Abstract
Background
The relationship of atrial fibrillation (AF) with coronary artery disease (CAD) is well established, yet it is often missed. There is evidence of myocardial ischemia on stress imaging in AF patients in the absence of obstructive CAD. In this prospective cohort, we studied the angiographic profiles of non-valvular AF patients.
Methods
The study was a nonrandomized, prospective, single-center observational study of consecutive patients of persistent non-valvular AF. Patients symptomatic for AF despite optimal medical therapy for 3 months were recruited and all underwent coronary angiograms (CAG). Patients with prior history of CAD were excluded.
Results
A total of 70 patients were followed for a mean duration of 12 ± 1.4 months. The mean age of the study group was 66.07 (±11.49) years. Hypertension was the commonest comorbidity seen in 74% patients. Obstructive CAD was present in 32 (46%) patients, non-obstructive (<50% stenosis) CAD in 17 (24%) patients and normal coronaries in 21 (30%) patients. Overall 49 (70%) patients had evidence of CAD. Amongst patients without obstructive CAD, slow flow was seen in 16 (42%) patients. Lower baseline ejection fraction, lower haemoglobin & albumin levels and higher creatinine levels was associated with increased mortality. In patients without obstructive CAD, hospitalizations for fast ventricular rate were significantly increased in those having slow flow on CAG (p = 0.005).
Conclusions
Majority (70%) of our patients had evidence of atherosclerotic CAD on CAG. A large proportion of patients without obstructive CAD had slow flow on CAG.
Keywords: Atrial fibrillation, Coronary angiography, Coronary artery disease, Coronary slow flow, Fast ventricular rate
Highlights
-
•
Coexistent coronary artery disease is common in symptomatic non-valvular atrial fibrillation (AF).
-
•
Revascularization was needed in 35.7% of patients.
-
•
Lower ejection fraction, haemoglobin and serum albumin levels correlated with worse outcomes.
-
•
Coronary slow-flow is highly prevalent in AF patients (42%) and has a bearing of future events.
-
•
Hospitalizations for fast ventricular rate were significantly increased in those having slow flow.
1. Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrythmia worldwide characterized by irregular fibrillatory waves on electrocardiogram with an overall prevalence of around 1–2%, though the prevalence increases with age and is around 10% over the age of 80 years.1 AF shares a unique relation with coronary artery disease (CAD) right from the similar risk factors and comorbidities, to similar pathophysiology; inflammation being common to both, and the unique therapeutic and prognostic implications when the two diseases coexist.2
The prevalence of CAD in AF patients varies widely in between 18% and 46.5% in different studies.3,4 There is enough evidence that these patients have inducible myocardial ischemia even in absence of obstructive CAD.5 Coronary slow flow has independently shown to be associated with myocardial ischemia in the absence of obstructive CAD and its significance in AF patients remains to be studied.6 AF and myocardial ischemia may be more closely linked than previously appreciated and hence warrants further research. Despite tremendous progress in management of AF, certain questions remain unanswered. The impact of microvascular ischemia on AF management is one such area and further research will shed light whether it will have a bearing on AF management in future. With this in background, we aimed to study the relationship of non-valvular AF and CAD.
2. Methods
The study was a nonrandomized, prospective, single-center observational study of consecutive patients of persistent non-valvular AF presenting to outpatient cardiology department of a tertiary care center of North India. We aimed to study the clinical and angiographic profiles of patients with AF and to determine the significance of association of baseline parameters with significant CAD. The study protocol is in line with ethical guidelines of the Declaration of Helsinki and was approved by the institute's ethical committee (INT/IEC/2019/000758 dated 09/04/2019). Informed written consent was obtained from all patients or their legal representatives.
2.1. Patient selection
The study enrolled patients over a period of 2 years (January 2019 to January 2021).
Inclusion criteria.
-
1)
Age >18 years
-
2)
Persistent AF
-
3)
NYHA class ≥ II (angina/dyspnea/palpitations) despite goal directed medical therapy (GDMT) ≥3 months
Exclusion criteria.
