Abstract
This study explored whether maltreatment moderates the association of polygenic risk for ADHD. Because individuals with low polygenic scores (PGS) for ADHD were previously shown to have better than expected functional outcomes (i.e., cognitive, mental health, social-emotional) than individuals with middle or high ADHD PGS, we hypothesized low ADHD PGS may confer a protective effect from maltreatment in the development of ADHD. Data were from participants with phenotypic and genotypic data in the National Longitudinal Study of Adolescent to Adult Health (Add Health; n=4,722). ADHD PGS were generated from the most recent genome-wide association study on ADHD and categorized into three groups (i.e., low, medium, high) using empirically determined cut-points. A maltreatment factor score was derived from five forms of self-reported maltreatment experiences prior to age 18. ADHD PGS and maltreatment were positively associated with ADHD symptoms, as expected. However, no interaction between ADHD PGS and maltreatment on ADHD symptoms was detected. Despite the increase in predictive power afforded by PGS, the lack of an interaction between ADHD PGS and maltreatment on ADHD symptoms converges with an emerging body of PGS studies that have also failed to detect PGS-environment interplay in mental disorders. We discuss possible reasons for this pattern of results and offer alternative methods for future research in understanding gene-environment interactions.
Keywords: Polygenic scores, ADHD, gene-environment interaction, maltreatment, Add Health
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder that affects 5–10% of children and young adults globally (Danielson et al., 2018). Maltreatment is associated with a broad range of mental disorders, such as internalizing disorders, PTSD, externalizing behaviors, and substance use (Copeland et al., 2018; Proctor et al., 2017; Warrier et al., 2021; Yoon, 2017). In particular, maltreatment is also a potent risk factor in the development of ADHD (González et al., 2019), though as with most negative outcomes, not all maltreated individuals go on to develop ADHD (Tabone et al., 2011; Yoon, 2018). The current study leverages powerful genetic methods using genome-wide information to test the hypothesis that polygenic liability for ADHD might moderate the negative effects of maltreatment in the development of ADHD in a large, population-based dataset.
Genes play a substantial role in the origins of ADHD. Twin studies suggest that approximately 70–80% of the variance in ADHD can be attributed to broad-band genetic differences among individuals (i.e., heritability) that are consistent across the lifespan (Faraone & Larsson, 2019). Genome-wide association studies (GWAS) have shown that common genetic variants via single nucleotide polymorphisms (SNPs) account for a substantial part of this heritability, although individual SNPs themselves only contribute a small to negligible amount of the variance in ADHD (Demontis et al., 2019; Pettersson et al., 2019). While the most recent GWAS on ADHD identified novel genes such as the FOXP2 (Demontis et al., 2019), which is also linked to speech and language problems, many previously examined candidate genes for ADHD did not emerge in GWAS, such as DRD4, DAT1 and MAOA (Brookes et al., 2006; Gizer et al., 2009). As GWAS has shown ADHD to be a highly polygenic disorder, researchers are increasingly using approaches that aggregate the effects of common genetic variants (i.e., polygenic scores; PGS) to characterize one’s genetic risk for the disorder. Traditionally, PGS represent each person’s genetic liability based on the weighted sum of their alleles across the variants that contribute to the trait in question. Higher ADHD PGS are consistently and robustly associated with ADHD symptoms and diagnosis across a wide range of populations, including clinical as well as population-based samples (see meta-analysis by Li & He, 2021). Importantly, the primary use of PGS is not to identify relevant genes or to reveal biological mechanisms, but rather, to better predict the heritable phenotype in question.
Crucially, ADHD PGS may reflect more than simply one’s genetic risk for ADHD. Using data from the National Longitudinal Study of Adolescent to Adult Health (Add Health), Li (2019b) found that individuals at the lowest end of the ADHD PGS distribution (i.e., in the bottom 20th percentile) had a 17–19% lower risk for ADHD, but they also had significantly higher IQ and educational attainment, and lower risks for other mental (e.g., fewer depressive symptoms) and physical health outcomes (e.g., lower BMI) compared to individuals in the middle (i.e., 21st to 79th percentiles) and highest ends of the ADHD PGS distribution (i.e., top 80th percentile). These results suggest that a low burden of genetic risk for ADHD may have beneficial or possibly protective effects on various functional outcomes (Li, 2019b). This finding also leads to a follow-up inquiry as it pertains to gene-environment interplay – can having a low ADHD PGS also protect against the negative effects of adverse environments, such as maltreatment, in the development of ADHD? The current study addresses this question, which is understudied for ADHD using contemporary genetic frameworks (Gould et al., 2018; Palladino et al., 2019).
In addition to genetic risk factors, maltreatment is also associated with the development of ADHD (Briscoe-Smith & Hinshaw, 2006; Sanderud et al., 2016). The association of maltreatment and ADHD is consistently observed across the types of maltreatment experienced (e.g., neglect, abuse), sex of participants, sampling methods (e.g., nationally-representative sample, community sample, online survey), and retrospective versus prospective studies (Clayton et al., 2018; González et al., 2019; Stern et al., 2018). However, less known is the extent to which ADHD PGS may moderate the negative effects of maltreatment on ADHD. Prior ADHD studies on gene-environment interactions have focused on candidate genes and their interactive effects on various adverse childhood environments (e.g., childhood trauma, and negative parenting behavior) (Li & Lee, 2012; Nigg et al., 2010; Park et al., 2017). However, candidate gene-environment interaction studies are now viewed with skepticism due to their focus on only a single or a few candidate genes that likely have small to null effects on complex phenotypes (Dick et al., 2015). There remains a critical lack of gene-environment interaction studies that have utilized contemporary and more powerful genetic methods (i.e., PGS) in ADHD research (Gould et al., 2018; Palladino et al., 2019)
The current study used data from Add Health to examine whether ADHD PGS moderates the association between maltreatment and ADHD symptoms. Based on prior work showing that individuals with low ADHD PGS may not only have lower rates of ADHD, but also have better cognitive performance and mental and physical health outcomes compared to individuals with medium and high ADHD PGS (Li, 2019b), we hypothesized that individuals with low ADHD PGS would be protected from the negative effects of maltreatment (as it pertains to their ADHD symptoms) relative to those with medium and high ADHD PGS.
Method
Participants
Add Health began as a prospective longitudinal sample of adolescents from grades 7–12 who were recruited from high schools across the United States. Data were collected from adolescents, their caregivers, peers at school, school administrators, siblings, and romantic partners across five Waves: Wave I (1994–1995, grades 7–12, N=20,745), Wave II (1995–1996, grades 8–12, N = 14,738), Wave III (2001–2002, ages 18–26, N = 15,197), Wave IV (2007–2008, ages 24–32, N = 15,701) and Wave V (2016–2018, ages 34–43, N=12,000). Written consent was acquired from parents or legal guardians and participants themselves when they were younger than 18, and only from participants after they were 18 years of age or older (see Add Health study design for additional details: https://addhealth.cpc.unc.edu/documentation/study-design/). To account for potential population stratification effects, as well as to reduce concerns regarding the poorer predictive performance of health-related PGS when applied to non-European ancestry populations (Duncan et al., 2019), the current analysis focused on Add Health individuals of European ancestry for whom phenotypic and genetic data were available (n=4,722). See Table 1 for descriptive statistics of key demographic variables and other study variables.
