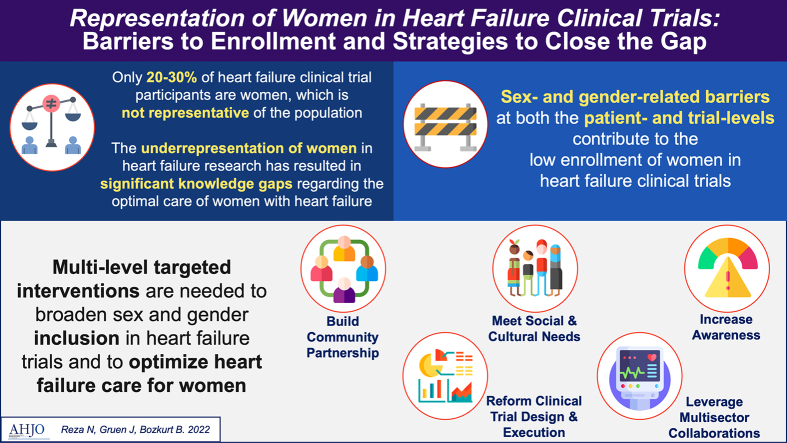
Abstract
Heart failure is a significant public health burden that differentially impacts women. Important sex- and gender-based differences in HF risk factors, presentation, and treatment exist, and the generation of high-quality evidence is critical to elucidate these differences. Despite the remarkable growth of the heart failure clinical research enterprise over the last four decades, women remain underrepresented in heart failure clinical trials relative to the population prevalence of heart failure in women. This disparity has resulted in significant knowledge gaps regarding the optimal care of women with heart failure. In this review, we summarize the existing literature regarding the participation of women in heart failure clinical trials. Additionally, we explain the evidence surrounding sex- and gender-specific barriers to enrollment in heart failure clinical trials and describe interventions that should be implemented throughout the clinical trial lifespan to achieve sex and gender parity.
Keywords: Heart failure, Female, Sex characteristics, Clinical trials, Patient participation, Diversity
Graphical abstract
1. Introduction
Women comprise half of the population of adults with heart failure (HF) in the United States (U.S.) [1]. Approximately half of the HF population has HF with preserved ejection fraction (HFpEF), and the prevalence of HFpEF at any given age is higher in women [2]. While the lifetime risk for HF with reduced ejection fraction (HFrEF) is lower in women compared with men, approximately 40% of patients with HFrEF are women [3]. Moreover, sex- and gender-based differences have been demonstrated across the spectrum of HF risk factors, pathophysiology, diagnosis, treatment, and outcomes of care [4]. Achieving equitable HF care for women necessitates a robust evidence base; however, women have been significantly underrepresented in HF clinical research.
The exclusion of women from clinical research in the U.S. is rooted in federal policy. In 1977, the U.S. Food and Drug Administration (FDA) outlined the exclusion of “women with childbearing potential” from phase I and early phase II clinical trials, partially in response to the devastating birth defects associated with maternal thalidomide use during pregnancy [5]. This guideline was interpreted broadly to include any woman capable of becoming pregnant in this definition [6]. Over the next decade, regulatory bodies increasingly realized that this lack of sex- and gender-specific data in biomedical research severely limited the generalizability of research and quality of care.
In the 1980s, newer National Institutes of Health (NIH) policies specifically encouraged the inclusion of women and performance of sex-stratified analyses in clinical research; however, U.S. governmental agencies still found that women were significantly underrepresented across many biomedical research domains, and especially in studies of cardiovascular disease [7]. In 1993, the FDA formally rescinded the 1977 policy, and the U.S. Congress codified the NIH's inclusion policies into law through a section in the NIH Revitalization Act of 1993 entitled “Women and Minorities as Subjects in Clinical Research” [8]. This law, along with a new guideline jointly issued by the NIH and U.S. Office of Research on Women's Health, mandated (1) the inclusion of women in all NIH-funded clinical research, (2) that trials be designed to elucidate sex-based differences, (3) and the creation of dedicated outreach programs to recruit and retain women as clinical trial participants. Despite these efforts, early investigations of the impact of these federal policies continued to demonstrate the underrepresentation of women in mixed-gender phase III or IV cardiovascular RCTs funded by the NIH [9]. This disparity persists such that even today, a significant proportion of contemporary HF trials do not adequately report sex/gender-specific subgroup data [10].
Furthermore, under-representation of women in clinical trials have resulted in misinterpretation of under-powered subgroup analyses in which women appeared to have differential benefit and side effect profiles from therapies such as angiotensin-converting enzyme inhibitors or implantable cardioverter defibrillators [11], [12], [13], [14], [15]. Most of these erroneous interpretations have since been corrected with meta-analyses demonstrating benefit with guideline directed medical therapy in women [16]. Similarly, when women were referred late for cardiovascular interventions with a higher symptom and disease burden, clinical outcomes were usually worse [17]. This created the misperception of a higher risk precluding referral for interventional therapies for women and widening therapeutic disparities [18].
