase in glucose metabolism in response to fluoxetine relative to placebo | Lateral anterior cingulate | ||||
| Hippocampus/parahippocampal gyrus | |||||
| Cuneate gyrus | |||||
| 8. Manzouri and Savic (2018a) | MRI2 | Cortical thickness | HoM < HeM | R | Cuneus |
| HoM > HeM | L | Precuneus | |||
| HoM > HeM | R | Rostral-anterior cingulate | |||
| HoF vs. HeF | ns | ||||
| Subcortical volume | HoM & HoF vs. HeM & HeF | ns | |||
| fMRI1 | Resting state; functional connectivity of the DMN | HoM & HoF < HeM & HeF | mPFC/ACC | ||
| Precuneus | |||||
| DTI2 | Fractional anisotropy | HoM & HoF vs. HeM & HeF | ns | ||
| 9. Manzouri & Savic (2018b) | MRI2 | Cortical thickness | HoM > HeM | R | ACC |
| R | Superior frontal gyrus | ||||
| R | Precuneus | ||||
| L | Occipito-temporal cortex | ||||
| L | Superior parietal cortex | ||||
| HoM < HeM | R | Cuneus | |||
| Cortical thickness; covariation in cortical thickness of precuneus (seed) | HoM > HeM | ACC | |||
| Mid cingulate | |||||
| Occipital cortex | |||||
| Inferior frontal cortex | |||||
| Insular cortex | |||||
| Surface area | HoM < HeM | L | Cuneus | ||
| Subcortical volume | HoM vs. HeM | ns | |||
| fMRI1 | Resting state; functional connectivity of the DMN | HoM < HeM | Posterior cingulate and precuneus | ||
| Resting state; functional connectivity of the left thalamus (seed) | HoM > HeM | L | Mediodorsal thalamus nucleus, anterior hypothalamus | ||
| Resting state; functional connectivity of the right thalamus (seed) | HoM > HeM | R | Mediodorsal thalamus nucleus, left pulvinar, anterior hypothalamus | ||
| Resting state; functional connectivity of the hypothalamus (seed) | HoM vs. HeM | ns | |||
| 10. Ponseti et al. (2007) | MRI2 | Global brain volume | HoM > HeM | Cerebrospinal fluid | |
| HoF vs. HeF | ns | ||||
| Regional grey matter volume | HoM vs. HeM | ns | |||
| HoF < HeF | L | Perirhinal cortex | |||
| L | Ventral premotor cortex | ||||
| L | Cerebellum | ||||
| R | Cerebellum | ||||
| R | Perirhinal cortex | ||||
| 11. Savic & Lindström (2008) | PET1 | Resting state; functional connectivity of the left amygdala (seed) | HoM > HeM | ACC | |
| R | Amygdala/ insula | ||||
| L | Parahippocampus | ||||
| HoM < HeM | L | Parietal cortex/ posterior cingulate cortex | |||
| HoF < HeF | R | Anterior cingulate (subcallosum) | |||
| R | Amygdala/ insula | ||||
| HoF > HeF | ns | ||||
| Resting state; functional connectivity of the right amygdala (seed) | HoM > HeM | ACC | |||
| Hypothalamus + portion of left amygdala | |||||
| HoM < HeM | ns | ||||
| HeF > HoF | L | Amygdala | |||
| HoF > HeF | L | Frontopolar cortex | |||
| R | Parietal cortex | ||||
| MRI2 | Hemisphere volume asymmetry | HeM and HoF had significant hemisphere asymmetry as compared to HoM and HeF who did not have asymmetry. | |||
| 12. Wang et al. (2020) | MRI/DTI2 | Anatomical connectome analysis | HoM < HeM | L | Connectivity between left postcentral gyrus and left supramarginal gyrus |
| 13. Witelson et al. (2008) | MRI2 | Corpus callosum volume | HoM > HeM | ns (trending) isthmus |
HeM = heterosexual males; HeF = heterosexual females; HoM = homosexual males; HoF = homosexual females. GNC = gender non-conforming, GC = gender conforming, ACC = anterior cingulate cortex, mPFC = medial prefrontal cortex, DMN= default mode network. 1Denotes functional neuroimaging results. 2Denotes structural neuroimaging results. aCoding based on results presented in Figure 2/Table 2 in Hu et al. (2014).
3. Results
3.1. Study selection
The PRISMA Flow Chart is depicted in Figure 1. In all, 610 records retrieved by the systematic database search were screened based on titles and abstracts, of which 586 records were excluded. After full-text assessment (k = 31) 13 studies were included in the final sample. References of excluded studies together with reasons for exclusion are provided in the Supplemental Material section.
3.2. Study characteristics
Demographic characteristics of each study included in the review are reported in Table 2. Seven studies were conducted in Europe (Sweden: 5, Germany: 1, Poland: 1), three in North America (US: 2, Canada: 1), and three in China. Overall sample sizes ranged from 15 to 140 (Md = 80). Five studies included only male participants and eight studies reported on samples with mixed sex composition. Twelve studies included adult participants, whereas one study included adolescents. Race and/or ethnicity was only reported in three studies (mixed ethnic composition in one study and White/Caucasian participants only in two studies respectively). All studies included heterosexual control groups and only included participants without any mental illness diagnoses. Brain imaging techniques that were utilized included structural MRI (k = 7), fMRI (k = 6), PET (k = 2), and DTI (k = 3; see Table 3).
Regarding assessment of sexual orientation, eleven studies used a variant of the Kinsey Scale (Kinsey, Pomeroy, & Martin, 1948), one study used an adapted version of the Sell Assessment of sexual orientation (Sell, 1996), and one study assessed sexual attraction by means of an item from the National Longitudinal Study on Adolescent Health (Chen & Chantala, 2014).
With regard to the Kinsey Scale in particular, four studies corroborated the scale scores using interviews or questionnaires assessing several dimensions of sexual orientation (i.e. attraction, fantasy, behaviour). Kinsey Scale scores (0 = ‘exclusively heterosexual’ to 6 = ‘exclusively homosexual’; Kinsey et al., 1948) ranged from 0 to 2 for heterosexual participants and from 3 to 6 for sexual minority participants. As per current APA guidelines (American Psychological Association, 2020), we will utilize the term ‘sexual minorities’, more specifically ‘gay’, ‘lesbian’ and ‘bisexual’ when appropriate, as opposed to ‘homosexual’ which has been and continues to be associated with negative stereotypes, pathology, and the reduction of people’s identities to their sexual behaviour. When referring to participants from studies using a variant of the Kinsey Scale in particular, we use the term ‘lesbian/gay’ when referring to samples with score ranges from 5 to 6 and ‘sexual minorities’ in remaining cases (i.e. score ranges from 3 to 6).
3.3. Narrative synthesis of findings
3.3.1. The neural correlates of minority stress
The current systematic review revealed a significant void in the current minority stress neuroimaging knowledge base. Only 1 of the 13 studies meeting inclusion criteria for this review (see Tables 2 and 3) directly report on levels of minority stress (Eckstrand et al., 2019), whereas the remaining 12 studies focus on investigating the neurobiological basis of sexual orientation. Eckstrand et al. (2019) found that victimization was associated with higher interpersonal depressive symptoms among male and female sexual minority and heterosexual adolescents. Here, as compared to heterosexual adolescents, sexual minority adolescents had decreased neural responses to social rewards among areas contained within the dorsal default-mode network (DMN), salience network, and social processing regions that are associated with depressive symptoms (Eckstrand et al., 2019). Specifically, as compared with heterosexual adolescents, sexual minority adolescents exhibited decreased activation in the right medial prefrontal cortex (mPFC), the left anterior insula, and the right temporoparietal junction (TPJ) in response to being liked. Here, lower response in the right TPJ, and higher activation in the anterior insula, were associated with greater depressive symptoms (Eckstrand et al., 2019). However, in this small sample of sexual minority adolescents (n= 8), violent victimization did not moderate associations between depressive symptoms and neural activity during reward processing. Further, victimization levels did not differ between the sexual minority and heterosexual adolescent groups, where Eckstrand and colleagues suggest that this may be due to their small sample size and the utilization of scales that were not developed specifically to measure aspects of sexual minority-related stress, where their measurements focused primarily on violent victimization.
3.3.2. Studies investigating the neural correlates of sexual orientation
The remaining 12 of the 13 studies meeting inclusion criteria for the current review did not directly report on levels of minority stress; notably, however, they identify important neural correlates that are unique to sexual minority as compared to heterosexual groups. Critically, although these 12 studies aimed to examine the neural underpinnings of sexual orientation, they may have indirectly investigated the neural correlates of minority stress, given i) the chronic/systemic insidious nature of minority stressors that sexual minorities experience (Dürrbaum & Sattler, 2020; Hatzenbuehler, 2009; Pachankis, 2015; Plöderl & Tremblay, 2015), and ii) the fact that this construct was not controlled for within the statistical analyses of these studies. One cannot conclude, therefore, that the results of these studies are exclusively related to sexual orientation, as experiences of minority stress would highly confound the results. A narrative synthesis of important themes is provided below, where a comprehensive reporting of these results can be found in Tables 2 and 3, and in the Supplemental Material section.
3.3.2.1. Functional neuroimaging findings
Four studies investigated resting-state fMRI differences between sexual minority men and women and heterosexuals, and together suggest alterations within the DMN among sexual minorities. Manzouri and Savic (2018b) found decreased resting-state DMN functional connectivity among gay men as compared to heterosexual men within major hubs of the DMN: the posterior cingulate cortex (PCC) and the precuneus. In a related study with overlapping samples, Manzouri & Savic (2018a) also found that gay men and lesbian women had decreased DMN resting-state functional connectivity to the mPFC/anterior cingulate cortex (ACC) and precuneus, as compared to heterosexual men and women. Additionally, among sexual minority men, Hu et al. (2014) found decreased DMN resting-state activation (fALFF) within the bilateral postcentral gyrus, left lingual gyrus, right pallidum, right superior temporal gyrus, left cuneus, and the left inferior frontal gyrus, as compared to heterosexual men. This study also found decreased DMN functional connectivity of the left postcentral gyrus and left cuneus among gay men as compared to heterosexuals. Interestingly, Hu et al. (2014) also found that gay men as compared to heterosexual men displayed increased resting-state activation within the right dl/dmPFC (right middle frontal gyrus), the right anterior cerebellar lobe, and the left inferior parietal gyrus, which are areas involved in the central executive network (CEN) and emotion regulation. Similarly, Hu et al. (2013) also found aberrant resting-state network regional homogeneity (ReHo) and functional connectivity in sexual minority as compared to heterosexual men among multiple cortical, subcortical, and midbrain structures (Hu et al., 2013).
Furthermore, one study reported altered resting-state amygdala functional connectivity – a key area of the salience network – among sexual minorities as compared to heterosexuals using PET (Savic & Lindström, 2008). Briefly, lesbian women displayed increased amygdala resting-state functional connectivity to the left frontopolar cortex, and right parietal cortex, and decreased amygdala connectivity to the right ACC, bilateral amygdala, and right insula as compared to heterosexual women (Savic & Lindström, 2008). Additionally, gay men were found to display increased amygdala resting-state functional connectivity to the bilateral ACC, bilateral amygdala, right insula, left parahippocampus, and left hypothalamus, with decreased amygdala connectivity to the left PCC/parietal cortex as compared to heterosexual men (Savic & Lindström, 2008). Similarly, Manzouri and Savic (2018b) also found increased resting-state connectivity among gay men between the thalamus and the hypothalamus, pulvinar, and mediodorsal thalamic nucleus, as compared to heterosexual men (Manzouri & Savic, 2018b).
Finally, during a mental rotation task, gender-conforming heterosexual men as compared to gender non-conforming gay men displayed increased activation in the right angular gyrus, and the bilateral middle temporal gyrus, where no differences were found when comparing gender conforming heterosexual and gay men, and no differences in performance on the mental rotation task were detected (Folkierska-Żukowska et al., 2020).
3.3.2.2. Structural neuroimaging findings
In the current review, 8 of the 13 studies examined structural differences between sexual minorities and heterosexuals. More specifically, Wang et al. (2020) examined white matter tractography via DTI using graph theoretical-based anatomical connectome analyses. Results revealed decreased connectivity between the left postcentral gyrus and left supramarginal gyrus in sexual minority men as compared to heterosexual men (Wang et al., 2020). In this study, cognitive performance (via the Wisconsin Card Sorting Test) was found to be significantly poorer among sexual minority men as compared to heterosexual men, although this was not associated with group differences in connectivity (Wang et al., 2020).
In addition, three studies (Abé, Johansson, Allzén, & Savic, 2014; Manzouri & Savic, 2018a, 2018b) found significant group differences in terms of cortical thickness/subcortical volumes when comparing gay men to heterosexual men. Abé et al. (2014) found using MRI decreased cortical thickness (in the right inferior temporal gyrus, right lateral orbitofrontal cortex, right pars triangularis, right lingual gyrus, right cuneus, and right pericalcarine cortex) and subcortical volumes (bilateral thalamus) in gay men as compared to heterosexual men (Abé et al., 2014). In two related studies with overlapping samples Manzouri & Savic (2018b, 2018a) found using MRI both increased cortical thickness (in the right ACC, right superior frontal gyrus, bilateral precuneus, left occipitotemporal cortex, and left superior parietal cortex), as well as decreased cortical thickness (in the right cuneus) and surface area (in the left cuneus) among gay men as compared to heterosexual men (Manzouri & Savic, 2018b, 2018a). Here, cortical thickness of the precuneus seed (involved in the DMN) significantly covaried with thickness of salience network regions including the insula cortex, ACC, mid-cingulate, and occipital cortex among gay men as compared to heterosexual men (Manzouri & Savic, 2018b).
Furthermore, one MRI study (Ponseti et al., 2007) reported significant group differences in grey matter volume between lesbian and heterosexual women. Specifically lesbian women displayed decreased grey matter volumes (in the left perirhinal cortex, left ventral premotor cortex, bilateral cerebellum, and right perirhinal cortex) as compared to heterosexual women (Ponseti et al., 2007).
