Abstract
Objectives
Associations between cognitive decline and depression have been inconclusive. We examined 1) whether sleep quality mediates these relationships and 2) which factor of sleep quality mediates these relationships.
Methods
This study utilized baseline data from the 2018 West China Health and Aging Trend study (WCHAT), a large cohort data-set that including participants aged over 50 years old. We defined depression using the 15-item Geriatric Depression Scale (GDS-15). Cognitive status was measured using the Short Portable Mental Status Questionnaire (SPMSQ) and sleep quality was assessed using the Pittsburgh sleep quality index (PSQI). Direct relationships between cognitive decline, sleep quality and depression were assessed using multiple linear regression. Mediation models and structural equation model (SEM) pathway analysis were used to test the mediating role of specific aspects of sleep (e.g., quality, duration) in the relationship between cognitive decline and depression.
Results
Of 6828 participants aged 50 years old or older, the proportion of depression was 17.4%. Regression analysis indicated a total association between cognitive scores (β = 0.251, 95% CI 0.211 to 0.290, p < 0.001) and depression status. After adjusted PSQI scores, the association between cognitive scores and depression status was still significant (β = 0.242, 95% CI 0.203 to 0.281, p < 0.001), indicating a partial mediation effect of sleep quality. Mediation analysis verified sleep quality partially mediate the associations between cognitive decline and depression (indirect effect estimate = 0.0308, bootstrap 95% CI 0.023 to 0.040; direct effect estimate = 0.3124, bootstrap 95% CI 0.269 to 0.350). And daytime dysfunction had a highest mediation effect with a proportion of mediation up to 14.6%.
Conclusions
Sleep quality partially mediated the relationship between cognitive decline and depression. Daytime dysfunction had a highest mediation effect. Further research is necessary to examine the effects of sleep quality on the relationship of cognitive decline and depression.
Keywords: Cognitive decline, Depression, Mediation analysis, Sleep quality
Introduction
With an aging population, late-life depression has been a severe health problem in rural China which was a major cause of global disability and suicide, and associated with cardiovascular diseases and mortality [1]. Recent research reported the prevalence of depression was 15.5% in China in a large cross-sectional study which included 19,379 healthcare workers from 25 provinces [2]. However, in old people, the prevalence of depression was higher. It was found that 17.1% of males and 23.1% of females in 950 participants aged≥60 years from 22 locations in China were identified as having depressive symptoms [3]. Many studies have identified that behavioral and psychosocial factors, such as alcohol abuse, smoking, sleep disturbance, physical inactivity, unhealthy eating habits, and stressful events, and sociodemographic factors, such as low income, unemployment, low education level, and low social support, are mostly related with an increased risk of depression [4, 5].
Besides these risk factors, cognitive decline was found to be associated with depression in the recent years. It was found that cognitive impairment may be one of the more practically important aspects of depression [6]. And depression was also a risk factor for Alzheimer’s disease [7]. Older adults who were diagnosed for the first time with depression after 65 years of age, showed a stronger association with cognitive impairment (OR = 6.65, 95% CI 2.390 to 10.900, p < 0.01) [8]. What’s more, sleep quality was both related with cognitive decline and depression [9]. Approximately half of older people report sleep disturbances, which are associated with various health conditions. Specifically, components of poorer sleep quality and greater sleep disturbance were related to worse sustained attention scores, while increased sleep latency and daytime sleepiness were associated with greater frequency and seriousness of forgetting [10]. Night sleep disturbances (OR = 1.95, 95% CI 1.170 to 3.250) and daytime sleepiness (OR = 1.93, 95% CI 1.160 to 3.200) were also associated with depression [11]. Particularly, a study found that daytime sleepiness and poor efficiency were significantly associated with loss of interest; and poor satisfaction, daytime sleepiness, mid-sleep time, and efficiency were significantly associated with having at least one depressive symptom [12].
Both the quality of sleep and cognitive decline impact depression while they influence each other. However, the underlying mechanisms are not yet clear. In our study, we hypothesized that participants with depression would have poorer scores on cognitive tests and that this association would be mediated by sleep quality. This leads us to explore the mediating effect of sleep quality between cognitive decline and depression in older adults and further test the components of sleep quality in this relationship.
Method
Study design and sample population
Our study was a cross-sectional analysis obtaining baseline data of the west China health and aging trend (WCHAT) study which was conducted from 2018 to 2020 [13]. The research was approved by the Ethical Review Committee of West China Hospital with the committee’s reference number 2017(445) and the registration number is ChiCTR 1,800,018,895. All methods were performed in accordance with the relevant guidelines and regulations. Our method of sampling is multi-stage cluster sampling as follows and the response rate was 50.2% in the baseline data collection:
1. According to the distribution of Chinese ethnicities, select the gathering places of the major ethnic gathering provinces in the west China: Sichuan, Yunnan, Guizhou, and Xinjiang. 2. Select ethnic gathering places in each province: Chengdu (Ethnic Han), Maoxian (Ethnic Qiang), Zoige (Ethnic Tibetan), Kangding (Ethnic Tibetan), Mianning (Ethnic Yi), Zhenyuan (Ethnic Miao), Kunming (Ethnic Hui), Akto county (Ethnic Uyghur and ethnic Kirgiz), Dali (Ethnic Bai), Shiling (Ethnic Yi), Miyi (Ethnic Lisu). 3. Consider the topographical characteristics of various ethnic regions, scattered living, and choose a county with convenient transportation and concentrated living. A county medical institution was selected as survey site. 4. In the selected counties, some adjacent towns are randomly selected. 5. Local government carried out publicity in advance in villages and villagers voluntarily signed up for the project.
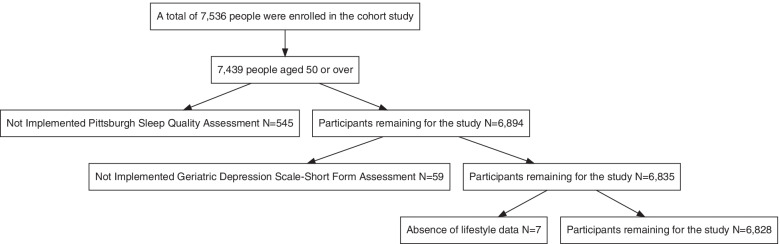
In our study, participants aged 50 years old or older were enrolled. Participants were recruited by the local government and asked verbally by the researchers about their willingness to take part in the study. Before investigation, informed consent was signed and obtained by each participant. Initially, 7536 participants were enrolled. Then we excluded subjects who were under 50 years old and 97 participants were excluded. Then we kept on excluding 545 subjects without doing sleep quality assessment. Besides, 59 participants were excluded without doing depression assessment. After that, 7 subjects were excluded without life style data. Therefore, 6828 participants were analyzed in our study (Fig. 1).
