Abstract
Women face a disproportionate burden of stroke mortality and disability. Biologic sex and sociocultural gender both contribute to differences in stroke risk factors, assessment, treatment and outcomes. There are substantial differences in the strength of association of stroke risk factors as well as female-specific risk factors. Moreover, there are differences in presentation, response to treatment and stroke outcomes in women. This review outlines current knowledge of impact of sex and gender on stroke, as well as delineates research gaps and areas for future inquiry.
Keywords: Stroke, Cardiovascular Disease, Ischemic Stroke, Risk Factors, Women, Sex, Gender
Introduction
Women face a disproportionate burden of stroke mortality and disability. They have substantial differences in the strength of association of stroke risk factors as well as female-specific risk factors. Moreover, there are differences in presentation, response to treatment and stroke outcomes in women. We have undertaken this review to highlight current knowledge as well as research gaps and areas for future inquiry (see Central Illustration).
Central illustration:

The impact of sex and and gender on stroke (Biologic sex is shown in red, gender in blue, combined in purple)
IV rtPA intravenous recombinant tissue plasminogen activator. APO: adverse pregnancy outcomes
Sex/gender
Most of the published data on stroke has not explicitly separated sex and gender, and likely incorporates both influences. Sex refers to the biological characteristics of individuals including genetic, biologic and physiologic expression. In contrast, gender is a social construct that includes gender identify, expression, roles and stereotypes for female, male and gender diverse people.1 Gender also relates to power, economic resources and healthcare access, all of which can also influence health. While neither sex nor gender are binary, most data collection in trials and cohorts has been binary, and sex and gender identity have not been collected separately. Additionally, quantification of gender traits is evolving and usually not available. Thus, many of the findings for “sex differences” may represent the combined impact of both sex and gender, and gendered exposures may contribute to observed differences between men and women.2 Moreover, gender inter-relates to and intersects with race/ethnicity, sexuality, age, geography, and other attributes, so intersectional research approaches are needed. A few studies have looked the impact of gender-related characteristics on CHD outcomes3 and health status2 in cis-gender women, but none are available for stroke to the best of our knowledge. Transgender data are also limited.4–6 For the remainder of this review, we will use the term sex differences, but stress the limitations listed above.
Stroke epidemiology
In 2019, the most current year available, stroke was the second leading cause of death worldwide, as well as the second cause of disability-adjusted life years.7 In the US in 2019, stroke was the 3rd leading cause of death in women, compared with 5th in men.8 Women accounted for 57.1% of stroke deaths in 2019, with stroke accounting for 6.2% of all female deaths, while comprising 4.4% of all male deaths.8 In total, Approximately 55,000 more fatal strokes occur in women each year than men.9 In the 2015 Greater Cincinnati Northern Kentucky Stroke Study (GCNKSS), stroke case fatality in women exceeded that of men for the first time, even after adjustment for age.10, 11 As show in Figure 1, strokes comprise a greater percentage of deaths in women than men throughout the adult lifespan (Lichtman et al, unpublished data).
Figure 1.
Sex- and age-specific ranking, percentage, and total number of deaths attributed to cerebrovascular diseases in 2015
Data from: National Vital Statistics System (NVSS), 2015. LCWK1: Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 2015. https://www.cdc.gov/nchs/data/dvs/LCWK1_2015.pdf. Accessed October 13, 2021.
Globally, the lifetime risk of stroke (from age 25 onward) is 25.1% in women and 24.7% in men, but there is substantial regional variation.12 There are regional differences with the highest lifetime risks for women in Eastern Europe and East Asia (36.5% and 36.3%, respectively).12 In the US, the lifetime risk of stroke is higher in women (20–21%), than in men (14–17%), for a 55 year old individual.13 Stroke is more likely to be the first manifestation of cardiovascular disease in women, whereas in men coronary heart disease is more common.14 The social context for women who experience stroke is important as well. Age at onset of stroke is on average 4–6 years older in women than men15,10 Moreover, women are more likely to be widowed, unmarried or living alone, and have a higher degree of disability in their activities of daily living than men at the time of their stroke.15
Sex is related to differences in stroke type incidence. Women have a higher prevalence and incidence of intracranial aneurysms, and substantially higher incidence of subarachnoid hemorrhage compared to men, while men have higher rates of intracerebral hemorrhage.7, 11 Across the lifespan, recent data from Canada shows that the risk ischemic stroke and transient ischemic attack (TIA) is higher for women than men <30 years, higher in men for midlife, and then equal above 80 years of age.7 In the oldest category (>85 years),7, 16 there is some suggestion that women have higher incidence of stroke than men.15 Significant disparities by race/ethnicity have been documented, particularly in older women. In the Northern Manhattan Study (NOMAS), Black and Hispanic women age 70 and older had a 76–77% higher risk of stroke than white women after adjusting for age, education and insurance status, a difference not seen in men.17 In data from the Women’s Health Initiative (WHI), Black women have a 47% higher risk of stroke than White women after adjustment for age, with much of the risk mediated by stroke risk factors and socioeconomic factors.18 Over the past several decades, stroke incidence has been declining, with relatively similar changes in both men and women. The Atherosclerosis Risk in Communities (ARIC) study documented substantial decline in stroke incidence among individuals age 65 year and older from 1987–2017, with similar declines in women and men.19 Similarly in the GCNKSS, incidence rates declined for both women and men from 1993/1994 to 2015.10
Impact of stroke
Given the aging of the US population and rises in life expectancy, in 2013, the American Heart Association and American Stroke Association projected that by 2030 the prevalence of stroke in the US would be 3.9%. Moreover, the economic burden of stroke was projected to rise by 129% from 2013 to 2030, with an increase in the direct economic burden of stroke-related medical costs, from $71.6 billion in 2013 to $184.1 billion in 2030.20 Given the predominance of women in these older age groups, this impact will be disproportionately in women.
Risk factors for stroke
Since previously published guidelines and scoping reviews, additional data have advanced our knowledge of sex differences in key stroke risk factors.21–23 See Figure 2 for an overview of risk factors.
Figure 2:
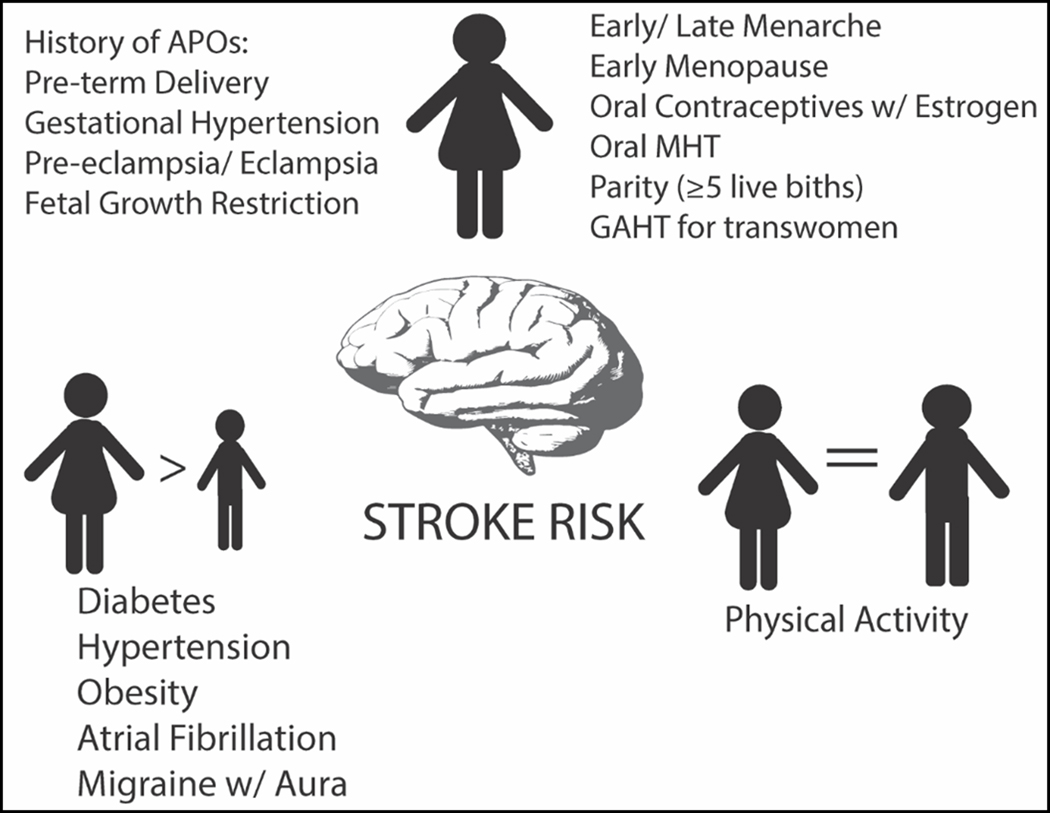
Sex differences in Stroke Risk Factors; size of figures indicates differences in strength of association between given risk factors and stroke; MHT: menopausal hormone therapy; GHT: gender-affirming hormone therapy
The lifetime risk of stroke is higher for women than men, with a 1 in 4 risk of stroke for women after age 25.12 The change in stroke risk with age varies by sex; stroke incidence is higher in women than men for those less than 30 years,7 while rates are higher in men than women during mid-life and either equal by sex or higher among women beginning in the eighth decade.7, 10, 24 Data on female-to-male incidence rate ratios during the reproductive years conflict.25 Further research is needed to determine contributors to the rising risk for women after menopause (and reversal of risk ratio relative to men) and whether factors affecting vascular dysfunction such as sex hormone levels and biologic age might be novel targets for stroke prevention. Recently published data conflict as to whether endogenous levels of age-varying sex hormones including sex hormone binding globulin, estradiol, and testosterone are independently associated with stroke.26, 27
Key modifiable risk factors that appear to affect risk differentially by sex include diabetes and hypertension. Diabetes is more strongly associated with incident ischemic stroke in women compared with men, with a more pronounced difference for type 1 diabetes.23, 28, 29 Ischemic stroke risk also begins rising at lower fasting blood glucose levels for women than men, even after adjustment for treatment with oral medications or insulin.30 More research is needed to understand the biologic mechanisms underlying these sex differences. For now, early and consistent screening for diabetes mellitus is warranted.31
For hypertension, although some previous data indicate no consistent sex difference in the association between increasing systolic blood pressure and stroke risk,14 recently published data from U.S. and international cohorts demonstrate a stronger association between hypertension and risk of incident total and ischemic stroke in women compared with men, adjusted for use of anti-hypertensives.28, 32, 33 Longitudinal data in the U.S. have revealed a faster rise in blood pressure for women than men starting in the third decade, supporting a sexual dimorphism in the contribution of blood pressure to cardiovascular disease and stroke later in life.34 While data from recent large-scale observational studies indicates that stroke risk becomes significant at a lower threshold of systolic blood pressure for women than men (120 vs. 150 mm Hg, respectively), more prospective and ideally clinical trial data will be needed before sex-specific targets are integrated into guidelines.35
Elevated body mass index (BMI), clinical obesity (BMI ≥30 kg/m2), and higher waist-to-hip ratio are associated with higher risk of total and ischemic stroke in both sexes, with a stronger association in women than men.28 The relationship between obesity and intracerebral hemorrhage (ICH) is more complex, with some evidence for a protective effect in women, but studies also indicate this varies by sex, with data from the UK biobank demonstrating an inverse association between BMI and risk of ICH in women but a positive association in men.28, 36, 37
Atrial fibrillation is a modifiable risk factor for both women and men with data demonstrating a higher risk of stroke and all-cause mortality in women with atrial fibrillation compared with men, and female sex has been incorporated as a risk factor in the CHADS2VASc2 score for decision making about anticoagulation.23 Women older than 65 with atrial fibrillation are at an especially high risk for subsequent stroke events.38, 39 Sex and gender disparities in use of oral anticoagulants for stroke prevention have been previously reported despite data demonstrating similar effectiveness as well as rates of intracranial hemorrhage (ICH) between women and men. Additional surveillance is needed now that direct oral anticoagulants (DOAC) are being used more frequently.39 Of note, although women were underrepresented in the four major trials comparing DOAC to warfarin (comprising 35 to 40% of participants) and formal sex comparisons were not presented, safety and efficacy for DOACs appear similar in women and men39 with clinically negligible differences between DOACS.40 Consistent with prior disparities in treatment of atrial fibrillation, newer data demonstrating lower rates of cardiac monitoring for the detection of arrhythmias following ischemic stroke in women are concerning and should be investigated further.23, 39 Ongoing research on the optimal time point for restarting anticoagulation in patients with atrial fibrillation and ICH should include sex-disaggregated data as well.
Stroke risk associated with lipid profiles is nuanced and differs between stroke subtypes but does not differ consistently by sex. Recent prospective data show an increased risk of ischemic stroke with increasing total cholesterol, LDL, and triglycerides as well as increased risk with increasing LDL/HDL ratio.41–43 The relationship between lipid profiles and incident ICH is less well understood, with prospective data demonstrating an elevated risk of ICH risk with low triglyceride and LDL levels (<70) in women and elevated ICH risk associated with lower total and LDL cholesterol in both women and men in the UK biobank.28, 44
Lifestyle factors including physical activity also reduce risk of stroke, although most data do not support a substantial sex difference in the impact of physical activity on stroke risk. More data are needed on the optimal level of physical activity for women compared with men given known sex differences in performance of strenuous physical activity among young and middle-aged adults as well as sex differences in activity limitations and frailty among older populations.45
Female-specific risk factors
Female-specific risk factors to consider include those that are directly modifiable as well as factors like reproductive lifespan that are less directly modifiable but potentially important for risk prediction.