3.3.2.3. Other neuroimaging studies
Finally, Kinnunen, Moltz, Metz, and Cooper (2004) examined metabolic differences of fluoxetine (a selective serotonin reuptake inhibitor) in gay men as compared to heterosexual men. Here, fluoxetine was administered as a challenge to the serotonergic systems and cerebral metabolic changes were measured with fluorodeoxyglucose positron emission tomography (FDG-PET). Briefly, results revealed unique glucose metabolism in response to fluoxetine within the prefrontal cortex, hypothalamus, cingulate cortex, cuneus, and hippocampus in sexual minority men and compared to heterosexual men (Kinnunen et al., 2004).
4. Discussion
The purpose of this systematic review was to identify the neural correlates of minority stress among sexual minorities. Our results indicate a highly concerning void in the current knowledge base, where only 1 of the 13 studies meeting inclusion criteria for our review examined associations between neural dynamics and levels of minority stress among sexual minorities. The remaining 12 neuroimaging studies aimed to uncover the neurobiological basis of sexual orientation. Indeed, of these limited neuroimaging studies that compared brain activation between sexual minority/majority groups, none directly examined the neural correlates of minority stress. This suggests that the interpretation of previous results in many neuroimaging studies among sexual minority groups are highly confounded, as levels of minority stress exposure were not included in the analysis. Additionally, associations between neural dynamics and mental health burdens have not previously been investigated among sexual minorities. Notably, the neuroimaging studies meeting inclusion criteria for our review also had several methodological limitations, including both overlapping and small sample sizes, as well as poor demographic characterization. Of importance, uncovering neural circuits of minority stress among sexual minorities is of high research priority given the mental health disparities that sexual minorities face globally (Dürrbaum & Sattler, 2020; Hatzenbuehler, 2009; Pitoňák, 2017; Plöderl & Tremblay, 2015; Russell & Fish, 2016; Sattler et al., 2017).
4.1. Neuroimaging themes in the current knowledge base – identifying potential minority stress pathways within intrinsic connectivity networks
In the current systemic review, we identified several important themes which together may point towards critical future directions of research in the field of minority stress and neuroimaging.
4.1.1. Default-mode network
The DMN, with core nodes within the PCC, precuneus, mPFC, and hippocampus (Buckner, Andrews-Hanna, & Schacter, 2008; Menon, 2011; Qin & Northoff, 2011) was found to repeatedly display unique activity and connectivity patterns among sexual minorities as compared to heterosexuals (Eckstrand et al., 2019; Hu et al., 2013, 2014; Manzouri & Savic, 2018b, 2018a). Specifically, Eckstrand et al. (2019) found that as compared to heterosexual adolescents, sexual minority adolescents had decreased neural responses to social reward within areas involved in the dorsal DMN (right mPFC), as well as socioaffective processing regions (anterior insula and temporoparietal junction) that were found to be associated with depressive symptoms (Eckstrand et al., 2019). Furthermore, blunted neural responses in the right TPJ – a region implicated in perspective-taking and processing social information – was associated with higher interpersonal depressive symptom severity (Eckstrand et al., 2019). Indeed, sexual minority adolescents are a population at high risk for experiencing symptoms of depression, and are more likely than heterosexual youth to have experienced social stressors, including interpersonal victimization and rejection (Burton et al., 2013). Manzouri and Savic (2018a, 2018b) also found decreased DMN resting-state functional connectivity among gay men and lesbian women as compared to heterosexual men and women within major hubs of the DMN (e.g. mPFC/ACC, PCC and precuneus). Additionally, cortical thickness of the precuneus (involved in the DMN) significantly covaried with thickness of salience network regions including the insula, ACC, and mid-cingulate, among gay men as compared to heterosexual men (Manzouri & Savic, 2018b). Several studies in the current review also reported altered DMN/resting-state network activation (fALFF), regional homogeneity (ReHo), and functional connectivity, among sexual minority men as compared to heterosexual men (Hu et al., 2013, 2014).
The DMN is the foundational basis of the ‘sense-of-self’, and is involved in autobiographical memory, self-referential processing, and social cognition, and additionally has been shown to be highly implicated in PTSD, anxiety, and affective disorders (Frewen et al., 2020; Lanius et al., 2015, 2020; Menon, 2011; Renner et al., 2015). Critically, the DMN orchestrates key social cognitive functions, where stress-related alterations to such pathways among sexual minorities (i.e. disruptions in attachment, low agency, social submission, poor social communication, and low self-knowledge) have been proposed to be associated with negative mental health outcomes (Pachankis, 2015; Qin & Northoff, 2011; Spreng, Mar, & Kim, 2008). In parallel, traumatic events and chronic stress can profoundly impact the sense-of-self (Lanius et al., 2020). In the aftermath of traumatic experiences, decreased DMN functional connectivity among individuals with PTSD is hypothesized to be related to modified and often negative self-referential thoughts, as well as to altered social cognition and autobiographical narratives (Fenster et al., 2018; Lanius et al., 2020; Nicholson et al., 2020; Yehuda et al., 2015). Indeed, DMN functional connectivity at rest has been shown previously to be negatively correlated to PTSD symptoms (Akiki, Averill, & Abdallah, 2017; Akiki et al., 2018; Koch et al., 2016; Lanius et al., 2015; Patel, Spreng, Shin, & Girard, 2012; Yehuda et al., 2015). Unfortunately, studies reporting unique patterns of DMN functional connectivity among sexual minorities have largely attributed these findings to the neurobiological basis of sexual orientation, where the chronic effects of minority stress were often ignored. Indeed, experiences of discrimination, violence, microaggressions, internalized homonegativity, shame, rejection-related cognitions, and concealment, may significantly affect DMN-related social cognition, autobiographical memory, and self-referential processing. Here, we hypothesize that that altered DMN activation and functional connectivity among sexual minorities may be associated with individual experiences of minority stress and chronic oppression, where future research is needed to confirm these hypotheses.
4.1.2. Salience network
The current review also identified several studies reporting alterations within the salience network among sexual minorities as compared to heterosexuals. The salience network, including the insula, amygdala, dorsal ACC, and the brainstem, is heavily involved in threat detection, fight-or-flight responding, and monitoring of personally salient environmental stimuli (Dosenbach et al., 2007; Menon, 2011; Seeley et al., 2007). Here, several neuroimaging studies within our review demonstrated altered activation and functional connectivity patterns of the insula and the amygdala among sexual minorities as compared to heterosexuals (Eckstrand et al., 2019; Savic & Lindström, 2008). Eckstrand et al. (2019) found that as compared to heterosexual adolescents, sexual minority adolescents had decreased neural responses to social reward within the left anterior insula, where activity within this region was further associated with depressive symptoms. Hu et al. (2013) also found increased ReHo within the bilateral midbrain at rest among sexual minority men as compared to heterosexual men. Additionally, Savic and Lindström (2008) found altered resting-state amygdala functional connectivity among sexual minorities as compared to heterosexuals (Savic & Lindström, 2008). Here, gay men were found to display increased amygdala resting-state functional connectivity to the bilateral ACC, bilateral amygdala, right insula, left parahippocampus, and left hypothalamus, with decreased amygdala connectivity to the left PCC/parietal cortex as compared to heterosexual men (Savic & Lindström, 2008). Additionally, lesbian women evidenced increased amygdala resting-state functional connectivity to the left frontopolar cortex, and right parietal cortex, and decreased amygdala connectivity to the right anterior cingulate, bilateral amygdala, and right insula as compared to heterosexual women (Savic & Lindström, 2008). These findings may suggest unique amygdala and salience network functional connectivity patterns among sexual minority men vs. women. Additional studies are required to elucidate the unique effects of minority stress exposure on intrinsic connectivity networks as a function of biological sex and gender given the divergent findings reported by Savic and Lindström (2008) between lesbian women and gay men. Importantly, previous research has also shown that the basolateral and centromedial complexes of the amygdala, as well as the anterior, mid, and posterior subregions of the insula, subserve unique functions within the salience network and additionally display unique functional connectivity patterns among individuals with PTSD with significant associations with dissociation and relieving symptoms (Harricharan et al., 2019; Nicholson et al., 2015, 2016). Hence, future research is also needed to examine further the effects of minority stress exposure on amygdala complex and insula subregion functional connectivity.
The salience network has been shown to be both hyperactive and dysregulated in PTSD and heavily correlated to trauma-related symptoms; additionally, the salience network has also been shown to be implicated in anxiety and affective disorders as well as suicide ideation (Cisler et al., 2014; Daniels et al., 2010; Harricharan et al., 2019; Koch et al., 2016; Menon, 2011; Nicholson et al., 2020; Patel et al., 2012; Schwartz, Ordaz, Ho, & Gotlib, 2019). More specifically, disruptions in salience network activity and connectivity involving the amygdala, insula, and the brainstem periaqueductal grey (PAG), have been shown to be associated with trauma-related symptoms, including hyperarousal, hypervigilance, avoidance, dissociation, relieving, and defence processing (Allen, 2020; Akiki et al., 2017; Harricharan et al., 2016, 2019; McCurry, Frueh, Chiu, & King-Casas, 2020; Nicholson et al., 2020; Sripada et al., 2012; Terpou et al., 2020; Yehuda et al., 2015). In parallel, hyperarousal, hypervigilance, and avoidance symptoms are part of the negative valence pathway that is hypothesized to transdiagnostically mediate associations between experiences of minority stress and negative mental health outcomes (Pachankis, 2015).
Minority stress theory suggests that systemic societal oppression as well as interpersonal experiences of discrimination and stigmatization from an early age may compromise the health of sexual minorities through several shared neuropsychological pathways of PTSD and stress-related disorders (Fenster et al., 2018; Hatzenbuehler, 2009; Pachankis, 2015; Robinson & Rubin, 2016; Yehuda et al., 2015). In direct support of this, Clark, Miller, and Hegde (2018) found that levels of discrimination were correlated to salience network dynamics (including both exacerbated amygdala activity and altered amygdala functional connectivity) in a diverse sample of racial/ethnic and sexual minorities (43% female; 72% African American; 23% Hispanic; 32% homosexual/bisexual; sub-analyses and results specific to the sexual minority group not reported). Specifically, greater discrimination exposure was associated with higher levels of bilateral amygdala resting-state activity (Clark et al., 2018). Furthermore, increased discrimination was also associated with stronger functional connectivity between the left amygdala and several salience network (e.g. left anterior insula, bilateral ACC, thalamus, and caudate), and DMN (bilateral mPFC) areas. Interestingly, higher discrimination exposure was associated with higher levels of current stress, depression, anxiety, and PTSD-related symptoms (Clark et al., 2018). Similarly, it has also been shown that rejection-related cognitions are associated with increased salience network dorsal ACC activity in response to disapproving facial expressions, albeit the sexual orientation of participants was not reported (Burklund, Eisenberger, & Lieberman, 2007). Additionally, processing highly negative social stigmas has been shown previously to be associated with increased salience network activity (Krendl, Macrae, Kelley, Fugelsang, & Heatherton, 2006). Taken together, we hypothesize that exposure to minority stressors (including discrimination and stigmatization) results in altered activity and functional connectivity within the salience network, which may reflect changes to neural circuits involved in threat-sensitivity, stress-reactivity, defence processing and fear-conditioning. Speculatively, this may constitute the neural basis of positive and negative valence minority stress pathways (Pachankis, 2015) subserving hypervigilance, hyperarousal, avoidance, approach motivation, and reward learning among sexual minorities, which may then mediate negative mental health outcomes in these populations. Future research is required to examine the dynamic interaction between minority stress, alterations in the salience network, and mental health outcomes among sexual minorities.
4.1.3. Central executive network
The CEN is a frontoparietal and cerebellar network centred around the dlPFC, and is involved in higher-order executive functioning including the cognitive control of thought, emotion regulation, and emotional awareness (Etkin, Büchel, & Gross, 2015; Koechlin & Summerfield, 2007; Menon, 2011; Seeley et al., 2007). Critically, exposure to chronic stigma-related stress can result in maladaptive coping/emotion regulation, which is associated with heightened risk for mental health burdens among sexual minorities (Inzlicht, McKay, & Aronson, 2006; Pachankis, 2015). Indeed, sexual minority adolescents can exhibit patterns of emotion dysregulation (both rumination and poor emotional awareness) as compared to heterosexuals, where emotion dysregulation specifically has been shown to mediate the relationship between sexual minority status and symptoms of depression and anxiety (Hatzenbuehler et al., 2008). In the current review, Hu et al. (2014) found increased resting-state activation in the right dl/dmPFC (right middle frontal gyrus), the right anterior cerebellum, and the left posterior parietal cortex (PPC; left inferior parietal gyrus) among gay men as compared to heterosexual men. Critically, these areas are known to be involved in emotion overmodulation in PTSD (i.e. exacerbated prefrontal cortex top-down regulation on hyperactive limbic structures), and have repeatedly been shown to be associated with emotional numbing and dissociation symptom profiles (Lanius et al., 2010; Nicholson et al., 2020; Rabellino, Densmore, Théberge, McKinnon, & Lanius, 2018). This may suggest increased recruitment of emotion regulation areas within the CEN during the resting-state among gay men (which may influence resting-state DMN self-referential processing and social cognition). This is in line with Hatzenbuehler’s PMF, which highlights that stigma-related stressors trigger alterations in emotion regulation, cognitive processes, and social/interpersonal problems (together heavily related to the CEN and DMN) that then serve to mediate the association between experiencing minority stress and mental health outcomes (Hatzenbuehler, 2009). Additionally, in the context of stigmatizing social climates, sexual minorities might develop poor emotion regulation strategies, including an inability to trust emotions as valid sources of their wants, needs, and desires (Pachankis, 2015). Although the scientific evidence is highly preliminary, these studies highlight the role of emotion regulation networks with respect to potential mechanisms by which minority stressors can result in negative mental health outcomes. As such, future studies are warranted to investigate the role of the CEN with respect to risk and resiliency for mental health burdens among sexual minorities.
