Abstract
The impaired ability to mount an effective immune response to vaccination leaves immunosuppressed patients at higher risk of severe COVID-19 infection. This retrospective study aimed to evaluate COVID-19 seroconversion and antibody titers for patients on immune modulating therapies compared to those not on disease modifying therapy (DMT). As expected, individuals on B-cell depletion therapies (BCDT) and those on sphingosine 1-phosphate (S1P) modulators had an impaired humoral response to mRNA vaccination. We observed variable seroconversion depending on the type of B-cell depleting medication, with a smaller percentage of seroconversion in patients on infused BCDT (iBCDT, ocrelizumab and rituximab) compared to ofatumumab. The humoral response to vaccination was not impaired for individuals on natalizumab or for untreated MS patients. These observations may influence DMT selection during the COVID-19 era.
1. Introduction
The introduction of safe and effective vaccines against COVID-19 represents a major scientific victory, emerging from the shadow of the pandemic. Nevertheless, immune compromised patients are less able to mount an appropriate immune response to vaccination, leaving this vulnerable population at risk for severe infections (Bar-Or et al., 2020; van Assen et al., 2010; Iannetta et al., 2021; Smets et al., 2021; Tallantyre et al., 2022; Georgieva et al., 2021). B-cell depleting medications, commonly used to treat patients with multiple sclerosis (MS) as well as other autoimmune diseases, may put individuals at greater risk for severe COVID-19 infection, as well as impair their humoral response to vaccination (Bar-Or et al., 2020; Waldman et al., 2021; Conte, 2021). More information about how DMT impact vaccination responses is needed to develop effective management strategies for MS patients (Baker et al., 2020). This retrospective study aimed to evaluate COVID-19 antibody responses after vaccination for patients on high efficacy immune modulating therapies compared with untreated patients.
2. Methods
Subjects: We performed a retrospective chart review of patients (N = 90) cared for by a single provider at the Yale University Multiple Sclerosis Center who had systematically checked COVID-19 spike antibody levels among patients on immunomodulatory therapy. Data was extracted on 10/25/2021 and covered the time period between December 2020 – October 2021. All patients had undergone vaccination with a COVID-19 vaccine cleared for Emergency Use Authorization by the FDA and had COVID-19 spike protein antibodies measured for clinical purposes > 2 weeks after vaccination. We included all DMTs used by > 1 patient, including patients who initiated DMT before or within 2 weeks of their second dose of COVID-19 vaccination. Patients were categorized into those receiving iBCDT (N = 54; ocrelizumab (N = 44) and rituximab (N = 10)), subcutaneous B-cell depletion with ofatumumab (N = 7 for Fig. 1 ; N = 4 in quantitative analysis), natalizumab (N = 5), the S1P modulators (N = 4; fingolimod (N = 3) and siponimod (N = 1)), and controls who were not on DMTs (N = 23). All participants were ≥ 18 years old, and diagnosed with MS, clinically isolated syndrome (CIS), neuromyelitis optica spectrum disorder (NMOSD), autoimmune encephalitis, or were undergoing workup for MS. Most (78%) COVID-19 spike antibody testing utilized the DiaSorin Liasion chemiluminescence assay; only these patients’ data were included for quantitative analysis of mean spike antibody titers. Qualitative COVID spike antibody seropositivity was determined based on test-specific lab reference ranges. When available, CD19 lymphocyte counts were measured at the same time as COVID-19 antibodies.
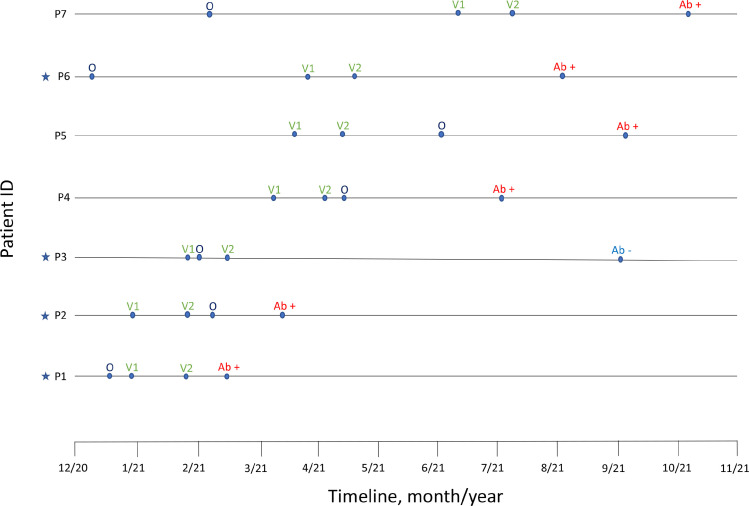
Fig. 1.
