Abstract
A hallmark pathological feature of the neurodegenerative diseases amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) is the depletion of RNA-binding protein TDP-43 from the nucleus of neurons in the brain and spinal cord1. A major function of TDP-43 is as a repressor of cryptic exon inclusion during RNA splicing2–4. Single nucleotide polymorphisms in UNC13A are among the strongest hits associated with FTD and ALS in human genome-wide association studies5,6, but how those variants increase risk for disease is unknown. Here we show that TDP-43 represses a cryptic exon-splicing event in UNC13A. Loss of TDP-43 from the nucleus in human brain, neuronal cell lines and motor neurons derived from induced pluripotent stem cells resulted in the inclusion of a cryptic exon in UNC13A mRNA and reduced UNC13A protein expression. The top variants associated with FTD or ALS risk in humans are located in the intron harbouring the cryptic exon, and we show that they increase UNC13A cryptic exon splicing in the face of TDP-43 dysfunction. Together, our data provide a direct functional link between one of the strongest genetic risk factors for FTD and ALS (UNC13A genetic variants), and loss of TDP-43 function.
Subject terms: RNA splicing, Amyotrophic lateral sclerosis, Neurodegeneration, Transcriptomics
TDP-43 controls an exon splicing event in UNC13A that results in the inclusion of a cryptic exon associated with frontotemporal dementia and amyotrophic lateral sclerosis.
Main
TDP-43, encoded by the TARDBP gene, is an abundant, ubiquitously expressed RNA-binding protein that normally localizes to the nucleus. It has a role in fundamental RNA-processing activities, including RNA transcription, alternative splicing and RNA transport7. A major splicing regulatory function of TDP-43 is to repress the inclusion of cryptic exons during splicing2,8–10. Unlike normal conserved exons, these cryptic exons occur in introns and are normally excluded from mature mRNAs. When TDP-43 is depleted from cells, these cryptic exons get spliced into messenger RNAs, often introducing frame shifts and premature termination, or even reduced RNA stability. However, the key cryptic splicing events that are integral to disease pathogenesis remain unknown.
STMN2—which encodes stathmin 2, a regulator of microtubule stability—is the gene whose expression is most significantly reduced when TDP-43 is depleted from neurons3,4. STMN2 harbours a cryptic exon (exon 2a) that is normally excluded from the mature STMN2 mRNA. The first intron of STMN2 contains a TDP-43 binding site. When TDP-43 is lost or its function is impaired, exon2a gets incorporated into the mature mRNA. Exon 2a harbours a stop codon and a polyadenylation signal—this results in truncated STMN2 mRNA and eightfold reduction3 of stathmin 2. Aberrant splicing and reduced stathmin 2 levels seem to be a major feature of sporadic and familial cases of ALS (except those with SOD1 mutations)3,4 and in frontotemporal lobar degeneration (FTLD) due to TDP-43 proteinopathy11 (FTLD-TDP). The discovery of STMN2 cryptic exon splicing in ALS and FTLD-TDP highlights a key mRNA target—we aimed to identify other possible mRNA targets.
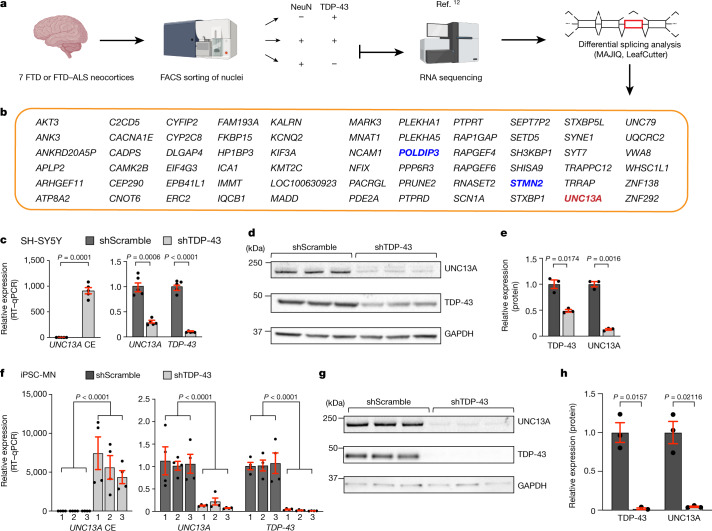
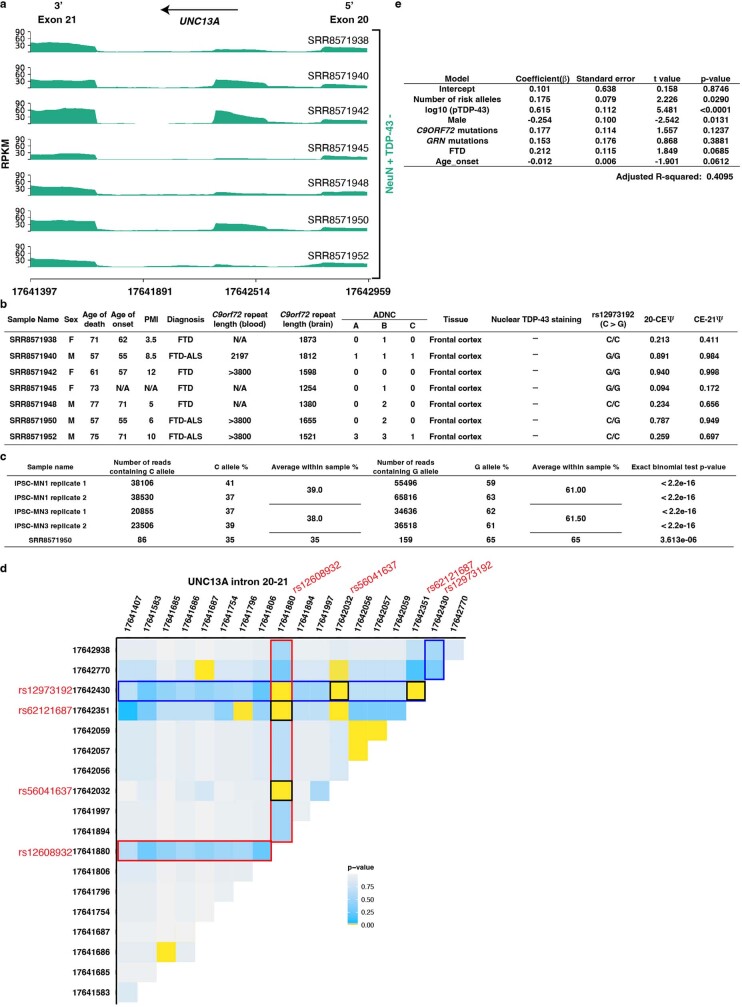
To discover cryptic splicing targets regulated by TDP-43 that may also have a role in disease pathogenesis, we used a recently generated RNA sequencing (RNA-seq) dataset12. To generate this dataset, fluorescence-activated cell sorting (FACS) was used to enrich neuronal nuclei with and without TDP-43 from postmortem brain tissue from patients with FTD and ALS (FTD–ALS); RNA-seq was performed to compare the transcriptomic profiles of TDP-43-positive and TDP-43-negative neuronal nuclei. This identified several differentially expressed genes12. We re-analysed the data to identify novel alternative splicing events affected by the loss of nuclear TDP-43. We performed splicing analyses using two pipelines, MAJIQ13 and LeafCutter14, designed to detect novel splicing events (Fig. 1a). We identified 266 alternative splicing events (P(ΔΨ > 0.1) > 0.95; where ΔΨ signifies changes of local splicing variations between two conditions) with MAJIQ and 152 with LeafCutter (P < 0.05). There were 66 alternatively spliced genes in common between the two analyses (Fig. 1b), probably because each tool uses different definitions for transcript variations and different criteria to control for false positives (Supplementary Note 1). These genes have at least one region that both tools identified to be alternatively spliced (Supplementary Table 1). Among the alternatively spliced genes identified by both tools were STMN2 and POLDIP3, both of which have been extensively validated as bona fide targets of splicing by TDP-433,4,11,15.
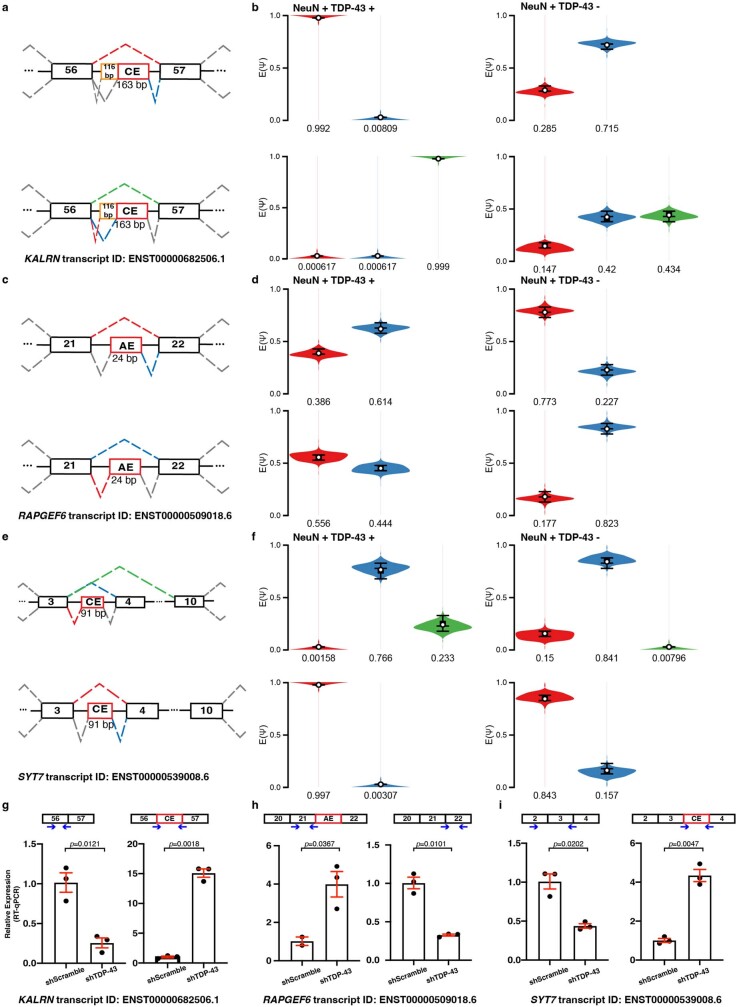
Fig. 1. Nuclear depletion of TDP-43 causes CE inclusion in UNC13A RNA and reduced expression of UNC13A protein.
a, Splicing analyses were performed on RNA-sequencing results from TDP-43-positive and TDP-43-negative neuronal nuclei isolated from frontal cortices of seven patients with FTD or FTD–ALS. Some illustrations were created with BioRender.com. b, Sixty-six alternatively spliced genes identified by both MAJIQ (P(ΔΨ > 0.1) > 0.95) and LeafCutter (P < 0.05). Genes in blue are previously validated TDP-43 splicing targets3,4,11,15. c, f, RT–qPCR confirmed inclusion of CE in UNC13A mRNA upon TDP-43 depletion in SH-SY5Y cells (n = 5 cell culture experiments for each condition; two sided-Welch two-sample t-test; mean ± s.e.m.) (c) and in 3 independent lines of iPSC-MNs (n = 2 independent cell culture experiments, each with 2 technical replicates for each iPSC-MN) (f). RPLP0 was used to normalize RT–qPCR. Three-way ANOVA; mean ± s.e.m. TDP-43 is also known as TARDBP. d, e, g, h, Immunoblotting for UNC13A and TDP-43 protein levels in SH-SY5Y cells (d; quantified in e) and iPSC-MNs (g; quantified in h) treated with scramble (shScramble) or TDP-43 shRNA (n = 3 independent cell culture experiments for each condition). GAPDH served as a loading control. Two-sided Welch two-sample t-test, mean ± s.e.m. Gel source data are shown in Supplementary Fig. 1a, b.
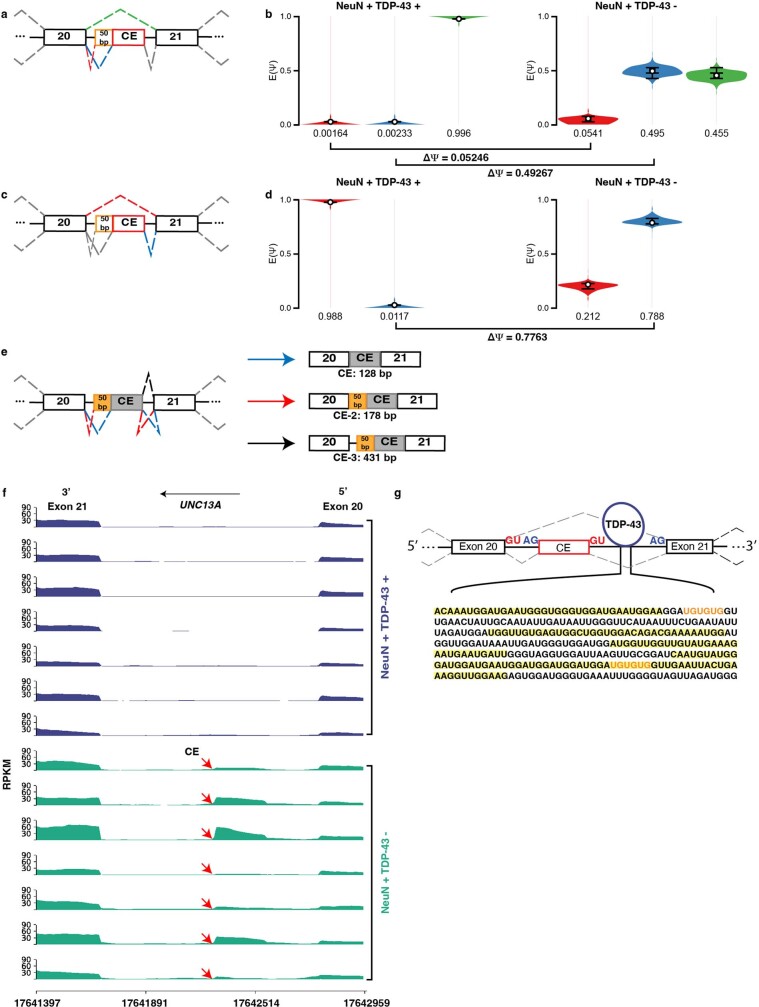
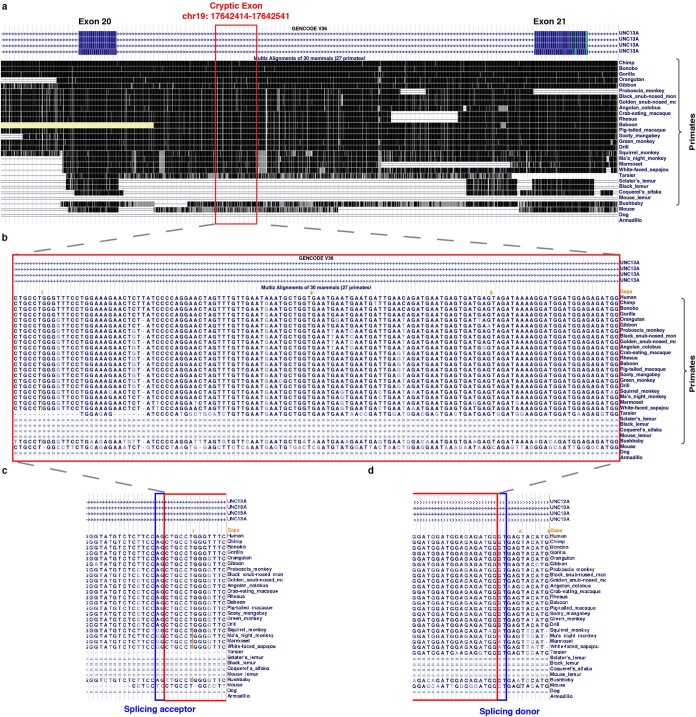
UNC13A was one of the genes with the most significant levels of alternative splicing (MAJIQ ΔΨ = 0.779; LeafCutter ΔΨ = 0.8360; P < 0.0001) in neurons with nuclear TDP-43 depletion (Fig. 1b). Depletion of TDP-43 resulted in the inclusion of a 128-bp or a 178-bp cryptic exon between the canonical exons 20 and 21 (hg38; chr19: 17642414–17642541 (128bp); chr19: 17642591–17642414 (178 bp)) (Extended Data Fig. 1a–e, Supplementary Note 2). The two cryptic exons share the same 3′ end but the 178-bp cryptic exon is 50 bp longer than the 128-bp cryptic exon at the 5′ end. The cryptic exons were almost completely absent in wild-type neuronal nuclei (Fig. 1f) and are not present in any of the known human isoforms of UNC13A16. Furthermore, analysis of ultraviolet cross-linking and immunoprecipitation (iCLIP) data for TDP-4317 provides evidence that TDP-43 binds directly to the intron harbouring these cryptic exons (shown by mapped reads) (Extended Data Fig. 1g). Because of the much higher abundance of the 128-bp cryptic exon in the RNA-seq data that we analysed, we focused our analyses on this cryptic exon, which we refer to as CE. Intron 20–21 of UNC13A and the CE sequence are conserved among most primates (Extended Data Fig. 2a, b) but not in mouse (Extended Data Fig. 2c, d), similar to STMN2 and other cryptic splicing targets of TDP-432–4. Together, these results suggest that TDP-43 functions to repress the inclusion of a cryptic exon in the UNC13A mRNA.