In support of these findings, it has been hypothesized that restoring aberrant neural dynamics within aforementioned intrinsic connectivity networks (ICNs), including the default-mode, salience, and central executive networks, is a critical future direction for the treatment of trauma and stress-related disorders (Lanius et al., 2015; Menon, 2011). Importantly, the studies reviewed here show preliminary, emerging evidence that ICNs may be functionally disrupted due to experiences of minority stress (Figure 2), paralleling years of empirical research in the field of traumatic stress (Patel et al., 2012; Yehuda et al., 2015). Critically, further research is needed to delineate diagnostic vs. transdiagnostic ICN models and how aberrant organization and functioning of ICNs may relate to risk and resilience for mental health outcomes among sexual minorities. Indeed, it is now sufficiently clear that DMN, SN, and CEN dysfunctions are major and consistent features of psychological disorders that transdiagnostically alter autobiographical memory, self-related mental processes, and social cognition (DMN), threat, fear, and interoceptive processing (SN), and executive functioning (CEN), respectively (Menon, 2011). This approach supports the shift towards developing links between core features of symptoms rather than syndromes, among psychological conditions (Menon, 2011) and may help to elucidate the neurobiological association between minority stress exposure and psychosocial syndemics (Pachankis, 2015).
Figure 2.
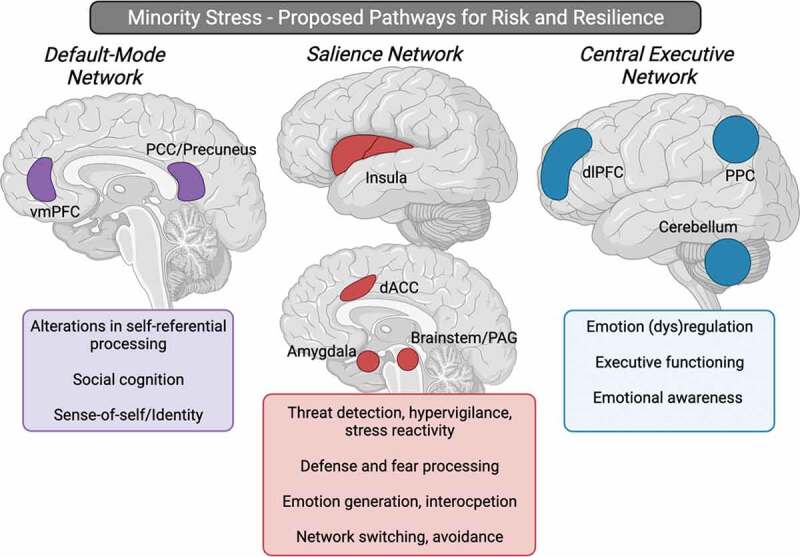
Proposed minority stress neural network pathways that may be associated with risk and resilience for psychopathology. The default-mode network, with key hubs within the vmPFC, and PCC/precuneus, may be associated with alterations in self-referential processing, social cognition, and sense-of-self/identity following minority stress exposure. The salience network, involving the insula, dACC, amygdala, and brainstem, may display altered functioning following minority stress exposure, subserving changes in threat detection, hypervigilance, and stress reactivity, as well as defence and fear processing, emotion generation, interoception, and avoidance. The central executive network, with key hubs involving the dlPFC, PPC, and the cerebellum may be involved in alterations observed in emotion regulation, executive functioning, and emotional awareness following minority stress exposure. Taken together, these alterations within intrinsic connectivity networks in the brain may be associated with the disproportionally higher rates of transdiagnostic psychopathology observed among sexual minorities (such as increased PTSD, depression, anxiety, suicidality, and substance use). Abbreviations; vmPFC = ventromedial prefrontal cortex, PCC = posterior cingulate cortex, dACC = dorsal anterior cingulate cortex, PAG = periaqueductal grey, dlPFC = dorsolateral prefrontal cortex, PPC = posterior parietal cortex. Figure created with BioRender.com.
5. Future research agenda
5.1. Chronic stress, trauma and PTSD
Profound disparities exist among sexual minorities with respect to i) heightened risk for developing PTSD, and ii) exposure to potentially traumatic events due to their marginalized identities, including interpersonal violence, discrimination, and psychological abuse across the lifetime (Balsam, Rothblum, & Beauchaine, 2005; Roberts, Rosario, Corliss, Koenen, & Austin, 2012). As compared to heterosexual individuals, sexual minorities have increased exposure to partner physical victimization and psychological abuse in adulthood, which may be related to minority stressors such as internalized homonegativity (Balsam et al., 2005). Moreover, it has been reported that sexual minority women are more than twice as likely to experience sexual assault in adulthood as compared to heterosexual women, where more than 1 in 10 sexual minority males have been shown to experience sexual assault in adulthood (Balsam et al., 2005). A recent systematic review also reports that the lifetime prevalence rate of sexual assault ranges from 16–85% in lesbian and bisexual women, and from 12–54% in gay and bisexual men (Rothman & Baughman, 2011). In parallel, there exists a large body of evidence that indicates elevated rates of comorbid PTSD, mood disorders, anxiety disorders, substance use disorders, and suicide ideation and attempts among both sexual minority adults and adolescents across the lifetime (Dürrbaum & Sattler, 2020; Pachankis, 2015; Pitoňák, 2017; Plöderl & Tremblay, 2015; Russell & Fish, 2016; Wawrzyniak & Sabbag, 2018).
In relation, insidious trauma refers to the ongoing experiences of discrimination and fear that may lead to PTSD (Root, 1992), without the presence of a specific identifiable traumatic event to instigate the disorder (Robinson & Rubin, 2016; Szymanski & Balsam, 2011). For example, homo- or bi-negative microaggressions and traumatic stress symptoms have been shown to be highly correlated, demonstrating the critical impact of insidious trauma among sexual minorities (Robinson & Rubin, 2016). Furthermore, it has been shown previously that both heterosexist discrimination and heterosexist hate crime victimization were significant positive predictors of lesbian women’s PTSD symptoms (Szymanski & Balsam, 2011). As such, it has been proposed that the diagnostic criteria for PTSD should be expanded to include insidious trauma related to oppression (Holmes, Facemire, & Da Fonseca, 2016; Roberts et al., 2012). Furthermore, internalized heterosexism has been shown to positively predict PTSD symptomatology among sexual minorities. Importantly, shame-related withdrawal tendencies have been shown to have a mediating role between increased internalized heterosexism and PTSD symptoms among trauma-exposed sexual minority women (Straub et al., 2018). Critically, both chronic experiences of minority stress and severe trauma/PTSD have been shown to result in altered HPA axis functioning. Specifically, sexual minority young adults who were raised in highly stigmatizing environments as adolescents evidenced a blunted cortisol response following social stress, compared to those from low-stigma environments (Hatzenbuehler & McLaughlin, 2014). Indeed, insidious trauma related to growing up in highly stigmatizing environments that target sexual minorities may exert biological effects that are similar to PTSD (Hatzenbuehler & McLaughlin, 2014), where PTSD and other forms of severe trauma are similarly related to basal hypocortisolism (Yehuda et al., 2015).
As previously mentioned, there is emerging preliminary evidence that points towards overlapping neural correlates of PTSD and minority stress pathways with respect to ICN dynamics as well as underlying psychological mechanisms (e.g. stress reactivity, avoidance, hypervigilance, disrupted attachment, social cognition/interpersonal difficulties, emotion dysregulation, and alterations in the sense-of-self). As such, future research is urgently needed to examine minority stress pathways in the brain and elucidate further how exposure to minority stressors may increase risk or resiliency for PTSD and trauma-related symptoms.
5.2. Shame, guilt and moral injury
Future research exploring the associations between minority stress, insidious trauma, and PTSD, should consider the mediating role of moral affective experiences such as shame, guilt, betrayal, and moral injury. In line with Hatzenbuehler’s (2009) PMF of minority stress, moral emotions such as shame, guilt, betrayal, and anger are psychological mediators that increase vulnerability to adverse psychiatric outcomes and have been consistently shown to result from minority stress exposure, thereby impacting neural functioning in ways consistent with other chronic stressors (Hequembourg & Dearing, 2013; Mereish & Paul Poteat, 2015; Straub et al., 2018). For example, both distal (e.g. discrimination) and proximal (e.g. internalized homophobia) sexual minority stressors have been associated with experiences of shame and anger and, in turn, have been linked to psychiatric sequelae including depression, anxiety, problematic substance use, and PTSD (Hequembourg & Dearing, 2013; Mereish & Paul Poteat, 2015; Straub et al., 2018). Specifically, shame-related behaviours have been shown to have a mediating role with respect to associations between minority stress and PTSD symptoms among sexual minority women (Straub et al., 2018). Within the broader PTSD literature, chronic interpersonal violence has been shown to generate intense feelings of shame, rather than fear, as the primary affective trauma response (Saraiya & Lopez-Castro, 2016). Importantly, the resolution of trauma-related guilt and shame has been shown to systematically predict PTSD symptom resolution over the course of front-line exposure therapies with and without cognitive rescripting (Bub & Lommen, 2017; Øktedalen, Hoffart, & Langkaas, 2015).
In cases where minority stress is chronic (i.e. insidious trauma) and/or severe, it is conceivable that such experiences may represent a morally injurious event. Moral injury is characterized by profound psychological, spiritual, and social suffering following a perceived moral transgression (Drescher et al., 2011; Jinkerson, 2016; Litz et al., 2009). Existing literature describes the experience of moral injury as most often occurring after the perpetration of a moral violation (e.g. by committing or failing to prevent an immoral act), or, after being betrayed in a morally violating way by a trusted individual or organization in power (e.g. betrayal by a caregiver, leader or institution, including betrayals resultant from exposure to a highly stigmatizing environment) (Drescher et al., 2011; Litz et al., 2009).
Moral injury can lead to a host of adverse consequences that parallel those resulting from minority stress, including negative moral affect (e.g. guilt, shame, and anger), as well as behavioural and interpersonal challenges such as loss of trust in oneself and others, existential conflict, self-harm, and social problems (Jinkerson, 2016). Consequently, moral injury has been linked to several psychiatric outcomes that are associated with minority stressors and known to disproportionately impact sexual minority populations, including depression, anxiety, substance use, suicidality, and PTSD, where moral injury has notably been identified as an important mediator between exposure to stress/trauma and the subsequent development of psychopathology (Barnes, Hurley, & Taber, 2019; Griffin et al., 2019; McEwen, Alisic, & Jobson, 2020; Williamson, Stevelink, & Greenberg, 2018). Furthermore, ‘Sanctuary Trauma’ (sometimes referred to as ‘Sanctuary Harm’ or ‘Organizational Trauma’) represents a related, yet relatively understudied concept (Silver, 1985, 1986). Specifically, sanctuary trauma refers to circumstances wherein an individual who has suffered a severe stressor encounters additional trauma instead of the supportive and protective environment that was expected (Silver, 1985). Here, sanctuary trauma has the potential to compound existing traumas, exacerbating feelings of betrayal, isolation, powerlessness and hopelessness (Esaki et al., 2013; Robins, Sauvageot, Cusack, Suffoletta-Maierle, & Frueh, 2005; Silver, 1985, 1986). Sanctuary trauma is unique because it is perpetrated by institutions that are expected to provide care. Although extant studies have investigated sanctuary trauma within the context of psychiatric settings, military personnel, and first responders, we propose it is reasonable and appropriate to extend this concept to minority stress. For example, abuse, neglect, or other traumatic exposure sustained towards sexual minorities at the community level, as well as within systems of care (i.e. when disclosing one’s sexual orientation), may represent pathways to sanctuary trauma that uniquely and disproportionately affect sexual minorities.
Indeed, moral injury (and by extension, sanctuary trauma) shares many clinical features with PTSD (e.g. overlapping determinants and symptom profiles), albeit the two have distinct symptom profiles and neural underpinnings (Barnes et al., 2019; Lloyd et al., 2020; Sun et al., 2019) despite often co-occurring together (Barnes et al., 2019). As such, future research should consider whether minority stressors (such as internalized homonegativity, chronic oppression, and discrimination) represent a chronic challenge that, in extreme cases, may result in moral injury. More specifically, and consistent with Hatzenbuehler’s (2009) PMF, research should examine not only the unique impact of moral emotions like guilt or shame, but also how the clustering of affective symptoms into a syndrome like moral injury may mediate the relation between minority stress and psychopathology in sexual minorities. To our knowledge, no studies examining minority stress and psychopathology to date have attempted to explore the contributions of more chronic, severe, or syndromal presentations of morally relevant affect, such as that resulting from a moral injury.
5.3. Measurement of sexual orientation within neuroimaging studies
Interestingly, eleven out of thirteen studies within our review used a variant of the Kinsey Scale (Kinsey et al., 1948) to assess sexual orientation. Ground-breaking at the time of its conception (Quinan, 2016), the Kinsey Scale locates sexual orientation (defined as encompassing both sexual experiences as well as psychosexual reactions) on a linear continuum ranging from 0 (exclusively heterosexual) to 6 (exclusively homosexual) and is commonly administered via a self-report, Likert-typed scale.
Notwithstanding its historical importance for the field of sexology and it being the psychometric basis for a vast body of research, the scale has received criticism from researchers and research participants alike. Longstanding scholarly criticism of the Kinsey Scale includes its conflation (or omission) of different aspects of sexual orientation, the assumed linearity of the scale (rather than being multidimensional), as well as its polarity that considers heterosexual and – using Kinsey’s terminology – homosexual desires as trade-offs of one another (e.g. Quinan, 2016; Sell, 1997, for in-depth discussions). Face validity studies highlight further shortcomings of the Kinsey Scale for adequately capturing sexual and gender minority participants’ sexual orientation, particularly so for plurisexual and/or gender minority participants (Galupo, Mitchell, & Davis, 2018; Galupo, Mitchell, Grynkiewicz, & Davis, 2014).
The Kinsey Scale’s popularity may partially be rooted in its easy administration and (originally unintended) potential to categorize research participants into distinct groups (e.g. exclusively heterosexual or lesbian/gay; Galupo et al., 2014; van Anders, 2015). This might explain its frequent use within studies assessing sexual orientation-related structural or functional differences within the brain that are conducted using exclusively heterosexual or lesbian/gay participants (e.g. Kinnunen et al., 2004). However, neuroimaging studies aiming at identifying neural correlates of minority stress in particular (as a consequence of belonging to a socially marginalized group) may benefit from a more nuanced assessment of sexual orientation.
