Summary
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal of all human malignancies. PDAC precursor lesions, invasive primary PDAC, and metastatic PDAC each display distinct morphologies that reflect unique biology. This ‘biomorphology’ is determined by a complex neoplastic history of clonal phylogenetic relationships, geographic locations, external environmental exposures, intrinsic metabolic demands, and tissue migration patterns. Understanding the biomorphological evolution of PDAC progression is not only of academic interest but also of great practical value. Applying this knowledge to surgical pathology practice facilitates the correct diagnosis on routine H&E stains without additional ancillary studies in most cases. Here I provide a concise overview of the entire biomorphological spectrum of PDAC progression beginning with initial neoplastic transformation and ending in terminal distant metastasis. Most biopsy and resection specimens are currently obtained prior to treatment. As such, our understanding of untreated PDAC biomorphology is mature. The biomorphology of treated PDAC is less defined but will assume greater importance as the frequency of neoadjuvant therapy increases. Although this overview is slanted towards pathology, it is written so that pathologists, clinicians, and scientists alike might find it instructive for their respective disciplines.
Keywords: Pancreatic cancer, pancreatic ductal adenocarcinoma, pathology, morphology, biology
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is both a genetic and metabolic disease that evolves through specific steps of progression under the selective pressures of fibroinflammatory microenvironments. Because early detection is not yet a reality, PDAC is still routinely encountered at all steps of its natural evolutionary history in fully developed histological form. Large macroscopic cystic precursor lesions are detected and resected with increasing frequency. Both microscopic [pancreatic intraepithelial neoplasia (PanIN)] and macroscopic [intraductal papillary mucinous neoplasm (IPMN), mucinous cystic neoplasm (MCN)] precursors may be difficult to distinguish from ‘cancerisation’ of pancreatic ducts in surgical resection specimens. IPMNs can even extend out of the pancreas and mimic ampullary adenomas on biopsies. Precursor lesions that undergo malignant transformation must contend with a dense desmoplastic stroma that is a hallmark morphological feature of this disease. This places extreme metabolic demands on PDAC cells as they struggle to proliferate, migrate, and survive within the thick stroma. As primary PDAC grows silently in the pancreas over time, morphologically divergent subclonal populations evolve that are easily appreciated on haematoxylin and eosin (H&E) examination of resection specimens. Some of these subclones will seed widespread distant metastasis. Needle biopsies of metastatic PDAC have become more common as the desperate search for elusive therapeutically actionable targets continues. These biopsies can be diagnostically challenging since the hallmark desmoplastic stroma of primary PDAC is often poorly formed or absent altogether in distant metastases. Thus, pathologists are routinely tasked with accurately diagnosing and/or staging PDAC in all its fully developed glory across all steps of neoplastic progression. Familiarity with the biological basis for how and why morphology evolves as it does during this progression is a ‘killer app’ in the skillset of any surgical pathologist. That is because it provides an intuitive understanding of disease behaviour that instills low magnification gestalt into the art of routine diagnostic practice.
THE BIOMORPHOLOGY OF PDAC PRECURSORS
Like other cancers, PDAC evolves through a stepwise genetic progression sequence that can be recognised on H&E by distinct histomorphologies.1 Most cases arise sporadically through acquisition of somatic mutations in pancreatic ductal or acinar epithelial cells. Some or all these somatic mutations are thought to arise in the setting of continuous or episodic fibroinflammatory conditions that injure native ducts and acini, especially chronic pancreatitis.2,3 This hypothesis is supported by high penetrance of PDAC in patients with hereditary pancreatitis4 and in genetically engineered mouse models (GEMMs) subjected to experimental pancreatitis.2 It is thought that fibroinflammatory environments apply selective pressures that favour acquisition of somatic mutations in a core set of cancer ‘driver’ genes that support pancreatic ductal neoplasia. Indeed, the same core set of driver genes is recurrently mutated in PDACs from different patients. That is because functional mutations in these specific genes increase fitness (growth and/or survival) of epithelial cells during otherwise lethal or senescent fibroinflammatory injury conditions.3
KRAS: the signature PDAC genetic driver
The signature PDAC genetic driver is the KRAS oncogene. Activating KRAS mutations are present in approximately 90% of PDAC patients5 and represent one of the earliest genetic events during neoplastic transformation.1,3,6 Oncogenic KRAS mutations are selected in part because the KRAS gene product participates in signal transduction pathways that activate a variety of adaptive outputs that help epithelial cells cope during stressful conditions (such as pancreatitis).3,6,7 One such adaptation is acinar to ductal metaplasia (ADM)8,9 (Fig. 1A). ADM may be adaptive because acinar cells (which secrete digestive enzymes) are converted into metaplastic ducts presumably as an attempt to shunt pools of tissue-damaging acinar secretions around obstructed or damaged native ducts. ADMs themselves are not neoplastic. Nevertheless, they can display histological features vaguely reminiscent of neoplasia including angulated glands lined by immature cells with nuclear atypia (Fig. 1B) that may exceed that of well-differentiated PDAC (Fig. 1C). The morphological overlap between ADM and neoplasia may reflect partially de-differentiated states, shared RAS signalling, or a combination of both.
Fig. 1.
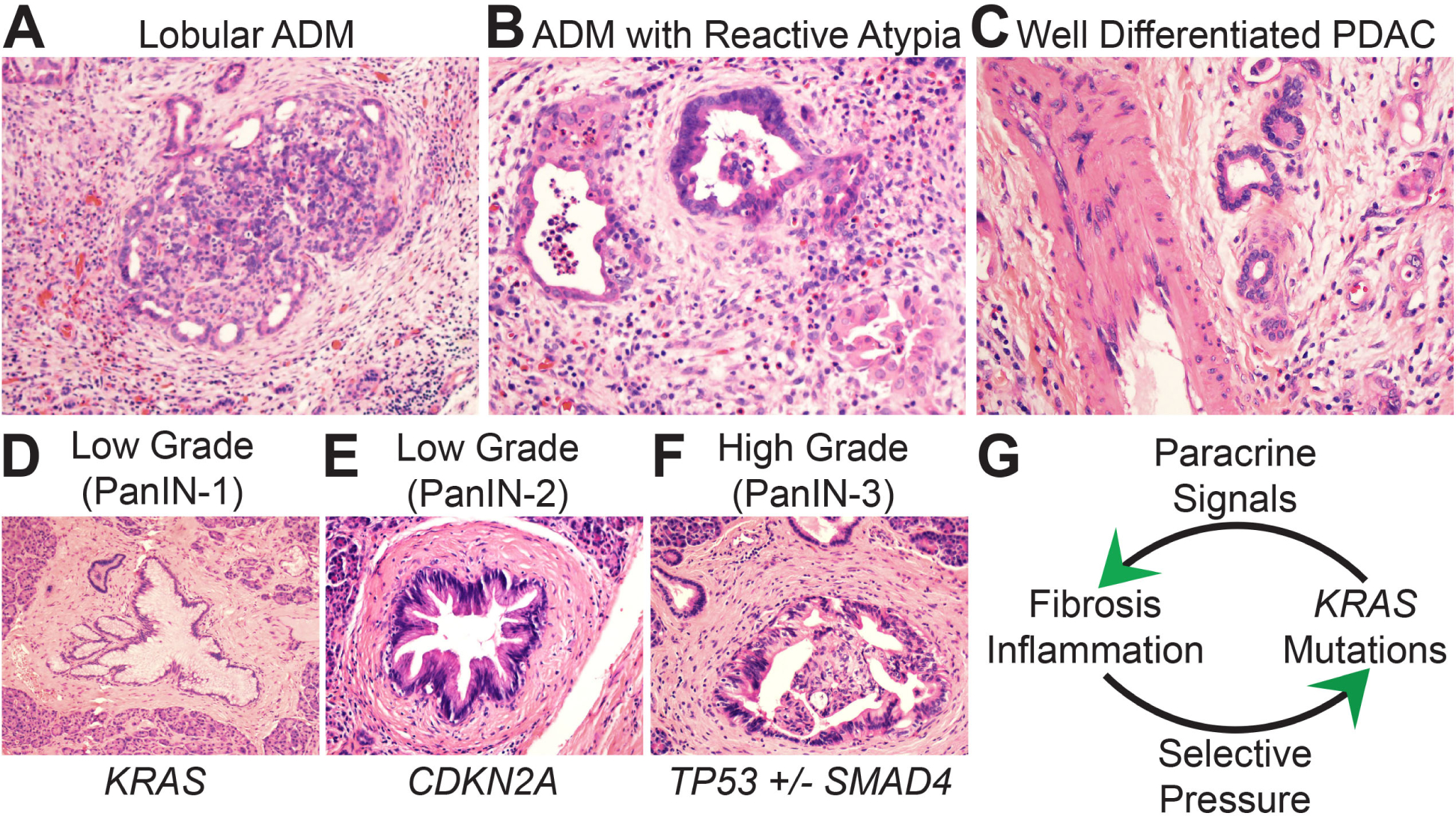
Biomorphology of PDAC precursor lesions. (A) At low magnification acinar to ductal metaplasia (ADM) maintains a lobular architecture surrounded by a fibroinflammatory stroma. Metaplastic ducts are seen on the periphery and residual acinar units on the interior. (B) At high magnification metaplastic ducts show morphology that overlaps with malignancy including luminal necrosis, nuclear atypia, and jagged glandular outlines. (C) The ADM ductal units in B are more atypical than this bland-appearing PDAC that is infiltrating adjacent to a large vessel. (D–F) Dysplasia increases as genetic drivers accumulate during precursor progression. (G) Gene:environment positive feedback maintains precursor lesions.