The purpose of this review is to summarize the existing literature on the representation of women in HF clinical trials, describe barriers to the enrollment of women as participants, and propose strategies to increase trial-level and patient-level representation of women in HF research. Herein, the term sex is defined as a biological variable determined by characteristics encoded in DNA, whereas the term gender refers to the social constructs and traits linked to biological sex assignment and is what is typically collected in clinical trials [10], [19].
2. Enrollment of women in heart failure clinical trials
In the 1980s–1990s, women comprised approximately 20–30% of participants in landmark trials investigating beta-blockers, renin-angiotensin-aldosterone inhibitors, vasodilators, and digoxin in HFrEF [20]. Early women leaders in HF recognized the implications of the underrepresentation of women in HF clinical trials, including the potential for under-recognition of important differences in pathophysiology, clinical presentation, and outcomes [14], [20]. It became clear that targeted benchmarking and focused inquiry into the causes of low enrollment of women in HF clinical trials were needed.
In 2000, Harris and Douglas sought to investigate whether the aforementioned federal efforts had been successful in increasing the enrollment of women in cardiovascular clinical trials conducted by the National Heart, Lung, and Blood Institute (NHLBI). They conducted an analysis of the gender composition of all cardiovascular clinical trials conducted by the NHLBI between 1965 and 1998 and found that of 17,349 subjects enrolled in 6 HF trials, 26% were women [21]. This was a strikingly low proportion when benchmarked against the estimated 43% population prevalence of HF in women at the time. The proportion of women enrolled in HF trials did not significantly change over the 13-year study period. The authors concluded that researchers, funding agencies, and policy makers must better collaborate to improve the representation of women in cardiovascular clinical trials, as efforts to date had achieved limited success.
As the corpus of cardiovascular clinical research grew, subsequent studies published throughout the 2000s, 2010s, and early 2020s serially catalogued the participation of women in HF trials. Much debate ensued regarding the definition of representation and its relationship to scientific validity [22], [23]. Given the lack of a universally accepted method for determining representation and challenges in establishing the sex-specific prevalence estimates of HF, multiple studies performed during this period used the participation to prevalence ratio (PPR), the proportion of women in a trial relative to the proportion of women in the disease population, to quantify trial representation. A PPR of <0.8 has generally been used to indicate the underrepresentation of women [24].
Heiat et al. performed the first analysis focused on the enrollment of women in HF clinical trials and examined 59 randomized controlled trials (RCTs) published between January 1985 and December 1999 [25]. Women constituted 21% of participants overall, significantly less than the proportion of women in the general HF population and also of NIH-funded study participants (50% estimated from the National Health and Nutrition Examination Survey, p < 0.001). Four trials excluded women outright, and 8 additional trials excluded women with childbearing potential. Notably, over the 14-year study period, the exclusion of women per trial protocol occurred less frequently, heralding an evolution beyond the paternalistic practices of prior decades. Gong et al. extended the survey period and found that in 64 HF RCTs published between 1986 and 2015 in 3 high-impact medical journals, the percentage of enrolled women was 28.4% [26]. A focused study of 264 HF publications, including RCTs and observational studies, published in 11 peer-reviewed journals in 2013 similarly demonstrated that women comprised 29% of participants [27].
The creation of ClinicalTrials.gov [28], a 2000 collaborative NIH and FDA effort to establish a clinical trials information registry, provided a rich resource for more advanced sex/gender-based inquiries into clinical trial designs, protocols, and results. Jin et al. systematically assessed the participation of women in 740 completed cardiovascular trials registered in ClinicalTrials.gov between 2010 and 2017, including 102 trials in HF, and compared characteristics across 8 major cardiovascular disease subtypes [29]. HF trials had nearly the lowest woman to man participant ratio and had the lowest PPR (0.48) of the 8 subtypes — findings that were largely unchanged compared to nearly two decades earlier [21].
With trial-level data more readily available, predictors of women's enrollment in HF trials were able to be identified. Participant age at trial enrollment repeatedly emerged as a relevant association. A 1-year increase in the mean age of participants was associated with a 5% increase in the enrollment of women in the Heiat et al. analysis [25]. This association between increasing age at trial enrollment and higher enrollment rates was also demonstrated in a systematic review of 325 landmark cardiovascular trials published between 1997 and 2009 [30]. For HF trials in this study, the authors estimated an increase in women's enrollment by 4.2% for every 5-year increment in age at enrollment. Although challenging to delineate age- versus sex-related effects, these findings suggested that excluding elderly individuals from clinical trials could thereby indirectly result in the exclusion of women as well.