In this vein, we suggest clearly differentiating between sexual identity, behaviour, and attraction in neuroimaging studies with sexual minorities (for best-practice items, see Badgett, 2009) and based on the research question at hand. For example, investigations into identity-based minority stress should incorporate measures of sexual identity rather than behaviours or attraction. Furthermore, studies with sexual minority youth should pay particular attention to a developmentally appropriate assessment of sexual orientation (see e.g. Schrager, Steiner, Bouris, MacApagal, & Brown, 2019). Moving forward, we caution against using polar (i.e. heterosexual and homosexual on opposing ends), continuum-based measures of sexual orientation (such as the Kinsey scale) in neuroimaging linear regression designs. The assumed linearity of sexuality therein (ranging from heterosexual to homosexual) would effectively mask the well-established and heightened risk of bi- or other plurisexual people (located on the midpoints of the scale) for adverse mental health outcomes (Ross et al., 2018; Salway et al., 2019), thus rendering a potentially vulnerable group analytically invisible.
5.4. Elucidating risk and resiliency for mental illness
A well-known criticism of research conducted within a minority stress framework is its emphasis on risk rather than resiliency-promoting factors (Frost, 2017; Meyer, 2015; Prendergast & MacPhee, 2018; Vaughan et al., 2014). This imbalance may hamper the derivation of therapeutic interventions, as less is known about why sexual minorities are not burdened by adverse mental health outcomes or even thrive despite their marginalized societal identities (Hill & Gunderson, 2015).
Empirical evidence on resilience in the face of (minority) stress and mental health burdens in sexual minority populations has indeed been slower to accumulate (Vaughan et al., 2014). However, a sizable body of qualitative research has identified unique positive factors associated with being a sexual minority which extend beyond the mere absence of discrimination, victimization, or stigmatization. These include aspects related to social support (e.g. belonging to a community, creating families of choice, having strong connections with others, and serving as a role model), increased self-knowledge and empathy (e.g. living an authentic life, having empathy for others, and promoting social justice), and being less constrained by societal roles and definitions (e.g. with regard to gender or relationships) (Riggle, Whitman, Olson, Rostosky, & Strong, 2008; Rostosky, Riggle, Pascale-Hague, & McCants, 2010). Importantly, these factors have been linked to aspects of mental health and well-being in quantitative designs (Petrocchi et al., 2020; Riggle, Rostosky, Mohr, Fingerhut, & Balsam, 2014; Rostosky, Cardom, Hammer, & Riggle, 2018).
Resilience-based models for sexual minorities (Hill & Gunderson, 2015; Kwon, 2013; Prendergast & MacPhee, 2018) highlight important general sources of resilience related to minority stress and subsequently mental health. Specifically, social support (in particular identity-affirming social support), emotion regulation and emotional openness, and personality traits (e.g. optimism), have been proposed to buffer against the negative mental health effects of minority stress, such as discrimination, reactivity to prejudice, and internalized homonegativity (Hill & Gunderson, 2015; Kwon, 2013; Prendergast & MacPhee, 2018). In addition, dyadic coping has been found to protect against minority stress, specifically sexual orientation discrimination, in same-gender couples (Randall, Tao, Totenhagen, Walsh, & Cooper, 2017; Randall, Totenhagen, Walsh, Adams, & Tao, 2017).
While we were not able to identify any studies assessing neural correlates of these resiliency-promoting factors among sexual minorities, their neurobiological basis has been documented empirically within the general population (see Feder, Nestler, & Charney, 2009; Kashdan & Rottenberg, 2010; Southwick & Charney, 2012; Wu et al., 2013, for reviews). Thus, future research should strive to assess similar mechanisms within sexual minority populations while also identifying possible neural correlates of resiliency that are specific to sexual minorities (e.g. a positive self-identity; Rostosky et al., 2018).
5.5. Racial and ethnic minority stress
The results of this systematic review have also revealed a distinct gap in the current knowledge base, such that no neuroimaging study meeting inclusion criteria examined directly the unique experiences of intersecting minority identities. Critically, mental health burdens also disproportionately affect racial and ethnic minorities (Berger & Sarnyai, 2015; Hatzenbuehler, 2009). Of importance, BIPOC sexual minorities face unique challenges and experiences of discrimination due to their multidimensional minority status, where capturing the intersectionality of one’s identity is imperative (Balsam et al., 2011; Parra & Hastings, 2018). Intersectionality refers to the multidimensional nature of one’s identity based on social categories pertaining to race, gender, sex, and sexual orientation (Bartlett & Kennedy, 1991). Within intersectionality, social categories do not exist independently, but rather interact with each other and result in both overlapping and distinct systems of discrimination. For instance, LGBTQ-BIPOC can experience differential forms of discrimination, due to their multidimensional minority status (Singh, 2017). These forms of discrimination may include heterosexism, racial discrimination, and a combination of both (Drazdowski et al., 2016; Singh, 2017). Indeed, it is important to note that the lived experience of minority oppression is often unique among LGBT-BIPOC (e.g. cis-gendered Black lesbian compared to cis-gendered White lesbian), as a result of unequal power, privilege, and standing that is inherent within systemic social categories (Balsam et al., 2011; Singh, 2017).
Neuroimaging research specific to racial and ethnic minority stress has advanced somewhat further as compared to sexual orientation minority stress and has critical implications for adopting an intersectional approach moving forward. In line with the ICN findings reviewed above, Masten, Telzer, & Eisenberger (2011) investigated the neural correlates of attributing negative social treatment to racial discrimination. Here, participants who appeared to be more distressed exhibited increased activation in salience network regions implicated in social rejection and pain-related processing (e.g. anterior insula and dACC), with concomitant decreases in activation within emotion regulation regions (e.g. lateral PFC and dmPFC), during exclusion as compared to inclusion conditions. Interestingly, when participants attributed exclusion to racial discrimination, there was less neural activity in salience network regions associated with social distress and threat perception (e.g. dACC) and greater activity in neural regions associated with emotional regulation (Masten et al., 2011). This finding may provide support for resiliency functions of external attributions (i.e. that discrimination is resulting from other’s prejudicial views), and may in turn buffer against negative emotions during the discriminatory event, providing a temporary coping mechanism in such situations and reducing negative affect and self-blame. Elsewhere, it has been shown that social marginalization impacts neural responding in the ACC, which results in altered salience attribution and hypervigilance with regard to race-related discrepancies (Fourie, Stein, Solms, Gobodo-Madikizela, & Decety, 2019). Similarly, Han et al. (2020) also report associations between salience network connectivity and self-report experiences of discrimination in a sample of 124 Black adult participants. Specifically, they found that greater self-reported experiences of discrimination were associated with stronger functional connectivity between the left insula and bilateral intracalcarine cortex, as well as weaker functional connectivity between the right insula and left supplementary motor cortex. Regarding ethnic discrimination, Akdeniz et al. (2014) found altered salience network and limbic activation during social stress processing. Here, increased perigenual (p)ACC activity was detected during social stress processing in ethnic minority individuals, which is a limbic system regulation area (Davidson & Mcewen, 2012; Etkin, Egner, Peraza, Kandel, & Hirsch, 2006; LeDoux, 2000). Additionally, social stress processing was also associated with increased functional coupling of the pACC to the dACC, which is a salience network area involved in the detection and resolution of conflict, error, negative emotion, and stress (Critchley, Melmed, Featherstone, Mathias, & Dolan, 2002; Ochsner et al., 2006; Pezawas et al., 2005).
Taken together, given that individuals with multiple stigmatized identities may be the most susceptible to the impact of oppression (Dale & Safren, 2019), it is of critical importance that future directions of research investigate the intersection of minority stressors from a neuroimaging perspective and engage deeply with the lived experience of marginalized individuals (Furman, Singh, Darko, & Wilson, 2018). Indeed, it is imperative that the field places a focus on investigating these complex social structures in a multidimensional way. Failure to consider such social determinants of health, including intersecting minority stressors, may have severe consequences such as developing exclusionary disease models which are not generalizable to the wider population, thereby facilitating treatment inequality and inefficacy (Harnett, 2020).
5.6. Gender minority stress
At a societal level, the predominant belief that biological sex and gender identity are synonymous continues to prevail, with gender identity being assumed based on sex assigned at birth (Sloan, Berke, & Shipherd, 2017). Relatedly, sexual orientation is also often assumed based on biological sex and these idealizations have been echoed throughout past research (Jacobson & Joel, 2019). Indeed, biological sex, gender identity, and sexual orientation are often conflated, and assumed to follow binary, heteronormative ideologies (Jacobson & Joel, 2019; Jordan-Young & Rumiati, 2012). These assumptions and beliefs are in contrast to research suggesting that sexual orientation and gender identity are both distinct and multidimensional (Savin-Williams, 2016), highlighting the need for a more nuanced, inclusive approach.
At an individual level, these hetero- and cis-normative beliefs serve to dismiss, invalidate, and/or alienate transgender and gender non-conforming (TGNC) individuals, whose experienced gender is incongruent and/or exists beyond the binary of biological sex (Hendricks & Testa, 2012; Sloan et al., 2017). Emerging evidence suggests TGNC individuals experience unique forms of minority stressors based on their gender identity and expression that is qualitatively different from the experience of sexual minority stressors (Tan, Treharne, Ellis, Schmidt, & Veale, 2019). For example, Su et al. (2016) found that among LGBTQ+ individuals, transgender identities were associated with higher incidence of discrimination and increased rates of depression and attempted suicide relative to cisgender identities (Su et al., 2016). Prevalence rates also suggest that TGNC individuals are more likely to experience violence, including both physical and sexual violence, relative to cisgender and/or sexual minority individuals (Tan et al., 2019).
Consequently, extending Meyer’s (2003) original Minority Stress Theory, the Gender Minority Stress Theory (GMST) postulates that TGNC individuals experience unique distal and proximal stressors (Testa, Habarth, Peta, Balsam, & Bockting, 2015). Here, distal stressors include gender related discrimination (e.g. difficulty accessing appropriate healthcare), victimization (e.g. harassment), rejection (e.g. familial or peer), and experiences of non-affirmation of identity (e.g. being misgendered) (Hendricks & Testa, 2012; Jäggi et al., 2018; Testa et al., 2015). Proximal stressors that TGNC might experience include internalized transphobia (e.g. negative beliefs about being TGNC), negative expectations (e.g. anticipating rejection), and nondisclosure (e.g. concealing gender identity, altering behaviour) (Hendricks & Testa, 2012; Jäggi et al., 2018; Testa et al., 2015). Moreover, given the complexities of navigating an invalidating, cisnormative environment, many TGNC individuals experience a multitude of other minority stressors, which interact with one another (Jäggi et al., 2018), creating a vulnerability and increased likelihood of emerging mental health concerns (Griffin, Casanova, Eldridge-Smith, & Stepleman, 2019 Hendricks & Testa, 2012).
With regard to neuroimaging studies among gender minorities, the vast majority of investigations have focused on the aetiology of TGNC identities, denoting almost a complete lack of research on how experiences of minority stress related to gender affect brain structure and function. Nevertheless, group comparisons between transgender and cisgender individuals report functional and anatomical differences among similar areas that have been found to be associated with traumatic experiences among sexual minority groups (Feusner et al., 2017; Hahn et al., 2015; Spizzirri et al., 2018). For instance, Uribe et al. (2020) found decreased functional connectivity in superior parietal regions, as part of the salience network and the CEN in transgender men, transgender women, and cisgender women, as compared to cisgender men. Additionally, transgender men were found to display weaker functional connectivity as compared to cisgender men within the salience network, and weaker inter-network connectivity between the salience network, DMN, and CEN. Interestingly, no statistical differences were observed among transgender men/women and cisgender women, which may point towards important parallels with respect to gender-related stressors experienced by transgender men/women and cisgender women (Uribe et al., 2020).
To the best of our knowledge, Mueller, Wierckx, Boccadoro, and T’Sjoen (2018) conducted the first investigation examining the neural correlates of ostracism related to social exclusion and re-inclusion among gender minorities. This study found that during the re-inclusion stage of the paradigm, transgender men and women had stronger activity in the vACC as compared to cisgender women, indicating persisting effects of exclusion among gender minorities (Mueller et al., 2018). Functional connectivity analyses during exclusion showed that transgender men had stronger vACC functional connectivity with the inferior parietal lobule and the left middle and superior frontal gyri as compared to cisgender men, which may suggest altered emotion regulation processing in response to ostracism. Additionally, transgender men had stronger functional connectivity of the vACC during re-inclusion with the superior and medial frontal gyrus, as compared to cisgender women. Mueller et al.’s (2018) findings are considered a representation of the impact of social ostracism on brain function among transgender people, potentially highlighting the neural effects of social-stressors, prejudice, and discrimination regarding stigmatized gender identities.
6. A minority mosaic framework for neuroimaging research
Capturing the intersectional nature of minority stress is critical in order to better characterize how the brain adapts as a result of these experiences, and in turn, how this may influence both resilience and psychological injury. Investigating the neurobiological basis of minority stress exposure requires a framework that considers the multidimensional nature of individual identities, especially in nonbinary instances. We suggest a novel theoretical framework designed to inform future directions of neuroimaging research in the field of minority stress. Here, we propose the minority mosaic framework for investigating the neurobiological basis of minority stress, which emphasizes that the experiences of sexual minorities must be understood as intersecting with other racial, ethnic, gender, and social identities.
A mosaic can be defined as a combination of diverse elements, which together, form a coherent image. The minority mosaic framework considers each minority identity element as a unique mosaic tile (Figure 3). This framework calls into action the fact that all diverse elements (i.e. tiles) representing one’s minority identity (i.e. mosaic) need to be considered in order to fully understand the neurobiological basis of minority stress. Metaphorically, the tiles of a ‘minority mosaic’ come together to form an individual’s identity, analogous to individual tiles forming a coherent image within a mosaic. Additionally, tiles pertaining to minority identity elements can take on different shapes and sizes. In this way, it is possible to represent multidimensional identities and associated unique experiences with minority stressors from a variety of perspectives (i.e. capturing experiences of racial discrimination, internalized homonegativity, violence, and microaggressions that are associated with a particular minority mosaic). Importantly, the minority mosaic framework does not solely consider how an individual’s numerous identities intersect, but further considers unique experiences that are associated with a person’s multidimensional identity, as well individual differences in the psychological processes/pathways that mediate response to such stressors (Hatzenbuehler, 2009; Pachankis, 2015). As such, this framework also acknowledges the unique lived experiences within minority groups.
