Abstract
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) infection is associated with a diverse spectrum of neurological complications during the acute and postacute stages. The pathogenesis of these complications is complex and dependent on many factors. For accurate and consistent interpretation of experimental data in this fast-growing field of research, it is essential to use terminology consistently. In this article, we outline the distinctions between neuroinvasiveness, neurotropism, and neurovirulence. Additionally, we discuss current knowledge of these distinct features underlying the pathogenesis of SARS-CoV-2-associated neurological complications. Lastly, we briefly discuss the advantages and limitations of different experimental models, and how these approaches can further be leveraged to advance the field.
Keywords: coronavirus, nervous system, brain, viral infection, neuroinflammation, pathogenesis
Challenges in studying different aspects of the pathogenesis of SARS-CoV-2-associated CNS disease
A variety of neurological complications have been associated with coronavirus disease 2019 (COVID-19) in humans. The spectrum of these neurological complications is not fully understood, but it is clear that a substantial proportion of individuals have neurological complications during the acute and/or postacute stage [1,2]. In the acute stage, these complications include anosmia, cerebrovascular events, altered mental state, peripheral neuropathies, and encephalopathies [3,4]. Although frequencies vary, studies show that up to 80% of patients hospitalized with COVID-19 have neurological manifestations during the acute stage of disease [4]. Neurological symptoms during the postacute stage, which belong to the spectrum of complications associated with long COVID, are observed after not only moderate to severe, but also mild self-limiting respiratory disease (as defined in [5]). Several studies have shown that 30–60% of all patients still exhibit symptoms 6 months after disease onset, including neurological and psychiatric complications, such as intracranial hemorrhage, parkinsonism, cognitive impairment, and sleep disorders [6., 7., 8.]. The impact of the increasing prevalence of long COVID, in particular with neurological symptoms, is not yet clear but is thought to carry long-term consequences and significant socioeconomic burdens.
A comprehensive understanding of the pathogenesis of neurological sequelae of SARS-CoV-2 is lacking but, given the range of these complications, there is likely to be more than one underlying mechanism. Possible mechanisms that may contribute to the pathogenesis of SARS-CoV-2-associated CNS diseases include hypoxia, immune-mediated damage, coagulation problems, and viral invasion into the CNS [9., 10., 11.]. The proclivity of SARS-CoV-2 to enter the nervous system and its ability to infect and replicate in CNS cells have been studied extensively, with sometimes seemingly contradicting findings. However, (i) a viral infection is not a static event, because the anatomical location of active virus replication may evolve over time and eventually disappear; (ii) the course and severity of infection vary between individuals; and (iii) CNS cell types are highly diverse and comprise many different subpopulations of neurons and non-neural cell types, with often distinct cell-intrinsic antiviral immunity [12].
To understand how virus invasion and associated responses contribute to the pathogenesis of SARS-CoV-2-associated CNS diseases, it is important to distinguish between neuroinvasiveness (see Glossary), neurotropism, and neurovirulence. Unfortunately, these terms are not used consistently in the literature, leading to ambiguity of the conclusions across studies examining SARS-CoV-2 infection in the CNS. Thus, we emphasize that these definitions should be used correctly, as defined in much of the long-standing literature in the field of neuroinflammation, and as summarized in the Glossary. In this context, we discuss current knowledge of these different aspects underlying the pathogenesis of SARS-CoV-2 infection. For neuroinvasiveness and neurovirulence, we focus on in vivo findings in humans and in experimental animal models, because the latter can provide more detailed insights into the temporal kinetics of a SARS-CoV-2 infection. In vitro studies are included when neurotropism is discussed. Finally, we discuss different in vivo and in vitro models that can be used to study the neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2.
Neuroinvasiveness
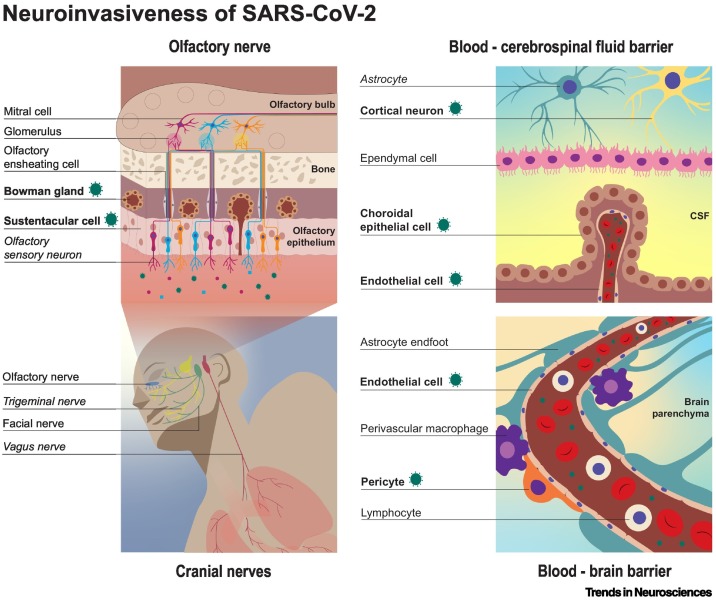
Neuroinvasiveness refers to the ability of a virus to enter the PNS or CNS. Viruses can access the CNS through peripheral nerves and/or via the hematogenous route (Figure 1 ). SARS-CoV-2 may enter the CNS through nerve endings of cranial nerves (CNs) that innervate the respiratory tract, followed by axonal (either anterograde or retrograde) transport of the virus to the CNS. For hematogenous spread, the virus needs to spill over into the circulation (viremia) and subsequently cross the blood–brain barrier (BBB) and/or blood–cerebrospinal fluid barrier (B-CSFB) [13,14] (see Box 1 ).
Figure 1.
Possible neuroinvasive routes of severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2).
