Abstract
The bacterial domain produces numerous types of sphingolipids with various physiological functions. In the human microbiome, commensal and pathogenic bacteria use these lipids to modulate the host inflammatory system. Despite their growing importance, their biosynthetic pathway remains undefined since several key eukaryotic ceramide synthesis enzymes have no bacterial homologue. Here we used genomic and biochemical approaches to identify six proteins comprising the complete pathway for bacterial ceramide synthesis. Bioinformatic analyses revealed the widespread potential for bacterial ceramide synthesis leading to our discovery of the first known Gram-positive species to produce ceramides. Biochemical evidence demonstrated that the bacterial pathway operates in a different order than in eukaryotes. Furthermore, phylogenetic analyses support the hypothesis that the bacterial and eukaryotic ceramide pathways evolved independently.
Introduction
Sphingolipids are found ubiquitously in eukaryotes from fungi, to plants, to animals. By contrast, this class of lipids has been identified in only a handful of bacterial taxa1. Within this small group of sphingolipid-producing bacteria there is a tremendous variety of acyl chain length and degree of saturation, acyl chain hydroxylation, and lipid headgroups. This structural diversity is paralleled by a wide range of physiological roles for sphingolipids including modulation of host-microbe interactions2,3, protection from bacteriophage4, bacterial life cycle and sporulation5, and microbial predation6. Deeper investigations into the mechanistic roles of sphingolipids in bacterial physiology and host-microbe interactions have been hampered by a lack of knowledge of their biosynthetic pathway. Due to their importance in human health and disease7, it is not surprising that the eukaryotic biosynthesis pathway has been elucidated in tremendous detail8. By contrast, bacteria do not appear to have homologous enzymes, except for serine palmitoyltransferase (Spt) which performs the initial conserved step in ceramide synthesis9,10.
To date, the presence of predicted spt genes is the only indication that a bacterial species may synthesize sphingolipids11. However, the presence of a predicted Spt alone is not a particularly reliable indicator of sphingolipid production because there is a high degree of similarity between members of the larger family of α-oxoamine synthases that are involved in heme and biotin synthesis. Here, we identified and characterized the remainder of the bacterial ceramide synthetic pathway. We note that during the preparation of this manuscript, the same set of genes were identified in C. crescentus12. While the independent identification of this biosynthetic pathway corroborates the importance of these genes in ceramide synthesis, our biochemical data suggested a different function for these enzymes than that originally proposed12. Furthermore, the elucidation of the bacterial ceramide synthesis pathway enabled us to perform a bioinformatic screen that led to the identification of 17 taxonomic classes, including the first Gram-positive bacteria, with the potential to synthesize sphingolipids. Lipid profiling of one of these Actinobacteria provided the first demonstration of Gram-positive bacterial ceramides, validated our bioinformatic approach, and suggested that bacterial sphingolipid synthesis occurs across a wide range of organisms. Surprisingly, the bacterial enzymes are not phylogenetically related to those in eukaryotes and the biosynthetic steps occur in a different order than in eukaryotes. These findings support the independent evolution of ceramide production in bacteria and eukaryotes.
Results
Characterization of serine palmitoyltransferase (Spt)
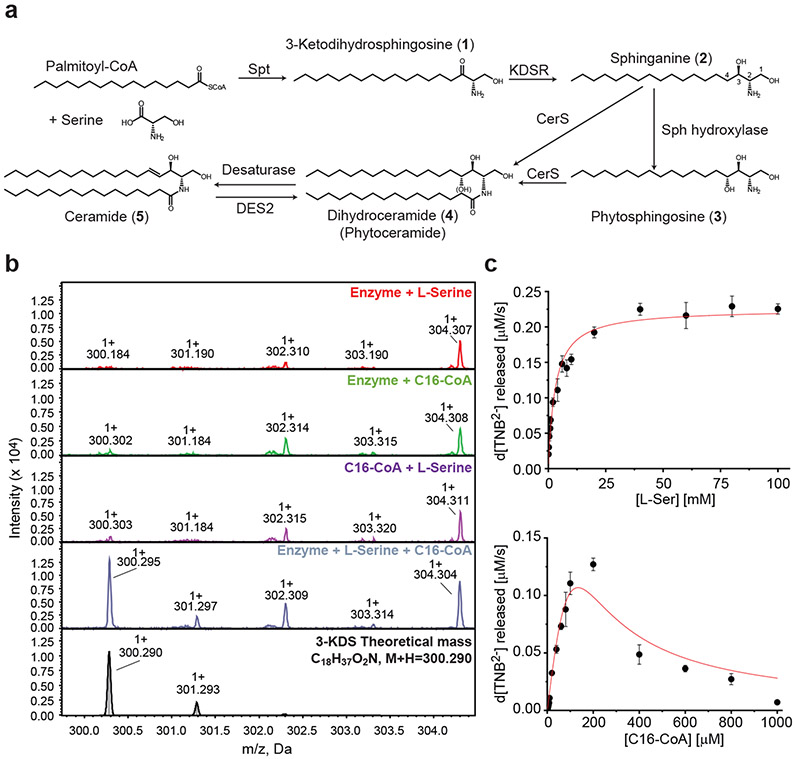
The first step in de novo ceramide synthesis, which is conserved between eukaryotes and prokaryotes, is the decarboxylative, Claisen-like condensation of palmitoyl-coenzyme A (palmitoyl-CoA) and L-serine into 3-ketosphinghanine (3-KDS, 1) (Fig. 1a)13. In C. crescentus, we previously identified ccna_01220 as a putative Spt since the Δccna_01220 strain had no detectable ceramide4. His-tagged recombinant CCNA_01220 was purified from E. coli (see Extended Data Fig. 1a-b); it displayed Spt activity, and the production of 3-KDS was detected by matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) (Fig. 1b). Kinetic analyses yielded Km values of 110.40 ± 13.37 μM and 2.98 ± 0.25 mM for C16:0-CoA and L-serine, respectively (Fig. 1c), which are comparable to the values determined for Sphingomonas paucimobilis and human Spt14-16. C. crescentus Spt also condensed serine and C16:1-CoA with similar kinetic parameters (see Extended Data Fig. 1c-d). Liquid chromatography/electrospray ionization-tandem mass spectrometry (LC/ESI-MS/MS) analysis of the ceramide molecule from C. crescentus showed that C16:0 was preferentially used as the Spt substrate to form the long-chain base (see Extended Data Fig. 1e). In some organisms, such as the gut commensal Bacteroides thetaiotaomicron, 1-deoxysphingolipids can be detected when Spt uses alanine as a substrate rather than serine2. Similarly, a C. crescentus serine auxotroph (ΔserA), produced 1-deoxyceramide (see Extended Data Fig. 1f-g).
Figure 1. CCNA_01220 is a functional serine palmitoyltransferase (Spt).
(a) The schematic depicts the eukaryotic ceramide synthesis pathway. In some organisms, phytoceramides (6) are produced by adding a hydroxyl group (OH) to the sphingoid base (3) (Sph) or to ceramide (5) (DES2). (b) Recombinant Spt was incubated with the indicated substrates for 1 hr and reaction products were analyzed by MALDI-MS. The final panel shows a theoretical mass spectrum for the expected product, 3-KDS. (c) Kinetic analyses of Spt determined the Km for L-serine (upper) and C16:0-CoA (lower) (n=3; data are presented as mean +/− SD).
Genetic screen identifies the ceramide synthesis pathway
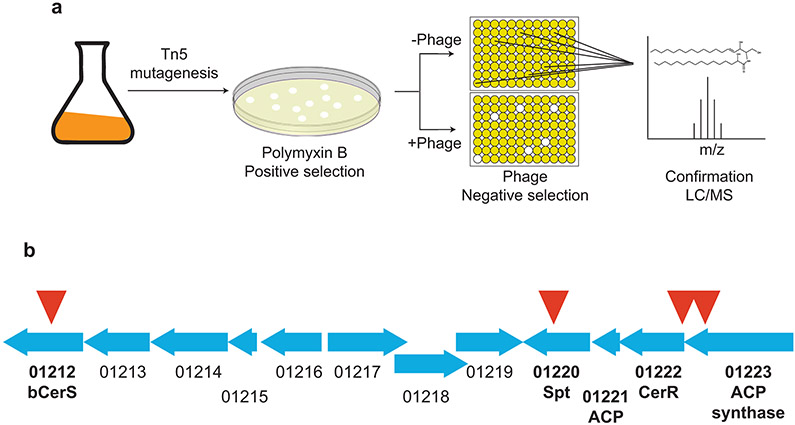
In the absence of ceramides, C. crescentus becomes resistant to the cationic antimicrobial peptide, polymyxin B (PMX) and increasingly sensitive to bacteriophage Φcr304. Using these two phenotypes of ceramide depletion, we performed a transposon mutagenesis screen incorporating both positive and negative selection to identify candidate genes involved in ceramide synthesis (see Extended Data Fig. 2A, detailed in Materials and Methods). Transposon insertions in cells displaying both selection phenotypes were mapped (see Supplementary Table 1 and Extended Data Fig. 2b) and multiple insertions were found in the spt gene validating this approach. These transposon mutants served as a platform for identifying ceramide synthesis enzymes as described below.