A few more recent studies evaluated HF trial enrollment through the lens of novel drug approvals. In 2018, Scott et al. examined women's participation in trials supporting new FDA drug applications from January 1, 2005, to September 15, 2015 [24]. Of the 57 trials of 35 drugs examined across 6 areas of cardiovascular disease, 3 drugs were studied in HFrEF. The proportion of women among trial participants was lowest in the HFrEF trials at 24%, and PPR was lowest in the HFrEF trials at 0.5–0.6. Khan et al. corroborated these findings in their analysis of the participation of women in pivotal trials supporting approval of cardiometabolic drugs from January 2008 to December 2017 (PPR for HF = 0.58) [31].
Contemporary analyses of HF trial enrollment sought to stratify participation by HF phenotype. Tahhan et al. provided an update of Heiat et al.'s work focused on HF trials and examined enrollment trends in 118 HF trials published between 2001 and 2016 [32]. Overall, 27% of enrolled participants in these trials were women, and this proportion did not significantly change over the 15-year study period. Chronic HFpEF trials enrolled more women (58%) compared with trials in HFrEF (24%) or acute HF (32%; p < 0.001 for comparison), and these enrollment proportions were all lower than the estimated disease prevalence of each respective HF phenotype. A higher proportion of women were enrolled in trials that were conducted in North America compared with other global regions (p < 0.003 for comparison); in trials funded by the NIH/NHLBI compared with other funding mechanisms (p < 0.004 for comparison); and in trials testing nondrug, noninvasive therapies (p = 0.05). Again, a higher mean age of participants was also significantly associated with greater women enrollment (p = 0.004). In a separate HFpEF-focused analysis, Tahhan et al. systematically reviewed trials published between 2001 and 2016 and found that women comprised 55% of overall trial participants, commensurate with the estimated prevalence of HFpEF in the U.S. [33].
Overall, the proportion of women enrolled in HF clinical trials has remained stagnant at approximately 20–30% over the past 4 decades. When compared with the available population prevalence estimates of HF and its subtypes among women, HF trial participants have not been representative of real-world HF populations. However, these prevalence estimates can vary widely depending on the populations sampled, and this challenges the ability to confidently and quantitatively assess the degree of women's underrepresentation. The majority of HF clinical trials have been performed in patients with HFrEF, and as such, there is now a robust evidence base for medical and interventional therapies that decrease morbidity and prolong life in this disease. Women comprise the majority of patients with HFpEF, and almost no trial-tested interventions have been shown to reduce its morbidity and mortality [34]. While women have been enrolled in HFpEF clinical trials at higher proportions than in HFrEF clinical trials, there remains a dearth of effective pharmacological or device therapy to improve the symptom burden or life expectancy of a substantial number of women with HF. Recent benchmarking efforts have focused on identifying predictors of greater trial participation by women, and further emphasis here is urgently needed.
3. Inclusion of women in trials supporting heart failure practice guidelines
In the last decade, inventive analyses of HF trials restricted to those cited in support of major society practice guidelines have yielded additional insight into predictors of trial participation. These cited studies usually reflect landmark trials that inform level of evidence for guideline recommendations. A 2014 study examined the enrollments of the 230 RCTs cited in the American College of Cardiology/American Heart Association 2009 Focused Update and the 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults [35] and compared female representation in trials cited across atrial fibrillation and unstable angina/non–ST-segment elevation myocardial infarction guidelines [36]. Although the inclusion of women in HF trials had improved from the 1980s to 2000s, female representation was lowest in HF guideline citations at 29%, despite an estimated population prevalence of HF of 47%.
From this focus on guidelines, provocative associations between the composition of HF trial leadership and the inclusion of women as trial participants emerged. In an analysis of 118 phase II-IV HF clinical trials published between January 2001 and December 2016, Reza et al. found that HF trial publications with a woman as first or senior author were associated with a higher proportion of women trial participants, and trial publications authored by a larger proportion of women enrolled higher proportions of women participants (r = 0.39, p < 0.001) [37]. The proportion of women authors per trial was the only significant independent predictor of woman participant enrollment after adjusting for the number of participants, number of sites, region of enrollment, funding mechanism, and trial intervention (β = 0.31, p < 0.001). This association between woman lead author and higher proportion of women enrollment was also demonstrated by Gong et al. [26], although their study included trials of coronary artery disease, vascular disease, and arrhythmia, as well.