Extended Data Fig. 1. Splicing analysis using MAJIQ demonstrates inclusion of cryptic exon between exon 20 and exon 21 of UNC13A.
(a, b) Depletion of TDP-43 introduces two alternative 3′ splicing acceptors in the intron 20–21: one is near chr19:17642591(ΔΨ = 0.05246) and the other one is near chr19:17642541(ΔΨ = 0.49267). (c, d) An alternative 5′ splicing donor is also introduced near chr19:17642414 (ΔΨ = 0.7763). See Supplementary Note 2. (a, c) Splice graphs showing the inclusion of the cryptic exon (CE) between exon 20 and exon 21 of UNC13A. (b, d) Violin plots corresponding to (a and c) respectively. Each violin in (b and d) represents the posterior probability distribution of the expected relative inclusion (PSI or Ψ) for the color matching junction in the splice graph. The tails of each violin represent the 10th and 90th percentile. The box represents the interquartile range with the line in the middle indicating the median. The white circles mark the expected PSI (E[Ψ]). The change in the relative inclusion level of each junction between two conditions is referred to as ΔΨ or ΔPSI13. (e) The three versions of cryptic exons resulting from the loss of TDP-43. (f) Visualization of RNA-sequencing alignment between exon 20 and exon 21 in UNC13A (hg38). Libraries were generated as described in Fig. 1a. CE, cryptic exon. (g) iCLIP for TDP-43 (from Tollervey et al.17) indicates that TDP-43 binds to intron 20–21. The sequence shown is an example of a region in intron 20-21 that is frequently bound by TDP-43 (shown by mapped reads from ERR039843, ERR039845 and ERR039855).
Extended Data Fig. 2. Intron 20-21 of UNC13A is conserved among most primates.
The Primates Multiz Alignment & Conservation track on UCSC49 genome browser (http://genome.ucsc.edu) includes 30 mammals, 27 of which are primates. (a) Exon 20 and exon 21 of UNC13A is well conserved among mammals. The location of the 128 bp cryptic exon is highlighted in red. However, intron 20-21 (a), the cryptic exon (b), and the splicing acceptor site (highlighted in blue) upstream of the cryptic exon (c) and splicing donor site (highlighted in blue) downstream of the cryptic exon (d) are only conserved in primates.
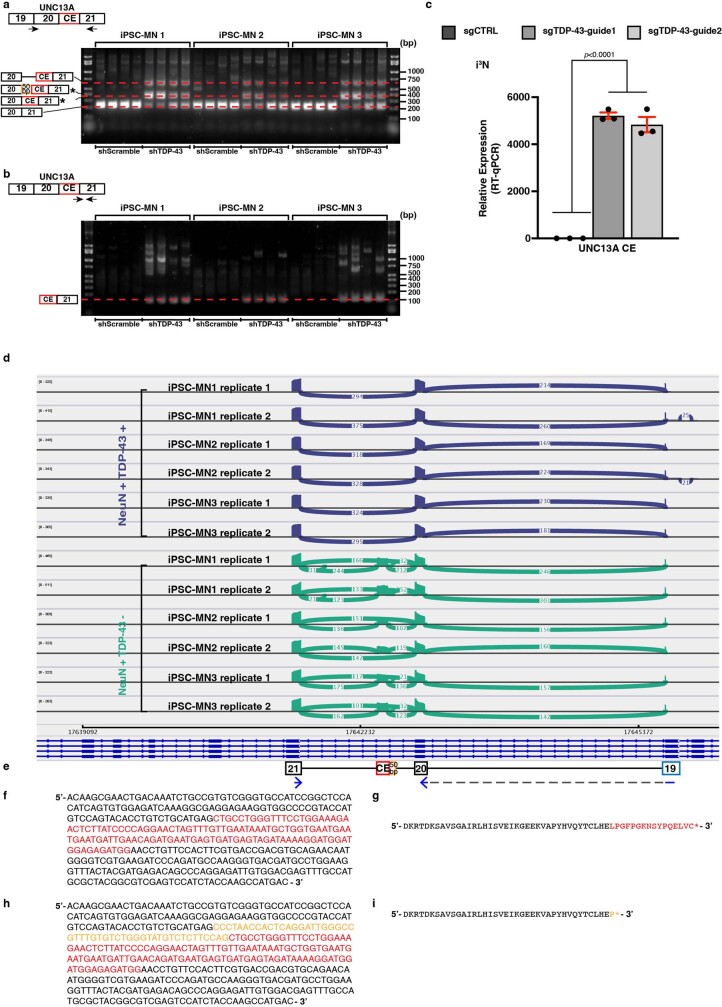
To determine whether TDP-43 directly regulates this UNC13A cryptic splicing event, we used short hairpin RNA (shRNA) to reduce TDP-43 levels in SH-SY5Y cells and quantitative PCR with reverse transcription (RT–qPCR) to detect CE inclusion in UNC13A transcript. CE was present in cells with TDP-43 depletion but not in cells treated with control shRNA (Fig. 1e). Along with the increase in CE, there was a corresponding decrease in the amount of the canonical UNC13A transcript upon TDP-43 depletion (Fig. 1c). By immunoblotting, we also observed a marked reduction in UNC13A protein in TDP-43-depleted cells (Fig. 1d, e). Reducing levels of TDP-43 in motor neurons derived from induced pluripotent stem cells (iPSC-MNs) (Fig. 1f–h, Extended Data Fig. 3a, b, Supplementary Table 3) and excitatory neurons (i3Ns) derived from human induced pluripotent stem (iPS) cells (Extended Data Fig. 3c) also resulted in CE inclusion and a reduction in UNC13A mRNA and protein. We confirmed insertion of the cryptic exon sequences into the mature transcript by amplicon sequencing of the product of reverse transcription with PCR (RT–PCR) (Extended Data Fig. 3d; see Supplementary Note 2) and demonstrated that the insertions introduce premature stop codons (Extended Data Fig. 3f–i), consistent with the observed decrease in UNC13A protein. UNC13A gene expression is probably regulated at multiple levels beyond simply inclusion of the cryptic exons. Other aspects of the cryptic exon-inclusion event (for example, aberrant peptides produced from it) could cause defects, although we do not yet have evidence that such peptides are produced. Thus, lowering levels of TDP-43 in human cells and neurons causes inclusion of CE in the UNC13A transcript, resulting in decreased UNC13A protein.
Extended Data Fig. 3. Depletion of TDP-43 from iPSC derived motor neurons (iPSC-MNs) and iPSC derived neurons (i3Ns) leads to cryptic exon inclusion in UNC13A.
(a) RT-PCR confirmed the expression of the cryptic exon-containing UNC13A splice variant upon TDP-43 depletion in three independent iPSC-MNs (n = 4 independent cell culture experiments for each condition). Gel picture shows results from all 4 experiments performed. In addition to the splice variant containing the 128 bp and 178 bp cryptic exons, we also detected inclusion of the complete intron upstream of the cryptic exon (Fig. 4e, Supplementary Note 2). The 128 bp and 178 bp cryptic exons cannot be distinguished here but they are detected through amplicon sequencing the corresponding band (d). The PCR products represented by each band are marked to the left of each gel. The location of the PCR primer pair used is shown on top of each gel image. (b) The PCR primer pairs spanning the cryptic exon and exon 21 junction confirms cryptic exon inclusion only occurs upon TDP-43 knockdown. For gel source data, see Supplementary Fig. 1c, d. (c) RT-qPCR analyses confirmed the inclusion of UNC13A cryptic exon upon TDP-43 depletion in iPSC derived neurons (i3Ns). TDP-43 was depleted by expressing two different sgRNAs: sgTDP-43-guide1 and sgTDP-43-guide2 in i3Ns stably expressing CRISPR inactivation machinery (CRISPRi). RPLP0 and GAPDH were used to normalize RT-qPCR. (n = 3 independent cell culture experiments for each condition; Ordinary one-way ANOVA with Dunnett’s multiple comparisons test, mean ± s.e.m.). (d) Sashimi plot visualization of the alignment (hg38) of the amplicon (2 × 250 bp) sequencing reads of the sequences amplified using primers (blue) shown in (e). Both the 128 bp and the 178 bp cryptic exons were supported by the sequencing reads. (e) Schematic of the exons amplified by the primers (blue). (f, h) DNA sequence of the 128 bp and 178 bp cryptic exons and their flanking exons. The sequences are color coded according to (e). (g, i) The amino acid sequences correspond to the DNA sequences in (f, h). The asterisks indicate stop codons are encountered.
UNC13A belongs to a family of genes originally discovered in Caenorhabditis elegans and was named on the basis of the uncoordinated (unc) movements exhibited by animals with mutations in these genes18, owing to deficits in neurotransmitter release. UNC13A encodes a large multidomain protein that is expressed in the nervous system, where it localizes to most synapses in the central nervous system and neuromuscular junctions, and has an essential role in the vesicle priming step, prior to synaptic vesicle fusion19–22. UNC13A is an essential neuronal protein because mice lacking Unc13a (also called Munc13-1) exhibit functional deficits at glutamatergic synapses, demonstrated by a lack of fusion-competent synaptic vesicles, and die within a few hours of birth21. Our data suggest that depletion of TDP-43 leads to loss of this critical synaptic protein. As well as UNC13A, several other genes encoding synaptic proteins are mis-spliced upon TDP-43 depletion (Fig. 1b). We validated the splicing events in three of these genes (KALRN, RAPGEF6 and SYT7) in induced pluripotent stem (iPS) cell-derived neurons (iNs) using RT–qPCR (Extended Data Fig. 4), providing evidence that disruption of synaptic function could be a major mechanism in the pathogenesis of ALS and FTD.
Extended Data Fig. 4. Validation of additional splicing targets.
(a–f) Depletion of TDP-43 introduces cryptic exons into KALRN mRNA and SYT7 mRNA; In RAPGEF6, TDP-43 depletion leads to a decrease in the usage of the exon AE (for alternative exon) between exon 21 and exon 22 of the isoform ENST0000509018.6. Exon AE does exist in some other isoforms of RAPGEF6, indicating the depletion of TDP-43 could lead to changes in isoform composition. (b, d, f) Violin plots corresponding to (a, c, e), respectively. Each violin in (b, d, f) represents the posterior probability distribution of the expected relative inclusion (PSI or Ψ) for the color matching junction in the splice graph. The tails of each violin represent the 10th and 90th percentile. The box represents the interquartile range with the line in the middle indicating the median. The white circles mark the expected PSI (E[Ψ]). The change in the relative inclusion level of each junction between two conditions is referred to as ΔΨ or ΔPSI13. (g–i) RT-qPCR analyses confirmed changes in exon usage upon TDP-43 depletion in iPSC-derived neurons. RPLP0 and GAPDH were used to normalize RT-PCR. (n = 3 independent cell culture experiments for each condition, two sided-Welch Two Sample t-test, mean ± s.e.m.).
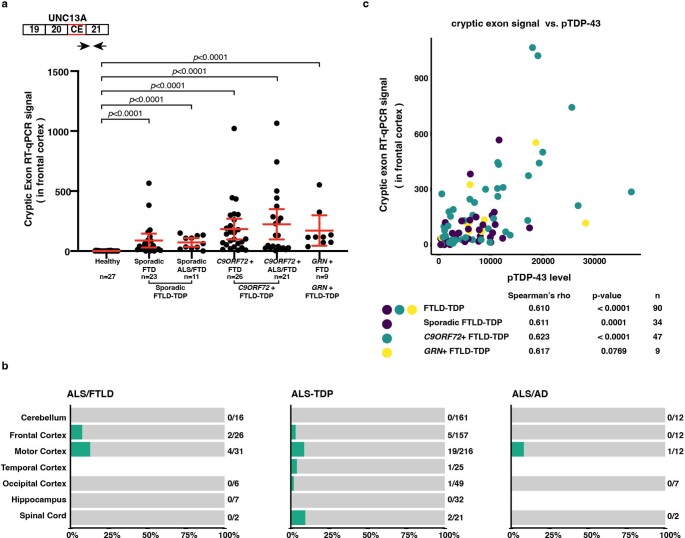
To extend our analysis of UNC13A cryptic exon inclusion to a larger collection of patient samples, we first analysed a series of 117 frontal cortex brain samples from the Mayo Clinic Brain Bank using RT–qPCR and a pair of primers that detects the shared 3′ end of the cryptic exons. We found a significant increase in UNC13A cryptic exon in the frontal cortices of patients with FTLD-TDP compared with healthy controls (Fig. 2a, Extended Data Fig. 5a). Next, we analysed brain samples from the New York Genome Center (NYGC). We interrogated RNA-seq data from 1,151 tissue samples from 413 individuals (with multiple tissues per individual; see Supplementary Note 3), 330 of whom are patients with ALS or FTD. We detected UNC13A splice variants in nearly 50% of the frontal and temporal cortical tissues donated by patients with neuropathologically confirmed FTLD-TDP (Fig. 2b) and in some of the patients with ALS whose pathology has not been confirmed (Extended Data Fig. 5b). Notably, we did not observe UNC13A splice variants in any of the samples from patients with FTLD associated with FUS (FTLD-FUS) (n = 9) or TAU (FTLD-TAU) (n = 18) or ALS associated with SOD1 (ALS-SOD1) (n = 22), nor in any of the control samples (n = 197) (Fig. 2b). Using the same criteria, we detected the known STMN2 cryptic exon3,4 in tissues from these patients, and the majority of the UNC13A splice variant containing tissues also contained the STMN2 splice variant (Supplementary Table 2). Hyperphosphorylated TDP-43 (pTDP-43) is a key feature of the pathology of these diseases1. We found a strong association between higher levels of pTDP-43 and higher levels of UNC13A CE inclusion in patients with FTLD-TDP (Spearman’s ρ = 0.610, P < 0.0001) (Extended Data Fig. 2c). Thus, UNC13A CE inclusion is a robust and specific facet of pathobiology in TDP-43 proteinopathies.
