Abstract
Aims
This study aims to assess differences in severity of short-term (<1 year) and long-term (≥1 year) adverse CV outcomes after PCI in insulin-treated vs. non-insulin-treated diabetes mellitus (DM) patients.
Methods
A systematic search on Pubmed and Embase led to the incorporation of 29 studies that compared post-percutaneous coronary interventional outcomes in insulin-treated and non-insulin-treated diabetes mellitus. Diabetes mellitus (type 2) was defined as fasting blood glucose (FBG) level of >7.0 mmol/L or with an oral glucose tolerance test (OGTT) level of >11.1 mmol/L at least on two separate occasions. Adverse CV outcomes were assessed in insulin-treated and non-insulin-treated DM after the PCI procedure considered for the analyses were mortality, MACE, TLR, TVR, MI, stent thrombosis, target lesion failure (TLF), and need for-post PCI CABG. Data were pooled and analyzed using Review Manager 5.3, and risk ratios (RR) with respective 95% confidence intervals (CI) were calculated.The statistical analyses were carried out by Review Manager v.5.3, and the data were pooled using a random-effects model. Risk ratios (RRs) with 95% confidence intervals (CI) were reported along with forest plots. The chi-square test was performed to assess for differences between the subgroups. Heterogeneity across studies was evaluated using Higgins I2 statistics. Visual inspection of the funnel plot and Begg's regression test were used to assess publication bias.
Results
A total of 40,527 patients (11742 in the Insulin-treated diabetes mellitus group and 28785 in the non-insulin-treated DM group) who underwent PCI were included. The pooled analysis of short-term follow up outcomes preceding PCI demonstrated a significantly higher risk of mortality (RR = 1.75 [1.24,2.47]; p = 0.002), MI (RR = 1.81[1.14,2.87]; p = 0.01], stent thrombosis (RR = 1.63[1.13, 2.35]; p = 0.009) and target lesion revascularization (TLR) (RR = 1.29[1.02,1.63]; p = 0.03) in insulin-treated DM patients. Similarly, analysis of long-term follow-up studies depicted a significantly higher risk mortality (RR = 1.55 [1.22, 1.97]; p = 0.0003), MI (RR = 1.63 [1.35, 1.97]; p=<0.00001), MACE (R = 1.47 [1.31, 1.65]; p=<0.00001), stent thrombosis (RR = 1.54 [1.19,1.99]; p = 0.001), TLR (RR = 1.40 [1.18, 1.66]; p = 0.0001), target vessel revascularization (TVR) (RR = 1.35 [1.11, 1.64]; p = 0.003) in insulin-treated DM group after PCI versus non-insulin-treated DM patients.
Conclusion
Despite a tremendous technical success rate of multi-vessel stenting, people living with diabetes who were being treated with insulin had higher long-term, and short-term mortality rates, MI, TLR, TVR, and stroke compared to people living with diabetes who were being treated with means other than insulin and are more prone to detrimental cardiovascular outcomes.
Keywords: Cardiovascular outcomes, PCI, Insulin treated DM, Non-insulin-treated DM, Mortality, Major adverse cardiovascular events
Abbreviations
- TLR
Target lesion revascularization
- TVR
Target vessel revascularization
- TLF
Target lesion failure
- CABG
Coronary artery bypass graft
- PCI
Percutaneous intervention
- DM
Diabetes mellitus
- CV
Cardiovascular outcomes
1. Introduction
As the current consumer lifestyle is becoming increasingly sedentary, the world faces an epidemic of ‘diabetes mellitus (DM)’ with more than 171 million people affected worldwide.1 It is one of the most common chronic medical conditions known for its progressive nature; DM poses a severe public health challenge in the twenty-first century. Initially, DM is managed through dietary modification and oral hypoglycemic drugs; an intensification of therapy is generally required as the disease progresses over the years rendering insulin addition to the regimen a necessity.2 It is well-established that cardiovascular (CV) complications are the leading cause of morbidity and mortality in people living with diabetes; hence standard practice involves aggressive risk factor control, yet approximately 20–30% of patients require percutaneous coronary interventions (PCI) at some point during the disease.3
There is currently conflicting evidence about the severity of adverse CV outcomes following PCI in diabetic populations treated with insulin compared to those with diabetes who have not received insulin therapy.4, 5, 6 Several studies have shown that the risk of adverse outcomes like target lesion failure (TLF) and target lesion revascularization (TLR) after PCI is higher in insulin-treated people living with diabetes than in non-insulin-treated people living with diabetes.3,6 However, other studies have concluded that the increased risk of adverse cardiovascular (CV) outcomes is only present until propensity score has been adjusted. Moreover, the frequency of stent thrombosis and mortality rates after stent placement were also found to be comparable in both groups i.e. insulin-treated patients living with diabetes and non-insulin-treated patients living with diabetes.7,8
Therefore, with the aim to standardize the issue, conclude the current debate and, further evaluate the risk of wide range of CV events in insulin-treated versus non-insulin-treated patients living with diabetes, we assessed short-term (<1 year) and long-term (≥1 year) adverse CV outcomes after PCI in both the aforementioned groups. We also aimed at analyzing the protective effects of insulin in DM population undergoing PCI.The primary adverse CV outcomes were death, major adverse cardiac effects (MACE), TLR, TVR, myocardial infarction (MI), and stent thrombosis, while the secondary outcome was stroke.
2. Methods
2.1. Data sources and search strategy
The preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines were followed in this meta-analysis. All study types, including randomized controlled trials (RCTs), comparative studies, registries, cohort studies, and observational studies were searched electronically (without any language and time restrictions) on Pubmed and Embase, using the search string: ‘diabetes OR ‘insulin-treated diabetes mellitus OR non-insulin treated diabetes mellitus AND percutaneous coronary intervention/PCI OR PCI coronary stent AND clinical outcomes’ OR results'.
The term ‘angioplasty’ has also been used to enhance this search further. In addition, the reference list of retrieved trials, previous meta-analysis, and review articles was manually screened to identify any relevant studies.
2.2. Study selection
Inclusion criteria: Randomized controlled trials (RCTs), comparative studies, registries, cohort studies, and observational studies that reported occurrences of any adverse CV outcomes (including but not limited to death) for insulin-treated and non-insulin-treated DM patients after PCI, irrespective of the types of stents implanted, were eligible for inclusion in the studies (observational and RCTs). The studies reporting either a short-term follow-up (<1 year) or a long-term follow-up (≥1 year) after PCI were eligible for our analyses. Case reports, literature reviews, and studies not reporting comparable data for both groups were excluded.
Exclusion criteria: Studies in which adverse clinical outcomes were not among the clinical endpoints, were meta analysis, case studies, or letter to editors, were excluded. Studies in which the control group/non-insulin-treated DM patients were absent were not included. Studies that did not incorporate data with discontinuous variables or data which could easily be converted to discontinuous variables were not eligible. Duplicate studies were also removed.
