Abstract
Background
Cyclosporin A has been used for patients with primary biliary cirrhosis, but the therapeutic responses in randomised clinical trials have been heterogeneous.
Objectives
To assess the beneficial and harmful effects of cyclosporin A for patients with primary biliary cirrhosis.
Search methods
Relevant randomised clinical trials were identified by searching The Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, The Chinese Biomedical Database, and LILACS, and manual searches of bibliographies to June 2006. We contacted authors of trials and the company producing cyclosporin A.
Selection criteria
Randomised clinical trials comparing cyclosporin A with placebo, no intervention, or another drug were included irrespective of blinding, language, year of publication, and publication status.
Data collection and analysis
Our primary outcomes were mortality, and mortality or liver transplantation. Dichotomous outcomes were reported as relative risk (RR) and if appropriate, Peto odds ratio with 95% confidence interval (CI). Continuous outcomes were reported as weighted mean difference (WMD) or standardised mean difference (SMD). We examined intervention effects by random‐effects and fixed‐effect models.
Main results
We identified three trials with 390 patients that compared cyclosporin A versus placebo. Two of them were assessed methodologically adequate with low‐bias risk. Cyclosporin A did not significantly reduce mortality risk (RR 0.92, 95% CI 0.59 to 1.45), and mortality or liver transplantation (RR 0.85, 95% CI 0.60 to 1.20). Cyclosporin A significantly improved pruritus (SMD ‐0.38, 95% CI ‐0.63 to ‐0.14), but not fatigue. Cyclosporin A significantly reduced alanine aminotransferase (WMD ‐41 U/L, 95% CI ‐63 to ‐18) and increased serum albumin level (WMD 1.66 g/L, 95% CI 0.26 to 3.05). Significantly more patients experienced adverse events in the cyclosporin A group than in the placebo group, especially renal dysfunction (Peto odds ratio 5.56, 95% CI 2.52 to 12.27) and hypertension (SMD 0.88, 95% CI 0.27 to 1.48).
Authors' conclusions
We found no evidence supporting or refuting that cyclosporin A may delay death, death or liver transplantation, or progression of primary biliary cirrhosis. Cyclosporin A caused more adverse events than placebo, like renal dysfunction and hypertension. We do not recommend the use of cyclosporin A outside randomised clinical trials.
Plain language summary
Cyclosporin A was without significant effects on mortality, liver transplantation, or progression of primary biliary cirrhosis, and patients given cyclosporin A experienced more adverse events
Primary biliary cirrhosis (PBC) is a chronic disease of the liver that is characterised by destruction of bile ducts. Estimates of annual incidence range from 2 to 24 people per million population, and estimates of prevalence range from 19 to 240 people per million population. PBC primarily affects middle‐aged women. The forecast for the symptomatic patient after diagnosis is between 10 and 15 years. The cause of PBC is unknown, but the dynamics of the disease resemble the group 'autoimmune disease'. Therefore, one might expect a noticeable effect of administering an immune repressing drug (immunosuppressant). This review evaluates all clinical data on the immunosuppressant cyclosporin A for PBC.
The findings in this review are based on three clinical trials with 390 patients. The drug cyclosporin A was tested against placebo. The primary findings of the review are that cyclosporin A has no effect on survival or progression of the disease (cirrhosis development). Patients given cyclosporin A experienced more adverse events than patients given placebo, especially renal dysfunction and hypertension. There was significant improvement in itching (pruritus) and liver biochemistry, which were secondary outcome measures.
We cannot recommend the use of cyclosporin A outside randomised clinical trials.
Background
Primary biliary cirrhosis is a chronic liver disease of unknown aetiology. Ninety per cent of patients with primary biliary cirrhosis are females and the majority are diagnosed after the age of 40 years (James 1981). Over the past 30 years, substantial increases in the prevalence of primary biliary cirrhosis has been observed (Kim 2000). Primary biliary cirrhosis is now a frequent cause of liver morbidity, and patients with primary biliary cirrhosis are significant users of health resources, including liver transplantation (Prince 2003). Primary biliary cirrhosis is diagnosed on the basis of the triad: antimitochondrial antibodies, found in over 95% of patients with primary biliary cirrhosis (Fregeau 1989; Lacerda 1995; Invernizzi 1997; Turchany 1997; Mattalia 1998); abnormal liver function tests that are typically cholestatic (with raised activity of alkaline phosphatases being the most frequently seen abnormality); and characteristic liver histological changes (Scheuer 1967) in the absence of extrahepatic biliary obstruction (Kaplan 1996).
Patients with primary biliary cirrhosis have been subjected to many drugs. Ursodeoxycholic acid (a bile acid) is the most extensively used drug in these patients (Verma 1999). Other drugs have been immunomodulatory and other agents, such as colchicine (Warnes 1987; Vuoristo 1995; Poupon 1996; Gong 2005b), prednisolone (Mitchison 1992; Prince 2005), chlorambucil (Hoofnagle 1986), azathioprine (Heathcote 1976; Christensen 1985), D‐penicillamine (Dickson 1985; Neuberger 1985; Gong 2004), methotrexate (Kaplan 1991; Lindor 1995; Gong 2005a), or cyclosporin A (Minuk 1988; Wiesner 1990; Gong 2005c).
Cyclosporin A has proved effective in preventing immune‐mediated rejection of a variety of transplanted human allografts (Cohen 1984) and has been shown to produce clinical improvement in a number of autoimmune conditions (Tugwell 1990). Cyclosporin A is a cyclic endecapeptide of fungal origin. It alters lymphokine production so that the T‐helper‐inducer subpopulations are attenuated, T‐cell help required for B‐cell activation is blocked, cytotoxic T‐cell generation is attenuated, and T‐suppressor cell subpopulations are expanded (Harris 1987). Thus, cyclosporin A would appear a potential ideal agent to modify the immunologic irregularities in primary biliary cirrhosis (James 1983). Since 1980, when Routhier showed beneficial effects of cyclosporin A on serum aspartate transaminase and alkaline phosphatases in six patients with primary biliary cirrhosis (Routhier 1980), several randomised clinical trials have been carried out with different results (Minuk 1988; Wiesner 1990). We could not identify any meta‐analyses or systematic reviews on the beneficial and harmful effects of cyclosporin A in primary biliary cirrhosis.
Objectives
To systematically assess the beneficial and harmful effects of cyclosporin A for patients with primary biliary cirrhosis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials irrespective of blinding, language, year of publication, and publication status. We excluded studies using quasi‐randomisation (for example, allocation by date of birth).
