Abstract
This article comments on:
Di Marzo M, Ebeling Viana V, Banfi C, Cassina V, Corti R, Herrera-Ubaldo H, Babolin N, Guazzotti A, Kiegle E, Gregis V, de Folter S, Sampedro J, Mantegazza F, Colombo L, Ezquer I. 2022. Cell wall modifications by α-XYLOSIDASE1 are required for the control of seed and fruit size. Journal of Experimental Botany 73, 1499–1515.
Keywords: Biomechanics, cell wall remodelling, climate change, diaspore heteromorphism, dispersal, dormancy, fruit and seed traits, hemicellulose
The developmental transition from flowers to the mature diaspores (seeds or fruits) depends on cell growth and differentiation (Finch-Savage et al., 2006; Balanza et al., 2016). The plant cell wall is a dynamic nanoscale network for which the classical model and role of xyloglucan–cellulose tethers in wall structure and cell growth was challenged by recent results from genetics, biomechanics, and advanced imaging (Moulia, 2013; Cosgrove, 2018; B. Zhang et al., 2021). Xyloglucan (XyG), the predominant hemicellulose, is composed of a β-1,4-glucan backbone that is consecutively substituted with α-1,6-linked xylosyl residues (Frankova et al., 2013; Pauly et al., 2016). Di Marzo et al. (2022) demonstrated that the MADS-box transcription factor SEEDSTICK (STK) specifically controls seed and fruit biology by α-xylosidase (XYL) mediated XyG remodelling.
Specific cell wall remodelling is decisive for generating the diversity in morphological, biomechanical, and physiological traits of dispersed diaspores during seed and fruit development (Steinbrecher et al., 2017; Landrein et al., 2019; Seale et al., 2020; Arshad et al., 2021; Huss et al., 2021). It is of similar importance in the control of germination timing via dormancy, seed responses to abiotic stresses including heat (thermoinhibition), and seedling growth required for plant establishment and survival in a particular environment (Finch-Savage et al., 2006; Shigeyama et al., 2016; Finch-Savage et al., 2017). A representative structural unit of XyG is composed of four β-1,4-linked glucose molecules (backbone) of which three have α-1,6-linked xylose side chains in Arabidopsis thaliana (XXXG; see Box 1 for nomenclature). The xylosyl residues are often modified with β-1,2-linked galactosyl residues which may be additionally α-1,2-linked with fucosyl residues (Box 1). A machinery of specific glycosyl transferases, transglycosidases, and hydroxylases generates the diversity in XyG structures, with XyG α-1,6-xyosyltransferases (XXTs) adding αXyl residues, and α-xylosidases (αXYLs) cleaving xyloysl residues from the non-reducing end of XyG cell wall components and XyG oligosaccharides (Frankova et al., 2013; Pauly et al., 2016; B. Zhang et al., 2021). Interestingly, while XyG-deficient A. thaliana xxt mutants exhibit only minor morphological phenotype changes, xyl1 mutants lacking α-xylosidase enzyme activity exhibit altered XyG side chains, free XyG oligosaccharide accumulation, and specific phenotypic defects during reproduction, seed dispersal, germination, and seedling growth. Di Marzo et al. (2022) demonstrate that the expression of the XYL1 gene is directly regulated in developing seeds and fruits by the STK transcription factor.