Use of oral contraceptive pills is associated with stroke in young women; one recent systematic review and meta-analysis demonstrated an odds ratio of 2.47 for use of any type of oral contraceptive, although when broken down by formulation, combined oral contraceptives remained significant while progesterone-only pills were not associated with increased risk.46
For menopausal hormone therapy, despite clinical trial data demonstrating increased total and ischemic stroke among women taking oral menopausal hormone therapy (either estrogen alone or estrogen combined with progestin), more recent data suggest that use of low-dose transdermal estrogen formulations may effectively treat menopausal symptoms without increasing stroke risk.47, 48 Recent guidelines recommend that menopausal hormone therapy may be considered for women less than 60 years old or within 10 years of the onset of menopause without other contraindications in the case of vasomotor menopausal symptoms or other menopausal symptoms.49, 50
Factors affecting lifetime estrogen exposure, defined as the time from menarche to menopause, are associated with elevated stroke risk; in a recent meta-analysis, a reproductive lifespan of <30 years was associated with a 75% increase in risk compared to 36 to 38 years.51 This association seems to be primarily driven by premature <40 years old) or early (40–44 years) menopause, though early or late menarche also appears to increase risk of stroke.51 These data are consistent across recent prospective studies.46, 52–54
Other reproductive health variables associated with stroke risk include parity and breast feeding. Data on parity suggests elevated stroke among women with ≥5 live births compared with 1 or 2 live-births, though in some studies, adjusting for confounding variables, particularly BMI, eliminated this association.55, 56 With regards to breast feeding, recent data from the Women’s Health Initiative demonstrated a 23% reduction in risk of incident stroke among women who reported ever breast feeding, and the reduction appeared to increase with longer duration of reported breast feeding.57
Adverse pregnancy outcomes, including preterm delivery, gestational hypertension, preeclampsia and fetal growth restriction, have been consistently associated with increased long-term risk of cardiovascular disease, including stroke, in the mother.58 Recent data from the Women’s Health Initiative showed a consistent association between history of adverse pregnancy outcomes and future atherosclerotic cardiovascular disease, including stroke.59 Some studies have showed triple the risk of future stroke (excluding peripartum stroke) in patients with a history of preeclampsia.60 Other adverse pregnancy outcomes have been associated with increased risk of future stroke, including fetal growth restriction (30% increased stroke risk),61 gestational hypertension (80% increased risk)60 and preterm delivery (65% higher risk).62 Furthermore, some studies demonstrate an association between history of hypertensive disorders of pregnancy and future vascular cognitive impairment.63, 64 Putative mechanisms of these associations including adverse pregnancy outcome-induced immunological intolerance and a heightened inflammatory state, causing long term effects on the maternal vasculature;65 alternatively, a high-risk maternal vascular phenotype may predispose to both adverse pregnancy outcomes and future cerebrovascular disease.
Understanding stroke risk factors in transgender individuals, particularly the use of gender affirming hormones, is of critical importance, but current knowledge remains limited. In a cohort study of over 4960 transgender women matched with >96,000 cisgender men and women, transgender women had an elevated stroke risk compared with ciswomen (HR 1.9 [95%CI 1.3–2.6]), with an even greater increase in risk among transwomen who initiated estrogen treatment.4 For those using hormones, risk appeared to be the greatest after 6 years of follow-up (HR 4.1 (95%CI 1.5–11.4) compared with cisgender women.4 Ischemic stroke risk for transgender men did not appear to be increased when compared to cisgender men or women.4 These data are largely supported by smaller studies and reviews, but there is a clear need for high quality, prospective studies with appropriate control subjects to elucidate the risks of long-term sex steroid use to better counsel trans- individuals of all ages and stages of transition.5, 6
As we enter an era of increasingly personalized medicine, applying sex-specific knowledge of stroke risk factors is the first step toward more effective, more targeted stroke prevention strategies.
Acute cerebrovascular complications in pregnancy and postpartum
Incidence of maternal stroke.
Stroke, including ischemic stroke, subarachnoid hemorrhage, and ICH, is among the most feared complications of pregnancy, accounting for 8.2% of US maternal deaths yearly.66 While stroke is rare, occurring in approximately 30 per 100,000 deliveries,67 the incidence has increased over the last 30 years, particularly in the postpartum period.42, 68–70 Some groups are at far higher risk; in patients with preeclampsia, stroke may occur in up to 1 in 500 pregnancies.71
Risk factors.
Hypertensive disorders of pregnancy, including chronic or gestational hypertension, preeclampsia/eclampsia, or the HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, are among the most important maternal stroke risk factors.72–74 Other notable risk factors include migraine,75 thrombophilias,76 congenital or acquired heart disease,77 and infections.78 Migraine confers increased risk of hypertensive disorders of pregnancy and maternal stroke, with some studies showing as high as 15-fold higher stroke risk in pregnant patients with migraine.69, 75 A recent retrospective study of 3 million births in California found a 6.8-fold higher risk of stroke in pregnant patients with migraine; a mediation analysis found approximately one quarter of the increased risk was mediated by higher risk of hypertensive disorders of pregnancy.79 As with other adverse pregnancy outcomes, Black patients experience disproportionately high pregnancy-related stroke rates, independent of socioeconomic status and vascular risk factors.70, 80–82
Timing.
While stroke can occur at any time during pregnancy or puerperium, the postpartum period confers the highest per-day risk of stroke, with the highest risk in the immediate peripartum and up to two weeks postpartum.67, 69, 83, 84
Stroke mechanisms and pathophysiology.
Maternal stroke due to atherosclerotic disease is extremely rare. In contrast to the general population, where 87% of strokes are ischemic,9 up to half of maternal strokes are due to subarachnoid or ICH.42 Common stroke mechanisms seen in pregnant and postpartum patients include cardioembolism, carotid or vertebral artery dissection, infarction or hemorrhage due to cerebral venous sinus or cortical vein thrombosis, reversible cerebral vasoconstriction syndrome causing vasospasm-related ischemia or convexity subarachnoid hemorrhage, hypertensive ICH, and rupture of vascular anomalies such as intracranial aneurysms, arteriovenous malformations, or moyamoya vasculopathy.67, 84–88 The unique and complex physiology of pregnancy and its complications, particularly hypertensive disorders of pregnancy, contributes to an increased risk of all of these acute cerebrovascular disorders (Figure 3).
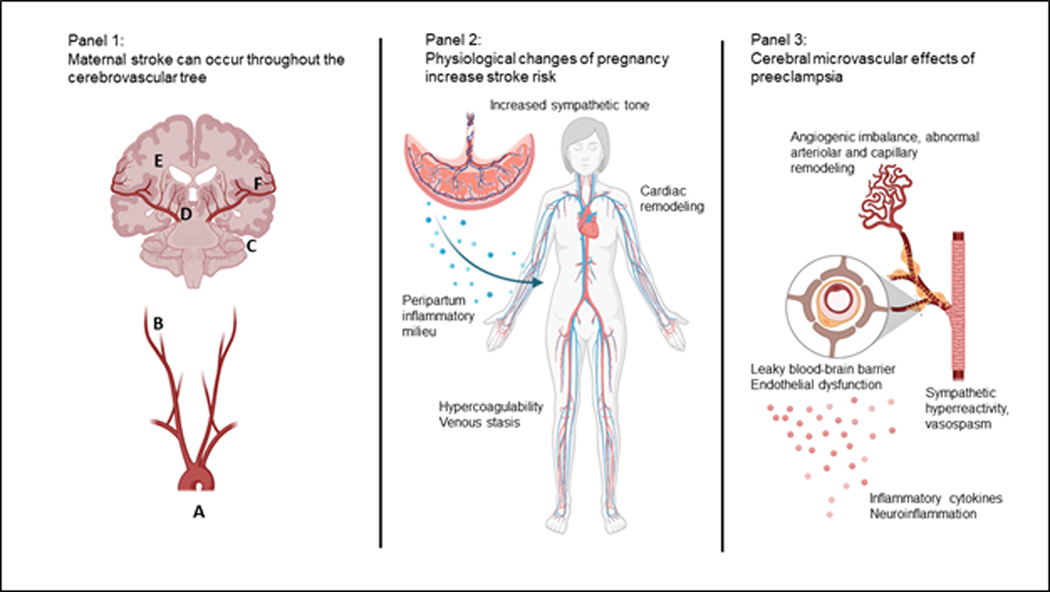
Figure 3:
Panel 1: Stroke in pregnancy or puerperium can occur due to pathological mechanisms throughout the cerebrovascular tree, including cardioembolism (A), cervical artery dissection (B), venous sinus thrombosis (C), rupture of vascular malformations (D), hypertensive intraparenchymal hemorrhage (E), or reversible cerebral vasoconstriction syndrome causing intracranial vasospasm and/or convexity subarachnoid hemorrhage (F).
Panel 2: Physiological changes of pregnancy affecting the cardiovascular, hematological, and immune systems may all increase stroke risk. Pathological complications of pregnancy such as peripartum cardiomyopathy or preeclampsia exacerbate this risk.
Panel 3: At the microvascular level, pathophysiological changes associated with preeclampsia may contribute to increased stroke risk, including neuroinflammation, leakiness of the blood-brain barrier, abnormal cerebral autoregulation, and sympathetic hyperreactivity.
Created with BioRender.com
The pregnancy “stress test.”
Pregnancy puts enormous strain on the maternal cardiovascular system, with cardiac output increasing by up to 50% by the end of gestation in response to fetal demand.89 In addition, pregnancy-related hormonal changes lead to systemic vasodilation, increased vascular compliance and venous stasis.90 Hypercoagulability is also part of the adaptive physiology of pregnancy, including increased levels of von Willebrand factor, factor V, factor VIII, and fibrinogen, acquired resistance to activated protein C, and placentally-produced anti-fibrinolytics (plasminogen activator inhibitors 1 and 2). Thus, even normal pregnancy is characterized by Virchow’s triad of venous stasis, hypercoagulability, and delivery-associated vascular damage, increasing the risk of thromboembolism.91 This risk is magnified in patients with preexisting cardiac conditions or comorbidities that put them at risk for arrhythmias or cardiomyopathy.
Immunomodulation and inflammation.
Inflammatory cytokines play crucial roles in implantation and parturition.92 In the first trimester, a highly inflammatory milieu predominates, with increased levels of pro-inflammatory T helper (Th)-1 activity, interleukin-6 (IL-6), C-reactive protein (CRP) and tumor necrosis factor alpha (TNFα), all of which affect endothelial function and may increase thrombotic risk.93, 94 The second trimester is characterized by immune quiescence, but near delivery, upregulation of NF-κB results in a robust inflammatory cytokine response and induction of labor.95 Dysregulation of this normal immunomodulatory response, with resulting inflammation-induced endothelial dysfunction, has been implicated in the pathophysiology of preterm birth96 and preeclampsia,97 and may be a driver of maternal stroke risk.
Hypertensive disorders of pregnancy and maternal stroke.
Preeclampsia and related hypertensive disorders of pregnancy affect nearly 1 in 10 US pregnancies.98 While the complex pathophysiology of preeclampsia is beyond the scope of this review, placental ischemia plays a critical role, leading to widespread maternal endothelial dysfunction as well as a procoagulant, pro-inflammatory, anti-angiogenic, and hyperreactive sympathetic state.99, 100 In addition to being highly associated with peripartum cardiomyopathy,101 preeclampsia has direct effects on the cerebral vasculature, including increased blood-brain barrier permeability, abnormal arteriolar remodeling, and sympathetic vasomotor activation.102–106 Thus, the preeclamptic milieu may predispose to stroke via both indirect (hypercoagulability, inflammation, angiogenic imbalance, and endothelial dysfunction) and direct mechanisms (peripartum cardiomyopathy-induced cardioembolism, venous sinus thrombosis, arterial dissection, reversible cerebral vasoconstriction syndrome, and hypertensive ICH). Interestingly, the use of low dose aspirin during pregnancy reduces the incidence of preeclampsia in high-risk patients by more than 50%,107 and is recommended by the US Preventive Services Task Force after 12 weeks gestation in patients at high risk of preeclampsia.108 Exactly how aspirin reduces preeclampsia risk is unknown, with multiple mechanisms proposed.109–111 In addition, it is not known whether people who have experienced preeclampsia should continue taking aspirin after pregnancy as primary prevention for long-term cardiovascular and cerebrovascular disease; however, some observational evidence has suggested a possible benefit of aspirin use for stroke risk reduction in this population.78
Outcomes.
Maternal outcomes after stroke depend on the stroke mechanism. Pregnant patients with acute ischemic strokes treated with reperfusion therapy (thrombolysis or mechanical thrombectomy) have similarly good outcomes compared to non-pregnant patients, with few complications,73, 112, 113 prompting both the American Heart Association/American Stroke Association and Canada Heart and Stroke to recommend consideration of these therapies in pregnant patients with disabling stroke symptoms.114, 115 Outcomes after maternal hemorrhagic stroke, particularly ICH, are substantially worse, often leading to death or severe disability.74, 84, 116–120 Patients with hypertensive disorders of pregnancy also have worse outcomes after stroke, compared to those without, regardless of stroke mechanism.72, 121, 122
Gaps in knowledge and future directions.
Despite being a major cause of severe maternal morbidity and maternal mortality, maternal stroke continues to be poorly understood, especially in the postpartum period. Many epidemiological studies are based on large administrative datasets using billing data, while studies that include detailed chart review and validation are small and may suffer from selection bias. Our understanding of the intriguing relationship between migraine, preeclampsia, and maternal stroke remains limited. There is an urgent need for more translational, clinical, and rigorous population-based studies, particularly prospective or registry-based studies, to better characterize the mechanisms and predictors of maternal stroke. Robust collaboration across disciplines, specifically between researchers in cerebrovascular and cardiovascular disease, neurobiology, maternal-fetal medicine, and placentology, will be needed to address these gaps in knowledge and reduce maternal cerebrovascular morbidity and mortality.
Stroke presentation and diagnosis
Early recognition of stroke symptoms is the first step in ensuring rapid investigations to confirm the diagnosis, understand prognosis, and institute treatment. A systematic review of 15,721 patients found that about 9% of cerebrovascular events are initially not diagnosed as such in the emergency department and a large population-based cross-sectional study on this issue found that men had 25% lower odds of misdiagnosis than women.123, 124 Misdiagnosis leads to missed opportunities for treatment, rehabilitation, and secondary prevention to improve stroke outcomes and reduce disability.
Cerebrovascular diseases have a wide spectrum of disease severity, ranging from TIA to severe strokes with disabling motor, visual, or language deficits among other symptoms. Diagnosing TIA or non-disabling stroke is particularly challenging because symptoms may be vague and signs on physical examination are often subtle.125 A Japanese study using data from a nationwide clinical registry found that 16% of patients with patients with TIA report non-focal symptoms, particularly in posterior circulation events.126
A common teaching pearl in clinical practice is that women are more likely than men to report “atypical” stroke symptoms. Indeed, prior work has shown that women are more likely than men to report non-focal neurological symptoms, such as headaches, fatigue, cognitive changes, generalized malaise or weakness.127–129 However, the absolute difference in symptoms by sex was small and these studies focused on individual symptoms even though patients with TIA or stroke commonly report a constellation of symptoms. A recent study found that even though non-focal symptoms were common in patients presenting with TIA or minor stroke, over 90% of patients reported some form of focal symptoms, defined as deficits involving motor, sensory, vision, or speech (aphasia or dysarthria) function, and importantly, there were no sex differences in presenting symptoms.130 Thus, it is important to recognize that non-focal neurologic symptoms, when reported with focal ones, should not discourage clinicians from investigating further for stroke.