Types of participants
Patients with primary biliary cirrhosis, ie, patients having at least two of the following: elevated serum activity of alkaline phosphatases (or other markers of intrahepatic cholestasis), and/or a positive result for serum mitochondrial antibody, and/or liver biopsy findings diagnostic for or compatible with primary biliary cirrhosis.
Types of interventions
Administration of any dose of cyclosporin A versus placebo or no intervention or other drugs. Co‐interventions were allowed as long as the intervention arms of the randomised clinical trial received similar co‐interventions.
Types of outcome measures
Primary outcome measures
Mortality.
Mortality or liver transplantation.
Secondary outcome measures
Pruritus: number of patients without improvement of pruritus or pruritus score.
Fatigue: number of patients without improvement of fatigue or fatigue score.
Incidence of liver complications: number of patients developing variceal bleeding, ascites, hepatic encephalopathy, jaundice, or hepato‐renal syndrome.
Liver biochemistry: serum (s‐)bilirubin; s‐alkaline phosphatases; s‐gamma‐glutamyltransferase; s‐aspartate aminotransferase; s‐alanine aminotransferase; s‐albumin; s‐cholesterol (total); plasma immunoglobulins.
Liver biopsy: worsening of liver histological stage or score.
Quality of life: physical functioning (ability to carry out activities of daily living such as self‐care and walking around), psychological functioning (emotional and mental well‐being), social functioning (social relationships and participation in social activities), and perception of health, pain, and overall satisfaction with life.
Adverse events (excluding mortality and liver transplantation). The adverse event is defined as any untoward medical occurrence in a patient in either of the two arms of the included randomised clinical trials, which did not necessarily have a causal relationship with the treatment, but did, however, result in a dose reduction, discontinuation of treatment, or registration of the advent as an adverse event/side effect (ICH‐GCP 1997).
Cost‐effectiveness: the estimated costs connected with the interventions were to be weighed against any possible health gains.
Search methods for identification of studies
We identified relevant randomised clinical trials by searching The Cochrane Hepato‐Biliary Group Controlled Trials Register, which involves hand searches of major hepatology journals and conference proceedings, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, The Chinese Biomedical Database, and LILACS (Royle 2003). The search strategies are given in Appendix 1 with the time span of the searches.
We tried to identify further trials by reading the reference lists of the identified publications. We wrote to the principal authors of the identified trials and to the researchers active in the field to inquire about additional randomised clinical trials they might know of. We also contacted the pharmaceutical company, Novartis, producer of cyclosporin A, to obtain any unidentified or unpublished randomised clinical trials.
Data collection and analysis
We performed a meta‐analysis following the protocol (Gong 2005c) and the recommendations given by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006) and the Cochrane Hepato‐Biliary Group Module (Gluud 2007).
Data extraction Two authors (YG and EC) independently evaluated whether the identified trials fulfilled the inclusion criteria. We listed the excluded trials in 'Characteristics of excluded studies' with the reasons for exclusion. YG extracted data and EC validated the data extraction. Disagreements were resolved by discussion with CG. We wrote to the authors of the included trials and asked them to specify the data of interest, if they had not been reported clearly in the publications.
Assessment of methodological quality of included trials We assessed the methodological quality of the randomised clinical trials using four components (Schulz 1995; Moher 1998; Kjaergard 2001). High‐quality trials, ie, trials with low‐bias risk, were considered adequate on two out of the first three components.
Generation of the allocation sequence
Adequate, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice was considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure;
Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not described.
Allocation concealment
Adequate, if the allocation of patients involved a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes;
Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described;
Inadequate, if the allocation sequence was known to the investigators who assigned participants.
Blinding (or masking)
Adequate, if the trial was described as double blind and the method of blinding involved identical placebo or active drug;
Unclear, if the trial was described as double blind, but the method of blinding was not described;
Not performed, if the trial was not double blind.
Follow‐up
Adequate, if the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals;
Unclear, if the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated;
Inadequate, if the number or reasons for dropouts and withdrawals were not described.
Characteristics of patients Number of patients randomised; patient inclusion and exclusion criteria; mean (or median) age; sex ratio; histological stage; number of patients lost to follow‐up.
Characteristics of interventions Type, dose, and form of cyclosporin A intervention; type of intervention in the control group and collateral interventions (if any); duration of treatment and follow‐up.
Characteristics of outcomes All outcomes were extracted from each included trial. We analysed the outcome measures at maximum follow‐up.
Statistical methods We used the statistical package RevMan Analyses 1.0 (RevMan 2003) provided by The Cochrane Collaboration. We presented dichotomous data as relative risk (RR) with 95% confidence interval (CI). Peto odds ratio (OR) was used to combine rare event data (less than 5%). We presented continuous outcome measures by weighted mean differences (WMD) with 95% CI. We used standardised mean differences (SMD) to combine dichotomous data and continuous data on pruritus, fatigue, and blood pressure (Higgins 2006).
We examined intervention effects by using both a random‐effects model (DerSimonian 1986) and a fixed‐effect model (Mantel 1959) with the significant level set at P < 0.05. If the results of the two analyses concurred, we presented only the results of the fixed‐effect model. In case of discrepancies of the two models, we reported the results of both models. We explored the presence of statistical heterogeneity by chi‐squared test with significance set at P < 0.10 and measured the quantities of heterogeneity by I2 (Higgins 2002) .
Due to small number of trials included, we did not perform subgroup analysis, sensitivity analysis, and statistical tests to explore publication bias and other biases, which were planned in the protocol (Gong 2005c).
Results
Description of studies
We identified a total of 269 references through electronic searches of The Cochrane Hepato‐Biliary Group Controlled Trials Register (n = 61), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (n = 54), MEDLINE (n = 31), EMBASE (n = 45), Science Citation Index Expanded (n = 35), The Chinese Biomedical CD Database (n = 43), and LILACS (n = 0). We excluded 254 duplicates and clearly irrelevant references by reading abstracts. Accordingly, 15 references were retrieved for further assessment. Of these, we excluded nine because they were non‐randomised clinical studies or observational studies. The remaining six references referred to three randomised clinical trials involving 390 patients with primary biliary cirrhosis, which fulfilled our inclusion criteria. The publication year of the trials ranged from year 1988 to 1993. All trials were published as full papers.
All the trials compared cyclosporin A versus placebo. The formulation included was the original one, not microemulsion and topical emulsion. The mean age of the patients was about 52 years. The majority of the patients were women (women/men: 338/52). Slightly more patients had stage III or IV than stage I or II (178/154). The dose of cyclosporin A was 2.5, 3, or 4 mg/kg/day. The duration of treatment and follow‐up varied from one to three years (See 'Characteristics of included studies').
