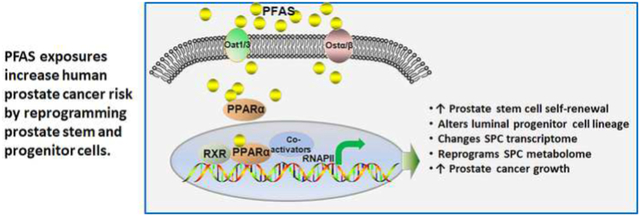
Abstract
Per- and polyfluorinated alkyl substances (PFAS) are a large family of widely used synthetic chemicals that are environmentally and biologically persistent and present in most individuals. Chronic PFAS exposure have been linked to increased prostate cancer risk in occupational settings, however, underlying mechanisms have not been interrogated. Herein we examined exposure of normal human prostate stem-progenitor cells (SPCs) to 10 nM PFOA or PFOS using serial passage of prostasphere cultures. Exposure to either PFAS for 3–4 weeks increased spheroid numbers and size indicative of elevated stem cell self-renewal and progenitor cell proliferation. Transcriptome analysis using single-cell RNA sequencing (scRNA-seq) showed 1) SPC expression of PPARs and RXRs able to mediate PFAS effects, 2) the emergence of a new cell cluster of aberrantly differentiated luminal progenitor cells upon PFOS/PFOA exposure, and 3) enrichment of cancer-associated signaling pathways. Metabolomic analysis of PFAS-exposed prostaspheres revealed increased glycolytic pathways including the Warburg effect as well as strongly enrichment of serine and glycine metabolism which may promote a pre-malignant SPC fate. Finally, growth of in vivo xenografts of tumorigenic RWPE-2 human prostate cells, shown to contain cancer stem-like cells, was markedly enhanced by daily PFOS feeding to nude mice hosts. Together, these findings are the first to identify human prostate SPCs as direct PFAS targets with resultant reprogrammed transcriptomes and metabolomes that augment a preneoplastic state and may contribute to an elevated prostate cancer risk with chronic exposures.
Keywords: prostate, stem cell, progenitor cell, PFAS, PFOS, PFOA
Graphical Abstract
1. INTRODUCTION
Per- and polyfluoroalkyl substances (PFAS) are a family of chemicals containing long hydrophobic (8-carbon) chains fully saturated with fluorine atoms (i.e., perfluoroalkyl chains) and a hydrophilic polar functional group [1]. These chemicals are thermally stable and lipid- and water-repelling, prompting their extensive use since the 1970s in household products such as carpets, Gortex products, and non-stick cookware. Such widespread usage has led to accumulation of these persistent organic pollutants in the environment [2–4]. Two PFAS, perflurooctanoate acid (PFOA) and perfluorooctanesulfonic acid (PFOS), are the most abundant in the environment and are a major source of exposure to humans through food and drinking water. Unfortunately, these and newer PFAS substitutes (e.g., GenEx) do not readily degrade and are commonly referred to as “forever chemicals”. An estimated 99% of Americans are exposed to and/or have these chemicals in their body [5–7]. Occupational PFAS exposures and those living near production facilities or contamination sites, including military bases, are associated with the highest body burdens [8]. Wastewater treatment plants are a major sink for PFAS by receiving PFAS-impacted wastewater. Landfill leachate, wastewater effluent, biosolids application, and groundwater recharge using surface runoff re-introduces PFAS to water sources including drinking water. Crucially, PFAS exposures can exert adverse health outcomes in humans. In a recent large report from the Center for Disease Control, diseases linked to chronic PFAS exposures include liver damage, cardiovascular abnormalities, altered immune function, and developmental and reproductive disorders based largely on epidemiology studies supported by laboratory research [9]. Additional studies have linked PFAS exposures to type 2 diabetes [10, 11], thyroid disruption [12] and cancers, particularly testicular and kidney cancer [13, 14]. The U.S. Environmental Protection Agency recently added PFAS to the 2021 Toxic Release Inventory [15], and the International Agency for Research on Cancer [16] has listed PFOA as a Class 2B carcinogen.
Prostate cancer is the most commonly diagnosed non-cutaneous cancer in American men and the second leading cause of cancer-related deaths [17], although its etiology remains elusive. While cancers are primarily a result of germline and/or somatic mutations, considerable evidence has identified environmental contributions to carcinogenesis in multiple organs including prostate cancer [18]. As the prostate and prostate cancer are androgen-dependent and influenced by multiple other hormones, it is logical that endocrine disrupting chemicals (EDCs) that mimic or interfere with hormone action may influence prostate growth and carcinogenic risk. Indeed, this has been shown for several EDCs including bisphenol A (BPA), arsenic, cadmium, dioxin, several pesticides, and PCBs, among others [19]. Of relevance to the present research, several epidemiology studies have indicated that prostate cancer risk and mortality increase with chronic PFAS exposure in both occupational settings and in men living near high contamination zones [20–23]. Men with a familial history of prostate cancer were particularly affected, suggesting a gene-environment interaction in disease promotion [23]. Despite this evidence, there are no laboratory studies that identify cellular targets or mechanistic data on the molecular underpinnings of PFAS chemicals in the prostate.
The prostate epithelium contains three cell types: luminal (majority), basal, and neuroendocrine (infrequent). These cells derive from rare stem cell population(s) [24, 25] that, during adult tissue replenishment, undergo asymmetric cell division to self-renew and generate bipotent progenitors that lineage commit and, upon amplification, differentiate to mature epithelial lineages. Further, prostate cancer contains cancer stem-like cells that continuously seed and maintain tumor growth [26, 27]. EDCs have been shown to target stem cells, which are compelling cancer targets in organisms during development and cancer progression [18, 28, 29]. Previous work from our laboratory has identified the prostate epithelial stem-progenitor cell (SPC) population as direct hormone and EDC targets, with environmentally relevant doses of BPA perturbing self-renewal and lineage commitment, altering gene transcription and reprogramming the SPC epigenome to increase carcinogenic risk [30–33] and environmentally relevant levels of arsenic increasing stem cell numbers and impairing the autophagy-lysosome pathway with subsequent transformation of SPCs [34].
In this context, the goals of the present study were to determine if PFAS exposures can target the human prostate SPC, modify their behaviors and in so doing, influence prostate cancer risk. For these studies, we employed primary prostate epithelial cells from normal organ donors as well as the cancerous human prostate cell line RWPE-2. We interrogated the human SPC for peroxisome proliferator-activated receptors (PPARs) and their obligate partners, retinoid X receptors (RXRs) known to mediate many PFAS effects [35–37], and serially passaged prostate SPC using spheroid culture in the presence of 10 nM PFOA or PFOS to evaluate their self-renewal capacity and proliferative responses to chronic exposure at environmentally relevant levels. Lineage commitment alterations were evaluated by scRNA-seq and PFAS-targeted gene pathways were identified. The PFAS-exposed metabolome of the SPC populations were next interrogated using gas chromatography - mass spectroscopy (GC/MS). Finally, the effects of PFOS exposure on RWPE-2 derived prostate tumors in vivo were examined. Together, the findings implicate prostate SPCs as direct PFAS targets and raise the intriguing possibility that PFAS-induced reprogramming contributes to their transformation and tumor-initiating capacity.
2. MATERIALS AND METHODS
2.1. Prostate Epithelial Cells and Prostasphere Culture
Primary normal human prostate epithelial cells (PrECs) were obtained from young (19–21 years of age) disease-free organ donors (Lonza, Walkersville, MD, USA) at passage 2–3 and cultured at 37 °C, 5% CO2 in ProstaLife Epithelial Cell Growth Medium (PrEGM) (LifeLine Cell Technology, Frederick, MD, USA) on fibronectin-coated flasks. Prostaspheres were cultured from PrECs as previously described and confirmed to be clonally derived spheroids of stem/progenitor cells [38–40]. Briefly, 1 × 105 PrEC cells were resuspended in 1:1 PrEGM:Matrigel (Cat# CB356238, Corning/BD Biosciences, Corning, NY, USA) and the suspension plated around the bottom well edge of 12-well plates. After the cell-Matrigel mix solidified, 1 mL PrEGM with or without 10 nM of PFOS or PFOA (Sigma-Aldrich, St. Louis, MO, USA; Cat #77282 for PFOS; Cat#171468 for PFOA) was added and replenished every 48 hr. Prostaspheres were cultured for 1 wk at 37 °C in 5% CO2 and serially passaged for 3–4 wks to provide extended PFOS/PFOA exposure. For serial passage, prostaspheres were harvested from Matrigel by digestion with 2 mL dispase (StemCell Technologies, Vancouver, BC, Canada) for 30 min and the spheres dispersed into single cells by digestion with 1 mL 0.05% trypsin-EDTA (Cat# 25300120, Gibco/Fisher Scientific, Waltham, MA, USA). Similar cell numbers for each treatment group (4 × 105) were re-plated in Matrigel culture to form the next generation spheroids. PFAS-exposed and control prostaspheres were serially passaged up to 6 generations. Prostasphere number and size at the end of each 7-day culture period were assessed using an automated digital image processing algorithm as previously detailed [31].