4. Barriers to the enrollment of women in heart failure trials
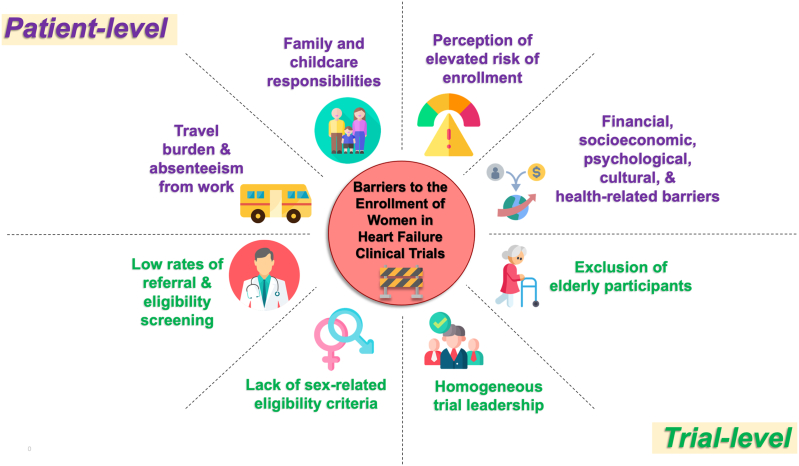
Many sex- and gender-based barriers to the enrollment of women in HF trials have been suggested, but few have been rigorously studied. A subanalysis of the Scott et al. study exploring 5 trials with available screening data suggested that the lower number of women enrolled in these trials was predominantly a reflection of the lower number of women referred for participation screening [24]. These authors concluded that failure to screen an adequate number of potential women participants and address barriers to women's enrollment are principal factors contributing to the underrepresentation of women in trials.
Whitelaw et al. conducted a systematic review of 317 HF RCTs published between January 2000 and May 2019 in high impact journals to identify trial-level characteristics associated with the underrepresentation of women participants relative to the sex-specific distribution of HFrEF [38]. Nearly 75% of these HF trials under-enrolled women, as benchmarked by a threshold of <32% enrolled women subjects. Sex-related eligibility criteria; ambulatory recruitment; trial coordination in North America, Europe and Asia; drug and device/surgery interventions; and trial leadership by men were each independently associated with under-enrollment of women participants. Harrison et al. retrospectively analyzed survey data from 97 women who were invited to participate in at least one of four HF studies but declined, and found that the four major deterrents to patients' participation were (1) lack of interest, (2) lack of time, (3) poor health, and (4) travel burden [39]. There were no significant differences by gender in patient-dependent or study-dependent reasons for refusal. Furthermore, as women comprise a larger proportion of older patients with HF, non-inclusion of elderly participants may influence their enrollment.
In addition to these data from HF trials, poor awareness of heart failure, difficulty accessing study sites; family and child care responsibilities; financial, cultural and socioeconomic barriers; and concerns about participation risk have been hypothesized as obstacles to women's participation in clinical trials [24]. Ambulatory women with heart failure are less likely to be referred for initial and follow-up cardiology consultations compared with men [40], leading to decreased access to heart failure investigators and clinical trials. A study of participation in cardiovascular trials revealed that women perceive a greater risk from clinical trial participation and are more reluctant to consider participation compared with men [41]. Women are more likely to seek external sources of input regarding decisions, to have their decisions influenced by others, and take more time to make decisions [42], characteristics which also impact willingness to participate.
A summary of patient- and trial-level barriers to the enrollment of women in clinical trials is shown in Fig. 1.
Fig. 1.
Patient- and trial-level barriers to the enrollment of women in heart failure clinical trials.
5. Strategies to increase the enrollment of women in heart failure trials
Knowledge of these barriers provides opportunities for personalized and targeted trial-level and patient-level interventions throughout the clinical trial design, execution, and follow up processes.
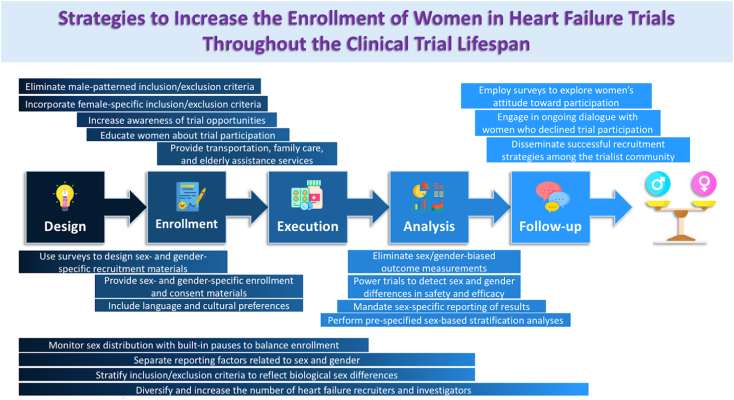
First, women must be made aware of opportunities to participate in clinical trials and must better understand the process of clinical trial participation. Women utilize different decision-making paradigms compared with men, and a personalized approach to education and consent may be needed. The efficacy of sex- and gender-specific trial enrollment materials incorporating psychological, socioeconomic, and cultural factors should be evaluated. Women must also have access to support for enrollment and participation, including resources for transportation to and from study sites and coverage for child and family care during trial visits. Recruitment of older patients has been shown to be associated with higher rates of enrollment of women [30], so particular assistance for elderly subjects should be incorporated. Almost as importantly, registries of women who decline participation in clinical trials should be maintained to identify driving factors regarding refusal decisions.
