Abstract
Impairment of the circadian rhythm promotes lung inflammation and fibrosis in pre-clinical models. We aimed to examine whether short and/or long sleep duration and other markers of sleep-wake patterns are associated with a greater burden of lung parenchymal abnormalities on computed tomography among adults. We cross-sectionally examined associations of sleep duration captured by actigraphy with interstitial lung abnormalities (n=1,111) and high attenuation areas (n=1,416) on CT scan in the Multi-Ethnic Study of Atherosclerosis at Exam 5 (2010–2013). We adjusted for potential confounders in logistic and linear regression models for interstitial lung abnormalities and high attenuation area, respectively. High attenuation area models were also adjusted for study site, lung volume imaged, radiation dose, and stratified by body mass index. Secondary exposures were self-reported sleep duration, sleep fragmentation index, sleep midpoint, and chronotype. The mean age of those with longer sleep duration (≥8 hours) was 70 years and the prevalence of interstitial lung abnormalities was 14%. Increasing actigraphy-based sleep duration among participants with ≥8 hours of sleep was associated with a higher adjusted odds of interstitial lung abnormalities (odds ratio of 2.66 per 1-hour increment, 95% CI 1.42 to 4.99). Longer sleep duration and higher sleep fragmentation index were associated with more high attenuation area on CT among participants with a body mass index<25 kg/m2 (p-value for interaction<0.02). Self-reported sleep duration, later sleep midpoint, and evening chronotype were not associated with outcomes. Actigraphy-based longer sleep duration and sleep fragmentation were associated with a greater burden of lung abnormalities on CT scan.
Keywords: sleep duration, actigraphy, interstitial lung disease
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a chronic form of interstitial lung disease (ILD) that leads to respiratory failure and death (Lederer & Martinez, 2018). Current treatments are limited in their therapeutic scope (Flaherty et al., 2019; King et al., 2014) as IPF and other fibrotic ILDs remain among the most common indications for lung transplantation. Repetitive parenchymal lung injury followed by aberrant wound healing that leads to irreversible scarring is believed to drive pulmonary fibrosis (Zoz, Lawson, & Blackwell, 2011). Identification of risk factors that cause recurrent harm and impair recovery may further our understanding of the underlying biology of this disease and inform prognostic and therapeutic strategies.
Under-studied potential risk factors for ILD are sleep and circadian disturbances. Prior studies have largely focused on the role obstructive sleep apnea may have in pulmonary fibrosis (Kim et al., 2017; Lancaster et al., 2009). However, other sleep-related pathologies may be critical in ILD including sleep disturbances or patterns that disrupt circadian rhythms. Short and long sleep durations (potential markers of circadian misalignment) have been linked to systemic inflammation and a higher risk of cardiovascular disease and mortality (Cappuccio, Cooper, D’Elia, Strazzullo, & Miller, 2011). While circadian rhythms have not been comprehensively investigated in adults with ILD, murine models and genome-wide association studies suggest that both dysregulated sleep-wake patterns and circadian rhythm lead to disturbances in lipid metabolism (Noordam et al., 2019), adipogenesis, and collagen homeostasis (Chang et al., 2020; Cunningham et al., 2020), all of which are relevant to the pathogenesis of pulmonary fibrosis (Kim et al., 2020; Podolanczuk et al., 2017). However, prior studies that examined sleep duration with ILD relied on self-reporting (Cho, Teoh, Roberts, & Wheatley, 2019), which correlates poorly with objective assessments like actigraphy (Jackson, Patel, Jackson, Lutsey, & Redline, 2018).
Developments in computed tomography (CT)-based methods that detect lung parenchymal abnormalities (ex. reticular opacities, traction bronchiectasis) have facilitated investigations that bridge pre-clinical evidence of pathological mechanisms in pulmonary fibrosis to humans. These imaging-based methods include interstitial lung abnormalities (ILA) (Putman et al., 2016) and high attenuation areas (HAA) (Podolanczuk et al., 2016), which may capture evidence of early interstitial changes that precede the development of fulminant fibrosing disease. This is supported by their associations with ILD-related hospitalization and death, biomarkers of fibrogenesis, a lower forced vital capacity, genetic variants linked to a higher risk of ILD, a higher prevalence among 1st-degree adult relatives of patients with ILD, and correlations with lung biopsies (Hatabu et al., 2020; Hunninghake et al., 2013; Hunninghake et al., 2020; Manichaikul et al., 2017; Miller et al., 2018).
We hypothesized that long and/or short sleep duration captured by actigraphy would be associated with a greater burden of lung parenchymal abnormalities on CT scan in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort. We explored associations of lung CT findings with other markers of sleep and circadian rhythm available from actigraphy or questionnaire, including self-reported sleep duration, sleep fragmentation index, sleep midpoint, and chronotype.
METHODS
Study Design
We used data from MESA, a National Heart, Lung, and Blood Institute-sponsored longitudinal cohort study that was originally designed to examine subclinical cardiovascular disease (Bild et al., 2002). MESA enrolled 6,814 community-dwelling adults from six U.S. communities between 2000 and 2002 (Exam 1) who were the ages of 45 to 84 years and free of clinical cardiovascular disease. MESA Sleep was an ancillary study that was performed in conjunction with MESA Exam 5 between 2010 and 2013. All MESA participants were invited to participate in MESA Sleep, which collected polysomnography, actigraphy, and sleep questionnaires during an in-home examination. Inclusion and exclusion criteria have been previously described (Chen et al., 2015). Briefly, at Exam 5 all MESA participants were invited to participate in the MESA Sleep Ancillary study which consisted of PSG, actigraphy, and sleep questionnaire data collected. Participants who reported regular use of nocturnal oxygen, oral devices, or positive airway pressure devices were not invited. Of the eligible 3,879 participants, 2,261 enrolled in MESA Sleep.
Actigraphy
The Actiwatch Spectrum (PA, USA) was worn by participants on the non-dominant wrist for seven consecutive days. Data were scored in 30 second epochs as sleep or wake using the Actiware Sleep software (version 5.59) by a central sleep reading center (Brigham and Women’s Hospital, Boston, MA, USA). Total sleep duration was calculated as the sum of epochs scored as sleep in each main sleep interval averaged over all days with valid recordings, as described before (Chen et al., 2015). Sleep intervals were manually identified based on sleep diary, light sensor, and self-actuated event marker. Calculation of sleep midpoint was defined as the clock time halfway between sleep onset and sleep offset, averaged over recording nights. An average sleep midpoint before 5:00 AM was defined as an “earlier midpoint” and past 5:00 AM was defined as a “later midpoint”(Roenneberg et al., 2007). The Sleep Fragmentation Index was determined by the sum of the sleep period percentage spent with movement and the percentage of the number of immobile phases that were less than 1 minute long (Knutson, Van Cauter, Zee, Liu, & Lauderdale, 2011). Intra and inter-scorer reliability was assessed with intra-class correlation coefficients. In the study, coefficients were greater than 90% for sleep duration and sleep midpoint.
Polysomnography
A 15-channel monitor (Compumedics Somte System; Compumedics Ltd., Abbotsford, AU) was used to conduct full in-home single-night polysomnography examinations as previously detailed (Chen et al., 2015). Recordings were scored by registered polysomnologists by a central reading center (Brigham and Women’s Hospital, Boston, MA, USA) using similar methods as the Sleep Heart Health Study (Redline et al., 1998; Whitney et al., 1998). The apnea-hypopnea index (AHI, events/hour) was calculated as the average number of all apneas and hypopneas associated with a 4% desaturation per hour of sleep (Chen et al., 2015).