Fig. 2. UNC13A CE inclusion in human TDP-43 proteinopathies.
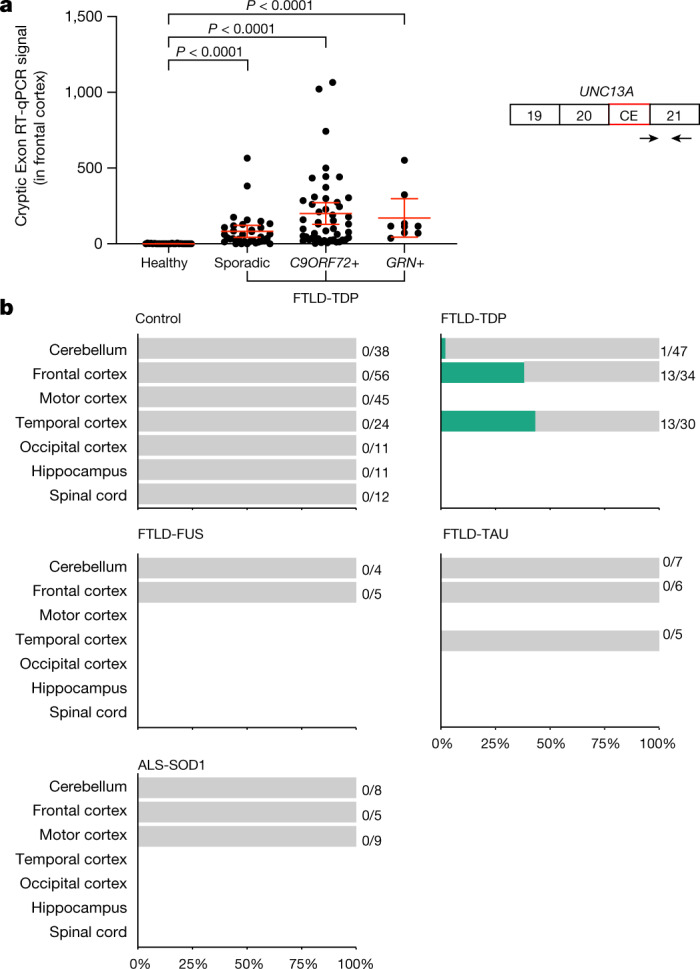
a, UNC13A CE expression level is increased in the frontal cortices of patients with FTLD-TDP. GAPDH and RPLP0 were used to normalize the RT–qPCR (two-tailed Mann–Whitney test, mean ± 95% confidence interval). The schematic to the right shows the localization of the primer pair (arrows) used for the RT–qPCR assay. Healthy: n = 27; sporadic FTLD-TDP: n = 34; C9ORF72+ FTLD-TDP: n = 47; GRN+ FTLD-TDP: n = 9. b, UNC13A CE is detected in nearly 50% of frontal cortical tissues and temporal cortical tissues from neuropathologically confirmed FTLD-TDP patients in bulk RNA-sequencing from the NYGC ALS Consortium cohort. CE is absent in tissues from healthy controls and patients with FTLD-FUS, FTLD-TAU or ALS-SOD1.
Extended Data Fig. 5. UNC13A cryptic exon inclusion is detected in disease relevant tissues of FTLD-TDP, ALS/FTLD, ALS-TDP and ALS/AD patients, and is correlated with phosphorylated TDP-43 levels in frontal cortices of FTLD-TDP patients.
(a) UNC13A cryptic exon expression level is significantly increased in the frontal cortices of patients with FTD and ALS/FTD clinical diagnoses (Mayo Clinic Brain Bank). GAPDH and RPLP0 were used to normalize RT-qPCR (the sample size of each group is listed under the corresponding group; two-tailed Mann-Whitney test, mean ± 95% confidence interval). The schematic on top shows the localization of the primer pair (arrows) used for the RT-qPCR assay. (b) UNC13A splice variants are observed in ALS patients with unconfirmed pathology. ALS-FTLD refers to patients who have concurrent FTD and ALS. ALS patients were categorized based on whether they carry SOD1 mutations (ALS-SOD1 (Fig. 2b) vs. ALS-TDP). ALS-AD refers to ALS patients with suspected Alzheimer’s disease. The diagnoses of these patients (NYGC) are not neuropathologically confirmed. Therefore, it is unclear whether TDP-43 mislocalization is present. (c) UNC13A cryptic exon signal is positively correlated with phosphorylated TDP-43 levels in frontal cortices of FTLD-TDP patients in Mayo Clinic Brain Bank (Spearman’s rho = 0.610, n = 90, p-values were calculated by one-sided t-test). Data points are colored according to patients’ reported genetic mutations. The correlation within each genetic mutation group and the corresponding p-value and sample size is also shown.
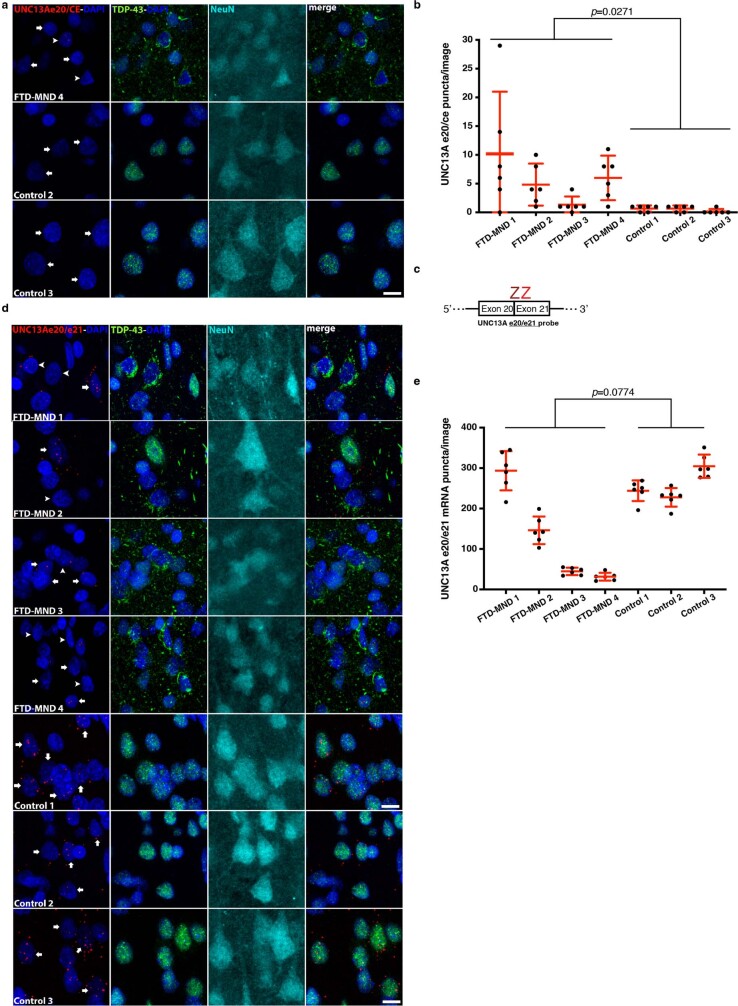
To visualize the UNC13A CE within single cells in the human brain, we designed custom BaseScope in situ hybridization probes that specifically bind to the exon 20–CE junction (Fig. 3a) or the exon 20–exon 21 junction (Extended Data Fig. 6c). We used these probes for in situ hybridization, combined with immunofluorescence for NeuN (to detect neuronal nuclei) and TDP-43. We stained sections from the medial frontal pole of four patients with FTLD-TDP and three controls (Supplementary Table 4). In neurons showing loss of nuclear TDP-43 and accompanying cytoplasmic TDP-43 inclusions, we observed UNC13A CE-containing mRNA splice variants in the nucleus (Fig. 3b, Extended Data Fig. 6a). We observed between one and four CE-containing mRNA puncta per nucleus. We did not observe puncta in the cytoplasm, perhaps because CE introduces a premature stop codon, which could lead to nonsense-mediated decay. Controls, however, had universally normal nuclear TDP-43 staining and showed no evidence of UNC13A cryptic splicing (Fig. 3b, Extended Data Fig. 6b). Next, we sought to determine whether TDP-43 nuclear depletion is associated with reduced expression of canonical UNC13A mRNA. In control brain tissue, UNC13A mRNA was widely expressed in neurons across cortical layers (Extended Data Fig. 6d, e). In patients, we saw a trend for reduced UNC13A mRNA in neurons showing TDP-43 pathology compared with neighbouring neurons with normal nuclear TDP-43 (Extended Data Fig. 6d, e), consistent with the RT–qPCR data (Fig. 1c, f). These findings suggest that cryptic splicing of UNC13A is absent from controls and, in patients, is seen exclusively in neurons showing depleted nuclear TDP-43.
Fig. 3. UNC13A cryptic splicing is associated with loss of nuclear TDP-43 in patients with FTD and motor neuron disease.
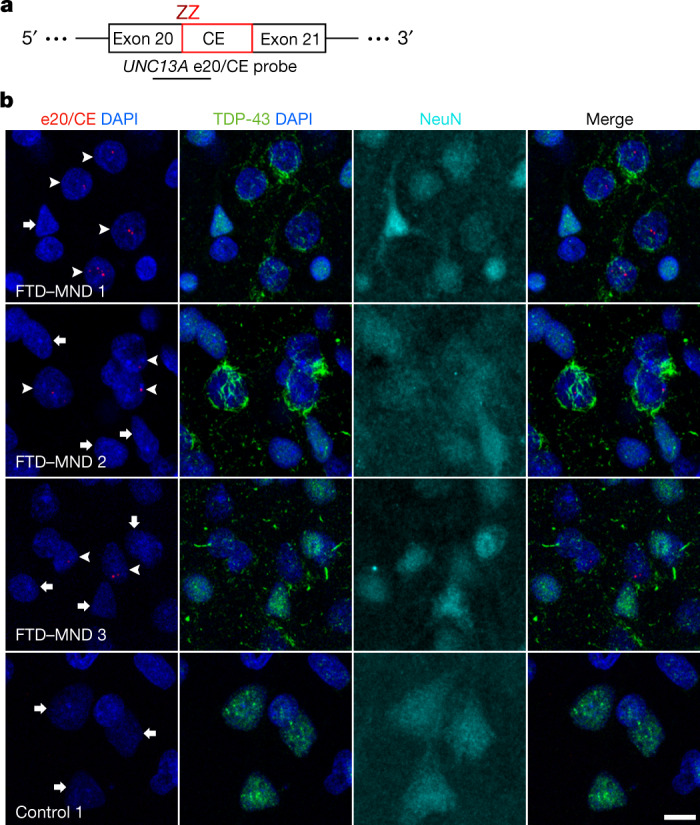
a, The design of the UNC13A e20/CE BaseScope probe targeting the alternatively spliced UNC13A transcript. Each Z binds to the transcript independently, and both must be in close proximity for successful signal amplification, ensuring binding specificity. b, BaseScope in situ hybridization using the UNC13A e20/CE probe, combined with immunofluorescence for TDP-43 and NeuN, was performed on sections from the medial frontal pole of patients with FTD and motor neuron disease (FTD–MND) and healthy controls. Representative images illustrate the presence of UNC13A CE (arrowheads) in neurons showing depletion of nuclear TDP-43. Neurons with normal nuclear TDP-43 in patients and controls show no CE signal (arrows). Images are maximum intensity projections of a confocal image z-stack. Scale bar, 10 µm. Images representative of six non-overlapping images from each individual. We optimized UNC13A probes on two cases and two controls in three separate experiments, with similar findings.
Extended Data Fig. 6. UNC13A cryptic splicing is associated with loss of nuclear TDP-43 in patients with FTD and motor neuron disease (MND).
(a) Additional patients and control subjects used in the study (but not shown in Fig. 3), demonstrating UNC13A cryptic splicing. Scale bar equals 10 µm. (b) Quantification of UNC13A cryptic exon BaseScope™ in situ hybridization. Six non-overlapping Z-stack images from layer 2–3 of medial frontal pole were captured, per subject, using a 63X oil objective and flattened into a maximum intensity projection image. Puncta counts per image were derived using the “analyze particle” plugin in ImageJ. Each data point represents the number of UNC13A cryptic exon puncta in a single image. Cryptic exon quantity varies between patients but always exceeds the technical background of the assay, as observed in controls. (n = 6 non-overlapping Z-stack images; Linear mixed model, mean ± s.d.). (c) The design of the UNC13A e20/e21 BaseScope™ probe targeting canonical UNC13A transcript. Each “Z” binds to the transcript independently. Both “Z”s must be in close proximity for successful signal amplification, ensuring binding specificity. (d) Representative images showing expression of UNC13A mRNA in layer 2–3 neurons from the medial frontal pole using the probe shown in (b). UNC13A mRNA expression is restricted to neurons (arrows) and is decreased in cells exhibiting TDP-43 nuclear depletion. Arrowheads represent neurons with loss of nuclear TDP-43 and accompanying cytoplasmic inclusions, and arrows indicate neurons with normal nuclear TDP-43. Images are maximum intensity projections of a confocal image Z-stack. Scale bar equals 10 µm. (e) Quantification of UNC13A mRNA BaseScope™ in situ hybridization. UNC13A mRNA puncta were quantified as described in (b). Each data point represents the number of UNC13A mRNA puncta in a single image. This suggests some variability in UNC13A mRNA levels potentially attributable to technical or biological factors. More importantly, the control UNC13A mRNA levels suggest that failure to detect UNC13A cryptic exons in controls is not due to nonspecific RNA degradation. There is variability of UNC13A mRNA detected per sample but we observe a trend of reduced UNC13A mRNA in patient samples compared to controls. (Linear mixed model, mean ± s.d.). The final BaseScope™ experimental run was performed once involving all the cases and controls. Six non-overlapping images were captured from each individual, and representative images are shown. UNC13A probes were first optimized by testing them on 2 cases and 2 controls in 3 separate pilot experiments, showing similar findings.