Table 1.
Descriptive statistics comparing high, medium, and low ADHD PGS groups
| ADHD PGS Groups (low, medium and high) | |||||
|---|---|---|---|---|---|
| Low(n=1,181) | Medium(n=2,360) | High(n=1,181) | F or Chi-square | p-value | |
| ADHD symptoms (M, SD) | 2.78(sd=3.38) | 3.22(sd=3.62) | 3.45(sd=3.89) | F(2, 4719)=10.39 | <.001 |
| Psychological neglect (%) | 83(7.52%) | 187(8.37%) | 77(6.87%) | χ2=2.50 | .287 |
| Physical neglect (%) | 48(4.21%) | 123(5.39%) | 75(6.56%) | χ2=6.19 | .045 |
| Physical abuse (%) | 135(11.89%) | 328(14.37%) | 180(15.73%) | χ2=7.22 | .027 |
| Sexual abuse (%) | 44(3.83%) | 90(3.91%) | 60(5.19%) | χ2=3.66 | .160 |
| Psychological abuse (%) | 123(10.46%) | 324(13.90%) | 167(14.29%) | χ2=9.93 | .007 |
| Parental education (0–9) | 6.010(sd=2.12) | 5.810(sd=2.12) | 5.670(sd=2.14) | F(2,4260)=6.91 | .001 |
| Less than HS (0–3) | 90(7.62%) | 208(8.81%) | 120(10.16%) | - | - |
| HS (4–5) | 319(27.01%) | 684(28.98%) | 371(31.41%) | - | - |
| Some college (6–7) | 338(28.62%) | 698(29.58%) | 331(28.03%) | - | - |
| College + (8–9) | 325(27.52%) | 522(22.12%) | 257(21.76%) | - | - |
| Household income (in thousands) | 53.90(sd=48.42) | 53.06(sd=56.17) | 49.93(sd=48.17) | F(2,4230)=.23 | .797 |
| Age | 15.99(sd=1.39) | 16.04(sd=1.38) | 15.97(sd=1.40) | F(2,4719)=.65 | .520 |
| # Female (%) | 628(53.17%) | 1276(54.07%) | 660(55.88%) | χ2=1.85 | .397 |
F-statistics were computed from a one-way ANOVA for continuous outcomes; Pearson Chi-squared tests (df=2) were conducted for binary outcomes
Measures
ADHD Symptoms.
Participants retrospectively self-reported on their own childhood ADHD symptoms at Wave III. Participants responded to 17 items on a 4-point Likert scale, describing how often a symptom “best describes your behavior when you were (between 5 and 12)”. Responses are dichotomized as presence (i.e., ‘sometimes’, ‘often’, ‘very often’), or absence (i.e., ‘never’) of that symptom. The symptoms were keyed to the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013). ADHD total symptoms were computed by summing the number of positively endorsed symptoms.
Maltreatment.
Maltreatment was retrospectively self-reported by participants at Wave III and Wave IV. Participants rated on a 6-point Likert scale regarding the frequency of five types of maltreatment experiences during childhood (i.e., prior to age 18), including physical abuse (“being slapped, hit or kicked by parents or other adult caregivers”), sexual abuse (“parents or adult caregivers touched you in a sexual way, forced you to touch him or her in a sexual way, or forced you to have sexual relations”), emotional abuse (“a parent or other adult caregiver say things that really hurt your feelings or made you feel like you were not wanted or loved”), physical neglect (“parents or other adult caregivers not taken care of your basic needs, such as keeping you clean or providing food or clothing”), and emotional neglect (“parents or other adult caregivers left you home alone when an adult should have been with you”). Possible responses range from 1 (i.e., one time) to 5 (i.e., over ten times), and 6 represents “never happened”. A response of 6 was recoded to 0. Each maltreatment item was then dichotomized into presence or absence using a cut-off score for each item as established in a previous study (Brumley et al., 2019). Specifically, each type of maltreatment was present (versus absent) if its reported frequency was three or more times for physical abuse, one or more times for sexual abuse, ten or more times for emotional abuse, two or more times for physical neglect, and ten or more times for emotional neglect. Per empirical precedent (Brumley et al., 2019), a latent factor score for maltreatment was computed (see Dimension Reduction section for details).
Dimension Reduction
We computed a latent factor score for maltreatment based on a confirmatory factor analysis of the frequencies of the five types of maltreatment. Maximum likelihood estimation was used for the CFA. Model fit was evaluated by comparative fit index (CFI), Tucker-Lewis index (TLI), and the root mean squared error of approximation (RMSEA). CFI and TLI values greater than .90 and RMSEA values less than .06 generally indicate “acceptable” model fit (Hu & Bentler, 1999). A one-factor model fit the data well (CFI=.94, TLI=.87, RMSEA=.05, χ2(5)=169.81). Individuals whose maltreatment factor score was 1 standard deviation higher than the mean had 1.13 times more ADHD symptoms (OR=1.13, se=.03, 95%CI=[1.07, 1.19], χ2(1)=20.62, p<.001). The maltreatment latent factor score had higher explanatory power compared to the cumulative composite of maltreatment in a separate study using the same Add Health data (Brumley et al., 2019). In the current study, the maltreatment factor score explained 4.78% of the variance in ADHD symptoms, whereas the cumulative composite explained 4% of the variance, after accounting for covariates (see Statistical Analyses section for details of the covariates).
Genotyping and Quality Control
Saliva samples were collected from consenting participants at Wave IV (96% of all Wave IV participants). Genotyping was done on two Illumina platforms (i.e., Omni-Quad BeadChip and Omni2.5-Quad BeadChip), and quality control (QC) procedures were carried out. Filtering by missing call rate of .02, Hardy-Weinberg Equilibrium of .0001, and minor allele frequency of .01, 346,754 single nucleotide polymorphisms (SNPs) carried through to imputation. European genetic samples were imputed on Release 1 of the Human Reference Consortium (HRS r1.1). Additional details of the quality control are available online (https://www.cpc.unc.edu/projects/addhealth/documentation/guides/AH_GWAS_QC.pdf)
Polygenic Scores (PGS) for ADHD
Per convention (International Schizophrenia Consortium et al., 2009), ADHD PGS for each Add Health individual were computed as the sum of the number of alleles weighted by their GWAS effect sizes. We employed the most recent and largest ADHD GWAS to date, a case-control meta-analysis that consisted of 55,374 children and adults (20,183 cases and 35,191 controls) from 12 studies of mixed (but predominantly European) ancestries (Demontis et al., 2019). The largest cohort among these 12 studies is a population-based case-control cohort in Denmark (iPSYCH; 14,584 cases and 22,492 controls). The other 11 case-control or trio samples were collected in Europe, Canada, United States and China, and aggregated by the Psychiatric Genomic Consortium (PGC). ADHD case status was determined based on International Classification of Diseases, tenth revision (ICD-10) in iPSYCH, and semi-structured clinical interviews (e.g., Schedule for Affective Disorders and Schizophrenia for School-Age Children, K-SADS) in the other 11 cohorts. As mentioned previously, because we focused on the European-ancestry subset of Add Health, we computed ADHD PGS based on the European-ancestry subset of the GWAS (totaling 19,099 cases and 34,194 controls) (Demontis et al., 2019).