Timeline of ofatumumab initiation (O), vaccination (V1 – first dose, V2 – second dose), and COVID-19 antibody testing (Ab + indicating positive status and Ab – indicating negative status) among all providers at our center. Stars indicate which patients met defined inclusion criteria and were included in Table 1 and the computational analysis.
In addition to the above cohort, we reviewed all patients at our center who were treated with ofatumumab and had anti-spike antibodies for COVID-19 measured (N = 7). The Yale University Institutional Review Board approved the study protocols.
2.1. Data analysis
Statistical tests were performed using R-Studio statistics software (R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2018) and summary statistics are reported in Table 1 . Time from infusion to vaccination was calculated for iBCDT, but this could not be determined for ofatumumab due to the monthly self-administration schedule. Mean antibody levels were compared across DMT groups using one-way ANOVA. Linear regression modeling was performed using stepwise model selection and Akaike Information Criteria (StepAIC function for R) to generate models with the minimal set of optimal features. Fisher exact test and Chi Square test of independence were used to evaluate frequency of antibody positivity among all subjects stratified by DMT use. Student's T-test was utilized to evaluate difference in mean antibody levels between various DMTs compared to controls, and between iBCDT and ofatumumab.
Table 1.
Cohort demographics. Seropositivity based on lab standards; mean COVID antibody calculations were limited to those whose tests were performed using the DiaSorin Liason chemiluminescence assay (n = 70/90). OCR = ocrelizumab, RTX = rituximab, S1P = fingolimod and Siponimod. Definite COVID infection defined as PCR or rapid antigen test positive.
| All B cell depleting therapy (OCR, RTX, OFT) | OCR | RTX | Ofatumumab (OFT) | Natalizumab | S1P | Control | |
|---|---|---|---|---|---|---|---|
| N | 58 | 44 | 10 | 4 | 5 | 4 | 23 |
| COVID antibody titer, mean (sd) | 57.1 (114) N = 44 |
45.1 (94) N = 35 | 19.5 (38) N = 7 |
400 (0) N = 2 |
158.2 (53) N = 5 | 69 (72) N = 4 | 223 (82) N = 17 |
| Seropositivity (%) | 23/58 (40%) | 19/44 (43%) | 1/9 (11%) | 3/4 (75%) | 5/5 (100%) | 2/4 (50%) | 23/23 (100%) |
| History of definite COVID infection | 6/58 (10%) | 4/44 (9%) | 1/10 (10%) | 1/4 (25%) | 0/5 | 0/4 | 0/23 |
| CD19+ Lymphocytes (%) | 0.74 (1.9) | 0.91 (2.2) | 0.25 (0.48) | 0.06 (0.05) | 10.6 (2.5) | ||
| Absolute CD19+ Count | 15.6 (52.2) | 19.9 (59.2) | 1.8 (3.4) | 1.0 (1) | 213 (95) | ||
| Time from vaccination to titer, mean (sd) | 105 days (65) | 109 days (67) | 78 days (52) | 123 days (73) | 115 days (78) | 120 (88) | 143 days (69) |
| Days from infusion to vaccination, mean (sd) | 85 (83) | 63 (69) | |||||
| Days from start of DMT to vaccination, mean, (sd) | 727 (598) | 652 (374) | 1339 (963) | 13 (54) | 575 (560) | 655 (787) | |
|
Type of vaccine Moderna, N (%) Pfizer, N (%) J&J, N(%) |
24 (41%) 33 (57%) 1 (2%) |
17 (39%) 26 (59%) 1 (2%) |
4 (40%) 6 (60%) 0 |
3 (75%) 1 (25%) 0 |
2 (40%) 3 (60%) 0 |
1 (25%) 3 (75%) 0 |
12 (52%) 11 (48%) 0 |
3. Results
On average, COVID-19 spike antibody titers were significantly lower for those on BCDT compared to the control group (defined as untreated patients) (p < 0.001) (Table 1). When infused BCDT and ofatumumab were considered separately, antibody levels for iBCDT were significantly lower than those for patients on subcutaneous BCDT (p < 0.001 for ofatumumab compared to rituximab and to ocrelizumab). Seroconversion rates varied depending on type of anti-CD20 monoclonal antibody, with rituximab having the lowest seroconversion rate (11%) and ofatumumab having the highest seroconversion rate (75%). All patients on BCDT were fully B-cell depleted at the time of COVID antibody measurement. There were few natural COVID-19 infections reported, and all occurred in subjects on B-cell therapies. Two subjects on ocrelizumab and one on rituximab remained antibody negative after infection and vaccination, while the other subjects (two on ocrelizumab and one on ofatumumab) had positive antibodies after infection and vaccination (Table 1). We additionally sought to determine if the response to vaccination in ofatumumab was solely due to the short duration of treatment. In our cohort, one subject was initiated on ocrelizumab shortly (2 weeks) before vaccination; this patient did mount an antibody response albeit with a low titer which could not be directly compared to our ofatumumab patients’ titers given different lab analysis. Because only a small number of patients on ofatumumab were available in the initial cohort, we expanded our chart review to include all patients at our Center who had been exposed to this medication around the time of their vaccination (Fig. 1). One additional patient was identified who had ofatumumab exposure prior to vaccination; they subsequently mounted an antibody response. Moreover, several patients were identified who initiated ofatumumab > 2 weeks after vaccination; all of these maintained a positive spike antibody response (Fig. 1).