-
1)
Asymptomatic patients
-
2)
≥ Moderate valvular heart disease
-
3)
Established CAD or past history of myocardial infarction
-
4)
Not willing for angiography
-
5)
Having contraindications for angiography
Symptomatic patients >18 years with persistent AF were recruited and all underwent electrocardiograms (ECG), echocardiograms, and blood investigations including complete blood counts, renal and hepatic parameters and thyroid function tests.
2.2. Coronary angiography
CAG was performed by a single trained interventional cardiologist. All angiograms were assessed separately by 2 interventional cardiologists. Angiograms were classified as having obstructive CAD (≥50% stenosis), non-obstructive CAD (<50% stenosis) or having normal coronaries without any evidence of atherosclerosis. Those without obstructive CAD underwent further analysis to seek alternate mechanism that could lead to myocardial ischemia. The prevalence of slow flow in the coronary arteries was also analyzed as a manifestation of endothelial dysfunction. Slow flow in the epicardial coronaries was defined as corrected TIMI frame count greater than 27 (at cinefilming 30 frames per second (fps) and heart rate 60-110bpm) after correction for vessel length. Frame count starts at first frame where dye fully enters the artery touching both borders at origin of the artery. The last frame of the count is when the dye enters distal landmark branch which is the distal bifurcation of left anterior descending (LAD), distal bifurcation of the longest obtuse marginal in left circumflex artery (LCX) and the first branch of posterolateral artery in right coronary artery (RCA).6,7
All patients received GDMT as per guidelines and revascularization was performed where indicated. The medical therapy aimed at 3 of the main therapeutic goals:- Anticoagulation guided by the individual stroke and bleeding risk, better symptom control (our default strategy was rate control) and the last was modification and control of underlying cardiovascular risk factors: hypertension, diabetes, obesity, thyroid illness, heart failure etc. Decision to anticoagulate was based upon patients CHA2DS2-VASc and HAS-BLED scores following the recent AHA/ACC and ESC guidelines for management of AF.8,9
2.3. Statistical analysis
All data was prospectively collected by trained physicians (study authors) and entered into a spreadsheet (Microsoft Excel 2016™; Microsoft corporation, USA). Statistical analysis was done via statistical package for social sciences (SPSS Inc., version 23.0™; IBM corporation, Chicago, USA). Continuous variables were summarized as mean ± standard deviation and categorical variables as frequencies (%). The comparison between two groups with continuous variables was done using Wilcoxon–Mann–Whitney test or Student's t-test and for categorical variables using chi-square or Fisher exact test. All p values are two-tailed and a statistical value of 0.05 or less was considered significant.
3. Results
3.1. Baseline clinical characteristics
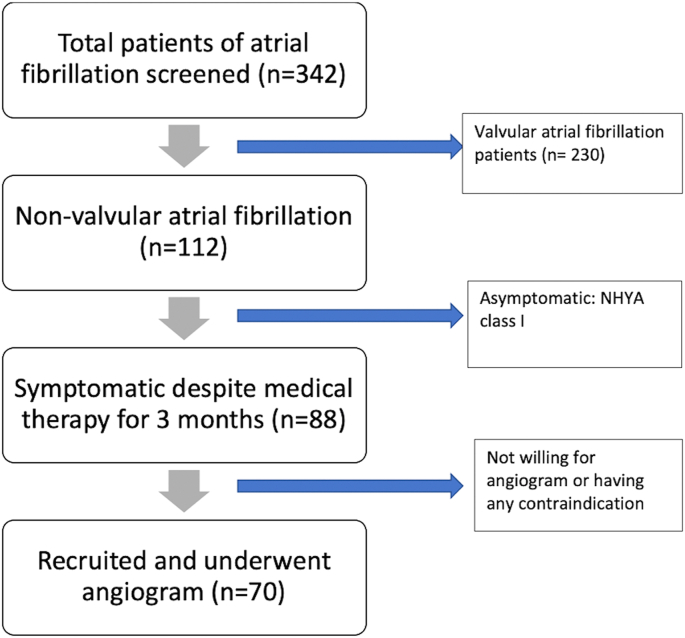
A total of 70 symptomatic patients underwent CAG and were followed up for a minimum of 9 months (Fig. 1). The mean age was 66.07 ± 11.496 years (range 33–88 years). Hypertension was the commonest comorbidity present in 52 patients (74.3%) followed by obesity in 25 (35.7%) and diabetes in 21 patients (30%). All patients were having symptoms of severity NYHA Class ≥ II at the time of enrolment into the study. Dyspnoea on exertion was the commonest symptom present in 61 patients (87.1%) followed by palpitations in 29 patients (41.4%). Only 13 patients (18.6%) had angina as a symptom. The mean left ventricular ejection fraction (LVEF) of the study population was 46.2% (±13.7) and 22 patients (31.4%) had severe LV systolic dysfunction (LVEF≤30%) at presentation. Baseline patient characteristics are shown in Table 1.
