Abstract
Nirmatrelvir–ritonavir (NMV/r) is now being used to treat high-risk patients with mild to moderate COVID-19. This article provides advice to clinicians regarding recognition of medications likely to interact with NMV/r and suggests approaches to managing such drug–drug interactions. An algorithm is provided to assist in decision making.
Nirmatrelvir–ritonavir (NMV/r) is increasingly available for use in high-risk patients with mild to moderate COVID-19 (1) who are likely to be receiving other medications. The use of ritonavir to boost plasma concentrations of nirmatrelvir through inhibition of cytochrome P450 (CYP) 3A4 confers a high potential for clinically significant drug–drug interactions (DDIs). Although HIV specialists have more than 2 decades of experience with ritonavir boosting (2), the 5-day treatment with oral NMV/r will be primarily prescribed by practitioners lacking such experience. The most important restriction to use of NMV/r will be DDIs. Moreover, limited management options (home isolation, accessing investigations, short window for intervention, and difficulties of dose adjustment) and the risk for unrecognized DDIs or their toxicities present challenges. Treatment guidance must be clear, simple, unambiguous, and pragmatic, balancing the risk for harm from DDIs with a short course of ritonavir against unnecessary denial of NMV/r treatment. We brought together expert clinicians and antiviral pharmacologists from Europe and North America to synthesize evidence-based, pragmatic recommendations for managing DDIs with NMV/r.
Safe prescribing of NMV/r requires full medication reconciliation, including over-the-counter medicines, herbals, and recreational drugs. Interactions between NMV/r and comedications can be screened using specialized resources, such as the National Institutes of Health COVID-19 treatment guidelines (3) or the University of Liverpool's website on COVID-19 drug interactions (4). Because these resources are comprehensive but not exhaustive, it is essential to understand when to anticipate a clinically relevant DDI with NMV/r by considering the metabolic pathways of co-administered medications.
Recognition of Medications Likely to Significantly Interact With NMV/r
Ritonavir inhibition of CYP3A4, and also the transporter P-glycoprotein, means that significant DDIs can be expected with comedications that are predominantly metabolized by CYP3A4 and/or have a narrow therapeutic index (such as tacrolimus) or are sensitive substrates of P-glycoprotein (such as digoxin). The contribution of CYP3A4 to the overall disposition of a drug determines the magnitude of a DDI, which tends to be mitigated for drugs with multiple metabolic pathways (5). For instance, ritonavir increases simvastatin (exclusive CYP3A4 metabolism) by 100-fold and is contraindicated (5); temporarily pausing simvastatin therapy will not cause clinical harm. Inhibition of CYP3A4 by ritonavir takes several days to resolve, and paused treatment with comedications should be restarted 3 days after the last dose of NMV/r (6).
Conversely, no clinically significant DDIs are expected between NMV/r and drugs metabolized by CYP2D6 (including most antidepressants) because a low dose of ritonavir weakly inhibits this enzyme (7). Ritonavir also induces several other drug-metabolizing enzymes (7), but induction is unlikely to be clinically relevant because it does not reach maximal effect during the short NMV/r treatment course.
Strong enzyme inducers (for example, rifampicin, carbamazepine, enzalutamide, and St. John's wort) can significantly reduce NMV/r exposure, potentially jeopardizing its efficacy. Because of persistent induction after withdrawal of an inducer, DDIs with strong inducers cannot be avoided and require the use of an alternative COVID-19 treatment.
Strategies for Managing Potentially Clinically Relevant DDIs
Clinically relevant DDIs with NMV/r can be managed in 4 ways: 1) preemptive pausing of the comedication therapy; 2) monitoring or dose adjustment of comedications (not usually feasible); 3) patient counseling with symptom-driven withdrawal of medications where appropriate, such as with antihypertensives; or 4) choice of an alternative treatment of SARS-CoV-2 infection. Which route is chosen depends on patient characteristics and the risk–benefit balance of modifying treatment of the comorbid condition. Access to specialist consultation where available is helpful. In settings where complex management strategies are not possible, where DDIs cannot be mitigated, or where the effectiveness of NMV/r may be impaired, an alternative COVID-19 treatment should be used. The Figure provides an algorithm for the management of DDIs with NMV/r. The following sections outline some of the key questions to consider when managing DDIs.
Figure. Flow diagram to assess management of nirmatrelvir–ritonavir DDIs.
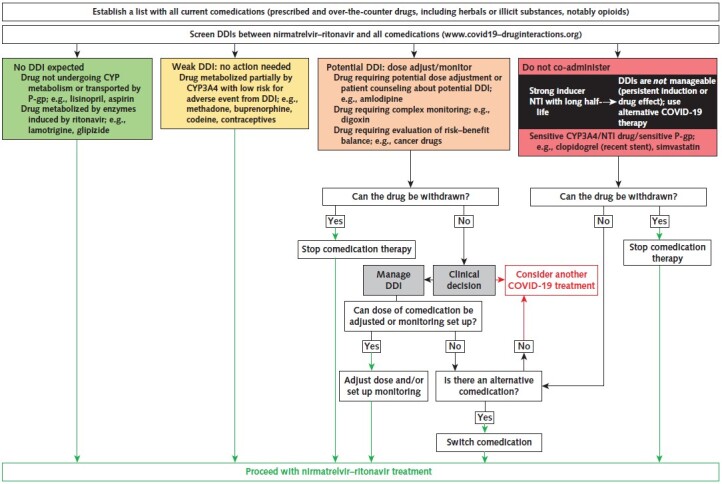
The inhibitory effect of ritonavir takes several days to resolve. Thus, paused comedication therapy should be restarted 3 d after the last dose of nirmatrelvir–ritonavir. The same timeline applies for comedications whose dosage has been adjusted during nirmatrelvir–ritonavir treatment. CYP = cytochrome; DDI = drug–drug interaction; NTI = narrow therapeutic index; P-gp = P-glycoprotein.
