ABSTRACT
Background
Children with Autism Spectrum Disorders (ASDs) tend to be selective in their food intake, which may compromise their diet quality. While ASD diagnoses capture severe levels of impairment, autistic traits vary on a continuum throughout the population. Yet, little is known about how autistic traits relate to diet quality at the population level.
Objectives
This study examines the association between autistic traits in early childhood and diet quality in mid-childhood and explores the mediating role of food selectivity.
Methods
Participants were children (n = 4092) from the population-based Generation R Study. Parents reported their child's autistic traits at 1.5, 3, and 6 years; food selectivity at 4 years; and food intake at 8 years, from which a diet quality score was derived. Associations of autistic traits and the autistic trait trajectory (identified using Latent Class Growth Modelling) with diet quality were examined using multiple linear regression models. The indirect effect of food selectivity in the association between autistic traits at 1.5 years and diet quality was examined using mediation analysis.
Results
Autistic traits were associated with diet quality (e.g., 1.5 years: β = −0.09; 95% CI: −0.13 to −0.06). Two classes captured the autistic trait trajectories from 1.5 to 6 years: children with “low and stable” (95%) and “high and increasing” (5%) mean scores. Children in the high and increasing group had poorer diet quality than those in the low and stable group (β = −0.28; 95% CI: −0.44 to −0.11). Food selectivity mediated the association between autistic traits at 1.5 years and diet quality at 8 years (βindirect = −0.03; 95% CI: −0.03 to −0.02).
Conclusions
Autistic traits in early childhood are associated with poorer diet quality in mid-childhood, and food selectivity appears to mediate this association. Interventions intended to optimize nutrition in children with elevated autistic traits may integrate behavioral strategies to support parents’ responding to their child's food selectivity.
Keywords: autism, autistic traits, picky eating, food selectivity, child, diet quality, latent class growth modelling, mediation, trajectories
Introduction
The prevalence of feeding (i.e., eating) problems in children with Autism Spectrum Disorders (ASDs) is 5-fold that of neurotypical children (1), culminating in limited dietary variety (2), nutrient inadequacy (3, 4), and overweight (5, 6). The Diagnostic and Statistical Manual of Mental Disorders (DSM) 5 characterizes ASDs as neurodevelopmental conditions that manifest in social communication deficits and restrictive and repetitive behavioral patterns (7). Approximately 1% of the global population has a clinical ASD diagnosis (8), yet autistic traits are also distributed on a continuum in the general population (9). An ASD diagnosis represents the extreme end of this continuum; however, this condition is heterogeneous in presentation. Most nutrition-related research focuses on comparing the diets of children with ASD to those of neurotypical children. Such approaches overlook autistic traits as a continuous dimension, and may underrepresent children with subclinical ASD or girls with ASD, who may be more difficult to identify (10).
Research to date shows that children with ASD tend to have lower intakes of individual nutrients compared to their neurotypical peers. A meta-analysis showed that children with ASD consume less protein, calcium, phosphorus, selenium, vitamin D, thiamine, riboflavin, vitamin B12, and ω-3 (11). While this may indicate potential nutritional concerns, individuals consume combinations of foods, rather than discrete nutrients, which have an interactive and cumulative effect on their health and disease risk (12). However, assessments of the whole diet, such as dietary patterns or quality, are generally absent from the autism literature. Diet quality in childhood, which is broadly measured by an individual's adherence to dietary guidelines, is prospectively associated with metabolic and mental health outcomes (13). Thus, a more complete examination of diet quality is needed to understand how autistic traits relate to diet, particularly in the general pediatric population.
Ritualistic tendencies, sensory processing issues, and insistence on sameness are features of autistic traits that may predispose children to food selectivity (or picky eating) (14). Food selectivity describes a child's aversion to certain tastes, textures, colors, types, or brands of food, resulting in a restricted variety of food intake (15). While food selectivity is common in neurotypical children, food selectivity is more prevalent, more severe, and more enduring in children with ASD (1). Food selectivity may explain variation in diet quality in children with ASD (16), and yet food selectivity is an underrecognized and undertreated component of ASD (17). Testing the role of food selectivity in the ASD-diet association could assist in establishing a conceptual theory (18) to inform the development of nutrition interventions. Therefore, the current study aims to examine the association between child autistic traits and diet quality (aim 1), and to explore the potential mediating role of food selectivity in this association (aim 2).
Methods
Study design and population
The study is embedded in the Generation R Study, a population-based cohort on health and development from fetal life onwards (19). All pregnant women living in Rotterdam, the Netherlands, with an expected delivery date between April 2002 and January 2006 were invited to participate (N = 9778; participation rate: 61%). The study has been approved by the Medical Ethical Committee of Erasmus Medical Center Rotterdam. Written informed consent was obtained from parents of all children.
Full consent for participation up to 8 years was obtained from parents for 6625 children. Participants with data on at least 1 autistic trait score at 1.5, 3, or 6 years (n = 5962) were considered for inclusion in the analyses to address aim 1. Of these participants, 4092 completed diet quality data at 8 years. Compared to this current analytical sample, children excluded due to missing values on diet or autistic traits (n = 2533) were more likely to be non-Dutch, had a lower birthweight, and had a higher BMI at 6 years, and their mothers were younger and had lower levels of education (all P values ≤ 0.001). To address aim 2, data were available for 3360 participants who had complete data on the autistic trait score at 1.5 years, food selectivity at 4 years, and diet quality at 8 years (see Supplemental Figure 1 for a study participant flowchart).