Chronic pancreatitis is stressful to epithelial cells because they are forced to regenerate under hostile conditions. Fibrosis interferes with microvascular delivery of oxygen and nutrients. This forces native and metaplastic ducts to regenerate under hypoxia and famine.3,7,10 Fibrotic conditions also attract inflammatory cells that can damage regenerating glands. KRAS signalling activates several unique adaptations that promote growth, survival, and ultimately neoplastic transformation of both native11 and metaplastic12,13 ductal epithelial cells. These adaptations may include so-called ‘professional scavenger’ pathways that counteract starvation by extracting what macromolecules are available from the stromal matrix and converting them into the minimal essential metabolites that fuel cellular metabolism and antioxidant defenses.7,10,14–23 Coalescence of mitochondria into ‘supercomplexes’ likewise maximises efficient oxygen consumption during hypoxia.24 Endocytosis-coupled digestion of cell-surface neoantigens promotes immune evasion.25 Signal transduction pathways that stimulate epithelial cell proliferation simultaneously suppress effector T-cell killing of regenerating ducts.26–30 Thus, there is strong selective pressure for pancreatic epithelial cells to acquire activating KRAS mutations, professional scavenger pathways, and immune evasion capabilities under fibroinflammatory conditions.3,7 If clinical or subclinical injury/pancreatitis persists long enough for an oncogenic gain-of-function mutation to emerge by chance during epithelial cell proliferation, then that ‘fortuitous’ cell gains fitness advantages over wild-type neighbours and outcompetes them through natural selection. The result is neoplastic transformation.2,11,12,31,32
Precursor progression: the core set of PDAC genetic drivers
KRAS-driven neoplastic transformation most often persists as microscopic (<0.5 cm) in situ PanIN precursor lesions that sprout within interlobular side branches of the pancreatic ductal system. Oncogenic KRAS also contributes to neoplastic cystogenesis (IPMN, MCN) in cooperation with oncogenic GNAS mutations33–35 but independently of metaplasia.36 Both native duct regeneration and ADM are reversible and low numbers of KRAS mutated epithelial cells can be outcompeted and cleared by wild-type counterparts if systemic pancreatic injury resolves in a timely fashion.37 PanINs that persist may do so in part because oncogenic KRAS assumes control over an injury-induced epigenetic program that instructs the mutated epithelial cells to secrete protective fibroinflammatory cytokines.38–41 If injury persists long enough, epithelial cells carrying the mutant KRAS alleles will proliferate beyond a ‘PanIN-permissive’ numerical threshold. At this threshold, the epithelial paracrine secretions are sufficient to construct a localised rim of fibroinflammatory tissue around the mutated epithelial cells (Fig. 1D–F). This creates a gene:environment ‘positive feedback’ interaction (Fig. 1G) that allows mutated cells to persist as PanINs even in the absence of systemic pancreatitis through self-reinforcing cross-talk: (1) the newly constructed fibroinflammatory rim only favours survival of epithelial cells with mutant KRAS alleles; (2) the mutated cells themselves secrete paracrine signals that maintain the rim of fibroinflammatory tissue. From a conceptual standpoint, mutant KRAS is the ‘selfish gene’ of pancreatitis.
Although selected at high frequency, KRAS mutations alone are weakly oncogenic. An analogous example in the colon is KRAS-driven hyperplastic polyps, which are tiny and harmless. Like hyperplastic polyps, PanINs that remain under the sole influence of oncogenic KRAS are benign, well-differentiated, and remain confined to their side branch site(s) of origin. They display the morphological features of low-grade dysplasia (LGD; formerly PanIN-1 and -2 lesions; Fig. 1D,E).42 Slow cell cycle rates retain ducts in a flat or undulating monolayer of polarised columnar epithelial cells, although some stratification may occur due to modest increases in cell numbers over time. Because cells spend most of their time in the G0 phase of the cell cycle the cytosol remains expanded with side branch (gastric)-type mucins. Nuclear atypia is mild or absent due to minimal amounts of (epi)genomic instability.
Autopsy studies indicate that the vast majority of PanINs remain low grade.43 However, KRAS addiction acts as a gateway for the gradual1 or punctuated44 acquisition of additional driver gene alterations in some precursors. These are recurrent mutations and/or allelic losses targeting specific tumour suppressor genes3 (most commonly CDKN2A,45,46 TP53,47 and SMAD448). The precise environmental selective pressures that favour acquisition of these additional drivers are not well defined. Survival during genotoxic stress and/or an evolutionary license to test drive copy number imbalances may be important since inactivation of CDKN2A and especially TP53 eliminates cell cycle checkpoints that normally guard against genome instability.3 TP53 hits may also further facilitate immunosuppression.49 Mutations and/or losses in SMAD4 (also known as DPC448) are also observed in 40–55% of PDACs. The selective pressures favouring SMAD4 loss-of-function probably include surviving potentially lethal aspects of stromal TGF-β signalling when neoplastic cells undergo epithelial-to-mesenchymal transition (EMT) and/or migrate through stromal matrix barriers.50,51 The additional drivers also correlate with the ability to spread to other regions of the ductal system. Intraductal spread occurs by anchorage-dependent lateral spreading along basement membrane-lined surfaces and by anchorage-independent floating of detached cells through luminal secretions.52 Collectively, inactivation of cell cycle checkpoints,53 induction of (epi)genomic instability,54–56 and enhanced mobility52 combine to produce the morphological features of high-grade dysplasia (HGD; formerly PanIN-3 lesions; Fig. 1F). Increased cell cycle rates cause cells to increase in number, crowd, and project papillary or cribriform outgrowths into the luminal space. Less time in the G0 phase of the cell cycle reduces cytosolic mucin production. Epigenetic reprogramming and genome instability cause nuclear atypia with loss of polarity and atypical mitotic figures. Increased mobility manifests as budding of cell clusters into the luminal space. Data indicate that genetic inactivation of CDKN2A is a relatively early event in the progression to HGD (Fig. 1D) whereas inactivation of TP53 and SMAD4 are late events that contribute to malignant transformation57,58 (Fig. 1F). Thus, the core set of PanIN genetic drivers required for HGD and subsequent malignant transformation (invasive adenocarcinoma) includes activating KRAS mutations with various combinations of inactivating CDKN2A, TP53, and/or SMAD4 genetic hits. The core set of genetic drivers in neoplastic cystic precursors includes additional hits in GNAS and RNF43.35
Neoplastic migrations: intraductal spread and surface colonisation
Intraductal migration is a trait acquired by precursor lesions52,59 that is retained by invasive PDAC that re-colonises native pancreatic ducts.60 As such, multi-focal involvement of the ductal system is commonly observed for both cystic precursor lesions and PDAC. This at least partially explains the high rate of local recurrence after surgical resections with negative margins. For frozen section evaluation of IPMN resection margins, only the presence of HGD (or invasion) at the margin warrants further surgical intervention.61 For PDAC resections, the presence of intraductal HGD (especially if circumferential involvement) may best be considered a positive margin since it likely represents re-colonisation of the ducts.60 Intraductal spread may even extend out of the pancreas into the periampullary ducts (Fig. 2A,B) and onto the intestinal mucosal surfaces to mimic an adenoma (Fig. 2C,D). The pathology report for initial ampullary mass biopsies should include a comment suggesting imaging studies to rule out spread from an adjacent IPMN or PDAC. Finally, the ability to spread along surfaces and within lumens extends to nerves (Fig. 2E,F) and muscular vessels,62 respectively. These proclivities should always be considered when evaluating surgical margins and small biopsies.
Fig. 2.
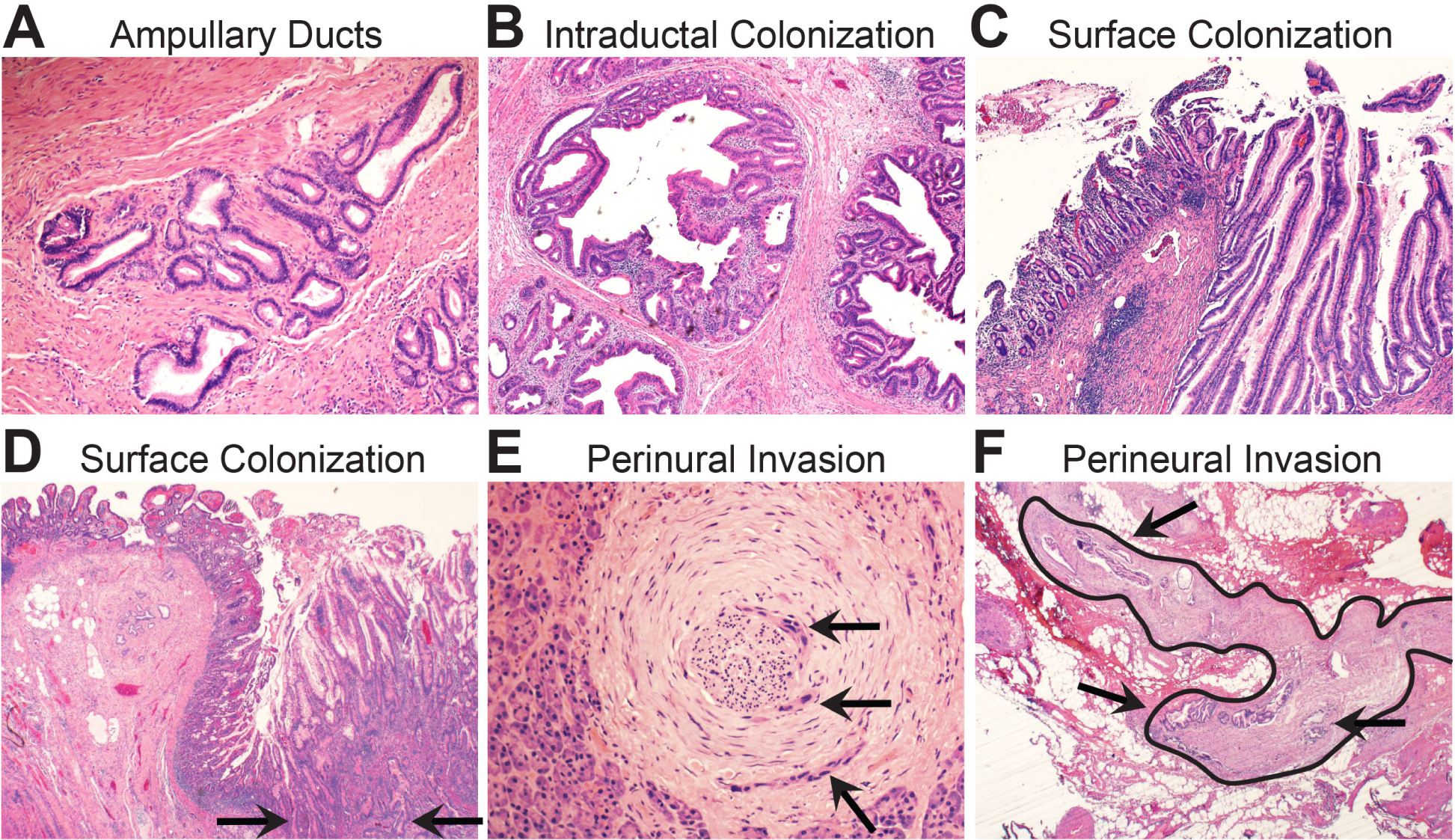
Biomorphology of neoplastic surface spread. (A) Periampullary ducts from the pancreas are situated within the ampullary wall muscle bundles. (B) Intraductal spread of invasive PDAC into the periampullary lumens. (C) An intestinal type intraductal papillary mucinous neoplasm (IPMN) has spread through the periampullary ducts and colonised the surface of the ampullary mucosa. This will mimic an ampullary villous adenoma on a biopsy. (D) Invasive PDAC has invaded directly through the wall of the duodenum (arrows) and colonised the duodenal mucosal surface. This may also simulate a surface adenoma on a biopsy. (E) High power magnification shows focal perineural invasion (arrows) in otherwise normal appearing pancreatic parenchyma. This was a grossly negative resection margin located 1 cm from the primary tumour mass. (F) Low power magnification of peripancreatic fibroadipose tissues shows a large nerve (outlined) with perineural invasion (arrows). This was a frozen section of a resection margin with no grossly identified tumour mass.