Sleep Questionnaire
A self-reported questionnaire adapted from the Hispanic Community Health Study/Study of Latinos questionnaire was used (Redline et al., 2014). Average self-reported sleep duration was computed by the difference between reported wake-up and bedtimes on weekdays and, separately, weekends. We used the weekday self-reported duration for the purposes of this study. The modified Horne-Östberg Morningness-Eveningess Questionnaire (MEQ) was used to determine chronotype for each participant (MEQ≤11 for eveningness and MEQ>11 for non-eveningness) (Horne & Ostberg, 1976).
CT Scan Measurements
Full lung non-contrast CT scans were performed at Exam 5 using the MESA Lung/SPIROMICS protocol (Sieren et al., 2016). ILA (present/absent) was assessed by one of five trained radiologists and defined as reticular abnormalities, honeycombing, non-emphysematous cysts, traction bronchiectasis, and/or ground-glass abnormalities with at least 5% of nondependent lung involvement (Hatabu et al., 2020). Scans with focal or unilateral reticular or ground-glass abnormalities or patch ground-glass abnormalities were labeled “indeterminate” and excluded from the analysis. HAA and percent emphysema were measured using the Pulmonary Analysis Software Suite at the University of Iowa’s Advanced Pulmonary Physiomic Imaging Laboratory as lungs were semi-automatically segmented and corrected by a trained technician. HAA and percent emphysema were defined as the percentage of voxels having attenuation values between −600 and −250 Hounsfield units (HU) (Podolanczuk et al., 2016) and below −950 HU (Barr et al., 2010), respectively.
Statistical Analysis
We initially considered categorizing actigraphy-based sleep duration based on recommendations for ideal sleep duration. However, these recommendations are largely based on studies that used self-reported sleep questionnaires, which have shown poor concordance with objective measures of sleep duration (Hirshkowitz et al., 2015; Jackson et al., 2018). Because our a priori hypothesis was that long and/or short duration would be linked to interstitial lung abnormalities, we used generalized additive models (GAMs) to plot the unadjusted association of sleep duration with ILA. We took this approach in order to divide the cohort into subgroups based on potential inflection points on the x-axis where the slope of the linear relationship changes.1 We used logistic regression models to examine the cross-sectional association of sleep duration as a continuous measure with ILA within each subgroup. Therefore, results are reported per 1-hour increment in sleep duration. Model 1 was unadjusted and Model 2 was adjusted for covariates that are potentially linked to sleep duration and ILD including age, sex, race/ethnicity, smoking status (never, former, and current), cigarette pack-years, education attainment, body mass index (BMI, kg/m2), AHI, percent emphysema, statin use, total intentional exercise, and anti-depressant use (a proxy for depressed mood). Due to potential confounding by concomitant co-morbidities leading to short or prolonged sleep duration, we also adjusted for systolic and diastolic blood pressures and diabetes (self-reported at Exam 5) (Whelton et al., 2018).
For HAA, which is a lung density-based quantitative assessment, body habitus may have a more confounding effect compared with ILA which is assessed qualitatively by a trained radiologist (Kliment et al., 2015). Therefore, we used GAMs by BMI category subgroup (<25 kg/m2, 25–30 kg/m2, ≥30 kg/m2) to identify inflection points in our HAA analysis. We then used linear regression models to examine associations of sleep duration with HAA by BMI subgroup. We transformed HAA as (−1/HAA2), which we have designated as “eta” (H), to model HAA to meet assumptions of linearity in our linear regression models (Kim et al., 2020). Model 1 was adjusted for study site, radiation dose, and lung volume imaged a priori (Podolanczuk et al., 2016). Model 2 was additionally adjusted for the same covariates as ILA model 2. We also included BMI (categorical) and “BMI (categorical) × sleep duration” interaction term in our HAA model because we analyzed by BMI subgroup. We used similar ILA and HAA regression models with other secondary exposure variables which included self-reported sleep duration, sleep fragmentation index (continuous), sleep midpoint (before vs. after 5:00 AM), and chronotype (non-evening vs. evening). For sleep fragmentation index model, results are reported per one log-unit increment of the index.
We performed exploratory stratified analysis to examine whether the associations of actigraphy-based sleep duration with ILA were modified by other sleep metrics (sleep fragmentation index, sleep midpoint, and chronotype). In other words, we were interested in whether the association of sleep duration varied in backgrounds of different sleep continuity, sleep timing, and timing preference. We tested for effect modification with the likelihood ratio test of models with and without interaction terms. Given the exploratory and hypothesis-generating nature of the stratified analyses, we did not adjust for multiple comparisons in our statistical testing. We used multiple imputation with chained equations for missing covariate data. We used SAS V9.4 (SAS Institute, Cary, NC, USA) for our analyses.
RESULTS
Study Participants
There were 1,949 MESA participants with actigraphy, polysomnography, and sleep questionnaire data, of whom 1,111 and 1,416 had Exam 5 CT ILA and HAA assessments, respectively (Figure 1). Baseline characteristics of participants with ILA assessments overall and by actigraphy-based sleep duration of less than 6 hours, 6 to 8 hours, and greater than 8 hours are summarized in Table 1. Characteristics of participants with HAA assessments are summarized in Table S1. MESA participants with ≥8 hours of sleep were older and had a higher prevalence of statin and antidepressant use, and a higher proportion were women.
Figure 1.
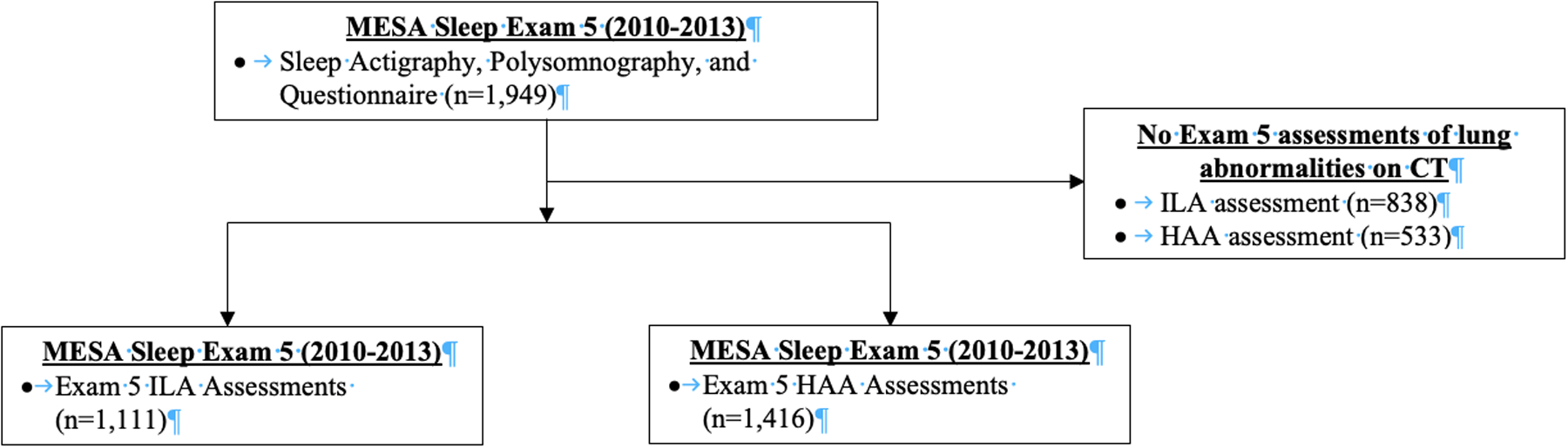
Flow diagram of MESA participants with actigraphy-based sleep duration and interstitial lung abnormalities and high attenuation areas assessments.
Table 1.