Measures
Autistic traits
At child ages (mean ± SD) 1.5 ± 0.1, 3.0 ± 0.1, and 6.1 ± 0.4 years, parents completed the Child Behavior Checklist (CBCL)/1.5–5 (20). We deliberately chose the CBCL/1.5–5 version, as the questionnaires were sent to the families before children turned 6 (57% of children were aged 5 years when the CBCL was completed). The 13-item, DSM-oriented Pervasive Developmental Problems subscale was used as an indicator of autistic traits. This scale has been shown to discriminate between groups of children with ASD, children with other developmental disorders, and children without clinical diagnoses (21). Parents responded to items on a 3-point Likert scale from 0 (never) to 2 (often). Items were summed to produce an overall score. All autistic trait scores from the CBCL (20) were used to examine the association between early autistic traits and diet quality in mid-childhood (aim 1). The autistic trait score at age 1.5 years was used to examine temporal associations with food selectivity at 4 years and diet quality at 8 years (aim 2).
At child age 6 years, parents also completed the 18-item short-form Social Responsiveness Scale (SRS) (22). The SRS is an autism screening questionnaire that provides a valid quantitative measure of (sub)clinical autistic traits. Items cover all autism domains of the DSM-5 and show good diagnostic validity (23, 24). Parents rated items on a 4-point Likert scale from 0 (never true) to 3 (almost always true). Items were summed to produce an overall SRS score. The SRS scale can also be further divided into subscales underpinning specific domains of autistic traits, including social cognition (5 items), social communication (8 items), and autistic mannerisms (5 items).
Diet quality
At child age 8.1 ± 0.2 years, parents completed a validated semi-quantitative FFQ (25). Parents were asked about their child's frequency and quantity of intake for a total of 71 food items in the prior 4 weeks. These food items were selected based on national survey data for children (26). Additional questions concerning the types and brands of foods and preparation methods were included for 27 food items to facilitate better estimation of nutrient intake. Information on the frequencies, types, and portion sizes of foods was converted into grams consumed per day using SAS VoVris (Vovris V2.4, TNO, 1999–2006). To quantify diet quality based on the gap between recommended and actual intakes, a diet quality score reflecting adherence to age-specific Dutch dietary guidelines was developed in Generation R in early (27) and mid-childhood (28). The score used in the current study reflected the dietary guidelines based on mid-childhood (28) and included the following 10 components: fruit (≥150 g/d; 2 items), vegetables (≥150 g/d; 2 items), whole grains (≥90 g/d; 2 items), fish (≥60 g/wk; 6 items), legumes (≥84 g/wk; 1 item), nuts (≥15 g/d; 2 items), dairy (≥300 g/d; 3 items), oils and soft or liquid margarines (≥30 g/d; 1 item), sugar-containing beverages (≤150 g/d; 4 items), and high-fat and processed meat (≤250 g/wk; 4 items). The ratio of the reported intake to recommended intake was calculated for each component (e.g., a vegetable intake of 90g/d would result in a score of 0.6, which is 90 g divided by the recommended 150 g). Sugar-containing beverages and meat were reverse coded. Individual component scores above 1 were truncated to 1 to align with the minimum recommended intake, indicating the best adherence to that food component according to the Dutch dietary guidelines (27). Individual component scores were then summed into a diet quality score ranging from 0 to 10, with a higher score indicating better dietary quality (27, 28).
Food selectivity
At child age 4.0 ± 0.1 years, food selectivity was measured via the 6-item “food fussiness” subscale (items shown in Supplemental Table 1) from the parent-reported Children's Eating Behaviour Questionnaire (CEBQ) (29). The CEBQ has good test-retest reliability and internal consistency (29). Items were anchored on a 5-point Likert scale from 1 (never) to 5 (always) and summed.
Covariates
Several possible confounders are considered in the analyses. Information on child sex and birth weight was obtained from hospital/midwife registries. Child ethnicity (categorized as Dutch or non-Dutch) was based on the country of birth of both biological parents, which was obtained at enrolment. Children of both parents born in the Netherlands were considered to have a Dutch ethnic background. For children who had 1 parent born outside of the Netherlands, the country of birth of that parent determined the child's ethnicity. If both parents were born abroad, the country of birth of the mother determined the ethnicity of the child. Children's height and weight were measured by research assistants at the research center visit at 6 years and converted into a sex- and age-adjusted BMI z-score using Dutch reference growth curves (30). Maternal age and education level were assessed by a postal questionnaire during pregnancy. Previous research in the Netherlands has shown that maternal education is the strongest socioeconomic correlate of child dietary patterns (31); therefore, maternal education was used a proxy for socioeconomic status in the current analysis. Energy intake was measured with the FFQ at 8 years and calculated using the Dutch Food Composition Table (NEVO 2001).