Risk of bias in included studies
None of the trials, except Lombard 1993, had adequate generation of the allocation sequence. Allocation concealment was adequate in two trials (Minuk 1988; Lombard 1993) and unclear in Wiesner 1990. Blinding was adequate in all trials. Follow‐up was adequately reported in all the trials. In total, 74 patients (19%) were lost to follow‐up: 46 (23%) patients in the cyclosporin A group and 28 (15%) in the placebo group. None of the trials reported a sample size estimate. Lombard 1993 reported that they used intention‐to‐treat analyses. Overall, two trials were regarded as low‐bias risk trials (Minuk 1988; Lombard 1993).
Effects of interventions
Mortality Three trials with 390 patients provided data to estimate the risk of mortality of cyclosporin A versus placebo (Comparison 01‐01). Compared with placebo, cyclosporine A did not significantly affect mortality (15% versus 17%). The relative risk was 0.92 (95% CI 0.59 to 1.45).
Mortality or liver transplantation Compared with placebo, cyclosporine A did not significantly affect mortality or liver transplantation (22% versus 27%) (Comparison 01‐02). The relative risk of mortality or liver transplantation was 0.85 (95% CI 0.60 to 1.20).
Pruritus, fatigue, and liver complications Cyclosporin A significantly improved pruritus (SMD ‐0.38, 95% CI ‐0.63 to ‐0.14), but did not significantly have an affect on fatigue (SMD ‐0.35, 95% CI ‐1.16 to 0.46). We were not able to locate data on liver complications because of poor reporting.
Liver biochemical and histological outcomes Regarding liver biochemistry (Comparison 01‐105 to 01‐10), cyclosporin A appeared to decrease the levels of s‐bilirubin, s‐alanine aminotransferase, and s‐alkaline phosphatases except for the levels of immunoglobulin M. Cyclosporin A also increased s‐albumin compared to the placebo group. Lombard et al used log transformed data on serum bilirubin, alkaline phosphatases, and aminotransferase for comparisons which prevented us from combining the data from all the three trials (Lombard 1993). Wiesner et al reported data on liver biopsy: histologic progression to at least one more stage and increased or unaltered portal inflammation (Wiesner 1990). There was no significant difference between cyclosporin A and placebo (Comparison 01‐10).
Adverse events In the largest trial (Lombard 1993), 34 out of 176 patients given cyclosporin A had adverse events that led to permanent discontinuation of the treatment versus 18 out of 173 patient given placebo (RR 1.86, 95% CI 1.09 to 3.16). All the three trials reported on other adverse events not necessitating permanent discontinuation of treatment (RR 1.41, 95% CI 1.15 to 1.73). The risks of such adverse events were significantly increased in the cyclosporin A treated patients. Among the adverse events, cyclosporin A significantly increased the risk of renal dysfunction (Peto OR 5.56, 95% CI 2.52 to 12.27). Cyclosporine significantly increased the blood pressure (SMD 0.88, 95% CI 0.27 to 1.48) as defined by a rise in the diastolic pressure above 5 mmHg since the previous visit (Lombard 1993) or an increase of ≥ 25 mmHg in the systolic pressure or ≥ 12 mmHg in the diastolic pressure (Wiesner 1990).
Quality of life and cost‐effectiveness None of the trials examined specific quality‐of‐life scales or cost‐effectiveness.
Regarding the subgroup and sensitivity analyses, they were not done because of the limited number of trials.
Discussion
Cyclosporin A did not significantly influence the risk of mortality or liver transplantation in patients with primary biliary cirrhosis, nor did it delay liver histological progression. Cyclosporin A seemed to ameliorate the patients' pruritus, but not fatigue. Cyclosporin A appeared to decrease the concentration of serum bilirubin and the activities of alanine aminotransferase and alkaline phosphatases. Patients given cyclosporin A experienced significantly more adverse events, especially renal dysfunction and hypertension.
To our knowledge, only three trials have been conducted to evaluate the effects of cyclosporin A for patients with primary biliary cirrhosis. Therefore, this systematic review has a major limitation: the small number of trials included (Ioannidis 2001). Furthermore, all the trials had shorter follow‐up than the estimated median survival of primary biliary cirrhosis, ie, 10 years to 15 years (Prince 2003). Therefore, it is difficult to detect a significant difference on mortality or liver transplantation.
Patients given cyclosporin A had not significantly lower risk of death and liver transplantation. Since two of the trials had a short trial duration (Minuk 1988; Wiesner 1990), few patients died during the period. In the largest trial by Lombard et al, patients were treated and followed up to six years. A total of 30 patients in the cyclosporin A group died and an additional 14 patients required liver transplantation, compared with 31 deaths and 15 transplants in the placebo group (Lombard 1993). When we combined the data, we found no significant difference on deaths and/or liver transplantations between the two groups. The heterogeneity was moderate (I2 = 41.4%) in spite of the disparity on trial duration. Lombard et al found a survival benefit (including death or liver transplantation) only after adjustment for a seemly imbalance in pretreatment variables (Lombard 1993). However, they did not find the same beneficial effect when adjustment was not applied (logrank P = 0.63). Furthermore, they did not confirm a beneficial effect in reducing the risk of death only ‐ neither without nor with the adjustment (logrank P = 0.87; Cox model P = 0.14). Therefore, we are not convinced of a beneficial effect of cyclosporin A on patients' survival and liver transplantation.
It seems that cyclosporin A improved the symptom of pruritus, which is one of the major complaints of the disease. But this finding should be interpreted with great caution. First of all, the pooling method here is based on an assumption that the underlying distribution of the pruritus score in each treatment group follows a logistic distribution, which might not be the case. Secondly, since pruritus is a subjective assessment, depending on patient's threshold and physician's experience, the potential improvement caused by cyclosporin A needs to be further investigated. We cannot exclude that blinding might have been broken in the trials because of, eg, occurrence of adverse events. This actually happened in the Wiesner 1990 trial. Such unblinding might have biased the assessment of pruritus (Schulz 1995; Kjaergard 2001).
Cyclosporin A seems to have beneficial effect in reducing the activity of alanine aminotransferase and in increasing serum albumin level. The variety of reporting did not allow us to integrate the data on serum bilirubin and alkaline phosphatases, which were found to be improved in Wiesner 1990 and Lombard 1993 trials. None of the three trials have found that cyclosporin A delayed the histological progression (including the assessment of inflammation or fibrosis).
