Abstract
Cellular metabolism orchestrates the intricate use of tissue fuels for catabolism and anabolism to generate cellular energy and structural components. The emerging field of immunometabolism highlights the importance of cellular metabolism for the maintenance and activities of immune cells. Macrophages are embryo- or adult bone marrow-derived leukocytes that are key for healthy tissue homeostasis but can also contribute to pathologies such as metabolic syndrome, atherosclerosis, fibrosis or cancer. Macrophage metabolism has largely been studied in vitro. However, different organs contain diverse macrophage populations that specialize in distinct and often tissue-specific functions. This context specificity creates diverging metabolic challenges for tissue macrophage populations to fulfill their homeostatic roles in their particular microenvironment and conditions their response in pathological conditions. Here, we outline current knowledge on the metabolic requirements and adaptations of macrophages located in tissues during homeostasis and selected diseases.
Keywords: Tissue macrophages, metabolism, homeostasis, pathology, tissue regeneration
Subject terms: Monocytes and macrophages, Innate immunity, Inflammation
Introduction
Macrophages are tissue-resident immune cells that act as important immune sentinels and concomitantly execute vital homeostatic tasks to ensure tissue integrity and functionality. Indeed, macrophages are present in virtually every organ of the body. They colonize tissues to form self-maintaining populations, can be replenished by circulating monocytes following insults or are constantly differentiating from infiltrating monocytes. However, the compositions and requirements of different microenvironments and niches differ notably, demanding distinct functions from their resident macrophages [1]. Seminal studies have shown that the identity of macrophage populations is imprinted by the residing tissue, which is evidenced by the expression of tissue-associated signature transcription factors [2–6]. Indeed, after loss of resident macrophages, incoming monocytes are reprogrammed by environmental macrophage niches to adopt the transcriptional programs that define the resident population [7–9]. These mostly tissue-specific transcriptional programs are vital for the functions, maintenance and phenotypes of tissue macrophages. Interestingly, several of these signature macrophage transcription factors regulate fundamental metabolic features [2–6].
In vitro studies in the context of pro- or anti-inflammatory activation highlight the metabolic plasticity of macrophages, which can completely rewire aspects of their cellular metabolism—bioenergetics, nutrient usage, generation of metabolites/cellular building blocks, etc.—depending on the task at hand [10, 11]. These observations suggest that tissue-resident macrophage identity requires a certain metabolic state that may depend on the availability of metabolites or nutrients and, importantly, facilitates their tissue-specific function. Moreover, disease can change the tissue microenvironment and consequently affect macrophage metabolism and function; in turn, macrophage metabolism may be key for disease resolution or progression.
Cellular metabolism is a complex network that is essential for cellular fitness and consists of catabolic processes (degradation of nutrients, predominantly for metabolite or energy generation in mitochondria) and anabolic processes (use of metabolites for synthesis of cellular structures). In short, tissue fuels (such as glucose, lipids or amino acids) are converted into metabolites (such as pyruvate, tricarboxylic acid [TCA] cycle intermediates, fatty acids or free cholesterol) by several metabolic reactions (such as glycolysis, lipolysis or glutaminolysis). These cellular metabolites are either fully oxidized in mitochondria (for example, via the TCA cycle, fatty acid oxidation [FAO] and oxidative phosphorylation [OXPHOS] by the electron transport chain [ETC]), used for the production of cellular building blocks (for example, in amino acid, nucleotide or fatty acid synthesis [FAS]) or released from the cells to prevent toxicity (for example, lactate or excess cholesterol).
Here, we discuss current knowledge on the cellular metabolism of diverse macrophage populations that reside in different tissues, how it supports their homeostatic activities and contributes to defining their identities. We focus on macrophages that have been studied in vivo with similar or context-specific metabolic needs, such as populations in the lung, spleen, liver, peritoneum, brain and bone. In addition, while the metabolism of tissue macrophages during inflammation has been recently reviewed [12], we explain the metabolic and functional adaptations of these cells in selected examples of different types of chronic pathologies in which macrophages are key for disease progression.
The in vitro paradigm of macrophage metabolism
A large body of literature has focused on macrophage metabolism in vitro [11, 13]. In these studies, resting macrophage colony stimulating factor (M-CSF)-induced bone marrow-derived macrophages (BMDMs) are referred to as M0 macrophages and undergo stimuli-specific differentiation into a range of polarization states, simplistically summarized as M1 at one extreme and M2 at the other [14–16] (Fig. 1A).
