ABSTRACT
Background
Approximately 10% of adolescents worldwide are overweight or obese, hence the urgent and universal need to elucidate possible mechanisms that lead to obesity in the adolescent population.
Objectives
We examined the hypothalamic metabolism and its relationship with physical development in obese and eutrophic adolescents.
Methods
We performed a case-control study with 115 adolescents between 11 and 18 years of age, to compare obese (BMI z-score ≥ 2) and nonobese individuals (eutrophic controls; BMI z-score ≤ 1). The following hypothalamic metabolite ratios were examined as primary outcomes: glutamate/creatine (Cr), the sum of glutamate and glutamine/Cr, N-acetylaspartate (NAA)/Cr, myoinositol/Cr, and total choline/Cr (glycerophosphocholine + phosphocholine/Cr), quantified by magnetic resonance spectroscopy. BMI z-scores, pubertal status, and scores on the Yale Food Addiction Scale, the Binge Eating Scale, and the Child Depression Inventory were assessed as secondary outcomes. Pearson coefficients (r) or nonparametric Spearman correlation (rho) analyses were performed between hypothalamic metabolite ratios and other parameters, such as BMI z-scores, physical development, food habits, depression symptoms, and serum protein concentrations (cytokines, hormones, and neuropeptides).
Results
Adolescents with obesity showed a lower hypothalamic NAA/Cr ratio (0.70 ± 0.19) compared to their eutrophic counterparts (0.84 ± 0.20; P = 0.004). The NAA/Cr ratio was negatively correlated with BMI z-scores (r = −0.25; P = 0.03) and serum insulin (rho = −0.27; P = 0.04), C-peptide (rho = −0.26; P = 0.04), amylin (r = −0.27; P = 0.04), ghrelin (rho = −0.30; P = 0.02), and neuropeptide Y (r = −0.27; P = 0.04). Also, the NAA/Cr ratio was positively correlated with circulating IL-8 levels (rho = 0.26; P = 0.04).
Conclusions
High BMI z-scores are associated with lower hypothalamic NAA/Cr ratios. The negative correlations found between the NAA/Cr ratio and serum cytokines, hormones, and neuropeptides suggest a broad cross-talk linking hormonal imbalances, neurohumoral alterations, and hypothalamic functions in adolescents with obesity.
Keywords: spectroscopy, neuroimaging, adolescent obesity, hypothalamus, brain damage
Introduction
Approximately 10% of adolescents worldwide are overweight or obese (1), hence the urgent and universal need to address the consequences of this disease on the physical development of adolescents. Equivocal dietary choices lead to obesity and comorbidities, including diabetes, cardiovascular disease, and an increased frequency of cancers (2). Furthermore, global forecasts predict an increasing rate of obesity, which will negatively affect future health and economic policies (3). Obesity in early life is associated with more health problems in adulthood (1); thus, preventing obesity in childhood and adolescence is critical. However, several studies conducted in the United States (1, 4) have reported that obesity rates remain high and that no significant improvements were observed between the periods of 2003–2004 and 2011–2012 (4). In Brazil, the most recent nationwide survey conducted in 2021 by the Ministry of Health estimated that 6.4 million children are overweight. Of these, over 3.1 million have already evolved to obesity (based on the BMIs of children in primary Health Care System records). In other words, 28% of Brazilian children are overweight and 13.2% are obese (5, 6). Interestingly, significant differences were found among different socioeconomic classes, with underprivileged children showing lower obesity rates than children in higher social classes (2.5% compared with 10.6%, respectively) (7). It is well known that diet plays a prominent role in the etiology of obesity, and appetite control and eating behaviors involve a complex network of neural systems. However, less is known about the role of the adolescent hypothalamus in these processes.
The hypothalamus is associated with important basic functions, such as the control of homeostasis, regulation of energy balance, reproduction, and food intake (8). This brain region is highly sensitive to peripheral metabolic mediators and integrates various neural, hormonal, and nutritional signals (9). Thus, the hypothalamus is critical in regulating the metabolism, appetite, weight, and body composition (10). Studies with MRI in obese adults reported decreased signal intensity in T2-weighted images of the mediobasal hypothalamus, suggesting gliosis (11), as well as decreased hypothalamic connectivity, as measured by diffusion tensor imaging (12). However, most of the literature focuses solely on other brain structures, like the hippocampus.
The hippocampus and the hypothalamus play similar roles in appetite control (13). Previous studies suggest that hippocampal neurons rely on neurohormonal signals to orchestrate a response to achieve energy balance through adaptive behavioral outcomes (14). Several rodent studies showed that excessive caloric intake via diets high in lipids and sugars reduces hippocampal function, plasticity, and neurogenesis (14–16). In humans, previous studies reported an inverse relationship between BMI and hippocampal N-acetylaspartate (NAA), indicating that a higher BMI is associated with lower hippocampal NAA concentration levels (13). Recent proton magnetic resonance spectroscopy studies also showed that a higher BMI is associated with decreased content of neurochemical markers of neuronal integrity (NAA) in several brain regions, like the hippocampus (17). Moreover, in adults with intact cognitive functions, obesity is associated with altered cerebral neurochemical profiles, increased myoinositol (mI) levels, and decreased NAA/creatine (Cr) and glutamate (Glu)/Cr ratios in the hippocampal region (18, 19).