The human prostate cancer cell line RWPE-2 (RWPE-kRAS) was obtained from ATCC (American Type Culture Collection, Manassas, VA, USA) and cultured at 37 °C, 5% CO2 in Gibco Keratinocyte SFM 1X growth media with glutamine (Cat# 17005042, Gibco/Fisher Scientific, Waltham, MA, USA). Prior to reaching confluence, the 2D cultured cells were transferred to 3D culture in Matrigel as described above to enrich for the cancer stem cell population. After 7 days in spheroid culture, some spheroids were switched to organoid culture medium (PrEGM containing 5% charcoal-stripped fetal bovine serum (Gibco/Fisher Scientific, Waltham, MA, USA), 1 nM Y-27632 (Apebio Technology, Houston, TX. USA) and 1 nM R1881 (Sigma-Aldrich, St. Louis, MO, USA)) and continued for 14 days.
2.2. Prostate Cancer Xenografts
RWPE-2 2D cultures were used for tumor xenografts in 4-week-old athymic nude male mice (Jackson Laboratory, Bar Harbor, ME, USA). Animal experiments and protocols were approved by the University of Illinois at Urbana-Champaign (IACUC Protocol #20159) and NIH standards for the use and care of animals were followed. Mice were fed AIN93M diet (Envigo Teklad Diets, Madison, WI, USA) ad libitum. RWPE-2 cells (2 × 106/graft) mixed in Matrigel were injected to the left and right flanks of the mice (N=8 mice/group) under anesthesia. Silastic tubes (1 cm, inside diameter 1.02 mm × outer diameter 2.16 mm; Dow Corning Corp, Midland, MI, USA) packed with testosterone (Sigma-Aldrich, St. Louis, MO, USA) were implanted to provide sufficient hormone for graft growth in the hypogonadal nude mice [38]. Animals were administered PFOS (Sigma-Aldrich, St. Louis, MO, USA) daily by oral gavage at 10 mg/kg BW. Animal weights were monitored twice weekly and tumor size measurements were performed 3x/week using a digital caliper and calculated using the formula V=0.5 × length × width2 [41]. Animals were euthanized at 5.5 wks according to IACUC regulations for maximum allowable tumor size.
2.3. Single-Cell Capture by the 10X Genomics Platform and Single-Cell RNA-Sequencing (sc-RNA-seq) Using NovaSeq 6000
PFAS-exposed and control passage 3 spheroids were dispersed into a single-cell suspension as described above. Single cells were separated and captured at a 10X Genomics station using Chromium Next GEM Single Cell 3ʹ v3 Reagent Kits (10X Genomics, CG000183, Pleasanton, CA, USA). After GEM generation and barcoding, mRNA was reverse transcribed into cDNA, and further amplified for library construction followed by RNA-seq using NovaSeq 6000 SP (Illumina, San Diego, CA, USA) at the University of Illinois Keck Center. Each lane of the sequencer generated 1.6 billion reads, 0.4 billion reads per sample, 0.1 million reads (paired-end, 28 nt + 91 nt) for each single-cell. We validated ~3500 single-cell cDNA libraries from each sample and ~7,500 genes per cell for data analysis.
ScRNA-seq data was aligned against hg38 reference genome sequence using 10X Cellranger pipeline. The aligned dataset was imported into Seurat package (Satijalab.org). Pre-processing of raw data included filtering out cell debris, unhealthy singlets, and potential doublets by setting cutoff based on the distribution of the feature RNA abundance (cutoff range was from 2000 to 7500; each data point indicates a singlet or single cell) and the percentage cutoff of mitochondrial genes within each data point was 10%. Post-filtered single cell feature expression values were normalized by the total expression through a global-scaling normalization method “LogNormalize”. The scale factor was set at the default value of 10,000. Subsequent feature selection was performed with default setting (nfeature = 2000) to gain a subset of features that reflect high cell-to-cell variation of the dataset. The expression value of each gene was scaled so that data points were centralized around the origin, and the variance across these data points equals to 1. Linear dimensional reduction was performed on the subset of feature genes selected by principal component analysis (PCA). Next, a resampling test inspired by the Jackstraw procedure was utilized to determine the PC number to include. The data points or single cells were finally clustered based on PCs determined previously using a graph-based clustering approach [42]. Pre-defined PCs were introduced to the clustering analysis to plot uniform manifold approximation and projection (UMAP) for improved cluster visualization. Stem cell cluster identification was based on previously identified biomarkers [40].
Gene set enrichment analysis (GSEA) was performed on normalized gene expression data generated by RNA-seq using the Broad Institute’s Molecular Signatures Database (gsea-msigdb.org) [43]. Analyses were run using the gene expression data against two gene sets: C2: curated gene sets and CP: BIOCARTA: BioCarta gene sets. The false discovery rate (FDR) was calculated by comparing the actual data with 1000 Monte Carlo simulations. The familywise error rate is a conservative correction that seeks to ensure that reported results do not include any false-positive gene sets.
2.4. GC/MS-based metabolic profiling of prostaspheres
Cell metabolites were extracted from prostaspheres serially passaged for 4 weeks in vehicle, PFOA or PFOS (10nM) using a 1:2:1 mixture of acetonitrile (Sigma-Aldrich, St. Louis, MO, USA):isopropanol (Fisher Scientific, Waltham, MA, USA):water. Extracts were sent to the University of Illinois at Urbana-Champaign’s Metabolomics Core Facility to detect and quantify metabolites using GC/MS. Metabolic profiles were obtained from an Agilent GC-MS system (Agilent 7890 gas chromatograph, Agilent 5975 MSD, and HP 7683B autosampler, Lexington, MA, USA).
The spectra of all chromatogram peaks were evaluated using the AMDIS 2.71 (chemdata.nist.org) and a custom-built database with 460 unique metabolites. All known artificial peaks were identified and removed before data mining. Individual metabolomic data sets for each treatment were separated and grouped into files to make comparisons between treatment conditions using MetaboAnalyst software (metaboanalyst.ca) [44]. Sample class annotations consisted of Veh vs. PFOA, Veh vs. PFOS and Veh vs. PFOA/PFOS combined (PFAS). Files were uploaded to the Enrichment Analysis tool of MetaboAnalyst software version 5.0. Data were not normalized, transformed, or scaled but were compared to the SMPDB reference metabolome, which represents metabolite values from normal metabolic human pathways. The top 25 enriched metabolic pathways and associated metabolites were retrieved along with their p-values and enrichment ratios.
2.5. Statistics
Spheroid data were analyzed using GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA, USA) and presented as mean ± SEM. Statistically significant differences were assessed by Student’s t-test or, with multiple groups, ANOVA followed by Tukey-Kramer post-hoc tests. For xenograft tumor growth rate data over time, a two-way ANOVA followed by Tukey’s multiple comparison test was used. For all data, P < 0.05 was considered significant.
3. RESULTS
3.1. Chronic PFAS exposures augment prostate stem and progenitor cell self-renewal and proliferation
To determine the effects of PFAS on primary SPCs grown from disease-free human prostate primary epithelial cell cultures, we studied two unique properties of stem and progenitor cells: self-renewal and differentiation. The sphere formation assay enriches for stem cells based on their unique capability to survive and clonally expand in 3D serum-free culture while differentiated primary cells undergo apoptosis or senescence. In addition, spheroid serial passage demonstrates their ability to maintain and expand a self-renewing stem cell population [45]. The number and size of prostaspheres represents stem cell self-renewal and daughter progenitor cell proliferation capacity, respectively [38, 39]. Prostaspheres were exposed to an environmentally relevant level (10 nM) of PFOA or PFOS through 3–4 serial passages to model chronic exposure. With 3–4 weeks of PFAS exposure, a significant increase in total sphere number was observed in response to either PFAS indicating augmentation of stem cell symmetric self-renewal (Fig. 1A, B). Size analysis by digital imaging revealed significant increases in small (40–60 μm), medium (60–80 μm) and large sized (>80 μm) spheres, with the large-size increases indicative of increased progenitor cell proliferative. This together suggests that both prostate stem and daughter progenitors are direct PFAS targets.
Figure 1: Effects of PFAS exposures on prostasphere formation and size.