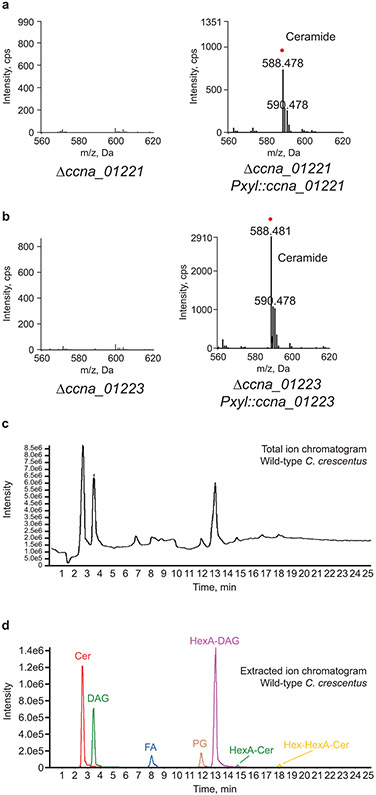
Spt enzymes vary in their preferred acyl-CoA substrate; some enzymes utilize fatty acyl-CoA thioesters whereas others use an acyl-chain bound as a thioester to an acyl carrier protein (ACP). Our genetic screening identified ccna_01223, a putative acyl-CoA synthetase, which adds CoA to a long-chain fatty acid. Additionally, inspection of neighboring genes revealed a candidate ACP (ccna_01221). This genomic arrangement of the Spt, ACP, and ACP-synthetase is similar to that seen in Sphingomonas wittichii17. Although no transposon insertions were identified in ccna_01221, this was not surprising given that the gene is only 261 bp and would therefore have a low probability of containing an insertion. Deletion of either gene resulted in a total loss of ceramides (see Extended Data Fig. 3a-b) and both deletions could be complemented by ectopic expression of the respective gene (see Extended Data Fig. 3a-b). Total ion and extracted ion chromatograms confirm that the deletions did not disrupt global lipid production (see Extended Data Fig. 3c-d and Supplementary Table 3).
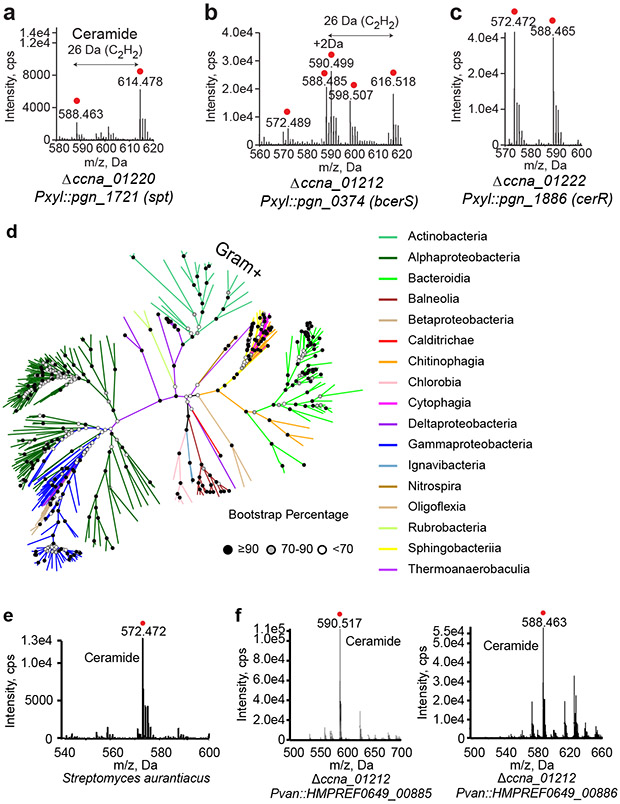
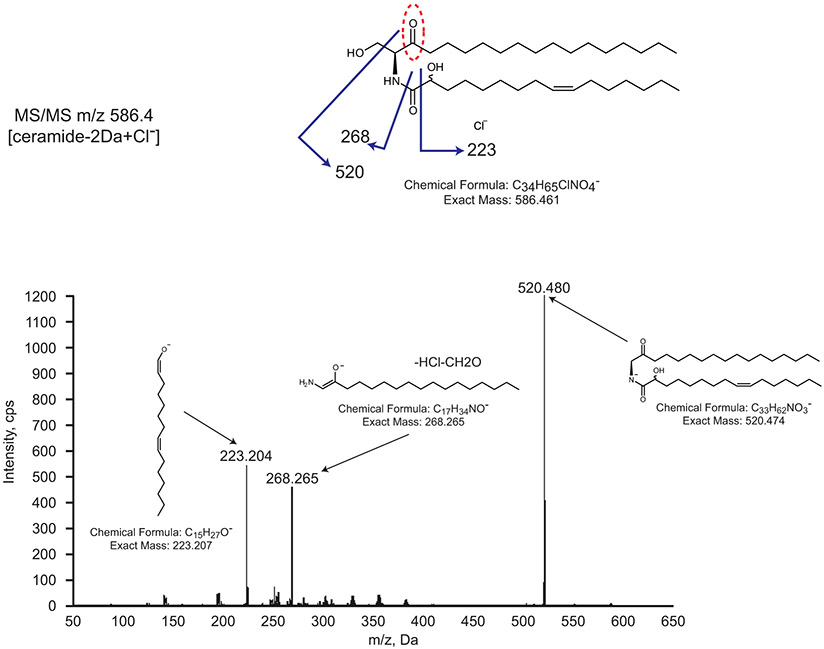
In eukaryotes, the second synthetic step is the reduction of 3-KDS to sphinganine (2), which is catalyzed by a NADPH-dependent 3-ketodihydrosphinghanine reductase (KDSR) (Fig. 1a). Among our transposon insertions, ccna_01222 was annotated as an NADH ubiquinone-oxidoreductase. Deletion of ccna_01222 resulted in a ceramide molecule with a mass reduction of 2 Da (Fig. 2a), corresponding to a loss of two hydrogens. Tandem mass spectrometry (MS/MS) analysis confirmed the retention of the oxidized double bond on the 3-KDS derived sphingoid base (see Extended Data Fig. 4). Complementation of the ccna_01222 deletion restored ceramide reduction (Fig. 2a). These data suggest that the bacterial reductase acts after the second acyl chain is added to 3-KDS. This contrasts with the eukaryotic pathway where deletion of KDSR generally leads to an accumulation of 3-KDS substrate and prevents downstream reactions18. There have been reports of 3-KDS being acylated directly by ceramide synthase in eukaryotic cells; however, this was only observed when Spt was highly overexpressed and cells were provided with excess serine and palmitate19. Since the bacterial reductase uses oxidized ceramide (oxCer) as a substrate, we have named CCNA_01222 Ceramide Reductase (CerR).
Figure 2. A genetic screen identified ceramide synthesis enzymes.
(a-d) Negative ion ESI/MS shows the [M + Cl]− ions of the lipids emerging at 2 to 3 min. Ceramide species are labelled with a red dot and the modified lipid moiety is designated with a red-dashed oval. Note that MS/MS analysis of ceramide from C. crescentus shows that the desaturation occurs on the acyl chain (see Extended Data Fig. 1e); however, we have not determined the precise position of the double bond. In this, and all subsequent figures, the structural cartoons only indicate which acyl chain is desaturated, but not the exact position of the double bond. Relative quantification of all the major lipid species for each mass spectrum is available in Supplementary Table 3. (a) Lipids were extracted from wild-type, Δccna_01222, and ccna_01222-complemented cells. (b) Lipids were extracted from Δccna_01212 and ccna_01212-complemented cells. (c) Recombinant CCNA_01212 was incubated with 40 μM 3-KDS and 50 μM C16:0-CoA for 1 hr and the reaction product was analyzed by normal phase LC/ESI-MS in negative ion mode. (d) Lipids were extracted from Δccna_00202 and ccna_00202-complemented cells. (e) Based on the MS data above, we propose the following model for bacterial ceramide synthesis. The genes comprising this synthetic pathway are in close proximity in the genome (see Extended Data Fig. 2b). (f) Cells expressing the indicated fluorescently-tagged proteins were grown overnight in the presence of inducer. GspG-mCherry and TAT-mCherry are control inner-membrane and periplasmic proteins, respectively. Control and permeabilized cells were imaged by fluorescence microscopy to monitor the loss of fluorescence upon permeabilization. The results are the overlay of phase and fluorescent images. Scale bar = 5 μm.