Fig. 1.
Flow diagram showing the patient recruitment process during the study.
Table 1.
Baseline patient characteristics.
| Variable | Frequency |
|---|---|
| Mean Age in years (±SD) | 66.07 (±11.496) |
| Sex, n (%) | |
| Female | 27 (38.6%) |
| Male | 43 (61.4%) |
| Mean BMI (kg/m2) (±SD) | 24.98 (±3.72) |
| Co-morbidities, n (%) | |
| Diabetes mellitus | 21 (30%) |
| Hypertension | 52 (74.3%) |
| CKD | 10 (14.3%) |
| Past history of CVA | 7 (10%) |
| Smoking | 14 (20%) |
| Significant alcohol intake ( | 26 (37.1%) |
| Dyslipidemia | 18 (25.7%) |
| Obesity (BMI >25 kg/m2) | 25 (35.7%) |
| Anemia | 36 (51.4%) |
| Presenting complaints, n (%) | |
| Dyspnea on exertion | 61 (87.1%) |
| Palpitations | 29 (41.4%) |
| Exertional chest pain | 13 (18.6%) |
| Dizziness/presyncope | 12 (17.1%) |
| Echocardiographic parameters | |
| Mean LVEF (%) (±SD) | 46.2 (±13.7) |
| Normal (50%–70%) | 29 (41.4%) |
| Mild dysfunction (40%–50%) | 7 (10%) |
| Moderate dysfunction (30%–40%) | 12 (17.1%) |
| Severe dysfunction (<30%) | 22 (31.4%) |
| Mean CHA2DS2-VASc score ±SD) | 3.16 (±1.28) |
| Mean HAS-BLED score (±SD) | 2.16 (±1.28) |
| Drug usage, n (%) | Frequency |
| Aspirin | 50 (71.4%) |
| DAPT (aspirin + P2Y12 inhibitors) | 32 (45.7%) |
| ACE inhibitors/ARBs | 37 (50.7%) |
| Beta blockers | 67 (90.4%) |
| Flecainide | 1 (1.4%) |
| Calcium channel blockers | 5 (6.8%) |
| Amiodarone | 16 (21.9%) |
| Digoxin | 4 (5.5.%) |
| Mineralocorticoid receptor antagonists | 27 (37%) |
| Parenteral iron | 9 (12.3%) |
| Anticoagulation, n (%) | |
| None | 20 (28.6%) |
| Vitamin K antagonists | 20 (28.6%) |
| NOACs | 30 (42.8%) |
| Dabigatran | 22 (31.4%) |
| Rivaroxaban | 5 (7.1%) |
| Apixaban | 3 (4.3%) |
| CHA2DS2-VASc 1 | 8 (11.4%) |
| Appropriate anticoagulation use (CHA2DS2-VASc ) | 50/62 (80.6%) |
Abbreviations: CKD; chronic kidney disease, CVA; cerebro-vascular accident, BMI; body mass index, LVEF; left-ventricular ejection-fraction, DAPT; dual-antiplatelet therapy, ACE; angiotensin convertase enzyme, ARB; angiotensin receptor blocker, NOAC; newer oral anticoagulant agents.
3.2. Angiographic profile
All 70 patients were taken up for CAG after informed consent. Evidence of atherosclerotic CAD was seen in a total of 49 (70%) of the patients while only 21 (30%) patients had normal coronaries. Amongst those with atherosclerotic disease, 32 (45.7%) patients had obstructive CAD while 17 (24.3%) had non obstructive CAD. Table 2 summarizes the angiographic profiles. Overall, 21 (30%) of patients underwent PCI and another 4 (5.7%) of patients were referred for CABG.