ADHD PGS was computed in PRSice2 software in R (Choi & O’Reilly, 2019). We applied a p-value threshold of 1.0, as this included all available genetic information while downweighing SNPs with null or marginal effect sizes. Furthermore, this threshold specifically avoids “cherry picking” via biased model selection and arbitrary p-value threshold cutoffs based on variances explained (Li & He, 2021). “Low”, “medium” and “high” ADHD PGS were derived in the following way. Following prior studies (Fang et al., 2019; Li, 2019b), we compared five pairs of percentile cut-points (i.e., lowest group being 5%, 10%, 15%, 20%, and 25%). ADHD PGS were dummy coded according to each pair of cut-points (e.g., 0–5% was low group and coded as 0, 6–94% was medium group and coded as 1, 95%–100% was high group and coded as 2). ADHD symptom counts were regressed on the dummy coded PGS variable, covarying out the effects of sex, age and 10 genetic PCs. The pairs of cut-points that provided the best model fit (by percent of variance explained in ADHD symptoms) were chosen as the optimal cut-points for “low”, “medium” and “high” PGS. Model fit statistics that we considered included AIC, BIC and r2. Statistical significance was evaluated by Wald Chi-square test, and Likelihood Ratio Test (LRT). Omnibus test statistics as well as model fit indices are presented in Table 2, where the best-fitting PGS cut-points were at the lowest 25th, middle 26th–75th, and highest 75th percentiles. As expected, dummy-coded ADHD PGS were significantly associated with ADHD symptoms (LRT(2)=9.58, p=.008; χ2(2)=8.95, p=.011; r2=.084). In post-hoc group comparison by PGS percentile groups, where the lowest 25th PGS percentile served as the reference group, individuals in the medium (26th–75th) percentile group exhibited higher log of expected number of symptoms (b=.19, 95%CI=[.06, .31]; OR=1.21, 95%CI=[1.06, 1.37], p=.008), and individuals in the high (top 75th) percentile group also exhibited higher log of expected number of symptoms by (b=.21, 95%CI=[.04, .37]; OR=1.23, 95%CI=[1.04, 1.44], p=.013).
Table 2.
ADHD PGS cut-point model fit statistics
| Percentile | LRT(df=2) | p | Wald(df=2) | p | AIC | BIC | R2 |
|---|---|---|---|---|---|---|---|
| 5% | 3.79 | .151 | 5.94 | .051 | 20518.22 | 20678.43 | .09 |
| 10% | .38 | .827 | .40 | .820 | 20539.27 | 20699.48 | .08 |
| 15% | 1.62 | .445 | 1.58 | .454 | 20532.16 | 20692.37 | .08 |
| 20% | 5.25 | .072 | 4.97 | .083 | 20525.59 | 20685.80 | .09 |
| 25% | 9.58 | .008 | 8.95 | .011 | 20512.97 | 20673.17 | .08 |
LRT Likelihood ratio test, comparing the model with dummy coded PGS and a model with only covariates; Wald Wald Chi-squared test comparing the model with the dummy coded PGS and a model without the PGS; AIC Akaike Information Criterion; BIC: Bayesian Information Criterion; R2 R-squared statistics based on the correlation between predicted and observed ADHD symptom count
Statistical Analyses
First, ADHD symptom counts were regressed on ADHD PGS (i.e., low, medium and high groups derived as described in the previous section) and the maltreatment latent factor score (i.e., henceforth referred to as the maltreatment score), covarying out effects of sex, and age, parental education and parental income at Wave I. We also covaried the first 10 genetic principal components (PCs) in our models to further account for the possibility of population stratification within the Add Health European-ancestry subsample. We controlled for parental education and parental income because they have been shown to associate with offspring ADHD (Russell et al., 2016). After testing the main effects model, we added a term for the interaction between ADHD PGS and the maltreatment score. A Wald chi-square test was used to determine the statistical significance of each of predictor. For ADHD PGS and the interaction term, significant chi-square statistics were followed with post-hoc analyses of between-group comparisons. To account for overdispersion and zero-inflation in the outcome variable (i.e., ADHD symptoms), zero-inflated negative binomial models were fit for all analyses. All models were adjusted for sample weights, region stratification, and treated schools as the cluster variable for multi-level modeling. To account for Type I error, we also controlled for false discovery rate (FDR) in our hypothesis testing. Finally, we conducted secondary analyses using a continuous ADHD PGS to check the robustness of our results that utilized a categorical ADHD PGS (in the event that categorizing PGS may have limited the variance in this variable).
Results
Descriptive Statistics
Descriptive statistics (by PGS categories) are provided in Table 1. As expected, there were significant differences among the three PGS groups in ADHD symptoms (F(2, 4719)=10.39, p<.001). Individuals in the high PGS group had more ADHD symptoms relative to low PGS group (Δ symptoms=.67, 95% CI=[.37, .96], p<.001). Group differences in the maltreatment score were significant as well (F(2,4291)=3.08, p=.046); the high PGS group had higher maltreatment scores relative to the low PGS group (Δ maltreatment=.09, 95%CI=[.01, .18], p=.033). Significant group differences were also detected in physical neglect, physical abuse, and psychological abuse, but not for psychological neglect or sexual abuse. That is, individuals with high PGS were more likely to report physical neglect (OR=1.60, 95%CI=[1.11, 2.34], p=.028), physical abuse (OR=1.38, 95%CI=[1.09, 1.77], p=.016), and psychological abuse (OR=1.43, 95%CI=[1.12, 1.84], p=.005). Parental education level also differed across the three PGS groups (F(2,4260)=6.91, p=.001) such that those in the low PGS group had parents with higher educational levels compared to those with higher PGS. Household income, age and biological sex did not differ by PGS groups.
Interaction of ADHD PGS and Maltreatment Score on ADHD Symptoms
In the main effects only model, ADHD PGS were positively associated with ADHD symptoms (χ2(1)=11.34, p=.003). As expected, those in the high PGS group had 1.29 time more ADHD symptoms than those in the low PGS group (OR=1.29, s.e.=.10, 95%CI=[1.10, 1.51], p=.004). The standardized maltreatment score was also positively associated with ADHD symptoms (OR=1.14, s.e.=03, χ2(1)=24.90, p<.001). The main effects model provided a significantly better fit to the data than a model with just the covariates alone (LRT(3)=34.859, p<.001; Table 3). However, the interaction between ADHD PGS and maltreatment did not predict ADHD symptoms (χ2(2)=1.38, p=.502) (see Figure 1). The full model with the interaction of ADHD PGS and maltreatment score provided no improvement to model fit (ΔLRT(2)=1.35, ΔAIC=2.65, ΔBIC=15.89, ΔR2=.001; Table 3). Critically, these results remain similar after we statistically account for the significant association between ADHD PGS and maltreatment factor score (by regressing maltreatment on ADHD PGS and covariates and using the residuals in downstream analyses; results available upon request).
Table 3.