2.3. Outcomes and definitions
Patients living with diabetes (Type 2 DM) were defined as patients with a fasting blood glucose (FBG) level of >7.0 mmol/L or with an oral glucose tolerance test (OGTT) level of >11.1 mmol/L at least on two separate occasions. DM patients were divided into insulin-treated (requiring insulin) and non-insulin-treated (not on insulin but may or may not be taking oral hypoglycemics) DM patients in this study.
Outcomes considered for the analyses were mortality, MACE, TLR, TVR, MI, stent thrombosis, target lesion failure (TLF), and need for-post PCI CABG. The primary study investigators’ definition was accepted for all outcomes. Ambiguous outcome terms have been elaborated.
Major adverse cardiac effects (MACEs) were a composite of death of cardiac or procedure-related origin, MI, and/or, revascularization after stents implantation. Target lesion revascularization (TLR) was defined as clinically indicated percutaneous or surgical revascularization of the index lesion, and target vessel revascularization (TVR) concerned the vessel affected. Target lesion failure (TLF) was a composite of clinically driven TLR, MI, or cardiac death related to the target vessel. If it could not be determined with certainty whether an MI or death is related to the target vessel, and at the same time if no other specific reasons can be given, it was considered as a case of TLF. Revascularization was clinically indicated if there was >70% diameter stenosis on angiography or >50% stenosis together with a positive stress test or ischemic symptoms.
2.4. Data extraction and assessment of study quality
All identified articles were exported to Endnote Reference Library X8.1 (Clarivate Analytics, Philadelphia, Pennsylvania) to remove duplicates. The articles were carefully assessed by two independent reviewers, and only those studies that met the eligibility were selected. Studies were narrowed down based on titles and abstracts, and full-texts were read for final inclusion. In case of any discrepancy, a consultation was carried out by a third party. Data related to baselines and outcomes were extracted in a predesigned form. The modified Cochrane Collaboration's risk of bias tool was used to assess the quality of published RCTs,9 observational studies were assessed using New Castle Ottawa scale.10
2.5. Statistical analysis
All statistical analyses were carried out by Review Manager v.5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The data were pooled using a random-effects model, and risk ratios (RRs) with 95% confidence intervals (CI) were reported along with forest plots. The chi-square test was performed to assess for differences between the subgroups. Heterogeneity across studies was evaluated using Higgins I2 statistics, and a value of 25%–50% was considered mild, 50%–75% as moderate, and >75% as severe heterogeneity. Potential causes of heterogeneity were explored by carrying out subgroup analysis. Visual inspection of the funnel plot and Begg's regression test were used to assess publication bias. A p-value <0.05 was considered significant in all cases.
2.6. Ethical approval
No approval was required from the ethical review board as this was an analysis of publicly available data.
3. Results
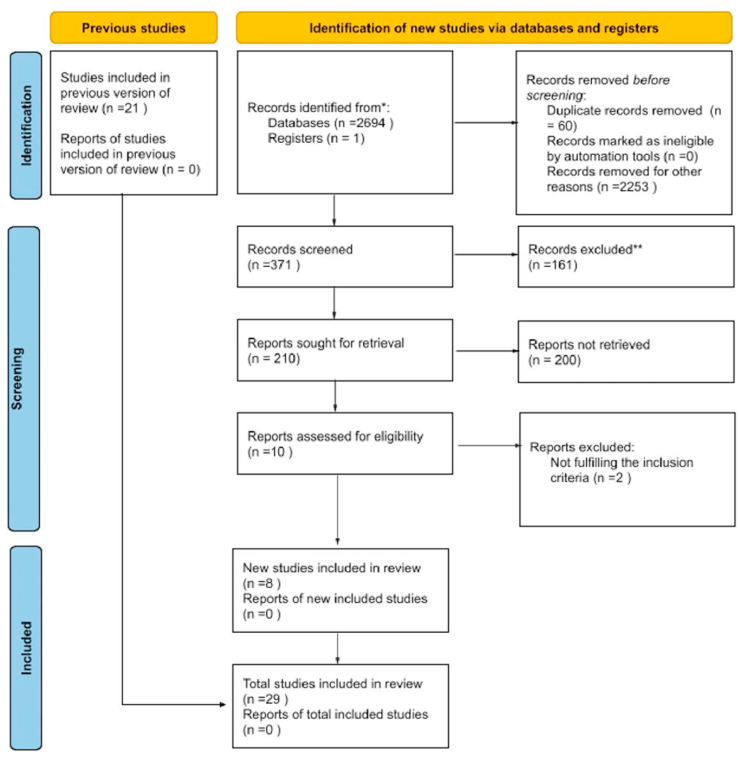
A total of 2695 articles were identified from the initial literature search. After the exclusion of duplicated articles and based on title and abstract, a total of 29 studies (18 observational and 11 RCTs) were included in this meta-analysis(Fig. 1).
Fig. 1.
Prisma Flow chart.
3.1. Baseline characteristics
The 29 studies included a total of 40,527 patients (11,742 in the Insulin-treated diabetes mellitus group and 28,785 in the non-insulin-treated DM group) who underwent PCI. Details about baseline characteristics, comorbidities, and previous surgeries according to types of study are given in Table 1, Table 2, Table 3, Table 4.
Table 1.
Baseline demographics of observational studies.