Although previous research suggests an important association between the hippocampal composition and obesity, little is known about the association between the hypothalamic metabolism [Glu/Cr, sum of glutamate and glutamine (Glx)/Cr, NAA/Cr, mI/Cr, and glycerophosphocholine (GPC) + phosphocholine (PCh)/Cr ratios], body weight, physical development, and food habits in adolescents. Therefore, our aim was to assess the hypothalamic metabolism in adolescent obesity.
We hypothesized that adolescent obesity would be associated with a decrease in the NAA/Cr ratio in the hypothalamus, a sign of synaptic loss and neurodegeneration. Furthermore, we investigated the association between the hypothalamic metabolism and BMI, physical development, and food habits in obese and eutrophic adolescents.
Methods
Study design
This is a case-control study to evaluate the association of the hypothalamic metabolism, body weight, physical development, and food habits, comparing adolescents with obesity and their eutrophic counterparts of both sexes. This study is described per the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines (20).
Ethics
The Institutional Review Board of the Santa Casa de São Paulo Hospital approved the study (CAAE: 24552413.2.0000.5479). Informed assent and consent forms were signed by adolescents and their parents/guardians, respectively, before the implementation of any study protocol.
Participants
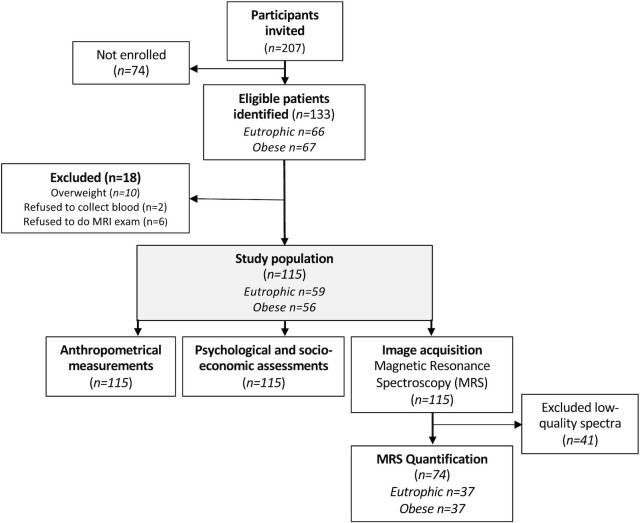
We collected data from the Childhood Obesity Outpatient Clinic of the Santa Casa de São Paulo. Participant recruitment started in May 2015, and follow-up ended in September 2016. We included adolescents between the ages of 11 and 18; the eutrophic group (control) had a BMI z-score ≤ 1 and the obesity group had a BMI z-score ≥ 2. We excluded subjects presenting neurological disorders, head trauma, or ferromagnetic objects in the body, including orthodontic appliances that could affect safety and/or the quality of the MRI exam. We excluded subjects with substance dependence or abuse, as well any psychiatric disorders diagnosed using the Kiddie Schedule for Affective Disorders and Schizophrenia questionnaire (see flowchart in Figure 1) (21).
FIGURE 1.
Flowchart of the MRS study. MRS, magnetic resonance spectroscopy.
Anthropometric variables
Systolic and diastolic blood pressure values were measured with a sphygmomanometer. Age at menarche was recorded for female participants. Pubertal status was measured using the Tanner staging system, a scale of physical development in children, adolescents, and adults. The scale defines physical measurements of development based on external primary and secondary sex characteristics, such as the size of breasts and genitals, testicular volume, and the development of pubic hair (22). To prevent adolescents from feeling uncomfortable, Tanner staging was not performed via physical examination; instead, participants were asked to identify figures that best resembled their pubic development stage.
Psychological and socio-economic assessments
The Yale Food Addiction Scale (YFAS) and the Binge Eating Scale (BES) were assessed as described by Simoes et al. (23). The Child Depression Inventory (CDI) was assessed via a 27-item self-reported measure that assesses the presence of subsyndrome depressive symptoms in children and adolescents from ages 7 to 17 (24). The Brazilian Economic Classification was employed to classify participants according to socio-economic strata (25).
Image acquisition
Magnetic resonance spectroscopy (MRS) was performed using a 3.0T whole-body magnet (Intera Achieva, Philips) in the Department and Institute of Radiology at the University of São Paulo (InRad HC-FMUSP) between 07:00 and 07:30 (the patients were in a fasted state during the exam). The Point Resolved single voxel (PRESS) technique was used with an echo time (TE)/ repetition time (TR) of 35/4000 ms and 160 repetitions. The nominal voxel size of the spectrum was 2 × 2 × 2 cm3 and was located at the height of the hypothalamus, with 4 saturation bars placed within the limits of the water-voxel overlap with NAA-voxel to reduce the effect of the chemical shift (Supplemental Figure 1).
MRS quantification
LCModel (version 6.3) was adopted to quantify metabolite levels of Glu, Glx, NAA, mI, total choline (Cho) as the sum of GPC and PCh, and Cr (26). An unsuppressed water signal was used as an internal reference. To ensure the accuracy of the measurements obtained, only metabolite results with Cramer-Rao lower bound (CRLB) values of less than 20% were considered (27). In the same way, spectra of low spectral resolution (frequency width at half maximum > 0.1 ppm) and those with a signal to noise ratio below 5 were excluded from the analysis (Supplemental Table 1). We report metabolite concentrations as the ratio to Cr, since absolute values are usually considered less reliable, as they are more susceptible to partial volume effects than ratios over Cr.