Model statistics for the interaction between ADHD PGS and maltreatment in predicting ADHD symptoms
| Model | Model Term | OR(s.e.) | Wald(df) | p | LRT(df) | p | AIC | BIC | R2 |
|---|---|---|---|---|---|---|---|---|---|
| Main | 34.86(3) | <.001 | 15076.95 | 15278.84 | .14 | ||||
| ADHD PGS | 11.34(2) | .003 | |||||||
| medium | 1.21(.08) | ||||||||
| high | 1.29(.10) | ||||||||
| MaltFS | 1.14(.03) | 24.90(1) | <.001 | ||||||
| Full | 36.21(5) | <.001 | 15079.60 | 15293.73 | .14 | ||||
| ADHD PGS | 12.05(2) | .002 | |||||||
| medium | 1.23(.09) | ||||||||
| high | 1.28(.11) | ||||||||
| MaltFS | 1.16(.05) | 11.99(1) | <.001 | ||||||
| ADHD PGS * MaltFS | 1.38(2) | .502 | |||||||
| medium | .10(.05) | ||||||||
| high | 1.01(.05) |
All models controlled for age, sex, parental education and parental income at Wave I. MaltFS was standardized before model fitting
MaltFS maltreatment factor score; LRT: Likelihood ratio test, comparing a model with the predictors and a model with only covariates
Fig. 1.
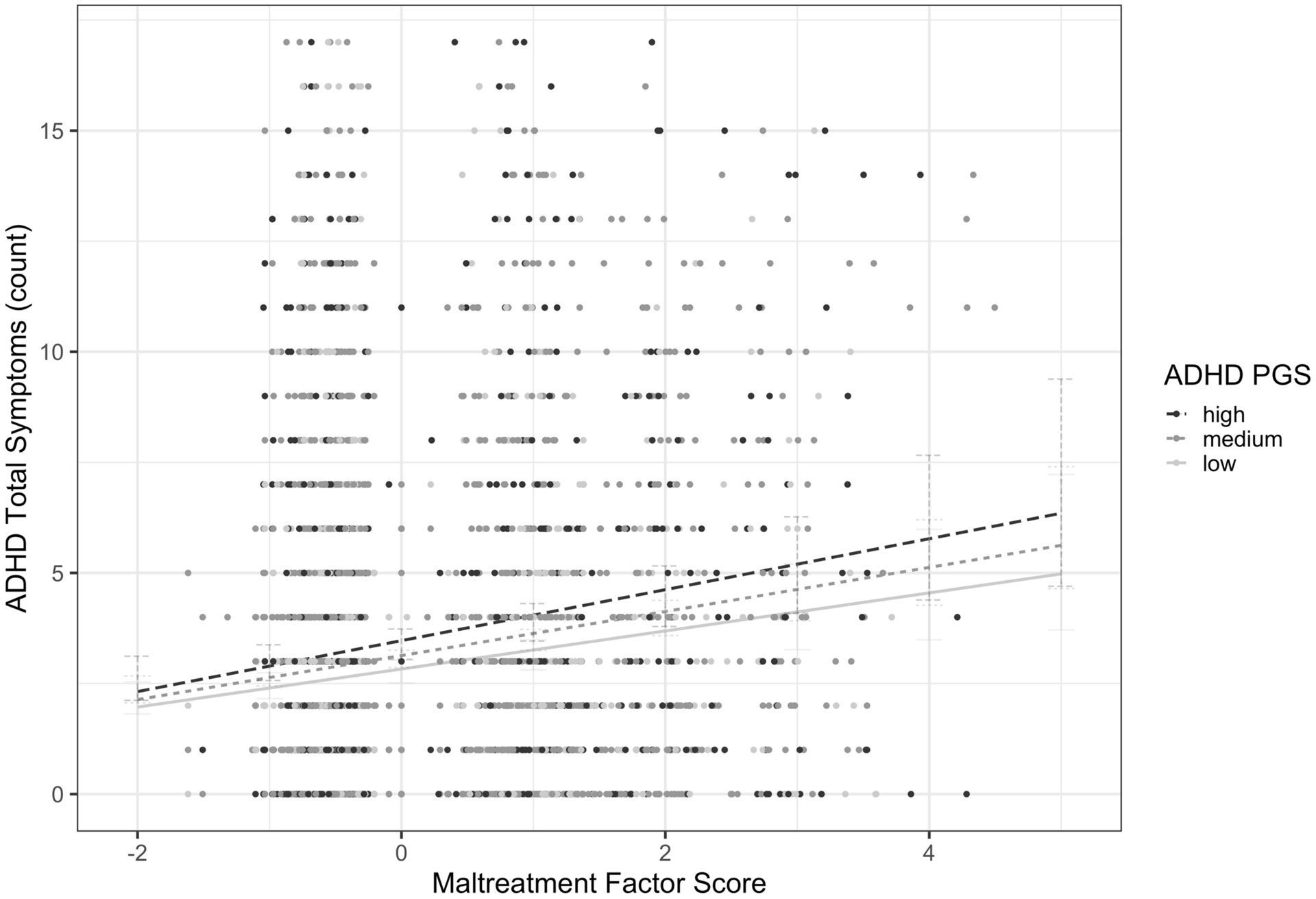
Non-significant interaction between ADHD PGS and maltreatment on ADHD symptoms
Secondary Analysis with a Continuous ADHD PGS
To account for the possible loss of variance with a categorical ADHD PGS (i.e., low, medium and high groups) in our prior models, we tested parallel analytic models using a more conventional continuous ADHD PGS instead. The results from these models were entirely consistent with the models that used the categorical ADHD PGS.
In the main effects only model, the standardized continuous ADHD PGS were positively associated with ADHD symptoms (OR=1.08, s.e.=.03, χ2(2)=8.58, p=.003). The standardized maltreatment score was also positively associated with ADHD symptoms (OR=1.13, s.e.=.03, χ2(1)=21.45, p<.001). The full model with the interaction term provided minimal improvement in model fit (ΔLRT(1)=.16, ΔAIC=1.84, ΔBIC=7.96, ΔR2<.001; Table 4) over the main effects only model. The interaction between ADHD PGS and the maltreatment score was not significant (OR=1.01, s.e.=.02, χ2(2)=.16, p=.691). Statistically accounting for the association between ADHD PGS and maltreatment only led to minimal changes in the results (available upon request).
Table 4.
Model statistics for the interaction between ADHD PGS (continuous) and maltreatment factor score
| Model | Model Term | OR(s.e.) | Wald | p | LRT(df) | p | AIC | BIC | R2 |
|---|---|---|---|---|---|---|---|---|---|
| Main | 41.29(4) | <.001 | 15078.74 | 15243.33 | .13 | ||||
| ADHD PGS | 1.08(.03) | 8.58 | .003 | ||||||
| MaltFS | 1.13(.03) | 21.45 | <.001 | ||||||
| Full | 41.44(5) | <.001 | 15079.98 | 15251.29 | .13 | ||||
| ADHD PGS | 1.08(.03) | 8.00 | .005 | ||||||
| MaltFS | 1.13(.03) | 21.24 | <.001 | ||||||
| ADHD PGS * MaltFS | 1.01(.02) | .16 | .691 |
All models controlled for age, sex, parental education and parental income at Wave I; ADHD PGS and MaltFS were standardized before model fitting
MaltFS maltreatment factor score; LRT Likelihood ratio test, comparing a model with the predictors and a model with only covariates
Discussion
Following on prior findings (Li, 2019b) using Add Health data, the current study tested the hypothesis that individuals with low ADHD PGS may be protected from the negative effects of maltreatment (as it pertains to their ADHD symptoms) relative to those with medium and high ADHD PGS. While we found positive associations of ADHD PGS and maltreatment on ADHD symptoms, no interaction between the two were detected.