Baseline characteristics of the analytical sample
| Characteristic | Overall (n=1,111) | Less than 6 hours (n=199) | 6 to 8 hours (n=625) | Greater than 8 hours (n=287) |
|---|---|---|---|---|
| 7 (1) | 5 (1) | 7 (1) | 9 (1) | |
| Age (years) | 68 (9) | 68 (10) | 67 (9) | 70 (9) |
| Female | 54% | 40% | 52% | 67% |
| Race/ethnicity | ||||
| White | 35% | 19% | 36% | 43% |
| Asian | 14% | 18% | 12% | 16% |
| Black | 28% | 44% | 28% | 18% |
| Hispanic | 23% | 19% | 24% | 23% |
| Smoking status | ||||
| Never Smoker | 51% | 46% | 52% | 53% |
| Former Smoker | 42% | 44% | 43% | 39% |
| Current Smoker | 7% | 10% | 5% | 8% |
| AHI (events/hr) | 14 (16) | 19 (18) | 13 (15) | 14 (17) |
| BMI (kg/m2) | 29 (6) | 30 (6) | 29 (5) | 28 (6) |
| Systolic blood pressure (mmHg) | 123 (20) | 124 (22) | 122 (19) | 124 (19) |
| Diastolic blood pressure (mmHg) | 69 (10) | 70 (11) | 69 (10) | 68 (10) |
| Statin use | 36% | 28% | 38% | 38% |
| Antidepressant use | 9% | 10% | 7% | 13% |
| Diabetes | 16% | 17% | 16% | 15% |
| Total intentional exercise (min/wk) | 2,952 (4,060) | 3,146 (3,888) | 3,091 (4,483) | 2,591 (3,097) |
| Education attainment | ||||
| Less than high school | 14% | 10% | 14% | 18% |
| High school | 45% | 45% | 46% | 44% |
| College and beyond | 41% | 45% | 40% | 38% |
| Interstitial lung abnormalities | 12% | 11% | 11% | 14% |
Continuous variables presented as mean (standard deviation) and categorical variables presented as percentages
Actigraphy-Based Sleep Duration
The continuous association between actigraphy-based sleep duration and ILA was U-shaped (p-value for non-linearity < 0.001) (Figure 2). Inflection points appeared to be at 6 and 8 hours. Among participants with a sleep duration less than 6 hours, shorter sleep duration was associated with a higher odds for ILA. In other words, in individuals with a sleep duration less than 6 hours per night, each 1-hour decrement was associated with a 1.54 unadjusted odds for ILA (95% CI 1.02 to 2.32); however, this association was attenuated after covariate adjustment. Participants with greater than 8 hours of sleep had an unadjusted odds ratio for ILA of 2.21 (95% CI 1.36 to 3.58) per 1-hour increased sleep duration increment, which did not appreciably change with covariate adjustment. No association was observed between sleep duration and odds of ILA among participants with a sleep duration of 6 to 8 hours (Table 2).
Figure 2.
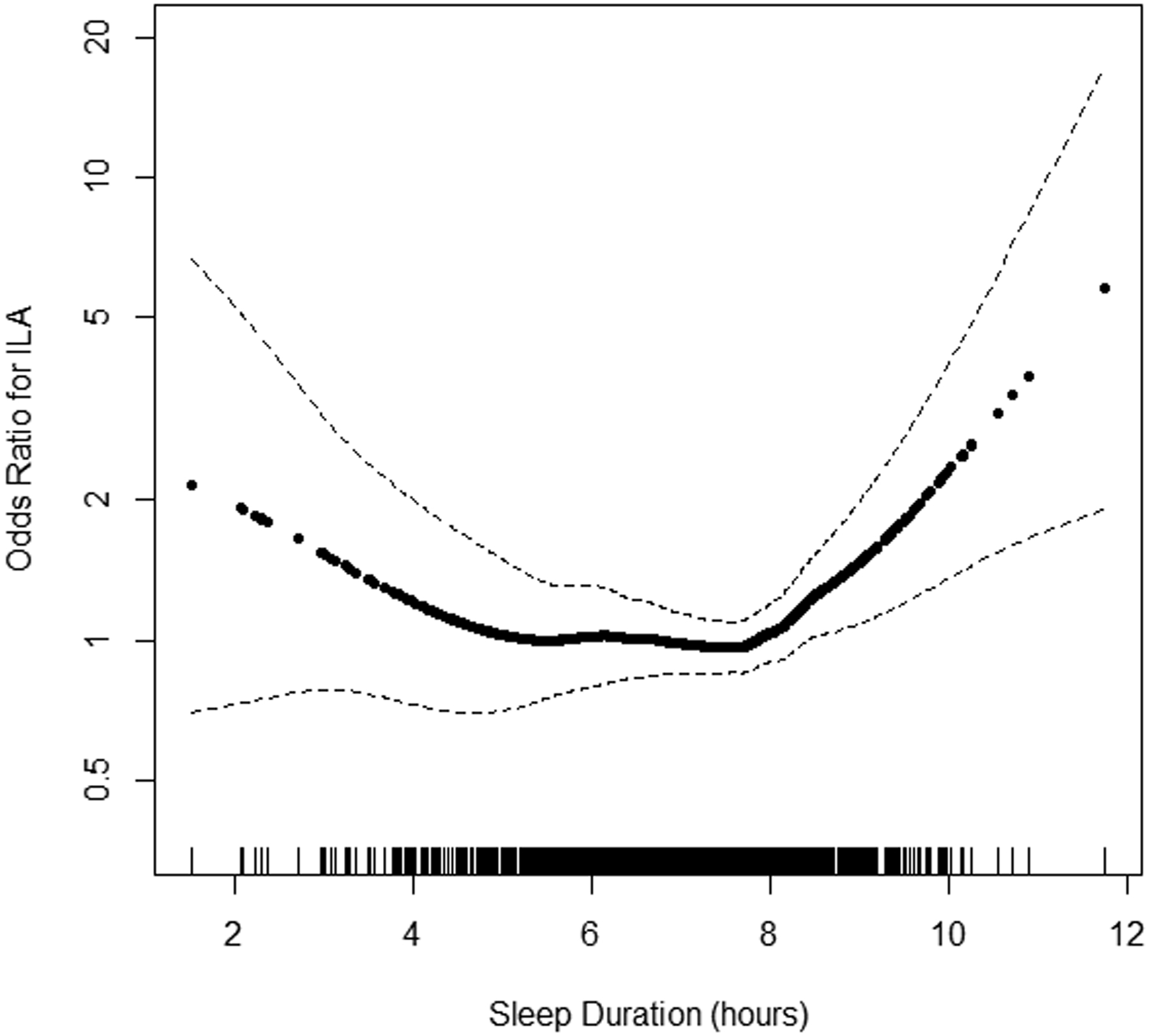
Continuous association of actigraphy-based sleep duration with odds ratio for interstitial lung abnormalities (n=1,111). P-values for linearity = 0.36 and for non-linearity <0.001. Model is unadjusted. The solid line is the overall effect estimate, thin dashed lines represent 95% confidence interval bands. Each vertical hashmark along the x-axis represents an individual participant.
Table 2.