Fig. 1.
Flow chart of study participants. Initially, 7536 participants were enrolled. Then we excluded subjects who were under 50 years old and 97 participants were excluded. Then we kept on excluding 545 subjects without doing sleep quality assessment. Besides, 59 participants were excluded without doing depression assessment. After that, 7 subjects were excluded without life style data. Therefore, 6828 participants were analyzed in our study
Data collection
We used electronic questionnaire and instruments to collect the required data. All the foreign questionnaires have been verified in China. All interviewers were medical students who were trained on collecting questionnaire data through face to face, one-on-one personal interviews. Other anthropometric and bioimpedance measurements were collected by trained technicians [13]. Depression was assessed using the 15-item Geriatric Depression Scale (GDS-15). The scale, which contains 15 items that require only a yes/no answer, is the most widely used scale for the detection of depression and scores ≥5 indicate depression [14]. Sleep quality was assessed using the Pittsburgh sleep quality index (PSQI). The questionnaire included 7 components and component scores range from 0 to 3 and a global score ranging from 0 to 21, with higher scores indicating worse sleep quality. Mostly, scores > 5 are considered as poor self-reported sleep quality [15]. Cognitive status was measured using a 10-item Short Portable Mental Status Questionnaire (SPMSQ). For SPMSQ scoring, 0 ~ 2 indicated complete cognitive function, 3 ~ 4 indicated mild cognitive functional impairment, 5 ~ 7 indicated moderate cognitive function impairment, and 8 ~ 10 indicated severe cognitive function impairment and this assessment should be based on the education level [16]. Specifically, if the education level was primary school and below, 0 to 3 errors were considered as good cognitive function; 4 to 5 errors were considered as mild cognitive decline; 6 to 8 errors were considered as moderate cognitive decline and 9 to 10 errors were considered as severe cognitive decline. If the education level was middle school, 0 to 2 errors were considered as good cognitive function; 3 to 4 errors were considered as mild cognitive decline; 5 to 7 errors were considered as moderate cognitive decline and 8 to 10 errors were considered as severe cognitive decline. If the education level was high school and above, 0 to 1 errors were considered as good cognitive function; 2 to 3 errors were considered as mild cognitive decline; 4 to 6 errors were considered as moderate cognitive decline and 7 to 10 errors were considered as severe cognitive decline [16]. The anxiety status was measured by the (GAD-7) instrument and the scores ≥5 was considered as having anxiety. Dancing was measured by asking dancing frequency and time of duration. Smoking was measured by asking smoking frequency, number of cigarettes and whether to quit smoking. Drinking alcohol was measured by asking the frequency, amount and whether to stop alcohol consumption. Drinking tea was measured by asking the frequency, type of tea and whether to stop tea drinking. These life style factors were found to be related with depression in most studies. Other baseline demographic information included age, gender, occupation, educational level, ethnic groups background. A medical history of chronic disease was self-reported. These disease conditions included hypertension, diabetes, osteoarthrosis, coronary heart disease, tumor, deafness and having two or more disease was considered as comorbidity.
Statistical analysis
Information was processed and analyzed using R version 4.0.2. Characteristics of baseline data were presented with mean values, standard deviation (SD), and frequencies. Differences between the categories of depression and the variables studied were analyzed through analysis of variance (ANOVA) in the continuous variables and the chi squared test on categorical variables [17]. Direct effects of cognitive decline (operationalized by SPMSQ scores) and poor sleep quality (operationalized by PSQI scores) on depression were assessed using three multi-variable regression models that included relevant variables from previous covariate analyses. 95% confidence intervals were generated for all regression coefficients. Mediation hypotheses of 7 components of PSQI questionnaire and PSQI total score on the relationship between cognitive decline and depression were done using the bias-corrected bootstrap method with 6828 samples to calculate confidence intervals (95%). The results were statistically significant with p < 0.05. An indirect effect was considered significant when the confidence interval did not include zero. Besides, path analysis of 7 components of PSQI questionnaire was shown in the SEM framework which was done using a SEM package in R version 4.0.2 [18].
Results
Overall, we enrolled 6828 participants (2562 men and 4266 women) aged 50 years old or older in the study. The mean age of the group was 62.43 ± 8.28 years. Table 1 shows descriptive characteristics of the participants according to depression assessment. The prevalence of depression according to the GDS-15 was 17.4%, with a higher prevalence of depression in women than in men and a lower prevalence in Han compared to other ethnic groups. Subjects with depression tended to be farmers and has a lower educational level (p < 0.001). It was observed that individuals in the depression group presented higher scores in subjective sleep quality, sleep latency, habitual sleep efficiency, sleep disturbance, used sleep medication, daytime dysfunction and PSQI total scores (p < 0.001). Subjects who enjoy dancing, drinking tea and smoking has a lower prevalence of depression (p < 0.001). And subjects with anxiety or cognitive decline has a higher prevalence of depression (p < 0.001). Besides, subjects with coronary heart disease (CHD), osteoarthrosis, deafness has a higher prevalence of depression (p < 0.05).
Table 1.
