Abstract
Major developments in research into the azole class of antifungal agents during the 1990s have provided expanded options for the treatment of many opportunistic and endemic fungal infections. Fluconazole and itraconazole have proved to be safer than both amphotericin B and ketoconazole. Despite these advances, serious fungal infections remain difficult to treat, and resistance to the available drugs is emerging. This review describes present and future uses of the currently available azole antifungal agents in the treatment of systemic and superficial fungal infections and provides a brief overview of the current status of in vitro susceptibility testing and the growing problem of clinical resistance to the azoles. Use of the currently available azoles in combination with other antifungal agents with different mechanisms of action is likely to provide enhanced efficacy. Detailed information on some of the second-generation triazoles being developed to provide extended coverage of opportunistic, endemic, and emerging fungal pathogens, as well as those in which resistance to older agents is becoming problematic, is provided.
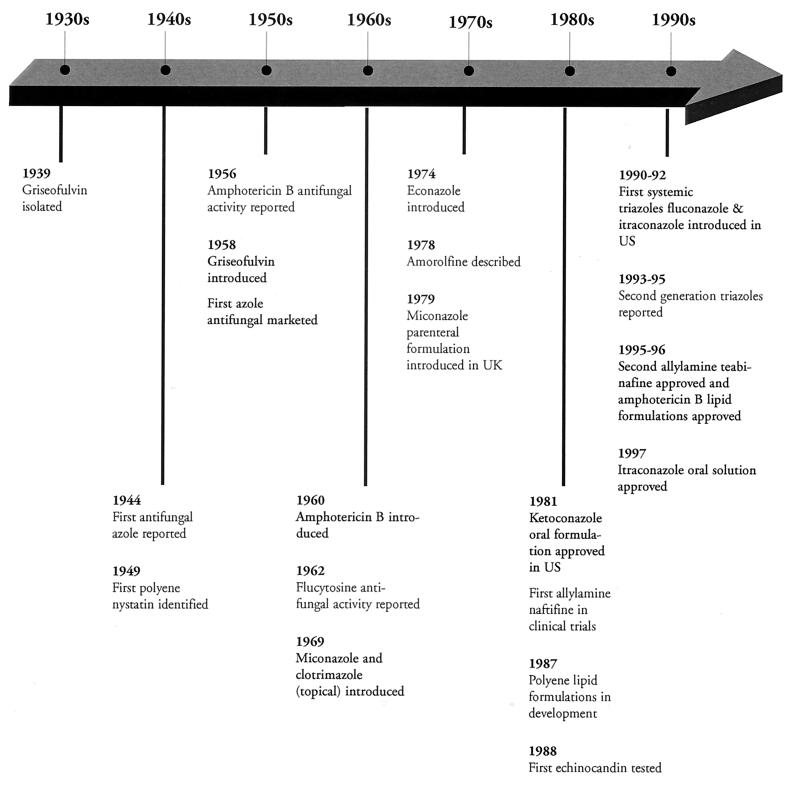
Although the first agent with antifungal activity, griseofulvin, was isolated in 1939 (28) and the first azole and polyene antifungal agents were reported in 1944 and 1949, respectively (85), it was not until 1958 that oral griseofulvin and topical chlormidazole became available for clinical use (Fig. 1) (13, 21, 28, 41, 85, 94, 103, 109, 132, 133, 151, 250, 274). The introduction of griseofulvin was followed in 1960 by that of amphotericin B (109), which is still the “gold standard” for the treatment of severe systemic mycoses (94). Two topical azole antifungal agents, miconazole and clotrimazole, were introduced in 1969; this was followed by the introduction of econazole in 1974 (109) and a parenteral formulation of miconazole in the late 1970s (21, 103). Today, these three agents remain the mainstay of topical therapy for many dermatophytoses.
FIG. 1.
Key events in antifungal drug development.
Progress in the development of both topical and systemic antifungal agents lagged, due in part to the intensive research efforts in the area of antibacterial therapy which began in the 1940s following the large-scale production of penicillin and also to the relatively low incidence of serious fungal infections compared with that of bacterial infections. By 1980, members of the four major classes of antifungal agents—polyenes, azoles, morpholines, and allylamines—had been identified, yet the only new drug introduced for the treatment of systemic fungal infections was oral ketoconazole (132). It would be more than 10 years before either fluconazole or itraconazole became available for the treatment of systemic mycoses (13).
During the 1980s and 1990s, the marked increase in the population of immunocompromised or severely ill individuals as the result of the spread of human immunodeficiency virus (HIV) infection, the increased use of immunosuppressive agents in association with organ transplants, chemotherapy, and improved life-saving medical techniques necessitating indwelling catheters led to a substantial increase in the occurrence of serious fungal infections (94, 95). Consequently, although it takes 10 to 12 years to bring a new drug to market and the cost of doing so has risen steadily (to $359 million in 1994) (20), the growing need for antifungal agents, particularly those for the treatment of systemic mycoses, has made this a worthwhile area of pharmaceutical research.
Advances made during the 1990s led to the introduction of a new allylamine, terbinafine, for the treatment of dermatophytoses and new lipid formulations of amphotericin B with improved safety profiles (95, 132). In addition, new classes of antifungal agents such as the candins (pneumocandins and echinocanidins), the nikkomycins, and the pradamicins-benanomicins are being studied (88, 95, 132). However, with 15 different marketed drugs worldwide (85, 132), the azoles are currently the most widely used and studied class of antifungal agents. This article focuses on the clinically important azole antifungal agents currently marketed in the United States and some promising new agents under development.
MECHANISM OF ACTION
For a detailed discussion of the mechanism of action of the azoles and other antifungal agents, the reader is referred to the recent review articles by Georgopapadakou and Walsh (94) and White et al. (269). A brief overview is provided here.
Azole antifungal agents prevent the synthesis of ergosterol, a major component of fungal plasma membranes, by inhibiting the cytochrome P-450-dependent enzyme lanosterol demethylase (also referred to as 14α-sterol demethylase or P-450DM) (94, 95, 136, 269). This enzyme also plays an important role in cholesterol synthesis in mammals (136). When azoles are present in therapeutic concentrations, their antifungal efficacy is attributed to their greater affinity for fungal P-450DM than for the mammalian enzyme (136). Exposure of fungi to an azole causes depletion of ergosterol and accumulation of 14α-methylated sterols (94, 95, 136). This interferes with the “bulk” functions of ergosterol in fungal membranes and disrupts both the structure of the membrane and several of its functions such as nutrient transport and chitin synthesis (94). The net effect is to inhibit fungal growth (136). Ergosterol also has a hormone-like (“sparking”) function in fungal cells, which stimulates growth and proliferation (94, 269). This function may be disrupted when ergosterol depletion is virtually complete (>99%) (94).
CLINICALLY IMPORTANT AZOLE ANTIFUNGAL AGENTS
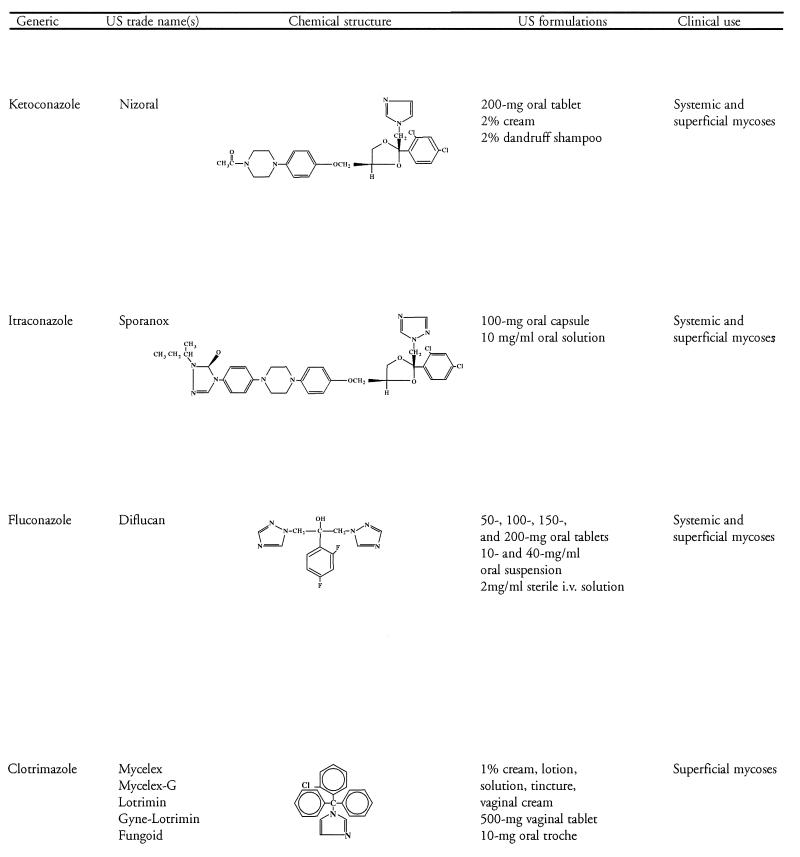
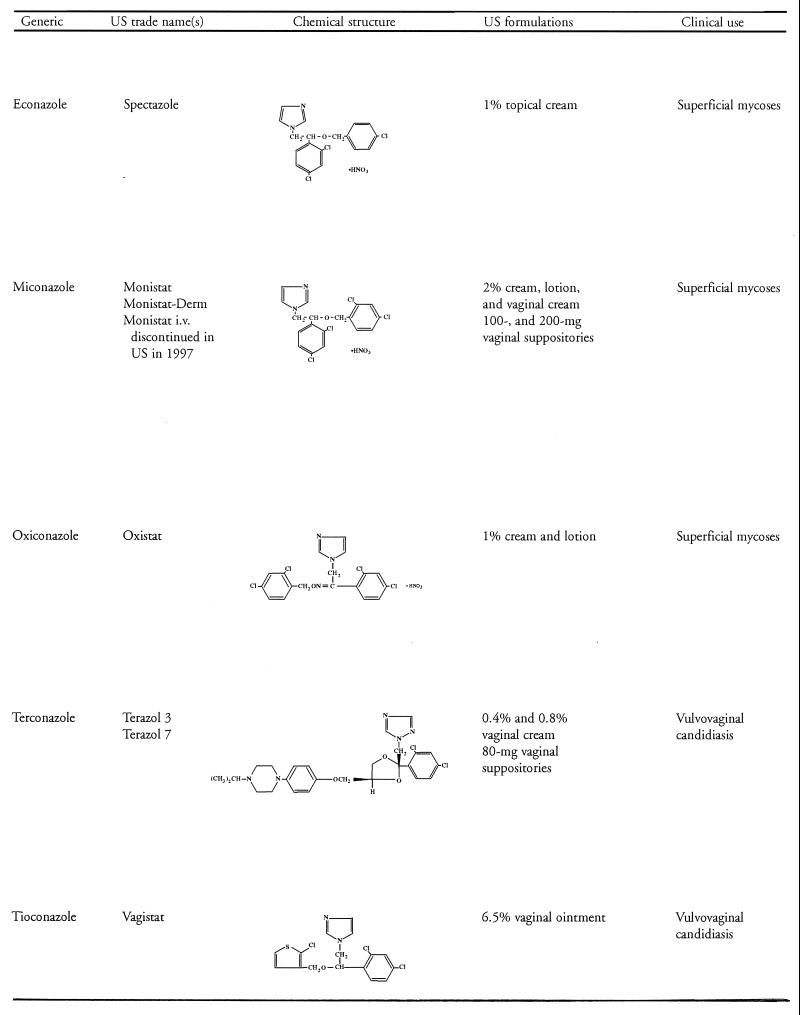
The azole antifungal agents in clinical use contain either two or three nitrogens in the azole ring and are thereby classified as imidazoles (e.g., ketoconazole and miconazole, clotrimazole) or triazoles (e.g., itraconazole and fluconazole), respectively. With the exception of ketoconazole, use of the imidazoles is limited to the treatment of superficial mycoses, whereas the triazoles have a broad range of applications in the treatment of both superficial and systemic fungal infections. Another advantage of the triazoles is their greater affinity for fungal rather than mammalian cytochrome P-450 enzymes, which contributes to an improved safety profile. Table 1 shows the generic and U.S. trade names, chemical structures, U.S. formulations, and general clinical uses of the clinically important azole antifungal agents currently marketed in the United States. Until the recent approval of itraconazole oral solution, only the itraconazole capsule formulation was available. Throughout this review, where studies pertain to the oral solution, it has been identified as such. Otherwise, “itraconazole” is used to refer to studies with the capsule formulation.
TABLE 1.
Azole antifungal agents
SYSTEMIC MYCOSES
Although the polyene amphotericin B remains the agent of choice for the treatment of most life-threatening systemic mycoses (18, 92, 246), ketoconazole, fluconazole, and itraconazole have a variety of uses in the treatment of disseminated infections due to opportunistic and endemic fungal pathogens. Ketoconazole and itraconazole (Table 1) share a number of pharmacokinetic characteristics. Both are available in oral (p.o.) formulations, whose absorption is affected by gastric acidity and food. By contrast, fluconazole is available in both intravenous (i.v.) and p.o. formulations, and absorption is neither dependent on gastric acidity nor affected by food (47). Because of its relatively low lipophilicity and low degree of protein binding (∼12%), fluconazole distributes readily into aqueous body fluids such as the cerebrospinal fluid (CSF) (99). On the other hand, although penetration of ketoconazole and itraconazole into aqueous fluids is negligible due to their high lipophilicity and high degree of protein binding (>99%), these azoles achieve concentrations in fatty tissues and exudates such as pus that are severalfold greater than the concomitant concentrations in serum (54, 101, 259). The therapeutic uses of these three azoles, based primarily on the published recommendations of experts in the field rather than the indications approved by the Food and Drug Administration (FDA), are listed in Table 2 and will be expanded upon in subsequent sections.
TABLE 2.
Azole antifungal agents for systemic mycoses
| Clinical use | Ketoconazole | Itraconazole | Fluconazole |
|---|---|---|---|
| Aspergillosis | √a | ||
| Candidiemia | √bc | √ | |
| Cryptococcosis | √d | √ | |
| Blastomycosis | √ade | √a | √d |
| Histoplasmosis | √ade | √a | √b |
| Coccidioidomycosis | √a | √ | √ |
| Paracoccidioidomycosis | √d | √ | √b |
| Sporotrichosis | √ | √b | |
| Pseudallescheriasis | √d | √ |
Not for meningeal infection.
Promising, but clinical studies needed.
Lack of i.v. formulation at present limits use in seriously ill patients.
Second-line azole.
Nonimmunosuppressed hosts only.
Opportunistic Fungal Infections
Aspergillosis, candidemia, and cryptococcosis have become increasingly prevalent as the population of immunocompromised or seriously ill individuals has increased (94, 95). None of these opportunistic fungal infections are responsive to p.o. ketoconazole (15).
Aspergillosis.
Amphotericin B remains the agent of choice for the initial therapy of invasive aspergillosis (16, 54, 110, 161, 259), although a 1990 review of the literature by Denning and Stevens showed that the overall response rate was only 55% (54). Moreover, the therapeutic response to amphotericin B in immunocompromised patients is generally poor (16). Itraconazole is the only currently marketed azole that appears to be a useful alternative (53, 144), and it has been approved by the FDA as a second-line agent for the treatment of pulmonary or extrapulmonary aspergillosis in patients who are refractory to or intolerant of amphotericin B (132). Clinically, itraconazole is often used as consolidation therapy in seriously ill patients who have responded to initial therapy with amphotericin B and as initial therapy in those with less severe infections (132).
When used for primary therapy, the response rate with itraconazole is slightly better than that with amphotericin B, and the most favorable outcomes are seen in patients with pulmonary infections and in solid-organ transplant recipients; aspergillosis patients with HIV infection are the least responsive to itraconazole (53, 103, 131). A recent analysis of the compassionate-use data on itraconazole therapy of invasive aspergillosis according to the National Institute of Allergy and Infectious Diseases (NIAID)-Mycoses Study Group (MSG) criteria provides further support for its use as initial therapy (245). Most of the 125 patients suitable for analysis, all but 8 of whom had underlying diseases, were treated with 200 to 400 mg of itraconazole per day; only 16 patients received less than 200 mg/day, and only 15 received more than 400 mg/day. The mean duration of itraconazole therapy was 209 days (median, 121 days; range, 3 to 1,657 days); only 15 patients received treatment for less than 2 weeks. A complete response was achieved in 34 patients (27%), 45 (36%) improved, 20 (15%) were unchanged, and 26 (21%) worsened. However, if the response rates were based on only patients who received at least 2 weeks of treatment, the corresponding percentages are 30, 39, 16, and 14% and are better than the 39% rate of complete or partial response achieved in 30 of 76 patients treated in an earlier study by the NIAID-MSG (53). Moreover, in the present analysis, a complete response or improvement was observed in 72% of 11 patients who received bone marrow transplants (55). Patients treated for less than 2 weeks and those with widely disseminated disease or infection of the sinuses or central nervous system (CNS) had a less favorable therapeutic outcome than the remaining patients. Interestingly, the outcomes of patients who had received prior therapy with amphotericin B alone or in combination with other agents such as flucytosine (5-FC) (112 patients) and those who had not (13 patients) were not significantly different, regardless of whether aspergillosis was pulmonary or extrapulmonary. These data provide further support for the effectiveness of p.o. itraconazole for the treatment of a substantial number of patients with invasive aspergillosis. The lack of an i.v. dosage form and the presence of drug interactions are major disadvantages of the use of itraconazole in treating invasive aspergillosis, particularly in patients who have received bone marrow transplants (53, 103, 131).
Clinical data indicate that initial administration of itraconazole antagonizes the antifungal activity of subsequent amphotericin B against Aspergillus fumigatus (153, 232). Thus, amphotericin B-azole combinations are not used clinically to treat patients with invasive aspergillosis. For a detailed review of in vitro data and clinical experience of amphotericin B-azole combinations, the reader is referred to the review by Sugar (246).
With regard to the prevention of invasive aspergillosis, a number of antifungal regimens have been suggested for neutropenic patients (16, 34, 147), but only itraconazole oral solution has been investigated in a large-scale, placebo-controlled clinical trial (51). The p.o. administration of nystatin, amphotericin B, ketoconazole or fluconazole is not effective (147). Because respiratory system colonization occurs before the development of invasive aspergillosis, nasal instillation or inhalation of amphotericin B has been suggested (16, 147). However, the results of controlled clinical trials with intranasal amphotericin B have differed and have not demonstrated efficacy in preventing invasive aspergillosis (16, 147). Although intranasal administration of amphotericin B is well tolerated (16), aerosol inhalation is associated with side effects such as cough, bad taste, and nausea, which led to discontinuation in 23% of treatment cycles in a study of neutropenic cancer patients (147).
Itraconazole is the only azole at present that may be considered for primary prophylaxis against aspergillosis (147). A recent randomized, double-blind trial compared itraconazole oral solution with placebo for the prophylaxis of fungal infections in 405 neutropenic patients with hematological malignancy (51). All patients also received p.o. nystatin (500,000 IU four times a day [q.i.d.]) and ciprofloxacin (500 mg twice a day [b.i.d.]). In an intent-to-treat analysis, proven and suspected deep fungal infections, defined as those requiring empirical therapy with i.v. amphotericin B, occurred in 48 (24%) of 201 patients treated with itraconazole oral solution and 68 (33%) of 204 patients treated with placebo (P = 0.035). Of the five proven deep fungal infections documented in patients receiving itraconazole, four were due to Aspergillus spp. and one was due to Candida albicans. Study medication was stopped in 33 members (16%) of the group receiving itraconazole and in 28 members (14%) of the group receiving placebo because of adverse events, details of which were not provided. In another study in patients with hematological malignancies, mortality was lower in those who received prophylaxis with itraconazole (200 mg/day) plus intranasal amphotericin B (10 mg/day) than in untreated historical controls (147). Further investigation of this regimen is warranted, particularly in view of the reported antagonism between itraconazole and amphotericin B. Until more clinical data are available, itraconazole or amphotericin B may be considered to prevent invasive aspergillosis in patients who have undergone bone marrow transplantation or who have hematologic malignancies and should be administered until the patient has recovered from neutropenia or graft-versus-host disease and/or cytomegalovirus infection and corticosteroid therapy has been discontinued (147). To prevent relapse during all future periods of immunosuppression in patients who have previously had invasive aspergillosis, administration of itraconazole or i.v. amphotericin B is recommended (147).
As noted by Walsh et al. (260), not all neutropenic patients benefit from antifungal prophylaxis. Variables such as the extent of neutropenia, the underlying cause of neutropenia and its status, and numerous host factors affect the risk of deep fungal infection. Neutropenic patients with relapsed neoplastic disease or hematologic malignancy and those who have received allogeneic bone marrow transplants are at increased risk of deep fungal infection. With regard to invasive aspergillosis, protracted granulocytopenia, use of corticosteroids, and prior infection with Aspergillus complicating neutropenia are known to increase the risk of infection (260). In addition to these host factors, environmental factors play an increasing role in the increased risk of invasive fungal seen at many centers specializing in cancer treatment. Thus, antifungal prophylaxis with amphotericin B or a triazole can be recommended routinely for all neutropenic patients. However, because mortality is high among AIDS patients who develop invasive aspergillosis, lifelong secondary prophylaxis with either amphotericin B or itraconazole is recommended following primary therapy with amphotericin B and adjunctive surgery (161).
Candidemia.
(i) Fluconazole.
The management of serious infections due to Candida spp., which are becoming increasingly prevalent, is problematic because of the increasing incidence of non-albicans species (1, 188) and the emergence of non-albicans isolates resistant to both amphotericin B and the newer azoles (172). Recently, a panel of 22 international experts in the management of Candida infections held a consensus conference to develop general recommendations for the prevention and treatment of these infections (74). The triazoles fluconazole and itraconazole were among the antifungal agents considered by the participants. Fluconazole, but not itraconazole, is approved by the FDA for the treatment of systemic Candida infections. However, itraconazole has been approved elsewhere for use in deep (nonmucosal) Candida infections and was therefore included among the available therapeutic options (74). Infections considered by the experts included candidemia, candiduria, hepatosplenic candidiasis (chronic disseminated candidiasis), candidal endophthalmitis, and candidal peritonitis. Management strategies were based not only on the site of Candida infection but also on other factors such as neutropenia, susceptibility of the Candida isolate, general condition of the patient, and whether the patient had undergone bone marrow or solid organ transplantation.
An overview of the consensus conference recommendations is provided in Table 3 (74). With regard to specific infections, the experts agreed that any patient with candidemia should receive antifungal therapy and that i.v. catheters should be removed or changed. Fluconazole was recommended as primary therapy for candidemia in stable neutropenic and nonneutropenic patients in whom C. krusei was unlikely and who had received no prior treatment with fluconazole. The experts chose fluconazole over amphotericin B mainly because it is less toxic. Amphotericin B was the agent of choice when infection was due to a fluconazole-resistant organism or C. krusei and was recommended for the continued treatment of patients who develop candidemia while receiving fluconazole. For unstable neutropenic and nonneutropenic patients, most of the experts preferred amphotericin B with or without 5-FC; however, for unstable patients with no prior fluconazole therapy and in whom infection due to C. krusei was unlikely, five participants chose fluconazole alone and five others chose fluconazole in combination with amphotericin B. For the management of candidemia after solid-organ transplant, the experts selected fluconazole as the initial therapy for stable patients who had not received fluconazole previously and amphotericin B for stable patients who had received fluconazole but differed in their opinions about the appropriate regimen for unstable patients (see Table 3) (74).
TABLE 3.
International Consensus Conference: general recommendations for management of severe candidal infectionsa
| Type of candidal infection | Recommendationb (no. of votes/no. of investigators voting) for use of:
|
||||||
|---|---|---|---|---|---|---|---|
| FLU | AmBc | AmB lipid | FLU + AmBc | AmB + 5-FC | FLU + 5-FC | ITRA | |
| Candidemia | |||||||
| Nonneutropenic | |||||||
| Stable, C. krusei unlikely, no prior FLU therapy | 20/20 | ||||||
| Stable, received FLU for >2 days | 3/20 | 10/20 | 7/20 | ||||
| Unstable, C. krusei unlikely, no prior FLU therapy | 5/20 | 4/20 | 2/20 | 5/20 | 4/20 | ||
| Neutropenic | |||||||
| Stable, uncomplicated candidemia, no prior triazole therapy, no sites of hematogenously seeded infection or other forms of deep candidal infection | 17/20 | 3/20 | |||||
| Stable, non-albicans species identified, or positive blood culture in patient receiving azole therapy | 5/18 | 13/18 | |||||
| C. glabrata | |||||||
| C. krusei | 18/18 | ||||||
| Unstable or evidence of deep-organ candidal infection | 2/20 | 1/20 | 2/20 | 4/20 | 10/20 | 1/20 | |
| After solid-organ transplant | |||||||
| Stable, no prior FLU therapy | 18/18 | ||||||
| Stable, prior FLU therapy | 18/18 | ||||||
| Unstable | 7/18 | 4/18 | 2/18 | 1/18 | 4/18 | ||
| Candiduria | |||||||
| Non-krusei cystitis | 19/20 | 1/20d | |||||
| Presumed upper urinary tract infection, non-krusei Candida species | 16/18 | 1/18e | 1/18 | ||||
| Candida peritonitis | 11/18 | 1/18 | 4/18f | 2/18 | |||
| Chronic disseminated candidiasis (formerly hepatosplenic), patient no longer neutropenic | 11/18 | 1/18 | 4/18 | 2/18 | |||
| Candida endophthalmitis | |||||||
| Uncomplicated (lesions not advancing rapidly, relatively small, not localized in area of macula) | 11/18 | 1/18 | 6/18 | ||||
| Enlarging lesion or threatening macula | 18/18 | ||||||
Adapted from reference 74 with permission of the publisher.
FLU, fluconazole; AmB, amphotericin B; ITRA, itraconazole.
Standard formulation.
Bladder irrigation.
Intravenous.
AmB initially, followed by FLU.
Other serious Candida infections for which a regimen containing fluconazole either alone or in combination with amphotericin B or 5-FC was the first choice were non-C. krusei cystitis or presumed upper urinary tract infection, Candida peritonitis, chronic disseminated candidiasis in patients who are no longer neutropenic, and uncomplicated Candida endophthalmitis. For patients with asymptomatic (without pyuria) candiduria, who do not have diabetes mellitus or genitourinary abnormalities and have not undergone renal transplantation, the experts agreed that no antifungal therapy is warranted because colonization is very common, often related to use of antibiotics or catheters, and can usually be cleared by their removal. For asymptomatic Candida colonization of the urinary tract or biliary tree following solid-organ transplantation, fluconazole was selected when the species was not C. krusei or C. glabrata and low-dose amphotericin B with or without 5-FC was selected when these species were present.
With regard to fluconazole dosage, the recommendation was 400 mg/day for stable nonneutropenic patients and 800 mg/day for unstable neutropenic patients (74, 104). The i.v. route was preferred for the initiation of fluconazole therapy in unstable patients and/or those with unreliable gastrointestinal absorption (74). For the treatment of neutropenic and nonneutropenic children with life-threatening invasive candidiasis, the recommended dosage of fluconazole was 6 mg/kg b.i.d., assuming normal renal function.
Although itraconazole was a possible alternative for severe Candida infections in stable patients, lack of an i.v. formulation in the United States, erratic p.o. absorption in this patient population, and the absence of efficacy data from large, well-controlled trials argued against its use (74). The only use found for itraconazole by one participant was to treat presumed upper urinary tract infection when caused by species of Candida other than C. krusei. However, the new cyclodextrin formulation, which increases the absorption of itraconazole and is being developed for i.v. administration, may lead to its future use in the treatment of serious Candida infections (104).
Further support for the use of fluconazole in serious Candida infections was provided by three studies, the results of which were published after the consensus conference (4, 6, 7). A matched cohort study compared therapeutic outcomes in 45 cancer patients with hematogenous candidiasis treated with fluconazole (200 to 600 mg/day) and 45 patients treated with amphotericin B (0.3 to 1.2 mg/kg/day) (7). The patients were matched for age, sex, status of underlying disease, use of antibiotics and growth factors, duration of treatment, presence and removal of central venous catheters, disseminated disease, and concomitant infections. The two antifungal regimens produced similar response rates at 48 h and 5 days, and at the end of therapy the overall response rates were 73% for fluconazole-treated patients and 71% for amphotericin B-treated patients. No differences in survival rates or causes of death were found. In both groups, the end-of-treatment response was higher for patients infected with C. albicans (78 and 74%, respectively) than for those infected with non-albicans species (68% for both drugs). The percentage of patients developing toxic effects was significantly greater with amphotericin B than with fluconazole (67 and 9%, respectively; P < 0.0001). Adverse events commonly associated with amphotericin B treatment were nephrotoxicity (40%), hypokalemia (27%), fever (16%), and chills (11%). No nephrotoxicity was observed in fluconazole-treated patients. Side effects associated with fluconazole therapy were hepatotoxicity, hypokalemia, fever, and chills, which occurred in 4, 4, 4, and 2% of patients, respectively (7).
The equivalent efficacy and better tolerability of fluconazole compared with amphotericin B were also documented in a prospective, randomized, multicenter study involving 164 patients (including 60 with neutropenia) with a variety of invasive Candida infections, including candidemia, urinary tract infection, intra-abdominal infection, postoperative wound infection, cholangitis, and pericarditis (4). Of 142 evaluable patients, 86 had documented candidemia or organ infection and 56 had presumed fungal infection. A presumptive diagnosis of candidiasis was made only for patients who were neutropenic or had undergone surgery. Patients received either fluconazole (400 mg/day) or amphotericin B (25 to 50 mg/day for nonneutropenic patients; 0.67 mg/kg/day for neutropenic patients). The overall response rates at the end of therapy were 66% (44 of 67 patients) in the amphotericin B group and 64% (48 of 75 patients) in the fluconazole group, and they remained similar when categorized according to site of infection and pathogen. Survival rates at the end of therapy were 87% in the amphotericin B group and 88% in the fluconazole group. Significantly more drug-related adverse events occurred among patients who received amphotericin B than among those who received fluconazole (37 and 5%, respectively; P < 0.0001). Nephrotoxicity occurred in 22 patients (28%) who received amphotericin B but in only 1 patient who received fluconazole.
Fluconazole and amphotericin B also appear to be equally effective in the treatment of candidemia in cancer patients, regardless of the degree of neutropenia, according to a retrospective review of medical records for 471 patients who experienced 476 independent episodes of candidemia (6). Some of these patients were included in the two earlier studies of fluconazole and amphotericin B; 35 were in the randomized comparison (4), and 90 were in the matched-cohort study (7). Although the main objective of the retrospective study was to identify factors affecting outcome, the relative efficacy of fluconazole and amphotericin B was analyzed by using a matched-pair analysis with a Monte Carlo simulation. The average success rates with fluconazole and amphotericin B over a total of 300 simulation trials generating an average of 82 matched pairs of patients were 71 and 73%, respectively, which were not significantly different statistically (P = 0.38 to 1.00). According to the investigators, although the retrospective nature of the study is a major limitation, there are no plans to conduct randomized, comparative trials of fluconazole and amphotericin B. Therefore, assessments of the relative efficacy of these two antifungal agents in this clinical setting will, of necessity, rely on observational data such as these.
(ii) Combination therapy.
As noted previously, some participants in the consensus conference on the management of severe candidal infections chose a regimen of fluconazole in combination either with amphotericin B or 5-FC in certain situations (Table 3) (74). However, the use of amphotericin B in combination with an azole is controversial because of the potential for antagonism based on their modes of action (95). With regard to fluconazole-amphotericin B, the antagonism demonstrated in some in vitro studies has not been observed in vivo in experimental animal models (95, 229, 246, 247). In a murine model of invasive candidiasis due to a fluconazole-resistant strain, fluconazole-amphotericin B was more effective than fluconazole alone and was equivalent to amphotericin B alone in protecting both immunocompromised and healthy immune-normal mice from infection and in decreasing fungal burden (247). A subsequent study of neutropenic-mouse and infective-endocarditis rabbit models found no antagonism but, rather, indifference with fluconazole-amphotericin B (229). In mice, fluconazole-amphotericin B and amphotericin B alone significantly prolonged survival compared to untreated controls and both were more effective than fluconazole alone in clearing Candida from the kidneys. However, fluconazole-amphotericin B and fluconazole alone both were more effective than amphotericin B alone in decreasing the fungal burden in the brain. In rabbits, both fluconazole-amphotericin B and amphotericin B alone were highly effective in decreasing Candida concentrations in cardiac vegetations.
An explanation for the lack of in vivo antagonism between amphotericin B and fluconazole seen in these animal infection models was suggested recently by Ghannoum (95), who noted that factors other than binding to ergosterol play a role in antifungal susceptibility to amphotericin B and that fluconazole-treated cells appear to retain an adequate amount of ergosterol to permit amphotericin B binding. The question whether the same lack of antagonism between these antifungal agents that has been observed in animal models will be seen clinically should be answered when the results of the ongoing NIAID-MSG trial (74) and other clinical investigations of the fluconazole-amphotericin B combination in the treatment of systemic mycoses (95, 103, 229) become available.