Neuroinvasiveness refers to the ability of a virus to enter the CNS or PNS, regardless of whether the virus specifically infects, or replicates in, cells of the nervous system. Cranial nerves (CNs), particularly the olfactory nerve, are suspected to contribute to the neuroinvasiveness of SARS-CoV-2. Other possibilities for neuroinvasion of SARS-CoV-2 are through hematogenous spread via either the blood–brain barrier (BBB) or the blood–cerebrospinal fluid barrier (B-CSFB). Virus antigen or viral RNA (indicated by the green corona-like icon) have been detected in the cell types or structures marked in bold. Potential sites of viral replication, with inconsistent evidence to date, are formatted in italics.
Box 1. Neuroinvasiveness of respiratory viruses.
Neurological complications are associated with many respiratory virus infections, including influenza A viruses, Enterovirus-D68, measles virus, respiratory syncytial virus, and human coronaviruses [SARS-CoV-1, Middle East respiratory syndrome (MERS), and human coronavirus (HCoV)-229E, -OC43, -NL63, and -HKU1] [13,100., 101., 102.]. Respiratory viruses can access the CNS via peripheral nerves, including CNs that innervate the respiratory tract, or via the hematogenous route. For coronaviruses (e.g., HCoV-OC43 and -229E, and SARS-CoV-1), evidence suggests that virus entry into the CNS occurs preferentially via the olfactory nerve [103,104]. Influenza A viruses can use both the olfactory nerve and other CNs that innervate the respiratory tract, such as the trigeminal [105] and vagus nerves [106]. Enterovirus-D68 is thought to use peripheral nerves by transaxonal transport in motor neurons [107,108].
Hematogenous virus spread into the CNS can occur via different mechanisms. For example, respiratory syncytial virus and measles virus can infect blood leukocytes, which transmigrate through the BBB into the CNS (acting as a ‘Trojan Horse’) [109]. Hematogenous spread to the brain might also occur in the case of coronaviruses. While this has not been studied extensively, different strains of coronaviruses have been shown to infect myeloid cells, and cell-free virus has been detected in the blood (viremia) [110,111].
Alt-text: Box 1
The human respiratory tract, which is the primary replication site for SARS-CoV-2, is innervated by several CNs. The nasal cavity is innervated by the trigeminal nerve (CN V) and olfactory nerve (CN I); the upper respiratory tract by the facial (CN VII) and glossopharyngeal (CN IX) nerve; and the lower respiratory tract by the vagus nerve (CN X). There is increasing evidence that SARS-CoV-2 can use these CNs to enter the CNS. Studies in humans and experimental animal models provided evidence for virus invasion along the olfactory nerve by the detection of viral RNA or viral protein in sustentacular cells in the olfactory mucosa and, to a lesser extent, in olfactory sensory neurons (OSNs) (Figure 1) (discussed in more detail in the section ‘Neurotropism’) [8,15., 16., 17., 18., 19., 20.]. Whether SARS-CoV-2 is transported to the CNS transaxonally in OSNs or through diffusion via channels formed by olfactory ensheathing cells along the olfactory nerve remains unclear. CNS invasion via the trigeminal nerve has been suggested in humans, as viral RNA has been detected in the trigeminal ganglion, suggesting virus spread via nerve endings to the soma of sensory neurons [16,21., 22., 23., 24.]. Evidence in humans for virus invasion through the vagus nerve has been provided by the detection of SARS-CoV-2 proteins by immunohistochemistry in vagus nerve fibers [23,25]. To our knowledge, no evidence for virus invasion via the glossopharyngeal or facial nerves has yet been reported.
SARS-CoV-2 is able to disseminate into the circulation, which could result in subsequent virus spread through the BBB or B-CSFB into the CNS. In patients with COVID-19, SARS-CoV-2 viral RNA [26., 27., 28.] and viral particles [26] have been detected in the blood or serum (referred to as RNAemia and viremia, respectively). The BBB, among its various functions, protects neural tissues from pathogens in the circulation. There is currently no conclusive evidence on whether SARS-CoV-2 can cross the BBB, but SARS-CoV-2 antigens have been detected in small vessel endothelial cells in humans and animal studies [16,29,30]. In addition, in vivo damage of the basement membrane of the BBB has been observed in mice and hamsters after SARS-CoV-2 infection [31] and, in in vitro BBB models, SARS-CoV-2 can cross the endothelial cell layer [31,32]. Whether this results in virus spread into the brain parenchyma in vivo is unknown. SARS-CoV-2 infection of choroid plexus epithelial cells, and subsequent invasion into the CNS via the B-CSFB, has been suggested in several studies [31,33]. Altogether, evidence for virus invasion into the CNS via the BBB or B-CSFB is limited, but studies suggest that such invasion might occur, at least in a subset of patients.
The route of virus entry into the CNS likely influences disease manifestation. For example, Bell’s palsy, which some studies have suggested to be associated with SARS-CoV-2 infection, could be the result of virus invasion via the facial nerve [34]; virus infection along the olfactory nerve could result in anosmia [16., 17., 18.,35]; and spread to the CNS via viremia and/or infection of brain microvascular endothelial cells may result in intracerebral hemorrhage [36,37]. Whether each of these infection routes is directly associated with specific disease manifestation requires more in-depth studies. A major goal for future work is to obtain a deeper understanding of the frequency and routes of CNS invasion by SARS-CoV-2, as well as how these potential routes of entry contribute to the observed diversity of CNS manifestations (see Outstanding questions).
Outstanding questions.
Which route(s) can SARS-CoV-2 use to enter the CNS, and how does the route of entry contribute to the subsequent virus spread and/or disease outcomes?
What is the frequency of SARS-CoV-2 entry into the CNS?
What are the cell-specific responses in the CNS after SARS-CoV-2 infection?
What are the different cell-autonomous mechanisms that can lead to the neurovirulence of SARS-CoV-2 during the acute and postacute phases?
