Abstract
A promising strategy that emerged in tissue engineering is to incorporate two-dimensional (2D) materials into polymer scaffolds, producing materials with desirable mechanical properties and surface chemistries, which also display broad biocompatibility. Black phosphorus (BP) is a 2D material that has sparked recent scientific interest due to its unique structure and electrochemical characteristics. In this study, BP nanosheets (BPNSs) were incorporated into a cross-linkable oligo[poly(ethylene glycol) fumarate] (OPF) hydrogel to produce a new nanocomposite for bone regeneration. BPNSs exhibited a controllable degradation rate coupled with the release of phosphate in vitro. MTS assay results together with live/dead images confirmed that the introduction of BPNSs into OPF hydrogels enhanced MC3T3-E1 cell proliferation. Moreover, the morphology parameters indicated better attachments of cells in the BPNSs containing group. Immunofluorescence images as well as intercellular ALP and OCN activities showed that adding a certain amount of BPNSs to OPF hydrogel could greatly improve differentiation of pre-osteoblasts on the hydrogel. Additionally, embedding black phosphorous into a neutral polymer network helped to control its cytotoxicity, with optimal cell growth observed at BP concentrations as high as 500 ppm. These results reinforced that the supplementation of OPF with BPNSs can increase the osteogenic capacity of polymer scaffolds for use in bone tissue engineering.
Keywords: 2D materials, Black phosphorus, Hydrogel, Osteogenesis, Phosphate
1. Introduction
Bone is the second most commonly transplanted tissue worldwide, with a diverse array of clinical uses, including the replacement of tissue lost due to fracture, infection, congenital diseases, or tumors.1–3 While autologous bone graft remains the most common method of treatment for bone loss, the limited supply of acceptable grafting sites and the prevalence of donor site morbidity continue to motivate the development of effective alternatives.4 To address these problems, promising new strategies within tissue engineering have been developed to replace bone grafts, including the development of synthetic polymers that can be implanted in the body.5–9 However, while attractive due to their cost and reproducibility, developing materials that are also biocompatible with desired mechanical and chemical properties to support bone cell attachment, growth, and mineralization remains a significant challenge.
One material that holds great promise for bone tissue engineering is oligo [poly(ethylene glycol) fumarate] (OPF), a synthetic polymer that is biodegradable and injectable,10–12 with previous success reported in both cartilage tissue and nerve regeneration.13,14 Through the formation of a cross-linked hydrogel, OPF can act as a scaffolding matrix capable of promoting cell adhesion, proliferation, and differentiation, while also degrading predictably. However, like other materials based on poly(ethylene glycol) (PEG), cell attachment and proliferation on OPF are often low, in large part due to the smooth surface topography and the formation of a hydrated surface layer that interferes with protein adsorption.12 To address this drawback, two-dimensional (2D) materials with desirable properties can be incorporated into the OPF polymer network, altering the physical properties of the polymer and effectively tailoring its use for a particular application.15–17
Black phosphorus (BP) is a 2D material that has attracted attention within tissue engineering due to its unique structure, mechanical and optical properties, electron-transfer capacity, and electrical conductivity.18–22 Relevant to bone tissue engineering, BP is biodegradable in vivo, producing a series of nontoxic intermediates, such as phosphoric acid, phosphorous acid, and hypophosphorous acid.23–25 Phosphate, the primary component of phosphoric acid, is absorbed by bone producing cells (osteoblasts), promoting the synthesis of cell membranes, nucleic acid, and adenosine triphosphate,26 as well as facilitating bone mineralization.27 In addition to cellular responses, BP has also been used in the treatment of bone tumors in photothermal therapy (PTT),28,29 although the high concentrations (>50 ppm) used have been reported to be cytotoxic.30 For this reason, the concentration of BP that cells interact with ultimately determines its applications in regenerative medicine.28
The degradation intermediates and physical characteristics of BP make it an attractive addition to synthetic polymers that normally exhibit low native levels of cell proliferation. Additionally, embedding BP into a cross-linked OPF polymer network could act to limit the direct interaction between BP and cells, effectively minimizing the material’s cytotoxicity while also allowing for phosphate generation.28,33 This approach could also account for the inherent instability of BP; encapsulating the material within the OPF network could effectively increase the stability of BP and protect it from degradation in ambient conditions.34 Under this scenario, the phosphate would then be released as the entire network degrades after implantation, promoting cellular growth on the surface of the material.
In the current study, BP was processed into BP nanosheets (BPNSs), before the fabrication of a series of OPF@BPNSs hydrogels to evaluate their osteogenic capacity in vitro (Figure 1a). The morphological features of BPNSs and hydrogels were determined using transmission electron microscopy (TEM), scanning electron microscopy (SEM), and atomic force microscopy (AFM), while the mechanical properties of each hydrogel were investigated using dynamic mechanical analysis (DMA). Further, the degradation of BPNSs and resulting phosphate release were also measured (Figure 1b). OPF@BPNSs hydrogels were then cultured with MC3T3 mouse pre-osteoblast cells to assess their cytocompatibility and osteoconductivity, with the goal of determining the optimal BPNS concentration that supports cell attachment, distribution, proliferation, and differentiation (Figure 1c).
Figure 1.
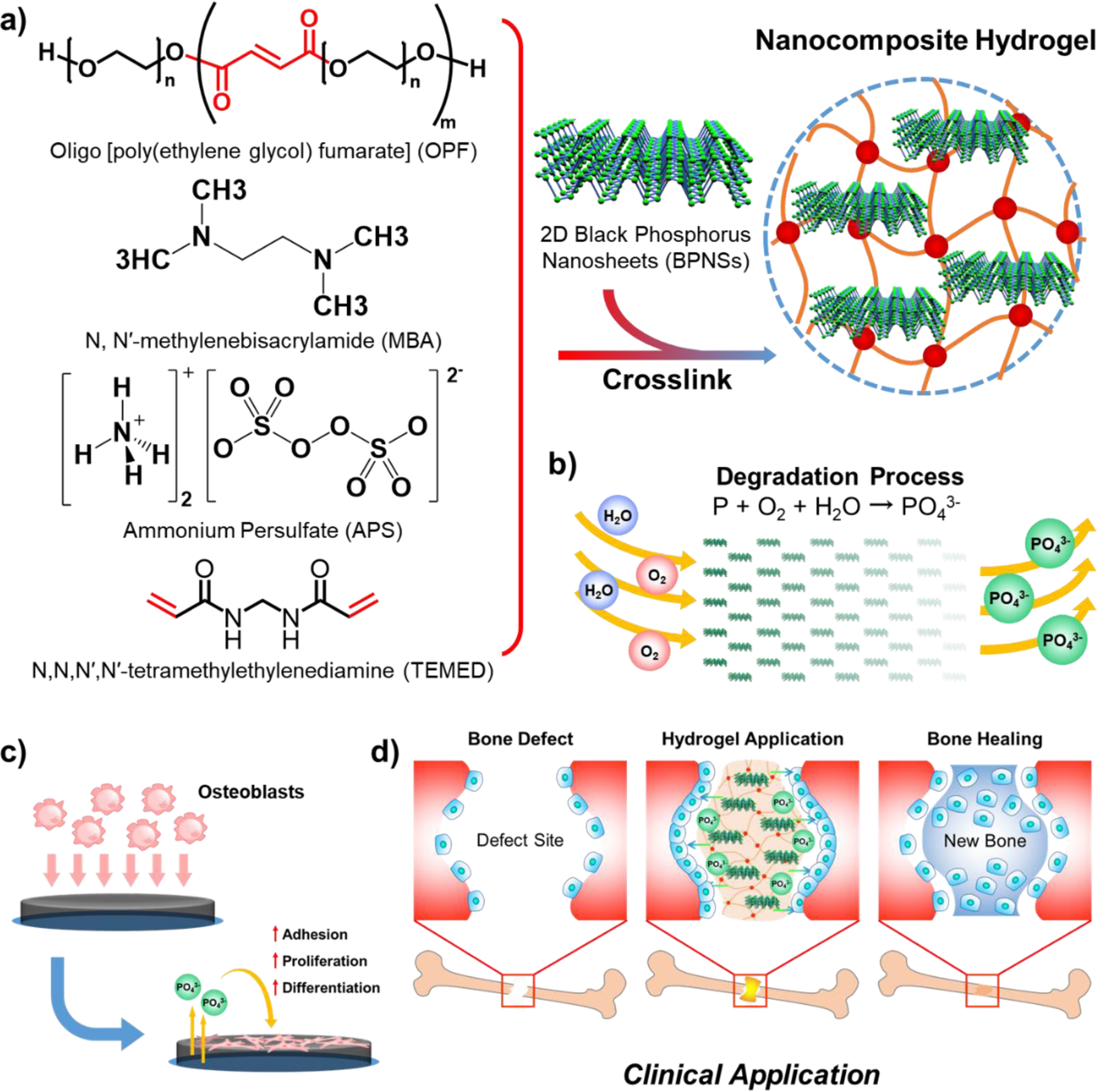
a) Structure of OPF polymer and schematic illustration of the fabrication process of OPF@BPNSs hydrogels. b) Continuous phosphate release as BPNSs degrade. c) Schematic illustration of cellular behavior after the application of OPF@BPNSs hydrogels. d) Schematic for clinical application of OPF@BPNSs hydrogels.
2. Materials and Methods
2.1. Preparation of BPNSs
BPNSs were synthesized by a liquid exfoliation method as described previously.35,36 Briefly, 10 mg of bulk BP (ACS Material, LLC, Pasadena, CA) was dispersed into 10 mL of nitrogen-flushed double-distilled water (ddH2O), and then sonicated in an ice bath sonicator (Elmasonic S10, Elma Schmidbauer GmbH, Germany) for 10 hours. The obtained suspension was centrifuged at 1500 rpm for 10 minutes to remove the residual unexfoliated precipitate, and the resulting BPNSs containing supernatant was collected for use in further experiments.
2.2. Characterization of BPNSs
AFM.