Sample characteristics stratified by depressed status (N = 6828)
| Characters | Total n = 6828 |
Depressed n = 1185(17.4) |
Non-depressed n = 5643(82.6) |
p |
|---|---|---|---|---|
| Ethnic group, n(%) | < 0.001 | |||
| Han | 2472(36.2) | 338(28.52) | 2134(37.82) | |
| Others | 4356(63.8) | 847(71.48) | 3509(62.18) | |
| Gender, n(%) | < 0.001 | |||
| Male, n(%) | 2562(37.52) | 367(30.97) | 2195(38.9) | |
| Female, n(%) | 4266(62.48) | 818(69.03) | 3448(61.1) | |
| Age, mean ± SD | 62.43 ± 8.28 | 62.78 ± 8.50 | 62.36 ± 8.23 | 0.1245 |
| Age group, n(%) | 0.2511 | |||
| 50–59 | 2697(39.5) | 454(38.31) | 2243(39.75) | |
| 60–69 | 2723(39.88) | 463(39.07) | 2260(40.05) | |
| 70–79 | 1215(17.79) | 235(19.83) | 980(17.37) | |
| 80 + | 193(2.83) | 33(2.78) | 160(2.84) | |
| Occupation | < 0.001 | |||
| Farmers | 4456(65.26) | 910(76.79) | 3546(62.84) | |
| Others | 2372(34.74) | 275(23.21) | 2097(37.16) | |
| Educational level, n(%) | < 0.001 | |||
| No formal education | 1897(27.78) | 434(36.62) | 1463(25.93) | |
| Elementary school | 2318(33.95) | 425(35.86) | 1893(33.55) | |
| Middle school | 1468(21.5) | 200(16.88) | 1268(22.47) | |
| High school and above | 1145(16.75) | 126(10.63) | 1019(18.04) | |
| Dwelling status, n(%) | 0.006 | |||
| Solitude | 339(4.96) | 78(6.58) | 261(4.63) | |
| Non-solitude | 6489(95.04) | 1107(93.42) | 5382(95.37) | |
| Subjective Sleep Quality, n(%) | < 0.001 | |||
| 0 score | 1243(18.2) | 163(13.76) | 1080(19.14) | |
| 1 score | 3597(52.68) | 584(49.28) | 3013(53.39) | |
| 2 score | 1688(24.72) | 351(29.62) | 1337(23.69) | |
| 3 score | 300(4.39) | 87(7.34) | 213(3.77) | |
| Sleep Latency, n(%) | < 0.001 | |||
| 0 score | 1451(21.25) | 172(14.51) | 1279(22.67) | |
| 1 score | 2654(38.87) | 425(35.86) | 2229(39.5) | |
| 2 score | 1940(28.41) | 431(36.37) | 1509(26.74) | |
| 3 score | 783(11.47) | 157(13.25) | 626(11.09) | |
| Sleep Duration, n(%) | 0.0563 | |||
| 0 score | 3336(48.86) | 550(46.41) | 2786(49.37) | |
| 1 score | 1546(22.64) | 263(22.19) | 1283(22.74) | |
| 2 score | 1228(17.98) | 225(18.99) | 1003(17.77) | |
| 3 score | 718(10.52) | 147(12.41) | 571(10.12) | |
| Habitual Sleep Efficiency, n(%) | < 0.001 | |||
| 0 score | 4800(70.3) | 763(64.39) | 4037(71.54) | |
| 1 score | 1041(15.25) | 214(18.06) | 827(14.66) | |
| 2 score | 425(6.22) | 81(6.84) | 344(6.1) | |
| 3 score | 562(8.23) | 127(10.72) | 435(7.71) | |
| Sleep Disturbance, n(%) | < 0.001 | |||
| 0 score | 258(3.78) | 25(2.11) | 233(4.13) | |
| 1 score | 4422(64.76) | 676(57.05) | 3746(66.38) | |
| 2 score | 2053(30.07) | 446(37.64) | 1607(28.48) | |
| 3 score | 95(1.39) | 38(3.21) | 57(1.01) | |
| Used Sleep Medication, n(%) | < 0.001 | |||
| 0 score | 6630(97.1) | 1131(95.44) | 5499(97.45) | |
| 1 score | 80(1.17) | 14(1.18) | 66(1.17) | |
| 2 score | 50(0.73) | 15(1.27) | 35(0.62) | |
| 3 score | 68(1) | 25(2.11) | 43(0.76) | |
| Daytime Dysfunction, n(%) | < 0.001 | |||
| 0 score | 2815(41.23) | 307(25.91) | 2508(44.44) | |
| 1 score | 2125(31.12) | 424(35.78) | 1701(30.14) | |
| 2 score | 1392(20.39) | 298(25.15) | 1094(19.39) | |
| 3 score | 496(7.26) | 156(13.16) | 340(6.03) | |
|
PSQIa, mean(SD) median, (Q1-Q3) |
6.16(3.29) 6(4–8) |
7.18(3.52) 7(4–9) |
5.95(3.20) 5(4–8) |
< 0.001 |
| Dancing status, n(%) | 0.0295 | |||
| Yes | 1346(19.71) | 206(17.38) | 1140(20.2) | |
| No | 5482(80.29) | 979(82.62) | 4503(79.8) | |
| Smoking history, n(%) | < 0.001 | |||
| Yes | 1315(19.26) | 179(15.11) | 1136(20.13) | |
| No | 5513(80.74) | 1006(84.89) | 4507(79.87) | |
| Drinking alcohol history, n(%) | 0.2663 | |||
| Yes | 1315(19.26) | 214(18.06) | 1101(19.51) | |
| No | 5513(80.74) | 971(81.94) | 4542(80.49) | |
| Drinking tea history, n(%) | < 0.001 | |||
| Yes | 3042(44.55) | 471(39.75) | 2571(45.56) | |
| No | 3786(55.45) | 714(60.25) | 3072(54.44) | |
| Anxiety status, n(%) | < 0.001 | |||
| Yes | 1490(21.82) | 530(44.73) | 960(17.01) | |
| No | 5338(78.18) | 655(55.27) | 4683(82.99) | |
|
Cognitive scorea, mean(SD) median (Q1-Q3) |
1.13 ± 1.50 1(0–2) |
1.64 ± 1.85 1(0–3) |
1.03 ± 1.39 1(0–1) |
< 0.001 |
| Cognitive status, n(%) | < 0.001 | |||
| No cognitive decline | 5776(84.59) | 871(73.5) | 4905(86.92) | |
| Mild cognitive decline | 755(11.06) | 207(17.47) | 548(9.71) | |
| Moderate to severe cognitive decline | 297(4.35) | 107(9.03) | 190(3.37) | |
| Hypertension, n(%) | 0.757 | |||
| Yes | 1736(25.42) | 306(25.82) | 1430(25.34) | |