Statistical methods
Data are expressed as means ± SDs. Categorical variables were analyzed using chi-squared tests. Mammary development and gonad development were analyzed within the respective gender categories. Normality was checked using the Kolmogorov-Smirnov test in continuous variables and comparisons were conducted through an ANOVA using unpaired t-tests (parametric) or Mann-Whitney U (nonparametric) tests, when appropriate. Pearson (parametric coefficient) or Spearman correlations (nonparametric coefficient) were conducted to evaluate the association between spectroscopy metabolite ratios and BMI z-scores and BES, YFAS, and CDI scores. The data obtained in the present study were correlated with circulating levels of cytokines, hormones, and neuropeptides measured in the same cohort by Simoes et al. (23, 28). Results are reported as predicted means with 95% CIs, and significance was considered at P values < 0.05. All analyses were performed using Graphpad Prism 7.0.
Results
Participants
Table 1 reports the characteristics of the entire cohort. A total of 115 subjects, who were 13.9 ± 1.93 years old on average and 52.2% of whom were female, were divided into 2 groups: a eutrophic group (control) with BMI z-scores ≤ 1 and an obesity group with BMI z-scores ≥ 2. Potential confounders, such as age and sex, presented no differences in the distribution between groups (P > 0.05). Adolescents with obesity presented with higher systolic blood pressure (122 ± 14.3 mmHg) compared to their eutrophic counterparts (108 ± 8.44 mmHg; P < 0.001). No differences were found regarding pubic hair development (P = 0.35), mammary development (P = 0.17), or gonad development (P = 0.07). The average menarche age among females was 11.6 ± 1.19 years. Adolescents with obesity showed higher scores on the YFAS (P = 0.02) and the BES (P = 0.001), indicating addictions to high-fat and/or high-sugar foods and eating disorders, respectively, when compared to normal weight controls.
TABLE 1.
Anthropometrics, psychological, and socio-economic variables of the entire cohort1
| Characteristics | Eutrophic,2n = 59 | Obese,3n = 56 | Missing values, n | P value |
|---|---|---|---|---|
| Age, y | 14.2 (± 1.91) | 13.6 (± 1.91) | 0 | 0.09 |
| Sex, male/female | 26/33 | 29/27 | 0 | 0.41 |
| Blood pressure, mm Hg | ||||
| Systolic | 107 (± 8.44) | 122 (± 14.3) | 11 | <0.001 |
| Diastolic | 74.2 (± 10.4) | 76.3 (± 14.0) | 12 | 0.17 |
| Age at menarche, y | 11.7 (± 1.27) | 11.5 (± 1.12) | 7 | 0.52 |
| Mammary development | 7 | 0.17 | — | |
| Stage 2 | 3 (10%) | 1 (4%) | — | |
| Stage 3 | 11 (38%) | 8 (33%) | — | |
| Stage 4 | 13 (45%) | 8 (33%) | — | |
| Stage 5 | 2 (7%) | 7 (29%) | — | |
| Gonad development | 13 | 0.07 | — | |
| Stage 1 | 0 (0%) | 1 (5%) | — | |
| Stage 2 | 3 (13%) | 5 (26%) | — | |
| Stage 3 | 10 (44%) | 3 (16%) | — | |
| Stage 4 | 5 (22%) | 9 (47%) | — | |
| Stage 5 | 5 (22%) | 1 (5%) | — | |
| Pubic hair development | 20 | 0.35 | — | |
| Stage 1 | 3 (6%) | 0 (0%) | — | |
| Stage 2 | 8 (15%) | 7 (16%) | — | |
| Stage 3 | 20 (39%) | 13 (30%) | — | |
| Stage 4 | 16 (31%) | 15 (35%) | — | |
| Stage 5 | 5 (10%) | 8 (19%) | — | |
| Psychological assessments | ||||
| CDI score | 10.3 (± 5.44) | 10.1 (± 5.32) | 0 | 0.85 |
| YFAS score | 1.67 (± 1.89) | 2.29 (± 1.75) | 0 | 0.02 |
| BES score | 6.81 (± 6.17) | 10.3 (± 6.49) | 0 | 0.001 |
| Socio-economic classification | 0 | 0.87 | — | |
| Upper class | 1 (2%) | 2 (4%) | — | |
| Middle class | 31 (53%) | 28 (50%) | — | |
| Lower class | 27 (46%) | 26 (46%) | — | |
Data are presented as means (± SDs). Significance between the groups was tested using a chi-squared test for categorical variables and an unpaired t-test or Mann-Whitney U test for continuous variables. BES, Binge Eating Scale; CDI, Children Depression Inventory; YFAS, Yale Food Addiction Scale.
Eutrophic participants presented BMI z-scores ≤ 1.
Obese patients presented BMI z-scores ≥ 2.