AFM was utilized to assess the morphology and layer height of BPNSs using previously published protocols.31,37 In brief, the fabricated BPNS sample (~5 μg/mL) was dropped onto the top of a fresh-surface mica disc (Ted Pella, Redding, CA) and dried under flowing nitrogen. Nanoscale images (512 × 512 pixel resolution) were taken by a Nanoscope IV PicoFroce Multimode AFM (Bruker, Camarillo, CA), using the machine’s contact mode at room temperature.
SEM.
Fabricated BPNSs (~50 μg/mL) were dried naturally, mounted on an aluminum stub, and sputter-coated with gold-palladium for 60 seconds. The morphological structure of BPNSs was then viewed on a scanning electron microscope (S-4700, Hitachi Instruments, Tokyo, Japan) at a voltage of 5 kV.
TEM.
TEM images of the BPNS samples (~50 μg/mL) were acquired on a transmission electron microscope (JEOL-1400, JEOL Inc., Japan) at 80 kV voltage.
Spectroscopy.
Ultraviolet-Visible (UV-Vis) spectroscopy was conducted by a UV-Vis absorbance microplate reader (SpectraMax Plus 384, Molecular Devices, Sunnyvale, CA) to measure the optical absorbance of BPNS solutions in the range of 240–1000 nm.
2.3. Preparation of OPF@BPNSs hydrogels
OPF was synthesized as previously described.38,39 Briefly, 50 g of PEG with a number average molecular weight (Mn) of 1000 g mol−1 was dissolved in 500 mL of methylene chloride and purged in an ice bath under nitrogen for 10 min, followed by dropwise addition of 0.9 mol triethylamine (TEA, Aldrich, Milwaukee, WI) per mol PEG and 1.8 mol fumaryl chloride (Acros, Pittsburgh, PA) per mol PEG. The reaction mixture was then stirred for 48 hours at room temperature, followed by the removal of methylene chloride via evaporation and crystallization of OPF in chilled ethyl acetate.
OPF@BPNSs hydrogels were formed by chemical crosslinking of dried OPF polymer, BPNS solution, N,N′-methylenebisacrylamide (MBA), ammonium persulfate (APS), and N,N,N′,N′-tetramethylethylenediamine (TEMED) (Figure 1a). APS was used as the initiator to generate free radicals and initiate the chemical crosslinking by passing the free radicals to the OPF chain and opening the double bonds in the OPF polymer chain. The activated OPF chain further passed the free radicals to inactivated polymer chains and started the polymerization. Meanwhile, TEMED accelerated the generation of free radicals from APS and speeded up the polymerization process. MBA, which contains two double bonds in the end, functionalized as the crosslinker to connect the activated polymer chains into one network and form the hydrogel (Figure 1a). In a typical example, 1 g of OPF polymer and 36 mg of MBA were completely dissolved in 2 mL of BPNSs/ddH2O solution with different concentrations of BPNSs (0, 100, 200, 500, and 1000 ppm). Subsequently, 0.1 mL of APS solution (1 g in 2 mL of ddH2O) and 0.1 mL of TEMED solution (1 mL in 2 mL of ddH2O) were added, and the mixture was immediately transferred to 0.8 mm-thick silicone rubber molds sandwiched between two glass plates. Molds were kept at room temperature for 8 hours to allow the hydrogels to fully cross-link. After removal from the molds and swelling in ddH2O overnight, hydrogels were punched into disc-shaped specimens using a cork borer (~9 mm in diameter and ~0.8 mm in thickness) for further use. Due to possible degradation of BPNSs,45 these hydrogels were used immediately following preparation. Based on the different BPNS concentrations (0, 100, 200, 500, and 1000 ppm) used to dissolve the OPF polymer before cross-linking, the subsequently fabricated hydrogels were designated as OPF@BPNSs 0, OPF@BPNSs 100, OPF@BPNSs 200, OPF@BPNSs 500, and OPF@BPNSs 1000, respectively.
2.4. Characterization of OPF@BPNSs hydrogels
Microscopy.
The morphological features of hydrogels were recorded by a digital camera and digital light microscope (Axiovert 25, Carl Zeiss, Germany).
SEM.
SEM images, corresponding element mapping, and energy dispersive X-ray spectroscopy (EDS) spectra of hydrogels dried by lyophilization for 3 days were taken on a scanning electron microscope (S-4700, Hitachi Instruments, Tokyo, Japan).
DMA.
The disc-shaped hydrogels were tested on a dynamic mechanical analyzer (RSA-G2, TA Instruments, New Castle, DE). The compressive strain-stress curves were obtained at a compression rate of 0.02 mm/s until failure. The compressive modulus of each hydrogel was calculated from the slope of the linear region in the stress-strain curves.
BPNS Degradation and Phosphate Release.
Hydrogel discs were separately immersed in 1 mL of ddH2O within wells of a 24-well plate and kept under two temperatures (room temperature or 37 °C). The solution within each well was changed daily with fresh ddH2O. The phosphate release kinetics of hydrogels was determined by measuring the phosphate ion concentration in the exchanged solution using a phosphate assay kit (ab65622, Abcam, Cambridge, UK). The absorbance at 650 nm was read on the UV-Vis absorbance microplate reader and the phosphate concentration was determined using a standard curve method. In the calculation of degradation ratio, the total phosphorous amount was quantified according to the concentration BPNSs/ddH2O solution used in crosslinking and the area ratio of each disc to the all fabricated hydrogel.
2.5. Pre-osteoblasts cell growth on OPF@BPNSs hydrogels
Cell Culture.
MC3T3 cells were cultured in Minimum Essential Medium Alpha without ascorbic acid (MEM-α, Gibco 32571–036), supplemented with 10% fetal bovine serum (FBS, Gibco 10437–028) and 0.5% penicillin-streptomycin (Pen-Strep, Gibco 15140–122), and maintained in an incubator with 5% CO2 and 95% relative humidity at 37 °C. Prior to cell culture, OPF@BPNSs hydrogel discs were sterilized in a 70% ethanol solution for 2 hours and immersed in phosphate-buffered saline (PBS) for 2 days with at least five PBS changes to remove any residual cross-linking agents and sol fraction. Then the hydrogel discs were transferred into 48-well tissue culture polystyrene (TCPS) plates, firmly set on the bottom of each well, and pretreated with culture medium for an additional 2 hours. MC3T3 cells were seeded onto hydrogels and TCPS control wells at a density of 30,000 cells per cm2.
Cell Proliferation.
After 1, 4, and 7 days of culture, cell proliferation was evaluated by measuring the cell density in each well using the MTS assay (CellTiter 96, Promega, Madison, WI). The optical absorbance at 490 nm read by the UV-Vis absorbance microplate reader was directly proportional to the number of living cells in culture. The cell viability was also determined by the LIVE/DEAD Cell Imaging Kit (Invitrogen), imaging stained cells with a digital Axiovert 25 Zeiss light microscope. Cells in 3 different views in each group were counted to quantify the cell viability. At 4 days post-seeding, 20 cells were randomly selected from the images of each group, followed by the analysis of the morphological parameters such as cell area, circularity, and pseudopodium length using ImageJ software.
Immuno-fluorescence Imaging.
Fluorescence images of MC3T3 cells growing on the hydrogels were obtained at 4 days post-seeding. Cells were fixed with 4% paraformaldehyde (PFA) solution at room temperature for 10 minutes and permeabilized by 0.2% Triton X-100 solution at room temperature for another 10 min, prior to being immersed in 1% bovine serum albumin (BSA)/PBS solution at 37 °C for 30 minutes to block non-specific binding sites. Cells were then incubated with anti-vinculin-FITC antibody (1:50 in PBS, Sigma-Aldrich Co., Milwaukee, WI) for 1 hour to visualize cellular focal adhesion and with rhodamine-phalloidin (RP, 1:200 in PBS, Cytoskeleton Inc, Denver, CO) for 1 hour to stain the cell cytoskeleton. Cell nuclei were finally labeled by 4’,6-diamidino-2-phenylindole (DAPI) at 37 °C for 10 min. The stained MC3T3 cells were immediately imaged using the fluorescent microscope.
Alkaline phosphatase (ALP) activity.
After 7, 14, and 21 days of culture, the medium of each well was collected and cell number was determined after trypsinization. The ALP concentration released in the medium was measured using a QuantiChrome™ Alkaline Phosphatase Assay Kit (DALP-250, BioAssay Systems, Hayward, CA), and the results were then normalized to the cell number.
Osteocalcin (OCN) content.
The OCN concentration released in the medium was quantified after culturing MC3T3 cells on the hydrogels for 7, 14, and 21 days, using the Mouse Osteocalcin Enzyme Immunoassay Kit (J64239, Alfa Aesar, Haverhill, MA). The results were also normalized to the cell number of each well. ALP activity and OCN content were reported as fold change as compared to the OPF treatment value on day 7 as a control.
2.6. Statistical analysis
Sample number one in the degradation experiment is three in proliferation study, and four in ALP activity or OCN content study. GraphPad Prism 7 (La Jolla, California, USA) was used for statistical analysis. One-way analysis of variance (ANOVA) was used to determine the differences between the groups, and further multiple comparisons were conducted using Tukey’s honest significant difference test. A p-value of < 0.05 was considered to be significantly different.
3. Results and Discussion
3.1. Characterization of fabricated BPNSs
BP bulk materials were dispersed in oxygen-free ddH2O to prevent BP from oxidation and degradation during sonication,36 and the mixture was sonicated in an ice bath for 10 hours to break up BP into nano-scale materials with even distribution in the solution.41,42 After exfoliation, the morphology of BPNSs was characterized by SEM, TEM, and AFM. As demonstrated in SEM images (Figure 2a–b), the prepared nanosheets were homogeneous in shape and consistent in size, with no other impurities detected in the solution. TEM images in Figure 2c clearly illustrated that the exfoliated nanosheets were in a single layer and free-standing with an approximate lateral size of 400 nm. AFM imaging was able to show the topographic morphology of BPNSs and the thickness determined by the cross-sectional analysis was approximately 40 nm (Figure 2d–f), which was consistent with previous studies,35,43 indicating successful exfoliation.
