Abstract
Background
Psychological well-being and quality of life (QoL) are important outcomes of lifestyle interventions, as a positive impact may favour long-term maintenance of behaviour change.
Objective
This study investigated the effect of a behavioural intervention for adopting and maintaining an active lifestyle on psychological well-being and health-related QoL in individuals with type 2 diabetes.
Methods
Three hundred physically inactive and sedentary patients were randomized 1:1 to receive 1 month’s theoretical and practical counselling once a year (intervention group, INT) or standard care (control group, CON) for 3 years. Psychological well-being and QoL, assessed using the World Health Organization (WHO)-5 and the 36-Item Short Form (SF-36) questionnaire, respectively, were pre-specified secondary endpoints. The primary endpoint was sustained behaviour change, as assessed by accelerometer-based measurement of physical activity (PA) and sedentary time.
Results
WHO-5 and SF-36 physical and mental component summary (PCS and MCS) scores increased progressively in the INT group and decreased in the CON group, resulting in significant between-group differences (WHO-5: mean difference 7.35 (95% confidence interval (CI) 3.15–11.55), P = 0.0007; PCS 4.20 (95% CI 2.25–6.15), P < 0.0001; MCS 3.04 (95% CI 1.09–4.99), P = 0.0025). Percentage of participants with likely depression decreased in the INT group and increased in the CON group. PA volume changes were independently associated with WHO-5 changes, which were significantly higher in participants who accumulated > 150 min·wk−1 of moderate-to-vigorous intensity PA versus those who did not (13.06 (95% CI 7.51–18.61), P < 0.0001), whereas no relationship was detected for QoL.
Conclusion
A counselling intervention that was effective in promoting a sustained change in PA and sedentary behaviour significantly improved psychological well-being and QoL.
Trial Registration
ClinicalTrials.gov; NCT01600937; 10 October 2012.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40279-021-01556-0.
Key Points
| In this randomized clinical trial that included 300 patients, an intervention that was effective in promoting a sustained change in physical activity and sedentary behaviour significantly improved scores of psychological well-being and physical and mental components of health-related quality of life. |
| The percentage of participants with (likely) depression decreased in the intervention group and increased in the control group. |
| Changes in psychological well-being and prevalence of depression, but not those in quality of life, were related to changes in physical activity volume. |
Introduction
Diabetes is known to be associated with depression, anxiety and other psychological distress or pathology as well as with reduced health-related quality of life (QoL). An average twofold increased prevalence of depression has in fact been reported in people with type 1 diabetes (T1D) and type 2 diabetes (T2D), based on either clinical diagnosis or self-report of depressive symptoms [1, 2]. Longitudinal prospective population-based studies have shown that this detrimental association is bi-directional. On the one hand, depression was found to confer an increased risk of incident diabetes [3, 4] by inducing diabetogenic health behaviours and possibly triggering common biological pathways [5], including increased counter-regulatory hormone release and action, alterations in glucose transport, and enhanced immunoinflammatory activation [6]. On the other hand, diabetes was shown to favour the development of depression [4], at least partly because of the psychological distress due to the burden of living with a demanding chronic disease and its disabling complications [7]. In addition, both diabetes and depression are associated to worse health scores [8] and reduced health-related QoL [9].
The dangerous association of depression (or depressive symptoms) and reduced QoL with diabetes is negatively linked to disease self-management through decreased compliance with diabetes monitoring and treatment [10, 11], ultimately resulting in poor glycaemic control [12] and increased likelihood to develop long-term diabetic complications [13]. This, in turn, may adversely affect psychological health [14, 15] and QoL [16], thus generating a vicious cycle resulting in worse disease outcomes. However, the relationship with diabetes self-management can also be the other way around, as recommended actions (e.g., glucose monitoring, insulin injections, exercise) and restrictions (e.g., dietary, sitting) may generate either negative or positive emotions that would limit or favour, respectively, adherence to disease monitoring and treatment, which is a critical issue in people with T2D [17] and involves all aspects, including compliance with physical activity (PA)/exercise and sedentary behaviour recommendations [18].
Two meta-analyses that investigated the effect of (mainly supervised) training programs of aerobic, resistance or combined exercise on QoL, symptoms of depression and anxiety, and emotional well-being found mixed results [19, 20]. However, in the Italian Diabetes and Exercise Study (IDES), we reported that physical and mental QoL scores improved with supervised aerobic and resistance exercise training [21] in a volume-dependent manner [22]. Compared to supervised exercise programs, counselling interventions targeting both PA/exercise and sedentary time (SED-time) may be more suitable for promoting a sustained behaviour change in T2D patients. Unfortunately, there are no data on whether these interventions are effective in improving well-being and QoL, thus favouring long-term maintenance of behaviour change.
In the IDES_2, we showed that a behavioural intervention compared with standard care resulted in a sustained increase in PA and decrease in SED-time over 3 years in individuals with T2D, associated with improvements in physical fitness and cardiovascular risk profile [23]. Here, we report on the effect of the intervention on psychological well-being and health-related QoL, which was a pre-specified secondary endpoint of this trial.
Methods
The design and methods have been detailed elsewhere [23, 24] and are briefly reported here.
Design
The IDES_2 was an open-label, assessor-blinded, parallel, superiority randomized clinical trial that assessed the efficacy of a behavioural intervention strategy in increasing PA and reducing SED-time in patients with T2D.
Patients
The main entry criterion was T2D of ≥ 1-year duration. Additional requirements were: age 40–80 years; body mass index (BMI) 27–40 kg/m2; physical inactivity and sedentary lifestyle for at least 6 months; ability to walk 1.6 km without assistance; and eligibility after cardiologic evaluation.