Do host factors (e.g., sex and age), or underlying comorbidities (e.g., diabetes or obesity) contribute to the severity of acute and postacute SARS-CoV-2-associated neurological complications and, if so, how?
What are risk factors for the development of acute and postacute SARS-CoV-2-associated CNS disease?
Are there differences among SARS-CoV-2 variants, in particular variants of concern, in their neuroinvasiveness, neurotropism, and neurovirulence?
Is SARS-CoV-2 unique among respiratory viruses in its ability to invade the CNS or in the frequency it does so? Is it unique in its ability infect certain cell types of the CNS, or in its ability to cause specific CNS complications?
Can infection with SARS-CoV-2 exacerbate prior underlying neurodegenerative or neuropsychiatric diseases?
Alt-text: Outstanding questions
Neurotropism
Neurotropism refers to the ability of a virus to infect and replicate in cells of the nervous system. Several reports investigated the cell tropism of SARS-CoV-2, including studies that examined its tropism along the olfactory nerve. The olfactory mucosa comprises OSNs, sustentacular cells, basal cells, and Bowman glands. In the submucosa, axon bundles of OSNs are enveloped by olfactory ensheathing cells (glial cells with Schwann-like properties) that form tunnels through the cribriform plate to the olfactory bulb. SARS-CoV-2 antigens or RNA have been frequently detected in sustentacular cells and Bowman glands in postmortem tissue from patients with COVID-19 [16,17], and in experimentally inoculated hamsters, mice, and ferrets (Figure 1) [17., 18., 19.,29,38., 39., 40.]. A few studies have found evidence for SARS-CoV-2 infection in OSNs early after inoculation in experimentally inoculated hamsters [17,18,40] or patients with COVID-19 with chronic anosmia [17]. However, in other studies, virus antigen in OSNs was not detected [20,29]. Whether these inconsistencies are related to differences in the time post infection, or reflect pathophysiological heterogeneity among individuals is not fully understood.
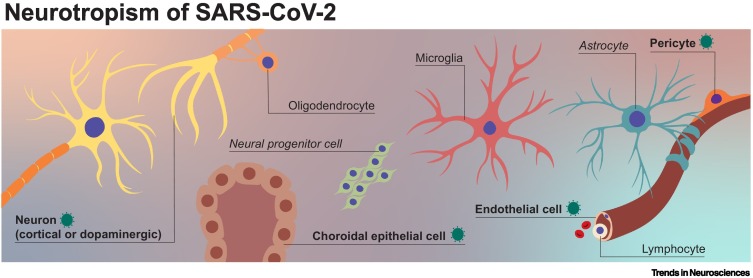
Once inside the CNS, the virus is exposed to different cell types, including various subtypes of neuron, glial lineage cell (oligodendrocyte precursor cells, oligodendrocytes, astrocytes, and ependymal cells), microglia, cells of the choroid plexus, and neurovascular cells, including vascular endothelial cells and pericytes (Figure 2 ). The receptor angiotensin converting enzyme-2 (ACE-2) is expressed in several brain locations, including the choroid plexus and olfactory bulb [41., 42., 43.]. Cell types expressing ACE2 include excitatory and inhibitory neurons, and some non-neuronal cells, such as astrocytes, oligodendrocytes, and endothelial cells [41,44]. In humans, autopsies revealed SARS-CoV-2 antigens in the brain parenchyma and cortical neurons of some patients, while SARS-CoV-2 viral RNA has been detected in the substantia nigra [16,29]. Additionally, animal studies suggest that dopaminergic neurons and, to a lesser extent, cortical neurons, microglia, and astrocytes are susceptible to SARS-CoV-2 infection [29,45,46]. Human pluripotent stem cell (hPSC)-derived 2D cultures and 3D organoids (models reviewed in [47]) have been used to investigate the cell tropism of SARS-CoV-2 in vitro [48]. In general, studies suggest that different cell types, including dopaminergic neurons, cortical neurons, brain microvascular endothelial cells, and choroidal epithelial cells, are susceptible to SARS-CoV-2 infection, but, among these different cell types, there are differences in the permissiveness for SARS-CoV-2 (i.e., its ability to produce progeny virus) [29,49., 50., 51., 52., 53., 54., 55., 56.]. Studies in choroid plexus organoids showed that choroidal epithelial cells are permissive for SARS-CoV-2 infection [50,51,54., 55., 56., 57., 58.]. Currently, there is no in vivo or in vitro evidence, to our knowledge, for productive infection of neural progenitor cells with SARS-CoV-2, and some studies have provided evidence arguing against this possibility [52,53,56].
Figure 2.
Neurotropism of severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2).
Neurotropism refers to the ability of viruses to infect and replicate in cells of the nervous system. Cells of the CNS include neurons, glial cells (e.g., astrocytes and oligodendrocytes), microglia, choroid plexus cells, and cells of the neurovascular system (such as vascular endothelial cells and pericytes). SARS-CoV-2 virus antigen or viral RNA has been detected in the cell types formatted in bold.
In humans and in animal models, SARS-CoV-2 virus or virus antigen has been detected in endothelial cells of the brain [16]. Evidence for productive infection of primary endothelial cells derived from different organs is scarce, and in vitro studies showed that SARS-CoV-2 infects endothelial cells only when ACE2 is artificially overexpressed [59,60]. In principle, abortive infection (cell infection without follow-up production of progeny virus) of endothelial cells [59,60] or pericytes [61] could weaken the BBB, which could make it more susceptible to virus invasion or result in microvascular complications.
Most studies examining SARS-CoV-2 infection of CNS cell types have shown that infection is restricted to a subset of cells and that virus replication is often inefficient or even abortive. Despite this inefficient or abortive replication, infection is likely associated with changes in cellular functioning and responses. One of the mechanisms underlying altered cellular function following SARS-CoV-2 infection involves cellular senescence, which may affect both infected and bystander cells [45].