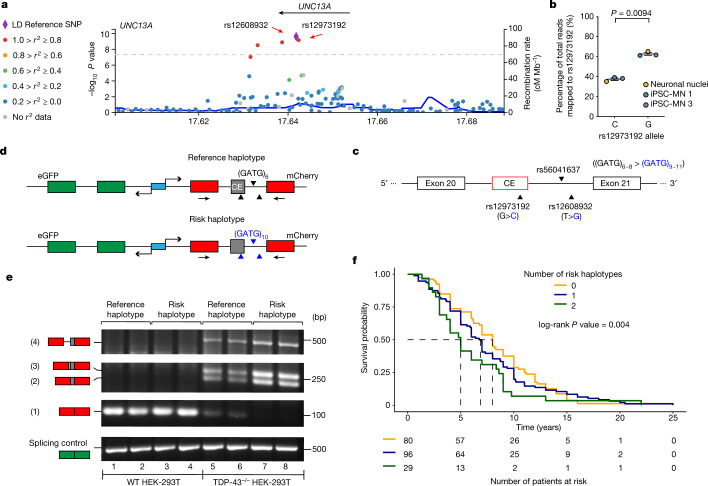
UNC13A is one of the top hits for ALS and FTD–ALS in multiple genome-wide association studies5,6,23–26 (GWAS). Single nucleotide polymorphisms (SNPs) in UNC13A are associated with increased risk of sporadic ALS5 and sporadic FTLD-TDP pathology, especially type B, the subtype associated with FTD–ALS6. In addition to increasing susceptibility to ALS, SNPs in UNC13A are associated with shorter survival in patients with ALS27–30. But the mechanism by which genetic variation in UNC13A increases risk for ALS and FTD is unknown. Notably, the two most significantly associated SNPs, rs12608932 (A>C) and rs12973192 (C>G), are both located in the same intron that we found harbours CE, with rs12973192 located in CE itself (Fig. 4a, c). This immediately suggested that these SNPs (or other nearby genetic variations tagged by these SNPs) might make UNC13A more vulnerable to CE inclusion upon TDP-43 depletion. To test this, we analysed the percentage of RNA-seq reads that mapped to intron 20–21 that support the inclusion of CE (Extended Data Fig. 7a, b). Among the seven patients included in the initial splicing analysis (Fig. 1a), two out of three who were homozygous (G/G) and the one patient who was heterozygous (C/G) for the risk allele at rs12973192, showed inclusion of CE in almost every UNC13A mRNA that was mapped to intron 20–21. By contrast, the three other patients who were homozygous for the reference allele (C/C) showed much less inclusion of CE (Extended Data Fig. 7a, b). Another way to directly assess the effect of the UNC13A risk alleles on CE inclusion is to measure allele imbalance in RNAs from individuals who are heterozygous for the risk allele. Two of the iPSC-MN lines that we used to detect CE inclusion upon TDP-43 knockdown (iPSC-MN1 and iPSC-MN3; Fig. 1h) are heterozygous (C/G) at rs12973192. We performed amplicon sequencing of the RT–PCR product that spans CE and analysed the allele distribution from these two samples (Extended Data Fig. 3d, e) as well as the one patient sample from the original RNA-seq dataset (Fig. 1a) that is heterozygous (C/G) at rs12973192 (Extended Data Fig. 7b). We found a significant difference between the percentage of risk and reference alleles in the spliced variant, with higher inclusion of the risk allele (Fig. 4b, Extended Data Fig. 7c).
Fig. 4. UNC13A risk haplotype associated with ALS or FTD susceptibility potentiates CE inclusion when TDP-43 is dysfunctional.
a, LocusZoom plot showing SNPs associated with ALS or FTD in UNC13A. SNPs are coloured on the basis of levels of linkage equilibrium; SNPs rs12608932 and rs12973192 are in strong linkage disequilibrium (LD). b, There is a higher percentage inclusion of the risk allele (G) at rs12973192 in the UNC13A splice variant (n = 3 biologically independent samples; two-sided paired t-test; mean ± s.e.m.). Quantification in Extended Data Fig. 7c. c, Location of rs56041637 relative to the two known FTD–ALS GWAS hits and UNC13A CE. d, Design of UNC13A CE minigene reporter constructs and location of the primer pair used for RT–PCR. Black (reference alleles) and blue (risk alleles) triangles represent the genetic variants as shown in c. e, Splicing of minigene reporters was assessed in wild-type (WT) and TDP-43−/− HEK 293T cells. In addition to the inclusion of CE (2), some splice variants showed inclusion of one of the other two cryptic splicing products (3 and 4) (Extended Data Figs. 1a–e, 3a, Supplementary Note 2). The risk haplotype-carrying minigene showed an almost complete loss of canonical splicing product (1) and an increase in alternatively spliced products (2, 3 and 4). n = 2 independent cell culture experiments for each condition. f, Top, survival curves of FTLD-TDP patients stratified on the basis of the number of risk haplotypes. Heterozygous (1) and homozygous (2) patients had shorter survival time after disease onset (n = 205, Mayo Clinic Brain Bank; score (log-rank) test, P = 0.004). Dashed lines mark median survival for each genotype. Risk haplotype effect is modelled additively using Cox multivariable analysis adjusted for genetic mutations, sex and age at onset. Bottom, risk table. Summary results of the analysis are shown in Extended Data Fig. 10b.
Extended Data Fig. 7. Levels of UNC13A cryptic exon inclusion are influenced by the number of risk haplotypes.
(a) Visualization of RNA-Seq alignment between exon 20 and exon 21 of UNC13A. RNA-Seq libraries were generated from TDP-43-negative neuronal nuclei as described in Fig. 1a. (b) Samples that are heterozygous (C/G) or homozygous (G/G) at rs12973192 have higher relative inclusion (Ψ) of the cryptic exon except for SRR8571945. Information about the patients were obtained from Liu et al. 12. (c) Percentages of C and G alleles in the UNC13A spliced variants in TDP-43 depleted iPSC-MNs and SRR8571950 neuronal nuclei. Exact binomial test was done for each replicate to test whether the observed difference in percentages differ from what was expected if both alleles are equally included in the cryptic exon. (d) rs56041637 and rs62121687 are in strong linkage disequilibrium with both GWAS hits in intron 20-21 of UNC13A (Method). Along the axes of the heatplot are all loci that show variation among the 297 patients from Answer ALS in July 2020. Each tile represents the p-value from the corresponding Chi-Square test. P-value < 0.05 are shown in yellow and others are shown in blue or gray. Red and blue blocks highlight the associations of rs12608932 and rs12973192 with other genetic variants in intron 20-21 respectively. Significant associations common to both are circled in black. (e) The summary results of multiple linear regression modeling the effects of the number of UNC13A risk alleles on the abundance of UNC13A cryptic exon inclusion measured by RT-qPCR. A multivariable model was derived adjusting for phosphorylated TDP-43 levels (pTDP-43), sex, known genetic mutations, disease types, and the age of onset. As shown in Extended Data Fig. 5c, pTDP-43 levels have a strong effect on the abundance of UNC13A cryptic exon inclusion. Normality of residuals is tested by Shapiro-Wilk normality test (p-value = 0.2014).
Given this evidence for an effect of the risk allele on CE inclusion, we extended our analysis by genotyping patients with FTLD-TDP harbouring CE (n = 86) in the Mayo Clinic Brain Bank dataset for the UNC13A risk alleles at rs12973192 and rs12608932. Because these two SNPs are in high linkage disequilibrium in the European population31, we consider them to be on the same haplotype. Thus, we refer to the haplotype that contains reference alleles at both SNPs as the reference haplotype, and the haplotype that contains risk alleles as the risk haplotype. We excluded the one patient who is homozygous for the reference allele (C/C) at rs12973192 but heterozygous (A/C) at rs12608932. The remaining patients (n = 85) have exactly the same number of risk alleles at both loci, indicating that it’s very likely that they are carriers of the reference haplotype or the risk haplotype. Using a multiple linear regression model, we found a strong positive correlation (β = 0.175, P = 0.0290) between the number of risk haplotypes and the abundance of UNC13A CE inclusion measured by RT–qPCR (Extended Data Fig. 7e). Together, these data suggest that genetic variation in UNC13A that increases risk for ALS and FTD in humans promotes CE inclusion upon nuclear depletion of TDP-43.
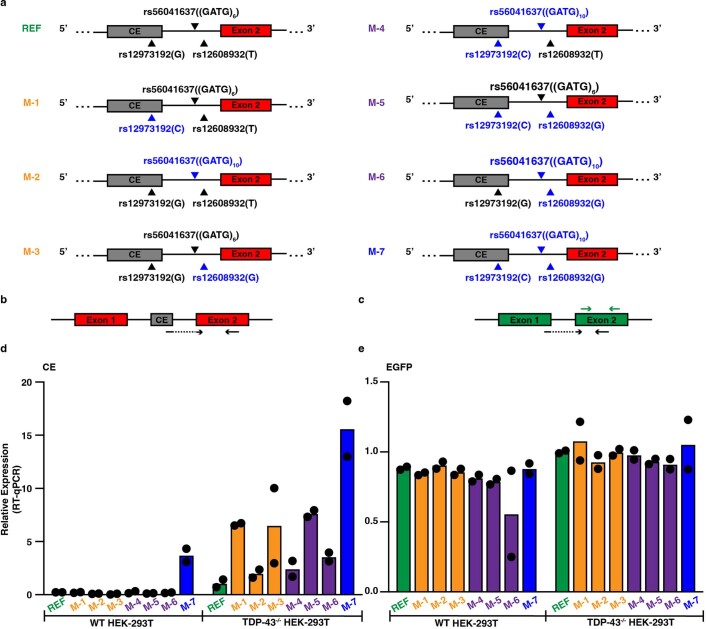
GWAS SNPs typically do not cause the trait but rather ‘tag’ other neighbouring genetic variation32. Thus, a major challenge in human genetics is to go from a GWAS hit to identifying the causative genetic variation that increases risk for disease33. A LocusZoom34 plot (Fig. 4a) generated using results from an ALS GWAS35 suggests that the strongest association signal on UNC13A is indeed in the region surrounding the two lead SNPs (rs12973192 and rs12608932). To identify other genetic variants in intron 20–21 that might also cause risk for disease by influencing CE inclusion but were not included in the original GWAS, we analysed genetic variants identified in whole genome sequencing data of 297 ALS patients of European descent (Answer ALS; https://www.answerals.org). We searched for novel genetic variants that could be tagged by the two SNPs by looking for other loci in intron 20–21 that are in linkage disequilibrium with both rs12608932 and rs12973192. We found one that fit these criteria: rs56041637 (Extended Data Fig. 7d). rs56041637 is a CATC-repeat insertion in intron 20–21. Most frequently there are six CATC repeats in this region. In the patient dataset, we observed that patients who are homozygous for the risk alleles at both rs12608932 and rs12973192 tend to have 3 to 5 additional CATC repeats; patients who are homozygous for reference alleles at both rs12608932 and rs12973192 tend to have only 0 to 2 additional repeats (Fig. 4c; CATC is shown as GATG because UNC13A is on the reverse strand). Thus, in addition to the two lead GWAS SNPs (rs12608932 and rs12973192), we now nominate rs56041637 as potentially contributing to risk for disease by making UNC13A more vulnerable to CE inclusion when TDP-43 is depleted from the nucleus.
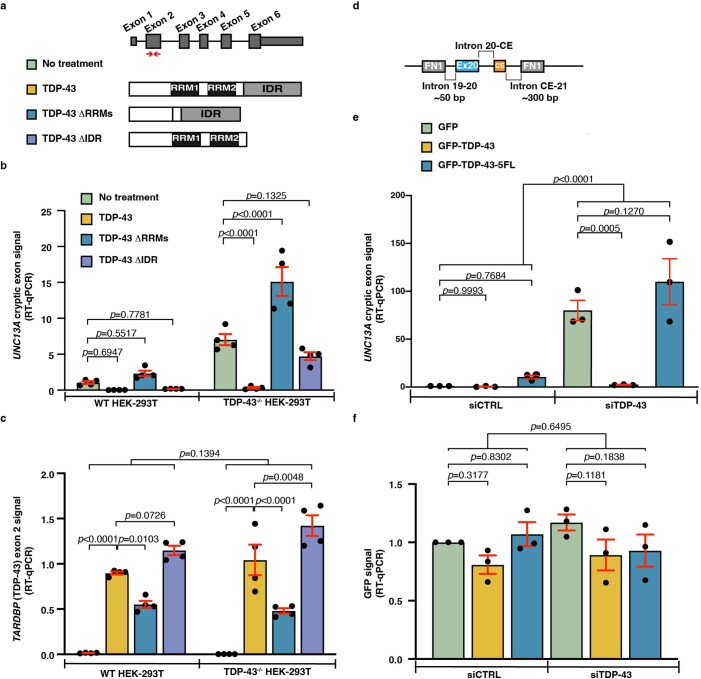
To directly test whether these three variants in UNC13A—which are part of the risk haplotype—increase CE inclusion upon TDP-43 depletion, we synthesized minigene reporter constructs (Fig. 4d). The reporter uses a bidirectional promoter to co-express eGFP containing a canonical intron and mCherry that is interrupted by UNC13A intron 20–21 from either the reference haplotype or the risk haplotype. Because UNC13A is on the reverse strand, the reference alleles and the risk alleles are the reverse complement of the genotypes reported on dbSNP—for example, in intron 20–21 of UNC13A, the reference allele at rs12973192 is G and the corresponding risk allele is C. We transfected wild-type and TDP-43-knock-out (TDP43−/−) HEK 293T cells36 with each minigene reporter construct. Using RT–PCR, we found that both versions of intron 20–21 were efficiently spliced out in wild-type cells (Fig. 4e, lanes 1–4). However, in TDP43−/− cells there was a decrease in completely intron-free splicing products and a concomitant increase in cryptic splicing products (Fig. 4e, lanes 5–6). Of note, in TDP-43–/– cells transfected with the minigene construct harbouring the risk haplotype in the intron, there was an even greater decrease in complete intron 20–21 splicing, and a proportional increase in cryptic splicing products (Fig. 4e, lanes 7–8; see Supplementary Note 2). The transcript levels of the eGFP control remained constant across all conditions, verifying equal reporter expression levels and the integrity of the splicing machinery independent of TDP-43.