Figure 3.
Minority mosaic framework. Mosaic tiles represent various minority identity elements, where the mosaic as a whole captures the complex and multidimensional nature of one’s identity. Arrows represent minority stress experiences that can be associated with unique minority mosaics. Bends in the arrows represent individual differences in psychological mediators and pathways that alter one’s response to such minority stressors. Taken together, these experiences can then result in resiliency (i.e. strength through adversity) and/or psychological injury.
Moving forward, it is critical that varying levels of experience with minority stressors be measured and analysed in a multidimensional way in neuroimaging studies, where great caution should be taken as to not silo identities during univariate group-level analyses based on isolated demographic factors such as sexual orientation. Advances in neuroimaging techniques have radically changed the way neuroscientists address questions related to the neurobiological basis of chronic stress. Importantly, multivariate machine learning approaches can be used to elucidate neuroimaging biomarkers that reveal how multidimensional minority experiences influence both chronic stress pathways and risk/resiliency for mental health burdens (Nicholson et al., 2018, 2020). Machine learning models afford the ability to evaluate highly complex sources of information (i.e. a minority mosaic with multiple minority identity tiles and various unique experiences associated with this mosaic) and allow for powerful predictions on the individual level. In this way, complex experiences of minority stressors related to intersecting identities (i.e. unique minority mosaics) can serve as predictive features in machine learning models to predict altered brain activity within ICNs and stress pathways. Furthermore, graph theoretical network-based analyses can be utilized to examine closeness and centrality of mosaic tiles.
Additionally, by utilizing a minority mosaic framework, it is possible to extend beyond binary conceptualizations of identity and examine minority stress from a basis that is actually generalizable in real-world settings. Critically, non-binary identities (e.g. multiracial, pansexual, and gender non-conforming identities) are known to experience unique challenges due to normative monocultural, monoracial, cis-gendered, and heteronormative discourse. This phenomenon has recently been termed ‘border identity stress’ (Jackson, Scheer, Chang, Loopuift, & Franco, 2021), and pertains to the unique stress that an individual experiences as a result of transgressing binary/categorical definitions of racial, sexual, and gender identities (Albuja, Gaither, Sanchez, Straka, & Cipollina, 2019). Indeed, compared to both heterosexual and lesbian/gay individuals, bisexual individuals are at an increased risk for developing mood disorders and substance use (Ross et al., 2018; Salway et al., 2019; Sarno, Newcomb, Feinstein, & Mustanski, 2020), and experience additional negative regard from within sexual minority communities (Ault, 1996; Sarno et al., 2020). Furthermore, individuals who identify as, for example, bisexual, multiracial, and gender non-binary experience the unique mental strain known as border identity stress (Albuja et al., 2019; Bratter & Gorman, 2011; Hovey, 2000; Jackson et al., 2021; Torres, 2010). By creating space for multidimensional identities, the minority stress framework aims to break the borders of normative monocultural, monoracial, cis-gendered, and heteronormative themes that are currently highly persistent and problematic in field of neuroimaging.
In all, the primary tenets of the minority mosaic framework include, i) representing multidimensional identities (pertaining to sexual, racial, ethnic, gender, and social identities) as tiles that together form a minority mosaic, ii) capturing unique experiences of minority stress that are associated with individual minority mosaics/intersecting identities, iii) incorporating multivariate approaches into neuroimaging studies in order to evaluate highly complex sources of information that are embedded within an individual’s unique mosaic pattern and associated lived-experience, and iv) predicting the influence of stressors associated with minority mosaics on both resiliency and psychological injury. Taken together, it is crucial that the neurobiological basis of such multidimensional experiences with minority stressors be elucidated, in order to define biomarkers that reveal how these minority identities become disproportionately affected by mental health burdens.
7. Conclusion
The purpose of this systematic review was to identify the neural correlates of minority stress among sexual minorities. Only 1 of 13 studies eligible for inclusion examined minority stress directly, where all other studies focused on investigating the neurobiological basis of sexual orientation. Here, we highlight important themes that suggest minority stress exposure may be associated with decreased activation and functional connectivity within the default-mode network, a brain network that plays a critical role in how we experience our sense-of-self and how we interact with others. Furthermore, we present preliminary evidence related to aberrant neural dynamics within the salience network (involved in threat detection and fear processing) and the central executive network (related to executive functioning and emotion regulation). Critically, these themes parallel the neural adaptations commonly observed among individuals with PTSD and supports the inclusion of insidious forms of trauma related to minority stress within diagnostic models of PTSD. Collectively, experiences of minority stress among sexual minorities may have several shared neuropsychological pathways as PTSD and stress-related disorders. In summary, there currently exists a limited number of neuroimaging studies directly investigating the neural correlates of minority stress, where additional research is urgently needed to examine the psycho-social basis of observed group differences. Here, we outline a detailed research agenda and propose a novel minority mosaic framework designed to inform future directions of minority stress neuroimaging research from an intersectional lens. Of importance, uncovering neural circuits of minority stress among sexual minorities is of high research priority given the mental health disparities that sexual minorities face globally.
Supplementary Material
Funding Statement
This work was supported by the LGBT Purge Fund, Canada, and the Centre of Excellence for PTSD, Royal Ottawa Hospital, Canada. Additionally, Andrew A. Nicholson has received funding support from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Individual Fellowship [grant agreement No. 897709]. Magdalena Siegel’s research is supported by a Marietta-Blau-Grant funded by the Austrian Federal Ministry of Education, Science and Research and processed by the OeAD, Austria’s Agency for Education and Internationalisation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Data availability statement
The authors confirm that the data supporting the findings of this review are available within the supplementary material systematic coding file.
References
References marked with an asterisk (*) were included in the systematic review.
- *Abé, C., Johansson, E., Allzén, E., & Savic, I. (2014). Sexual orientation related differences in cortical thickness in male individuals. PLoS ONE, 9(12), 1–33. doi: 10.1371/journal.pone.0114721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdeniz, C., Tost, H., Streit, F., Haddad, L., Wüst, S., Schäfer, A., … Meyer-Lindenberg, A. (2014). Neuroimaging evidence for a role of neural social stress processing in ethnic minority-associated environmental risk. JAMA Psychiatry, 71(6), 672–680. doi: 10.1001/jamapsychiatry.2014.35 [DOI] [PubMed] [Google Scholar]
- Akiki, T. J., Averill, C. L., & Abdallah, C. G. (2017). A network-based neurobiological model of PTSD: Evidence from structural and functional neuroimaging studies. Current Psychiatry Reports, 19(11). doi: 10.1007/s11920-017-0840-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki, T. J., Averill, C. L., Wrocklage, K. M., Scott, J. C., Averill, L. A., Schweinsburg, B., … Abdallah, C. G. (2018). Default mode network abnormalities in posttraumatic stress disorder: A novel network-restricted topology approach. NeuroImage, 176(February), 489–498. doi: 10.1016/j.neuroimage.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuja, A. F., Gaither, S. E., Sanchez, D. T., Straka, B., & Cipollina, R. (2019). Psychophysiological stress responses to bicultural and biracial identity denial. Journal of Social Issues, 75(4), 1165–1191. doi: 10.1111/josi.12347 [DOI] [Google Scholar]
- Allen, M. (2020). Unravelling the neurobiology of interoceptive inference. Trends in Cognitive Sciences, 24(4), 265–266. doi: 10.1016/j.tics.2020.02.002 [DOI] [PubMed] [Google Scholar]
- American Psychological Association . (2020). Publication manual of the American Psychological Association (7th ed.). Washington: Author. doi: 10.1037/0000165-000 [DOI] [Google Scholar]
- Ault, A. (1996). Ambiguous identity in an unambiguous sex/gender structure: The case of bisexual women. The Sociological Quarterly, 37(3), 449–463. doi: 10.1111/j.1533-8525.1996.tb00748.x [DOI] [Google Scholar]
- Badgett, M. V. L. (2009, November). Best practices for asking questions about sexual orientation on surveys. http://www.escholarship.org/uc/item/706057d5
- Balsam, K., Molina, Y., Beadnell, B., Simoni, J., & Walters, K. (2011). Measuring multiple minority stress: The LGBT people of color microaggressions scale. Cultural Diversity and Ethnic Minority Psychology, 17(2), 163. doi: 10.1037/a0023244.Measuring [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam, K. F., Rothblum, E. D., & Beauchaine, T. P. (2005). Victimization over the life span: A comparison of lesbian, gay, bisexual, and heterosexual siblings. Journal of Consulting and Clinical Psychology, 73(3), 477–487. doi: 10.1037/0022-006X.73.3.477 [DOI] [PubMed] [Google Scholar]
- Barnes, H. A., Hurley, R. A., & Taber, K. H. (2019). Moral injury and PTSD: Often co-occurring yet mechanistically different. Journal of Neuropsychiatry and Clinical Neurosciences, 31(2), A4–103. doi: 10.1176/appi.neuropsych.19020036 [DOI] [PubMed] [Google Scholar]
- Bartlett, K.T., and Kennedy, R. (Eds.). (1991). Feminist legal theory: Readings in law and gender (1st ed.). Routledge. 10.4324/9780429500480 [DOI] [Google Scholar]
- Berg, R. C., Munthe-Kaas, H. M., & Ross, M. W. (2016). Internalized homonegativity: A systematic mapping review of empirical research. Journal of Homosexuality, 63(4), 541–558. doi: 10.1080/00918369.2015.1083788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, M., & Sarnyai, Z. (2015). “More than skin deep”: Stress neurobiology and mental health consequences of racial discrimination. Stress, 18(1), 1–10. doi: 10.3109/10253890.2014.989204 [DOI] [PubMed] [Google Scholar]
- Bratter, J. L., & Gorman, B. K. (2011). Does multiracial matter? A study of racial disparities in self-rated health. Demography, 48(1), 127–152. doi: 10.1007/s13524-010-0005-0 [DOI] [PubMed] [Google Scholar]
- Bub, K., & Lommen, M. J. J. (2017). The role of guilt in posttraumatic stress disorder. European Journal of Psychotraumatology, 8(1), 1407202. doi: 10.1080/20008198.2017.1407202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain ’ s default network anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 38, 1–38. doi: 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- *Burke, S. M., Manzouri, A. H., & Savic, I. (2017). Structural connections in the brain in relation to gender identity and sexual orientation. Scientific Reports, 7(1), 1–12. doi: 10.1038/s41598-017-17352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burklund, L. J., Eisenberger, N. I., & Lieberman, M. D. (2007). The face of rejection: Rejection sensitivity moderates dorsal anterior cingulate activity to disapproving facial expressions. Social Neuroscience, 2(3–4), 238–253. doi: 10.1080/17470910701391711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, C. M., Marshal, M. P., Chisolm, D. J., Sucato, G. S., & Friedman, M. S. (2013). Sexual minority-related victimization as a mediator of mental health disparities in sexual minority youth: A longitudinal analysis. Journal of Youth and Adolescence, 42(3), 394–402. doi: 10.1007/s10964-012-9901-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, C. L., Wang, K., & Pachankis, J. E. (2019). Psychotherapy for the spectrum of sexual minority stress: Application and technique of the ESTEEM treatment model Charles. Cognitive and Behavioral Practice, 26(2), 285–299. doi: 10.1016/j.cbpra.2017.05.001.Psychotherapy [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho, A. F., Lewis, R. J., Derlega, V. J., Winstead, B. A., & Viggiano, C. (2011). Internalized sexual minority stressors and same-sex intimate partner violence. Journal of Family Violence, 26(7), 501–509. doi: 10.1007/s10896-011-9384-2 [DOI] [Google Scholar]
- Chen, P., & Chantala, K. (2014). Guidelines for analyzing Add Health Data. Chapel Hill, NC: Carolina Population Center, University of North Carolina at Chapel Hill. [Google Scholar]
- Cisler, J. M., Steele, J. S., Lenow, J. K., Smitherman, S., Everett, B., Messias, E., & Kilts, C. D. (2014). Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: An exploratory fMRI study. Journal of Psychiatric Research, 48(1), 47–55. doi: 10.1016/j.jpsychires.2013.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, U., Miller, E., & Hegde, R. (2018). Experiences of discrimination are associated with greater resting amygdala activity and functional connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging, 3(4), 367. doi: 10.1016/j.bpsc.2017.11.011.Experiences [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. M., Rodriguez-Seijas, C., Feinstein, B. A., Taylor, C. B., & Newman, M. G. (2016). Rejection sensitivity as a transdiagnostic risk factor for internalizing psychopathology among gay and bisexual men. Psychology of Sexual Orientation and Gender Diversity, 3(3), 259–264. doi: 10.1037/sgd0000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley, H. D., Melmed, R. N., Featherstone, E., Mathias, C. J., & Dolan, R. J. (2002). Volitional control of autonomic arousal: A functional magnetic resonance study. NeuroImage, 16(4), 909–919. doi: 10.1006/nimg.2002.1147 [DOI] [PubMed] [Google Scholar]