| Study and year | Study type | Study center | Follow up period | Total No. of patients (n) |
Mean Age (year) |
Male n (%) |
|||
|---|---|---|---|---|---|---|---|---|---|
| ITDM | Non-ITDM | ITDM | Non-ITDM | ITDM | Non-ITDM | ||||
| Biswas, S., 20216 | Pros.obs | Australia | 1 year | 1111 | 3468 | 65.2 ± 11.3 | 67.1 ± 11.2 | 741 (66.7) | 2531 (73.0) |
| Pepe, M., 201911 | Pros.obs | Italy | 1 year | 83 | 248 | 73.01 ± 9.7 | 69.2 ± 10.0 | 61 (73.5) | 188 (75.8) |
| Pi, S. H., 201812 | Pros.obs | Korea | 1 year | 617 | 1169 | 65.2 ± 9.6 | 65.2 ± 9.6 | 346 (56.1) | 848 (72.5) |
| Schofer, J., 200013 | Retr.obs | Germany | 6 months | 48 | 177 | 60 ± 9 | 62 ± 9 | 34 (71) | 136 (77) |
| Stien, B., 199514 | Pros.obs | USA | In-Hospital | 352 | 781 | 58 ± 11 | 60 ± 10 | 187 (53.1) | 516 (66.1) |
| Chandrasekhar, J., 201815 | Retr.obs | USA | 1 year | 2313 | 5737 | 64.86 ± 10.59 | 65.76 ± 10.62 | 1360 (58.7) | 3886 (67.7) |
| Tada, T., 201116 | Pros.obs | Japan | 3 years | 996 | 3404 | 66.7 | 67.9 | 667 (67) | 2587 (76) |
| Nakamura, M., 201017 | Pros.obs | Japan | 3 years | 200 | 647 | 66.2 | 67.2 | 13240 (66.2) | 488 (75.4) |
| Mulukutla, S. R., 200918 | Pros.obs | Pennsylvania | 1 year | 817 | 1749 | 63.5 | 64 | 414 (50.7) | 1076 (61.5) |
| Kumar, R., 200719 | Pros.obs | USA | 9 months | 115 | 182 | 62 | 67 | 71 (62) | 122 (67) |
| Mehran, R., 200420 | Pros.obs | USA | in hospital | 63 | 66 | 63 ± 11 | 66 ± 10 | 49 (77) | 51 (77) |
| Abizaid, A., 199821 | Retr.obs | Washington | For 1 year | 97 | 151 | 63 ± 12 | 63 ± 11 | 48 (49.5) | 96 (63.6) |
| Akin, I., 201022 | Pros.obs | Germany | 1 year | 581 | 1078 | 66.9 ± 9.4 | 66.6 ± 9.4 | 380 (65.4) | 809 (75) |
| Antoniucci, D., 200423 | Pros.obs | Italy | 6months | 84 | 82 | 69 ± 12 | 68 ± 11 | 55 (65) | 60 (73) |
| Jain, A. K., 201024 | Retr.obs | Multicenter | 12 months | 682 | 2039 | 66.57 ± 9.85 | 64.90 ± 1 0.23 | 424 (62.2) | 1463 (71.8) |
| Kuchukulanti, P. K., 201025 | Pros.obs | USA | 6 months | 265 | 586 | 65.1 | 65.1 | 160 (60.5) | 355 (60.5) |
| Konishi, Y., 201626 | Pros. obs | Tokyo | At 1 year | 199 | 575 | 68.3 ± 8.9 | 69.7 ± 9.4 | 121 (60.8) | 442 (76.9) |
Pros.obs: Prospective observational; Retr.obs: Retrospective observational.
Table 2.
Comorbidities of patients in observational studies.
| Study and year | Hypertension n (%) |
Dyslipidemia n (%) |
Chronic Kidney Disease n (%) |
Prior MI n (%) |
Prior PCI n (%) |
Smoker n (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITDM | Non-ITDM | ITDM | Non-ITDM | ITDM | Non-ITDM | ITDM | Non-ITDM | ITDM | Non-ITDM | ITDM | Non-ITDM | |
| Biswas, S., 2021 | 947 (85.3) | 2875 (83) | 930 (83.8) | 2833 (81.8) | 595 (54.7) | 2404 (71.1) | 485 (43.7) | 1161 (33.5) | 179 (16.6) | 578 (16.9) | ||
| Pepe, M., 2019 | 70 (84.3) | 216 (87.1) | 49 (59.0) | 157 (63.3) | 28 (33.7) | 41 (16.5) | 29 (34.9) | 60 (24.2) | 29 (34.9) | 68 (27.4) | 27 (32.5) | 97 (39.1) |
| Pi, S. H., 2018 | 475 (77.4) | 855 (73.3) | 298 (49.3) | 449 (38.6) | 330 (53.5) | 296 (25.6) | 64 (10.4) | 148 (12.7) | 124 (20.1) | 169 (14.5) | 110 (18.2) | 299 (25.8) |
| Schofer, J., 2000 | 35 (73) | 132 (75) | 31 (65) | 128 (72) | 24 (50) | 67 (38) | 6 (13) | 35 (20) | ||||
| Stien, B., 1995 | 200 (56.8) | 492 (63.0) | 145 (41.2) | 276 (35.3) | ||||||||
| Chandrasekhar, J., 2018 | 2260 (97.7) | 5573 (97.1) | 2238 (96.8) | 5577 (97.2) | 1060 (48.1) | 1648 (29.8) | 258 (11.2) | 720 (12.6) | ||||
| Tada, T., 2011 | 757 (76) | 2655 (78) | 285 (28.6) | 1026 (30.1) | 513 (51.5) | 1704 (50.1) | 159 (16) | 715 (21) | ||||
| Nakamura, M., 2010 | 136 (68) | 466 (72) | 116 (58) | 414 (64) | 87 (43.5) | 272 (42.0) | 24 (12.0) | 126 (19.5) | ||||
| Mulukutla S. R., 2009 | 693 (84.8) | 1452 (83) | 654 (80.0) | 1352 (77.3) | 368 (45.0) | 311 (17.80) | 572 (70.1) | 1062 (60.7) | 138 (16.9) | 339 (19.4) | ||
| Kumar, R., 2007 | 108 (94.0) | 169 (93.0) | 102 (89.0) | 167 (92.0) | 30 (26) | 30 (16) | 65 (57) | 72 (40) | 13 (11) | 15 (8) | ||
| Mehran, R., 2004 | 45 (71) | 44 (67) | 13 (20) | 9 (13) | 30 (48) | 36 (54) | 28 (45) | 20 (31) | 7 (11) | 5 (8) | ||
| Abizaid, A., 1998 | 71 (73) | 103 (68) | 58 (60) | 97 (64) | 53 (54.5) | 82 (54.5) | 60 (61.4) | 86 (57) | 48 (49.0) | 73.4 (48.6) | ||
| Akin, I., 2010 | 537 (92.4) | 1003 (93) | 471 (81.0) | 900 (83.50) | 145 (24.9) | 323 (30.0) | 198 (34.1) | 326 (30.2) | 280 (48.2) | 467 (43.3) | 87 (14.9) | 208 (19.3) |
| Antoniucci, D., 2004 | 34 (40) | 35 (43) | 25 (30) | 25 (30) | 14 (17) | 13 (15.9) | 17 (20) | 21 (26) | ||||
| Jain, A. K., 2010 | 560 (82.1) | 1580 (77.5) | 463 (67.9) | 1380 (67.7) | 44 (6.5) | 47 (2.3) | 244 (35.8) | 652 (32) | 200 (29.3) | 479 (23.5) | 95 (13.9) | 367 (18) |
| Kuchukulanti, P. K., 2010 | 236 (89) | 522 (89) | 235 (88.5) | 519 (88.5) | 42 (16) | 94 (16) | ||||||
| Konishi, Y., 2016 | 152 (76.4) | 446 (77.6) | 159 (79.9) | 487 (84.7) | 99 (49.8) | 139 (24.2) | 75 (37.7) | 172 (29.9) | 94 (47.2) | 217 (37.7) | 37 (18.6) | 108 (18.8) |
Table 3.