Hypothalamic metabolite levels in adolescents
Out of a total of 115 spectra obtained, only 74 spectra (37 per group) fulfilled the established quality criteria and were considered for further analysis. The high number of excluded low-quality spectra (n = 41) is related to the location of the hypothalamus, which is very difficult to shim (homogenization of the B0 magnetic field) and will often lead to a nonoptimal equipment adjustment. MRS quality parameters, such as the frequency width at half maximum, signal to noise ratio, and CRLB for each metabolite, were calculated (Supplemental Table 1), and no differences in terms of MRS quality were observed between groups.
An exploratory analysis considering the entire cohort demonstrated that adolescents with obesity presented lower NAA/Cr ratios relative to eutrophic adolescents (P = 0.004; Table 2). Glu/Cr, Glx/Cr, mI/Cr, and GPC + PCh/Cr ratios did not present differences between groups.
TABLE 2.
Results of proton magnetic resonance spectroscopy in the hypothalamus of the entire cohort1
| Ratios | Eutrophic,2n = 37 | Obese,3n = 37 | P value |
|---|---|---|---|
| Glu/Cr | 1.05 (± 0.17) | 1.03 (± 0.32) | 0.85 |
| Glx/Cr | 1.73 (± 0.36) | 1.78 (± 0.41) | 0.59 |
| NAA/Cr | 0.84 (± 0.20) | 0.70 (± 0.19) | 0.004 |
| mI/Cr | 0.96 (± 0.18) | 0.99 (± 0.16) | 0.49 |
| GPC + PCh/Cr | 0.34 (± 0.04) | 0.33 (± 0.03) | 0.19 |
Data presented as Mean (± SD). Significance between the groups was tested using Unpaired T test. Cr, creatine; Glu, glutamate; Glx, sum of glutamate and glutamine; GPC, glycerophosphocholine; mI, myo-inositol; NAA, N-acetylaspartate; PCh, phosphocholine.
Eutrophic participants presented BMI z-scores ≤ 1.
Obese patients presented BMI z-scores ≥ 2.
Significant differences in hypothalamic levels of NAA, as well as the BMI z-scores of the entire cohort, were considered for correlation analyses, which showed significant negative linearity between BMI z-scores and the NAA/Cr ratio (P = 0.03), indicating that lower hypothalamic levels of NAA are associated with increased BMI z-scores (Figure 2). However, no correlations were found between the hypothalamic NAA content and physical development, food habits, or depression symptoms (Supplemental Table 2).
FIGURE 2.
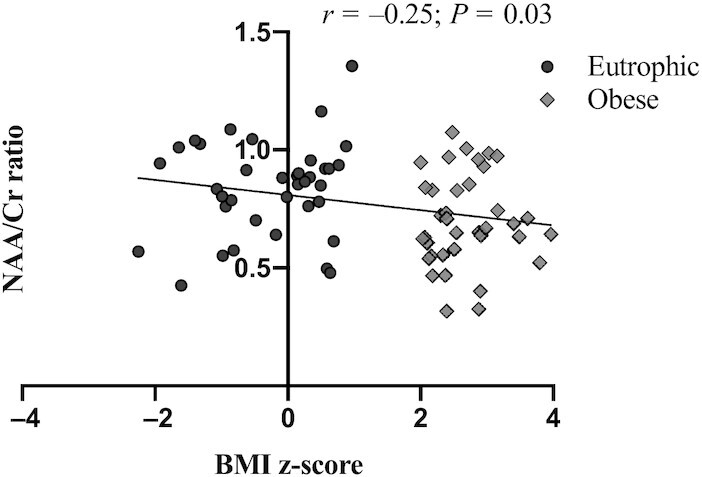
BMI z-score correlations to NAA/Cr ratio within the entire cohort. Significant differences were tested using Pearson coefficients (r) within the entire cohort [eutrophic (n = 37) and obese (n = 37)]. Cr, creatine; NAA, N-acetylaspartate; r, correlation coefficient.
Relationship between circulating factors and hypothalamic metabolites
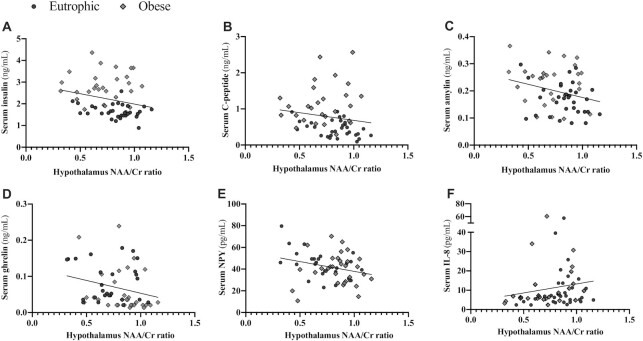
To evaluate the cross-talk between serum protein concentrations and central metabolites, correlation analyses were performed for NAA/Cr and circulating cytokines, hormones, or neuropeptides (Table 3). There was a negative correlation between the NAA/Cr ratio and insulin (rho = −0.27; P = 0.04; Figure 3A), C-peptide (rho = −0.26; P = 0.04; Figure 3B), amylin (r = −0.27; P = 0.04; Figure 3C), and ghrelin (rho = −0.30; P = 0.02; Figure 3D). Furthermore, the NAA/Cr ratio was negatively correlated with the neuropeptide concentration (neuropeptide Y; r = −0.27; P = 0.04; Figure 3E) and positively correlated with cytokine levels (IL-8; rho = 0.26; P = 0.04; Figure 3F). No other correlations were detected between NAA/Cr and circulating factors.