Randomization and Blinding
In three tertiary referral outpatient Diabetes Clinics in Rome, patients were randomized 1:1 to either an intervention (INT) group, receiving theoretical and practical exercise counselling, or a control (CON) group, receiving only general physician recommendations for increasing daily PA and decreasing SED-time.
Randomization was stratified by centre and, within each centre, by age (< 65 vs. ≥ 65 years) and diabetes treatment (non-insulin vs. insulin), using a permuted-block randomization SAS 9.4 software that randomly varies the block size (range 4–8).
Patients from both groups received the same treatment regimen, including dietary prescription, to achieve glycaemic, lipid, blood pressure (BP) and body-weight targets, according to current guidelines. Treatment was adjusted at each visit using a pre-specified algorithm.
Physicians, exercise specialists, and participants were not blinded, whereas assessors of PA/SED-time, biochemical parameters and well-being/QoL were blinded to group assignment.
Intervention
The intervention in the INT group consisted of one individual theoretical counselling session, held by a diabetologist, plus eight twice-weekly individual theoretical and practical counselling sessions, held by a certified exercise specialist, once a year for 3 years (i.e., at months 1, 13 and 25).
Outcome Measures
Co-primary endpoints were changes from baseline in total PA volume (metabolic equivalents (METs) h·wk−1), time spent in light-intensity PA (LPA, h·day−1) and moderate-to-vigorous-intensity PA (MVPA, min·day−1), and SED-time (h·day−1) over the 3-year period. Secondary endpoints were changes from baseline in physical fitness, modifiable cardiovascular factors and scores, and well-being/QoL. Here, we report on the effect of intervention on psychological well-being and health-related QoL, whereas results for the other endpoints have been presented in a previous publication [23].
Psychological Well-Being and Health-Related Quality of Life (QoL)
At baseline and at 4, 12, 16, 24, 28 and 36 months of follow-up, psychological well-being and health-related QoL were assessed using the World Health Organization-5 (WHO-5) Well-Being Index and the 36-Item Short Form Health Survey (SF-36), respectively.
The WHO-5 Well-Being Index is a five-item questionnaire developed from the WHO-10 Well-Being Index, which is suitable for use in patients with T1D and T2D [25]. Each of the five positively worded items is scored on a six-point Likert scale ranging from 0 to 5 and is transformed to a score from 0 (worst) to 100 (best). The scale has adequate validity both as an outcome measure in clinical trials and as a screening tool for depression, with a score of ≤ 50 suggesting suboptimal well-being and the need for further testing and a score ≤ 28 representing likely depression [26].
The SF-36, which was previously validated in patients with T2D [27], is a 36-item multipurpose survey that evaluates eight fields of life, which are further grouped into the physical component summary (PCS) and the mental component summary (MCS) [28]. These aggregated scores are converted into norm-based scores (mean, 50; SD, 10), with higher scores indicating a more favourable physical functioning and psychological well-being [28].
Other Measurements
At baseline and every 4 months thereafter, PA volume, LPA, MVPA and SED-time were measured using an accelerometer (MyWellness Key, Technogym, Cesena, Italy) and a daily diary for non-accelerometer recordable activities.
At the same time points, the modifiable cardiovascular risk factors BMI, waist circumference, fasting plasma glucose; glycated haemoglobin (HbA1c); triglycerides; total, HDL and LDL cholesterol; systolic and diastolic BP; and high-sensitivity C-reactive protein were assessed using standard methods, and coronary heart disease (CHD) and stroke 10-year risk scores were calculated using the United Kingdom Prospective Diabetes Study (UKPDS) risk engine.
At baseline and every year thereafter, participants were evaluated for physical fitness by assessing cardiorespiratory fitness (as maximal oxygen uptake, VO2max), strength and flexibility by maximal treadmill exercise test, isometric test and bending test, respectively.
Statistical Analysis
Sample size calculation for the primary endpoint has been reported elsewhere [23].
The nonparametric Mann–Whitney test was utilized for between-group comparisons of baseline measures, as appropriate. Frequency (n) and percentage (%) of participants in the two groups presenting at each time point with the WHO-5 score indicating optimal or suboptimal well-being or likely depression were compared using Pearson’s χ2 test.
The superiority of the intervention versus standard care on psychological well-being and health-related QoL was assessed by mixed models for repeated measures in the whole cohort and in participants stratified by age (< 65 and ≥ 65 years) and gender. The overall estimated scores for WHO-5, PCS, MCS and individual SF-36 domains during the 3-year follow-up were assessed by contrasts generated from mixed models, and results are expressed as estimated mean difference and 95% Wald confidence interval (CI). Models for repeated measures with an autoregressive correlation type matrix make an assumption of missing at random and account for both missingness at random and potential correlation within participants, as they allow evaluating all individuals, including those with incomplete data [23]. In addition, score changes detected in the 50 individuals (49 from the INT and one from the CON group) who became physically active, i.e., accumulated ≥ 150 min·wk−1 of MVPA throughout the 3-year period, were compared with those in the remaining 250 participants by mixed models for repeated measures.
Linear regression analyses were applied to assess the independent correlates of baseline to end-of-study changes in WHO-5, PCS and MCS scores. Age, gender, baseline value of each score and either baseline levels (Model 1) or baseline to end-of-study changes (Model 2) of BMI, HbA1c, systolic BP and either physical fitness (VO2max and lower body muscle strength) or activity (PA volume and SED-time) parameters were included as covariates in the regression models. Logistic regression analysis was applied to assess the independent correlates of likely depression (WHO-5 scores ≤ 28) using the covariates indicated above.
All P values < 0.05 were considered statistically significant. Statistical analyses were performed using SAS software release 9.4 (Cary, NC, USA) and R version 4.0.0.