| No | 5092(74.58) | 879(74.18) | 4213(74.66) | |
| Diabetes, n(%) | 0.8324 | |||
| Yes | 500(7.32) | 89(7.51) | 411(7.28) | |
| No | 6328(92.68) | 1096(92.49) | 5232(92.72) | |
| Coronary heart disease (CHD), n(%) | 0.0114 | |||
| Yes | 276(4.04) | 64(5.4) | 212(3.76) | |
| No | 6552(95.96) | 1121(94.6) | 5431(96.24) | |
| Osteoarthrosis, n(%) | 0.0387 | |||
| Yes | 756(11.07) | 152(12.83) | 604(10.7) | |
| No | 6072(88.93) | 1033(87.17) | 5039(89.3) | |
| Tumour, n(%) | 0.4499 | |||
| Yes | 49(0.72) | 11(0.93) | 38(0.67) | |
| No | 6779(99.28) | 1174(99.07) | 5605(99.33) | |
| Deafness, n(%) | 0.0081 | |||
| Yes | 49(0.72) | 16(1.35) | 33(0.58) | |
| No | 6779(99.28) | 1169(98.65) | 5610(99.42) | |
| Disease comorbidity, n(%) | 0.5049 | |||
| Yes | 1560(22.85) | 280(23.63) | 1280(22.68) | |
| No | 5268(77.15) | 905(76.37) | 4363(77.32) |
Note. Means ± standard deviation was shown. Others = other nationalities including Zhuang, Manchu, Hui, Mongolia, Tujia nationalities. Data are shown using % or mean (standard deviation). P values were calculated with chi-squared tests and Student’s t tests for categorical and continuous variables, respectively.a These variables are presented as median (interquartile range)
Table 2 showed the results of three multiple linear regression analysis in three models. Model 1 was multiple linear regression analysis between depression status and cognitive score. Model 2 was multiple linear regression analysis between depression status and cognitive scores adjusted by PSQI scores. Model 3 was multiple linear regression analysis between PSQI scores and cognitive scores. All the three models adjusted related covariates including gender, age, and ethnic group, life styles (dancing, smoking and drinking tea), chronic diseases (deafness, CHD and osteoarthrosis) and anxiety. In model 1, the results from regression analysis indicated a significant association between cognitive scores (β = 0.251, 95% CI 0.211 to 0.290, p < 0.001) and depression status. Model 2 showed that after adjusted PSQI scores, the association between cognitive scores and depression status was still significant (β = 0.242, 95% CI 0.203 to 0.281, p < 0.001), indicating a partial mediation effect of sleep quality. While model 3 showed a significant association between cognitive scores (β = 0.101, 95% CI 0.047 to 0.154, p < 0.001) and PSQI scores.
Table 2.
Associations between cognitive status and sarcopenia in adults aged over 50 years old
| Outcome variable | Model 1: Depressed | Model 2: Depressed | Model 3: PSQI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | |
| PSQI | – | – | – | 0.088 | < 0.001 | 0.071 to 0.106 | – | – | – |
| Cognitive score | 0.251 | < 0.001 | 0.211 to 0.29 | 0.242 | < 0.001 | 0.203 to 0.281 | 0.101 | < 0.001 | 0.047 to 0.154 |
| Ethnic group: Han | − 0.236 | < 0.001 | − 0.353 to − 0.119 | − 0.281 | < 0.001 | − 0.398 to − 0.165 | 0.509 | < 0.001 | 0.349 to 0.669 |
| Gender: Female | − 0.094 | 0.207 | − 0.241 to 0.052 | − 0.191 | 0.011 | − 0.338 to − 0.044 | 1.094 | < 0.001 | 0.893 to 1.295 |
| Age | −0.002 | 0.568 | −0.009 to 0.005 | − 0.005 | 0.195 | − 0.012 to 0.002 | 0.029 | < 0.001 | 0.019 to 0.038 |
| Educational: Elementary school | −0.066 | 0.369 | −0.211 to 0.078 | −0.066 | 0.367 | −0.21 to 0.078 | − 0.003 | 0.975 | − 0.201 to 0.195 |
| Educational: Middle school | −0.21 | 0.016 | −0.381 to − 0.039 | −0.193 | 0.026 | −0.363 to − 0.023 | −0.19 | 0.111 | −0.424 to 0.044 |
| Educational: High school and above | −0.563 | < 0.001 | −0.761 to − 0.364 | −0.522 | < 0.001 | − 0.719 to − 0.324 | −0.463 | 0.001 | −0.735 to − 0.19 |
| Occupation: Farmers | 0.447 | < 0.001 | 0.312 to 0.581 | 0.433 | < 0.001 | 0.3 to 0.567 | 0.152 | 0.106 | −0.032 to 0.336 |
| Dancing, Yes | −0.207 | 0.004 | −0.348 to − 0.066 | −0.182 | 0.011 | −0.322 to − 0.042 | −0.289 | 0.003 | −0.482 to − 0.096 |
| Smoking: Yes | − 0.177 | 0.035 | − 0.342 to − 0.013 | −0.203 | 0.015 | −0.367 to − 0.04 | 0.292 | 0.011 | 0.067 to 0.518 |
| Drinking tea: Yes | −0.096 | 0.095 | −0.208 to 0.017 | −0.101 | 0.077 | −0.213 to 0.011 | 0.059 | 0.456 | −0.096 to 0.213 |
| Anxiety: Yes | 1.828 | < 0.001 | 1.695 to 1.962 | 1.689 | < 0.001 | 1.554 to 1.824 | 1.574 | < 0.001 | 1.392 to 1.757 |
| Deafness: Yes | 0.561 | 0.084 | −0.076 to 1.197 | 0.425 | 0.188 | −0.208 to 1.057 | 1.535 | 0.001 | 0.664 to 2.406 |