TABLE 3.
Serum protein levels and NAA/Cr ratio correlations within the entire cohort1
| NAA/Cr | rho | P value | |
|---|---|---|---|
| r | |||
| Serum hormones | |||
| Insulin | — | −0.27 | 0.04 |
| Leptin | — | −0.07 | 0.61 |
| C-peptide | — | −0.26 | 0.04 |
| Amylin | −0.27 | — | 0.04 |
| Glucagon | — | −0.19 | 0.15 |
| GLP-1 | −0.11 | — | 0.43 |
| GIP | — | −0.12 | 0.36 |
| Ghrelin | — | −0.30 | 0.02 |
| Serum neuropeptides | |||
| α-MSH | — | 0.26 | 0.08 |
| β-Endorphin | — | 0.18 | 0.22 |
| Neurotensin | — | 0.16 | 0.24 |
| Oxytocin | — | 0.13 | 0.36 |
| Orexin | — | 0.13 | 0.36 |
| MCH | — | 0.04 | 0.75 |
| NPY | −0.27 | — | 0.04 |
| Serum cytokines | |||
| L1β | — | 0.07 | 0.59 |
| IL-6 | — | 0.08 | 0.57 |
| IL-8 | — | 0.26 | 0.04 |
| IL-10 | — | 0.06 | 0.66 |
Significant differences were tested using Pearson coefficients (r) or nonparametric Spearman correlation (rho) within the entire cohort [eutrophic (n = 37) and obese (n = 37)]. α-MSH, α-melanocyte-stimulating hormone; Cr, creatine; GIP, gastric inhibitory polypeptide; GLP-1, glucagon-like peptide-1; MCH, melanin-concentrating hormone; NAA, N-acetylaspartate; NPY, neuropeptide Y.
FIGURE 3.
NAA/Cr ratio, cytokines, hormones, and neuropeptides’ significant correlations within the entire cohort. (A) Serum insulin and hypothalamus NAA/Cr ratio correlation; (B) serum C-peptide and hypothalamus NAA/Cr ratio correlation; (C) serum amylin and hypothalamus NAA/Cr ratio correlation; (D) serum ghrelin and hypothalamus NAA/Cr ratio correlation; (E) serum neuropeptide Y and hypothalamus NAA/Cr ratio correlation; and (F) serum IL8 and NAA/Cr ratio correlation. Significant differences were tested using Pearson coefficients (r) or nonparametric Spearman correlation (rho) within the entire cohort [eutrophic (n = 37) and obese (n = 37)]. Significance was considered at a P value < 0.05. Cr, creatine; NAA, N-acetylaspartate.
Discussion
To the best of our knowledge, this is the first study evaluating the relationship of the hypothalamic metabolite composition (Glu/Cr, Glx/Cr, NAA/Cr, mI/Cr, and GPC + PCh/Cr ratios) with body weight, physical development, and food habits in adolescents. Higher BMI z-scores were associated with lower NAA/Cr ratios in the hypothalamus, a brain area that has been deemed crucial for weight homeostasis.
The NAA/Cr ratio is considered an indicator of functional integrity, neuronal density, and overall brain activity (29). Consequently, a decrease in the NAA/Cr ratio is considered a sign of synaptic loss and neurodegeneration (29). The NAA presence is high in neurons, since it is an active component of various processes, including myelination, myelin repair, lipid metabolism, osmoregulation, and neuronal signaling (30). NAA is also a key component of the regulation of the oligodendrocyte metabolism during brain development and in situations involving brain damage. The findings thus suggest that obese adolescents may be at risk of developing impairments in these processes, with possible long-term consequences.
The finding that high BMI z-scores were associated with lower NAA/Cr ratios corroborates studies reporting that the pathogenesis of obesity involves increased adiposity, which is linked to progressively compromised weight loss capacity (31, 32). The impaired connectivity and hypothalamic gliosis observed among obese individuals may possibly contribute to cognitive dysfunction, weight gain, and metabolic disease (33–35). The literature stresses that decreased NAA/Cr ratios may function as a marker for the reduction of neuronal dysfunction and dopaminergic neurotransmission, which are involved in the regulation of memory, attention, learning, and executive functioning (36, 37). Spectroscopic studies in obese adolescents demonstrated lower levels of NAA and Cho in relation to their eutrophic counterparts, but no significant differences were observed in Cr, NAA/Cr, or Cho/Cr in the frontal lobe and hippocampus (38). These findings are noteworthy, as alterations frequently associated with obesity, such as insulin resistance, may also be related to lower NAA (39). Similarly, our data demonstrate a negative correlation between NAA/Cr and insulin. Furthermore, C-peptide (released during insulin cleavage) and amylin were also negatively correlated with NAA/Cr, indicating that increased circulating hormone concentrations could contribute to the severity of hypothalamic impairments (lower NAA/Cr ratios) in patients with higher z-scores. Nonetheless, the specific mechanism driving such a disruption is unknown (40, 41). We hypothesize that insulin resistance leads to increased levels of circulating cytokines, with the potential to cross the blood-brain barrier and modulate NAA concentrations. The same can be postulated in regard to augmented levels of circulating glucose (18). We also describe a positive correlation between IL-8 and NAA/Cr ratios.