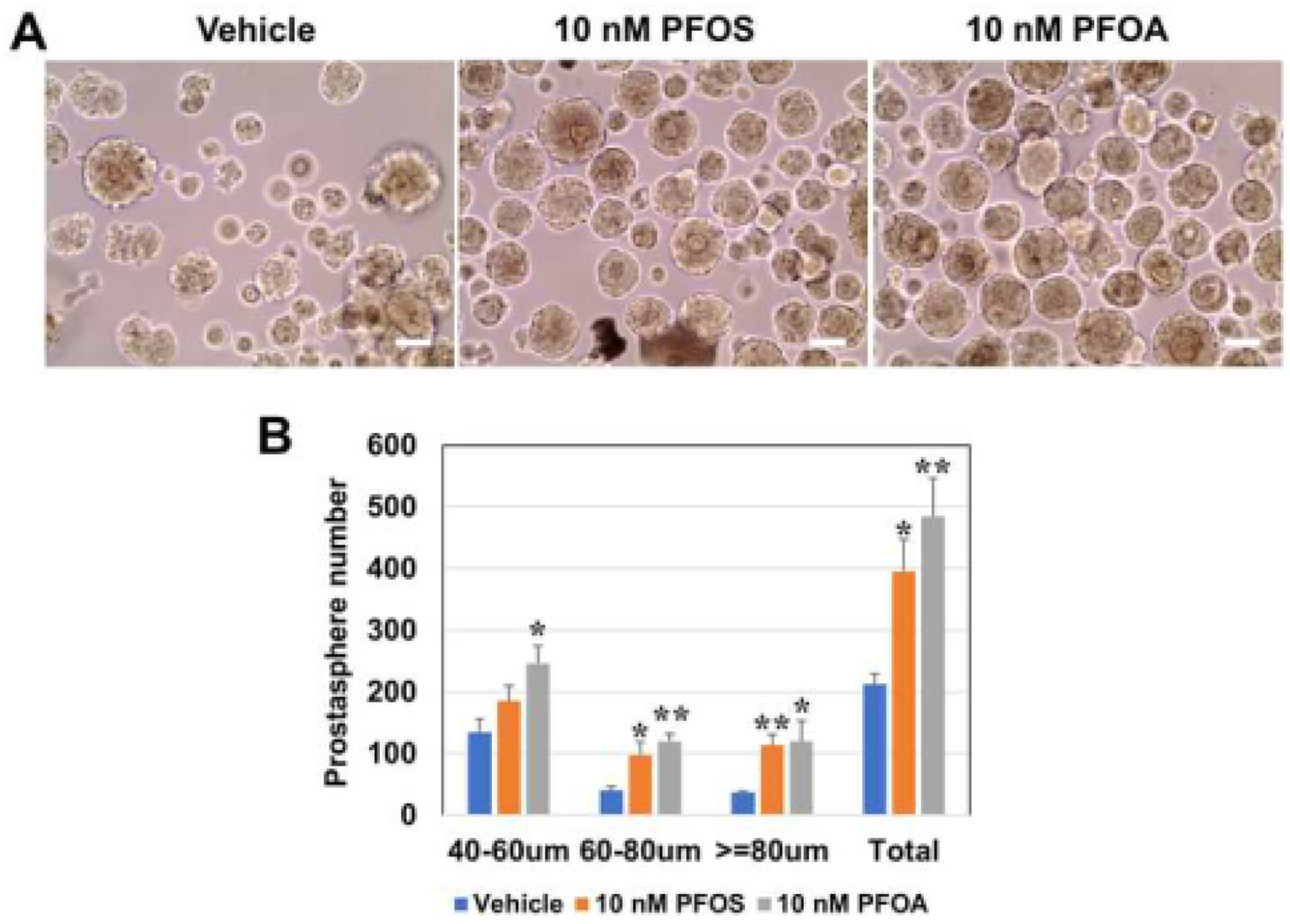
A: Human PrEC-derived prostaspheres were culture in 3–4 serial passages (1 week each) in the absence or presence of 10 nM of PFOS or PFOA or in vehicle as controls. White bar = 50μm. B: Total spheroid numbers and sizes across 20 μm increments were quantitated by an automated digital image processing algorithm [38]. Treatment with either PFAS compound markedly increased prostasphere numbers formed as well as their size across the size spectrum, indicating that prostate stem/progenitor cells are the direct PFAS cell targets. N=4; bars represent the mean ± SEM. * Indicates P<0.05 and ** indicates P<0.01 versus vehicle.
3.2. RNA-sequencing identifies PPAR and RXR expression in prostate stem and progenitor cells
PFAS can activate cellular responses in many target tissues by engaging PPARs with PFOA and PFOS predominantly activating the alpha isoform (PPARα) compared to the gamma or delta isoform (PPARγ, PPARδ) [35–37]. As such, we examined prostaspheres for gene expression of PPARs and RXRs, their obligate binding partners, using scRNA-seq and bulk RNA-sequencing. Transcriptome analysis identified two cell clusters in spheroids: a smaller one enriched in prostate stemness genes, PITX1 and SOX2 [40], and a larger cluster of prostate progenitor cells (Fig. 2A). scRNA-seq of prostaspheres revealed that both stem and progenitor cell populations solidly expressed PPARα while PPARγ and PPARδ expression were comparatively lower (Fig. 2B) suggesting PPARα as the dominant PPAR. We next interrogated a previously derived [40] deep RNA-seq data set from FACS-sorted prostasphere stem and progenitor populations to identify average reads for the PPARs and RXRs. We confirmed that all three PPARs were expressed in stem and progenitor cells, although using this approach, the expression levels were similar for PPARα, PPARγ and PPARδ (Fig. 2C). Importantly, RXRα and RXRβ, but not RARγ, were present in both populations. Levels of PPAR and RXR were just below expression levels for glucocorticoid receptor (GR), the highest expressed nuclear receptor family member in prostasphere cells. Together, these data indicate that the prostate stem and progenitor cell populations have the necessary receptors to mediate PFAS actions within the cells.
Figure 2: Human prostate stem and progenitor cells express PPARs and RXRs.
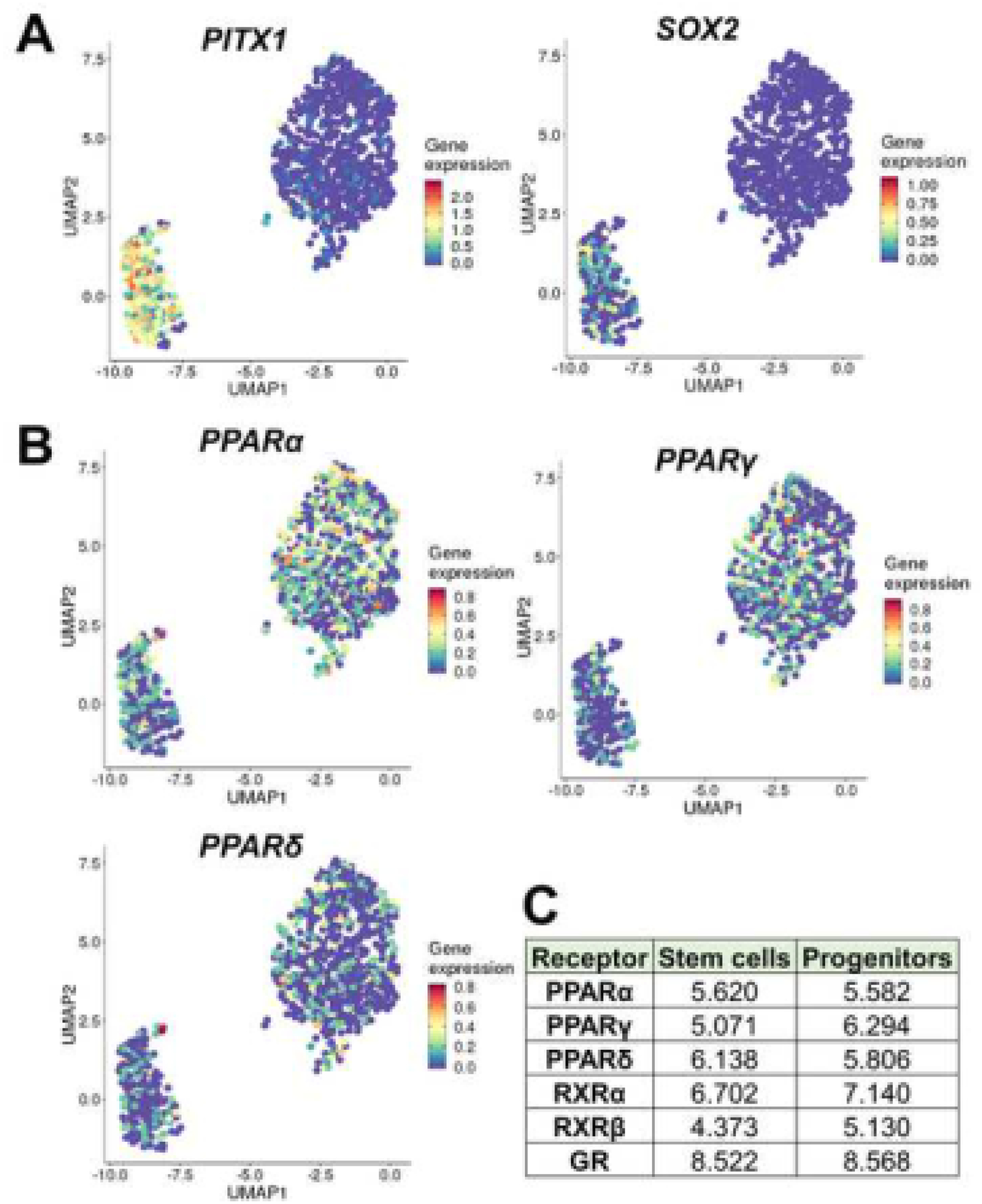
A-B: Disease-free human primary PrECs were transferred to 3D Matrigel culture to form prostaspheres and after 3 serial passages, scRNA-seq was used to identify relative gene expression in the stem and progenitor cell populations. A: UMAP clustering was used to identify 2 major cell clusters with expression of PITX1 and SOX2, known prostate stem cell genes, enriched in the smaller cluster thus identifying the minor population of stem cells. The larger cluster expressed early differentiation genes associated with prostate progenitor cells [40]. B: Expression levels of PPARα, PPARγ and PPARδ were identified in both the stem and progenitor populations with PPARα and PPARγ at somewhat higher levels than PPARδ. C: RNA-seq reads of PPARs and RXRs in prostaspheres sorted for stem and progenitor populations by FACS followed by deep RNA-seq as previously described [63]. PPARα and PPARδ levels were similar between the stem and progenitor populations while PPARγ expression was higher in progenitor cells, similar to scRNA-seq data. RXRα was expressed at higher levels that RXRβ, and approached levels of GR, the top expressed nuclear receptor.