Associations of Actigraphy-Based Sleep Duration with ILA
| Sleep Duration | No. Participants | Odds Ratio for ILA (95% CI) per 1-hour increment | P-value | P-value for Interaction |
|---|---|---|---|---|
| Model 1 | ||||
| Less than 6 hours* | 199 | 1.54 (1.02 to 2.32) | 0.04 | |
| 6 to 8 hours | 625 | 0.74 (0.47 to 1.15) | 0.18 | |
| Greater than 8 hours | 287 | 2.21 (1.36 to 3.58) | 0.001 | |
| Model 2 | ||||
| Less than 6 hours* | 199 | 1.13 (0.66 to 1.92) | 0.66 | |
| 6 to 8 hours | 625 | 0.70 (0.42 to 1.15) | 0.18 | |
| Greater than 8 hours | 287 | 2.66 (1.42 to 4.99) | 0.002 | |
| Stratified Analysis | ||||
| Fragmentation Index | ||||
| Less than 6 hours | 0.34 | |||
| Tertile 1 | 53 | 1.64 (0.58 to 4.62) | 0.35 | |
| Tertile 2 | 72 | 0.92 (0.27 to 3.09) | 0.79 | |
| Tertile 3 | 74 | 0.56 (0.22 to 1.44) | 0.29 | |
| 6 to 8 hours | 0.06 | |||
| Tertile 1 | 206 | 0.46 (0.17 to 1.22) | 0.12 | |
| Tertile 2 | 214 | 0.43 (0.19 to 0.97) | 0.04 | |
| Tertile 3 | 205 | 1.61 (0.67 to 3.89) | 0.29 | |
| Greater than 8 hours | 0.52 | |||
| Tertile 1 | 111 | 4.63 (1.47 to 14.54) | 0.01 | |
| Tertile 2 | 85 | 2.30 (0.98 to 5.42) | 0.06 | |
| Tertile 3 | 91 | 2.05 (0.56 to 7.59) | 0.28 | |
| Chronotype | ||||
| Less than 6 hours | 0.91 | |||
| Non-evening | 182 | 0.85 (0.50 to 1.46) | 0.56 | |
| Evening | 17 | 1.90 (0.05 to 67.00) | 0.72 | |
| 6 to 8 hours | 0.80 | |||
| Non-evening | 571 | 0.69 (0.41 to 1.18) | 0.17 | |
| Evening | 54 | 0.87 (0.17 to 4.43) | 0.87 | |
| Greater than 8 hours | 0.70 | |||
| Non-evening | 269 | 2.64 (1.37 to 5.07) | 0.004 | |
| Evening | 18 | 4.46 (0.27 to 74.87) | 0.30 | |
| Sleep Midpoint | ||||
| Less than 6 hours | 0.64 | |||
| Before 5:00 AM | 148 | 0.96 (0.50 to 1.85) | 0.91 | |
| After 5:00 AM | 51 | 0.49 (0.13 to 1.82) | 0.28 | |
| 6 to 8 hours | 0.97 | |||
| Before 5:00 AM | 557 | 0.70 (0.40 to 1.20) | 0.19 | |
| After 5:00 AM | 68 | 0.81 (0.24 to 2.72) | 0.74 | |
| Greater than 8 hours | 0.02 | |||
| Before 5:00 AM | 268 | 3.71 (1.80 to 7.63) | <0.001 | |
| After 5:00 AM | 19 | 0.38 (0.03 to 4.90) | 0.46 | |
Model 1: Unadjusted
Model 2: Adjusted for age, sex, race/ethnicity, smoking status, cigarette pack-years, body mass index, percent emphysema, systolic and diastolic blood pressures, statin use, apnea-hypopnea index, total intentional exercise, education attainment, diabetes, antidepressant use.
