Abstract
Introduction
It is unclear if SARS-CoV-2 has affected people living with HIV (PLWH) more.
Methods
We compared SARS-CoV-2 testing, test positivity, hospitalisation, intensive care unit (ICU) admission, and mortality between PLWH and the general HIV-negative population of Catalonia, Spain from March 1 to December 15, 2020.
Results
SARS-CoV-2 testing was lower among PLWH 3556/13,142 (27.06%) compared to the general HIV-negative population 1,954,902/6,446,672 (30.32%) (p < 0.001) but test positivity was higher among PLWH (21.06% vs. 15.82%, p < 0.001). We observed no significant differences between PLWH and the general population in terms of hospitalisation (13.75% vs. 14.97%, p = 0.174) and ICU admission (0.93% vs. 1.66%, p = 0.059). Among positive cases, we found a lower mortality rate among PLWH compared to the general population (1.74% vs 3.64%, p = 0.002).
Conclusion
PLWH tested less frequently for SARS-CoV-2, had a higher test positivity, similar ICU admission and hospitalisation rates, and lower SARS-CoV-2-associated mortality compared to the general HIV-negative population.
Keywords: SARS-CoV-2, COVID-19, HIV, AIDS, Epidemiological surveillance
Abstract
Introducción
No está claro si el SARS-CoV-2 ha afectado más a las personas que viven con VIH (PVV).
Métodos
Se compararon los test realizados de SARS-CoV-2, la positividad de la prueba, la hospitalización, los ingresos en la unidad de cuidados intensivos (UCI), las tasas de mortalidad entre PVV y la población general de Cataluña desde el 1 de marzo hasta el 15 de diciembre de 2020.
Resultados
Los test realizados de SARS-CoV-2 fueron menos entre PVV 3.556/13.142 (27.06%) comparado con la población general de Cataluña 1.954.902/6.446.672 (30,32%) (p < 0,001), pero la positividad de la prueba de SARS-CoV-2 fue mayor entre las PVV (21,06 vs. 15,82%; p < 0,001). No se observaron diferencias estadísticamente significativas entre PVV y la población general en cuanto a hospitalizaciones (13,75 vs. 14,97%; p = 0,174) e ingresos en la UCI (0,93 vs. 1,66%; p = 0,059). Entre los casos positivos, se encontró una menor tasa de mortalidad entre las PVV en comparación con la población general (1,74 vs. 3,64%; p = 0,002).
Conclusiones
Las PVV fueron testadas menos frecuentemente por SARS-CoV-2 que la población general, tuvieron una tasa de positividad más elevada, tasas similares de hospitalización e ingresos en la UCI, y menos mortalidad asociada al SARS-CoV-2.
Palabras clave: SARS-CoV-2, COVID-19, VIH, Sida, Vigilancia epidemiológica
Introduction
It is unclear if the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected people living with HIV (PLWH) more.1 Current data on testing and incidence of SARS-CoV-2 in this population is conflicting.2, 3 Intuitively, PLWH should have more opportunities to test for SARS-CoV-2 because this population is considered a high-risk group. However, PLWH had limited access to healthcare and were not routinely tested for SARS-CoV-2 during the current pandemic.4 Testing in Spain was based on the presence of signs and symptoms of coronavirus disease 2019 (COVID-19), chronic comorbidities, older age, and contact tracing.4
Initial studies demonstrated no vivid evidence of poorer disease course among PLWH infected with SARS-CoV-2 compared to HIV-negative individuals.5 As more data become available, large studies have reported poorer outcomes for HIV/SARS-CoV-2 co-infected persons.5 Older age and the presence of underlying chronic conditions like hypertension, diabetes, cardiovascular disease, obesity, and chronic respiratory diseases have been linked with severe COVID-19 outcomes.1 With PLWH in Europe aging and disproportionately affected by comorbidities,6 they could experience poorer prognosis than HIV-negative individuals. The potential enhanced susceptibility of PLWH to SARS-CoV-2 and severe outcomes from the co-infection has become a matter of concern.7
We compared SARS-CoV-2 testing, test positivity, hospitalisation, intensive care unit (ICU) admission, and mortality between PLWH and the general HIV-negative population of Catalonia, Spain.