Another combination that has shown synergy in vitro against C. albicans, including fluconazole-resistant strains, and other Candida species is an azole plus the allylamine antifungal agent terbinafine (10, 225). This combination was tested in vitro in two studies, both of which used checkerboard broth microdilution techniques (10, 225). In one study, the fluconazole-terbinafine combination was effective in vitro against most types of resistant isolates, including 5 of 5 nonresistant C. albicans, 17 of 19 fluconazole-resistant C. albicans, and 5 of 9 C. glabrata isolates (225). The other study tested activity against a C. albicans isolate obtained during azole therapy from an AIDS patient with oropharyngeal candidiasis (OPC) (10). Synergy, usually indicated by a fourfold decrease in the MIC, was observed in 40% of fluconazole-terbinafine interactions and 43% of itraconazole-terbinafine interactions. Even when the interaction was only additive, a decrease of at least twofold in the MICs of both drugs was observed in 100% of fluconazole-terbinafine interactions and 76% of itraconazole-terbinafine interactions. Additive effects of the azole-terbinafine combinations also were observed against C. krusei (10). Whether these azole-terbinafine combinations will be useful clinically in the treatment of invasive candidiasis awaits investigation.
The combination of a fluoroquinolone, such as trovafloxacin or ciprofloxacin, and either amphotericin B or fluconazole appears to enhance the antifungal activity of the latter agents in invasive hematogenously disseminated candidiasis in mice (249). Although the fluoroquinolones did not enhance the in vitro activity of either amphotericin B or fluconazole against C. albicans, C. tropicalis, or other fungi when tested by a checkerboard broth microdilution technique, in mice infected i.v. with a fluconazole-resistant strain of C. albicans, the combination of either fluoroquinolone with fluconazole was more effective than fluconazole alone in prolonging survival. Use of trovafloxacin in combination with a suboptimal dose of amphotericin B also improved survival. These data suggest that fluoroquinolones may be useful as adjuncts to antifungal agents.
(iii) Prophylaxis.
Prophylactic administration of a systemic antifungal agent, usually fluconazole, is reserved for selected patients considered to be at high risk of candidemia (74). Although prophylaxis is not routinely recommended for nonneutropenic patients, fluconazole might be appropriate for those in whom the risk of candidemia is increased. The example given in the international consensus conference report is “ … a patient has received antibacterial therapy for >14 days, has indwelling intravascular lines in place, is receiving hyperalimentation fluids, has had Candida isolated from two or more sites, and has undergone complicated intraabdominal surgery” (74). For neutropenic patients who have not undergone bone marrow or organ transplants (i.e., those with leukemia or who are hospitalized in surgical intensive care units), participants in the international consensus conference were reluctant to recommend widespread prophylaxis because of limited data on efficacy and concerns about the development of azole resistance (74). For high-risk neutropenic patients with C. albicans colonization at multiple sites, Lortholary and Dupont reported that low-dose fluconazole (≥50 mg/day) is appropriate (147). However, as discussed in greater detail below in the section dealing with resistance, most reports of fluconazole resistance in non-HIV-infected patients have involved non-albicans species of Candida, mainly C. krusei and C. glabrata, which generally emerged in patients given low-dose (≤200-mg/day) fluconazole prophylaxis either alone or in combination with amphotericin B (2, 172, 220, 269). A shift in the species of Candida causing bloodstream infections has also been found in cancer patients receiving fluconazole prophylaxis with higher doses, usually 400 mg/day (1). A retrospective review of medical records of 474 cancer patients who experienced 479 episodes of hematogenous candidiasis between 1988 and 1992 showed that fluconazole prophylaxis was associated with a relative decrease in the proportion of bloodstream infections caused by C. albicans and C. tropicalis and an increase in the proportion of infections due to C. krusei and C. glabrata. These findings provide further support for the recommendation to limit fluconazole prophylaxis to high-risk patients, such as those with neutropenia, and to administer the drug only during periods when the patient is at highest risk, in order to minimize selection of these less susceptible species (1).
There is general agreement that bone marrow transplant recipients should receive prophylaxis with fluconazole at a dosage of 400 mg/day given p.o. or i.v. (74, 147). For patients who have received lung or heart-lung transplants, the international consensus conference participants suggest administration of fluconazole for 10 to 14 days posttransplantation (74). There is no definitive recommendation for prophylaxis following liver transplantation; however, fluconazole is considered the appropriate prophylactic agent (74, 147). A review of experience in patients with fulminant hepatic failure who underwent liver transplantation at one center suggests that fluconazole prophylaxis (100 mg/day) begun at the time of admission reduces the occurrence of invasive C. albicans infections (138). Among 72 patients who underwent transplant prior to routine use of fluconazole prophylaxis, there were 6 deaths due to Candida infection. By contrast, among 45 patients who received liver transplants after routine use of fluconazole prophylaxis was begun, there were 3 deaths due to fungal infections, all associated with organisms not susceptible to fluconazole (Aspergillus spp. and Mucor sp.). No invasive C. albicans infections occurred. Two other recent reports suggest that prophylaxis with either low-dose amphotericin B (10 to 20 mg/day) (146) or liposomal amphotericin B (AmBisome; 1 mg/kg/day) (252) also decreases the rate of invasive fungal infections in liver transplant recipients. With regard to pancreatic transplantation, fluconazole prophylaxis at a dosage of 400 mg/day begun perioperatively and continued for 7 days postoperatively appears to decrease the rate of intraabdominal infection (14). In a retrospective study, fungal infection rates were 6 and 10%, respectively, among 108 patients given fluconazole prophylaxis and 327 patients not given prophylaxis (P = 0.2) (14). Because the incidence of severe Candida infection is low following kidney transplantation, no antifungal prophylaxis is recommended (147).
Cryptococcosis.
For cryptococcosis, the therapeutic regimen is based on the site and severity of infection and the patient’s immune status. Treatment options for both pulmonary cryptococcosis and disseminated cryptococcal infection in immunocompetent patients include amphotericin B with or without 5-FC, which is the preferred regimen for patients with severe infection, or p.o. fluconazole (92). One study suggests that fluconazole may be as effective as amphotericin B with or without 5-FC in the treatment of both extrameningeal and meningeal cryptococcosis in HIV-negative patients (70). In a retrospective analysis of 83 HIV-negative patients treated in France between January 1985 and December 1992, cure rates at the end of initial therapy with amphotericin B (usually with 5-FC) or fluconazole (≤400 mg/day) were 74 and 68%, respectively, among patients with meningeal infection and 75 and 93%, respectively, among those with nonmeningeal infection (70). There are no general recommendations at present for the treatment of extraneural cryptococcosis in HIV-infected patients (161).
For acute cryptococcal meningitis, amphotericin B alone or in combination with 5-FC is the drug of choice for immunocompromised patients as well as immunocompetent patients who are severely ill or in whom infection is rapidly progressing (15, 58, 62, 92, 161). For HIV-infected patients with acute cryptococcal meningitis, an initial course of amphotericin B with or without 5-FC followed by lifelong maintenance therapy with p.o. fluconazole in a dosage of 200 to 400 mg given once daily (q.d.) appears to be effective in preventing relapse (12, 15, 58, 62, 157). Fluconazole is highly effective in preventing the recurrence of cryptococcal infection (26) and is more effective than maintenance with once-weekly amphotericin B (200) or once-daily itraconazole (170, 226). A 400-mg dose of fluconazole appears to be needed if a shorter course of amphotericin B is used as primary therapy (170). An alternative regimen of high-dose amphotericin B (≥0.7 mg/kg/day) with or without 5-FC administered for 2 weeks followed by consolidation therapy with 400 mg of either fluconazole or itraconazole per day for 8 to 10 weeks is under investigation (198). A preliminary analysis of the results of a U.S. study by the NIAID-MSG and AIDS Clinical Trial Group found that this regimen improved survival (the mortality rate was <8%) (198). Fluconazole and itraconazole were equally effective in controlling cryptococcal infection (198). In another study in which high-dose amphotericin B (1.0 mg/kg/day) with or without 5-FC (100 to 150 mg/kg/day) was followed by lifelong maintenance with 300 mg of fluconazole or itraconazole per day, a 94% response rate was achieved and there were no deaths due to cryptococcal infection (50). Only three relapses occurred during a mean observation period of 10.7 months. Currently, most experts recommend amphotericin B (≥0.7 mg/kg) plus 5-FC for 2 to 3 weeks followed by fluconazole (400 mg p.o.) for 8 to 10 weeks (15, 161, 198).
Both fluconazole and itraconazole have been investigated as alternatives for the initial therapy of cryptococcal meningitis, mainly in HIV-infected patients, because they are less toxic and better tolerated than is amphotericin B alone or combined with 5-FC (47, 58, 131, 157, 161). According to Powderly (196), initial studies suggested that at a daily dose of 200 to 400 mg, both triazoles were effective for the treatment of cryptococcal meningitis in HIV-infected patients; about 60% of previously untreated patients responded favorably. Most clinicians prefer fluconazole because of problems with absorption of itraconazole in HIV-infected patients. At present, fluconazole (400 mg p.o. q.d.) may be used for the initial therapy of neurologically intact patients with mild disease who are thought to have a favorable prognosis (15, 92, 157). Because fluconazole doses of 200 to 400 mg sterilize the CSF more slowly than does i.v. amphotericin B (≥0.3 mg/kg) (median time to first negative CSF culture, 64 and 42 days, respectively) (227), higher fluconazole doses (≥800 mg/day) have been studied as salvage therapy in HIV-infected patients who failed to respond to prior therapy for cryptococcal meningitis (19) and as primary therapy (115, 159). In a recent trial, 6 (54.5%) of 14 HIV-infected patients treated with fluconazole (800 to 1,000 mg/day given i.v. for 3 weeks and then p.o.) showed clinical success after 10 weeks of therapy, and at the end of therapy 8 patients (72.7%) had responded (159). With this high-dose regimen, the median time to sterilization of the CSF was 33.5 days. High-dose fluconazole was well tolerated; treatment was not interrupted and the dose was not decreased because of side effects. Further clinical trials are needed to establish whether high-dose fluconazole will be an alternative to a lower-dose regimen preceded by 2 to 3 weeks of treatment with amphotericin B with or without 5-FC.
Combinations of fluconazole with either amphotericin B or 5-FC have been studied in animal infection models and are undergoing clinical investigation for the treatment of cryptococcal meningitis (95, 229). Studies of the fluconazole–5-FC combination with and without added amphotericin B in murine models of cryptococcal meningitis (57, 61, 140, 171) have demonstrated that fluconazole markedly enhances the fungicidal activity of 5-FC against Cryptococcus spp. (140, 171) and that the fluconazole–5-FC combination is synergistic in vivo (171), improves the survival of infected mice (140, 171), and is effective in cases where monotherapy with either drug was ineffective and in cases in which the infecting isolate was resistant to fluconazole (171).
A consistent observation in these studies was that when higher doses of fluconazole were used, it was possible to lower the dose of 5-FC while maintaining a maximal therapeutic effect (61, 140, 171). In one study, the severity of meningitis was varied by delaying the onset of therapy from 3 to 7 days. As meningitis became more severe, the dose of fluconazole in the combination had to be increased (61). Among mice treated initially on day 3 or 5 postinfection, 100% survival was achieved with fluconazole at >5 mg/kg/day regardless of the dose of 5-FC. Mice not treated until day 7 postinfection required higher fluconazole doses (>25 mg/kg/day) for 75 to 100% survival, regardless of the dose of 5-FC. In this latter group with more severe meningitis, a maximal fungicidal effect was achieved with a combination of either fluconazole (45 mg/kg/day) and 5-FC (60 to 90 mg/kg/day) or fluconazole (>45 mg/kg/day) and 5-FC (30 to 120 mg/kg/day). When the triple combination of fluconazole plus 5-FC and amphotericin B (given as the amphotericin B colloidal dispersion, ABCD) was tested in mice infected with a C. neoformans isolate from an AIDS patient with cryptococcal meningitis who responded promptly to treatment with fluconazole–5-FC, 100% survival was achieved, regardless of the dose of the combination (57). Without added ABCD, 100% survival was achieved with the fluconazole–5-FC combination when the fluconazole dose was 20 mg/kg/day or more, regardless of the 5-FC dose. Maximum antifungal effect was achieved with fluconazole (≥30 mg/kg/day) and 5-FC (20 to 60 mg/kg/day) plus ABCD (5.0 to 7.5 mg/kg/day). Weight changes were not associated with the dose of 5-FC. The animals maintained weight while on the triple combination when the fluconazole dose was ≥10 mg/kg/day and the ABCD dose was 5.0 or 7.5 mg/kg/day; however, weight loss occurred at the highest dosages of fluconazole and ABCD (≥40 and 7.5 mg/kg/day, respectively). In this model, the best therapeutic effect was achieved with higher doses of fluconazole in combination with low to moderate doses of 5-FC and ABCD.
Clinical trials evaluating the effectiveness of amphotericin B-fluconazole combinations in the treatment of cryptococcal meningitis (95, 229) and candidemia (74, 95, 103) are in progress, and no results have been published to date. The NIAID-MSG halted a clinical trial of fluconazole–5-FC in the treatment of cryptococcal meningitis in patients without AIDS when the disease progressed in some patients (103). Whether this patient population will benefit from the combination is not known. Preliminary results from a Ugandan clinical trial comparing p.o. fluconazole alone and fluconazole–5-FC in AIDS-related cryptococcal meningitis showed that use of a higher dose of fluconazole (1200 mg/day) in combination with a lower dose of 5-FC (100 mg/day) did not increase either survival or toxicity (253). Overall, the findings in the first 100 patients treated showed that the addition of 5-FC for the first 4 weeks of a 10-week course of fluconazole therapy (either 1,200 or 400 mg/day) increased the rate of CSF sterilization and decreased the time to CSF sterilization but increased toxicity. Survival rates after 10 weeks were comparable with fluconazole alone (43.2%; 16 of 37 patients) and fluconazole–5-FC (46.2%; 18 of 39 patients). The results of a prospective, open-label clinical trial by the California Collaborative Treatment Group in which 32 AIDS patients with cryptococcal meningitis were treated p.o. with a combination of fluconazole (400 mg/day) and 5-FC (150 mg/kg/day) showed that use of the combination improved clinical success rates compared to those achieved with either fluconazole or amphotericin B monotherapy (141). Because of concern that it might have an additive effect on 5-FC toxicity, especially granulocytopenia, zidovudine was withheld during the initial 6 weeks of treatment. According to Kaplan-Meier estimates, the clinical success rate was 63%, and CSF cultures were sterile after 10 weeks in 75% of the 32 patients. The median time needed for CSF sterilization was 23 days. Six surviving patients considered therapeutic failures at or before 10 weeks responded to amphotericin B clinically, and cryptococci were eradicated from the CSF. Most patients experienced toxicity related to 5-FC, and treatment was discontinued due to dose-limiting side effects in 9 patients (28%). However, 96% of patients tolerated at least 2 weeks of treatment; 89% tolerated at least 4 weeks; 71% tolerated at least 6 weeks; and 62% tolerated 10 weeks. These findings provided the basis for the randomized, controlled clinical trial of this combination currently in progress.
Although not recommended routinely, prophylaxis could be considered for patients at increased risk of developing cryptococcal infection, particularly those with HIV infection (12, 103, 147, 161, 198). Cryptococcal infections account for about 6% of AIDS-defining illnesses (198), and patients with CD4+-cell counts of less than 50 to 100/mm3 are at greatest risk (12, 147). Recent clinical evaluations of primary prophylaxis in HIV-infected patients have shown that fluconazole provides protection against cryptococcal infection, particularly in those with CD4+-cell counts below 50/mm3 (147, 199). In the ACTG study, in over 400 patients with CD4+ cell counts below 200/mm3 who were monitored for a median of 35 months, cryptococcal infection occurred in 2 of 217 patients receiving fluconazole (200 mg/day) compared with 15 of 211 patients receiving clotrimazole troches (10 mg five times daily) (199). Among patients with CD4+-cell counts of 50/mm3 or less, the differences between treatments were especially evident. In this group, the estimated 2-year cumulative risk of cryptococcal infection was 1.6% for those receiving fluconazole and 9.9% for those receiving clotrimazole (P = 0.02), whereas the corresponding risk estimates for those with higher CD4+ cell counts were 0.8 and 4.3%, respectively (P = 0.04). Fluconazole was also effective in reducing the frequency of Candida infections, although 10.6% of patients receiving the drug experienced at least one episode during the study. Fluconazole had no effect on survival.
A concern is that routine use of fluconazole prophylaxis may lead to breakthrough infections with fluconazole-resistant Candida. A review of blood and venous-catheter cultures obtained from neutropenic patients over a 6-month period documented Candida fungemia in 8 of 76 bone marrow transplant recipients receiving fluconazole (271). C. glabrata was the species isolated from six of the eight patients in whom fungemia developed.
Although fluconazole prophylaxis should be considered for HIV-infected patients with CD4+ cell counts below 50/mm3 (12, 161), the U.S. Public Health Service/Infectious Diseases Society of America (USPHS/IDSA) task force (256) and other experts do not recommend the routine use of fluconazole for the primary prevention of cryptococcal infections in all patients with AIDS for a number of reasons, including failure to demonstrate a survival benefit, the risk of selecting fluconazole-resistant Candida, and cost-benefit considerations (103, 147, 161, 198).
Endemic Mycoses
Although the endemic mycoses are restricted to certain geographical areas, they are evolving into opportunistic infections that may be encountered elsewhere (147). Immunocompromised individuals (e.g., transplant recipients, HIV-infected persons) residing in areas of endemic infection tend to develop primary infections, whereas reactivation of infection occurs among those who have left the regions (121). Immunocompromised individuals are especially susceptible to all but paracoccidioidomycosis, and those with depleted CD4+ cell counts are at high risk of disseminated infection and CNS involvement. Disseminated coccidioidomycosis has been considered to be an AIDS-defining event since 1987, and 10% of HIV-infected individuals living in an area of endemic infection will develop active infection each year (243). Likewise, histoplasmosis affects 2 to 5% of AIDS patients living in areas of endemic infection in the United States and up to 25% of those in certain cities (262). Among organ transplant recipients, histoplasmosis is the most common endemic fungal infection; coccidioidomycosis occurs primarily in those who have received kidney, heart, and heart-lung transplants (147). Blastomycosis is rarely found in either patients with AIDS or transplant recipients (27, 147), and paracoccidioidomycosis and sporotrichosis generally occur in individuals with normal immune systems (208, 212).
Coccidioidomycosis.
When Coccidioides immitis infection in an individual with an intact immune system is limited to the lungs, antifungal therapy generally is not needed unless the illness is severe; however, disseminated extrapulmonary infection should be treated in most cases (243). When there is no CNS involvement, extrapulmonary infection may be treated i.v. with amphotericin B (total dose, 1 to 2.5 g) or p.o. with either itraconazole (200 mg b.i.d. with food) or fluconazole (400 to 600 mg q.d.). The efficacy of these triazoles in the treatment of nonmeningeal coccidioidomycosis was evaluated by the NIAID-MSG in separate multicenter, open-label trials (35, 106). Itraconazole (100 to 400 mg/day; 400 mg/day in most patients) was administered to 49 patients for up to 39 months, including 3 immunosuppressed (non-HIV-infected) patients and 29 patients who had relapsed or failed to respond to prior therapy either with amphotericin B or ketoconazole (106). Among 44 clinically evaluable patients who completed therapy, the remission rate was 57% (25 patients), based on a 50% reduction in the pretreatment score (i.e., assessments of lesion number and size, symptoms, culture, and serologic titer). Patients improved slowly, with few achieving remission before 10 months; the rate of response was similar for patients with soft tissue, chronic pulmonary, and osteoarticular disease. At the end of the study, 21 of the 25 patients remained in clinical remission. Four relapses occurred at 4, 4.5, 7, and 21 months among 11 patients after therapy for at least 12 months. In the other study, 78 patients with coccidioidomycosis received fluconazole (200 to 400 mg/day) for up to 1 year, including 7 patients with HIV infection and 48 patients who had experienced prior episodes of coccidioidomycosis (35). Among 75 patients evaluated for efficacy, a satisfactory response (defined as any reduction of baseline abnormality by month 4 and at least a 51% reduction by month 8) was achieved in 12 (86%) of 14 patients with skeletal disease, 22 (55%) of 40 patients with chronic pulmonary disease, and 16 (76%) of 21 patients with soft tissue disease. Forty-one patients who received fluconazole (400 mg/day) for at least part of their treatment were monitored posttreatment for a median of 235 days (range, 21 to 595 days), during which time reactivation of infection occurred in 15 patients (37%). The investigators commented that higher doses of fluconazole should be evaluated, particularly for patients with chronic pulmonary disease. Although some patients with extrapulmonary coccidioidomycosis may respond to ketoconazole (400 mg/day), the likelihood of relapse appears to be even greater than that following treatment with either itraconazole or fluconazole (15, 243). A comparison of these two triazoles for the initial therapy of nonmeningeal coccidioidomycosis is ongoing (243). Although the optimal duration of oral azole therapy for nonmeningeal cryptococcal infections remains to be determined, in view of the tendency of coccidioidomycosis to recur after the completion of therapy, Stevens recommends continued administration for 6 months after the disappearance of disease (243).
For immunocompetent patients with CNS involvement, the traditional therapeutic regimen consists of intra-CSF and systemic amphotericin B plus intrathecal corticosteroid (243). However, patients may respond to p.o. therapy with either itraconazole or fluconazole, which avoids amphotericin B-related toxicity and the need for intra-CSF administration (90, 243, 255). Clinical experience with itraconazole is limited to a prospective, nonrandomized, multicenter study of 10 patients with active chronic coccidioidal meningitis refractory to standard therapy with intra-CSF and i.v. amphotericin B and in some cases to ketoconazole (7 patients), miconazole (2 patients), and fluconazole (1 patient) (255). Neither concurrent illness nor immunodeficiency was considered to be contributing to the meningeal infection. Itraconazole treatment (300 to 400 mg/day) was initiated in five patients while they were receiving intra-CSF amphotericin B and was the sole therapy in the remaining patients. Seven of eight evaluable patients who were monitored on therapy for a median duration of 10 months (range, 6 to 42 months) have responded, including four of five receiving itraconazole alone. The single failure occurred in a patient who was intolerant of intra-CSF amphotericin B and failed to respond to intra-CSF miconazole (20 mg/day) in combination with fluconazole (100 mg/day) and then itraconazole.
Fluconazole, with which there is more experience, has become the preferred initial treatment for patients with coccidioidal meningitis (15, 132). In an uncontrolled multicenter trial, investigators from the NIAID-MSG evaluated fluconazole treatment in 50 patients with coccidioidal meningitis, including 9 with HIV infection and 3 receiving immunosuppressive therapy (90). Of these, 25 patients had relapsed following prior therapy with either intrathecal amphotericin B (24 patients) or ketoconazole (1 to 2 g/day) (1 patient). Once-daily treatment with fluconazole (400 mg p.o.) was frequently begun without hospitalization, and most patients were treated on an ambulatory basis. Of 47 evaluable patients, 37 (79%) responded to fluconazole therapy, including 6 of 9 HIV-infected patients who survived for 9 to 26 months. These six patients and four others subsequently died of unrelated causes; of the 27 survivors, 25 continued to receive fluconazole therapy for 2 to 4 years (median, 38 months). Of the 10 patients who failed to respond to fluconazole (400 mg/day), 2 were switched to intrathecal amphotericin B, 2 with HIV infection died, and 6 received fluconazole (800 mg/day) for 15 to 20 months; 4 of these showed improvement. Most responses were noted within the first 4 months of therapy, and response rates were not affected by prior therapy or the presence of HIV infection or hydrocephalus. Symptomatic improvement occurred despite the persistence of CSF pleocytosis and other CSF abnormalities in 15 of 20 responders who were monitored for at least 20 months. The one patient who discontinued fluconazole therapy after responding subsequently relapsed. Ketoconazole is not recommended for use in patients with coccidioidal meningitis. According to Tucker et al. (255), high-dose regimens have been effective in selected patients but are associated with considerable toxicity.
HIV-infected patients with severe coccidioidal infections are treated initially with i.v. amphotericin B (157, 161), and Masur (157) suggests a dosage of 0.5 to 1.0 mg/kg/day for at least 8 weeks. Although not the preferred agent for initial therapy, fluconazole (400 to 800 mg/day) is an alternative to amphotericin B (157). Primary therapy is followed by lifelong suppression with either fluconazole (200 to 600 mg/day) or itraconazole (100 to 200 mg b.i.d.) (157, 161, 256), although itraconazole triazole has not undergone clinical evaluation for this use (147). Ketoconazole is not effective for long-term suppressive therapy in immunocompromised patients, and some HIV-infected patients have developed coccidioidal infection while receiving the drug for other infections (147). Although the value of antifungal prophylaxis against coccidioidomycosis in HIV-infected patients has yet to be determined, it should be considered for those who are serologically positive for Coccidioides (147). Fluconazole (200 mg/day) is being evaluated for this use in regions of endemic infection in the United States (262).
Histoplasmosis.
Asymptomatic or mildly symptomatic acute pulmonary infection due to Histoplasma capsulatum in patients with normal immune status generally does not require antifungal therapy (29). Amphotericin B is the drug of choice for severe or life-threatening infections, for H. capsulatum meningitis, for patients unresponsive to or relapsing after azole therapy (27, 29, 75, 132, 196), and for the initial therapy of moderate to severe infection in HIV-infected patients (161). The azoles are alternatives to amphotericin B for the initial therapy of patients with milder infections. Following primary therapy with amphotericin B or an azole, most patients require lifelong maintenance therapy to prevent relapse.
Itraconazole is the drug of choice for the primary therapy of non-life-threatening, nonmeningeal histoplasmosis in patients without HIV infection (27, 132), and it may also be effective as primary therapy or lifelong suppression in patients who have received solid-organ transplants (110). Nonrandomized, multicenter, open-label studies demonstrated the effectiveness of itraconazole for the primary treatment of more indolent forms of disseminated histoplasmosis in patients with intact immune systems (63) and in those with underlying HIV infection (264). In a trial by the NIAID-MSG, 37 patients, 1 of whom had AIDS, were treated for nonmeningeal, non-life-threatening histoplasmosis with itraconazole (200 to 400 mg/day) (63). The clinical success rates were 81% (30 of 37 patients) overall and 86% (30 of 35 patients) in those who were treated for more than 2 months (median duration, 9.0 months). All 10 patients with disseminated extrapulmonary histoplasmosis and 13 (65%) of 20 patients with chronic cavitary pulmonary infection responded to itraconazole. Of the remaining five patients, two had persistent infection after 3 months of itraconazole therapy and three, treated for 6 to 7 months, relapsed 1, 3, and 12 months after the treatment was discontinued, respectively. An equally good response rate was achieved in AIDS patients treated for a first episode of histoplasmosis with itraconazole (300 mg b.i.d. for 3 days and then 200 mg b.i.d. for 12 weeks) (264). Of 59 evaluable patients, 50 (85%) responded. An inability to achieve therapeutic concentrations of itraconazole plasma (≥2 μg/ml, measured by bioassay) was thought to have contributed to a lack of response in two patients, and another patient with histoplasma meningitis was considered to have failed to respond when fungemia and meningitis persisted for 2 weeks. An analysis of long-term follow-up data for patients who responded to itraconazole and had completed a median of 12 months of maintenance on the drug was reportedly in progress (264). Currently, the suggested itraconazole regimens for pulmonary infection are 200 mg/day for 3 to 6 weeks in acute infection and 200 to 400 mg/day for 6 to 12 months in chronic or subacute pulmonary infection (29). For HIV-infected patients with very mild histoplasmosis, the suggested itraconazole regimen is 600 mg/day for 3 days and then 400 mg/day for 10 weeks, followed by chronic maintenance with 200 to 400 mg/day (157, 161).
Limited experience in one study suggests that fluconazole might be useful in the treatment of disseminated histoplasmosis in immunocompetent patients (59). Fluconazole appears to be less effective than itraconazole for the initial therapy of AIDS patients (27, 75, 196, 235) but might be considered for those who are intolerant of itraconazole. A retrospective review of experience with these triazoles in the treatment of AIDS patients with disseminated histoplasmosis at one center found a greater number of remissions in patients who received itraconazole (7 of 12 patients) than in those who received fluconazole (3 of 10 patients) (235). The mean duration of itraconazole therapy was 24 months (range, 0.1 to 36 months), and the mean duration of fluconazole therapy was 12 months (range, 1.25 to 24 months). Itraconazole (400 mg/day) was the initial therapy in 9 of the 12 patients. One of the remaining patients had received amphotericin B initially, and two others had received fluconazole. Fluconazole (100 mg/day) was the initial therapy in four of five patients who received this dosage and in two of five patients who received 400 to 800 mg/day; the remaining patients initially received amphotericin B. The response to fluconazole did not differ in the two dosage groups. Two of the three treatment failures in the itraconazole group and one of the six failures in the fluconazole group occurred following an interruption in triazole therapy because of associated illness.
Although weekly or biweekly amphotericin B is effective for chronic suppressive therapy (157, 196), itraconazole is preferred for this use (27, 132, 256). One regimen suggested for HIV-infected patients with life-threatening histoplasmosis is amphotericin (15 mg/kg/day) for 2 weeks followed by itraconazole (400 mg/day) for 12 weeks and then lifelong maintenance with itraconazole (200 mg/day) (75). Others recommend a higher dose (200 mg b.i.d.) for itraconazole maintenance (152). The effectiveness of itraconazole in preventing relapse of disseminated histoplasmosis in HIV-infected patients was demonstrated in a multicenter, open-label clinical trial by the NIAID-MSG (265). Maintenance therapy with itraconazole (200 mg b.i.d.) was given to 42 AIDS patients with disseminated histoplasmosis who had responded to induction therapy with amphotericin B in a dosage of 15 mg/kg given over 4 to 12 weeks. Two patients (5%) relapsed during a median follow-up of 109 weeks. One patient withdrew from the study at 8 weeks and died of histoplasmosis 18 weeks later while not receiving maintenance. The other patient did not comply with itraconazole therapy and was considered a possible relapse. The median survival in this study was 109 weeks.
Fluconazole appears to be less effective than itraconazole for lifelong maintenance. In a retrospective nonrandomized trial, the relapse rate was 12% (9 of 76) in patients who received fluconazole maintenance at dosages of 100 to 400 mg/day following successful induction therapy with amphotericin B, itraconazole, or fluconazole (177). Unlike the itraconazole study, in which patients began maintenance therapy within 6 weeks after induction therapy (265), 30% of the patients in this study had received other forms of maintenance before starting fluconazole. All nine patients who relapsed had received amphotericin B induction therapy, and four of the relapses occurred in patients maintained on fluconazole (100 mg/day). Median survival from the start of induction in the 76 patients was 94 weeks; survival was significantly better for patients who received more than 1 g of amphotericin B for induction and additional amphotericin B for maintenance before starting fluconazole maintenance than for those who received less than 1 g of amphotericin B and no additional drug prior to fluconazole maintenance (156 and 74 weeks, respectively; P < 0.02). These investigators considered chronic suppressive therapy of histoplasmosis with fluconazole (≥200 mg/day) moderately effective and an option for patients in whom itraconazole is inappropriate (because of drug interactions, malabsorption, side effects).
Although ketoconazole (400 mg/day) has been effective in nonmeningeal (i.e., disseminated and chronic pulmonary) infection in immunocompetent patients (15, 29), relapse rates are high (27). HIV-infected patients with histoplasmosis respond poorly to ketoconazole (161), and ketoconazole is not effective for chronic suppressive therapy (29, 196). Its use has largely been replaced by fluconazole and itraconazole (132).
With regard to primary prophylaxis, a placebo-controlled trial of itraconazole conducted in AIDS patients living in cities with a high incidence of histoplasmosis demonstrated a protective effect (147). Other studies have shown that low-dose fluconazole (100 to 200 mg/day) does not protect HIV-infected patients against Histoplasma (147), and a patient receiving fluconazole 50 mg/day for thrush developed signs of histoplasmosis (235).
Blastomycosis.