Baseline demographics of randomized controlled trials.
| Study and year | Study type | Study center | Follow up period | Total No. of patients (n) |
Mean Age (year) |
Male n (%) |
|||
|---|---|---|---|---|---|---|---|---|---|
| ITDM | Non-ITDM | ITDM | Non-ITDM | ITDM | Non-ITDM | ||||
| Bangalore, S., 20167 | RCT | Not specified | 1 year | 747 | 1083 | 58.52 ± 8.63 | 58.27 ± 9.52 | 530 (71.0) | 847 (78.2) |
| Witzenbichler, B., 201127 | RCT | Multicenter | At 1 year | 159 | 434 | 64.5 | 64.5 | 117 (73.4) | 319 (73.4) |
| Kappetein, A. P., 201328 | RCT | USA | 5 years | 89 | 142 | 65.4 | 65.4 | 63 (71) | 101 (71) |
| Beneduce, A., 20193 | RCT | Italy | 1 year | 113 | 372 | 68 ± 9 | 69 ± 9 | 82 (72.5) | 307 (82.5) |
| Kalkman, D. N., 201729 | RCT | Netherlands | 1 year | 64 | 117 | 64.8 ± 9.4 | 68.6 ± 9.4 | 43 (67.2) | 86 (72.9) |
| Stone, G. W., 201130 | RCT | USA | 2 years | 494 | 1375 | 63.8 | 63.8 | 312 (63.2) | 869 (63.2) |
| Dangas, G. D. 201431 | RCT | Not specified | 5 years and 1 month | 602 | 1248 | 62.55 ± 9.2 | 63.25 ± 9 | 368 (61.1) | 954 (76.4) |
| Hermiller, J. B, 200432 | RCT | Not specified | 1 year | 105 | 213 | 62.2 | 62.2 | 67 (63.5) | 135 (63.5) |
| Kereiakes, D. J, 201033 | RCT | Not specified | 12 months | 314 | 826 | 63.3 | 63.3 | 199 (63.3) | 523 (63.3) |
| Kirtane, A. J., 200934 | RCT | USA | 1 year | 137 | 319 | 64 | 64 | 83 (60.4) | 193 (60.4) |
| Kirtane, A. J. 20088 | RCT | Not specified | 4 years | 265 | 562 | 63 | 63 | 171 (64.7) | 364 (64.7) |
| Moussa, I., 200435 | RCT | USA | During 1 year | – | – | – | – | – | – |
RCT: Randomized controlled trial.
Table 4.
Comorbidities of patients in randomized controlled trials.
| Study and year | Hypertension n (%) |
Dyslipidemia n (%) |
Chronic Kidney Disease n (%) |
Prior MI n (%) |
Prior PCI n (%) |
Smoker n (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITDM | Non-ITDM | ITDM | Non-ITDM | ITDM | Non-ITDM | ITDM | Non-ITDM | ITDM | Non-ITDM | ITDM | Non-ITDM | |
| Bangalore,S.,2016 | 490 (65.6) | 727 (67.1) | 569 (76.2) | 843 (77.8) | 277 (37.1) | 465 (42.9) | 82 (11.0) | 63 (5.8) | 92 (12.3) | 181 (16.7) | ||
| Witzenbichler, B., 2011 | 115 (72.3) | 314 (72.3) | 96 (60.3) | 262 (60.3) | 27 (16.7) | 72 (16.7) | 26 (16.5) | 72 (16.5) | 90 (56.8) | 247 (56.8) | ||
| Kappetein, A. P., 2013 | 62 (70) | 99 (70) | 73 (82) | 116 (82) | 29 (32) | 45 (32) | 14 (16) | 22.7 (16) | ||||
| Beneduce, A., 2019 | 100 (88) | 301(81) | 90 (24) | 258 (69) | 38 (34) | 78 (21) | 35 (31) | 97 (26) | 60 (53) | 186 (50) | 19 (17) | 108 (29) |
| Kalkman, D. N., 2017 | 51 (79.7) | 94 (79.7) | 51 (79.7) | 80 (67.8) | 10 (15.6) | 15 (12.7) | 23 (35.9) | 38 (32.2) | 26 (40.6) | 42 (35.6) | 12 (18.8) | 20 (16.9) |
| Stone, G. W., 2011 | 411 (83.1) | 1143 (83.1) | 392 (79.4) | 1092 (79.4) | 97 (19.6) | 270 (19.6) | ||||||
| Dangas, G. D. 2014 | 175 (29.0) | 166 (13.3) | 308 (51.1) | 644 (51.6) | 217 (36) | 362 (29) | ||||||
| Hermiller, J. B, 2004 | 85 (81.1) | 173 (81.1) | 75 (71) | 151 (71) | ||||||||
| Kereiakes, D. J, 2010 | 273 (87) | 719 (87) | 259 (82.5) | 681 (82.5) | 57 (18.3) | 151 (18.3) | ||||||
| Kirtane, A. J., 2009 | 240 (90.6) | 509 (90.6) | 231 (87.1) | 490 (87.1) | 143 (54.1) | 304 (54.1) | ||||||
| Kirtane, A. J. 2008 | 112 (82.1) | 262 (82.1) | 101 (74) | 236 (74) | 25 (18.4) | 59 (18.4) | ||||||
| Moussa, I., 2004 | – | – | – | – | – | – | – | – | – | – | – | – |
3.2. Assessment of baseline characteristics
When baseline characteristics were pooled, we found a significant probability of non-insulin treated DM patients being males (RR = 0.89[0.87, 0.92]; p=<0.00001) and smokers (RR = 0.89[0.83.0.97]; p = 0.006). However, for insulin-treated DM patients we found a significant probability of comorbidities like chronic kidney disease (RR = 1.17[1.09, 1.27]; p=<0.00001), previous MI (RR = 1.11[1.03, 1.20]; p = 0.008) and previous PCI (RR = 1.17[1.06, 1.30]; p = 0.003. All the remaining characteristics were insignificant between both groups. Differences in key baseline between insulin-treated DM patients and non-insulin-treated DM patients undergoing PCI are represented in (Table 5).
Table 5.
Pooled baseline demographics comparing insulin-treated DM group versus non-insulin-treated DM group.
| Baseline Characteristics | Insulin-treated | Non-insulin-treated | Insulin-treated DM vs non-insulin-treated DM (95% CI) | p-value |
|---|---|---|---|---|
| Age (mean ± SD) | 62.3 ± 26.5 | 62.8 ± 51.2 | WMD = −0.59 [-1.22, 0.030] | 0.06 |
| Male | 11742 | 28785 | RR = 0.89 [0.87, 0.92] | <0.00001 |
| Hypertension | 11559 | 28785 | RR = 1.01 [0.98, 1.04] | 0.65 |
| Dyslipidemia | 9791 | 23352 | RR = 1.00 [0.98, 1.02] | 0.91 |
| Chronic Kidney disease | 4028 | 10541 | RR = 1.17 [1.09, 1.27] | <0.0001 |
| Prior Myocardial Infarction | 7619 | 18520 | RR = 1.11 [1.03, 1.20] | 0.008 |
| Prior PCI | 4407 | 10645 | RR = 1.17 [1.06, 1.30] | 0.003 |
| Smoker | 11222 | 5422 | RR = 0.89 [0.83, 0.97] | 0.006 |
DM, Diabetes millets; RR, relative risks; WMD, weighted mean difference; CI, confidence interval.