Methods
We conducted a cross-sectional study leveraging data from the PISCIS cohort of PLWH in Catalonia (Spain). PISCIS is a prospective, multicentre, observational, population-based cohort that monitors hospital utilisation, reception of antiretroviral therapy (ART), and clinical status of approximately 80% of all PLWH aged ≥ 16 years in Catalonia since January 1, 1998. PISCIS data was linked with data from public health care system and disease surveillance registries through the Public Data Analysis for Health Research and Innovation Project of Catalonia (PADRIS)8 to obtain data on SARS-CoV-2 testing and associated outcomes. This work was conducted according to the Declaration of Helsinki as revised in 2013. The PISCIS cohort study has been approved by the Institutional Review Board of Germans Trias i Pujol Hospital, Badalona, Spain (EO-11-108). Patient information obtained from PADRIS was anonymized and deidentified before the analysis.
Aggregated data on the SARS-COV-2 testing, test positivity, related hospitalisation, ICU admission, and death for the general HIV-negative population within the same study period for individuals aged ≥ 16 years was obtained from COVID-19 epidemiological monitoring registry via the Agency for Health and Quality Assessment of Catalonia (AQuAS).
The study period was from March 1, 2020 to December 15, 2020. We used acute SARS-CoV-2 infections confirmed by real-time reverse transcription polymerase chain reaction (rRT-PCR) test or rapid antigen test (RAT). We excluded PLWH from the general population before the analysis.
We calculated SARS-CoV-2 test positivity per the proportion of the population that tested for SARS-CoV-2. Among SARS-CoV-2 positive cases, we determined the rates per 100 persons of hospitalisation, ICU admission, and mortality.
We used the Z-test to compare testing, test positivity, hospitalisation, ICU admission, and mortality between the two populations. We presented our findings using the onion plots concept.
Results
PLWH in our population were predominantly male (81.7%) with a median age of 47.6 years. More than half of PLWH were men who have sex with men (MSM) (52.9%) and 34.8% were of non-Spanish origin. Median CD4 count was 692.5 cells/mm3 and 81.9% had undetectable plasma HIV-RNA (≤50 copies/ml). Regarding treatment, 94.1% of PLWH were on ART with majority receiving tenofovir (57.4%). PLWH had been diagnosed with HIV for a median duration of 12.0 years and 34.2% were without chronic comorbidities (Table 1 ).
Table 1.
Characteristics of HIV-positive individuals with and without SARS-CoV-2 diagnosis in Catalonia, Spain.
| People living with HIV (PLWH) No. (%) |
||||
|---|---|---|---|---|
| All | SARS-CoV-2 negative | SARS-CoV-2 positive | p value | |
| Characteristic | (n = 13,142) | (n = 12,393) | (n = 749) | |
| Sex | ||||
| Male | 10,739 (81.7) | 10,121 (81.7) | 618 (82.5) | 0.59 |
| Female | 2402 (18.3) | 2271 (18.3) | 131 (17.5) | |
| Missing | 1 (0.01) | 1 (0.01) | 0 (0) | |
| Age, median (IQR), y | 47.6 (39.6–54.9) | 47.8 (39.8–55.0) | 43.5 (37.0–52.7) | <0.001 |