Results
A total of 449 patients were assessed for eligibility from October 2012 to February 2014 in the three centres and, after excluding 149 patients for various reasons, the remaining 300 were randomized to CON or INT group (n = 150 each) (Fig. 1). The baseline features of study participants have been reported elsewhere [23].
Fig. 1.
Study flow chart. Flow of participants through the study. CON control group, INT intervention group, F females, M males
As previously reported [23], the percentages of session attendance and study completion were > 90% and 89%, respectively, and adverse events did not differ between groups and were unrelated to PA. The number of participants who filled in the questionnaires is shown in Fig. 1.
Co-Primary and Other Secondary Outcomes
The main trial outcomes have been reported elsewhere [23]. Briefly, as compared with CON, INT participants showed an increase over time in PA volume, MVPA and LPA, and a reciprocal decrease in SED-time. Significant between-group differences were maintained throughout the study, though difference in MVPA diminished ~ twofold during the third year. These changes were associated with sustained improvements in physical fitness, including VO2max, lower body strength and flexibility, and modifiable cardiovascular risk factors/scores, including fasting plasma glucose, systolic BP, and total and fatal CHD 10-year risk scores, in INT versus CON participants.
Psychological Well-Being
The WHO-5 scores did not differ between the two groups at baseline (Table 1); thereafter, they increased in the INT and decreased in the CON participants (Fig. 2a). Over the 3-year period, participants in the INT group perceived a significantly higher state of psychological well-being compared to those in the CON group (7.35 (95% CI 3.15–11.55), P = 0.0007) (Fig. 2a). Between-group differences over time were similar in participants aged < 65 and ≥ 65 years (Online Supplementary Material (OSM) Fig. 1a, b); conversely, though the trend was similar in the two sexes, differences between INT and CON participants were significant only in females, who showed much lower baseline WHO scores (OSM Fig. 1c, d).
Table 1.
Baseline values of WHO-5 and SF-36 scores by study arm
| Variable | CONT | INT | P* |
|---|---|---|---|
| WHO-5 | 51.6 ± 21.9 | 53.7 ± 20.4 | 0.48 |
| SF-36 | |||
| Physical functioning | 46.3 ± 9.2 | 46.9 ± 8.4 | 0.72 |
| Limitation physical | 47.1 ± 10.3 | 47.2 ± 9.9 | 0.90 |
| Bodily pain | 43.8 ± 10.8 | 46.2 ± 10.4 | 0.05 |
| General health | 38.4 ± 10.9 | 39.8 ± 9.3 | 0.33 |
| Emotional/mental health | 41.6 ± 11.0 | 43.8 ± 10.5 | 0.08 |
| Limitation emotional | 50.7 ± 8.9 | 49.5 ± 10.2 | 0.50 |
| Social functioning | 40.8 ± 9.7 | 43.0 ± 9.0 | 0.04 |
| Energy/vitality | 46.1 ± 9.6 | 47.7 ± 8.6 | 0.28 |
| PCS | 44.2 ± 9.1 | 45.4 ± 8.0 | 0.29 |
| MCS | 44.8 ± 9.0 | 45.9 ± 9.4 | 0.25 |
Values are mean ± SD
WHO World Health Organization, SF-36 36-Item Short Form, PCS physical component summary, MCS mental component summary, CON control group, INT intervention group
*Mann–Whitney U test
Fig. 2.
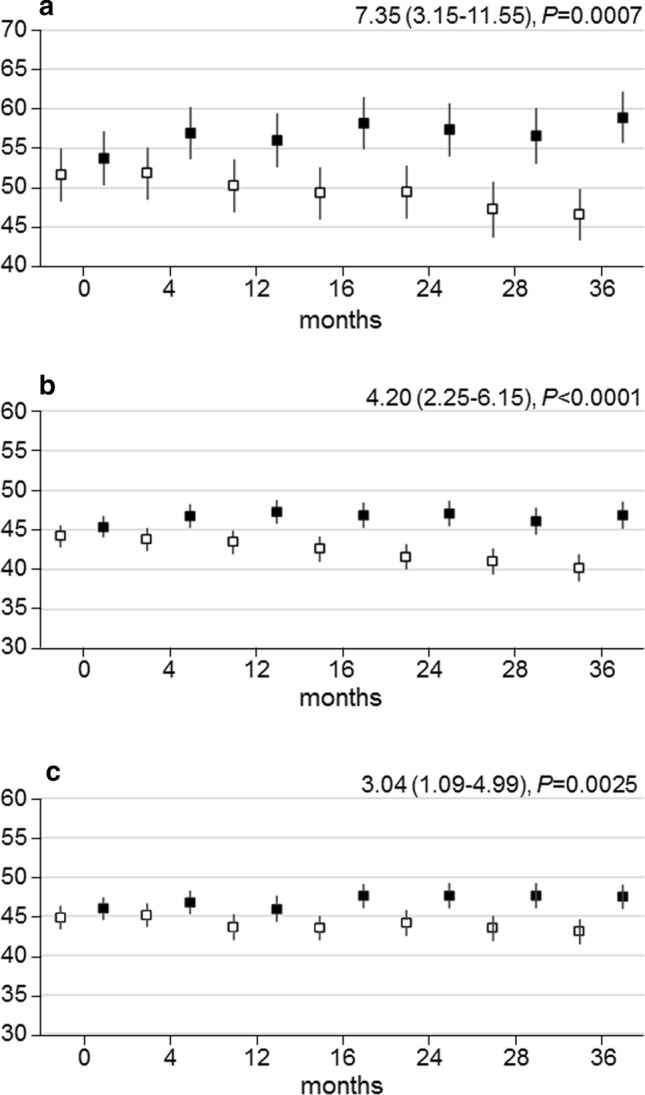
WHO-5, PCS and MCS scores over time. Change over time in WHO-5 (a), PCS (b) and MCS (c) scores in participants in the INT (black boxes) versus CON (white boxes) group. Data [estimated mean with 95% confidence interval (CI)] were calculated on the basis of questionnaires filled in at scheduled visits. The analyses are based on a mixed model for repeated measurements, taking into account within-participant correlation. P values were calculated with a mixed model for repeated measurements. Estimated mean differences over time with 95% CIs and P values between INT and CON are reported at the top right. WHO World Health Organization, PCS physical component summary, MCS mental component summary, CON control group, INT intervention group
The percentage of participants with likely depression decreased in the INT group and increased in the CON group during the 3-year period, with statistically significant between-group differences after 1 year from randomization (Table 2). Again, the effect of intervention was similar in participants aged < 65 and ≥ 65 years (OSM Table 1) and lower in males than in females, who showed a much greater prevalence of likely depression and, to a lesser extent, of sub-optimal well-being (OSM Table 2).