Our review shows a benefit from treatment with cyclosporin A on pruritus and liver biochemistry and poses the question as to whether the shown benefits statistically outweigh the adverse events. Lombard et al reported that more patients in the cyclosporin A group experienced adverse events warranting discontinuation and that the proportion of patients with discontinuation was significantly higher than in the placebo group (Lombard 1993). Most of the adverse events were renal impairment, hypertension, and infective episodes. All the three trials reported adverse events not necessitating permanent discontinuation of treatment. Patients given cyclosporin A experienced significantly more adverse events with the majority being hirsutism, increased blood pressure, and a slight increase in viral or bacterial infection occurrence.
For cyclosporin A, nephrotoxicity and hypertension are adverse events of major concerns. We have, therefore, also extracted the data on these adverse events. Our analyses show that significantly more patients given cyclosporin A had renal dysfunction as defined by creatinine persistently above 141 µmol/L (Wiesner 1990; Lombard 1993). In a majority of the patients, reducing the dose or discontinuing cyclosporin A temporarily was associated with the resolution of the adverse events. On the other hand, no dynamic renal function tests were undertaken in the trials, and it must be conceded that serum creatinine elevation probably underestimates the incidence of nephrotoxicity. Our result demonstrates that cyclosporin A treated patients significantly increased blood pressure. In general, hypertension was easily controlled with medical therapy when indicated (Wiesner 1990).
Authors' conclusions
Implications for practice.
Despite improvements in pruritus and liver biochemical variables, cyclosporin A did not delay the progression to death or liver transplantation, or to an advanced histological stage. In addition, patients given cyclosporin A experienced more adverse events, especially renal dysfunction and hypertension. We do not recommend the use of cyclosporin A outside randomised clinical trials.
Implications for research.
Further randomised clinical trials need to investigate the short‐term and long‐term effects of cyclosporin A on progression of the disease, need for liver transplantation, and survival. The potential benefits in pruritus and liver biochemistry also need to be further investigated. Future trials need to be closely monitored because of the adverse events, especially renal dysfunction and hypertension. Future trials ought to be reported according to the recommendations of the CONSORT Group (http://www.consort‐statement.org/).
What's new
| Date | Event | Description |
|---|---|---|
| 17 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We primarily extend our acknowledgements to the patients who took part in and the investigators who designed and conducted the reviewed trials. We thank Genald Minuk for providing supplementary information. Dimitrinka Nikolova, Nader Salas, and Styrbjørn Birch, all from The Cochrane Hepato‐Biliary Group, are thanked for expert assistance during the preparation of this review.
Appendices
Appendix 1. Search Strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | June 2006. | 'primary biliary cirrhosis' and 'cyclosporin A' |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 2, 2006. | #1 = LIVER CIRRHOSIS BILIARY: MESH #2 = primary and biliary and cirrhosis #3 = primary biliary cirrhosis #4 = pbc #5 = #1 or #2 or #3 or #4 #6 = CYCLOSPORIN A: MESH #7 = IMMUNOSUPPRESSIVE AGENTS: MESH #8 = cyclosporins #9 = #6 or #7 or #8 #10 = #5 and #9 |
| MEDLINE | 1966 to June 2006. | #1 = LIVER‐CIRRHOSIS‐BILIARY: MESH #2 = primary and biliary and cirrhosis #3 = primary biliary cirrhosis #4 = PBC #5 = #1 or #2 or #3 or #4 #6 = CYCLOSPORIN A: MESH #7 = IMMUNOSUPPRESSIVE AGENTS: MESH #8 = cyclosporin* #9 = immunosuppressive agent* #10 = #6 or #7 or #8 or #9 #11 = #5 and #10 #12 = random* or placebo* or blind* or meta‐analysis #13 = #11 and #12 |
| EMBASE | 1980 to June 2006. | #1 = PRIMARY‐BILIARY‐CIRRHOSIS: MESH #2 = BILIARY‐CIRRHOSIS: MESH #3 = primary and biliary and cirrhosis #4 = primary biliary cirrhosis #5 = PBC #6 = #1 or #2 or #3 or #4 or #5 #7 = CYCLOSPORIN A: MESH #8 = IMMUNOSUPPRESIVE AGENTS: MESH #9 = cyclosporin* #10 = immunosuppressive agent* #11 = #7 or #8 or #9 or #10 #12 = #6 and #11 #13 = random* or placebo* or blind* or meta‐analysis #14 = #12 and #13 |
| Science Citation Index Expanded (http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame) | 1945 to June 2006. | #1 = TS=(primary biliary cirrhosis OR PBC) #2 = TS=(cyclosporine OR cyclosporin*) #3 = #2 AND #1 #4 = TS=(random* OR blind* OR placebo* OR meta‐analysis) #5 = #4 AND #3 |
| LILACS | 1982 to June 2006. | #1 = (primary and biliary and cirrhosis) or (primary biliary cirrhosis) #2 = primary biliary cirrhosis #3 = cyclosporin A #4 = (#1 OR #2) AND # 3 |
| Chinese Biochemical CD Database | 1979 to June 2006. | #1 = LIVER‐CIRRHOSIS‐BILIARY: MESH #2 = primary and biliary and cirrhosis #3 = primary biliary cirrhosis #4 = PBC #5 = #1 or #2 or #3 or #4 #6 = CYCLOSPORIN A: MESH #7 = IMMUNOSUPPRESSIVE AGENTS: MESH #8 = cyclosproin* #9 = immunosuppressive agent* #10 = #6 or #7 or #8 or #9 #11 = #5 and #10 #12 = random* or placebo* or blind* or meta‐analysis #13 = #11 and #12 |
Data and analyses
Comparison 1. Cyclosporin A versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.59, 1.45] |
| 2 Mortality and/or liver transplantation | 3 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.20] |
| 3 Pruritus score and number of patients with the improvements | 3 | SMD (Fixed, 95% CI) | ‐0.38 [‐0.63, ‐0.14] | |
| 4 Fatigue score and number of patients with the improvements | 2 | SMD (Fixed, 95% CI) | ‐0.35 [‐1.16, 0.46] | |
| 5 Bilirubin (µmol/L) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Change from baseline (untransformed) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐17.1 [‐27.70, ‐6.50] |
| 5.2 Change from baseline (logtransformed) | 1 | 349 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐0.84, ‐0.00] |
| 5.3 Final measurement | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 16.1 [‐55.18, 87.38] |
| 6 Alanine aminotransferase (U/L) | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐40.55 [‐63.38, ‐17.71] |
| 6.1 Change from baseline | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐48.0 [‐72.56, ‐23.44] |
| 6.2 Final measurement | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [‐55.03, 69.03] |
| 7 Alkaline phosphatases (U/L) (change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Untransformed | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐711.0 [‐1063.41, ‐358.59] |
| 7.2 Logtransformed | 1 | 349 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.44, 0.00] |
| 8 Immunoglobulin M (g/L) | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐2.71, 0.62] |
| 8.1 Change from baseline | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐4.08 [‐6.17, ‐1.99] |
| 8.2 Final measurement | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [1.45, 6.95] |
| 9 Serum albumin (g/L) | 3 | 388 | Mean Difference (IV, Fixed, 95% CI) | 1.66 [0.26, 3.05] |
| 9.1 Change from baseline | 2 | 378 | Mean Difference (IV, Fixed, 95% CI) | 2.07 [0.61, 3.52] |
| 9.2 Final measurement | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐3.50 [‐8.65, 1.65] |
| 10 Histologic assessment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Histologic progression to at least one more stage | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.30, 1.37] |
| 10.2 Worsend/unaltered portal inflammation | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.10, 0.76] |
| 11 Adverse event | 3 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.23, 1.81] |
| 11.1 Permanent discontinuation of treatment | 1 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.09, 3.16] |
| 11.2 Not necessitating permanent discontinuation of treatment | 3 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.15, 1.73] |
| 12 Renal dysfunction | 2 | 378 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.56 [2.52, 12.27] |