To define the effect of each individual risk variant on splicing, we generated six additional minigene reporters, each carrying a different combination of the reference and risk alleles (individually, two at a time, or all three). Using RT–qPCR, we found that the risk variant in CE (rs12973192) had the strongest effect on CE inclusion in cells lacking TDP-43. The other variants also contributed to mis-splicing, but in a non-additive way, and the largest effect was with the construct harbouring all three risk variants (Extended Data Fig. 8). Expression of full-length TDP-43 rescued the splicing defects, whereas an RNA binding-deficient mutant did not (Extended Data Fig. 9). Together, these results provide direct functional evidence that TDP-43 regulates splicing of UNC13A intron 20–21 and that genetic variants associated with ALS and FTD susceptibility in humans potentiate cryptic exon inclusion when TDP-43 is dysfunctional.
Extended Data Fig. 8. The impact of variants at rs12973192, rs12608932 and rs56041637 on splicing.
(a) Diagrams showing the design of the UNC13A minigene reporter constructs used to assess the impact of the variants at each locus. The complete design of the reporter construct is shown in Fig. 4d. For clarity, the mCherry and GFP exons that are closest to the promoter (blue in Fig. 4d) are labeled as exon 1, and the downstream exon are labeled as exon 2. REF is the reporter that carries the reference haplotype. M-1 to M-3 carry a single risk variant. M-4 to M-6 carry two risk variants. M-7 carries all three variants, the risk haplotype. (b, c) The locations of the RT-qPCR primer pairs used to detect the inclusion of the cryptic exon (b) and the splicing of EGFP (c, shown in black). (d, e) The expression level of the cryptic exon (d) or the splicing of EGFP (e) in each condition is calculated with reference to the expression level of cryptic exon or the splicing of EGFP from the WT construct in TDP-43-/- HEK-293T cells. The expression of the reporter construct measured using a pair of primers aligned to the second exon of EGFP (c, shown in green) was used to normalize RT-qPCR. The cryptic exon expression levels of each pair of reporters expressed within the same cell line were compared. The splicing of EGFP remained constant across all conditions, verifying equal reporter expression levels and the integrity of the splicing machinery independent of TDP-43.
Extended Data Fig. 9. TDP-43-dependent minigene splicing reporter assay in HEK293T cells and HeLa cells.
(a) Schematic of various TDP-43 overexpression constructs used in HEK293T cells. RRM1&RRM2: RNA recognition motifs 1 & 2; IDR: intrinsically disordered region; adapted from50. The RT-qPCR primers (red arrows) for measuring the expression levels of the TDP-43 overexpression constructs are mapped to the second exon of TARDBP. The primer pair can detect all the TDP-43 overexpression constructs, including the endogenous TDP-43. Since the HEK293T TDP-43 knock-out cells do not have TDP-4336, using the primers does not interfere with measurement of TDP-43 construct expression levels in TDP-43-/- HEK293T. (b) Expression of full-length TDP-43 rescued the splicing defects in HEK293T. TDP-43 lacking both RRMs (TDP-43 ∆RRMs) exacerbates the splicing defects and TDP-43 lacking the IDR (TDP-43 ∆IDR) has a much weaker rescue effect compared to full length TDP-43. (c) The expression levels of the second exon of TDP-43 across different conditions in HEK293T measured by RT-qPCR. The expression levels differ significantly, possibly due to the autoregulation of TDP-4351. Despite the variability, the full length TDP-43 is significantly better at reducing the cryptic exon in UNC13A compared to TDP-43 ∆RRMs and TDP-43 ∆IDR. (n = 4 independent cell culture experiments for each condition in (b, c)). (d) Schematic of the pTB UNC13A minigene construct in HeLa cells. The pTB UNC13A minigene construct containing UNC13A cryptic exon sequence and the flanking sequences upstream (from 50 bp at the of end of intron 19 to the cryptic exon) and downstream (~300 bp intron 20) were expressed using the pTB vector, which we have previously used to study TDP-43 splicing regulation of other TDP-43 targets47. (e) Depletion of TDP-43 by siRNA in HeLa cells resulted in inclusion of the cryptic exon, which was rescued by expressing an siRNA-resistant form of TDP-43 (GFP-TDP-43) but not by an RNA-binding deficient mutant TDP-43 (GFP-TDP-43-5FL). (f) RT-qPCR of GFP demonstrating expressions of the constructs are similar across different conditions. (n = 3 independent cell culture experiments in (e, f)). GAPDH and RPLP0 were used to normalize RT-qPCR in (c) and (f). For all RT-PCR analysis, two-way ANOVA with Dunnett’s ’s multiple comparisons test was used for all RT-qPCR analysis, mean ± s.e.m.).
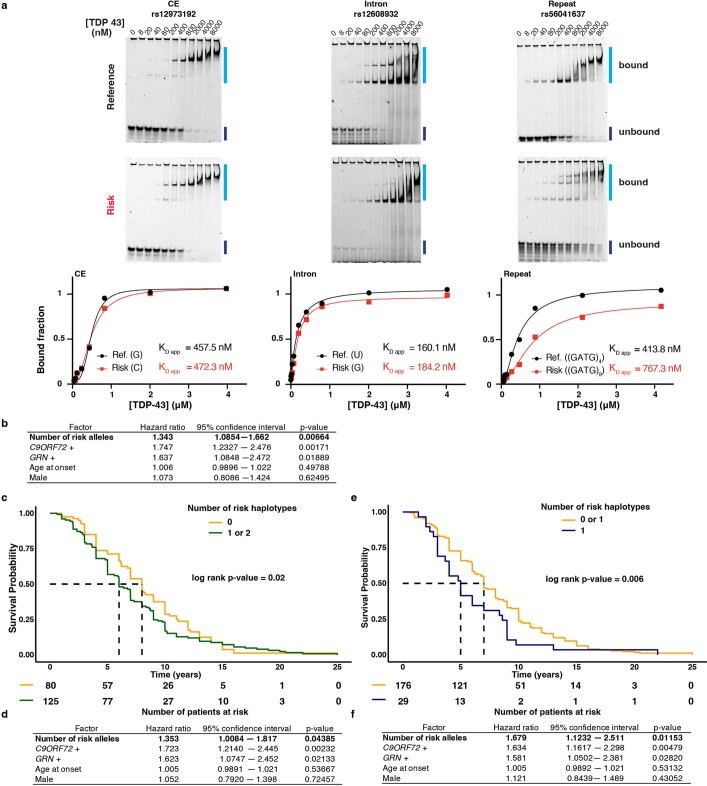
To directly test whether the risk variants affected TDP-43 binding, we performed quantitative electrophoretic mobility shift assays (EMSA). We incubated radioactively labelled RNA probes containing reference or risk versions of sequences within the UNC13A CE or intronic region with recombinant full-length TDP-43. This resulted in a mobility shift, indicating that TDP-43 can bind to these sequences (Extended Data Fig. 10a). TDP-43 binds the reference and risk versions of the CE probe with similar affinity but the intronic risk allele results in a minor reduction in affinity (Extended Data Fig. 10a). TDP-43 had lower affinity for the probe containing the risk allele at rs56041637 compared with the probe containing the reference allele (Fig. 4g). Overall, TDP-43 has a much higher affinity for the intron sequence compared with the exon or repeat sequence. The diminished binding affinity of TDP-43 to risk alleles of the intron and repeat sequence may contribute to the increased cryptic splicing found in ALS and FTD. We note that these in vitro binding results are somewhat different from those reported in the accompanying Article by Brown and colleagues37. Our use of full-length TDP-43, which can be prone to aggregation (although our maltose-binding protein (MBP)-tagged TDP-43 is soluble) but contains additional domains that are important for TDP-43 function, differs from the one used by Brown and colleagues, which contains only the TDP-43 RNA-recognition motifs (RRMs). Future studies will be required to explore how TDP-43 regulates the cryptic splicing of UNC13A and other splicing targets and the effect of different genetic variations on TDP-43 binding in vivo.
Extended Data Fig. 10. UNC13A risk haplotype is associated with diminished binding affinity of TDP-43 and reduced survival time of FTLD-TDP patients.
(a) Reference vs. risk allele RNA binding assay using electrophoretic mobility shift assay (EMSA). Exponentially increasing concentrations (0–8 μM) of purified TDP-43 were incubated with 1 nM Cy5 labeled RNA substrate for the reference and risk alleles of CE (rs12973192), intron (rs12608932), and repeat sequences (rs56041637) (see Methods). 8 mM values were excluded in plots due to significant aggregation at this concentration. The total bound population was quantified at each TDP-43 condition and used to plot the binding curve and calculate the apparent binding affinity, KD app. Experiments were performed three times for introns and two times for exons and repeats, which produced similar results. For gel source data, see Supplementary Fig. 1g. (b) UNC13A risk haplotype is associated with reduced survival time of FTLD-TDP patients. Summary results of Cox multivariable analysis (adjusted for genetic mutations, sex and age at onset) of an additive model. (c, e) Survival curves of FTLD-TDP patients (n = 205, Mayo Clinic Brain Bank), according to a dominant model (c) and a recessive model (e) and their corresponding risk tables. Summary results of Cox multivariable analysis (adjusted for genetic mutations, sex and age at onset) of a dominant model (d) and a recessive model (f). Both the dominant model (c, d) and the recessive model (e, f) show that the presence of the risk haplotype can reduce the survival of FTLD-TDP patients. Dashed lines mark the median survival for each genotype. Log rank p-values were calculated using Score (logrank) test. (b, d, f) The significance of each factor was calculated by Wald test.
To examine whether these SNPs affect survival in patients with FTLD-TDP, we evaluated the association of the risk haplotype with survival time after disease onset using data from the Mayo Clinic Brain Bank (n = 205). Using Cox multivariable analysis adjusting for other factors known to influence survival (genetic mutations, sex and age at onset), the risk haplotype was associated with survival time under an additive model (Fig. 4f). The number of risk haplotypes an individual carries was a strong prognostic factor (Extended Data Fig. 10b). The association remained significant under a dominant model (Extended Data Fig. 10c, d) and a recessive model (Extended Data Fig. 10e, f), indicating that carrying the risk haplotype reduces patient survival time after disease onset, consistent with previous analyses27–30. Thus, as suggested by previous studies28,30,38, genetic variants in UNC13A that increase cryptic exon inclusion are associated with decreased survival in patients.
Here we have found that TDP-43 regulates a cryptic splicing event in the FTD–ALS risk gene UNC13A. The most significant genetic variants associated with disease risk are located within the intron harbouring the cryptic exon itself. Brain samples from patients with FTLD-TDP carrying these SNPs exhibited more UNC13A CE inclusion than those from patients with FTLD-TDP lacking the risk alleles. These risk alleles seem insufficient to cause CE inclusion because CE is not detected extensively in RNA-seq data from healthy control samples16 (GTEx) and our functional studies indicate that TDP-43 dysfunction is required for substantial CE inclusion. Instead, the UNC13A risk alleles exert a TDP-43 loss-of-function-dependent disease-modifying effect. We propose that UNC13A risk alleles might act as a kind of Achilles’ heel, not causing problems until TDP-43 becomes dysfunctional. The discovery of a novel TDP-43-dependent cryptic splicing event in a bona fide FTD–ALS risk gene opens up a multitude of new directions for validating UNC13A as a biomarker and therapeutic target in ALS and FTD. This cryptic exon inclusion event—similar to that of STMN23,4—is not conserved in mouse, so will require studies in human neuron models to test whether blocking UNC13A cryptic splicing is sufficient to rescue phenotypes associated with loss of TDP-43 function. It is possible that a full rescue of TDP-43 function will require restoration of more than one cryptic splicing target (for example, STMN2, UNC13A and perhaps some of the others (Fig. 1b)). But the human genetics data (Fig. 4f) showing a dose-dependent decrease in survival in individuals carrying UNC13A risk alleles indicate that UNC13A is a key target of TDP-43. We note that UNC13A is more highly expressed in the frontal cortex (transcripts per million (TPM) = 530.2) than in the spinal cord16 (TPM = 35.54). One picture that might emerge is that the cryptic target STMN2 could have a key role in lower motor neurons in the spinal cord, whereas UNC13A could have a key role in the brain. Perhaps some combination of effects could contribute to ALS or FTD. It remains unknown why TDP-43 pathology is associated with ALS, FTLD-TDP, or even other aging-related neuropathological changes39. TDP-43-dysfunction-related cryptic splicing plays out across the diverse regional and neuronal landscape of the human brain. It is tempting to speculate that in addition to STMN2 and UNC13A, there could be specific portfolios of other important cryptic exon splicing events (and genetic variations that increase or decrease susceptibility to some of these events) that contribute to heterogeneity in clinical manifestation of TDP-43 dysfunction.
Methods
All materials used in this study are available upon request.
RNA-seq alignment and splicing analysis
The detailed pipeline v2.0.1 for RNA-seq alignment and splicing analysis is available on https://github.com/emc2cube/Bioinformatics/sh_RNAseq.sh. FASTQ files were downloaded from the Gene Expression Omnibus (GEO) database (GSE126543). Adaptors in FASTQ files were removed using trimmomatic (0.39) (ILLUMINACLIP:TruSeq3-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:36). The quality of the resulting files was evaluated using FastQC (v0.11.9). RNA-seq reads were mapped to the human (hg38) using STAR v2.7.3a following ENCODE standard options, read counts were generated using RSEM v1.3.1, and differential expression analysis was performed in R v4.0.2 using the DESeq2 package v1.28.140.
Splicing analysis
MAJIQ
Alternative splicing events were analysed using MAJIQ (2.2) and VOILA13. In brief, uniquely mapped, junction-spanning reads were used by MAJIQ with the following parameters: ‘majiq build -c config–min-intronic-cov 1–simplify’, to construct splice graphs for transcripts by using the UCSC transcriptome annotation (release 82) supplemented with de novo detected junctions. Here, de novo refers to junctions that were not in the UCSC transcriptome annotation but had sufficient evidence in the RNA-seq data (–min-intronic-cov 1). Distinct local splice variations (LSVs) were identified in gene splice graphs, and the MAJIQ quantifier ‘majiq psi’ estimated the fraction of each junction in each LSV, denoted as percent spliced in (PSI or Ψ), in each RNA-seq sample. The changes in each junction’s PSI (ΔPSI or ΔΨ) between the two conditions (TDP-43-positive neuronal nuclei versus TDP-43-negative neuronal nuclei) were calculated by using the command ‘majiq deltapsi’. The gene splice graphs and the posterior distributions of PSI and ΔPSI were visualized using VOILA.
LeafCutter
LeafCutter is available as commit 249fc26 on https://github.com/davidaknowles/leafcutter. Using RNA-seq reads aligned as previously described, reads that span exon–exon junctions and map with a minimum of 6 nt into each exon were extracted from the alignment (bam) files using filter_cs.py with the default settings. Intron clustering was performed using the default settings in leafcutter_cluster.py. Differential excision of the introns between the two conditions (TDP-43-positive neuronal nuclei versus TDP-43-negative neuronal nuclei) were calculated using leafcutter_ds.R.
Sashimi plot
RNA-seq densities along the exons were plotted using the sashimi_plot function included in the MISO package (misopy 0.5.4). In the sashimi plot, introns are scaled down by a factor of 15 and exons are scaled down by a factor of 5. RNA-seq read densities across exons are scaled by the number of mapped reads in the sample and are measured in RPKM units. Slight modifications were made to plot_gene.py and plot_settings.py within the sashimi_plot directory of the MISO package to highlight the RNA-seq density plot. The modified sashimi_plot directory is available at (https://github.com/rosaxma/TDP-43-UNC13A-2021).