- Dale, S. K., & Safren, S. A. (2019). Gendered racial microaggressions predict posttraumatic stress disorder symptoms and cognitions among Black women living with HIV. Psychological Trauma: Theory, Research, Practice, and Policy, 11(7), 685–694. doi: 10.1037/tra0000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, J. K., Mcfarlane, A. C., Bluhm, R. L., Moores, K. A., Richard Clark, C., Shaw, M. E., … Lanius, R. A. (2010). Switching between executive and default mode networks in posttraumatic stress disorder: Alterations in functional connectivity. Journal of Psychiatry and Neuroscience, 35(4), 258–266. doi: 10.1503/jpn.090010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, R. J., & Mcewen, B. S. (2012). Social influences on neuroplasticity: Stress and interventions to promote well-being. Nature Neuroscience, 15(5), 689–695. doi: 10.1038/nn.3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach, N. U. F., Fair, D. A., Miezin, F. M., Cohen, A. L., Wenger, K. K., Dosenbach, R. A. T., … Petersen, S. E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. doi: 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazdowski, T. K., Perrin, P. B., Trujillo, M., Sutter, M., Benotsch, E. G., & Snipes, D. J. (2016). Structural equation modeling of the effects of racism, LGBTQ discrimination, and internalized oppression on illicit drug use in LGBTQ people of color. Drug and Alcohol Dependence, 159, 255–262. doi: 10.1016/j.drugalcdep.2015.12.029 [DOI] [PubMed] [Google Scholar]
- Drescher, K. D., Foy, D. W., Kelly, C., Leshner, A., Schutz, K., & Litz, B. (2011). An exploration of the viability and usefulness of the construct of moral injury in war veterans. Traumatology, 17(1), 8–13. doi: 10.1177/1534765610395615 [DOI] [Google Scholar]
- Dürrbaum, T., & Sattler, F. A. (2020). Minority stress and mental health in lesbian, gay male, and bisexual youths: A meta-analysis. Journal of LGBT Youth, 17(3), 298–314. doi: 10.1080/19361653.2019.1586615 [DOI] [Google Scholar]
- Dyar, C., Feinstein, B. A., Eaton, N. R., & London, B. (2018). The mediating roles of rejection sensitivity and proximal stress in the association between discrimination and internalizing symptoms among sexual minority women. Archives of Sexual Behavior, 47(1), 205–218. doi: 10.1007/s10508-016-0869-1 [DOI] [PubMed] [Google Scholar]
- Eaton, D. K., Kann, L., Kinchen, S., Shanklin, S., Ross, J., Hawkins, J., … Centers for Disease Control and Prevention (CDC) . (2010). Youth risk behavior surveillance - United States, 2009. MMWR Surveillance Summaries, 59(5), 1–142. [PubMed] [Google Scholar]
- Eckstrand, K. L., Flores Jr, L. E., Cross, M., Silk, J. S., Allen, N. B., Healey, K. L., … Forbes, E. E. (2019). Social and non-social reward processing and depressive symptoms among sexual minority adolescents. Frontiers in Behavioral Neuroscience, 13(September), 1–9. doi: 10.3389/fnbeh.2019.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaki, N., Benamati, J., Yanosy, S., Middleton, J. S., Hopson, L. M., Hummer, V. L., & Bloom, S. L. (2013). The sanctuary model: Theoretical framework. Families in Society, 94(2), 87–95. doi: 10.1606/1044-3894.4287 [DOI] [Google Scholar]
- Etkin, A., Büchel, C., & Gross, J. J. (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16(11), 693–700. doi: 10.1038/nrn4044 [DOI] [PubMed] [Google Scholar]
- Etkin, A., Egner, T., Peraza, D. M., Kandel, E. R., & Hirsch, J. (2006). Resolving emotional conflict: A role for the Rostral anterior cingulate cortex in modulating activity in the Amygdala. Neuron, 51(6), 871–882. doi: 10.1016/j.neuron.2006.07.029 [DOI] [PubMed] [Google Scholar]
- Feder, A., Nestler, E. J., & Charney, D. S. (2009). Psychobiology and molecular genetics of resilience. Nature Reviews Neuroscience, 10(6), 446–457. doi: 10.1038/nrn2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein, B. A. (2020). The rejection sensitivity model as a framework for understanding sexual minority mental health. Archives of Sexual Behavior, 49(7), 2247–2258. doi: 10.1007/s10508-019-1428-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein, B. A., Goldfried, M. R., & Davila, J. (2012). The relationship between experiences of discrimination and mental health among lesbians and gay men: An examination of internalized homonegativity and rejection sensitivity as potential mechanisms. Journal of Consulting and Clinical Psychology, 80(5), 917–927. doi: 10.1037/a0029425 [DOI] [PubMed] [Google Scholar]
- Fenster, R. J., Lebois, L. A. M., Ressler, K. J., & Suh, J. (2018). Brain circuit dysfunction in post-traumatic stress disorder: From mouse to man. Nature Reviews Neuroscience, 19(9), 535–551. doi: 10.1038/s41583-018-0039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner, J. D., Lidström, A., Moody, T. D., Dhejne, C., Bookheimer, S. Y., & Savic, I. (2017). Intrinsic network connectivity and own body perception in gender dysphoria. Brain Imaging and Behavior, 11(4), 964–976. doi: 10.1007/s11682-016-9578-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flentje, A., Heck, N. C., Brennan, J. M., & Meyer, I. H. (2020). The relationship between minority stress and biological outcomes: A systematic review. Journal of Behavioral Medicine, 43(5), 673–694. doi: 10.1007/s10865-019-00120-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flentje, A., Kober, K. M., Carrico, A. W., Neilands, T. B., Flowers, E., Heck, N. C., & Aouizerat, B. E. (2018). Minority stress and leukocyte gene expression in sexual minority men living with treated HIV infection. Brain, Behavior, and Immunity, 70, 335–345. doi: 10.1016/j.bbi.2018.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkierska-Żukowska, M., Rahman, Q., Marchewka, A., Wypych, M., Droździel, D., Sokołowski, A., & Dragan, W. (2020). Male sexual orientation, gender nonconformity, and neural activity during mental rotations: An fMRI study. Scientific Reports, 10(1), 1–14. doi: 10.1038/s41598-020-74886-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie, M. M., Stein, D. J., Solms, M., Gobodo-Madikizela, P., & Decety, J. (2019). Effects of early adversity and social discrimination on empathy for complex mental states: An fMRI investigation. Scientific Reports, 9(1). doi: 10.1038/s41598-019-49298-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen, P., Schroeter, M. L., Riva, G., Cipresso, P., Fairfield, B., Padulo, C., … Northoff, G. (2020). Neuroimaging the consciousness of self: Review, and conceptual-methodological framework. Neuroscience and Biobehavioral Reviews, 112(January), 164–212. doi: 10.1016/j.neubiorev.2020.01.023 [DOI] [PubMed] [Google Scholar]
- Frost, D. M. (2017). The benefits and challenges of health disparities and social stress frameworks for research on sexual and gender minority health. Journal of Social Issues, 73(3), 462–476. doi: 10.1111/josi.12226 [DOI] [Google Scholar]
- Furman, E., Singh, A., Darko, N., & Wilson, C. (2018). Activism, intersectionality, and community psychology: The way in which Black lives matter Toronto helps us to examine White supremacy in Canada’s LGBTQ community. Community Psychology in Global Perspective, 4(2), 34–54. doi: 10.1285/i24212113v4i2p34. [DOI] [Google Scholar]
- Galupo, M. P., Mitchell, R. C., & Davis, K. S. (2018). Face validity ratings of sexual orientation scales by sexual minority adults: Effects of sexual orientation and gender identity. Archives of Sexual Behavior, 47(4), 1241–1250. doi: 10.1007/s10508-017-1037-y [DOI] [PubMed] [Google Scholar]
- Galupo, M. P., Mitchell, R. C., Grynkiewicz, A. L., & Davis, K. S. (2014). Sexual minority reflections on the Kinsey Scale and the Klein sexual orientation grid: Conceptualization and measurement. Journal of Bisexuality, 14(3–4), 404–432. doi: 10.1080/15299716.2014.929553 [DOI] [Google Scholar]
- Griffin, J. A., Casanova, T. N., Eldridge-Smith, E. D., & Stepleman, L. M. (2019). Gender minority stress and health perceptions among transgender individuals in a small metropolitan southeastern region of the United States. Transgender Health, 4(1), 247–253. doi: 10.1089/trgh.2019.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, B. J., Purcell, N., Burkman, K., Litz, B. T., Bryan, C. J., Schmitz, M., … Maguen, S. (2019). Moral injury: An integrative review. Journal of Traumatic Stress, 32(3), 350–362. doi: 10.1002/jts.22362 [DOI] [PubMed] [Google Scholar]
- Gusenbauer, M., & Haddaway, N. R. (2020). Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Research Synthesis Methods, 11(2), 181–217. doi: 10.1002/jrsm.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn, A., Kranz, G. S., Küblböck, M., Kaufmann, U., Ganger, S., Hummer, A., … Lanzenberger, R. (2015). Structural connectivity networks of transgender people. Cerebral Cortex, 25(10), 3527–3534. doi: 10.1093/cercor/bhu194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, S. D., Lamar, M., Fleischman, D., Kim, N., Bennett, D. A., Lewis, T. T., … Barnes, L. L. (2020). Self-reported experiences of discrimination in older black adults are associated with insula functional connectivity. Brain Imaging and Behavior. doi: 10.1007/s11682-020-00365-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett, N. G. (2020). Neurobiological consequences of racial disparities and environmental risks: A critical gap in understanding psychiatric disorders. Neuropsychopharmacology, 45(8), 1247–1250. doi: 10.1038/s41386-020-0681-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harricharan, S., Nicholson, A. A., Thome, J., Densmore, M., McKinnon, M. C., Théberge, J., … Lanius, R. A. (2019, September). PTSD and its dissociative subtype through the lens of the insula: Anterior and posterior insula resting‐state functional connectivity and its predictive validity using machine learning. Psychophysiology, 2018, 1–23. doi: 10.1111/psyp.13472 [DOI] [PubMed] [Google Scholar]
- Harricharan, S., Rabellino, D., Frewen, P. A., Densmore, M., Théberge, J., Mckinnon, M. C., … Lanius, R. A. (2016, June). fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain and Behavior, 1–16. doi: 10.1002/brb3.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler, M. L. (2009). How does sexual minority stigma “Get Under the Skin”? A psychological mediation framework. Psychological Bulletin, 135(5), 707–730. doi: 10.1037/a0016441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler, M. (2017). Advancing research on structural stigma and sexual orientation disparities in mental health among youth. Journal of Clinical Child & Adolescent Psychology, 46(3), 463. doi: 10.1080/15374416.2016.1247360.Advancing [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler, M. L., & Link, B. G. (2014). Introduction to the special issue on structural stigma and health. Social Science & Medicine, 103, 1–6. doi: 10.1016/j.socscimed.2013.12.017 [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler, M. L., & McLaughlin, K. A. (2014). Structural stigma and hypothalamic-pituitary-adrenocortical axis reactivity in lesbian, gay, and bisexual young adults. Annals of Behavioral Medicine, 47(1), 39–47. doi: 10.1007/s12160-013-9556-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler, M. L., McLaughlin, K. A., & Nolen-Hoeksema, S. (2008). Emotion regulation and internalizing symptoms in a longitudinal study of sexual minority and heterosexual adolescents. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 49(12), 1270–1278. doi: 10.1111/j.1469-7610.2008.01924.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks, M. L., & Testa, R. J. (2012). A conceptual framework for clinical work with transgender and gender nonconforming clients: An adaptation of the minority stress model. Professional Psychology, Research and Practice, 43(5), 460–467. doi: 10.1037/a0029597 [DOI] [Google Scholar]
- Hequembourg, A. L., & Dearing, R. L. (2013). Exploring shame, guilt, and risky substance use among sexual minority men and women. Journal of Homosexuality, 60(4), 615–638. doi: 10.1080/00918369.2013.760365.Exploring [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, C. A., & Gunderson, C. J. (2015). Resilience of lesbian, gay, and bisexual individuals in relation to social environment, personal characteristics, and emotion regulation strategies. Psychology of Sexual Orientation and Gender Diversity, 2(3), 232–252. doi: 10.1037/sgd0000129 [DOI] [Google Scholar]
- Holmes, S. C., Facemire, V. C., & Da Fonseca, A. M. (2016). Expanding criterion a for posttraumatic stress disorder: Considering the deleterious impact of oppression. Traumatology, 22(4), 314–321. doi: 10.1037/trm0000104 [DOI] [Google Scholar]
- Hovey, J. D. (2000). Acculturative stress, depression, and suicidal ideation in Mexican immigrants. Cultural Diversity & Ethnic Minority Psychology, 6(2), 134–151. doi: 10.1037/1099-9809.6.2.134 [DOI] [PubMed] [Google Scholar]
- Hu, S., Xu, D., Peterson, B., Wang, Q., He, X., Hu, J., … Xu, Y. (2013). Association of cerebral networks in resting state with sexual preference of homosexual men: A study of regional homogeneity and functional connectivity. PLoS ONE, 8(3). doi: 10.1371/journal.pone.0059426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, S., Xu, D., Peterson, B. S., Wang, Q., Lai, J., Hu, J., … Xu, Y. (2014). Differing default mode network activities in men with homosexual or heterosexual preferences. Journal of Sexual Medicine, 11(10), 2474–2484. doi: 10.1111/jsm.12639 [DOI] [PubMed] [Google Scholar]
- Inzlicht, M., McKay, L., & Aronson, J. (2006). Stigma as ego depletion: How being the target of prejudice affects self-control. Psychological Science, 17(3), 262–269. doi: 10.1111/j.1467-9280.2006.01695.x [DOI] [PubMed] [Google Scholar]
- Jackson, S. D., Scheer, J. R., Chang, C., Loopuift, C. C., & Franco, M. G. (2021). A mixed methods experience sampling approach to measuring border identity stress. Walking between worlds: Border identity stress among Biracial, bisexual, & gender non-binary people. In International Congress of Psychology, Prague, Czech Republic. [Google Scholar]