Neurovirulence
Neurovirulence indicates the ability of a viral infection to cause CNS pathology, independently from the ability of the virus to invade, or infect cells of, the CNS. There is substantial evidence that a SARS-CoV-2 infection can cause various neurological pathologies and neuropsychiatric symptoms during both the acute and postacute stage. The neurovirulent potential of SARS-CoV-2 is not restricted to cases of severe disease, and patients with mild or severe disease can develop neurological complications. Several mechanisms have been hypothesized to contribute to the neurovirulence of SARS-CoV-2, including virus invasion into the CNS, dysregulated systemic inflammatory responses, hypoxia, and autoimmune responses (Figure 3 ).
Figure 3.
Neurovirulence.
Neurovirulence refers to the ability of a virus infection to cause pathology in the CNS that contribute to the development of clinical disease of the nervous system, independent of its ability to invade the CNS and infect cells of the CNS. Lesions or inflammatory responses associated with severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) infection are illustrated.
The neurovirulent potential of SARS-CoV-2 has been examined in postmortem brain tissue and in vivo animal models, and by radiological studies during the acute and postacute stages of COVID-19. Radiological studies suggest morphological changes, especially in the olfactory bulb (edema and microbleeding) [62., 63., 64., 65., 66.], as well as loss of gray matter in the parahippocampal gyrus, lateral orbitofrontal cortex, and insula [66].
Anosmia has often been associated with SARS-CoV-2 infection, at least with the initial variants of the virus, but the underlying pathology is not fully understood. The mechanisms underlying olfactory dysfunction may involve a complex and possibly long-lasting interplay of dysregulated immune responses in the olfactory mucosa and the olfactory bulb, as well as virus-induced lesions along the olfactory tract [18,35]. In hamsters and mice, there is evidence for focal destruction of the olfactory mucosa, associated with an influx of inflammatory cells [19]. Autopsy findings from patients with COVID-19 found focal atrophy and infiltrating CD45+ leukocytes, CD4+ T cells, CD8+ T cells, and activated macrophages in the olfactory mucosa in a subset of patients [16,17,67., 68., 69.].
Several studies have found evidence for CNS inflammation after SARS-CoV-2 infection. In humans, activated microglia were found in the olfactory bulb, midbrain (specifically, in the substantia nigra), hindbrain, dorsal motor nucleus of the vagus nerve, and the pre-Bötzinger complex in the medulla [70]. Furthermore, multifocal microgliosis and astrogliosis were reported in older patients [71], although it could not be ruled out that these were associated with host factors [72]. Perivascular and parenchymal infiltrations of CD8+ cytotoxic T cells and macrophages have been reported postmortem in patients with COVID-19 and in intranasally inoculated mice at 6 days post inoculation [61,71,73]. Extensive inflammatory responses, such as astrogliosis, activation of microglia, and perivascular cuffing of T cells, were detected postmortem in both white and gray matter of patient brains regardless of their COVID-19 disease severity, a finding that appeared most pronounced in the cranial medulla oblongata and olfactory bulb [74]. Furthermore, evidence for acute hypoxic injury was observed in the cerebrum and cerebellum, with loss of neurons in the cerebral cortex, hippocampus, and cerebellar Purkinje cell layer [75]. In mice that express human ACE2 in their respiratory tract, SARS-CoV-2 infection triggered microglial activation and hypomyelination in the subcortical white matter and impaired neurogenesis in the hippocampus [76]. In hamsters, SARS-CoV-2 infection triggers microglial and T cell activation in the olfactory bulb [18,35].
Neurovascular injuries and lesions in the vasculature of the CNS have been detected in patients hospitalized with COVID-19 [65,77,78]. Postmortem analyses on the CNS from patients who died with COVID-19 showed thinning of the basal lamina of endothelial cells and congested blood vessels with fibrinogen leakage, suggestive of microhemorrhages [70]. Studies in patients with COVID-19 and in animal models, including hamsters and K18-hACE2 mice, suggested that SARS-CoV-2 infection of brain vascular endothelial can lead to endothelial cell death and formation of string vessels in the cortex [30]. Additionally, perivascular infiltration of CD3+, CD8+ T cells, and macrophages, as well as the presence of hypertrophic astrocytes and activated microglia, were detected [16,17,68,69].
The underlying neurovirulent pathologies of SARS-CoV-2 are diverse, and it is plausible that there is not one sole mechanism triggering these changes. Furthermore, it is likely that host factors, such as sex and age, or underlying diseases, complicate the picture of the diverse CNS complications associated with SARS-CoV-2 infection (see Outstanding questions).
Models and techniques to study the neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2
Studying the pathogenesis in human samples and human postmortem tissue can reveal important insights into neurological complications associated with SARS-CoV-2 infection, albeit with limitations. First, samples are collected after disease onset; thus, the early phases of the infection are not captured in this approach. Second, sequential sampling is often difficult and, in most cases (except postmortem studies), samples can only be taken from the CSF or from outside the CNS. Lastly, there is substantial heterogeneity in human cohorts, including differences in age, sex, comorbidities, and immune status. Complementing studies in humans, in vivo animal models and in vitro hPSC approaches can assist in elucidating pathological mechanisms during the acute and postacute stages of the disease as well as various aspects of virus–host cell interactions. However, extrapolating from animal data or human cellular modeling data to human clinical situations comes with limitations, which should be carefully considered.