Results are reported per 1-hour decrement in sleep duration
Sleep fragmentation index and chronotype did not modify associations between sleep duration and ILA (i.e., there was no statistical evidence of interaction; Table 2). Among participants with a sleep duration greater than 8 hours, sleep midpoint modified its association with ILA (p-value for interaction=0.02). For every 1-hour increment in sleep duration greater than 8 hours, there was a 3.71 odds of ILA (95% CI 1.80 to 7.63) among those with an earlier sleep midpoint compared with 0.38 (95% CI 0.03 to 4.90) among those with a later sleep midpoint.
Continuous associations of actigraphy-based sleep duration with HAA by BMI subgroup are shown in Figures 3A–C. The inflection point describing the association between HAA and sleep duration appeared to be at 7 hours among participants with a BMI<25 kg/m2. Among participants with a sleep duration less than 7 hours, incremental changes in sleep duration were not associated with HAA among the BMI subgroups in unadjusted and adjusted analyses (p-value for BMI interaction ≥0.22). Among participants with a sleep duration greater than 7 hours and after adjustment for covariates, a 1-hour increased increment was associated with a change in HAA of 33.13 (95% CI 13.16 to 53.09), −8.56 (95% CI −27.01 to 9.89), and −20.75 (95% CI −39.16 to −2.34) among those with a BMI<25 kg/m2, 25 to 30 kg/m2, ≥30 kg/m2, respectively (p-value for BMI interaction<0.001) (Table 3).
Figure 3.
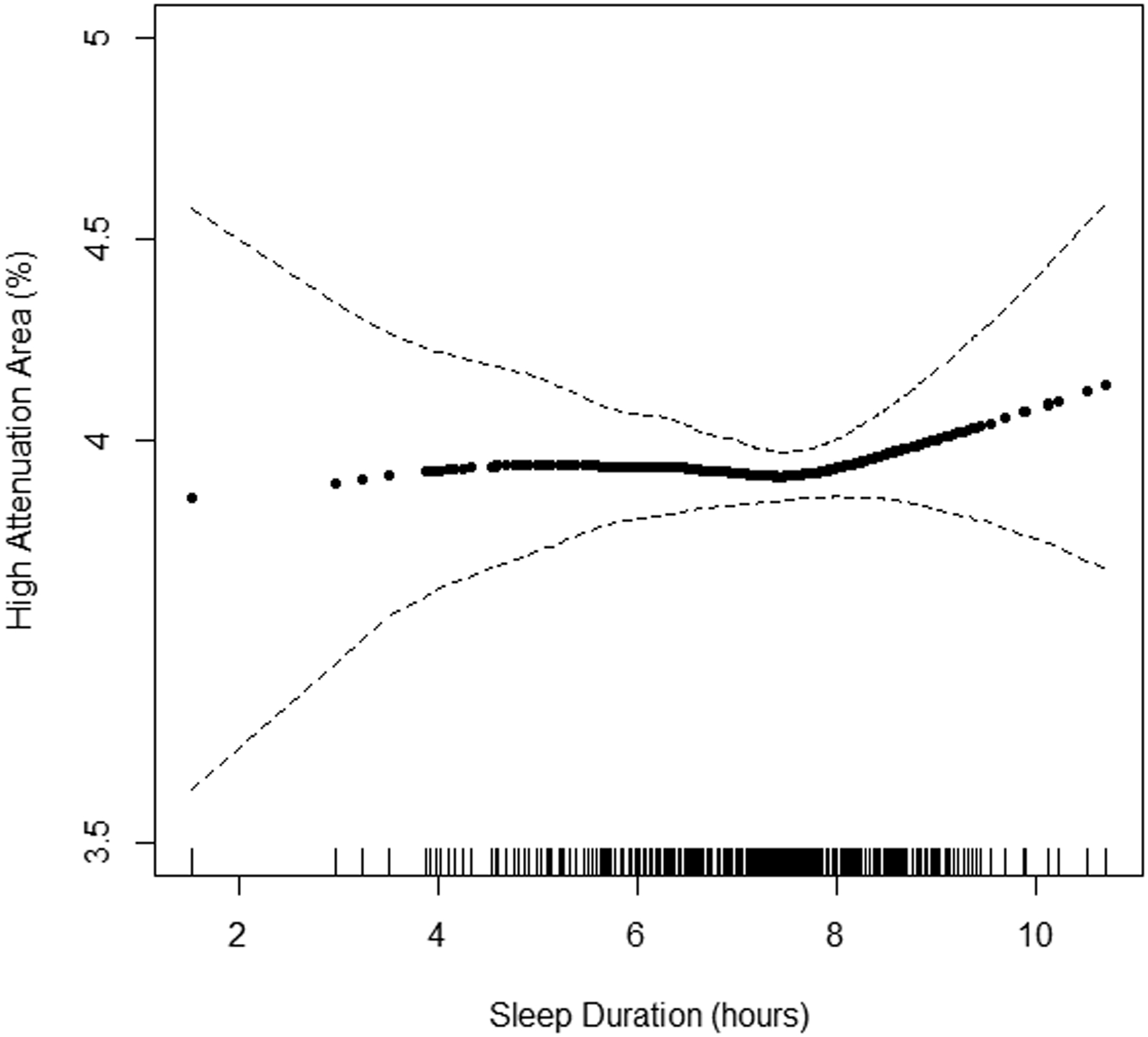
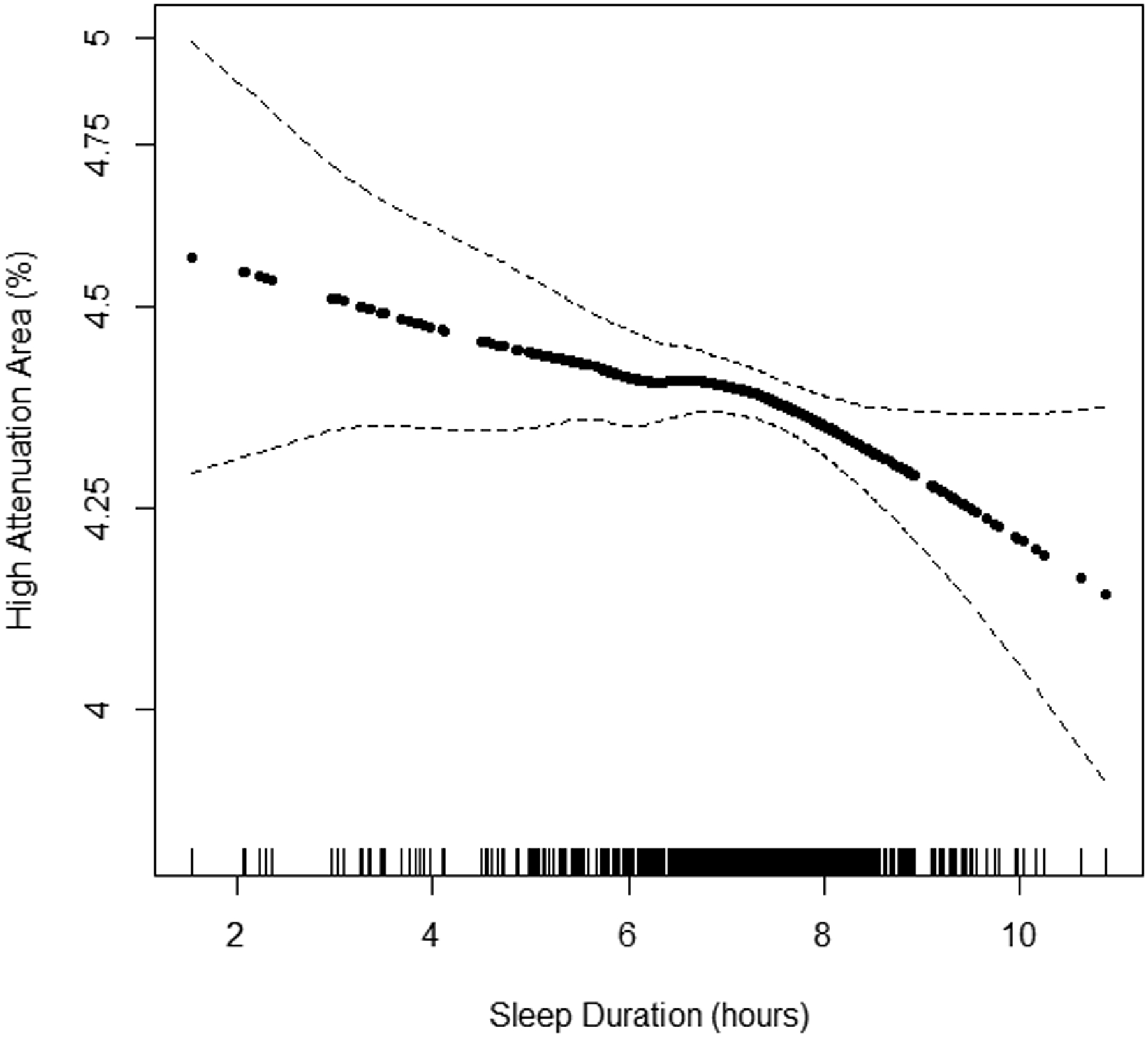
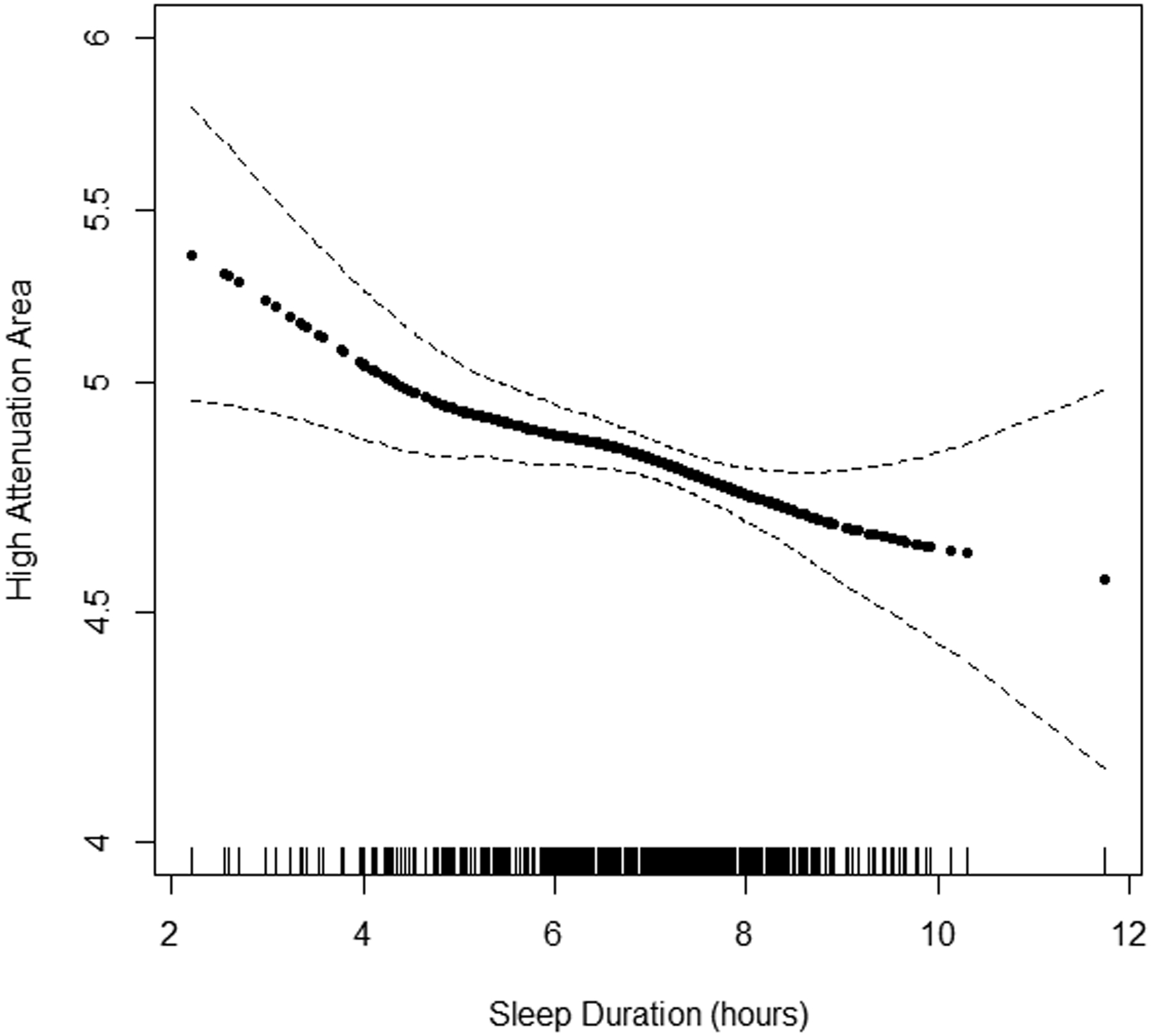
Continuous association of actigraphy-based sleep duration with high attenuation areas by body mass index (BMI) subgroup: A) BMI<25 kg/m2 (n=371), B) BMI 25–30 kg/m2 (n=537), and C) BMI ≥30 kg/m2 (n=508). P-values for linearity were A) 0.68, B) 0.02, C) 0.001. P-values for non-linearity were A) 0.37, B) 0.49, and C) 0.72. Models adjusted for study site, lung volume imaged, and radiation dose. The solid line is the overall effect estimate, thin dashed lines represent 95% confidence interval bands. Each vertical hashmark along the x-axis represents an individual participant.