Despite the drastic increase in predictive power afforded by the PGS approach (relative to the candidate gene approach), we did not find evidence to support our hypothesis that ADHD PGS moderates one’s sensitivity to maltreatment in relation to ADHD symptoms. This finding is consistent with another recent study that also found no significant interaction between ADHD PGS and a range of environmental risk factors including parental mental disorder history, education, working status and income in ADHD in a large case-control sample in Denmark (Østergaard et al., 2020). Other researchers have also reported either null or generally inconsistent findings between PGS and environmental risk factors on mental disorders more broadly (Lewis & Vassos, 2020). For example, a meta-analysis of major depressive disorder (MDD) found no interaction involving childhood trauma and MDD PGS (Peyrot et al., 2018), while another study found a negative interaction between MDD PGS and childhood trauma (Mullins et al., 2016) (i.e., lower MDD PGS associated with enhanced negative effects of childhood adversity on MDD). Yet another recent study found no interaction between schizophrenia PGS and childhood adversity in other psychotic disorders (Trotta et al., 2016). Our finding adds to a growing body of psychiatric genetics literature that even the use of more powerful PGS approaches may not reliably detect gene-environment interaction effects for mental disorders as previously expected (Dick et al., 2015).
We use our findings to advance alternative considerations for gene-environment interaction studies of ADHD and mental disorders more broadly. For example, gene-environment interactions may be better elucidated if we focused on endophenotypes, which are more proximal to the etiology of ADHD and possibly even more susceptible to environmental influences than mental disorders per se (Gottesman & Gould, 2003). In ADHD research, studies have shown that response inhibition moderated psychosocial risks, such that individuals with faster response inhibition are less likely to develop ADHD and ODD in the presence of family adversity compared to those with slower response inhibition (Nigg et al., 2007). Similarly, in a sample enriched with children with ADHD, slower response inhibition increased the negative associations of low social preference and high relational aggression in childhood with future exposure to intimate partner violence, compared to faster response inhibition (Youn et al., 2019). As neuropsychological endophenotypes like response inhibition are also quite heritable (Crosbie et al., 2013), it stands to reason that genetic factors underlying response inhibition may also help to shed light on how environmental influences can impact the expression of downstream consequences of poor response inhibition, which not only include ADHD, but also substance abuse (Groman et al., 2009), bipolar disorder (Roberts et al., 2013), and autism spectrum disorder (Geurts et al., 2014). Future studies might benefit from investigating PGS for endophenotypes in gene-environment interaction models of related mental disorders.
Additionally, conventional PGS may not represent “pure” genetic risks for psychopathology in the first place, making them a challenge to interpret in gene-environment models. Genetic variants identified in GWAS are variants that were inherited from parents that convey a direct (i.e., biologically mediated) role on the offspring’s phenotype. However, emerging evidence from family-based studies indicate that GWAS estimates also incorporate the effects of non-inherited genotypes from both parents as well (Kong et al., 2018; Young et al., 2019). This is referred to as “genetic nurture” because uninherited parental genotypes can impact one’s phenotype indirectly through the effects of the home environment. Thus, conventional PGS for mental disorders may have already incorporated critical environmental signals via indirect effects from non-transmitted parental genotypes, which may confound or possibly attenuate potential gene-environment interaction effects. For example, a recent study found that only a direct effect ADHD PGS was associated with ADHD (and not the indirect effect PGS), suggesting that genetic risk in ADHD is mainly (although not entirely) accounted for by the transmitted genotypes from parents rather than non-transmitted parental genotypes (de Zeeuw et al., 2020). Future PGS-environment interaction studies should separately compute the direct and the indirect effects of mental disorder PGS. Although large-scale and genetically informed family-based datasets are still relatively rare, new statistical methods are becoming available for disentangling direct and indirect genetic effects in GWAS (e.g., Wu et al., 2020). Both direct and indirect effect PGS could be promising variables to examine in gene-environment interactions. Although the indirect effect PGS did not exhibit a significant main effect in de Zeeuw et al., (2020), it remains unclear if additional exogenous, non-familial exposures could moderate its effect on behavior. Additionally, unraveling the mechanisms that explain direct and indirect PGS effects is critical to understanding etiology and intergenerational transmission of ADHD (Branje et al., 2020). Potential mechanisms that explain direct and indirect PGS pathways include specific neural pathways (e.g., ventral striatum) or psychosocial factors such as parenting behaviors (Li & Lee, 2012; Thorell et al., 2012; Tibu et al., 2016). This work has yet to emerge, which is sensible given how recently direct and indirect effect PGS were described (Kong et al., 2018).
A few study limitations are also noteworthy. First, we limited our analyses to individuals of European ancestry. Some scholars suggest that trans-ancestral PGS predictions may misrepresent the true association between genetic risk and a wide range of phenotypes given that non-European discovery sample sizes are comparatively small and underpowered relative to European sample sizes (e.g., Duncan et al., 2019). We await more diverse and larger genotyped samples in non-European ancestry samples to better address the growing racial-ethnic disparity in psychiatric genetics research. Second, we did not test for sex differences in the interactive effects of ADHD PGS and maltreatment given that our investigation was likely underpowered to do so. There are clear sex differences in the prevalence of ADHD (e.g., Carbonneau et al., 2020), and while Martin et al (2018) recently showed no differences in mean ADHD PGS between males and female cases, there may be sex-specific ADHD PGS × environment interactions for ADHD. Third, maltreatment was retrospectively self-reported by participants. Retrospective self-reports of maltreatment may be influenced by recall bias or inaccurate reporting, which could contribute to null or false findings (Osborn & Widom, 2020; von Wirth et al., 2020). On the other hand, while substantiated records of maltreatment may offer additional validity, these records come at the cost of likely underreporting (i.e., many instances of maltreatment go unreported), detection bias, and relatively low concordance with self-reports (Finkelhor et al., 2014; Osborn & Widom, 2020; Swahn et al., 2006; Widom et al., 2015). Therefore, our results should be interpreted with caution given the challenges in measuring maltreatment. Fourth, childhood ADHD was also retrospectively self-reported when the respondents were young adults. Retrospective reporting of childhood ADHD could be influenced by concurrent (adult) symptoms of ADHD, in addition to other recall biases (Gomez et al., 2020; Miller et al., 2010). Therefore, the PGS-ADHD association we found could be reflective of unique (e.g., persistent forms) of ADHD during adulthood (Agnew-Blais et al., 2021). Finally, we note that Add Health only assessed ADHD at a single time point, thus precluding our ability to examine trajectories of ADHD development over time. Developmental considerations in gene-environment interaction studies are important, yet understudied in this literature (Li, 2019a)
In summary, our study found no evidence of an interaction between ADHD PGS and maltreatment in ADHD symptoms in a sample of nationally representative adolescents followed into adulthood. Despite the increase in predictive power that PGS approaches afford, our findings highlight the continued complexity of studying gene-environment interactions for mental disorders, including ADHD. We are optimistic that approaches that leverage existing theories with new methods, including a focus on endophenotypes and disentangling genetic nurture from GWAS effects, will aid future work in understanding gene-environment interplay.