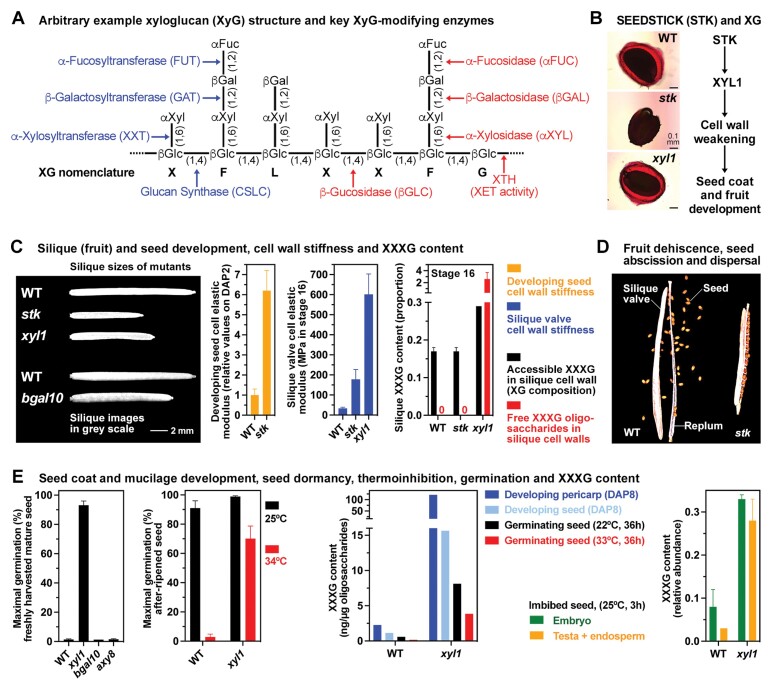
Box 1. Xyloglucan remodelling and cell wall biomechanics during Arabidopsis thaliana seed and fruit biology.
Specific XyG remodelling by a battery of enzymes (A) has profound roles during reproduction, seed dispersal, and germination (B–E). The control of reproduction by the MADS-box transcription factor STK is achieved in part by αXYL-mediated cell wall remodelling (B) combined with other pathways which may differ between seed and fruit development (see cited references and figure 7 in Di Marzo et al., 2022). The control of silique growth (C) by STK, for example, requires XYL1 with a reduced silique size and increased valve cell wall stiffness in both the stk and the xyl1 mutant. There were no obvious morphological phenotype changes observed in axy8 and bglc1 mutants. In contrast to this, bgal and xyl1 mutants exhibited specific seed- and fruit-associated phenotype changes. As for the xyl1 mutant, reduced silique elongation growth was also observed in the bgal10 mutant (Sampedro et al., 2012); however, in contrast to the non-dormant xyl1 mutant seeds, the seeds of bgal10 mutants are dormant. The seeds of bgal6 (mum2) (Dean et al., 2007), stk (Ezquer et al., 2016), and stk/xyl1 mutants are impaired in mucilage production (B), whereas xyl1 mutant seeds have wild-type (WT) phenotype and produce mucilage (Di Marzo et al., 2022). As in the xyl1 mutant, increased cell wall stiffness (C) was also observed in developing seeds of the stk mutant (Ezquer et al., 2016) and may lead to its smaller seed size as well as the defects in seed coat development in that stk, but not xyl1, mutant seeds are impaired in mucilage production (B) and impaired seed abscission [D; from Balanza et al. (2016) with permission (https://doi.org/10.1242/dev.135202)] required for seed dispersal (Balanza et al., 2016). STK seems to achieve this via the MUM2 gene encoding a βGAL6 involved in pectin and possibly also XyG remodelling (Dean et al., 2007; Ezquer et al., 2016). The bgal10 mutant is also reduced in silique growth (C), impaired in seed mucilage production, and XyG remodelling (Sampedro et al., 2012). The production of dormant seeds (E) is not affected in the bgal10 and axy8 (the AXY8 gene encodes an αFUC) mutants, but xyl1 mutant seeds are non-dormant (Sechet et al., 2016). Interestingly, the non-dormant xyl1 mutant seeds are thermoinhibition resistant (E) and have increased hypocotyl cell wall stiffness in creep-extension analysis (Shigeyama et al., 2016). Altered XyG in cell walls and the accumulation of free XyG oligosaccharides (C, E) were associated with the altered fruit and seed phenotypes of the xyl1 (Iglesias et al., 2006; Sampedro et al., 2010; Günl and Pauly, 2011; Sechet et al., 2016; Shigeyama et al., 2016; Di Marzo et al., 2022), bgal10 (Sampedro et al., 2012), axy8 (Günl et al., 2011), and bglc1 (Sampedro et al., 2017) mutants. DAP, days after pollination.