Many patients with mild pulmonary infection due to Blastomyces dermatitidis recover spontaneously without antifungal therapy and require only prolonged follow-up (27). For seriously ill patients, those with CNS involvement, and HIV-infected patients, amphotericin B is the drug of choice (27, 37, 161, 196). A clinical trial by the NIAID-MSG demonstrated that itraconazole (200 to 400 mg/day) was highly effective in the treatment of nonmeningeal, non-life-threatening pulmonary and extrapulmonary blastomycosis in patients without HIV infection (63). Treatment was successful overall in 43 (90%) of 48 patients, and the percentage cured rose to 95% (38 of 40 patients) among those treated for more than 2 months. The median duration of itraconazole therapy in these 38 responders was 6.2 months (range, 3.0 to 24.4 months), and the median interval from the end of therapy to the last posttreatment examination was 11.9 months (range, 0.0 to 25.1 months). Most relapses of blastomycosis occur within a few months after the completion of therapy, but only one patient treated for more than 2 months relapsed. This patient, who had cutaneous blastomycosis, was markedly immunosuppressed due to a splenectomy and prolonged corticosteroid therapy for autoimmune hemolytic anemia. Based on their findings, the investigators concluded that itraconazole (200 mg/day) was highly effective for most patients with nonmeningeal, non-life-threatening forms of blastomycosis and that the itraconazole dosage should be increased to 400 mg/day for those with progressive or persistent infection. Recently, Bradsher (27) commented that itraconazole (200 mg/day for 6 months) should replace amphotericin B for less seriously ill, compliant patients with nonmeningeal infection but cautioned that patients have developed CNS blastomycosis while receiving either itraconazole or ketoconazole.
Experience with fluconazole in the treatment of blastomycosis is limited and suggests that this triazole is not as effective as itraconazole (27, 182, 196). A multicenter, randomized pilot study by the NIAID-MSG demonstrated that fluconazole (200 and 400 mg/day) was moderately effective in the treatment of patients with non-life-threatening, non-CNS blastomycosis, including six patients who had failed to respond to treatment with ketoconazole or amphotericin B or who had relapsed when therapy was discontinued (182). Fluconazole therapy was successful in 15 (65%) of 23 evaluable patients, including the 6 patients who had failed to respond to prior antifungal therapy. For the 15 patients who responded to fluconazole, the median duration of therapy was 6.7 months and the median duration of posttreatment follow-up was 11.3 months. Success rates with the 200- and 400-mg/day doses of fluconazole were 62% (8 of 13 patients) and 70% (7 of 10 patients), respectively, suggesting that the higher dose was more effective; however, the numbers of patients were too small to establish this conclusively. The investigators consider fluconazole a useful alternative for patients who fail to respond to standard antifungal agents and commented that others have successfully treated CNS blastomycosis with fluconazole.
As is the case for other endemic mycoses, use of ketoconazole has been supplanted by that of itraconazole and fluconazole (27, 37, 132). Itraconazole (200 to 400 mg/day) is also used for long-term suppression of blastomycosis in HIV-infected patients (161, 196), although in their review, Lortholary and Dupont (147) stated that no clear recommendations could be made about either primary prophylaxis or long-term suppression.
Paracoccidioidomycosis.
Although usually restricted to tropical and subtropical areas, infection due to Paracoccidioides brasiliensis may be encountered elsewhere among former residents of these areas. It is a unique systemic mycosis, because it can be treated with sulfonamides either alone or in combination with trimethoprim (208). These drugs are administered for 3 to 5 years to prevent relapse (208). Both ketoconazole and itraconazole are also effective in the treatment of paracoccidioidomycosis; itraconazole is preferred over ketoconazole because the duration of therapy is shorter (6 months and 12 to 18 months, respectively), the daily dosage is lower (100 and 200 to 400 mg, respectively), there are fewer relapses (3 to 5% and 10%, respectively), and risk of drug interactions or hepatic toxicity is minimal (208). Fluconazole (200 to 400 mg/day) was effective in 27 of 28 immune-normal patients with paracoccidioidomycosis who were treated in a multinational study (59). Most patients (19 of 28) were treated for 6 months or less, and of the 16 responders who had posttreatment follow-up, only 7 were monitored for at least 1 year. The only relapse occurred 24 months posttreatment in a patient with cutaneous and oral infection who was treated for only 2 months. Larger numbers of patients will have to be treated and observed for several years thereafter before the usefulness of fluconazole in treating this deep mycosis can be determined (59, 208).
Sporotrichosis.
Like paracoccidioidomycosis, infections caused by Sporothrix schenckii occur mainly in tropical and subtropical regions (212). Because it is more expensive, itraconazole (100 to 200 mg/day) is considered an alternative to treatment with a saturated solution of potassium iodide but is used for the initial treatment of extracutaneous sporotrichosis in a dose of 200 to 400 mg/day (212). The effectiveness of itraconazole in the treatment of lymphocutaneous, articular/osseous, or pulmonary sporotrichosis was demonstrated in a clinical trial by the NIAID-MSG, in which 27 adults received 30 courses of itraconazole therapy (100 to 600 mg/day) lasting 3 to 18 months (234). Eleven patients had failed to respond to prior therapy, which included ketoconazole in six patients and fluconazole in two others. In 25 of the 30 courses of treatment, patients responded to itraconazole (usually 200 or 400 mg/day). Seven patients who responded to itraconazole relapsed 1 to 7 months posttreatment, two of whom were improving with additional itraconazole therapy. One responder was lost to follow-up after 10 months of itraconazole therapy, 3 responders continued to receive itraconazole, and 14 remained disease free during follow-up periods ranging from 6 to 42 months. Experience with fluconazole in the treatment of sporotrichosis is limited. In a multinational study, 13 of 19 patients with cutaneous or lymphocutaneous sporotrichosis responded to fluconazole (200 to 400 mg/day) (59). Of the 13 patients, 10 responded within 6 months and had remained free of infection for at least 8 months (10 of 13 patients) to 1 year (6 of 10 patients). Only one patient who was treated for only 1 month relapsed 2 months posttherapy. These investigators recommended that patients who respond to fluconazole during the first 6 months of therapy be treated for a minimum of 6 to 12 months. For the treatment of extracutaneous sporotrichosis, both fluconazole (200 to 400 mg/day) and ketoconazole (400 to 800 mg/day) appear to be less effective than itraconazole (212). The efficacy of all three drugs in the treatment of advanced pulmonary sporotrichosis has yet to be established; some patients have responded, and others have not (212). No reports of the use of these azoles in meningeal infection due to S. schenckii have been published (212, 234, 272).
Other Systemic Mycoses
Experience with the azoles in other systemic mycoses is limited but suggests that ketoconazole and itraconazole may be useful in the treatment of more indolent cases of infection due to Pseudallescheria boydii or Penicillium marneffei (17). In addition, a child who developed invasive sinonasal infection with Scopulariopsis candida while undergoing treatment for non-Hodgkin’s lymphoma responded to prolonged treatment with a combination of amphotericin B (total dose, 3,000 g for 5 months), itraconazole (500 mg/day for 6 months), and granulocyte colony-stimulating factor; cancer chemotherapy was withheld (137). Neither ketoconazole, itraconazole, nor fluconazole has demonstrated good efficacy in the treatment of mucormycosis (47).
SUPERFICIAL MYCOSES
As shown in Table 4, a number of topical and several oral azole antifungal agents are used in the treatment of a variety of superficial mycotic infections. It should be noted that topical azoles other than those listed in Table 4 are available as well. A detailed discussion of each individual infection and antifungal agent is beyond the scope of this review; a general overview follows. For additional information, the reader is referred to the guidelines of care for superficial mycotic infections of the skin that were developed by the American Academy of Dermatology’s Guidelines/Outcomes Committee and published in 1996 and from which much of the information on clinical uses provided in Table 4 was obtained (64–69).
TABLE 4.
Oral and selected topical azole antifungal agents for superficial mycoses
| Clinical use | Orala
|
Oral/topicala
|
Topical onlya
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| ITRA | FLUC | KETO | CLOT | ECON | MICO | OXIC | TERCb | TIOCb | |
| Mucocutaneous candidiasis | |||||||||
| Oropharyngeal | √c | √ | √d | √e | |||||
| Esophageal | √c | √ | √d | ||||||
| Vaginal/genital | √ | √g | √d | √g | √ | √g | √h | √h | |
| Cutaneous | √f | √ | √ | √ | √ | √ | √ | ||
| Chronic | √f | √ | √ | ||||||
| Onychomycosis | |||||||||
| Candida | √ | √ | √ (T)a | √ | √ | ||||
| Dermatophytes | √ | √ | √ | √ | √ | √ | √ | √fh | |
| Dermatophytoses | |||||||||
| Tinea corporis/cruris | √ | √ | √ | √ | √ | √ | √ | ||
| Tinea pedis | √f | √f | √ | √ | √ | √ | √ | ||
| Tinea capitis/barbae | √f | √f | √ | √ | √ | √ | √ | ||
| Pityriasis (tinea) versicolor | √f | √f | √ | √ | √ | √ | √ | ||
ITRA, itraconazole; FLUC, fluconazole; KETO, ketoconazole; CLOT, clotrimazole; ECON, econazole; MICO, miconazole; OXIC, oxiconazole; TERC, terconazole; TIOC, tioconazole; (T), topical only.
For vaginal use only.
Oral solution.
Second-line azole.
Troches.
Promising.
CDC-recommended regimen.
Nail lacquer.
Mucocutaneous Candidiasis
Many forms of mucocutaneous candidiasis respond well to either topical or systemic therapy with a variety of azole antifungal agents (Table 4) (64). Among the systemic agents, use of ketoconazole for conditions requiring prolonged treatment has been largely replaced by that of fluconazole because of concerns about hepatotoxicity with ketoconazole. This is especially true in patients who have received solid-organ transplants, because of the interaction between ketoconazole and cyclosporin (110). Fluconazole has been used widely to treat mucocutaneous candidiasis, particularly in HIV-infected patients; problems with absorption in this patient population have limited the use of itraconazole p.o. tablets. However, in 1997 itraconazole cyclodextrin p.o. solution with enhanced drug absorption was approved by the FDA for the treatment of OPC and esophageal candidiasis.
Oropharyngeal and esophageal candidiasis.
OPC is a frequent problem in HIV-infected patients and usually precedes esophageal candidiasis (75, 197). Up to 90% of AIDS patients will experience at least one episode of OPC, and up to 50% may develop esophageal infection (197). Recurrent OPC is common, affecting as many as 50% of patients (197). OPC can be treated with both topical and systemic agents (3, 64, 108, 197), although relapse tends to be more rapid following topical therapy (3, 197). For initial and/or milder episodes of OPC, usually seen in patients with early HIV infection, topical antifungal agents such as nystatin pastilles or oral solution, clotrimazole troches, or miconazole buccal gel (available in Europe) are usually effective (3, 72). However, OPC is generally more severe in those with advanced HIV infection, and systemic azole therapy may be required (3). All patients with esophageal candidiasis should be treated with a systemic antifungal agent (197). Although both ketoconazole oral tablets and itraconazole oral capsules are effective in the treatment of oral and esophageal candidiasis in HIV-infected patients, their use in patients with more advanced HIV infection has been limited because of problems with drug absorption (75, 103, 220).
Fluconazole, which is effective in both OPC and esophageal candidiasis (99, 102), has become the standard agent for the treatment of these infections (75, 203). From 1988 to date, more than 20 million patients in the United States, including 500,000 with AIDS, have received fluconazole (data on file, Pfizer Pharmaceuticals, New York, N.Y.). Clinical resistance to fluconazole has emerged among patients with AIDS and OPC, and Rex et al. (220) estimate that approximately 5% of patients with advanced AIDS and OPC or esophageal candidiasis will eventually fail to respond to fluconazole therapy. However, in the absence of inherently resistant Candida species such as C. krusei or C. glabrata, contributory anatomic factors, interactions with concomitant therapies, or poor compliance with the regimen, therapeutic failures are rare and are usually associated with underlying neutropenia and the use of relatively low doses (≤200 mg/day) (220).
Fluconazole therapy remains effective, at least initially, for most patients with AIDS and OPC, and those who ultimately fail to respond to standard doses may respond to 800 mg/day (220). An alternative may be to switch patients who become unresponsive to fluconazole tablets to the oral suspension (156). A pharmacokinetic study comparing drug concentrations in saliva and plasma following administration of fluconazole capsules and oral suspension found that although concentrations in plasma and areas under the concentration-time curve (AUCs) were comparable for the two formulations, the mean AUC0–24 in saliva was greater with the oral suspension than with the capsules (89.13 ± 23.42 and 69.27 ± 12.89 mg/h/liter, respectively) (135). Clinically, Martins and Rex recently reported that two patients with advanced AIDS (CD4+-cell counts below 10/mm3) and OPC refractory to fluconazole tablets (200 mg/day) responded rapidly when switched to fluconazole oral suspension (100 mg b.i.d.) (156).
The use of itraconazole for the treatment of OPC and esophageal candidiasis has been limited to a large extent by the variable absorption of the drug following ingestion of the capsule formulation. This is particularly the case in patients with more advanced HIV infection, who are afflicted by dysphagia and hypochlorhydria and are invariably receiving concomitant drug therapy (111). However, an itraconazole oral solution in hydroxypropyl-β-cyclodextrin with improved absorption was approved by the FDA in February 1997 for the treatment of OPC and esophageal candidiasis. Compared to the capsule formulation, itraconazole absorption from the oral solution was increased by 30% in normal volunteers (107, 270) and trough concentrations in patients with chemotherapy-induced neutropenia were twice as great (98). Higher concentrations of itraconazole and its active metabolite hydroxyitraconazole in serum have also been recorded in HIV-infected patients (32, 221) and contribute to the enhanced efficacy of the oral solution (32). Administration of a 5-mg/kg dose of itraconazole oral solution either q.d. or b.i.d. to bone marrow transplant recipients or patients receiving chemotherapy for acute myeloid leukemia provides adequate concentrations in serum for antifungal prophylaxis (201, 202).
A topical effect of the itraconazole oral solution is also thought to contribute to the more favorable clinical response of patients with OPC compared with the response to the capsule formulation (72, 221). In 23 HIV-infected adults with oral candidiasis treated with 100 mg of itraconazole oral solution given b.i.d. 10 min after breakfast and dinner for 14 days, effective concentrations (>250 ng/ml) of drug were achieved in plasma at day 4 (221). Concentrations of itraconazole in saliva were highest 2 h after ingestion, and mean concentrations in excess of 250 ng/ml persisted at 4 h. A comparison of pharmacokinetic data for 12 patients with CD4+-cell counts of >200/mm3 and no AIDS and 11 patients with CD4+-cell counts of <100/mm3 and AIDS showed that the relative bioavailability of itraconazole from the oral solution was not affected by the stage of HIV infection (221). In both groups of patients, marked clinical improvement was evident within 3 to 4 days after the start of therapy and complete cure was achieved in 6.4 days in all 12 patients with no AIDS and in 6.9 days in 9 of 11 patients with AIDS; the remaining 2 AIDS patients were markedly improved. Based on the rapid therapeutic response, together with the high concentrations of itraconazole found in saliva, the investigators suggest that the oral solution has a topical action (221). Dupont et al. (72) believe that the topical effect of itraconazole solution is greater than the systemic effect for two reasons: (i) a clinical response is evident within a few days, whereas steady-state concentrations in serum after administration of itraconazole capsules are not achieved for 2 weeks, and (ii) although itraconazole has a long half-life, patients relapse quickly following the completion of therapy.
Multicenter clinical trials have shown that itraconazole oral solution is superior to clotrimazole troches for the treatment of OPC in immunocompromised patients (mainly those with HIV infection or AIDS) (164), and as effective as oral fluconazole in the treatment of HIV-infected patients with OPC (107) or esophageal candidiasis (270). In these studies, patients rinsed their mouths vigorously with the oral solution for several seconds before swallowing and did not rinse afterward, contributing to any topical effect. Compared to clotrimazole troches (five 10-mg troches per day), a 14-day course of itraconazole oral solution (200 mg once daily) achieved significantly higher rates of mycological eradication (32 and 60%, respectively P < 0.001) and overall response, defined as a negative culture plus clinical cure or improvement (30 and 53%, respectively; P = 0.006) (164). Relapse during the first month posttreatment occurred in 46% of 39 patients responding to itraconazole and 60% of 21 patients responding to clotrimazole, with the median time to relapse being 31 days and 28 days, respectively. Another open-label study in 179 patients with HIV infection or AIDS demonstrated that itraconazole oral solution (200 mg once daily for either 7 or 14 days) was equivalent to oral fluconazole (100 mg once daily for 14 days) for most efficacy parameters. Complete eradication of lesions was achieved in 87% of patients treated with itraconazole for 7 days, 86% of those treated with itraconazole for 14 days, and 97% of those treated with fluconazole (107). Approximately half of the patients in each treatment group relapsed during the first month posttreatment. The results of a randomized, double-blind comparative study in 126 immunocompromised patients with esophageal candidiasis showed that itraconazole oral solution was clinically and mycologically equivalent to oral fluconazole (270). Both drugs were administered in a dosage of 100 to 200 mg once daily for 3 to 8 weeks; treatment was continued for an additional 2 weeks following resolution of symptoms. A clinical response (cured or improved) was achieved in 94% (50 of 53 patients) in the itraconazole group and 91% (52 of 57 patients) in the fluconazole group; the corresponding rates of mycological eradication were 92% (45 of 49 patients) and 78% (40 of 51 patients), respectively. Relapse within 30 days after completing treatment occurred in 8 patients (18%) treated with itraconazole and 12 patients (27%) treated with fluconazole; median times to relapse were not significantly different.
Itraconazole oral solution has proven to be effective for HIV-infected patients with OPC clinically refractory to fluconazole (32, 33, 72, 81, 195), although a small percentage (∼30%) of patients with isolates cross-resistant to itraconazole fail to respond (32, 33). In a preliminary study in 24 patients with pseudomembranous OPC refractory to treatment with 200 to 400 mg of fluconazole per day for 7 to 10 days, treatment with itraconazole oral solution (100 mg b.i.d.) (duration not stated) cured 21 patients and markedly improved the condition of the remaining 3 patients (72). No follow-up data were provided. These investigators noted that in their experience, treatment of such patients with 200 to 400 mg of encapsulated itraconazole per day has been of limited value. In a multicenter, open-label trial, 78 HIV or AIDS patients with OPC who had failed to respond to treatment for 14 days or longer with at least 200 mg of fluconazole per day within the 14 days preceding the study were treated with itraconazole (100 mg b.i.d.) for 14 days, which was continued for an additional 14 days if the patient had not responded (81). Among 74 patients with positive mycological cultures at study entry, clinical improvement was achieved in 52 (70%), with complete clearing of lesions in 41 (55%). However, mycological eradication was documented at day 14 or day 28 in only 8 (11%) of the patients, although 17 (23%) had mycological cure, defined as yeast quantification of ≤20 CFU/ml. This was not unexpected, because clinical improvement despite the persistence of yeast is frequently seen in AIDS patients with recurrent OPC (204). All 22 responders who were monitored posttreatment relapsed an average of 13 days after clinical cure, according to Kaplan-Meier estimates. A similar overall clinical response rate of 65% (22 of 34 patients) was observed following a 14-day course of itraconazole solution (100 mg b.i.d.) in another prospective, open-label study in HIV-infected patients failing to respond to ≥100 mg of fluconazole per day for ≥10 days (195). However, 4 (36%) of 11 responders with posttreatment follow-up relapsed within 2 months. Itraconazole cross-resistance (defined as an MIC of >10 mg/liter) was implicated in the failure of therapy with itraconazole oral solution in four patients with C. albicans infection. Likewise, two of four patients who had failed to respond to prior therapy with itraconazole capsules (200 to 300 mg/day) failed to respond to itraconazole oral solution; the remaining two responded (one completely and one partially).
Studies relating clinical outcome with itraconazole oral solution to concentrations in serum and in vitro susceptibility in HIV and AIDS patients with risk factors for or documented clinical resistance to fluconazole (32, 33) used the Odds relative growth microplate method for in vitro studies (181) rather than the National Committee for Clinical Laboratory Standards (NCCLS) approved method for yeasts (169). With Odds’ method, a cutoff relative growth in itraconazole of 68% was correlated with failure to respond clinically to itraconazole oral solution (32, 33). Among patients with itraconazole-susceptible isolates, clinical failure was associated with failure to achieve an itraconazole concentration in serum in excess of 1,000 ng/ml on day 7 of treatment (as measured by high-performance liquid chromatography), and none of the patients with itraconazole-resistant isolates responded to treatment (32). An assessment of the response of HIV-infected patients with OPC to itraconazole oral solution (200 mg b.i.d. for 7 days) according to in vitro susceptibility showed that although the rate of clinical clearance of candidiasis in patients with fluconazole-susceptible isolates was 100% (28 of 28 patients), it was only 60% (43 of 72 patients) in patients with a fluconazole-resistant isolate and prior clinical failure to respond to fluconazole therapy (33). Cross-resistance to itraconazole was documented in 29 (30%) of 96 fluconazole-resistant Candida isolates and in 38% of C. glabrata isolates, but all fluconazole-susceptible isolates remained susceptible to itraconazole. All five C. krusei isolates were resistant to fluconazole but remained susceptible to itraconazole.
Overall, itraconazole oral solution has been well tolerated, with a side effects profile similar to that of fluconazole (107, 270). The adverse events most commonly reported by patients receiving itraconazole oral solution were nausea, vomiting, diarrhea, and abdominal pain (81, 107, 164, 195, 270). In two studies, patients complained about the taste of the itraconazole oral solution (98, 195), which did not appear to interfere with compliance among HIV-infected patients (195) but which was associated with nausea immediately after ingestion in patients with chemotherapy-induced neutropenia (98).
The USPHS/IDSA recommends chronic suppressive fluconazole therapy for HIV-infected adults or adolescents who have experienced one or more episodes of esophageal candidiasis (256). However, most experts do not recommend lifelong antifungal prophylaxis for OPC or esophageal candidiasis, unless the patient is profoundly immunocompromised (CD4+-cell count, ≤50/mm3), because of concerns about the emergence of resistance and because that most episodes of infection respond clinically to topical or oral antifungal therapy (12, 147, 256). For patients in whom prophylaxis is indicated, maintenance with 50 to 200 mg/day or 150 to 200 mg/week (147, 199, 204, 210, 233) decreases the rate of subsequent relapse following resolution of an episode of OPC. Options for patients who relapse repeatedly are topical clotrimazole or nystatin, or fluconazole, ketoconazole, or itraconazole (147, 256), which may be administered either intermittently or continuously. As experience with itraconazole oral solution becomes greater, this formulation may also prove particularly useful for long-term suppressive therapy. Continuous administration of antifungal prophylaxis appears to be preferable to intermittent administration, because several controlled trials have shown lower relapse rates among patients receiving drug continuously (119, 204, 210).
As noted previously, there is a concern that resistance will develop during fluconazole prophylaxis. A recent comparative study by the NIAID/ACTG in patients with advanced HIV infection demonstrated that prophylaxis with fluconazole (200 mg/day) was more effective than clotrimazole troches (10 mg five times daily) in decreasing not only superficial fungal infections but also cryptococcosis, esophageal candidiasis, and invasive mycoses (199). However, 10.6% of patients receiving the drug experienced at least one episode of candidiasis (either proved or presumed) during follow-up. This suggests the possible development of fluconazole resistance, but no data on resistance patterns were obtained in this trial.
Other investigators have monitored the development of resistance in oral isolates from patients receiving fluconazole prophylaxis during controlled clinical trials (119, 210). In one study, HIV-positive patients (CD4+-cell count, <350/mm3) received either intermittent or continuous fluconazole prophylaxis (200 mg/day) following treatment of an acute episode of OPC (210). Over a median follow-up of 11 months, oral samples for culture were obtained weekly during OPC episodes and quarterly for surveillance. Microbiological resistance (defined in this study as a fourfold increase in the MIC to ≥16 μg/ml) was noted in 9 (56%) of 16 patients receiving continuous fluconazole and in 13 (46%) of 28 receiving intermittent therapy (P = 0.75). Nevertheless, therapeutic responses to fluconazole (up to 800 mg/day) for the treatment of recurrent OPC were excellent in 42 of 44 patients. Another study compared oral isolates from HIV-infected patients (CD4+-cell count, <400/mm3) who had received either continuous (19 patients) or intermittent (11 patients) fluconazole for at least 6 months as maintenance therapy for cryptococcal meningitis or to prevent OPC or esophageal candidiasis with those from matched controls who had not received fluconazole during the preceding 6 months (119). Compared with matched controls, significantly more (P < 0.001) patients receiving continuous fluconazole had sterile cultures; none of those receiving intermittent fluconazole had sterile cultures. Moreover, both groups of patients receiving fluconazole were more likely to have non-albicans species of Candida isolated, and these isolates, as well as C. albicans isolates, were more likely to be associated with high fluconazole MICs (i.e., ≥16 μg/ml). However, none of the patients receiving continuous fluconazole had symptoms of oroesophageal candidiasis whereas five patients receiving intermittent fluconazole had signs and/or symptoms of OPC.
These studies suggest that fluconazole prophylaxis can select for non-albicans species of Candida and that these species, as well as C. albicans isolates, are more likely to show in vitro resistance to fluconazole. However, this increased in vitro resistance has not translated into clinical resistance thus far.
Vulvovaginal candidiasis.
An estimated 75% of women will experience at least one episode of vulvovaginal candidiasis (VVC) during their lifetime (73). VVC is often associated with conditions such as diabetes mellitus, antibiotic therapy, and pregnancy, but many women have no predisposing factors (36, 73). Although retrospective data reported during the early years of the AIDS pandemic suggested that the prevalence of VVC was increased in HIV-infected women compared to noninfected women, an association has yet to be proven; prospective, controlled studies to resolve this issue are in progress (36, 268). Moreover, the response of VVC to standard antifungal therapy appears to be similar among HIV-infected and HIV-negative women (36). In the 1998 guidelines issued by the Centers for Disease Control and Prevention (CDC), VVC is now classified as either uncomplicated “ … ie, mild-to-moderate, sporadic, nonrecurrent disease in a normal host with normally susceptible C. albicans” or complicated “ … ie, severe local or recurrent VVC in an abnormal host (eg, VVC in a patient who has uncontrolled diabetes or infection caused by a less susceptible fungal pathogen such as Candida glabrata).” Treatment for 7 days or less is effective for uncomplicated VVC, but complicated infection requires 10 to 14 days of therapy with either a topical or p.o. azole (36).
Current treatment recommendations for uncomplicated VVC issued by the CDC apply to both HIV-infected and HIV-negative women (Table 5) (36) and probably would be effective for the treatment of this infection in children and adolescents (259). Intravaginal therapy with an azole antifungal agent is more effective than topical nystatin and provides clinical and mycological cure in 80 to 90% of patients who complete the full course of therapy (36, 238). Two of the recommended intravaginal regimens, 500-mg clotrimazole vaginal tablet and 6.5% tioconazole ointment, require only a single application, which should optimize compliance. Other intravaginal regimens require 3 or 7 days of therapy. Intravaginal azole therapy is generally well tolerated, but Sobel’s experience indicates that mild to moderate vulvovaginal burning occurs more often than is generally thought (238).
TABLE 5.
1998 CDC recommended regimens for uncomplicated vulvovaginal candidiasisa
| Intravaginal agents |
| Butoconazole 2% cream 5 g intravaginally for 3 daysbc |
| or |
| Clotrimazole 1% cream 5 g intravaginally for 7–14 daysbc |
| or |
| Clotrimazole 100-mg vaginal tablet for 7 daysb |
| or |
| Clotrimazole 500-mg vaginal tablet, one tablet in a single applicationb |
| or |
| Miconzole 2% cream 5 g intravaginally for 7 daysbc |
| or |
| Miconazole 200 mg vaginal suppository, one suppository daily for 3 daysbc |
| or |
| Miconazole 100 mg vaginal suppository, one suppository daily for 7 daysbc |
| or |
| Nystatin 1000,000-unit vaginal tablet, one tablet daily for 14 days |
| or |
| Tioconazole 6.5% ointment 5 g intravaginally in a single applicationbc |
| or |
| Terconazole 0.4% cream 5 g intravaginally for 7 days,b |
| or |
| Terconazole 0.8% cream 5 g intravaginally for 3 days,b |
| or |
| Terconazole 80 mg vaginal suppository, one suppository daily for 3 daysb |
| Oral agent |
| Fluconazole 150 mg oral tablet, one tablet in single dose |
Adapted from reference 36.
These creams and suppositories are oil based and might weaken latex condoms and diaphragms. Refer to condom product labeling for additional information.
Over-the-counter preparation.
Many women prefer oral therapy, and the CDC recommends fluconazole (150 mg) given in a single dose (36), the oral azole regimen approved by the FDA in June 1994. Clinical trials have shown this regimen to be as effective as intravaginal therapy with clotrimazole (100 mg for 7 days or 200 mg for 3 days) (207, 239) or miconazole (400 mg for 3 days) (251). Alternatives are itraconazole (single day) or ketoconazole, although use of ketoconazole is limited because of potential hepatotoxicity (36, 238). With regard to itraconazole, a placebo-controlled trial demonstrated that oral itraconazole (200 mg/day) was as effective as clotrimazole vaginal tablets (200 mg/day) for the treatment of acute VVC (241). One week after completing treatment, 46 (96%) of 48 patients treated with itraconazole and 17 (77%) of 22 patients treated with clotrimazole were asymptomatic or significantly improved clinically and 35 (73%) and 16 (95%) of these patients, respectively, had negative mycological cultures. Four weeks posttreatment, the clinical failure rate with itraconazole was lower than that with clotrimazole (17% [8 of 48 patients] and 30% [6 of 20 patients], respectively), although the difference was not statistically significant (P > 0.05; β = 0.81). Mycological eradication rates were similar, being 89% (31 of 35 patients) with itraconazole and 83% (15 of 18 patients) with clotrimazole.
Recurrent VVC, defined as four or more symptomatic episodes within 1 year, is experienced by fewer than 5% of women, most of whom have no obvious predisposing factors (36, 242). For these patients, the CDC recommends that maintenance therapy for at least 6 months begin immediately after 10 to 14 days of intensive initial therapy (36). A 100-mg p.o. dose of ketoconazole once daily for up to 6 months has been found to decrease the frequency of VCC recurrences, and a weekly fluconazole regimen is being investigated clinically (36). In two multicenter, placebo-controlled trials, administration of a single 150-mg p.o. dose of fluconazole monthly for 4 or 12 months provided effective prophylaxis against recurrent candidiasis in one study (237). With prophylactic administration for 4 months, VVC recurrence rates were 39 and 53%, respectively, among women who received fluconazole or placebo (P = 0.051). Among women receiving prophylaxis for 12 months, the recurrence rate (42%) in the fluconazole group was significantly (P = 0.02) lower than that (68%) in the placebo group. The median times to recurrence with fluconazole prophylaxis for either 4 or 12 months (153 days and >363 days, respectively) were significantly longer than those with placebo (83 and 182 days, respectively); P values at 4 and 12 months were 0.036 and 0.007, respectively. Weekly application of 0.8% terconazole cream may also provide effective prophylaxis. In one clinical trial, 22 women were treated initially for an acute episode of VVC with 0.8% terconazole cream for 3 days, which was extended for an additional 3 days if the patient remained symptomatic after the first 3 days (242). This was followed by suppressive therapy with one applicator of 0.8% terconazole cream once weekly for an additional 26 weeks. One patient discontinued treatment after 16 weeks due to vaginal irritation, and another patient was lost from the study. During 26 weeks of prophylaxis, 10 (50%) of 20 women experienced 14 episodes of vaginal symptoms, 5 of which were attributable to genital pathogens including Candida (4 episodes) and bacterial vaginosis (1 episode). After the prophylactic phase of the study, 14 (78%) of 18 women monitored for an additional 26 weeks experienced a recurrence of VVC, a statistically significantly higher incidence (P < 0.001, Fisher’s exact test) than that observed during maintenance therapy.
Sex partners of women diagnosed with VVC do not receive antifungal therapy routinely unless they have symptomatic balanitis or penile dermatitis (36). Therapeutic options include a 1- to 2-week course of a topical antifungal agent such as clotrimazole, econazole, ketoconazole, or miconazole or p.o. treatment with fluconazole or itraconazole (64, 240). With regard to oral azoles, a recent randomized, open-label, parallel-group, multicenter study of 157 men with candidal balanitis found a single 150-mg p.o. dose of fluconazole to be as effective as topical 1% clotrimazole cream applied twice daily for 7 days (240). Approximately 90% of patients in each treatment group had responded clinically and mycologically when examined 8 to 11 days after the start of treatment. The investigators commented that single-dose p.o. therapy was more convenient for the patients than multiple-dose topical therapy, which may have improved patient acceptance and compliance; 12 of 15 patients previously treated with topical antifungal agents stated a preference for p.o. fluconazole.
Cutaneous candidiasis.
Cutaneous candidiasis may be generalized or limited to interdigital areas or the hair follicles (73). In HIV-infected patients, areas commonly involved are the groin, axilla, and/or inframammary region (3). Unless infection is widespread or refractory or occurs in an immunocompromised patient, topical azoles are generally effective when given alone or in combination with a short course of topical corticosteroid to reduce inflammation (64). More severe infections require systemic therapy with a p.o. azole or possibly terbinafine (64).