In vivo models
Several animal models have been established to study the pathogenesis of SARS-CoV-2 infection in vivo, including models in mice, hamsters, and, to a lesser extent, ferrets and non-human primates (NHPs) [79,80]. Mice are not naturally sensitive to SARS-CoV-2 replication, but transgenic expression of human ACE2 or transduction of mice with adenovirus or adeno-associated viruses expressing human ACE2 sensitizes mice to SARS-CoV-2 infection. Although the tissue and cell ACE2 expression in these models differs from that in humans, these mice models have been used to study the effect of both virus replication [79., 80., 81., 82.] and inflammation-induced changes [73,83,84] in the brain. Although the observations cannot be extrapolated directly to humans, they provide important insights into the different mechanisms that could contribute to the large spectrum of neurological complications following SARS-CoV-2 infection.
Experimental inoculation of ferrets or NHPs with SARS-CoV-2 generally results in only mild respiratory disease, with limited evidence for CNS invasion [81,85,86]. However, these models could shed light on changes in the CNS during the acute and postacute stage of mild SARS-CoV-2 infection [87].
Experimental inoculation of hamsters results in more severe respiratory disease, with virus being detected in extrapulmonary tissues, including the CNS. Studies in Syrian hamsters detected viral RNA in the CNS as well as inflammation and changes in the neurovascular system [17,30]. Although the Syrian hamster has been used most frequently in hamster studies, it was recently shown that, in Roborovski dwarf hamsters, inbred hamsters, transgenic hamsters expressing human ACE2, and obese hamsters, SARS-CoV-2 infection resulted in more severe disease and systemic spread, including virus spread to the CNS [88., 89., 90., 91.].
The different in vivo models enable studies of the neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2 during the course of infection, including before disease onset and the postacute phase. In our opinion, these models provide a unique opportunity to study the impact of virus dose, sex, age, obesity, as well as other risk factors and comorbidities on the pathogenesis during the acute and postacute stages in a controlled manner, which is challenging in humans. However, there are differences in the course of disease among the in vivo models and, thus, it is important to choose the most suitable model for specific research questions.
In vitro models
hPSC-based models are ideal tools to investigate the interaction between SARS-CoV-2 and cells of the CNS and PNS. These models, either 2D or 3D, allow the investigation of neural and non-neural cells from different CNS regions, which are known to display extensive heterogeneity in their gene expression profiles, functionality, and immunological status. Moreover, hPSC-derived sensory neurons can be used to address the question of whether and which sensory neuron subtypes are susceptible to SARS-Co-2 infection, and whether they facilitate transaxonal transport. Due to their scalability, hPSC-based models are particularly well suited for large-scale compound screening and whole-genome screens to dissect virus–host interactions.
Methodological considerations
When studying SARS-CoV-2 infection in different cells and/or tissues, it is important to differentiate between susceptibility or permissiveness. Susceptibility can be shown by the detection of viral antigens (using immunofluorescence or immunohistochemistry) or by the detection of viral RNA (using in situ hybridization, qPCR, or RNA-seq). When these analyses are performed on tissue sections from in vivo models, the location of virus antigen can directly be associated with histological lesions. Nonetheless, these assays require the use of adequate controls, such as isotype and omission-negative controls, and preferentially the inclusion of two methods to ensure true detection of infection. Ideally, permissiveness is determined in vitro by the detection of an increase in infectious virus over time by determining the tissue culture infectious dose (TCID) or plaque-forming units (PFU). Alternatively, the detection of virus antigen or RNA in vivo, together with the location and presence of histological lesions, detection of virus particles by electron microscopy, or isolation of infectious virus, are also suggestive of active virus replication.
Concluding remarks
Currently available data show that SARS-CoV-2 has neuroinvasive potential, that its neurotropism is limited, and that it can be neurovirulent in at least a subgroup of patients. This concurs with observations from the clinic, where the impact of SARS-CoV-2-associated CNS complications appears limited during the acute phase, but more prominent during the postacute phase. Reports of severe disease during the acute phase, such as encephalitis, exist, but these are rare compared with the number of people infected [92,93]. However, the percentage of patients with SARS-CoV-2-associated CNS impairments during the postacute phase, which are part of the wide spectrum of complications associated with long COVID, can be up to 30–60% [5., 6., 7.].
CNS complications observed during the acute and postacute stages of SARS-CoV-2 infection are diverse, which might be related, at least in part, to host factors, comorbidities, immune status of the host, virus variants, or other variables [94., 95., 96.]. Host factors or comorbidities that affect the risk of developing severe disease from COVID-19 include age, sex, metabolic status, or pre-existing neurological conditions. Whether each of these factors influences the development of CNS disease and, if so how, is not fully understood. In addition, potential differences among SARS-CoV-2 variants in terms of their neuroinvasiveness, neurotropism, or neurovirulence remain to be further investigated, although it has already been shown that SARS-CoV-2 variants differ in the pathogenesis of respiratory disease [97,98]. The possible emergence of future variants could add additional complexity to the issue. Furthermore, the risk of developing CNS diseases after infection with SARS-CoV-2 might change after vaccination or prior to SARS-CoV-2 infection. It has already been shown that vaccination partly protects against the development of long COVID [99]. Other variables that might influence the development of CNS disease include, for example, the infection dose and primary replication site of the virus [73].
As both the acute and postacute disease burdens of COVID-19 continue to increase, there is a pressing need to better understand the contribution of all factors that influence the course of disease and how they contribute to CNS complications (see Outstanding questions). Much remains to be learned about the underlying mechanisms leading to SARS-CoV-2-induced neuropathology. In vitro and in vivo models, together with analyses in patients, can reveal important insights into the neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2 variants within different environmental settings and host factors.
Acknowledgments
Acknowledgments
We apologize to our colleagues whose work we could not cite due to space limitations. D.V.R. is supported by fellowships from The Netherlands Organization for Scientific Research (VIDI contract 91718308) and a EUR fellowship. O.H. is supported by fellowships from the Warren Alpert Foundation, the Encephalitis Society, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. This work was also supported by the Netherlands Organ-on-Chip Initiative, an NWO Gravitation project (024.003.001) funded by the Ministry of Education, Culture and Science of the Government of The Netherlands (to S.A.K. and F.M.S.D.V.), a Dutch ZonMw More-Knowledge-with-Fewer-Animals (MKMD) Create2Solve grant (114025201; to S.A.K. and F.M.S.D.V.), and by an Erasmus MC Human Disease Model Award to F.M.S.D.V.