Box 1 summarizes seed- and fruit-associated morphological, biochemical, biomechanical, and physiological changes of xyl1 and stk mutants, including reduced silique elongation growth and increased cell wall stiffness in both, as well as altered XyG side chains, accumulation of free XXXG oligosaccharides, lack of seed dormancy, and increased seed thermotolerance of the xyl1 mutant (Sampedro et al., 2010; Günl et al., 2011; Sechet et al., 2016; Shigeyama et al., 2016; Di Marzo et al., 2022). Likewise, results from bgal10, bgal6 (mum2), axy8, and bglc1 mutants are presented which have reduced β-galactosidase, α-fucosidase, and β-glucosidase enzyme activities, respectively. They all have cell wall XyG with altered side chains and free XyG oligosaccharide accumulation (Iglesias et al., 2006; Dean et al., 2007; Günl et al., 2011; Sampedro et al., 2012, 2017). XYL1 and the transcriptional regulation of its expression by STK plays a major role in the control of seed and fruit mechanical properties by XyG remodelling (Box 1); however, depending on the specific process or tissue, other interacting pathways may dominate.
An integrated approach combining genetics with biomechanical and image analysis appears to be important for advancing our understanding of XyG remodelling and cell wall mechanics in seed and fruit biology (Sechet et al., 2016; Shigeyama et al., 2016; Di Marzo et al., 2022). Using atomic force microscopy (AFM) to analyse silique valve cell wall stiffness, Di Marzo et al. (2022) demonstrate that developmentally regulated XYL1 gene expression is required for maintaining wall integrity during silique growth. Using creep-extension analysis with elongating stem segments, Shigeyama et al. (2016) reported that xyl1 mutant cell wall stiffness was higher than in wild-type plants. This work also demonstrated that epidermal cells of xyl1 mutant siliques are longitudinally shorter and horizontally enlarged, a finding which fits with the increased cell wall stiffness in xyl1 mutant siliques reported by Di Marzo et al. (2022). Although different biomechanical methods were used, in both cases the same conclusion about the role of αXYL in controlling cell wall mechanical properties (stiffness) was obtained. Interestingly, the silique elongation growth is reduced in XyG-deficient xxt1/xxt2 mutants (Sechet et al., 2016), and the cell wall stiffness tested by microtensile assays of hypocotyls was also decreased compared with the wild type (Cavalier et al., 2008). The importance of the right balance in XyG remodelling enzymes (Box 1) seems crucial, and both XXT-mediated incorporation and αXYL-mediated removal of xylosyl residues can lead to the same biomechanical changes.
The αXYL-catalysed cleavage of xylosyl residues from the non-reducing ends of cell wall XyG chains and XyG oligosaccharides has been shown to be the limiting step in XyG oligosaccharide degradation (Iglesias et al., 2006; Shigeyama et al., 2016; Sampedro et al., 2017). Released XyG oligosaccharides can also alter cell wall properties by incorporation catalysed by XyG endotransglycosylase (XET) enzyme activity (Box 1). In grass caryopses, this may lead to coleorhiza-enforced dormancy due to tissue stiffening (Holloway et al., 2021) and in tomato and other endospermic seeds tissue to weakening of the micropylar endosperm (Finch-Savage et al., 2006; Steinbrecher et al., 2017). XyG oligosaccharides were also proposed to directly or indirectly mediate cell wall signalling which can result in altered hormonal biosynthesis or signalling (Frankova et al., 2013; Pauly et al., 2016; Sechet et al., 2016; Shigeyama et al., 2016; B. Zhang et al., 2021). The structure of XyG differs between plant species especially in diversity of the side chains; however, despite this, conservation in XyG remodelling mechanisms and enzymes was also established (Pauly et al., 2016; Rubianes et al., 2019; Holloway et al., 2021). Mutants in XyG remodelling enzymes, such as in STK and XYL1 in the work of Di Marzo et al. (2022), are indeed highly suited to advance our understanding of the mechanisms of cell wall biochemistry and biomechanics (Box 1).