Cell culture
SH-SY5Y (ATCC) cells were grown in DMEM/F12 media supplemented with Glutamax (Thermo Scientific), 10% fetal bovine serum and 10% penicillin–streptomycin at 37 °C, 5% CO2. For shRNA treatments, cells were plated on day 0, transduced with shRNA on day 2 followed by media refresh on day 3, and collected for readout (RT–qPCR and immunoblotting) on day 6. HEK 293T TDP-43 knockout cells and parent HEK 293T cells were generated as described36. The cells were cultured in DMEM medium (Gibco 10564011) supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin, 2 mM l-glutamine (Gemini Biosciences) and 1× MEM non-essential amino acids solution (Gibco) at 37 °C, 5% CO2.
iPS cell maintenance and differentiation into iPSC-MNs
iPS cell lines were obtained from public biobanks (GM25256-Corriell Institute; NDS00262, NDS00209-NINDS) and maintained in mTeSR1 media (StemCell Technologies) on Matrigel (Corning). iPS cells were fed daily and split every 4–7 days using ReLeSR (StemCell Technologies) according to the manufacturer’s instructions. Differentiation of iPS cells into motor neurons was carried out as previously described41. In brief, iPS cells were dissociated and placed in ultra-low adhesion flasks (Corning) to form 3D spheroids in media containing DMEMF12/Neurobasal (Thermo Fisher), N2 Supplement (Thermo Fisher), and B-27 Supplement-Xeno free (Thermo Fisher). Small molecules were added to induce neuronal progenitor patterning of the spheroids, (LDN193189, SB-431542, Chir99021), followed by motor neuron induction (with retinoic acid, Smo agonist and DAPT). After 14 days, neuronal spheroids were dissociated with Papain and DNAse (Worthington Biochemical) and plated on poly-d-lysine/laminin coated plates in Neurobasal Medium (Thermo Fisher) containing neurotrophic factors (BDNF, GDNF and CNTF; R&D Systems). For viral transductions, neuronal cultures were incubated for 18 h with media containing lentivirus particles for shScramble, or shTDP-43. Infection efficiency of over 90% was assessed by RFP expression. Neuronal cultures were analysed for RNA and protein 7 days post transduction.
shRNA cloning, lentiviral packaging, and cellular transduction for detecting the UNC13A splice variant
shRNA sequences were originated from the Broad GPP Portal (TDP-43: AGATCTTAAGACTGGTCATTC, scramble: GATATCGCTTCTACTAGTAAG). To clone, complementary oligonucleotides were synthesized to generate 4-nt overhangs, annealed and ligated into pRSITCH (Tet inducible U6) or pRSI16 (constitutive U6) (Cellecta). Ligations were transformed into Stbl3 chemically competent cells (Thermo Scientific) and grown at 30 °C. Large scale plasmid generation was performed using Maxiprep columns (Promega), with purified plasmid used as input for lentiviral packaging with second generation packaging plasmids psPAX2 and pMD2.G (Cellecta), transduced with Lipofectamine 2000 (Invitrogen) in Lenti-X 293T cells (Takara). Viral supernatant was collected at 48 and 72 h post transfection and concentrated using Lenti-X Concentrator (Takara). Viral titer was established by serial dilution in relevant cell lines and readout of percentage of BFP+ cells by flow cytometry, with a dilution achieving a minimum of 80% BFP+ cells selected for experiments.
Immunoblotting
SH-SY5Y cells and iPSCs-MNs were transfected and treated as above before lysis. Cells were lysed in ice-cold RIPA buffer (Sigma-Aldrich R0278) supplemented with a protease inhibitor cocktail (Thermo Fisher 78429) and phosphatase inhibitor (Thermo Fisher 78426). After pelleting lysates at maximum speed on a table-top centrifuge for 15 min at 4 °C, bicinchoninic acid (Invitrogen 23225) assays were conducted to determine protein concentrations. 60 μg (SH-SY5Y) and 30 μg (iPSCs-MNs) protein of each sample was denatured for 10 min at 70 °C in LDS sample buffer (Invitrogen NP0008) containing 2.5% 2-mercaptoethanol (Sigma-Aldrich). These samples were loaded onto 4–12% Bis–Tris gels (Thermo Fisher NP0335BOX) for gel electrophoresis, then transferred onto 0.45-μm nitrocellulose membranes (Bio-Rad 162-0115) at 100 V for 2 h using the wet transfer method (Bio-Rad Mini Trans-Blot Electrophoretic Cell 170-3930). Membranes were blocked in Odyssey Blocking Buffer (LiCOr 927-40010) for 1 h then incubated overnight at room temperature in blocking buffer containing antibodies against UNC13A (1:500, Proteintech 55053-1-AP), TDP-43 (1:1,000, Abnova H00023435-M01), or GAPDH (1:1,000, Cell Signaling Technologies 5174S). Membranes were subsequently incubated in blocking buffer containing horseradish peroxidase (HRP)-conjugated anti-mouse IgG (H+L) (1:2,000, Fisher 62-6520) or HRP-conjugated anti-rabbit IgG (H+L) (1:2,000, Life Technologies 31462) for 1 h. ECL Prime kit (Invitrogen) was used for development of blots, which were imaged using ChemiDox XRS+ System (Bio-Rad). The intensity of bands was quantified using Fiji, and then normalized to the corresponding controls.
RNA extraction, cDNA synthesis and RT–qPCR or RT–PCR for detecting the UNC13A splice variant in iPSC-MNs
Total RNA was extracted using RNeasy Micro kit (Qiagen) per manufacturer’s instructions, with lysate passed through a QIAshredder column (Qiagen) to maximize yield. RNA was quantified by Nanodrop (Thermo Scientific), with 75 ng used for cDNA synthesis with SuperScript IV VILO Master Mix (Thermo Scientific). Quantitative PCR was run with 6 ng cDNA input in a 20 µl reaction using PowerTrack SYBR Green Master Mix (Thermo Scientific) with readout on a QuantStudio 6 Flex using standard cycling parameters (95 °C for 2 min, 40 cycles of 95 °C for 15s and 60 °C for 60 s), followed by standard dissociation (95 °C for 15 s at 1.6 °C s−1, 60 °C for 60 s at 1.6 °C s−1, 95 °C for 15 s at 0.075 °C s−1). ΔΔCt was calculated with the housekeeper gene RPLP0 as control and relevant shScramble as reference; measured Ct values greater than 40 were set to 40 for visualizations. See Supplementary Table 6 for primers.
PCR was conducted with 15 ng cDNA input in a 100 µl reaction using NEBNext Ultra II Q5 Master Mix (New England Biolabs), with the following cycling parameters: initial denaturation: 98 °C for 30 s; 40 cycles: 98 °C for 10 s, 64 °C for 30 s, 72 °C for 20 s; final extension: 72 °C for 2 min. The resulting products were visualized on a 1.5% TAE gel. See Supplementary Table 6 for primers.
Human iPS cell-derived neurons for detecting UNC13A splice variants
cDNA was available from CRISPRi-i3Neuron iPS cells (i3N) generated from our previous publication11, in which TDP-43 is downregulated to about 50%. RT–qPCR was performed using SYBR GreenER qPCR SuperMix (Invitrogen). Samples were run in triplicate, and RT–qPCR reactions were run on a QuantStudio 7 Flex Real-Time PCR System (Applied Biosystems). Relative quantification was determined using the ∆∆Ct method and normalized to the endogenous controls RPLP0 and GAPDH. We normalized relative transcript levels for wild-type UNC13A to that of the neurons treated with control sgRNA (mean set to 1). See Supplementary Table 6 for primers.
Cell culture for validating additional splicing events in iPS cell-derived neurons
We used an induced neuron (iN) system previously established for rapidly differentiating human iPS cells into functional cortical neurons42. In brief, iPS cells (without disease mutation) were cultured using feeder-free conditions on Matrigel (Fisher Scientific CB-40230) using mTeSR1 media (Stemcell Technologies 85850). Cells were transduced with a Tet-On induction system that allows expression of the transcription factor NGN2. Cells were dissociated on day 3 of differentiation and replated on Matrigel-coated tissue culture plates in Neurobasal Medium (Thermo Fisher) containing neurotrophic factors, BDNF and GDNF (R&D Systems) with viral transductions for shScramble or shTDP-43. RNA and protein were extracted 7 days after transduction.
shRNA cloning, lentiviral packaging, and cellular transduction for validating additional splicing events
The lentiviral plasmid targeting TARDBP (Millipore-Sigma TRCN0000016038) and Scramble (CAACAAGATGAAGAGCACCAA) were packaged using third generation packaging plasmids (pMDLg/pRRE, pRSV-Rev, pMD2.G) and transduced with Lipofectamine 3000 (Invitrogen) into HEK 293T cells cultured under standard conditions (DMEM, 10% FBS, penicillin–streptomycin). Viral supernatant was collected at 48 and 72 h post-transfection and concentrated 1:100 using Lenti-X Concentrator (Takara).
RNA extraction, cDNA synthesis and RT–qPCR for validating additional splicing events
Total RNA was extracted using RNeasy Micro kit (Qiagen) and reverse transcribed into cDNA using High-Capacity cDNA Reverse Transcription Kits (Invitrogen). Quantitative PCR was run with 2 ng cDNA input in a 10 µl reaction using PowerTrack SYBR Green Master Mix (Thermo Scientific) with readout on a QuantStudio 6 Flex using standard cycling parameters. ΔΔCt was calculated with RPLP0 or GAPDH as housekeeper gene controls and relevant shScramble transduced condition as reference; measured Ct values greater than 40 were set to 40 for visualizations. See Supplementary Table 6 for primers used for detecting mis-spliced transcripts and normal splicing transcripts, and primers used for internal controls.
Amplicon sequencing of the splice variants
Splice variants in iPSC-MNs were established by PCR amplification from UNC13A exon 19 to exon 21 (UNC13A_19_21 FWD 5′–3′= CAACCTGGACAAGCGAACTG, UNC13A_19_21 RVS 5′-3′= GGGCTGTCTCATCGTAGTAAAC). Resulting products were purified using Wizard SV Gel and PCR Clean-Up columns (Promega) and submitted for NGS (Amplicon EZ, Genewiz). Adaptors in FASTQ files were removed using trimmomatic (0.39) (ILLUMINACLIP:TruSeq3-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:36). The quality of the resulting files was then evaluated using FastQC (v0.11.9). The sequencing reads were then mapped to the human (hg38) using STAR v2.7.3a following ENCODE standard options. Uniquely mapped reads were then filtered for using the command ‘samtools view -b -q 255’. The Sashimi Plot were then generated using the sashimi plot function in IGV (2.8.0) with the minimum junction coverage set to 20.
Post-mortem brain tissues for detecting UNC13A splice variant
Post-mortem brain tissues from patients with FTLD-TDP and cognitively normal control individuals were obtained from the Mayo Clinic Florida Brain Bank. Diagnosis was independently ascertained by trained neurologists and neuropathologists upon neurological and pathological examinations, respectively. Written informed consent was given by all participants or authorized family members and all protocols were approved by the Mayo Clinic Institution Review Board and Ethics Committee. Complementary DNA (cDNA) obtained from 500 ng of RNA (RIN ≥ 7.0) from medial frontal cortex was available from a previous study, as well as matching pTDP-43 data from the same samples43. Following standard protocols, RT–qPCR was conducted using SYBR GreenER qPCR SuperMix (Invitrogen) for all samples in triplicates. RT–qPCR reactions were run in a QuantStudio 7 Flex Real-Time PCR System (Applied Biosystems). Relative quantification was determined using the ∆∆Ct method and normalized to the endogenous controls RPLP0 and GAPDH. We normalized relative transcript levels to that of the healthy controls (mean set to 1). See Supplementary Table 6 for primers.
Quantification of UNC13A splice variants in bulk RNA sequencing
RNA-seq data generated by NYGC ALS Consortium cohort were downloaded from the NCBI Gene Expression Omnibus (GEO) database (GSE137810, GSE124439, GSE116622 and GSE153960). We used the 1658 available and quality-controlled samples classified as described11. After pre-processing and aligning the reads to human (hg38) as described previously, we estimated the expression of the full-length UNC13A using RSEM (v1.3.2). PCR duplicates were removed using MarkDuplicates from Picard Tools (2.23.0) using the command ‘MarkDuplicates REMOVE_DUPLICATES=true CREATE_INDEX=true’. We then filtered for uniquely mapped reads using the command ‘samtools view -b -q 255’. Reads that span either exon 19–exon 20 junction, exon 20–CE junction, CE–exon 21 junction or exon 20–exon 21 junction were quantified using bedtools (2.27.1) using the command ‘bedtools intersect -split’. Because of the relatively low level of expression of UNC13A in post-mortem tissues and the heterogeneity of the tissues, it is possible that not all tissues have enough detectable UNC13A for us to detect the splice variants. Since UNC13A contains more than 40 exons and RNA-seq coverages of mRNA transcripts are often not uniformly distributed44, we looked at reads spanning the exon 19–exon 20 junction, which is included in both the canonical isoform variant and the splice variant, and there is a strong correlation (Pearson’s r = 0.99) between the numbers of reads mapped to the exon 19–exon 20 junction and the exon 20–exon 21 junction. We observed that samples that have at least 2 reads spanning either exon 20–CE junction or CE–exon 21 junction have at least either UNC13A TPM = 1.55 or 20 reads spanning exon 19– exon 20 junction. Therefore, we selected the 1,151 samples that had a TPM ≥ 1.55, or at least 20 reads mapped to the exon 19–exon 20 junction as samples suitable for UNC13A splice variant analysis.
In situ hybridization UNC13A CE analysis in postmortem brain samples
Patients and diagnostic neuropathological assessment
Postmortem brain tissue samples used for this study were obtained from the University of California San Francisco (UCSF) Neurodegenerative Disease Brain Bank (Supplementary Table 4). Supplementary Table 4 provides demographic, clinical, and neuropathological information. Consent for brain donation was obtained from subjects or their surrogate decision makers in accordance to the Declaration of Helsinki, and following a procedure approved by the UCSF Committee on Human Research. Brains were cut fresh into 1 cm thick coronal slabs, and alternate slices were fixed in 10% neutral buffered formalin for 72 h. Blocks from the medial frontal pole were dissected from the fixed coronal slabs, cryoprotected in graded sucrose solutions, frozen, and cut into 50 µm thick sections as described previously45. Clinical and neuropathological diagnosis were performed as described previously45. Subjects were selected on the basis of clinical and neuropathological assessment. Patients selected had a primary clinical diagnosis of behavioural variant frontotemporal dementia (bvFTD) with or without amyotrophic lateral sclerosis or motor neuron disease and a neuropathological diagnosis of FTLD-TDP, type B. We excluded subjects if they had a known disease-causing mutation, post-mortem interval ≥ 24 h, Alzheimer’s disease neuropathologic change > low, Thal amyloid phase > 2, Braak neurofibrillary tangle stage > 4, CERAD neuritic plaque density > sparse, and Lewy body disease > brainstem predominant45.