| CHD: Yes | 0.09 | 0.526 | −0.187 to 0.367 | −0.043 | 0.762 | −0.319 to 0.234 | 1.498 | < 0.001 | 1.119 to 1.877 |
| Osteoarthrosis, Yes | −0.074 | 0.405 | −0.249 to 0.101 | −0.177 | 0.047 | −0.352 to − 0.003 | 1.167 | < 0.001 | 0.928 to 1.406 |
| Constant | 2.309 | < 0.001 | 1.802 to 2.817 | 2.06 | < 0.001 | 1.554 to 2.566 | 2.826 | < 0.001 | 2.132 to 3.519 |
| Observations | 6828 | 6828 | 6828 | ||||||
| R2 | 0.1675 | 0.1796 | 0.1271 | ||||||
| Adjusted R2 | 0.1656 | 0.1777 | 0.1251 | ||||||
| Residual standard error | 2.249 (df = 6812) | 2.233 (df = 6811) | 3.077 (df = 6812) | ||||||
| F Statistic (df; P value) | 91.36 (df = (15, 6812);P value< 0.001) | 93.22 (df = (16,6811);P value< 0.001) | 66.1 (df = (15,6812); P value< 0.001) | ||||||
Note. Model 1: multiple linear regression analysis between depressed and cognitive score, Model 2: multiple linear regression analysis between depressed and cognitive score adjusted by PSQI, Model 3: multiple linear regression analysis between PSQI and cognitive score
Adjusted by gender, age, and ethnic group, life styles (dancing, smoking and drinking tea), chronic diseases (deafness, CHD, osteoarthrosis) and anxiety
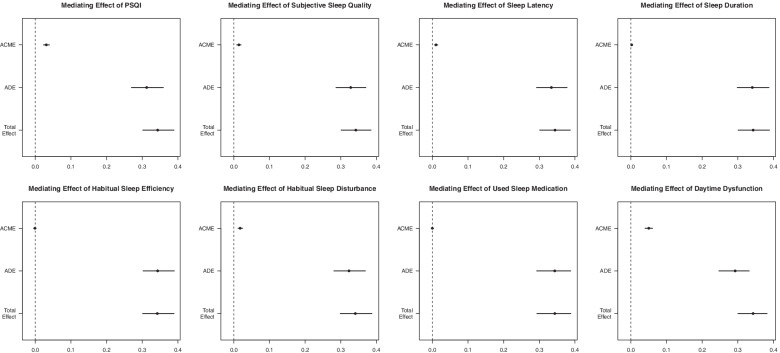
Table 3 showed the relative total, direct and indirect effects for the mediating role of sleep quality on the relationship between cognitive decline and depression in mediation models. Our mediation hypothesis was confirmed because bootstrapping revealed significant relative indirect effects for depression (ACME = 0.0308, 95% CI 0.023 to 0.040), indicating that sleep quality mediated the association between cognitive decline and depression. And most of sleeping components also has mediation effect like subjective sleep quality (ACME = 0.0145, 95% CI 0.009 to 0.020), sleep latency (ACME = 0.010, 95% CI 0.006 to 0.020), sleep duration (ACME = 0.0026, 95% CI 0.005 to 0.010), sleep disturbance (ACME = 0.018, 95% CI 0.012 to 0.020) and daytime dysfunction (ACME = 0.0503, 95% CI 0.040 to 0.060). Among these components, daytime dysfunction had a highest mediation effect with a proportion of mediation up to 14.56%. And these mediation effects were also shown in Fig. 2.
Table 3.
Mediation models: relative total, direct and indirect effects for the mediating role of sleeping quality on the relationship between cognitive decline and depression
| Mediator Variable | β | P-value | 95% CI | |
|---|---|---|---|---|
| PSQI | ACME | 0.0308 | < 0.001 | 0.0231 to 0.04 |
| ADE | 0.3124 | < 0.001 | 0.2692 to 0.35 | |
| Total Effect | 0.3432 | < 0.001 | 0.2976 to 0.39 | |
| Prop. Mediated | 0.0885 | < 0.001 | 0.0686 to 0.12 | |
| Subjective Sleep Quality | ACME | 0.0145 | < 0.001 | 0.0092 to 0.02 |
| ADE | 0.3281 | < 0.001 | 0.2873 to 0.37 | |
| Total Effect | 0.3426 | < 0.001 | 0.3025 to 0.39 | |
| Prop. Mediated | 0.0419 | < 0.001 | 0.0274 to 0.06 | |
| Sleep Latency | ACME | 0.0103 | < 0.001 | 0.0061 to 0.02 |
| ADE | 0.3331 | < 0.001 | 0.2913 to 0.37 | |
| Total Effect | 0.3435 | < 0.001 | 0.3028 to 0.38 | |
| Prop. Mediated | 0.0296 | < 0.001 | 0.0173 to 0.05 | |
| Sleep Duration | ACME | 0.0026 | 0.008 | 0.0005 to 0.01 |
| ADE | 0.3408 | < 0.001 | 0.2964 to 0.38 | |
| Total Effect | 0.3433 | < 0.001 | 0.3016 to 0.39 | |
| Prop. Mediated | 0.0071 | 0.008 | 0.0015 to 0.02 | |
| Habitual Sleep Efficiency | ACME | −0.0007 | 0.68 | −0.0039 to 0 |
| ADE | 0.3443 | < 0.001 | 0.2985 to 0.39 | |
| Total Effect | 0.3436 | < 0.001 | 0.2974 to 0.39 | |
| Prop. Mediated | −0.0024 | 0.68 | −0.0117 to 0.01 | |
| Sleep Disturbance | ACME | 0.018 | < 0.001 | 0.0123 to 0.02 |
| ADE | 0.325 | < 0.001 | 0.2803 to 0.37 | |
| Total Effect | 0.343 | < 0.001 | 0.297 to 0.39 | |
| Prop. Mediated | 0.0518 | < 0.001 | 0.0357 to 0.07 | |
| Used Sleep Medication | ACME | 0.0002 | 0.91 | −0.0033 to 0 |
| ADE | 0.3445 | < 0.001 | 0.3006 to 0.38 | |
| Total Effect | 0.3446 | < 0.001 | 0.3015 to 0.38 | |
| Prop. Mediated | 0.0006 | 0.91 | −0.0101 to 0.01 | |
| Daytime Dysfunction | ACME | 0.0503 | < 0.001 | 0.0403 to 0.06 |
| ADE | 0.2924 | < 0.001 | 0.2519 to 0.34 | |
| Total Effect | 0.3428 | < 0.001 | 0.3035 to 0.39 | |
| Prop. Mediated | 0.1456 | < 0.001 | 0.1162 to 0.18 |
Note. ACME, average causal mediation effects (indirect effect); ADE, average direct effects; Prop. Mediated, the mediator variable explains the percentage of the association between cognitive and depressed
Fig. 2.