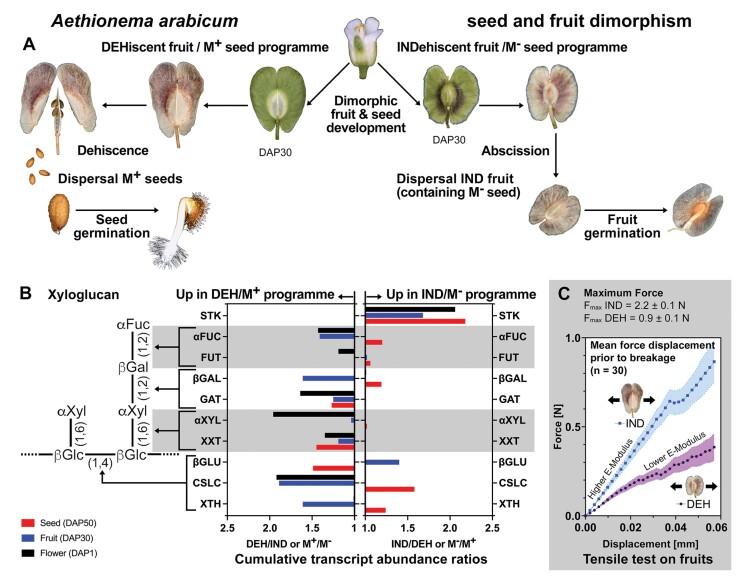
Within the Brassicaceae, the dimorphic diaspores of Aethionema arabicum offer another interesting approach into cell wall biology during reproduction (Box 2). In Ae. arabicum, the developmental control and plasticity of fruit and seed morphs is associated with morphological, biomechanical, gene expression, and physiological differences between the morphs (Lenser et al., 2016; Wilhelmsson et al., 2019; Arshad et al., 2029, 2021). Comparing the distinct seed and fruit morphs of heteromorphic species therefore provides very interesting systems for future research into cell wall biochemistry and biomechanics including for XyG remodelling enzymes (Box 2).
Box 2. Biomechanics and XyG remodelling enzymes during Aethionema arabicum fruit and seed dimorphism.
Heteromorphic species can produce seed and fruit morphs that are distinct in dispersal, germination, morphology, and physical properties (Lenser et al., 2016). The dimorphic species Aethionema arabicum naturally exhibits the production of two different seed and fruit morphs on the same plant (A). In addition to this interesting developmental control, it exhibits phenotypic plasticity in that the ratios and numbers are controlled by environmental cues during reproduction. Comparative transcriptome analysis of the dimorphic fruit and seed developmental programme revealed differences in transcription factor and downstream gene expression (Wilhelmsson et al., 2019; Arshad et al., 2021). This includes the transcript abundances of STK and XyG remodelling enzymes (B), and suggests that XyG may differ between the fruits and seed coats of the two morphs. Aethionema arabicum develops a larger dehiscent fruit (DEH) with 2–4 M+ seeds (with mucilage) and a smaller indehiscent fruit (IND) with a single non-mucilaginous (M–) seed (A). Fruit opening in IND fruits needs significantly higher forces than in DEH fruits. When the linear regions of individual force displacement curves (the part prior to breakage) are compared (C), IND fruits (separation area 0.86±0.03mm2) show a faster increase in force per mm and therefore a higher elastic modulus than DEH fruits (separation area 6.94±0.14mm2) (Arshad et al., 2019). Dimorphic fruits with distinct cell wall architecture are ideal model systems to investigate the effects of cell wall polysaccharide composition and dynamics on seed and fruit size, as well as their biomechanical properties and developmental patterns.
Environmental conditions play a key role in seed and fruit biology (Finch-Savage et al., 2017; Fernandez-Pascual et al., 2019). Temperature during reproduction can shift the ratios and numbers of the Ae. arabicum fruit and seed morphs (Lenser et al., 2016). Temperature and photoperiod contribute to population fitness by affecting seed coat cell wall properties (thickness, proanthocyanidin content) and thereby dormancy in other species (Finch-Savage et al., 2006; Mizzotti et al., 2014; MacGregor et al., 2015; Fernández Farnocchia et al., 2021). The cell wall is a highly dynamic and adjustable structure, and its biomechanical properties are determined by specific cell wall compositions for which new modelling approaches are being pursued (B. Zhang et al., 2021; Y, Zhang et al., 2021). Integrating molecular work with morphological and biomechanical analysis, as exemplified by Di Marzo et al. (2022), and further with such novel modelling approaches are promising prospects for future research into this fascinating topic.