3.3. Chronic PFAS exposures alter luminal progenitor cell differentiation
To examine hierarchical cell clusters within the stem cell-derived prostaspheres, PrEC-derived prostaspheres were serially passaged without or with 10 nM of PFOS or PFOA and passage 3 spheroids were dispersed for scRNA-seq using the 10X Genomics platform. Transcriptome analysis by UMAP plots revealed cell sub-clusters in the PFOS (5 clusters) and PFOA (8 clusters) exposed prostaspheres integrated with respective vehicle-exposed prostaspheres (Fig. 3A, B). Importantly, UMAP plots that identify the vehicle cells vs PFOS, or PFOA-exposed cells revealed the appearance of a unique cell cluster in PFAS-exposed spheroids (Fig. 3C, D). Both the PFOS-exposed targeted cluster 3 (Fig. 3A, C) and PFOA-exposed targeted cluster 5 (Fig. 3B, D) express luminal keratin genes KRT8/18, lack stemness and basal keratin gene expression [40] and show similarities to cluster 0 in vehicle-exposed spheres (Fig. 3E, F) which are characterized as early-stage luminal progenitor cells. This suggests that PFAS-exposure results in an aberrant differentiation program of luminal cells at the progenitor cell stage.
Figure 3: PFOS and PFOA reprogram progenitor cell differentiation in exposed prostaspheres.

Prostaspheres were serially passaged in the absence or presence of 10nM PFOS or PFOA. Passage 3 spheroids were dispersed for scRNA-seq using the 10X Genomics platform. A-B: UMAP plots of integrated PFOS and vehicle (A) and PFOA and Vehicle (B) transcriptomes identified 5 and 8 unique cell clusters, respectively. C-D: The same UMAP plots as in A and B are highlighted for vehicle-exposed cells (blue) and PFOS (C) or PFOA (D) exposed cells (pink). Green arrows point to unique cell clusters that emerge in the PFAS-exposed spheroids that are not present in the vehicle treated prostaspheres. E-F: PFOS exposure (E) introduced cluster 3 (A&C) while PFOA exposure introduced cluster 5 (B&D). Dot plots of keratin gene expression analysis reveal that both PFAS-targeted cell clusters express luminal keratin genes KRT8/18, lack stemness (KRT80, 16A) and basal (KRT5) keratin gene expression and most closely align with cluster 0 in vehicle-exposed spheres, characterized as early-stage luminal progenitor cells.
GSEA of genes expressed in PFOA and PFOS-exposed prostaspheres versus their respective vehicle controls revealed significant enrichment of pathways involved in cell replication including E2F targets, G2M checkpoint and mitotic spindle in both PFAS exposure groups which supports the increased proliferative responses observed in the spheroids (Fig 4). In addition, both PFAS exposures significantly increased TNFα via NFκB pathways and k-RAS signaling in spheroids, both connected to carcinogenic pathways. The top 20 enriched signaling pathways for each chemical highlight these as well as inflammatory-associated pathways (IL-2, IL-6, TGFβ, inflammatory response) and metabolic pathways (glycolysis, oxidative phosphorylation) (Table 1).
Figure 4: The top 5 enriched gene signaling pathways in prostaspheres by PFOA and PFOS exposures.
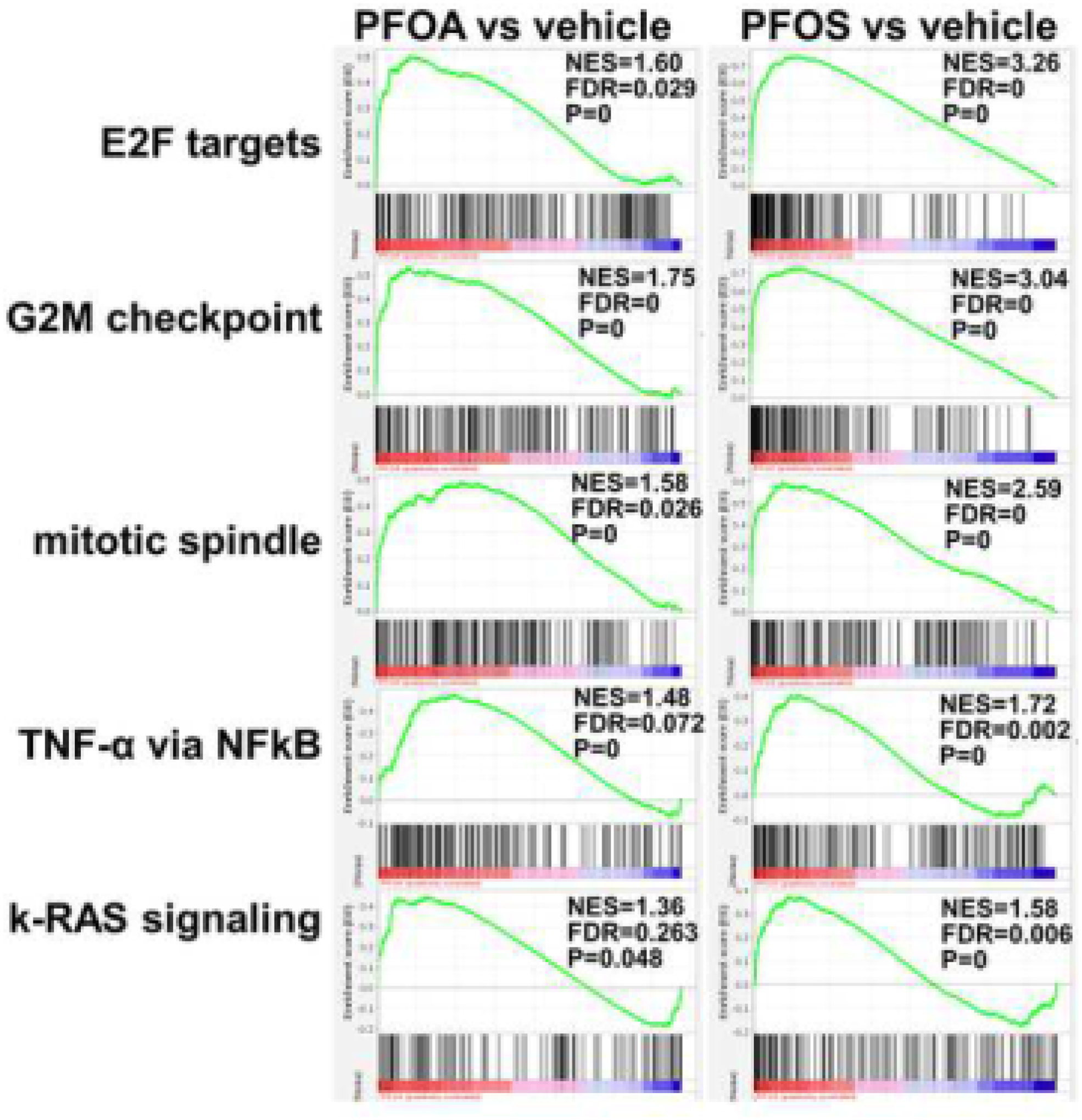
GSEA of scRNA-seq transcriptomes were used to identify the top common gene signaling pathways enriched in prostaspheres exposed for 3 wks to PFOA (left, red) or PFAS (right, red) as compared to vehicle treatments (blue). Exposure to either PFAS significantly upregulated cell cycle pathways including E2F targets, G2M checkpoint and mitotic spindle which aligns with the increased cell proliferation observed in Figure 1. Enhancement of oncogenic pathways, including NFκB and k-RAS signaling, suggesting that these PFAS exposures have potential for initiating progenitor cell transformation. NES = normalized enrichment score, FDR = false discovery rate.