Figure 2.
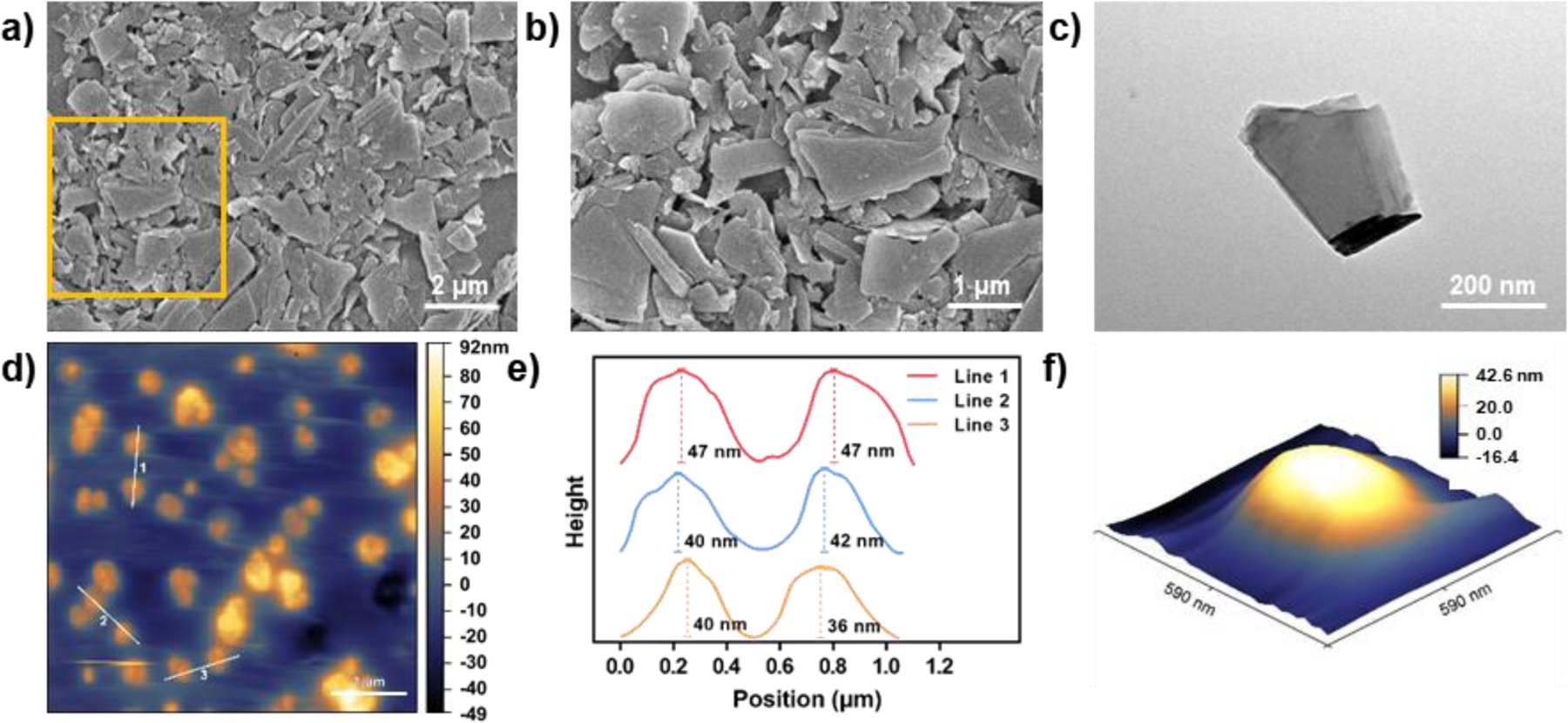
SEM images of BPNSs at a) lower (10000x) and b) higher magnifications (20000x). c) TEM image of BPNSs. d) AFM image of highly-dispersed BPNSs. e) BPNS thickness measurement based on three lines in (d). f) 3D reconstruction of a representative BPNS in (d).
3.2. Morphological and mechanical properties of OPF@BPNSs hydrogels
Unmodified OPF hydrogel discs were colorless and transparent under gross observation (Figure 3a), and appeared uniform and impurity-free under optical microscopy (Figure 3b). For the four types of hydrogels containing BPNSs, OPF was uniformly dissolved in BPNS solutions prior to cross-linking, such that the BPNSs were evenly distributed throughout the fabricated hydrogels. These four types of hydrogels turned opaque with grey color and became dark to varying degrees (Figure 3a). In line with the gross observation, the density of the black nanosheets within these hydrogels also gradually increased as the concentration of BPNSs increased with uniform dispersion (Figure 3b), confirming the successful incorporation of BPNSs into the polymeric network.
Figure 3.
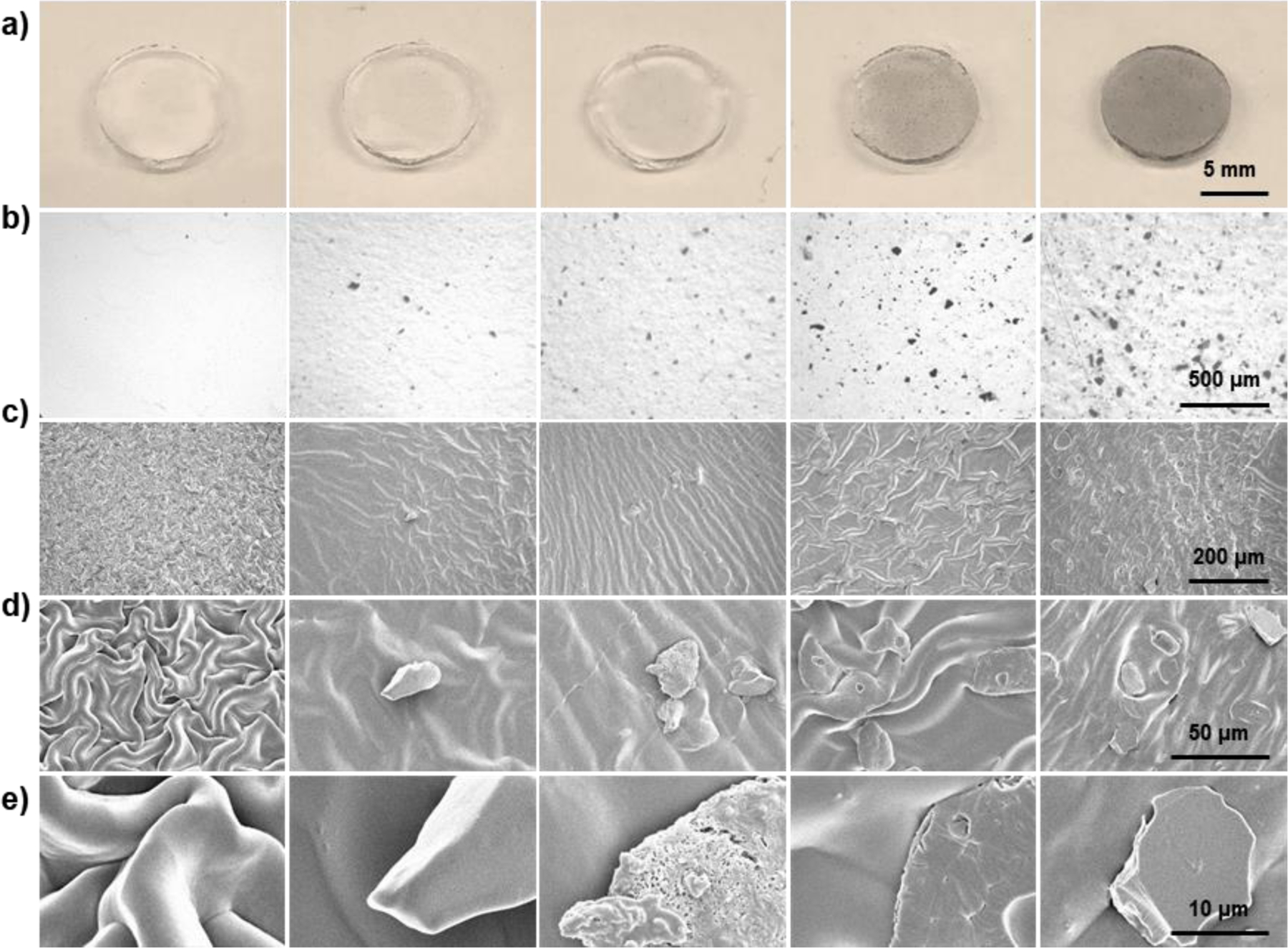
Morphological characterization of OPF@BPNSs hydrogels. a) Digital photographs, b) microscopic images, c) 200x SEM images, d) 1000x SEM images, and e) 5000x SEM images of OPF@BPNSs hydrogels. From left to right: OPF@BPNSs 0, OPF@BPNSs 100, OPF@BPNSs 200, OPF@BPNSs 500, and OPF@BPNSs 1000.
Dried OPF hydrogels demonstrated a ridge-like structure as a result of shrinkage due to lyophilization prior to SEM (Figure 3c,d).44 The hydrogel surfaces appeared smooth under high-magnification SEM (Figure 3e). The incorporation of BPNSs did not change the surface topography of the hydrogels when compared with pure OPF but scattered nanosheets were found over the ridge-like structure (Figure 3c). Few pieces of nanosheets could be detected on the 1000X images of OPF@BPNSs 100 hydrogel, while about ten pieces could be seen in the OPF@BPNSs 1000 hydrogel (Figure 3d). At 5000X magnification (Figure 3e), nanosheets were shown to be coated with the cross-linked polymer to varying degrees, exhibiting various shapes and sizes. Pieces with poor polymer coating demonstrated sharp margins, while polymer-encapsulated pieces showed smooth edges. Some nanosheets located on the surface of the hydrogel were corroded and oxidized resulting in porous structures (Figure 3e).