Acknowledgements
We acknowledge the financial support of our research projects into sustainable seed technologies by the Biotechnology and Biological Sciences Research Council (BBSRC, grant no. BB/V017462/1 and BB/S016112/1) and SeedAdapt BB/M00192X/1. For expert information about seed dormancy and biomechanics visit ‘The Seed Biology Place’ www.seedbiology.eu.
References
- Arshad W, Lenser T, Wilhelmsson PKI, et al. 2021. A tale of two morphs: developmental patterns and mechanisms of seed coat differentiation in the dimorphic diaspore model Aethionema arabicum (Brassicaceae). The Plant Journal 107, 166–181. [DOI] [PubMed] [Google Scholar]
- Arshad W, Sperber K, Steinbrecher T, Nichols B, Jansen VAA, Leubner-Metzger G, Mummenhoff K.. 2019. Dispersal biophysics and adaptive significance of dimorphic diaspores in the annual Aethionema arabicum (Brassicaceae). New Phytologist 221, 1434–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanza V, Roig-Villanova I, Marzo M Di, Masiero S, Colombo L.. 2016. Seed abscission and fruit dehiscence required for seed dispersal rely on similar genetic networks. Development 143, 3372–3381. [DOI] [PubMed] [Google Scholar]
- Cavalier DM, Lerouxel O, Neumetzler L, et al. 2008. Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. The Plant Cell 20, 1519–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. 2018. Diffuse growth of plant cell walls. Plant Physiology 176, 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean GH, Zheng HQ, Tewari J, et al. 2007. The Arabidopsis MUM2 gene encodes a beta-galactosidase required for the production of seed coat mucilage with correct hydration properties. The Plant Cell 19, 4007–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo M, Ebeling Viana V, Herrera-Ubaldo H, et al. 2022. Cell wall modifications by α-XYLOSIDASE1 are required for the control of seed and fruit size. Journal of Experimental Botany 73, 1499–1515. [DOI] [PubMed] [Google Scholar]
- Ezquer I, Mizzotti C, Nguema-Ona E, et al. 2016. The developmental regulator SEEDSTICK controls structural and mechanical properties of the Arabidopsis seed coat. The Plant Cell 28, 2478–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández Farnocchia RB, Benech-Arnold RL, Mantese A, Battla D.. 2021. Optimization of next-generation emergence timing of Amaranthus hybridus is determined through seed dormancy modulation by the maternal environment. Journal of Experimental Botany 72, 4283–4297. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pascual E, Mattana E, Pritchard HW.. 2019. Seeds of future past: climate change and the thermal memory of plant reproductive traits. Biological Reviews 94, 439–456. [DOI] [PubMed] [Google Scholar]
- Finch-Savage WE, Footitt S.. 2017. Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments. Journal of Experimental Botany 68, 843–856. [DOI] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G.. 2006. Seed dormancy and the control of germination. New Phytologist 171, 501–523. [DOI] [PubMed] [Google Scholar]
- Frankova L, Fry SC.. 2013. Biochemistry and physiological roles of enzymes that cut and paste plant cell-wall polysaccharides. Journal of Experimental Botany 64, 3519–50. [DOI] [PubMed] [Google Scholar]
- Günl M, Neumetzler L, Kraemer F, de Souza A, Schultink A, Pena M, York WS, Pauly M.. 2011. AXY8 encodes an alpha-fucosidase, underscoring the importance of apoplastic metabolism on the fine structure of Arabidopsis cell wall polysaccharides. The Plant Cell 23, 4025–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günl M, Pauly M.. 2011. AXY3 encodes a alpha-xylosidase that impacts the structure and accessibility of the hemicellulose xyloglucan in Arabidopsis plant cell walls. Planta 233, 707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway T, Steinbrecher T, Perez M, Seville A, Stock D, Nakabayashi K, Leubner-Metzger G.. 2021. Coleorhiza-enforced seed dormancy: a novel mechanism to control germination in grasses. New Phytologist 229, 2179–2191. [DOI] [PubMed] [Google Scholar]