Table 1.
Top 20 enriched gene signaling pathways in PFOA- and PFOS-exposed prostaspheres compared to Vehicle controls.
| PFOA vs Vehicle | PFOS vs Vehicle |
|---|---|
| G2M_CHECKPOINT | E2F_TARGETS |
| E2F_TARGETS | G2M_CHECKPOINT |
| MITOTIC_SPINDLE | MYC_TARGETS_V1 |
| TNFA_SIGNALING_VIA_NFKB | MITOTIC_SPINDLE |
| KRAS_SIGNALING_DN | MYC_TARGETS_V2 |
| IL6_JAK_STAT3_SIGNALING | EPITHELIAL_MESENCHYMAL_TRANSITION |
| SPERMATOGENESIS | DNA_REPAIR |
| ESTROGEN_RESPONSE_EARLY | TNFA_SIGNALING_VIA_NFKB |
| PANCREAS_BETA_CELLS | OXIDATIVE_PHOSPHORYLATlON |
| GLYCOLYSIS | KRAS_SIGNALING_UP |
| UV_RESPONSE_UP | SPERMATOGENESIS |
| IL2_STATS_SIGNALING | ALLOGRAFT_REJECTION |
| ALLOGRAFT_REJECTION | MTORC1_SIGNALING |
| MYOGENESIS | APICAL_JUNCTION |
| ESTROGEN_RESPONSE_LATE | ANGIOGENESIS |
| UV_RESPONSE_DN | INFLAMMATORY_RESPONSE |
| NOTCH_SIGNALING | IL6_JAK_STAT3_SIGNALING |
| HEDGEHOG_SIGNALING | TGF_BETA_SIGNALING |
| HYPOXIA | IL2_STAT5_SIGNALING |
| INFLAMMATORY_RESPONSE | COMPLEMENT |
RNA-seq followed by GSEA was used to identify the top 20 gene pathways enriched in prostaspheres exposed to 10 nM PFOA or PFOS through 3 serial passages as compared to vehicle controls. All pathways listed were considered significant with a false discovery rate (FDR) < 0.25.
3.4. PFAS alters the prostasphere metabolome
PPARs play key roles in regulating cellular metabolism and since the transcriptome data indicated PFAS-induced shifts in metabolic pathways, we next analyzed the prostasphere metabolites following 4 weeks of exposure to PFOA or PFOS using GC/MS. The top enriched metabolite set for the PFAS exposures compared to vehicle were glycine and serine metabolism with enhancement of glucose metabolism through the Warburg effect (Fig. 5A). The top individual metabolites significantly enhanced by the combined PFAS exposures are shown in Fig. 5B and include glycerol, glutamic acid, citric acid, urea, serine, alanine, and glucose. It is important to note that PFOS and PFOA, while overall producing a glycolytic state had distinct profiles for the individual metabolites with PFOA exposure resulting in the greatest changes over a wider range of metabolites as compared to PFOS (Table 2). Further, GSEA of the scRNA-seq data identified genes involved in glycolysis as significantly upregulated by PFOA exposure (Fig. 5C). Together, these findings show that PFAS exposures drive a metabolome shift that favors altered SPC proliferation and cell fate decisions and reprograms a metabolome in prostate SPCs that can act as a determinant in the formation of a malignant state.
Figure 5: Enriched metabolites in PFAS-exposed prostaspheres.
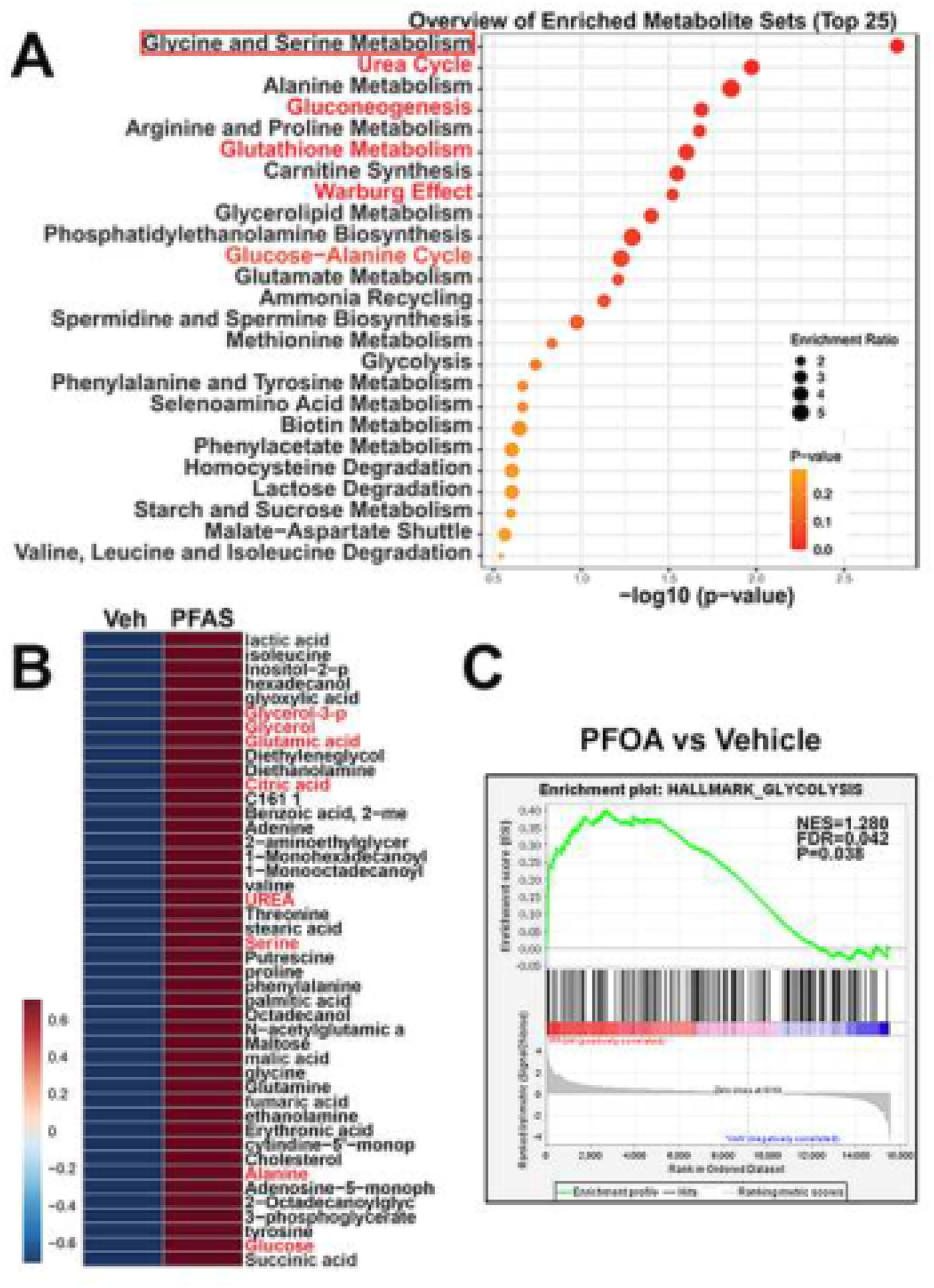
PrEC-derived prostaspheres were cultured through 4 serial passages in the absence or presence of 10 nM of PFOS or PFOA. Passage 4 spheres were analyzed by GC-MS for all metabolites including amino acids. A: The top 25 enriched metabolite sets in PFAS-exposed spheroids compared to vehicles reveals glycine and serine metabolism as the top enriched metabolites. Other markedly enriched metabolite sets include urea, gluconeogenesis, glutathione metabolism, the glucose-alanine cycle, alanine, arginine and proline metabolism and metabolites involved in the Warburg effect. B: A heat map of the most enriched individual metabolites in PFAS vs vehicle exposed spheroids. C: GSEA of transcriptome data in PFOA vs vehicle-exposed prostaspheres reveals a significant enrichment of genes involved in glycolysis following PFOA exposure.