3.3. Quality assessment and publication bias
The quality assessment of studies using the New Castle Ottawa scale depicted a significantly low risk of bias in all the included observational studies (Supplementary Table S1). Assessment of RCTs by Cochrane tool showed fair to good quality results (Supplementary Table S2). The funnel plots showed no publication bias for both short and long-term follow-up outcomes (Supplementary Figure S1 and S2), which was confirmed by Begg's test. The details of Begg's test for all outcomes is given in (Table 6).
Table 6.
Results of Begg's test of publication bias for short and long term outcomes.
| Outcomes | Begg's p- value for short term | Begg's p-value for short term |
|---|---|---|
| Mortality | 0.458 | 0.547 |
| MI | 0.404 | 0.054 |
| MACE | 0.573 | 0.411 |
| TLR | 0.091 | 0.951 |
| TVR | 0.089 | 0.788 |
| TVF | – | 0.174 |
| Stent thrombosis | 0.142 | 0.681 |
| CABG | 0.117 | 0.602 |
| Stroke | – | 0.117 |
MI: Myocardial infarction; MACE: Major adverse cardiac effects; TLR: Target lesion revascularization, TVR: Target vessel revascularization, TLF: Target lesion failure, CABG: coronary artery bypass grafting.
3.4. Cardiovascular outcomes of PCI
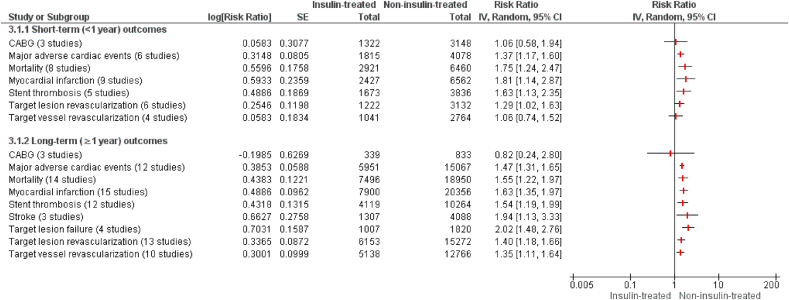
The results of all meta-analyses have been summarized in Fig. 2.
Fig. 2.
Short and long term follow-up cardiovascular outcomes of insulin-treated DM group versus non-insulin-treated DM group undergoing percutaneous intervention (PCI).
3.4.1. Short-term (<1 year)
Short-term outcomes were reported in 12 studies. The pooled analysis of short term follow up outcomes (<1 year) preceding PCI demonstrated a significantly higher risk of mortality (RR = 1.75[1.24,2.47]; p = 0.002), MI (RR = 1.81[1.14,2.87]; p = 0.01], MACE (RR = 1.37[1.17,1.60]; p = 0.001), stent thrombosis (RR = 1.63[1.13,2.35]; p = 0.009) and TLR (RR = 1.29[1.02,1.63]; p = 0.03) in insulin-treated DM patients as compared to non-insulin-treated DM group. Conversely, no significant differences were observed between both the groups in the risk of CABG (RR = 1.06 [0.58, 1.94]; p = 0.84) and TVR (RR = 1.06 [0.74, 1.52]; p = 0.75) following PCI. The results of short term outcomes are illustrated in Fig. 2. Supplementary Table S3 and supplementary Figure S3 contain individual outcome results for short-term.
3.4.2. Long-term (≥1 year)
Similarly, the pooled analysis of long-term follow-up (≥1 year) studies depicted a significantly higher risk of cardiovascular events in the insulin-treated DM group versus the non-insulin-treated DM group except post-PCI need for CABG. The risk for mortality (RR = 1.55 [1.22, 1.97]; p = 0.0003), MI (RR = 1.63 [1.35, 1.97]; p=<0.00001), MACE (R = 1.47 [1.31, 1.65]; p=<0.00001), stent thrombosis (RR = 1.54 [1.19,1.99]; p = 0.001), TLR (RR = 1.40 [1.18, 1.66]; p = 0.0001), TVR (RR = 1.35 [1.11, 1.64]; p = 0.003), TLF (RR = 2.02 [1.48, 2.76]; p=<0.00001) and stroke (RR = 1.94 [1.13, 3.33]; p = 0.02) were significantly higher in insulin-treated DM group after PCI as compared to non-insulin-treated DM patients. However, no significant distinction was discerned in both the groups in the risk of post-PCI CABG (RR = 0.82 [0.24, 2.80]; p = 0.74). The results for the long term outcomes have been illustrated in Fig. 2. Supplementary Table S4 and Supplementary Figure S4 contain individual outcome results for long-term.
3.5. Subgroup analysis
Subgroup analysis was performed to check whether Bifurcation lesion and American Heart Association (ACC/AHA) class B2/C lesion influence the results produced. The individual angiographic characteristics for each study are given in Supplementary Table S5. It was noted that no significant difference was observed in any CV outcomes among the subgroups (with and without bifurcation lesion; with and without class B2/C lesion) except that risk of TVR was significantly higher in patients having ACC/AHA class B2/C lesion (RR = 1.28[1.08, 1.51]; p = 0.02). The details of other subgroup analyses are given in Table 7.
Table 7.
Subgroup analysis by the presence of Bifurcation lesion for cardiovascular outcomes after PCI.