Acknowledgements
J.J.L was supported in part by a core grant to the Waisman Center from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P50HD105353) and the Wisconsin Alumni Research Foundation. J.J.L. and Q.H. were also supported by the UW-Madison Center for Human Genomics and Precision Medicine.
Funding
Add Health is directed by Robert A. Hummer and funded by the National Institute on Aging cooperative agreements U01 AG071448 (Hummer) and U01AG071450 (Aiello and Hummer) at the University of North Carolina at Chapel Hill. Waves I-V data are from the Add Health Program Project, grant P01 HD31921 (Harris) from Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), with cooperative funding from 23 other federal agencies and foundations. Add Health GWAS data were funded by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grants R01 HD073342 (Harris) and R01 HD060726 (Harris, Boardman, and McQueen). Investigators thank the staff and participants of the Add Health Study for their important contributions. Add Health was designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill.
Footnotes
Ethics Approval
This study has been approved by the University of Wisconsin-Madison Education and Social/Behavioral Science IRB (Protocol #2015–1189, Title: Add Health Study).
Consent to Participate
Add Health participants provided written informed consent for participation in all aspects of Add Health in accordance with the University of North Carolina School of Public Health Institutional Review Board guidelines that are based on the Code of Federal Regulations on the Protection of Human Subjects 45CFR46: https://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html. Additional information can be found on Add Health’s webpage under FAQs: (https://addhealth.cpc.unc.edu/documentation/frequently-asked-questions/).
References
- Agnew-Blais JC, Belsky DW, Caspi A, Danese A, Moffitt TE, Polanczyk GV, Sugden K, Wertz J, Williams B, Lewis CM, & Arseneault L (2021). Polygenic Risk and the Course of Attention-Deficit/Hyperactivity Disorder From Childhood to Young Adulthood: Findings From a Nationally-Representative Cohort. Journal of the American Academy of Child & Adolescent Psychiatry. 10.1016/j.jaac.2020.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: Author. https://www.psychiatry.org/psychiatrists/practice/dsm [Google Scholar]
- Branje S, Geeraerts S, de Zeeuw EL, Oerlemans AM, Koopman-Verhoeff ME, Schulz S, Nelemans S, Meeus W, Hartman CA, Hillegers MHJ, Oldehinkel AJ, & Boomsma DI (2020). Intergenerational transmission: Theoretical and methodological issues and an introduction to four Dutch cohorts. Developmental Cognitive Neuroscience, 45, 100835. 10.1016/j.dcn.2020.100835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe-Smith AM, & Hinshaw SP (2006). Linkages Between Child Abuse and Attention-Deficit/Hyperactivity Disorder in Girls: Behavioral and Social Correlates. Child Abuse & Neglect, 30(11), 1239–1255. 10.1016/j.chiabu.2006.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N, Anney R, Aneey R, Franke B, Gill M, Ebstein R, Buitelaar J, Sham P, Campbell D, Knight J, Andreou P, Altink M, Arnold R, Boer F, … Asherson P (2006). The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: Association signals in DRD4, DAT1 and 16 other genes. Molecular Psychiatry, 11(10), 934–953. 10.1038/sj.mp.4001869 [DOI] [PubMed] [Google Scholar]
- Brumley LD, Brumley BP, & Jaffee SR (2019). Comparing cumulative index and factor analytic approaches to measuring maltreatment in the National Longitudinal Study of Adolescent to Adult Health. Child Abuse & Neglect, 87, 65–76. 10.1016/j.chiabu.2018.08.014 [DOI] [PubMed] [Google Scholar]
- Carbonneau M, Demers M, Bigras M, & Guay M-C (2020). Meta-Analysis of Sex Differences in ADHD Symptoms and Associated Cognitive Deficits. Journal of Attention Disorders, 1087054720923736. 10.1177/1087054720923736 [DOI] [PubMed] [Google Scholar]
- Choi SW, & O’Reilly PF (2019). PRSice-2: Polygenic Risk Score software for biobank-scale data. GigaScience, 8(7). 10.1093/gigascience/giz082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton K, Lee JB, Cheung K, Theule J, & Henrikson B (2018). Quantifying the Relationship between Attention-Deficit/Hyperactivity Disorder and Experiences of Child Maltreatment: A Meta-Analysis. Child Abuse Review, 27(5), 361–377. 10.1002/car.2530 [DOI] [Google Scholar]
- Copeland WE, Shanahan L, Hinesley J, Chan RF, Aberg KA, Fairbank JA, van den Oord EJCG, & Costello EJ (2018). Association of Childhood Trauma Exposure With Adult Psychiatric Disorders and Functional Outcomes. JAMA Network Open, 1(7), e184493. 10.1001/jamanetworkopen.2018.4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosbie J, Arnold P, Paterson A, Swanson J, Dupuis A, Li X, Shan J, Goodale T, Tam C, Strug LJ, & Schachar RJ (2013). Response Inhibition and ADHD Traits: Correlates and Heritability in a Community Sample. Journal of Abnormal Child Psychology, 41(3), 497–507. 10.1007/s10802-012-9693-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, & Blumberg SJ (2018). Prevalence of Parent-Reported ADHD Diagnosis and Associated Treatment Among U.S. Children and Adolescents, 2016. Journal of Clinical Child and Adolescent Psychology : The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 47(2), 199–212. 10.1080/15374416.2017.1417860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeeuw EL, Hottenga J-J, Ouwens KG, Dolan CV, Ehli EA, Davies GE, Boomsma DI, & van Bergen E (2020). Intergenerational Transmission of Education and ADHD: Effects of Parental Genotypes. Behavior Genetics. 10.1007/s10519-020-09992-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, Baldursson G, Belliveau R, Bybjerg-Grauholm J, Bækvad-Hansen M, Cerrato F, Chambert K, Churchhouse C, Dumont A, Eriksson N, Gandal M, Goldstein JI, Grasby KL, Grove J, … Neale BM (2019). Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nature Genetics, 51(1), 63–75. 10.1038/s41588-018-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Keller MC, Adkins A, Aliev F, Monroe S, Hewitt JK, Kendler KS, & Sher KJ (2015). Candidate Gene–Environment Interaction Research: Reflections and Recommendations. Perspectives on Psychological Science, 10(1), 37–59. 10.1177/1745691614556682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan L, Shen H, Gelaye B, Meijsen J, Ressler K, Feldman M, Peterson R, & Domingue B (2019). Analysis of polygenic risk score usage and performance in diverse human populations. Nature Communications, 10(1), 1–9. 10.1038/s41467-019-11112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Scott L, Song P, Burmeister M, & Sen S (2019). Genomic prediction of depression risk and resilience under stress. Nature Human Behaviour. 10.1038/s41562-019-0759-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, & Larsson H (2019). Genetics of attention deficit hyperactivity disorder. Molecular Psychiatry, 24(4), 562–575. 10.1038/s41380-018-0070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelhor D, Vanderminden J, Turner H, Hamby S, & Shattuck A (2014). Child maltreatment rates assessed in a national household survey of caregivers and youth. Child Abuse & Neglect, 38(9), 1421–1435. 10.1016/j.chiabu.2014.05.005 [DOI] [PubMed] [Google Scholar]