EDS and element mapping were performed on these five hydrogels. As displayed in Figure 4a, element mapping indicated the homogeneous distribution of carbon (C) and oxygen (O) on the hydrogel surfaces, while phosphorus (P) was highly concentrated in the areas where nanosheets were located. The introduction of phosphorus into the OPF polymer network may modulate the osteogenic capability of the hydrogels due to potential phosphate release. The corresponding EDS spectra also proved that phosphorus was not contained in pure OPF but only in BPNSs incorporated hydrogels (Figure 4b).
Figure 4.
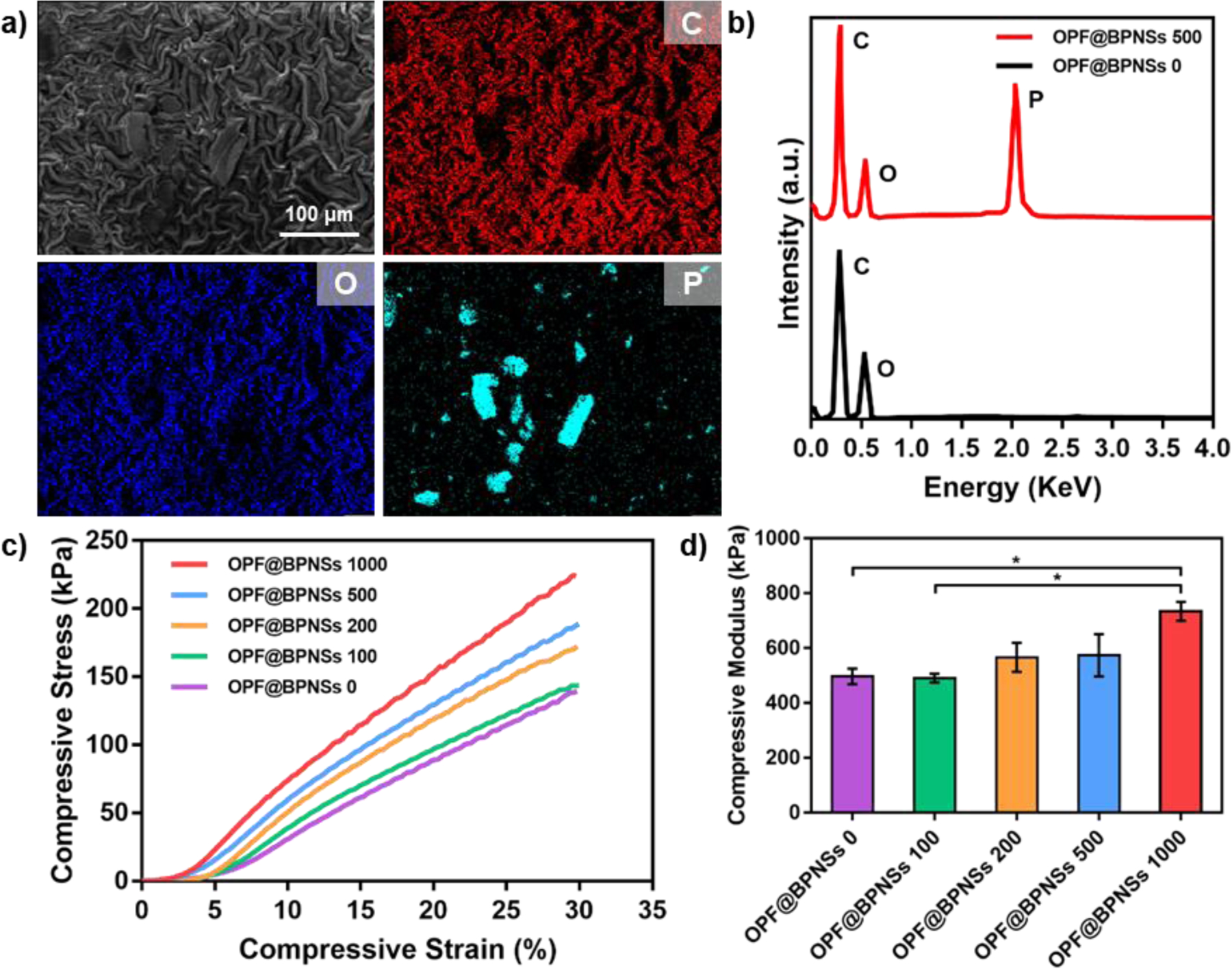
Compositional and mechanical characterization of OPF@BPNSs hydrogels. a) Corresponding element mappings of C, O, and P on OPF@BPNSs 500 hydrogel. b) EDS results of OPF@BPNSs 0 and OPF@BPNSs 500. c) Compressive stress-strain curves and (d) compressive moduli of the five types of hydrogels (*: p < 0.05).
Figure 4c showed the stress-strain curves obtained from DMA tests. The calculated compressive moduli indicated increasing values of 497.0 ± 28.3, 490.5 ± 16.3, 566.5 ± 53.0, 574.5 ± 77.1, and 734.5 ± 34.7 kPa for OPF@BPNSs 0, OPF@BPNSs 100, OPF@BPNSs 200, OPF@BPNSs 500, and OPF@BPNSs 1000, respectively (Figure 4d). The higher concentrations of BPNSs resulted in higher compressive modulus values. This may be due to the embedded BPNSs acting as supports in the process of cross-linking and thus tightening the OPF network, resulting in more complete cross-linking and a higher elastic modulus.
3.3. BPNS degradation and phosphate release
Phosphorus is not only an essential mineral element primarily used for cell proliferation and differentiation,26 but also an important component regulating bone mineralization by optimizing the calcium-phosphorus ratio.27,45 Under prolonged exposure to atmospheric oxygen, BPNSs oxidize and degrade gradually, leading to the release of H3PO4, H3PO3, H3PO2 and so on.40 Phosphorus can then be absorbed by cells in the form of phosphate and fulfill its specific biological functions. BPNSs-containing hydrogels could therefore enhance pre-osteoblast cell growth.
The degradation kinetics of BPNSs at both room and body temperature (37 °C) was studied by immersing the hydrogels in ddH2O with water change every day. As illustrated in Figure 5a, the color of OPF@BPNSs 1000 hydrogels faded gradually after two weeks of immersion, suggesting a corresponding decrease in the BPNS content of the hydrogel. During this process, BPNSs reacted with H2O and O2 and the released phosphates were quantified to obtain the degradation kinetics of BPNSs.
Figure 5.
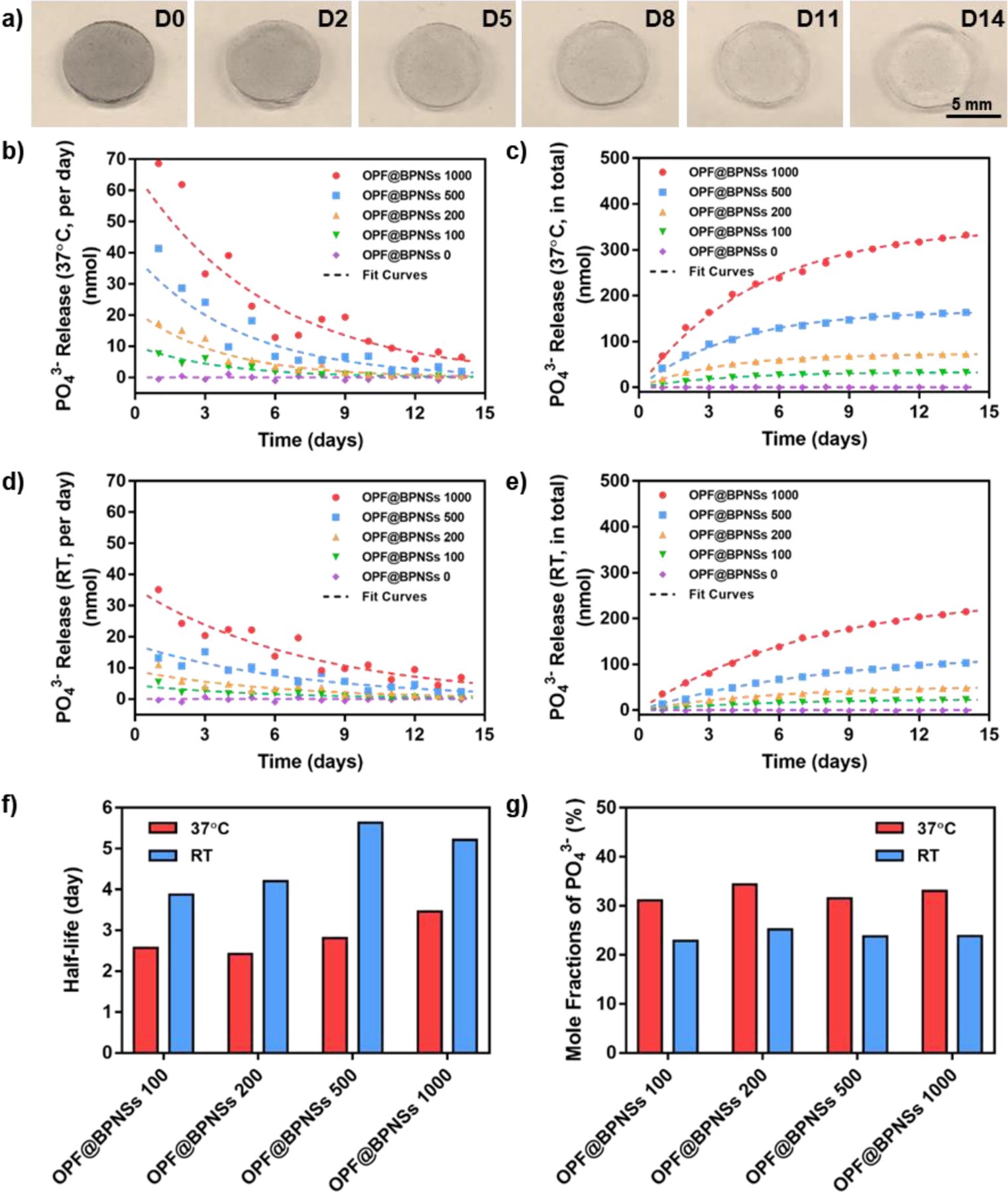
Degradation of BPNSs and subsequent release of phosphate. a) Digital photographs of OPF@BPNSs 1000 hydrogel immersed in ddH2O for 0, 2, 5, 8, 11, and 14 days. b) Daily and c) cumulative phosphate release from OPF@BPNSs hydrogels at 37 °C. d) Daily and e) cumulative phosphate release from OPF@BPNSs hydrogels at room temperature. f) Half-lives of phosphate release from different OPF@BPNSs hydrogels. g) Mole conversion ratio of BPNSs into phosphates in OPF@BPNSs hydrogels. RT: room temperature.