| Country of origin | <0.001 | |||
| Spain | 8564 (65.2) | 8162 (65.9) | 402 (53.7) | |
| Outside Spain | 4576 (34.8) | 4229 (34.1) | 347 (46.3) | |
| Unknown | 2 (0.02) | 2 (0.02) | 0 (0) | |
| HIV transmission route | <0.001 | |||
| PWID | 1876 (14.3) | 1817 (14.7) | 59 (7.9) | |
| MSM | 6870 (52.3) | 6425 (51.8) | 445 (59.4) | |
| Male heterosexual | 1768 (13.5) | 1680 (13.6) | 88 (11.8) | |
| Female hetero/homo/bisexual | 1714 (13.0) | 1609 (13.0) | 105 (14.0) | |
| Other | 787 (6.0) | 741 (6.0) | 46 (6.1) | |
| Missing | 127 (1.0) | 121 (1.0) | 6 (0.8) | |
| Years since HIV diagnosis, median (IQR) | 12.0 (7.0–18.7) | 12.1 (7.1–18.9) | 10.3 (6.1–15.7) | <0.001 |
| Recent CD4 count (cells/mm3), median (IQR) | 692.5 (500.0–917.0) | 692.0 (500.0–917.0) | 695.5 (483.5–912.3) | 0.65 |
| CD4/CD8 ratio, median (IQR) | 0.9 (0.6–1.2) | 0.9 (0.6–1.2) | 0.9 (0.6–1.2) | 0.26 |
| HIV-RNA(recent)a | 0.19 | |||
| Detectable | 1068 (8.1) | 1017 (8.2) | 51 (6.8) | |
| Undetectable | 10,758 (81.8) | 10,136 (81.8) | 622 (83.0) | |
| Missing | 1316 (10.0) | 1240 (10.0) | 76 (10.2) | |
| Chronic comorbidities | ||||
| Number of chronic comorbidities | 0.83 | |||
| 0 | 4489 (34.2) | 4231 (34.1) | 258 (34.5) | |
| 1 | 3434 (26.1) | 3247 (26.2) | 187 (25.0) | |
| 2 | 2244 (17.1) | 2106 (17.0) | 138 (18.4) | |
| 3 | 1325 (10.1) | 1253 (10.1) | 72 (9.6) | |
| ≥ 4 | 1650 (12.6) | 1556 (12.6) | 94 (12.6) | |
| Years on ART, median (IQR) | 9.4 (5.1–15.0) | 9.5 (5.1–15.2) | 7.8 (4.6–12.9) | <0.001 |
| Backbone ART | 0.79 | |||
| TAF | 6606 (50.2) | 6215 (50.2) | 391 (52.2) | |
| TDF | 939 (7.2) | 891 (7.2) | 48 (6.4) | |
| ABC + 3TC | 3820 (29.1) | 3594 (29.0) | 226 (30.2) | |
| Other | 670 (5.1) | 630 (5.1) | 40 (5.3) | |
| Missing | 1107 (8.4) | 1063 (8.6) | 44 (5.9) | |
Abbreviations: SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; IQR, interquartile range; PWID, people who inject drugs; MSM, men who have sex with men; ART, antiretroviral therapy; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ABC, abacavir; 3TC, lamivudine.
Undetectable HIV/RNA plasma viral load was defined as values ≤50 copies/ml.
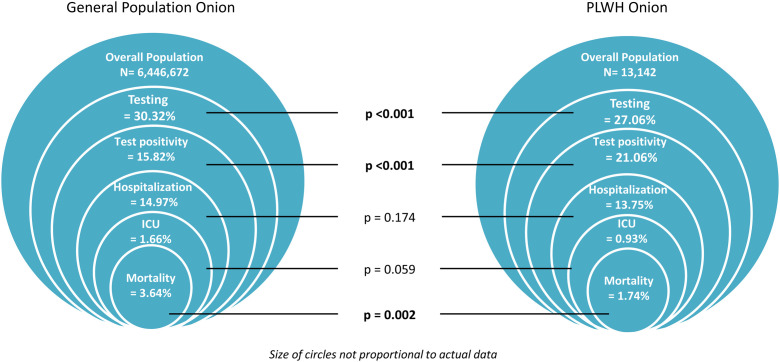
SARS-CoV-2 testing was lower among PLWH 3556/13,142 (27.06%) compared to the general HIV-negative population 1,954,902/6,446,672 (30.32%) (p < 0.001). SARS-CoV-2 test positivity was however higher among PLWH 749/3556 (21.06%, 95% confidence interval [95% CI]: 19.72–22.40) compared to the general population 309,359/1,954,902 (15.82%, 95% CI: 15.76–15.87) (p < 0.001). We observed no significant differences between PLWH and the general population in terms of hospitalisation [103/749 (13.75%, 95% CI: 11.29–16.22) vs 46,318/309,359 (14.97%, 95% CI: 14.85–15.10) (p = 0.174)] and ICU admission [7/749 (0.93%, 95% CI: 0.25–1.62) vs 5140/309,359 (1.66%, 95% CI: 1.62–1.71 p = 0.059)]. Among positive cases, we found a lower mortality rate among PLWH 13/749 (1.74%, 95% CI: 0.80–2.67) compared to the general population 11,272/3,093,599 (3.64%, 95% CI: 3.58–3.71) (p = 0.002) (Fig. 1 ).