As expected, patients on S1P modulators demonstrated an attenuated spike antibody response compared to controls, while natalizumab-treated patients did not (p = 0.01, p = 0.06).
A linear regression model controlling for DMT, CD19 lymphocyte subset count, vaccine brand, time since vaccination, and duration of DMT found ofatumumab use (t-value 5.1) and CD19 lymphocyte count (t-value 4.4) to be significant predictors of COVID-19 antibody levels (p-value < 0.001, R2 = 0.65).
4. Discussion
The COVID-19 pandemic has introduced new uncertainty regarding optimal treatment of autoimmune inflammatory disease. Although BCDT are highly effective in controlling disease activity, their use increases risk of severe COVID (Simpson-Yap et al., 2021; MP Sormani et al., 2021a) and attenuates humoral vaccine responses (Fagni et al., 2021). Practitioners must therefore weigh the long-term risks of neurologic damage with the short-term risks of COVID-19 infection and sequelae. We observed heterogeneous antibody responses among patients taking different types of BCDT, with a smaller percentage of seroconversion in patients on iBCDT compared to ofatumumab, consistent with emerging data presented at ECTRIMS 2021 (Karussis et al., 2021). Natalizumab-treated patients maintained a strong antibody response to vaccination.
While provocative, these data require replication in larger, prospective cohorts. We had access to only a small cohort of patients taking ofatumumab, and many had initiated treatment shortly before or around the same time as their COVID-19 vaccination. The vaccine response among ofatumumab patients could therefore be related to B-cell populations that had not yet been impacted by the medication. We expect that the antibody response to vaccination will depend on the overall level of B-cell depletion (Ram et al., 2021; Moor et al., 2021). This may be approximated by the time since last treatment for infused medications (Leandro et al., 2006). There were not significant differences between the time since last ocrelizumab and rituximab treatments and vaccination in our cohort. Since ofatumumab is self-administered every 28 days, the time since last drug exposure should be comparable among patients taking that medication. Seroconversion may also depend on the time between vaccination and testing. All patients in our cohort had antibodies checked ≥ 2 weeks after vaccination, and there were not significant between-group differences in elapsed time between vaccination and antibody testing. Antibody status may be impacted by type of vaccine; emerging data suggested that B-cell depleted patients were more likely to seroconvert after mRNA-1273 (Moderna) vaccine (MP Sormani et al., 2021b), while data from the Israeli cohort corroborates that ofatumumab seroconversion rates remain higher than iBCDT in the setting of (BNT162b2-Pfizer) COVID-19 vaccination (Karussis et al., 2021). A larger proportion of patients in the ofatumumab group received mRNA-1273 (Moderna) vaccine compared to the iBCDT group in our cohort. Since the COVID-19 spike antibody test cannot differentiate between natural infection and vaccination, the contribution of prior infection to antibody levels is unknown, and additional work to further elucidate the effect of natural infection on antibody level and impact of type of vaccine in this patient population is warranted. The relative contribution of COVID antibodies to clinical immunity is not yet clear, as both humoral and cellular immune responses are elicited by infection and vaccination (Apostolidis et al., 2021).