| 13 Increased blood pressure | 3 | SMD (Fixed, 95% CI) | 0.88 [0.27, 1.48] |
1.1. Analysis.
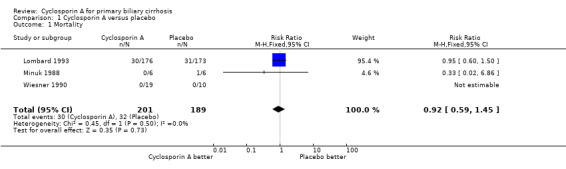
Comparison 1 Cyclosporin A versus placebo, Outcome 1 Mortality.
1.2. Analysis.
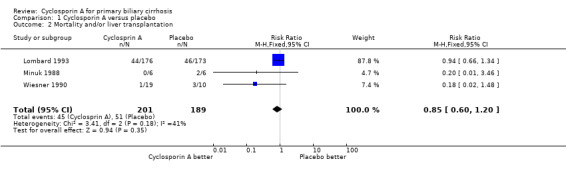
Comparison 1 Cyclosporin A versus placebo, Outcome 2 Mortality and/or liver transplantation.
1.3. Analysis.
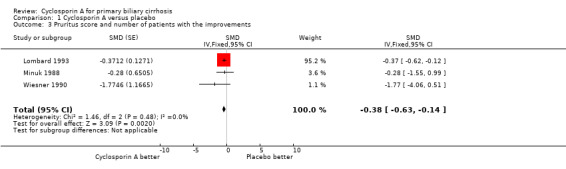
Comparison 1 Cyclosporin A versus placebo, Outcome 3 Pruritus score and number of patients with the improvements.
1.4. Analysis.
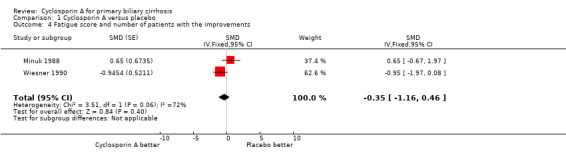
Comparison 1 Cyclosporin A versus placebo, Outcome 4 Fatigue score and number of patients with the improvements.
1.5. Analysis.
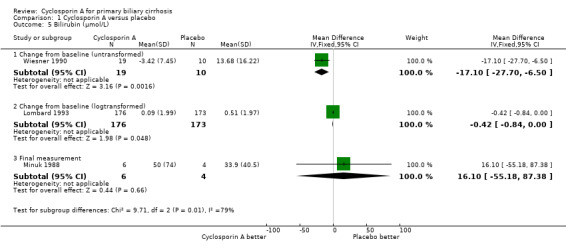
Comparison 1 Cyclosporin A versus placebo, Outcome 5 Bilirubin (µmol/L).
1.6. Analysis.
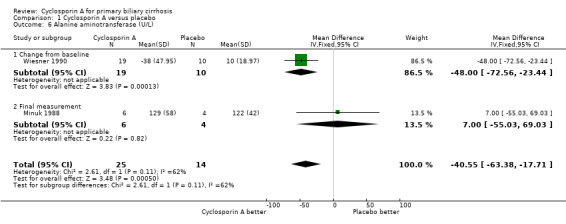
Comparison 1 Cyclosporin A versus placebo, Outcome 6 Alanine aminotransferase (U/L).
1.7. Analysis.
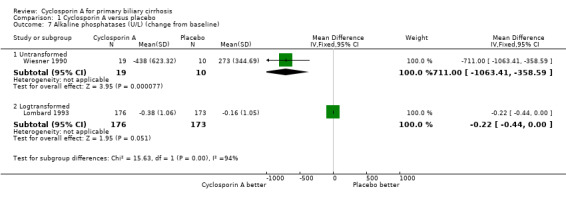
Comparison 1 Cyclosporin A versus placebo, Outcome 7 Alkaline phosphatases (U/L) (change from baseline).
1.8. Analysis.
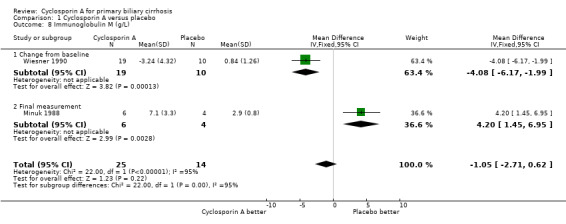
Comparison 1 Cyclosporin A versus placebo, Outcome 8 Immunoglobulin M (g/L).
1.9. Analysis.
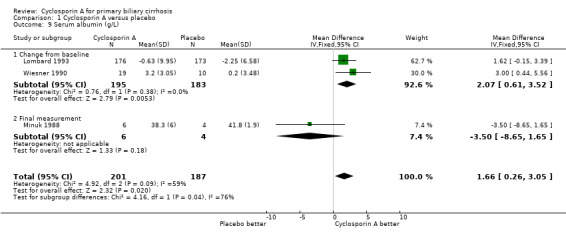
Comparison 1 Cyclosporin A versus placebo, Outcome 9 Serum albumin (g/L).
1.10. Analysis.
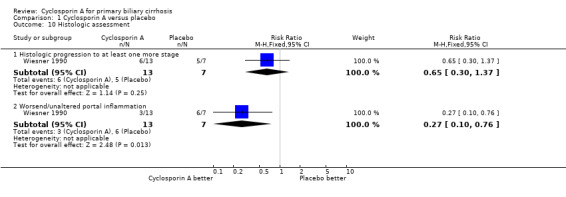
Comparison 1 Cyclosporin A versus placebo, Outcome 10 Histologic assessment.