Table 2.
Angiographic profiles of atrial fibrillation patients.
| Variable | Frequency |
|---|---|
| Pattern of CAD, n (%) | |
| Obstructive CAD | 32 (45.7%) |
| Non obstructive CAD (<50% stenosis) | 17 (24.3%) |
| Normal coronaries | 21 (30%) |
| Coronary involvement pattern (Obstructive CAD), n (%) | |
| Left main | 2 (2.9%) |
| LAD | 29 (41.4%) |
| LCX | 20 (28.6%) |
| RCA | 22 (31.4%) |
| Amongst obstructive CAD, n (%) | |
| Single vessel disease | 7 (10%) |
| Double vessel disease | 11 (15.7%) |
| Triple vessel disease | 14 (20%) |
| Chronic total occlusion | 4 (5.7%) |
| Severe calcification | 7 (10%) |
| Percutaneous intervention | 21 (30%) |
| Coronary artery bypass referral | 4 (5.7%) |
| No obstructive CAD cohort, n (%) | Total = 38 |
| Slow flow | 16 (42.1%) |
| Slow flow territory, n (%) | (total = 16) |
| LAD only | 4 (25%) |
| RCA only | 2 (12.5%) |
| LCX only | 0 |
| All territories | 10 (62.5%) |
Abbreviations: CAD; coronary artery disease, LAD; left anterior descending artery, LCX; left circumflex artery, RCA; right coronary artery.
Post revascularisation majority of patients reported an improvement in their EuroQoL scores. Out of the 25 (35.7%) of patients who underwent revascularization the EuroQoL score improved by at least one grade in 14 (56%) out of 25 patients though most patients still continued to remain in AF post revascularization. Only 2 (8%) out of the 25 had reverted to sinus rhythm postintervention and continued to do so 9 months into follow up. The mean LVEF of the 25 patients was 45.6% (±7.25) preintervention and 46.6% (±7.70) postintervention, which was statistically non-significant (p = 0.09).
The CAGs of 38 (54.3%) patients who did not have obstructive CAD, were further analyzed to look into any evidence of slow flow. Slow flow in one or more coronary territory was seen in 16 patients (42.1%).
3.3. Outcomes and predictors of outcome
Patients were followed up for a mean duration of 12 ± 1.4 months after CAG. Table 3 summarizes the outcomes at the end of study period. Overall 6 (8.6%) of patients died in the follow-up period. The cause of death was due to sudden cardiac death (SCD) in 3 patients and major stroke in the rest of the 3 patients. The patients who died during the follow-up had a lower mean LVEF, lower haemoglobin, lower mean albumin values and higher mean creatinine values. Also, there was a significant lower usage of new oral anticoagulants (NOACs), beta blockers and angiotensin converting enzyme inhibitors (ACEi)/Angiotensin receptor blockers (ARBs) amongst these individuals. Table S1 summarizes the difference between survivors and non survivors. A total of 17 (24.3%) patients required hospitalization for heart failure. Across all variables, no statistically significant difference was observed between the 2 groups (Table S2). Lower albumin levels (3.80 ±0 .42 gm/dL vs 4.0 ± 0.36 gm/dL; p = 0.06) were observed in the group requiring heart failure related hospitalizations, however it did not reach statistical significance.
Table 3.
Follow-up data.
| Outcomes | |
|---|---|
| Total patients, n (%) | Total = 70 |
| Total deaths | 6 (8.6%) |
| Heart failure hospitalizations | 17 (24.3%) |
| Hospitalizations for Fast ventricular rate | 16 (22.9%) |
| Strokes | 6 (8.6%) |
| Major bleeds | 3 (4.3%) |
Rate control was initially attempted in all patients. However 20 (28.5%) patients remained symptomatic or had difficulty in controlling heart rate and subsequently were subjected to rhythm control strategy. Catheter ablation was not considered given the limited success and significant recurrence rate in patients with persistent AF. Echocardiographic LVEF assessment was achieved in 54 patients at the end of 9 month follow up. The mean LVEF in these 54 patients increased from 48.1% (±13.5) to 50.27% (±11.23) at 9 months, though it was not statistically significant (p = 0.18). Those patients who had a heart rate >110/min and significant non-tolerable symptoms necessitating visit to cardiac emergency were considered hospital admission and stabilization. Patients who did not have symptoms disturbing routine activities and a fast ventricular rate were managed medically on OPD basis.