Mediation effects of PQSI and 7 sleeping elements in the relationship between cognitve decline and depression. The mediation effects are as following: PSQI (ACME = 0.0308, 95%CI 0.0231 to 0.04), subjective sleep quality (ACME = 0.0145, 95% CI 0.009 to 0.020), sleep latency (ACME = 0.010, 95% CI 0.006 to 0.020), sleep duration (ACME = 0.0026, 95% CI 0.005 to 0.010), habitual Sleep Efficiency (ACME = -0.0007, 95%CI − 0.0039 to 0), sleep disturbance (ACME = 0.018, 95% CI 0.012 to 0.020), used sleep medication (ACME = 0.0002, 95%CI − 0.0033 to 0) and daytime dysfunction (ACME = 0.0503, 95% CI 0.040 to 0.060)
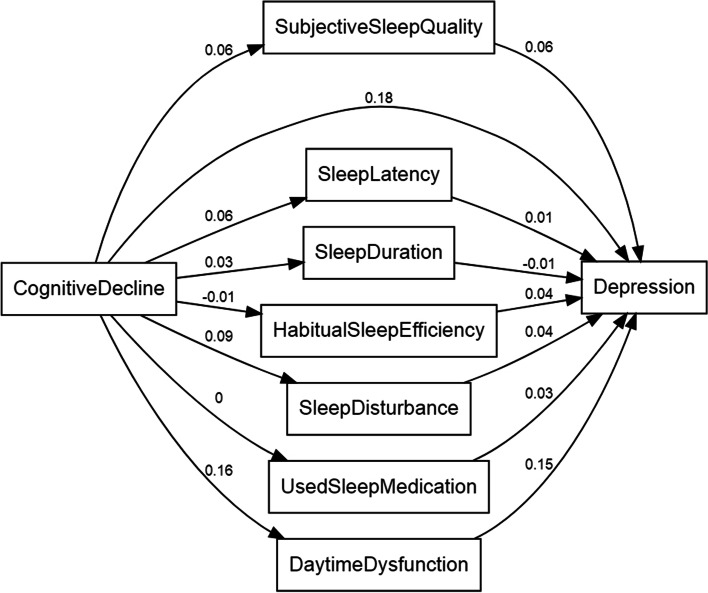
We then performed path analysis using the structural equation model (SEM) framework(Chi-square statistic = 1736.2, GFI = 0.939, TLI = 0.688, RMSEA = 0.098). As shown in Fig. 3, SEM pathway analysis showed that the correlation between cognitive decline and depression was positive (SEM co-efficient: 0.18). 7 components of PSQI assessment were also shown different correlation between cognitive decline and depression. Most correlation was positive, while only the correlation between sleep duration and depression was negative (SEM co-efficient: − 0.01) and the correlation between cognitive decline and habitual sleep efficiency was negative (SEM co-efficient: − 0.01). These results further confirmed the association between cognitive decline, sleep quality and depression.
Fig. 3.
Path analysis of the sleeping quality’s mediation effects using the structural equation model (SEM) framework. SEM pathway analysis showed that the correlation between cognitive decline and depression was positive (SEM co-efficient: 0.18). 7 components of PSQI assessment were also shown in Fig. 3
Discussion
The current study evaluated the mediating role of sleep quality in the relationship between cognitive decline and depression. Several mechanisms have been proposed to explain how sleep quality impacts both cognition and depression. 60 to 70% of people with cognitive impairment or dementia have sleep disturbances. Research has shown that poor sleep quality as measured by the PSQI is associated with multiple markers of metabolic dysfunction, including insulin resistance which is related with bad performance on executive function tasks among older adults [19, 20]. Furthermore, several mouse models have demonstrated strong relationships between diet induced insulin resistance and memory dysfunction [21, 22]. Besides, good sleep plays a protective role in human emotional homeostasis and regulation [23]. And in depressed individuals, dysregulated sleep was often-reported [24]. As many as 90% of patients with depression will have sleep quality complaints [25]. It was reported that as many as 24 to 58% of individuals with sleep disordered breathing (eg, obstructive sleep apnea) meet the criteria for depression [26]. And it was found that among all the symptoms of depression, sleep problems are the most common (13.6%). Compared to those without sleep problems, people with sleep problems have the highest relative odds (7.6 times) of having a new major depressive episode next year. Thus, sleep disturbance was associated with having more depressive symptoms [27]. Another mechanism through which poor sleep could affect both cognitive function and depression is through oxidative stress. Higher levels of oxidative stress biomarkers were found in patients with bad sleep quality [28]. In addition, sleep deprivation is related with an increased rate of oxidative pentose phosphate pathway activity [29]. High levels of oxidative stress has also been implicated in depression among older adults [30] as well as those with cognitive decline [31]. Our study demonstrates that bad sleep quality partially mediates the effects of cognitive decline on depression in older adults. Our results thus contribute to the current knowledge by providing evidence that improving sleep quality may ameliorate the negative impact of cognitive decline on depression.
Among the 7 components of sleeping assessment, we found that daytime dysfunction had a highest mediation effect with a proportion of mediation up to 14.56%, the following was sleep disturbance which had a mediation effect of 5.18% and subjective sleep quality which had a mediation effect of 4.19%. This was consistent with previous studies. A recent research found that the effects of sleep disturbance, subjective sleep quality and daytime dysfunction scores were most obvious on anxiety in the elderly aged 60 years and older in China, and the ORs (95%CI) were 4.63 (3.55–6.04), 2.75 (2.33–3.23) and 2.50 (2.19–2.86), respectively [32]. An earlier study also found that symptoms of short sleep duration, daytime sleepiness and sleep disturbances are independently related to anxiety while the use of sleep medication is independently associated to depression in a random sample of 2393 individuals aged 65 years or older [33]. Another longitudinal study found that short sleep duration, especially on weekdays, was significantly associated with subsequent depressive (OR = 0.86, 95%CI 0.80 to 0.92) [34]. Besides, shorter sleep duration has been found to be associated with a greater rate of ventricular enlargement, which similarly reflects loss of brain volume [35]. And sleep disturbances were studied to be linked to cortical thinning, a marker of cortical atrophy found in many dementia subtypes [36, 37]. As we discussed before, numerous studies provide findings indicating the remarkable relationship between sleep alterations and depression. Our study found the most three relevant components (eg. daytime dysfunction, sleep disturbance, subjective sleep quality) mediated the relationship between cognitive decline and depression, which might be the target to focus on improving sleep quality.