1.11. Analysis.
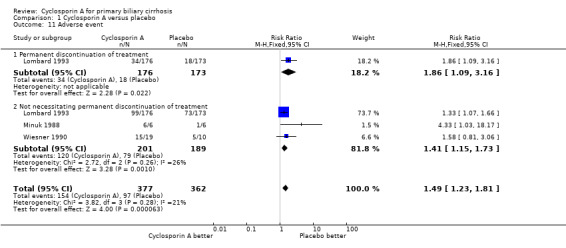
Comparison 1 Cyclosporin A versus placebo, Outcome 11 Adverse event.
1.12. Analysis.
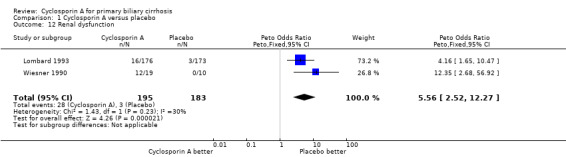
Comparison 1 Cyclosporin A versus placebo, Outcome 12 Renal dysfunction.
1.13. Analysis.
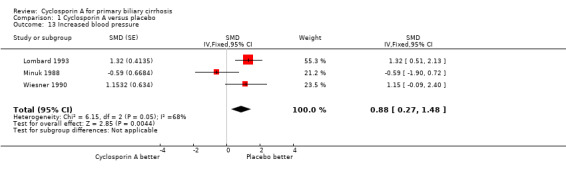
Comparison 1 Cyclosporin A versus placebo, Outcome 13 Increased blood pressure.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lombard 1993.
| Methods | Generation of the allocation sequence: a schedule of block randomisation ‐ considered adequate. Allocation concealment: a 'blinded' investigator ‐ considered adequate. Blinding: patients and investigators ‐ considered adequate. Follow‐up: 40 in cyclosporin A group and 25 in placebo group were lost to follow‐up ‐ considered adequate. |
|
| Participants | Country: UK. Mean age: 53.9 years in cyclosporin A group, 54.2 years in placebo group. Female/Male: 298/51. PBC stage status: stage I/II: 62 in cyclosporin A group, 71 in placebo group; stage III/IV: 87 in cyclosporin A group, 71 in placebo group. | |
| Interventions | Cyclosporin A: 3 mg/kg/day (n = 176); Placebo (n = 173). Median follow‐up: 928 days (range 6 to 2146 days). | |
| Outcomes | (1) Mortality and liver transplantation. (2) Clinical outcomes and liver biochemical variables. (3) Adverse events. | |
| Notes | (1) Two types of analysis were presented: the first one was on death (the end point) and liver transplantation censored at time of transplantation; the second one combined death and liver transplantation. (2) Correspondence sent to the author on 8 June 2005. No reply was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Minuk 1988.
| Methods | Generation of the allocation sequence: unclear. Allocation concealment: sealed envelopes ‐ considered adequate. Blinding: patients ‐ considered adequate. Follow‐up: no one lost to follow‐up ‐ considered adequate. |
|
| Participants | Country: Canada. Mean age: 50.7 years in cyclosporin A group, 58.6 years in placebo group. Female/Male: 11/1 PBC stage status: stage I/II: 3 in cyclosporin A group, 2 in placebo group; stage III/IV: 3 in cyclosporin A group, 4 in placebo group. | |
| Interventions | Cyclosporin A: 2.5 mg/kg/day (n = 6); Placebo (n = 6). Treatment: one year Posttreatment follow‐up: 6 months. | |
| Outcomes | (1) Mortality and liver transplantation. (2) Clinical outcomes and liver biochemical variables. (3) Histological assessment. (4) Adverse events. | |
| Notes | (1) Correspondence sent to the author on 8 June 2005. His email with information on methodological quality was received on the same day. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Wiesner 1990.
| Methods | Generation of the allocation sequence: unclear. Allocation concealment: unclear. Blinding: patients and investigators were planned to be 'blinded'. However, the assessment of the 'blinding' effectiveness revealed that a considerable unblinding did occur, so we considered it inadequate. Follow‐up: 6 in cyclosporin A group and 3 in placebo group were lost to follow‐up ‐ we considered it adequate. |
|
| Participants | Country: US. Mean age: 45.5 years in cyclosporin A group, 48.0 years in placebo group. Female/Male: 29/0 PBC stage status: stage I/II: 11 in cyclosporin A group, 5 in placebo group; stage III/IV: 8 in cyclosporin A group, 5 in placebo group. | |
| Interventions | Cyclosporin A: 4 mg/kg/day (n = 19); Placebo (n = 10). Median follow‐up: 2.7 years. | |
| Outcomes | (1) Mortality and liver transplantation. (2) Clinical outcomes and liver biochemical variables. (3) Histological assessment. (4) Adverse events. | |
| Notes | (1) This trial only included precirrhotic patients with primary biliary cirrhosis. (2) It was a preliminary report of first 29 patients out of 59. (3) Correspondence sent to the author on 8 June 2005. No reply was received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chau 2001 | The authors described the histological patterns of rejection in liver transplant recipients using induction therapies with cyclosporin and tacrolimus monotherapy compared with standard triple therapy as historical control. |
| Dmitrewski 1996 | The authors have examined the liver allograft biopsies taken at 1 and 2 years after transplantation from patients receiving either FK‐506 or cyclosporin as part of a multi‐centre trial. The objective was to study the recurrence of primary biliary cirrhosis in the liver allograft. |
| McMichael 1993 | A randomised concentration‐controlled clinical trial was performed to discover important concentration response relationships of FK‐506, a potent immunosuppressive agent for prevention and treatment of graft rejection. |
| McMichael 1996 | This is a computer‐guided randomised concentration‐controlled trials of tacrolimus in autoimmunity: multiple sclerosis and primary biliary cirrhosis. |
| Mueller 1995 | In the present study, 121 patients, 61 randomly assigned to FK‐506‐ and 60 assigned to cyclosporin A‐based immunosuppression, were analysed according to the primary diagnosis for liver transplantation. |
| Robert 2003 | A clinical review article to discuss the specific treatment to primary biliary cirrhosis. |
| Sanchez 2003 | Data were obtained from prospectively maintained liver‐transplant database and evaluated statistically to determine the recurrence of primary biliary cirrhosis. |
| Slitzky 1990 | A clinical review article discussing the approaches to the treatment of primary biliary cirrhosis. |
| von Graffenried 1985 | In this paper, the authors reported the presently available experience with regard to renal function in patients with autoimmune diseases treated with cyclosporin A. |
Contributions of authors
YG performed the searches, selected trials for inclusion, wrote to authors and pharmaceutical companies, performed data extraction and data analyses, and drafted the protocol and the review. EC validated data extraction and revised the protocol and the review. CG formulated the idea of this review and revised the protocol, arbitrated disagreements on data extraction, validated data analyses, and revised the protocol and the review.