Statistical analyses
All analyses were performed in R (version 3.5.3; R Foundation for Statistical Computing). Two-sided statistical significance was set as α <0.05. For all scales used in the analysis, <25% of items missing were allowed and weighted sum scores were calculated and then standardized for comparative purposes. Assumptions were checked before all analyses were performed, and the main outcome of interest (diet quality) was normally distributed. Correlations between the main variables of interest were explored using Pearson's correlations. For aim 1, multiple linear regression models were run to examine the association between the autistic trait score at each assessment age [1.5, 3, and 6 years; CBCL (20)] and the diet quality score at 8 years. To examine consistency in results across measures, analyses with the CBCL autistic trait score at 6 years were rerun with the SRS at 6 years, which is a more specific and detailed screening tool used to assess ASD. We also created trajectories of the 3 repeated CBCL autistic trait assessments using Latent Class Growth Models (LCGM) in the lcmm package (32). LCGMs were estimated with a class-specific intercept and slope for each assessment wave, beginning with a single latent class and increasing the number of classes by 1 until the best-fitting model to explain the data was identified. Both linear and quadratic trajectories were examined. Best fit was determined by selecting the model with the lowest Bayesian information criterion, high posterior probabilities for each class, and at least 5% of individuals assigned to 1 class. Then, a linear regression model was run to examine the association between the autistic trait trajectories and diet quality score at 8 years. For each of the regression models with autistic trait scores at different ages/trajectories, 2 models were run. Model 1 adjusted for child sex, age, and energy intake, and Model 2 additionally adjusted for child ethnicity, birth weight, BMI z-score (6 years), maternal age at recruitment, and maternal education. The models adjusted for energy intake to control for over- or underconsumption (33, 34). To examine whether the association between the autistic trait score/trajectories and diet quality score was driven by particular food component scores, post hoc analyses were conducted with separate multiple linear regressions, adjusting for covariates. Missing values on covariates were imputed using multiple imputation by chained equations using 20 imputed data sets in the mice package (35).
To address aim 2, a structural equation modeling analysis in the lavaan package (36) was used to estimate the indirect effects of autistic traits at 1.5 years and diet quality at 8 years through food selectivity at 4 years, adjusting for covariates. The model was estimated using maximum likelihood estimation to obtain the bootstrapped estimates (with 5000 resamples) and 95% CIs for the total, indirect, and direct effects. Missing values on covariates were imputed using Full Information Maximum Likelihood. Post hoc analyses were performed to examine the indirect effects of food selectivity on the association between autistic traits at 1.5 years and specific food components at 8 years. Only food component scores significantly associated with autistic traits at 1.5 years and with food selectivity were examined in this step. Post hoc analyses were rerun with the autistic trait score at 3 years.
Sensitivity analyses included rerunning all analyses in children with a Dutch ethnic background only, as the FFQ was validated in children with a Dutch ethnic background (25). Children who also met the screening cutoff on the SRS were excluded as a sensitivity analysis. Based on the developer's recommended screening cutoffs in population-based settings [weighted mean scores of 1.078 for boys and 1.000 for girls (37)], 51 (1.2%) of children in the sample met the cutoff (34 boys and 17 girls). All analyses were also rerun using nonimputed data to test whether the methods used to deal with missing data affected the results.
Results
Imputed participant sociodemographic characteristics are shown in Table 1 (nonimputed characteristics are available in Supplemental Table 2). Descriptions of the main variables of interest are shown in Table 2. Pearson correlations showed that autistic traits at 1.5, 3, and 6 years were all negatively associated with diet quality at 8 years and positively associated with food selectivity at 4 years (all P values < 0.001; Supplemental Table 3).
TABLE 1.
Imputed sample characteristics (N = 4092)1
| Characteristics | |
|---|---|
| Child | |
| Sex, % boys | 2027 (49.4) |
| Birthweight, g | 3445.0 ± 570.0 |
| Ethnicity, % Dutch | 2829 (69.1) |
| BMI z-score,2 6 years | 0.2 ± 0.8 |
| Age at diet quality assessment, years | 8.1 ± 0.2 |
| Energy intake,3 kcal/d | 1483.8 ± 371.5 |
| Mother | |
| Age at inclusion, y | 31.8 ± 4.4 |
| Educational level4 | |
| High | 1422 (34.8) |
| Mid-high | 1066 (26.1) |
| Mid-low | 1095 (26.8) |
| Low | 509 (12.4) |
Values are shown as the mean ± SD for continuous variables or frequency (%) for categorical variables.
Sex- and age-adjusted BMI z-score, calculated using Dutch Reference growth curves (30).
Dervied from the FFQ at 8 years.
Education levels include: low (<3 years of secondary school), mid-low (>3 years of secondary school; intermediate vocational training; first year of higher vocational training), mid-high (higher vocational training; Bachelor's degree), and high (university level).
TABLE 2.
Descriptives of study variables
| Scale | Mean ± SD | α | N | |
|---|---|---|---|---|
| Autistic traits score1 (CBCL/1.5–5) | 0 to 25 | — | — | — |
| 1.5 years | — | 1.6 ± 1.9 | 0.61 | 3629 |
| 3 years | — | 1.9 ± 2.1 | 0.67 | 3679 |
| 6 years | — | 2.1 ± 2.3 | 0.71 | 3898 |
| Autistic traits score (SRS),2 6 years | 0 to 54 | 3.8 ± 4.1 | 0.78 | 3651 |
| Social cognition | 0 to 15 | 1.8 ± 1.8 | 0.46 | 3641 |
| Social communication | 0 to 24 | 1.5 ± 2.1 | 0.68 | 3648 |
| Autistic mannerisms | 0 to 15 | 0.5 ± 1.2 | 0.68 | 3651 |
| Diet quality score,3 8 years | 0 to 10 | 4.5 ± 1.2 | — | 4092 |
| Food selectivity,4 4 years | 5 to 30 | 17.8 ± 4.9 | 0.90 | 3360 |
Pervasive Developmental Problems subscale from the Child Behavior Checklist/1.5–5 (20). Abbreviations: α, Cronbach's alpha; CBCL, Child Behavior Checklist; SRS, Social Responsiveness Scale.
SRS (22).
Developed by van der Velde et al. (28).
Food fussiness subscale of the Children's Eating Behaviour Questionnaire (29).