In situ hybridization and immunofluorescence
To detect single RNA molecules, a BaseScope Red Assay kit (ACDBIO, USA) was used. One 50 µm thick fixed frozen tissue section from each subject was used for staining. Experiments were performed under RNase-free conditions as appropriate. Probes that target the transcript of interest, UNC13A, specific to either the mRNA (exon 20–exon 21 junction) or the cryptic exon containing spliced target (exon 20–cryptic exon junction) were used. Positive (Homo Sapiens PPIB) and negative (Escherichia coli DapB) control probes were also included. In situ hybridization was performed based on vendor specifications for the BaseScope Red Assay kit. In brief, frozen tissue sections were washed in PBS and placed under an LED grow light (HTG Supply, LED-6B240) chamber for 48 h at 4 °C to quench tissue autofluorescence. Sections were quickly rinsed in PBS and blocked for endogenous peroxidase activity. Sections were transferred on to slides and dried overnight. Slides were subjected to target retrieval and protease treatment and advanced to ISH. Probes were detected with TSA Plus-Cy3 (Akoya Biosciences), and subjected to immunofluorescence staining with antibodies to TDP-43 (rabbit polyclonal, Proteintech, RRID: AB_615042, dilution 1:4,000, catalogue (cat.) no. 10782-2-AP) and NeuN (Guinea pig polyclonal, Synaptic Systems, dilution 1:500; cat. no. 266004), and counterstained with DAPI (Life Technologies) for nuclei.
Image acquisition and analysis
Z-stack images were captured using a Leica SP8 confocal microscope with an 63× oil immersion objective (1.4 NA). For RNA probes, image capture settings were established during initial acquisition based on PPIB and DAPB signal and remained constant across UNC13A probes and subjects. TDP-43 and NeuN image capture settings were modified based on staining intensity differences between cases. For each case, 6 non-overlapping Z-stack images were captured across cortical layers 2–3. RNA puncta for the UNC13A cryptic exon were quantified using the ‘analyze particle’ plugin in ImageJ. In brief, all images were adjusted for brightness using similar parameters and converted to maximum intensity Z-projections, images were adjusted for auto-threshold (intermodes), and puncta were counted (size: 6-infinity, circularity: 0–1).
Linkage disequilibrium analysis
Recalibrated VCF files of 297 ALS patients of European descent generated by GATK HaplotypeCallers were downloaded from Answer ALS in July 2020 (https://www.answerals.org). VCFtools (0.1.16) were used to filter for sites that are in intron 20–21. The filtered VCF files were merged using BCFtools (1.8). Since there are sites that contain more than 2 alleles, we tested for genotype independence using the chi-squared statistics by using the command ‘vcftools–geno-chisq–min-alleles 2–max-alleles 8’. We found two additional SNPs, rs56041637 (P < 0.0001 with rs12608932, P < 0.0001 with rs12973192), and rs62121687 (P < 0.0001 with rs12608932, P < 0.0001 with rs12973192) that are in linkage disequilibrium with both. However, since rs62121687 was included in a GWAS and has a P-value35 of 0.0186585, we excluded it from further analysis.
Determination of rs12608932 and rs12973192 SNP genotype in human postmortem brain
Genomic DNA (gDNA) was extracted from human frontal cortex using Wizard Genomic DNA Purification Kit (Promega), according to the manufacturer’s instructions. TaqMan SNP genotyping assays were performed on 20 ng of gDNA per assay, using a commercial pre-mixture consisting of a primer pair and VIC or FAM-labelled probes specific for each SNP (cat. no. 4351379, assay ID 43881386_10 for rs12608932 and 11514504_10 for rs12973192, Thermo Fisher Scientific), and run on a QuantStudio 7 Flex Real-Time PCR system (Applied Biosystems), according to the manufacturer’s instructions. The PCR programs were 60 °C for 30 s, 95 °C for 10 min, 40 cycles of 95 °C for 15 s and, 60 °C (rs12973192) or 62.5 °C for 1 min (rs12608932), and 60 °C for 30 s.
Splicing reporter assay
Minigene constructs were designed in silico, synthesized by GenScript and sub-cloned into a vector with the GFP splicing control. HEK 293T TDP-43 knockout cells and the parent HEK 293T cells were seeded into standard P12 tissue culture plates (at 1.6 × 105 cells per well), allowed to adhere overnight, and transfected with the indicated splicing reporter constructs (400 ng per well) using Lipofectamine 3000 transfection reagent (Invitrogen). Each reporter comprised one of the splicing modules (shown in Fig. 4d), which is expressed from a bidirectional promoter. Twenty-four hours after transfection, RNA was extracted from these cells using PureLink RNA Mini Kit (Life Technologies) according to the manufacturer’s protocol, with on-column PureLink DNase (Invitrogen) treatment. The RNA was reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Invitrogen) according to the manufacturers’ instructions. PCRs were performed using OneTaq 2X Master Mix with Standard Buffer (NEB) with the following cycling parameters: denaturation: 94 °C for 30 s; 30 cycles: 94 °C for 20 s, 54 °C for 30 s, 68 °C for 30 s; final extension: 68 °C for 5 min on a Mastercycler Pro (Eppendorf) thermocycler PCR machine. PCR products were separated by electrophoresis on a 1.5% TAE gel and imaged ChemiDox XRS+ System (Bio-Rad). See Supplementary Table 6 for primers.
Assay to assess the effect of variants at rs12973192, rs12608932 and rs56041637 on splicing
Additional minigene constructs shown in Extended Data Fig. 8 were either generated using site-directed mutagenesis (New England Biolabs, E0554S) or synthesized by GenScript, and sub-cloned into the vector with the GFP splicing control. HEK 293T TDP-43 knockout cells and the parent HEK 293T cells were seeded into standard P12 tissue culture plates (at 5 × 105 cells per well), allowed to adhere overnight and transfected with the indicated splicing reporter constructs (400 ng per well) using Lipofectamine 3000 transfection reagent (Invitrogen). Twenty-four hours after transfection, RNA was extracted from these cells using PureLink RNA Mini Kit (Life Technologies) according to the manufacturer’s protocol, with on-column PureLink DNase treatment. The RNA was reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Invitrogen) according to the manufacturers’ instructions. The UNC13A cryptic exon signal was measured using a pair of primers that detect the junction of the CE and the immediately downstream mCherry exon. The splicing of eGFP was measured using a pair of primers that detect the junction of the first and second exons of eGFP. A pair of primers that mapped within the second exon of eGFP was used to measure the transfection efficiency of the splicing reporter construct and was used as a normalizer. ΔΔCt was calculated using the cryptic exon signal level or the splicing of eGFP in the HEK 293T TDP-43 knockout cells expressing the reference haplotype-carrying reporter as reference. See Supplementary Table 6 for primers.
Rescue of UNC13A splicing using TDP-43 overexpression constructs
HEK 293T TDP-43 knockout cells and the parent (wild-type) HEK 293T cells were seeded into standard P12 tissue culture plates (at 5 × 105 cells per well), allowed to adhere overnight and transfected with the splicing reporter construct carrying the reference haplotype (400 ng per well; Fig. 4e) and the indicated TDP-43 overexpression constructs (600 ng per well) using Lipofectamine 3000 transfection reagent (Invitrogen). Twenty-four hours after transfection, RNA was extracted from these cells using PureLink RNA Mini Kit (Life Technologies) according to the manufacturer’s protocol, with on-column PureLink DNase treatment. The RNA was reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Invitrogen) according to the manufacturers’ instructions. Quantitative PCR was run with 8 ng cDNA input in a 10 µl reaction using PowerTrack SYBR Green Master Mix (Thermo Scientific) with readout on a QuantStudio 6 Flex using standard cycling parameters.
The UNC13A cryptic exon signal was measured using a pair of primers that detect the junction of the CE and the mCherry exon immediately downstream of it. A pair of primers that are mapped within the second exon of eGFP was used to measure the transfection efficiency of the splicing reporter construct, and was used as a normalizer. ΔΔCt was calculated using the cryptic exon signal level in the wild-type HEK 293T cells without TDP-43 overexpression constructs as reference. See Supplementary Table 6 for primers.
The expression levels of the overexpression constructs were measured using a pair of primers that detect the second exon of TDP-43. The primers do detect the endogenous TDP-43 but since the HEK 293T TDP-43 knockout cells do not have TDP-43 expression as shown previously36, using the primers do not interfere with the measurement of the expression levels of TDP-43 constructs in the knockout cells. ΔΔCt was calculated using the TDP-43 expression level in the HEK 293T TDP-43 knockout cells with full length TDP-43 overexpression constructs as reference. RPLP0 and GAPDH were used as internal controls. See Supplementary Table 6 for primers.
Generation of pTB UNC13A minigene construct
The pTB UNC13A minigene construct containing the human UNC13A cryptic exon sequence and the nucleotide flanking sequences upstream (50 bp at the of end of intron 19, the entirety of exon 20, and the entirety of intron 20 sequence upstream of the cryptic exon) and downstream (approximately 300-bp intron 20) of the cryptic exon were amplified from human genomic DNA using the following primers: FWD 5′–3′, AGGTCATATGCACTGCTATAGTGGGAAGTTC and RVS 5′–3′, CTTACATATGTAATAACTCAACCACACTTCCATC; and subcloned into the NdeI site of the pTB vector. We have previously used a similar approach to study TDP-43 splicing regulation of other TDP-43 targets46 .
Rescue of UNC13A splicing using the pTB minigene and TDP-43 overexpression constructs
HeLa cells were grown in Opti-MEM I Reduced Serum Medium, GlutaMAX Supplement (Gibco) plus 10% fetal bovine serum (Sigma), and 1% penicillin/streptomycin (Gibco). For double-transfection and knockdown experiments, cells were first transfected with 1.0 µg of pTB UNC13A minigene construct and 1.0 µg of one of the following plasmids: GFP, GFP-TDP-43 or GFP-TDP-43 5FL constructs to express GFP-tagged TDP-43 proteins have been previously described46,47, in serum-free media and using Lipofectamine 2000 following the manufacturer’s instructions (Invitrogen). Four hours following transfection, media was replaced with complete media containing siLentfect (Bio-Rad) and siRNA complexes (AllStars Neg. Control siRNA or siRNA against TARDBP 3′ untranslated region, a region not included in the TDP-43 overexpression constructs) (Qiagen) following the manufacturer’s protocol. Cycloheximide (Sigma) was added at a final concentration of 100 µg ml−1 at 6 h prior to collecting the cells. Then RNA was extracted from the cells using TRIzol Reagent (Zymo Research), following the manufacturer’s instructions. Approximately 1 µg of RNA was converted into cDNA using the High Capacity cDNA Reverse Transcription Kit with RNA inhibitor (Applied Biosystems). The RT–qPCR assay was performed on cDNA (diluted 1:40) with SYBR GreenER qPCR SuperMix (Invitrogen) using QuantStudio7 Flex Real-Time PCR System (Applied Biosystems). All samples were analysed in triplicates. The RT–qPCR program was as follows: 50 °C for 2 min, 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. For dissociation curves, a dissociation stage of 95 °C for 15 s, 60 °C for 1 min and 95 °C for 15 s was added at the end of the program. Relative quantification was determined using the ∆∆Ct method and normalized to the endogenous controls RPLP0 and GAPDH. We normalized relative transcript levels for wild-type UNC13A and GFP to that of the control siRNA condition (mean set to 1). See Supplementary Table 6 for primers.
In vitro TDP-43 binding studies
Cloning
The plasmid encoding TDP43 as a C-terminal MBP-tagged protein (TDP43–MBP–His6) was purchased from Addgene (#104480).
Bacterial growth and protein expression
The wild-type TDP-43 expression plasmid was transformed into E. coli One Shot BL21 Star (DE3) cells (ThermoFisher). Transformed E. coli were grown at 37 °C in 1 l of LB media supplemented with 0.2% dextrose and 50 μg ml−1 kanamycin until absorbance at 600 nm reached 0.5–0.6. The culture was then incubated at 4 °C for 30–45 min. TDP-43 expression was induced with 1 mM IPTG for 16 h at 4 °C. Cells were collected by centrifugation.
Recombinant TDP-43 purification
Wild-type TDP-43–MBP was purified as described48. In brief, cell pellets were resuspended in lysis buffer 1 M NaCl, 20 mM Tris (pH 8.0), 10 mM imidazole, 10% glycerol and 2.5 mM 2-mercaptoethanol and supplemented with cOmplete, EDTA-free protease inhibitor cocktail tablets (Roche) then lysed via sonication. Cell lysates were centrifuged at 31,400g at 4 °C for 1 h, filtered, then purified with FPLC using a XK 50/20 column (Cytiva) packed with Ni-NTA agarose beads (Qiagen) which were equilibrated in lysis buffer. TDP-43 was recovered via a 0–80% gradient elution using 1 M NaCl, 20 mM TrisHCl (pH 8.0), 10 mM imidazole, 10% glycerol and 2.5 mM 2-mercaptoethanol as the base buffer and 1 M NaCl, 20 mM TrisHCl (pH 8.0), 500 mM imidazole, 10% glycerol, and 2.5 mM 2-mercaptoethanol as the elution buffer. Eluted protein was concentrated using Amicon Ultra-15 centrifugal filters, MWCO 50 kDa (Millipore), filtered and further purified with size-exclusion chromatography using a 26/600 Superdex 200 pg column (Cytiva) equilibrated with 300 mM NaCl, 20 mM TrisHCl (pH8.0) and 1 mM DTT. The second out of three peaks, as evaluated by absorbance at 280 nm, was collected, spin concentrated as before, aliquoted, flash frozen in liquid N2, and stored at −80 C until further use. Protein concentrations were determined using absorbance at 280 nm (Nanodrop) and purity was determined by running samples on a 4–20% SDS–PAGE gel and visualized with Coomassie stain.
Electrophoresis mobility shift assay
EMSA was used to compare TDP-43 binding to the reference and risk RNA sequences for reference and risk alleles of CE (rs12973192), intron (rs12608932), and repeat sequences (rs56041637) (see Supplementary Table 5). Increasing TDP-43 concentrations ranging from 0–4 mM were incubated with a constant 1 nM concentration of RNA in buffer (50 mM Tris-HCl, pH 7.5, 100 mM KCl, 2 mM MgCl2, 100 mM β−mercaptoethanol, 0.1 mg ml−1 BSA) for 30 min at room temperature. RNA is dual-labelled (Cy3 and Cy5) and contains an 18-nucleotide partial duplex on the 3′ end. Reactions were mixed with loading dye and run on a 6% non-denaturing polyacrylamide gel and imaged using fluorescence mode (Cy5) on a Typhoon scanner. Bound fractions were determined using the Analyze Gel plugin in ImageJ and normalized to the total intensity per lane. Apparent binding affinities were calculated using the ‘Specific binding with Hill slope’ function in Graphpad.