Table 2.
Metabolite enrichment ratios for PFOA- or PFOS-exposed prostaspheres over Vehicle controls.
| Metabolites | PFOA/Vehicle | PFOS/Vehicle | PFAS/Vehicle |
|---|---|---|---|
| urea | 4.434 | 1.871 | 3.153 |
| serine | 3.451 | 2.099 | 2.775 |
| maltose | 3.578 | 1.563 | 2.571 |
| glycerol-3-p | 2.840 | 2.082 | 2.461 |
| diethanolamine | 2.355 | 0.000 | 1.177 |
| glucose | 2.269 | 2.011 | 2.140 |
| β-alanine | 2.554 | 1.695 | 2.125 |
| glutamine | 2.081 | 2.077 | 2.079 |
| proline | 2.385 | 1.717 | 2.051 |
| glyoxylic acid | 2.607 | 1.475 | 2.041 |
| benzoic acid, 2-methyl | 2.409 | 1.654 | 2.032 |
| threonine | 2.376 | 1.568 | 1.972 |
| pyroglutamic acid | 2.296 | 1.581 | 1.938 |
| ornithine | 2.862 | 0.941 | 1.901 |
| 1 -monooctadecanoylglycerol | 2.239 | 1.339 | 1.789 |
| 1 -monohexadecanoylglycerol | 2.239 | 1.237 | 1.738 |
| succinic acid | 1.841 | 1.553 | 1.697 |
| ethanolamine | 2.053 | 1.325 | 1.689 |
| palmitic acid | 1.990 | 1.378 | 1.684 |
| stearic acid | 2.021 | 1.336 | 1.679 |
| tetradecanoic acid | 2.252 | 1.063 | 1.658 |
| phenylalanine | 1.885 | 1.382 | 1.633 |
| alanine | 2.121 | 1.080 | 1.601 |
| lactic acid | 1.956 | 1.240 | 1.598 |
| leucine | 1.785 | 1.345 | 1.565 |
| glycerol | 2.014 | 1.095 | 1.555 |
| valine | 1.672 | 1.329 | 1.501 |
Metabolite reads from GC-MS analysis of prostaspheres exposed to 10 nM PFOA or PFOS for 4 serial passages were normalized to reads from respective vehicle control groups to derive enrichment ratios for each treatment group. Enrichment ratios for the combined PFAS (PFOA + PFOS) chemicals were also normalized to the combined Vehicle control groups. Enrichment ratios for individual metabolites above 1.50 in the PFAS column are shown.
3.5. Exposure to PFOS increases RWPE-2 xenograft tumor growth in vivo
RWPE-2 cells are a tumorigenic prostate cell line derived from the benign human prostate epithelial cell line RPWE-1 through stable transfection with oncogenic k-RAS [46]. Cultured RPWE-2 cells contain a minor population of cancer stem-like cells that can be enriched in 3-D spheroid culture (Fig. 6A, a&b). When transferred to organoid culture conditions, tumoroids result with enrichment of cancer stem-like cells, identified with high KRT13 expression (Fig. 6A, c&d) [39, 40]. To evaluate the effect of PFOS exposures on tumor growth in vivo, RWPE-2 xenografts were established in athymic nude mice gavaged daily with vehicle or with 10 mg/kg BW PFOS. Over a 40-day period, tumor growth in PFOS-fed mice was significantly greater as compared to controls at each time point (Fig. 6B), with a final average volume of 710 mm3 vs 400 mm3 in the vehicle-fed mice.
Figure 6: PFOS exposures enhance RWPE-2 tumor growth in vivo.
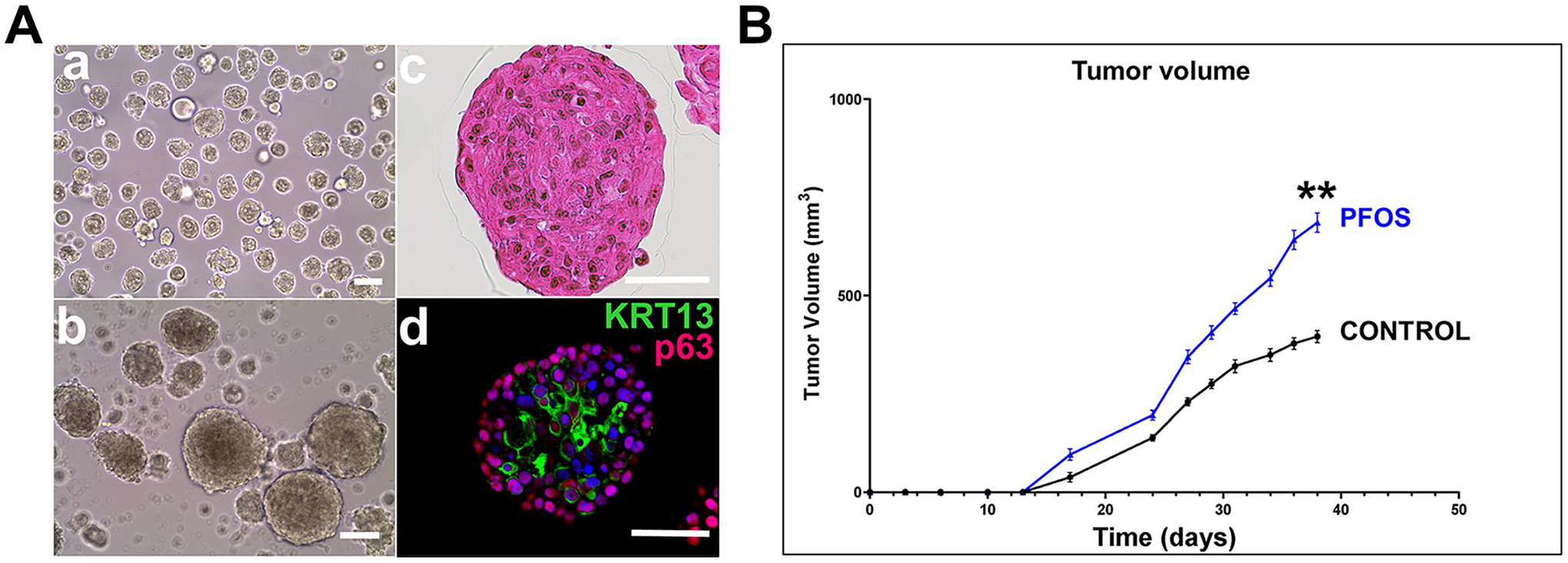
A: Tumorigenic RWPE-2 cells grown in 3D Matrigel culture form spheroids from resident stem-like cancer cells that exist in 2D cultures (a & b). When day 7 spheres are transferred to organoid culture, tumoroids form over 14 days as evidenced by abnormal-shaped nuclei with prominent nucleoli (c). Whole-mount immunocytochemistry identifies basal-like p63+ cells in the periphery and a high proportion of cancer stem-like KRT13+ cells centrally (d). White bar = 50μm. B: RWPE-2 cells were established as xenografts in athymic nude mice given vehicle or 10mg/kg BW PFOS daily. Tumor growth measured over 40 days revealed a significant increase in tumor growth in PFOS-fed mice compared to vehicle controls. N=8 mice/group. ** P<0.0001 versus control.
4. DISCUSSION
In the present study, we identify for the first time that PFAS compounds directly target the human prostate SPC population and in so doing, may contribute to increased carcinogenesis and tumor growth in the prostate gland. Specifically, prolonged exposure of prostaspheres derived from disease-free human prostate epithelial cells to an environmentally relevant level of either PFOS or PFOA significantly increased spheroid numbers with a constant cell number seeded with each serial passage. Since prostaspheres are largely established by resident stem cells under the culture conditions employed herein [39], a consistent increase in spheroid numbers indicates a stimulation of stem-cell symmetric self-renewal in the presence of PFAS that permits stem cell expansion. This is particularly relevant since cancer risk strongly correlates with the number of normal stem cell divisions across multiple tissues, including the prostate [47, 48]. In addition, PFAS exposures led to an increase in larger sized spheres denoting enhanced proliferation of progenitor cells as they comprise the cell majority within prostaspheres [39]. Previous studies have shown that other EDCs, including BPA, dioxin and inorganic arsenic likewise target the human prostate SPCs, likely due to their expression of multiple steroid receptors that make them EDC targets [31, 34, 49]. In the present study, expression of PPARS and RXRs were identified in human prostate SPC with strong levels of PPARα and RXRα which is crucial since many PFAS effects are mediated through PPARα and an RXR binding partner [35–37]. Together, this sets the stage for initiation of downstream signaling effects that alter the SPC phenotypes and behaviors observed herein.