Table 3.
Associations of Actigraphy-Based Sleep Duration with HAA
| Less than 7 hours sleep duration | Greater than 7 hours sleep duration | |||||
|---|---|---|---|---|---|---|
| Sleep Duration | No. Participants | Change in HAA per 1 hour increment in sleep duration (95% CI) | P-value | No. Participants | Change in HAA per 1 hour increment in sleep duration (95% CI) | P-value |
| Model 1 | ||||||
| Less than 25 kg/m2 | 69 | 14.45 (−12.03 to 40.94) | 0.28 | 302 | 22.98 (−3.91 to 49.88) | 0.09 |
| 25–30 kg/m2 | 132 | −12.92 (−31.21 to 5.38) | 0.17 | 405 | −12.94 (−37.77 to 11.89) | 0.31 |
| Greater than 30 kg/m2 | 121 | −9.93 (−27.04 to 7.19) | 0.26 | 387 | −23.33 (−48.14 to 1.48) | 0.07 |
| Model 2 | ||||||
| Less than 25 kg/m2 | 69 | 3.28 (−16.96 to 23.52) | 0.75 | 302 | 33.13 (13.16 to 53.09) | 0.001 |
| 25–30 kg/m2 | 132 | 6.91 (−7.16 to 20.97) | 0.34 | 405 | −8.56 (−27.01 to 9.89) | 0.36 |
| Greater than 30 kg/m2 | 121 | −0.74 (−13.58 to 12.09) | 0.91 | 387 | −20.75 (−39.16 to −2.34) | 0.03 |
Model 1: adjusted for study site, radiation dose, lung volume imaged.
Model 2: Model 1 + age, sex, race/ethnicity, smoking status, cigarette pack-years, percent emphysema, systolic and diastolic blood pressures, statin use, apnea-hypopnea index, total intentional exercise, education attainment, diabetes, and antidepressant use.
P-values for BMI interaction (Model 1): 0.22 (less than 7 hours sleep duration); 0.04 (greater than 7 hours sleep duration)
P-values for BMI interaction (Model 2): 0.72 (less than 7 hours sleep duration); <0.001 (greater than 7 hours sleep duration)
Self-reported sleep duration was not associated with ILA in adjusted models (Tables S2). Longer self-reported sleep duration among participants who reported sleeping less than 7 hours and have a BMI ≥30 kg/m2 was associated with less HAA (Table S3). This association was no longer significant after adjusting for covariates.
Additional Sleep and Chronotype Markers
Sleep fragmentation index, sleep midpoint after 5:00 AM, and evening chronotype were each not associated with ILA or HAA in the overall sample (Table S4–S5). A higher sleep fragmentation index was associated with more HAA among those with a BMI<25 kg/m2 (p-value for BMI interaction = 0.02). A one natural log unit increment in sleep fragmentation index was associated with an HAA of 52.10 (95% CI 16.15 to 88.04) after adjustment for covariates (Table S3).
DISCUSSION
Among community-dwelling adults, there was a U-shaped association between actigraphy-assessed sleep duration and ILA in unadjusted analyses, where shorter sleep duration from 6 hours and less and increasing sleep duration greater than 8 hours were associated with more ILA. However, in fully adjusted analyses, only the association between increasing sleep duration beyond 8 hours per night remained associated with ILA. Among participants with a BMI<25 kg/m2 and ≥30 kg/m2, increasing sleep duration beyond 7 hours was associated with more and less HAA, respectively. Associations were not explained by consideration of ILD-related risk factors such as age, smoking, sex, and medication use. Higher sleep fragmentation index was associated with more HAA among adults with a BMI <25 kg/m2. In contrast, self-reported sleep duration, later sleep midpoint, and evening chronotype were not associated with CT lung abnormalities.
Longer sleep duration has been identified as a risk factor for multiple chronic health conditions and increased mortality (Cappuccio et al., 2011). One hypothesis is that these associations reflect residual confounding by factors such as depression, cardiovascular disease, and lower socioeconomic status (Stamatakis & Punjabi, 2007). Adults with longer sleep durations have poorer health and social determinants that may put them at higher risk for worse clinical outcomes. Adults with ILD suffer from poor sleep quality and continuity as they have less deep sleep and reduced sleep efficiency (Cho et al., 2019). This link between perturbed sleep continuity and ILD may be driven by co-morbidity confounders (ex. mood disorders, smoking history). In our study, MESA participants with an actigraphy-based sleep duration ≥8 hours were older and had a higher prevalence of statin and anti-depressant use. However, a positive association between longer sleep and ILA persisted after adjusting for multiple confounders, including AHI, and was not modified by sleep fragmentation or chronotype in stratified analyses.
Emerging data also have identified associations between long sleep duration and a higher odds of pulmonary fibrosis in the UK Biobank Cohort, even after accounting for risk factors including age and smoking (Cunningham et al., 2020). Variation in sleep duration may reflect key differences in circadian programming (Aeschbach et al., 2003). Individuals with prolonged sleep duration may experience misalignment between the timing of the biological clock with the timing of sleep and other activities (ex. physical activity and food intake), resulting in metabolic dysfunction and inflammation. Disruption of processes regulated by the human circadian clock, such as lipid homeostasis (Podolanczuk et al., 2017) and adipogenesis (Kim et al., 2020), which are both implicated in the pathogenesis of ILD, may have partly driven our findings. A prior genome-wide interaction study revealed significant interactions between prolonged sleep duration and variants linked to lipid-related genes, supporting the biologic plausibility of abnormal sleep-wake rhythms and patterns on diseases mediated by impaired lipid metabolism (Noordam et al., 2019). Adipokines, such as adiponectin, have been postulated to have a contributory role in ILD pathogenesis (Kim et al., 2020), as their gene expression and transcription demonstrate 24-hour rhythmicity with impairment of their signaling induced by mutations of key clock proteins, ARNTL and CLOCK (Gomez-Abellan et al., 2010).