Table 2.
Percentage of participants with optimal and suboptimal psychological well-being and likely depression in the whole cohort and by study arm
| Months | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 4 | 12 | 16 | 24 | 28 | 36 | |
| Optimal | |||||||
| Total | 174 (58.0) | 181 (60.3) | 164 (59.2) | 153 (58.6) | 158 (60.1) | 147 (58.1) | 159 (59.8) |
| CON | 85 (56.7) | 85 (56.7) | 74 (52.9) | 60 (46.5) | 71 (53.4) | 61 (47.7) | 63 (47.7) |
| INT | 89 (59.3) | 96 (64.0) | 90 (65.7) | 93 (70.5) | 87 (66.9) | 86 (68.8) | 96 (71.6) |
| Suboptimal | |||||||
| Total | 75 (25.0) | 76 (25.3) | 67 (24.2) | 64 (24.5) | 56 (21.3) | 58 (22.9) | 62 (23.3) |
| CON | 36 (24.0) | 39 (26.0) | 38 (27.1) | 39 (30.2) | 30 (22.6) | 35 (27.3) | 36 (27.3) |
| INT | 39 (26.0) | 37 (24.7) | 29 (21.2) | 25 (18.9) | 26 (20.0) | 23 (18.4) | 26 (19.4) |
| Likely depression | |||||||
| Total | 51 (17.0) | 43 (14.3) | 46 (16.6) | 44 (16.9) | 49 (18.6) | 48 (19.0) | 45 (16.9) |
| CON | 29 (19.3) | 26 (17.3) | 28 (20.0) | 30 (23.3) | 32 (24.1) | 32 (25.0) | 33 (25.0) |
| INT | 22 (14.7) | 17 (11.3) | 18 (13.1) | 14 (10.6) | 17 (13.1) | 16 (12.8) | 12 (9.0) |
| N | 300 | 300 | 277 | 261 | 263 | 253 | 266 |
| p CON vs INT* | 0.556 | 0.272 | 0.086 | < 0.0001 | 0.039 | 0.002 | < 0.0001 |
Values are n (%)
CON control group, INT intervention group
*Pearson’s χ2 test
The 50 participants who reached or exceeded the recommended MVPA level of 150 min·wk−1 over the 3-year follow-up perceived a higher state of psychological well-being compared to those who did not (13.06 (95% CI 7.51–18.61), P < 0.0001); this difference became statistically significant after 28 months from randomization (Fig. 3).
Fig. 3.
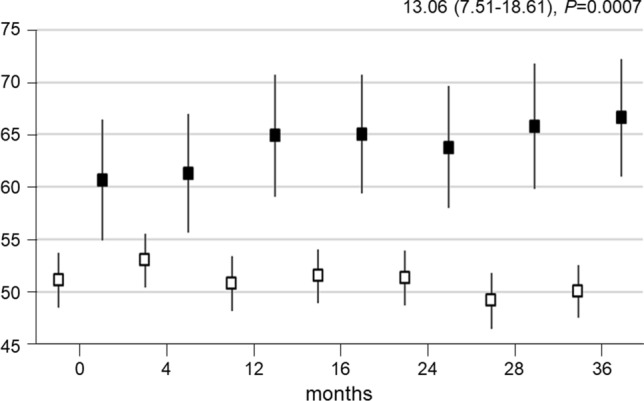
WHO-5 scores over time by physically active status. Change over time in WHO-5 scores in participants accumulating (black boxes) and not accumulating (white boxes) 150 min·wk−1 of MVPA. Data (estimated mean with 95% CI) were calculated on the basis of questionnaires filled in at scheduled visits. The analyses are based on a mixed model for repeated measurements, taking into account within-participant correlation. P values were calculated with a mixed model for repeated measurements. Estimated mean differences over time with 95% CIs and P values between INT and CON are reported at the top right. WHO World Health Organization, CON control group, INT intervention group
Higher baseline WHO-5 value, VO2max, PA volume and SED-time as well as baseline to end-of-study changes in VO2max, lower body strength and PA volume were independent predictors of higher baseline to end-of-study changes in WHO-5 scores; most of these variables were also independent predictors of likely depression (OSM Table 3).