Innovative strategies to recruit and enroll women in cardiovascular clinical trials are evolving. Sisk et al. demonstrated successful enrollment of a gender and racially/ethnically diverse patient population into an RCT testing ambulatory HF care by using a culturally sensitive stepwise recruitment approach employed across different practice settings [43]. Their blueprint, incorporating language preferences, remuneration, transportation, and appropriate pre-enrollment education, can be tested as a scalable model for other HF trials. An industry-led initiative called Women Opt-In for Heart Research (WIN-Her; Boston Scientific Corporation, Marlborough, Massachusetts) is using qualitative and quantitative surveys to explore women's attitudes toward participation in clinical trials. This feedback is being used to design sex- and gender-specific educational materials to support enrollment in the ASAP-TOO (ClinicalTrials.gov Identifier: NCT02928497) and MADIT S-ICD (ClinicalTrials.gov Identifier: NCT02787785) trials [29]. Direct-to-participant enrollment, digital recruitment and follow up, and community based participatory research structures are modern outreach techniques that may be effective for recruiting women and should be further studied. Best practices that result in the successful recruitment of a representative population of women should be disseminated among the HF trialist community.
Changes in clinical trial design have also been proposed as approaches to increase enrollment of women in HF trials. Sex-specific considerations that can be incorporated into statistical analysis plans include (1) powering trials to detect statistically significant sex and gender differences in safety and efficacy endpoints; (2) performing pre-specified sex-based stratification analyses and adjusted analyses with an interaction term for sex; (3) enforcing gender parity of subject enrollment; (4) eliminating sex/gender-biased outcomes measurements; and (5) mandating sex-specific reporting of results. However, because of variations in disease prevalence, indications for intervention, and challenges in patient recruitment, there are no definitive FDA or NIH mandates regarding the design of cardiovascular clinical trials. Women enrolled in the PARAGON-HF (Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor with Angiotensin Receptor Blocker Global Outcomes in Heart Failure and Preserved Left Ventricular Ejection Fraction) comprised 52% of the trial population, among the highest proportion of enrolled women in contemporary HF trials, and demonstrated a more favorable treatment effect in women in a prespecified subgroup analysis (rate ratio, 0.73 [95% CI, 0.59–0.90] in women; 1.03 [95% CI, 0.84–1.25] in men; p interaction = 0.017) with the predominant attributable benefit due to a greater reduction in HF hospitalization [44], [45]. A recent systematic review of 261 primary HF RCTs published in nine high-impact journals from 2008 to 2017 demonstrated poor credibility and inconsistent claims of subgroup effects, indicating a persistent challenge in deriving accurate sex-specific conclusions from contemporary HF trials [46]. PARAGON-HF exemplified the importance of incorporating sex stratification as a prespecified subgroup analysis and serves as an example for other large, multicenter HF clinical trials.
Importantly, as the number of woman clinical trial participants increases, we must ensure that trial populations accurately represent the composition and experience of real-world populations of women with HF. Strategies to accomplish this include (1) monitoring sex distribution during trial recruitment with built-in pauses to balance enrollments; (2) eliminating male-patterned inclusion and exclusion criteria; (3) incorporating female-specific risk factors into inclusion criteria; (4) stratifying common inclusion and exclusion criteria that reflect biological sex differences, such as age at HF diagnosis, body size, estimated glomerular filtration rate, childbearing potential, and biomarkers, as examples; (5) separating reporting factors relating to sex from those relating to gender; and (6) expanding trial eligibility beyond binary sex/gender definitions [47]. In the first patient-level comparison of trial and real-world patients with HF by sex, Greene et al. examined the characteristics of patients enrolled in the ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) trial with those enrolled in the Get With The Guidelines-Heart Failure (GWTG-HF) registry [48]. Compared with women patients enrolled in ASCEND-HF, women patients in GWTG-HF who met eligibility criteria to participate in ASCEND-HF had higher rates of 30-day readmission (p ≤ 0.02). The investigators also found that the sex distributions of participants during the transition from trial-eligible to trial-enrolled were distinctly different, suggesting that individual patient and local investigator decisions are particularly influential in the enrollment of women in clinical trials. Improving the representation of women in HF trials may require parallel efforts to ensure patient-level representativeness.