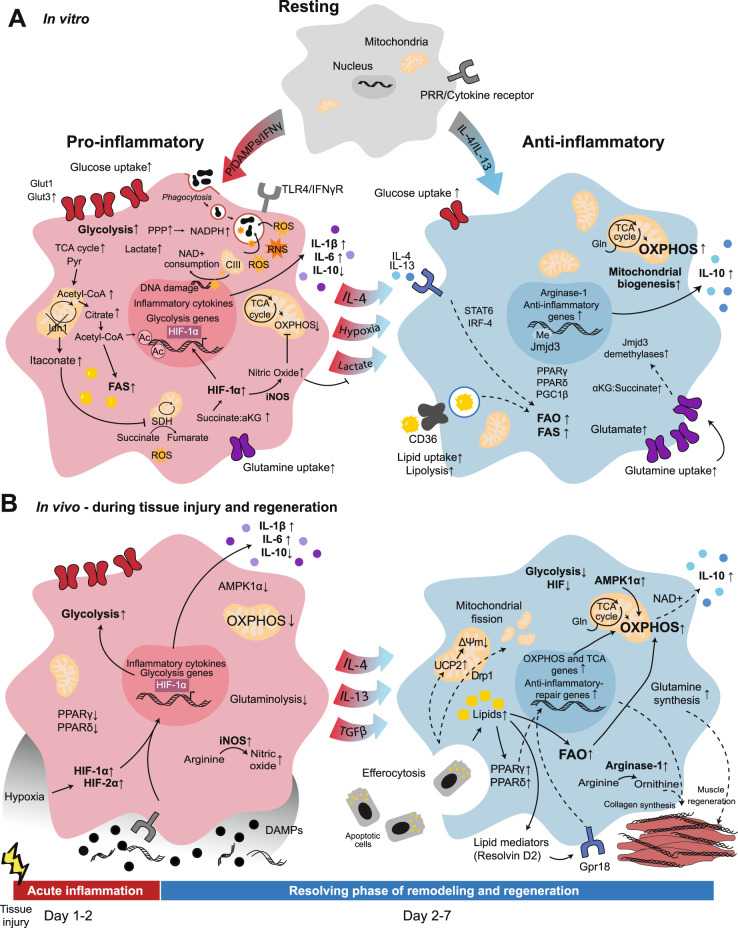
Fig. 1.
Metabolic rearrangement in macrophage polarization to proinflammatory or alternatively activated macrophages in vitro and in vivo. A In vitro, resting macrophages can be activated by various pathogen- or danger-associated molecular patterns (PAMPs or DAMPs), and cytokines polarize to classically activated proinflammatory M1 macrophages or alternatively activated anti-inflammatory M2 macrophages. B Upon in vivo tissue injury, damaged cell debris is released into the extracellular microenvironment, and an inflammatory response is mounted. Next, upon clearance of cell debris and DAMPs, the response changes to promote resolution of inflammation. Both in vitro (A left) and in vivo (B left), proinflammatory polarization has been associated with enhanced glycolytic metabolism; however, the majority of the related information has been elucidated in vitro. Both in vitro (A right) and in vivo (B right), increases in OXPHOS, FAO and glutaminolysis are associated with alternatively activated macrophages. Ac, Acetylation; CIII, complex III; Drp1, dynamin-related protein 1; Gln, Glutamine; Gpr18, G protein-coupled receptor 18; Me, Methylation; PRR, pattern recognition receptor; Pyr, Pyruvate; UCP2, mitochondrial uncoupling protein 2; ΔΨm, mitochondrial membrane potential. Solid lines: direct relationships; dashed lines: indirect relationships. Black circles: DAMPs; blue circles: anti-inflammatory cytokines; purple circles: proinflammatory cytokines; orange stars: ROS and RNS; black irregular ovals: phagocytosed particles
Classically activated “proinflammatory” (M1) macrophages are generated via Toll-like receptor (TLR) stimulation with agonists such as lipopolysaccharide (LPS) and/or cytokines such as interferon (IFN)γ [17, 18]. M1 macrophages are characterized by glycolytic metabolism, inducible nitric oxide synthase (iNOS) expression, and the production of proinflammatory cytokines [14, 19] (Fig. 1A). Upon TLR ligation, glucose is taken up, and glycolysis increases [16, 20, 21]. Elevated amounts of glycolytic intermediates promote the pentose phosphate pathway (PPP), which supports nicotinamide adenine dinucleotide phosphate (NADPH) generation for nucleotide biosynthesis and reactive oxygen species (ROS) production [19, 22]. Increased glycolysis leads to enhancement of lactate production and entry of glucose-derived pyruvate into the TCA cycle [23, 24]. This is accompanied by two breaks within the TCA cycle [24, 25]. Isocitrate dehydrogenase (IDH)-1, the enzyme that converts isocitrate to α-ketoglutarate (αKG), is downregulated, allowing accumulation of citrate and synthesis of itaconate [23, 25–27]. Citrate is converted into acetyl-coenzyme A (CoA), which is used first for de novo histone acetylation and inflammatory gene transcription and second as a substrate in FAS, leading to fatty acid accumulation required for expansion of cell membranes and increased protein synthesis [19, 24, 28–31]. Itaconate is one of the most highly upregulated metabolites upon M1 macrophage activation [32–35]. Itaconate inhibits succinate dehydrogenase (SDH; complex II of the ETC), inhibiting the conversion of succinate to fumarate, leading to the second break in the TCA cycle and succinate accumulation [25, 34–37]. Succinate accumulation stabilizes hypoxia-inducible factor (HIF)-1α, promoting a second wave of sustained metabolic reprogramming via gene expression programs [19, 38]. This second phase is characterized by a decrease in mitochondrial respiration and a dependence on glycolysis, similar to the “Warburg effect” described in cancer cells [19, 39]. Itaconate has also been shown to modulate macrophage cytokine production independently of succinate accumulation [40]. Succinate oxidation by SDH promotes