In addition to its influence on these metabolic pathways, an impaired circadian clock may contribute to ILD risk more directly via effects on fibrogenesis and collagen production in experimental murine models. Deficiency in the 19th exon of the clock gene (Clock Δ19) leads to an accumulation of collagen and alteration of the redox pathway via regulation of NRF2 in mice (Chang et al., 2020). Furthermore, deletion of NR1D1 (REVERBα) leads to an increased fibrotic response and accumulation of α-smooth muscle actin positive myofibroblasts in bleomycin-exposed mouse models mediated by reduced expression of the downstream transcription factor, TBPL1 (Cunningham et al., 2020). Among the clock genes, REVERBα is highly expressed in human lung tissue from adults with IPF.
Our study adds to these murine and cell-based studies by linking objectively estimated long sleep duration with two unique CT-based assessments of lung parenchymal changes in humans. Discordance between the actigraphy-based and self-reported sleep duration findings is consistent with previous work that has compared these tools (Jackson et al., 2018). Sleep fragmentation and chronotype did not modify the association been sleep duration and ILA; however, power to detect small to modest interactions is limited. Longer sleep duration was more strongly associated with ILA among those with an earlier sleep midpoint. Earlier sleep timing is seen with aging, and it is possible that earlier sleep placement in this sample is a marker of more advanced chronological aging and subsequent susceptibility to the biological effects of circadian misalignment when sleep is prolonged. However, delayed phase is more commonly associated with chronic health conditions, and given the small number of individuals with long sleep duration and later sleep timing, these subgroup analyses are prone to type I error and we caution over-interpretation of this finding.
In addition to its association with ILA overall, we found that actigraphy-based longer sleep duration was differentially associated with HAA by BMI subgroup. Longer sleep was associated with more HAA on CT among those with a BMI<25 kg/m2, whereas an inverse association was observed in those with a BMI ≥30 kg/m2. A potential reason for this may be the confounding effects that body habitus (i.e., soft tissue and atelectasis) has on CT lung densities (Kliment et al., 2015). Compared with ILA, HAA may capture other characteristics not related to ILD which include atelectasis, increased attenuation from soft tissue, and reduced inspiratory volumes. This may be why we found a stronger association in the hypothesized direction among those with a lower BMI that is consistent with our ILA outcome. Alternatively, sleep continuity and circadian alignment may have distinct influences on lung injury resolution and remodeling by body habitus type. Further research and development in CT methods to account for the effects of body habitus on lung densities (ex. texture-based analysis) may help elucidate the complex relationship between sleep, early lung injury/scarring, and body habitus.
In addition to prolonged sleep duration, we found that a higher sleep fragmentation index was strongly associated with more HAA on CT scan in participants with a BMI <25 kg/m2. The sleep fragmentation index measures the frequency of transitions from sleep to wakefulness, which usually is accompanied by altered sleep stage distributions. Changes in sleep continuity and depth can result in abnormalities in autonomic nervous system activation and elevated norepinephrine levels, resulting in elevations in blood pressure and abnormalities in cortisol secretion and glucose metabolism (Knutson et al., 2011; Mezick et al., 2009). These sympathetic system-related stresses triggered by disruption of sleep continuity may be pertinent to the lung (Heindl, Lehnert, Criee, Hasenfuss, & Andreas, 2001). The absence of effect modification by sleep fragmentation on duration and ILA suggests that measurement of sleep duration and fragmentation may capture unique causal pathways to lung injury that are not entirely overlapping or synergistic and require further investigation.
Although prior research suggests harmful effects of shorter sleep duration, later sleep midpoint, and evening chronotype, they were not associated with either ILA or HAA in our study in adjusted analyses. Many other population-based studies also have not shown consistent associations between short sleep duration and chronic cardiometabolic outcomes, potentially due to the variable reasons for short sleep across populations (Kwok et al., 2018). We did not have information on circadian phase, which would have better defined the influence of circadian markers on lung outcomes.
Our study has several limitations. Analyses were cross-sectional and reverse causality cannot be excluded. Our outcomes of ILA and HAA are possibly surrogate radiologic markers of subclinical disease in a general population cohort, which may attenuate the possible effect of reversal causation. While we carefully selected potential confounders to adjust for in our models, we cannot rule out the effect of residual confounding on our findings. Although the MESA cohort is racially and ethnically diverse and is derived from multiple U.S. communities, the generalizability of our findings is limited to older adults. Whether prolonged sleep duration is associated with a progression to fulminant ILD was not addressed by our study as our analyses were cross-sectional. Although ILA and HAA are strongly associated with future ILD-related clinical events and genetic variants associated with ILD risk, it remains unknown what proportion of adults with these radiologic abnormalities will progress to fulminant disease and is an ongoing area of research (Hatabu et al., 2020). We acknowledge that our findings may still be due to chance. Particularly with our stratified analyses due to the multiple comparisons and smaller subgroup sizes. Taking into account the above limitations, we strongly caution extrapolating any clinical implications of this finding as longitudinal studies and trials that examine the effect of sleep duration on risk and progression of ILD are needed.
In conclusion, longer sleep duration was cross-sectionally associated with a greater burden of lung inflammation and scarring on CT scan. Future prospective cohort studies and sleep behavior modification interventional trials will further elucidate the potential role abnormal sleep-wake patterns have in the pathogenesis of ILD.
Supplementary Material
Acknowledgement
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding:
The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study was supported by grants R01-HL077612, RC1-HL100543, and R01-HL093081, from the National Heart, Lung, and Blood Institute (NHLBI). The MESA Lung Fibrosis Study was supported by grants R01-HL103676 from NHLBI. MESA is supported by the NHLBI in collaboration with MESA investigators. Support for MESA is provided by NHLBI contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001881, and DK06349. The MESA Sleep Study was supported by R01-HL-135818. JSK was supported by the Pulmonary Fibrosis Foundation Scholars Award and K23-HL-150301 from the NHLBI. AJP was supported by K23-HL-140199 from the NHLBI. JB is a Medical Research Council (MR/T032529/1) transition support fellow and is also supported by the NIHR Manchester Biomedical Research Centre and British Lung Foundation. SR was supported by NHLBI R35-HL-135818.
Conflict of Interest Statement:
JSK reports grants from the Pulmonary Fibrosis Foundation Scholars Award and NIH. AJP reports grants from the NIH during the conduct of the study. AJP receives personal fees from Regeneron, grants from American Lung Association, and personal fees from Boehringer Ingelheim, outside the submitted work. RGB reports grants from the NIH and COPD Foundation. SR reports grants from the NIH during the conduct of the study and has received consulting fees from Eisai Inc., Respicardia, Apnimed, and Jazz Pharma unrelated to this work.