Another potential reason why the more recent study did not find sex differences in stroke symptoms is because the authors included all patients with suspected TIA or minor stroke referred to stroke neurologists.130 Most prior work on the topic of stroke symptoms first identified patients with TIA or stroke and subsequently compared the symptoms reported by women and men.127–129 Given that misdiagnosis is more common in women than men, this approach could lead to selection bias. Investigators in cardiology took a similar approach to examine the nature of chest pain in 1,941 patients with suspected acute coronary syndrome presenting to an emergency department, regardless of final diagnosis.131 Although it is often said that women experience “atypical” chest pain, the authors found that using their approach, “typical” chest pain was in fact more common in women than men, and it also had greater predictive value for myocardial infarction in women.
In addition, sex differences in comorbid conditions may influence the clinical diagnosis. In the Diagnosis of Uncertain Origin Benign Transient Events (DOUBT) study, a prospective cohort study of patients with transient or minor neurological events, women were more likely than men to report a history of migraine (33% women and 17% men) and recent stressors (19% women and 12% men), which are two conditions that may lower the clinical suspicion of stroke.132 Indeed, migraine aura and somatoform disorder were among the most common stroke mimic diagnosis in DOUBT. On the other hand, there are known sex-specific vascular risk factors, such as hypertensive disorders of pregnancy and premature menopause, yet these conditions are not routinely included in the history-taking for a vascular event.52, 100 Further studies are needed to understand whether there are differences in how women describe their symptoms compared to men, provider-level biases when evaluating symptoms described by women and men, and whether sex-specific cardiovascular disease guidelines may reduce gaps in care.133
Even with a detailed history physical examination, ambiguous clinical scenarios are unavoidable. In a prospective multi-national cohort study of 1,028 patients with acute low-risk transient or minor stroke symptoms, the authors found that routine use of MRI brain within 8 days of symptom onset led to a revision in diagnosis in 30% of study participants, either from stroke mimic to TIA/stroke or vice versa.132 A subsequent sex-stratified analysis of this cohort found that women were more likely than men to be initially diagnosed as stroke mimic, but among 496 patients initially diagnosed with stroke mimic, men and women were equally likely to have acute infarct on MRI brain (5% of women and 8% of men, adjusted odds ratio 0.56, 95% confidence interval [0.26, 1.21]).134 Researchers from the Netherlands similarly showed that up to a quarter of patients clinically diagnosed with non-ischemic “transient neurological attacks” have an acute infarct on MRI.135 In the context of these studies, a brain MRI was mandated by the study protocol, but in clinical practice, advanced imaging is frequently only pursued when there is a clinical suspicion of stroke. Furthermore, prior studies have shown that women, even after being diagnosed with stroke, were less likely than men to be investigated with the standard diagnostic tests and women admitted to hospital with stroke are less likely to receive defect-free care.135, 136 As shown in Figure 4, sex differences in investigations for suspected stroke can perpetuate the higher risk of misdiagnosis in women.
Figure 4. Case study: 54-year-old woman with a history of migraine with aura presents with 15 minutes of right arm heaviness and hand clumsiness.
This occurred during a stressful work meeting. She needed to concentrate to speak at the meeting, but she does not think her colleagues noticed anything. She experienced a headache afterwards. She presents to medical attention.
In summary, any sex differences in stroke symptoms, if present, are likely to be small and we should move away from the male-centric narrative that women overall are more likely to report “atypical” symptoms of cardiovascular disease compared to men. Instead, the focus should be on improving diagnosis through establishing standardized investigations for suspected acute cerebrovascular events, implementing strategies to reduce sex differences in investigations and care, and routinely inquiring about sex-specific risk factors for stroke.
Acute treatment of stroke
Ischemic stroke
Intravenous thrombolysis with recombinant tissue plasminogen activator (IV rtPA) and endovascular thrombectomy are evidence-based treatments for acute ischemic stroke that improve outcomes in carefully selected individuals. However, there continues to be controversy as to whether sex differences exist in access, efficacy, and safety of these treatments.
A meta-analysis of 24 studies, published between 2008 and 2018, reported on sex-specific usage rates of IV rtPA use in ischemic stroke.137 These studies were a mix of hospital-based, registry-based and administrative data studies. While there was some heterogeneity between studies, the analysis found that women had a 13% lower odds of receiving IV rtPA treatment compared to men (summary unadjusted OR 0.87 [95% CI, 0.82–0.93]). These sex differences in IV rtPA use in ischemic stroke differed across regions and were observed in European (summary unadjusted OR was 0.82 [95% CI, 0.78–0.85]) and United States-based (0.85 [95% CI, 0.75–0.96]) studies but not in studies from Asia (0.98 [95% CI, 0.94–1.03]) and Germany.137,138 Thus, further research into the causes of lower rtPA treatment in women, and effective strategies to improve equity, is needed. Given the high incidence of ischemic stroke in women, treatment differences in IV rtPA use are likely to translate into a large number of untreated women. In addition, because women have worse stroke outcomes than men,139 but receive comparable treatment benefits,137 this missed treatment opportunity could have significant consequences for disability status in women.
Secondary analysis of large, international, randomized controlled trials comparing IV rtPA to placebo in ischemic stroke have not shown any sex differences in clinical outcome.140 Being post-hoc analysis, these studies were not specifically designed to test sex differences in efficacy; however, the findings are reassuring for both women and men. Symptomatic ICH is one of the most serious adverse effects of IV rtPA. In the Safe Implementation of Treatments in Stroke-International Stroke Thrombolysis Register (SITS-ISTR),141 a total of 45,079 patients treated with IV rtPA were recorded from 2002 to 2011. In the multivariate analysis, after adjustment for confounding variables such as age, stroke severity and time to treatment), men had 19% higher odds of symptomatic ICH (OR of 1.25 [95% CI 1.04–1.51] with women as referent).
Several studies have suggested higher rates of endovascular thrombectomy in women with ischemic stroke than men. Sex differences in ischemic stroke treatment were explored in a nationwide administrative database in Germany where over 1.11 million patients were hospitalized for first or recurrent ischemic stroke from 2013 to 2017. During the 5-year study period, women had an overall 26% (95% CI, 22%–30%) higher chance of receiving endovascular thrombectomy. This higher endovascular thrombectomy utilization in women compared to men was significantly higher across all age groups.142 Similar findings were seen in a retrospective, serial, cross-sectional study of over 4 million patients in the United States, where women were 20% more likely to be treated with endovascular thrombectomy compared with men in the period 2016 to 2017.143 The reasons for this sex disparity in endovascular thrombectomy use is not known but women with atrial fibrillation are less often treated for stroke prevention which may lead to the great number of large vessel cardioembolic strokes in women and higher need for endovascular thrombectomy.
Whether there are sex differences in endovascular thrombectomy efficacy is controversial. In the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN trial)144 of 500 patients (42% women), the treatment effect (modified Rankin score at 90 days) was null in women (OR 0.99 [95% CI 0.60–1.66]) compared to protective in men (OR 2.39 [95% CI 1.55–3.68]). Women in the intervention group were also more likely to die, as well as have serious adverse events, than men in MR CLEAN. In a later meta-analysis of individual patient data from five randomized trials of endovascular thrombectomy after large-vessel ischemic stroke (including MR CLEAN), no sex differences were found in the benefit of the intervention on 90-day functional outcomes, nor on serious adverse events.145 Additionally, in the combined cohorts SWIFT (Solitaire Flow Restoration With the Intention for Thrombectomy), STAR (Solitaire Flow Restoration Thrombectomy for Acute Revascularization), and SWIFT PRIME (Solitaire Flow Restoration With the Intention for Thrombectomy as Primary Endovascular Treatment) where 389 eligible patients were treated with endovascular stroke thrombectomy (55% women), women were found to have had more disability-adjusted life years after endovascular thrombectomy compared to men, 10.6 versus 8.5 years (P<0.001). These findings diminished with increasing age.146
In summary, women are more likely to receive endovascular thrombectomy, although it is not clear whether this is due to differences in stroke types and presentations. There are no sex differences in efficacy and safety; however, women may gain more optimal life years after this procedure. More research on optimizing the use of revascularization treatment in men and women is required.
The most recent guidelines on the management of carotid artery stenosis recommend carotid endarterectomy in symptomatic patients with ≥ 50% stenosis and in asymptomatic patients ≥60% stenosis.147, 148 Carotid artery stenting can be considered for symptomatic patients with stenosis of ≥ 50% at high risk for carotid endarterectomy for anatomic or medical reasons147 or for patients < 70 years old with symptomatic ≥ 50% carotid stenosis.148 These recommendations warrant a review on the impact of sex on these surgical and endovascular treatments of carotid stenosis. Not only are these guidelines based on randomized trials performed over a decade ago, before best medical therapy or technological advances were yet to be refined, but the trials have also underrepresented women (<35% women enrolled)149, 150 and reported higher periprocedural risk of for any stroke, myocardial infarction, or death in women undergoing carotid artery stenting compared to carotid endarterectomy. In a pooled analysis of symptomatic patients in large, randomized trials, sex differences were examined in the carotid artery stenting -to- carotid endarterectomy risk for any stroke or death during the 120-day periprocedural period and stroke.151 The authors found that there was significant heterogeneity in the 4 completed revascularization trials and pooling of the studies was not appropriate. No clear conclusions can be drawn regarding carotid artery stenting versus carotid endarterectomy. These findings further strengthen the need to adequately power trials to allow reliable sex-disaggregated analysis and conclusions about the treatment for carotid artery stenosis in women.
Intracerebral hemorrhage
Unlike ischemic stroke, there are no medical treatments definitively proven in randomized clinical trials to improve outcomes for ICH.152 The primary goals of acute management are to stabilize the patient to ensure they survive the initial insult by managing blood pressure, reversing anticoagulant-associated hemorrhage and neurosurgical intervention. The Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage trials 1 and 2, randomized patients to intensive (target <140mmHg within 1 hour) or guideline-recommended blood pressure lowering treatment. The interventions showed no differences in the primary outcome of death or major disability. In a post-hoc analysis of the 3233 patients in the pooled studies,153 the 37% women were significantly older, had higher stroke severity and smaller hematoma volumes compared to men. There was no effect of sex on the combined endpoint of death or major disability, hematoma growth or effect of randomized blood pressure lowering treatment.
Nearly twenty percent of all ICH cases are associated with anticoagulant use.152 Discontinuing the offending agent and reversing anticoagulation is key to improving outcomes. Idarucizumab154 is a fragment of humanized monoclonal antibody that binds dabigatran and rapidly reverses its anticoagulant activity. At this time, only one trial on the reversal of direct oral anticoagulation has included anticoagulant-associated ICH. This multicenter, prospective, single cohort study enrolled 503 patients in 39 countries with 53 (17.6%) having ICH. The median maximum percentage reversal within 4 hours after the administration of idarucizumab was 100%. The reversal occurred independent of age, sex, renal function, and dabigatran concentration at baseline; however, sex-specific outcomes for patients with ICH were not reported.
Sex differences in ICH treatment effects have been less often reported compared to ischemic stroke—particularly for access to and surgical treatment outcomes after ICH. Disaggregating results by sex, especially in interventional trials, is vital for generalizability of results to both sexes.
Stroke Outcomes
As the US population ages, it is estimated that by 2050, there will be an excess of 68,000 stroke deaths in women as compared with men, with the greatest excess seen in women 85 years of age or older.155 Studies have reported variable results for stroke case fatality by sex, with many providing little evidence of a difference, some showing higher case fatality, and others reporting lower case fatality for women.9, 155–161 Overall, these studies suggest that baseline differences in age, stroke characteristics, and cerebrovascular risk factors account for much of the observed sex differences in case fatality. Age-adjusted mortality rates are commonly reported, but because sex differences are strongly modified by age, they can mask the complex relation of sex differences across age groups and can hide the burden of stroke mortality for elderly women.
As shown in Figure 1, using the most recent national data on stroke death rates per 100,000 population from the Centers for Disease Control and Prevention (CDC), Lichtman and colleagues graphed the total number of cerebrovascular deaths, the percentage of total deaths due to cerebrovascular disease, and the ranking of cause of death for cerebrovascular diseases for women and men. The total number of deaths due to cerebrovascular diseases for women exceeded those for men starting from the age of 75 years and remained substantially higher for women across all older age groups. Cerebrovascular deaths comprised a greater proportion of total deaths for women as compared with men for all age groups and was ranked higher as a leading cause of death for women than men until the age of 70 years.
Studies have generally reported poorer functional recovery and lower quality of life after stroke for women compared to men.21, 155, 160–169 Moreover, women fared worse in the measured dimensions of pain/discomfort, anxiety/depression, fatigue, and mobility than men.15, 21, 155, 160, 161, 169, 170 Individual-level factors such as advanced age, poorer pre-stroke function, comorbidities, lower social support, and an increased likelihood of being widowed have been hypothesized as contributors to sex and gender differences in functional outcomes and quality of life, yet adjustment for these factors does not fully explain the observed differences in outcomes for women and men.155, 160, 169 There remains a need to explore the role of access to clinical care and rehabilitation services as well as social, mental health, and socioeconomic factors that may be contributing to observed sex and gender differences in these outcomes. Few studies have addressed sex differences in cognitive impairment after stroke. A focused review included 5 studies that found mixed results, with some showing a higher risk among men and others showing a higher risk among women.169 None of the studies were population-based. Similarly, there is a paucity of population-based studies that report sex differences in recurrent stroke events. A study of 8900 adult ischemic stroke patients in a rural Pennsylvania population did not find a sex difference in 5-year survival after controlling for age and comorbid conditions.158
Depression is common following a stroke, affecting up to one-third of stroke survivors.171 Across the adult lifespan, depression is nearly twice as common in women than men.172 Studies have found a higher prevalence of poststroke depression in women than men.21, 155, 169, 170, 173–175 A systematic review of the prevalence of poststroke depression in 45 publications between 1982 and 2006 found the prevalence of PSD was 78% higher among women as compared with men.175 A more recent review of sex differences identified 10 papers that reported poststroke depression.169 In the 8 papers that reported unadjusted associations between sex and PSD, women had a higher likelihood of poststroke depression (OR range 1.27–3.15 and HR 3.52) or a higher mean number of poststroke depression symptoms. Women remained significantly more likely to have higher prevalence, incidence, or increased number of symptoms of depression than men after multivariable adjustment for factors such as age, stroke severity, and activity limitation. The majority of studies to date have not been population-based or specifically designed to examine sex differences in poststroke depression. A study of 786 patients from the population-based Brain Attack Surveillance in Corpus Christi Project assessed sex differences in poststroke depression at 90 days after first-ever stroke using the 8-item Patient Health Questionnaire.174 The study did not find a statistically significant sex difference in poststroke depression after accounting for attrition. However, the study reported significant effect modification of the association between sex and poststroke depression such that women on medication for depression at the time of stroke were significantly protected against poststroke depression, whereas women with a history of depression who were not on medication showed an elevated risk compared with men. The results highlight that depression among stroke survivors is heterogeneous and multifactorial with sex differences throughout the disease course before and after a stroke. Because untreated depression after stroke can lead to a poorer prognosis, reduced quality of life, and increased mortality, enhanced screening and the development of interventions to prevent poststroke depression are needed, particularly for women and those with pre-stroke depression not undergoing treatment for depression at the time of the stroke.169, 171, 174, 175
In summary, research shows that women experience worse outcomes after stroke than men regarding mortality, quality of life, poststroke depression, and activity limitations. Differences are partly explained by women’s poorer health at the time of the index stroke event, advanced age, and greater stroke severity. Data on how aspects of access and quality of clinical care, pre-stroke health, mental health, social isolation, loneliness, social support, or socioeconomic status may be contributing to worse outcomes for women are limited.169, 176 Rigorous population-based studies are needed to explore these potential factors for sex differences in outcomes, including designs that can study the bidirectional nature between sex and these factors.