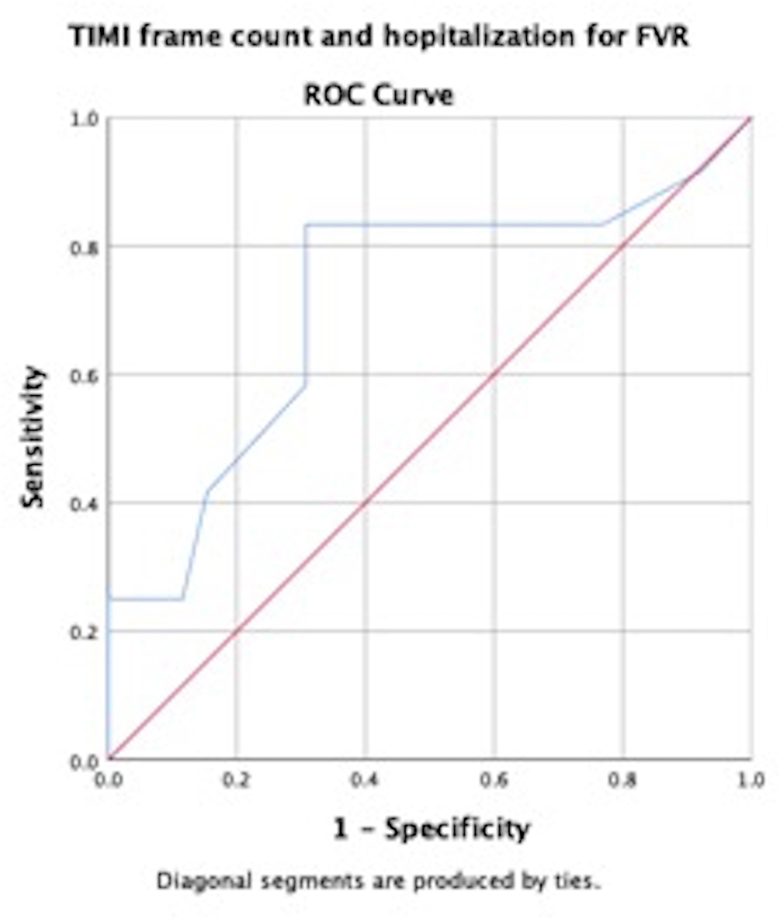
Hospitalizations were observed in 16 (22.9%) patients. Patients having slow flow on CAG had significantly increased FVR hospitalization as compared to those who did not have slow flow (p = 0.005). Accordingly, the mean TIMI frame count (35.3 ± 11 vs 25.8 ± 8.9; p = 0.008) was higher in patients being admitted for FVR compared to those not requiring hospitalization for FVR. TIMI frame count >31 had a sensitivity of 83% and a specificity of 69% for predicting hospitalizations for FVR (AUROC = 0.71) Fig. S1. Use of parenteral iron therapy for correction of iron deficiency anaemia also resulted in fewer FVR hospitalizations (p = 0.05) (Table S3).
Cerebrovascular accident (CVA) occurred in 6 patients (8.6%) on follow up; all were ischaemic stroke. Three patients (4.3%) were on warfarin, and all of them had sub-therapeutic international normalized ratio (INR) at the time of event. Another 3 patients were on NOACs, 2 (2.8%) of them on dabigatran and 1 (1.4%) on rivaroxaban, but again all of them were non-compliant to treatment regimen during the weeks preceding the event. At the end of follow-up only 38 (54.2%) were compliant to anticoagulation therapy with adequate INR monitoring. Major bleed was seen in 3 (4.3%) patients, requiring blood transfusion or any other intervention. One patient on dabigatran had gastrointestinal bleed and the other two other patients were on warfarin and had haematuria.