- Geurts HM, Bergh, van den SFWM, & Ruzzano L (2014). Prepotent Response Inhibition and Interference Control in Autism Spectrum Disorders: Two Meta-Analyses. Autism Research, 7(4), 407–420. 10.1002/aur.1369 [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, & Waldman ID (2009). Candidate gene studies of ADHD: A meta-analytic review. Human Genetics, 126(1), 51–90. 10.1007/s00439-009-0694-x [DOI] [PubMed] [Google Scholar]
- Gomez R, Stavropoulos V, & Watson S (2020). Measurement Invariance Across Adult Self-Ratings of Current and Retrospective Childhood ADHD Symptoms. Journal of Psychopathology and Behavioral Assessment, 42(3), 475–487. 10.1007/s10862-020-09802-x [DOI] [Google Scholar]
- González RA, Vélez-Pastrana MC, McCrory E, Kallis C, Aguila J, Canino G, & Bird H (2019). Evidence of concurrent and prospective associations between early maltreatment and ADHD through childhood and adolescence. Social Psychiatry and Psychiatric Epidemiology, 54(6), 671–682. 10.1007/s00127-019-01659-0 [DOI] [PubMed] [Google Scholar]
- Gottesman II, & Gould TD (2003). The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. American Journal of Psychiatry, 160(4), 636–645. 10.1176/appi.ajp.160.4.636 [DOI] [PubMed] [Google Scholar]
- Gould KL, Coventry WL, Olson RK, & Byrne B (2018). Gene-Environment Interactions in ADHD: The Roles of SES and Chaos. Journal of Abnormal Child Psychology, 46(2), 251–263. 10.1007/s10802-017-0268-7 [DOI] [PubMed] [Google Scholar]
- Groman SM, James AS, & Jentsch JD (2009). Poor response inhibition: At the nexus between substance abuse and attention deficit/hyperactivity disorder. Neuroscience & Biobehavioral Reviews, 33(5), 690–698. 10.1016/j.neubiorev.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- International Schizophrenia Consortium, Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, & Sklar P (2009). Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature, 460(7256), 748–752. 10.1038/nature08185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A, Thorleifsson G, Frigge ML, Vilhjalmsson BJ, Young AI, Thorgeirsson TE, Benonisdottir S, Oddsson A, Halldorsson BV, Masson G, Gudbjartsson DF, Helgason A, Bjornsdottir G, Thorsteinsdottir U, & Stefansson K (2018). The nature of nurture: Effects of parental genotypes. Science, 359(6374), 424–428. 10.1126/science.aan6877 [DOI] [PubMed] [Google Scholar]
- Lewis CM, & Vassos E (2020). Polygenic risk scores: From research tools to clinical instruments. Genome Medicine, 12(1), 44. 10.1186/s13073-020-00742-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ (2019a). Assessing phenotypic and polygenic models of ADHD to identify mechanisms of risk for longitudinal trajectories of externalizing behaviors. Journal of Child Psychology and Psychiatry, 60(11), 1191–1199. 10.1111/jcpp.13071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ (2019b). The positive end of the polygenic score distribution for ADHD: A low risk or a protective factor? Psychological Medicine, 1–10. 10.1017/S0033291719003039 [DOI] [PubMed] [Google Scholar]
- Li JJ, & He Q (2021). Polygenic Scores for ADHD: A Meta-Analysis. Research on Child and Adolescent Psychopathology. 10.1007/s10802-021-00774-4 [DOI] [PubMed] [Google Scholar]
- Li JJ, & Lee SS (2012). Association of Positive and Negative Parenting Behavior with Childhood ADHD: Interactions with Offspring Monoamine Oxidase A (MAO-A) Genotype. Journal of Abnormal Child Psychology, 40(2), 165–175. 10.1007/s10802-011-9553-z [DOI] [PubMed] [Google Scholar]
- Martin J, Walters RK, Demontis D, Mattheisen M, Lee SH, Robinson E, Brikell I, Ghirardi L, Larsson H, Lichtenstein P, Eriksson N, Agee M, Alipanahi B, Auton A, Bell RK, Bryc K, Elson SL, Fontanillas P, Furlotte NA, … Neale BM (2018). A Genetic Investigation of Sex Bias in the Prevalence of Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry, 83(12), 1044–1053. 10.1016/j.biopsych.2017.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Newcorn JH, & Halperin JM (2010). Fading Memories: Retrospective Recall Inaccuracies in ADHD. Journal of Attention Disorders, 14(1), 7–14. 10.1177/1087054709347189 [DOI] [PubMed] [Google Scholar]
- Mullins N, Power RA, Fisher HL, Hanscombe KB, Euesden J, Iniesta R, Levinson DF, Weissman MM, Potash JB, Shi J, Uher R, Cohen-Woods S, Rivera M, Jones L, Jones I, Craddock N, Owen MJ, Korszun A, Craig IW, … Lewis CM (2016). Polygenic interactions with environmental adversity in the aetiology of major depressive disorder. Psychological Medicine, 46(4), 759–770. 10.1017/S0033291715002172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J, Nikolas M, & Burt SA (2010). Measured Gene by Environment Interaction in Relation to Attention-Deficit/Hyperactivity Disorder (ADHD). Journal of the American Academy of Child and Adolescent Psychiatry, 49(9), 863–873. 10.1016/j.jaac.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J, Nikolas M, Friderici K, Park L, & Zucker RA (2007). Genotype and Neuropsychological Response Inhibition as Resilience Promoters for ADHD, ODD, and CD under Conditions of Psychosocial Adversity. Development and Psychopathology, 19(3), 767–786. 10.1017/S0954579407000387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M, & Widom CS (2020). Do documented records and retrospective reports of childhood maltreatment similarly predict chronic inflammation? Psychological Medicine, 50(14), 2406–2415. 10.1017/S0033291719002575 [DOI] [PubMed] [Google Scholar]
- Østergaard SD, Trabjerg BB, Als TD, Climent CA, Privé F, Vilhjálmsson BJ, Bækvad-Hansen M, Bybjerg-Grauholm J, Hougaard DM, Nordentoft M, Werge T, Demontis D, Mortensen PB, Børglum AD, Mors O, & Agerbo E (2020). Polygenic risk score, psychosocial environment and the risk of attention-deficit/hyperactivity disorder. Translational Psychiatry, 10(1), 1–11. 10.1038/s41398-020-01019-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino V, McNeill R, Reif A, & Kittel-Schneider S (2019). Genetic risk factors and gene–environment interactions in adult and childhood attention-deficit/hyperactivity disorder. Psychiatric Genetics, 29(3), 63–78. 10.1097/YPG.0000000000000220 [DOI] [PubMed] [Google Scholar]
- Park S, Kim B-N, Kim J-W, Shin M-S, Yoo HJ, & Cho S-C (2017). Interactions Between Early Trauma and Catechol-O-Methyltransferase Genes on Inhibitory Deficits in Children With ADHD. Journal of Attention Disorders, 21(3), 183–189. 10.1177/1087054714543650 [DOI] [PubMed] [Google Scholar]