Conclusion: Gaps in research
Despite substantial progress in examining sex differences in stroke as well as specific factors influencing risk and outcomes in women, significant research gaps remain (Table). Overall, sex and gender have often been combined in stroke research; thus, delineating the impact of biologic sex from the social impact of gender-related factors needs to continue. With regard to risk factors, the effects of gender-affirming hormone therapy on risk of stroke need to better quantified, particularly for transgender women. While a number of female-specific risk factors have been noted, no currently available stroke prediction models incorporate female-specific risk factors. Despite the higher long-term risk of stroke among individuals with a history of adverse pregnancy outcomes, including hypertensive disorders of pregnancy, preterm delivery and fetal growth retardation, we lack specific screening and treatment guidelines to reduce this risk. To reduce misdiagnosis in women, research is needed to understand whether women describe their symptoms differently, whether provider-level biases influence the interpretation of symptoms described by women and men, and whether strategies to reduce sex differences in investigations and care may reduce gaps in diagnosis. With regard to treatment, although women comprise more than half of strokes, the inclusion of women in clinical trials of stroke treatments has been lower.177, 178 Methods to achieve better representation of women in stroke clinical trials should include carefully scrutinizing inclusion criteria and their impact on gender balance in trials, avoiding age-based exclusion criteria, and increasing the number of women who lead stroke clinical trials.179 Further, it is essential that clinical trials and registries present sex disaggregated data and stratified results. Given older ages and higher disability prior to stroke and worse outcomes after stroke for women, the role of access to clinical care and rehabilitation services as well as social, mental health, and socioeconomic factors that may contribute to these observed sex differences are needed. Better knowledge of the impact of sex and gender on stroke will improve outcomes for all individuals.
Table.
Research gaps to eliminate disparities in stroke by sex and gender.
| Research area | Research gaps |
|---|---|
| Stroke epidemiology | • Delineation of the separate effects of biologic sex and sociocultural gender |
| Stroke risk factors | • Delineating the separate effects of biologic sex and sociocultural gender • Prediction models with female-specific risk factors • CVD prevention for women with a history of adverse pregnancy outcomes: screening and treatment guidelines, as well as understanding biologic mechanisms |
| Stroke diagnosis | • Research on strategies to reduce misdiagnosis in women, as well as in investigation and care |
| Stroke treatment | • Inclusion of women in clinical trials consistent with their stroke incidence • Increasing the number of women who lead clinical trials • Presentation of sex disaggregated data and stratified results from all clinical trials • Investigation of sex differences in outcomes with endovascular therapy, carotid artery stenting and carotid endarterectomy |
| Stroke outcomes | • Improved understanding of the factors contributing to worse outcomes after stroke in women • Interventions to improve outcomes including disability and post-stroke depression |
Acknowledgments
Funding
Dr. Rexrode is supported by the National Institutes of Health, National Heart Lung Blood Institute (R01 HL34594 and HL153725).
Dr Madsen is supported by National Institutes of Health, National Heart Lung Blood Institute (K23 HL140081).
Dr. Miller is supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke (K23NS107645, R01NS122815-01), the National Institutes of Health National Institute on Aging (R21AG069111) and the Gerstner Family Foundation (Gerstner Scholars Program).
Dr. Carcel acknowledges the support of the National Heart Foundation of Australia (Postdoctoral fellowship 102741).
Dr. Lichtman is supported by the National Institutes of Health National Institute on Aging (R01 AG056628).
Nonstandard Abbreviations and Acronyms:
- ICH
intracerebral hemorrhage
- BMI
body mass index
- IV rtPA
intravenous thrombolysis with recombinant tissue plasminogen activator
- TIA
transient ischemic attack
Footnotes
Disclosures: Dr. Miller received personal compensation for expert testimony regarding maternal stroke from the following firms: Finch McCranie, LLP (2020); Argionis & Associates, LLC (2019–2021); Heyl, Royster, Voelker & Allen (2020); Ketcham, Eide, Telan & Meltz (2021). Dr. Miller received personal compensation from Elsevier, Inc for editorial work on Handbook of Clinical Neurology, Vols 171 and 172 (Neurology of Pregnancy); 2020.
References
- 1.Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, De Vries GJ, Epperson CN, Govindan R, Klein SL, Lonardo A, Maki PM, McCullough LD, Regitz-Zagrosek V, Regensteiner JG, Rubin JB, Sandberg K, Suzuki A. Sex and Gender: Modifiers of Health, Disease, and Medicine. Lancet. 2020;396(10250):565–82. Epub 2020/08/24. doi: 10.1016/s0140-6736(20)31561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norris CM, Johnson NL, Hardwicke-Brown E, McEwan M, Pelletier R, Pilote L. The Contribution of Gender to Apparent Sex Differences in Health Status among Patients with Coronary Artery Disease. J Womens Health (Larchmt). 2017;26(1):50–7. Epub 2016/07/12. doi: 10.1089/jwh.2016.5744. [DOI] [PubMed] [Google Scholar]
- 3.Pelletier R, Khan NA, Cox J, Daskalopoulou SS, Eisenberg MJ, Bacon SL, Lavoie KL, Daskupta K, Rabi D, Humphries KH, Norris CM, Thanassoulis G, Behlouli H, Pilote L. Sex Versus Gender-Related Characteristics: Which Predicts Outcome after Acute Coronary Syndrome in the Young? J Am Coll Cardiol. 2016;67(2):127–35. Epub 2016/01/23. doi: 10.1016/j.jacc.2015.10.067. [DOI] [PubMed] [Google Scholar]
- 4.Getahun D, Nash R, Flanders WD, Baird TC, Becerra-Culqui TA, Cromwell L, Hunkeler E, Lash TL, Millman A, Quinn VP, Robinson B, Roblin D, Silverberg MJ, Safer J, Slovis J, Tangpricha V, Goodman M. Cross-Sex Hormones and Acute Cardiovascular Events in Transgender Persons: A Cohort Study. Ann Intern Med. 2018;169(4):205–13. Epub 2018/07/11. doi: 10.7326/m17-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maraka S, Singh Ospina N, Rodriguez-Gutierrez R, Davidge-Pitts CJ, Nippoldt TB, Prokop LJ, Murad MH. Sex Steroids and Cardiovascular Outcomes in Transgender Individuals: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2017;102(11):3914–23. Epub 2017/09/26. doi: 10.1210/jc.2017-01643. [DOI] [PubMed] [Google Scholar]
- 6.Connelly PJ, Marie Freel E, Perry C, Ewan J, Touyz RM, Currie G, Delles C. Gender-Affirming Hormone Therapy, Vascular Health and Cardiovascular Disease in Transgender Adults. Hypertension. 2019;74(6):1266–74. Epub 2019/10/28. doi: 10.1161/hypertensionaha.119.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vyas MV, Silver FL, Austin PC, Yu AYX, Pequeno P, Fang J, Laupacis A, Kapral MK. Stroke Incidence by Sex across the Lifespan. Stroke. 2021;52(2):447–51. Epub 2021/01/26. doi: 10.1161/strokeaha.120.032898. [DOI] [PubMed] [Google Scholar]
- 8.Heron M. Deaths: Leading Causes for 2019. Natl Vital Stat Rep. 2021;70(9):1–114. Epub 2021/09/15. [PubMed] [Google Scholar]
- 9.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation. 2021;143(8):e254–e743. Epub 2021/01/28. doi: 10.1161/cir.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 10.Madsen TE, Khoury JC, Leppert M, Alwell K, Moomaw CJ, Sucharew H, Woo D, Ferioli S, Martini S, Adeoye O, Khatri P, Flaherty M, De Los Rios La Rosa F, Mackey J, Mistry E, Demel SL, Coleman E, Jasne A, Slavin SJ, Walsh K, Star M, Broderick JP, Kissela BM, Kleindorfer DO. Temporal Trends in Stroke Incidence over Time by Sex and Age in the Gcnkss. Stroke. 2020;51(4):1070–6. Epub 2020/02/23. doi: 10.1161/strokeaha.120.028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rehman S, Sahle BW, Chandra RV, Dwyer M, Thrift AG, Callisaya M, Breslin M, Phan HT, Otahal P, Gall S. Sex Differences in Risk Factors for Aneurysmal Subarachnoid Haemorrhage: Systematic Review and Meta-Analysis. J Neurol Sci. 2019;406:116446. Epub 2019/09/16. doi: 10.1016/j.jns.2019.116446. [DOI] [PubMed] [Google Scholar]
- 12.Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, Abajobir AA, Abate KH, Abd-Allah F, Abejie AN, Abyu GY, Ademi Z, Agarwal G, Ahmed MB, Akinyemi RO, Al-Raddadi R, Aminde LN, Amlie-Lefond C, Ansari H, Asayesh H, Asgedom SW, Atey TM, Ayele HT, Banach M, Banerjee A, Barac A, Barker-Collo SL, Bärnighausen T, Barregard L, Basu S, Bedi N, Behzadifar M, Béjot Y, Bennett DA, Bensenor IM, Berhe DF, Boneya DJ, Brainin M, Campos-Nonato IR, Caso V, Castañeda-Orjuela CA, Rivas JC, Catalá-López F, Christensen H, Criqui MH, Damasceno A, Dandona L, Dandona R, Davletov K, de Courten B, deVeber G, Dokova K, Edessa D, Endres M, Faraon EJA, Farvid MS, Fischer F, Foreman K, Forouzanfar MH, Gall SL, Gebrehiwot TT, Geleijnse JM, Gillum RF, Giroud M, Goulart AC, Gupta R, Gupta R, Hachinski V, Hamadeh RR, Hankey GJ, Hareri HA, Havmoeller R, Hay SI, Hegazy MI, Hibstu DT, James SL, Jeemon P, John D, Jonas JB, Jóźwiak J, Kalani R, Kandel A, Kasaeian A, Kengne AP, Khader YS, Khan AR, Khang YH, Khubchandani J, Kim D, Kim YJ, Kivimaki M, Kokubo Y, Kolte D, Kopec JA, Kosen S, Kravchenko M, Krishnamurthi R, Kumar GA, Lafranconi A, Lavados PM, Legesse Y, Li Y, Liang X, Lo WD, Lorkowski S, Lotufo PA, Loy CT, Mackay MT, Abd El Razek HM, Mahdavi M, Majeed A, Malekzadeh R, Malta DC, Mamun AA, Mantovani LG, Martins SCO, Mate KK, Mazidi M, Mehata S, Meier T, Melaku YA, Mendoza W, Mensah GA, Meretoja A, Mezgebe HB, Miazgowski T, Miller TR, Ibrahim NM, Mohammed S, Mokdad AH, Moosazadeh M, Moran AE, Musa KI, Negoi RI, Nguyen M, Nguyen QL, Nguyen TH, Tran TT, Nguyen TT, Anggraini Ningrum DN, Norrving B, Noubiap JJ, O’Donnell MJ, Olagunju AT, Onuma OK, Owolabi MO, Parsaeian M, Patton GC, Piradov M, Pletcher MA, Pourmalek F, Prakash V, Qorbani M, Rahman M, Rahman MA, Rai RK, Ranta A, Rawaf D, Rawaf S, Renzaho AM, Robinson SR, Sahathevan R, Sahebkar A, Salomon JA, Santalucia P, Santos IS, Sartorius B, Schutte AE, Sepanlou SG, Shafieesabet A, Shaikh MA, Shamsizadeh M, Sheth KN, Sisay M, Shin MJ, Shiue I, Silva DAS, Sobngwi E, Soljak M, Sorensen RJD, Sposato LA, Stranges S, Suliankatchi RA, Tabarés-Seisdedos R, Tanne D, Nguyen CT, Thakur JS, Thrift AG, Tirschwell DL, Topor-Madry R, Tran BX, Nguyen LT, Truelsen T, Tsilimparis N, Tyrovolas S, Ukwaja KN, Uthman OA, Varakin Y, Vasankari T, Venketasubramanian N, Vlassov VV, Wang W, Werdecker A, Wolfe CDA, Xu G, Yano Y, Yonemoto N, Yu C, Zaidi Z, El Sayed Zaki M, Zhou M, Ziaeian B, Zipkin B, Vos T, Naghavi M, Murray CJL, Roth GA. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N Engl J Med. 2018;379(25):2429–37. Epub 2018/12/24. doi: 10.1056/NEJMoa1804492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The Lifetime Risk of Stroke: Estimates from the Framingham Study. Stroke. 2006;37(2):345–50. [DOI] [PubMed] [Google Scholar]
- 14.Leening MJ, Ferket BS, Steyerberg EW, Kavousi M, Deckers JW, Nieboer D, Heeringa J, Portegies ML, Hofman A, Ikram MA, Hunink MG, Franco OH, Stricker BH, Witteman JC, Roos-Hesselink JW. Sex Differences in Lifetime Risk and First Manifestation of Cardiovascular Disease: Prospective Population Based Cohort Study. BMJ. 2014;349:g5992. Epub 2014/11/19. doi: 10.1136/bmj.g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender Differences in Stroke Incidence and Poststroke Disability in the Framingham Heart Study. Stroke. 2009;40(4):1032–7. Epub 2009/02/13. doi: 10.1161/strokeaha.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gokhale S, Caplan LR, James ML. Sex Differences in Incidence, Pathophysiology, and Outcome of Primary Intracerebral Hemorrhage. Stroke. 2015;46(3):886–92. Epub 2015/02/07. doi: 10.1161/strokeaha.114.007682. [DOI] [PubMed] [Google Scholar]