Aim 1: associations between autistic traits and diet quality
Multiple linear regression analyses showed that autistic trait scores at 1.5, 3, and 6 years were negatively associated with diet quality at 8 years (Table 3). Estimates were also similar in magnitude between the total SRS score (22) at 6 years and diet quality (e.g., model 2: β = −0.09; 95% CI: −0.13 and −0.05). Individual SRS subscales were also associated with diet quality; social cognition showed the strongest relationship (β = −0.10; 95% CI: −0.14 and −0.06) in the fully adjusted models (model 2), followed by social communication (β = −0.07; 95% CI: −0.11 and −0.03) and autistic mannerisms (β = −0.04; 95% CI: −0.08 and −0.005). In the LCGM analysis, the model with 2 linear trajectories showed an optimal fit. Trajectories included children with low and stable (n = 3885; 95%) and high and increasing autistic traits (n = 207; 5%; Figure 1). Children in the high and increasing group were more likely to be boys, to be Dutch, and to have younger mothers (all P values < 0.02). No other covariates were associated with the autistic trait trajectory. Approximately 73% of children who met the clinical cutoff score on the SRS (22) at 6 years were in the high and increasing group. Children in the high and increasing group had greater mean food selectivity scores than children in the low and stable group (19.5 compared with 17.7, respectively; P < 0.001). Furthermore, children in the high and increasing group had diet quality scores that were, on average, 0.28 SD points lower than children in the low and stable group (Table 3). In the post hoc analyses, autistic trait scores were associated with food components in the expected directions (Supplemental Table 4). Effect estimates showed that associations between autistic traits and diet quality were mainly explained by lower fruit, vegetable, whole grain, fish, and oil and soft margarine scores.
TABLE 3.
Multiple linear regression analyses showing the associations between autistic traits at various ages and diet quality at age 8 years1
| Autistic traits2 | Autistic trait trajectory3 | |||
|---|---|---|---|---|
| Model outcome: Diet quality at 8 years4 | 1.5 years, n = 3629 | 3 years, n = 3679 | 6 years, n = 3898 | Low and stable (referent group) compared with high and increasing |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Model 1 | −0.12 (−0.16 to −0.08) | −0.09 (−0.12 to −0.05) | −0.10 (−0.12 to −0.04) | −0.31 (−0.48 to −0.16) |
| Model 2 | −0.09 (−0.13 to −0.06) | −0.06 (−0.10 to −0.03) | −0.09 (−0.12 to −0.05) | −0.28 (−0.44 to −0.11) |
Values are pooled standardized regression coefficients and 95% CIs. All estimate P values are < 0.001. Model 1 adjusts for the child's energy intake, sex, and age. Model 2 additionally adjusts for child ethnicity, birth weight, BMI z-score (6 years), maternal age at recruitment, and education.
Pervasive Developmental Problems subscale from the Child Behavior Checklist/1.5–5 (20).
3Latent Class Growth Analysis categories: low and stable (n = 3885; 95%) and high and increasing (n = 207; 5%).
Developed by van der Velde et al. (28).
FIGURE 1.
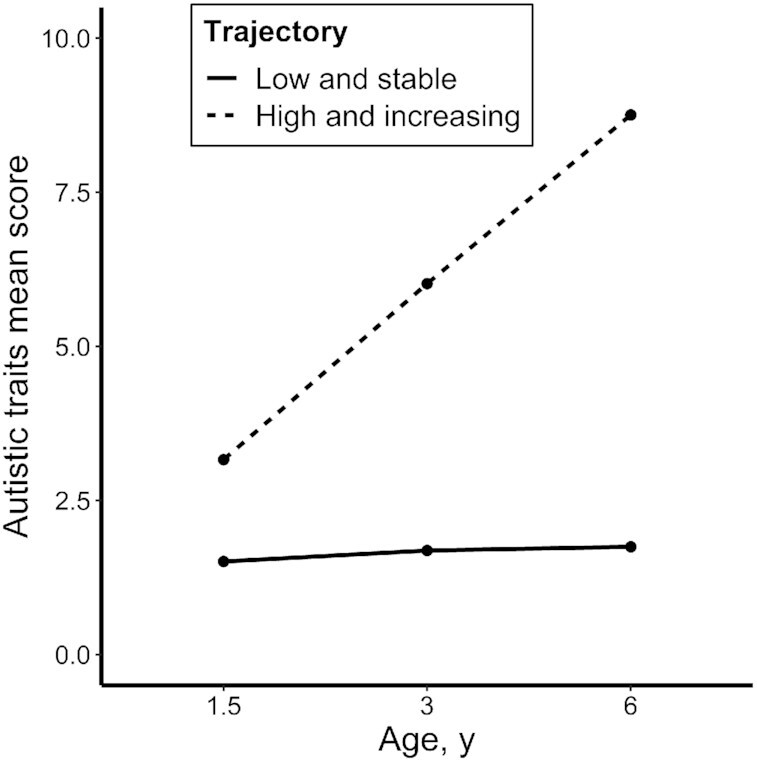
Mean scores of autistic traits across child age by each Latent Class Growth Analysis trajectory (N = 4092). The categories were low and stable (n = 3885; 95%) and high and increasing (n = 207; 5%). Autistic traits were measured at each time point via the Pervasive Developmental Problems subscale of the Child Behavior Checklist/1.5–5 (20).