Finally, factors external to HF trial design and execution are also targets for improvement. Globalization of HF clinical research will require improvement in sex-specific epidemiological reporting of HF from regions outside Europe and North America [49]. Industry collaborations to develop sex-specific investigational devices may improve the enrollment of women in device and procedural trials, which have a particular paucity of women subjects [50]. While the causal relationship between HF trial authorship and number of women trial participants remains elusive, diversifying and increasing the number of HF trial recruiters and investigators is independently needed [37], [51], [52], [53]. The FDA Office of Women's Health provides a wealth of resources on recruiting and retaining women in clinical research [54], and engagement with these materials should be mandatory for HF clinical trialists and investigators.
A summary of strategies to increase the enrollment of women in heart failure trials throughout the clinical trial lifespan is shown in Fig. 2.
Fig. 2.
Strategies to increase the enrollment of women in the heart failure trials throughout the clinical trial lifespan. Individual strategies are illustrated spanning across the central steps of a clinical trial where applicable.
6. Conclusions
Heart failure remains a leading cause of morbidity and mortality worldwide, and nearly half of adults with HF in the U.S. are women. Despite the urgent need to generate high-quality, generalizable evidence to guide HF practice, women remain underrepresented in HF clinical trials, with little improvement over the last three decades. Several predictors of greater trial participation by women — such as older age at enrollment, trial funding from government sources, and woman trial leadership — have been identified; however, these associations require further studies. Sex- and gender-based enrollment barriers persist throughout the clinical trial lifespan, and directed trial-level and patient-level interventions to rectify these disparities are needed. Multisector collaborations among federal, academic, community, and industry partners should be leveraged to close these gaps and optimize HF care for all.
Funding
N.R. was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health [KL2TR001879]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CRediT authorship contribution statement
Nosheen Reza: Conceptualization, manuscript drafting, critical revision, visualization. Jadry Gruen: Visualization. Biykem Bozkurt: Conceptualization, manuscript drafting, critical revision, supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Contributor Information
Nosheen Reza, Email: nosheen.reza@pennmedicine.upenn.edu.
Jadry Gruen, Email: jadry.gruen@pennmedicine.upenn.edu.
Biykem Bozkurt, Email: bbozkurt@bcm.edu.
References
- 1.Virani S.S., et al. Heart disease and stroke Statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Dunlay S.M., Roger V.L., Redfield M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017;14(10):591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 3.Desai R.J., et al. Epidemiologic characterization of heart failure with reduced or preserved ejection fraction populations identified using medicare claims. Am. J. Med. 2021;134(4):e241–e251. doi: 10.1016/j.amjmed.2020.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Lam C.S.P., et al. Sex differences in heart failure. Eur. Heart J. 2019;40(47):3859–3868c. doi: 10.1093/eurheartj/ehz835. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy C.R. Historical background of clinical trials involving women and minorities. Acad. Med. 1994;69(9):695–698. doi: 10.1097/00001888-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Liu K.A., Mager N.A.D. Women’s involvement in clinical trials: historical perspective and future implications. Pharm. Pract. (Granada) 2016;14(1):708. doi: 10.18549/PharmPract.2016.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merkatz R.B. Inclusion of women in clinical trials: a historical overview of scientific, ethical, and legal issues. J. Obstet. Gynecol. Neonatal. Nurs. 1998;27(1):78–84. doi: 10.1111/j.1552-6909.1998.tb02594.x. [DOI] [PubMed] [Google Scholar]
- 8.I. of M. (US) C. on E. and L. I. R. to the I. of W. in C. Studies. Mastroianni A.C., Faden R., Federman D. National Academies Press; US: 1994. NIH Revitalization Act of 1993 Public Law 103-43.https://www.ncbi.nlm.nih.gov/books/NBK236531/ Available: [Google Scholar]