The results showed that BPNS degradation was logarithmic, with the majority of phosphates released during the first 9 days in solution, reaching a plateau after that period. In the 37 °C environment, the daily phosphate release rate of OPF@BPNSs 100, OPF@BPNSs 200, OPF@BPNSs 500, and OPF@BPNSs 1000 followed the fitting equations of 10.19e−0.273t, 21.39e−0.270t, 38.93e−0.221t, and 66.00e−0.176t, respectively (Figure 5b). By accumulating the daily amounts, the resulting cumulative phosphate release was 33.05(1 - e−0.269t), 73.32(1 - e−0.286t), 167.70(1 - e−0.246t), and 350.80(1 - e−0.200t), respectively (Figure 5c). As no phosphorus was present in the neutral OPF treatment, PO43− release from OPF@BPNSs 0 was negligible. Further analysis suggested that the degradation half-life of BPNSs in OPF@BPNSs 100 was 2.57 days, while OPF@BPNSs 1000 was 3.47 days (Figure 5f). This result showed that hydrogels with more BPNSs displayed a lower degradation rate, which could be explained by the inhibitory effect on further degradation by the relatively higher concentration of PO43− and more surface passivation. After comparing with the total phosphorus amount contained in single hydrogel discs, 31.1%−34.4% of phosphorus in the hydrogels was converted into phosphate (Figure 5g), which was consistent with previous studies,24,25 with the mole fractions showing no significant difference between different groups.
The degradation behavior of BPNSs at room temperature was in consonance with that in 37 °C. The daily PO43− release from OPF@BPNSs 100, OPF@BPNSs 200, OPF@BPNSs 500, and OPF@BPNSs 1000 followed the fitting equations of 4.45e−0.173t, 9.08e−0.161t, 17.33e−0.136t, and 35.55e−0.133t, respectively (Figure 5d), while the accumulated release fitted the equations of 24.40(1 - e−0.179t), 53.68(1 - e−0.165t), 126.65(1 - e−0.123t), and 254.14(1 - e−0.133t), respectively (Figure 5e). The degradation rates of BPNSs decreased at higher concentrations, with half-lives of 3.87 days, 4.20 days, 5.64 days, and 5.21 days determined for OPF@BPNSs 100, OPF@BPNSs 200, OPF@BPNSs 500, and OPF@BPNSs 1000 hydrogels, respectively (Figure 5f), which were significantly slower than that at 37 °C. In addition, the mole fractures of PO43− at room temperature were 22.9%−25.2% (Figure 5g), also relatively lower than that at 37 °C.
Hence, the oxidative degradation rate of BPNSs increased both with the increase of temperature and the decrease of BPNS content, as the half-life of BPNSs was higher in the high BPNSs concentration group. At 37 °C, 31.1%−34.4% phosphorus was converted into phosphates, which was gradually released to the surrounding environment at a logarithmic rate and then absorbed and utilized by the surrounding cells for proliferation and differentiation.
3.4. MC3T3 cell proliferation on OPF@BPNSs hydrogels
In vitro study was conducted by culturing pre-osteoblast MC3T3 cell line derived from mouse calvaria46 on OPF@BPNSs hydrogels to evaluate the osteogenic capability of each formulation. MTS results in Figure 6b showed that the positive TCPS control group demonstrated higher absorbance than the five types of hydrogels, indicating higher rates of cell proliferation. When compared with pure OPF hydrogel, OPF@BPNSs 500 group showed significantly higher MTS absorbance at 4 days post-seeding, and the adsorption increased gradually as the concentration of BPNSs increased from 100 ppm to 500 ppm. However, when BPNS concentration increased from 500 ppm to 1000 ppm, the absorbance decreased substantially, suggesting that BPNSs may be cytotoxic when concentrations exceed this threshold. At 7 days post-seeding, a similar absorbance trend was observed with the highest MTS absorbance displayed in the OPF@BPNSs 500 treatment.
Figure 6.
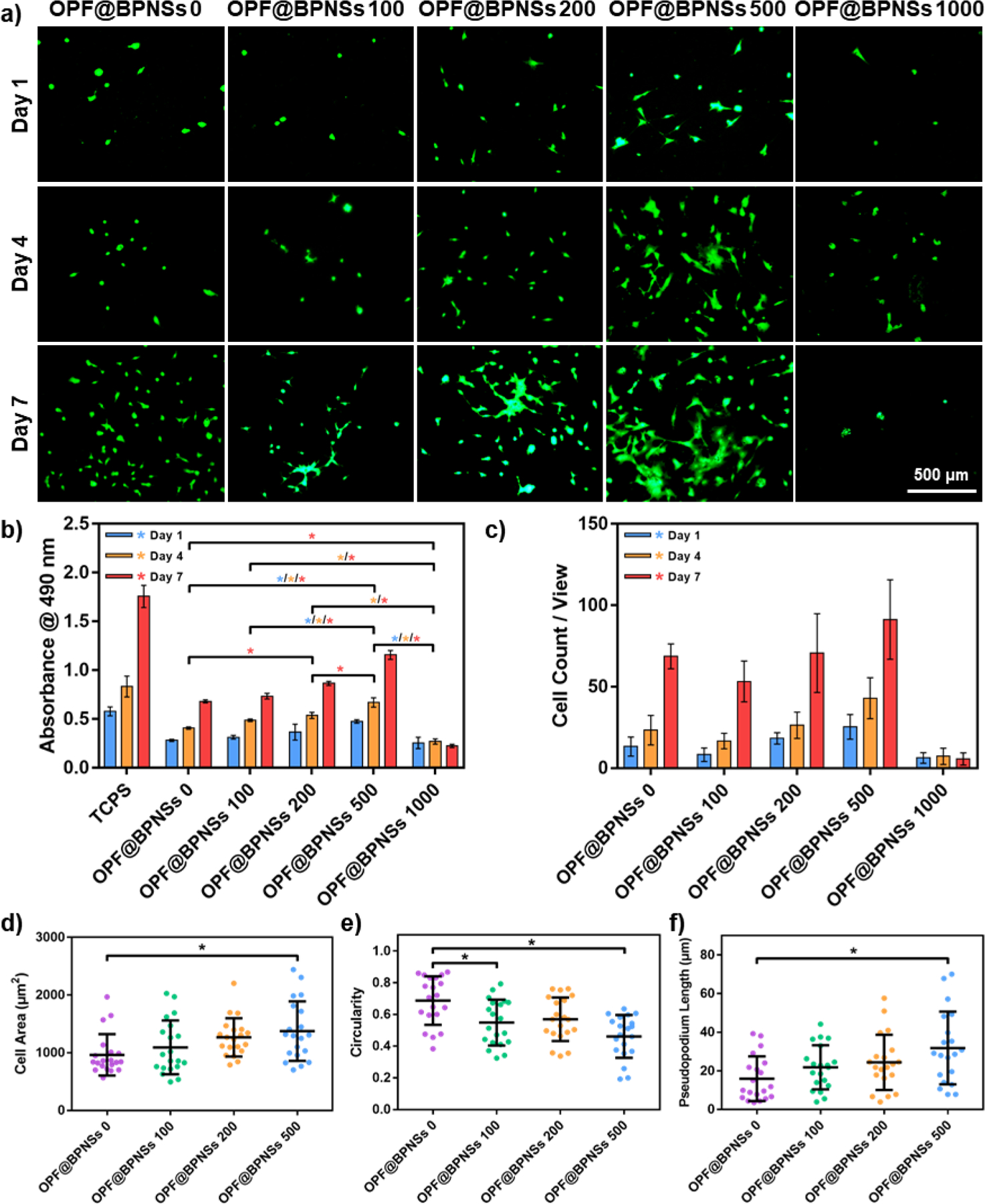
Proliferation of MC3T3 cells on OPF@BPNSs hydrogels. a) Live/Dead staining images of MC3T3 cells on OPF@BPNSs hydrogels after 1, 4, and 7 days post-seeding. b) MTS absorbance of MC3T3 cells at 1, 4, and 7 days post-seeding on the five types of hydrogels (*: p < 0.05). TCPS served as the positive control. c) Cell count results in each view in Live/Dead staining images. d) Cellular spreading area, e) circularity and f) pseudopodium length calculated using 20 single cells at 4 days post-seeding on four types of hydrogels (*: p < 0.05).
The quantity and morphology of MC3T3 cells were further determined by live/dead cell assays. As demonstrated in Figure 6a, the density of cells on the OPF@BPNSs 500 hydrogel at 1-day post-seeding, indicating that the BPNS concentration may influence not only the cellular proliferation but also the cell adhesion. The number of cells in a single microscopic field increased with time and reached a maximum on the OPF@BPNSs 500 hydrogel at 7 days post-seeding. However, cell number and growth rate on OPF@BPNSs 1000 group were significantly lower than the OPF@BPNSs 500 group. The MTS assay results together with live/dead images confirmed that the introduction of BPNSs into OPF hydrogels enhanced MC3T3 cell proliferation, with an optimal concentration at 500 ppm.