Aim 2: mediation of autistic traits and diet quality through food selectivity
Food selectivity was negatively associated with diet quality, adjusting for all covariates (β = −0.23; 95% CI: −0.27 and −0.20). This inverse association between food selectivity and diet quality was mainly driven by low scores on fruit, vegetables, fish, legumes, and nuts (Supplemental Table 5). The mediation analysis showed a significant, indirect effect between the autistic trait score at 1.5 years and diet quality score at 8 years through food selectivity at 4 years (indirect effect: β = −0.02; 95% CI: −0.03 and −0.01; Figure 2). Post hoc mediation analyses were run with fruit and vegetable scores as the outcomes, as these were the only food components associated with autistic trait score at 1.5 years and food selectivity at 4 years. The indirect effect of food selectivity significantly mediated associations between autistic traits and fruit scores, as well as autistic traits and vegetable scores (Supplemental Table 6). These analyses were repeated using the assessment of autistic traits at 3 years, and the findings were similar (data not shown). In the sensitivity analyses (rerunning analyses in children with a Dutch ethnic background only, excluding children above the SRS screening cutoff, and rerunning the analyses with nonimputed data), all estimates were in a similar direction and reflected similar effect sizes (data not shown).
FIGURE 2.
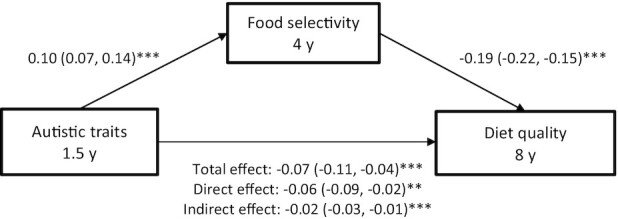
Mediation pathway showing the indirect relationship between child autistic traits and diet quality through food selectivity. **P < 0.01; ***P < 0.001. Values represent the standardized coefficients (95% CIs) for each pathway, adjusted for the child's energy intake, sex, age, ethnicity, birth weight, BMI z-score (6 years), maternal age at recruitment, and education (n = 3360). Autistic traits were assessed using the Pervasive Developmental Problems subscale from the Child Behavior Checklist/1.5–5 (20). Food selectivity assessed using the food fussiness subscale from the Children's Eating Behaviour Questionnaire (29). Diet quality was assessed using methods outlined in van der Velde et al. (28).
Discussion
The current study provides support for an inverse association between early expression of autistic traits and diet quality in mid-childhood in a population-based cohort, with food selectivity appearing to play a key role in this association. A higher diet quality score in this cohort has previously been associated with increased intakes of micronutrients, plant protein, dietary fiber, and ω-3 fatty acids and a decreased intake of saturated fat (28). Findings are therefore in the expected direction as based on clinical research examining nutrient intakes in children diagnosed with ASD (3). Findings may also be suggestive of a phenotypic overlap between autistic traits and eating disorder traits, such as Avoidant Restrictive Food Intake Disorder symptoms (38) or other eating disturbances (39), which may manifest through food selectivity and are evident in dietary patterns. This expanded understanding of autistic traits and diet within a community sample contributes to advancing the early detection of children at risk of poor diet quality, and can inform the development of early dietary interventions.
Dietary patterns are established in childhood and track into adulthood (40); thus, careful monitoring of food intake in children with elevated autistic-like behaviors is needed from a young age. The current research suggests that while there is a linear association between autistic traits and diet quality, children with initially high levels of autistic traits at a young age (toddlers) that increase up to 6 years may be at particular risk of poor diet quality. ASD screening tools could assist in identifying elevated autistic-like behaviors. However, these tools are typically designed to prospectively diagnose ASD (41), rather than to flag and monitor children with subclinical levels of autistic traits. Therefore, purpose-built screening tools to identify children with elevated autistic traits could assess concurrent problematic eating behaviors like food selectivity, as well as a history of feeding problems beginning in the milk feeding period (42). Such a tool could assist with implementing timely interventions before dietary habits become entrenched.
Addressing food selectivity may be a key ingredient in the delivery of dietary interventions tailored for children with elevated autistic traits. These children may be delayed in “growing out” of a picky eating stage or may not transition out of picky eating at all, and therefore they require careful approaches to management. Parent-mediated interventions are central to the success of dietary interventions, as parents provide food and structure mealtimes within the home. A previous study from the same cohort showed that parental monitoring, a structure-related feeding practice characterized by keeping track of what and how much their child eats, is associated with increased diet quality (43). Early-stage trials examining nutrition education delivered to parents of children with ASD who were exhibiting food selectivity appear promising (44, 45), although whether these interventions could be adapted to children with subclinical autistic traits should be explored. Parents caring for children with elevated autistic traits may experience mealtimes as a significant source of stress (46). Considering that eating or feeding difficulties are evident from infancy (42, 47), the emotional climate of mealtimes and parents’ distress in response to children's food rejection may be ingrained by mid-childhood; thus, early interventions are required. Supporting parents’ responsiveness to their child's needs, abilities, and level of communication at mealtimes, while managing their expectations, could be investigated as a strategy to improve diet quality.
Current findings suggest that clinicians working with young children who present with elevated autistic traits could be alert to associations with poor diet quality. These associations appeared to be primarily driven by inadequate fruit, vegetable, and fish intakes, which showed the largest effect sizes with autistic traits. This may be partly explained by a preference for more uniform, bland, and neutral-colored foods in children with ASD (48). Children with ASD also tend to have sensory sensitivities, such as atypical sensory oral processing, which may contribute to a lower or reduced variety of fruit and vegetable intakes (49). Sensory sensitivities could also contribute to reduced fish intake in children with elevated autistic traits, although fish intake has been less well studied compared to fruit and vegetable intakes. Furthermore, fresh food products tend to be inconsistent in sensory characteristics across offerings (e.g., the ripeness of a banana), and children with ASD tend to have a preference for predictability (50). A more in-depth study of the food preferences and eating idiosyncrasies of children with elevated autistic traits could inform early intervention strategies to improve diet quality.