Sources of support
Internal sources
Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Denmark.
External sources
S.C. Van Foundation, Denmark.
Declarations of interest
None known. We have no affiliations or financial contracts with companies producing the drugs examined in this review.
Edited (no change to conclusions)
References
References to studies included in this review
Lombard 1993 {published data only}
- Lombard M, Portmann B, Neuberger J, Williams R, Tygstrup N, Ranek L, et al. Cyclosporin A treatment in primary biliary cirrhosis: results of a long‐term placebo controlled trial. Gastroenterology 1993;104:519‐26. [DOI] [PubMed] [Google Scholar]
- Robson SC, Neuberger JM, Williams R. The influence of cyclosporine A therapy on sex hormone levels in pre‐ and post‐menopausal women with primary biliary cirrhosis. Journal of Hepatology 1994;21:412‐4. [DOI] [PubMed] [Google Scholar]
Minuk 1988 {published data only}
- Hanley DA, Ayer LM, Gundberg CM, Minuk GY. Parameters of calcium metabolism during a pilot study of cyclosporin A in patients with symptomatic primary biliary cirrhosis. Clinical and Investigative Medicine 1991;14(4):282‐7. [PubMed] [Google Scholar]
- Minuk GY, Bohme CE, Burgess E, Hershfield NB, Kelly JK, Shaffer EA, et al. Pilot study of cyclosporin A in patients with symptomatic primary biliary cirrhosis. Gastroenterology 1988;95:1356‐63. [DOI] [PubMed] [Google Scholar]
- Parsons HG, Thirsk JE, Frohlich J, Dias V, Minuk GY. Effect of cyclosporin A on serum lipids in primary biliary cirrhosis patients. Clinical and Investigative Medicine 1989;12(6):386‐91. [PubMed] [Google Scholar]
Wiesner 1990 {published data only}
- Wiesner RH, Ludwig J, Lindor KD, Jorgensen RA, Baldus WP, Homburger HA, et al. A controlled trial of cyclosporine in the treatment of primary biliary cirrhosis. New England Journal of Medicine 1990;322(20):1419‐24. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chau 2001 {published data only}
- Chau TN, Quaglia A, Rolles K, Burroughs AK, Dhillon AP. Histological patterns of rejection using oral microemulsified cyclosporine and tacrolimus (FK 506) as monotherapy induction after orthotopic liver transplantation. Liver 2001;21:329‐34. [DOI] [PubMed] [Google Scholar]
Dmitrewski 1996 {published data only}
- Dmitrewski J, Hubscher SG, Mayer AD, Neuberger M. Recurrence of primary biliary cirrhosis in the liver allograft: the effect of immunosuppression. Journal of Hepatology 1996;24:253‐7. [DOI] [PubMed] [Google Scholar]
McMichael 1993 {published data only}
- McMichael J, Lieberman R, Doyle H, McCauley J, Thiel D, Thomson A. Computer‐guided concentration‐controlled trials in autoimmune disorders. Therapeutic Drug Monitoring 1993;15:510‐3. [DOI] [PubMed] [Google Scholar]
McMichael 1996 {published data only}
- McMichael J, Lieberman R, McCauley J, Irish W, Marino I, Doyle H. Computer‐guided randomized concentration‐controlled trials of tacrolimus in autoimmunity: multiple sclerosis and primary biliary cirrhosis. Therapeutic Drug Monitoring 1996;18(4):435‐7. [DOI] [PubMed] [Google Scholar]
Mueller 1995 {published data only}
- Mueller AR, Platz KP, Bechstein WO, Blumhardt G, Christe W, Hopf U, et al. The optimal immunosuppressant after liver transplantation according to diagnosis: Cyclosporine A or FK506?. Clinical Transplantation 1995;9:176‐84. [PubMed] [Google Scholar]
Robert 2003 {published data only}
- Robert L, Carithers Jr. Primary biliary cirrhosis: specific treatment. Clinical Liver Disease 2003;7:923‐39. [DOI] [PubMed] [Google Scholar]
Sanchez 2003 {published data only}
- Sanchez EQ, Levy MF, Goldstein RM, Fasola CG, Tillery GW, Netto GJ. The changing clinical presentation of recurrent primary biliary cirrhosis after liver transplantation. Transplantation 2003;76:1583‐8. [DOI] [PubMed] [Google Scholar]
Slitzky 1990 {published data only}
- Slitzky BE, Ouellette GS, Boyer JL. Approaches to the treatment of primary biliary cirrhosis ‐ a status report. Gastroenterology International 1990;3(3):134‐9. [Google Scholar]
von Graffenried 1985 {published data only}
- Graffenried B, Harrison WB. Renal function in patients with autoimmune diseases treated with cyclosporine. Transplantation Proceedings 1985;17(4 Suppl 1):215‐31. [PubMed] [Google Scholar]
Additional references
Christensen 1985
- Christensen E, Neuberger J, Crowe J, Altman DG, Popper H, Portmann B, et al. Beneficial effect of azathioprine and prediction of prognosis in primary biliary cirrhosis. Final results of an international trial. Gastroenterology 1985;89:1084‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cohen 1984
- Cohen DJ, Loertscher R, Runin MF, Tilney NL, Carpenter CB, Strom TB. Cyclosporine: a new immunosuppressive agent for organ transplantation. Annals of Internal Medicine 1984;101:667‐82. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dickson 1985
- Dickson ER, Fleming TR, Wiesner RH, Baldus WP, Fleming CR, Ludwig J, et al. Trial of penicillamine in advanced primary biliary cirrhosis. New England Journal of Medicine 1985;312(16):1011‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fregeau 1989
- Fregeau D, Water J, Danner D, Ansart T, Coppel R, Gershwin M. Antimitochondrial antibodies AMA of primary biliary cirrhosis PBC recognize dihydrolipoamide acyltransferase and inhibit enzyme function of the branched chain alpha ketoacid dehydrogenase complex. Faseb Journal 1989;3:A1121. [MEDLINE: ] [PubMed] [Google Scholar]
Gluud 2007
- Gluud C, Nikolova D, Klingenberg SL, Als‐Nielsen B, D'Amico G, Davidson B, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2007, Issue 2. Art. No.: LIVER.