| Outcomes | Bifurcation lesion |
p value subgroups | I2 (%) | ACC/AHA lesion B2/BC |
p value subgroups | I2 (%) | ||
|---|---|---|---|---|---|---|---|---|
| With Bifurcation lesion |
Without Bifurcation lesion |
With ACC/AHA lesion B2/BC |
Without ACC/AHA lesion B2/BC |
|||||
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |||||
| All-cause mortality | 1.69 [1.37, 2.08] | 1.65 [1.40, 1.94] | 0.85 | 0 | 1.80 [1.46, 2.21] | 1.64 [1.38, 1.96] | 0.52 | 0 |
| Myocardial Infarction (MI) | 1.76 [1.33, 2.33] | 1.44 [1.17, 1.78] | 0.26 | 19.9 | 1.78 [1.31, 2.42] | 1.42 [1.21, 1.66] | 0.20 | 37.9 |
| Major adverse cardiac effects (MACEs) | 1.45 [1.14, 1.84] | 1.46 [1.32, 1.61] | 0.97 | 0 | 1.44 [1.23, 1.69] | 1.41 [1.24, 1.61] | 0.83 | 0 |
| Stent thrombosis | 1.66 [1.29, 2.13] | 1.35 [0.90, 2.02] | 0.39 | 0 | 1.57 [1.09, 2.26] | 1.57 [1.21, 2.03] | 0.98 | 0 |
| Target lesion failure (TLF) | 2.25 [1.33, 3.80] | 2.00 [1.21, 3.31] | 0.75 | 0 | 2.44 [1.57, 3.79] | 1.69 [1.09, 2.61] | 0.25 | 25.0 |
| Target vessel revascularization (TVR) | 1.46 [1.05, 2.02] | 1.20 [0.97, 1.49] | 0.33 | 0 | 1.46 [1.23, 1.75] | 1.03 [0.81, 1.31] | 0.02 | 81.4 |
| Target lesion revascularization (TLR) | 1.53 [1.35, 1.74] | 1.23 [0.93, 1.63] | 0.16 | 49.6 | 1.63 [1.38, 1.93] | 1.28 [1.03, 1.59] | 0.09 | 66.1 |
| Coronary Artery Bypass Graft (CABG) | 1.30 [0.71, 2.38] | 0.72 [0.27, 1.92] | 0.31 | 1.3 | 0.71 [0.31, 1.60] | 1.04 [0.43, 2.51] | 0.53 | 0 |
3.6. Propensity matched/adjusted data analysis
Adjusted short-term mortality and MACE were reported by two studies only. However, a significant difference in mortality was observed between insulin-treated DM group and non-insulin-treated DM group (aOR = 1.78 [1.28, 2.49]; p = 0.0007). Whereas, for short-term MACE, no significant difference was observed between both the groups (aOR = 1.84 [0.93, 3.64]; p = 0.08). The forest plots of these outcomes are illustrated in Supplementary Figure S5.
Three studies reported adjusted long-term mortality, and a significant difference in mortality was observed between insulin-treated DM group and non-insulin-treated DM group (aHR = 1.46 [1.15, 1.86]; p = 0.002). However no significant differences were observed between both the groups in adjusted long-term MI (aHR = 1.13 [0.75, 1.71]; P = 0.58), adjusted long-term MACE (aHR = 1.02 [0.96, 1.09]; p = 0.45) and adjusted long-term stent thrombosis (aHR = 1.56 [0.87.2.80]; p = 0.14). Moreover, adjusted long-term TLR was reported in three studies which demonstrated a significant difference between the insulin-treated DM group and non-insulin-treated DM group (aHR = 1.46 [1.15,1.86]; p = 0.002). The forest plots of these outcomes are shown in Supplementary Figure S6.
4. Discussion
Our meta-analysis consisting of 29 comparative studies, in essence, demonstrated the following main findings: (i) Treatment with insulin was associated with higher rates of mortality, MI, MACE, stent thrombosis, TLR, TVR, and stroke on both short-term and long-term basis; (ii) There was no significant difference in the need for post-PI CABG between the two groups; and (iii) The presence of bifurcation lesions or class B2/C lesions had no significant effect on the cardiovascular outcomes post-PCI. These findings are consistent with the previous meta-analyses.4
Considerable efforts have been made to understand the reasons behind this significantly higher rate of adverse CV outcomes in insulin-treated DM patients after PCI. Firstly, insulin-treated DM patients have worse clinical outcomes regardless of the treatment regimen, either due to more advancing disease in these patients or an adverse effect of this insulin therapy.31 This impression is backed by our analysis which shows patients with insulin-treated DM had a significantly higher rate of comorbidities like MI, hypertension, dyslipidemia, chronic kidney disease, and previous PCI history. Typically, insulin therapy is implemented in a more advanced stage of diabetes.36 Therefore, a higher rate of detrimental outcomes should be foreseen in these complicated patients after PCI. By the same token, it has been seen that although insulin controls diabetes-induced hyperglycemia, it can also boost pro-inflammatory response by macrophages and stimulate hormonal activation of signal transduction pathways, thus accelerating the progression of atherogenesis by disturbing the balance between the synthesis and release of endothelial mediators.37,38 Apart from this, studies show that treatment with insulin has been associated with increased platelet aggregation,19 thus contributing to the higher rates of stent re-thrombosis in patients treated with insulin. Another reason that can be linked to the increased risk of adverse outcomes in insulin-treated DM patients is obesity leading to treatment-resistant diabetes and the greater prevalence of family history of coronary as well as peripheral arterial disease in these patients.34
Similar to this meta-analysis, previously, several meta-analyses of large-scale trials have shown that patients with insulin-treated DMare associated with worse adverse cardiovascular outcomes than patients with non-insulin-treated T2DM following PCI.39,4 A meta-analysis of 21 studies published by Bundhun et al comparing the adverse outcomes in patients with insulin-treated DM and non-insulin-treated T2DM showed that both short-term and long-term adverse outcomes were significantly more likely ininsulin-treated DM subgroup following PCI.4 In concordance to that, another study showed that adverse outcomes after PCI were significantly higher in the long-term follow-up period compared to short-term follow-up in patients with ITDM.38
Nevertheless, a few studies have published results that were different from this meta-analysis. A study conducted by Zhuo et al showed that although the risk of stent thrombosis was higher in short-term follow-up, it was not significantly higher in long-term follow-up period in patients with insulin-treated DM.1 Another study concluded that the risk of stent thrombosis did not differ significantly across the groups.40
A study published by Beneduce et al showed that the rate of TLR and TLF in patients with insulin-treated DM and non-insulin-treated T2DM were comparable. However, ITDM patients showed higher rates of cardiac deaths.3 Similarly, a meta-analysis conducted in patients with insulin-treated DM found that mortality associated with cardiac causes was significantly higher than non-cardiac related mortality following PCI procedure.39
Moreover, a study that followed participants over an 11-year follow-up found that the rate of worse outcomes of PCI was higher in diabetic women than in diabetic men. Thus, treatment with insulin might not be the only reason for the worse outcomes seen in patients living with diabetes following PCI.41
Compared to the earlier studies, the strength of this study is the performance of subgroup analysis and the increment in the number of the outcomes as well a larger sample size, making results more robust. Nevertheless, there are a few limitations to this study. Firstly, the size of the population of the diabetic group was small in all individual studies. Secondly, like the previous meta-analysis3 non-insulin-treated T2DM patients had patients on different oral medications; the difference in the dose and medication class could have contributed to some undesirable heterogeneity. In addition to these limitations, we found great variability in the definition of MACE in the studies included in our analysis as well as other similar studies. This disparity in definition may have led to inaccurate determination of the concerned outcome. Finally, our study may have been affected by the factor that different stents were used on patients who were observed during the study.