Can the Comedication Be Withdrawn?
Where possible, therapy with contraindicated comedications should be paused temporarily. If this is not appropriate—for example, if discontinuation poses too much risk, such as stopping clopidogrel treatment within 6 weeks of coronary stenting (8)—then alternatives to NMV/r should be considered. For example, a switch from clopidogrel to prasugrel reverses the deleterious effect of DDIs on platelet aggregation (9) and, if feasible, should be considered in the 6-week period of highest risk after coronary stenting (8). For other clinical scenarios, a brief reduction in clopidogrel exposure may be acceptable.
Can DDI Management Allow NMV/r Treatment?
Most DDIs can be managed safely by temporarily withdrawing the problematic comedication. In some circumstances, DDIs cannot be prevented by this approach, either because of persisting effects (for example, residual induction with carbamazepine or rifampicin) or a very long half-life of the comedication (for example, amiodarone), potentially resulting in inadequate exposure to nirmatrelvir or toxic drug levels, respectively. An alternative COVID-19 treatment would be considered in such situations.
Can Arrangements for Monitoring or Dose Adjustment Be Established?
Short-term arrangements for monitoring or dose adaptation are challenging to implement. For example, digoxin concentrations are expected to be significantly increased by ritonavir through inhibition of P-glycoprotein (10). Management of this DDI is not straightforward, requiring individualization of digoxin dosage or a dosing schedule based on the treatment indication and the patient's renal function. Without digoxin therapeutic drug monitoring, this interaction requires evaluation of the risk–benefit balance. Although more stringent dosage measures may apply to drugs with a narrow therapeutic index, dosage adjustments may be optional for drugs whose DDI can be easily managed (such as amlodipine). A special situation exists for patients already receiving a drug combination containing the pharmacokinetic booster cobicistat or low-dose ritonavir (that is, patients receiving certain antiretrovirals). For these patients, the additional ritonavir in NMV/r for 5 days is unlikely to cause significant intolerance, and continuation of antiretroviral therapy is recommended.
Challenges
Not all potential DDIs result in harms, and prescribers must weigh the risk–benefit balance of co-administration in each case. Additional challenges posed by the need to isolate and avoid unnecessary attendance at health care facilities limit our ability to manage these DDIs. However, the greatest challenges are longstanding: incomplete ascertainment of all medications taken and underrecognition of the existence of DDIs. As NMV/r is rolled out across different health care settings, we recommend use of up-to-date resources, such as the Liverpool DDI checker (www.covid19-druginteractions.org).
Footnotes
This article was published at Annals.org on 1 March 2022.
References
- 1. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. 22 December 2021. Accessed at www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19 on 17 January 2022.
- 2. Boffito M , Back D , Gatell JM . Twenty years of boosting antiretroviral agents: where are we today. AIDS. 2015;29:2229-33. [PMID: ] doi: 10.1097/QAD.0000000000000800 [DOI] [PubMed] [Google Scholar]
- 3. National Institutes of Health. The COVID-19 Treatment Guidelines Panel's statement on potential drug-drug interactions between ritonavir-boosted nirmatrelvir (Paxlovid) and concomitant medications. Updated 30 December 2021. Accessed at www.covid19treatmentguidelines.nih.gov/therapies/statement-on-paxlovid-drug-drug-interactions on 17 January 2022.
- 4.COVID-19 Drug Interactions. University of Liverpool; 2022. Accessed at www.covid19-druginteractions.org on 17 January 2022.
- 5. Stader F , Kinvig H , Battegay M , et al. Analysis of clinical drug-drug interaction data to predict magnitudes of uncharacterized interactions between antiretroviral drugs and comedications. Antimicrob Agents Chemother. 2018;62. [PMID: ] doi: 10.1128/AAC.00717-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stader F , Khoo S , Stoeckle M , et al. Stopping lopinavir/ritonavir in COVID-19 patients: duration of the drug interacting effect [Letter]. J Antimicrob Chemother. 2020;75:3084-3086. [PMID: ] doi: 10.1093/jac/dkaa253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marzolini C , Gibbons S , Khoo S , et al. Cobicistat versus ritonavir boosting and differences in the drug-drug interaction profiles with co-medications. J Antimicrob Chemother. 2016;71:1755-8. [PMID: ] doi: 10.1093/jac/dkw032 [DOI] [PubMed] [Google Scholar]
- 8. Valgimigli M , Bueno H , Byrne RA , et al; ESC Scientific Document Group. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213-260. [PMID: ] doi: 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 9. Marsousi N , Daali Y , Fontana P , et al. Impact of boosted antiretroviral therapy on the pharmacokinetics and efficacy of clopidogrel and prasugrel active metabolites. Clin Pharmacokinet. 2018;57:1347-1354. [PMID: ] doi: 10.1007/s40262-018-0637-6 [DOI] [PubMed] [Google Scholar]
- 10. Ding R , Tayrouz Y , Riedel KD , et al. Substantial pharmacokinetic interaction between digoxin and ritonavir in healthy volunteers. Clin Pharmacol Ther. 2004;76:73-84. [PMID: ] [DOI] [PubMed] [Google Scholar]