Chronic mucocutaneous candidiasis.
Chronic mucocutaneous candidiasis (CMC), a chronic, recurrent form of candidiasis affecting the skin, nails, and mucous membranes, is an immunologic disease (73, 134). In most patients, the major immunologic defect is the inability of T lymphocytes to respond effectively to stimulation with Candida antigen (73, 134). Individuals who develop the disease in infancy or early childhood have the most severe immunologic defects (134). Topical azole therapy for skin and mucous membranes involvement is of limited value (73) but may be useful as an adjunct to systemic treatment with fluconazole, ketoconazole, or itraconazole (64). Prolonged treatment is needed, and unless it is combined with immunostimulating procedures, most patients will relapse within a few weeks or months after azole therapy is discontinued (73, 134). According to Kirkpatrick (134), patients treated with Candida transfer factor in combination with a systemic antifungal agent have experienced remissions of CMC lasting more than 10 years.
Onychomycosis
Most nail infections are caused by dermatophytes (Trichophyton spp. and Epidermophyton floccosum), followed by yeasts (mainly C. albicans) and, rarely, saprophytic moulds (111, 223). Although a number of topical agents are active against dermatophytes (Table 4), they seldom cure fungal nail infections when used as monotherapy, even with prolonged application (43, 65, 111, 145). However, use of topical antifungal agents may slow the spread of infection in patients who are unwilling or unable to take p.o. antifungal agents (76). Preliminary data suggest that nail lacquers containing either amorolfine (43, 49) or tioconazole (65) may offer increased efficacy. Until the availability of p.o. azoles, griseofulvin was the primary p.o. agent for the treatment of onychomycosis due to dermatophytes but not Candida spp. or saprophytes (117, 145, 223). Cure rates in fingernail infections approach 80% after 6 to 9 months of griseofulvin therapy, but 50% or less of toenail infections respond even to prolonged therapy for 12 to 18 months (223). With ketoconazole, it became possible to treat onychomycosis due to Candida as well as dermatophytes, but prolonged administration was still necessary because, like griseofulvin, ketoconazole must be administered until the nail plate is clear (145, 223). In fingernail infections, ketoconazole was slightly more effective than griseofulvin; however, the response in toenail infections remained inadequate (∼50% cure rate) (223). Because of the risk of hepatotoxicity with prolonged therapy, p.o. ketoconazole is no longer used for fungal nail infections (223). Until recently, chemical or surgical avulsion of infected nails was used in conjunction with topical or systemic therapy in an attempt to increase effectiveness (43, 145); it is rarely performed now (65).
Today, griseofulvin and ketoconazole have been replaced by p.o. terbinafine and p.o. itraconazole, both of which are approved in the United States for the treatment of onychomycosis due to dermatophytes. A meta-analysis of 18 published studies by Arikian et al. (8) suggest that itraconazole is more effective in the treatment of toenail onychomycosis than either griseofulvin or ketoconazole but is less effective than terbinafine. For fingernail onychomycosis, a meta-analysis of 16 studies showed that terbinafine was the most effective treatment, followed by ketoconazole, itraconazole, and griseofulvin (8). However, an advantage of itraconazole is that it is effective in the treatment of nail infection due to Candida as well as dermatophytes (111, 224) whereas the activity of p.o. terbinafine is limited to dermatophyte infections (65, 145, 223, 224). Terbinafine is administered p.o. in a dosage of 250 mg q.d. for 6 weeks in patients with fingernail infections and for 12 weeks in patients with toenail infections. Similarly, the recommended itraconazole regimen for the treatment of toenail onychomycosis with or without fingernail involvement is 200 mg q.d. for 12 weeks. A pulse regimen consisting of two 1-week treatment periods with 200 mg b.i.d. separated by a 3-week drug-free period effectively treats fingernail onychomycosis. Pulse therapy with itraconazole is effective, because the drug persists in therapeutic concentrations in fingernails for up to 3 months and in toenails for up to 6 months after the completion of therapy (111). Results of a recent randomized, open trial in 50 patients with mycologically confirmed onychomycosis due mainly to Trichophyton rubrum suggest that pulse therapy is also effective for toenail infections (48). Response rates were comparable among patients treated with either three or four pulses of itraconazole administered in a dosage of 200 mg b.i.d. for the first 7 days of each month. Detectable amounts of itraconazole were present in the distal ends of the nails after the first pulse, reached therapeutic concentrations (>250 ng/g) with further therapy, and persisted at levels in excess of 300 ng/g for several months after the completion of therapy. At follow-up 1 year after the start of treatment, clinical response rates (cure or marked improvement) were 88% (22 of 25 patients) in the three-pulse group and 84% (21 of 25 patients) in the four-pulse group; mycological cure rates (negative KOH preparation and negative culture) were 80% (20 of 25 patients) in both groups. The itraconazole regimen of four pulses of 200 mg b.i.d. for the first 7 days of each month has also been reported to be efficacious in the treatment of onychomycosis in patients with HIV infection (3).
Another promising alternative for the treatment of onychomycosis is oral fluconazole, which may be effective against infection due to both dermatophytes and C. albicans (9, 65, 117, 139, 145). Detectable levels of fluconazole are present in the nails within 48 h (116), and in a study of normal subjects a mean concentration of 1.5 mg/kg was achieved in fingernails with administration of 50 mg/day for 2 weeks (102). Clinical experience with fluconazole in onychomycosis is limited. An open, noncomparative study in 20 patients with onychomycosis due to T. rubrum evaluated once-weekly administration of fluconazole (150 mg) for up to 12 months (mean duration, 9.3 months) (139). The 39 most severely affected toenails and 7 fingernails were used to assess therapeutic efficacy. Clinical and mycological cures were achieved for all fingernails and 92% of toenails, and respective cure rates 6 months after the completion of treatment were 100 and 83%. Administration of fluconazole (100 mg per day for 24 weeks) clinically and mycologically cured dermatophyte fingernail infection in three of five healthy adults who had failed to respond to prior therapy with topical or oral antifungal agents. Another patient who remained mycologically positive at the end of treatment was clinically clear and culture negative at the 3-month posttreatment follow-up examination (125). In another study, a 100-mg dose of fluconazole administered every other day for 4 to 8 months was successful in the treatment of toenail infection in two patients who had failed to respond to prolonged therapy with p.o. griseofulvin (44). Like itraconazole, fluconazole has successfully treated onychomycosis in patients with HIV infection (3, 76). Once-weekly treatment with 150 mg of fluconazole effectively treated fingernail onychomycosis in a renal transplant patient (165). Marked improvement was noted at 3 months, treatment and complete resolution occurred after 5 months of treatment, and the patient remained free of infection at 2 months posttreatment. No adjustments in cyclosporin A dosage were necessary during fluconazole therapy. In another study, 11 patients with moderate-to-severe onychomycosis were treated with 200 mg (1 patient) or 300 mg (8 patients) of fluconazole once weekly or with 100 or 200 mg of fluconazole every other day (2 patients) (9). None of the patients underwent concurrent nail avulsion, but eight did use topical antimycotic agents (e.g., 1% creams containing terbinafine, oxiconazole, or econazole) as adjuncts. All six patients with toenail infection, which was caused by T. rubrum in four patients, were cured clinically after once-weekly fluconazole therapy lasting for 20 to 36 weeks (mean time to cure, 25.7 weeks). Any concurrent tinea pedis also was cured, as was concomitant fingernail onychomycosis. Five patients with fingernail infection only, which was caused by C. albicans in three patients, C. parapsilosis in one patient, and Fusarium solani in one patient, were clinically cured following treatment with fluconazole either once weekly (three patients) or every other day (two patients). The mean duration of therapy for fingernail onychomycosis was 15.8 weeks (range, 8 to 28 weeks). Cultures were not repeated posttreatment to document the mycological response. At the time of their report, Assaf and Elewski (9) commented that all patients remained clinically clear of infection but that most had only completed therapy 2 to 3 months earlier. One patient with fingernail infection due to F. solani remained clear 14 months after completing therapy.
Dermatophytoses
Many dermatophytoses respond well to topical antifungal therapy; however, more extensive or severe disease may require systemic therapy. Prolonged treatment (∼6 weeks) with an oral antifungal agent generally is needed to cure tinea capitis and tinea barbae; topical antifungal preparations may be used as adjuncts (68, 84). Negative cultures were achieved in 93% of 42 patients with tinea capitis due to Trichophyton tonsurans or Microsporum canis following treatment with itraconazole, the usual regimen being 100 mg/day for 5 weeks (143). Likewise, 89% of children included in a multicenter, noncomparative study were cured 2 months after completing treatment with itraconazole (5 mg/kg/day) for 6 weeks (49). Noninflammatory lesions of tinea corporis, tinea cruris, and tinea pedis generally respond to topical treatment with one of the azoles or other topical antifungal agents, but inflammatory lesions may require p.o. therapy, particularly if the infection is chronic or has failed to respond to prior topical antifungal agents or the patient is immunosuppressed (69). Among symptomatic, HIV-infected patients, tinea pedis is the most common dermatophytosis, followed by onychomycosis and tinea cruris (3). Moreover, most cases of tinea corporis in this patient population are actually an extension of tinea cruris infection (3). Tinea cruris and tinea corporis in AIDS patients require systemic p.o. therapy with one of the azoles, whereas most cases of uncomplicated tinea pedis will respond to topical treatment with a broad-spectrum azole (3).
A number of comparative trials have shown that itraconazole is more effective than griseofulvin in the treatment of tinea corporis, tinea cruris, and tinea pedis, and two studies comparing 15- to 30-day treatment with itraconazole (100 mg/day) or fluconazole (50 mg/day) suggest that the efficacy of these triazoles is similar (111). According to Degreef and De Doncker (49), the duration of therapy can be shortened by increasing the daily dose of itraconazole to 200 mg. Mycological cure and a clinical response at the end of follow-up (time not specified) were achieved in 89 and 97%, respectively, of patients treated for tinea corporis and tinea cruris with itraconazole (200 mg/day) administered for 1 week. For the treatment of tinea pedis, itraconazole (200 mg/day) is effective when administered once daily for 2 weeks or twice daily for 1 week (50). One study of patients with “dry-type” tinea pedis found that 95% had responded clinically at 4 weeks posttreatment and 85% were mycologically cured (49).
Pityriasis (tinea) versicolor generally responds to topical antifungal therapy (67), but 60 to 80% of infections recur (231). Savin (231) recommends a short course of a p.o. azole such as itraconazole (200 mg/day for 7 days) or fluconazole (400 mg/day for 3 days). A 400-mg dose of ketoconazole also is effective when administered once or repeated monthly (49). For HIV-infected patients, Aly and Berger (3) recommend p.o. ketoconazole administered as a 400-mg dose for 1 to 3 days and repeated monthly thereafter. If Malassezia folliculitis is present, a regimen of p.o. ketoconazole (200 mg/day) for 10 to 14 days is suggested (3). Because relapse is common among patients with pityriasis versicolor, the effect of itraconazole prophylaxis was studied in 125 patients who had responded to initial treatment with itraconazole (200 mg/day) for 7 days and were then randomized 1 month later to treatment with a single 200-mg dose of itraconazole or placebo given once monthly for 5 months (111). Clinical and mycological recurrences were recorded significantly more often in patients receiving placebo (11%) than in those receiving itraconazole (29%).
IN VITRO SUSCEPTIBILITY TESTING
In 1997, NCCLS released the approved version (M27-A) of standardized broth macrodilution and microdilution methods for the antifungal susceptibility testing of yeasts (169). The M27 method was proposed in 1992, after two multicenter studies provided data on critical factors affecting the reproducibility of testing from one laboratory to another, including end-point definition, inoculum size and preparation, incubation time and temperature, and media (191, 194). For a detailed discussion of these and other issues that were addressed in the course of development of the method, the reader is referred to the 1993 reviews by Rex et al. (219) and Sheehan et al. (236). Subsequent studies identified suitable quality control isolates (189, 218), showed that there was good agreement between broth macrodilution and microdilution methods (80), established that incubation at 35°C for 48 and 72 h provided the most consistent results for Candida spp. and Cryptococcus neoformans, respectively (87), and demonstrated that the 48-h microdilution MIC was in close agreement with the macrodilution MIC (80). A summary of the M27 methodology is provided in Table 6 (217). The interlaboratory reproducibility with this method for the antifungal susceptibility testing of yeasts compares well with that achieved for the testing of antibacterial susceptibility (217).
TABLE 6.
Summary of the M27 method developed by the NCCLSa
| Factor | Implementation in the M27 methodology |
|---|---|
| Methodology | Broth macrodilution, 1-ml final volume; or broth microdilution, 0.2-ml final volume |
| Medium | RPMI 1640 containing 0.165 M MOPSb (pH 7.0) |
| Fungal inoculum | (0.5–2.5) × 103 organisms |
| Incubation temp | 35°C |
| Incubation time | 48 h (Candida species) or 72 h (Cryptococcus neoformans) |
| End point | Amphotericin B, optically clear tube; azoles and 5-FC, 80% reduction in turbidity by comparison with growth control |
| Drug and quality control (QC) isolates | Two QC isolates and corresponding QC ranges established via the M23 procedure are specified for amphotericin B, flucytosine, ketoconazole, itraconazole, and fluconazole |
Adapted from reference 217 with permission of the publisher.
Morpholinepropanesulfonic acid.
Once standardized methodology was developed, members of the NCCLS Subcommittee for Antifungal Susceptibility Testing investigated interpretive breakpoints for antifungal agents (217). Interpretive guidelines for testing the susceptibility of Candida isolates to fluconazole and itraconazole developed by the Subcommittee after its analysis of data packages submitted to the NCCLS by the manufacturers and incorporated in the approved method appear in Table 7 (169). Factors considered for each drug included MIC and outcome data (mainly for OPC in AIDS patients), pharmacology, correlation between MICs and results of animal studies, and clinical data correlating MIC with outcome. For details of the Subcommittee’s analysis, the reader is referred to their 1997 publication in Clinical Infectious Diseases (217). Overall, the fluconazole data were consistent with the view that both MIC and drug dose contribute to the therapeutic response to this triazole and that, regardless of the actual MIC, C. krusei is intrinsically resistant to the drug. For itraconazole, the key factors were the MIC and itraconazole level in plasma rather than the dose; data from studies of OPC in AIDS patients suggest that the therapeutic response may be enhanced when itraconazole levels in plasma exceed 0.5 μg/ml (217).
TABLE 7.
Interpretive guidelines for susceptibility testing in vitro of Candida speciesa
| Antifungal agent | Breakpointb (μg/ml) for Candida against agent
|
|||
|---|---|---|---|---|
| Susceptible (S) | Susceptible-dose dependent (S-DD)c | Intermediate (I) | Resistant (R) | |
| Fluconazolee | ≤8 | 16–32 | —d | ≥64 |
| Itraconazolef | ≤0.125 | 0.25–0.5 | — | ≥1 |
Adapted from reference 169 with permission of the publisher. The interpretive data are valid only if the methodology in M27-A is followed. The current standard may be obtained from NCCLS, 940 West Valley Road, Suite 1400, Wayne, PA 19087.
Shown are the breakpoints for Candida species against the indicated agents. If MICs are measured on a scale that yields results falling between categories, the next higher category is implied. Thus, an isolate with a fluconazole MIC of 12.5 μg/ml would be placed in the S-DD category. These breakpoints were adopted at a meeting of the subcommittee held June 1, 1996 in Reston, VA. They are considered tentative for 1 year and are open for comments.
Susceptibility is dependent on achieving the maximal possible level in blood. For fluconazole, doses of 400 mg/day or more may be required in adults with normal renal function and body habitus. For itraconazole, measures to ensure adequate drug absorption and concentrations of >0.5 μg/ml in plasma may be required for an optimal response.
—, the susceptibility of these isolates is not certain, and the available data do not permit them to be clearly categorized as either susceptible or resistant.
For fluconazole, these guidelines are based substantially on experience with mucosal infections but are consistent with the limited information for invasive infectious due to Candida spp. Isolates of C. krusei are assumed to be intrinsically resistant to fluconazole, and the MICs for these isolates should not be interpreted on the basis of this scale. It is also pertinent that the 8-μg/ml upper boundary for the range of susceptibility to fluconazole is not known with certainty; the data would permit selection of either 4 or 8 μg/ml for this cutoff.
For itraconazole, the data are based entirely on experience with mucosal infections and data supporting breakpoints for invasive Candida infections are not available.
A number of future modifications of the M27-A method are under investigation. To provide for isolates exhibiting trailing growth when tested against azole antifungal agents, the method established 48 h as the appropriate time for reading MICs for Candida spp. and the end-point criterion as an 80% reduction in growth (215). However, the correlation between MICs and outcome in vivo may be improved by shortening the incubation time to 24 h (215, 217) and by relaxing the end-point criterion, at least for fluconazole, to the lowest drug concentration producing a 50% reduction in growth (215). This was investigated in a recent study in which Candida isolates showing trailing growth when tested against fluconazole were ranked in order of in vivoresponse in a murine model of invasive candidiasis (215). For three of the six isolates tested, the M27-A criterion overestimated resistance. However, a better correlation between the MIC and the in vivo response was obtained by using the criterion of a 50% reduction in growth after 24 h of incubation. Another strategy used with the broth microdilution method disperses trailing growth by mechanical agitation of the microdilution tray before the MIC is read (5). A multilaboratory study evaluated this technique when it was used to test C. albicans and C. neoformans against fluconazole, 5-FC, and amphotericin B (5). Additional modifications to the M27 microdilution method used in this study were an increased total volume, a reduced incubation time of 24 h for C. albicans and 48 h for C. neoformans, and a 10-fold greater inoculum. End-point criteria were a reduction in growth of at least 75% before agitation or of at least 50% after agitation. All aspects of the method showed good to excellent reproducibility, regardless of drug, organisms, or end-point criterion. Trailing growth was not completely eliminated by agitation, but the technique did make it possible for most observers to interpret the end point, particularly for fluconazole.
Colorimetric and spectrophotometric tests are also under investigation as a means of decreasing the subjective nature of the NCCLS methods, although the cost of the spectrophotometric equipment for turbidimetric testing puts it beyond the budget of many clinical laboratories (78). A colorimetric test incorporating the oxidation-reduction indicator Alamar Blue (Accumed/Sensititre/Alamar, Westlake, Ohio) to enhance the readability of broth microdilution end points compared favorably with the NCCLS broth microdilution method when used to determine the antifungal susceptibility of 600 clinical yeast isolates to amphotericin B, fluconazole, and 5-FC; overall agreement between methods was 95% or greater for all three antifungal agents at 24 h (190). An advantage of the colorimetric microdilution method is that it can be automated (190). Another simple and relatively low-cost method under investigation is an adaptation of the Etest for antifungal susceptibility testing. A plastic strip containing a defined continuous gradient of antifungal drug is placed on the surface of inoculated RPMI agar and then incubated overnight or for 24 h for Candida spp. and C. glabrata or for 48 to 72 h for Cryptococcus neoformans (46). Etest strips containing amphotericin B, fluconazole, 5-FC, itraconazole, and ketoconazole are currently available for investigational purposes (78). Studies comparing the Etest MICs of the three azoles for these yeast species with the NCCLS broth macrodilution and microdilution MICs showed that there was good general agreement between methods (46, 77). However, one group recently reported large discrepancies between Etest and NCCLS microdilution MICs and commented that the Etest was difficult to read for azole antifungal drugs (97).
The NCCLS method has limited ability to identify isolates of Candida and Cryptococcus resistant to amphotericin B (96). By substituting antibiotic medium 3 broth buffered to pH 5 or 7 for RPMI 1640 medium buffered to pH 7, Rex et al. (214) were able to discriminate between amphotericin B-resistant and -susceptible isolates of Candida. Further studies to identify suitable quality control isolates and to establish interlaboratory reproducibility are in progress (214). However, the Etest may provide a simpler alternative. A comparison of the Etest and the NCCLS broth macrodilution method with 91 clinical Candida isolates found that agreement within ±2 dilutions between the two methods was 95% for fluconazole and 96 to 97% for amphotericin B (261). Moreover, the Etest was able to identify amphotericin B-resistant isolates on glucose-supplemented RPMI 1640 agar as well as on undefined antibiotic medium 3.
Adaptations of the NCCLS broth macro- and microdilution methods for yeasts are being evaluated for the antifungal susceptibility testing of filamentous fungi (78, 79). At present, routine susceptibility testing of yeasts and other fungi is not recommended (193, 217). However, experts believe that susceptibility testing of Candida spp. may help guide therapy when AIDS patients with oropharyngeal candidiasis or some patients with invasive candidiasis fail to respond to azole therapy (193, 269).
ISSUES IN ANTIFUNGAL DRUG RESISTANCE
For details of the cellular and molecular mechanisms of azole resistance described to date for C. albicans, many of which are probably operative in other species of fungi, the reader is referred to the recent review by White et al. (269). This section provides a brief overview of current knowledge about resistance in the clinical setting, which has been reported for each of the currently marketed oral azole antifungal agents (269). Most reviews have focused on azole resistance in Candida spp., and the majority of reports have discussed fluconazole resistance because this drug has been the most widely used azole. However, fluconazole-resistant clinical isolates of C. neoformans (257) and H. capsulatum (266) and itraconazole-resistant clinical isolates of A. fumigatus (55) have been documented recently. The focus here is on Candida spp. because it has been studied extensively.
Candida spp. resistant in vitro to azoles have been encountered most often in AIDS patients with OPC, and a limited number of studies have correlated in vitro resistance with clinical failure to respond to treatment in this patient population (96, 217, 220, 269). Recent reports suggest that in this clinical setting, approximately 30% of patients with advanced AIDS will develop azole-resistant Candida infection (152, 211). Azole resistance, usually involving C. albicans, typically occurs in patients with CD4+ cell counts of <50/mm3 who were previously exposed to azoles and in whom the total cumulative dose of azole has been 10 g or greater (211, 269). Generally the same strain of C. albicans is repeatedly isolated and is associated with progressively increasing MICs over repeated courses of azole therapy, the dose of which has been gradually increased to achieve a therapeutic response (220). A fluconazole dose of 100 mg/day begins to be less effective in AIDS patients with OPC when the MIC for the infecting Candida isolate reaches 8 μg/ml and is generally ineffective at an MIC of 16 μg/ml. Higher doses of 400 to 800 mg/day may effectively treat the infection until the MIC is 64 μg/ml or higher (96). Loss of a fluconazole-resistant C. albicans isolate which emerged during treatment of OPC with intermittent fluconazole (50 to 200 mg three times weekly or more) was reported in a patient following resolution of clinical signs of infection after itraconazole treatment (130). In a fluconazole-naive patient believed to have acquired a C. albicans strain with decreased susceptibility to fluconazole (MIC, >16 μg/ml by the NCCLS method) from his sexual partner, OPC failed to respond to fluconazole (200 mg/day) initially but a subsequent episode which occurred after loss of this strain did respond (118). Non-C. albicans isolates resistant to fluconazole, including C. glabrata, C. tropicalis, C. parapsilosis, C. kefyr, and C. krusei, have been recovered from AIDS patients with OPC and have been associated with therapeutic failure in some cases (220). Typically, these species have been isolated from patients who have received prior courses of azole therapy, and increased MICs usually have been associated with clinical failures (220).
Most reports of azole resistance in non-HIV-infected patients have involved infections with non-albicans species of Candida, such as C. krusei, which are intrinsically resistant to fluconazole (220, 269). The largest number of failures have been found in patients who developed fungemia during fluconazole therapy or in whom preexisting fungemia failed to respond to fluconazole (2, 22, 176, 220). One or more risk factors were present in patients who developed fungemia while receiving fluconazole, including leukemia, lymphoma, solid tumor, bone marrow transplant, diabetes mellitus, neutropenia, i.v. catheters, or high-dose steroid therapy (220). Many of these patients were given fluconazole prophylactically in low doses (≤200 mg/day) either alone or in combination with amphotericin B, and C. krusei and C. glabrata were the predominant isolates (2, 172, 220). An increased incidence of infections due to C. krusei and C. glabrata has been noted in cancer patients, bone marrow transplant recipients, and patients in surgical intensive care units; however, whether this is related to the use of fluconazole or to other factors such as neutropenia is not known (269). In one retrospective review of all cultures positive for Candida over a 5-year period (1987 to 1991), cases of invasive C. krusei candidiasis were identified in eight non-HIV-infected, immunocompromised patients, seven of whom were colonized with this yeast before infection was diagnosed (127). All eight patients received amphotericin B, which was initiated in seven patients before invasive candidiasis was diagnosed, and four patients also received 5-FC while hospitalized. None of the eight patients received fluconazole. Nosocomial transmission of a clonal strain of C. krusei rather than use of fluconazole for antifungal prophylaxis was the apparent cause of an outbreak of C. krusei fungemia among patients in the hematology and oncology unit of one large hospital (25, 178).
Fluconazole-resistant C. albicans has been reported rarely in non-HIV-infected patients (269); however, Nolte et al. (176) recently described two patients with leukemia who developed fungemia due to fluconazole- and amphotericin B-resistant C. albicans after approximately 2 weeks of fluconazole prophylaxis (400 mg/day) and empirical amphotericin B therapy (0.5 mg/kg/day). An antifungal susceptibility study of 232 bloodstream Candida isolates obtained from nonneutropenic patients during treatment for candidemia with either fluconazole (400 mg/day) or amphotericin B (0.5 to 0.6 mg/kg/day) found no correlation between high MICs of either antifungal drug and clinical outcome (213, 216). These investigators suggested that factors such as failure to exchange intravascular catheters may have had a greater effect on the therapeutic outcome in these patients.
According to White et al. (269), risk factors for the development of resistant C. albicans in response to azole exposure in bone marrow transplant recipients are being studied. A recently completed surveillance study of yeast isolates from patients with leukemia or lymphoma and those undergoing bone marrow or solid-organ transplants failed to find an association between prior antifungal therapy and the gastrointestinal carriage of a new, drug-resistant organism (25). Use of fluconazole at the medical center began in 1990, and this drug accounted for 90% of subsequent azole use though 1997. Azole resistance was first documented in 1993, after 3 years of continuous use of fluconazole. However, the susceptibility of C. albicans to fluconazole, ketoconazole, and miconazole has remained stable since then, and C. albicans has remained the predominant species, accounting for 70 of 101 colonizing yeast isolates recovered from 97 non-AIDS, immunocompromised patients between 1994 and 1997. C. glabrata accounted for 15 isolates, C. krusei accounted for 8, and C. tropicalis accounted for 7. The rates of resistance among patients who had previously received antifungal therapy and those who had not were comparable (26 and 20%, respectively). The following MICs were used to define susceptible strains: fluconazole, ≤10 μg/ml; ketoconazole, miconazole, or itraconazole, ≤5 μg/ml. The overall susceptibility of C. albicans during 1994 to 1997 remained stable, ranging from 77 to 90% for fluconazole, 92 to 100% for ketoconazole, and 73% to 95% for miconazole, with invasive isolates showing comparable if not better susceptibility. Although itraconazole was used infrequently, 17 and 12% of C. albicans isolates tested in 1996 and 1997, respectively, were resistant to this agent.
There have been isolated reports of fluconazole-resistant Candida spp. in non-HIV-infected patients in other clinical settings (220, 269), including 17 patients who had never been treated with azole antifungal agents (100). In addition to the occurrences reviewed by Rex et al. (220) and White et al. (269), there have been reports of fluconazole-refractory C. albicans candiduria in an infant with postsurgical sepsis and an indwelling urinary catheter (120), C. parapsilosis infection of a prosthetic knee joint which failed to respond to prolonged fluconazole therapy despite isolation of susceptible isolates (267), and development of C. famata peritonitis refractory to fluconazole in a 76-year-old man receiving continuous ambulatory peritoneal dialysis for chronic nephropathy (205).
The impact of the azole dosing regimen on the development of resistance remains to be determined. Experience suggests that use of these antifungal agents to prevent OPC may favor the emergence of resistance (269). Whether the development of resistance can be reduced by continuous administration of a high dose of azole for a short course of therapy remains to be determined. Fluconazole resistance has been reported with continuous and intermittent use, with daily and weekly prophylaxis, and with sporadic single doses in patients with AIDS (269). An ongoing multicenter study by the MSG and the ACTG is evaluating some of these factors as they relate to azole use in HIV-infected patients (269).
AZOLE ANTIFUNGAL AGENTS UNDER DEVELOPMENT
A number of promising new azole and triazole derivatives are currently being developed by several pharmaceutical companies. Three of these agents—voriconazole, ER-30346, and D0870—are derivatives of fluconazole, and another agent, SCH 56592, is a hydroxylated analogue of itraconazole. All of these new agents are active following p.o. administration; voriconazole and T-8581 may also be administered i.v., and SCH 56592 shows topical activity. With the exception of D0870, UR-9746, and UR-9751, which show little activity against Aspergillus spp., the new triazole derivatives possess potent, broad-spectrum antifungal activity. These agents have promise for the treatment of a wide variety of superficial and systemic infections due to both opportunistic and endemic fungal pathogens. As of December 1997, only voriconazole was in phase III clinical trials; SCH 56592 was in phase I/II studies, and the other new azoles were in the preliminary stages of development; and D0870 had been withdrawn from development. Thus, any proposed clinical uses are based for the most part on in vitro data and the results of in vivo studies in experimental animal infection models. The chemical structures, proposed routes of administration, and potential clinical uses of these compounds are listed in Table 8.
TABLE 8.
Important new azole antifungal agents under development
Voriconazole
Voriconazole (UK-109,496) is a new triazole derivative of fluconazole discovered by Pfizer that is currently undergoing phase III clinical trials (60). Compared to fluconazole, voriconazole exhibits 1.6- and 160-fold greater inhibition of ergosterol P-450-dependent 14α-demethylase in C. albicans and A. fumigatus lysates, respectively (124). Studies with C. albicans and C. krusei have shown that voriconazole inhibits ergosterol synthesis in a dose-dependent fashion and is more effective than fluconazole in this regard (228).
In vitro activity.
Voriconazole exhibits potent, wide-spectrum activity against clinically important fungal pathogens such as Candida spp. (11, 222), Aspergillus spp. (40, 163), and Cryptococcus neoformans (39, 173); dimorphic fungi such as B. dermatitidis, Coccidioides immitis, and H. capsulatum (158); and also emerging and less common mold pathogens including several species of Fusarium and Penicillium marneffei (206).
When tested against 249 isolates including C. albicans, C. guilliermondii, C. krusei, C. kefyr, C. parapsilosis, and C. tropicalis, voriconazole was 10 to 100 times more potent than fluconazole (11). The enhanced activity of voriconazole was especially evident for C. krusei and C. guilliermondii, most strains of which were relatively resistant to fluconazole (MIC90s, 64 and 124 μg/ml, respectively) but much more susceptible to voriconazole (MIC90s, 0.5 and 4.0 μg/ml, respectively). Voriconazole also demonstrates good activity against C. albicans strains with decreased susceptibility to fluconazole (11, 222). Rhunke et al. (222) determined the susceptibility of 105 C. albicans oral isolates from HIV-infected patients to voriconazole by using the broth microdilution technique proposed by the NCCLS in 1995 (M27-T) (168). Against 75 fluconazole-resistant (MIC, ≥25 μg/ml) and 30 fluconazole-sensitive (MIC, <25 μg/ml) C. albicans isolates, voriconazole MICs ranged from 0.09 to 3.12 μg/ml and ≤0.048 to 0.78 μg/ml, respectively. Voriconazole MICs for fluconazole-resistant isolates were significantly higher than those for fluconazole-susceptible isolates (P < 0.001). In another study, voriconazole was 4- to 16-fold more active than fluconazole and 2- to 8-fold more active than itraconazole against Candida species, including C. krusei and C. glabrata (39). Isolates that were highly resistant to both fluconazole and itraconazole also exhibited cross-resistance to voriconazole, consistent with common mechanisms of resistance.
Incubation time has an effect on the voriconazole MIC for some Candida isolates. Lozano-Chiu et al. (148) tested the activity of voriconazole against 173 pathogenic bloodstream and oral Candida isolates by the NCCLS microdilution method (169). Although MICs for most isolates were similar at 24 and 48 h, trailing growth produced voriconazole MICs for some isolates that were much higher at 48 h than at 24 h. Overall, the modal voriconazole MIC was ≤0.008 μg/ml at both 24 and 48 h for isolates for which the fluconazole MICs were ≤8 μg/ml. However, for isolates for which the fluconazole MICs were ≥16 μg/ml, the modal voriconazole MIC was 0.125 μg/ml at 24 h and 0.25 μg/ml at 48 h. This difference was especially evident for C. tropicalis isolates, for which the voriconazole modal MIC was ≤0.008 μg/ml at 24 h and >4 μg/ml at 48 h.