The relevance of the present study resides in demonstrating that patients with higher BMI z-scores also show signs of an altered hypothalamic metabolism, as inferred by lower NAA/Cr ratios. Considering the hypothalamus as the major center of appetite control and metabolism regulation, the correlations found between NAA/Cr and serum cytokines, hormones, and neuropeptides may suggest an intricate cross-talk, linking hormonal imbalances, neurohumoral alterations, and hypothalamic functions in adolescents with obesity.
A previous report (42) suggests that lower metabolite ratios associated with higher BMIs may not reflect real physiological changes, but rather the lower quality of the spectrum in the presence of thicker layers of subcutaneous fat. In our study, we showed that the MRS qualities for both groups were comparable, reinforcing the idea that our findings represent real metabolic changes. In agreement with this, a recent in vitro study also showed that the presence of a fat layer does not affect MRS quantification (43).
While our study fills a gap in the literature, it has the limitations that are usually associated with an observational design. Firstly, our metabolite measurements were not validated through agreement across different measurements, thus introducing potential measurement bias. Secondly, we did not follow the participants longitudinally to evaluate whether these associations could be modified over time. Thirdly, the described correlations may be considered relatively weak. Nevertheless, the novelty consists of a comprehensive and concomitant analysis of variables within the same population, thus achieving clinical relevance. Finally, although our sample was among the largest in the literature, the final number of participants can be deemed relatively small. This fact can be explained by the rigid quality control applied to our MRS data, which left us with only 74 samples out of 115 participants, which is considered acceptable for further data analysis. Future studies should take this reduction into consideration when planning data collection, as image quality can be affected by the surrounding body tissue and cerebrospinal fluid.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all patients and their families who made the research possible at Children Obesity Outpatient Clinic of the Santa Casa de Misericordia Hospital in São Paulo. The authors’ responsibilities were as follows – TMGN, ES, MCGO, RRU: conceived of and designed the study; TMGN, ES: performed the statistical analysis; TMGN, ES, JC-L: conducted molecular experiments; TMGN, ES, ELdBC, PB, NAdC, FLdSD, MdGMM, VHOO, TZdSO, DACV, GBF, CK: collected samples; TMGN, ES, MCLS, MCGO, RRU: wrote the manuscript; ELdBC, PB, NAdC, FLdSD, MdGMM, VHOO, TZdSO, DACV, GBF, CK, JC-L: analyzed the data and revised the manuscript; MCLS, MCGO, RRU: supervised the study; and all authors: read and approved the final manuscript.
Notes
This work was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP; grant number 2012/21677-6; 2012/50079-0).
Author disclosures: The authors report no conflicts of interest.
Supplemental Figure 1 and Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
TMGN and ES contributed equally to this work.
Abbreviations used: BES, Binge Eating Scale; CDI, Child Depression Inventory; Cho, total choline; Cr, creatine; CRLB, Cramer-Rao lower bound; Glu, glutamate; Glx, sum of glutamate and glutamine; GPC, glycerophosphocholine; mI, myoinositol; MRS, magnetic resonance spectroscopy; NAA, N-acetylaspartate; PCh, phosphocholine; YFAS, Yale Food Addiction Scale.
Contributor Information
Thaysa Mara Gazzotto Neves, Mental Health Department, Santa Casa de Sao Paulo School of Medical Sciences, São Paulo, Brazil.
Estefania Simoes, Cancer Metabolism Research Group, University of São Paulo, São Paulo, Brazil.
Maria Concepcíon García Otaduy, Department of Radiology (LIM-44), Faculty of Medicine, University of São Paulo, São Paulo, Brazil.
Elie Leal de Barros Calfat, Mental Health Department, Santa Casa de Sao Paulo School of Medical Sciences, São Paulo, Brazil.
Pâmela Bertolazzi, Mental Health Department, Santa Casa de Sao Paulo School of Medical Sciences, São Paulo, Brazil.
Naomi Antunes da Costa, Neuroimaging Laboratory (LIM-21), Institute Psychiatry, University of São Paulo, São Paulo, Brazil.
Fábio Luís de Souza Duran, Neuroimaging Laboratory (LIM-21), Institute Psychiatry, University of São Paulo, São Paulo, Brazil.
Joanna Correia-Lima, Cancer Metabolism Research Group, University of São Paulo, São Paulo, Brazil.
Maria da Graça Morais Martin, Department of Radiology (LIM-44), Faculty of Medicine, University of São Paulo, São Paulo, Brazil.
Marília Cerqueira Leite Seelander, Cancer Metabolism Research Group, University of São Paulo, São Paulo, Brazil; Department of Surgery and LIM 26, Hospital das Clínicas, University of São Paulo, São Paulo, Brazil.
Victor Henrique Oyamada Otani, Mental Health Department, Santa Casa de Sao Paulo School of Medical Sciences, São Paulo, Brazil.
Thais Zélia dos Santos Otani, Mental Health Department, Santa Casa de Sao Paulo School of Medical Sciences, São Paulo, Brazil.