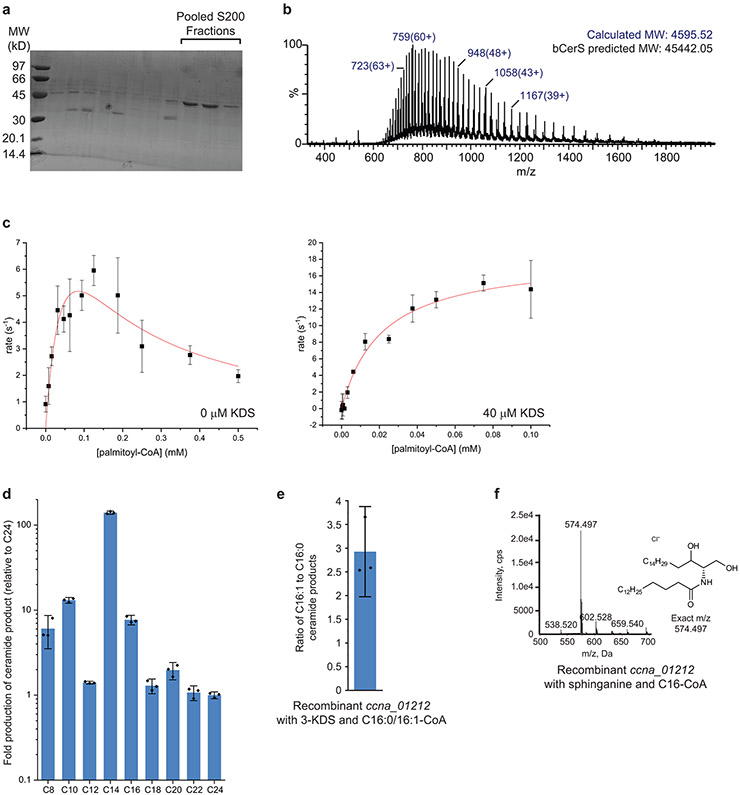
In eukaryotes, the second acyl chain is attached to the sphingoid backbone by ceramide synthase (CerS) to form dihydroceramide (4) (Fig. 1a). Analysis of our transposon hits pointed to ccna_01212 as a potential CerS. In C. crescentus, this gene is annotated as a dATP pyrophosphohydrolase; however, closely related genes identified by BLAST have a variety of annotations including Gcn5-related N-acetyltransferase (GNAT). Deletion of ccna_01212 led to a complete loss of ceramides (Fig. 2b), consistent with its role in ceramide synthesis. To confirm the enzymatic activity of CCNA_01212, recombinant protein purified from E. coli (see Extended Data Fig. 5a-b) was incubated with the substrates 3-KDS and palmitoyl-CoA. LC/ESI-MS/MS analysis of the reaction product identified the expected ceramide molecule (Fig. 2c). Kinetic analyses showed that CCNA_01212 constitutively hydrolyzed acyl-CoA even in the absence of 3-KDS, and the reaction exhibits substrate inhibition (see Extended Data Fig. 5c). Upon the addition of 3-KDS, the reaction proceeded according to Michaelis-Menten kinetics and we determined the Km,app for C16:0-CoA to be 21.3 ± 4.1 μM (see Extended Data Fig. 5c). Based on these data we have named CCNA_01212 bacterial Ceramide Synthase (bCerS). Since eukaryotic CerS enzymes have specific acyl-chain specificities20, we assessed the substrate preference of C. crescentus bCerS. Recombinant bCerS was incubated with 3-KDS and an equimolar mixture of acyl-CoA substrates ranging from C8-C24, and the relative amount of the respective products was monitored by LC/ESI-MS. C. crescentus had the highest in vitro activity with C14 and showed very little activity with acyl-CoA thioesters of 18 carbons or longer (see Extended Data Fig. 5d). In vivo, the C16 ceramide product is most abundant rather than C14 (see Extended Data Fig. 1e); this may reflect the fact that C16 accounts for 30% of the fatty acid content of the cell, whereas C14 is only 2%21. Our experiments demonstrating bCerS activity with acyl-CoA as a substrate are consistent with ceramide synthase activity; however, given that the acyl-CoA is readily hydrolyzed by bCerS to a free fatty acid, we cannot rule out the possibility that bCerS ligates the fatty acid to 3-KDS via a reverse-ceramidase-like mechanism22.
A common ceramide modification found in fungi, plants, some animal tissues, and bacteria is the addition of a hydroxyl group to form phytoceramides (6) (Fig. 1a). In plants, sphingoid base hydroxylase 1/2 (Sbh1/2) adds a hydroxyl group to sphinganine to form phytosphingosine (3) prior to the addition of the second acyl chain23. In mammals, DES2 has dual Δ4-desaturase and C-4 hydroxylase activities enabling the hydroxylation of ceramide (5) to phytoceramide24. C. crescentus does not have a homologue of Sbh1/2; DES2 has some homology to the fatty acid desaturase CCNA_03535, though deletion of ccna_03535 did not abolish ceramide hydroxylation. We did not identify any putative hydroxylases in our transposon screen; however, we hypothesized that this modification may not have a strong effect on one or both of our selection phenotypes. To focus on genes that promoted PMX-resistance only, we searched the Fitness Browser database25 and found that the disruption of ccna_00202, a DesA-family fatty acid desaturase/hydroxylase, results in PMX resistance. Deletion of ccna_00202 led to a ceramide molecule with a mass reduction of 16 Da corresponding to the loss of a hydroxyl group (Fig. 2d). Complementation of ccna_00202 restored ceramide hydroxylation (Fig. 2d). Tandem MS/MS data are consistent with the hydroxylation occurring on C2 of the acyl chain (see Extended Data Fig. 4). For this reason, we have named CCNA_00202 Ceramide Hydroxylase (CerH). Examination of the mass spectra of the CerR deletion mutant showed that hydroxylation remains in the absence of reduction (Fig. 2a). Mechanistically, this suggests that CerH can use either DHC or oxCer as a substrate, or that acyl chain hydroxylation occurs upstream of oxCer reduction.
The ceramide in C. crescentus has a monounsaturated acyl chain (see Extended Data Fig. 1e). Acyl chain desaturation has also been reported in Sphingomonas species26. Attempts to identify a desaturase were unsuccessful. C. crescentus encodes at least four DesA-family desaturases (ccna_00203, ccna_01515, ccna_01743, and ccna_03535). Deletion of each of these genes individually or in combination had no effect on ceramides. While there could be other, yet unidentified, desaturases, we hypothesized that C. crescentus bCerS may have a preference for monounsaturated acyl-CoA substrates. In vitro, recombinant bCerS had a 3-fold greater preference for C16:1-CoA over C16:0-CoA as a substrate (see Extended Data Fig. 5e); the saturated and monounsaturated C16 fatty acids are present in equal amounts in vivo21. C. crescentus encodes two orthologues each of FabA/FabB which are involved in the synthesis of monounsaturated fatty acids27. These four genes are all essential28 which precluded direct tests of our hypothesis. Since many bacteria produce fully saturated ceramides2,5, it appears that this modification is not a universal feature of bacterial ceramide synthesis.
The observations of oxCer in the ΔcerR strain (Fig. 2a) and the ability of bCerS to use 3-KDS as a substrate (Fig. 2c) suggested that the order of the synthetic pathway in bacteria is different than that in eukaryotes. Based on the MS data, we can propose the following model for bacterial ceramide synthesis (Fig. 2e). Spt condenses serine with an acyl-thioester (either acyl-ACP or acyl-CoA) to produce 3-KDS. bCerS uses 3-KDS and a second palmitoyl-CoA to generate oxCer which is subsequently reduced to ceramide by CerR. We considered the possibility that CerR may work upstream of bCerS, as in eukaryotes. In this case, an alternative model is that bCerS can use either 3-KDS or sphinganine as a substrate leading to oxCer or ceramide as the final product, respectively. In vitro assays using recombinant bCerS show that the enzyme can use either substrate (Fig. 2c and Extended Data Fig. 5f). To determine which pathway was more likely to occur in vivo, we used fluorescently tagged proteins to infer the subcellular localization of the synthetic enzymes. Incubation of bacteria with chloroform-saturated Tris buffer results in the preferential permeabilization of the outer membrane and leakage of soluble periplasmic proteins29 (Fig. 2f). Using this approach with the three core ceramide synthesis genes showed that Spt and bCerS retained fluorescence while the CerR signal was entirely lost (Fig. 2f). Consistent with the purification of recombinant Spt and bCerS as soluble proteins, these imaging studies suggest that Spt and bCerS are cytoplasmic whereas CerR is a soluble periplasmic protein. Though we cannot rule out other mechanisms, the spatial separation of these proteins in vivo is consistent with our model in which bCerS acts upstream of CerR (Fig. 2e).
Bacterial ceramides can be modified in a variety of species-specific manners including fatty acid hydroxylation (Fig. 2d), acyl chain branching2, and head group modification by phosphorylation2 or glycosylation4. In C. crescentus, we previously identified two sphingolipid glycosyltransferases4 and here we report the discovery of a ceramide hydroxylase CerH (Fig. 2d).
Bioinformatic identification of ceramide producing species
The identification of Spt, bCerS, and CerR as the core bacterial ceramide synthetic enzymes in C. crescentus presented the opportunity to find orthologues in other species and perform a bioinformatic screen for additional potential ceramide producers. Sphingolipids have been isolated from the oral pathogen Porphyromonas gingivalis3. The spt gene (pgn_1721) has been identified3 and BLAST analysis of bCerS and CerR suggested that PGN_0374 and PGN_1886 are the respective orthologues despite having limited homology (PGN_0374: 25% identical, 43% similar; PGN_1886: 23% identical, 43% similar). Additionally, unlike in C. crescentus, the proposed P. gingivalis genes are not in the same genomic locus. To test their functionality, we complemented C. crescentus deletion strains with the corresponding P. gingivalis genes. Complementation with spt (pgn_1721)30 yielded both the expected C. crescentus ceramide (m/z 588.465 Da) as well as a ceramide with two additional methylene units (m/z 614.478 Da) (Fig. 3a). These data are consistent with previous studies showing that P. gingivalis has a substrate preference for 17, 18, and 19-carbon fatty-acyl-CoA substrates31.
Figure 3. Bioinformatic analysis identifies a wide range of potential ceramide-producing bacteria.
(a-c) Deletions of the spt (a), bcerS (b), and cerR (c) genes in C. crescentus were complemented with the indicated homologues from P. gingivalis. Lipids extracted from these strains were analyzed by normal phase LC/ESI-MS in negative ion mode. [M + Cl]− ions of the ceramide species emerging at 2 to 3 min are shown with ceramide species labelled with a red dot. (d) Bacterial species with homologues to all three ceramide synthesis enzymes are clustered by the overall homology of the 3 proteins (see Methods). Bootstrap percentage values are indicated by shaded circles at each node. Branches are colored by taxonomic class and Gram-positive Actinobacteria are labeled. (e) Negative ion ESI/MS shows the [M + Cl]− ions of the ceramide species (emerging at 2 to 3 min) extracted from S. aurantiacus. Ceramide is labelled with a red dot. Determination of the ceramide structure by MS/MS is provided in Extended Data Fig. 7b. (f) Deletion of bcerS in C. crescentus was complemented by two bcerS orthologues from P. buccae. [M + Cl]− ions of the ceramide species emerging at 2 to 3 min are shown with ceramide species labelled with a red dot. Determination of the ceramide structures by MS/MS is provided in Extended Data Figs. 7c-d.
Complementation of ΔbcerS with pgn_0374 restored ceramide synthesis (Fig. 3b); a variety of ceramide molecules were observed suggesting that, like Spt, the P. gingivalis bCerS has different substrate preferences than the C. crescentus orthologue. Lastly, P. gingivalis cerR (pgn_1886) was able to rescue ceramide reduction (Fig. 3c). Together, these results demonstrate that screening for organisms with this set of genes could yield a broader set of bacteria with the potential to synthesize ceramides.