4. Discussion
The current study is amongst the few prospective studies showcasing the coronary angiographic profile of patients with non-valvular AF and its association with short term survival. The average age of diagnosis of AF is a decade younger in India compared to the western population largely owing to the increased burden of rheumatic heart disease in the country. Also, larger number of patients have persistent/permanent AF and there is a greater likelihood of stroke compared to the western data.10 With improvement in health care system and better survival in India, the prevalence of Non-valvular AF is projected to increase owing to an increase in elderly population. This coupled with poor implementation of GDMT will translate into significant morbidity and mortality in our country.11 Access to therapeutic options including electrophysiology studies and ablation is limited and medical management remains the main stay of treatment in the vast majority of this population,12 and hence diagnosing contributory factors that initiate and perpetuate AF is essential. Myocardial ischemia is closely related to AF but is often underrecognized. Co-existent CAD is often missed due to similar presentation of these two disease entities. Diagnosing co-existent CAD is important as optimal medical therapy (statins/antiplatelets/antianginals etc.) and revascularization where indicated can decrease the morbidity and mortality associated with AF.13,14 CAD and AF can not only promote each other but also worsen the preexisting condition. CAD leads to AF progression by affecting blood supply to atrial myocardium and conductive tissue and affecting re-entry, aberrant ectopic activity and neural remodelling. On the other hand AF can promote and worsen CAD by leading to mismatch of blood supply and oxygen consumption, initiating atherosclerosis and increased likelihood of thrombosis.13
The baseline parameters of our study match to that of prior literature. The mean age here was 66 ± 11 yrs which is similar to the western registries and is around a decade higher than prior Indian registries, which predominately comprised of rheumatic heart disease population. In IHRS-AF registry, the mean age was around 54 years, but the recent Kerala AF registry had mean age of 65 years as it had predominantly non-valvular cohort. Hypertension, the commonest comorbidity present in about 74% of our population was similar to the international RELIASE-AF and GARFIELD AF registries where hypertension was present in 78% and 76% of the patients respectively,15,16 but is higher compared to Indian IHRS-AF and Kerala AF registries where hypertension was seen in 31% and 53% respectively.17,18 Education regarding nature and management of various risk factors and comorbidities contributing to AF were given to all patients. Since alcohol consumption is a well-known risk factor for AF, patients were counselled regarding avoidance of alcohol.19 Of the 26 patients counselled, 19 (73.1%) reported to have stopped alcohol intake within 2 weeks of follow up. The rest continued to take alcohol but reported decrease in amount compared to prior consumption. The mean CHA2DS2-VASc score of our patient population was 3.16 (±1.28) reflecting a population at higher ischemic risk.
The most important finding of our study is a much higher prevalence of CAD (70%) compared to the previously published literature and amongst these about 2/3rd of these had obstructive CAD. In the study by Kralev et al, which also included symptomatic due to AF, the incidence of CAD in patients undergoing first time CAG was around 34%,20 the incidence in the AFFIRM study was around 38%.4 However a study by Nucifora et al showed the prevalence of CAD by computed tomography coronary angiography to be around 82% which more closely resembled our data.21 This could reflect the increased prevalence of comorbidities in our study group. Triple vessel involvement was the commonest pattern and we also found all three vessels (LAD, LCx and RCA) were equally involved in our study. However, previous studies have which showed AF to be more common when RCA and LCX was involved especially when proximal to atrial branches.20 This reflects that besides ischemia of the atria, ventricular myocardial ischemia and resultant decreased ventricular compliance and increased LV end diastolic pressure may be leading to atrial remodelling and predisposing to AF.22
The mortality rate in our group (8.6%) was higher than international data available (1.6–4.2%).23,24 Studies from India on the mortality rate our lacking. In general patients with AF have a 3.67 fold higher risk of death than age and sex matched general population.25 The increased mortality in our group could be explained by the higher comorbidities and higher prevalence of CAD both of which contribute adversely to the mortality. The mean CHA2DS2-VASc score of our population was 3.16 (±1.28) reflecting a population with higher cardiovascular risk factors. In a study by Khumri et al increasing CHA2DS2-VASc score was associated with not only higher likelihood of stroke but also death.26 Lower mean LVEF, deranged serum creatinine and lower serum albumin were independently associated with increased mortality. Lower serum albumin has previously been linked to increased incidence of AF, and our study highlights its adverse impact on prognosis in AF patients.27 Increased SCD could have been due to non-adherence to medications and underusage of implantable cardioverter defibrillator. Usage of ACEi/ARBs and beta blockers was associated with improved survival. These drugs have proven benefit in patients with low LVEF, however mortality benefit in AF patients with normal LVEF has not been demonstrated. Since ours was a small study group with a mixed cohort, larger randomized studies are needed to confirm this observation.