Strengths of this research include the population-based study design with repeated measures of autistic traits, which mitigates some limitations of clinical studies. Families with an interest in child nutrition may opt into diet-related clinical research, therefore introducing self-selection bias, while the population-based design of the Generation R Study reduces the likelihood of this bias. However, parents reported their child's autistic traits, food selectivity, and food intake, which may introduce shared-reporter bias or social desirability bias. To some extent, parents of school-age children may not be aware of their child's actual intake due to some meals being consumed away from home or at school. However, Dutch children bring food from home to school, and therefore parents in the current study may have a good overview of what their children eat, or at least what they are offered, at meals. The diet quality score was developed in this specific cohort based on Dutch dietary guidelines, which may limit the generalizability of results outside of the Netherlands. Although Dutch guidelines are comparable to those of other countries, some food groups may be absent for children from a non-Dutch ethnic background. The diet quality measure did not include foods which were not mentioned in the dietary guidelines (i.e., sweets, cakes, crisps), and therefore further investigation into the displacement of healthy foods compared to energy-dense and nutrient-poor foods could not be examined, except for sugar-sweetened beverages and processed meats. Differences in sociodemographic characteristics between participants who were included and excluded in the current study (due to missing data on autistic traits or dietary intakes) may suggest that findings cannot be generalized beyond socioeconomically homogenous populations. Furthermore, ASD is a heterogeneous condition, and future research could consider examining idiosyncrasies of diets related to subclinical autistic traits.
The current study demonstrates a graded association between the expression of autistic traits in early childhood and poorer diet quality in mid-childhood at the population level, and food selectivity may be a potential mechanism explaining this association. Clinicians providing dietary counselling to families with children who may be developmentally vulnerable may consider providing additional support for parents regarding appropriately and safely responding to food selectivity. Future research could investigate food selectivity as a central focus point of behavioral or nutrition interventions in young children with elevated autistic-like behaviors. Supporting parents’ management of difficult mealtime behaviors may be a realistic goal for dietary management.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—HAH, PWJ, YM, and TV: designed and conducted the research; HAH: performed the statistical analyses and has primary responsibility for the final content; HAH, YM, and PWJ: wrote the paper; GCD and TV: critically reviewed the manuscript for important intellectual content; and all authors: read and approved the final manuscript.
Notes
The general design of the Generation R Study is made possible by financial support from the Erasmus Medical Center and the Erasmus University Rotterdam; the Netherlands Organization for Health Research and Development (ZonMW); the Netherlands Organisation for Scientific Research (NWO); the Ministry of Health, Welfare, and Sport; and the Ministry of Youth and Families. This project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement (No. 707404 to HAH). The current study was also made possible by a grant from the Netherlands Organization for Health Research and Development (Mental Health Care Research Program, Fellowship 636320005 to PWJ).
Author disclosures: YM is supported by China Scholarship Council (CSC) PhD Fellowship for her PhD study in Erasmus Medical Center, Rotterdam, the Netherlands (scholarship file number 201806240125; http://www.csc.edu.cn/). All other authors report no conflicts of interest.
The opinions expressed in this document reflect only the author's view. The European Commission is not responsible for any use that may be made of the information it contains. All sources of support had no involvement or restrictions regarding the current manuscript.
Supplemental Figure 1 and Supplemental Tables 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ASD, Autism Spectrum Disorder; CBCL, Child Behavior Checklist; CEBQ, Children's Eating Behaviour Questionnaire; DSM, Diagnostic and Statistical Manual of Mental Disorders; LCGM, Latent Curve Growth Model; SRS, Social Responsiveness Scale.
Contributor Information
Holly A Harris, Department of Child & Adolescent Psychiatry/Psychology, Erasmus MC, University Medical Center, Rotterdam, The Netherlands; Generation R Study, Erasmus MC, University Medical Center, Rotterdam, The Netherlands.
Yuchan Mou, Generation R Study, Erasmus MC, University Medical Center, Rotterdam, The Netherlands; Department of Epidemiology, Erasmus MC, University Medical Center, Rotterdam, The Netherlands.
Gwen C Dieleman, Department of Child & Adolescent Psychiatry/Psychology, Erasmus MC, University Medical Center, Rotterdam, The Netherlands.
Trudy Voortman, Department of Epidemiology, Erasmus MC, University Medical Center, Rotterdam, The Netherlands; Division of Human Nutrition and Health, Wageningen University & Research, Wageningen, The Netherlands.
Pauline W Jansen, Department of Child & Adolescent Psychiatry/Psychology, Erasmus MC, University Medical Center, Rotterdam, The Netherlands; Department of Psychology, Education and Child Studies, Erasmus University Rotterdam, Rotterdam, The Netherlands.
Data Availability
Data described in the manuscript, code book, and analytic code can be made available upon request to datamanagementgenr@erasmusmc.nl and will be discussed in the Generation R Study Management Team.