Gong 2004
- Gong Y, Frederiksen SL, Gluud C. D‐penicillamine for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No.: CD004789. DOI: 10.1002/14651858.CD004789.pub2. [DOI] [PMC free article] [PubMed]
Gong 2005a
Gong 2005b
- Gong Y, Gluud C. Colchicine for primary biliary cirrhosis: a systematic review of randomised clinical trials. American Journal of Gastroenterology 2005;100:1876‐85. [DOI] [PubMed] [Google Scholar]
Gong 2005c
- Gong Y, Christensen E, Gluud C. Cyclosporin A for primary biliary cirrhosis. (Protocol) Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No.: CD005526. DOI: 10.1002/14651858.CD005526. [DOI] [PMC free article] [PubMed]
Harris 1987
- Harris DT, Kozumbo WJ, Cerutti PA, Cerottini JC. Mechanism of cyclosporin A induced immunosuppression. Cell Immunology 1987;109:104‐14. [DOI] [PubMed] [Google Scholar]
Heathcote 1976
- Heathcote J, Ross A, Sherlock S. A prospective controlled trial of azathioprine in primary biliary cirrhosis. Gastroenterology 1976;70(5 Pt 1):656‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. The Cochrane Library, Issue 4, 2006. Chichester, UK: John Wiley & sons, Ltd. [Google Scholar]
Hoofnagle 1986
- Hoofnagle JH, Davis GL, Schafer DF, Peters M, Avigan MI, Pappas SC, et al. Randomized trial of chlorambucil for primary biliary cirrhosis. Gastroenterology 1986;91(6):1327‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation of Technical Requiements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1997. [Google Scholar]
Invernizzi 1997
- Invernizzi P, Crosignani A, Battezzati PM, Covini G, De‐Valle G, Larghi A, et al. Comparison of the clinical features and clinical course of antimitochondrial antibody‐positive and negative primary biliary cirrhosis. Hepatology 1997;25(5):1090‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ioannidis 2001
- Ioannidis JPA, Lau J. Evolution of treatment effects over time: empirical insight from recursive cumulative metaanalyses. Proceedings of the National Academy of Sciences of the United States of America 2001;98(3):831‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
James 1981
- James O, Macklon AF, Waston AJ. Primary biliary cirrhosis ‐ a revised clinical spectrum. Lancet 1981;1(8233):1278‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
James 1983
- James SP, Hoofnagle J, Strober W, Jones EA. Primary biliary cirrhosis: a model autoimmune disease. Annals of Internal Medicine 1983;99:500‐12. [DOI] [PubMed] [Google Scholar]
Kaplan 1991
- Kaplan MM, Knox TA. Treatment of primary biliary cirrhosis with low‐dose weekly methotrexate. Gastroenterology 1991;101:1332‐8. [DOI] [PubMed] [Google Scholar]
Kaplan 1996
- Kaplan MM. Primary biliary cirrhosis. New England Journal of Medicine 1996;335(21):1570‐80. [DOI] [PubMed] [Google Scholar]
Kim 2000
- Kim WR, Lindor KD, Locke GR 3rd, Therneau TM, Homburger HA, Batts KP, et al. Epidemiology and natural history of primary biliary cirrhosis in a U.S. community. Gatroenterology 2000;119:1631‐6. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lacerda 1995
- Lacerda MA, Ludwig J, Dickson ER, Jorgensen RA, Lindor KD. Antimitochondrial antibody‐negative primary biliary cirrhosis. American Journal of Gastroenterology 1995;90(2):247‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Lindor 1995
- Lindor KD, Dickson ER, Jorgensen RA, Anderson ML, Wiesner RH, Gores GJ, et al. The combination of ursodeoxycholic acid and methotrexate for patients with primary biliary cirrhosis: the results of a pilot study. Hepatology 1995;22(4 Pt 1):1158‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of National Cancer Institute 1959;22:719‐48. [PubMed] [Google Scholar]
Mattalia 1998
- Mattalia A, Quaranta S, Leung PS, Bauducci M, Van‐de‐Water J, Calvo PL. Characterization of antimitochondrial antibodies in health adults. Hepatology 1998;27(3):656‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mitchison 1992
- Mitchison HC, Palmer JM, Bassendine MF, Watson AJ, Record CO, James OF. A controlled trial of prednisolone treatment in primary biliary cirrhosis. Three‐year results. Journal of Hepatology 1992;15(3):336‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Neuberger 1985
- Neuberger J, Christensen E, Portmann B, Caballeria J, Rodes J, Ranek L, et al. Double blind controlled trial of D‐penicillamine in patients with primary biliary cirrhosis. Gut 1985;26(2):114‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poupon 1996
- Poupon RE, Huet PM, Poupon R, Bonnand AM, Nhieu JT, Zafrani ES, et al. A randomized trial comparing colchicine and ursodeoxycholic acid combination to ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1996;24(5):1098‐103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prince 2003
- Prince MI, James OFW. The epidemiology of primary biliary cirrhosis. Clinics in Liver Disease 2003;7:795‐819. [DOI] [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
Routhier 1980
- Routhier G, Epstein O, Janossy G, Thomas HC, Sherlock S. Effects of cyclosporin A on suppressor and induceer T lymphocytes in primary biliary cirrhosis. Lancet 1980;2:1223‐6. [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Scheuer 1967
- Scheuer P. Primary biliary cirrhosis. Proceedings of the Royal Society of Medicine 1967;60(12):1257‐60. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tugwell 1990
- Tugwell P, Bombardier C, Gent M, Bennett KJ, Bensen WG, Carette S, et al. Low dose cyclosporin versus placebo in patients with rheumatoid arthritis. Lancet 1990;335:1051‐5. [DOI] [PubMed] [Google Scholar]
Turchany 1997
- Turchany JM, Uibo R, Kivik T, Van‐de‐Water J, Prindiville T, Coppel RL, et al. A study of antimitochondrial antibodies in a random population in Estonia. American Journal of Gastroenterology 1997;92(1):124‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Verma 1999
- Verma A, Jazrawi RP, Ahmed HA, Northfield TC. Prescribing habits in primary biliary cirrhosis: a national survey. European Journal of Gastroenterology and Hepatology 1999;11(8):817‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vuoristo 1995
- Vuoristo M, Farkkila M, Karvonen AL, Leino R, Lehtola J, Makinen J, et al. A placebo‐controlled trial of primary biliary cirrhosis treatment with colchicine and ursodeoxycholic acid [see comments]. Gastroenterology 1995;108(5):1470‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Warnes 1987
- Warnes TW, Smith A, Lee FI, Haboubi NY, Johnson PJ, Hunt L. A controlled trial of colchicine in primary biliary cirrhosis: trial design and preliminary report. Jounal of Hepatology 1987;5(1):1‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