proinflammatory responses in LPS-treated cells and induces mitochondrial ROS (mtROS) generation [26, 37, 41]. M1-induced mtROS are recruited to the phagosome to enhance bacterial killing, but they also induce oxidative DNA damage, activating NAD-consuming poly-(ADP-ribose) polymerase (PARP) enzymes and leading to M1 macrophage reliance on the NAD+ salvage pathway [42–44]. M1-induced nitric oxide (NO) is antimicrobial but also inhibits the ETC to reduce mitochondrial respiration [27, 45, 46]. Mitochondrial dysfunction due to NO decreases the adenosine triphosphate (ATP):adenosine diphosphate (ADP) ratio in cells, which has been shown to dampen inflammatory responses [47]. However, NO can also prevent M2 repolarization of inflammatory macrophages, potentially impeding the transition to the resolution phase of the immune response [46–48].
Alternatively activated “anti-inflammatory” (M2) macrophages differentiate in response to interleukin (IL)-4 or IL-13 and are characterized by increased mitochondrial respiration, anti-inflammatory cytokine production, and high arginase-1 expression [18, 49, 50] (Fig. 1A). M2 macrophages promote T helper cell (Th)2 responses and the resolution of inflammation [51]. Increased arginase expression in M2 macrophages leads to increases in the activity of ornithine and polyamine biosynthesis pathways. The polyamine spermidine has been shown to hypusinate translation factor eukaryotic initiation factor 5 A, facilitating the expression of mitochondrial proteins required for OXPHOS-dependent M2 differentiation [52]. The energetic profile of these cells is characterized by increased expression of genes related to fatty acid uptake, transport, and oxidation and increased uptake of both glucose and fatty acids in culture [51]. While M1 macrophages increase glycolysis within a very short time frame, M2 macrophages do so only at later time points and do not rely on glycolysis for their differentiation [53, 54]. Instead, M2 macrophages utilize glutamine and FAO to support their metabolic demands upon IL-4 sensing [53, 55]. One-third of TCA carbons are glutamine-derived in M2 cells, whereas one-fifth are glutamine-derived in M1 macrophages [25, 56]. Endocytosis of triacylglycerol-containing lipoproteins is driven by increased CD36 expression on M2 macrophages, dependent on the transcription factors signal transducer and activator of transcription (STAT)6, peroxisome proliferator-activated receptor gamma (PPAR)γ, PPARδ, PGC-1β (a PPAR coactivator), and interferon regulatory factor (IRF)4 [51, 54, 55, 57, 58] (Fig. 1A). An increase in both the uptake of exogenous lipids and FAS supports enhanced FAO and mitochondrial biogenesis, resulting in a higher oxygen consumption rate (OCR) in M2 macrophages than in M0 and M1 macrophages [55]. However, several studies using FAO inhibitors or genetic models that prevent FAO have shown no reliance on FAO for M2 polarization, making it unclear whether the metabolic changes observed in M2 macrophages are responsible for immune polarization or if they are a consequence [46, 59, 60]. Glutaminolysis promotes αKG accumulation, leading to activation of Jumonji domain-containing protein D3 (JMJD3) demethylases [56]. JMJD3-dependent histone demethylation on M2-specific gene promoters is responsible for M2 polarization [25, 56, 61, 62]. IL-4 signaling also activates Akt, which is responsible for increased de novo histone acetylation at M2-specific genes [63]. Whether this activation depends on mammalian target of rapamycin (mTOR) complex (mTORC) 1 or 2 appears to be context-dependent [54, 63, 64]. Glutamine also supports uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) synthesis and subsequent N-glycosylation of lectin and mannose receptors required for pathogen recognition [25].