Two non-exclusive downstream actions were characterized in the present studies; PFAS-induced alterations in the transcriptome and metabolome. Using scRNA-seq to identify transcriptomic shifts in SPCs as a function of extended PFAS exposures, we first observe that both PFOS and PFOA result in the emergence of a unique progenitor cell cluster not found in the vehicle-treated spheres. Recent work from our laboratory identified the lineage hierarchy of human prostate SPCs based on cell-type specific shifts in KRT gene profiles during lineage commitment and differentiation [40]. Using these markers to interrogate the new cell clusters that appear upon PFOS or PFOA exposures, they identify as altered early-stage luminal progenitor cells. This is of noteworthy since aberrant luminal cell differentiation may underpin an increased carcinogenic risk in the prostate [50, 51]. Further analysis of the spheroid transcriptome ascertained a significant PFAS-induced upregulation of gene signaling pathways involved in proliferative potential (G2M checkpoint, mitotic spindle, E2F targets) and oncogenesis (k-RAS and MYC signaling, TNFα via NFκB, IL-6-JAK-STAT3, TGFβ and inflammatory signaling). Enhancement of these gene sets in the SPC pool is of special note in the context of a recent report that identified a gene signature in therapeutic-resistant metastatic prostate cancer having stemness-associated pathways including IL-6-JAK-STAT, TGFβ and inflammatory signaling [52]. Collectively, these findings support the potential for early transformation of the prostate SPC population to a pre-malignant state by PFAS and, according to the cancer stem cell hypothesis [53–55], augment the risk for prostate cancer.
Since PPARs are key signaling regulators of cellular metabolism, we interrogated the effects of PFAS exposures on the SPC metabolome. Results identified enhancement of glycine and serine metabolism and enhanced glucose metabolism through the Warburg effect, among other changes in response to PFOA and PFOS exposures. This is further supported by GSEA identification of enhanced expression of glycolysis pathway genes in SPCs exposed to PFOA. To our knowledge, these are the first studies to identify metabolome shifts in prostate SPCs in response to environmental chemical exposures. Metabolic factors are known to be critical in regulating the balance between stem cell self-renewal and differentiation [56] as well as drivers of carcinogenesis and tumor progression [57]. Cultured pluripotent stem cells are reliant on exogenous glucose and glutamine and engage in aerobic glycolysis, consuming high levels of glucose and secreting lactate [58], a phenomenon that is reduced with early differentiation. Similar properties have also been reported for cancer stem cells compared to non-stem cancer cells [59]. As such, further enhancement of glycolysis and lactate production by PFOA in cultured prostaspheres may underlie the increased self-renewal capability in prostate stem cells as well as differentiation perturbation. Another critical stem cell self-renewal/differentiation regulator recently identified in epidermal pre-malignant stem cells is amino acid availability, specifically serine synthesis from glucose [60]. There, activation of glucose flux to serine is a rate-limiting step in pre-malignant stem cell growth [61] suggesting that the metabolic profile of a stem cell is a determinant in the formation of a malignant state. Elevated serine and glycine metabolism have also been reported for prostate cancer stem-like cells [59]. This is particularly relevant since the present studies identified increased serine and glycine metabolism as the top enriched metabolic set by far in prostaspheres exposed to PFOS over 4 weeks of serial passage. Whether this can serve to promote a pre-malignant prostate SPC fate remains to be determined, however it is tempting to speculate that enhanced levels of serine and other amino acids and heightened serine/glycine metabolism due to PFAS exposures may augment stem cell self-renewal, alter normal luminal progenitor cell differentiation, and enhance the tumor-initiating capacity of prostate SPCs.
To directly test the ability of PFAS exposures to modify prostate cancer growth, human-derived RWPE-2 cells (transformed by k-RAS) were grafted into nude mice and daily exposure to PFOS significantly increased tumor growth over a 40-day period. While this treatment directly targets the cancer cells to alter their metabolome and enhance their proliferation [62], we also provide evidence that RWPE-2 cells contain a cancer-stem-like population that is enriched in tumoroid growth. Thus, it is possible that PFOS-driven enhancement of tumor growth may be in part driven through effects of PFOS on the cancer stem cell population as well.
In summary, the present study provides novel evidence that PFAS exposures can directly target human prostate stem and progenitor cells, increasing their self-renewal and proliferative potential and altering luminal progenitor cell differentiation. This is underpinned, in part, by alterations in the SPC transcriptome and metabolome towards pathways that resemble a pre-malignant stem-progenitor cell fate. Since in vivo PFOS exposure was capable of accelerating tumorous xenograft growth from RWPE-2 cells that harbor a cancer stem-like cell population, we propose that direct PFOS effects on the cancer stem cells may contribute to tumor growth and progression. Together, these findings help to understand the reported increase in prostate cancer incidence and mortality observed in PFAS-exposed populations and support the need for additional research on these epidemiologic observations balanced with mechanistic studies to delineate human prostate cancer risks from chronic PFAS exposures.
Acknowledgements
This research was supported by NIH grants P30 ES-027792 (GSP and WYH), R01-ES02207 (GSP) and R01-CA172220 (GSP and WYH), the National Institute of Food and Agriculture grant ILLU-698-909 (ZME) and the Michael Reese Research and Educational Foundation (GSP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: The authors have nothing to disclose.
REFERENCES
- 1.Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 2006; 40:32–44. [DOI] [PubMed] [Google Scholar]
- 2.Oakes KD, Benskin JP, Martin JW, Ings JS, Heinrichs JY, Dixon DG, Servos MR. Biomonitoring of perfluorochemicals and toxicity to the downstream fish community of Etobicoke Creek following deployment of aqueous film-forming foam. Aquat Toxicol 2010; 98:120–129. [DOI] [PubMed] [Google Scholar]
- 3.Rappazzo KM, Coffman E, Hines EP. Exposure to Perfluorinated Alkyl Substances and Health Outcomes in Children: A Systematic Review of the Epidemiologic Literature. Int J Environ Res Public Health 2017; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karásková P, Venier M, Melymuk L, Bečanová J, Vojta Š, Prokeš R, Diamond ML, Klánová J. Perfluorinated alkyl substances (PFASs) in household dust in Central Europe and North America. Environ Int 2016; 94:315–324. [DOI] [PubMed] [Google Scholar]
- 5.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol 2011; 45:8037–8045. [DOI] [PubMed] [Google Scholar]
- 6.Calafat AM, Kato K, Hubbard K, Jia T, Botelho JC, Wong LY. Legacy and alternative per- and polyfluoroalkyl substances in the U.S. general population: Paired serum-urine data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int 2019; 131:105048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser AJ, Webster TF, Watkins DJ, Strynar MJ, Kato K, Calafat AM, Vieira VM, McClean MD. Polyfluorinated compounds in dust from homes, offices, and vehicles as predictors of concentrations in office workers’ serum. Environ Int 2013; 60:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agency USEP. Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA). EPA - Technical Fact Sheet 2017; EPA 505-F-17–001.
- 9.CDC. Toxicological Profile for Perfluoroalkyls, 2021. Center for Disease Control : Agency for Toxic Substances and Disease Registry; 2021; CAS# 335–67-1, 1763–23-1, 355–46-4, 375–95-1.. [PubMed] [Google Scholar]
- 10.Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P. Plasma Concentrations of Perfluoroalkyl Substances and Risk of Type 2 Diabetes: A Prospective Investigation among U.S. Women. Environ Health Perspect 2018; 126:037001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birru RL, Liang HW, Farooq F, Bedi M, Feghali M, Haggerty CL, Mendez DD, Catov JM, Ng CA, Adibi JJ. A pathway level analysis of PFAS exposure and risk of gestational diabetes mellitus. Environ Health 2021; 20:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coperchini F, Croce L, Ricci G, Magri F, Rotondi M, Imbriani M, Chiovato L. Thyroid Disrupting Effects of Old and New Generation PFAS. Front Endocrinol (Lausanne) 2020; 11:612320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanifer JW, Stapleton HM, Souma T, Wittmer A, Zhao X, Boulware LE. Perfluorinated Chemicals as Emerging Environmental Threats to Kidney Health: A Scoping Review. Clin J Am Soc Nephrol 2018; 13:1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steenland K, Winquist A. PFAS and cancer, a scoping review of the epidemiologic evidence. Environ Res 2021; 194:110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. https://www.epa.gov/toxics-release-inventory-tri-program.