Multiple independent LGD and HGD precursor lesions may develop at different locations within the ductal system during a human lifespan.57,63 Individual precursors may also be polyclonal mixtures themselves.64 Intraductal migrations by one of these lesions35,52 or invasive PDAC60 (that has re-colonised the ducts) can eliminate (poly)clonal heterogeneity. This occurs because neoplastic cells from one lesion can migrate and colonise multiple other locations.52 If the pioneering neoplastic cells are more fit (better at growing and/or surviving) than the stationary neoplastic cells at those sites, the pioneers will have a competitive advantage that results in a ‘clonal sweep’.3,52 If clonal sweeps are common events prior to clinical diagnosis, then the full spectrum of therapeutically actionable genetic drivers can theoretically be detected in untreated patients by sequencing tumour tissue from a single biopsy in most cases.65
THE BIOMORPHOLOGY OF PRIMARY PDAC
Malignant transformation of a benign precursor lesion is pathologically defined as invasion through the ductal basement membrane into the surrounding pancreatic parenchyma. This initiates a period of primary tumour growth in the pancreas. PDAC is the ‘silent killer’ due to the presence of a clinically asymptomatic primary tumour within a poorly accessible retroperitoneal location. Sequencing studies indicate that primary PDACs grow over a period of years prior to clinical presentation.52,66 From a clinical oncology perspective this is unexpected considering the aggressive behaviour of this disease. From a pathobiology perspective this is expected since thickly fibrotic tumours often grow slowly even if malignant (desmoids for example), whereas loosely fibrotic tumours may grow rapidly even if benign (nodular fasciitis for example). The period of silent growth is extremely important because it presents an elusive ‘window of opportunity’ for early detection and cure prior to the development of locally advanced disease or metastasis.67 Early detection is currently only possible for the small subset of patients with cystic precursors detected incidentally by imaging or those who present with painless jaundice from small PDACs that arise near the common bile duct and obstruct it soon after invasion. Development of early detection technology is justifiably the focus of intense research that will impact both anatomical and clinical pathology practice in the future. Promising technologies include but are not limited to endoscopic collection of pancreatic fluids for biomarker testing,68 ‘liquid biopsies’ of biomarkers in the systemic circulation,69,70 clinical risk assessment algorithms, and imaging-based screening programs for high-risk populations.71–73
The desmoplastic stroma: a hallmark feature of primary PDAC
Matched sequencing studies from the same individual patient(s) indicate that the core set of genetic driver alterations acquired during progression to HGD probably also represents the full complement required to support invasive primary tumour growth.3 Beyond those genetic requirements, the precise physical mechanisms whereby cells from intraductal HGD precursor lesions invade through the ductal basement membrane barrier remain incompletely understood. Proteolytic digestion of matrix, prolapse through matrix weaknesses, active migration through matrix, and spread onto periductal investing structures (especially nerves,74–76 Fig. 3A) are possible. Irrespective of the mechanism, invasive primary tumour growth appears to be clonally initiated through a bottleneck of neoplastic cells that carry the core set of PDAC genetic drivers (the ‘parental clone’).66
Fig. 3.
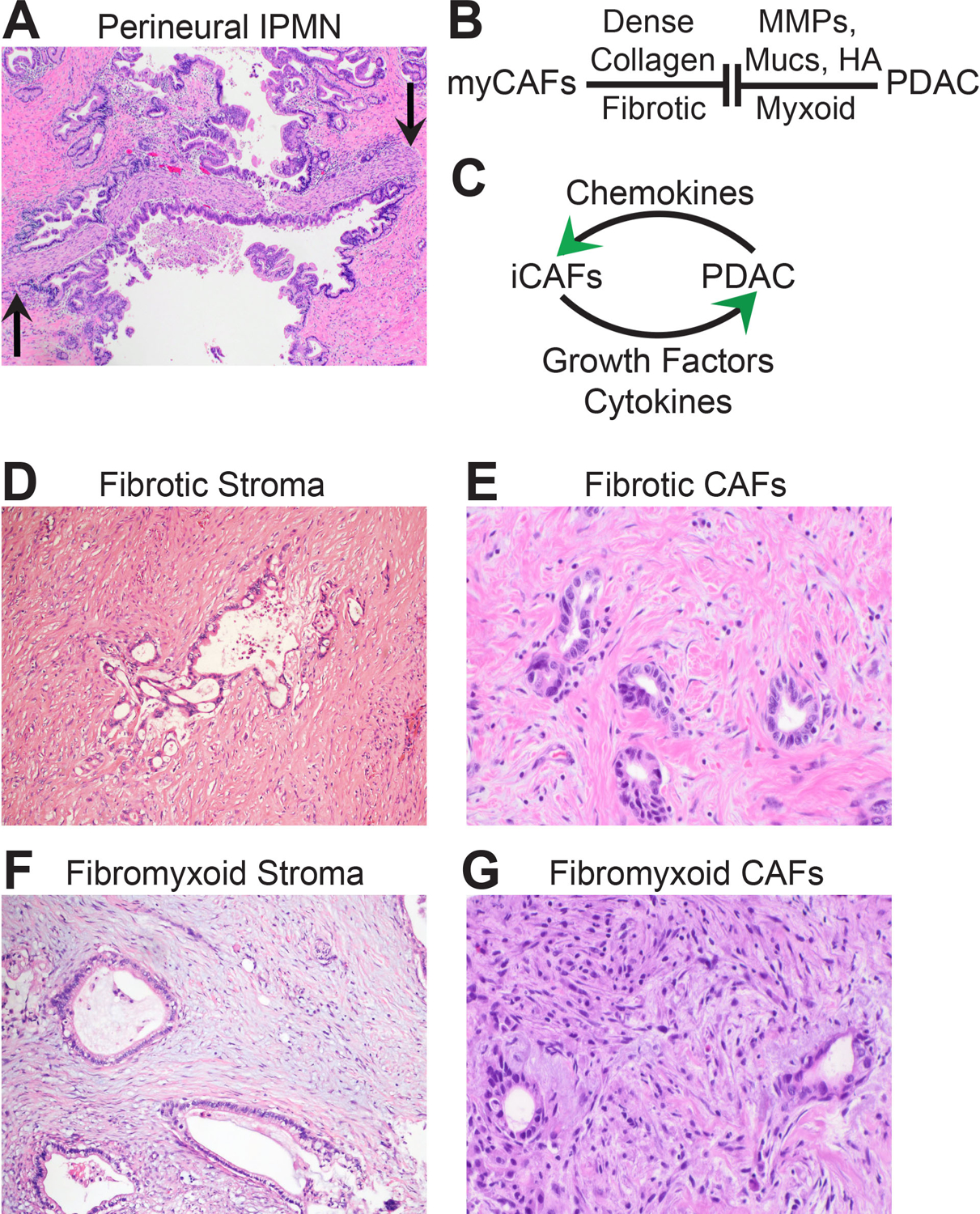
Biomorphology of primary PDAC and the hallmark desmoplastic stroma. (A) An intraductal papillary mucinous neoplasm (IPMN) has grown into a large nerve (flanked by arrows). The neoplastic epithelium has anchored onto the nerve. Is this an event that facilitates malignant transformation? (Slide kindly shared by M. Garcia-Buitrago.) (B) The schematic depicts secretory matrix opposition between myofibroblast-type cancer-associated fibroblasts (myCAFs) and PDAC that occurs within the tumour stroma. (C) The schematic depicts secretory matrix cooperation between inflammatory-type cancer-associated fibroblasts (iCAFs) and PDAC that occurs within the tumour stroma. (D) Low power magnification shows a large PDAC gland encased within densely fibrotic stroma. (E) High power magnification of CAFs residing in fibrotic areas. These could represent myCAFs. (F) Low power magnification shows PDAC glands encased within a partially fibromyxoid stroma. (G) High power magnification of CAFs residing in fibromyxoid areas. Patrols of inflammatory cells are also often in fibromyxoid stroma, although they are ineffective at controlling the PDAC invaders.