In the live/dead images, twenty single MC3T3 cells in each group at 4 days post-seeding were haphazardly selected and the morphology parameters including cell area, circularity, and pseudopodium length of these cells were quantified using ImageJ software. Since the cells on OPF@BPNSs 1000 hydrogels were too few to be counted, this group was not included in further comparisons. As indicated in Figure 6d, the cells on OPF@BPNSs 0 hydrogel showed limited spreading with an average cell area of 965 ± 359 μm2 and the cells growing on OPF@BPNSs 200 and OPF@BPNSs 500 hydrogels exhibited enlarged cell areas with values of 1268 ± 331 μm2 and 1376 ± 515 μm2, respectively. The value of OPF@BPNSs 500 was statistically greater than the pure OPF group, indicating that BPNSs were beneficial for the spreading of cells.
The circularity of cells, defined as the degree of the cellular shape approaching a round circle, was also measured.47 The circularity is close to 1.0 when the cellular shape tends to be round, whereas the near-zero value is for cells with a linear shape. As noted in Figure 6e, cells on pure OPF hydrogels had a higher circularity distribution with average values of 0.69 ± 0.15, suggesting a more rounded cellular shape. After incorporation of BPNSs, decreased circularities with values of 0.55 ± 0.14 and 0.57 ± 0.14 were detected for OPF@BPNSs 100 and OPF@BPNSs 200, respectively. The lowest circularity value was observed for cells on OPF@BPNSs 500 hydrogel with an average of 0.46 ± 0.13.
The pseudopodium length of the cells on the four types of OPF@BPNSs hydrogels was also evaluated. Pseudopodium is a temporary cellular structure being used for motility and ingesting nutrients.48 Long pseudopodium is an indication that the cells growing well on the surface are differentiating.49 On the neutral OPF hydrogel, cells developed pseudopodia with an average length of 15.97 ± 11.59 μm. In contrast, with the addition of BPNSs, the cells on the functionalized hydrogels produced significantly longer pseudopodia with average values of 21.85 ± 11.39 μm and 24.45 ± 14.28 μm for OPF@BPNSs 100 and OPF@BPNSs 200, respectively. As the most suitable material for cell growth, hydrogels with BPNS concentration of 500 ppm supported the longest pseudopodia in cells with a value of 31.85 ± 18.83 μm. All of the above trends affirmed that the incorporation of BPNSs into OPF hydrogels could remarkably promote the growth of MC3T3 cells on the OPF hydrogels.
At 4 days post-seeding, MC3T3 cells growing on the hydrogels were fixed, stained, and imaged. Fluorescence images of the cells were presented in Figure 7. F-actin was stained red by rhodamine−phalloidin, vinculin stained green by anti-vinculin-FITC, and cell nuclei stained blue by DAPI. The merged images provided a clearer comparison of cellular morphologies on these hydrogels. An obvious trend was observed wherein MC3T3 cells were attached on the OPF@BPNSs 500 hydrogels with significantly more development of F-actin and vinculin. F-actin is essential for cellular functions such as mobility and contraction of cells during cell division,50,51 while vinculin is also a cytoskeletal protein associated with cell adhesion.52 The development of F-actin and vinculin demonstrated healthy adhesion, division, and differentiation of MC3T3 pre-osteoblast cells on the functionalized hydrogels. Therefore, adding a certain amount of BPNSs to OPF hydrogel could greatly improve cell compatibility and would be enormously beneficial for bone tissue engineering.
Figure 7.
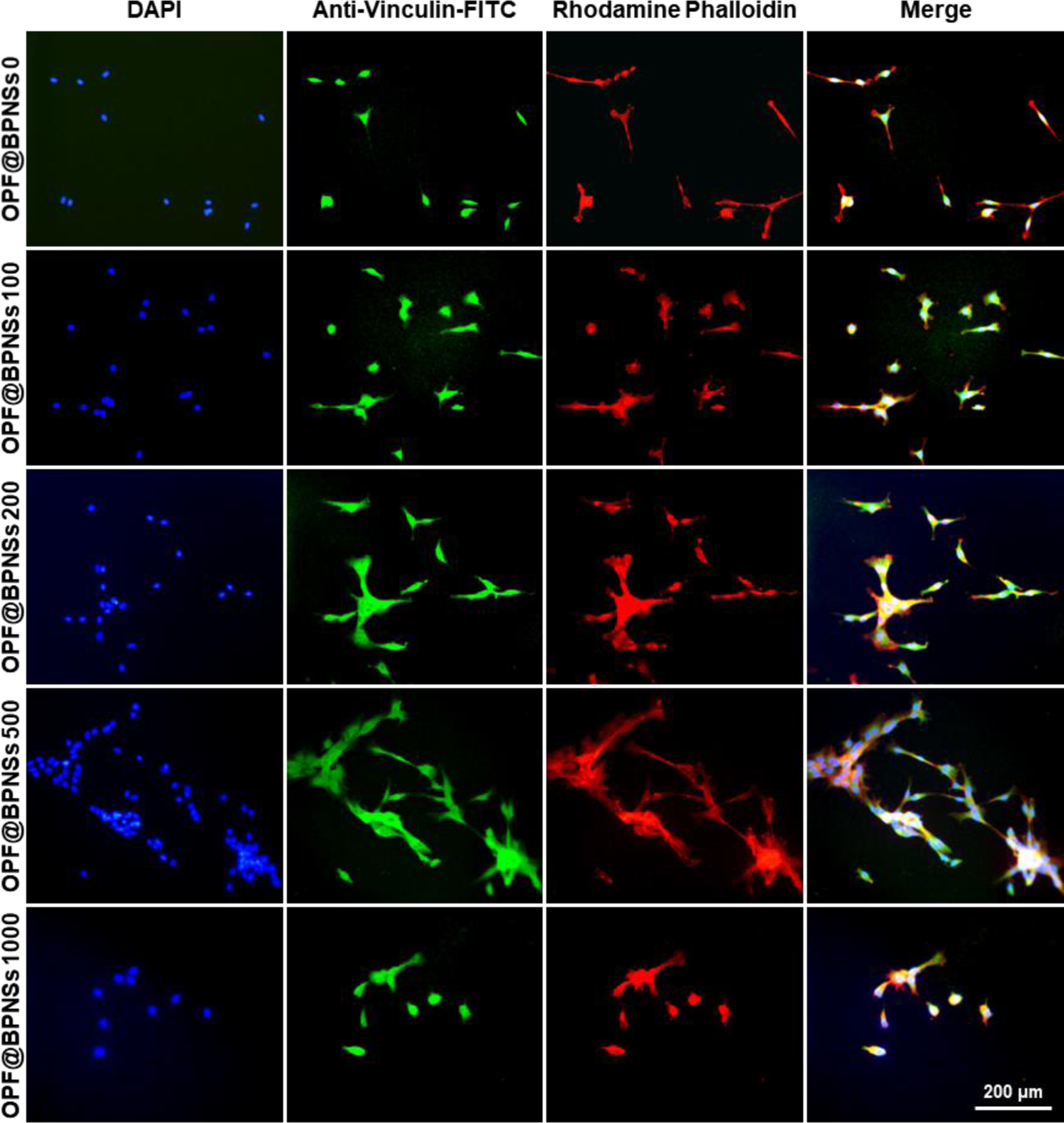
Immunofluorescence images of MC3T3 cells on five types of hydrogels after 4 days of culture. F-actin was stained red by rhodamine−phalloidin, vinculin stained green by anti-vinculin-FITC, and cell nuclei stained blue by DAPI
3.4. MC3T3 cell differentiation on OPF@BPNSs hydrogels
Osteogenic differentiation of MC3T3 cells was also affected by the introduction of BPNSs. ALP and OCN activities, two typical differentiation markers, were quantified after 7, 14, and 21 days of culture. As shown in Figure 8, ALP activity was elevated after 14 days in most of the groups, with relative ALP activity of OPF@BPNSs 500 group being much higher than pure OPF hydrogel and OPF@BPNSs 100 group. In contrast to ALP activity, OCN secretion peak was recorded at 21 days, and both OPF@BPNSs 200 and OPF@BPNSs 500 groups demonstrated a significant increase in cellular OCN secretion when compared to the pure OPF group. Similar to the results of cell proliferation, the ALP and OCN activities in the OPF@BPNSs 1000 group was relatively low, demonstrating that excessively high BPNSs concentration in the hydrogels are detrimental to the osteogenesis of MC3T3 cells. Taken together, these results suggested that an appropriate increase of BPNSs concentration in the OPF has the potential to enhance the differentiation of pre-osteoblasts on the hydrogel.
Figure 8.