Statistical methods
Survival curves were compared using the coxph function in the survival (3.1.12) R package, which fits a multivariable Cox proportional hazards model that contains sex, reported genetic mutations and age at onset, and performs a score (log-rank) test. Effect sizes are reported as the hazard ratios. Proportional Hazards assumptions were tested using cox.zph function. The survival curves were plotted using ggsurvplot in suvminer (v.0.4.8) R package. Linear mixed effects models were analysed using lmerTest R package (3.1.3). Statistical analyses were performed using R (version 4.0.0), or Prism 8 (GraphPad), which were also used to generate graphs.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-022-04424-7.
Supplementary information
This file contains Supplementary Tables 3–5 and Supplementary Notes 1–3.
Uncropped images of immunoblots, PCR gels, and images of EMSA presented in this study.
List of genes alternatively spliced in the absence of TDP-43 identified by both MAJIQ and LeafCutter
Detection of STMN2 and UNC13A splice variants in bulk RNA-sequencing of patient tissues from the NYGC ALS Consortium cohort
List of all the primers used in the study
Acknowledgements
This work was supported by NIH grants R35NS097263(10) (A.D.G.), R35NS097273(17) (L.P.), U54NS123743 (A.D.G., L.P., M.P. and W.W.S.), P01NS084974 (L.P.), R01NS104437 (W.W.S.), RF1NS120992 (M.P.), F32 NS116208-02 (C.M.R.), T32 GM007231 (G. Mekonnen), 2T32AG047126-06A1 (T.A.), F32GM139268 (L.G.), RF1AG071326 (S. Myong.), RF1NS113636 (S. Myong), the Robert Packard Center for ALS Research at Johns Hopkins (L.P., J.S. and A.D.G.), P30AG06267 (R.C.P.), U01AG006786 (R.C.P.), 2T32HG000044-21 NIHGRI training grant (X.R.M.), the Brain Rejuvenation Project of the Wu Tsai Neurosciences Institute (A.D.G.), Target ALS (J.S.), Amyotrophic Lateral Sclerosis Association (J.S.), and the Office of the Assistant Secretary of Defense for Health Affairs through the Amyotrophic Lateral Sclerosis Research Program (W81XWH-20-1-0242 to J.S.). G.K. is supported by a fellowship from the Stanford Knight-Hennessy Scholars Program. A.B. is supported by a Fulbright Future Scholarship. Y.K. is supported by Milton Safenowitz Postdoctoral Fellowship Program from the Amyotrophic Lateral Sclerosis Association (21-PDF-582). S.P. is supported by a BrightFocus ADR Grant (A2020279F). T.A. is supported by a fellowship from the Takeda Science Foundation. The UCSF Neurodegenerative Disease Brain Bank receives funding support from NIH grants P30AG062422, P01AG019724, U01AG057195 and U19AG063911, as well as the Rainwater Charitable Foundation and the Bluefield Project to Cure FTD. Some of the computing for this project was performed on the Sherlock cluster. We would like to thank Stanford University and the Stanford Research Computing Center for providing computational resources and support that contributed to these research results.
Extended data figures and tables
Source data
Author contributions
X.R.M., M.P., L.P. and A.D.G. designed the experiments. X.R.M. and A.D.G. wrote the paper. X.R.M., M.P., Y.K. performed experiments and analysed data. S.C.V. and W.W.S. performed and analysed the UNC13A BaseScope experiments on patient samples. G.K. performed the UNC13A immunoblotting experiments. C.M.R. helped perform RT–PCR analyses. F.H., D.W.W., K.K., G.M., S.M., N.S. and E.M.G. performed TDP-43 knockdown experiments and analysed data. A.B. and H.B.S. performed some of the minigene reporter assays. C.G. analysed additional TDP-43 target genes. T.A. generated and analysed induced neurons for TDP-43 knockdown experiments. B.B.C. helped with UNC13A human genetics analyses. G.M., L.G., J.D.R., J.S. and S.M. performed TDP-43 RNA-binding studies and analysed data. K.J-W., C.N.C. and S.P. generated TDP-43 splicing reporter constructs. B.O., N.R.G-R., B.F.B., D.S.K., R.C.P. and D.W.D. contributed patient samples.
Peer review
Peer review information
Nature thanks Noa Lipstein, Magdalini Polymenidu and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The amplicon sequencing data has been deposited in the Gene Expression Omnibus (GEO) at GSE182976. RNA-seq data for splicing analysis is available at GSE126543. RNA-seq data generated by NYGC ALS Consortium cohort is available at GSE137810, GSE124439, GSE116622 and GSE153960. Source data are provided with this paper.
Code availability
All codes used in this study are available at (https://github.com/rosaxma/TDP-43-UNC13A-2021) and (10.5281/zenodo.5770954).
Competing interests
A.D.G. is a scientific founder of Maze Therapeutics. X.R.M. served as a consultant for Maze Therapeutics. M.P. and L.P. serve as consultants for Target ALS. F.H., B.B.C., D.W.W., K.K., G. Miller, S. Mekhoubad, N.S. and E.G. are employees of Maze Therapeutics, which has filed a patent (63/171,522) on methods to modulate splicing of UNC13A.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: X. Rosa Ma, Mercedes Prudencio, Yuka Koike
Contributor Information
Leonard Petrucelli, Email: petrucelli.leonard@mayo.edu.
Aaron D. Gitler, Email: agitler@stanford.edu
Extended data
is available for this paper at 10.1038/s41586-022-04424-7.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-022-04424-7.
References
- 1.Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 2.Ling JP, Pletnikova O, Troncoso JC, Wong PC. TDP-43 repression of nonconserved cryptic exons is compromised in ALS–FTD. Science. 2015;349:650–655. doi: 10.1126/science.aab0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melamed Z, et al. Premature polyadenylation-mediated loss of stathmin-2 is a hallmark of TDP-43-dependent neurodegeneration. Nat. Neurosci. 2019;22:180–190. doi: 10.1038/s41593-018-0293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klim JR, et al. ALS-implicated protein TDP-43 sustains levels of STMN2, a mediator of motor neuron growth and repair. Nat. Neurosci. 2019;22:167–179. doi: 10.1038/s41593-018-0300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Es MA, et al. Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat. Genet. 2009;41:1083–1087. doi: 10.1038/ng.442. [DOI] [PubMed] [Google Scholar]
- 6.Diekstra FP, et al. C9orf72 and UNC13A are shared risk loci for amyotrophic lateral sclerosis and frontotemporal dementia: A genome-wide meta-analysis. Ann. Neurol. 2014;76:120–133. doi: 10.1002/ana.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagier-Tourenne C, Polymenidou M, Cleveland DW. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum. Mol. Genet. 2010;19:46–64. doi: 10.1093/hmg/ddq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donde A, et al. Splicing repression is a major function of TDP-43 in motor neurons. Acta Neuropathol. 2019;138:813–826. doi: 10.1007/s00401-019-02042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun M, et al. Cryptic exon incorporation occurs in Alzheimer’s brain lacking TDP-43 inclusion but exhibiting nuclear clearance of TDP-43. Acta Neuropathol. 2017;133:923–931. doi: 10.1007/s00401-017-1701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong YH, et al. Tdp-43 cryptic exons are highly variable between cell types. Mol. Neurodegener. 2017;12:13. doi: 10.1186/s13024-016-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prudencio M, et al. Truncated stathmin-2 is a marker of TDP-43 pathology in frontotemporal dementia. J. Clin. Invest. 2020;130:6080–6092. doi: 10.1172/JCI139741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu EY, et al. Loss of nuclear TDP-43 is associated with decondensation of LINE retrotransposons. Cell Rep. 2019;27:1409–1421. doi: 10.1016/j.celrep.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaquero-Garcia J, et al. A new view of transcriptome complexity and regulation through the lens of local splicing variations. eLife. 2016;5:e11752. doi: 10.7554/eLife.11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YI, et al. Annotation-free quantification of RNA splicing using LeafCutter. Nat. Genet. 2018;50:151–158. doi: 10.1038/s41588-017-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiga A, et al. Alteration of POLDIP3 splicing associated with loss of function of TDP-43 in tissues affected with ALS. PLoS ONE. 2012;7:e43120. doi: 10.1371/journal.pone.0043120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carithers LJ, et al. A novel approach to high-quality postmortem tissue procurement: the GTEx project. Biopreserv. Biobank. 2015;13:311–319. doi: 10.1089/bio.2015.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tollervey JR, et al. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat. Neurosci. 2011;14:452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:95–104. doi: 10.1093/genetics/77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipstein N, et al. Synaptic UNC13A protein variant causes increased neurotransmission and dyskinetic movement disorder. J. Clin. Invest. 2017;127:1005–1018. doi: 10.1172/JCI90259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng L, Kaeser PS, Xu W, Südhof TC. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of munc13. Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Augustin I, Rosenmund C, Südhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- 22.Böhme MA, et al. Active zone scaffolds differentially accumulate Unc13 isoforms to tune Ca2+ channel-vesicle coupling. Nat. Neurosci. 2016;19:1311–1320. doi: 10.1038/nn.4364. [DOI] [PubMed] [Google Scholar]
- 23.Nicolas A, et al. Genome-wide analyses identify KIF5A as a novel ALS gene. Neuron. 2018;97:1268–1283. doi: 10.1016/j.neuron.2018.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Placek K, et al. UNC13A polymorphism contributes to frontotemporal disease in sporadic amyotrophic lateral sclerosis. Neurobiol. Aging. 2019;73:190–199. doi: 10.1016/j.neurobiolaging.2018.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pottier C, et al. Genome-wide analyses as part of the international FTLD–TDP whole-genome sequencing consortium reveals novel disease risk factors and increases support for immune dysfunction in FTLD. Acta Neuropathol. 2019;137:879–899. doi: 10.1007/s00401-019-01962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Blitterswijk M, et al. Genetic modifiers in carriers of repeat expansions in the C9ORF72 gene. Mol. Neurodegener. 2014;9:38. doi: 10.1186/1750-1326-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidal-Taboada JM, et al. UNC13A confers risk for sporadic ALS and influences survival in a Spanish cohort. J. Neurol. 2015;262:2285–2292. doi: 10.1007/s00415-015-7843-z. [DOI] [PubMed] [Google Scholar]
- 28.Yang B, et al. UNC13A variant rs12608932 is associated with increased risk of amyotrophic lateral sclerosis and reduced patient survival: a meta-analysis. Neurol. Sci. 2019;40:2293–2302. doi: 10.1007/s10072-019-03951-y. [DOI] [PubMed] [Google Scholar]
- 29.Tan H, et al. The distinct traits of the UNC13A polymorphism in amyotrophic lateral sclerosis. Ann. Neurol. 2020;88:796–806. doi: 10.1002/ana.25841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diekstra FP, et al. UNC13A is a modifier of survival in amyotrophic lateral sclerosis. Neurobiol. Aging. 2012;33:630.e3–630.e8. doi: 10.1016/j.neurobiolaging.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 31.Auton A, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaid DJ, Chen W, Larson NB. From genome-wide associations to candidate causal variants by statistical fine-mapping. Nat. Rev. Genet. 2018;19:491–504. doi: 10.1038/s41576-018-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallagher MD, Chen-Plotkin AS. The post-GWAS era: from association to function. Am. J. Hum. Genet. 2018;102:717–730. doi: 10.1016/j.ajhg.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruim RJ, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2011;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Rheenen W, et al. Genome-wide association analyses identify new risk variants and the genetic architecture of amyotrophic lateral sclerosis. Nat. Genet. 2016;48:1043–1048. doi: 10.1038/ng.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt HB, Barreau A, Rohatgi R. Phase separation-deficient TDP43 remains functional in splicing. Nat. Commun. 2019;10:4890. doi: 10.1038/s41467-019-12740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown, A.-L. et al. TDP-43 loss and ALS-risk SNPs drive mis-splicing and depletion of UNC13A. Nature10.1038/s41586-022-04436-3 (2022). [DOI] [PMC free article] [PubMed]
- 38.Chiò A, et al. UNC13A influences survival in Italian amyotrophic lateral sclerosis patients: a population-based study. Neurobiol. Aging. 2013;34:357.e1–5. doi: 10.1016/j.neurobiolaging.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson PT, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain. 2019;142:1503–1527. doi: 10.1093/brain/awz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maury Y, et al. Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nat. Biotechnol. 2015;33:89–96. doi: 10.1038/nbt.3049. [DOI] [PubMed] [Google Scholar]
- 42.Bieri G, et al. LRRK2 modifies α-syn pathology and spread in mouse models and human neurons. Acta Neuropathol. 2019;137:961–980. doi: 10.1007/s00401-019-01995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prudencio M, et al. Repetitive element transcripts are elevated in the brain of C9orf72 ALS/FTLD patients. Hum. Mol. Genet. 2017;26:3421–3431. doi: 10.1093/hmg/ddx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen KD, Brenner SE, Dudoit S. Biases in Illumina transcriptome sequencing caused by random hexamer priming. Nucleic Acids Res. 2010;38:e131. doi: 10.1093/nar/gkq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nana AL, et al. Neurons selectively targeted in frontotemporal dementia reveal early stage TDP-43 pathobiology. Acta Neuropathol. 2019;137:27–46. doi: 10.1007/s00401-018-1942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prudencio M, et al. Misregulation of human sortilin splicing leads to the generation of a nonfunctional progranulin receptor. Proc. Natl Acad. Sci. USA. 2012;109:21510–21515. doi: 10.1073/pnas.1211577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang YJ, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc. Natl Acad. Sci. USA. 2009;106:7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hallegger M, et al. TDP-43 condensation properties specify its RNA-binding and regulatory repertoire. Cell. 2021;184:4680–4696. doi: 10.1016/j.cell.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kent WJ, et al. The Human Genome Browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seyfried NT, et al. Multiplex SILAC analysis of a cellular TDP-43 proteinopathy model reveals protein inclusions associated with SUMOylation and diverse polyubiquitin chains. Mol. Cell. Proteomics. 2010;9:705–718. doi: 10.1074/mcp.M800390-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ayala YM, et al. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J. 2011;30:277–288. doi: 10.1038/emboj.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Supplementary Tables 3–5 and Supplementary Notes 1–3.
Uncropped images of immunoblots, PCR gels, and images of EMSA presented in this study.
List of genes alternatively spliced in the absence of TDP-43 identified by both MAJIQ and LeafCutter
Detection of STMN2 and UNC13A splice variants in bulk RNA-sequencing of patient tissues from the NYGC ALS Consortium cohort
List of all the primers used in the study
Data Availability Statement
The amplicon sequencing data has been deposited in the Gene Expression Omnibus (GEO) at GSE182976. RNA-seq data for splicing analysis is available at GSE126543. RNA-seq data generated by NYGC ALS Consortium cohort is available at GSE137810, GSE124439, GSE116622 and GSE153960. Source data are provided with this paper.
All codes used in this study are available at (https://github.com/rosaxma/TDP-43-UNC13A-2021) and (10.5281/zenodo.5770954).