- Jacobson, R., & Joel, D. (2019). Self-reported gender identity and sexuality in an online sample of cisgender, transgender, and gender-diverse individuals: An exploratory study. Journal of Sex Research, 56(2), 249–263. doi: 10.1080/00224499.2018.1523998 [DOI] [PubMed] [Google Scholar]
- Jäggi, T., Jellestad, L., Corbisiero, S., Schaefer, D. J., Jenewein, J., Schneeberger, A., … Garcia Nuñez, D. (2018). Gender minority stress and depressive symptoms in transitioned Swiss transpersons. BioMed Research International, 2018, 1–10. doi: 10.1155/2018/8639263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinkerson, J. D. (2016). Defining and assessing moral injury: A syndrome perspective. Traumatology, 22(2), 122–130. doi: 10.1037/trm0000069 [DOI] [Google Scholar]
- Jordan-Young, R., & Rumiati, R. I. (2012). Hardwired for sexism? Approaches to sex/gender in neuroscience. Neuroethics, 5(3), 305–315. doi: 10.1007/s12152-011-9134-4 [DOI] [Google Scholar]
- Kashdan, T. B., & Rottenberg, J. (2010). Psychological flexibility as a fundamental aspect of health. Clinical Psychology Review, 30(4), 865–878. doi: 10.1016/j.cpr.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen, L. H., Moltz, H., Metz, J., & Cooper, M. (2004). Differential brain activation in exclusively homosexual and heterosexual men produced by the selective serotonin reuptake inhibitor, fluoxetine. Brain Research, 1024(1–2), 251–254. doi: 10.1016/j.brainres.2004.07.070 [DOI] [PubMed] [Google Scholar]
- Kinsey, A. C., Pomeroy, W. R., & Martin, C. E. (1948). Sexual behavior in the human male. Philadelphia: Saunders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, S. B. J., van Zuiden, M., Nawijn, L., Frijling, J. L., Veltman, D. J., & Olff, M. (2016). Aberrant resting-state brain activity in posttraumatic stress disorder: A meta-analysis and systematic review. Depression and Anxiety, 14(January), n/a–n/a. doi: 10.1002/da.22478 [DOI] [PubMed] [Google Scholar]
- Koechlin, E., & Summerfield, C. (2007). An information theoretical approach to prefrontal executive function. Trends in Cognitive Sciences, 11(6). doi: 10.1016/j.tics.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Krendl, A. C., Macrae, C. N., Kelley, W. M., Fugelsang, J. A., & Heatherton, T. F. (2006). The good, the bad, and the ugly: An fMRI investigation of the functional anatomic correlates of stigma. Social Neuroscience, 1(1), 5–15. doi: 10.1080/17470910600670579 [DOI] [PubMed] [Google Scholar]
- Kwon, P. (2013). Resilience in lesbian, gay, and bisexual individuals. Personality and Social Psychology Review, 17(4), 371–383. doi: 10.1177/1088868313490248 [DOI] [PubMed] [Google Scholar]
- Lanius, R. A., Frewen, P. A., Tursich, M., Jetly, R., & Mckinnon, M. C. (2015). Restoring large-scale brain networks in PTSD and related disorders: A proposal for neuroscientifically-informed treatment interventions. European Journal of Psychotraumatology, 1, 1–12. doi: 10.3402/ejpt.v6.27313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. A., Terpou, R. A., & Mckinnon, M. C. (2020). The sense of self in the aftermath of trauma: Lessons from the default mode network in posttraumatic stress disorder network in posttraumatic stress disorder. European Journal of Psychotraumatology, 11(1), 1807703. doi: 10.1080/20008198.2020.1807703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. a., Vermetten, E., Loewenstein, R. J., et al. (2010). Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. American Journal of Psychiatry, 167, 640–647. doi: 10.1176/appi.ajp.2009.09081168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux, J. E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23(1), 155–184. doi: 10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- Litz, B. T., Stein, N., Delaney, E., Lebowitz, L., Nash, W. P., Silva, C., & Maguen, S. (2009). Moral injury and moral repair in war veterans: A preliminary model and intervention strategy. Clinical Psychology Review, 29(8), 695–706. doi: 10.1016/j.cpr.2009.07.003 [DOI] [PubMed] [Google Scholar]
- Lloyd, C. S., Nicholson, A. A., Densmore, M., Théberge, J., Neufeld, R. W. J., Jetly, R., … Lanius, R. A. (2020, November). Shame on the brain: Neural correlates of moral injury event recall in posttraumatic stress disorder. Depression and Anxiety. doi: 10.1002/da.23128 [DOI] [PubMed] [Google Scholar]
- Manzouri, A., & Savic, I. (2018a). Cerebral sex dimorphism and sexual orientation. Human Brain Mapping, 39(3), 1175–1186. doi: 10.1002/hbm.23908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzouri, A., & Savic, I. (2018b). Multimodal MRI suggests that male homosexuality may be linked to cerebral midline structures. PLoS ONE, 13(10), 1–19. doi: 10.1371/journal.pone.0203189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten, C. L., Telzer, E. H., & Eisenberger, N. I. (2011). An fMRI investigation of attributing negative social treatment to racial discrimination. Journal of Cognitive Neuroscience, 23(5), 1042–1051. doi: 10.1162/jocn.2010.21520 [DOI] [PubMed] [Google Scholar]
- McCurry, K. L., Frueh, B. C., Chiu, P. H., & King-Casas, B. (2020). Opponent effects of hyperarousal and re-experiencing on affective habituation in posttraumatic stress disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(2), 203–212. doi: 10.1016/j.bpsc.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, C., Alisic, E., & Jobson, L. (2020). Moral injury and mental health: A systematic review and meta-analysis. Traumatology. doi: 10.1037/trm0000287 [DOI] [Google Scholar]
- Menon, V. (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. doi: 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Mereish, E. H., & Paul Poteat, V. (2015). A relational model of sexual minority mental and physical health: The negative effects of shame on relationships, loneliness, and health. Journal of Counseling Psychology, 62(3), 425–437. doi: 10.1037/cou0000088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, I. H. (2003). Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: Conceptual issues and research evidence. Psychological Bulletin, 129(5), 674–697. doi: 10.1037/0033-2909.129.5.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, I. H. (2015). Resilience in the study of minority stress and health of sexual and gender minorities. Psychology of Sexual Orientation and Gender Diversity, 2(3), 209–213. doi: 10.1037/sgd0000132 [DOI] [Google Scholar]
- Meyer, I. H. (2016). Does an improved social environment for sexual and gender minorities have implications for a new minority stress research agenda? Psychology of Sexualities Review, 7(1), 81. [PMC free article] [PubMed] [Google Scholar]
- Miller, G. E., Chen, E., & Zhou, E. S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133(1), 25–45. doi: 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G.; The PRISMA Group . (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr, J. J., & Fassinger, R. E. (2003). Self-acceptance and self-disclosure of sexual orientation in lesbian, gay, and bisexual adults: An attachment perspective. Journal of Counseling Psychology, 50(4), 482–495. doi: 10.1037/0022-0167.50.4.482 [DOI] [Google Scholar]
- Mueller, S. C., Wierckx, K., Boccadoro, S., & T’Sjoen, G. (2018). Neural correlates of ostracism in transgender persons living according to their gender identity: A potential risk marker for psychopathology? Psychological Medicine, 48(14), 2313–2320. doi: 10.1017/S0033291717003828 [DOI] [PubMed] [Google Scholar]
- Newcomb, M. E., & Mustanski, B. (2010). Internalized homophobia and internalizing mental health problems: A meta-analytic review. Clinical Psychology Review, 30(8), 1019–1029. doi: 10.1016/j.cpr.2010.07.003 [DOI] [PubMed] [Google Scholar]
- Nicholson, A., Densmore, M., Frewen, P. A., et al. (2015). The dissociative subtype of posttraumatic stress disorder: Unique resting-state functional connectivity of basolateral and centromedial Amygdala Complexes. Neuropsychopharmacology. Published online. doi: 10.10138/npp.2015.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, A. A., Densmore, M., McKinnon, M., Neufeld, R. W. J., Frewen, P., Theberge, J., … Lanius, R. A. (2018). Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: A multimodal neuroimaging approach. Psychological Medicine. doi: 10.1017/S0033291718002866 [DOI] [PubMed] [Google Scholar]
- Nicholson, A. A., Harricharan, S., Densmore, M., Neufeld, R. W. J., Ros, T., McKinnon, M. C., … Lanius, R. A. (2020). Classifying heterogeneous presentations of PTSD via the default mode, central executive, and salience networks with machine learning. NeuroImage: Clinical, 27(April), 102262. doi: 10.1016/j.nicl.2020.102262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, A. A., Sapru, I., Densmore, M., et al., (2016). Unique insula subregion resting-state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Research: Neuroimaging, 250, 61–72. doi: 10.1016/j.pscychresns.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N., Ludlow, D. H., Knierim, K., Hanelin, J., Ramachandran, T., Glover, G. C., & Mackey, S. C. (2006). Neural correlates of individual differences in pain-related fear and anxiety. Pain, 120(1–2), 69–77. doi: 10.1016/j.pain.2005.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øktedalen, T., Hoffart, A., & Langkaas, T. F. (2015). Trauma-related shame and guilt as time-varying predictors of posttraumatic stress disorder symptoms during imagery exposure and imagery rescripting—A randomized controlled trial. Psychotherapy Research, 25(5), 518–532. doi: 10.1080/10503307.2014.917217 [DOI] [PubMed] [Google Scholar]
- Pachankis, J. E. (2007). The psychological implications of concealing a stigma: A cognitive-affective-behavioral model. Psychological Bulletin, 133(2), 328–345. doi: 10.1037/0033-2909.133.2.328 [DOI] [PubMed] [Google Scholar]
- Pachankis, J. E. (2015). A transdiagnostic minority stress treatment approach for gay and bisexual men’s syndemic health conditions. Archives of Sexual Behavior, 44(7), 1843–1860. doi: 10.1007/s10508-015-0480-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachankis, J. E., Mahon, C. P., Jackson, S. D., Fetzner, B. K., & Bränström, R. (2020). Sexual orientation concealment and mental health: A conceptual and meta-analytic review. Psychological Bulletin, 146(10), 831–871. doi: 10.1037/bul0000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra, L. A., & Hastings, P. D. (2018). Integrating the neurobiology of minority stress with an intersectionality framework for LGBTQ-Latinx populations. New Directions for Child and Adolescent Development, (161), 91–108. doi: 10.1002/cad.20244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, R., Spreng, R. N., Shin, L. M., & Girard, T. A. (2012). Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 36(9), 2130–2142. doi: 10.1016/j.neubiorev.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Petrocchi, N., Pistella, J., Salvati, M., Carone, N., Laghi, F., & Baiocco, R. (2020). I embrace my LGB identity: Self-reassurance, social safeness, and the distinctive relevance of authenticity to well-being in Italian lesbians, gay men, and bisexual people. Sexuality Research and Social Policy, 17(1), 75–86. doi: 10.1007/s13178-018-0373-6 [DOI] [Google Scholar]
- Pezawas, L., Meyer-Lindenberg, A., Drabant, E. M., Verchinski, B. A., Munoz, K. E., Kolachana, B. S., … Weinberger, D. R. (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience, 8(6), 828–834. doi: 10.1038/nn1463 [DOI] [PubMed] [Google Scholar]
- Pitoňák, M. (2017). Mental health in non-heterosexuals: Minority stress theory and related explanation frameworks review. Mental Health and Prevention, 5(October 2016), 63–73. doi: 10.1016/j.mhp.2016.10.002 [DOI] [Google Scholar]
- Plöderl, M., & Tremblay, P. (2015). Mental health of sexual minorities. A systematic review. International Review of Psychiatry, 27(5), 367–385. doi: 10.3109/09540261.2015.1083949 [DOI] [PubMed] [Google Scholar]
- Ponseti, J., Siebner, H. R., Klöppel, S., Wolff, S., Granert, O., Jansen, O., … Bosinski, H. A. (2007). Homosexual women have less grey matter in perirhinal cortex than heterosexual women. PLoS ONE, 2(8), 1–6. doi: 10.1371/journal.pone.0000762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast, S., & MacPhee, D. (2018). Family resilience amid stigma and discrimination: A conceptual model for families headed by same-sex parents. Family Relations, 67(1), 26–40. doi: 10.1111/fare.12296 [DOI] [Google Scholar]
- Purdie-Vaughns, V., & Eibach, R. P. (2008). Intersectional invisibility: The distinctive advantages and disadvantages of multiple subordinate-group identities. Sex Roles, 59(5–6), 377–391. doi: 10.1007/s11199-008-9424-4 [DOI] [Google Scholar]
- Qin, P., & Northoff, G. (2011). NeuroImage How is our self related to midline regions and the default-mode network? NeuroImage, 57(3), 1221–1233. doi: 10.1016/j.neuroimage.2011.05.028 [DOI] [PubMed] [Google Scholar]
- Quinan, C. L. (2016). Kinsey Scale. In The Wiley Blackwell encyclopedia of gender and sexuality studies (Vol. 2, Issue 2, pp. 1–3). Hoboken, NJ: John Wiley & Sons, Ltd. doi: 10.1002/9781118663219.wbegss555 [DOI] [Google Scholar]
- Rabellino, D., Densmore, M., Théberge, J., McKinnon, M. C., & Lanius, R. A. (2018). The cerebellum after trauma: Resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Human Brain Mapping, 39(8), 3354–3374. doi: 10.1002/hbm.24081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall, A. K., Tao, C., Totenhagen, C. J., Walsh, K. J., & Cooper, A. N. (2017). Associations between sexual orientation discrimination and depression among same-sex couples: Moderating effects of dyadic coping. Journal of Couple and Relationship Therapy, 16(4), 325–345. doi: 10.1080/15332691.2016.1253520 [DOI] [Google Scholar]
- Randall, A. K., Totenhagen, C. J., Walsh, K. J., Adams, C., & Tao, C. (2017). Coping with workplace minority stress: Associations between dyadic coping and anxiety among women in same-sex relationships. Journal of Lesbian Studies, 21(1), 70–87. doi: 10.1080/10894160.2016.1142353 [DOI] [PubMed] [Google Scholar]