References
- 1. Sharp WG, Berry RC, McCracken C, Nuhu NN, Marvel E, Saulnier CA, Klin A, Jones W, Jaquess DL. Feeding problems and nutrient intake in children with Autism Spectrum Disorders: a meta-analysis and comprehensive review of the literature. J Autism Dev Disord. 2013;43(9):2159–73. [DOI] [PubMed] [Google Scholar]
- 2. Emond A, Emmett P, Steer C, Golding J. Feeding symptoms, dietary patterns, and growth in young children with Autism Spectrum Disorders. Pediatrics. 2010;126(2):e337–42. [DOI] [PubMed] [Google Scholar]
- 3. Johnson CR, Turner K, Stewart PA, Schmidt B, Shui A, Macklin E, Reynolds A, James J, Johnson SL, Courtney PM. Relationships between feeding problems, behavioral characteristics and nutritional quality in children with ASD. J Autism Dev Disord. 2014;44(9):2175–84. [DOI] [PubMed] [Google Scholar]
- 4. Suarez MA, Nelson NW, Curtis AB. Longitudinal follow-up of factors associated with food selectivity in children with Autism Spectrum Disorder. Autism. 2014;18(8):924–32. [DOI] [PubMed] [Google Scholar]
- 5. Wallace GL, Llewellyn C, Fildes A, Ronald A. Autism Spectrum Disorder and food neophobia: clinical and subclinical links. Am J Clin Nutr. 2018;108(4):701–7. [DOI] [PubMed] [Google Scholar]
- 6. Bandini LG, Curtin C, Phillips S, Anderson SE, Maslin M, Must A. Changes in food selectivity in children with Autism Spectrum Disorder. J Autism Dev Disord. 2017;47(2):439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Psychological Association . Diagnostic and Statistical Manual of Mental Disorders–5. Washington (DC): American Psychological Association; 2013. [Google Scholar]
- 8. Lord C, Brugha TS, Charman T, Cusack J, Dumas G, Frazier T, Jones EJH, Jones RM, Pickles A, State MWet al. Autism Spectrum Disorder. Nat Rev Dis Primers. 2020;6(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robinson EB, St Pourcain B, Anttila V, Kosmicki JA, Bulik-Sullivan B, Grove J, Maller J, Samocha KE, Sanders SJ, Ripke Set al. Genetic risk for Autism Spectrum Disorders and neuropsychiatric variation in the general population. Nat Genet. 2016;48(5):552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in Autism Spectrum Disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466–74. [DOI] [PubMed] [Google Scholar]
- 11. Esteban-Figuerola P, Canals J, Fernández-Cao JC, Arija Val V. Differences in food consumption and nutritional intake between children with Autism Spectrum Disorders and typically developing children: a meta-analysis. Autism. 2019;23(5):1079–95. [DOI] [PubMed] [Google Scholar]
- 12. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 13. Dalwood P, Marshall S, Burrows TL, McIntosh A, Collins CE. Diet quality indices and their associations with health-related outcomes in children and adolescents: an updated systematic review. Nutr J. 2020;19(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zickgraf HF, Richard E, Zucker NL, Wallace GL. Rigidity and sensory sensitivity: independent contributions to selective eating in children, adolescents, and young adults. J Clin Child Adolesc Psychology. 2020;1–3. [DOI] [PubMed] [Google Scholar]
- 15. van Dijk MWG, Buruma ME, Blijd-Hoogewys EMA. Detecting feeding problems in young children with Autism Spectrum Disorder. J Autism Dev Disord. 2021;51:4115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zimmer MH, Hart LC, Manning-Courtney P, Murray DS, Bing NM, Summer S. Food variety as a predictor of nutritional status among children with autism. J Autism Dev Disord. 2012;42(4):549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matson JL, Fodstad JC. The treatment of food selectivity and other feeding problems in children with Autism Spectrum Disorders. Res Autism Spectr Disord. 2009;3(2):455–61. [Google Scholar]
- 18. Fairchild AJ, McDaniel HL. Best (but oft-forgotten) practices: mediation analysis. Am J Clin Nutr. 2017;105:1259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaddoe VW, Mackenbach JP, Moll HA, Steegers EA, Tiemeier H, Verhulst FC, Witteman JC, Hofman A. The Generation R Study: design and cohort profile. Eur J Epidemiol. 2006;21(6):475–84. [DOI] [PubMed] [Google Scholar]
- 20. Achenbach T, Rescorla L. Manual for the ASEBA Preschool Forms & Profiles. Burlington (VT): University of Vermont, Research Center for Children, Youth, & Families; 2000. [Google Scholar]
- 21. Levy SE, Rescorla LA, Chittams JL, Kral TJ, Moody EJ, Pandey J, Pinto-Martin JA, Pomykacz AT, Ramirez AJ, Reyes N. ASD screening with the Child Behavior Checklist/1.5-5 in the study to explore early development. J Autism Dev Disord. 2019;49(6):2348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, Reich W. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33(4):427–33. [DOI] [PubMed] [Google Scholar]
- 23. Hampton J, Strand PS. A review of level 2 parent-report instruments used to screen children aged 1.5–5 for autism: a meta-analytic update. J Autism Dev Disord. 2015;45(8):2519–30. [DOI] [PubMed] [Google Scholar]
- 24. Constantino JN, Lavesser PD, Zhang YI, Abbacchi AM, Gray T, Todd RD. Rapid quantitative assessment of autistic social impairment by classroom teachers. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1668–76. [DOI] [PubMed] [Google Scholar]
- 25. Dutman AE, Stafleu A, Kruizinga A, Brants HAM, Westerterp KR, Kistemaker C, Meuling WJA, Alexandra GR. Validation of an FFQ and options for data processing using the doubly labelled water method in children. Public Health Nutr. 2011;14(03):410–7. [DOI] [PubMed] [Google Scholar]