Declaration of interests
The authors declare no conflicts of interest
Glossary
- Neuroinvasiveness
ability of a virus to enter either the PNS or CNS.
- Neurotropism
ability of a virus to infect and replicate in cells of the nervous system. Cells of the nervous system include neurons, glial cells (e.g., astrocytes, oligodendrocytes, and oligodendrocyte precursor cells), microglial cells, meningeal cells, choroid plexus cells, and cells of the neurovascular system (such as vascular endothelial cells and pericytes).
- Neurovirulence
ability of a virus infection to cause pathology in the CNS that contributes to the development of clinical disease of the nervous system independently of its ability to invade the CNS or infect cells of the CNS.
References
- 1.Misra S., et al. Frequency of neurologic manifestations in COVID-19: a systematic review and meta-analysis. Neurology. 2021;97:e2269–e2281. doi: 10.1212/WNL.0000000000012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero-Sánchez C.M., et al. Neurologic manifestations in hospitalized patients with COVID-19. Neurology. 2020;95 doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varatharaj A., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou S.H.-Y., et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19—a report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi R.T., et al. Mild or moderate Covid-19. N. Engl. J. Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 6.Taquet M., et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neurology T.L. Long COVID: understanding the neurological effects. Lancet Neurol. 2021;20:247. doi: 10.1016/S1474-4422(21)00059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blomberg B., et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021;27:1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zubair A.S., et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77:1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jafari Khaljiri H., et al. Comprehensive review on neuro-COVID-19 pathophysiology and clinical consequences. Neurotox. Res. 2021;39:1613–1629. doi: 10.1007/s12640-021-00389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han Y., et al. Neuropsychiatric manifestations of COVID-19, potential neurotropic mechanisms, and therapeutic interventions. Transl. Psychiatry. 2021;11:499. doi: 10.1038/s41398-021-01629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmer B., et al. Human iPSC-derived trigeminal neurons lack constitutive TLR3-dependent immunity that protects cortical neurons from HSV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E8775–E8782. doi: 10.1073/pnas.1809853115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludlow M., et al. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 2016;131:159–184. doi: 10.1007/s00401-015-1511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyuncu O.O., et al. Virus infections in the nervous system. Cell Host Microbe. 2013;13:379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai M., et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. U. S. A. 2020;117:16587–16595. doi: 10.1073/pnas.2009799117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meinhardt J., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 17.de Melo G.D., et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 2021;13:eabf8396. doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zazhytska M., et al. Non-cell autonomous disruption of nuclear architecture as a potential cause of COVID-19 induced anosmia. Cell. 2022 doi: 10.1016/j.cell.2022.01.024. Published online February 2, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryche B., et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun. 2020;89:579–586. doi: 10.1016/j.bbi.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan M., et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184:5932–5949. doi: 10.1016/j.cell.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagre A., et al. SARS-CoV-2 infection, neuropathogenesis and transmission among deer mice: Implications for spillback to New World rodents. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messlinger K., et al. Activation of the trigeminal system as a likely target of SARS-CoV-2 may contribute to anosmia in COVID-19. Cephalalgia Int. J. Headache. 2021;42:176–180. doi: 10.1177/03331024211036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Weyhern C.H., et al. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina-Gil J., et al. Trigeminal neuralgia as the sole neurological manifestation of COVID-19: a case report. Headache J. Head Face Pain. 2021;61:560–562. doi: 10.1111/head.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulfamante G., et al. Brainstem neuropathology in two cases of COVID-19: SARS-CoV-2 trafficking between brain and lung. J. Neurol. 2021;268:4486–4491. doi: 10.1007/s00415-021-10604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs J.L., et al. SARS-CoV-2 viremia is associated with COVID-19 severity and predicts clinical outcomes. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab686. Published online August 10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y., et al. SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes. J. Clin. Invest. 2021;131 doi: 10.1172/JCI148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Riel D., et al. Temporal kinetics of RNAemia and associated systemic cytokines in hospitalized COVID-19 patients. mSphere. 2021;6 doi: 10.1128/mSphere.00311-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song E., et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021;218 doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wenzel J., et al. The SARS-CoV-2 main protease Mpro causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat. Neurosci. 2021;24:1522–1533. doi: 10.1038/s41593-021-00926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L., et al. SARS-CoV-2 crosses the blood–brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal. Transduct. Target. Ther. 2021;6:337. doi: 10.1038/s41392-021-00719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krasemann S., et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Reports. 2022;17:307–320. doi: 10.1016/j.stemcr.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomes I., et al. SARS-CoV-2 infection of the central nervous system in a 14-month-old child: A case report of a complete autopsy. Lancet Reg. Heal. Am. 2021;2 doi: 10.1016/j.lana.2021.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamaki A., et al. Incidence of Bell palsy in patients with COVID-19. JAMA Otolaryngol. Neck Surg. 2021;147:767–768. doi: 10.1001/jamaoto.2021.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frere J.J., et al. SARS-CoV-2 infection results in lasting and systemic perturbations post recovery. BioRxiv. 2022 doi: 10.1101/2022.01.18.476786. Published online January 20, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Margos N.P., et al. Intracerebral hemorrhage in COVID-19: a narrative review. J. Clin. Neurosci. 2021;89:271–278. doi: 10.1016/j.jocn.