- Huss JC, Gierlinger N.. 2021. Functional packaging of seeds. New Phytologist 230, 2154–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias N, Abelenda JA, Rodino M, Sampedro J, Revilla G, Zarra I.. 2006. Apoplastic glycosidases active against xyloglucan oligosaccharides of Arabidopsis thaliana. Plant & Cell Physiology 47, 55–63. [DOI] [PubMed] [Google Scholar]
- Landrein B, Ingram G.. 2019. Connected through the force: mechanical signals in plant development. Journal of Experimental Botany 70, 3507–3519. [DOI] [PubMed] [Google Scholar]
- Lenser T, Graeber K, Cevik OS, et a l.. 2016. Developmental control and plasticity of fruit and seed dimorphism in Aethionema arabicum. Plant Physiology 172, 1691–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor DR, Kendall SL, Florance H, Fedi F, Moore K, Paszkiewicz K, Smirnoff N, Penfield S.. 2015. Seed production temperature regulation of primary dormancy occurs through control of seed coat phenylpropanoid metabolism. New Phytologist 205, 642–652. [DOI] [PubMed] [Google Scholar]
- Mizzotti C, Ezquer I, Paolo D, et al. 2014. SEEDSTICK is a master regulator of development and metabolism in the Arabidopsis seed coat. PLoS Genetics 10, e1004856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulia B. 2013. Plant biomechanics and mechanobiology are convergent paths to flourishing interdisciplinary research. Journal of Experimental Botany 64, 4617–33. [DOI] [PubMed] [Google Scholar]
- Pauly M, Keegstra K.. 2016. Biosynthesis of the plant cell wall matrix polysaccharide xyloglucan. Annual Review of Plant Biology 67, 235–59. [DOI] [PubMed] [Google Scholar]
- Rubianes D, Valdivia ER, Revilla G, Zarra I, Sampedro J.. 2019. Xyloglucan exoglycosidases in the monocot model Brachypodium distachyon and the conservation of xyloglucan disassembly in angiosperms. Plant Molecular Biology 100, 495–509. [DOI] [PubMed] [Google Scholar]
- Sampedro J, Gianzo C, Iglesias N, Guitian E, Revilla G, Zarra I.. 2012. AtBGAL10 is the main xyloglucan beta-galactosidase in Arabidopsis, and its absence results in unusual xyloglucan subunits and growth defects. Plant Physiology 158, 1146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro J, Pardo B, Gianzo C, Guitian E, Revilla G, Zarra I.. 2010. Lack of alpha-xylosidase activity in Arabidopsis alters xyloglucan composition and results in growth defects. Plant Physiology 154, 1105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro J, Valdivia ER, Fraga P, Iglesias N, Revilla G, Zarra I.. 2017. Soluble and membrane-bound beta-glucosidases are involved in trimming the xyloglucan backbone. Plant Physiology 173, 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale M, Nakayama N.. 2020. From passive to informed: mechanical mechanisms of seed dispersal. New Phytologist 225, 653–658. [DOI] [PubMed] [Google Scholar]
- Sechet J, Frey A, Effroy-Cuzzi D, et al. 2016. Xyloglucan metabolism differentially impacts the cell wall characteristics of the endosperm and embryo during Arabidopsis seed germination. Plant Physiology 170, 1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyama T, Watanabe A, Tokuchi K, S Toh, Sakurai N, Shibuya N, Kawakami N.. 2016. α-Xylosidase plays essential roles in xyloglucan remodelling, maintenance of cell wall integrity, and seed germination in Arabidopsis thaliana. Journal of Experimental Botany 67, 5615–5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher T, Leubner-Metzger G.. 2017. The biomechanics of seed germination. Journal of Experimental Botany 68, 765–783. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson PKI, Chandler JO, Fernandez-Pozo N, et al. 2019. Usability of reference-free transcriptome assemblies for detection of differential expression: a case study on Aethionema arabicum dimorphic seeds. BMC Genomics 20, ARTN 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Y Gao, Zhang L, Zhou Y.. 2021. The plant cell wall: biosynthesis, construction, and functions. Journal of Integrative Plant Biology 63, 251–272. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yu JY, Wang X, Durachko DM, Zhang SL, Cosgrove DJ.. 2021. Molecular insights into the complex mechanics of plant epidermal cell walls. Science 372, 706–711. [DOI] [PubMed] [Google Scholar]