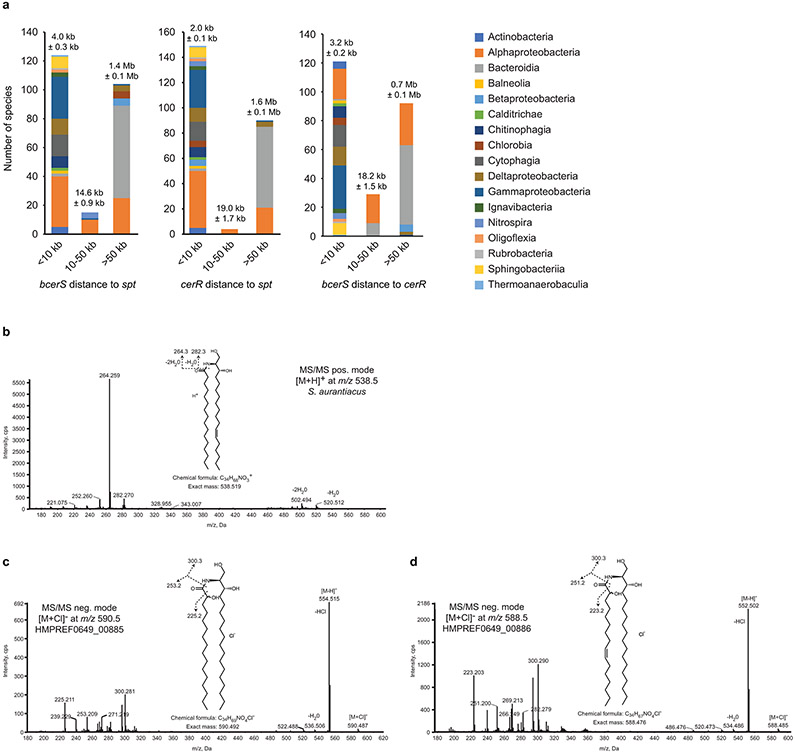
A BLAST analysis of Spt, bCerS and CerR against the NCBI prokaryotic representative genomes database (5,700+ representative bacterial organisms; see Materials and Methods and Supplementary Data 1 for detailed search parameters and E-value cutoffs) identified 272 organisms, belonging to 17 taxonomic classes, containing orthologues of all three genes (Fig. 3d, Supplementary Data 1, and Extended Data Fig. 6). Analysis of the distance between these core genes showed that, in most clades, the genes were within 10 kb of one another (see Extended Data Fig. 7a). However, among the Bacteroides these genes were found scattered throughout the genome (see Extended Data Fig. 7a); this is consistent with the high degree of chromosomal plasticity and genomic rearrangements associated with these organisms32. Whereas all previously identified ceramide producers are Gram-negative organisms, our bioinformatic analysis suggested that several Gram-positive Actinobacteria may be competent for ceramide synthesis. Lipidomic analyses confirmed the presence of dihydroceramide in Streptomyces aurantiacus, providing the first evidence of ceramide lipids in Gram-positive bacteria (Fig. 3e). MS/MS analysis of S. aurantiacus showed that, in contrast to C. crescentus, fatty acid desaturation occurred on the long-chain base (LCB) (see Extended Data Fig. 7b). Based on phylogenetic clustering of the individual ceramide synthesis genes (Extended Data Fig. 6), as well as the fact that the three genes are found in a putative operon, it is possible that the ceramide synthesis cassette may have been acquired in these Actinobacteria by horizontal gene transfer from Deltaproteobacteria.
In eukaryotes, organisms often have multiple CerS isoforms with distinct fatty acyl-CoA specificities. For example, the six human CerS isoforms enable the synthesis of ceramides with acyl chain lengths of 14-26 carbons20. We performed a bioinformatic search to identify bacterial species with multiple bCerS isoforms and found 22 candidates among the Alphaproteobacteria, Bacteroidia, Balneolia, Chitinophagia, and Rhodothermia (see Supplementary Data 2). In most cases, the two bCerS homologues were far apart on the chromosome making it impossible to know whether the two proteins were truly bCerS isoforms or simply similar N-acetyltransferases. One exception was Prevotella buccae, where the two candidate bcerS genes were encoded immediately next to one another (HMPREF0649_00885 and HMPREF0649_00886; 57% identical and 74% similar). We complemented the C. crescentus ΔbcerS strain with each of the P. buccae isoforms and found that while both could rescue ceramide synthesis, each enzyme produced distinct lipid products (Fig. 3f). HMPREF0649_00885 preferred a fully saturated LCB substrate while HMPREF0649_00886 used a desaturated LCB (see Extended Data Fig. 7c-d).
Independent evolution of ceramide production in bacteria
While the Spt enzyme catalytic residues are conserved between eukaryotic and bacterial species (see Extended Data Fig. 8), neither CerR nor bCerS share obvious homology to KDSR or CerS, respectively. The closest eukaryotic homologue to CerR is NADH dehydrogenase 1A subcomplex subunit 9 (NDUF9A), which is a component of Complex I in the mitochondrial oxidative phosphorylation pathway. Conversely, the bacterial relatives of KDSR are annotated as short-chain dehydrogenases33. While both reductase families have conserved catalytic and NAD-binding sites34 (see Extended Data Fig. 9), human KDSR and C. crescentus CerR are only 27% similar and 14% identical. Phylogenetic clustering of these proteins showed that CerR evolved its ceramide reductase activity convergently, arising from NDUF9A-related genes (Fig. 4a).
Figure 4. Phylogenetic analysis indicates convergent evolution of ceramide synthesis.
Unrooted trees built using the maximum likelihood method show the distance between eukaryotic and bacterial ceramide synthesis genes as well as their closest homologues. Bootstrap percentage values are indicated by shaded circles at each node. (a) Bacterial CerR is most closely related to eukaryotic and bacterial proteins of the NDUF9A family, a subunit of mitochondrial Complex I. By contrast, Eukaryotic KDSR is homologous to bacterial short-chain dehydrogenases, unrelated to CerR. (b) Bacterial bCerS is part of a larger family of GNAT acyltransferases, which are, in turn closely related to eukaryotic Gcn5 proteins. By contrast, eukaryotic CerS proteins are distant from the Gcn5-related proteins.
Similarly, bCerS is a member of the GNAT family of acyltransferases. Bacteria have a variety of GNAT proteins which are closely related to eukaryotic Gcn5 acyltransferases. Phylogenetic analysis demonstrated that bCerS is a subgroup of bacterial GNATs whereas the eukaryotic CerS is only distantly related to Gcn5 family proteins (Fig. 4b). Indeed, eukaryotic CerS has a highly conserved Lag1P domain35,36 which is not found in any of the bCerS proteins (see Extended Data Fig. 10). These phylogenetic analyses, coupled with the proposed reordering of the synthetic pathway (Fig. 1a and 2f-g), demonstrate that ceramide synthesis evolved independently in bacteria and eukaryotes.
Discussion
Since the discovery of sphingolipids in the late 19th century by Johann L.W. Thudichum, thousands of publications have demonstrated these lipids to be ubiquitous throughout Eukarya; to date, there are over 500 published headgroup variants and acyl chain modifications among this lipid group (LIPID MAPS,37). The diversity of sphingolipids reflects the multifunctional roles of these molecules: the sphingoid backbone provides structural integrity to the cell membrane, the headgroups are involved in lipid-mediated interactions, and some sphingolipid-derivatives function as intracellular second messengers7.
The list of bacterial sphingolipid-producing species is comparatively short but growing. Their presence in several taxa begs the question of ‘how widespread could this lipid be?’. Our results identify the key enzymes required for bacterial ceramide synthesis. While the Spt enzyme is homologous between prokaryotes and eukaryotes, bCerS and CerR are unique to bacteria. We note a recent publication also identified these genes as being involved in ceramide synthesis in C. crescentus12. The authors proposed, without direct evidence, that these proteins carry out the same functions as their eukaryotic counterparts (see Reference 12, Figure 11). However, our biochemical analyses demonstrate that CerR and bCerS have unique enzymatic activities and the sequence of synthetic reactions in the bacterial pathway is likely different than that in eukaryotes (Fig. 1a and 2f). The previous findings regarding bacterial ceramide synthesis are actually consistent with our proposed pathway; their thin-layer chromatography (TLC) analysis of the CerR deletion yielded an unidentified band which is likely oxCer (see Reference 12, Figure 9, Δ1164, top-most band). Additionally, ectopic expression of Spt and CerR in E. coli did not yield sphinganine, while expression of Spt and bCerS did lead to the production of the fast migrating sphingolipid species (see Reference 12, Supplementary Figure 4). As this molecule does not occur in eukaryotes, they lacked a TLC standard to confirm the identity of this band leading to a challenge in interpreting the data.
Though our data support a mechanism in which bCerS directly adds an acyl chain to 3-KDS, we note that the metabolic intermediate sphinganine has been detected in B. thetaiotaomicron2. We have tried several lipid extraction methods as well as stable-isotope labeling but have never detected sphinganine in C. crescentus. While it is possible that the ceramide synthesis pathway operates differently in Bacteroides, given the conservation of this enzyme as well as the ability of the P. gingivalis enzyme to complement the deletion in C. crescentus that seems less likely. An alternative hypothesis is that Bacteroides has a ceramidase enzyme, which is lacking in C. crescentus, that hydrolyzes ceramide to sphinganine. Indeed, a bioinformatic search identified two linear amide CN-hydrolases (pfam PF02275; of which ceramidases are members) in B. thetaiotaomicron. One of these enzymes hydrolyzes bile acids, however, the function of the second homologue is unknown38. C. crescentus does not have a homologue of these proteins. Additionally, B. thetaiotaomicron can assimilate and metabolize sphinganine from the host gut to produce ceramide lipids39. Not surprisingly, the details of SL biosynthesis vary in different bacteria and a working hypothesis is that that their environmental niches provide an opportunity for the utilization of particular SL metabolic pathways. Further characterization of the structures and mechanisms of these enzymes will be necessary to elucidate their physiological functions.