QoL
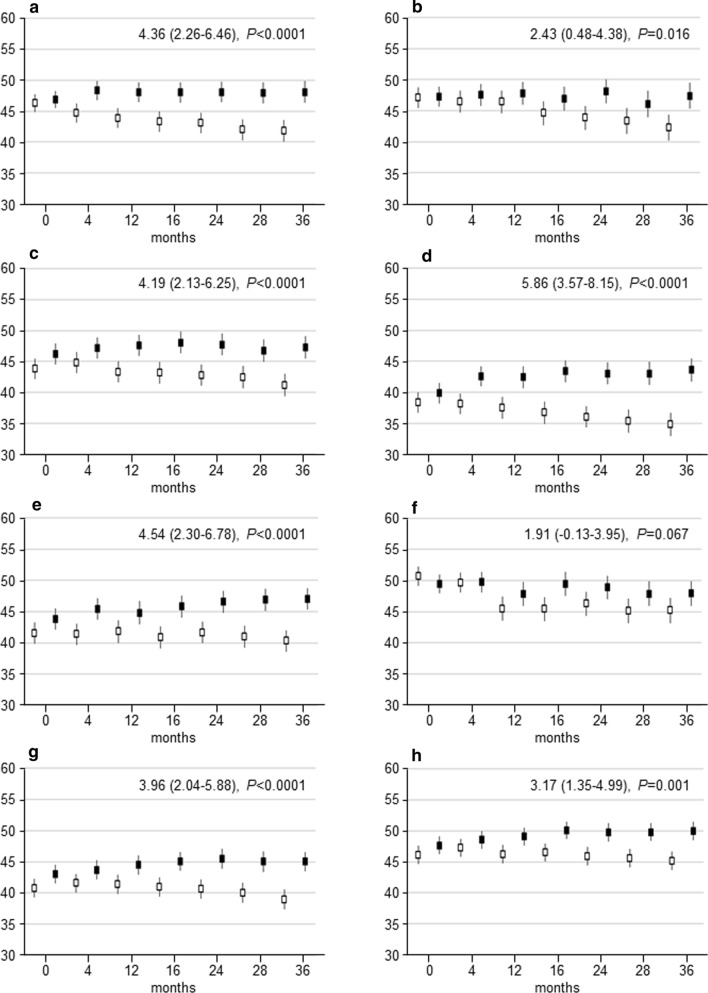
Baseline SF-36 scores did not differ between the two groups, except for a slightly higher social functioning score in the INT versus CON participants (Table 1). Gradual and sustained improvements in PCS and MCS were observed in the INT group, whereas these scores worsened progressively in the CON group, resulting in significant between-group differences over the 3-year period (PCS: 4.20 (95% CI 2.25–6.15), P < 0.0001; MCS: 3.04 (95% CI 1.09–4.99), P = 0.0025) (Fig. 2b, c). Significant differences were also detected in all physical and mental domains except for the domain score ‘limitation emotional’ (Fig. 4). The effect of intervention on PCS and MCS was similar in participants aged < 65 years and those aged ≥ 65 years and in males and females, though between-group differences over time were not significant in older individuals and females for MCS (OSM Fig. 2).
Fig. 4.
Physical and mental domain scores over time. Change over time in SF-36 scores in physical functioning (a), limitation physical (b), bodily pain (c), general health (d), emotional/mental health (e), limitation emotional (f), social functioning (g) and energy/vitality (h) in participants in the INT (black boxes) versus CON (white boxes) group. Data [estimated mean with 95% confidence interval (CI)] were calculated on the basis of questionnaires filled in at scheduled visits. The analyses are based on a mixed model for repeated measurements, taking into account within-participant correlation. P values were calculated with a mixed model for repeated measurements. Estimated mean differences over time with 95% CIs and P values between INT and CON are reported at the top right. SF-36 36-Item Short Form, CON control group, INT intervention group
No statistically significant differences were found between participants accumulating or not accumulating ≥ 150 min·wk−1 of MVPA throughout the 3-year period (not shown).
Higher baseline PCS and MCS values were independent predictors of higher baseline to end-of-study changes in PCS and MCS scores, respectively; in addition, higher baseline BMI and baseline to end-of-study changes in HbA1c were independent predictors of higher PCS and MCS scores, respectively (OSM Table 4).
Discussion
Psychological well-being and QoL are considered important treatment goals in people with diabetes, in addition to traditional medical outcomes [29]. Moreover, they represent main factors for achieving optimal diabetes self-management [10, 11], including the adoption and maintenance of an active lifestyle, which in turn might affect psychological factors [19, 20].
Effects of Intervention on Well-Being/QoL
We have shown that a behavioural intervention, which was successful in promoting a sustained increase in PA and decrease in SED-time, was associated with a progressive improvement in psychological well-being and health-related QoL and reduction in the number of participants with likely depression, whereas opposite trends were observed in the CON group.
These findings add substantially to our understanding of the relationships between PA/exercise and well-being/QoL. In fact, prior publications reported almost invariably on exercise intervention studies in which participants were engaged in aerobic and/or resistance training sessions in gym facilities under the supervision of exercise specialists. In the IDES, we found a positive effect of a supervised aerobic and resistance training program of 1 year’s duration on all components of health-related QoL [21]. Likewise, a 2-year multicomponent exercise intervention showed improved physical and mental health-related QoL in older adults with T2D [30]. However, two meta-analyses reported mixed results, because many other studies failed to detect a positive effect of supervised exercise programs [19, 20]. These contrasting findings might be related to the reported U-shaped relationship between diabetes-specific health behaviours, including diet and exercise, and perceived burden [31], i.e., after a certain threshold, the more demanding the lifestyle program, the greater the burden patients perceive. Conversely, in the IDES_2, participants received a 1-month counselling intervention each year aimed at promoting a behaviour change during the remaining 11 months by encouraging them to perform PA on their own and to reduce (and interrupt) SED-time as much as possible. This approach could have been less burdensome as it resulted in less striking increases in PA, especially MVPA, and, therefore, it may have had a more favourable impact on QoL, compared to some, more demanding, supervised exercise programs. The counselling intervention of the IDES_2 was in fact effective in improving psychological well-being and health-related QoL and, in turn, this positive impact on psychosocial factors may have contributed to maintenance of behaviour change in the long term.