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leasure A.C., et al. Intracerebral hemorrhage in patients with COVID-19: an analysis from the COVID-19 Cardiovascular Disease Registry. Stroke. 2021;52:e321–e323. doi: 10.1161/STROKEAHA.121.034215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sia S.F., et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Everett H.E., et al. Intranasal infection of ferrets with SARS-CoV-2 as a model for asymptomatic human infection. Viruses. 2021;13:113. doi: 10.3390/v13010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang A.J., et al. Severe acute respiratory syndrome coronavirus 2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clin. Infect. Dis. 2021;73:e503–e512. doi: 10.1093/cid/ciaa995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen R., et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front. Neurol. 2021;11 doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukiw W.J., et al. SARS-CoV-2 infectivity and neurological targets in the brain. Cell. Mol. Neurobiol. 2022;42:217–224. doi: 10.1007/s10571-020-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fodoulian L., et al. SARS-CoV-2 receptors and entry genes are expressed in the human olfactory neuroepithelium and brain. iScience. 2020;23 doi: 10.1016/j.isci.2020.101839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahalingam R., et al. Single-cell RNA sequencing analysis of SARS-CoV-2 entry receptors in human organoids. J. Cell. Physiol. 2020;236:2950–2958. doi: 10.1002/jcp.30054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen S., et al. SARS-CoV-2 infection causes dopaminergic neuron senescence. Res. Sq. 2021 doi: 10.21203/rs.3.rs-513461/v1. Published online May 21, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferren M., et al. Hamster organotypic modeling of SARS-CoV-2 lung and brainstem infection. Nat. Commun. 2021;12:1–17. doi: 10.1038/s41467-021-26096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harschnitz O., Studer L. Human stem cell models to study host–virus interactions in the central nervous system. Nat. Rev. Immunol. 2021;21:441–453. doi: 10.1038/s41577-020-00474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramani A., et al. Neurotropic effects of SARS-CoV-2 modeled by the human brain organoids. Stem Cell Reports. 2021;16:373–384. doi: 10.1016/j.stemcr.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramani A., et al. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020;39 doi: 10.15252/embj.2020106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacob F., et al. Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell. 2020;27:937–950. doi: 10.1016/j.stem.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pellegrini L., et al. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell. 2020;27:951–961. doi: 10.1016/j.stem.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bauer L., et al. Replication kinetics, cell tropism, and associated immune responses in SARS-CoV-2- and H5N1 virus-infected human induced pluripotent stem cell-derived neural models. mSphere. 2021;6 doi: 10.1128/mSphere.00270-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bullen C.K., et al. Infectability of human BrainSphere neurons suggests neurotropism of SARS-CoV-2. ALTEX. 2020;37:665–671. doi: 10.14573/altex.2006111. [DOI] [PubMed] [Google Scholar]
- 54.McMahon C.L., et al. SARS-CoV-2 targets glial cells in human cortical organoids. Stem Cell Reports. 2021;16:1156–1164. doi: 10.1016/j.stemcr.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C., et al. ApoE-isoform-dependent SARS-CoV-2 neurotropism and cellular response. Cell Stem Cell. 2021;28:331–342. doi: 10.1016/j.stem.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang B.-Z., et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;30:928–931. doi: 10.1038/s41422-020-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang L., et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–136. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yi S.A., et al. Infection of brain organoids and 2D cortical neurons with SARS-CoV-2 pseudovirus. Viruses. 2020;12:1004. doi: 10.3390/v12091004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nascimento Conde J., et al. Recombinant ACE2 expression is required for SARS-CoV-2 to infect primary human endothelial cells and induce inflammatory and procoagulative responses. MBio. 2020;11 doi: 10.1128/mBio.03185-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schimmel L., et al. Endothelial cells are not productively infected by SARS-CoV-2. Clin. Transl. Immunol. 2021;10 doi: 10.1002/cti2.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bocci M., et al. Infection of brain pericytes underlying neuropathology of COVID-19 patients. Int. J. Mol. Sci. 2021;22:11622. doi: 10.3390/ijms222111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aragão M.F.V.V., et al. Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. Am. J. Neuroradiol. 2020;41:1703–1706. doi: 10.3174/ajnr.A6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laurendon T., et al. Bilateral transient olfactory bulb edema during COVID-19–related anosmia. Neurology. 2020;95:224–225. doi: 10.1212/WNL.0000000000009850. [DOI] [PubMed] [Google Scholar]
- 64.Gutierrez Amezcua J.M., et al. COVID-19-induced neurovascular injury: a case series with emphasis on pathophysiological mechanisms. SN Compr. Clin. Med. 2020;2:2109–2125. doi: 10.1007/s42399-020-00598-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radmanesh A., et al. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;297:E223–E227. doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Douaud G., et al. Brain imaging before and after COVID-19 in UK Biobank. medRxiv. 2021 doi: 10.1101/2021.06.11.21258690. Published online August 18, 2021. [DOI] [Google Scholar]
- 67.Morbini P., et al. Ultrastructural evidence of direct viral damage to the olfactory complex in patients testing positive for SARS-CoV-2. JAMA Otolaryngol. Neck Surg. 2020;146:972–973. doi: 10.1001/jamaoto.2020.2366. [DOI] [PubMed] [Google Scholar]
- 68.Vaira L.A., et al. Olfactory epithelium histopathological findings in long-term coronavirus disease 2019 related anosmia. J. Laryngol. Otol. 2020;134:1123–1127. doi: 10.1017/S0022215120002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kirschenbaum D., et al. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet. 2020;396:166. doi: 10.1016/S0140-6736(20)31525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee M.-H., et al. Microvascular injury in the brains of patients with Covid-19. N. Engl. J. Med. 2021;384:481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matschke J., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cosentino G., et al. Neuropathological findings from COVID-19 patients with neurological symptoms argue against a direct brain invasion of SARS-CoV-2: a critical systematic review. Eur. J. Neurol. 2021;28:3856–3865. doi: 10.1111/ene.15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fumagalli V., et al. Administration of aerosolized SARS-CoV-2 to K18-hACE2 mice uncouples respiratory infection from fatal neuroinvasion. Sci. Immunol. 2021;7:eabl9929. doi: 10.1126/sciimmunol.abl9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schurink B., et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Solomon I.H., et al. Neuropathological features of Covid-19. N. Engl. J. Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fernández-Castañeda A., et al. Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. BioRxiv. 2022 doi: 10.1101/2022.01.07.475453. Published online January 10, 2022. [DOI] [Google Scholar]