Questions regarding how to most effectively vaccinate immune compromised individuals have been amplified by the pandemic, and further study is needed to clarify the above findings. Clinicians and patients continue to face many decisions regarding choice of immunotherapy, type and frequency of vaccination and lifestyle changes for patients requiring immunosuppressive therapy. These preliminary results raise the possibility that humoral vaccine responses may be variable among the anti-CD20 monoclonal antibodies, which was not previously expected. If confirmed, these findings may influence the choice of DMT in this era of COVID-19.
Disclosures
Elle Levit MD has no disclosures.
Erin Longbrake, MD PhD has received research funding from NIH K23107624, Race to Erase MS, Genentech and CTSA Grant number KL2 TR001862. She has received honoraria for consulting for Genentech, Genzyme, and Alexion.
Sharon S Stoll DO, MS has served on scientific advisory boards for Hoffmann-La Roche Ltd, Genentech, Inc., Forepont Capital Partners, TG Therapeutics, Horizon, Bristol Myers Squibb and received research support from BeCare MS Link and MedDay Pharmaceuticals. She has also received compensation for consulting services, served on scientific advisory board and received speaker honorarium for Roche Genentech, Biogen, Sanofi Genzyme, Novartis, and Alexion. She is also CEO of Global Consultant MD. Dr Stoll also serves on the steering committee of Horizon and Roche Genentech pharmaceuticals.
References
- Apostolidis S.A., Kakara M., Painter M.M., et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021 doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Roberts C.A.K., Pryce G., et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin. Exp. Immunol. 2020;202:149–161. doi: 10.1111/cei.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Calkwood J.C., Chognot C., et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95:e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte W. B-Cell depleters attenuate the humoral response to SARS-CoV2 vaccines in multiple sclerosis patients: a case control study. Mult. Scler. Relat. Disord. 2021 doi: 10.1016/j.msard.2021.103413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagni F., Simon D., Tascilar K., et al. COVID-19 and immune-mediated inflammatory diseases: effect of disease and treatment on COVID-19 outcomes and vaccine responses. Lancet Rheumatol. 2021 doi: 10.1016/S2665-9913(21)00247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva Z.G., Dӧffinger R., Kumararatne D., Coles A.J., McCarthy C. Diminished seroconversion following a single SARS-CoV-2 vaccine in ocrelizumab-treated relapsing-remitting multiple sclerosis patients. Mult. Scler. 2021 doi: 10.1177/13524585211046786. 13524585211046786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetta M., Landi D., Cola G., et al. T-cell responses to SARS-CoV-2 in multiple sclerosis patients treated with ocrelizumab healed from COVID-19 with absent or low anti-spike antibody titers. Mult. Scler. Relat. Disord. 2021;55 doi: 10.1016/j.msard.2021.103157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karussis D., Karni A., Milo R., Staun-Ram E., Miller A. Humoral immune response to COVID-19 mRNA (BNT162b2-Pfizer) vaccine in patients with multiple sclerosis. Mult. Scler. J. 2021:766–767. ECTRIMS 2021 – Late Breaking News ePoster P940. [Google Scholar]
- Leandro M.J., Cambridge G., Ehrenstein M.R., Edwards J.C. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheumatol. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- Moor M.B., Suter-Riniker F., Horn M.P., et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021;3:e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram R., Hagin D., Kikozashvilli N., et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy-a single-center prospective cohort study. Transplant. Cell. Ther. 2021;27:788–794. doi: 10.1016/j.jtct.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Yap S., Brouwer E.D., Kalincik T., et al. 1298 Associations of DMT therapies with COVID-19 severity in multiple sclerosis. Int. J. Epidemiol. 2021;50(Supplement_1):168–604. [Google Scholar]
- Smets I., Reyes S., Baker D., Giovannoni G. Blunted vaccines responses after ocrelizumab highlight need for immunizations prior to treatment. Mult. Scler. Relat. Disord. 2021;50 doi: 10.1016/j.msard.2021.102851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89:780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Inglese M., Schiavetti I., et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre E.C., Vickaryous N., Anderson V., et al. COVID-19 vaccine response in people with multiple sclerosis. Ann. Neurol. 2022;91(1):89–100. doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Assen S., Holvast A., Benne C.A., et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheumatol. 2010;62:75–81. doi: 10.1002/art.25033. [DOI] [PubMed] [Google Scholar]
- Waldman R.A., Creed M., Sharp K., et al. Toward a COVID-19 vaccine strategy for patients with pemphigus on rituximab. J. Am. Acad. Dermatol. 2021;84:e197–e198. doi: 10.1016/j.jaad.2020.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]