The intervention was also effective in reducing the prevalence of likely depression among the study participants, consistent with an isotemporal substitution study showing that replacing even small amounts of SED-time with LPA may reduce depression in older adults [32].
Of note, the effects of the behavioural counselling on well-being and QoL were even more striking when considering that both deteriorated in the CON group, consistent with the known worsening of these scores associated with diabetes [4, 8, 9]. Furthermore, as already known [33, 34], prevalence of likely depression was much higher in females than in males, a finding that may explain why the opposite effects of intervention and standard care on WHO scores were more pronounced in women than in men.
Predictors of Well-Being/QoL Changes
An intriguing finding is that the effect of the intervention on psychological well-being was related to increases in PA volume and achievement of a physically active status, whereas that on QoL was not. Changes in PA volume (and related changes in fitness parameters) were in fact independently associated with changes in WHO-5 scores, which were significantly higher in participants who accumulated ≥ 150 min·wk−1 of MVPA over the 3-year follow-up, compared to those who did not. Conversely, no relationships with PA/sedentary behaviour or fitness parameters were detected for health-related QoL. This finding is in contrast with our previous IDES report in T2D patients engaged in a 1-year supervised exercise program [22]. It is also at odds with two cross-sectional surveys in older adults reporting higher physical QoL scores in individuals meeting MVPA recommendations compared to those who did not [35] and significantly better PCS if replacing SED-time or LPA with MVPA [36]. In the present study, the intervention per se seems to be important for the improvements in QoL measures, regardless of its efficacy in terms of increase in PA volume (and decrease in SED-time). This interpretation is consistent with a previous report in individuals with knee and hip osteoarthritis participating in an educational exercise program, who showed increased QoL despite no change in PA and SED-time [37]. However, our finding might also reflect the U-shaped relationship between PA and perceived burden of adherence to lifestyle recommendations [31], with individuals maintaining a more active and less sedentary lifestyle for such a long period of time showing a negative (or less positive) impact on QoL compared with those who responded to the intervention with a more modest change in behaviour.
The independent associations of changes in PCS with baseline BMI and of changes in MCS with changes in HbA1c are difficult to explain as they seem to suggest that higher body weight at the start and deterioration of glycaemic control during the study impacted favourably on QoL. Moreover, the finding that well-being and QoL changes were related to baseline scores supports the concept that prior psychosocial status is a major determinant of the impact of diabetes burden, including implementation of lifestyle measures, on perception of physical and mental health.
Strengths and Limitations
The main strengths of this study concern the application of an intervention targeting both PA and SED-time, the specific training of investigators, the long study duration, and the large sample size. Other strengths include the use of validated questionnaires for assessing psychological well-being and health-related QoL, together with objective (accelerometer-based) measurement of PA/SED-time and concurrent assessment of physical fitness and cardiovascular risk profile.
This study has several limitations. First, generalizability requires further investigation and validation in different cohorts or settings. Second, as the WHO-5 Well-Being Index is only a screening tool for depression, which requires additional tests for diagnosis and classification [38], results regarding the impact of intervention on the prevalence of depression should be interpreted as exploratory. Third, results might have been affected by unmeasured confounders, for instance diet, that were not considered in the data analysis, though patients received dietary prescriptions and adherence to diet was verified at intermediate visits.
Conclusion
A counselling intervention that was effective in producing a sustained behavioural change with increased PA and reduced SED-time significantly improved psychological well-being and health-related QoL and decreased the proportion of participants with likely depression, compared with standard care, which was associated with worsening of all scores. Changes in psychological well-being and prevalence of likely depression, but not those in PCS and MCS, were related to changes in PA volume.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients and the IDES_2 Investigators (see ESM for the complete list) for participating in this study and Philippa Mungra for help with manuscript preparation.
The IDES_2 Investigators: Steering Committee: Giuseppe Pugliese, Department of Clinical and Molecular Medicine, University of Rome La Sapienza, and Diabetes Unit, Sant’Andrea University Hospital, Rome, Italy (Principal Investigator); Stefano Balducci, Metabolic Fitness Association O.N.L.U.S., Monterotondo, Rome, Italy (Co-Investigator); Massimo Sacchetti, Department of Human Movement and Sport Sciences, University of Rome ‘Foro Italico’, Rome, Italy; Silvano Zanuso, Centre for Applied Biological & Exercise Sciences, Faculty of Health & Life Sciences, Coventry University, Coventry, United Kingdom; Patrizia Cardelli, Department of Clinical and Molecular Medicine, University of Rome La Sapienza, and Laboratory of Clinical Chemistry, Sant’Andrea University Hospital, Rome, Italy; Antonio Nicolucci, Centre for Outcomes Research and Clinical Epidemiology (CORESEARCH), Pescara, Italy.
Participating centers: Diabetes Clinics: Giuseppe Pugliese, Maria Cristina Ribaudo, Elena Alessi, Martina Vitale, Tiziana Cirrito, Lucilla Bollanti, Francesco G. Conti, Diabetes Unit, Sant’Andrea University Hospital, Rome, Italy. Nicolina Di Biase, Filomena La Saracina, Diabetes Unit, Fatebenefratelli San Pietro Hospital, Rome, Italy. Stefano Balducci, Mario Ranuzzi, Jonida Haxhi, Valeria D’Errico, Diabetes Unit, Health District, Monterotondo, Rome, Italy.
Metabolic Fitness Centres: Massimo Sacchetti, Giorgio Orlando, Department of Human Movement and Sport Sciences, University of Rome ‘Foro Italico’, Rome, Italy. Luca Milo, Roberto Milo, Centre for the Study of Metabolism, Rome, Italy. Gianluca Balducci, Enza Spinelli, Metabolic Fitness Association, Monterotondo, Rome, Italy.