References
- Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, & Wehr TA (2003). A longer biological night in long sleepers than in short sleepers. J Clin Endocrinol Metab, 88(1), 26–30. doi: 10.1210/jc.2002-020827 [DOI] [PubMed] [Google Scholar]
- Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, … Watson KE (2010). Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med, 362(3), 217–227. doi: 10.1056/NEJMoa0808836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, … Tracy RP (2002). Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol, 156(9), 871–881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, & Miller MA (2011). Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J, 32(12), 1484–1492. doi: 10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- Chang J, Garva R, Pickard A, Yeung CC, Mallikarjun V, Swift J, … Kadler KE (2020). Circadian control of the secretory pathway maintains collagen homeostasis. Nat Cell Biol, 22(1), 74–86. doi: 10.1038/s41556-019-0441-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcantara C, … Redline S (2015). Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep, 38(6), 877–888. doi: 10.5665/sleep.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JG, Teoh A, Roberts M, & Wheatley J (2019). The prevalence of poor sleep quality and its associated factors in patients with interstitial lung disease: a cross-sectional analysis. ERJ Open Res, 5(3). doi: 10.1183/23120541.00062-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham PS, Meijer P, Nazgiewicz A, Anderson SG, Borthwick LA, Bagnall J, … Blaikley JF (2020). The circadian clock protein REVERBalpha inhibits pulmonary fibrosis development. Proc Natl Acad Sci U S A, 117(2), 1139–1147. doi: 10.1073/pnas.1912109117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, … Investigators, I. T. (2019). Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med, 381(18), 1718–1727. doi: 10.1056/NEJMoa1908681 [DOI] [PubMed] [Google Scholar]
- Gomez-Abellan P, Gomez-Santos C, Madrid JA, Milagro FI, Campion J, Martinez JA, … Garaulet M (2010). Circadian expression of adiponectin and its receptors in human adipose tissue. Endocrinology, 151(1), 115–122. doi: 10.1210/en.2009-0647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatabu H, Hunninghake GM, Richeldi L, Brown KK, Wells AU, Remy-Jardin M, … Lynch DA (2020). Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society. Lancet Respir Med, 8(7), 726–737. doi: 10.1016/S2213-2600(20)30168-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindl S, Lehnert M, Criee CP, Hasenfuss G, & Andreas S (2001). Marked sympathetic activation in patients with chronic respiratory failure. Am J Respir Crit Care Med, 164(4), 597–601. doi: 10.1164/ajrccm.164.4.2007085 [DOI] [PubMed] [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, … Adams Hillard PJ (2015). National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health, 1(1), 40–43. doi: 10.1016/j.sleh.2014.12.010 [DOI] [PubMed] [Google Scholar]
- Horne JA, & Ostberg O (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol, 4(2), 97–110. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1027738 [PubMed] [Google Scholar]
- Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, … Schwartz DA (2013). MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med, 368(23), 2192–2200. doi: 10.1056/NEJMoa1216076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake GM, Quesada-Arias LD, Carmichael NE, Martinez Manzano JM, Poli De Frias S, Baumgartner MA, … Rosas IO (2020). Interstitial Lung Disease in Relatives of Patients with Pulmonary Fibrosis. Am J Respir Crit Care Med, 201(10), 1240–1248. doi: 10.1164/rccm.201908-1571OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CL, Patel SR, Jackson WB 2nd, Lutsey PL, & Redline S (2018). Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: Multi-Ethnic Study of Atherosclerosis. Sleep, 41(6). doi: 10.1093/sleep/zsy057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Anderson MR, Podolanczuk AJ, Kawut SM, Allison MA, Raghu G, … Giles JT (2020). Associations of Serum Adipokines With Subclinical Interstitial Lung Disease Among Community-Dwelling Adults: The Multi-Ethnic Study of Atherosclerosis (MESA). Chest, 157(3), 580–589. doi: 10.1016/j.chest.2019.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Podolanczuk AJ, Borker P, Kawut SM, Raghu G, Kaufman JD, … Lederer DJ (2017). Obstructive Sleep Apnea and Subclinical Interstitial Lung Disease in MESA. Ann Am Thorac Soc. doi: 10.1513/AnnalsATS.201701-091OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TE Jr., Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, … Group, A. S. (2014). A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med, 370(22), 2083–2092. doi: 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- Kliment CR, Araki T, Doyle TJ, Gao W, Dupuis J, Latourelle JC, … Hunninghake GM (2015). A comparison of visual and quantitative methods to identify interstitial lung abnormalities. BMC Pulm Med, 15, 134. doi: 10.1186/s12890-015-0124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E, Zee P, Liu K, & Lauderdale DS (2011). Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care, 34(5), 1171–1176. doi: 10.2337/dc10-1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok CS, Kontopantelis E, Kuligowski G, Gray M, Muhyaldeen A, Gale CP, … Mamas MA (2018). Self-Reported Sleep Duration and Quality and Cardiovascular Disease and Mortality: A Dose-Response Meta-Analysis. J Am Heart Assoc, 7(15), e008552. doi: 10.1161/JAHA.118.008552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster LH, Mason WR, Parnell JA, Rice TW, Loyd JE, Milstone AP, … Malow BA (2009). Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest, 136(3), 772–778. doi: 10.1378/chest.08-2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer DJ, & Martinez FJ (2018). Idiopathic Pulmonary Fibrosis. N Engl J Med, 379(8), 797–798. doi: 10.1056/NEJMc1807508 [DOI] [PubMed] [Google Scholar]
- Manichaikul A, Wang XQ, Sun L, Dupuis J, Borczuk AC, Nguyen JN, … Lederer DJ (2017). Genome-wide association study of subclinical interstitial lung disease in MESA. Respir Res, 18(1), 97. doi: 10.1186/s12931-017-0581-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall M, Kamarck TW, Buysse DJ, Owens JF, & Reis SE (2009). Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology, 34(9), 1346–1354. doi: 10.1016/j.psyneuen.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ER, Putman RK, Vivero M, Hung Y, Araki T, Nishino M, … Hunninghake GM (2018). Histopathology of Interstitial Lung Abnormalities in the Context of Lung Nodule Resections. Am J Respir Crit Care Med, 197(7), 955–958. doi: 10.1164/rccm.201708-1679LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordam R, Bos MM, Wang H, Winkler TW, Bentley AR, Kilpelainen TO, … Redline S (2019). Multi-ancestry sleep-by-SNP interaction analysis in 126,926 individuals reveals lipid loci stratified by sleep duration. Nat Commun, 10(1), 5121. doi: 10.1038/s41467-019-12958-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolanczuk AJ, Oelsner EC, Barr RG, Hoffman EA, Armstrong HF, Austin JH, … Lederer DJ (2016). High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study. Eur Respir J, 48(5), 1442–1452. doi: 10.1183/13993003.00129-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolanczuk AJ, Raghu G, Tsai MY, Kawut SM, Peterson E, Sonti R, … Lederer DJ (2017). Cholesterol, lipoproteins and subclinical interstitial lung disease: the MESA study. Thorax, 72(5), 472–474. doi: 10.1136/thoraxjnl-2016-209568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, … Investigators, C. O. (2016). Association Between Interstitial Lung Abnormalities and All-Cause Mortality. JAMA, 315(7), 672–681. doi: 10.1001/jama.2016.0518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, … Kiley JP (1998). Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep, 21(7), 759–767. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11300121 [PubMed] [Google Scholar]
- Redline S, Sotres-Alvarez D, Loredo J, Hall M, Patel SR, Ramos A, … Daviglus ML (2014). Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med, 189(3), 335–344. doi: 10.1164/rccm.201309-1735OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, & Merrow M (2007). Epidemiology of the human circadian clock. Sleep Med Rev, 11(6), 429–438. doi: 10.1016/j.smrv.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Sieren JP, Newell JD Jr., Barr RG, Bleecker ER, Burnette N, Carretta EE, … Group, S. R. (2016). SPIROMICS Protocol for Multicenter Quantitative Computed Tomography to Phenotype the Lungs. Am J Respir Crit Care Med, 194(7), 794–806. doi: 10.1164/rccm.201506-1208PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis KA, & Punjabi NM (2007). Long sleep duration: a risk to health or a marker of risk? Sleep Med Rev, 11(5), 337–339. doi: 10.1016/j.smrv.2007.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, … Wright JT Jr. (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 138(17), e426–e483. doi: 10.1161/CIR.0000000000000597 [DOI] [PubMed] [Google Scholar]
- Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, … Nieto FJ (1998). Reliability of scoring respiratory disturbance indices and sleep staging. Sleep, 21(7), 749–757. doi: 10.1093/sleep/21.7.749 [DOI] [PubMed] [Google Scholar]
- Zoz DF, Lawson WE, & Blackwell TS (2011). Idiopathic pulmonary fibrosis: a disorder of epithelial cell dysfunction. Am J Med Sci, 341(6), 435–438. doi: 10.1097/MAJ.0b013e31821a9d8e [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