Invasion of the parental clone into the pancreatic stroma activates resident pancreatic stellate cells (PSCs), initiates secretion of extracellular matrix, and triggers recruitment of inflammatory cells.77 This constitutes the ‘desmoplastic stroma’ that is a hallmark feature of primary PDAC.78 The desmoplastic stroma bears some similarities to chronic pancreatitis79,80 and chronic pancreatitis is usually present in areas away from the tumour mass (possibly due to large duct obstruction). Activated PSCs proliferate and differentiate into at least two distinct types of ‘cancer-associated fibroblasts’ (CAFs).81,82 Myofibroblast-type CAFs (myCAFs, Fig. 3B) emerge in juxtaposition to invading glands and secrete densely collagenous extracellular matrix82 that restrains PDAC progression.83–85 To combat this, the malignant cells secrete matrix metalloproteinases, mucins, and hyaluronic acid,86 which may synergise to partially solubilise the dense collagen matrix. Malignant cells also secrete chemokines that instruct PSCs to differentiate into inflammatory-type CAFs (iCAFs, Fig. 3C). iCAFs return the favour by secreting growth factors and immunosuppressive cytokines that enhance fitness of the same PDAC cells that secreted the chemokines to the iCAFs.29,41,82 Depending on the densities of proteinaceous collagens and aqueous proteoglycans, the stroma extracellular matrix ranges from scar-like (dense collagen, Fig. 3D,E) to fibromyxoid (collagen mixed with mucins and/or hyaluronic acid, Fig. 3F,G). In fibrotic areas, CAFs are slender spindle cells with hyperchromatic nuclei situated in between wavy collagen bundles (Fig. 3E). In fibromyxoid areas, CAFs ‘open’ into more plump stellate cells (Fig. 3G). Immunohistochemistry for smooth muscle α-actin can distinguish myCAFs (high expression) from iCAFs (low expression). Beyond CAFs, stromal inflammation is present especially in fibromyxoid areas. Neutrophils form intraluminal microabscesses within the PDAC glands. Scattered collections of macrophages and eosinophils coalesce around contents that spill from ruptured malignant ducts. Lymphocytes patrol aimlessly through the stromal matrix and fail to home toward the malignant invaders. Stromal matrix composition,84,87,88 immunosuppressive cytokines,26,27,38 myeloid-derived (immune) suppressor cells,28,30,89 and endocytosis of PDAC neoantigens25,90 likely all contribute to tumour defense against lymphocyte attack. Indeed, PDAC is notoriously ‘cold’ to immunotherapy.91
The desmoplastic stroma may occupy up to 80–90% of the total tumour volume. Some features of this specialised stroma undoubtedly contribute to PDAC progression.87,88,92,93 Such advantages may come at considerable cost, since stromal pressures collapse the already sparse numbers of arterioles, capillaries, and venules that feed and drain the tumour mass.94 The result is sluggish delivery of systemically circulating oxygen, nutrients, and xenobiotics with impaired clearance of waste and toxic metabolite byproducts.17,95 Therefore, primary PDACs grow and evolve within a hostile ecosystem that is nutrient-deprived, hypoxic, and toxic. Perhaps because of shared similarities with chronic pancreatitis,79 the same stress response scavenger pathways active in precursor lesions remain adaptive within the desmoplastic stroma. The extreme microenvironment also impacts treatment effectiveness. Collapsed capillaries impair delivery of systemically administered chemotherapy into the tumour mass.94,96,97 Chemotherapy that does enter the tumour is forced to compete with macrophage-secreted pyrimidines that block uptake of therapy into the malignant cells.98 Thus, the stromal microenvironment is also hostile to oncologists.
PDAC evolution: divergent morphological subclones
Primary PDAC evolves as it grows within the pancreas.66,99 This culminates in a heterogeneous mixture of phylogenetically related subclonal populations that evolved from a common ancestor (the parental clone) yet occupy geographically distinct regions of the primary tumour mass. Geographic subclones often possess divergent H&E morphologies, differentiation states, stroma content, transcriptomic profiles,100 and in some cases malignant tendencies including metastatic competence.101–103 Morphological evidence of subclonal evolution is therefore readily appreciated on routine H&E examination of well-sampled PDAC resection or autopsy specimens (Fig. 4A). Unusual morphological variants reported in the literature also represent geographic subclones. Because they each descended from the parental clone, all subclones within a primary PDAC share the same core set of genetic drivers. Additional mutations that distinguish subclones from each other are ‘passenger’ events that do not encode additional malignant properties.3,65 However, there are additional genetic drivers beyond the core set that occur at lower frequencies across patients.3 These genetic ‘backseat’ drivers encode nuanced yet selectable phenotypes that are not required for malignancy per se. They instead influence evolutionary trajectories and malignant propensities as briefly introduced below.
Fig. 4.
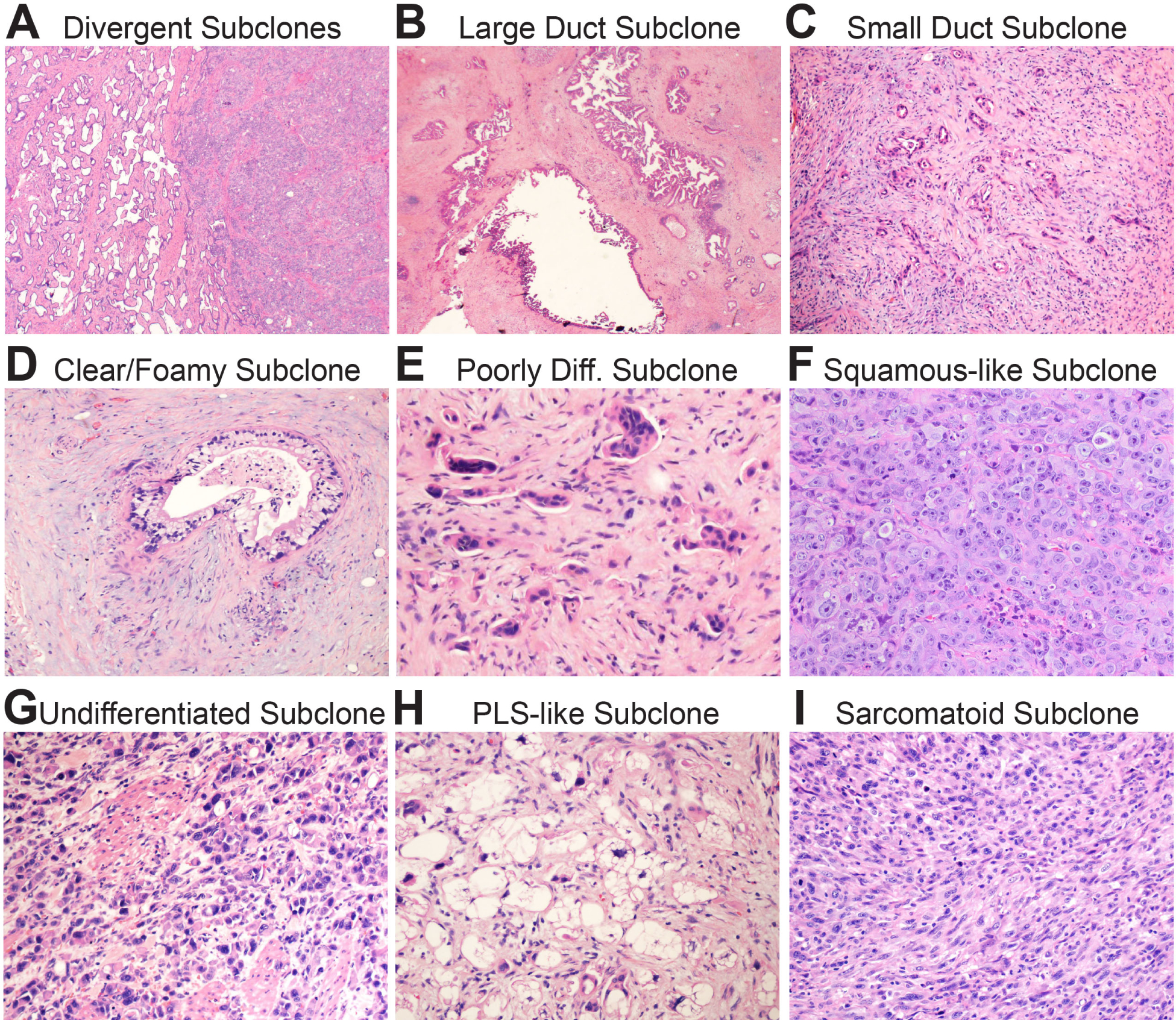
Biomorphology of subclonal evolution. (A) Low power magnification shows a well-differentiated (classic) glandular subclone on the left and a poorly differentiated squamous-like subclone on the left. Note the sharp boundary between them. (B) Low power magnification of a large duct subclone. The ducts are often as large as the main pancreatic duct. (C) Low power magnification of a small duct subclone. The ducts are so small they may be difficult to see at low power. (D) High power magnification of a clear/foamy gland subclone with characteristic pale cytoplasm and raisinoid nuclei. (E) High power magnification of a poorly differentiated subclone. Note small clusters and single cells infiltrating in the stroma. (F) High power magnification of a squamous-like subclone. The tumour in this example grows as sheets of poorly differentiated pink cells with little intervening stroma. These subclones usually express patchy p63 by immunohistochemistry. (G) High power magnification of an undifferentiated subclone. The cells are discohesive and do not form glands or nests. (F) High power magnification of a pleomorphic liposarcoma (PLS)-like subclone. This is a variant I have occasionally noticed in practice. (G) High power magnification of a sarcomatoid subclone. The overtly malignant spindle cells are tightly packed with little intervening stroma.