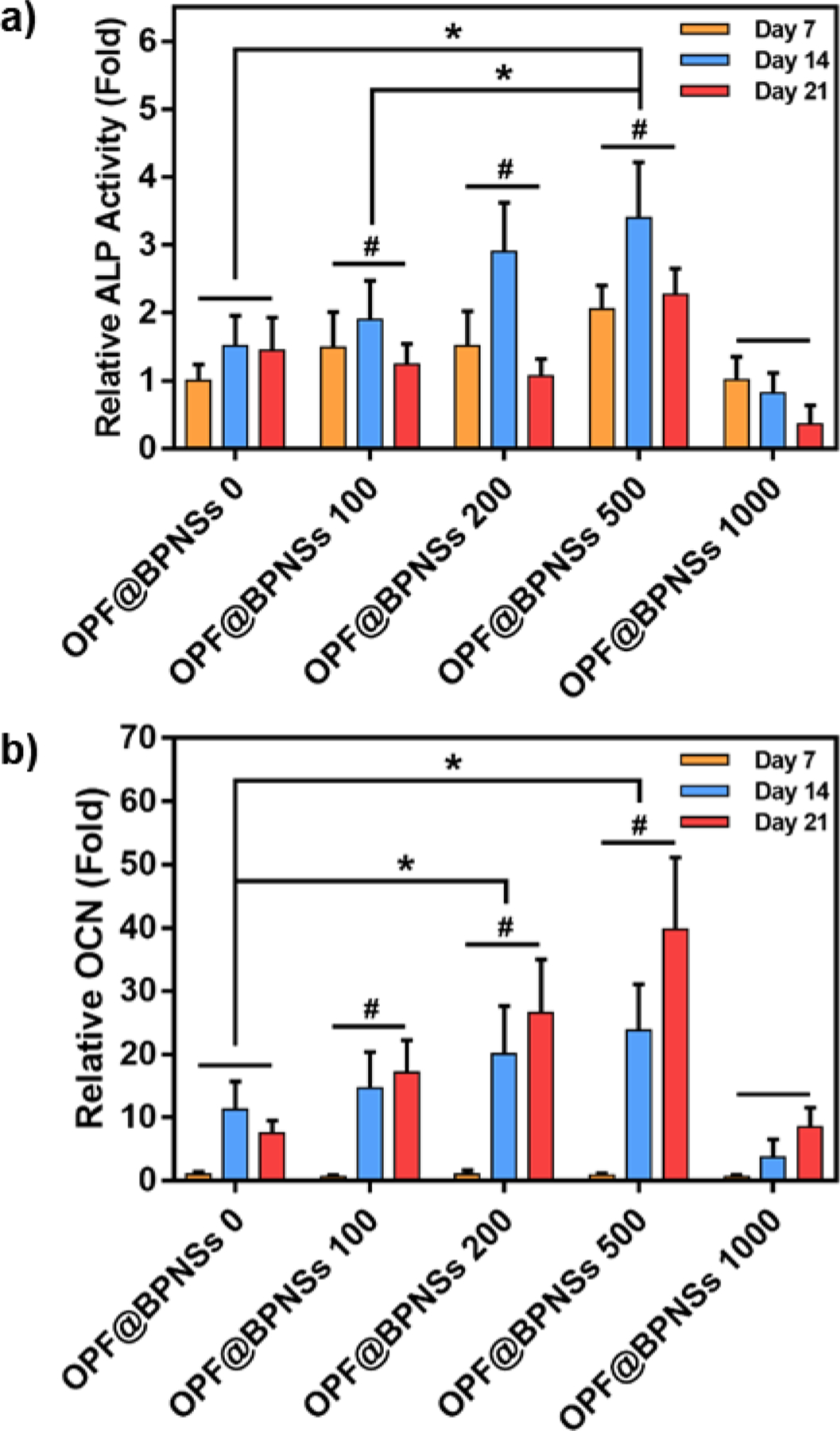
a) Relative ALP activity (fold change) and b) relative OCN secretion (fold change) of MC3T3 cells on five types of hydrogels after 7, 14, and 21 days of culture (*: p < 0.05, #: p < 0.05 compared to OPF@BPNSs 1000 group).
4. Conclusion
Phosphorus is a necessary substance for cells, which can be released during the process of BPNS degradation and absorbed by surrounding cells for further metabolism in the form of phosphate. In the OPF@BPNSs system developed in this study, embedding BPNSs into OPF hydrogels was shown to enhance the surface properties of the hydrogels and improve their biomechanical capability. The degradation behavior of BPNSs was controlled, at a dynamic and logarithmic rate, coupled with the moderate release of phosphate. A complete degradation process continued for an additional two to three weeks, which was sufficient for the cells to accomplish the initial adhesion and growth. Hence, the addition of BPNSs helped to increase the cytocompatibility and osteogenic capacity of the material, leading to an increase in the proliferation and differentiation of pre-osteoblast cells. Embedding BPNSs in an OPF hydrogel functioned to limit the amount of direct contact with cells and BPNSs at a concentration of 500 ppm showed optimized cellular compatibility in the OPF hydrogel platform. In conclusion, BPNSs at certain concentrations enhanced MC3T3 cell behavior including spreading, distribution, proliferation, and differentiation on the OPF hydrogels. The OPF@BPNSs system could potentially find application in the restoration of bone defects.
Acknowledgments
This work was supported by the National Institutes of Health Grant R01 AR56212 and AR75037.
Footnotes
Conflicts of interest
The authors declare no competing financial interest.
References
- [1].Ho-Shui-Ling A; Bolander J; Rustom LE; Johnson AW; Luyten FP; Picart C Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162, DOI: 10.1016/j.biomaterials.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lu Y; Li L; Zhu Y; Wang X; Li M; Lin Z; Hu X; Zhang Y; Yin Q; Xia H; Mao C Multifunctional Copper-Containing Carboxymethyl Chitosan/Alginate Scaffolds for Eradicating Clinical Bacterial Infection and Promoting Bone Formation. ACS Appl Mater Interfaces 2018, 10(1), 127–138, DOI: 10.1021/acsami.7b13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bollen L; Dijkstra SPD; Bartels RHMA; de Graeff A; Poelma DLH; Brouwer T; Algra PR; Kuijlen JMA; Minnema MC; Nijboer C; Rolf C; Sluis T; Terheggen MAMB; van der Togt-van Leeuwen ACM; van der Linden YM; Taal W Clinical management of spinal metastases-The Dutch national guideline. Eur J Cancer 2018, 104, 81–90, DOI: 10.1016/j.ejca.2018.08.028. [DOI] [PubMed] [Google Scholar]
- [4].Banwart JC; Asher MA; Hassanein RS Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine (Phila Pa 1976) 1995, 20(9), 1055–1060. [DOI] [PubMed] [Google Scholar]
- [5].Liu X; Miller AL; Fundora KA; Yaszemski MJ; Lu L Poly(ε-caprolactone) Dendrimer Cross-Linked via Metal-Free Click Chemistry: Injectable Hydrophobic Platform for Tissue Engineering. ACS Macro Lett 2016, 5(11), 1261–1265, DOI: 10.1021/acsmacrolett.6b00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Guo J; Liu X; Miller AL; Waletzki BE; Yaszemski MJ; Lu L Novel porous poly(propylene fumarate-co-caprolactone) scaffolds fabricated by thermally induced phase separation. J Biomed Mater Res A 2017, 105(1), 226–235, DOI: 10.1002/jbm.a.35862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Henry MG; Cai L; Liu X; Zhang L; Dong J; Chen L; Wang Z; Wang S Roles of hydroxyapatite allocation and microgroove dimension in promoting preosteoblastic cell functions on photocured polymer nanocomposites through nuclear distribution and alignment. Langmuir 2015, 31(9), 2851–2860, DOI: 10.1021/la504994e. [DOI] [PubMed] [Google Scholar]
- [8].Cai L; Foster CJ; Liu X; Wang S Enhanced bone cell functions on poly(ε-caprolactone) triacrylate networks grafted with polyhedral oligomeric silsesquioxane nanocages. Polymer 2014, 55(16), 3836–3845, DOI: 10.1016/j.polymer.2014.06.057. [DOI] [Google Scholar]
- [9].Lasprilla A; Martinez GA; Lunelli BH; Jardini AL; Filho RM Poly-lactic acid synthesis for application in biomedical devices - a review. Biotechnol Adv 2012, 30(1), 321–328, DOI: 10.1016/j.biotechadv.2011.06.019. [DOI] [PubMed] [Google Scholar]
- [10].Kinard LA; Kasper FK; Mikos AG Synthesis of oligo(poly(ethylene glycol) fumarate). Nat Protoc 2012, 7(6), 1219–1227, DOI: 10.1038/nprot.2012.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jo S; Shin H; Shung AK; Fisher JP; Mikos AG Synthesis and Characterization of Oligo(poly(ethylene glycol) fumarate) Macromer. Macromolecules 2001, 34(9), 2839–2844, DOI: 10.1021/ma001563y. [DOI] [Google Scholar]
- [12].Dadsetan M; Giuliani M; Wanivenhaus F; Brett Runge M; Charlesworth JE; Yaszemski MJ Incorporation of phosphate group modulates bone cell attachment and differentiation on oligo(polyethylene glycol) fumarate hydrogel. Acta Biomater 2012, 8(4), 1430–1439, DOI: 10.1016/j.actbio.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dadsetan M; Knight AM; Lu L; Windebank AJ; Yaszemski MJ Stimulation of neurite outgrowth using positively charged hydrogels. Biomaterials 2009, 30(23–24), 3874–3881, DOI: 10.1016/j.biomaterials.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dadsetan M; Szatkowski JP; Yaszemski MJ; Lu L Characterization of photo-cross-linked oligo[poly(ethylene glycol) fumarate] hydrogels for cartilage tissue engineering. Biomacromolecules 2007, 8(5), 1702–1709, DOI: 10.1021/bm070052h. [DOI] [PubMed] [Google Scholar]
- [15].Dadsetan M; Liu Z; Pumberger M; Giraldo CV; Ruesink T; Lu L; Yaszemski MJ A stimuli-responsive hydrogel for doxorubicin delivery. Biomaterials 2010, 31(31), 8051–8062, DOI: 10.1016/j.biomaterials.2010.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Olthof MGL; Tryfonidou MA; Liu X; Pouran B; Meij BP; Dhert WJA; Yaszemski MJ; Lu L; Alblas J; Kempen DHR Phosphate Functional Groups Improve Oligo[(Polyethylene Glycol) Fumarate] Osteoconduction and BMP-2 Osteoinductive Efficacy. Tissue Eng Part A 2018, 24(9–10), 819–829, DOI: 10.1089/ten.TEA.2017.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Olthof MGL; Kempen DHR; Liu X; Dadsetan M; Tryfonidou MA; Yaszemski MJ; Dhert WJA; Lu L Bone morphogenetic protein-2 release profile modulates bone formation in phosphorylated hydrogel. J Tissue Eng Regen Med 2018, 12(6), 1339–1351, DOI: 10.1002/term.2664. [DOI] [PubMed] [Google Scholar]
- [18].Kou L; Chen C; Smith SC Phosphorene: Fabrication, Properties, and Applications. J Phys Chem Lett 2015, 6(14), 2794–2805, DOI: 10.1021/acs.jpclett.5b01094. [DOI] [PubMed] [Google Scholar]
- [19].Jing Y; Tang Q; He P; Zhou Z; Shen P Small molecules make big differences: molecular doping effects on electronic and optical properties of phosphorene. Nanotechnology 2015, 26(9), 095201, DOI: 10.1088/0957-4484/26/9/095201. [DOI] [PubMed] [Google Scholar]
- [20].Xu F; Ma H; Lei S; Sun J; Chen J; Ge B; Zhu Y; Sun L In situ TEM visualization of superior nanomechanical flexibility of shear-exfoliated phosphorene. Nanoscale 2016, 8(28), 13603–13610, DOI: 10.1039/c6nr02487d. [DOI] [PubMed] [Google Scholar]
- [21].Fei R; Faghaninia A; Soklaski R; Yan JA; Lo C; Yang L Enhanced thermoelectric efficiency via orthogonal electrical and thermal conductances in phosphorene. Nano Lett 2014, 14(11), 6393–6399, DOI: 10.1021/nl502865s. [DOI] [PubMed] [Google Scholar]