Daniel Augusto Corrêa Vasques, Mental Health Department, Santa Casa de Sao Paulo School of Medical Sciences, São Paulo, Brazil.
Geraldo Busatto Filho, Neuroimaging Laboratory (LIM-21), Institute Psychiatry, University of São Paulo, São Paulo, Brazil.
Cristiane Kochi, Pediatrics Department, Santa Casa de Sao Paulo School of Medical Sciences, São Paulo, Brazil.
Ricardo Riyoiti Uchida, Mental Health Department, Santa Casa de Sao Paulo School of Medical Sciences, São Paulo, Brazil.
Data Availability
Data described in the manuscript, code book and analytic code will be made available upon request to the corresponding author Estefania.simoesfer@gmail.com.
References
- 1. Collins CE, Watson J, Burrows T. Measuring dietary intake in children and adolescents in the context of overweight and obesity. Int J Obes. 2010;34(7):1103–15. [DOI] [PubMed] [Google Scholar]
- 2. Aicr W. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. Continuous Update Project Expert Report 2018. Third Expert Report. World Cancer Research Fund/American Institute for Cancer Research; 2018. Available at: dietandcancerreport.org [Google Scholar]
- 3. Gortmaker SL, Swinburn BA, Levy D, Carter R, Mabry PL, Finegood DT, Huang T, Marsh T, Moodie ML. Changing the future of obesity: Science, policy, and action. Lancet North Am Ed. 2011;378(9793):838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fradkin C, Yunes MAM. Childhood obesity in Brazil: Lessons to be learned from the Northern hemisphere. Educ Ciência e Cult. 2014;19:117–22. [Google Scholar]
- 6. Ferreira CM, Reis ND, Castro A de O, Höfelmann DA, Kodaira K, Silva MT, Galvao TF. Prevalence of childhood obesity in Brazil: Systematic review and meta-analysis. J Pediatr (Rio J). 2021;97(5):490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Oliveira AMA, Cerqueira Ede M, de Oliveira AC. [Prevalence of overweight and childhood obesity in Feira de Santana-BA: Family detection vs. clinical diagnosis]. J Pediatr (Rio J). 2003;79(4):325–8. [DOI] [PubMed] [Google Scholar]
- 8. Gabriela Pop M, Crivii C, Opincariu I. Hypothalamus in Health and Diseases (IntechOpen). Anatomy and function of the hypothalamus. 2016, Available from doi: 10.5772/intechopen.80728 [Google Scholar]
- 9. Schwartz MW, Porte D. Diabetes, obesity, and the brain. Science. 2005;307(5708):375–9. [DOI] [PubMed] [Google Scholar]
- 10. Toda C, Santoro A, Kim JD, Diano S. POMC neurons: From birth to death. Annu Rev Physiol. 2017;79(1):209–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kreutzer C, Peters S, Schulte DM, Fangmann D, Türk K, Wolff S, van Eimeren T, Ahrens M, Beckmann J, Schafmayer Cet al. . Hypothalamic inflammation in human obesity is mediated by environmental and genetic factors. Diabetes. 2017;66(9):2407–15. [DOI] [PubMed] [Google Scholar]
- 12. Puig J, Blasco G, Daunis-I-Estadella J, Molina X, Xifra G, Ricart W, Pedraza S, Fernández-Aranda F, Fernández-Real JM. Hypothalamic damage is associated with inflammatory markers and worse cognitive performance in obese subjects. J Clin Endocrinol Metab. 2015;100(2):E276–81. [DOI] [PubMed] [Google Scholar]
- 13. Coplan JD, Fathy HM, Abdallah CG, Ragab SA, Kral JG, Mao X, Shungu DC, Mathew SJ. Reduced hippocampal N-acetyl-aspartate (NAA) as a biomarker for overweight. Neuroimage Clin. 2014;4:326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol. 2007;7(6):613–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci. 2004;101(29):10827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2007;182(1):57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaur S, Birdsill AC, Steward K, Pasha E, Kruzliak P, Tanaka H, Haley AP. Higher visceral fat is associated with lower cerebral N-acetyl-aspartate ratios in middle-aged adults. Metab Brain Dis. 2017;32(3):727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gazdzinski S, Millin R, Kaiser LG, Durazzo TC, Mueller SG, Weiner MW, Meyerhoff DJ. BMI and neuronal integrity in healthy, cognitively normal elderly: A proton magnetic resonance spectroscopy study. Obesity. 2010;18(4):743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzales MM, Tarumi T, Eagan DE, Tanaka H, Vaghasia M, Haley AP. Indirect effects of elevated body mass index on memory performance through altered cerebral metabolite concentrations. Psychosom Med. 2012;74(7):691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Prev Med. 2007;45(4):247–51. [DOI] [PubMed] [Google Scholar]
- 21. Young ME, Bell ZE, Fristad MA. Validation of a brief structured interview: The Children's Interview for Psychiatric Syndromes (ChIPS). J Clin Psychol Med Settings. 2016;23(4):327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanner J. Growth at adolescence, with a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. Oxford, United Kingdom: Blackwell Scientific; 1962. 325. [Google Scholar]