- 16.IARC. Some chemicals used as solvents and in polymer manufacture International Agency for Cancer Research: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 2017; 110. [Google Scholar]
- 17.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021; 71:7–33. [DOI] [PubMed] [Google Scholar]
- 18.Xie L, Prins GS. Environmental Factors in Cancer Risk. In: Heber D (ed.) Nutritional Oncology: Nutrition in Cancer Prevention, Treatment, and Survivorship: CRC Press; 2021. [Google Scholar]
- 19.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 2015; 36:E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilliland FD, Mandel JS. Mortality among employees of a perfluorooctanoic acid production plant. J Occup Med 1993; 35:950–954. [DOI] [PubMed] [Google Scholar]
- 21.Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect 2013; 121:1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eriksen KT, Sørensen M, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K, Raaschou-Nielsen O. Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population. J Natl Cancer Inst 2009; 101:605–609. [DOI] [PubMed] [Google Scholar]
- 23.Hardell E, Kärrman A, van Bavel B, Bao J, Carlberg M, Hardell L. Case-control study on perfluorinated alkyl acids (PFAAs) and the risk of prostate cancer. Environ Int 2014; 63:35–39. [DOI] [PubMed] [Google Scholar]
- 24.Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single cell. Nature 2008; 456:804–808. [DOI] [PubMed] [Google Scholar]
- 25.Blackwood JK, Williamson SC, Greaves LC, Wilson L, Rigas AC, Sandher R, Pickard RS, Robson CN, Turnbull DM, Taylor RW, Heer R. In situ lineage tracking of human prostatic epithelial stem cell fate reveals a common clonal origin for basal and luminal cells. J Pathol 2011; 225:181–188. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Li Q, Liu X, Liu C, Liu R, Rycaj K, Zhang D, Liu B, Jeter C, Calhoun-Davis T, Lin K, Lu Y, et al. Defining a Population of Stem-like Human Prostate Cancer Cells That Can Generate and Propagate Castration-Resistant Prostate Cancer. Clin Cancer Res 2016; 22:4505–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vummidi Giridhar P, Williams K, VonHandorf AP, Deford PL, Kasper S. Constant Degradation of the Androgen Receptor by MDM2 Conserves Prostate Cancer Stem Cell Integrity. Cancer Res 2019; 79:1124–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopras E, Potluri V, Bermudez ML, Williams K, Belcher S, Kasper S. Actions of endocrine-disrupting chemicals on stem/progenitor cells during development and disease. Endocr Relat Cancer 2014; 21:T1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prins GS, Calderon-Gierszal EL, Hu WY. Stem Cells as Hormone Targets That Lead to Increased Cancer Susceptibility. Endocrinology 2015; 156:3451–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 2006; 66:5624–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, Huang K, Nelles JL, Ho SM, Walker CL, Kajdacsy-Balla A, van Breemen RB. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 2014; 155:805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho SM, Cheong A, Lam HM, Hu WY, Shi GB, Zhu X, Chen J, Zhang X, Medvedovic M, Leung YK, Prins GS. Exposure of Human Prostaspheres to Bisphenol A Epigenetically Regulates SNORD Family Noncoding RNAs via Histone Modification. Endocrinology 2015; 156:3984–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calderon-Gierszal EL, Prins GS. Directed Differentiation of Human Embryonic Stem Cells into Prostate Organoids In Vitro and its Perturbation by Low-Dose Bisphenol A Exposure. PLoS One 2015; 10:e0133238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie L, Hu WY, Hu DP, Shi G, Li Y, Yang J, Prins GS. Effects of Inorganic Arsenic on Human Prostate Stem-Progenitor Cell Transformation, Autophagic Flux Blockade, and NRF2 Pathway Activation. Environ Health Perspect 2020; 128:67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maloney EK, Waxman DJ. trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol Appl Pharmacol 1999; 161:209–218. [DOI] [PubMed] [Google Scholar]
- 36.Buhrke T, Kibellus A, Lampen A. In vitro toxicological characterization of perfluorinated carboxylic acids with different carbon chain lengths. Toxicol Lett 2013; 218:97–104. [DOI] [PubMed] [Google Scholar]
- 37.Takacs ML, Abbott BD. Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol Sci 2007; 95:108–117. [DOI] [PubMed] [Google Scholar]
- 38.Hu WY, Shi GB, Lam HM, Hu DP, Ho SM, Madueke IC, Kajdacsy-Balla A, Prins GS. Estrogen-initiated transformation of prostate epithelium derived from normal human prostate stem-progenitor cells. Endocrinology 2011; 152:2150–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu WY, Hu DP, Xie L, Li Y, Majumdar S, Nonn L, Hu H, Shioda T, Prins GS. Isolation and functional interrogation of adult human prostate epithelial stem cells at single cell resolution. Stem Cell Res 2017; 23:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu WY, Hu DP, Xie L, Nonn L, Lu R, Abern M, Shioda T, Prins GS. Keratin Profiling by Single-Cell RNA-Sequencing Identifies Human Prostate Stem Cell Lineage Hierarchy and Cancer Stem-Like Cells. Int J Mol Sci 2021; 22:8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 1989; 24:148–154. [DOI] [PubMed] [Google Scholar]
- 42.Macosko E, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas A, Kamitaki N, Martersteck EM, Trombetta J, Weitz D, et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell Biochem Funct 2015; 161:1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005; 102:15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, Gauthier C, Jacques P, Li S, Xia J. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res 2021; 49:W388–w396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xin L, Lukacs RU, Lawson DA, Cheng D, Witte ON. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells 2007; 25:2760–2769. [DOI] [PubMed] [Google Scholar]
- 46.Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis 1997; 18:1215–1223. [DOI] [PubMed] [Google Scholar]
- 47.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015; 347:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 2017; 355:1330–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu WY, Shi GB, Hu DP, Nelles JL, Prins GS. Actions of estrogens and endocrine disrupting chemicals on human prostate stem/progenitor cells and prostate cancer risk. Mol Cell Endocrinol 2012; 354:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Germann M, Wetterwald A, Guzmán-Ramirez N, van der Pluijm G, Culig Z, Cecchini MG, Williams ED, Thalmann GN. Stem-like cells with luminal progenitor phenotype survive castration in human prostate cancer. Stem Cells 2012; 30:1076–1086. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Grogan TR, Hieronymus H, Hashimoto T, Mottahedeh J, Cheng D, Zhang L, Huang K, Stoyanova T, Park JW, Shkhyan RO, Nowroozizadeh B, et al. Low CD38 Identifies Progenitor-like Inflammation-Associated Luminal Cells that Can Initiate Human Prostate Cancer and Predict Poor Outcome. Cell Rep 2016; 17:2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alumkal JJ, Sun D, Lu E, Beer TM, Thomas GV, Latour E, Aggarwal R, Cetnar J, Ryan CJ, Tabatabaei S, Bailey S, Turina CB, et al. Transcriptional profiling identifies an androgen receptor activity-low, stemness program associated with enzalutamide resistance. Proc Natl Acad Sci U S A 2020; 117:12315–12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001; 414:105–111. [DOI] [PubMed] [Google Scholar]
- 54.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005; 65:10946–10951. [DOI] [PubMed] [Google Scholar]
- 55.Tu SM, Zhang M, Wood CG, Pisters LL. Stem Cell Theory of Cancer: Origin of Tumor Heterogeneity and Plasticity. Cancers (Basel) 2021; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 2015; 518:413–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab 2016; 23:27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Intlekofer AM, Finley LWS. Metabolic signatures of cancer cells and stem cells. Nat Metab 2019; 1:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aguilar E, Marin de Mas I, Zodda E, Marin S, Morrish F, Selivanov V, Meca-Cortés Ó, Delowar H, Pons M, Izquierdo I, Celià-Terrassa T, de Atauri P, et al. Metabolic Reprogramming and Dependencies Associated with Epithelial Cancer Stem Cells Independent of the Epithelial-Mesenchymal Transition Program. Stem Cells 2016; 34:1163–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cable J, Pourquié O, Wellen KE, Finley LWS, Aulehla A, Gould AP, Teleman A, Tu WB, Garrett WS, Miguel-Aliaga I, Perrimon N, Hooper LV, et al. Metabolic decisions in development and disease-a Keystone Symposia report. Ann N Y Acad Sci 2021. [DOI] [PubMed] [Google Scholar]
- 61.Baksh SC, Todorova PK, Gur-Cohen S, Hurwitz B, Ge Y, Novak JSS, Tierney MT, Dela Cruz-Racelis J, Fuchs E, Finley LWS. Extracellular serine controls epidermal stem cell fate and tumour initiation. Nat Cell Biol 2020; 22:779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Imir OB, Kamisnky AZ, Zuo Q, Liu YJ, Singh R, Spinella MJ, Irudayaraj J, Hu WY, Prins GS, Madak Erdogan Z. Per- and Polyfluoroalkyl Substance Exposure combined with high-fat diet supports prostate cancer progression. Nutrients 2021; under review. [DOI] [PMC free article] [PubMed] [Google Scholar]