Voriconazole exhibits fungicidal activity against Aspergillus spp., with a minimum fungicidal concentration (MFC) of 0.34 μg/ml (124). When tested at a U.S. medical center against clinical isolates of A. fumigatus (n = 21), A. flavus (n = 10), and A. niger (n = 10) by a modification of the proposed NCCLS method (79), voriconazole MICs for all three isolates (MIC ranges, <0.03 to 0.5, 0.25 to 0.5, and 0.25 to 1.0 μg/ml, respectively) were lower than those of amphotericin B (MIC ranges, 0.5 to 2.0, 1.0 to 4.0, and 0.5 to 1.0 μg/ml) (163). Voriconazole was more active than itraconazole against strains of A. fumigatus and A. niger (itraconazole MIC ranges, <0.03 to 1.0 and 0.5 to 2.0 μg/ml, respectively) and slightly less active against strains of A. flavus (itraconazole MIC range, 0.125 to 0.25 μg/ml). Another study conducted in the Netherlands tested the in vitro activity of voriconazole, amphotericin B, and itraconazole against 151 A. fumigatus isolates, including 131 isolates from 65 patients and 20 isolates from the hospital environment (258). Against itraconazole-susceptible (MIC, 0.125 to 0.5 μg/ml) and -resistant (MIC, >64 μg/ml) isolates, the geometric mean (GM) MICs and ranges at 48 and 72 h, respectively, were 0.56 (0.28 to 8) and 0.78 (0.25 to 8) μg/ml for voriconazole, 0.25 (0.06 to 1) and 0.48 (0.125 to 8) μg/ml for itraconazole, and 0.91 (0.25 to 2) and 1.50 (1 to 4) μg/ml for amphotericin B. In another study, voriconazole exhibited good activity in vitro against 18 amphotericin B-resistant A. fumigatus isolates (154).
In two susceptibility studies using the NCCLS broth macrodilution method (M27-A), voriconazole was highly active against C. neoformans (39, 173). When tested against 33 clinical isolates of C. neoformans, 12% of which were associated with fluconazole MICs of >8 μg/ml, the MIC50 of voriconazole was 0.06 μg/ml (39). All isolates were inhibited by voriconazole at <0.125 μg/ml, indicating that there was no cross-resistance between fluconazole and voriconazole. In another study of 50 clinical isolates of fluconazole-susceptible (MIC, ≤8 μg/ml), fluconazole-susceptible dose-dependent (MICs, 16–32 μg/ml), and fluconazole-resistant (MIC, 64 μg/ml) C. neoformans, the MICs of voriconazole and itraconazole increased in parallel with those of fluconazole (173). Against fluconazole-susceptible isolates, the GM MIC of voriconazole was 0.07 μg/ml, which was significantly lower than that of itraconazole (0.14 μg/ml; P = 0.001). However, against fluconazole-susceptible dose-dependent isolates, voriconazole and itraconazole were equally active (MICs, 0.37 and 0.29 μg/ml, respectively). Likewise, for fluconazole-resistant isolates, the MICs of voriconazole and itraconazole were either 1 or 2 μg/ml. According to the investigators, this is the first report of such high MICs of these two triazoles for C. neoformans.
Voriconazole also shows potent in vitro activity against Fusarium spp. (40, 124). Using the NCCLS macrodilution method, adapted for molds, Clancy et al. (40) tested the activity of voriconazole and amphotericin B against 25 clinical isolates of Fusarium. Voriconazole MICs ranged from 0.25 to 2 μg/ml, with a GM MIC of 1.2 μg/ml and a MIC90 of 2 μg/ml. By contrast, 82% of Fusarium isolates were associated with amphotericin B MICs of ≥1 μg/ml, a level that cannot be achieved reliably in serum. In a comparison of the susceptibility of various pathogenic molds to voriconazole and itraconazole, voriconazole was more active in vitro than itraconazole against the six Fusarium isolates tested (206).
Other clinically important dimorphic fungi, as well as opportunistic molds and yeasts, are susceptible to voriconazole. McGinnis et al. found that in a comparison with amphotericin B, fluconazole, and itraconazole in an in vitro macrobroth dilution test based upon the current NCCLS tentative standards (169), voriconazole MICs were lower than fluconazole MICs for all species tested (Table 9) (158). They also found that the voriconazole MICs were lower than those of amphotericin B and itraconazole as well as fluconazole for P. boydii and C. immitis and that the MICs of voriconazole and itraconazole for P. marneffei were nearly identical. Using an agar dilution method in high-resolution medium, Radford et al. (206) found that the in vitro activity of voriconazole was greater than that of itraconazole against Acremonium kiliense, Lasiodiplodia theobromae, Scedosporium prolificans, Scopulariopsis brevicaulis, and Paecilomyces lilacinus but not Paecilomyces variotii. Both triazoles showed excellent in vitro activity against P. marneffei, and voriconazole showed good activity against other molds, including Bipolaris australiensis, Cladophialophora bantiana, several Exophiala and Fonsecaea spp., and several molds that cause eumycetoma, including Leptosphaeria senegalenis and Madurella mycetomatis. Voriconazole is similar to itraconazole with regard to activity in vitro against zygomycetes; most appear to be resistant to these triazoles (38, 101). In a study of in vitro activity, voriconazole MICs were 32 to >64 μg/ml for the following members of this class: Mucor ramosissimus, Rhizomucor pusillus, Cunninghamella bertholletiae, and Absidia corymbifera (38).
TABLE 9.
In vitro susceptibility to four antifungal drugsa
| Fungus | MIC range (μg/ml) (no. of isolates tested) of:
|
|||
|---|---|---|---|---|
| Voriconazole | Amphotericin B | Fluconazole | Itraconazole | |
| Blastomyces dermatitidis | s≤0.03–1 (37) | ≤0.03–1 (28) | 1–64 (30) | ≤0.03–1 (33) |
| Coccidioides immitis | ≤0.03–0.125 (38) | 0.125–1 (27) | 2–64 (29) | 0.125–2 (27) |
| Histoplasma capsulatum | ≤0.03–1 (39) | ≤0.03–2 (31) | ≤0.125–64 (29) | ≤0.03–8 (31) |
| Paracoccidioides brasiliensis | ≤0.03–2 (19) | 0.125–4 (14) | ≤0.125–64 (14) | ≤0.03–1 (14) |
| Penicillium marneffei | ≤0.03 (27) | ≤0.03–8 (25) | 1–8 (25) | ≤0.03–2 (25) |
| Pseudallescheria boydii | 0.06–1 (23) | 2–≥16 (23) | 4–64 (23) | 1–4 (23) |
| Sporothrix schenckii | 0.50–6 (32) | 0.025–2 (23) | 32–≥128 (23) | 0.25–4 (26) |
| Trichosporon beigelii group | ≤0.03–1 (24) | 0.125–≥16 (24) | 0.5–≥128 (24) | 0.125–2 (24) |
Adapted from reference 158.
Pharmacokinetics.
The pharmacokinetics of voriconazole following p.o. or i.v. administration were studied in the mouse, rat, rabbit, guinea pig, and dog (128, 129). In all species, apparent p.o. bioavailability was 60% or greater following single or multiple once-daily administration. Following multiple once-daily administration, systemic clearance was increased approximately fivefold in the mouse and rat and less than twofold in the guinea pig and dog; there was no increase in the rabbit. The volume of distribution was relatively constant at 1.7 ± 0.6 liters/kg. Mean values for terminal elimination half-life ranged from 1.0 to 1.6 h in the mouse, rat, and rabbit and were 3.4 h and 5.5 h, respectively, in the dog and guinea pig. It was concluded that in the mouse and rat, significant autoinduction of clearance with multiple dosing decreases systemic concentrations in these species. Voriconazole is a relatively high-clearance drug in these species, as well as in the rabbit, but not in either the guinea pig or the dog. Therefore, Jezequel et al. (128) consider the guinea pig the species of choice for tests of in vivo efficacy. By using whole-body autoradiography, voriconazole was shown to distribute rapidly and extensively through the tissues following i.v. administration to rats; however, most of the radioactivity had been excreted by 72 h. Steady-state concentrations of voriconazole in guinea pig CSF were approximately 50% of the concentrations in plasma, whereas those in CNS tissues were approximately twofold higher. Plasma protein binding in the mouse, rat, rabbit, guinea pig, and dog ranged from 51 to 67%. The amount of radioactivity excreted in the urine represented 71, 87, and 61% of the dose, respectively, in the rat, rabbit, and dog and only 45% of the dose in the mouse. The remainder of the dose was excreted in the feces, and in all species less than 7% of the dose was excreted as unchanged drug. Overall, voriconazole is extensively metabolized by the liver to more than nine metabolites, most of which were identified in the four animal species tested (129).
The pharmacokinetics of voriconazole in humans have been studied in approximately 300 volunteers and patients following i.v. and p.o. administration of single and multiple (for 10 to 30 days) doses (136, 184). Absorption was fairly rapid following p.o. administration; the time to maximum concentration of drug in serum (Tmax) was less than 2 h, and the mean half-life (t1/2) was about 6 h. Bioavailability was relatively high (up to 90%). Accumulation (up to eightfold) and a decrease in systemic clearance was observed with administration of multiple doses. The relatively low volume of distribution (2 liters/kg) suggests wide distribution of voriconazole throughout body fluids and tissues (184). Consistent with a plasma protein binding of 58%, the concentration of voriconazole in saliva was 65% of that in plasma. These findings demonstrate that the pharmacokinetics of voriconazole in human subjects are nonlinear, possibly as the result of saturable, first-pass metabolism and systemic clearance (184). In a separate investigation, the metabolism and excretion of voriconazole were studied following administration of a single p.o. or i.v. dose of radiolabeled drug after attainment of steady state with nonradiolabeled voriconazole given p.o. at a dosage of 200 mg twice daily or i.v. at a dosage of 3 mg/kg twice daily (185). Following administration by either route, total recovery of radioactivity during 6 days was greater than 90%, with about 78 to 88% being recovered in urine and 18 to 27% being recovered in feces. Less than 5% of the dose was excreted as unchanged drug; voriconazole was extensively metabolized to three major and five minor metabolites. According to Patterson et al. (185), three human liver microsomes participate in the metabolism of voriconazole: CYP2C9, CYP2C18, and CYP3A4.
Experimental in vivo activity.
In keeping with its potent antifungal activity in vitro, voriconazole was effective as a therapeutic agent in a number of animal models of fungal infections, including systemic candidiasis, pulmonary and intracranial cryptococcosis, systemic and pulmonary aspergillosis, and Aspergillus endocarditis. In neutropenic guinea pigs with systemic candidiasis, p.o. voriconazole was equivalent to p.o. fluconazole or itraconazole and superior to i.v. amphotericin B in decreasing the fungal load in kidney tissues of animals infected with C. albicans. Voriconazole was more active than fluconazole or itraconazole in animals infected with C. krusei, C. glabrata, or azole-resistant strains of C. albicans (254). Likewise, in guinea pig models of pulmonary and intracranial infections due to C. neoformans, voriconazole was as effective as fluconazole and itraconazole in decreasing the fungal load in lung and brain tissue, respectively but amphotericin B was ineffective in both models (122). In neutropenic guinea pigs with systemic aspergillosis, p.o. voriconazole given twice daily was curative (i.e., the tissue cure rate was 100%) and was significantly (P < 0.05) more effective than either itraconazole at the same dosage or amphotericin B given i.v. on alternate days (123). Similarly, in immunocompromised guinea pigs with pulmonary aspergillosis, voriconazole was significantly (P < 0.05) more effective than itraconazole at the same dosage; the respective cure rates were 50 and 11% (123). In another study, voriconazole was highly effective in the prevention and treatment of A. fumigatus endocarditis in guinea pigs and was more effective than itraconazole (155). As prophylaxis, voriconazole (10 mg/kg) given intraperitoneally (i.p.) twice daily prevented infection in 11 of 12 animals whereas itraconazole at the same dosage did not prevent infection in any animal. When infected guinea pigs were treated p.o. with either voriconazole or itraconazole at a dosage of 10 mg/kg twice daily for 7 days, the respective cure rates were 100 and 0%.
Although the pharmacokinetics of voriconazole in the rat and the rabbit are suboptimal as a result of its rapid metabolism in these species (128), voriconazole was effective against aspergillosis in these animal models (93, 163). In an immunosuppressed, temporarily leukopenic rabbit model of invasive aspergillosis, p.o. administration of voriconazole at 10 or 15 mg/kg every 8 h for 5 days (15 doses) beginning 24 h after lethal or sublethal challenge eliminated mortality and reduced the tissue burden of A. fumigatus 10- to 100-fold in the liver and kidneys and to a lesser extent in the lungs (93). Both dosages eliminated circulating antigen, and at the higher dosage no viable organisms were recovered from brain. Voriconazole had a t1/2 of 2.5 to 3 h in rabbits, and no accumulation was observed. In an experimental model of invasive pulmonary aspergillosis in the rat which mimics human infection, the survival rate was significantly greater among animals treated p.o. with voriconazole at a dosage of 30 mg/kg/day than among untreated controls (100 and 41.6%, respectively; P < 0.01) and those treated with the same dosage of itraconazole (75 and 41.6%, respectively; P < 0.10). The difference in survival with voriconazole and itraconazole was not statistically significant.
Clinical studies.
Data from phase II clinical trials indicate that voriconazole is a promising agent for the treatment of OPC, esophageal candidiasis, and acute and chronic invasive aspergillosis (52, 71, 136, 222). In a blinded, dose comparison study in AIDS patients with OPC, a favorable response was achieved in 80 to 100% of those treated with 200 mg of voriconazole given p.o. either q.d. or b.i.d.; a 50-mg dose given q.d. was less effective (136). There were no serious side effects. Transient, reversible visual disturbances, which appeared to be dose related, were reported by seven patients receiving either 50 mg or 200 mg q.d. and by 10 patients receiving 200 mg b.i.d.; only two of these patients discontinued treatment. Four other patients withdrew from treatment due to other adverse events possibly related to voriconazole. Rhunke et al. (222) achieved a favorable response in six patients with esophageal candidiasis refractory to fluconazole by administration of voriconazole at 200 mg p.o. twice daily. The voriconazole MICs for pretreatment C. albicans isolates from these patients were ≤0.39 μg/ml. Some posttreatment isolates were associated with voriconazole MICs of ≥3.12 μg/ml, suggesting that, as with fluconazole, decreased susceptibility to voriconazole may develop during treatment.
The therapeutic activity of voriconazole in acute and chronic invasive aspergillosis was evaluated in open, noncomparative studies (52, 71, 136). Immunocompromised patients with acute invasive aspergillosis, 72% of whom had failed to respond to prior therapy with amphotericin B or itraconazole, received voriconazole as follows: 6 mg/kg i.v. every 12 h (q12h) for two doses, then 3 mg/kg i.v. q12h for 6 to 27 days, followed by 200 mg p.o. twice daily for a total treatment duration of 4 to 24 weeks (52, 136). An interim analysis of data for 53 of 71 evaluable patients showed that treatment with voriconazole resulted in a favorable clinical response in 39 patients (74%). Six patients experienced mild to moderate visual disturbances but did not discontinue treatment and had no residual effects. Four patients were withdrawn from the study due to adverse events consisting of skin rash in one patient and elevated hepatic enzyme levels in three patients.
Nonneutropenic patients with chronic, invasive aspergillosis, approximately 50% of whom had failed to respond to prior treatment with either amphotericin B or itraconazole, were treated with voriconazole 200 mg p.o. twice daily for 4 to 24 weeks (71, 136). At an interim analysis, 13 of 25 patients treated with voriconazole had end-of-treatment assessments and were evaluated for efficacy; the mean duration of therapy was 12 weeks. Voriconazole achieved a favorable clinical response in 9 (69%) of the 13 evaluable patients. Of the 25 patients, 11 (44%) who had received voriconazole reported visual disturbances but continued treatment and 2 (8%) were withdrawn from the study due to elevations in hepatic enzyme levels.
Phase III clinical trials comparing voriconazole with established agents in the treatment of invasive aspergillosis and other life-threatening fungal infections are in progress.
SCH 56592
The new triazole SCH 56592 is a hydroxylated analogue of itraconazole with a 1,3-dioxolone skeleton rather than the tetrahydrofuran skeleton found in itraconazole (86, 88). Discovered by Schering-Plough, SCH 56592 is in phase I/II studies to assess safety, antifungal efficacy, and pharmacokinetics (136). Compared to itraconazole, SCH 56592 is equally active in inhibiting sterol C14 demethylation in C. albicans and is at least 10 times more potent in inhibiting lanosterol 14α-demethylase (CYP51) in A. fumigatus and A. flavus (162).
In vitro activity.
SCH 56592 shows potent broad-spectrum activity against primary opportunistic fungal pathogens including Candida spp. (142, 192), Cryptococcus neoformans (91, 187), and Aspergillus spp. (179), and other less common, emerging pathogens including dimorphic fungi, dermatophytes, zygomycetes, opportunistic moniliaceous molds and dematiaceous fungi (83), and Fusarium spp. (230).
SCH 56592 was tested against 103 isolates of Candida, including 87 clinical isolates, by using a microtiter modification of NCCLS method M27-T (142). SCH 56592 showed fungistatic activity that was slightly greater than that of itraconazole (GM MICs, 0.21 and 0.34 μg/ml, respectively) and much greater than that of fluconazole (GM MIC, 4.99 μg/ml). Both SCH 56592 and itraconazole showed good activity (MICs, ≤1 μg/ml) against 41 of 57 isolates for which the fluconazole MICs were elevated (≥8 μg/ml), especially C. norvegensis, C. guilliermondii, C. krusei, C. inconspicua, and most fluconazole-resistant C. albicans isolates. However, only 3 of 14 C. glabrata isolates were susceptible to SCH 56592 at <2 μg/ml, the breakpoint suggested by Pfaller et al. (192). In another study, the anticandidal activity of SCH 56592 was compared with that of itraconazole, fluconazole, amphotericin B, and 5-FC against 382 clinical isolates by using the broth microdilution version of NCCLS reference method M27-T (192). SCH 56592 was very active against most species tested, with MIC90s of 0.06 μg/ml for C. stellatoidea, 0.12 μg/ml for C. tropicalis, C. parapsilosis, and C. lusitaniae, 0.25 μg/ml for C. albicans, and 0.5 μg/ml for C. krusei and other Candida spp. (including C. guilliermondii, C. rugosa, and C. lipolytica). Against these species, SCH 56592 was 2- and 32-fold more active, respectively, than were amphotericin B and 5-FC. SCH 56592 was least active against C. glabrata (MIC90, 2.0 μg/ml). Compared to the other triazoles, the activity of SCH 56592 was similar to that of itraconazole and at least eightfold greater than that of fluconazole. Against 18 clinical isolates demonstrating substantial resistance to both fluconazole and itraconazole (MICs, ≥128 and ≥8 μg/ml, respectively), including 11 C. albicans, 1 C. tropicalis, and 6 C. glabrata isolates, SCH 56592 was not particularly active, with MICs of ≥2.0 μg/ml. According to Pfaller et al. (192), this finding suggests possible cross-resistance among strains that are highly resistant to azoles. Similar findings regarding the activity of SCH 56592 against various species of Candida that were not highly resistant to fluconazole (MICs, 0.125 to 32 μg/ml) were reported by Galgiani and Lewis (91), who used the broth macrodilution method proposed by the NCCLS. C. krusei and C. glabrata were the least susceptible species to either SCH 56592 or itraconazole, and the activity of SCH 56592 against C. parapsilosis, C. lusitaniae, C. albicans, and C. tropicalis was two- to fourfold greater than that of itraconazole. The 15 isolates of Cryptococcus neoformans tested in this study were highly susceptible to both triazoles. Galgiani and Lewis observed that for both itraconazole and SCH 56592, the concentration and type of solubilizing agent affected the activity of the drug against these two fungal pathogens. They also compared results obtained with the alternative broth microdilution method to those obtained by the broth macrodilution technique. As has been observed with other drugs, the MICs of SCH 56592 and itraconazole at 48 h were substantially higher than at 24 h by both methods and agreement between broth microdilution and broth macrodilution results was closest for the 24-h readings.
Results of another in vitro study suggest that against C. neoformans, SCH 56592 may possess fungicidal as well as fungistatic activity (187). Multiple isolates of C. neoformans, including strains from both AIDS and non-AIDS patients, were tested by the NCCLS method proposed in 1992 (M27-P) (167). MIC data showed SCH 56592 to be more active in vitro against all C. neoformans isolates tested than was either amphotericin B or fluconazole but not itraconazole. After 54 h of drug exposure, SCH 56592 exhibited fungicidal activity at 1 and 10 μg/ml, which appeared to be enhanced in the presence of serum and was greater than that exhibited by amphotericin B.
SCH 56592 is active against Aspergillus spp., with GM MICs approximately 3- and 20-fold lower than those of itraconazole and amphotericin B, respectively, when tested in a microtiter format (179). A. terreus was the most susceptible to SCH 56592 (GM MIC, 0.05 μg/ml), and A. flavus was the least susceptible (GM MIC, 0.22 μg/ml). In the drug concentration range tested (0.01 to 16 μg/ml), SCH 56592 showed fungicidal activity against 71% of the isolates tested. Moreover, Fothergill et al. (83) found that the mean minimum lethal concentration of SCH 56592 against A. fumigatus was comparable to that of amphotericin B (1.3 and 1.4 μg/ml, respectively). In another study, SCH 56592 showed good in vitro activity against three species of Fusarium (F. solani, F. oxysporum, and F. moniliforme) when tested by a broth macrodilution method based on NCCLS method M27-A modified for filamentous fungi (230). The MICs of SCH 56592 at 48 h were highest for F. solani (4 to >16 μg/ml), intermediate for F. oxysporum (0.5 to 8.0 μg/ml), and lowest for F. moniliforme (0.125 to 0.5 μg/ml). By contrast, the MICs of amphotericin B were consistent across the three species, being 1 to 4 μg/ml for F. solani and F. oxysporum and 2 to 4 μg/ml for F. moniliforme.
Endemic fungal pathogens including B. dermatitidis, H. capsulatum, and C. immitis show good susceptibility in vitro to SCH 56592 (149, 248, 263). When tested against 12 strains of B. dermatitidis by NCCLS method M27-P, SCH 56592 was more active than itraconazole, amphotericin B, and fluconazole (GM MICs, were ≤0.40, ≤1.97, ≤3.14, and 8.41 μg/ml, respectively) (248). The activity of SCH 56592 against 20 patient isolates of H. capsulatum was comparable to that of itraconazole (GM MICs, ≤0.14 and ≤0.19 μg/ml, respectively), and both were more active than amphotericin B (GM MIC, 0.56 μg/ml) (263). SCH 56592 showed both inhibitory and fungicidal activity against five clinical isolates of C. immitis, with MICs ranging from 0.39 to 3.13 μg/ml and minimal fungicidal concentrations (MFCs) ranging from 1.56 to 3.13 μg/ml (149). SCH 56592 also shows good activity against a broad range of filamentous fungi (83). The GM MICs determined by Fothergill et al. were 0.06 to 0.4 μg/ml for dimorphic fungi, 0.09 μg/ml for dermatophytes, 0.6 μg/ml for zygomycetes, 0.04 μg/ml for dematiaceous fungi, and 0.1 μg/ml for Aspergillus spp. The MICs of SCH 56592 were generally similar to those of itraconazole and lower than those of fluconazole.
Pharmacokinetics.
A preliminary pharmacokinetic evaluation of SCH 56592 was conducted following single-dose i.v. (in hydroxypropyl-β-cyclodextrin vehicle) and p.o. (suspension in methylcellulose) administration to mice, rats, dogs, and cynomolgus monkeys and p.o. administration to rabbits (174). Oral bioavailability was approximately 48% in rats and mice, and good bioavailability in rabbits was evidenced by a high AUC, similar to that observed in mice. Oral bioavailability was 24% in dogs and 12% in monkeys, and the t1/2s were 18 and 22 h, respectively. Following p.o. administration of single doses ranging from 10 to 120 mg/kg to dogs, the concentrations in serum increased in a dose-related fashion. In all five species, concentrations in serum 24 h after a single p.o. dose exceeded the MICs and MFCs, suggesting a once-daily regimen for clinical use. A subsequent study in dogs evaluated the bioavailability of SCH 56592 from capsule, tablet, and liquid dosage forms administered in a single dose of approximately 18 mg/kg (175). The drug was also administered i.v. as a solution in hydroxypropyl-β-cyclodextrin to determine the absolute bioavailability. When SCH 56592 was administered to fed dogs, its bioavailability from the three dosage forms ranged from 47 to 58% and the Tmax occurred at 11 to 12 h. Data from fasting dogs given either the tablet or capsule formulation showed that food increased bioavailability from the capsule by 7.5-fold, from 7.1% to 53%, but had no effect on that from the tablet.
Experimental in vivo activity.
SCH 56592 has demonstrated therapeutic efficacy in a number of animal models of fungal infections, including systemic, gastrointestinal, and vaginal candidiasis; systemic and pulmonary aspergillosis; cryptococcal meningitis; histoplasmosis; disseminated coccidioidomycosis; and topical dermatophyte infection with Trichophyton mentagrophytes. SCH 56592 was effective in preventing infection in murine models of systemic candidiasis and pulmonary aspergillosis (30, 31). A dose of 50 mg of SCH 56592 per kg administered 1.5 to 24 h prior to infection with C. albicans provided 100% protection, as did a dose of 100 mg/kg administered 72 h prior to infection with A. fumigatus or A. flavus. It was found that if therapy was delayed, higher doses of SCH 56592 were needed to provide the same degree of efficacy (30). For example, in mice infected with A. flavus, treatment with 20 mg of SCH 56592 per kg given once daily for 4 days starting on day 1 or 2 postinfection was as effective as treatment with 80 mg/kg starting on day 2 or 3 postinfection. No synergy or antagonism was noted when SCH 56592 was administered concomitantly with amphotericin B (1 or 5 mg/kg given subcutaneously) to mice infected with C. albicans or A. flavus. In a study of in vitro-in vivo correlations for candidiasis or aspergillosis, the method used to determine MICs affected the concentrations in serum needed to predict efficacy. When MICs were determined by non-NCCLS methods (in either Eagle’s Miminum Essential Medium or Sabouraud dextrose broth), the level of free, unbound SCH 56592 or itraconazole in serum had to equal or exceed the MIC for the organism, whereas for fluconazole, the level in serum had to be at least 10 times greater than the MIC (160). However, when MICs were determined in RPMI medium according to NCCLS guidelines, the levels of all three azoles in serum had to be 5 to 10 times greater than the MICs to predict efficacy.
SCH 56592 was also evaluated in immunocompromised-animal models of gastrointestinal candidiasis and disseminated/invasive aspergillosis (45, 180, 186). In mice with retrovirus-induced immunodeficiency, once-daily administration of SCH 56592 (1 or 5 mg/kg solubilized in methylcellulose) for 7 days beginning 100 days postinfection was effective in clearing the gastrointestinal mucosal candidiasis and was more effective than fluconazole, which failed to clear infection when administered at the same dose (45). Similarly, in mice immunosuppressed with cortisone acetate (100 mg/kg/day) administered subcutaneously for 3 days beginning 1 day before intranasal infection with A. fumigatus, treatment from days 1 through 5 with 10, 25, or 50 mg of SCH 56592 per kg p.o. significantly prolonged survival compared with controls (P < 0.05). Numbers of fungi in lung tissue were reduced with the 10- and 50-mg/kg doses of SCH 56592. Treatment with amphotericin B (3 or 6 mg/kg i.p.) was not effective in prolonging survival or reducing lung tissue counts. A temporarily neutropenic murine model of disseminated aspergillosis (lungs and kidneys) was used to compare SCH 56592 (5, 10, and 25 mg/kg p.o.), itraconazole (25 mg/kg p.o.), and amphotericin B (5 mg/kg i.p.) (180). Groups of mice infected with either an itraconazole-susceptible or an itraconazole-resistant strain of A. fumigatus were treated over a period of 10 days, beginning 24 h postinfection. Both isolates were fatal in 90% of untreated mice. In mice infected with the itraconazole-susceptible isolate, survival rates were 100% with itraconazole and the two highest doses of SCH 56592, 90% with the 5-mg/kg dose of SCH 56592, and 40% with amphotericin B. Survival rates in mice infected with the itraconazole-resistant isolate were 0% for itraconazole (25 mg/kg); 100, 60, and 20% for SCH 56592 (5, 10, and 25 mg/kg); and 50% for amphotericin B. In both itraconazole-susceptible and -resistant infections, the 25-mg/kg dose of SCH 56592 was significantly better than amphotericin B in reducing fungal burdens in the lungs and kidneys. A relationship between MICs and organ fungal load was evident; at the highest dose of SCH 56592, the difference between fungal burdens in lungs and kidneys in itraconazole-susceptible and -resistant infections was approximately 100-fold and the difference between MICs was about 50-fold (0.01 and 0.5 μg/ml, respectively). Thus, in vitro testing of SCH 56592 against Aspergillus spp. should have clinical relevance. Similar results were reported by Patterson et al. (186), who compared these three antifungal agents in an immunosuppressed, temporarily neutropenic rabbit model of invasive aspergillosis. Treatment with SCH 56592 (2.5 or 10 mg/kg/day p.o.), itraconazole (10 mg/kg/day p.o. in cyclodextrin solution), or amphotericin B (1.0 mg/kg per day i.v.) was begun 24 h after infection with A. fumigatus and lasted for 5 days. Mortality rates were 100% in untreated controls, 25 and 12.5% with 2.5- and 10-mg/kg doses of SCH 56592, respectively, and 50% with broth itraconazole and amphotericin B. Amphotericin B and the 10-mg/kg dose of SCH 56592 reduced fungal burden in the liver, lungs, kidneys, and brain; both were more effective than itraconazole in this regard.
Studies in animal models of endemic mycoses indicate that SCH 56592 warrants further evaluation in the treatment of these infections in humans. In a murine pulmonary model of histoplasmosis, survival rates at day 28 in mice infected with a lethal dose of H. capsulatum were 100% for those treated with SCH 56592 (0.25, 1, and 5 mg/kg/day), amphotericin B (5 mg/kg every other day), or itraconazole (75 mg/kg/day) and 44% for those treated with a lower dose of itraconazole (5 mg/kg/day) (263). Fungal burden studies with a sublethal inoculum showed that the degree of organ sterilization with SCH 56592 (1 mg/kg/day) was greater than that with amphotericin B (2 mg/kg every other day) or itraconazole (10 mg/kg/day) (263). SCH 56592 also exhibited potent activity in a murine model of disseminated coccidioidomycosis. Compared to controls, once-daily treatment with any of the following p.o. regimens begun 2 days postinfection and continued for 19 days was more effective in prolonging survival: SCH 56592 (0.5, 2, 10, or 25 mg/kg), itraconazole (10 or 100 mg/kg), and fluconazole (10 or 100 mg/kg) (150). The three drugs did not differ in their effect on survival, and all were significantly better (P < 0.001) than treatment with diluent or no treatment. However, the potency of SCH 56592 in reducing tissue burden in spleen, liver, and lungs was 700- to 20,000-fold greater than that of fluconazole and 50- to 200-fold greater than that of itraconazole, on a weight basis. Unlike fluconazole and itraconazole, the two highest doses of SCH 56592 (10 and 25 mg/kg) completely eradicated C. immitis from the organs of 6 of 10 and 9 of 9 surviving mice, respectively. SCH 56592 also was effective in a rabbit model of cryptococcal meningitis in which severe CSF leukopenia was induced with corticosteroid (187). Treatment with itraconazole suspension (in methylcellulose) at 20 or 80 mg/kg/day or fluconazole tablets (100-mg tablets scored) was administered as follows: 20-mg/kg dose given for 11 consecutive days beginning on day 4 postinfection, or 80-mg/kg dose given for 15 consecutive days starting on day 2 postinfection. After administration of either dose for several days, the concentrations of SCH 56592 in serum were 20 to 100 times greater than the MIC for the most resistant strain of C. neoformans studied in vitro (0.25 μg/ml) and the trough levels throughout the 24-h dosing interval remained approximately 30 to 115 times the MIC for the infecting strain (0.063 μg/ml). Although SCH 56592 was not detected in the CSF at 2, 6, and 24 h after administration of either dose, its fungicidal activity within the CSF was similar to that of fluconazole; low and high doses of each azole decreased fungal burden in CSF over the 2-week treatment period, although neither regimen sterilized the subarachnoid space of most animals.
In models of superficial fungal infection, the results of fungal cultures demonstrated that a single p.o. dose of 2.5 or 10 mg of SCH 56592 per kg was more effective than fluconazole in clearing vaginal candidiasis in hamsters and that topical application of SCH 56592 (0.25 or 0.5%) was superior to p.o. fluconazole, p.o. itraconazole, and topical miconazole in clearing T. mentagrophytes dermatophytosis in guinea pigs (183). Likewise, p.o. administration of SCH 56592 to guinea pigs with T. mentagrophytes infection was more effective than p.o. fluconazole and p.o. itraconazole in reducing fungal burden but was comparable to these azoles in improving lesion severity. Both culture and lesion severity results showed that topical administration of SCH 56592 (0.25%) was at least as effective as most commercially available antifungal creams (183).