- 23. Simoes E, Correia-Lima J, Calfat ELB, Otani TZDS, Vasques DAC, Otani VHO, Bertolazzi P, Kochi C, Seelaender M, Uchida RR. Sex-dependent dyslipidemia and neuro-humoral alterations leading to further cardiovascular risk in juvenile obesity. Front Nutr. 2021;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Figueras Masip A, Amador-Campos JA, Gómez-Benito J, del Barrio Gándara V. Psychometric properties of the Children's Depression Inventory in community and clinical sample. Span J Psychol. 2010;13(2):990–9. [DOI] [PubMed] [Google Scholar]
- 25. Kamazura W, Mazzon JA. Critérios de estratificação e comparação de classificadores socioeconômicos no Brasil. Rev Adm Empresas. 2016;56(1):55–70. [Google Scholar]
- 26. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–4. [DOI] [PubMed] [Google Scholar]
- 27. Kreis R. Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR Biomed. 2004;17(6):361–81. [DOI] [PubMed] [Google Scholar]
- 28. Simoes E, Correia-Lima J, Sardas L, Storti F, dos Santos Otani TZ, Vasques DAC, Otani VHO, Bertolazzi P, Kochi C, Seelaender Met al. . Sex dimorphism in inflammatory response to obesity in childhood. Int J Obes. 2021;45(4):879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calderón-Garcidueñas L, Mora-Tiscareño A, Melo-Sánchez G, Rodríguez-Díaz J, Torres-Jardón R, Styner M, Mukherjee PS, Lin W, Jewells V. A critical proton MR spectroscopy marker of Alzheimer's disease early neurodegenerative change: Low hippocampal NAA/Cr ratio impacts APOE ɛ4 Mexico City children and their parents. J Alzheimers Dis. 2015;48(4):1065–75. [DOI] [PubMed] [Google Scholar]
- 30. Moffett JR, Arun P, Ariyannur PS, Namboodiri AMA. N-acetylaspartate reductions in brain injury: Impact on post-injury neuroenergetics, lipid synthesis, and protein acetylation. Front Neuroenergetics. 2013;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guyenet SJ, Schwartz MW. Regulation of food intake, energy balance, and body fat mass: Implications for the pathogenesis and treatment of obesity. J Clin Endocrinol Metab. 2012;97(3):745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berkseth KE, Guyenet SJ, Melhorn SJ, Lee D, Thaler JP, Schur EA, Schwartz MW. Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: A combined immunohistochemical and magnetic resonance imaging study. Endocrinology. 2014;155(8):2858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thaler JP, Yi C-X, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KRet al. . Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dorfman MD, Thaler JP. Hypothalamic inflammation and gliosis in obesity. Curr Opin Endocrinol Diabetes Obes. 2015;22(5):325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schur EA, Melhorn SJ, Oh S-K, Lacy JM, Berkseth KE, Guyenet SJ, Sonnen JA, Tyagi V, Rosalynn M, De Leon Bet al. . Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity. 2015;23(11):2142–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wiguna T, Guerrero APS, Wibisono S, Sastroasmoro S. Effect of 12-week administration of 20-mg long-acting methylphenidate on Glu/Cr, NAA/Cr, Cho/Cr, and mI/Cr ratios in the prefrontal cortices of school-age children in Indonesia: A study using 1H magnetic resonance spectroscopy (MRS). Clin Neuropharmacol. 2012;35(2):81–5. [DOI] [PubMed] [Google Scholar]
- 37. Jessen F, Fingerhut N, Sprinkart AM, Kühn KU, Petrovsky N, Maier W, Schild HH, Block W, Wagner M, Träber F. N-acetylaspartylglutamate (NAAG) and N-acetylaspartate (NAA) in patients with schizophrenia. Schizophr Bull. 2013;39(1):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun J, Chen P, Bi C. 1H-MRS technique and spectroscopic imaging LCModel based adolescent obese metabolic syndrome research. Multimed Tools Appl. 2017;76(19):19491–505. [Google Scholar]
- 39. Baslow MH. N-acetylaspartate in the vertebrate brain: Metabolism and function. Neurochem Res. 2003;28(6):941–53. [DOI] [PubMed] [Google Scholar]
- 40. Karczewska-Kupczewska M, Tarasów E, NikoŁajuk A, Stefanowicz M, Matulewicz N, Otziomek E, Górska M, Straczkowski M, Kowalska I. The effect of insulin infusion on the metabolites in cerebral tissues assessed with proton magnetic resonance spectroscopy in young healthy subjects with high and low insulin sensitivity. Diabetes Care. 2013;36(9):2787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heurling K, Johansson E, Leuzy A. Disturbances in brain energy metabolism in insulin resistance and diabetes and Alzheimer's disease–Learnings from brain imaging biomarkers. Int Rev Neurobiol. 2020;154:111–30. [DOI] [PubMed] [Google Scholar]
- 42. Mon A, Abé C, Durazzo TC, Meyerhoff DJ. Effects of fat on MR-measured metabolite signal strengths: Implications for in vivo MRS studies of the human brain. NMR Biomed. 2013;26(12):1768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kyathanahally SP, Fichtner ND, Adalid V, Kreis R. Does superficial fat affect metabolite concentrations determined by MR spectroscopy with water referencing?. NMR Biomed. 2015;28(11):1543–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book and analytic code will be made available upon request to the corresponding author Estefania.simoesfer@gmail.com.