- 26. Centre Netherlands Nutrition . Results of the Dutch Food Consumption Survey 1997–1998 [Dutch: Zo eet Nederland: resultaten van de Voedselconsumptiepeiling 1997–1998]. The Hague (Netherlands): Netherlands Nutrition Centre [Dutch: Voedingscentrum]; 1998. [Google Scholar]
- 27. Voortman T, Kiefte-de Jong JC, Geelen A, Villamor E, Moll HA, de Jongste JC, Raat H, Hofman A, Jaddoe VWV, Franco OH. The development of a diet quality score for preschool children and its validation and determinants in the Generation R Study. J Nutr. 2015;145(2):306–14. [DOI] [PubMed] [Google Scholar]
- 28. van der Velde LA, Nguyen AN, Schoufour JD, Geelen A, Jaddoe VWV, Franco OH, Voortman T. Diet quality in childhood: the Generation R Study. Eur J Nutr. 2019;58(3):1259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children's Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42(7):963–70. [DOI] [PubMed] [Google Scholar]
- 30. Fredriks AM, van Buuren S, Burgmeijer RJ, Meulmeester JF, Beuker RJ, Brugman E, Roede MJ, Verloove-Vanhorick SP, Wit JM. Continuing positive secular growth change in the Netherlands 1955–1997. Pediatr Res. 2000;47(3):316–23. [DOI] [PubMed] [Google Scholar]
- 31. Rashid V, Weijs PJM, Engberink MF, Verhoeff AP, Nicolaou M. Beyond maternal education: socio-economic inequalities in children's diet in the ABCD cohort. PLoS One. 2020;15(10):e0240423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Proust-Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J Stat Softw. 2017;78(2):1–56. [Google Scholar]
- 33. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4):1220S–8S. [DOI] [PubMed] [Google Scholar]
- 34. Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R, Troiano RP, Bingham S, Schoeller DA, Schatzkin A, Carroll RJ. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol. 2003;158(1):14–21. [DOI] [PubMed] [Google Scholar]
- 35. Buuren Sv, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2010;45(3):1–67. [Google Scholar]
- 36. Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48(2):36. [Google Scholar]
- 37. Constantino JN. Social Responsiveness Scale (SRS), manual. Los Angeles (CA): Western Psychological Services; 2002. [Google Scholar]
- 38. Dovey TM, Kumari V, Blissett J, Mealtime Hostage Parent Science Gang . Eating behaviour, behavioural problems and sensory profiles of children with avoidant/restrictive food intake disorder (ARFID), Autistic Spectrum Disorders or picky eating: same or different?. Eur Psychiatry. 2019;61:56–62. [DOI] [PubMed] [Google Scholar]
- 39. Solmi F, Bentivegna F, Bould H, Mandy W, Kothari R, Rai D, Skuse D, Lewis G. Trajectories of autistic social traits in childhood and adolescence and disordered eating behaviours at age 14 years: a UK general population cohort study. J Child Psychol Psychiatry. 2021;62(1):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Craigie AM, Lake AA, Kelly SA, Adamson AJ, Mathers JC. Tracking of obesity-related behaviours from childhood to adulthood: a systematic review. Maturitas. 2011;70(3):266–84. [DOI] [PubMed] [Google Scholar]
- 41. Charman T, Gotham K. Measurement issues: Screening and diagnostic instruments for Autism Spectrum Disorders–lessons from research and practise. Child Adolesc Ment Health. 2013;18(1):52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van't Hof M, Ester WA, van Berckelaer-Onnes I, Hillegers MHJ, Hoek HW, Jansen PW. Do early-life eating habits predict later autistic traits? Results from a population-based study. Appetite. 2021;156:104976. [DOI] [PubMed] [Google Scholar]
- 43. Mou Y, Jansen PW, Raat H, Nguyen AN, Voortman T. Associations of family feeding and mealtime practices with children's overall diet quality: results from a prospective population-based cohort. Appetite. 2021;160:105083. [DOI] [PubMed] [Google Scholar]
- 44. Sharp WG, Burrell TL, Berry RC, Stubbs KH, McCracken CE, Gillespie SE, Scahill L. The autism managing eating aversions and limited variety plan vs parent education: a randomized clinical trial. J Pediatr. 2019;211:185–92.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johnson CR, Brown K, Hyman SL, Brooks MM, Aponte C, Levato L, Schmidt B, Evans V, Huo Z, Bendixen Ret al. Parent training for feeding problems in children with Autism Spectrum Disorder: initial randomized trial. J Pediatr Psychol. 2019;44(2):164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Curtin C, Hubbard K, Anderson SE, Mick E, Must A, Bandini LG. Food selectivity, mealtime behavior problems, spousal stress, and family food choices in children with and without Autism Spectrum Disorder. J Autism Dev Disord. 2015;45(10):3308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bolton PF, Golding J, Emond A, Steer CD. Autism Spectrum Disorder and autistic traits in the Avon Longitudinal Study of Parents and Children: precursors and early signs. J Am Acad Child Adolesc Psychiatry. 2012;51(3):249–60..e25. [DOI] [PubMed] [Google Scholar]
- 48. Mayes SD, Zickgraf H. Atypical eating behaviors in children and adolescents with autism, ADHD, other disorders, and typical development. Res Autism Spectr Disord. 2019;64:76–83. [Google Scholar]
- 49. Chistol LT, Bandini LG, Must A, Phillips S, Cermak SA, Curtin C. Sensory sensitivity and food selectivity in children with Autism Spectrum Disorder. J Autism Dev Disord. 2018;48(2):583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goris J, Brass M, Cambier C, Delplanque J, Wiersema JR, Braem S. The relation between preference for predictability and autistic traits. Autism Res. 2020;13(7):1144–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code can be made available upon request to datamanagementgenr@erasmusmc.nl and will be discussed in the Generation R Study Management Team.