Higher percentage (81%) of our patients were on anticoagulation according to the guidelines, which is in contrast to international and national registries in which only around 50% of patients were on appropriate anticoagulation.12,15,17 Also higher percentage of our patients were on NOACs compared to VKAs, which again resembles the western data and reflects the growing physician and patient preference for NOACs. Better survival observed in the NOAC group, could be explained by the fact that warfarin was used more frequently in the group with deranged renal functions which was independently associated with increased mortality.
Another important observation in our study was higher prevalence of slow flow (42%) in those without obstructive CAD as compared to general population of around 5%.28 Slow flow can cause myocardial ischemia independent of obstructive CAD.6 The fact that slow flow was associated with significantly increased hospitalization for fast ventricular rate merits consideration. Subclinical ischemia leads to electrical dispersion and promotes re-entry and alters neural remodelling which ultimately culminates in AF.13 Uncorrected ischemia could periodically be the trigger for episodes of AF with FVR.
The major limitation of the study was the small sample size of population and short term follow-up which limits the results of to be generalized to wider population. As a part of study the patient population was closely followed up which may not be always possible in real world setting. The lack of an age and comorbidities matched control arm is also a major limitation. Also, we were unable to perform non-invasive tests for demonstrating myocardial ischemia and correlate it with slow flow in those without obstructive CAD.
5. Conclusions
A significant proportion of patients of non-valvular AF who are symptomatic despite GDMT have associated CAD. Patients with AF are at high risk of stroke and mortality because of the low rates of compliance to anticoagulation therapy and adequate INR monitoring. Coronary slow flow can lead to myocardial ischemia independent of obstructive CAD. AF patients have a much higher prevalence of coronary slow flow (42% in our study) compared to the general population.
Patient consent
Written informed consent obtained from all participants.
Ethical approval
The study protocol conforms to the ethical guidelines of the declaration of Helsinki and was reviewed and cleared by the ethical committee of the institute.
Declaration of competing interest
None.
No financial relationships.
Acknowledgments
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2021.12.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
References
- 1.Colilla S., Crow A., Petkun W., Singer D.E., Simon T., Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol. 2013;112(8):1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Staerk L., Sherer J.A., Ko D., Benjamin E.J., Helm R.H. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. 2017;120(9):1501–1517. doi: 10.1161/CIRCRESAHA.117.309732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krahn A.D., Manfreda J., Tate R.B., Mathewson F.A., Cuddy T.E. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98(5):476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 4.Investigators Affirm. Baseline characteristics of patients with atrial fibrillation: the AFFIRM Study. Am Heart J. 2002;143(6):991–1001. doi: 10.1067/mhj.2002.122875. [DOI] [PubMed] [Google Scholar]
- 5.Elabbassi W., Chowdhury M.A., Liska B., Hatala R. vol. 2014. 2014. (Clinical Profile and Angiographic Findings Among Patients with Atrial Fibrillation Presenting for Selective Coronary Angiography). Health (N Y) [Google Scholar]
- 6.Wang X., Nie S.-P. The coronary slow flow phenomenon: characteristics, mechanisms and implications. Cardiovasc Diagn Ther. 2011;1(1):37. doi: 10.3978/j.issn.2223-3652.2011.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson C.M., Cannon C.P., Daley W.L., et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93(5):879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 8.Hindricks G., Potpara T., Dagres N., et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. 2021. [DOI] [PubMed] [Google Scholar]
- 9.January C.T., Wann L.S., Calkins H., et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2019;74(1):104–132. doi: 10.1016/j.jacc.2019.01.011. 2019. [DOI] [PubMed] [Google Scholar]