Coupled with phylogenetic analysis, our data support a model in which bacterial ceramide synthesis evolved convergently and independently of the eukaryotic pathway. This type of evolution, where identical substrates and products are metabolized by distinct enzymes, is well characterized particularly in plants. For example, 1) gibberellins (tetracyclic diterpenoid carboxylic acids) are produced by unique enzymes in plants and fungi40, 2) several independent enzymatic pathways are used to produce caffeine among plant species41, and 3) the UDP-glucosyltransferases and β-glucosidases used in the synthesis of benzoxazinoids evolved independently in different plant lineages42. One potential mechanism for the independent evolution of metabolic pathways is gene duplication and modification of substrate specificity. In the case of bacterial ceramide synthesis, the CerR protein in C. crescentus is related to the NDUF9A-domain protein CCNA_03718 (27% identical and 46% similar). In this study, we found that P. buccae encodes two bCerS proteins (57% identical and 74% similar) with unique substrate preferences (Fig. 3f).
Phylogenetic analysis of the three ceramide synthetic genes has identified a wide range of Gram-negative, as well as several Gram-positive, species with the potential to produce ceramides. These organisms occupy a range of habitats including aquatic, soil, and within animal hosts. Among the small subset of previously identified ceramide-producing species, these lipids play roles in outer membrane integrity43, defense against bacteriophage4, protection against extracellular stress12, suppression of host inflammation2, and their production can be developmentally regulated5. Furthermore, lipidomic analyses of these organisms has identified a wide range of ceramide species with varying acyl chain length and saturation, acyl chain hydroxylation, and head group glycosylation and phosphorylation; the consequences of these modifications are yet to be determined. By defining the microbial blueprint for ceramide synthesis, we now have a platform for dissecting the physiological functions of these lipids and for potentially engineering the production of novel sphingolipids.
Materials and Methods
Bacterial strains, plasmids, and growth conditions
The strains, plasmids, and primers used in this study are described in Supplementary Tables 4, 5, and 6, respectively. Strain construction details are available in a Supplementary Note. C. crescentus wild-type strain NA1000 and its derivatives were grown at 30 °C in peptone-yeast-extract (PYE) medium for routine culturing. To control serine concentration for the serine auxotrophic strain, C. crescentus was grown in Hutner-Imidazole-Glucose-Glutamate (HIGG) media46 with variable amounts of serine (0-10 mM). E. coli strains were grown at 37 °C in LB medium. When necessary, antibiotics were added at the following concentrations: kanamycin 30 μg/ml in broth and 50 μg/ml in agar (abbreviated 30:50) for E. coli and 5:25 for C. crescentus; tetracycline 12:12 E. coli and 1:2 C. crescentus; spectinomycin 50:50 E. coli and 25:100 C. crescentus; and ampicillin 50:100 E. coli. Gene expression was induced in C. crescentus with either 0.3% (w/v) xylose or 0.5 mM vanillate. Streptomyces aurantiacus was grown in International Streptomyces Project Synthetic Salts-Starch Medium (ISP4) at 30 °C.
Genetic screen for ceramide synthesis enzymes
A conjugation-competent and diaminopimelic acid (DAP) auxotrophic strain of E. coli (MFDpir,47) carrying the kanamycin-encoding mini-Tn5 plasmid pBAM1 (Addgene #60487,48) was grown overnight in LB media containing 0.3 mM DAP. Wild-type C. crescentus was grown overnight in PYE. In a microcentrifuge tube, 1 ml of C. crescentus was mixed with 100 μl of E. coli, the cells were washed once in PYE, and the final cell pellet was resuspended in 20 μl PYE. The concentrated cell sample was dropped onto a PYE agar plate containing 0.3 mM DAP and incubated at 30 °C for 6 hr. After incubation, the cells were scraped into 1 ml of PYE, vortexed, and spread onto PYE agar plates containing 25 μg/ml kanamycin and 200 μg/ml polymyxin B. In the absence of DAP, the donor E. coli strain could not grow, the kanamycin selected for transposon insertions, and the polymyxin B selected for potentially ceramide-deficient cells. Colonies were picked into duplicate 96-well plates containing 200 μl PYE per well +/− 2 μl bacteriophage ϕCr30. Each plate had one well of wild-type control and one well with no cells, as a control for contamination. Approximately 20 sets of duplicate plates were inoculated. Plates were incubated overnight at 30 °C and growth was measured in a BMG Labtech CLARIOstar plate reader by absorbance at 660 nm. The 94 phage-containing wells that had the lowest OD660 were considered potential hits. The corresponding wells from the non-infected plates were consolidated into new 96-well plates and treated +/− phage as above. Growth curves were acquired for 27 hr on a BMG Labtech CLARIOstar plate reader incubating at 30 °C with shaking. The ratio of the final to the maximal OD660 for each well was calculated and normalized to the wild-type control. Wells with a ratio less than that of the wild-type control were kept for further characterization. To determine the site of transposon insertion we used arbitrarily-primed PCR with primer pairs EKS153/S159 and EKS154/S160 as previously described48.
Cloning and purification of C. crescentus Spt
The ccna_01220 gene was amplified with primers EK1107/1108. A C-terminal 6-histidine tag expression vector (pET-28a, EMD Biosciences) was amplified with primers EK1131/1106 and the insert was ligated using HiFi Assembly (New England Biolabs). The resulting plasmid was transformed into E. coli BL21 (DE3) cells. One colony was grown overnight in Terrific Broth (TB)/kanamycin at 37 °C with shaking. The inoculant was diluted into TB/kanamycin to OD600 of 0.1. When the OD600 reached 0.8, protein expression was induced with 0.5 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG), and cultures were grown at 16 °C overnight. Cells were harvested by centrifugation at 5,000 x g for 7 min. The cell pellets were resuspended in 20 mM potassium phosphate buffer, pH 7.5, 250 mM NaCl, 30 mM imidazole and 25 μM pyridoxal phosphate (PLP). The cells were sonicated on ice (Soniprep 150, 10 cycles of 30 seconds on/30 seconds off), cell lysates were cleared by centrifugation at 24,000 x g for 40 min, and supernatants were filtered through a 0.45 μm filter. The recombinant Spt was purified using an Äkta FPLC system (Cytiva) and a HisTrap HP 1 ml Ni2+ column (Cytiva) with an imidazole gradient from 30 mM to 500 mM, followed by size exclusion chromatography (SEC) on a HiLoad 16/600 Superdex 200 preparatory grade column (Cytiva) with a buffer containing 20 mM potassium phosphate, pH 7.5, 250 mM NaCl, 10% (v/v) glycerol and 25 μM PLP. The purification was monitored by SDS-PAGE and Coomassie blue staining.
Cloning and purification of C. crescentus bCerS
The ccna_01212 gene was amplified with primers EK1199/1269 and cloned into the NdeI/HindIII site of plasmid pET-28a to generate an N-terminal 6-histidine tag. The construct was transformed into E. coli BL21 (DE3) competent cells. One colony was grown overnight in LB broth/kanamycin at 37 °C with shaking. The inoculant was diluted into LB/kanamycin to OD600 of 0.1. When the OD600 reached 0.8, protein expression was induced with 1 mM IPTG, and cultures were grown at 16 °C overnight. Cells were harvested by centrifugation at 5,000 x g for 7 min. The cell pellets were resuspended in 50 mM Tris-HCl, pH 8.0, 250 mM NaCl, and 30 mM imidazole. The cells were sonicated on ice (Soniprep 150, 10 cycles of 30 seconds on/30 seconds off), cell lysates were cleared by centrifugation at 24,000 x g for 40 min, and supernatants were filtered through a 0.45 μm filter. The recombinant bCerS was purified using an Äkta FPLC system (Cytiva) and a HisTrap HP 1 ml Ni2+ column (Cytiva) with an imidazole gradient from 10 mM to 500 mM, followed by size exclusion chromatography (SEC) on a HiLoad 16/600 Superdex 200 preparatory grade column (Cytiva) with a buffer containing 50 mM Tris-HCl, pH 8.0, 250 mM NaCl, and 10% (v/v) glycerol. The purification was monitored by SDS-PAGE and Coomassie blue staining.
Mass Spectrometry of recombinant proteins
Purified Spt and bCerS were analyzed in positive ion mode using a liquid chromatography system connected to a Waters Synapt G2 QTOF with an electrospray ionization (ESI) source. 10 μL of 10 μM protein was injected into a Phenomenex C4 3.6 μm column. The conditions for the qTOF were source temperature 120 °C, backing pressure 2 mbar, and sampling cone voltage 54V. The protein was eluted with a 12-minute gradient, starting at 5% acetonitrile with 0.1% formic acid to 95% acetonitrile. The resulting spectra were analyzed using MassLynx V4.1 software (Waters Corporation)
Determination of kinetic constants for C. crescentus Spt
Spt kinetic parameters were determined using a 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) assay as previously described16. The enzyme kinetic assay was carried out in a 96 well microtiter plate containing 0.4 mM DTNB, 1 μM Spt enzyme, 20 mM L-serine, 1-1000 μM C16:0/1-CoA for Km-C16:0/1-CoA determination or 0.4 mM DTNB, 1 μM Spt enzyme, 0.1-100 mM L-serine, 250 μM C16:0/1-CoA for Km-L-Ser determination in a buffer containing 100 mM HEPES, 250 mM NaCl, pH 7.0. The experiments were monitored in a BioTek Synergy HT plate reader at 412 nm in 1 min intervals for 60 minutes at 30 °C. The enzyme kinetic constants were calculated by fitting the Michaelis-Menten equation to a plot of reaction rate versus concentration using Origin 2019 (OriginLab).