The morphology of the parental clone, and most well to moderately differentiated subclones,3 falls within the spectrum of classic PDAC: pale to pink appearing pancreaticobiliary glands with jagged edges and incomplete lumens that haphazardly infiltrate through a densely fibrotic or fibromyxoid stromal matrix (Fig. 3D–G). Multiple morphologically indistinguishable classic-type subclones may populate a primary tumour. These can only be distinguished by sequencing passenger mutations, although gains and losses of the genetic backseat driver GATA6 (a pancreatic developmental transcription factor) strongly influence the probability that individual subclones will maintain or diverge from the classic morphology.3,100,104 Common well-differentiated subclones with variant morphologies include large duct, small duct, and clear/foamy duct subtypes. Although these variant subclones are recurrently observed across different patients, it has been suggested that they evolve by random genetic drift rather than natural selection.105 They are important to recognise because they can mimic other processes. As the name implies, large duct subclones form very large rounded or cystic glands with open lumens106 (Fig. 4B). They can be mistaken for cystic precursors or even the main pancreatic duct, especially on frozen sections. Small duct subclones grow as tiny, angulated glands that approximate the size of terminal side branches (Fig. 4C). They can closely resemble regenerating pancreatic ducts or ADMs. Foamy and clear duct subclones form glands lined by cells with a distinctively pale and/or microvesicular cytoplasm and a folded, hyperchromatic (‘raisinoid’) nucleus107,108 (Fig. 4D). They can masquerade as benign mucinous ducts when well-differentiated108 and as neuroendocrine tumours or metastatic clear cell carcinomas when poorly differentiated.107
Poorly differentiated subclones display a variety of morphologies. In pancreatic resection specimens such subclones virtually always co-exist with other more well-differentiated subclones. Poorly differentiated glandular PDACs109 are the most common of these. Glands either fuse into vaguely cribriform structures with focal mucin production or disperse into small discohesive nests (Fig. 4E). In either case, individual cells detach from the residual glands and infiltrate the stroma as single EMT-like cells or small cell clusters.109 Squamous-like subclones (also called ‘basal-like’) are composed of poorly differentiated, overtly malignant cells growing in solid nests or sheets (Fig. 4F). The cytosol is eosinophilic with vague to overt hints of squamous differentiation. Primary PDACs with genetic backseat drivers in components of the SWI/SNF110 or COMPASS111 chromatin remodelling complexes are prone to evolve squamous-like subclones102 since these hits impart epigenetic plasticity111,112 that increases the potential for ectopic TP63 expression.111,113 Squamous-like subclones may acquire additional subclone-specific genetic backseat drivers including MYC amplifications.102 MYC activates several metabolic adaptations that increase growth, survival, and metastatic efficiency. Entosis114 is one MYC-driven adaptation that highlights the nuanced nature of backseat driver selection: malignant cells that possess MYC amplifications cannibalise those that do not and therefore outcompete them.102 Other rarely encountered subclones include undifferentiated with or without (Fig. 4G) osteoclast-like giant cells,115,116 pleomorphic liposarcoma-like (PLS-like; Fig. 4H), sarcomatoid115 (Fig. 4I), and other extremely rare variants (signet ring and hepatoid, for example).
THE BIOMORPHOLOGY OF METASTATIC PDAC
Approximately 20–30% of PDAC patients develop either non-lethal oligometastatic disease or no metastases at all.103,109 These patients typically succumb to locally advanced disease secondary to primary tumour overgrowth.109 The remaining 70–80% of patients develop widely metastatic disease.109 These patients succumb to organ failure and cachexia secondary to innumerable distant (haematogenous) metastases that diffusely involve the liver and/or lungs.109 A subset of these patients also develops metachronous peritoneal carcinomatosis,109 which refers to small metastatic tumours implanted onto the surfaces of intra-abdominal (peritonealised) organs. Primary tumours, peritoneal metastases, and distant metastases each presents a characteristic morphology that reflects unique biology as detailed below. Lymph node metastases are also extremely common in pancreatic resection specimens. Although prognostically important, they are not themselves directly lethal and are omitted from this review for brevity.
Peritoneal metastasis: a reflection of primary PDAC
Unlike distant metastases, peritoneal metastases spread by detaching off the primary tumour mass and directly seeding the outer surface of surrounding intra-abdominal organs.117 Conceptually they are best regarded as metastatic ‘implants’ that sprout on top of intra-abdominal surfaces like weeds. They are often first detected during surgical procedures as small stellate scars (Fig. 5A) that were not visible on prior imaging studies. Common target sites include the serous lining of the inner abdominal wall, intestinal serosa and attached mesentery, capsular surface of the liver, diaphragm, ovarian serosa, and omentum. The detachment and direct seeding of surfaces is reminiscent of intraductal migrations within the primary tumour. Oxygen tensions and nutrient supplies on the outer surfaces of intra-abdominal target sites118 may simulate those within the primary pancreatic tumour as well. Thus, new adaptations may not be required for the development of peritoneal carcinomatosis.101,117
Fig. 5.
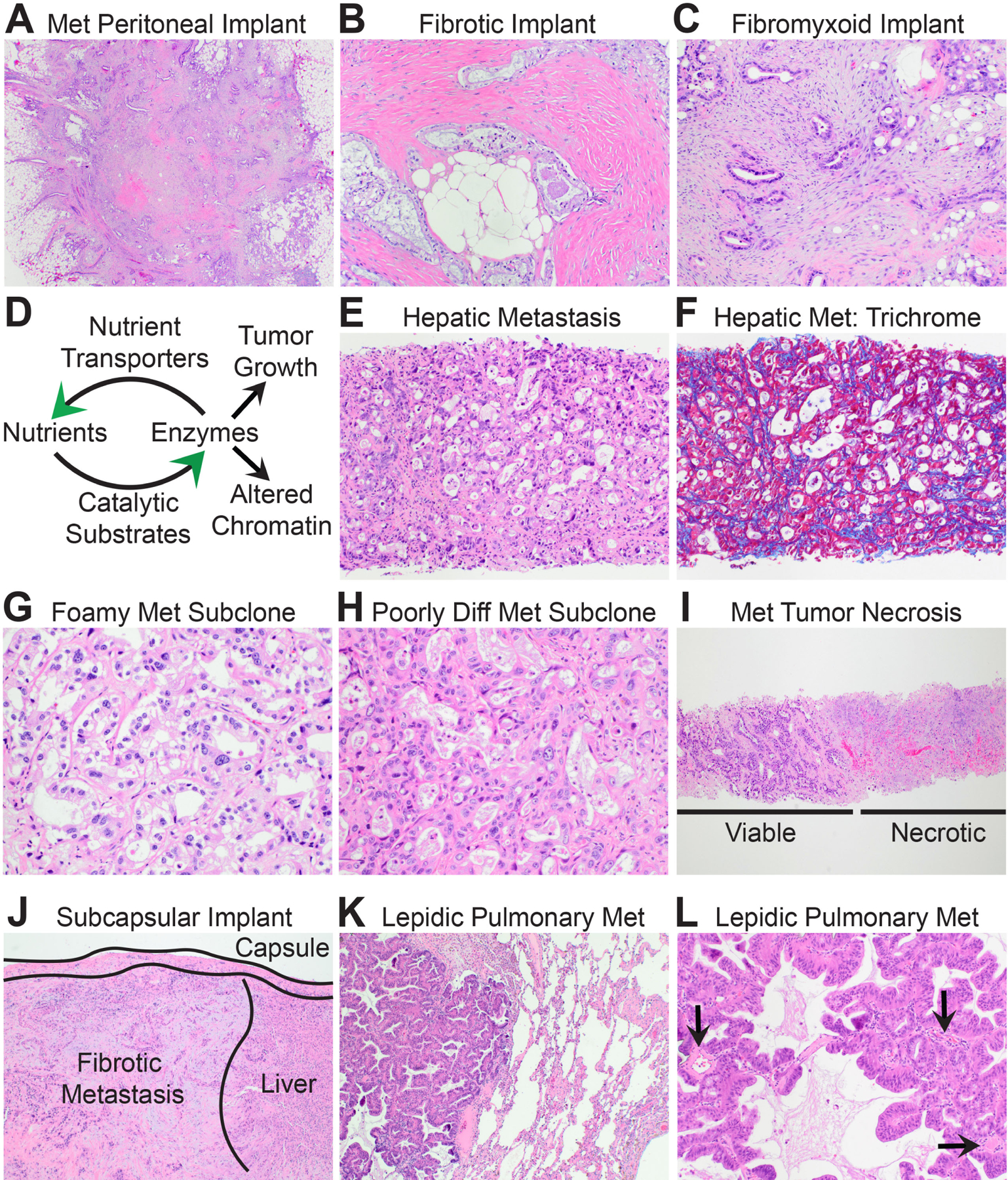
The biomorphology of metastatic PDAC. (A) Low power magnification shows that metastatic peritoneal implants resemble stellate scars. (B) Some peritoneal PDACs are encased within densely fibrotic stroma. (C) Others are encased within fibrofibromyxoid stroma. (D) Schematic illustrating positive feedback loops between nutrient transport systems and pro-tumourigenic biosynthetic enzymes that produce anabolic metabolites and/or reprogram chromatin for metastasis. (E) High power magnification of a distant metastatic PDAC with ‘biosynthetic’ morphology and an underdeveloped ‘delicate’ stroma. (F) Masson trichrome stain highlights the delicate pericellular fibrosis. (G) A liver metastasis seeded by a well-differentiated clear/foamy gland subclone forms back-to-back glands with delicate intervening stroma. (H) Likewise, a liver metastasis seeded by a poorly differentiated subclone is highly cellular with a delicate stroma. (I) In many instances, half of the core biopsy of a liver metastasis is viable tumour while the other half is necrotic. (J) Liver metastases may show well-developed fibrosis beneath the liver capsule. Such metastases are usually implants on the liver surface rather than true haematogenous metastases. (K) Low power magnification of a metastatic PDAC to the lung shows ‘lepidic’ growth within alveoli. (L) Like liver metastases, lepidic metastases develop a delicate stroma and glands are adjacent to native vessels (arrows).
Consistent with the above conjecture, the morphological, genetic, signal transduction, metabolic, and epigenetic features of primary PDACs are largely retained during peritoneal metastasis. Like primary tumours, peritoneal implants are polyclonal.119,120 They are also phylogenetically closely related to the parental clone that initiated invasive primary tumour growth.66,99,102 KRAS-driven dependencies101 and a desmoplastic stroma102,117 are accordingly retained. The implant stroma ranges from scar-like (Fig. 5B) to fibromyxoid (Fig. 5C). Our own unpublished studies indicate that the scavenger metabolic pathways likewise remain highly activated in metastatic peritoneal implants. A globally hypermethylated and condensed chromatin state is inherited from the primary101 and may contribute to the relatively small hyperchromatic nuclei seen on H&E (Fig. 5B,C). Peritoneal metastases and the primary tumour subclones that seed them both express high levels of thioredoxin-interacting protein (TXNIP).117 TXNIP is an intriguing multi-functional protein that stress response pathways employ to dampen toxic metabolite and inflammatory inputs.121 Based on the literature117,121–127 it is conceivable that TXNIP is co-opted by PDAC genetic drivers to stabilise or even accentuate adaptations that increase fitness within the desmoplastic stroma. Although much remains to be learned about the biology of peritoneal PDAC, the currently available data indicate that the biomorphology of peritoneal metastasis largely reflects that of the primary tumour.