According to the World Health Organization, depression is the leading cause of disability, affecting over 300 million people. Depression is also the commonest mental disorder in older adults worldwide, affecting 7% of the world’s older population and accounting for 5.7% of years lived with disability among adults aged over 60 years [38]. For many individuals with depression, the major impairment they experience is cognitive decline [39]. Our study found a high prevalence of depression that was 17.4% and after adjusting numerous confounders, the association between cognitive decline and depression was still significant. This is most likely regulated by several mechanisms. Firstly, depression’s duration has a significant impact on left hippocampal volume, indicating that the time since first depressive episode plays an important role in hippocampal degeneration which leading to cognitive decline [40]. And lower hippocampal volumes are associated with a poorer clinical outcome and more depressive episodes [41]. Secondly, accumulated evidence highlighting the major role of systemic inflammation, which were existed both in cognitive decline and depression [42]. Thirdly, as we discussed before, oxidative stress was a common mechanism in cognitive decline and depression [30, 31]. Thus, the relationship between cognitive decline and depression is complex and bidirectional. The ultimate goal of treatment in depression is fully functional recovery, and assessing patients for cognitive impairment and selecting treatments that address cognitive dysfunction.
There are several limitations in this study. Firstly, our sampling did not cover all the cities in west China. Secondly, our study design was a cross-sectional study. Thirdly, we conducted a centralized investigation and not a household survey. Furthermore, most of the participants who came to the site of investigation on their own were relatively healthy. Some bias were existed in the analysis. A critical next step would be to replicate this study with longitudinal data to establish the relationship. In addition, it would be crucial to examine if clinically established sleep interventions are able to prevent or reverse depression in cognitive decline adults.
Conclusions
In conclusion, our study demonstrated that the relationship between cognitive decline and depression was partially mediated by sleep quality. However, our study did not find that improving sleep quality in older adults with cognitive decline could counteract the progression of depression. Further research is necessary to examine the effects of sleep quality on the relationship of cognitive decline and depression.
Acknowledgements
We thank all the volunteers for the participation and personnel for their contribution in the WCHAT study.
Abbreviations
- WCHAT
West China Health and Aging Trend study
- GDS-15
15-item Geriatric Depression Scale
- SPMSQ
Short Portable Mental Status Questionnaire
- PSQI
Pittsburgh sleep quality index
- SEM
Structural equation model
- SD
Standard deviation
- ACME
Average causal mediation effects (indirect effect)
- ADE
Average direct effects
Authors’ contributions
XL and XX contributed to conceptualization, data collection, data curation, formal analysis, writing the original draft, and review and editing of the paper. FH contributed to data collection, data curation, and review and editing of the paper. QH contributed to data collection, data curation. LH contributed to data collection, data curation. XS contributed to data collection, data curation. GZ contributed to data collection, data curation. JY and BD contributed to study conceptualization, funding acquisition, investigation, methodology, project administration, supervision, and review and editing of the paper. All authors have read and approved the manuscript.
Funding
This work was funded by National Key R&D Program of China (2020YFC2008600 and 2020YFC2008603), the National Natural Science Foundation of China (No.82101653), the Fundamental Research Funds for the Central University (20826041D4046), Post-doc Coronavirus Epidemic Prevention and Control Fund (0040204153349), West China Hospital Postdoctoral Fund (2020HXBH011).
Availability of data and materials
The data-set generated and analyzed during the current study will be available two years later and is also available now from the corresponding author on a reasonable request.
Declarations
Ethics approval and consent to participate
Subjects (or their guardians) have given their written informed consent. The current research was approved by the Ethical Review Committee of West China Hospital with the committee’s reference number 2017(445) and the registration number is ChiCTR 1800018895. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not Applicable.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaolei Liu and Xin Xia contributed equally to this work.
References
- 1.Carney RM, Freedland KE. Depression and coronary heart disease. Nat Rev Cardiol. 2017;14(3):145-155. [DOI] [PubMed]
- 2.Yang X, Chen D. Geographical distribution and prevalence of mental disorders among healthcare workers in China: A cross-sectional country-wide survey: A cross-sectional study to assess mental disorders of healthcare workers in China. 2021. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Cui PY, Pan YY, Li YX, Waili N, Li Y. Association between housing environment and depressive symptoms among older people: a multidimensional assessment. 2021;21(1):259. [DOI] [PMC free article] [PubMed]
- 4.Meng X, D'Arcy C. The projected effect of increasing physical activity on reducing the prevalence of common mental disorders among Canadian men and women: a national population-based community study. Prev Med. 2013;56(1):59–63. doi: 10.1016/j.ypmed.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Fu TS, Lee CS, Gunnell D, Lee WC, Cheng AT. Changing trends in the prevalence of common mental disorders in Taiwan: a 20-year repeated cross-sectional survey. Lancet (London, England) 2013;381(9862):235–241. doi: 10.1016/S0140-6736(12)61264-1. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Qi S, Wang M, et al. Subtypes of depression characterized by different cognitive decline and brain activity alterations. J Psychiatr Res. 2021;138:413–419. doi: 10.1016/j.jpsychires.2021.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Sáiz-Vázquez O, Gracia-García P, Ubillos-Landa S. Depression as a Risk Factor for Alzheimer's Disease: A Systematic Review of Longitudinal Meta-Analyses. 2021;10(9). [DOI] [PMC free article] [PubMed]
- 8.Nazar G, Ulloa N, Martínez-Sanguinetti MA, et al. Association between cognitive impairment and depression in Chilean older adults. Rev Med Chil. 2020;148(7):947–955. doi: 10.4067/S0034-98872020000700947. [DOI] [PubMed] [Google Scholar]