- [22].Lv H; Lu W; Shao D; Sun Y Enhanced thermoelectric performance of phosphorene by strain-induced band convergence. Phys Rev B 2014, 90(8), 085433, DOI: 10.1103/PhysRevB.90.085433. [DOI] [Google Scholar]
- [23].Childers DL; Corman J; Edwards M; Elser JJ Sustainability challenges of phosphorus and food: solutions from closing the human phosphorus cycle. Bioscience 2011, 61(2), 117–124, DOI: 10.1525/bio.2011.61.2.6. [DOI] [Google Scholar]
- [24].Zhang T; Wan Y; Xie H; Mu Y; Du P; Wang D; Wu X; Ji H; Wan L Degradation Chemistry and Stabilization of Exfoliated Few-Layer Black Phosphorus in Water. J Am Chem Soc 2018, 140(24), 7561–7567, DOI: 10.1021/jacs.8b02156. [DOI] [PubMed] [Google Scholar]
- [25].Plutnar J; Sofer Z; Pumera M Products of Degradation of Black Phosphorus in Protic Solvents. ACS Nano 2018, 12(8), 8390–8396, DOI: 10.1021/acsnano.8b03740. [DOI] [PubMed] [Google Scholar]
- [26].Pasek M A role for phosphorus redox in emerging and modern biochemistry. Curr Opin Chem Biol 2018, 49, 53–58, DOI: 10.1016/j.cbpa.2018.09.018. [DOI] [PubMed] [Google Scholar]
- [27].LeGeros RZ Calcium phosphate-based osteoinductive materials. Chem Rev 2008, 108(11), 4742–4753, DOI: 10.1021/cr800427g. [DOI] [PubMed] [Google Scholar]
- [28].Yang B; Yin J; Chen Y; Pan S; Yao H; Gao Y; Shi J 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds: A Stepwise Countermeasure for Osteosarcoma. Adv Mater 2018, 30(10), 1705611, DOI: 10.1002/adma.201705611. [DOI] [PubMed] [Google Scholar]
- [29].Shao J; Ruan C; Xie H; Li Z; Wang H; Chu PK; Yu XF Black-Phosphorus-Incorporated Hydrogel as a Sprayable and Biodegradable Photothermal Platform for Postsurgical Treatment of Cancer. Adv Sci (Weinh) 2018, 5(5), 1700848, DOI: 10.1002/advs.201700848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Latiff NM; Teo WZ; Sofer Z; Fisher AC; Pumera M The Cytotoxicity of Layered Black Phosphorus. Chemistry 2015, 21(40), 13991–13995, DOI: 10.1002/chem.201502006. [DOI] [PubMed] [Google Scholar]
- [31].Liu X; Miller AL; Park S; Waletzki BE; Zhou Z; Terzic A; Lu L Functionalized Carbon Nanotube and Graphene Oxide Embedded Electrically Conductive Hydrogel Synergistically Stimulates Nerve Cell Differentiation. ACS Appl Mater Interfaces 2017, 9(17), 14677–14690, DOI: 10.1021/acsami.7b02072. [DOI] [PubMed] [Google Scholar]
- [32].Daly WT; Knight AM; Wang H; de Boer R; Giusti G; Dadsetan M; Spinner RJ; Yaszemski MJ; Windebank AJ Comparison and characterization of multiple biomaterial conduits for peripheral nerve repair. Biomaterials 2013, 34(34), 8630–8639, DOI: 10.1016/j.biomaterials.2013.07.086. [DOI] [PubMed] [Google Scholar]
- [33].Choi JR; Yong KW; Choi JY; Nilghaz A; Lin Y; Xu J; Lu X Black Phosphorus and its Biomedical Applications. Theranostics 2018, 8(4), 1005–1026, DOI: 10.7150/thno.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee HU; Lee SC; Won J; Son BC; Choi S; Kim Y; Park SY; Kim HS; Lee YC; Lee J Stable semiconductor black phosphorus (BP)@titanium dioxide (TiO2) hybrid photocatalysts. Sci Rep 2015, 5, 8691, DOI: 10.1038/srep08691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen L; Zhou G; Liu Z; Ma X; Chen J; Zhang Z; Ma X; Li F; Cheng HM; Ren W Scalable Clean Exfoliation of High-Quality Few-Layer Black Phosphorus for a Flexible Lithium Ion Battery. Adv Mater 2016, 28(3), 510–517, DOI: 10.1002/adma.201503678. [DOI] [PubMed] [Google Scholar]
- [36].Wang H; Yang X; Shao W; Chen S; Xie J; Zhang X; Wang J; Xie Y Ultrathin Black Phosphorus Nanosheets for Efficient Singlet Oxygen Generation. J Am Chem Soc 2015, 137(35), 11376–11382, DOI: 10.1021/jacs.5b06025. [DOI] [PubMed] [Google Scholar]
- [37].Zhou Z; Liu X; Wu W; Park S; Miller AL; Terzic A; Lu L Effective nerve cell modulation by electrical stimulation of carbon nanotube embedded conductive polymeric scaffolds. Biomater Sci 2018, 6(9), 2375–2385, DOI: 10.1039/c8bm00553b. [DOI] [PubMed] [Google Scholar]
- [38].Dadsetan M; Szatkowski JP; Yaszemski MJ; Lu L Characterization of photo-cross-linked oligo[poly(ethylene glycol) fumarate] hydrogels for cartilage tissue engineering. Biomacromolecules 2007, 8(5), 1702–1709, DOI: 10.1021/bm070052h. [DOI] [PubMed] [Google Scholar]
- [39].Dadsetan M; Hefferan TE; Szatkowski JP; Mishra PK; Macura SI; Lu L; Yaszemski MJ Effect of hydrogel porosity on marrow stromal cell phenotypic expression. Biomaterials 2008, 29(14), 2193–2202, DOI: 10.1016/j.biomaterials.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Huang Y; Qiao J; He K; Bliznakov S; Sutter E; Chen X; Luo D; Meng F; Su D; Decker J; Ji W; Ruoff RS; Sutter P Interaction of Black Phosphorus with Oxygen and Water. Chem Mater 2016, 28(22), 8330–8339, DOI: 10.1021/acs.chemmater.6b03592. [DOI] [Google Scholar]
- [41].Brent JR; Savjani N; Lewis EA; Haigh SJ; Lewis DJ; O’Brien P Production of few-layer phosphorene by liquid exfoliation of black phosphorus. Chem Commun (Camb) 2014, 50(87), 13338–13341, DOI: 10.1039/c4cc05752j. [DOI] [PubMed] [Google Scholar]
- [42].Zhang W; Wang YC; Li X; Song C; Wan L; Usman K; Fang J Recent Advance in Solution-Processed Organic Interlayers for High-Performance Planar Perovskite Solar Cells. Adv Sci (Weinh) 2018, 5(7), 1800159, DOI: 10.1002/advs.201800159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dhanabalan SC; Ponraj JS; Guo Z; Li S; Bao Q; Zhang H Emerging Trends in Phosphorene Fabrication towards Next Generation Devices. Adv Sci (Weinh) 2017, 4(6), 1600305, DOI: 10.1002/advs.201600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu X; Paulsen A; Giambini H; Guo J; Miller AL; Lin PC; Yaszemski MJ; Lu L A New Vertebral Body Replacement Strategy Using Expandable Polymeric Cages. Tissue Eng Part A 2017, 23(5–6), 223–232, DOI: 10.1089/ten.TEA.2016.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Boushell MK; Khanarian NT; LeGeros RZ; Lu HH Effect of ceramic calcium-phosphorus ratio on chondrocyte-mediated biosynthesis and mineralization. J Biomed Mater Res A 2017, 105(10), 2694–2702, DOI: 10.1002/jbm.a.36122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Czekanska EM; Stoddart MJ; Richards RG; Hayes JS In search of an osteoblast cell model for in vitro research. Eur Cell Mater 2012, 24, 1–17, DOI: 10.22203/eCM.v024a01. [DOI] [PubMed] [Google Scholar]
- [47].Peyton SR; Raub CB; Keschrumrus VP; Putnam AJ The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials 2006, 27(28), 4881–4893, DOI: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- [48].Bugyi B; Carlier MF Control of actin filament treadmilling in cell motility. Annu Rev Biophys 2010, 39, 449–470, DOI: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- [49].Chodniewicz D; Klemke RL Guiding cell migration through directed extension and stabilization of pseudopodia. Exp Cell Res 2004, 301(1), 31–37, DOI: 10.1016/j.yexcr.2004.08.006. [DOI] [PubMed] [Google Scholar]
- [50].Doherty GJ; McMahon HT Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annu Rev Biophys 2008, 37, 65–95, DOI: 10.1146/annurev.biophys.37.032807.125912. [DOI] [PubMed] [Google Scholar]
- [51].Shawky JH; Balakrishnan UL; Stuckenholz C; Davidson LA Multiscale analysis of architecture, cell size and the cell cortex reveals cortical F-actin density and composition are major contributors to mechanical properties during convergent extension. Development 2018, 145(19), dev161281, DOI: 10.1242/dev.161281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Geiger B; Spatz JP; Bershadsky AD Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 2009, 10(1), 21–33, DOI: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]