Distant metastasis: a sharp divergence from primary PDAC
Unlike peritoneal metastasis, distant metastasis is a multi-step cascade that requires malignant cells to directly invade blood vessels, disseminate in the circulation, seed foreign soils of other organs, and achieve successful metastatic outgrowth within the parenchyma of target organs. Although the genetic drivers are largely if not completely shared between primary PDAC and distant metastases in treatment naïve patients,66,99,128 the clinical and biological behaviours diverge sharply. The primary pancreatic tumour progresses silently over a period of years.3 Clinically relevant distant metastasis presents suddenly and progresses rapidly over a period of weeks to months (the metastatic ‘boom’). Primary tumour outgrowth culminates in a solitary mass lesion that locally invades into the duodenum or other adjacent structures.109 Distant metastatic outgrowth culminates in hundreds to thousands of treatment-resistant metastatic tumours that diffusely involve the liver and/or lungs.109 The primary tumour is a heterogeneous mixture of geographically distinct subclonal populations.66,99 Distant metastases are largely monoclonal3,119 and seeded by the latest evolving subclone(s) in the primary tumour.66 Primary tumours are renowned for their dense stroma. Distant metastases do not develop a similar dense stroma in most cases.117,129,130 These collective differences raise the possibility that unique metastasis-intrinsic adaptations may arise late in disease evolution to accelerate progression.66,101,117,131–138 If so, this might explain the terminal metastatic boom that is observed in patients prior to death.
Rapidly progressive tumour growth is metabolically demanding. Furthermore, the metabolic demands of metastasis itself are very different from those that support primary tumour growth.139 Primary PDACs rely on genetically encoded pro-survival adaptations to cope with extreme hypoxia and starvation.7,10 However, as the primary pancreatic mass remodels over time, some PDAC cells will pioneer new regions where stroma is unusually loose and/or well-vascularised. This provides oxygen, nutrients, and a convenient exit into the circulation. Other PDAC cells will chance encounter regions with medium to large muscular vessels that resist stromal collapse. These tumour cells can then directly invade into those vessels and colonise the intravascular lumens.62 Indeed, large (3–6 cm) primary tumours are often observed invading directly into the peripancreatic vessels when PDAC is first detected by imaging. PDAC cells that enter the hematogenous circulation disseminate into the portal venous system that drains partially oxygenated blood containing freshly digested nutrients from the intestines into the liver. Tumour cells that successfully seed the hepatic parenchyma along this route initially lodge within hepatic sinusoids where they temporarily cease to proliferate, quietly evade immune surveillance,140 and bathe in nutrient-replete blood. Thus, PDAC cells that successfully sprout distant metastatic tumours are exposed to nutrient-replete soils for some time prior to the metastatic boom observed in patients.
Because PDACs are genetically programmed for the nutrient-poor conditions of the primary tumour,7,14 malignant cells that pioneer nutrient-replete microenvironments must evolve new adaptations to convert the newly available nutrients into metabolites that fuel the metastatic boom. Glucose is a highly anabolic and pro-tumourigenic nutrient that is depleted in the primary tumour7,17,95 yet replete along hematogenous routes. Widely metastatic PDACs accordingly evolve unique metabolic adaptations117,132 that allow them to consume excessive amounts of glucose.117 The excess glucose is used to fuel biosynthetic enzymes101,117,132 that convert glucose-derived substrates into anabolic metabolites that support rapid tumour growth101 (Fig. 5D). In PDAC patients who develop widely metastatic disease the biomorphology of distant metastasis strongly reflects these unique adaptations. Because of this, the histological features of distant metastasis are morphologically distinct from primary and peritoneal PDACs, including depletion of the hallmark desmoplastic stroma, higher tumour cellularity, and an overtly malignant ‘biosynthetic’ cytology,102,117,129,130 as briefly described below.
The most common metastatic PDAC specimens in routine practice are core needle biopsies of liver lesions. The histology is often biphasic: one edge of the tissue core is viable (tumour periphery) while the other is necrotic (tumour core). The viable edge displays high cellularity with closely approximated glands separated by a delicate septal or pericellular ‘chicken wire’ pattern of fibrosis (Fig. 5E,F) that is reminiscent of steatohepatitis instead of primary tumour desmoplasia. The delicate fibrotic matrix is often invested with an open microvascular network as required for sustained delivery of glucose and other nutrients. Viable metastatic cells accordingly appear biosynthetic with plump cytoplasm (organelle expansion) and prominent nucleoli (protein synthesis) irrespective of differentiation state (Fig. 5G,H). The massive influx of glucose also generates metabolite byproducts (acetyl groups for example) that are deposited onto chromatin. These metabolites reprogram the PDAC epigenome into a globally ‘open’ chromatin state101,117,141 that is permissive for activation of the metastatic transcriptome.101,133 This manifests as enlarged irregular nuclei with pale to microvesicular chromatin containing visible chromocentres (Fig. 5G,H). Collectively, these unique ‘metaboloepigenetic’ adaptations may synergise with pre-existing genetic drivers23,101,117 and systemic immune conditions140 to ignite and/or fuel the metastatic boom.
If the needle samples the interior (core) of a hepatic distant metastasis, the edge opposite viable tumour is often necrotic (Fig. 5I). Central necrosis may result from overconsumption and/or exhaustion of nutrient supplies within the interior of the lesion as metastatic outgrowth rapidly expands in three dimensions. Such necrosis is far less common in primary tumours and virtually never seen in metastatic peritoneal implants. Necrotic regions within metastases may remodel over time if nutrients and growth rates decline142 prior to patient death. This is probably the case for the minority of hepatic distant metastases that appear densely fibrotic,143 although samplings of peritoneal implants on the capsular surface of the liver102,117 (Fig. 5J) and stochastic oligometastatic disease103,143 are other rare situations when dense fibrosis is encountered in liver metastases. Pulmonary metastases are less commonly encountered in practice but may resemble biosynthetic hepatic metastases in some cases. In others, they display a unique form of ‘lepidic’ growth within alveoli that mimics a primary bronchoalveolar carcinoma.144 The biology of lepidic metastasis is not well understood. Outgrowth probably also occurs under nutrient-replete conditions since the metastatic cells anchor along well-vascularised alveolar surfaces (Fig. 5K,L).
CONCLUSION
PDAC morphology progresses in step with the evolutionary biology of this disease. Epithelial cells that incidentally acquire oncogenic KRAS mutations during pancreatic injury gain fitness advantages under fibroinflammatory conditions. These cells may clonally expand to form LGD precursor lesions that self-sustain by encasing themselves within a rim of localised ‘pseudo pancreatitis’. Some precursors acquire the remaining core set of PDAC genetic drivers that trigger loss of cell cycle checkpoints, (epi)genomic instability, intraductal migratory capacity, and HGD. Clonal sweeps from HGD lesions reduce genetic heterogeneity and increase the probability of malignant transformation. Once the parental clone invades into the desmoplastic stroma the primary tumour slowly enlarges into a sizable mass lesion over a period of years. Stochastic tumour:stroma interactions remodel different geographic regions of the mass into independent ecosystems. A combination of genetic drift and natural selection within these different ecosystems allows the tumour to evolve, culminating in geographically divergent morphologic subclones. Subclones confined to dense stroma are genetically well-adapted and either grow relentlessly within the pancreas or detach and directly seed intra-abdominal surfaces (peritoneal carcinomatosis). Occasionally an ecosystem remodels into unusual terrain such as loosely fibrotic stroma with accessible vascular access. The unusual terrain presents new selective pressures that drive subclonal expansion of pioneering PDAC cells with genetic backseat drivers, unique metabolic adaptations, and immunosuppressive abilities that increase fitness and enhance metastatic efficiency. Once all adaptations are fully installed and operational, a widely metastatic boom occurs. The boom may occur in one of two ways: rapid metastatic outgrowth of many individual cells seeded from the same primary tumour subclone or rapid metastatic re-seeding by a highly fit founder cell(s) resulting in a clonal sweep within metastatic target organs. Either way, metastatic outgrowth is driven by an overtly malignant biosynthetic subclonal population that breaches the dense stromal barriers of the primary tumour. Rapid metastatic outgrowth requires fuel. Stroma-poor distant metastases gain access to nutrient-replete reservoirs that are harnessed to fuel their biosynthetic malignant traits. The central necrosis observed on core needle biopsies further implies that glutinous distant metastases are reluctant to acutely reactivate their genetically encoded scavenging defaults as they overconsume the natural resources of metastatic habitats.