- 9.Kim E.S.H., Carrigan T.P., Menon V. Enrollment of women in National Heart, Lung, and Blood Institute-funded cardiovascular randomized controlled trials fails to meet current federal mandates for inclusion. J. Am. Coll. Cardiol. 2008;52(8):672–673. doi: 10.1016/j.jacc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Vaduganathan M., et al. Do women and men respond similarly to therapies in contemporary heart failure clinical trials? JACC Heart Fail. 2019;7(3):267–271. doi: 10.1016/j.jchf.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ignaszewski M.T., Daugherty S.L., Russo A.M. Implantable cardioverter-defibrillators and cardiac resynchronization therapy in women. Heart Fail. Clin. 2019;15(1):109–125. doi: 10.1016/j.hfc.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Santangeli P., et al. Gender differences in clinical outcome and primary prevention defibrillator benefit in patients with severe left ventricular dysfunction: a systematic review and meta-analysis. Heart Rhythm. 2010;7(7):876–882. doi: 10.1016/j.hrthm.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 13.Os I., Bratland B., Dahlöf B., Gisholt K., Syvertsen J.O., Tretli S. Female sex as an important determinant of lisinopril-induced cough. Lancet. 1992;339(8789):372. doi: 10.1016/0140-6736(92)91694-4. [DOI] [PubMed] [Google Scholar]
- 14.Piña I.L. A better survival for women with heart failure? It’s not so simple. J. Am. Coll. Cardiol. 2003;42(12):2135–2138. doi: 10.1016/j.jacc.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Limacher M.C., Yusuf S. Cardiovascular Health and Disease in Women. LeJacq Communications; Greenwich, CT: 1993. Gender differences in presentation, morbidity and mortality in the studies of left ventricular dysfunction (SOLVD): a preliminary report; pp. 345–348. [Google Scholar]
- 16.Garg R., Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE inhibitor trials. JAMA. 1995;273(18):1450–1456. [PubMed] [Google Scholar]
- 17.Hsich E.M., et al. Should women receive left ventricular assist device support?: findings from INTERMACS. Circ. Heart Fail. 2012;5(2):234–240. doi: 10.1161/CIRCHEARTFAILURE.111.963272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albert C.M., et al. Sex differences in outcome after implantable cardioverter defibrillator implantation in nonischemic cardiomyopathy. Am. Heart J. 2008;156(2):367–372. doi: 10.1016/j.ahj.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 19.C. I. of H. R. Government of Canada How to integrate sex and gender into research - CIHR. 2018. https://cihr-irsc.gc.ca/e/50836.html Feb. 12. (accessed Nov. 11, 2021)
- 20.Lindenfeld J., Krause-Steinrauf H., Salerno J. Where are all the women with heart failure? J. Am. Coll. Cardiol. 1997;30(6):1417–1419. doi: 10.1016/s0735-1097(97)00343-4. [DOI] [PubMed] [Google Scholar]
- 21.Harris D.J., Douglas P.S. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N. Engl. J. Med. 2000;343(7):475–480. doi: 10.1056/NEJM200008173430706. [DOI] [PubMed] [Google Scholar]
- 22.Meinert C.L., Gilpin A.K. Enrollment of women in cardiovascular clinical trials. N. Engl. J. Med. 2000;343(26):1972. author reply 1972-1973, Dec. [PubMed] [Google Scholar]
- 23.Cheung A.M., Naglie G. Enrollment of women in cardiovascular clinical trials. N. Engl. J. Med. 2000;343(26):1972–1973. doi: 10.1056/NEJM200012283432617. [DOI] [PubMed] [Google Scholar]
- 24.Scott P.E., et al. Participation of women in clinical trials supporting FDA approval of cardiovascular drugs. J. Am. Coll. Cardiol. 2018;71(18):1960–1969. doi: 10.1016/j.jacc.2018.02.070. [DOI] [PubMed] [Google Scholar]
- 25.Heiat A., Gross C.P., Krumholz H.M. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch. Intern. Med. 2002;162(15):1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 26.Gong I.Y., et al. Temporal trends of women enrollment in major cardiovascular randomized clinical trials. Can. J. Cardiol. 2019;35(5):653–660. doi: 10.1016/j.cjca.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Pressler S.J. Women with heart failure are disproportionately studied as compared with prevalence: a review of published studies from 2013. J. Cardiovasc. Nurs. 2016;31(1):84–88. doi: 10.1097/JCN.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 28.ClinicalTrials.gov Background - ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/about-site/background
- 29.Jin X., Chandramouli C., Allocco B., Gong E., Lam C.S.P., Yan L.L. Women’s participation in cardiovascular clinical trials from 2010 to 2017. Circulation. 2020;141(7):540–548. doi: 10.1161/CIRCULATIONAHA.119.043594. [DOI] [PubMed] [Google Scholar]
- 30.Tsang W., Alter D.A., Wijeysundera H.C., Zhang T., Ko D.T. The impact of cardiovascular disease prevalence on women’s enrollment in landmark randomized cardiovascular trials: a systematic review. J. Gen. Intern. Med. 2012;27(1):93–98. doi: 10.1007/s11606-011-1768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan M.S., et al. Ten-year trends in enrollment of women and minorities in pivotal trials supporting recent US Food and Drug Administration approval of novel cardiometabolic drugs. J. Am. Heart Assoc. 2020;9(11) doi: 10.1161/JAHA.119.015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tahhan A.S., et al. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: a systematic review. JAMA Cardiol. 2018;3(10):1011–1019. doi: 10.1001/jamacardio.2018.2559. [DOI] [PubMed] [Google Scholar]