- 17.Gardener H, Sacco RL, Rundek T, Battistella V, Cheung YK, Elkind MSV. Race and Ethnic Disparities in Stroke Incidence in the Northern Manhattan Study. Stroke. 2020;51(4):1064–9. Epub 2020/02/23. doi: 10.1161/strokeaha.119.028806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiménez MC, Manson JE, Cook NR, Kawachi I, Wassertheil-Smoller S, Haring B, Nassir R, Rhee JJ, Sealy-Jefferson S, Rexrode KM. Racial Variation in Stroke Risk among Women by Stroke Risk Factors. Stroke. 2019;50(4):797–804. Epub 2019/03/15. doi: 10.1161/strokeaha.117.017759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koton S, Sang Y, Schneider ALC, Rosamond WD, Gottesman RF, Coresh J. Trends in Stroke Incidence Rates in Older Us Adults: An Update from the Atherosclerosis Risk in Communities (Aric) Cohort Study. JAMA Neurol. 2020;77(1):109–13. Epub 2019/10/01. doi: 10.1001/jamaneurol.2019.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, Saver JL, Trogdon JG. Forecasting the Future of Stroke in the United States: A Policy Statement from the American Heart Association and American Stroke Association. Stroke. 2013;44(8):2361–75. Epub 2013/05/24. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 21.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Piña IL, Reeves MJ, Rexrode KM, Saposnik G, Singh V, Towfighi A, Vaccarino V, Walters MR. Guidelines for the Prevention of Stroke in Women: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(5):1545–88. Epub 2014/02/08. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demel SL, Kittner S, Ley SH, McDermott M, Rexrode KM. Stroke Risk Factors Unique to Women. Stroke. 2018;49(3):518–23. Epub 2018/02/14. doi: 10.1161/strokeaha.117.018415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madsen TE, Howard VJ, Jiménez M, Rexrode KM, Acelajado MC, Kleindorfer D, Chaturvedi S. Impact of Conventional Stroke Risk Factors on Stroke in Women: An Update. Stroke. 2018;49(3):536–42. Epub 2018/02/14. doi: 10.1161/strokeaha.117.018418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard VJ, Madsen TE, Kleindorfer DO, Judd SE, Rhodes JD, Soliman EZ, Kissela BM, Safford MM, Moy CS, McClure LA, Howard G, Cushman M. Sex and Race Differences in the Association of Incident Ischemic Stroke with Risk Factors. JAMA Neurol. 2019;76(2):179–86. Epub 2018/12/12. doi: 10.1001/jamaneurol.2018.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leppert MH, Ho PM, Burke J, Madsen TE, Kleindorfer D, Sillau S, Daugherty S, Bradley CJ, Poisson SN. Young Women Had More Strokes Than Young Men in a Large, United States Claims Sample. Stroke. 2020;51(11):3352–5. Epub 2020/09/19. doi: 10.1161/strokeaha.120.030803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madsen TE, Luo X, Huang M, Park KE, Stefanick ML, Manson JE, Liu S. Circulating Shbg (Sex Hormone-Binding Globulin) and Risk of Ischemic Stroke: Findings from the Whi. Stroke. 2020;51(4):1257–64. Epub 2020/02/23. doi: 10.1161/strokeaha.120.028905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu J, Lin JH, Jiménez MC, Manson JE, Hankinson SE, Rexrode KM. Plasma Estradiol and Testosterone Levels and Ischemic Stroke in Postmenopausal Women. Stroke. 2020;51(4):1297–300. Epub 2020/02/23. doi: 10.1161/strokeaha.119.028588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters SAE, Carcel C, Millett ERC, Woodward M. Sex Differences in the Association between Major Risk Factors and the Risk of Stroke in the Uk Biobank Cohort Study. Neurology. 2020;95(20):e2715–e26. Epub 2020/10/18. doi: 10.1212/wnl.0000000000010982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters SA, Huxley RR, Woodward M. Diabetes as a Risk Factor for Stroke in Women Compared with Men: A Systematic Review and Meta-Analysis of 64 Cohorts, Including 775,385 Individuals and 12,539 Strokes. Lancet. 2014;383(9933):1973–80. Epub 2014/03/13. doi: 10.1016/s0140-6736(14)60040-4. [DOI] [PubMed] [Google Scholar]
- 30.Madsen TE, Long DL, Carson AP, Howard G, Kleindorfer DO, Furie KL, Manson JE, Liu S, Howard VJ. Sex and Race Differences in the Risk of Ischemic Stroke Associated with Fasting Blood Glucose in Regards. Neurology. 2021;97(7):e684–e94. Epub 2021/05/29. doi: 10.1212/wnl.0000000000012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Krist AH, Kubik M, Li L, Ogedegbe G, Owens DK, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB. Screening for Prediabetes and Type 2 Diabetes: Us Preventive Services Task Force Recommendation Statement. JAMA. 2021;326(8):736–43. Epub 2021/08/25. doi: 10.1001/jama.2021.12531. [DOI] [PubMed] [Google Scholar]
- 32.Peters SA, Huxley RR, Woodward M. Comparison of the Sex-Specific Associations between Systolic Blood Pressure and the Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis of 124 Cohort Studies, Including 1.2 Million Individuals. Stroke. 2013;44(9):2394–401. Epub 2013/07/04. doi: 10.1161/strokeaha.113.001624. [DOI] [PubMed] [Google Scholar]
- 33.Madsen TE, Howard G, Kleindorfer DO, Furie KL, Oparil S, Manson JE, Liu S, Howard VJ. Sex Differences in Hypertension and Stroke Risk in the Regards Study: A Longitudinal Cohort Study. Hypertension. 2019;74(4):749–55. Epub 2019/08/14. doi: 10.1161/hypertensionaha.119.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, Cheng S. Sex Differences in Blood Pressure Trajectories over the Life Course . JAMA Cardiol. 2020;5(3):19–26. Epub 2020/01/16. doi: 10.1001/jamacardio.2019.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji H, Niiranen TJ, Rader F, Henglin M, Kim A, Ebinger JE, Claggett B, Merz CNB, Cheng S. Sex Differences in Blood Pressure Associations with Cardiovascular Outcomes. Circulation. 2021;143(7):761–3. Epub 2021/02/16. doi: 10.1161/circulationaha.120.049360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rexrode K, Rundek T. Body Mass Index and Stroke in Uk Women: “Obesity Paradox” Revisited. Neurology. 2016;87(14):1432–3. Epub 2016/09/09. doi: 10.1212/wnl.0000000000003186. [DOI] [PubMed] [Google Scholar]
- 37.Kroll ME, Green J, Beral V, Sudlow CL, Brown A, Kirichek O, Price A, Yang TO, Reeves GK. Adiposity and Ischemic and Hemorrhagic Stroke: Prospective Study in Women and Meta-Analysis. Neurology. 2016;87(14):1473–81. Epub 2016/09/09. doi: 10.1212/wnl.0000000000003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sulzgruber P, Wassmann S, Semb AG, Doehner W, Widimsky P, Gremmel T, Kaski JC, Savarese G, Rosano GMC, Borghi C, Kjeldsen K, Torp-Pedersen C, Schmidt TA, Lewis BS, Drexel H, Tamargo J, Atar D, Agewall S, Niessner A. Oral Anticoagulation in Patients with Non-Valvular Atrial Fibrillation and a Cha2ds2-Vasc Score of 1: A Current Opinion of the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy and European Society of Cardiology Council on Stroke. Eur Heart J Cardiovasc Pharmacother. 2019;5(3):171–80. Epub 2019/05/24. doi: 10.1093/ehjcvp/pvz016. [DOI] [PubMed] [Google Scholar]
- 39.Kostopoulou A, Zeljko HM, Bogossian H, Ciudin R, Costa F, Heijman J, Kochhaeuser S, Manola S, Scherr D, Sohal M, Wakili R, Wolf M, Irfan G. Atrial Fibrillation-Related Stroke in Women: Evidence and Inequalities in Epidemiology, Mechanisms, Clinical Presentation, and Management. Clin Cardiol. 2020;43(1):14–23. Epub 2019/11/07. doi: 10.1002/clc.23284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moseley A, Doukky R, Williams KA, Jaffer AK, Volgman AS. Indirect Comparison of Novel Oral Anticoagulants in Women with Nonvalvular Atrial Fibrillation. J Womens Health (Larchmt). 2017;26(3):214–21. Epub 2016/11/22. doi: 10.1089/jwh.2016.5892. [DOI] [PubMed] [Google Scholar]
- 41.Gu X, Li Y, Chen S, Yang X, Liu F, Li Y, Li J, Cao J, Liu X, Chen J, Shen C, Yu L, Huang J, Lam TH, Fang X, He Y, Zhang X, Lu X, Wu S, Gu D. Association of Lipids with Ischemic and Hemorrhagic Stroke: A Prospective Cohort Study among 267 500 Chinese. Stroke. 2019;50(12):3376–84. Epub 2019/10/30. doi: 10.1161/strokeaha.119.026402. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Chan W-S, Ray JG, Kramer MS, Joseph KS. Stroke and Cerebrovascular Disease in Pregnancy. Stroke. 2019;50(1):13–20. doi: doi: 10.1161/STROKEAHA.118.023118. [DOI] [Google Scholar]
- 43.Zheng J, Sun Z, Zhang X, Li Z, Guo X, Xie Y, Sun Y, Zheng L. Non-Traditional Lipid Profiles Associated with Ischemic Stroke Not Hemorrhagic Stroke in Hypertensive Patients: Results from an 8.4 Years Follow-up Study. Lipids Health Dis. 2019;18(1):9. Epub 2019/01/10. doi: 10.1186/s12944-019-0958-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rist PM, Buring JE, Ridker PM, Kase CS, Kurth T, Rexrode KM. Lipid Levels and the Risk of Hemorrhagic Stroke among Women. Neurology. 2019;92(19):e2286-e94. Epub 2019/04/12. doi: 10.1212/wnl.0000000000007454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Siegrist J. Physical Activity and Risk of Cardiovascular Disease--a Meta-Analysis of Prospective Cohort Studies. Int J Environ Res Public Health. 2012;9(2):391–407. Epub 2012/04/04. doi: 10.3390/ijerph9020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okoth K, Chandan JS, Marshall T, Thangaratinam S, Thomas GN, Nirantharakumar K, Adderley NJ. Association between the Reproductive Health of Young Women and Cardiovascular Disease in Later Life: Umbrella Review. BMJ. 2020;371:m3502. Epub 2020/10/09. doi: 10.1136/bmj.m3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of Estrogen Plus Progestin on Stroke in Postmenopausal Women: The Women’s Health Initiative: A Randomized Trial. JAMA. 2003;289(20):2673–84. Epub 2003/05/29. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 48.Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and Oral Hormone Replacement Therapy and the Risk of Stroke: A Nested Case-Control Study. BMJ. 2010;340:c2519. Epub 2010/06/09. doi: 10.1136/bmj.c2519. [DOI] [PubMed] [Google Scholar]
- 49.Lee SR, Cho MK, Cho YJ, Chun S, Hong SH, Hwang KR, Jeon GH, Joo JK, Kim SK, Lee DO, Lee DY, Lee ES, Song JY, Yi KW, Yun BH, Shin JH, Chae HD, Kim T. The 2020 Menopausal Hormone Therapy Guidelines. J Menopausal Med. 2020;26(2):69–98. Epub 2020/09/08. doi: 10.6118/jmm.20000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The 2017 Hormone Therapy Position Statement of the North American Menopause Society. Menopause. 2017;24(7):728–53. Epub 2017/06/27. doi: 10.1097/gme.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 51.Mishra SR, Chung HF, Waller M, Dobson AJ, Greenwood DC, Cade JE, Giles GG, Bruinsma F, Simonsen MK, Hardy R, Kuh D, Gold EB, Crawford SL, Derby CA, Matthews KA, Demakakos P, Lee JS, Mizunuma H, Hayashi K, Sievert LL, Brown DE, Sandin S, Weiderpass E, Mishra GD. Association between Reproductive Life Span and Incident Nonfatal Cardiovascular Disease: A Pooled Analysis of Individual Patient Data from 12 Studies. JAMA Cardiol. 2020;5(12):1410–8. Epub 2020/09/17. doi: 10.1001/jamacardio.2020.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welten S, Onland-Moret NC, Boer JMA, Verschuren WMM, van der Schouw YT. Age at Menopause and Risk of Ischemic and Hemorrhagic Stroke. Stroke. 2021;52(8):2583–91. Epub 2021/06/04. doi: 10.1161/strokeaha.120.030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mishra SR, Chung HF, Waller M, Mishra GD. Duration of Estrogen Exposure During Reproductive Years, Age at Menarche and Age at Menopause, and Risk of Cardiovascular Disease Events, All-Cause and Cardiovascular Mortality: A Systematic Review and Meta-Analysis. Bjog. 2021;128(5):809–21. Epub 2020/09/24. doi: 10.1111/1471-0528.16524. [DOI] [PubMed] [Google Scholar]
- 54.Mishra SR, Waller M, Chung HF, Mishra GD. Association of the Length of Oestrogen Exposure with Risk of Incident Stroke in Postmenopausal Women: Insights from a 20-Year Prospective Study. Int J Cardiol. 2021;328:206–14. Epub 2020/12/16. doi: 10.1016/j.ijcard.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 55.Oliver-Williams C, Vladutiu CJ, Loehr LR, Rosamond WD, Stuebe AM. The Association between Parity and Subsequent Cardiovascular Disease in Women: The Atherosclerosis Risk in Communities Study. J Womens Health (Larchmt). 2019;28(5):721–7. Epub 2018/11/28. doi: 10.1089/jwh.2018.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vladutiu CJ, Meyer ML, Malek AM, Stuebe AM, Mosher A, Aggarwal S, Kleindorfer D, Howard VJ. Racial Differences in the Association between Parity and Incident Stroke: Results from the Reasons for Geographic and Racial Differences in Stroke Study. J Stroke Cerebrovasc Dis. 2017;26(4):749–55. Epub 11/07. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobson LT, Hade EM, Collins TC, Margolis KL, Waring ME, Van Horn LV, Silver B, Sattari M, Bird CE, Kimminau K, Wambach K, Stefanick ML. Breastfeeding History and Risk of Stroke among Parous Postmenopausal Women in the Women’s Health Initiative. J Am Heart Assoc. 2018;7(17):e008739. Epub 2018/10/30. doi: 10.1161/jaha.118.008739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parikh NI, Gonzalez JM, Anderson CAM, Judd SE, Rexrode KM, Hlatky MA, Gunderson EP, Stuart JJ, Vaidya D. Adverse Pregnancy Outcomes and Cardiovascular Disease Risk: Unique Opportunities for Cardiovascular Disease Prevention in Women: A Scientific Statement from the American Heart Association. Circulation. 2021;143(18):e902–e16. Epub 2021/03/30. doi: 10.1161/cir.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 59.Søndergaard MM, Hlatky MA, Stefanick ML, Vittinghoff E, Nah G, Allison M, Gemmill A, Van Horn L, Park K, Salmoirago-Blotcher E, Sattari M, Sealy-Jefferson S, Shadyab AH, Valdiviezo C, Manson JE, Parikh NI. Association of Adverse Pregnancy Outcomes with Risk of Atherosclerotic Cardiovascular Disease in Postmenopausal Women. JAMA Cardiol. 2020;5(12):1390–8. Epub 2020/09/17. doi: 10.1001/jamacardio.2020.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, Smith GN, Gore GC, Ray JG, Nerenberg K, Platt RW. Cardiovascular Disease-Related Morbidity and Mortality in Women with a History of Pregnancy Complications. Circulation. 2019;139(8):1069–79. Epub 2019/02/20. doi: 10.1161/circulationaha.118.036748. [DOI] [PubMed] [Google Scholar]
- 61.Riise HKR, Sulo G, Tell GS, Igland J, Nygård O, Iversen AC, Daltveit AK. Association between Gestational Hypertension and Risk of Cardiovascular Disease among 617 589 Norwegian Women. J Am Heart Assoc. 2018;7(10). Epub 2018/05/15. doi: 10.1161/jaha.117.008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu P, Gulati M, Kwok CS, Wong CW, Narain A, O’Brien S, Chew-Graham CA, Verma G, Kadam UT, Mamas MA. Preterm Delivery and Future Risk of Maternal Cardiovascular Disease: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2018;7(2). Epub 2018/01/18. doi: 10.1161/jaha.117.007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adank MC, Hussainali RF, Oosterveer LC, Ikram MA, Steegers EAP, Miller EC, Schalekamp-Timmermans S. Hypertensive Disorders of Pregnancy and Cognitive Impairment: A Prospective Cohort Study. Neurology. 2021;96(5):e709–e18. Epub 2021/01/01. doi: 10.1212/wnl.0000000000011363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basit S, Wohlfahrt J, Boyd HA. Pre-Eclampsia and Risk of Dementia Later in Life: Nationwide Cohort Study. BMJ. 2018;363:k4109. Epub 2018/10/20. doi: 10.1136/bmj.k4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng SB, Sharma S. Preeclampsia and Health Risks Later in Life: An Immunological Link. Semin Immunopathol. 2016;38(6):699–708. Epub 2016/06/25. doi: 10.1007/s00281-016-0579-8. [DOI] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System 2020. [June 22, 2021]. Available from: https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm.