T-8581
T-8581, a 2-fluorobutanamide derivative discovered by the Toyama group (Japan), is undergoing preclinical investigation. This triazole is nearly 20 times more water soluble than fluconazole (41.8 and 2.6 mg/ml, respectively) and is expected to be particularly useful for high-dose parenteral therapy (250).
In vitro activity.
T-8581 exhibits potent activity in vitro against Cryptococcus neoformans and many species of Candida (274). The IC80s (the lowest drug concentration reducing the optical density at 630 nm by 80% compared with the drug-free control) of T-8581, fluconazole, and itraconazole against 160 strains of eight species of fungi were determined by a broth microdilution method with RPMI 1640 (274). The activity of T-8581 against C. albicans, C. tropicalis, and C. parapsilosis was similar to that of fluconazole. GM IC80s of T-8581 for these three species of Candida were 0.218, 0.358, and 0.401 μg/ml, respectively. The GM IC80s of T-8581 for C. guilliermondii and C. krusei (1.19 and 10.2 μg/ml, respectively) were lower than those of fluconazole (4.00 and 18.8 μg/ml, respectively). Against C. glabrata, T-8581 was 3.6-fold less active than fluconazole (GM IC80s, 11.8 and 3.29 μg/ml, respectively). T-8581 was 2.3-fold less active than fluconazole against C. neoformans (GM IC80s, 9.28 and 4.00 μg/ml, respectively) and 3.4-fold more active against A. fumigatus (GM IC80s, 71.0 and 239 μg/ml, respectively). Against all eight fungal species tested, the GM IC80s of itraconazole were significantly superior to those of either T-8581 or fluconazole (P < 0.01).
Pharmacokinetics.
The pharmacokinetics of T-8581 were studied following p.o. and i.v. administration of single doses of 10 mg/kg to mice, rats, rabbits, and dogs (274). Following p.o. administration, peak concentrations in serum in these four species were 10.9, 10.5, 7.14, and 12.0 μg/ml, respectively, and drug was detectable after 24 h in the sera of all species except the mouse. Half-lives following p.o. administration ranged from 3.2 h in mice and 3.8 h in rats to 7.2 h in rabbits and 9.9 h in dogs. Likewise, the AUCs following p.o. and i.v. administration were lowest in mice (51.7 and 46.1 μg · h/ml) and rats (76.9 and 72.6 μg · h/ml), intermediate in rabbits (115 and 139 μg · h/ml), and highest in dogs (209 and 216 μg · h/ml). These AUC data suggest that absorption of T-8581 following p.o. administration was virtually complete in each species.
Experimental in vivo activity.
The therapeutic potential of T-8581 was evaluated in murine models of systemic candidiasis and aspergillosis and in a rabbit model of systemic aspergillosis (274). In the murine model, treatment with T-8581 or fluconazole (p.o. and i.p.) or itraconazole (p.o.) was initiated 2 h after infection with either C. albicans or A. fumigatus and continued for 6 days. A 0.5% methylcellulose vehicle was used for p.o. drug administration, and physiological saline was used for i.p. injection. In the systemic-candidiasis model, T-8581 and fluconazole showed similar therapeutic efficacy; the 50% effective doses (ED50s) were 0.412 mg/kg (p.o.) and 0.438 mg/kg (i.p.) for T-8581 and 0.392 mg/kg (p.o.) and 0.396 mg/kg (i.p.) for fluconazole. The ED50 of itraconazole (>320 mg/kg for p.o. administration) was more than 700 times higher than that of either T-8581 or fluconazole, possibly as a result of the 0.5% methylcellulose vehicle. Against systemic infection with A. fumigatus in mice, T-8581 was significantly more active than either fluconazole or itraconazole (Litchfield and Wilcoxon method; P value not stated). The ED50s for p.o. administration were 50.5 mg/kg for T-8581, 138 mg/kg for fluconazole, and >320 mg/kg for itraconazole. When given i.p., T-8581 was considerably more effective than fluconazole (ED50s, 59.2 and >20 mg/kg, respectively), due mainly to the limited solubility of fluconazole in physiological saline.
In the rabbit model of systemic aspergillosis, treatment was administered p.o. or i.v. once a day beginning 2 h postinfection and continuing for 6 days; mortality was monitored over 20 days. By day 5 all the control animals were dead. Treatment with T-8581 (20 or 40 mg/kg p.o.) was more effective in prolonging survival than was either fluconazole or itraconazole. With T-8581, survival rates at 20 days were 42.9% (p.o.) and 37.5% (i.v.) with the 20-mg/kg dose and 87.5% (p.o./i.v.) with the 40-mg/kg dose. By contrast, the survival rates among rabbits treated with fluconazole and itraconazole (40 mg/kg p.o.) were 14.3 and 37.5%, respectively. After i.v. administration of a dose of 20 mg/kg, both T-8581 and fluconazole prevented mortality significantly compared with control rabbits (P < 0.01 and P < 0.05, respectively).
Based on the promising pharmacokinetic profile of T-8581 and its potential effectiveness in systemic fungal infections caused by C. albicans and A. fumigatus, further studies are being conducted to evaluate the toxicity of this new triazole (274).
ER-30346 (BMS-207147)
ER-30346 is an oral triazole derivative of fluconazole that was discovered by Eisai Co., Ltd., Japan (88), and subsequently licensed to Bristol-Myers Squibb (Wallingford, Conn.) as BMS-207147 (89). The drug is currently undergoing preclinical investigation. Its inhibitory potency and binding affinity for yeast P-450-dependent 14α-demethylase are similar to those of itraconazole (114).
In vitro activity.
ER-30346 possesses potent broad-spectrum activity against important fungal pathogens, including Candida spp., A. fumigatus, and Cryptococcus neoformans, as well as most hyaline hyphomycetes (except Fusarium spp. and Pseudallescheria boydii), dermatophytes, and dematiaceous fungi (89, 113). It is not active against Sporothrix schenckii and zygomycetes (89). In a comparison of in vitro activity by the proposed NCCLS broth microdilution method (M27-P), ER-30346 was 4 to 64 times more active than itraconazole, fluconazole, and amphotericin B against C. albicans, C. parapsilosis, and C. glabrata; the MIC90s of ER-30346 were 0.025, 0.05, and 0.39 μg/ml, respectively (113). Against C. tropicalis, although the MIC90 of ER-30346 (12.5 μg/ml) was 4 to 8 times lower than that of itraconazole or fluconazole, it was 16 times higher than that of amphotericin B (0.78 μg/ml). Against C. neoformans the MIC90 of ER-30346 was 0.10 μg/ml; the MIC90 of itraconazole, the most active comparator, was fourfold higher (0.39 μg/ml). All isolates of A. fumigatus tested were resistant to fluconazole (MIC90, >100 μg/ml) but susceptible to ER-30346, itraconazole, and amphotericin B (MIC90s, 0.39, 0.78, and 1.56 μg/ml, respectively). ER-30346 also showed potent activity against the dermatophytes Trichophyton mentagrophytes, T. rubrum, Microsporum gypseum, and M. canis, which was 2 to 8 times greater than that of itraconazole and >32 times higher than that of fluconazole. Compared with amphotericin B, the activity of ER-30346 was comparable against M. canis and 8 to 16 times greater against T. mentagrophytes, T. rubrum, and Microsporum gypseum. For all fungi tested, strains showing decreased susceptibility in vitro to either itraconazole or fluconazole were also less susceptible to ER-30346 (113).
With the exception of the MIC90s for C. tropicalis and C. glabrata, the findings of Hata et al. (113) were confirmed by a subsequent study by the NCCLS broth macrodilution method (M27-A) modified for filamentous fungi (89). In this study the MIC90s for these two species of Candida were higher (≥16 μg/ml). Against C. krusei, which was not tested in the previous study, the MIC90 of ER-30346 was 0.5 μg/ml, 10-fold greater than that for C. albicans or C. parapsilosis, suggesting less intrinsic activity against this species. The activity of ER-30346 against Aspergillus was comparable to that of itraconazole. All strains of C. neoformans tested were susceptible to the three triazoles and amphotericin B. Unlike the previous study, in which ER-30346 was four times more active than itraconazole against C. neoformans, in this study itraconazole was twice as active as ER-30346 (MIC90s, 0.008 and 0.016 μg/ml, respectively). Against C. neoformans and many strains of Aspergillus spp., both agents displayed fungicidal activity. Against three of four strains of C. neoformans tested, the MFC producing a 95% reduction in the number of CFU of the final inoculum size per milliliter was <1 μg/ml for both ER-30346 and itraconazole. Against Aspergillus, the MFC producing a 90% reduction (MFC90) was <1 μg/ml for 7 of 14 strains when tested against ER-30346 and for 10 of 14 strains when tested against itraconazole.
Pharmacokinetics.
The pharmacokinetic parameters of ER-30346 have been studied following p.o. and i.v. administration of single doses to mice, rats, and dogs (113, 166). Following p.o. administration of 2 mg/kg, the bioavailability of ER-30346 was 48% in rats and 74% in dogs; Cmax was 0.15 and 0.32 μg/ml, respectively; and AUC was 2.2 and 3.5 μg · h/ml, respectively (166). In dogs, the mean elimination t1/2 after i.v. injection was 8.8 h. Over the dose range of 1 to 10 mg/kg p.o. in dogs (166) and 2 to 40 mg/kg p.o. in mice (113), linear increases in Cmax and AUC were observed. The presence of food enhanced absorption; following a 10-mg/kg dose, Cmax and AUC values in fasted dogs were substantially lower than those in fed animals (166). In another study, the pharmacokinetics of ER-30346 were compared with those of itraconazole following p.o. administration of single doses of 2, 10, and 40 mg/kg to groups of three mice (113). The mean peak concentrations of ER-30346 and itraconazole in serum were similar at 1 h (∼1.00 μg · h/ml), but Hata et al. note that this is sevenfold lower than the Cmax reported for a 10-mg/kg dose of fluconazole. ER-30346 but not itraconazole was detectable in serum at 12 h. This is consistent with the threefold greater half-life (4 and 1.4 h, respectively) and AUC (6.81 and 2.20 μg · h/ml, respectively) of ER-30346 compared with itraconazole. No ER-30346 was detected in the serum at 24 h.
Experimental in vivo activity.
The therapeutic efficacy of oral ER-30346 was compared with that of fluconazole and itraconazole in murine models of systemic and pulmonary infection due to Candida, Aspergillus, and Cryptococcus, as well as intracranial cryptococcosis and oral candidiasis (112, 113). In murine systemic candidiasis due to C. albicans, treatment with either ER-30346 or fluconazole (10.0 mg/kg p.o.) 1 h after infection was equally effective, and both triazoles delayed mortality significantly compared with the same dose of itraconazole (P < 0.05) (113). For the treatment of systemic cryptococcosis or aspergillosis, drugs were administered p.o. twice daily for 5 consecutive days and mortality was monitored for 21 and 14 days, respectively. In systemic cryptococcosis, ER-30346 and fluconazole showed comparable efficacy at doses of 8 and 32 mg/kg b.i.d., although the MIC of fluconazole for the infecting strain of C. neoformans was 125 times greater than that of ER-30346. Both doses of ER-30346 and fluconazole were significantly more effective than the corresponding doses of itraconazole in delaying mortality (P < 0.05). In the systemic aspergillosis model, the infecting strain of A. fumigatus was highly susceptible to both ER-30346 and itraconazole (MICs, 0.39 and 0.78 μg/ml, respectively) and resistant to fluconazole (MIC, >100 μg/ml). Treatment with ER-30346 (10 mg/kg b.i.d.) was significantly more effective than either fluconazole or itraconazole at the same dosage in delaying mortality (P < 0.05). However, at 40 mg/kg b.i.d., there was no difference between ER-30346 and itraconazole, and both were more significantly more effective than fluconazole (P < 0.05).
Murine studies of pulmonary candidiasis and aspergillosis were performed with immunosuppressed mice; immunocompetent mice were used for the pulmonary cryptococcosis model (112). Drug efficacy was determined on the basis of reduction in fungal burden in lung tissue. In the candidiasis model, infection caused by a fluconazole-susceptible strain of C. albicans was treated with drugs administered p.o. in doses of 0.625, 2.5, and 10 mg/kg given b.i.d. for 2 or 3 days; a 40-mg/kg dose was also used to treat infection due to a fluconazole-resistant strain. Against fluconazole-susceptible C. albicans pulmonary infections, reductions in the log number of CFU in the lungs were greater with increasing doses of ER-30346, fluconazole, and itraconazole. The efficacy of ER-30346 was comparable to that of fluconazole and greater than that of itraconazole. Against fluconazole-resistant C. albicans infection, only treatment with ER-30346 caused a significant reduction in fungal burden in lung tissue compared with controls (P < 0.05). Against pulmonary C. neoformans infections, which were treated with p.o. doses of 8 and 32 mg/kg b.i.d. for 3 days, ER-30346 and itraconazole showed dose-dependent therapeutic effects whereas both doses of fluconazole were equally effective. The fungal burden in the lungs of mice treated with the 32-mg/kg dose of ER-30346 was similar to that observed in mice treated with the 8-mg/kg dose of fluconazole. At both doses, ER-30346 and fluconazole were significantly more effective than itraconazole or control treatment (P < 0.05). In the pulmonary aspergillosis model, the infecting strains showed good susceptibility in vitro to ER-30346 (MIC range, 0.20 to 0.39 μg/ml) and itraconazole (MIC, 0.78 μg/ml) but were resistant to fluconazole (MIC, >100 μg/ml). ER-30346 and itraconazole were administered at 2, 8, or 32 mg/kg b.i.d. for 3 days; the dose of fluconazole was 32 mg/kg. As in the other pulmonary infection models, the effectiveness of ER-30346 and itraconazole was dose dependent. At the 32-mg/kg dose, the reduction in fungal burden with ER-30346 was significantly greater (P < 0.05) than that with fluconazole, itraconazole, and control treatment.
Hata et al. (112) also compared the efficacy of ER-30346, itraconazole, and fluconazole in a murine model of intracranial cryptococcosis and a rodent model of oral candidiasis. In the intracranial cryptococcosis model, healthy mice were treated with 8 mg/kg p.o. for 3 days and efficacy was based on determinations of fungal burden in brain tissue 24 h after the last dose of drug. Treatment with either ER-30346 or fluconazole significantly (P < 0.05) reduced the log number of CFU in brain tissue compared with itraconazole and control treatment; itraconazole did not reduce fungal burden compared with the control treatment. In the oral candidiasis model, rats were treated with tetracycline for 7 days prior to infection three times at 48-h intervals with a fluconazole-susceptible strain of C. albicans. Drugs were administered p.o. at 1 and 4 mg/kg q.d. for 3 days, and oral swabs were obtained 5 days after the last infection. Both doses of ER-30346 and fluconazole significantly (P < 0.05) reduced the log number of CFU in oral swabs compared with the control; the reduction with itraconazole was significant only at the higher dose. Overall, the efficacy of ER-30346 was comparable to that of fluconazole, and both were more effective than itraconazole.
On the basis of its broad antifungal activity in vitro and potent activity in animal models of fungal infections caused by C. albicans, C. neoformans, and A. fumigatus, further pharmacokinetic and toxicological evaluation of ER-30346 would be expected.
D0870
D0870, another derivative of fluconazole (88), was under development by Zeneca until March 1997 (273), at which time it was discontinued, possibly because of relatively weak in vitro activity against Aspergillus spp. compared to itraconazole (88). However, D0870 shows greater activity in vitro than itraconazole against most Candida spp., particularly C. tropicalis and C. parapsilosis, and slightly more activity against fluconazole-resistant isolates of C. albicans (273). Compared with fluconazole, D0870 is more active in vitro against B. dermatitidis and H. capsulatum and equally active against Coccidioides immitis (88). D0870 is excreted by nonrenal mechanisms, and its half-life in mice was estimated to be more than 10 times longer than that of fluconazole (50 and 4 h, respectively) (88). Studies in mice have also shown D0870 to be more toxic than fluconazole and suggest that the dosing interval must be sufficiently long to permit clearance of drug to nontoxic levels (88).
D0870 was more effective than fluconazole in murine models of systemic candidiasis due to fluconazole-susceptible and -resistant strains of C. albicans, disseminated C. tropicalis infection (including fluconazole-resistant strains), and hematogenous C. krusei infection in neutropenic mice (88, 105). In an estrogen-dependent murine model of C. albicans vaginal candidiasis, D0870 was 10 times more active than fluconazole against infection due to a fluconazole-susceptible isolate (82). However, to effectively clear infection due to a fluconazole-resistant C. albicans isolate, a 10-fold increase in the dose of D0870 to 25 mg/kg was required. Like fluconazole, D0870 was not effective against vaginal infection due to C. glabrata, even at 100 mg/kg per day (82). In animal models of endemic fungal infections, D0870 was more effective than fluconazole against C. neoformans systemic infection in immunocompetent and immunosuppressed mice and against pulmonary blastomycosis, systemic coccidioidomycosis, and systemic histoplasmosis in mice (41, 88).
A pilot clinical study evaluated the effectiveness of D0870 in the treatment of OPC in HIV-infected patients with no history of clinical failure of fluconazole (56). Three regimens were evaluated: a single 50-mg/kg dose on day 1 followed by 10 mg/day for 4 days; a single 100-mg initial dose on day 1 followed by 25 mg/day for 4 days; and a single 100-mg initial dose on day 1 followed by 10 mg/day for 5 days. Overall, clinical cure was achieved in 27 (77%) of 35 patients who completed treatment, clinical improvement was noted in 6 (17%), and 2 (6%) failed at the lowest dose. Within 2 weeks after stopping therapy, 10 (37%) of 27 patients followed up at either day 7 or 14 had relapsed. D0870 was well tolerated, and mild adverse events considered possibly related to treatment were noted in five patients.
According to Yamada et al. (273), although Zeneca has discontinued development of D0870, Mochida Pharmaceutical Co., Ltd. (Japan) is continuing its evaluation at lower doses than those used by Zeneca.
UR-9746 and UR-9751
Two new azole derivatives containing an N-morpholine ring, UR-9746 and UR-9751, are being developed by J. Uriach & Cia (Spain) (13). The in vitro antifungal activity of these two azoles was comparable to that of fluconazole when tested by broth macrodilution methods, including that proposed by the NCCLS (244). Of the three agents tested, UR-9751 was the most active against C. albicans and displayed activity comparable to that of fluconazole against other Candida species. All three drugs were active against C. neoformans, and H. capsulatum, but Aspergillus spp. was generally resistant. Although the MFCs of UR-9751, UR-9746, and fluconazole for C. immitis were >100 μg/ml, the isolate was inhibited by all three drugs (MICs, 3.13, 25, and 6.25 μg/ml, respectively).
Both UR-9751 and UR-9746 were highly active in murine models of cryptococcal meningitis (209), severe disseminated histoplasmosis (126), systemic coccidioidomycosis (42), and systemic candidiasis (13) and in a rodent model of vaginal candidiasis (13). Survival rates were 90 to 100% in mice with systemic C. albicans infection treated with 1 mg of UR-9746 or UR-9751 per kg given 1, 4, and 24 h postinfection (13). UR-9746 was slightly more effective than UR-9751, and both were superior to fluconazole. When the drugs were administered in a dosage of 0.25 mg/kg per day for 3 days following infection, cure rates at 10 and 15 days were 95 and 100%, respectively, with UR-9746, 40 and 60%, respectively, with UR-9751, and 10 and 25%, respectively, with fluconazole (13). In mice infected intracranially with C. neoformans, p.o. administration of 10 mg of UR-9751, UR-9746, or fluconazole per kg per day for 7 days significantly (P < 0.005) prolonged survival compared with controls, and UR-9746 also prolonged survival when given at 1 mg/kg per day (209). Against systemic cryptococcosis, UR-9746 was significantly more effective than either amphotericin B or fluconazole in delaying mortality (P < 0.05) and both azoles were more effective than amphotericin B in reducing fungal burden in the lungs, liver, and brain but not in the spleen (23, 24). UR-9751 was not included in this comparison. In another study in mice infected with H. capsulatum, the greatest reduction in fungal burden in the spleen was achieved with amphotericin B, followed by UR-9746 and then UR-9751; itraconazole failed to decrease the fungal burden (126).
The most extensive information on these two azole derivatives was provided by Clemons and Stevens (42), who studied their activity in vitro and in vivo against the Silveira strain of Coccidioides immitis. Tests of in vitro activity by a broth macrodilution method in defined medium showed the inhibitory activity of UR-9751 to be twice than that of fluconazole (MICs, 3.1 and 6.3 μg/ml, respectively) whereas UR-9746 was much less active (MIC, 25 μg/ml). Pharmacokinetic evaluations in healthy mice given a single 100-mg/kg dose found that peak bioactivity (expressed as concentration of the native drug) of 184 μg of UR-9746 per ml occurred at 8 h postdose. For UR-9751, the peak activity (34 μg/ml) occurred at 8 to 24 h postdose. Concentrations were higher following administration of 100 mg/kg/day for 19 days, and two peaks appeared at 1 and 48 h postdose. Both drugs showed bioactivity half-lives of >16 h; and inhibitory concentrations severalfold greater than MICs for C. immitis were maintained throughout the interval between doses. In mice with systemic coccidioidomycosis, UR-9746, UR-9751, and fluconazole showed dose-related efficacy. Survival was 100% with doses of 10 or 100 mg of UR-9746 per kg or 100 mg of UR-9751 per kg, 90% with 10 mg of UR-9751 per kg, and 80% with 100 mg of fluconazole per kg. However, in terms of recovery of C. immitis from the organs, none of the surviving mice treated with UR-9746, UR-9751, or fluconazole had complete clearance of organisms from the spleen, liver, or lungs. The two azoles were comparable in terms of prolonging survival and clearing infection, and both were about 10 times more effective than fluconazole, although twice-daily administration might increase its efficacy. Further studies with both UR-9746 and UR-9751 are anticipated.
FUTURE ROLE OF AZOLES IN ANTIFUNGAL THERAPY
Major developments in the azole class of antifungal agents during the 1990s have provided expanded options for the treatment of many opportunistic and endemic fungal infections. For over 20 years, amphotericin B was the mainstay of treatment of serious systemic mycoses. The first oral azole, ketoconazole, was introduced in the United States in 1981 and provided an alternative to amphotericin B for nonmeningeal, non-life-threatening infections and for outpatient treatment of histoplasmosis and blastomycosis (132). With the introduction in the United States of i.v. and p.o. fluconazole in 1990 and p.o. itraconazole in 1992, clinicians had safer, well-tolerated alternatives to both amphotericin B and ketoconazole.
Fluconazole has received widespread use for prophylaxis and treatment of candidal and cryptococcal infections, which are becoming increasingly prevalent as the population of immunocompromised patients grows, and as single-dose oral therapy for vulvovaginal candidiasis. Although less widely used than fluconazole in AIDS patients and bone marrow transplant recipients due to variable absorption, itraconazole has become the drug of choice for the treatment of histoplasmosis and blastomycosis. It is also used for the initial therapy of less severe cases of aspergillosis and for the treatment of onychomycosis due to both Candida and dermatophytes. An itraconazole oral solution with enhanced drug absorption was introduced in the United States in 1997 for the treatment of OPC and esophageal candidiasis. This formulation provides a therapeutic option for HIV-infected patients with OPC clinically refractory to fluconazole (32, 33, 72, 81, 195).
Expanded uses for the currently available azoles have been suggested. High-dose fluconazole (800 to 1,000 mg/day) may prove useful as salvage therapy for patients with cryptococcal meningitis who fail to respond to amphotericin B (19) and as primary therapy (115, 159). Other suggested uses of fluconazole include the initial therapy of nonmeningeal coccidioidomycosis (243), prophylaxis for C. immitis infection in serologically positive HIV-infected patients living in areas of endemic infection (262), treatment of disseminated histoplasmosis in immunocompetent patients (59), and treatment of paracoccidioidomycosis (59, 208) and onychomycosis (9, 65, 117, 139, 145). Currently recommended for lifelong secondary prophylaxis of Aspergillus infections in bone marrow transplant recipients and patients with hematologic malignancies (147, 161), itraconazole has been suggested as initial therapy for many patients with invasive aspergillosis (245), particularly if an i.v. formulation becomes available (103), and as primary prophylaxis (147). The itraconazole i.v. formulation with cyclodextrin that is being developed may also expand the role of this triazole in the treatment of systemic Candida infections (103).
Despite these advances, many fungal infections fail to respond to treatment. One approach to this problem is to use combinations of antifungal agents with different mechanisms of action. Clinical trials of the combination of fluconazole and amphotericin B for the treatment of both cryptococcal meningitis (95, 229) and severe candidemia (74, 95, 103) are in progress. Against C. neoformans, the combination of 5-FC and fluconazole improves survival in infected animals and is effective in cases where monotherapy with either agent has been ineffective, and in which infection was due to a fluconazole-resistant isolate (140, 171). Preliminary clinical trials in patients with cryptococcal meningitis indicate that this combination improves the therapeutic outcome but suggest that a high dose of fluconazole (∼1,200 mg/day) should be administered (140, 141, 253). A triple combination of amphotericin B–5-FC–fluconazole achieved 100% survival in mice infected with a C. neoformans isolate from an AIDS patient successfully treated for meningitis with fluconazole–5-FC (57). Other potential combinations include a triazole with terbinafine (10, 225), amphotericin B or fluconazole in combination with a fluoroquinolone such as trovafloxacin or ciprofloxacin (249), and an azole in combination with cytokines to improve the response in immunocompromised patients (74, 94, 103, 132).
Resistance to the available azole antifungal agents has become a major concern with their widespread use during the 1990s. A number of second-generation azoles and triazoles, including voriconazole, SCH-56592, T-8581, BMS-207147 (formerly ER-30346), and UR-0746 and UR-9751, are currently in various stages of development. All of the agents discussed in this review are active following p.o. administration. Voriconazole and T-8581 may also be given i.v., and SCH 56592 also shows topical activity. With the exception of D0870 and the two UR compounds, the second-generation agents exhibit broad-spectrum antifungal activity and are particularly promising for the treatment of infections due to Aspergillus spp. They also have the potential to extend coverage to include not only the typical opportunistic and endemic pathogens but also dimorphic and filamentous fungi and dematiaceous and hyaline molds, including P. marneffei and Fusarium spp. Both voriconazole and SCH 56592 are active in vitro against Candida spp. with decreased susceptibility to fluconazole (11, 38, 192, 222), although highly azole-resistant strains show apparent cross-resistance (38, 192).
SCH 56592 is undergoing phase I/II clinical trials, and no results were available at the time of this review. Initial findings in phase II clinical trials of voriconazole indicate that this triazole is promising for the treatment of OPC and esophageal candidiasis and acute and chronic invasive aspergillosis (222). Phase III clinical trials comparing voriconazole with established agents in the treatment of invasive aspergillosis and other life-threatening fungal infections are in progress.
CONCLUSIONS
In this review, we have described present and future uses of the currently available azole antifungal agents in the treatment of systemic and superficial fungal infections and provided a brief overview of the current status of in vitro susceptibility testing and the growing problem of clinical resistance to the azoles. Use of the currently available azoles in combination with other antifungal agents with different mechanisms of action is likely to provide enhanced efficacy. Detailed information on some of the second-generation azoles and triazoles being developed to provided extended coverage of opportunistic, endemic, and emerging fungal pathogens, as well as those in which resistance to older agents is becoming problematic, has been provided. A number of promising new broad-spectrum p.o. antifungal agents are on the horizon. If the tolerability of these agents is comparable to that of the first-generation triazoles, clinicians should soon have expanded options for the treatment of serious fungal infections.
ACKNOWLEDGMENTS
This review would not have been possible without the assistance of Clara Henson of the Pfizer Information Center and Debbie Vertulo, who provided extensive literature searches and obtained the relevant documents.