Abbreviations
- QoL
Quality of life
- PA
Physical activity
- IDES
Italian Diabetes and Exercise Study
- SED-time
Sedentary time
- INT
Intervention
- CON
Control
- BMI
Body mass index
- BP
Blood pressure
- LPA
Light-intensity PA
- MVPA
Moderate-to-vigorous-intensity PA
- METs
Metabolic equivalents
- HbA1c
Glycated haemoglobin
- CHD
Coronary heart disease
- UKPDS
United Kingdom Prospective Diabetes Study
- VO2max
Maximal oxygen uptake
- WHO
World Health Organization
- SF-36
36-Item short form
- PCS
Physical component summary
- MCS
Mental component summary
- CHD
Coronary heart disease
- UKPDS
United Kingdom Prospective Diabetes Study
- CI
Confidence interval
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Declarations
Funding
This work was supported by the Metabolic Fitness Association, Monterotondo, Rome, Italy. The sponsor had no role in the design and conduct of the study; collection, management and interpretation of the data; or preparation, review or approval of the manuscript, and decision to submit for publication.
Conflict of interest/Competing interests
All authors have completed and submitted the Conflicts of Interest Disclosure form. Antonio Nicolucci reported grants from Artsana, Astra-Zeneca, Eli Lilly, Novo Nordisk and Sanofi Aventis and personal fees from Eli Lilly and Novo Nordisk; Silvano Zanuso is an employee of Technogym; Stefano Balducci reported personal fees from Astra-Zeneca, Eli Lilly, Novo Nordisk and Takeda; Giuseppe Pugliese reported personal fees from Astra-Zeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dome, Mylan, Sigma-Tau and Takeda; no other disclosures were reported.
Ethics approval
The study protocol was approved by the Ethics Committee of Sant’Andrea University Hospital (Prot. n. 212/2012) and was conducted in accordance with the Declaration of Helsinki. The participants provided written informed consent.
Consent to participate
Participants provided written informed consent prior to participation.
Consent for publication
Not applicable.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author contributions
AN conceived and designed the study, performed statistical analysis, and reviewed/edited the manuscript; JH conceived and designed the study, researched data and drafted the manuscript; VDE, MS, GO, PC, MV, LB, FC and SZ researched data and contributed to the discussion; GL performed statistical analysis and contributed to the discussion; SB conceived and designed the study, researched data, and reviewed/edited the manuscript; GP conceived and designed the study, researched data and drafted the manuscript. All authors read and approved the final manuscript.
Footnotes
The members of the Italian Diabetes and Exercise Study 2 (IDES_2) Investigators are listed in the acknowledgements section.
Antonio Nicolucci and Jonida Haxhi contributed equally to this work.
Contributor Information
Giuseppe Pugliese, Email: giuseppe.pugliese@uniroma1.it.
for the Italian Diabetes and Exercise Study 2 (IDES_2) Investigators:
Giuseppe Pugliese, Stefano Balducci, Massimo Sacchetti, Silvano Zanuso, Patrizia Cardelli, Antonio Nicolucci, Giuseppe Pugliese, Maria Cristina Ribaudo, Elena Alessi, Martina Vitale, Tiziana Cirrito, Lucilla Bollanti, Nicolina Di Biase, Filomena La Saracina, Stefano Balducci, Mario Ranuzzi Jonida Haxhi, Valeria D’Errico, Massimo Sacchetti, Giorgio Orlando, Luca Milo, Roberto Milo, Gianluca Balducci, and Enza Spinelli
References
- 1.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 2.Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012;142(Suppl):S8–S21. doi: 10.1016/S0165-0327(12)70004-6. [DOI] [PubMed] [Google Scholar]
- 3.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49(5):837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 4.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31(12):2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabák AG, Akbaraly TN, Batty GD, Kivimäki M. Depression and type 2 diabetes: a causal association? Lancet Diabetes Endocrinol. 2014;2(3):236–245. doi: 10.1016/S2213-8587(13)70139-6. [DOI] [PubMed] [Google Scholar]
- 6.Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54(3):317–329. doi: 10.1016/S0006-3223(03)00569-9. [DOI] [PubMed] [Google Scholar]
- 7.Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol. 2015;3(6):450–460. doi: 10.1016/S2213-8587(15)00135-7. [DOI] [PubMed] [Google Scholar]
- 8.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 9.Goldney RD, Phillips PJ, Fisher LJ, Wilson DH. Diabetes, depression, and quality of life: a population study. Diabetes Care. 2004;27(5):1066–1070. doi: 10.2337/diacare.27.5.1066. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008;31(12):2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose M, Fliege H, Hildebrandt M, Schirop T, Klapp BF. The network of psychological variables in patients with diabetes and their importance for quality of life and metabolic control. Diabetes Care. 2002;25(1):35–42. doi: 10.2337/diacare.25.1.35. [DOI] [PubMed] [Google Scholar]
- 12.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 13.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26(10):2822–2828. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 14.Aikens JE, Perkins DW, Lipton B, Piette JD. Longitudinal analysis of depressive symptoms and glycemic control in type 2 diabetes. Diabetes Care. 2009;32(7):1177–1181. doi: 10.2337/dc09-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63(4):619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Wang RH, Lin KC, Hsu HC, Lee YJ, Shin SJ. Determinants for quality of life trajectory patterns in patients with type 2 diabetes. Qual Life Res. 2019;28(2):481–490. doi: 10.1007/s11136-018-2013-2. [DOI] [PubMed] [Google Scholar]