- 10.Raja D.C., Kapoor A. Epidemiology of atrial fibrillation-an Indian perspective. J Assoc Phys India. 2016;64(Suppl):7–10. [PubMed] [Google Scholar]
- 11.Narasimhan C., Verma J.S., Kishore A.R., et al. Cardiovascular risk profile and management of atrial fibrillation in India: real world data from RealiseAF survey. Indian Heart J. 2016;68(5):663–670. doi: 10.1016/j.ihj.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawhney J.P., Kothiwale V.A., Bisne V., et al. Risk profiles and one-year outcomes of patients with newly diagnosed atrial fibrillation in India: insights from the GARFIELD-AF Registry. Indian Heart J. 2018;70(6):828–835. doi: 10.1016/j.ihj.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang F., Wang Y. Coronary heart disease and atrial fibrillation: a vicious cycle. Am J Physiol Heart Circ Physiol. 2021;320(1):H1–H12. doi: 10.1152/ajpheart.00702.2020. [DOI] [PubMed] [Google Scholar]
- 14.Martin R., Bates M. Management of atrial fibrillation and concomitant coronary artery disease. Contin Cardiol Educ. 2017;3(2):47–55. [Google Scholar]
- 15.Chiang C.-E., Naditch-Brûlé L., Murin J., et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol. 2012;5(4):632–639. doi: 10.1161/CIRCEP.112.970749. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg B.A., Gao H., Shrader P., et al. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am Heart J. 2017;194:132–140. doi: 10.1016/j.ahj.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Vora A., Kapoor A., Nair M., et al. Clinical presentation, management, and outcomes in the Indian heart rhythm society-atrial fibrillation (IHRS-AF) registry. Indian Heart J. 2017;69(1):43–47. doi: 10.1016/j.ihj.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopalan B.C., Namboodiri N., Abdullakutty J., et al. Kerala Atrial Fibrillation Registry: a prospective observational study on clinical characteristics, treatment pattern and outcome of atrial fibrillation in Kerala, India, cohort profile. BMJ Open. 2019;9(7) doi: 10.1136/bmjopen-2018-025901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voskoboinik A., Kalman J.M., De Silva A., et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20–28. doi: 10.1056/NEJMoa1817591. [DOI] [PubMed] [Google Scholar]
- 20.Kralev S., Schneider K., Lang S., Süselbeck T., Borggrefe M. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0024964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nucifora G., Schuijf J.D., Tops L.F., et al. Prevalence of coronary artery disease assessed by multislice computed tomography coronary angiography in patients with paroxysmal or persistent atrial fibrillation. Circ Cardiovasc Imaging. 2009;2(2):100–106. doi: 10.1161/CIRCIMAGING.108.795328. [DOI] [PubMed] [Google Scholar]
- 22.Michniewicz E., Mlodawska E., Lopatowska P., Tomaszuk-Kazberuk A., Malyszko J. Patients with atrial fibrillation and coronary artery disease–double trouble. Adv Med Sci. 2018;63(1):30–35. doi: 10.1016/j.advms.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Olsson S. Executive Steering Committee of the SIIII. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362(9397):1691–1698. doi: 10.1016/s0140-6736(03)14841-6. [DOI] [PubMed] [Google Scholar]
- 24.Wyse D. Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators: a comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 25.Lee E., Choi E.-K., Han K.-D., et al. Mortality and causes of death in patients with atrial fibrillation: a nationwide population-based study. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0209687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khumri T.M., Idupulapati M., Rader V.J., Nayyar S., Stoner C.N., Main M.L. Clinical and echocardiographic markers of mortality risk in patients with atrial fibrillation. Am J Cardiol. 2007;99(12):1733–1736. doi: 10.1016/j.amjcard.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 27.Mukamal K.J., Tolstrup J.S., Friberg J., Grønbaek M., Jensen G. Fibrinogen and albumin levels and risk of atrial fibrillation in men and women (the Copenhagen City Heart Study) Am J Cardiol. 2006;98(1):75–81. doi: 10.1016/j.amjcard.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins B.M., Stavrakis S., Rousan T.A., Abu-Fadel M., Schechter E. Coronary slow flow–prevalence and clinical correlations. Circ J. 2012;76(4):936–942. doi: 10.1253/circj.cj-11-0959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.