As insulin-treated DM patients showed a significant increase in both short-term and long-term adverse outcomes following PCI, it is imperative for cardiothoracic surgeons to be vigilant. This necessitates an introduction of a pre-procedure protocol to rule out high-risk insulin-treated DM patients and minimize any risk factor that could precipitate an adverse outcome after the procedure. Alternatively, the increment in adverse outcomes faced by insulin-treated DM patients also warrants the need to devise secondary treatment strategies to replace PCI. Additionally, to increase the validity of the current findings, further studies are required to be done with similar kinds of stents being used in the study population and an equal number of patients across study groups i.e., insulin-treated DM patients and non-insulin-treated T2DM patients.
5. Conclusion
Insulin-treated DM patients showed a significant rise in short-term as well as long-term adverse outcomes following PCI, compared to non-insulin-treated DM patients indicating that PCI, otherwise a highly successful procedure, entails a poor prognosis in the diabetic population treated with insulin. Careful pre and post-PCI assessments are warranted for insulin-treated DM patients to reduce the risk of adverse CV outcomes.
Funding
None.
Declaration of competing interest
The authors declared no conflict of interests.
Acknowledgments
None.
Footnotes
All authors have approved and proofread the manuscript and gone through the tables and figures. All authors accept that all material in the manuscript is correct to the best of their knowledge.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2021.12.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhuo X., Zhang C., Feng J., et al. In-hospital, short-term and long-term adverse clinical outcomes observed in patients with type 2 diabetes mellitus vs non-diabetes mellitus following percutaneous coronary intervention: a meta-analysis including 139,774 patients. Medicine (Baltimore) 2019;98(8) doi: 10.1097/MD.0000000000014669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laimer M., Jenni S., Stettler C. Insulintherapiebei Typ-2-Diabetes: ein Review vom «Wann» übers «Wie» biszum «Warum». Praxis (Bern 1994) 2015;104(4):181–185. doi: 10.1024/1661-8157/a001924.PMID:25669222. [DOI] [PubMed] [Google Scholar]
- 3.Beneduce A., Ferrante G., Ielasi A., et al. One-year clinical outcome of biodegradable polymer sirolimus-eluting stent in diabetic patients: insight from the ULISSE registry (ULtimaster Italian multicenter all comerS Stent rEgistry) Catheter Cardiovasc Interv. 2020;96(2):255–265. doi: 10.1002/ccd.28694. [DOI] [PubMed] [Google Scholar]
- 4.Bundhun P.K., Li N., Chen M.-H. Adverse cardiovascular outcomes between insulin-treated and non-insulin treated diabetic patients after percutaneous coronary intervention: a systematic review and meta-analysis. Cardiovasc Diabetol. 2015;14(1):135. doi: 10.1186/s12933-015-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu S., Wang B., Liu W., Wu C., Huang J. The effects of insulin therapy on mortality in diabetic patients undergoing percutaneous coronary intervention. Ann Transl Med. 2021;9(16):1294. doi: 10.21037/atm-21-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswas S., Dinh D., Andrianopoulos N., et al. Comparison of long-term outcomes after percutaneous coronary intervention in patients with insulin-treated versus non-insulin treated diabetes mellitus. Am J Cardiol. 2021 doi: 10.1016/j.amjcard.2021.02.025. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 7.Bangalore S., Bhagwat A., Pinto B., et al. Percutaneous coronary intervention in patients with insulin-treated and non-insulin-treated diabetes mellitus: secondary analysis of the TUXEDO trial. JAMA Cardiol. 2016;1(3):266–273. doi: 10.1001/jamacardio.2016.0305. [DOI] [PubMed] [Google Scholar]
- 8.Kirtane A.J., Ellis S.G., Dawkins K.D., et al. Paclitaxel-eluting coronary stents in patients with diabetes mellitus: pooled analysis from 5 randomized trials. J Am Coll Cardiol. 2008;51(7):708–715. doi: 10.1016/j.jacc.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Higgins J.P., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. Published 2011 Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells G.A., Tugwell P., O'Connell D., et al. 2015. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Retrieved from. [Google Scholar]
- 11.Pepe M., Sardella G., Stefanini G.G., et al. Impact of insulin-treated and noninsulin-treated diabetes mellitus in all-comer patients undergoing percutaneous coronary interventions with polymer-free biolimus-eluting stent (from the RUDI-FREE registry) Am J Cardiol. 2019;124(10):1518–1527. doi: 10.1016/j.amjcard.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Pi S.H., Rhee T.M., Lee J.M., et al. Outcomes in patients with diabetes mellitus according to insulin treatment after percutaneous coronary intervention in the second-generation drug-eluting stent era. Am J Cardiol. 2018 Jun 15;121(12):1505–1511. doi: 10.1016/j.amjcard.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 13.Schofer J., Schlüter M., Rau T., et al. Influence of treatment modality on angiographic outcome after coronary stenting in diabetic patients: a controlled study. J Am Coll Cardiol. 2000 May;35(6):1554–1559. doi: 10.1016/s0735-1097(00)00574-x. [DOI] [PubMed] [Google Scholar]
- 14.Stein B., Weintraub W.S., Gebhart S.S., et al. Influence of diabetes mellitus on early and late outcome after percutaneous transluminal coronary angioplasty. Circulation. 1995 Feb 15;91(4):979–989. doi: 10.1161/01.cir.91.4.979. [DOI] [PubMed] [Google Scholar]
- 15.Chandrasekhar J., Dangas G., Baber U., et al. Impact of insulin treated and non-insulin-treated diabetes compared to patients without diabetes on 1-year outcomes following contemporary PCI. Cathet Cardiovasc Interv. 2020 Aug;96(2):298–308. doi: 10.1002/ccd.28841. [DOI] [PubMed] [Google Scholar]
- 16.Tada T., Kimura T., Morimoto T., et al. Comparison of three-year clinical outcomes after sirolimus-eluting stent implantation among insulin-treated diabetic, non–insulin-treated diabetic, and non-diabetic patients from j-Cypher registry. Am J Cardiol. 2011 Apr 15;107(8):1155–1162. doi: 10.1016/j.amjcard.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura M., Yokoi H., Hamazaki Y., et al. Impact of insulin-treated diabetes and hemodialysis on long-term clinical outcomes following sirolimus-eluting stent deploy- ment. Insights from a sub-study of the Cypher Stent Japan Post-Market- ing Surveillance (Cypher J-PMS) Registry. Circ J. 2010;74(12):2592–2597. doi: 10.1253/circj.cj-10-0179. [DOI] [PubMed] [Google Scholar]