- 33.Tahhan A.Samman, Vaduganathan M., Kumar S., Okafor M., Greene S.J., Butler J. Design elements and enrollment patterns of contemporary trials in heart failure with preserved ejection fraction: a systematic review. JACC Heart Fail. 2018;6(8):714–717. doi: 10.1016/j.jchf.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borlaug B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020;17(9):559–573. doi: 10.1038/s41569-020-0363-2. [DOI] [PubMed] [Google Scholar]
- 35.Hunt S.A., et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and Management of Heart Failure in adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 36.Sardar M.R., Badri M., Prince C.T., Seltzer J., Kowey P.R. Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA Intern. Med. 2014;174(11):1868–1870. doi: 10.1001/jamainternmed.2014.4758. [DOI] [PubMed] [Google Scholar]
- 37.Reza N., et al. Representation of women authors in international heart failure guidelines and contemporary clinical trials. Circ Heart Fail. 2020;13(8) doi: 10.1161/CIRCHEARTFAILURE.119.006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitelaw S., et al. Trial characteristics associated with under-enrolment of females in randomized controlled trials of heart failure with reduced ejection fraction: a systematic review. Eur. J. Heart Fail. 2021;23(1):15–24. doi: 10.1002/ejhf.2034. [DOI] [PubMed] [Google Scholar]
- 39.Harrison J.M., et al. Refusal to participate in heart failure studies: do age and gender matter? J. Clin. Nurs. 2016;25(7–8):983–991. doi: 10.1111/jocn.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook N.L., Ayanian J.Z., Orav E.J., Hicks L.S. Differences in specialist consultations for cardiovascular disease by race, ethnicity, gender, insurance status, and site of primary care. Circulation. 2009;119(18):2463–2470. doi: 10.1161/CIRCULATIONAHA.108.825133. [DOI] [PubMed] [Google Scholar]
- 41.Peterson E.D., Lytle B.L., Biswas M.S., Coombs L. Willingness to participate in cardiac trials. Am. J. Geriatr. Cardiol. 2004;13(1):11–15. doi: 10.1111/j.1076-7460.2004.01709.x. [DOI] [PubMed] [Google Scholar]
- 42.Lobato L., Bethony J.M., Pereira F.B., Grahek S.L., Diemert D., Gazzinelli M.F. Impact of gender on the decision to participate in a clinical trial: a cross-sectional study. BMC Public Health. 2014;14:1156. doi: 10.1186/1471-2458-14-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sisk J.E., Horowitz C.R., Wang J.J., McLaughlin M.A., Hebert P.L., Tuzzio L. The success of recruiting minorities, women, and elderly into a randomized controlled effectiveness trial. Mt. Sinai J. Med. 2008;75(1):37–43. doi: 10.1002/msj.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomon S.D., et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N. Engl. J. Med. 2019;381(17):1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 45.McMurray J.J.V., et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation. 2020;141(5):338–351. doi: 10.1161/CIRCULATIONAHA.119.044491. [DOI] [PubMed] [Google Scholar]
- 46.Khan M.S., et al. Reporting and interpretation of subgroup analyses in heart failure randomized controlled trials. ESC Heart Fail. 2021;8(1):26–36. doi: 10.1002/ehf2.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michos E.D., et al. Improving the enrollment of women and racially/ethnically diverse populations in cardiovascular clinical trials: an ASPC practice statement. Am. J. Prev. Cardiol. 2021;8 doi: 10.1016/j.ajpc.2021.100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greene S.J., et al. Representativeness of a heart failure trial by race and sex: results from ASCEND-HF and GWTG-HF. JACC Heart Fail. 2019;7(11):980–992. doi: 10.1016/j.jchf.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Groenewegen A., Rutten F.H., Mosterd A., Hoes A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020;22(8):1342–1356. doi: 10.1002/ejhf.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pacheco C., Bairey Merz C.N. Women in cardiovascular clinical trials-what are the barriers to address to improve enrollment? Can. J. Cardiol. 2019;35(5):552–554. doi: 10.1016/j.cjca.2019.03.018. May. [DOI] [PubMed] [Google Scholar]
- 51.Whitelaw S., et al. Characteristics of heart failure trials associated with under-representation of women as Lead authors. J. Am. Coll. Cardiol. 2020;76(17):1919–1930. doi: 10.1016/j.jacc.2020.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denby K.J., Szpakowski N., Silver J., Walsh M.N., Nissen S., Cho L. Representation of women in cardiovascular clinical trial leadership. JAMA Intern. Med. 2020;180(10):1382–1383. doi: 10.1001/jamainternmed.2020.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eliya Y., Whitelaw S., Thabane L., Voors A.A., Douglas P.S., Van Spall H.G.C. Temporal trends and clinical trial characteristics associated with the inclusion of women in heart failure trial steering committees: a systematic review. Circ Heart Fail. 2021;14(8) doi: 10.1161/CIRCHEARTFAILURE.120.008064. [DOI] [PubMed] [Google Scholar]
- 54.O. of the Commissioner . FDA; 2020. FDA Research, Policy, and Workshops on Women in Clinical Trials.https://www.fda.gov/science-research/womens-health-research/fda-research-policy-and-workshops-women-clinical-trials Available: [Google Scholar]