- 17.García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175–194. doi: 10.1007/s13300-013-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30(2):203–209. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 19.Cai H, Li G, Zhang P, Xu D, Chen L. Effect of exercise on the quality of life in type 2 diabetes mellitus: a systematic review. Qual Life Res. 2017;26(3):515–530. doi: 10.1007/s11136-016-1481-5. [DOI] [PubMed] [Google Scholar]
- 20.van der Heijden MM, van Dooren FE, Pop VJ, Pouwer F. Effects of exercise training on quality of life, symptoms of depression, symptoms of anxiety and emotional well-being in type 2 diabetes mellitus: a systematic review. Diabetologia. 2013;56(6):1210–1225. doi: 10.1007/s00125-013-2871-7. [DOI] [PubMed] [Google Scholar]
- 21.Nicolucci A, Balducci S, Cardelli P, Zanuso S, Pugliese G, Italian Diabetes Exercise Study (IDES) Investigators Improvement of quality of life with supervised exercise training in subjects with type 2 diabetes mellitus. Arch Intern Med. 2011;171(21):1951–1953. doi: 10.1001/archinternmed.2011.561. [DOI] [PubMed] [Google Scholar]
- 22.Nicolucci A, Balducci S, Cardelli P, et al. Relationship of exercise volume to improvements of quality of life with supervised exercise training in patients with type 2 diabetes in a randomised controlled trial: the Italian Diabetes and Exercise Study (IDES) Diabetologia. 2012;55(3):579–588. doi: 10.1007/s00125-011-2425-9. [DOI] [PubMed] [Google Scholar]
- 23.Balducci S, D'Errico V, Haxhi J, et al. Effect of a behavioral intervention strategy on sustained change in physical activity and sedentary behavior in patients with type 2 diabetes: the IDES_2 randomized clinical trial. JAMA. 2019;321(9):880–890. doi: 10.1001/jama.2019.0922. [DOI] [PubMed] [Google Scholar]
- 24.Balducci S, Sacchetti M, Haxhi J, et al. The Italian Diabetes and Exercise Study 2 (IDES-2): a long-term behavioral intervention for adoption and maintenance of a physically active lifestyle. Trials. 2015;16:569. doi: 10.1186/s13063-015-1088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bech P, Olsen LR, Kjoller M, Rasmussen NK. Measuring well-being rather than the absence of distress symptoms: a comparison of the SF-36 Mental Health subscale and the WHO-Five Well-being Scale. Int J Methods Psychiatr Res. 2003;12:85–91. doi: 10.1002/mpr.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topp CW, Østergaard SD, Søndergaard S, Bech P. The WHO-5 Well-Being Index: a systematic review of the literature. Psychother Psychosom. 2015;84(3):167–176. doi: 10.1159/000376585. [DOI] [PubMed] [Google Scholar]
- 27.Anderson RM, Fitzgerald JT, Wisdom K, Davis WK, Hiss RG. A comparison of global versus disease-specific quality-of-life measures in patients with NIDDM. Diabetes Care. 1997;20(3):299–305. doi: 10.2337/diacare.20.3.299. [DOI] [PubMed] [Google Scholar]
- 28.Ware JE., Jr SF-36 health survey update. Spine (Phila Pa 1976) 2000;25(24):3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association 4 Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S40–S52. doi: 10.2337/dc21-S004. [DOI] [PubMed] [Google Scholar]
- 30.Baptista LC, Dias G, Souza NR, Veríssimo MT, Martins RA. Effects of long-term multicomponent exercise on health-related quality of life in older adults with type 2 diabetes: evidence from a cohort study. Qual Life Res. 2017;26(8):2117–2127. doi: 10.1007/s11136-017-1543-3. [DOI] [PubMed] [Google Scholar]
- 31.Watkins KW, Connell CM, Fitzgerald JT, Klem L, Hickey T, Ingersoll-Dayton B. Effect of adults' self-regulation of diabetes on quality-of-life outcomes. Diabetes Care. 2000;23(10):1511–1515. doi: 10.2337/diacare.23.10.1511. [DOI] [PubMed] [Google Scholar]
- 32.Yasunaga A, Shibata A, Ishii K, Koohsari MJ, Oka K. Cross-sectional associations of sedentary behaviour and physical activity on depression in Japanese older adults: an isotemporal substitution approach. BMJ Open. 2018;8(9):e022282. doi: 10.1136/bmjopen-2018-022282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demmer RT, Gelb S, Suglia SF, et al. Sex differences in the association between depression, anxiety, and type 2 diabetes mellitus. Psychosom Med. 2015;77(4):467–477. doi: 10.1097/PSY.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deischinger C, Dervic E, Leutner M, et al. Diabetes mellitus is associated with a higher risk for major depressive disorder in women than in men. BMJ Open Diabetes Res Care. 2020;8(1):e001430. doi: 10.1136/bmjdrc-2020-001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiel DM, Al Sayah F, Vallance JK, Johnson ST, Johnson JA. Association between physical activity and health-related quality of life in adults with type 2 diabetes. Can J Diabetes. 2017;41(1):58–63. doi: 10.1016/j.jcjd.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Yasunaga A, Shibata A, Ishii K, et al. Replacing sedentary time with physical activity: effects on health-related quality of life in older Japanese adults. Health Qual Life Outcomes. 2018;16(1):240. doi: 10.1186/s12955-018-1067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jönsson T, Ekvall Hansson E, Thorstensson CA, Eek F, Bergman P, Dahlberg LE. The effect of education and supervised exercise on physical activity, pain, quality of life and self-efficacy—an intervention study with a reference group. BMC Musculoskelet Disord. 2018;19(1):198. doi: 10.1186/s12891-018-2098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association Publishing; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.