Assessing sphingolipid products using MALDI-TOF-MS
Spt reaction products were desalted using OMIX C4 pipette tips (Agilent) and eluted in 100% acetonitrile (ACN) containing 0.2% formic acid. 1 μL of first matrix seed (20 mg/ml alpha-cyano-4-hydroxycinnamic acid (CHCA) in methanol/acetone (2:3, v/v) was spotted onto a MTP 384 ground steel plate (Bruker) and left to air dry. The samples were mixed with the second matrix (20 mg/ml CHCA in 50% ACN within 0.25% trifluoroacetic acid (TFA)) in a 1:1 ratio, and 1 μL of the mixture was spotted on top of the CHCA-acetone layer and left to air-dry. The samples were analyzed in reflector mode using a calibrated Bruker UltrafleXtreme MALDI-TOF-mass spectrometer. The analysis was carried out in positive ion mode. The laser power was adjusted to provide optimum signal. Each sample was tested with 500 laser shots and each spectrum was a sum of over 5000 shots. Spectra were acquired over a range of m/z 200-1500. The data acquisition software used was Flex Control version 3.4. The data was analyzed using Data Analysis version 4.4 software.
Enzymatic activity assay for C. crescentus bCerS
The reactions were carried out with 2 μM bCerS, 40 μM 3-ketodihydrosphingosine (3-KDS) (dissolved in 0.1% v/v ethanol) and 50 μM C16-CoA for at least 1 hour in a buffer containing 20 mM HEPES, 25 mM KCl, 2 mM MgCl2, pH 7.5. The reaction products were extracted using the Bligh-Dyer method47 and characterized by LC/MS as described below. To assay bCerS substrate specificity, the reaction was carried out as above using equimolar amounts of C8-C24-CoA (total acyl-CoA concentration remained 50 μM).
Determination of kinetic constants for C. crescentus bCerS
bCerS kinetic parameters were determined using a DTNB assay as previously described above for Spt. The enzyme kinetic assay was carried out in a 96 well microtiter plate containing 1 mM DTNB, 1.2 mg/ml bCerS enzyme, 0 or 40 μM 3-KDS, and 1-500 μM C16:0-CoA for Km-C16:0-CoA determination in a buffer containing 100 mM HEPES, 150 mM NaCl, pH 7.5. The experiments were monitored in a BioTek Synergy HT plate reader at 412 nm in 30 second intervals for 60 minutes at 30 °C. The enzyme kinetic constants were calculated by fitting the Michaelis-Menten equation to a plot of reaction rate versus concentration using Origin 2019 (OriginLab).
Lipid extraction
C. crescentus strains were grown overnight (5 ml) and lipids were extracted by the method of Bligh and Dyer49. Cells were harvested in glass tubes at 10,000 x g for 30 min and the supernatant was removed. The cells were resuspended in 1 ml of water, 3.75 volumes of 1:2 (v/v) chloroform: methanol was added, and the samples were mixed by vortexing. Chloroform (1.25 volumes) and water (1.25 volumes) were added sequentially with vortexing to create a two-phase system and the samples were centrifuged at 200 x g for 5 minutes at room temperature. The bottom, organic phase was transferred to a clean tube with a Pasteur pipette and washed twice in “authentic” upper phase. Subsequently, the residual organic phase with the lipids was collected and dried under argon.
Lipid analysis by normal phase LC/ESI–MS/MS
Methods for lipid analysis by normal phase LC/ESI–MS/MS have been described50. Briefly, normal phase LC was performed on an Agilent 1200 Quaternary LC system equipped with an Ascentis Silica HPLC column, 5 μm, 25 cm × 2.1 mm (Sigma-Aldrich, St. Louis, MO) as described. The LC eluent (with a total flow rate of 300 μl/min) was introduced into the ESI source of a high resolution TripleTOF5600 mass spectrometer (Applied Biosystems, Foster City, CA). Instrumental settings for negative ion ESI and MS/MS analysis of lipid species were as follows: ion spray voltage (IS) = −4500 V; curtain gas (CUR) = 20 psi; ion source gas 1 (GSI) = 20 psi; declustering potential (DP) = −55 V; and focusing potential (FP) = −150 V. The MS/MS analysis used nitrogen as the collision gas. Data analysis was performed using Analyst TF1.5 software (Applied Biosystems, Foster City, CA). A list of the identified lipid species can be found in Supplementary Table 2. A representative total ion chromatogram (TIC) and its corresponding extracted ion chromatogram (EIC) is available in Extended Data Figs. 3c-d. The peak areas of the EICs of major lipid species are compiled in Supplementary Table 3.
Cell permeabilization and labeling
Chloroform-saturated Tris buffer was prepared by mixing 50 mM Tris, pH 7.4 with chloroform (70:30) and shaking the mixture at room temperature for 30 min. Cells to be permeabilized were collected via centrifugation (2 min at 6,000 x g, 4 °C) and resuspended in an equal volume of the aqueous phase of the chloroform-saturated Tris buffer. Resuspended cells were rocked for 45 min at room temperature and then washed twice in 50 mM Tris, pH 7.4 (via centrifugation for 10 min at 5,000 x g) to remove residual chloroform. Control cells were treated as above, but incubated in 50 mM Tris, pH 7.4 without chloroform.
Fluorescence microscopy
Cells harboring fluorescent fusions were induced overnight with 0.3% xylose and permeabilized as described above. The permeabilizes cells were spotted onto 1% agarose pads. Fluorescence microscopy was performed on a Nikon Ti-E inverted microscope equipped with a Prior Lumen 220PRO illumination system, CFI Plan Apochromat 100X oil immersion objective (NA 1.45, WD 0.13 mm), Zyla sCMOS 5.5-megapixel camera (Andor), and NIS Elements v, 4.20.01 for image acquisition.
Phylogenetic analysis of bacterial ceramide synthesis genes
Following our identification of Spt, bCerS, and CerR as key enzymes in ceramide synthesis in C. crescentus, we used BLASTP to find the closest protein homologues in the known ceramide producers P. gingivalis, M. xanthus, and B. stolpii (see Supplementary Data 1). Using each of these proteins as a query, we used TBLASTN to find related proteins in the NCBI prokaryotic representative genomes database (5,700+ representative bacterial organisms). E-value cutoffs were determined by performing a traditional BLASTP with each protein and getting the approximate E-value cutoff for the top 250 hits (see Supplementary Data 1). TBLASTN settings were chosen to only take the top hit for each organism, and we collected the organism name, taxonomic ID, sequence start and end position, strand orientation, and protein sequence. Following the TBLASTN searches, the data were combined and filtered to remove duplicates. We identified organisms that contained hits for all three target genes. To facilitate comparison of these organisms, we made in silico fusions by concatenating the Spt, CerR, and bCerS protein sequences. These fused sequences were aligned using MUSCLE aligner51. Phylogenetic trees were prepared using RAxML (Randomized Axelerated Maximum Likelihood version 8.2.12)52 with 100 bootstraps and a maximum-likelihood search. RAxML was run on the CIPRES Portal at the San Diego Supercomputer Center53. Similar phylogenetic analyses were performed for the individual enzymes (see Extended Data Fig. 6). The taxonomic class for each organism was retrieved from the NCBI taxonomy database using the R package taxize54. Phylogenetic trees were visualized in R using the packages ggtree55, ape56, treeio57, and ggplot258.
Phylogenetic analyses of ceramide synthesis genes
To get a representative set of sequences from across the eukaryotic domain, we used BLASTP on the NCBI server to find the top 500 eukaryotic hits for CerS from humans (CerS1, Accession P27544.1), Arabidopsis thaliana (Lag1P, Accession NP_001184985), and Saccharomyces cerevisiae (Lag1P, Accession AAA21579.1). The results were cleaned to remove duplicate hits; in order to compare roughly the same number of proteins in each group, 250 hits were chosen at random using the Linux “shuf” command. The same protocol was used for the following Spt and KDSR queries: human (Accessions NP_006406.1 and NP_002026.1), A. thaliana (Accessions NP_190447.1 and NP_187257), and S. cerevisiae (Accessions CAA56805.1 and P38342). The yeast CerS and KDSR proteins were then used to find the closest homologues in bacteria using BLASTP. Short-chain dehydrogenases similar to yeast KDSR were identified; by contrast, yeast CerS had a single partial match (45% query coverage, E-value=4.0) in Orenia metallireducens. C. crescentus CerR was used as a query to find the closest eukaryotic homologues, which identified NDUF9A proteins. A reverse-BLAST identified CCNA_03718 as the closest C. crescentus NDUF9A homologue, which was then used to identify other bacterial NDUF9A homologues. To trace the lineage of bCerS, we used the following protein queries to identify eukaryotic Gcn5 proteins: human (Accession AAC39769.1) and S. cerevisiae (Accession NP_011768.1). We identified bacterial GNAT proteins using C. crescentus bCerS as a BLASTP query to search the entire NCBI bacterial database. The eukaryotic Spt proteins were compared to the bacterial Spt proteins identified in Supplemental Data 1. Phylogenetic trees for the Spt, CerR/KDSR/NDUF9A, and CerS/Gcn5/GNAT/bCerS proteins were prepared with MUSCLE and RAxML and visualized with R as described above.
Extended Data
Extended Data Fig. 1. C. crescentus Spt can use a variety of substrates.