REFERENCES
- 1.Abi-Said D, Anaissie E, Uzun O, Raad I, Pinzcowski H, Vartivarian S. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin Infect Dis. 1997;24:1122–1128. doi: 10.1086/513663. [DOI] [PubMed] [Google Scholar]
- 2.Alangaden G, Chandrasekar P H, Bailey E, Khaliq Y the Bone Marrow Transplantation Team. Antifungal prophylaxis with low-dose fluconazole during bone marrow transplantation. Bone Marrow Transplant. 1994;14:919–924. [PubMed] [Google Scholar]
- 3.Aly R, Berger T. Common superficial fungal infections in patients with AIDS. Clin Infect Dis. 1996;22:S128–S132. doi: 10.1093/clinids/22.supplement_2.s128. [DOI] [PubMed] [Google Scholar]
- 4.Anaissie E J, Darouiche R O, Abi-Said D, Uzun O, Mera J, Gentry L O, Williams T, Kontoyiannis D P, Karl C L, Bodey G P. Management of invasive candidal infections: results of a prospective, randomized, multicenter study of fluconazole versus amphotericin B and review of the literature. Clin Infect Dis. 1996;23:964–972. doi: 10.1093/clinids/23.5.964. [DOI] [PubMed] [Google Scholar]
- 5.Anaissie E J, Paetznick V L, Ensign L G, Espinel-Ingroff A, Galgiani J N, Hitchcock C A, LaRocco M, Patterson T, Pfaller M A, Rex J H, et al. Microdilution antifungal susceptibility testing of Candida albicans and Cryptococcus neoformans with and without agitation: an eight-center collaborative study. Antimicrob Agents Chemother. 1996;40:2387–2391. doi: 10.1128/aac.40.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anaissie E J, Rex J H, Uzun O, Vartivarian S. Predictors of adverse outcome in cancer patients with candidemia. Am J Med. 1998;104:238–245. doi: 10.1016/s0002-9343(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 7.Anaissie E J, Vartivarian S E, Abi-Said D, Uzun O, Pinczowski H, Kontoyiannis D P, Khoury P, Papadakis K, Gardner A, Raad I I, et al. Fluconazole versus amphotericin B in the treatment of hematogenous candidiasis: a matched cohort study. Am J Med. 1996;101:170–176. doi: 10.1016/s0002-9343(96)80072-6. [DOI] [PubMed] [Google Scholar]
- 8.Arikian, S. R., T. R. Einarson, G. Kobelt-Nguyen, F. Schubert, and the Onymychosis Study Group. 1994. A multinational pharmacoeconomic analysis of oral therapies for onychomycosis. Br. J. Dermatol. 130(Suppl. 43):35–44. [DOI] [PubMed]
- 9.Assaf R R, Elewski B E. Intermittent fluconazole dosing in patients with onychomycosis: results of a pilot study. J Am Acad Dermatol. 1996;35:216–219. doi: 10.1016/s0190-9622(96)90327-8. [DOI] [PubMed] [Google Scholar]
- 10.Barchiesi F, Di Francesco L F, Scalise G. In vitro activities of terbinafine in combination with fluconazole and itraconazole against isolates of Candida albicans with reduced susceptibility to azoles. Antimicrob Agents Chemother. 1997;41:1812–1814. doi: 10.1128/aac.41.8.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barry A L, Brown S D. In vitro studies of two triazole antifungal agents (voriconazole [UK-109,496] and fluconazole) against Candida species. Antimicrob Agents Chemother. 1996;40:1948–1949. doi: 10.1128/aac.40.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartlett J G. Medical management of HIV infection. Baltimore, Md: Department of Infectious Diseases, Johns Hopkins University; 1998. [Google Scholar]
- 13.Bartroli J, Turmo E, Algueró M, Boncompte E, Vericat M L, García-Rafanell J, Forn J. Synthesis and antifungal activity of new azole derivatives containing an N-acylmorpholine ring. J Med Chem. 1995;38:3918–3932. doi: 10.1021/jm00020a005. [DOI] [PubMed] [Google Scholar]
- 14.Benedetti E, Gruessner A C, Troppmann C, Papalois B E, Sutherland D E R, Dunn D L, Gruessner R W G. Intra-abdominal fungal infections after pancreatic transplantation: incidence, treatment, and outcome. J Am Coll Surg. 1996;183:307–316. [PubMed] [Google Scholar]
- 15.Bennett J E. Antifungal agents. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 401–410. [Google Scholar]
- 16.Bennett J E. Aspergillus species. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2306–2311. [Google Scholar]
- 17.Bennett J E. Miscellaneous fungi, and Prototheca. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2389–2393. [Google Scholar]
- 18.Bennett J E. Antifungal agents. In: Hardman G J G, Limbird L E, Molenoff P B, Ruddon R W, Gilman A G, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 9th ed. New York, N.Y: McGraw-Hill Book Co.; 1996. pp. 1175–1190. [Google Scholar]
- 19.Berry A J, Rinaldi M G, Graybill J R. Use of high-dose fluconazole as salvage therapy for cryptococcal meningitis in patients with AIDS. Antimicrob Agents Chemother. 1992;36:690–692. doi: 10.1128/aac.36.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billstein S A. How the pharmaceutical industry brings an antibiotic drug to market in the United States. Antimicrob Agents Chemother. 1994;38:2679–2682. doi: 10.1128/aac.38.12.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodey, G. P. 1992. Azole antifungal agents. Clin. Infect. Dis. 14(Suppl. 1):S161–S169. [DOI] [PubMed]
- 22.Bonnotte B, Caillot D, Solary E, Lopez J, Bailly C, Casanovas O, Chavanet P, Bonnin A, Guy H. Breakthrough candidemias in patients with haematological malignancies during antifungal azole prophylaxis. A report of 20 cases. J Infect. 1994;8:59. . (Abstract.) [Google Scholar]
- 23.Bornay-Llinares F J, Mayol-Belda M S, Onrubia-Pintado J, Torres-Rodriguez J M. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Murine cryptococcosis. Tissular damage decreases after treatment with UR-9746, abstr. F88; p. 128. [Google Scholar]
- 24.Bornay-Llinares F J, Torres-Rodriguez J M. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Anticryptococcal activity of a new azole derivative (UR-9746) in a murine model, abstr. F86; p. 128. [Google Scholar]
- 25.Boschman C R, Bodnar U R, Tornatore M A, Obias A A, Noskin G A, Englund K, Postelnick M A, Suriano T, Peterson L R. Thirteen-year evolution of azole resistance in yeast isolates and prevalence of resistant strains carried by cancer patients at a large medical center. Antimicrob Agents Chemother. 1998;42:734–738. doi: 10.1128/aac.42.4.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozzette S A, Larsen R, Chiu J, Leal M A E, Jacobsen J, Rothman P, Robinson P, Gilbert G, McCutchan J A, Tilles J, et al. A controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:580–584. doi: 10.1056/NEJM199102283240902. [DOI] [PubMed] [Google Scholar]
- 27.Bradsher, R. W. 1996. Histoplasmosis and blastomycosis. Clin. Infect. Dis. 22(Suppl. 2):S102–S111. [DOI] [PubMed]
- 28.Budavari S, editor. The Merck index. Rahway, N.J: Merck & Co., Inc.; 1989. [Google Scholar]
- 29.Bullock W E. Histoplasma capsulatum. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2340–2353. [Google Scholar]
- 30.Cacciapuoti A, Loebenberg D, Frank D, Moss E L J, Menzel F, Michalski M, Norris C, Yaremko B, Hare R S, Miller G H. Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Effect of delayed or extended treatment with SCH56592 (SCH) or concomitant treatment with amphotericin B (AMB) in murine models of pulmonary aspergillosis and systemic candidiasis, abstr. F96; p. 116. [Google Scholar]
- 31.Cacciapuoti A, Parmegiani R, Loebenberg D, Antonacci B, Moss E L J, Menzel F J, Norris C, Hare R S, Miller G H. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Efficacy of SCH 56592 in pulmonary aspergillosis and candidiasis in mice, abstr. F66; p. 124. [Google Scholar]
- 32.Cartledge J D, Midgely J, Gazzard B G. Itraconazole solution: higher serum drug concentrations and better clinical response rates than the capsule formulation in acquired immunodeficiency syndrome patients with candidosis. J Clin Pathol. 1997;50:477–480. doi: 10.1136/jcp.50.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cartledge J D, Midgley J, Gazzard B G. Itraconazole cyclodextrin solution: the role of in vitro susceptibility testing in predicting successful treatment of HIV-related fluconazole-resistant and fluconazole-susceptible oral candidosis. AIDS. 1997;11:163–168. doi: 10.1097/00002030-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Castagnola, E., B. B, E. Montinaro, and C. Viscoli. 1996. Fungal infections in patients undergoing bone marrow transplantation: an approach to a rational management protocol. Bone Marrow Transplant. 18(Suppl. 2):97–106. [PubMed]
- 35.Catanzaro A, Galgiani J N, Levine B E, Sharkey-Mathis P K, Fierer J, Stevens D A, Chapman S W, Cloud G NIAID Mycoses Study Group. Fluconazole in the treatment of chronic pulmonary and nonmeningeal disseminated coccidioidomycosis. Am J Med. 1995;98:249–256. doi: 10.1016/s0002-9343(99)80371-4. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. 1998 guidelines for treatment of sexually transmitted diseases. Morbid Mortal Weekly Rep. 1998;47(RR-1):75–79. [PubMed] [Google Scholar]
- 37.Chapman S W. Blastomyces dermatitidis. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2353–2365. [Google Scholar]
- 38.Chin N-X, Weitzman I, Della-Latta P. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. In vitro antifungal activity of voriconazole alone and in combination with flucytosine against Candida species and other pathogenic fungi, abstr. E-84; p. 128. [Google Scholar]
- 39.Clancy C J, Yu C Y, Nguyen M H. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. In vitro activity of voriconazole against yeasts and comparison with fluconazole, abstr. E-88; p. 129. [Google Scholar]
- 40.Clancy C J, Yu Y C, Nguyen M H. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Comparison of in vitro activity of voriconazole and amphotericin B against filamentous fungi, abstr. E-89; p. 129. [Google Scholar]
- 41.Clemons K V, Hanson L H, Stevens D A. Activities of the triazole D0870 in vitro against murine blastomycosis. Antimicrob Agents Chemother. 1993;37:1177–1179. doi: 10.1128/aac.37.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clemons K V, Stevens D A. Efficacies of two novel azole derivatives each containing a morpholine ring, UR-9746 and UR-9751, against systemic murine coccidioidomycosis. Antimicrob Agents Chemother. 1997;41:200–203. doi: 10.1128/aac.41.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen P R, Scher R K. Topical and surgical treatment of onychomycosis. J Am Acad Dermatol. 1994;31:S74–S77. doi: 10.1016/s0190-9622(08)81273-x. [DOI] [PubMed] [Google Scholar]
- 44.Coldiron B. Recalcitrant onychomycosis of the toenails successfully treated with fluconazole. Arch Dermatol. 1992;128:909–910. [PubMed] [Google Scholar]
- 45.Cole G T, Seshan K R, Loebenberg D. Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Evaluation of SCH 56592 in clearance of GI mucosal candidiasis from mice with retroviral-induced immunodeficiency, abstr. F95; p. 116. [Google Scholar]
- 46.Colombo A L, Barchiesi F, McGough D A, Rinaldi M G. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for azole antifungal susceptibility testing. J Clin Microbiol. 1995;33:535–540. doi: 10.1128/jcm.33.3.535-540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Como J A, Dismukes W E. Oral azole drugs as systemic antifungal therapy. N Engl J Med. 1994;330:263–272. doi: 10.1056/NEJM199401273300407. [DOI] [PubMed] [Google Scholar]
- 48.DeDoncker P, Decroix J, Pierard G E, Roelant D, Woestenborghs R, Jacqmin P, Odds F, Heremans A, Dockx P, Roseeuw D. Antifungal pulse therapy for onychomycosis. A pharmacokinetic and pharmacodynamic investigation of monthly cycles of 1-week pulse therapy with itraconazole. Arch Dermatol. 1996;132:34–41. doi: 10.1001/archderm.132.1.34. [DOI] [PubMed] [Google Scholar]
- 49.Degreef J H, DeDoncker P R G. Current therapy of dermatophytosis. J Am Acad Dermatol. 1994;31:S25–S30. doi: 10.1016/s0190-9622(08)81263-7. [DOI] [PubMed] [Google Scholar]
- 50.deLalla F, Pellizzer G, Vaglia A, Manfrin V, Franzetti M, Fabris P, Stecca C. Amphotericin B as primary therapy for cryptococcosis in patients with AIDS: reliability of relatively high doses administered over a relatively short period. Clin Infect Dis. 1995;20:263–266. doi: 10.1093/clinids/20.2.263. [DOI] [PubMed] [Google Scholar]
- 51.Del Favero A, Menichetti F, Micozzi A, Bucaneve G, De Buele K, Martino P the GIMEMA Infection Program. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Antifungal prophylaxis in neutropenic patients (<1,000/mmc) with haematologic malignancy: a double-blind trial to compare itraconazole oral solution (IOS) with placebo (PLA), abstr. LM-84; p. 380. [Google Scholar]
- 52.Denning D, del Favero A, Gluckman E, Norflok D, Ruhnke M, Yonren S, Troke P, Sarantis N the Aspergillosis Study Group. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. UK-109,496, a novel, wide-spectrum triazole derivative for the treatment of fungal infections: clinical efficacy in acute invasive aspergillosis. abstr. F80; p. 126. [Google Scholar]
- 53.Denning D W, Lee J Y, Hostetler J S, Pappas P, Kauffman C A, Dewsnup D H, Galgiani J N, Graybill J R, Sugar A M, Catanzaro A, et al. NIAID Mycoses Study Group multicenter trial of oral itraconazole therapy for invasive aspergillosis. Am J Med. 1994;97:135–144. doi: 10.1016/0002-9343(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 54.Denning D W, Stevens D A. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis. 1990;12:1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- 55.Denning D W, Venkateswarlu K, Oakley K L, Anderson M J, Manning N J, Stevens D A, Warnock D W, Kelly S L. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1364–1368. doi: 10.1128/aac.41.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeWit S, Dupont B, Cartledge J D, Hawkins D A, Gazzard B G, Clumeck N, Denning D W. A dose comparison study of a new triazole antifungal (D0870) in HIV-positive patients with oral candidiasis. AIDS. 1997;11:759–763. doi: 10.1097/00002030-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Diamond D M, Bauer M, Daniel B E, Leal M A E, Johnson D, Williams B K, Thomas A M, Ding J C, Najvar L, Graybill J R, et al. Amphotericin B colloidal dispersion combined with flucytosine with or without fluconazole for treatment of murine cryptococcal meningitis. Antimicrob Agents Chemother. 1998;42:528–533. doi: 10.1128/aac.42.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diamond R D. Cryptococcus neoformans. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious disease. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2331–2340. [Google Scholar]
- 59.Diaz, M., R. Negroni, F. Montero-Gei, L. G. M. Castro, S. A. P. Sampaio, D. Borelli, A. Restrepo, L. Franco, J. L. Bran, E. G. Arathoon, et al. 1992. A pan-American 5-year study of fluconazole therapy for deep mycoses in the immunocompetent host. Clin. Infect. Dis. 14(Suppl. 1):S68–S76. [DOI] [PubMed]
- 60.Dickinson R P, Bell A S, Hitchcock C A, Narayanaswami S, Ray S J, Richardson K, Troke P F. Novel antifungal 2-aryl-1-(1H-1,2,4-triazol-1-yl)butan-2-ol derivatives with high activity against Aspergillus fumigatus. Bioorg Med Chem Lett. 1996;6:2031–2036. [Google Scholar]
- 61.Ding J C, Bauer M, Diamond D M, Leal M A E, Johnson D, Williams B K, Thomas A M, Majvar L, Graybill J R, Larsen R A. Effect of severity of meningitis on fungicidal activity of flucytosine combined with fluconazole in a murine model of cryptococcal meningitis. Antimicrob Agents Chemother. 1997;41:1589–1593. doi: 10.1128/aac.41.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dismukes, W. E. 1993. Management of cryptococcosis. Clin. Infect. Dis. 17(Suppl. 2):S507–S512. [DOI] [PubMed]
- 63.Dismukes W E, Bradsher Jr R W, Cloud G C, Kauffman C A, Chapman S W, George R B, Stevens D A, Girard W M, Saag M S, Bowles-Patton C, et al. Itraconazole therapy for blastomycosis and histoplasmosis. Am J Med. 1992;93:489–497. doi: 10.1016/0002-9343(92)90575-v. [DOI] [PubMed] [Google Scholar]
- 64.Drake L A, Dinehart S M, Farmer E R, Goltz R W, Graham G F, Hordinsky M K, Lewis C W, Pariser D M, Skouge J W, Webster S B, et al. Guidelines of care for superficial mycotic infections of the skin: mucocutaneous candidiasis. J Am Acad Dermatol. 1996;34:110–115. doi: 10.1016/s0190-9622(96)80136-8. [DOI] [PubMed] [Google Scholar]
- 65.Drake L A, Dinehart S M, Farmer E R, Goltz R W, Graham G F, Hordinsky M K, Lewis C W, Pariser D M, Skouge J W, Webster S B, et al. Guidelines of care for superficial mycotic infections of the skin: onychomycosis. J Am Acad Dermatol. 1996;34:116–121. doi: 10.1016/s0190-9622(96)80136-8. [DOI] [PubMed] [Google Scholar]
- 66.Drake L A, Dinehart S M, Farmer E R, Goltz R W, Graham G F, Hordinsky M K, Lewis C W, Pariser D M, Skouge J W, Webster S B, et al. Guidelines of care for superficial mycotic infections of the skin: piedra. J Am Acad Dermatol. 1996;34:122–124. doi: 10.1016/s0190-9622(96)80136-8. [DOI] [PubMed] [Google Scholar]
- 67.Drake L A, Dinehart S M, Farmer E R, Goltz R W, Graham G F, Hordinsky M K, Lewis C W, Pariser D M, Skouge J W, Webster S B, et al. Guidelines of care for superficial mycotic infections of the skin: pityriasis (tinea) versicolor. J Am Acad Dermatol. 1996;34:287–289. doi: 10.1016/s0190-9622(96)80136-8. [DOI] [PubMed] [Google Scholar]
- 68.Drake L A, Dinehart S M, Farmer E R, Goltz R W, Graham G F, Hordinsky M K, Lewis C W, Pariser D M, Skouge J W, Webster S B, et al. Guidelines of care for superficial mycotic infections of the skin: tinea capitis and tinea barbae. J Am Acad Dermatol. 1996;34:290–294. doi: 10.1016/s0190-9622(96)80137-x. [DOI] [PubMed] [Google Scholar]
- 69.Drake L A, Dinehart S M, Farmer E R, Goltz R W, Graham G F, Hordinsky M K, Lewis C W, Pariser D M, Skouge J W, Webster S B, et al. Guidelines of care for superficial mycotic infections of the skin: tinea corporis, tinea cruris, tinea faciei, tinea manuum, and tinea pedis. J Am Acad Dermatol. 1996;34:282–286. doi: 10.1016/s0190-9622(96)80135-6. [DOI] [PubMed] [Google Scholar]
- 70.Dromer, F., S. Mathoulin, B. Dupont, O. Brugiere, L. Letenneur, and the French Cryptococcosis Study Group. 1996. Comparison of the efficacy of amphotericin B and fluconazole in the treatment of cryptococcosis in human immunodeficiency virus-negative patients: retrospective analysis of 83 cases. Clin. Infect. Dis. 22(Suppl. 2):S154–S160. [DOI] [PubMed]
- 71.Dupont B, Denning D, Lode H, Youren S, Troke P, Sarantis N. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. UK-109,496, a novel, wide-spectrum triazole derivative for the treatment of fungal infections: clinical efficacy in chronic invasive aspergillosis, abstr. F81; p. 127. [Google Scholar]
- 72.Dupont, B. F., F. Dromer, and L. Improvisi. 1996. The problem of azole resistance in Candida. J. Mycol. Med. 6(Suppl. II):12–19.
- 73.Edwards J E J. Candida species. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2289–2306. [Google Scholar]
- 74.Edwards J E J, Bodey G P, Bowden R A, Büchner T, de Pauw B E, Filler S G, Ghannoum M A, Glauser M, Herbrecht R, Kauffman C A, et al. International conference for the development of a consensus on the management and prevention of severe candidal infections. Clin Infect Dis. 1997;25:43–59. doi: 10.1086/514504. [DOI] [PubMed] [Google Scholar]
- 75.Elewski B E, Hay R J. Cutaneous antifungal therapy in AIDS: summary of an international summit. Infect Dis Clin Pract. 1996;5:359–370. doi: 10.1093/clinids/23.2.305. [DOI] [PubMed] [Google Scholar]
- 76.Elmets C A. Management of common superficial fungal infections in patients with AIDS. J Am Acad Dermatol. 1994;31:S60–S63. doi: 10.1016/s0190-9622(08)81270-4. [DOI] [PubMed] [Google Scholar]
- 77.Espinel-Ingroff A. Etest for antifungal susceptibility testing of yeasts. Diagn Microbiol Infect Dis. 1994;19:217–220. doi: 10.1016/0732-8893(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 78.Espinel-Ingroff A. Antifungal susceptibility testing. Clin Microbiol Newsl. 1996;18:161–167. [Google Scholar]
- 79.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M, et al. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Espinel-Ingroff A, Kish C W J, Kerkering T M, Fromtling R A, Bartizal K, Galgiani J N, Villareal K, Pfaller M A, Gerarden T, Rinaldi M G, et al. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol. 1992;30:3138–3145. doi: 10.1128/jcm.30.12.3138-3145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fessel W J, Merrill K W, Ward D J, Moskovitz B L, Benken C, Oleka N, Grimwood H. Presented at the 4th Conference on Retroviruses and Opportunistic Infections, Washington, D.C., 22 to 26 January 1997. 1997. Itraconazole oral solution (IS) for the treatment of fluconazole-refractory oropharyngeal candidiasis (OC) in HIV-positive patients, abstract. [Google Scholar]
- 82.Fidel P L J, Cutright J L, Sobel J D. Efficacy of D0870 treatment of experimental Candida vaginitis. Antimicrob Agents Chemother. 1997;41:1455–1459. doi: 10.1128/aac.41.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fothergill A W, Sutton D A, Rinaldi M G. Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. An in vitro head-to-head comparison of Schering 56592, Amphotericin B, Fluconazole, and Itraconazole against a spectrum of filamentous fungi, abstr. F89; p. 115. [Google Scholar]
- 84.Frieden I J, Howard R. Tinea capitis: epidemiology, diagnosis, treatment, and control. J Am Acad Dermatol. 1994;31:S42–S46. doi: 10.1016/s0190-9622(08)81266-2. [DOI] [PubMed] [Google Scholar]
- 85.Fromtling R A. Overview of medically important antifungal azole derivatives. Clin Microbiol Rev. 1988;1:187–219. doi: 10.1128/cmr.1.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fromtling R A, Castaner J. SCH-56592. Drugs Future. 1996;21:160–166. [Google Scholar]
- 87.Fromtling R A, Galgiani J N, Pfaller M A, Espinel-Ingroff A, Bartizal K F, Bartlett M S, Body B A, Frey C, Hall G, Roberts G D, et al. Multicenter evaluation of a broth macrodilution antifungal susceptibility test for yeasts. Antimicrob Agents Chemother. 1993;37:39–45. doi: 10.1128/aac.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fung-Tomc J C, Bonner D P. Recent developments in pradimicin-benanomicin and triazole antibiotics. Exp Opin Investig Drugs. 1997;6:129–145. doi: 10.1517/13543784.6.2.129. [DOI] [PubMed] [Google Scholar]
- 89.Fung-Tomc J C, Huczko E, Minassian B, Bonner D P. In vitro activity of a new oral triazole, BMS-207147 (ER-30346) Antimicrob Agents Chemother. 1998;42:313–318. doi: 10.1128/aac.42.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galgiani J N, Catanzaro A, Cloud G A, Higgs J, Friedman B A, Larsen R A, Graybill J R the NIAID Mycoses Study Group. Fluconazole therapy for coccidioidal meningitis. Ann Inter Med. 1993;119:28–35. doi: 10.7326/0003-4819-119-1-199307010-00005. [DOI] [PubMed] [Google Scholar]
- 91.Galgiani J N, Lewis M L. In vitro studies of activities of the antifungal triazoles SCH56592 and itraconazole against Candida albicans, Cryptococcus neoformans, and other pathogenic yeast. Antimicrob Agents Chemother. 1997;41:180–183. doi: 10.1128/aac.41.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gallis, H. A. 1996. Amphotericin B: a commentary on its role as an antifungal agent and as a comparative agent in clinical trials. Clin. Infect. Dis. 22(Suppl. 2):S145–S147. [DOI] [PubMed]
- 93.George D, Miniter P, Andriole V T. Efficacy of UK-109496, a new azole antifungal agent, in an experimental model of invasive aspergillosis. Antimicrob Agents Chemother. 1996;40:86–91. doi: 10.1128/aac.40.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Georgopapadakou N H, Walsh T J. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279–291. doi: 10.1128/aac.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghannoum M A. Future of antimycotic therapy. Dermatol Ther. 1997;3:104–111. [Google Scholar]
- 96.Ghannoum M A, Rex J H, Galgiani J N. Susceptibility testing of fungi: current status of correlation of in vitro data with clinical outcome. J Clin Microbiol. 1996;34:489–495. doi: 10.1128/jcm.34.3.489-495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gibb A P, Thorsten C, Van Den Elzen H. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. No increase in frequency of antifungal resistance among yeasts isolated from sterile sites, Foothills Hospital, Calgary, 1993–1996, abstr. E-76; p. 127. [Google Scholar]
- 98.Glasmacher A, Hahn C, Molitor E, Kleinschmidt R, Marklein G. Presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 28 September to 1 October 1997. 1997. Itraconazole in antifungal prophylaxis: comparison of trough levels in neutropenic patients receiving capsules and oral solution, abstract. [Google Scholar]
- 99.Goa K L, Barradell L B. Fluconazole. An update of its pharmacodynamic and pharmacokinetic properties and therapeutic use in major superficial and systemic mycoses in immunocompromised patients. Drugs. 1995;50:658–690. doi: 10.2165/00003495-199550040-00007. [DOI] [PubMed] [Google Scholar]
- 100.Goff D A, Koletar S L, J B W, Barnishan J, Fass R J. Isolation of fluconazole-resistant Candida albicans from human immunodeficiency virus-negative patients never treated with azoles. Clin Infect Dis. 1995;20:77–83. doi: 10.1093/clinids/20.1.77. [DOI] [PubMed] [Google Scholar]
- 101.Grant S M, Clissold S P. Itraconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in superficial and systemic mycoses. Drugs. 1989;37:310–344. doi: 10.2165/00003495-198937030-00003. [DOI] [PubMed] [Google Scholar]
- 102.Grant S M, Clissold S P. Fluconazole: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycoses. Drugs. 1990;39:877–916. doi: 10.2165/00003495-199039060-00006. [DOI] [PubMed] [Google Scholar]
- 103.Graybill, J. R. 1996. The future of antifungal therapy. Clin. Infect. Dis. 22(Suppl. 2):S166–S178. [DOI] [PubMed]
- 104.Graybill J R. Editorial response: can we agree on the treatment of candidiasis? Clin Infect Dis. 1997;25:60–62. doi: 10.1086/514503. [DOI] [PubMed] [Google Scholar]
- 105.Graybill J R, Najvar L K, Holmberg J D, Luther M F. Fluconazole, D0870, and flucytosine treatment of disseminated Candida tropicalis infections in mice. Antimicrob Agents Chemother. 1995;39:924–929. doi: 10.1128/aac.39.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Graybill J R, Stevens D A, Galgiani J N, Dismukes W E, Cloud G A the NIAID Mycoses Study Group. Itraconazole treatment of coccidioidomycosis. Am J Med. 1990;89:282–290. doi: 10.1016/0002-9343(90)90339-f. [DOI] [PubMed] [Google Scholar]
- 107.Graybill J R, Vazques J, Darouiche R O, Morhart R, Greenspan D, Tuazon C, Wheat J, Carey J, Leviton I, Hewitt R G, et al. Randomized trial of itraconazole oral solution for oropharyngeal candidiasis in HIV/AIDS patients. Am J Med. 1998;104:33–39. doi: 10.1016/s0002-9343(97)00307-0. [DOI] [PubMed] [Google Scholar]
- 108.Greenspan D. Treatment of oropharyngeal candidiasis in HIV-positive patients. J Am Acad Dermatol. 1994;31:S51–S55. doi: 10.1016/s0190-9622(08)81268-6. [DOI] [PubMed] [Google Scholar]
- 109.Gupta A K, Sauder D N, Shear N H. Antifungal agents: an overview. I. J Am Acad Dermatol. 1994;30:677–698. [PubMed] [Google Scholar]
- 110.Hadley S, Karchmer A W. Fungal infections in solid organ transplant recipients. Infect Dis Clin North Am. 1995;9:1045–1074. [PubMed] [Google Scholar]
- 111.Haria M, Bryson H M, Goa K L. Itraconazole. A reappraisal of its pharmacological properties and therapeutic use in the management of superficial fungal infections. Drugs. 1996;51:586–620. doi: 10.2165/00003495-199651040-00006. [DOI] [PubMed] [Google Scholar]
- 112.Hata K, Kimura J, Miki H, Toyosawa T, Moriyama M, Katsu K. Efficacy of ER-30346, a novel oral triazole antifungal agent, in experimental models of aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1996;40:2243–2247. doi: 10.1128/aac.40.10.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hata K, Kimura J, Miki H, Toyosawa T, Nakamura T, Katsu K. In vitro and in vivo antifungal activities of ER-30346, a novel oral triazole with a broad antifungal spectrum. Antimicrob Agents Chemother. 1996;40:2237–2242. doi: 10.1128/aac.40.10.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hata K, Uneo J, Miki H, Toyosawa T, Katsu K, Nakamura T, Horie T. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. ER-30346, a novel antifungal triazole. III. In vitro activity and its mode of action, abstr. F92; p. 129. [Google Scholar]
- 115.Haubrich R H, Haghighat D, Bozzette S A, Tilles J, McCutchan J A the California Collaborative Treatment Group. High-dose fluconazole for treatment of cryptococcal disease in patients with human immunodeficiency virus infection. J Infect Dis. 1994;170:238–242. doi: 10.1093/infdis/170.1.238. [DOI] [PubMed] [Google Scholar]
- 116.Hay R J. Onychomycosis. Agents of choice. Dermatol Clin. 1993;11:161–169. [PubMed] [Google Scholar]
- 117.Hay R J. Dermatophytosis and other superficial mycoses. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2375–2386. [Google Scholar]
- 118.He X, Tiballi R N, Zarins L T, Bradley S F, Sangeorzan J A, Kauffman C A. Azole resistance in oropharyngeal Candida albicans strains isolated from patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1994;38:2495–2497. doi: 10.1128/aac.38.10.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heald A E, Cox G M, Schell W A, Bartlett J A, Perfect J R. Oropharyngeal yeast flora and fluconazole resistance in HIV-infected patients receiving long-term continuous versus intermittent fluconazole therapy. AIDS. 1996;10:263–268. doi: 10.1097/00002030-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 120.Hennequin C, Labenne M, Benkerrou M, Hambourg M. Fluconazole-resistant Candida albicans in an immunocompetent child. Clin Infect Dis. 1994;19:1179–1180. doi: 10.1093/clinids/19.6.1179. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 121.Hibberd, P. L., and R. H. Rubin. 1994. Clinical aspects of fungal infection in organ transplant recipients. Clin. Infect. Dis. 19(Suppl. 1):S33–S40. [DOI] [PubMed]
- 122.Hitchcock C A, Andrews B J, Lewis B G H, Troke P F. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. UK-109,496, a novel, wide-spectrum triazole derivative for the treatment of fungal infections: antifungal activity in experimental infections with Cryptococcus, abstr. F75; p. 126. [Google Scholar]
- 123.Hitchcock C A, Andrews R J, Lewis B G H, Troke P F. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. UK-109,496, a novel, wide-spectrum triazole derivative for the treatment of fungal infections: antifungal activity in experimental infections with Aspergillus, abstr. F74; p. 125. [Google Scholar]
- 124.Hitchcock C A, Pye G W, Oliver G P, Troke P F. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. UK-109,496, a novel, wide-spectrum triazole derivative for the treatment of fungal infections: antifungal activity and selectivity in vitro, abstr. F72; p. 125. [Google Scholar]
- 125.Hochman L G, Scher R K, Meyerson M S, Cohen J L, Holwell J E. The safety and efficacy of oral fluconazole in the treatment of onychomycosis. J Geriatr Dermatol. 1993;1:169–172. [Google Scholar]
- 126.Improvisi L, Garcia-Hermoso D, Provost F, Dromer F, Dupont B. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Evaluation of two novel antifungal agents, UR-9746 and UR-9751 in the treatment of experimental murine histoplasmosis, abstr. F89; p. 128. [Google Scholar]
- 127.Iwen P C, Kelly D M, Reed E C, Hinrichs S H. Invasive infection due to Candida krusei in immunocompromised patients not treated with fluconazole. Clin Infect Dis. 1995;20:342–347. doi: 10.1093/clinids/20.2.342. [DOI] [PubMed] [Google Scholar]
- 128.Jezequel S G, Clark M, Cole S, Evans K E, Wastall P. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. UK-109,496, a novel, wide-spectrum triazole derivative for the treatment of fungal infections: pre-clinical pharmacokinetics, abstr. F76; p. 126. [Google Scholar]
- 129.Jezequel S G, Clark M, Evans K E, Roffey S, Wastall P, Webster R. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. UK-109,496, a novel, wide-spectrum triazole derivative for the treatment of fungal infections: disposition in animals, abstr. F77; p. 126. [Google Scholar]
- 130.Johnson E M, Warnock D W, Luker J, Porter S R, Scully C. Emergence of azole drug resistance in 24 Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J Antimicrob Chemother. 1995;35:103–114. doi: 10.1093/jac/35.1.103. [DOI] [PubMed] [Google Scholar]
- 131.Kauffman, C. A. 1996. Role of azoles in antifungal therapy. Clin. Infect. Dis. 22(Suppl. 2):S148–S153. [DOI] [PubMed]
- 132.Kauffman C A, Carver P L. Antifungal agents in the 1990s. Current status and future developments. Drugs. 1997;53:539–549. doi: 10.2165/00003495-199753040-00001. [DOI] [PubMed] [Google Scholar]
- 133.Khardori N, Nguyen H, Stephens L C, Kalvakuntla L, Rosenbaum B, Bodey G P. Comparative efficacies of cliofungin ( Ly121019) and amphotericin B against disseminated Candida albicans infection in normal and granulocytopenic mice. Antimicrob Agents Chemother. 1993;37:729–736. doi: 10.1128/aac.37.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kirkpatrick C H. Chronic mucocutaneous candidiasis. J Am Acad Dermatol. 1994;31:S14–S17. doi: 10.1016/s0190-9622(08)81260-1. [DOI] [PubMed] [Google Scholar]
- 135.Koks C H W, Meenhorst P L, Hillebrand M J X, Bult A, Beijnen J H. Pharmacokinetics of fluconazole in saliva and plasma after administration of an oral suspension and capsules. Antimicrob Agents Chemother. 1996;40:1935–1937. doi: 10.1128/aac.40.8.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koltin Y, Hitchcock C A. Progress in the search for new triazole antifungal agents. Curr Opin Chem Biol. 1997;1:176–182. doi: 10.1016/s1367-5931(97)80007-5. [DOI] [PubMed] [Google Scholar]
- 137.Kriesel J D, Adderson E E, Gooch W M I, Pavia A T. Invasive sinonasal disease due to Scopulariopsis candida: case report and review of scopulariopsosis. Clin Infect Dis. 1994;19:317–319. doi: 10.1093/clinids/19.2.317. [DOI] [PubMed] [Google Scholar]