- 18.Mulukutla S.R., Vlachos H.A., Marroquin O.C., et al. Impact of drug-eluting stents among insulin-treated diabetic patients: a report from the national Heart, lung, and blood institute dynamic registry. JACC Cardiovasc Interv. 2008;1(2):139–147. doi: 10.1016/j.jcin.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar R., Lee T.T., Jeremias A., et al. Compari-son of outcomes using sirolimus-eluting stenting in diabetic versus non- diabetic patients with comparison of insulin versus non-insulin therapy in the diabetic patients. Am J Cardiol. 2007;100(8):1187–1191. doi: 10.1016/j.amjcard.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 20.Mehran R., Dangas G.D., Kobayashi Y., et al. Short- and long-term results after multivessel stenting in diabetic patients. J Am Coll Cardiol. 2004;43(8):1348–1354. doi: 10.1016/j.jacc.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Abizaid A., Kornowski R., Mintz G.S., et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol. 1998;32:584–589. doi: 10.1016/j.jjcc.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Akin I., Bufe A., Eckardt L., et al. Compari-son of outcomes in patients with insulin-dependent versus non-insulin dependent diabetes mellitus receiving drug-eluting stents (from the first phase of the prospective multicenter German DES.DE registry) Am J Cardiol. 2010;106(9):1201–1207. doi: 10.1016/j.amjcard.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 23.Antoniucci D., Valenti R., Migliorini A., et al. Impact of insulin requiring diabetes mellitus on effectiveness of reperfusion and outcome of patients undergoing primary percutane- ous coronary intervention for acute myocardial infarction. Am J Cardiol. 2004;93(9):1170–1172. doi: 10.1016/j.amjcard.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 24.Jain A.K., Lotan C., Meredith I.T., et al. Twelvemonth outcomes in patients with diabetes implanted with a zotarolimus eluting stent: results from the E-Five Registry. Heart. 2010;96(11):848–853. doi: 10.1136/hrt.2009.184150. 34. Kereiakes DJ, Cutlip DE, Applegate RJ, Wang J, Yaqub M, Sood P, et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuchulakanti P.K., Chu W.W., Torguson R., et al. Sirolimus-eluting stents versus Paclitaxel-eluting stents in the treatment of coronary artery disease in patients with diabetes mellitus. Am J Cardiol. 2006;98(2):187–192. doi: 10.1016/j.amjcard.2006.01.074. [DOI] [PubMed] [Google Scholar]
- 26.Konishi Y., Ashikaga T., Sasaoka T., et al. Comparison of outcomes after everolimus-eluting stent implantation in diabetic versus non-diabetic patients in the Tokyo-MD PCI study. J Cardiol. 2016 Mar 1;67(3):241–247. doi: 10.1016/j.jjcc.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Witzenbichler B., Mehran R., Guagliumi G., et al. Impact of diabetes mellitus on the safety and effectiveness of bivalirudin in patients with acute myocardial infarction undergoing primary angioplasty: analysis from the HORIZONS-AMI (Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2011 Jul;4(7):760–768. doi: 10.1016/j.jcin.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Kappetein A.P., Head S.J., Morice M.-C., et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing out- comes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardio Thorac Surg. 2013;43:1006–1013. doi: 10.1093/ejcts/ezt017. [DOI] [PubMed] [Google Scholar]
- 29.Kalkman D.N., Woudstra P., Den Heijer P., et al. One-year clinical outcomes in patients with insulin-treated diabetes mellitus and non-insulin-treated diabetes mellitus compared to non-diabetics after deployment of the bio-engineered COMBO stent. Int J Cardiol. 2017 Jan 1;226:60–64. doi: 10.1016/j.ijcard.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 30.Stone G.W., Kedhi E., Kereiakes D.J., et al. Differential clinical responses to everolimus-eluting and Paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011 Aug 23;124(8):893–900. doi: 10.1161/CIRCULATIONAHA.111.031070. Epub 2011 Aug 8. [DOI] [PubMed] [Google Scholar]
- 31.Dangas G.D., Farkouh M.E., Sleeper L.A., et al. Long-term outcome of PCI versus CABG in insulin and non-insulintreated diabetic patients: results from the FREEDOM trial. J Am Coll Cardiol. 2014;64(12):1189–1197. doi: 10.1016/j.jacc.2014.06.1182. [DOI] [PubMed] [Google Scholar]
- 32.Hermiller J.B., Raizner A., Cannon L., et al. Outcomes with the polymer-based paclitaxel-eluting TAXUS stent in patients with diabetes mellitus: the TAXUS-IV trial. J Am Coll Cardiol. 2005;45(8):1172–1179. doi: 10.1016/j.jacc.2004.10.075. [DOI] [PubMed] [Google Scholar]
- 33.Kereiakes D.J., Cutlip D.E., Applegate R.J., et al. Outcomes in diabetic and nondiabetic patients treated with everolimusor paclitaxel-eluting stents: results from the SPIRIT IV clinical trial (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) J Am Coll Cardiol. 2010;56(25):2084–2089. doi: 10.1016/j.jacc.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Kirtane A.J., Patel R., O'Shaughnessy C., et al. Clinical and angiographic outcomes in diabetics from the ENDEAVOR IV trial: randomized comparison of zotarolimus- and paclitaxel-eluting stents in patients with coronary artery disease. JACC Cardiovasc Interv. 2009;2(10):967–976. doi: 10.1016/j.jcin.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Moussa I., Leon M.B., Baim D.S., et al. Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated BxVelocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation. 2004;109(19):2273–2278. doi: 10.1161/01.CIR.0000129767.45513.71. [DOI] [PubMed] [Google Scholar]
- 36.Elezi S., Kastrati A., Pache J., et al. Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement. J Am Coll Cardiol. 1998;32(7):1866–1873. doi: 10.1161/01.CIR.0000109693.64957.20. [DOI] [PubMed] [Google Scholar]
- 37.Muniyappa R., Montagnani M., Koh K.K., Quon M.J. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 38.Potenza M.A., Addabbo F., Montagnani M. Vascular actions of insulin with implications for endothelial dysfunction. Am J Physiol Endocrinol Metab. 2009 Sep;297(3):E568–E577. doi: 10.1152/ajpendo.00297.2009. Epub 2009 Jun 2. [DOI] [PubMed] [Google Scholar]
- 39.Wang Q., Liu H., Ding J. Cardiac versus non-cardiac related mortality following percutaneous coronary intervention in patients with insulin-treated type 2 diabetes mellitus: a meta-analysis. Diabetes Ther. 2018;9(3):1335–1345. doi: 10.1007/s13300-018-0444-y. [Free PMC article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li N., Yang Y.G., Chen M.H. Comparing the adverse clinical outcomes in patients with non-insulin treated type 2 diabetes mellitus and patients without type 2 diabetes mellitus following percutaneous coronary intervention: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:238. doi: 10.1186/s12872-016-0422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-de-Andres A., Jimenez-García R., Hernandez-Barrera V., et al. National trends in utilization and outcomes of coronary revascularization procedures among people with and without type 2 diabetes in Spain (2001–2011) Cardiovasc Diabetol. 2014;13:3. doi: 10.1186/1475-2840-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.