- 67.Swartz RH, Cayley ML, Foley N, Ladhani NNN, Leffert L, Bushnell C, McClure JA, Lindsay MP. The Incidence of Pregnancy-Related Stroke: A Systematic Review and Meta-Analysis. Int J Stroke. 2017;12(7):687–97. Epub 2017/09/09. doi: 10.1177/1747493017723271. [DOI] [PubMed] [Google Scholar]
- 68.Kuklina EV, Tong X, Bansil P, George MG, Callaghan WM. Trends in Pregnancy Hospitalizations That Included a Stroke in the United States from 1994 to 2007: Reasons for Concern? Stroke. 2011;42(9):2564–70. Epub 2011/07/30. doi: 10.1161/strokeaha.110.610592. [DOI] [PubMed] [Google Scholar]
- 69.Karjalainen L, Tikkanen M, Rantanen K, Aarnio K, Korhonen A, Saaros A, Laivuori H, Gissler M, Ijäs P. Stroke in Pregnancy and Puerperium: Validated Incidence Trends with Risk Factor Analysis in Finland 1987–2016. Neurology. 2021;96(21):e2564–e75. Epub 2021/04/09. doi: 10.1212/wnl.0000000000011990. [DOI] [PubMed] [Google Scholar]
- 70.Limaye K, Patel A, Dave M, Kenmuir C, Lahoti S, Jadhav AP, Samaniego EA, Ortega-Gutièrrez S, Torner J, Hasan D, Derdeyn CP, Jovin T, Adams HP Jr., Leira EC. Secular Increases in Spontaneous Subarachnoid Hemorrhage During Pregnancy: A Nationwide Sample Analysis. J Stroke Cerebrovasc Dis. 2019;28(4):1141–8. Epub 2019/02/04. doi: 10.1016/j.jstrokecerebrovasdis.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 71.Miller EC, Gatollari HJ, Too G, Boehme AK, Leffert L, Marshall RS, Elkind MSV, Willey JZ. Risk Factors for Pregnancy-Associated Stroke in Women with Preeclampsia. Stroke. 2017;48(7):1752–9. Epub 2017/05/27. doi: 10.1161/strokeaha.117.017374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive Disorders and Pregnancy-Related Stroke: Frequency, Trends, Risk Factors, and Outcomes. Obstet Gynecol. 2015;125(1):124–31. Epub 2015/01/07. doi: 10.1097/aog.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leffert LR, Clancy CR, Bateman BT, Cox M, Schulte PJ, Smith EE, Fonarow GC, Kuklina EV, George MG, Schwamm LH. Treatment Patterns and Short-Term Outcomes in Ischemic Stroke in Pregnancy or Postpartum Period. Am J Obstet Gynecol. 2016;214(6):723.e1-.e11. Epub 2015/12/29. doi: 10.1016/j.ajog.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 74.Scott CA, Bewley S, Rudd A, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. Incidence, Risk Factors, Management, and Outcomes of Stroke in Pregnancy. Obstet Gynecol. 2012;120(2 Pt 1):318–24. Epub 2012/07/25. doi: 10.1097/AOG.0b013e31825f287c. [DOI] [PubMed] [Google Scholar]
- 75.Wabnitz A, Bushnell C. Migraine, Cardiovascular Disease, and Stroke During Pregnancy: Systematic Review of the Literature. Cephalalgia. 2015;35(2):132–9. Epub 2014/10/12. doi: 10.1177/0333102414554113. [DOI] [PubMed] [Google Scholar]
- 76.Croles FN, Nasserinejad K, Duvekot JJ, Kruip MJ, Meijer K, Leebeek FW. Pregnancy, Thrombophilia, and the Risk of a First Venous Thrombosis: Systematic Review and Bayesian Meta-Analysis. BMJ. 2017;359:j4452. Epub 2017/10/28. doi: 10.1136/bmj.j4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu P, Jordan KP, Chew-Graham CA, Coutinho T, Lundberg GP, Park KE, Chappell LC, Myint PK, Maas A, Mamas MA. Temporal Trends in Pregnancy-Associated Stroke and Its Outcomes among Women with Hypertensive Disorders of Pregnancy. J Am Heart Assoc. 2020;9(15):e016182. Epub 2020/08/05. doi: 10.1161/jaha.120.016182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller EC, Boehme AK, Chung NT, Wang SS, Lacey JV Jr., Lakshminarayan K, Zhong C, Woo D, Bello NA, Wapner R, Elkind MSV, Willey JZ. Aspirin Reduces Long-Term Stroke Risk in Women with Prior Hypertensive Disorders of Pregnancy. Neurology. 2019;92(4):e305–e16. Epub 2018/12/28. doi: 10.1212/wnl.0000000000006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bandoli G, Baer RJ, Gano D, Pawlowski LJ, Chambers C. Migraines During Pregnancy and the Risk of Maternal Stroke. JAMA Neurol. 2020;77(9):1177–9. Epub 2020/06/02. doi: 10.1001/jamaneurol.2020.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller EC, Zambrano Espinoza MD, Huang Y, Friedman AM, Boehme AK, Bello NA, Cleary KL, Wright JD, D’Alton ME. Maternal Race/Ethnicity, Hypertension, and Risk for Stroke During Delivery Admission. J Am Heart Assoc. 2020;9(3):e014775. Epub 2020/01/25. doi: 10.1161/jaha.119.014775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gyamfi-Bannerman C, Pandita A, Miller EC, Boehme AK, Wright JD, Siddiq Z, D’Alton ME, Friedman AM. Preeclampsia Outcomes at Delivery and Race. J Matern Fetal Neonatal Med. 2020;33(21):3619–26. Epub 2019/02/23. doi: 10.1080/14767058.2019.1581522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.James AH, Jamison MG, Brancazio LR, Myers ER. Venous Thromboembolism During Pregnancy and the Postpartum Period: Incidence, Risk Factors, and Mortality. Am J Obstet Gynecol. 2006;194(5):1311–5. Epub 2006/05/02. doi: 10.1016/j.ajog.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 83.Too G, Wen T, Boehme AK, Miller EC, Leffert LR, Attenello FJ, Mack WJ, DʼAlton ME, Friedman AM. Timing and Risk Factors of Postpartum Stroke. Obstet Gynecol. 2018;131(1):70–8. Epub 2017/12/08. doi: 10.1097/aog.0000000000002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoshida K, Takahashi JC, Takenobu Y, Suzuki N, Ogawa A, Miyamoto S. Strokes Associated with Pregnancy and Puerperium: A Nationwide Study by the Japan Stroke Society. Stroke. 2017;48(2):276–82. Epub 2016/12/29. doi: 10.1161/strokeaha.116.014406. [DOI] [PubMed] [Google Scholar]
- 85.Lee S, Kim Y, Navi BB, Abdelkhaleq R, Salazar-Marioni S, Blackburn SL, Bambhroliya AB, Lopez-Rivera V, Vahidy F, Savitz SI, Medhus A, Kamel H, Grotta JC, McCullough L, Chen PR, Sheth SA. Risk of Intracranial Hemorrhage Associated with Pregnancy in Women with Cerebral Arteriovenous Malformations. J Neurointerv Surg. 2021;13(8):707–10. Epub 2020/11/25. doi: 10.1136/neurintsurg-2020-016838. [DOI] [PubMed] [Google Scholar]
- 86.Salehi Omran S, Parikh NS, Poisson S, Armstrong J, Merkler AE, Prabhu M, Navi BB, Riley LE, Fink ME, Kamel H. Association between Pregnancy and Cervical Artery Dissection. Ann Neurol. 2020;88(3):596–602. Epub 2020/06/12. doi: 10.1002/ana.25813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song TJ, Lee KH, Li H, Kim JY, Chang K, Kim SH, Han KH, Kim BY, Kronbichler A, Ducros A, Koyanagi A, Jacob L, Kim MS, Yon DK, Lee SW, Yang JM, Hong SH, Ghayda RA, Kang JW, Shin JI, Smith L. Reversible Cerebral Vasoconstriction Syndrome: A Comprehensive Systematic Review. Eur Rev Med Pharmacol Sci. 2021;25(9):3519–29. Epub 2021/05/19. doi: 10.26355/eurrev_202105_25834. [DOI] [PubMed] [Google Scholar]
- 88.Aoyama J, Nariai T, Moriyama K, Hara S, Mukawa M, Inaji M, Tanaka Y, Miyasaka N, Taketoshi M. Clinical Characteristics of the Pregnancies and Deliveries of Patients with Moyamoya Disease: A Single-Center Analysis over Three Decades. Int J Stroke. 2021;16(5):526–33. Epub 2020/10/13. doi: 10.1177/1747493020963806. [DOI] [PubMed] [Google Scholar]
- 89.Bredy C, Mongeon FP, Leduc L, Dore A, Khairy P. Pregnancy in Adults with Repaired/Unrepaired Atrial Septal Defect. J Thorac Dis. 2018;10(Suppl 24):S2945-s52. Epub 2018/10/12. doi: 10.21037/jtd.2017.10.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanghavi M, Rutherford JD. Cardiovascular Physiology of Pregnancy. Circulation. 2014;130(12):1003–8. Epub 2014/09/17. doi: 10.1161/circulationaha.114.009029. [DOI] [PubMed] [Google Scholar]
- 91.Greer IA. Thrombosis in Pregnancy: Maternal and Fetal Issues. Lancet. 1999;353(9160):1258–65. Epub 1999/04/27. doi: 10.1016/s0140-6736(98)10265-9. [DOI] [PubMed] [Google Scholar]
- 92.Lappas M, Rice GE. Transcriptional Regulation of the Processes of Human Labour and Delivery. Placenta. 2009;30 Suppl A:S90–5. Epub 2008/11/18. doi: 10.1016/j.placenta.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Esenwa CC, Elkind MS. Inflammatory Risk Factors, Biomarkers and Associated Therapy in Ischaemic Stroke. Nat Rev Neurol. 2016;12(10):594–604. Epub 2016/09/13. doi: 10.1038/nrneurol.2016.125. [DOI] [PubMed] [Google Scholar]
- 94.Saghazadeh A, Rezaei N. Inflammation as a Cause of Venous Thromboembolism. Crit Rev Oncol Hematol. 2016;99:272–85. Epub 2016/01/27. doi: 10.1016/j.critrevonc.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 95.Gómez-Chávez F, Correa D, Navarrete-Meneses P, Cancino-Diaz JC, Cancino-Diaz ME, Rodríguez-Martínez S. Nf-Κb and Its Regulators During Pregnancy. Front Immunol. 2021;12:679106. Epub 2021/05/25. doi: 10.3389/fimmu.2021.679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prairie E, Côté F, Tsakpinoglou M, Mina M, Quiniou C, Leimert K, Olson D, Chemtob S. The Determinant Role of Il-6 in the Establishment of Inflammation Leading to Spontaneous Preterm Birth. Cytokine Growth Factor Rev. 2021;59:118–30. Epub 2021/02/09. doi: 10.1016/j.cytogfr.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 97.Stefańska K, Zieliński M, Jankowiak M, Zamkowska D, Sakowska J, Adamski P, Jassem-Bobowicz J, Piekarska K, Leszczyńska K, Świątkowska-Stodulska R, Kwiatkowski S, Preis K, Trzonkowski P, Marek-Trzonkowska N. Cytokine Imprint in Preeclampsia. Front Immunol. 2021;12:667841. Epub 2021/07/13. doi: 10.3389/fimmu.2021.667841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Centers for Disease Control and Prevention. Data on Selected Pregnancy Complications in the United States. 2019. [July 27, 2021]. Available from: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-complications-data.htm#hyper.