- 138.Kung N, Fisher N, Gunson B, Hastings M, Mutimer D. Fluconazole prophylaxis for high-risk liver transplant recipients. Lancet. 1995;345:1234–1235. [PubMed] [Google Scholar]
- 139.Kuokkanen K, Alava S. Fluconazole in the treatment of onychomycosis caused by dermatophytes. J Dermatol Treat. 1992;3:115–117. [Google Scholar]
- 140.Larsen R A, Bauer M, Weiner J M, Diamond D M, Leal M E, Ding J C, Rinaldi M G, Graybill J R. Effect of fluconazole on fungicidal activity of flucytosine in murine cryptococcal meningitis. Antimicrob Agents Chemother. 1996;40:2178–2182. doi: 10.1128/aac.40.9.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Larsen R A, Bozzette S A, Jones B E, Haghighat D, Leal M A, Forthal D, Bauer M, Tilles J G, McCutchan J A, Leedom J M. Fluconazole combined with flucytosine for treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1994;19:741–745. doi: 10.1093/clinids/19.4.741. [DOI] [PubMed] [Google Scholar]
- 142.Law D, Moore C B, Denning D W. Activity of SCH 56592 compared with those of fluconazole and itraconazole against Candida spp. Antimicrob Agents Chemother. 1997;41:2310–2311. doi: 10.1128/aac.41.10.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Legendre R, Escola-Macre J. Itraconazole in the treatment of tinea capitis. J Am Acad Dermatol. 1990;23:559–560. doi: 10.1016/0190-9622(90)70254-f. [DOI] [PubMed] [Google Scholar]
- 144.LeMoing V, Lortholary O, Casassus P, Guillevin L, Dupont B. Traitement des aspergilloses par l’itraconazole. Med Mal Infect. 1995;25:50–57. [Google Scholar]
- 145.Lesher J L J. Recent developments in antifungal therapy. Dermatol Clin. 1996;14:163–169. doi: 10.1016/s0733-8635(05)70337-5. [DOI] [PubMed] [Google Scholar]
- 146.Linden P, Kramer D J, Mazariegos G, Marsh W, Casavilla A, Pinna A, Fung J, Kusne S. Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Low-dose amphotericin B (LDA) for the prophylaxis of serious Candida infections in high-risk liver recipients, abstr. J47; p. 227. [Google Scholar]
- 147.Lortholary O, Dupont B. Antifungal prophylaxis during neutropenia and immunodeficiency. Clin Microbiol Rev. 1997;10:477–504. doi: 10.1128/cmr.10.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lozano-Chiu M, Nelson P W, Paetznick V, Rex J H. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Activity of voriconazole (VORI) vs. Candida: effects of incubation time, Candida species, and fluconazole (FLU) susceptibility, abstr. E-87; p. 129. [Google Scholar]
- 149.Lutz J E, Clemons K V, Aristizabal B H, Stevens D A. Activity of the triazole SCH 56592 against disseminated murine coccidioidomycosis. Antimicrob Agents Chemother. 1997;41:1558–1561. doi: 10.1128/aac.41.7.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lutz J E, Clemons K V, Stevens D A. Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Efficacy of SCH 56592 (SCH) in a murine model of systemic coccidioidomycosis, abstr. F100; p. 117. [Google Scholar]
- 151.Lyman C A, Walsh T J. Systemically administered antifungal agents. A review of their clinical pharmacology and therapeutic applications. Drugs. 1992;44:9–35. doi: 10.2165/00003495-199244010-00002. [DOI] [PubMed] [Google Scholar]
- 152.Maenza J R, Merz W G, Romagnoli M J, Keruly J C, Moore R D, Gallant J E. Infection due to fluconazole-resistant Candida in patients with AIDS: prevalence and microbiology. Clin Infect Dis. 1997;24:28–34. doi: 10.1093/clinids/24.1.28. [DOI] [PubMed] [Google Scholar]
- 153.Maesaki S, Kohno S, Kaku M, Koga H, Hara K. Effects of antifungal agent combinations administered simultaneously and sequentially against Aspergillus fumigatus. Antimicrob Agents Chemother. 1994;38:2843–2845. doi: 10.1128/aac.38.12.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Manavathu E K, Cutright J L, Chandrasekar P H. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. A comparative study of the in vitro susceptibility of amphotericin B-resistant isolates of Aspergillus fumigatus to voriconazole and itraconazole, abstr. E-85; p. 129. [Google Scholar]
- 155.Martin M V, Yates J, Hitchcock C A. Comparison of voriconazole (UK-109,496) and itraconazole in prevention and treatment of Aspergillus fumigatus endocarditis in guinea pigs. Antimicrob Agents Chemother. 1997;41:13–16. doi: 10.1128/aac.41.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Martins M D, Rex J H. Fluconazole suspension for oropharyngeal candidiasis unresponsive to tablets. Ann Intern Med. 1997;126:332–333. doi: 10.7326/0003-4819-126-4-199702150-00020. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 157.Masur H. Management of opportunistic infections associated with HIV infection. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 1280–1294. [Google Scholar]
- 158.McGinnis M R, Pasarell L, Sutton D A, Fothergill A W, Cooper C R J, Rinaldi M G. In vitro evaluation of voriconazole against some clinically important fungi. Antimicrob Agents Chemother. 1997;41:1832–1834. doi: 10.1128/aac.41.8.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Menichetti F, Fiorio M, Tosti A, Gatti G, Pasticci M B, Miletich F, Marroni M, Bassetti D, Pauluzzi S. High-dose fluconazole therapy for cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1996;22:838–840. doi: 10.1093/clinids/22.5.838. [DOI] [PubMed] [Google Scholar]
- 160.Miller G H, Lobenberg D, Antonacci B, Cacciapuoti A, Moss E L J, Menzel F J, Michalski M, Norris C, Parmegiani R, Yarosh-Tomaine T, et al. Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro-in vivo correlations for the azole antifungals SCH 56592 (56592), SCH39304 (39304), SCH51048 (51048), fluconazole (FLZ) and itraconazole (ITZ) in candidiasis and aspergillosis, abstr. F94; p. 116. [Google Scholar]
- 161.Minamoto G Y, Rosenberg A S. Fungal infections in patients with acquired immunodeficiency syndrome. Med Clin North Am. 1997;81:381–409. doi: 10.1016/s0025-7125(05)70523-x. [DOI] [PubMed] [Google Scholar]
- 162.Munayyer H, Shaw K J, Hare R S, Salisbury B, Heimark L, Pramanik B, Greene J R. Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. SCH 56592 is a potent inhibitor of sterol C14 demethylation in fungi, abstr. F92; p. 115. [Google Scholar]
- 163.Murphy M, Bernard E M, Ishimaru T, Armstrong D. Activity of voriconazole (UK-109,496) against clinical isolates of Aspergillus species and its effectiveness in an experimental model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 1997;41:696–698. doi: 10.1128/aac.41.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Murray P A, Koletar S L, Mallegol I, Wu J, Moskovitz B L. Itraconazole oral solution versus clotrimazole troches for the treatment of oropharyngeal candidiasis in immunocompromised patients. Clin Ther. 1997;19:471–480. doi: 10.1016/s0149-2918(97)80131-2. [DOI] [PubMed] [Google Scholar]
- 165.Nahas G T, Sisto M. Onychomycosis: successful treatment with once-weekly fluconazole. Dermatology. 1993;186:59–61. doi: 10.1159/000247304. [DOI] [PubMed] [Google Scholar]
- 166.Nakamura T, Sakai R, Sonoda J, Katoh T, Kaneko T, Horie T. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. ER-30346, a novel antifungal triazole. IV. Pharmacokinetic and toxicological studies in mice, rats, and dogs, abstr. F94; p. 129. [Google Scholar]
- 167.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeast. Proposed standard M27-P. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 168.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Tentative standard M27-T. NCCLS document M27T. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 169.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 170.Nelson M R, Fisher M, Cartledge J, Rogers T, Gazzard B G. The role of azoles in the treatment and prophylaxis of cryptococcal disease in HIV infection. AIDS. 1994;8:651–654. doi: 10.1097/00002030-199405000-00011. [DOI] [PubMed] [Google Scholar]
- 171.Nguyen M H, Najvar L K, Yu C Y, Graybill J R. Combination therapy with fluconazole and flucytosine in the murine model of cryptococcal meningitis. Antimicrob Agents Chemother. 1997;41:1120–1123. doi: 10.1128/aac.41.5.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nguyen M H, Peacock J E J, Morris A J, Tanner D C, Nguyen M L, Snydman D R, Wagener M M, Rinaldi M G, Yu V L. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 173.Nguyen M H, Yu C Y. In vitro comparative efficacy of voriconazole and itraconazole against fluconazole-susceptible and -resistant Cryptococcus neoformans isolates. Antimicrob Agents Chemother. 1998;42:471–472. doi: 10.1128/aac.42.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Noemir A, Kumari P, Hilbert M J, Loebenberg D, Cacciapuoti A, Menzel F J, Moss E J, Hare R, Miller G H, Cayen M N, et al. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Comparative pharmacokinetics of a new triazole antifungal agent, SCH 56592, in mice, rats, rabbits, dogs, and cynomolgus monkeys. abstr. F68; p. 124. [Google Scholar]
- 175.Noemir A A, Kumari P, Loebenberg D, Cacciapuoti A, Hare R, Miller G, Sangekar S, Mahashabde S, Sequeira J, Vadino W A, et al. Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Bioavailability of SCH 56592, a new broad spectrum triazole antifungal agent, from various formulations, abstr. F103; p. 117. [Google Scholar]
- 176.Nolte F S, Parkinson T, Falconer D J, Dix S, Williams J, Gilmore C, Geller R, Wingard J R. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob Agents Chemother. 1997;44:196–199. doi: 10.1128/aac.41.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Norris S, Wheat J, McKinsey D, Lancaster D, Katz B, Black J, Driks M, Baker R, Israel K, Traeger D, et al. Prevention of relapse of histoplasmosis with fluconazole in patients with the acquired immunodeficiency syndrome. Am J Med. 1994;96:504–508. doi: 10.1016/0002-9343(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 178.Noskin G A, Lee J, Hacek D M, Postelnick M, Reisberg B E, Stosor V, Weitzman S A, Peterson L R. Molecular typing for investigating an outbreak of Candida krusei. Diagn Microbiol Infect Dis. 1996;26:117–123. doi: 10.1016/s0732-8893(96)00204-0. [DOI] [PubMed] [Google Scholar]
- 179.Oakley K L, Moore C B, Denning D W. In vitro activity of SCH-56592 and comparison with activities of amphotericin B and itraconazole against Aspergillus spp. Antimicrob Agents Chemother. 1997;41:1124–1126. doi: 10.1128/aac.41.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Oakley K L, Morrissey G, Denning D W. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1504–1507. doi: 10.1128/aac.41.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Odds F C. Antifungal susceptibility testing of Candida spp. by relative growth measurement at single concentrations of antifungal agents. Antimicrob Agents Chemother. 1992;36:1727–1737. doi: 10.1128/aac.36.8.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pappas P G, Bradsher R W, Chapman S W, Kauffman C A, Dine A, Cloud G A, Dismukes W E the National Institute of Allergy and Infectious Diseases Mycoses Study Group. Treatment of blastomycosis with fluconazole: a pilot study. Clin Infect Dis. 1995;20:267–271. doi: 10.1093/clinids/20.2.267. [DOI] [PubMed] [Google Scholar]
- 183.Parmegiani R, Cacciapuoti A, Loebenberg D, Antonacci B, Norris C, Yarosh-Tomaine T, Michalski M, Hare R S, Miller G H. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Efficacy of SCH 56592 in topical infection models, abstr. F67; p. 124. [Google Scholar]
- 184.Patterson B E, Coates P E. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. UK-109,496, a novel, wide-spectrum triazole derivative for the treatment of fungal infections: pharmacokinetics in man, abstr. F78; p. 126. [Google Scholar]
- 185.Patterson B E, Roffey S, Jezequel S G, Jones B. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. UK-109,496, a novel, wide-spectrum tirazole derivative for the treatment of fungal infections: disposition in man, abstr. F79; p. 126. [Google Scholar]
- 186.Patterson T F, Kirkpatrick W R, Mcatee R K, Loebenberg D. Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Efficacy of SCH 56592 in a rabbit model of invasive aspergillosis, abstr. F98; p. 116. [Google Scholar]
- 187.Perfect J R, Cox G M, Dodge R K, Schell W A. In vitro and in vivo efficacies of the azole SCH56592 against Cryptococcus neoformans. Antimicrob Agents Chemother. 1996;40:1910–1913. doi: 10.1128/aac.40.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pfaller, M. A. 1996. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 22(Suppl. 2):S89–S94. [DOI] [PubMed]
- 189.Pfaller M A, Bale M, Buschelman G, Lancaster M, Espinel-Ingroff A, Rex J H, Rinaldi M G. Selection of candidate quality control isolates and tentative quality control ranges for in vitro susceptibility testing of yeast isolates by National Committee for clinical Laboratory Standards proposed standard methods. J Clin Microbiol. 1994;32:1650–1653. doi: 10.1128/jcm.32.7.1650-1653.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pfaller M A, Barry A L. Evaluation of a novel colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1994;32:1992–1996. doi: 10.1128/jcm.32.8.1992-1996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pfaller M A, Burmeister L, Bartlett M S, Rinaldi M G. Multicenter evaluation of four methods of yeast inoculum preparation. J Clin Microbiol. 1988;26:1437–1441. doi: 10.1128/jcm.26.8.1437-1441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pfaller M A, Messer S, Jones R N. Activity of a new triazole, Sch56592, compared with those of four other antifungal agents tested against clinical isolates of Candida spp. and Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1997;41:233–235. doi: 10.1128/aac.41.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pfaller M A, Rex J H, Rinaldi M G. Antifungal susceptibility testing: technical advances and potential clinical applications. Clin Infect Dis. 1997;24:776–784. doi: 10.1093/clinids/24.5.776. [DOI] [PubMed] [Google Scholar]
- 194.Pfaller M A, Rinaldi M G, Galgiani J N, Bartlett M S, Body B A, Espinel-Ingroff A, Fromtling R A, Hall G S, Hughes C E, Odds F C, et al. Collaborative investigation of variables in susceptibility testing of yeasts. Antimicrob Agents Chemother. 1990;34:1648–1654. doi: 10.1128/aac.34.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Phillips P, Zemcov J, Mahmood W, Montaner J S G, Craib K, Clarke A M. Itraconazole cyclodextrin solution for fluconazole-refractory oropharyngeal candidiasis in AIDS: correlation of clinical response with in vitro susceptibility. AIDS. 1996;10:1369–1376. doi: 10.1097/00002030-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 196.Powderly W G. Fungi. In: Broder S, Merigan T C Jr, Bolognesi D, editors. Textbook of AIDS medicine. Baltimore, Md: The Williams & Wilkins Co.; 1994. pp. 345–357. [Google Scholar]
- 197.Powderly W G. Resistant candidiasis. AIDS Res Hum Retroviruses. 1994;10:925–929. doi: 10.1089/aid.1994.10.925. [DOI] [PubMed] [Google Scholar]
- 198.Powderly, W. G. 1996. Recent advances in the management of cryptococcal meningitis in patients with AIDS. Clin. Infect. Dis. 22(Suppl. 2):S119–S123. [DOI] [PubMed]
- 199.Powderly W G, Finkelstein D M, Feinberg J, Frame P, He W, Ven der Horst C, Koletar S L, Eyster M E, Carey J, Waskin H, et al. A randomized trial comparing fluconazole with clotrimazole troches for the prevention of fungal infections in patients with advanced human immunodeficiency virus infection. N Engl J Med. 1995;332:700–705. doi: 10.1056/NEJM199503163321102. [DOI] [PubMed] [Google Scholar]
- 200.Powderly W G, Saag M S, Cloud G A, Robinson P, Meyer R D, Jacobson J M, Graybill J R, Sugar A M, McAuliffe V J, Follansbee S E, et al. A controlled trial of fluconazole or amphotericin B to prevent relapse of cryptococcal meningitis in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:793–798. doi: 10.1056/NEJM199203193261203. [DOI] [PubMed] [Google Scholar]
- 201.Prentice A G, Warnock D W, Johnson S A N, Phillips M J, Oliver D A. Multiple dose pharmacokinetics of an oral solution of itraconazole in autologous bone marrow transplant recipients. J Antimicrob Chemother. 1994;34:247–252. doi: 10.1093/jac/34.2.247. [DOI] [PubMed] [Google Scholar]
- 202.Prentice A G, Warnock D W, Johnson S A N, Taylor P C, Oliver D A. Multiple dose pharmacokinetics of an oral solution of itraconazole in patients receiving chemotherapy for acute myeloid leukaemia. J Antimicrob Chemother. 1995;36:657–663. doi: 10.1093/jac/36.4.657. [DOI] [PubMed] [Google Scholar]
- 203.Quart A, Espinel-Ingroff A, Reich D. Fluconazole for refractory oropharyngeal candidaisis in AIDS patients. AIDS Patient Care. 1995;9:56–59. [Google Scholar]
- 204.Quart A M, Espinel-Ingroff A, Steele Moore L, Reich D. Evaluation of fluconazole in the treatment of oropharyngeal candidiasis in patients with AIDS. Infect Med. 1996;13:708–713. [Google Scholar]
- 205.Quindós G, Cabrera F, del Carmen Arilla M, Burgos A, Ortiz-Vigón R, Cañón J L, Pontón J. Fatal Candida famata peritonitis in a patient undergoing continuous ambulatory peritoneal dialysis who was treated with fluconazole. Clin Infect Dis. 1994;18:658–660. doi: 10.1093/clinids/18.4.658. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 206.Radford S A, Johnson E M, Warnock D W. In vitro studies of activity of voriconazole (UK-109,496), a new triazole antifungal agent, against emerging and less-common mold pathogens. Antimicrob Agents Chemother. 1997;41:841–843. doi: 10.1128/aac.41.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Report of an International Multicenter Trial. A comparison of single-dose oral fluconazole with 3-day intravaginal clotrimazole in the treatment of vaginal candidiasis. Br J Obstet Gynaecol. 1989;96:226–232. doi: 10.1111/j.1471-0528.1989.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 208.Restrepo A. Paracoccidioides brasiliensis. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2386–2389. [Google Scholar]
- 209.Restrepo M, Najvar L, Barchiesi F, Graybill J R. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Treatment of murine cryptococcal meningitis with UR-9751 and UR-9746, abstr. F87; p. 128. [Google Scholar]
- 210.Revankar S G, Kirkpatrick W R, McAtee R K, Dib O P, Fothergill A W, Redding S W, Rinaldi M G, Hilsenbeck S G, Patterson T F. A randomized trial of continuous or intermittent therapy with fluconazole for oropharyngeal candidiasis in HIV-infected patients: clinical outcomes and development of fluconazole resistance. Am J Med. 1998;105:7–11. doi: 10.1016/s0002-9343(98)00137-5. [DOI] [PubMed] [Google Scholar]
- 211.Revankar S G, Kirkpatrick W R, McAtee R K, Dib O P, Fothergill A W, Redding S W, Rinaldi M G, Patterson T F. Detection and significance of fluconazole resistance in oropharyngeal candidiasis in human immunodeficiency virus-infected patients. J Infect Dis. 1996;174:821–827. doi: 10.1093/infdis/174.4.821. [DOI] [PubMed] [Google Scholar]
- 212.Rex J H. Sporothrix schenckii. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2321–2324. [Google Scholar]
- 213.Rex J H, Bennett J E, Sugar A M, Pappas P G, Van Der Horst C M, Edwards J E, Washburn R G, Scheld W M, Karchmer A W, Dine A P, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N Engl J Med. 1994;331:1325–1330. doi: 10.1056/NEJM199411173312001. [DOI] [PubMed] [Google Scholar]
- 214.Rex J H, Cooper C R J, Merz W G, Galgiani J N, Anaissie E J. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob Agents Chemother. 1995;39:906–909. doi: 10.1128/aac.39.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rex J H, Nelson P W, Paetznick V L, Lozano-Chiu M, Espinel-Ingroff A, Anaissie E J. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 1998;42:129–134. doi: 10.1128/aac.42.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rex J H, Pfaller M A, Barry A L, Nelson P W, Webb C D for the NIAID Mycoses Study Group and the Candidemia Study Group. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. Antimicrob Agents Chemother. 1995;39:40–44. doi: 10.1128/aac.39.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, et al. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 218.Rex J H, Pfaller M A, Lancaster M, Odds F C, Bolstrom A, Rinaldi M G. Quality control guidelines for National Committee for Clinical Laboratory Standards-recommended broth macrodilution testing of ketoconazole and itraconazole. J Clin Microbiol. 1996;34:816–817. doi: 10.1128/jcm.34.4.816-817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Reynes J, Bazin C, Ajana F, Datry A, Le Moing J-P, Chwetzoff E, Levron J-C. Pharmacokinetics of itraconazole (oral solution) in two groups of human immunodeficiency virus-infected adults with oral candidiasis. Antimicrob Agents Chemother. 1997;41:2554–2558. doi: 10.1128/aac.41.11.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rhunke M, Schmidt-Westhausen A, Trautmann M. In vitro activities of voriconazole (UK-109,486) against fluconazole-susceptible and -resistant Candida albicans isolates from oral cavities of patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1997;41:575–577. doi: 10.1128/aac.41.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Roberts D T. Oral therapeutic agents in fungal nail disease. J Am Acad Dermatol. 1994;31:S78–S81. doi: 10.1016/s0190-9622(08)81274-1. [DOI] [PubMed] [Google Scholar]
- 224.Roseeuw, D., and P. De Doncker. 1993. New therapeutic concepts for the treatment of onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2(Suppl. 1):S34–S38.
- 225.Ryder N S, Leitner I. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Synergy between terbinafine and fluconazole against azole- and multidrug-resistant Candida isolates, abstr. E-70; p. 126. [Google Scholar]
- 226.Saag M S, Cloud G C, Graybill J R, Sobel J, Tuazon C, Wiesinger B, Riser L, Moskovitz B L, Dismukes W E the NIAID Mycoses Study Group (MSG) Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Comparison of fluconazole (FLU) versus itraconazole (ITRA) as maintenance therapy of AIDS-associated cryptococcal meningitis (CM), abstr. I218; p. 244. [Google Scholar]
- 227.Saag M S, Powderly W G, Cloud G A, Robinson P, Grieco M H, Sharkey P K, Thompson S E, Sugar A M, Tuazon C U, Fisher J F, et al. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. N Engl J Med. 1992;326:83–89. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- 228.Sanati H, Belanger P, Fratti R, Ghannoum M. A new triazole, voriconazole (UK-109,496), blocks sterol biosynthesis in Candida albicans and Candida krusei. Antimicrob Agents Chemother. 1997;41:2492–2496. doi: 10.1128/aac.41.11.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sanati H, Ramos C F, Bayer A S, Ghannoum M A. Combination therapy with amphotericin B and fluconazole against invasive candidiasis in neutropenic-mouse and infective-endocarditis rabbit models. Antimicrob Agents Chemother. 1997;41:1345–1348. doi: 10.1128/aac.41.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sanche S E, Fothergill A W, Rinaldi M G. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Interspecies variation of the susceptibility of Fusarium species to Schering 56592 in vitro, abstr. E-66; p. 125. [Google Scholar]
- 231.Savin R. Diagnosis and treatment of tinea versicolor. J Fam Pract. 1996;43:127–132. [PubMed] [Google Scholar]
- 232.Schaffner A, Frick P G. The effect of ketoconazole on amphotericin B in a model of disseminated aspergillosis. J Infect Dis. 1985;151:902–910. doi: 10.1093/infdis/151.5.902. [DOI] [PubMed] [Google Scholar]
- 233.Schuman P, Capps L, Peng G, Vazquez J, El-Sadr W, Goldman A I, Alston B, Besch C L, Vaughn A, Thompson M A, Cobb M N, Kerkering T, Sobel J D for the Terry Beirn Community Programs for Clinical Research on AIDS. Weekly fluconazole for the prevention of mucosal candidiasis in women with HIV infection. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1997;126:689–696. doi: 10.7326/0003-4819-126-9-199705010-00003. [DOI] [PubMed] [Google Scholar]
- 234.Sharkey-Mathis P K, Kauffman C A, Graybill J R, Stevens D A, Hostetler J S, Cloud G, Dismukes W E the NIAID Mycoses Study Group. Treatment of sporotrichosis with itraconazole. Am J Med. 1993;95:279–285. doi: 10.1016/0002-9343(93)90280-3. [DOI] [PubMed] [Google Scholar]
- 235.Sharkey-Mathis P K, Velez J, Fetchick R, Graybill J R. Histoplasmosis in the acquired immunodeficiency syndrome (AIDS): treatment with itraconazole and fluconazole. J Acquired Immune Defic Syndr. 1993;6:809–819. [PubMed] [Google Scholar]
- 236.Sheehan, D. J., A. Espinel-Ingroff, L. Steele Moore, and C. D. Webb. 1993. Antifungal susceptibility testing of yeasts: a brief overview. Clin. Infect. Dis. 17(Suppl. 2):S494–S500. [DOI] [PubMed]
- 237.Sobel, J. D. 1992. Fluconazole maintenance therapy in recurrent vulvovaginal candidiasis. Int. J. Gynaecol. Obstet. 37(Suppl. 1):17–24.
- 238.Sobel J D. Controversial aspects in the management of vulvovaginal candidiasis. J Am Acad Dermatol. 1994;31:S10–S13. doi: 10.1016/s0190-9622(08)81259-5. [DOI] [PubMed] [Google Scholar]
- 239.Sobel J D, Brooker D, Stein G E, Thomason J L, Wermeling D P, Bradley B, Weinstein L the Fluconazole Vaginitis Study Group. Single oral dose fluconazole compared with conventional clotrimazole topical therapy of Candida vaginitis. Am J Obstet Gynecol. 1995;172:1263–1268. doi: 10.1016/0002-9378(95)91490-0. [DOI] [PubMed] [Google Scholar]
- 240.Stary A, Soeltz-Szoets J, Ziegler C, Kinghorn G R, Roy R B. Comparison of the efficacy and safety of oral fluconazole and topical clotrimazole in patients with candida balanitis. Genitourin Med. 1996;72:98–102. doi: 10.1136/sti.72.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Stein G E, Mummaw N. Placebo-controlled trial of itraconazole for treatment of acute vaginal candidiasis. Antimicrob Agents Chemother. 1993;37:89–92. doi: 10.1128/aac.37.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Stein G E, Mummaw N L, Schooley S L. Prevention of recurrent vaginal candidiasis with weekly terconazole cream. Ann Pharmacother. 1996;30:1080–1083. doi: 10.1177/106002809603001002. [DOI] [PubMed] [Google Scholar]
- 243.Stevens D A. Coccidioides immitis. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2365–2375. [Google Scholar]
- 244.Stevens D A, Devine M J, Martinez M, Aristizabal B. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. In vitro antifungal activity of novel azole derivatives with a morpholine ring, UR-9746 (U46) and UR-9751 (U51): comparison with fluconazole (F), abstr. F 85; p. 127. [Google Scholar]
- 245.Stevens D A, Lee J Y. Analysis of compassionate use itraconazole therapy for invasive aspergillosis by the NIAID Mycoses Study Group criteria. Arch Intern Med. 1997;157:1857–1862. [PubMed] [Google Scholar]
- 246.Sugar A M. Use of amphotericin B with azole antifungal drugs: what are we doing? Antimicrob Agents Chemother. 1995;39:1907–1912. doi: 10.1128/aac.39.9.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sugar A M, Hitchcock C A, Troke P F, Picard M. Combination therapy of murine invasive candidiasis with fluconazole and amphotericin B. Antimicrob Agents Chemother. 1995;39:598–601. doi: 10.1128/AAC.39.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sugar A M, Liu X-P. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. In vitro activity of SCH 56592 against Blastomyces dermatitidis (Bd), abstr. F65; p. 124. [Google Scholar]
- 249.Sugar A M, Liu X-P, Chen R-J. Effectiveness of quinolone antibiotics in modulating the effects of antifungal drugs. Antimicrob Agents Chemother. 1997;41:2518–2521. doi: 10.1128/aac.41.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Takashima K, Oki T. Azole antifungal agents. Exp Opin Ther Patients. 1996;6:645–654. [Google Scholar]
- 251.Timonen H. Shorter treatment for vaginal candidosis: comparison between single-dose oral fluconazole and three-day treatment with local micnoazole. Mycoses. 1992;35:317–320. doi: 10.1111/j.1439-0507.1992.tb00887.x. [DOI] [PubMed] [Google Scholar]
- 252.Tollemar J, Höckerstedt K, Ericzon B-G, Jalanko H, Ringden O. Liposomal amphotericin B prevents invasive fungal infections in liver transplant recipients. A randomized, placebo-controlled study. Transplantation. 1995;59:45–50. doi: 10.1097/00007890-199501150-00009. [DOI] [PubMed] [Google Scholar]
- 253.Tomberlin M G, Baingana G, Grant R, Mbidde E K, Sande M A. Program and Abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Fluconazole with and without flucytosine for AIDS related cryptococcal meningitis in Uganda, abstr. M71; p. 247. [Google Scholar]
- 254.Troke P F, Brammer K W, Hitchcock C A, Yonren S, Sarantis N. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. UK-109,496, a novel, wide-spectrum triazole derivative for the treatment of fungal infections: activity in systemic candidiasis models and early clinical efficacy in oropharyngeal candidiasis (OPC), abstr. F73; p. 125. [Google Scholar]
- 255.Tucker R M, Denning D W, Dupont B, Stevens D A. Itraconazole therapy for chronic coccidioidal meningitis. Ann Intern Med. 1990;112:108–112. doi: 10.7326/0003-4819-112-2-108. [DOI] [PubMed] [Google Scholar]
- 256.U.S. Public Health Service/Infectious Disease Society of America. 1995. Prevention of Opportunistic Infections Working Group. USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus: disease-specific recommendations. Clin. Infect. Dis. 21(Suppl. 1):S32–S43. [PubMed]
- 257.Venkateswarlu K, Taylor M, Manning N J, Rinaldi M G, Kelly S L. Fluconazole tolerance in clinical isolates of Cryptococcus neoformans. Antimicrob Agents Chemother. 1997;41:748–751. doi: 10.1128/aac.41.4.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Verweij P E, Mensink M, Rijs A J, Donnelly J P, Meis J F, Denning D W. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. In vitro activity of amphotericin B, itraconazole and voriconazole against 151 clinical and environmental Aspergillus fumigatus isolates, abstr. E-90; p. 129. [Google Scholar]
- 259.Walsh T J, Gonzalez C, Lyman C A, Chanock S J, Pizzo P A. Invasive fungal infectious in children: recent advances in diagnosis and treatment. Adv Pediatr Infect Dis. 1996;11:187–290. [PubMed] [Google Scholar]
- 260.Walsh T J, Hiemenz J, Pizzo P A. Editorial response: evolving risk factors for invasive fungal infections—all neutropenic patients are not the same. Clin Infect Dis. 1994;18:793–798. doi: 10.1093/clinids/18.5.793. [DOI] [PubMed] [Google Scholar]
- 261.Wanger A, Mills K, Nelson P W, Rex J H. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob Agents Chemother. 1995;39:2520–2522. doi: 10.1128/aac.39.11.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wheat J. Endemic mycoses in AIDS: a clinical review. Clin Microbiol Rev. 1995;8:146–159. doi: 10.1128/cmr.8.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wheat J, Bick C, Connolly P, Smedema M, Durkin M, Kohler S, Loebenberg D. Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Comparison of a new triazole, Schering 56592, with itraconazole and amphotericin B for treatment of murine histoplasmosis, abstr. F 101; p. 117. [Google Scholar]
- 264.Wheat J, Hafner R, Korzun A H, Limjoco M T, Spencer P, Larsen R A, Hecht F M, Powderly W the AIDS Clinical Trial Group. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Med. 1995;98:336–342. doi: 10.1016/s0002-9343(99)80311-8. [DOI] [PubMed] [Google Scholar]
- 265.Wheat J, Hafner R, Wulfsohn M, Spencer P, Squires K, Powderly W, Wong B, Rinaldi M, Saag M, Hamill R, et al. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1993;118:610–616. doi: 10.7326/0003-4819-118-8-199304150-00006. [DOI] [PubMed] [Google Scholar]
- 266.Wheat J, Marichal P, Vanden Bossche H, Le Monte A, Connolly P. Hypothesis on the mechanism of resistance to fluconazole in Histoplasma capsulatum. Antimicrob Agents Chemother. 1997;41:410–414. doi: 10.1128/aac.41.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.White A, Goetz M B. Candida parapsilosis prosthetic joint infection unresponsive to treatment with fluconazole. Clin Infect Dis. 1995;20:1068–1069. doi: 10.1093/clinids/20.4.1068. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 268.White, M. H. 1996. Is vulvovaginal candidiasis an AIDS-related illness? Clin. Infect. Dis. 22(Suppl. 2):S124–S127. [DOI] [PubMed]
- 269.White T C, Marr K A, Bowden R A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wilcox C M, Darouiche R O, Laine L, Moskovitz B L, Mallegol I, Wu J. A randomized, double-blind comparison of itraconazole oral solution and fluconazole tablets in the treatment of esophageal candidiasis, cyclodextrin. J Infect Dis. 1997;176:227–232. doi: 10.1086/514028. [DOI] [PubMed] [Google Scholar]
- 271.Wingard J R, Merz W G, Rinaldi M G, Miller C B, Karp J E, Saral R. Association of Torulopsis glabrata infections with fluconazole prophylaxis in neutropenic bone marrow transplant patients. Antimicrob Agents Chemother. 1993;37:1847–1849. doi: 10.1128/aac.37.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Winn R E, Anderson J, Piper J, Aronson N E, Pluss J. Systemic sporotrichosis treated with itraconazole. Clin Infect Dis. 1993;17:210–217. doi: 10.1093/clinids/17.2.210. [DOI] [PubMed] [Google Scholar]
- 273.Yamada H, Watanabe T, Kato K, Mochizuki H. Fungicidal mechanisms of action of D0870 against Cryptococcus neoformans under acidic conditions. Antimicrob Agents Chemother. 1997;41:2710–2713. doi: 10.1128/aac.41.12.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Yotsuji A, Shimizu K, Araki H, Fujimaki K, Nishida N, Hori R, Annen N, Yamamoto S, Hayakawa H, Imaizumi H, et al. T-8581, a new orally and parenterally active triazole antifungal agent: in vitro and in vivo evaluations. Antimicrob Agents Chemother. 1997;41:30–34. doi: 10.1128/aac.41.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]