- 77.Mao L., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oxley T.J., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muñoz-Fontela C., et al. Advances and gaps in SARS-CoV-2 infection models. PLoS Pathog. 2022;18 doi: 10.1371/journal.ppat.1010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muñoz-Fontela C., et al. Animal models for COVID-19. Nature. 2020;586:509–515. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Munster V.J., et al. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020;585:268–272. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tostanoski L.H., et al. Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters. Nat. Med. 2020;26:1694–1700. doi: 10.1038/s41591-020-1070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vidal E., et al. Chronological brain lesions after SARS-CoV-2 infection in hACE2-transgenic mice. Vet. Pathol. 2021 doi: 10.1177/03009858211066841. Published online December 27, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Olivarria G.M., et al. Microglia do not restrict SARS-CoV-2 replication following infection of the central nervous system of K18-human ACE2 transgenic mice. J. Virol. 2021;96 doi: 10.1128/jvi.01969-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rockx B., et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science (80-. ) 2020;368:1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi J., et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368:1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiao L., et al. The olfactory route is a potential way for SARS-CoV-2 to invade the central nervous system of rhesus monkeys. Signal. Transduct. Target. Ther. 2021;6:1–11. doi: 10.1038/s41392-021-00591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Golden J.W., et al. Hamsters expressing human angiotensin-converting enzyme 2 develop severe disease following exposure to SARS-CoV-2. MBio. 2022;13 doi: 10.1128/mbio.02906-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Port J.R., et al. Western diet increases COVID-19 disease severity in the Syrian hamster. BioRxiv. 2021 doi: 10.1101/2021.06.17.448814. Published online June 17 2021. [DOI] [Google Scholar]
- 90.Zhai C., et al. Roborovski hamster (Phodopus roborovskii) strain SH101 as a systemic infection model of SARS-CoV-2. Virulence. 2021;12:2430–2442. doi: 10.1080/21505594.2021.1972201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trimpert J., et al. The Roborovski dwarf hamster is a highly susceptible model for a rapid and fatal course of SARS-CoV-2 infection. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vandervorst F., et al. Encephalitis associated with the SARS-CoV-2 virus: a case report. Interdiscip. Neurosurg. 2020;22 doi: 10.1016/j.inat.2020.100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanchez C.V., et al. Autoimmune encephalitis after SARS-CoV-2 infection: case frequency, findings, and outcomes. Neurology. 2021;97:e2262–e2268. doi: 10.1212/WNL.0000000000012931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Velavan T.P., et al. Host genetic factors determining COVID-19 susceptibility and severity. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schneider W.M., et al. Genome-scale identification of SARS-CoV-2 and pan-coronavirus host factor networks. Cell. 2021;184:120–132. doi: 10.1016/j.cell.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Daniloski Z., et al. Identification of required host factors for SARS-CoV-2 infection in human cells. Cell. 2021;184:92–105. doi: 10.1016/j.cell.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Halfmann P.J., et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature. 2022 doi: 10.1038/s41586-022-04441-6. Published online January 21, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abdelnabi R., et al. The omicron (B.1.1.529) SARS-CoV-2 variant of concern does not readily infect Syrian hamsters. Antivir. Res. 2022;198 doi: 10.1016/j.antiviral.2022.105253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuodi P., et al. Association between vaccination status and reported incidence of post-acute COVID-19 symptoms in Israel: a cross-sectional study of patients tested between March 2020 and November 2021. MedRxiv. 2022 doi: 10.1101/2022.01.05.22268800. Published online January 17, 2022. [DOI] [Google Scholar]
- 100.Hixon A.M., et al. Understanding enterovirus D68-induced neurologic disease: a basic science review. Viruses. 2019;11:1–17. doi: 10.3390/v11090821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bohmwald K., et al. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abdelaziz O.S., Waffa Z. Neuropathogenic human coronaviruses: a review. Rev. Med. Virol. 2020;30 doi: 10.1002/rmv.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Desforges M., et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12:E14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suzuki M., et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117:272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park C.H., et al. The invasion routes of neurovirulent A/Hong Kong/483/97 (H5N1) influenza virus into the central nervous system after respiratory infection in mice. Arch. Virol. 2002;147:1425–1436. doi: 10.1007/s00705-001-0750-x. [DOI] [PubMed] [Google Scholar]
- 106.Matsuda K., et al. In vitro demonstration of neural transmission of avian influenza A virus. J. Gen. Virol. 2005;86:1131–1139. doi: 10.1099/vir.0.80704-0. [DOI] [PubMed] [Google Scholar]
- 107.Hixon A.M., et al. Contemporary circulating enterovirus D68 strains infect and undergo retrograde axonal transport in spinal motor neurons independent of sialic acid. J. Virol. 2019;93:1–18. doi: 10.1128/JVI.00578-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morrey J.D., et al. Causation of acute flaccid paralysis by myelitis and myositis in enterovirus-D68 infected mice deficient in interferon αβ/γ receptor deficient mice. Viruses. 2018;10:33. doi: 10.3390/v10010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Espinoza J.A., et al. Impaired learning resulting from respiratory syncytial virus infection. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9112–9117. doi: 10.1073/pnas.1217508110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Desforges M., et al. Activation of human monocytes after infection by human coronavirus 229E. Virus Res. 2007;130:228–240. doi: 10.1016/j.virusres.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arbour N., et al. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000;74:8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]