Fig. 1.
Diagram comparing testing, test positivity, and clinical outcomes of Coronavirus disease 2019 (COVID-19) between PLWH and in the general population of Catalonia (Spain) from March 1, 2020 to December 15, 2020. Abbreviations: PLWH: People living with HIV. ICU: intensive care unit. Rates are provided per 100 persons. Testing is the percentage (%) of the overall population. Hospitalisation, ICU, and mortality are given per 100 persons per number of SARS-CoV-2 infected persons. Z-test was used to compare testing, test positivity, hospitalisation, ICU admission, and mortality between the two populations.
Discussion
In our population-based assessment, SARS-CoV-2 testing was lower among PLWH with higher test positivity compared to the general HIV-negative population. In terms of hospitalisation and ICU admission, no significant differences were observed between the two groups. Mortality was lower among PLWH compared to the general population.
A large study from the USA reported higher testing rates among PLWH compared to HIV-negative individuals.3 The findings of our population-based assessment found otherwise. HIV-positive persons were not prioritised for SARS-CoV-2 testing in Spain.4 During the early days of the pandemic, testing was based on presenting signs and symptoms, comorbidities, age, and contact tracing.4 The fact that PLWH are not prioritized for SARS-CoV-2 testing could mask the real picture of the pandemic in this population. Testing is imperative because it helps to timely identify positive cases including asymptomatic and mildly symptomatic persons and isolate to control spread or initiate early treatment to avoid severe clinical outcomes.
Contrary to other studies that have reported a similar or lower9, 10 SARS-CoV-2 incidence among PLWH, our assessment found a higher test positivity in this group. The previously reported lower incidence was attributed to sheltering of PLWH due to perceived vulnerability.11 Besides sheltering, some studies have suggested that the low SARS-CoV-2 infection rates in this population is due to the potential protection from ART.12 The therapeutic effects of these anti-HIV agents against SARS-CoV-2 is however inconclusive.12 PLWH are disproportionately affected by social determinants including low socioeconomic levels and non-Spanish origin which have been associated with increased SARS-CoV-2 diagnosis in Spain and could partly explain higher test positivity among PLWH.13, 14
We found similar rates of COVID-19-associated hospitalisation and ICU admission with lower mortality among PLWH. Earlier studies reported no increased risks of severe outcomes from SARS-CoV-2 infection among PLWH compared to HIV-negative individuals.5 However, subsequent relatively larger studies showed higher risk of death among PLWH infected with COVID-19.5 The variation in the impact of HIV on COVID-19 outcomes could be explained by the differences in severe COVID-19 risk factors present in the study populations. The fact that PLWH are disproportionately affected by other heath determinants for worse COVID-19 outcomes makes it uncertain to access the excess risk of HIV on COVID-19.
Our analysis is limited by our inability to adjust for potential confounders including age and chronic comorbidities due to its ecological design as these factors could evidently impact COVID-19 outcomes. A matched analysis adjusted for these factors will be vital to understand the observed differences. Secondly, our analysis involves only individuals engaged in the public health system of Catalonia. Individuals who tested for SARS-CoV-2 in private health facilities, pharmacies, and outside Catalonia would not be captured in the PADRIS records. However, we do not expect this to qualitatively affect the results of our study as the first phases of the pandemic was handled by the public healthcare system with restricted movements to other regions and countries. Finally, we could be underestimating the SARS-CoV-2 diagnosis rates because we only included laboratory confirmed (rRT-PCR or RAT) acute infections in the analysis and moreover, only symptomatic individuals were offered testing at the beginning of the pandemic in Spain.
In summary, PLWH tested less frequently for SARS-CoV-2 infection than the general population, had a higher positivity rate, and similar rates of hospitalisation and ICU admission. Overall, SARS-CoV-2-associated mortality was lower among PLWH. Public health strategies should be employed to increase SARS-CoV-2 testing coverage among PLWH and further studies will be needed to understand the susceptibility of this population to SARS-CoV-2.