(a) Recombinant CCNA_01220 (Spt) was purified as described in the methods. TP is total protein extract. Recombinant protein was independently purified at least three times with similar purities as judged by Coomassie staining. (b) The identity of the purified Spt protein was confirmed by LC/ESI/MS. The peaks correspond to the relative abundances of the multiple-charged species of the protein analyte; the charge is indicated in parenthesis. (c-d) Kinetic analyses of CCNA_01220 determined the Km values for C16:1-CoA (0.045 ± 0.004 mM. Panel c) and L-serine (11.97 ± 2.18 mM. Panel d) (n=3; data are presented as mean +/− SD). (e) Positive- and negative-ion mode MS/MS analysis of ceramides from wild-type C. crescentus (see Fig. 2a) confirm that the desaturation and additional hydroxyl group are located on the acyl chain moiety. (f-g) Serine auxotrophic ΔserA cells were grown in HIGG media without serine, and lipids were isolated for MS/MS analysis. (f) Negative ion ESI/MS shows the [M + Cl]− ion of 1-deoxyceramide emerging at 2 to 3 min. The incorporation of alanine rather than serine is designated with a red-dashed oval. (g) MS/MS analysis of the parent ion confirmed the identity of the 1-deoxyceramide.
Extended Data Fig. 2. A transposon screen for ceramide-deficient mutants yielded multiples hits in the Spt genomic locus.
(a) The schematic illustrates the tandem positive/negative selection screen used to identify ceramide synthesis genes. Transposon mutants were initially screened for growth on polymyxin B. Resistant clones were then assayed for increased sensitivity to bacteriophage ϕCr30. The effects on ceramide production were assessed by LC/MS. (b) The sites of transposon insertions are indicated with a red triangle. Gene annotations are based on the results presented in Figures 1-3 and Extended Data Figures 3-5. The exact coordinates of the insertions are provided in Supplementary Table 1.
Extended Data Fig. 3. Acyl carrier protein and ACP-synthetase are required for ceramide synthesis in C. crescentus.
(a-b) ACP (ccna_01221) and ACP-synthetase (ccna_01223) deletion strains and the corresponding complementation strains were grown overnight in PYE with 0.3% (w/v) xylose. Negative ion ESI/MS shows the [M + Cl]− ions of the lipids emerging at 2 to 3 min. Ceramide species are labelled with a red dot. (c) A total ion chromatogram (TIC) and (d) the corresponding extracted ion chromatogram (EIC) of lipids from C. crescentus grown in PYE media show the major species present in the lipid extract.
Extended Data Fig. 4. MS/MS confirms the production of oxidized ceramide in the absence of CerR.
The oxCer lipid produced in the ΔcerR strain (Fig. 2a, middle panel) was subjected to MS/MS analysis. The fragment ions confirm the presence of two oxidized C═O bonds.
Extended Data Fig. 5. Purification of C. crescentus bCerS.
(a) bCerS was purified as described in the Materials and Methods. SDS-PAGE analysis confirmed protein purity following S200 size-exclusion chromatography. The indicated fractions were pooled for MS characterization. Recombinant protein was independently purified at least three times with similar purities as judged by Coomassie staining. (b) The identity of the purified bCerS protein was confirmed by LC/ESI/MS. The ions for two species were detected, which differed by 178 Da (top); this difference is due to spontaneous alpha-N-6-phosphogluconoylation which is commonly observed in His-tagged proteins expressed in E. coli41. The protein molecular weight was calculated using the relative abundances of the multiple-charged species of the protein analyte (bottom); the charges are indicated in parenthesis. (c) Kinetic analyses of CCNA_01212 in the absence (left) and presence (right) of 3-KDS determined the Km,app value for C16:0-CoA (21.3 ± 4.1 μM) (n=3, data are presented as mean +/− SD). (d) To determine the substrate specificity of C. crescentus bCerS, we incubated the recombinant enzyme with 40 μM 3-KDS and an equimolar mixture of fatty acid-CoA (C8-C24) with a total final concentration of 50 μM. The reaction proceeded for 1 hr and the reaction products were analyzed by ESI/MS. The integrated ion counts were normalized to C24 (n=3, data are presented as mean +/− SD). (e) To determine the acyl-chain saturation preference of C. crescentus bCerS, we incubated the recombinant enzyme with 40 μM 3-KDS and an equimolar mixture of C16:0-CoA and C16:1-CoA, with a total final concentration of 50 μM. The reaction proceeded for 1 hr and the reaction products were analyzed by NPLC-ESI/MS in the negative ion mode to determine the ratio of ceramide products produced (C16:0 product, [M+Cl]− at m/z 572.481; C16:1 product, [M+Cl]− at m/z 570.466) (n=3, data are presented as mean +/− SD). (f) Recombinant bCerS was incubated with 40 μM sphinganine and 50 μM C16:0-CoA for 1 hr and the reaction product was analyzed by ESI/MS.
Extended Data Fig. 6. Phylogeny of bacterial ceramide synthesis enzymes.
Unrooted phylogenetic trees were created for the individual Spt, CerR, and bCerS proteins from the organisms identified in Fig. 3d (left). A zoomed in view of the branches of the trees containing Actinobacteria show the close relationship between these Gram-positive organisms and Deltaproteobacteria (right). For each of the individual enzymes, the closest homologues for the Actinobacteria are found in Deltaproteobacteria. Bootstrap percentages are indicated by the filled circles at each node.
Extended Data Fig. 7. Ceramide synthesis orthologues.
(a) Using the genomic coordinates acquired during the phylogenetic analysis of bacterial ceramide genes, the distances between spt, bcerS, and cerR were calculated. The histogram columns are colored by taxonomic class. The numbers above the columns are the average distance ± the standard error of the mean. (b) MS/MS analysis of ceramides from S. aurantiacus confirm that the desaturation occurs on the long-chain base. (c) MS/MS analysis of ceramides produced by bCerS homologues from P. buccae (see Fig. 3f) show that HMPREF0649_00885 preferred fully saturated substrates, while HMPREF0649_00886 used a desaturated palmitoyl-CoA substrate.
Extended Data Fig. 8. Alignment of Spt across taxonomic domains suggests a common evolutionary ancestor.
Protein alignment of several eukaryotic and bacterial Spt enzymes was performed using Clustal Omega44. Previously characterized active site residues are indicated by (*)45.
Extended Data Fig. 9. Alignment of reductase enzymes suggests independent evolution of CerR and KDSR.
(a-b) Alignments of bacterial CerR proteins (a) or C. crescentus CerR and human KDSR (b) were done using Clustal Omega44. Green-highlighted residues are active site amino acids in KDSR34. The YxxxK motif is the reductase active site and the TGxxxGxG motif is the NAD binding site. Note that the bacterial NAD binding site, TGxxGFxG, is different from the eukaryotic site.
Extended Data Fig. 10. Alignment of ceramide synthase enzymes suggests independent evolution of bCerS and CerS.
(a) bCerS homologues were aligned using Clustal Omega44. (b) The conserved Lag1P domains from eukaryotic CerS proteins35,36 were aligned with Clustal Omega44. Active-site residues are highlighted in yellow. The Lag1P domain is absent from bCerS.
Supplementary Material
Data S1: TBLASTN hits and query parameters used to identify bacterial species with genes encoding Spt, bCerS, and CerR.
Data S2: Bacterial species with multiple bCerS homologues.
Acknowledgments:
The authors thank Carla Cugini (Rutgers University) for providing P. gingivalis genomic DNA for cloning. The authors also thank Anthony Geneva (Rutgers University-Camden) for helpful discussions. Funding was provided by National Science Foundation grants MCB-1553004 and MCB-2031948 (E.A.K.), National Institutes of Health grants GM069338 and R01AI148366 (Z.G.), and Biotechnology and Biological Sciences Research Council grants BB/M010996/1 and BB/T016841/1 (D.J.C.).
Footnotes
Competing interests: Authors declare no competing interests.
Data availability:
The raw data for Figure 1C and Extended Data Figures 1c-d, 5c-e, and 7a are provided as Microsoft Excel files in the supplementary information (Source Data 1-4). The data for the bioinformatic analyses was obtained from the following publicly available NCBI resources: NCBI Prokaryotic Representative Genomes: https://ftp.ncbi.nlm.nih.gov/genomes/GENOME_REPORTS/prok_representative_genomes.txt. Accession numbers for the proteins used for BLAST analyses are as follows. Bacterial Spt homologues: C. crescentus YP_002516593.1; P. gingivalis BAG34240; M. xanthus ABF87747; B. stolpii BAF73753. Bacterial bCerS homologues: C. crescentus YP_002516585.1; P. gingivalis BAG32893; M. xanthus ABF92629; B. stolpii WP_102243213. Bacterial CerR homologues: C. crescentus YP_002516595.1; P. gingivalis BAG34405; M. xanthus ABF87537; B. stolpii WP_102243212. Eukaryotic CerS homologues: Human P27544.1; A. thaliana NP_001184985; S. cerevisiae AAA21579.1. Eukaryotic Spt homologues: Human NP_006406.1; A. thaliana NP_190447.1; S. cerevisiae CAA56805.1. Eukaryotic KDSR homologues: Human NP_002026.1; A. thaliana NP_187257; S. cerevisiae P38342. Eukaryotic Gcn5 homologues: Human AAC39769.1 and S. cerevisiae NP_011768.1.