Conflicts of interest and sources of funding:
This work was supported by National Institutes of Health grant R01 CA222594. The author declares no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res 2000; 6: 2969–72. [PubMed] [Google Scholar]
- 2.Guerra C, Schuhmacher AJ, Cañamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 2007; 11: 291–302. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi A, Hong J, Iacobuzio-Donahue CA. The pancreatic cancer genome revisited. Nat Rev Gastroenterol Hepatol 2021; 18: 469–81. [DOI] [PubMed] [Google Scholar]
- 4.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst 1997; 89: 442–6. [DOI] [PubMed] [Google Scholar]
- 5.Hruban RH, van Mansfeld AD, Offerhaus GJ, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol 1993; 143: 545–54. [PMC free article] [PubMed] [Google Scholar]
- 6.Ying H, Dey P, Yao W, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 2016; 30: 355–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Encarnación-Rosado J, Kimmelman AC. Harnessing metabolic dependencies in pancreatic cancers. Nat Rev Gastroenterol Hepatol 2021; 18: 482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strobel O, Dor Y, Alsina J, et al. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology 2007; 133: 1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichert M, Rustgi AK. Pancreatic ductal cells in development, regeneration, and neoplasia. J Clin Invest 2011; 121: 4572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halbrook CJ, Lyssiotis CA. Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell 2017; 31: 5–19. [DOI] [PubMed] [Google Scholar]
- 11.Shi C, Pan FC, Kim JN, et al. Differential cell susceptibilities to Kras(G12D) in the setting of obstructive chronic pancreatitis. Cell Mol Gastroenterol Hepatol 2019; 8: 579–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habbe N, Shi G, Meguid RA, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci USA 2008; 105: 18913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liou GY, Döppler H, DelGiorno KE, et al. Mutant KRas-induced mitochondrial oxidative stress in acinar cells upregulates EGFR signaling to drive formation of pancreatic precancerous lesions. Cell Rep 2016; 14: 2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerk SA, Papagiannakopoulos T, Shah YM, Lyssiotis CA. Metabolic networks in mutant KRAS-driven tumours: tissue specificities and the microenvironment. Nat Rev Cancer 2021; 21: 510–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auciello FR, Bulusu V, Oon C, et al. A stromal lysolipid-autotaxin signaling axis promotes pancreatic tumor progression. Cancer Discov 2019; 9: 617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Commisso C, Davidson SM, Soydaner-Azeloglu RG, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013; 497: 633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamphorst JJ, Nofal M, Commisso C, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res 2015; 75: 544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamphorst JJ, Cross JR, Fan J, Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci USA 2013; 110: 8882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang S, Wang X, Contino G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev 2011; 25: 717–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sousa CM, Biancur DE, Wang X, et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 2016; 536: 479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Son J, Lyssiotis CA, Ying H, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013; 496: 101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying H, Kimmelman AC, Lyssiotis CA, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012; 149: 656–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrer A, Trefely S, Zhao S, et al. Acetyl-CoA metabolism supports multistep pancreatic tumorigenesis. Cancer Discov 2019; 9: 416–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollinshead KER, Parker SJ, Eapen VV, et al. Respiratory supercomplexes promote mitochondrial efficiency and growth in severely hypoxic pancreatic cancer. Cell Rep 2020; 33: 108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto K, Venida A, Yano J, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 2020; 581: 100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ischenko I, D’Amico S, Rao M, et al. KRAS drives immune evasion in a genetic model of pancreatic cancer. Nat Commun 2021; 12: 1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 2012; 21: 836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayne LJ, Beatty GL, Jhala N, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 2012; 21: 822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathew E, Collins MA, Fernandez-Barrena MG, et al. The transcription factor GLI1 modulates the inflammatory response during pancreatic tissue remodeling. J Biol Chem 2014; 289: 27727–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Velez-Delgado A, Mathew E, et al. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut 2017; 66: 124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gidekel Friedlander SY, Chu GC, Snyder EL, et al. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell 2009; 16: 379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flowers BM, Xu H, Mulligan AS, et al. Cell of origin influences pancreatic cancer subtype. Cancer Discov 2021; 11: 660–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ideno N, Yamaguchi H, Ghosh B, et al. GNAS(R201C) induces pancreatic cystic neoplasms in mice that express activated KRAS by inhibiting YAP1 signaling. Gastroenterology 2018; 155: 1593–607.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patra KC, Kato Y, Mizukami Y, et al. Mutant GNAS drives pancreatic tumourigenesis by inducing PKA-mediated SIK suppression and reprogramming lipid metabolism. Nat Cell Biol 2018; 20: 811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noë M, Niknafs N, Fischer CG, et al. Genomic characterization of malignant progression in neoplastic pancreatic cysts. Nat Commun 2020; 11: 4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang L, Desai R, Conrad DN, Commitment and oncogene-induced plasticity of human stem cell-derived pancreatic acinar and ductal organoids. Cell Stem Cell 2021; 28: 1090–104.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill W, Zaragkoulias A, Salvador-Barbero B, et al. EPHA2-dependent outcompetition of KRASG12D mutant cells by wild-type neighbors in the adult pancreas. Curr Biol 2021; 31: 2550–60.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McAllister F, Bailey JM, Alsina J, et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 2014; 25: 621–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alonso-Curbelo D, Ho YJ, Burdziak C, et al. A gene-environment-induced epigenetic program initiates tumorigenesis. Nature 2021; 590: 642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tape CJ, Ling S, Dimitriadi M, et al. Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell 2016; 165: 910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tape CJ, Ling S, Dimitriadi M, et al. Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell 2016; 165: 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basturk O, Hong SM, Wood LD, et al. A revised classification system and recommendations from the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol 2015; 39: 1730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuda Y, Furukawa T, Yachida S, et al. The prevalence and clinicopathological characteristics of high-grade pancreatic intraepithelial neoplasia: autopsy study evaluating the entire pancreatic parenchyma. Pancreas 2017; 46: 658–64. [DOI] [PubMed] [Google Scholar]
- 44.Notta F, Chan-Seng-Yue M, Lemire M, et al. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature 2016; 538: 378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schutte M, Hruban RH, Geradts J, et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res 1997; 57: 3126–30. [PubMed] [Google Scholar]
- 46.Caldas C, Hahn SA, da Costa LT, et al. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet 1994; 8: 27–32. [DOI] [PubMed] [Google Scholar]
- 47.Redston MS, Caldas C, Seymour AB, et al. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res 1994; 54: 3025–33. [PubMed] [Google Scholar]
- 48.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 1996; 271: 350–3. [DOI] [PubMed] [Google Scholar]
- 49.Siolas D, Vucic E, Kurz E, Hajdu C, Bar-Sagi D. Gain-of-function p53(R172H) mutation drives accumulation of neutrophils in pancreatic tumors, promoting resistance to immunotherapy. Cell Rep 2021; 36: 109578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.David CJ, Huang YH, Chen M, et al. TGF-β tumor suppression through a lethal EMT. Cell 2016; 164: 1015–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang YH, Hu J, Chen F, et al. ID1 mediates escape from TGFβ tumor suppression in pancreatic cancer. Cancer Discov 2020; 10: 142–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makohon-Moore AP, Matsukuma K, Zhang M, et al. Precancerous neoplastic cells can move through the pancreatic ductal system. Nature 2018; 561: 201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein WM, Hruban RH, Klein-Szanto AJ, Wilentz RE. Direct correlation between proliferative activity and dysplasia in pancreatic intraepithelial neoplasia (PanIN): additional evidence for a recently proposed model of progression. Mod Pathol 2002; 15: 441–7. [DOI] [PubMed] [Google Scholar]
- 54.Koorstra JB, Hong SM, Shi C, Widespread activation of the DNA damage response in human pancreatic intraepithelial neoplasia. Mod Pathol 2009; 22: 1439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hata T, Suenaga M, Marchionni L, et al. Genome-wide somatic copy number alterations and mutations in high-grade pancreatic intraepithelial neoplasia. Am J Pathol 2018; 188: 1723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morris JPt, Yashinskie JJ, Koche R, et al. α-Ketoglutarate links p53 to cell fate during tumour suppression. Nature 2019; 573: 595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hosoda W, Chianchiano P, Griffin JF, et al. Genetic analyses of isolated high-grade pancreatic intraepithelial neoplasia (HG-PanIN) reveal paucity of alterations in TP53 and SMAD4. J Pathol 2017; 242: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilentz RE, Iacobuzio-Donahue CA, Argani P, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res 2000; 60: 2002–6. [PubMed] [Google Scholar]
- 59.Pea A, Yu J, Rezaee N, et al. Targeted DNA sequencing reveals patterns of local progression in the pancreatic remnant following resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg 2017; 266: 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutchings D, Waters KM, Weiss MJ, et al. Cancerization of the pancreatic ducts: demonstration of a common and under-recognized process using immunolabeling of paired duct lesions and invasive pancreatic ductal adenocarcinoma for p53 and Smad4 expression. Am J Surg Pathol 2018; 42: 1556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adsay V, Mino-Kenudson M, Furukawa T, et al. Pathologic evaluation and reporting of intraductal papillary mucinous neoplasms of the pancreas and other tumoral intraepithelial neoplasms of pancreatobiliary tract: recommendations of Verona Consensus Meeting. Ann Surg 2016; 263: 162–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong SM, Goggins M, Wolfgang CL, et al. Vascular invasion in infiltrating ductal adenocarcinoma of the pancreas can mimic pancreatic intraepithelial neoplasia: a histopathologic study of 209 cases. Am J Surg Pathol 2012; 36: 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujikura K, Hosoda W, Felsenstein M, et al. Multiregion whole-exome sequencing of intraductal papillary mucinous neoplasms reveals frequent somatic KLF4 mutations predominantly in low-grade regions. Gut 2021; 70: 928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fischer CG, Beleva Guthrie V, Braxton AM, et al. Intraductal papillary mucinous neoplasms arise from multiple independent clones, each with distinct mutations. Gastroenterology 2019; 157: 1123–37.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reiter JG, Baretti M, Gerold JM, et al. An analysis of genetic heterogeneity in untreated cancers. Nat Rev Cancer 2019; 19: 639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010; 467: 1114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lennon AM, Wolfgang CL, Canto MI, et al. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res 2014; 74: 3381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suenaga M, Yu J, Shindo K, et al. Pancreatic juice mutation concentrations can help predict the grade of dysplasia in patients undergoing pancreatic surveillance. Clin Cancer Res 2018; 24: 2963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014; 6: 224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singhi AD, Wood LD. Early detection of pancreatic cancer using DNA-based molecular approaches. Nat Rev Gastroenterol Hepatol 2021; 18: 457–68. [DOI] [PubMed] [Google Scholar]
- 71.Goggins M, Overbeek KA, Brand R, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020; 69: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aslanian HR, Lee JH, Canto MI. AGA clinical practice update on pancreas cancer screening in high-risk individuals: expert review. Gastroenterology 2020; 159: 358–62. [DOI] [PubMed] [Google Scholar]