- Pettersson E, Lichtenstein P, Larsson H, Song J, Attention Deficit/Hyperactivity Disorder Working Group of the iPSYCH-Broad-PGC Consortium, A. S. D. W. G. of the iPSYCH-B.-P. C., Agrawal A, Børglum AD, Bulik CM, Daly MJ, Davis LK, Demontis D, Edenberg HJ, Grove J, Gelernter J, Neale BM, Pardiñas AF, Stahl E, Walters JTR, Walters R, … Polderman TJC (2019). Genetic influences on eight psychiatric disorders based on family data of 4 408 646 full and half-siblings, and genetic data of 333 748 cases and controls. Psychological Medicine, 49(7), 1166–1173. 10.1017/S0033291718002039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrot WJ, Van der Auwera S, Milaneschi Y, Dolan CV, Madden PAF, Sullivan PF, Strohmaier J, Ripke S, Rietschel M, Nivard MG, Mullins N, Montgomery GW, Henders AK, Heat AC, Fisher HL, Dunn EC, Byrne EM, Air TA, Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, … Penninx BWJH (2018). Does Childhood Trauma Moderate Polygenic Risk for Depression? A Meta-analysis of 5765 Subjects From the Psychiatric Genomics Consortium. Biological Psychiatry, 84(2), 138–147. 10.1016/j.biopsych.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor LJ, Lewis T, Roesch S, Thompson R, Litrownik AJ, English D, Arria AM, Isbell P, & Dubowitz H (2017). Child maltreatment and age of alcohol and marijuana initiation in high-risk youth. Addictive Behaviors, 75, 64–69. 10.1016/j.addbeh.2017.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G, Green MJ, Breakspear M, McCormack C, Frankland A, Wright A, Levy F, Lenroot R, Chan HN, & Mitchell PB (2013). Reduced Inferior Frontal Gyrus Activation During Response Inhibition to Emotional Stimuli in Youth at High Risk of Bipolar Disorder. Biological Psychiatry, 74(1), 55–61. 10.1016/j.biopsych.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Russell AE, Ford T, Williams R, & Russell G (2016). The Association Between Socioeconomic Disadvantage and Attention Deficit/Hyperactivity Disorder (ADHD): A Systematic Review. Child Psychiatry & Human Development, 47(3), 440–458. 10.1007/s10578-015-0578-3 [DOI] [PubMed] [Google Scholar]
- Sanderud K, Murphy S, & Elklit A (2016). Child maltreatment and ADHD symptoms in a sample of young adults. European Journal of Psychotraumatology, 7. 10.3402/ejpt.v7.32061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A, Agnew-Blais J, Danese A, Fisher HL, Jaffee SR, Matthews T, Polanczyk GV, & Arseneault L (2018). Associations between abuse/neglect and ADHD from childhood to young adulthood: A prospective nationally-representative twin study. Child Abuse & Neglect, 81, 274–285. 10.1016/j.chiabu.2018.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swahn MH, Whitaker DJ, Pippen CB, Leeb RT, Teplin LA, Abram KM, & McClelland GM (2006). Concordance Between Self-Reported Maltreatment and Court Records of Abuse or Neglect Among High-Risk Youths. American Journal of Public Health, 96(10), 1849–1853. 10.2105/AJPH.2004.058230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabone JK, Guterman NB, Litrownik AJ, Dubowitz H, Isbell P, English DJ, Runyan DK, & Thompson R (2011). Developmental Trajectories of Behavior Problems Among Children Who Have Experienced Maltreatment: Heterogeneity During Early Childhood and Ecological Predictors. Journal of Emotional and Behavioral Disorders, 19(4), 204–216. 10.1177/1063426610383861 [DOI] [Google Scholar]
- Thorell LB, Rydell A-M, & Bohlin G (2012). Parent–child attachment and executive functioning in relation to ADHD symptoms in middle childhood. Attachment & Human Development, 14(5), 517–532. 10.1080/14616734.2012.706396 [DOI] [PubMed] [Google Scholar]
- Tibu F, Sheridan MA, McLaughlin KA, Nelson CA, Fox NA, & Zeanah CH (2016). Disruptions of working memory and inhibition mediate the association between exposure to institutionalization and symptoms of attention deficit hyperactivity disorder. Psychological Medicine, 46(3), 529–541. 10.1017/S0033291715002020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta A, Iyegbe C, Di Forti M, Sham PC, Campbell DD, Cherny SS, Mondelli V, Aitchison KJ, Murray RM, Vassos E, & Fisher HL (2016). Interplay between schizophrenia polygenic risk score and childhood adversity in first-presentation psychotic disorder: A pilot study. PLoS ONE, 11(9). http://search.ebscohost.com/login.aspx?direct=true&AuthType=ip,uid&db=psyh&AN=2016-47244-001&site=ehost-live&scope=site [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wirth E, Mandler J, Breuer D, & Döpfner M (2020). The Accuracy of Retrospective Recall of Childhood ADHD: Results from a Longitudinal Study. Journal of Psychopathology and Behavioral Assessment. 10.1007/s10862-020-09852-1 [DOI] [Google Scholar]
- Warrier V, Kwong ASF, Luo M, Dalvie S, Croft J, Sallis HM, Baldwin J, Munafò MR, Nievergelt CM, Grant AJ, Burgess S, Moore TM, Barzilay R, McIntosh A, van IJzendoorn MH, & Cecil CAM (2021). Gene–environment correlations and causal effects of childhood maltreatment on physical and mental health: A genetically informed approach. The Lancet. Psychiatry, 8(5), 373–386. 10.1016/S2215-0366(20)30569-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS, Czaja SJ, & DuMont KA (2015). Intergenerational transmission of child abuse and neglect: Real or detection bias? Science, 347(6229), 1480–1485. 10.1126/science.1259917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhong X, Lin Y, Zhao Z, Chen J, Zheng B, Li JJ, Fletcher JM, & Lu Q (2020). Estimating genetic nurture with summary statistics of multi-generational genome-wide association studies. BioRxiv, 2020.10.06.328724. 10.1101/2020.10.06.328724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S (2017). Child maltreatment characteristics as predictors of heterogeneity in internalizing symptom trajectories among children in the child welfare system. Child Abuse & Neglect, 72, 247–257. 10.1016/j.chiabu.2017.08.022 [DOI] [PubMed] [Google Scholar]
- Yoon S (2018). Fostering Resilient Development: Protective Factors Underlying Externalizing Trajectories of Maltreated Children. Journal of Child and Family Studies, 27(2), 443–452. 10.1007/s10826-017-0904-4 [DOI] [Google Scholar]
- Youn C, Meza JI, & Hinshaw SP (2019). Childhood Social Functioning and Young Adult Intimate Partner Violence in Girls With and Without ADHD: Response Inhibition as a Moderator. Journal of Attention Disorders, 23(12), 1486–1496. 10.1177/1087054718778119 [DOI] [PubMed] [Google Scholar]
- Young AI, Benonisdottir S, Przeworski M, & Kong A (2019). Deconstructing the sources of genotype-phenotype associations in humans. Science, 365(6460), 1396–1400. 10.1126/science.aax3710 [DOI] [PMC free article] [PubMed] [Google Scholar]