Funding
This work was supported by the Fundació “la Caixa” [grant name: COVIHCAT]. The funder had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflicts of interest
J.M.M. reported receiving a personal 80:20 research grant from Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain, during 2017–21. For the remaining authors none was declared.
Acknowledgements
We thank the Public Data Analysis for Health Research and Innovation Program of Catalonia (PADRIS) and all healthcare workers from the PISCIS cohort collaborating hospitals for their support.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.eimc.2022.02.006.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Ambrosioni J., Blanco J.L., Reyes-urueña J.M., Davies M., Sued O., Marcos M.A., et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV. 2021;8:294–305. doi: 10.1016/S2352-3018(21)00070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saglietto A., Moirano G., Anselmino M., De Ferrari G.M. Higher testing coverage associated with a lower COVID-19 mortality rate: insights from Italian regions. Disaster Med Public Health Prep. 2020:1–3. doi: 10.1017/dmp.2020.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang J.J., Bruxvoort K., Chen L.H., Akhavan B., Rodriguez J., Hechter R.C. COVID-19 testing, characteristics, and outcomes among people living with HIV (PLWH) in an integrated health system. JAIDS J Acquir Immune Defic Syndr. 2021 doi: 10.1097/QAI.0000000000002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballester-Arnal R., Gil-Llario M.D. The virus that changed Spain: impact of COVID-19 on people with HIV. AIDS Behav. 2020;24:2253–2257. doi: 10.1007/s10461-020-02877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mellor M.M., Bast A.C., Jones N.R., Roberts N.W., Ordóñez-Mena J.M., Reith A.J.M., et al. Risk of adverse coronavirus disease 2019 outcomes for people living with HIV. AIDS. 2021;35:F1. doi: 10.1097/QAD.0000000000002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelchen-Matthews A., Ryom L., Borges Á.H., Edwards S., Duvivier C., Stephan C., et al. Aging and the evolution of comorbidities among HIV-positive individuals in a European cohort. AIDS. 2018;32:2405–2416. doi: 10.1097/QAD.0000000000001967. [DOI] [PubMed] [Google Scholar]
- 7.Brown L.B., Spinelli M.A., Gandhi M. The interplay between HIV and COVID-19: summary of the data and responses to date. Curr Opin HIV AIDS. 2021;16:63–73. doi: 10.1097/COH.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Generalitat de Catalunya . 2017. Agència de Qualitat i Avaluació Sanitàries de Catalunya. Programa públic d’analítica de dades per a la recerca i la innovació en salut a Catalunya – PADRIS [Internet], Barcelona Spain. Available from: https://aquas.gencat.cat/web/.content/minisite/aquas/publicacions/2017/Programa_analitica_dades_PADRIS_aquas2017.pdf. [Google Scholar]
- 9.Tesoriero J.M., Swain C-AE, Pierce J.L., Zamboni L., Wu M., Holtgrave D.R., et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.37069. e2037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J., Xie N., Hu X., Yan H., Ding J., Liu P., et al. Epidemiological, virological and serological features of COVID-19 cases in people living with HIV in Wuhan City: a population-based cohort study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spinelli M.A., Lynch K.L., Yun C., Glidden D.V., Peluso M.J., Henrich T.J., et al. SARS-CoV-2 seroprevalence, and IgG concentration and pseudovirus neutralising antibody titres after infection, compared by HIV status: a matched case–control observational study. Lancet HIV. 2021 doi: 10.1016/S2352-3018(21)00072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanella I., Zizioli D., Castelli F., Quiros-Roldan E. Tenofovir, another inexpensive well-known and widely available old drug repurposed for SARS-COV-2 infection. Pharmaceuticals. 2021;14:454. doi: 10.3390/ph14050454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollán M., Pérez-Gómez B., Pastor-Barriuso R., Oteo J., Hernán M.A., Pérez-Olmeda M., et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nomah D., Reyes-Urueña J., Díaz Y., Moreno S., Aceiton J., Bruguera A., et al. Sociodemographic, clinical, and immunological factors associated with SARS-CoV-2 diagnosis and severe COVID-19 outcomes in people living with HIV: a retrospective cohort study. Lancet HIV. 2021;3018:1–10. doi: 10.1016/S2352-3018(21)00240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.