- 73.Dbouk M, Brewer Gutierrez OI, Lennon AM, et al. Guidelines on management of pancreatic cysts detected in high-risk individuals: an evaluation of the 2017 Fukuoka guidelines and the 2020 International Cancer of the Pancreas Screening (CAPS) consortium statements. Pancreatology 2021; 21: 613–21. [DOI] [PubMed] [Google Scholar]
- 74.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012; 491: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sinha S, Fu YY, Grimont A, et al. PanIN neuroendocrine cells promote tumorigenesis via neuronal cross-talk. Cancer Res 2017; 77: 1868–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Banh RS, Biancur DE, Yamamoto K, et al. Neurons release serine to support mRNA translation in pancreatic cancer. Cell 2020; 183: 1202–18.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest 2007; 117: 50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dougan SK. The pancreatic cancer microenvironment. Cancer J 2017; 23: 321–5. [DOI] [PubMed] [Google Scholar]
- 79.Binkley CE, Zhang L, Greenson JK, et al. The molecular basis of pancreatic fibrosis: common stromal gene expression in chronic pancreatitis and pancreatic adenocarcinoma. Pancreas 2004; 29: 254–63. [DOI] [PubMed] [Google Scholar]
- 80.Engle DD, Tiriac H, Rivera KD, et al. The glycan CA19–9 promotes pancreatitis and pancreatic cancer in mice. Science 2019; 364: 1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Öhlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017; 214: 579–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Biffi G, Oni TE, Spielman B, et al. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov 2019; 9: 282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014; 25: 735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2015; 28: 831–3. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y, Kim J, Yang S, et al. Type I collagen deletion in αSMA(+) myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 2021; 39: 548–65.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mahlbacher V, Sewing A, Elsässer HP, Kern HF. Hyaluronan is a secretory product of human pancreatic adenocarcinoma cells. Eur J Cell Biol 1992; 58: 28–34. [PubMed] [Google Scholar]
- 87.Öhlund D, Franklin O, Lundberg E, Lundin C, Sund M. Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop. BMC Cancer 2013; 13: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laklai H, Miroshnikova YA, Pickup MW, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med 2016; 22: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Steele CW, Karim SA, Leach JDG, et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 2016; 29: 832–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Freed-Pastor WA, Lambert LJ, Ely ZA, et al. The CD155/TIGIT axis promotes and maintains immune evasion in neoantigen-expressing pancreatic cancer. Cancer Cell 2021; 39: 1342–60.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balachandran VP, Beatty GL, Dougan SK. Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology 2019; 156: 2056–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bachem MG, Schünemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 2005; 128: 907–21. [DOI] [PubMed] [Google Scholar]
- 93.Armstrong T, Packham G, Murphy LB, et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res 2004; 10: 7427–37. [DOI] [PubMed] [Google Scholar]
- 94.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012; 21: 418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sullivan MR, Danai LV, Lewis CA, et al. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. Elife 2019; 8: e44235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009; 324: 1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014; 159: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Halbrook CJ, Pontious C, Kovalenko I, et al. Macrophage-released pyrimidines inhibit gemcitabine therapy in pancreatic cancer. Cell Metab 2019; 29: 1390–9.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Makohon-Moore AP, Zhang M, Reiter JG, et al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat Genet 2017; 49: 358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Collisson EA, Bailey P, Chang DK, Biankin AV. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol 2019; 16: 207–20. [DOI] [PubMed] [Google Scholar]
- 101.McDonald OG, Li X, Saunders T, et al. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat Genet 2017; 49: 367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hayashi A, Fan J, Chen R, et al. A unifying paradigm for transcriptional heterogeneity and squamous features in pancreatic ductal adenocarcinoma. Nat Cancer 2020; 1: 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iacobuzio-Donahue CA, Litchfield K, Swanton C. Intratumor heterogeneity reflects clinical disease course. Nat Cancer 2020; 1: 3–6. [DOI] [PubMed] [Google Scholar]
- 104.Brunton H, Caligiuri G, Cunningham R, et al. HNF4A and GATA6 loss reveals therapeutically actionable subtypes in pancreatic cancer. Cell Rep 2020; 31: 107625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Noë M, Hong SM, Wood LD, et al. Pancreatic cancer pathology viewed in the light of evolution. Cancer Metastasis Rev 2021; 40: 661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bagci P, Andea AA, Basturk O, Jang KT, Erbarut I, Adsay V. Large duct type invasive adenocarcinoma of the pancreas with microcystic and papillary patterns: a potential microscopic mimic of non-invasive ductal neoplasia. Mod Pathol 2012; 25: 439–48. [DOI] [PubMed] [Google Scholar]
- 107.Kim L, Liao J, Zhang M, et al. Clear cell carcinoma of the pancreas: histopathologic features and a unique biomarker: hepatocyte nuclear factor-1β. Mod Pathol 2008; 21: 1075–83. [DOI] [PubMed] [Google Scholar]
- 108.Adsay V, Logani S, Sarkar F, Crissman J, Vaitkevicius V. Foamy gland pattern of pancreatic ductal adenocarcinoma: a deceptively benign-appearing variant. Am J Surg Pathol 2000; 24: 493–504. [DOI] [PubMed] [Google Scholar]
- 109.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009; 27: 1806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shain AH, Giacomini CP, Matsukuma K, et al. Convergent structural alterations define SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeler as a central tumor suppressive complex in pancreatic cancer. Proc Natl Acad Sci USA 2012; 109: E252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andricovich J, Perkail S, Kai Y, Casasanta N, Peng W, Tzatsos A. Loss of KDM6A activates super-enhancers to induce gender-specific squamous-like pancreatic cancer and confers sensitivity to BET inhibitors. Cancer Cell 2018; 33: 512–26.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miyabayashi K, Baker LA, Deschênes A, et al. Intraductal transplantation models of human pancreatic ductal adenocarcinoma reveal progressive transition of molecular subtypes. Cancer Discov 2020; 10: 1566–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Somerville TDD, Xu Y, Miyabayashi K, et al. TP63-mediated enhancer reprogramming drives the squamous subtype of pancreatic ductal adenocarcinoma. Cell Rep 2018; 25: 1741–55.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hayashi A, Yavas A, McIntyre CA, et al. Genetic and clinical correlates of entosis in pancreatic ductal adenocarcinoma. Mod Pathol 2020; 33: 1822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alguacil-Garcia A, Weiland LH. The histologic spectrum, prognosis, and histogenesis of the sarcomatoid carcinoma of the pancreas. Cancer 1977; 39: 1181–9. [DOI] [PubMed] [Google Scholar]
- 116.Westra WH, Sturm P, Drillenburg P, et al. K-ras oncogene mutations in osteoclast-like giant cell tumors of the pancreas and liver: genetic evidence to support origin from the duct epithelium. Am J Surg Pathol 1998; 22: 1247–54. [DOI] [PubMed] [Google Scholar]
- 117.Bechard ME, Smalling R, Hayashi A, et al. Pancreatic cancers suppress negative feedback of glucose transport to reprogram chromatin for metastasis. Nat Commun 2020; 11: 4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blessing AM, Santiago-O’Farrill JM, Mao W, et al. Elimination of dormant, autophagic ovarian cancer cells and xenografts through enhanced sensitivity to anaplastic lymphoma kinase inhibition. Cancer 2020; 126: 3579–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maddipati R, Stanger BZ. Pancreatic cancer metastases harbor evidence of polyclonality. Cancer Discov 2015; 5: 1086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McPherson A, Roth A, Laks E, et al. Divergent modes of clonal spread and intraperitoneal mixing in high-grade serous ovarian cancer. Nat Genet 2016; 48: 758–67. [DOI] [PubMed] [Google Scholar]
- 121.Wu N, Zheng B, Shaywitz A, et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell 2013; 49: 1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sullivan WJ, Mullen PJ, Schmid EW, et al. Extracellular matrix remodeling regulates glucose metabolism through TXNIP destabilization. Cell 2018, 175:117–32.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wilde BR, Ye Z, Lim TY, Ayer DE. Cellular acidosis triggers human MondoA transcriptional activity by driving mitochondrial ATP production. Elife 2019; 8: e40199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lerner AG, Upton JP, Praveen PV, et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab 2012; 16: 250–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oslowski CM, Hara T, O’Sullivan-Murphy B, et al. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab 2012; 16: 265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 2010; 11: 136–40. [DOI] [PubMed] [Google Scholar]
- 127.Nishiyama A, Matsui M, Iwata S, et al. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem 1999; 274: 21645–50. [DOI] [PubMed] [Google Scholar]
- 128.Reiter JG, Makohon-Moore AP, Gerold JM, et al. Minimal functional driver gene heterogeneity among untreated metastases. Science 2018; 361: 1033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Torphy RJ, Wang Z, True-Yasaki A, et al. Stromal content is correlated with tissue site, contrast retention, and survival in pancreatic adenocarcinoma. JCO Precis Oncol 2018; 2018: PO.17.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiang H, Torphy RJ, Steiger K, et al. Pancreatic ductal adenocarcinoma progression is restrained by stromal matrix. J Clin Invest 2020; 130: 4704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McDonald OG. Cancer metastasis: selectable traits without genetic constraints. Mol Cell Oncol 2020; 7: 1825910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bechard ME, Word AE, Tran AV, Liu X, Locasale JW, McDonald OG. Pentose conversions support the tumorigenesis of pancreatic cancer distant metastases. Oncogene 2018; 37: 5248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roe JS, Hwang CI, Somerville TDD, et al. Enhancer reprogramming promotes pancreatic cancer metastasis. Cell 2017; 170: 875–88.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Whittle MC, Izeradjene K, Rani PG, et al. RUNX3 controls a metastatic switch in pancreatic ductal adenocarcinoma. Cell 2015; 161: 1345–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cheung EC, DeNicola GM, Nixon C, et al. Dynamic ROS control by TIGAR regulates the initiation and progression of pancreatic cancer. Cancer Cell 2020; 37: 168–82.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jian Z, Cheng T, Zhang Z, et al. Glycemic variability promotes both local invasion and metastatic colonization by pancreatic ductal adenocarcinoma. Cell Mol Gastroenterol Hepatol 2018; 6: 429–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chiou SH, Risca VI, Wang GX, et al. BLIMP1 induces transient metastatic heterogeneity in pancreatic cancer. Cancer Discov 2017; 7: 1184–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Recouvreux MV, Moldenhauer MR, Galenkamp KMO, et al. Glutamine depletion regulates Slug to promote EMT and metastasis in pancreatic cancer. J Exp Med 2020; 217: e20200388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bergers G, Fendt SM. The metabolism of cancer cells during metastasis. Nat Rev Cancer 2021; 21: 162–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pommier A, Anaparthy N, Memos N, et al. Unresolved endoplasmic reticulum stress engenders immune-resistant, latent pancreatic cancer metastases. Science 2018; 360: eaao4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sherman MH, Yu RT, Tseng TW, et al. Stromal cues regulate the pancreatic cancer epigenome and metabolome. Proc Natl Acad Sci USA 2017; 114: 1129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aiello NM, Bajor DL, Norgard RJ, et al. Metastatic progression is associated with dynamic changes in the local microenvironment. Nat Commun 2016; 7: 12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Whatcott CJ, Diep CH, Jiang P, et al. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin Cancer Res 2015; 21: 3561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McDonald OG, Maitra A, Hruban RH. Human correlates of provocative questions in pancreatic pathology. Adv Anat Pathol 2012; 19: 351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