References
- 1.Harrison PJ, Dunn TM & Campopiano DJ Sphingolipid biosynthesis in man and microbes. Nat Prod Rep 35, 921–954 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown EM et al. Bacteroides-derived sphingolipids are critical for maintaining intestinal homeostasis and symbiosis. Cell Host Microbe 25, 668–680 e667 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moye ZD, Valiuskyte K, Dewhirst FE, Nichols FC & Davey ME Synthesis of sphingolipids impacts survival of Porphyromonas gingivalis and the presentation of surface polysaccharides. Front Microbiol 7, 1919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stankeviciute G, Guan Z, Goldfine H & Klein EA Caulobacter crescentus adapts to phosphate starvation by synthesizing anionic glycoglycerolipids and a novel glycosphingolipid. mBio 10, e00107–00119 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahrendt T, Wolff H & Bode HB Neutral and phospholipids of the Myxococcus xanthus lipodome during fruiting body formation and germination. Appl Environ Microbiol 81, 6538–6547 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaneshiro ES, Hunt SM & Watanabe Y Bacteriovorax stolpii proliferation and predation without sphingophosphonolipids. Biochem Biophys Res Commun 367, 21–25 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Hannun YA & Obeid LM Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol 19, 175–191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merrill AH Jr. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem Rev 111, 6387–6422 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikushiro H, Hayashi H & Kagamiyama H A water-soluble homodimeric serine palmitoyltransferase from Sphingomonas paucimobilis EY2395T strain. Purification, characterization, cloning, and overproduction. J Biol Chem 276, 18249–18256 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Yard BA et al. The structure of serine palmitoyltransferase; gateway to sphingolipid biosynthesis. J Mol Biol 370, 870–886 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Geiger O, Gonzalez-Silva N, Lopez-Lara IM & Sohlenkamp C Amino acid-containing membrane lipids in bacteria. Prog Lipid Res 49, 46–60 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Olea-Ozuna RJ et al. Five structural genes required for ceramide synthesis in Caulobacter and for bacterial survival. Environ Microbiol (2020). [DOI] [PubMed] [Google Scholar]
- 13.Wadsworth JM et al. The chemical basis of serine palmitoyltransferase inhibition by myriocin. J Am Chem Soc 135, 14276–14285 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Harrison PJ et al. Use of isotopically labeled substrates reveals kinetic differences between human and bacterial serine palmitoyltransferase. J Lipid Res 60, 953–962 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Xie T, Liu P, Wang L & Gong X Structural insights into the assembly and substrate selectivity of human SPT-ORMDL3 complex. Nat Struct Mol Biol 28, 249–257 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Raman MC et al. The external aldimine form of serine palmitoyltransferase: structural, kinetic, and spectroscopic analysis of the wild-type enzyme and HSAN1 mutant mimics. J Biol Chem 284, 17328–17339 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raman MC, Johnson KA, Clarke DJ, Naismith JH & Campopiano DJ The serine palmitoyltransferase from Sphingomonas wittichii RW1: An interesting link to an unusual acyl carrier protein. Biopolymers 93, 811–822 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Ren J et al. Quantification of 3-ketodihydrosphingosine using HPLC-ESI-MS/MS to study SPT activity in yeast Saccharomyces cerevisiae. J Lipid Res 59, 162–170 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng W et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta 1758, 1864–1884 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Tidhar R et al. Eleven residues determine the acyl chain specificity of ceramide synthases. J Biol Chem 293, 9912–9921 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow TC & Schmidt JM Fatty acid composition of Caulobacter crescentus. Microbiology 83, 369–373 (1974). [Google Scholar]
- 22.Okino N et al. The reverse activity of human acid ceramidase. J Biol Chem 278, 29948–29953 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Chen M, Markham JE, Dietrich CR, Jaworski JG & Cahoon EB Sphingolipid long-chain base hydroxylation is important for growth and regulation of sphingolipid content and composition in Arabidopsis. Plant Cell 20, 1862–1878 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omae F et al. DES2 protein is responsible for phytoceramide biosynthesis in the mouse small intestine. Biochem J 379, 687–695 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price MN et al. Mutant phenotypes for thousands of bacterial genes of unknown function. Nature 557, 503–509 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Kawahara K, Moll H, Knirel YA, Seydel U & Zähringer U Structural analysis of two glycosphingolipids from the lipopolysaccharide-lacking bacterium Sphingomonas capsulata. European Journal of Biochemistry 267, 1837–1846 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Feng Y & Cronan JE Escherichia coli unsaturated fatty acid synthesis: complex transcription of the fabA gene and in vivo identification of the essential reaction catalyzed by FabB. J Biol Chem 284, 29526–29535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christen B et al. The essential genome of a bacterium. Mol Syst Biol 7, 1–7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stankeviciute G et al. Differential modes of crosslinking establish spatially distinct regions of peptidoglycan in Caulobacter crescentus. Mol Microbiol 111, 995–1008 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Rocha FG et al. Porphyromonas gingivalis sphingolipid synthesis limits the host inflammatory response. J Dent Res 99, 568–576 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mun J et al. Structural confirmation of the dihydrosphinganine and fatty acid constituents of the dental pathogen Porphyromonas gingivalis. Org Biomol Chem 5, 3826–3833 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Nguyen M & Vedantam G Mobile genetic elements in the genus Bacteroides, and their mechanism(s) of dissemination. Mob Genet Elements 1, 187–196 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavanagh KL, Jornvall H, Persson B & Oppermann U Medium- and short-chain dehydrogenase/reductase gene and protein families : the SDR superfamily: functional and structural diversity within a family of metabolic and regulatory enzymes. Cell Mol Life Sci 65, 3895–3906 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyden LM et al. Mutations in KDSR cause Recessive Progressive Symmetric Erythrokeratoderma. Am J Hum Genet 100, 978–984 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kageyama-Yahara N & Riezman H Transmembrane topology of ceramide synthase in yeast. Biochem J 398, 585–593 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spassieva S et al. Necessary role for the Lag1p motif in (dihydro)ceramide synthase activity. J Biol Chem 281, 33931–33938 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Liebisch G et al. Update on LIPID MAPS classification, nomenclature and shorthand notation for MS-derived lipid structures. J Lipid Res (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao L et al. A selective gut bacterial bile salt hydrolase alters host metabolism. Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MT, Le HH & Johnson EL Dietary sphinganine is selectively assimilated by members of the mammalian gut microbiome. J Lipid Res 62, 100034 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tudzynski B Gibberellin biosynthesis in fungi: genes, enzymes, evolution, and impact on biotechnology. Appl Microbiol Biotechnol 66, 597–611 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Huang R, O'Donnell AJ, Barboline JJ & Barkman TJ Convergent evolution of caffeine in plants by co-option of exapted ancestral enzymes. Proc Natl Acad Sci U S A 113, 10613–10618 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dick R et al. Comparative analysis of benzoxazinoid biosynthesis in monocots and dicots: independent recruitment of stabilization and activation functions. Plant Cell 24, 915–928 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawasaki S et al. The cell envelope structure of the lipopolysaccharide-lacking gram-negative bacterium Sphingomonas paucimobilis. J Bacteriol 176, 284–290 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madeira F et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowther J et al. Role of a conserved arginine residue during catalysis in serine palmitoyltransferase. FEBS Lett 585, 1729–1734 (2011). [DOI] [PubMed] [Google Scholar]
Methods references
- 46.Poindexter JS Selection for nonbuoyant morphological mutants of Caulobacter crescentus. J Bacteriol 135, 1141–1145 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrieres L et al. Silent mischief: bacteriophage Mu insertions contaminate products of Escherichia coli random mutagenesis performed using suicidal transposon delivery plasmids mobilized by broad-host-range RP4 conjugative machinery. J Bacteriol 192, 6418–6427 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez-Garcia E, Calles B, Arevalo-Rodriguez M & de Lorenzo V pBAM1: an all-synthetic genetic tool for analysis and construction of complex bacterial phenotypes. BMC Microbiol 11, 38 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bligh EG & Dyer WJ A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37, 911–917 (1959). [DOI] [PubMed] [Google Scholar]
- 50.Guan Z, Katzianer D, Zhu J & Goldfine H Clostridium difficile contains plasmalogen species of phospholipids and glycolipids. Biochim Biophys Acta 1842, 1353–1359 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edgar RC MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5, 113 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stamatakis A RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller MA, Pfeiffer W & Schwartz T Creating the CIPRES Science Gateway for inference of large phylogenetic trees. 2010 Gateway Computing Environments Workshop (GCE), 1–8 (2010). [Google Scholar]
- 54.Chamberlain SA & Szocs E taxize: taxonomic search and retrieval in R. F1000Res 2, 191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu G Using ggtree to visualize data on tree-like structures. Curr Protoc Bioinformatics 69, e96 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Paradis E & Schliep K ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Wang LG et al. Treeio: An R Package for phylogenetic tree input and output with richly annotated and associated data. Mol Biol Evol 37, 599–603 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wickham H ggplot2: Elegant Graphics for Data Analysis. (Springer-Verlag; New York, 2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: TBLASTN hits and query parameters used to identify bacterial species with genes encoding Spt, bCerS, and CerR.
Data S2: Bacterial species with multiple bCerS homologues.
Data Availability Statement
The raw data for Figure 1C and Extended Data Figures 1c-d, 5c-e, and 7a are provided as Microsoft Excel files in the supplementary information (Source Data 1-4). The data for the bioinformatic analyses was obtained from the following publicly available NCBI resources: NCBI Prokaryotic Representative Genomes: https://ftp.ncbi.nlm.nih.gov/genomes/GENOME_REPORTS/prok_representative_genomes.txt. Accession numbers for the proteins used for BLAST analyses are as follows. Bacterial Spt homologues: C. crescentus YP_002516593.1; P. gingivalis BAG34240; M. xanthus ABF87747; B. stolpii BAF73753. Bacterial bCerS homologues: C. crescentus YP_002516585.1; P. gingivalis BAG32893; M. xanthus ABF92629; B. stolpii WP_102243213. Bacterial CerR homologues: C. crescentus YP_002516595.1; P. gingivalis BAG34405; M. xanthus ABF87537; B. stolpii WP_102243212. Eukaryotic CerS homologues: Human P27544.1; A. thaliana NP_001184985; S. cerevisiae AAA21579.1. Eukaryotic Spt homologues: Human NP_006406.1; A. thaliana NP_190447.1; S. cerevisiae CAA56805.1. Eukaryotic KDSR homologues: Human NP_002026.1; A. thaliana NP_187257; S. cerevisiae P38342. Eukaryotic Gcn5 homologues: Human AAC39769.1 and S. cerevisiae NP_011768.1.