- 99.Goulopoulou S. Maternal Vascular Physiology in Preeclampsia. Hypertension. 2017;70(6):1066–73. Epub 2017/10/25. doi: 10.1161/hypertensionaha.117.08821. [DOI] [PubMed] [Google Scholar]
- 100.Miller EC. Preeclampsia and Cerebrovascular Disease. Hypertension. 2019;74(1):5–13. Epub 2019/05/06. doi: 10.1161/hypertensionaha.118.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bello N, Rendon ISH, Arany Z. The Relationship between Pre-Eclampsia and Peripartum Cardiomyopathy: A Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2013;62(18):1715–23. Epub 2013/09/10. doi: 10.1016/j.jacc.2013.08.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reyes LM, Usselman CW, Davenport MH, Steinback CD. Sympathetic Nervous System Regulation in Human Normotensive and Hypertensive Pregnancies. Hypertension. 2018;71(5):793–803. Epub 2018/03/14. doi: 10.1161/hypertensionaha.117.10766. [DOI] [PubMed] [Google Scholar]
- 103.Warrington JP, Drummond HA, Granger JP, Ryan MJ. Placental Ischemia-Induced Increases in Brain Water Content and Cerebrovascular Permeability: Role of Tnf-Α. Am J Physiol Regul Integr Comp Physiol. 2015;309(11):R1425–31. Epub 2015/09/25. doi: 10.1152/ajpregu.00372.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Warrington JP, Fan F, Murphy SR, Roman RJ, Drummond HA, Granger JP, Ryan MJ. Placental Ischemia in Pregnant Rats Impairs Cerebral Blood Flow Autoregulation and Increases Blood-Brain Barrier Permeability. Physiol Rep. 2014;2(8). Epub 2014/08/30. doi: 10.14814/phy2.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schreurs MP, Houston EM, May V, Cipolla MJ. The Adaptation of the Blood-Brain Barrier to Vascular Endothelial Growth Factor and Placental Growth Factor During Pregnancy. Faseb j. 2012;26(1):355–62. Epub 2011/09/14. doi: 10.1096/fj.11-191916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnson AC. Physiology of the Cerebrovascular Adaptation to Pregnancy. Handb Clin Neurol. 2020;171:85–96. Epub 2020/08/02. doi: 10.1016/b978-0-444-64239-4.00004-7. [DOI] [PubMed] [Google Scholar]
- 107.Nicolaides KH. Aspirin Versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017;377(24):2400. Epub 2017/12/14. doi: 10.1056/NEJMc1713798. [DOI] [PubMed] [Google Scholar]
- 108.Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Kubik M, Li L, Ogedegbe G, Pbert L, Silverstein M, Simon MA, Stevermer J, Tseng CW, Wong JB. Aspirin Use to Prevent Preeclampsia and Related Morbidity and Mortality: Us Preventive Services Task Force Recommendation Statement. JAMA. 2021;326(12):1186–91. Epub 2021/09/29. doi: 10.1001/jama.2021.14781. [DOI] [PubMed] [Google Scholar]
- 109.Su MT, Wang CY, Tsai PY, Chen TY, Tsai HL, Kuo PL. Aspirin Enhances Trophoblast Invasion and Represses Soluble Fms-Like Tyrosine Kinase 1 Production: A Putative Mechanism for Preventing Preeclampsia. J Hypertens. 2019;37(12):2461–9. Epub 2019/07/25. doi: 10.1097/hjh.0000000000002185. [DOI] [PubMed] [Google Scholar]
- 110.Cadavid AP. Aspirin: The Mechanism of Action Revisited in the Context of Pregnancy Complications. Front Immunol. 2017;8:261. Epub 2017/04/01. doi: 10.3389/fimmu.2017.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mirabito Colafella KM, Neuman RI, Visser W, Danser AHJ, Versmissen J. Aspirin for the Prevention and Treatment of Pre-Eclampsia: A Matter of Cox-1 and/or Cox-2 Inhibition? Basic Clin Pharmacol Toxicol. 2020;127(2):132–41. Epub 2019/08/20. doi: 10.1111/bcpt.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Uy CE, Gosselin-Lefebvre S, Book AM, Field TS. Reperfusion Therapy for Acute Stroke in Pregnant and Post-Partum Women: A Canadian Survey. Can J Neurol Sci. 2021;48(3):344–8. Epub 2020/09/23. doi: 10.1017/cjn.2020.207. [DOI] [PubMed] [Google Scholar]
- 113.Dicpinigaitis AJ, Sursal T, Morse CA, Briskin C, Dakay K, Kurian C, Kaur G, Sahni R, Bowers C, Gandhi CD, Mayer SA, Al-Mufti F. Endovascular Thrombectomy for Treatment of Acute Ischemic Stroke During Pregnancy and the Early Postpartum Period. Stroke.0(0):STROKEAHA.121.034303. doi: doi: 10.1161/STROKEAHA.121.034303. [DOI] [PubMed]
- 114.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. Epub 2019/10/31. doi: 10.1161/str.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 115.Ladhani NNN, Swartz RH, Foley N, Nerenberg K, Smith EE, Gubitz G, Dowlatshahi D, Potts J, Ray JG, Barrett J, Bushnell C, Bal S, Chan WS, Chari R, El Amrani M, Gandhi S, Hill MD, James A, Jeerakathil T, Jin A, Kirton A, Lanthier S, Lausman A, Leffert LR, Mandzia J, Menon B, Pikula A, Poppe A, Saposnik G, Sharma M, Bhogal S, Smitko E, Lindsay MP. Canadian Stroke Best Practice Consensus Statement: Acute Stroke Management During Pregnancy. Int J Stroke. 2018;13(7):743–58. Epub 2018/07/20. doi: 10.1177/1747493018786617. [DOI] [PubMed] [Google Scholar]
- 116.Lappin JM, Darke S, Duflou J, Kaye S, Farrell M. Fatal Stroke in Pregnancy and the Puerperium. Stroke. 2018;49(12):3050–3. Epub 2018/12/21. doi: 10.1161/strokeaha.118.023274. [DOI] [PubMed] [Google Scholar]
- 117.Foo L, Bewley S, Rudd A. Maternal Death from Stroke: A Thirty Year National Retrospective Review. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):266–70. Epub 2013/10/17. doi: 10.1016/j.ejogrb.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 118.Meeks JR, Bambhroliya AB, Alex KM, Sheth SA, Savitz SI, Miller EC, McCullough LD, Vahidy FS. Association of Primary Intracerebral Hemorrhage with Pregnancy and the Postpartum Period. JAMA Netw Open. 2020;3(4):e202769. Epub 2020/04/15. doi: 10.1001/jamanetworkopen.2020.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hasegawa J, Ikeda T, Sekizawa A, Tanaka H, Nakata M, Murakoshi T, Katsuragi S, Osato K, Ishiwata I, Kinoshita K. Maternal Death Due to Stroke Associated with Pregnancy-Induced Hypertension. Circ J. 2015;79(8):1835–40. Epub 2015/05/29. doi: 10.1253/circj.CJ-15-0297. [DOI] [PubMed] [Google Scholar]
- 120.Ascanio LC, Maragkos GA, Young BC, Boone MD, Kasper EM. Spontaneous Intracranial Hemorrhage in Pregnancy: A Systematic Review of the Literature. Neurocrit Care. 2019;30(1):5–15. Epub 2018/02/25. doi: 10.1007/s12028-018-0501-4. [DOI] [PubMed] [Google Scholar]
- 121.Yoshimatsu J, Ikeda T, Katsuragi S, Minematsu K, Toyoda K, Nagatsuka K, Naritomi H, Miyamoto S, Iihara K, Yamamoto H, Ohno Y. Factors Contributing to Mortality and Morbidity in Pregnancy-Associated Intracerebral Hemorrhage in Japan. J Obstet Gynaecol Res. 2014;40(5):1267–73. Epub 2014/04/03. doi: 10.1111/jog.12336. [DOI] [PubMed] [Google Scholar]
- 122.Bateman BT, Schumacher HC, Bushnell CD, Pile-Spellman J, Simpson LL, Sacco RL, Berman MF. Intracerebral Hemorrhage in Pregnancy: Frequency, Risk Factors, and Outcome. Neurology. 2006;67(3):424–9. Epub 2006/08/09. doi: 10.1212/01.wnl.0000228277.84760.a2. [DOI] [PubMed] [Google Scholar]
- 123.Tarnutzer AA, Lee SH, Robinson KA, Wang Z, Edlow JA, Newman-Toker DE. Ed Misdiagnosis of Cerebrovascular Events in the Era of Modern Neuroimaging: A Meta-Analysis. Neurology. 2017;88(15):1468–77. Epub 2017/03/31. doi: 10.1212/wnl.0000000000003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Newman-Toker DE, Moy E, Valente E, Coffey R, Hines AL. Missed Diagnosis of Stroke in the Emergency Department: A Cross-Sectional Analysis of a Large Population-Based Sample. Diagnosis (Berl). 2014;1(2):155–66. Epub 2014/06/01. doi: 10.1515/dx-2013-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Castle J, Mlynash M, Lee K, Caulfield AF, Wolford C, Kemp S, Hamilton S, Albers GW, Olivot JM. Agreement Regarding Diagnosis of Transient Ischemic Attack Fairly Low among Stroke-Trained Neurologists. Stroke. 2010;41(7):1367–70. Epub 2010/05/29. doi: 10.1161/strokeaha.109.577650. [DOI] [PubMed] [Google Scholar]
- 126.Ishihara T, Sato S, Uehara T, Ohara T, Hayakawa M, Kimura K, Okada Y, Hasegawa Y, Tanahashi N, Suzuki A, Nakagawara J, Arii K, Nagahiro S, Ogasawara K, Uchiyama S, Matsumoto M, Iihara K, Toyoda K, Minematsu K. Significance of Nonfocal Symptoms in Patients with Transient Ischemic Attack. Stroke. 2018;49(8):1893–8. Epub 2018/07/18. doi: 10.1161/strokeaha.118.022009. [DOI] [PubMed] [Google Scholar]
- 127.Stuart-Shor EM, Wellenius GA, DelloIacono DM, Mittleman MA. Gender Differences in Presenting and Prodromal Stroke Symptoms. Stroke. 2009;40(4):1121–6. Epub 2009/02/13. doi: 10.1161/strokeaha.108.543371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jerath NU, Reddy C, Freeman WD, Jerath AU, Brown RD. Gender Differences in Presenting Signs and Symptoms of Acute Ischemic Stroke: A Population-Based Study. Gend Med. 2011;8(5):312–9. Epub 2011/09/20. doi: 10.1016/j.genm.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lisabeth LD, Brown DL, Hughes R, Majersik JJ, Morgenstern LB. Acute Stroke Symptoms: Comparing Women and Men. Stroke. 2009;40(6):2031–6. Epub 2009/02/21. doi: 10.1161/strokeaha.109.546812. [DOI] [PubMed] [Google Scholar]
- 130.Yu AYX, Penn AM, Lesperance ML, Croteau NS, Balshaw RF, Votova K, Bibok MB, Penn M, Saly V, Hegedus J, Zerna C, Klourfeld E, Bilston L, Hong ZM, Coutts SB. Sex Differences in Presentation and Outcome after an Acute Transient or Minor Neurologic Event. JAMA Neurol. 2019;76(8):962–8. Epub 2019/05/23. doi: 10.1001/jamaneurol.2019.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ferry AV, Anand A, Strachan FE, Mooney L, Stewart SD, Marshall L, Chapman AR, Lee KK, Jones S, Orme K, Shah ASV, Mills NL. Presenting Symptoms in Men and Women Diagnosed with Myocardial Infarction Using Sex-Specific Criteria. J Am Heart Assoc. 2019;8(17):e012307. Epub 2019/08/23. doi: 10.1161/jaha.119.012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Coutts SB, Moreau F, Asdaghi N, Boulanger JM, Camden MC, Campbell BCV, Demchuk AM, Field TS, Goyal M, Krause M, Mandzia J, Menon BK, Mikulik R, Penn AM, Swartz RH, Hill MD. Rate and Prognosis of Brain Ischemia in Patients with Lower-Risk Transient or Persistent Minor Neurologic Events. JAMA Neurol. 2019;76(12):1439–45. Epub 2019/09/24. doi: 10.1001/jamaneurol.2019.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.DeFilippis EM, Van Spall HGC. Is It Time for Sex-Specific Guidelines for Cardiovascular Disease? J Am Coll Cardiol. 2021;78(2):189–92. Epub 2021/07/10. doi: 10.1016/j.jacc.2021.05.012. [DOI] [PubMed] [Google Scholar]
- 134.Yu AYX, Hill MD, Asdaghi N, Boulanger JM, Camden MC, Campbell BCV, Demchuk AM, Field TS, Goyal M, Krause M, Mandzia J, Menon BK, Mikulik R, Moreau F, Penn AM, Swartz RH, Coutts SB. Sex Differences in Diagnosis and Diagnostic Revision of Suspected Minor Cerebral Ischemic Events. Neurology. 2021;96(5):e732–e9. Epub 2020/11/14. doi: 10.1212/wnl.0000000000011212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smith MA, Lisabeth LD, Brown DL, Morgenstern LB. Gender Comparisons of Diagnostic Evaluation for Ischemic Stroke Patients. Neurology. 2005;65(6):855–8. Epub 2005/09/28. doi: 10.1212/01.wnl.0000176054.72325.0f. [DOI] [PubMed] [Google Scholar]
- 136.Reeves MJ, Fonarow GC, Zhao X, Smith EE, Schwamm LH. Quality of Care in Women with Ischemic Stroke in the Gwtg Program. Stroke. 2009;40(4):1127–33. Epub 2009/02/13. doi: 10.1161/strokeaha.108.543157. [DOI] [PubMed] [Google Scholar]
- 137.Strong B, Lisabeth LD, Reeves M. Sex Differences in Iv Thrombolysis Treatment for Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Neurology. 2020;95(1):e11–e22. doi: 10.1212/WNL.0000000000009733. [DOI] [PubMed] [Google Scholar]
- 138.Bonkhoff AK, Karch A, Weber R, Wellmann J, Berger K. Female Stroke. Stroke. 2021;52(2):406–15. doi: doi: 10.1161/STROKEAHA.120.032850. [DOI] [PubMed] [Google Scholar]
- 139.Carcel C, Wang X, Sandset EC, Delcourt C, Arima H, Lindley R, Hackett ML, Lavados P, Robinson TG, Muñoz Venturelli P, Olavarría VV, Brunser A, Berge E, Chalmers J, Woodward M, Anderson CS. Sex Differences in Treatment and Outcome after Stroke. Neurology. 2019: 10.1212/WNL.0000000000008615. doi: . [DOI] [PubMed]
- 140.Bushnell C, Howard VJ, Lisabeth L, Caso V, Gall S, Kleindorfer D, Chaturvedi S, Madsen TE, Demel SL, Lee SJ, Reeves M. Sex Differences in the Evaluation and Treatment of Acute Ischaemic Stroke. Lancet Neurol. 2018;17(7):641–50. Epub 2018/06/20. doi: 10.1016/s1474-4422(18)30201-1. [DOI] [PubMed] [Google Scholar]