- Renner, F., Siep, N., Lobbestael, J., Arntz, A., Peeters, F. P. M. L., & Huibers, M. J. H. (2015). Neural correlates of self-referential processing and implicit self-associations in chronic depression. Journal of Affective Disorders, 186, 40–47. doi: 10.1016/j.jad.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Riggle, E. D. B., Rostosky, S. S., Mohr, J. J., Fingerhut, A. W., & Balsam, K. F. (2014). A multifactor lesbian, gay, and bisexual positive identity measure (LGB-PIM). Psychology of Sexual Orientation and Gender Diversity, 1(4), 398–411. doi: 10.1037/sgd0000057 [DOI] [Google Scholar]
- Riggle, E. D. B., Whitman, J. S., Olson, A., Rostosky, S. S., & Strong, S. (2008). The positive aspects of being a lesbian or gay man. Professional Psychology: Research and Practice, 39(2), 210–217. doi: 10.1037/0735-7028.39.2.210 [DOI] [Google Scholar]
- Rive, M. M., van Rooijen, G., Veltman, D. J., Mary, M. L., Schene, A. H., & Ruhé, H. G. (2013). Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience and Biobehavioral Reviews, 37(10), 2529–2553. doi: 10.1016/j.neubiorev.2013.07.018 [DOI] [PubMed] [Google Scholar]
- Roberts, A. L., Rosario, M., Corliss, H. L., Koenen, K. C., & Austin, S. B. (2012). Elevated risk of posttraumatic stress in sexual minority Youths: Mediation by childhood abuse and gender nonconformity. American Journal of Public Health, 102(8), 1587–1593. doi: 10.2105/AJPH.2011.300530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins, C. S., Sauvageot, J. A., Cusack, K. J., Suffoletta-Maierle, S., & Frueh, B. C. (2005). Consumers’ perceptions of negative experiences and “sanctuary harm” in psychiatric settings. Psychiatric Services, 56(9), 1134–1138. doi: 10.1176/appi.ps.56.9.1134 [DOI] [PubMed] [Google Scholar]
- Robinson, J. L., & Rubin, L. J. (2016). Homonegative microaggressions and posttraumatic stress symptoms. Journal of Gay & Lesbian Mental Health, 20(1), 57–69. doi: 10.1080/19359705.2015.1066729 [DOI] [Google Scholar]
- Root, M. P. (1992). Reconstructing the impact of trauma on personality development: A feminist perspective. In L. S. Brown, &, and M. Ballou(Eds.), Personality and psychopathology: Feminist reappraisals (pp. 229–266). New York, NY: Guilford Press. [Google Scholar]
- Ross, L. E., Salway, T., Tarasoff, L. A., MacKay, J. M., Hawkins, B. W., & Fehr, C. P. (2018). Prevalence of depression and anxiety among bisexual people compared to gay, lesbian, and heterosexual individuals: A systematic review and meta-analysis. Journal of Sex Research, 55(4–5), 435–456. doi: 10.1080/00224499.2017.1387755 [DOI] [PubMed] [Google Scholar]
- Rostosky, S. S., Cardom, R. D., Hammer, J. H., & Riggle, E. D. B. (2018). LGB positive identity and psychological well-being. Psychology of Sexual Orientation and Gender Diversity, 5(4), 482–489. doi: 10.1037/sgd0000298 [DOI] [Google Scholar]
- Rostosky, S. S., Riggle, E. D. B., Pascale-Hague, D., & McCants, L. E. (2010). The positive aspects of a bisexual self-identification. Psychology and Sexuality, 1(2), 131–144. doi: 10.1080/19419899.2010.484595 [DOI] [Google Scholar]
- Rothman, E. F., & Baughman, A. (2011). The prevalence of sexual assault against people who identify as Gay, Lesbian or Bisexual in the United States: A systematic review. Trauma Violence and Abuse, 12(2), 55–66. doi: 10.1177/1524838010390707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, S., & Fish, J. (2016). Mental health in lesbian, gay, bisexual, and transgender (LGBT) youth Stephen. Annual Review of Clinical Psychology, 12(1), 465–487. doi: 10.1146/annurev-clinpsy-021815-093153.Mental [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salway, T., Ross, L. E., Fehr, C. P., Burley, J., Asadi, S., Hawkins, B., & Tarasoff, L. A. (2019). A systematic review and meta-analysis of disparities in the prevalence of suicide ideation and attempt among bisexual populations. Archives of Sexual Behavior, 48(1), 89–111. doi: 10.1007/s10508-018-1150-6 [DOI] [PubMed] [Google Scholar]
- Saraiya, T., & Lopez-Castro, T. (2016). Ashamed and afraid: A scoping review of the role of shame in post-traumatic stress disorder (PTSD). Journal of Clinical Medicine, 5(11), 94. doi: 10.3390/jcm5110094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarno, E. L., Newcomb, M. E., Feinstein, B. A., & Mustanski, B. (2020). Bisexual men’s experiences with discrimination, internalized binegativity, and identity affirmation: Differences by partner gender. Archives of Sexual Behavior, 49(5), 1783–1798. doi: 10.1007/s10508-020-01712-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler, F. A., Zeyen, J., & Christiansen, H. (2017). Does sexual identity stress mediate the association between sexual identity and mental health? Psychology of Sexual Orientation and Gender Diversity, 4(3), 296–303. doi: 10.1037/sgd0000232 [DOI] [Google Scholar]
- Savic, I., & Lindström, P. (2008). PET and MRI show differences in cerebral asymmetry and functional connectivity between homo- and heterosexual subjects. Proceedings of the National Academy of Sciences of the United States of America, 105(27), 9403–9408. doi: 10.1073/pnas.0801566105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin-Williams, R. C. (2016). Sexual orientation: Categories or continuum? Commentary on Bailey et al. (2016). Psychological Science in the Public Interest, 17(2), 37–44. doi: 10.1177/1529100616637618 [DOI] [PubMed] [Google Scholar]
- Schrager, S. M., Steiner, R. J., Bouris, A. M., MacApagal, K., & Brown, C. H. (2019). Methodological considerations for advancing research on the health and wellbeing of sexual and gender minority youth. LGBT Health, 6(4), 156–165. doi: 10.1089/lgbt.2018.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, J., Ordaz, S. J., Ho, T. C., & Gotlib, I. H. (2019). Longitudinal decreases in suicidal ideation are associated with increases in salience network coherence in depressed adolescents. Journal of Affective Disorders, 245(September2018), 545–552. doi: 10.1016/j.jad.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(9), 2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell, R. L. (1996). The Sell Assessment of Sexual Orientation: Background and scoring. Journal of Gay, Lesbian and Bisexual Identity, 1, 295–310. https://link.springer.com/article/10.1007/BF03372244 [Google Scholar]
- Sell, R. L. (1997). Defining and measuring sexual orientation: A review. Archives of Sexual Behavior, 26(6), 643–658. doi: 10.1023/a:1024528427013 [DOI] [PubMed] [Google Scholar]
- Sherry, A. (2007). Internalized homophobia and adult attachment: Implications for clinical practice. Psychotherapy, 44(2), 219–225. doi: 10.1037/0033-3204.44.2.219 [DOI] [PubMed] [Google Scholar]
- Silver, S. M. (1985). Post-traumatic stress and the death imprint: The search for a new mythos. In Kelly W. E. (Ed.), Post-traumatic stress disorder and the war veteran patient. New York, NY: Brunner/Mazel. [Google Scholar]
- Silver, S. M. (1986). An inpatient program for post-traumatic stress disorder: Context as treatment. In Figley C. R. (Ed.), Trauma and its wake (Vol. 2; pp. 213–231). New York, NY: Brunner/ Mazel. [Google Scholar]
- Singh, A. A. (2017). Understanding trauma and supporting resilience with LGBT people of color. In K. Eckstrand & J. Potter (Eds.), Trauma, resilience, and health promotion in LGBT patients Cham: Springer. doi: 10.1007/978-3-319-54509-7_10 [DOI] [Google Scholar]
- Sloan, C. A., Berke, D. S., & Shipherd, J. C. (2017). Utilizing a dialectical framework to inform conceptualization and treatment of clinical distress in transgender individuals. Professional Psychology, Research and Practice, 48(5), 301–309. doi: 10.1037/pro0000146 [DOI] [Google Scholar]
- Southwick, S. M., & Charney, D. S. (2012). The science of resilience: Implications for the prevention and treatment of depression. Science, 338(6103), 79–82. doi: 10.1126/science.1222942 [DOI] [PubMed] [Google Scholar]
- Spizzirri, G., Duran, F. L. S., Chaim-Avancini, T. M., Serpa, M. H., Cavallet, M., Pereira, C. M. A., … Abdo, C. H. N. (2018). Grey and white matter volumes either in treatment-naïve or hormone-treated transgender women: A voxel-based morphometry study. Scientific Reports, 8(1), 1–10. doi: 10.1038/s41598-017-17563-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N., Mar, R. A., & Kim, A. S. N. (2008). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis (pp. 489–510). [DOI] [PubMed]
- Sripada, R. K., King, a. P., Welsh, R. C., et al. (2012). Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic Medicine, 911(35). doi: 10.1097/PSY.0b013e318273bf33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub, K. T., Mcconnell, A. A., & Messman-Moore, T. L. (2018). Internalized heterosexism and posttraumatic stress disorder symptoms: The mediating role of shame proneness among trauma-exposed sexual minority women. Psychology of Sexual Orientation and Gender Diversity, 5(1), 99–108. doi: 10.1037/sgd0000263 [DOI] [Google Scholar]
- Su, D., Irwin, J. A., Fisher, C., Ramos, A., Kelley, M., Mendoza, D. A. R., & Coleman, J. D. (2016). Mental health disparities within the LGBT population: A comparison between transgender and nontransgender individuals. Transgender Health, 1(1), 12–20. doi: 10.1089/trgh.2015.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, D., Phillips, R. D., Mulready, H. L., Zablonski, S. T., Turner, J. A., Turner, M. D., … Morey, R. A. (2019). Resting-state brain fluctuation and functional connectivity dissociate moral injury from posttraumatic stress disorder. Depression and Anxiety, 36(5), 442–452. doi: 10.1002/da.22883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski, D. M., & Balsam, K. F. (2011). Insidious trauma: Examining the relationship between heterosexism and Lesbians’ PTSD symptoms. Traumatology, 17(2), 4–13. doi: 10.1177/1534765609358464 [DOI] [Google Scholar]
- Tan, K. K. H., Treharne, G. J., Ellis, S. J., Schmidt, J. M., & Veale, J. F. (2019). Gender minority stress: A critical review. Journal of Homosexuality, 67(10), 1471–1489. doi: 10.1080/00918369.2019.1591789 [DOI] [PubMed] [Google Scholar]
- Terpou, B. A., Densmore, M., Théberge, J., Frewen, P., McKinnon, M. C., Nicholson, A. A., & Lanius, R. A. (2020). The hijacked self: Disrupted functional connectivity between the periaqueductal gray and the default mode network in posttraumatic stress disorder using dynamic causal modeling. NeuroImage: Clinical, 27, 102345. doi: 10.1016/j.nicl.2020.102345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa, R. J., Habarth, J., Peta, J., Balsam, K., & Bockting, W. (2015). Development of the gender minority stress and resilience measure. Psychology of Sexual Orientation and Gender Diversity, 2(1), 65–77. doi: 10.1037/sgd0000081 [DOI] [Google Scholar]
- Torres, L. (2010). Predicting levels of Latino depression: Acculturation, acculturative stress, and coping. Cultural Diversity & Ethnic Minority Psychology, 16(2), 256–263. doi: 10.1037/a0017357 [DOI] [PubMed] [Google Scholar]
- Uribe, C., Junque, C., Gómez-Gil, E., Abos, A., Mueller, S. C., & Guillamon, A. (2020). Brain network interactions in transgender individuals with gender incongruence. NeuroImage, 211(February), 116613. doi: 10.1016/j.neuroimage.2020.116613 [DOI] [PubMed] [Google Scholar]
- van Anders, S. M. (2015). Beyond sexual orientation: Integrating gender/sex and diverse sexualities via sexual configurations theory. Archives of Sexual Behavior, 44(5), 1177–1213. doi: 10.1007/s10508-015-0490-8 [DOI] [PubMed] [Google Scholar]
- Vanable, P. A., Carey, M. P., Blair, D. C., & Littlewood, R. A. (2006). Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS and Behavior, 10(5), 473–482. doi: 10.1007/s10461-006-9099-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, M. D., Parent, M. C., Tilghman, J. D., Miles, J., Lee, H. S., & Prokhorets, S. (2014). A content analysis of LGBT-themed positive psychology articles. Psychology of Sexual Orientation and Gender Diversity, 1(4), 313–324. doi: 10.1037/sgd0000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., Hu, J. B., Ji, G. J., Xu, D. R., Wang, D. D., Xi, C. X., … Hu, S. H. (2020). Executive function and its relation to anatomical connectome in homosexual and heterosexual men. Quantitative Imaging in Medicine and Surgery, 10(10), 1973–1983. doi: 10.21037/QIMS-19-821B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzyniak, A. J., & Sabbag, S. (2018). PTSD in the lesbian, gay, bisexual, and transgender (LGBT) population. Post-Traumatic Stress Disorder, 229–241. doi: 10.1093/med/9780190259440.001.0001 [DOI] [Google Scholar]
- Weinstein, T. L., & Li, X. (2016). The relationship between stress and clinical outcomes for persons living with HIV/AIDS: A systematic review of the global literature. AIDS Care - Psychological and Socio-Medical Aspects of AIDS/HIV, 28(2), 160–169. doi: 10.1080/09540121.2015.1090532 [DOI] [PubMed] [Google Scholar]
- Williamson, V., Stevelink, S. A. M., & Greenberg, N. (2018). Occupational moral injury and mental health: Systematic review and meta-analysis. British Journal of Psychiatry, 212(6), 339–346. doi: 10.1192/bjp.2018.55 [DOI] [PubMed] [Google Scholar]
- *Witelson, S. F., Kigar, D. L., Scamvougeras, A., Kideckel, D. M., Buck, B., Stanchev, P. L., … Black, S. (2008). Corpus callosum anatomy in right-handed homosexual and heterosexual men. Archives of Sexual Behavior, 37(6), 857–863. doi: 10.1007/s10508-007-9276-y [DOI] [PubMed] [Google Scholar]
- Wu, G., Feder, A., Cohen, H., Kim, J. J., Calderon, S., Charney, D. S., & Mathé, A. A. (2013). Understanding resilience. Frontiers in Behavioral Neuroscience, 7(6), 446–457. doi: 10.3389/fnbeh.2013.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda, R., Hoge, C. W., McFarlane, A. C., Vermetten, E., Lanius, R. A., Nievergelt, C. M., … Hyman, S. E. (2015). Post-traumatic stress disorder. Nature Reviews Disease Primers, 1(1), 15057. doi: 10.1038/nrdp.2015.57 [DOI] [PubMed] [Google Scholar]
- Young, K. D., Ph, D., and Siegle, G. J., et al. (2017, August). Randomized clinical trial of real-time fMRI amygdala neurofeedback for major depressive disorder: Effects on symptoms and autobiographical memory recall. doi: 10.1176/appi.ajp.2017.16060637 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this review are available within the supplementary material systematic coding file.