- 9.Leng M, Yin H, Zhang P, et al. Sleep Quality and Health-Related Quality of Life in Older People With Subjective Cognitive Decline, Mild Cognitive Impairment, and Alzheimer Disease. J Nerv Ment Dis. 2020;208(5):387–396. doi: 10.1097/NMD.0000000000001137. [DOI] [PubMed] [Google Scholar]
- 10.Siddarth P, Thana-Udom K, Ojha R, et al. Sleep quality, neurocognitive performance, and memory self-appraisal in middle-aged and older adults with memory complaints. Int Psychogeriatr. 2020;28:1–11. doi: 10.1017/S1041610220003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagnew B, Dagne H. Depression and Its Determinant Factors Among University of Gondar Medical and Health Science Students, Northwest Ethiopia: Institution-Based Cross-Sectional Study. 2020;16:839–45. [DOI] [PMC free article] [PubMed]
- 12.Furihata R, Saitoh K, Suzuki M, et al. A composite measure of sleep health is associated with symptoms of depression among Japanese female hospital nurses. Compr Psychiatry. 2020;97:152151. doi: 10.1016/j.comppsych.2019.152151. [DOI] [PubMed] [Google Scholar]
- 13.Hou L, Liu X, Zhang Y, et al. Cohort Profile: West China Health and Aging Trend (WCHAT). J Nutr Health Aging. 2020. [DOI] [PubMed]
- 14.Lim PP, Ng LL, Chiam PC, Ong PS, Ngui FT, Sahadevan S. Validation and comparison of three brief depression scales in an elderly Chinese population. Int J Geriatric Psychiatry. 2000;15(9):824–830. doi: 10.1002/1099-1166(200009)15:9<824::AID-GPS207>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Hou L, Xia X, et al. Prevalence of sarcopenia in multi ethnics adults and the association with cognitive impairment: findings from West-China health and aging trend study. 2020;20(1):63. [DOI] [PMC free article] [PubMed]
- 18.Cheung MW. metaSEM: an R package for meta-analysis using structural equation modeling. Front Psychol. 2014;5:1521. doi: 10.3389/fpsyg.2014.01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desideri G, Kwik-Uribe C, Grassi D, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) study. Hypertension (Dallas, Tex 1979) 2012;60(3):794–801. doi: 10.1161/HYPERTENSIONAHA.112.193060. [DOI] [PubMed] [Google Scholar]
- 20.Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30(2):219–223. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- 21.Stranahan AM, Norman ED, Lee K, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18(11):1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winocur G, Greenwood CE, Piroli GG, et al. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119(5):1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- 23.Tempesta D, Socci V, De Gennaro L, Ferrara M. Sleep and emotional processing. Sleep Med Rev. 2018;40:183–195. doi: 10.1016/j.smrv.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10(4):473–481. doi: 10.31887/DCNS.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254–1269. doi: 10.4088/JCP.v66n1008. [DOI] [PubMed] [Google Scholar]
- 26.Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry. 2003;64(10):1195–1200. doi: 10.4088/JCP.v64n1009. [DOI] [PubMed] [Google Scholar]
- 27.Eaton WW, Badawi M, Melton B. Prodromes and precursors: epidemiologic data for primary prevention of disorders with slow onset. Am J Psychiatry. 1995;152(7):967–972. doi: 10.1176/ajp.152.7.967. [DOI] [PubMed] [Google Scholar]
- 28.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 29.Everson CA, Laatsch CD, Hogg N. Antioxidant defense responses to sleep loss and sleep recovery. Am J Phys Regul Integr Comp Phys. 2005;288(2):R374–R383. doi: 10.1152/ajpregu.00565.2004. [DOI] [PubMed] [Google Scholar]
- 30.Gonçalves VF, Mendes-Silva AP, Koyama E, Vieira E, Kennedy JL, Diniz B. Increased levels of circulating cell-free mtDNA in plasma of late life depression subjects. J Psychiatr Res. 2021;139:25–29. doi: 10.1016/j.jpsychires.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed T, Braidy N. Editorial: From Oxidative Stress to Cognitive Decline - Towards Novel Therapeutic Approaches. Front Mol Neurosci. 2021;14:650498. doi: 10.3389/fnmol.2021.650498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi WY, Guo MH, Du P, et al. Association of sleep with anxiety in the elderly aged 60 years and older in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(1):13–19. doi: 10.3760/cma.j.issn.0254-6450.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Potvin O, Lorrain D, Belleville G, Grenier S, Préville M. Subjective sleep characteristics associated with anxiety and depression in older adults: a population-based study. Int J Geriatric Psychiatry. 2014;29(12):1262–1270. doi: 10.1002/gps.4106. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Du X, Guo Y, et al. The associations between sleep situations and mental health among Chinese adolescents: A longitudinal study. Sleep Med. 2021;13(82):71–77. doi: 10.1016/j.sleep.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Lo JC, Loh KK, Zheng H, Sim SK, Chee MW. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep. 2014;37(7):1171–1178. doi: 10.5665/sleep.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Möller C, Hafkemeijer A, Pijnenburg YAL, et al. Different patterns of cortical gray matter loss over time in behavioral variant frontotemporal dementia and Alzheimer's disease. Neurobiol Aging. 2016;38:21–31. doi: 10.1016/j.neurobiolaging.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Zarei M, Ibarretxe-Bilbao N, Compta Y, et al. Cortical thinning is associated with disease stages and dementia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2013;84(8):875–881. doi: 10.1136/jnnp-2012-304126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mlaki DA, Asmal L, Paddick SM. Prevalence and associated factors of depression among older adults in rural Tanzania. 2021. [DOI] [PubMed] [Google Scholar]
- 39.Culpepper L, Lam RW, McIntyre RS. Cognitive Impairment in Patients With Depression: Awareness, Assessment, and Management. J Clin Psychiatry. 2017;78(9):1383–1394. doi: 10.4088/JCP.tk16043ah5c. [DOI] [PubMed] [Google Scholar]
- 40.Hansen N, Singh A, Bartels C, et al. Hippocampal and Hippocampal-Subfield Volumes From Early-Onset Major Depression and Bipolar Disorder to Cognitive Decline. Front Aging Neurosci. 2021;13:626974. doi: 10.3389/fnagi.2021.626974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16(3):252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 42.Peiffer G, Underner M, Perriot J, Fond G. COPD, anxiety-depression and cognitive disorders: Does inflammation play a major role? Rev Mal Respir. 2021;38(4):357–371. doi: 10.1016/j.rmr.2021.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data-set generated and analyzed during the current study will be available two years later and is also available now from the corresponding author on a reasonable request.