- 141.Lorenzano S, Ahmed N, Falcou A, Mikulik R, Tatlisumak T, Roffe C, Wahlgren N, Toni D. Does Sex Influence the Response to Intravenous Thrombolysis in Ischemic Stroke?: Answers from Safe Implementation of Treatments in Stroke-International Stroke Thrombolysis Register. Stroke. 2013;44(12):3401–6. Epub 2013/11/01. doi: 10.1161/strokeaha.113.002908. [DOI] [PubMed] [Google Scholar]
- 142.Weber R, Krogias C, Eyding J, Bartig D, Meves SH, Katsanos AH, Caso V, Hacke W. Age and Sex Differences in Ischemic Stroke Treatment in a Nationwide Analysis of 1.11 Million Hospitalized Cases. Stroke. 2019;50(12):3494–502. doi: 10.1161/STROKEAHA.119.026723. [DOI] [PubMed] [Google Scholar]
- 143.Otite FO, Saini V, Sur NB, Patel S, Sharma R, Akano EO, Anikpezie N, Albright K, Schmidt E, Hoffman H, Gould G, Khandelwal P, Latorre JG, Malik AM, Sacco RL, Chaturvedi S. Ten-Year Trend in Age, Sex, and Racial Disparity in Tpa (Alteplase) and Thrombectomy Use Following Stroke in the United States. Stroke (1970). 2021;52(8):2562–70. doi: 10.1161/STROKEAHA.120.032132. [DOI] [PubMed] [Google Scholar]
- 144.de Ridder IR, Fransen PS, Beumer D, Berkhemer OA, van den Berg LA, Wermer MJ, Lingsma H, van Zwam WH, Roos YB, van Oostenbrugge RJ, Majoie CB, van der Lugt A, Dippel DW. Is Intra-Arterial Treatment for Acute Ischemic Stroke Less Effective in Women Than in Men? Interv Neurol. 2016;5(3–4):174–8. Epub 2016/10/27. doi: 10.1159/000447331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Davalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millan M, Davis SM, Roy D, Thornton J, Roman LS, Ribo M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG. Endovascular Thrombectomy after Large-Vessel Ischaemic Stroke: A Meta-Analysis of Individual Patient Data from Five Randomised Trials. Lancet. 2016;387(10029):1723–31. Epub 2016/02/24. doi: 10.1016/s0140-6736(16)00163-x. [DOI] [PubMed] [Google Scholar]
- 146.Sheth AS, Lee JS, Warach GS, Gralla OJ, Jahan MR, Goyal SM, Nogueira DR, Zaidat LO, Pereira LV, Siddiqui LA, Lutsep LH, Liebeskind LD, McCullough LL, Saver LJ. Sex Differences in Outcome after Endovascular Stroke Therapy for Acute Ischemic Stroke. Stroke. 2019;50(9):2420–7. doi: 10.1161/STROKEAHA.118.023867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated Society for Vascular Surgery Guidelines for Management of Extracranial Carotid Disease. J Vasc Surg. 2011;54(3):e1–31. Epub 2011/09/06. doi: 10.1016/j.jvs.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 148.Bonati LH, Kakkos S, Berkefeld J, de Borst GJ, Bulbulia R, Halliday A, van Herzeele I, Koncar I, McCabe DJ, Lal A, Ricco J-B, Ringleb P, Taylor-Rowan M, Eckstein H-H. European Stroke Organisation Guideline on Endarterectomy and Stenting for Carotid Artery Stenosis. European stroke journal. 2021;6(2):I-XLVII. doi: 10.1177/23969873211012121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rothwell PM, Goldstein LB. Carotid Endarterectomy for Asymptomatic Carotid Stenosis: Asymptomatic Carotid Surgery Trial. Stroke. 2004;35(10):2425–7. Epub 2004/08/28. doi: 10.1161/01.STR.0000141706.50170.a7. [DOI] [PubMed] [Google Scholar]
- 150.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for Symptomatic Carotid Stenosis in Relation to Clinical Subgroups and Timing of Surgery. Lancet. 2004;363(9413):915–24. Epub 2004/03/27. doi: 10.1016/s0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 151.Howard VJ, Algra A, Howard G, Bonati LH, Borst GJd, Bulbulia R, Calvet D, Eckstein H-H, Fraedrich G, Greving JP, Halliday A, Hendrikse J, Jansen O, Brown MM, Mas J-L, Ringleb PA, Brott TG. Absence of Consistent Sex Differences in Outcomes from Symptomatic Carotid Endarterectomy and Stenting Randomized Trials. Stroke. 2021;52(2):416–23. doi: doi: 10.1161/STROKEAHA.120.030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McGurgan IJ, Ziai WC, Werring DJ, Al-Shahi Salman R, Parry-Jones AR. Acute Intracerebral Haemorrhage: Diagnosis and Management. Practical Neurology. 2021;21(2):128–36. doi: 10.1136/practneurol-2020-002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sandset EC, Wang X, Carcel C, Sato S, Delcourt C, Arima H, Stapf C, Robinson T, Lavados P, Chalmers J, Woodward M, Anderson CS. Sex Differences in Treatment, Radiological Features and Outcome after Intracerebral Haemorrhage: Pooled Analysis of Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trials 1 and 2. European stroke journal. 2020. doi: 10.1177/2396987320957513. [DOI] [PMC free article] [PubMed]
- 154.Pollack CV, Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kam C-W, Kamphuisen PW, Kreuzer J, Levy JH, Royle G, Sellke FW, Stangier J, Steiner T, Verhamme P, Wang B, Young L, Weitz JI. Idarucizumab for Dabigatran Reversal — Full Cohort Analysis. New England Journal of Medicine. 2017;377(5):431–41. doi: 10.1056/NEJMoa1707278. [DOI] [PubMed] [Google Scholar]
- 155.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex Differences in Stroke: Epidemiology, Clinical Presentation, Medical Care, and Outcomes. Lancet Neurol. 2008;7(10):915–26. Epub 2008/08/30. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Phan HT, Gall S, Blizzard CL, Lannin NA, Thrift AG, Anderson CS, Kim J, Grimley R, Castley HC, Kilkenny MF, Cadilhac DA. Sex Differences in Causes of Death after Stroke: Evidence from a National, Prospective Registry. J Womens Health (Larchmt). 2021;30(3):314–23. Epub 2020/11/24. doi: 10.1089/jwh.2020.8391. [DOI] [PubMed] [Google Scholar]
- 157.Phan HT, Gall SL, Blizzard CL, Lannin NA, Thrift AG, Anderson CS, Kim J, Grimley R, Castley HC, Hand P, Cadilhac DA. Sex Differences in Care and Long-Term Mortality after Stroke: Australian Stroke Clinical Registry. J Womens Health (Larchmt). 2019;28(5):712–20. Epub 2019/03/23. doi: 10.1089/jwh.2018.7171. [DOI] [PubMed] [Google Scholar]
- 158.Lambert C, Chaudhary D, Olulana O, Shahjouei S, Avula V, Li J, Abedi V, Zand R. Sex Disparity in Long-Term Stroke Recurrence and Mortality in a Rural Population in the United States. Ther Adv Neurol Disord. 2020;13:1756286420971895. Epub 2021/01/09. doi: 10.1177/1756286420971895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Barker-Collo S, Bennett DA, Krishnamurthi RV, Parmar P, Feigin VL, Naghavi M, Forouzanfar MH, Johnson CO, Nguyen G, Mensah GA, Vos T, Murray CJ, Roth GA, Group GBDW, Group GBDSPE. Sex Differences in Stroke Incidence, Prevalence, Mortality and Disability-Adjusted Life Years: Results from the Global Burden of Disease Study 2013. Neuroepidemiology. 2015;45(3):203–14. Epub 2015/10/28. doi: 10.1159/000441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D, European BSoSCG. Sex Differences in the Clinical Presentation, Resource Use, and 3-Month Outcome of Acute Stroke in Europe: Data from a Multicenter Multinational Hospital-Based Registry. Stroke. 2003;34(5):1114–9. Epub 2003/04/12. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 161.Kapral MK, Fang J, Hill MD, Silver F, Richards J, Jaigobin C, Cheung AM, Investigators of the Registry of the Canadian Stroke N. Sex Differences in Stroke Care and Outcomes: Results from the Registry of the Canadian Stroke Network. Stroke. 2005;36(4):809–14. Epub 2005/02/26. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- 162.Wyller TB, Sødring KM, Sveen U, Ljunggren AE, Bautz-Holter E. Are There Gender Differences in Functional Outcome after Stroke? Clinical Rehabilitation. 1997;11(2):171–9. doi: 10.1177/026921559701100211. [DOI] [PubMed] [Google Scholar]
- 163.Roquer J, Campello AR, Gomis M. Sex Differences in First-Ever Acute Stroke. Stroke. 2003;34(7):1581–5. Epub 2003/06/14. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- 164.Glader EL, Stegmayr B, Norrving B, Terent A, Hulter-Asberg K, Wester PO, Asplund K, Riks-Stroke C. Sex Differences in Management and Outcome after Stroke: A Swedish National Perspective. Stroke. 2003;34(8):1970–5. Epub 2003/07/12. doi: 10.1161/01.STR.0000083534.81284.C5. [DOI] [PubMed] [Google Scholar]
- 165.Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A, International Stroke Trial Collaborative G. Influence of Gender on Baseline Features and Clinical Outcomes among 17,370 Patients with Confirmed Ischaemic Stroke in the International Stroke Trial. Neuroepidemiology. 2005;24(3):123–8. Epub 2005/01/08. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- 166.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The Influence of Gender and Age on Disability Following Ischemic Stroke: The Framingham Study. J Stroke Cerebrovasc Dis. 2003;12(3):119–26. Epub 2007/10/02. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 167.Lai SM, Duncan PW, Dew P, Keighley J. Sex Differences in Stroke Recovery. Prev Chronic Dis. 2005;2(3):A13. Epub 2005/06/21. [PMC free article] [PubMed] [Google Scholar]
- 168.Gargano JW, Reeves MJ, Paul Coverdell National Acute Stroke Registry Michigan Prototype I. Sex Differences in Stroke Recovery and Stroke-Specific Quality of Life: Results from a Statewide Stroke Registry. Stroke. 2007;38(9):2541–8. Epub 2007/08/04. doi: 10.1161/STROKEAHA.107.485482. [DOI] [PubMed] [Google Scholar]
- 169.Gall S, Phan H, Madsen TE, Reeves M, Rist P, Jimenez M, Lichtman J, Dong L, Lisabeth LD. Focused Update of Sex Differences in Patient Reported Outcome Measures after Stroke. Stroke. 2018;49(3):531–5. Epub 2018/02/14. doi: 10.1161/STROKEAHA.117.018417. [DOI] [PubMed] [Google Scholar]
- 170.Katzan IL, Thompson NR, Uchino K, Lapin B. The Most Affected Health Domains after Ischemic Stroke. Neurology. 2018;90(16):e1364-e71. Epub 2018/03/30. doi: 10.1212/WNL.0000000000005327. [DOI] [PubMed] [Google Scholar]
- 171.Towfighi A, Ovbiagele B, El Husseini N, Hackett ML, Jorge RE, Kissela BM, Mitchell PH, Skolarus LE, Whooley MA, Williams LS, American Heart Association Stroke C, Council on C, Stroke N, Council on Quality of C, Outcomes R. Poststroke Depression: A Scientific Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48(2):e30–e43. Epub 2016/12/10. doi: 10.1161/STR.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 172.Kuehner C. Why Is Depression More Common among Women Than among Men? Lancet Psychiatry. 2017;4(2):146–58. Epub 2016/11/20. doi: 10.1016/s2215-0366(16)30263-2. [DOI] [PubMed] [Google Scholar]
- 173.Eriksson M, Asplund K, Glader EL, Norrving B, Stegmayr B, Terent A, Asberg KH, Wester PO, Riks-Stroke C. Self-Reported Depression and Use of Antidepressants after Stroke: A National Survey. Stroke. 2004;35(4):936–41. Epub 2004/03/06. doi: 10.1161/01.STR.0000121643.86762.9a. [DOI] [PubMed] [Google Scholar]
- 174.Dong L, Sanchez BN, Skolarus LE, Stulberg E, Morgenstern LB, Lisabeth LD. Sex Difference in Prevalence of Depression after Stroke. Neurology. 2020;94(19):e1973–e83. Epub 2020/04/22. doi: 10.1212/WNL.0000000000009394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Poynter B, Shuman M, Diaz-Granados N, Kapral M, Grace SL, Stewart DE. Sex Differences in the Prevalence of Post-Stroke Depression: A Systematic Review. Psychosomatics. 2009;50(6):563–9. Epub 2009/12/10. doi: 10.1176/appi.psy.50.6.563. [DOI] [PubMed] [Google Scholar]
- 176.Boden-Albala B, Litwak E, Elkind MS, Rundek T, Sacco RL. Social Isolation and Outcomes Post Stroke. Neurology. 2005;64(11):1888–92. Epub 2005/06/16. doi: 10.1212/01.WNL.0000163510.79351.AF. [DOI] [PubMed] [Google Scholar]
- 177.Strong B, Pudar J, Thrift AG, Howard VJ, Hussain M, Carcel C, de Los Campos G, Reeves MJ. Sex Disparities in Enrollment in Recent Randomized Clinical Trials of Acute Stroke: A Meta-Analysis. JAMA Neurol. 2021;78(6):666–77. Epub 2021/04/27. doi: 10.1001/jamaneurol.2021.0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Carcel C, Woodward M, Balicki G, Koroneos GL, Sousa DA, Cordonnier C, Lukaszyk C, Thompson K, Wang X, Davies L, Bassi M, Anderson CS, Peters SA, Sandset EC. Trends in Recruitment of Women and Reporting of Sex Differences in Large-Scale Published Randomized Controlled Trials in Stroke. Int J Stroke. 2019;14(9):931–8. Epub 2019/05/28. doi: 10.1177/1747493019851292. [DOI] [PubMed] [Google Scholar]
- 179.Carcel C, Reeves M. Under-Enrollment of Women in Stroke Clinical Trials: What Are the Causes and What Should Be Done About It? Stroke. 2021;52(2):452–7. Epub 2021/01/26. doi: 10.1161/strokeaha.120.033227. [DOI] [PubMed] [Google Scholar]