Tissue macrophages and their metabolism in homeostasis
Macrophages resident in the lung
Alveolar macrophages (AMs, CD11c+ SiglecF+ CXC3R1−, Table 1) are derived from fetal liver monocytes and populate the alveolar spaces in the lung after birth, where they self-maintain during homeostasis. They phagocytose inhaled particles and perform immune surveillance, but their main physiological function is the clearance of the constantly renewed pulmonary surfactant [5, 6, 65–67]. Given that the surfactant contains ~90% lipids (mainly phospholipids and cholesterol) [68, 69], AMs are equipped for lipid catabolism and cholesterol handling [70] (Fig. 2A). Indeed, AM development from embryonic progenitors is dependent on granulocyte-macrophage (GM)-CSF- and transforming growth factor (TGF)β-mediated induction of the signature transcription factor PPARγ, a master regulator of lipid metabolism [66, 71, 72]. Alveolar type 2 epithelial cells appear to be the crucial source of GM-CSF for AM development in murine lung [73]. PPARγ-deficient AMs are dramatically reduced in number and accumulate intracellular lipids due to reduced lipid catabolism and FAO, increased cholesterol esterification and diminished cholesterol efflux. Notably, PPARγ loss alters the AM phenotype and its specific gene expression program, highlighting the fundamental importance of cellular lipid metabolism for AM identity [66, 71, 72]. Similarly, loss of BTB and CNC homology (BACH)2, a transcriptional repressor regulating immune cell development, in AMs leads to reduced expression of genes involved in lipid catabolism and transport as well as cholesterol metabolism [74, 75]. AMs also accumulate lipids in mice with enhanced activity of the transcriptional lipogenesis regulator sterol regulatory element-binding protein (SREBP)1 by loss of insulin-induced gene (INSIG)1 and INSIG2 [76]. Treatment with the SREBP1/2 inhibitor fatostatin actually decreases AM numbers in mice [77]. Consistently, SREBP1 and SREBP2 are mTOR targets, and mTOR deficiency or mTOR inhibition with rapamycin phenocopies the reductions in lung AM numbers through the regulation of lipid metabolism [77]. Likewise, deficiency of CCAAT-enhancer-binding protein (C/EBP)β, a transcription factor associated with adipocyte differentiation and lipid-induced inflammatory responses [78, 79], causes deregulated lipid metabolism and AM loss in mice [80]. Hence, a fine balance of cellular lipid metabolism appears to be vital for AM identity, function and survival [70] (Fig. 2A).
Table 1.
Overview of the main functions and metabolic features of tissue macrophage populations
| Macrophage type | Organ/system | Ontogeny | Main surface markers | Main functions | Main metabolic features | References |
|---|---|---|---|---|---|---|
| Alveolar macrophages | Lung | Fetal liver monocytes | CD11c+ SiglecF+ CXC3R1− | Surfactant clearance, phagocytosis of inhaled particles, immune sentinel functions | ↑OXPHOS/mitochondrial respiration, lipid catabolism, cholesterol handling (PPARγ, LXRα, C/EBPβ, VHL); ↓Glycolysis | [5, 6, 65–70, 74–83, 85–88] |
| Interstitial macrophages | Lung | Adult bone marrow/blood monocytes | CXC3R1+ CD11b+ SiglecF− | Control of pathogens and infections, immune sentinel functions | Upon Mtb infection: ↑Glycolysis; ↓Mitochondrial respiration, fatty acid or cholesterol metabolism | [5, 65, 87, 88] |
| Marginal zone macrophages | Spleen | Adult bone marrow/blood monocytes | SIGNR1+ | Removal of blood-borne antigens and pathogens | Their development and immune function is controlled by LXRα (and LXRβ) | [89–93] |
| Marginal metallophilic macrophages | Spleen | Adult bone marrow/blood monocytes | CD169+ Sialoadhesin+ | Removal of blood-borne antigens and pathogens | Their development and immune function is controlled by LXRα (and LXRβ) | [89–93] |
| Tingible body macrophages | Spleen | Adult bone marrow/blood monocytes | F4/80− CD68+ | B cell phagocytosis during germinal center reaction | To be investigated | [89, 90] |
| Red pulp macrophages | Spleen | Yolk sac and fetal liver progenitors | F4/80+ VCAM1+ CD11blo | Clearance of erythrocytes, platelets and blood pathogens; iron recycling; immune sentinel functions | ↑Iron metabolism (Spi-C, NRF2, HO-1), lipid and cholesterol handling (PPARγ, LXRα) | [3, 4, 66, 70, 89, 90, 95,99–107] |
| Liver capsular macrophages | Liver | Adult bone marrow/blood monocytes | F4/80+ CX3CR1+ MHCII+ | Immune surveillance, neutrophil recruitment | ↓Metabolic gene signatures compared with Kupffer cells | [94, 95, 98] |
| Kupffer cells | Liver | Fetal liver monocytes | F4/80+ Clec4F+ Tim4+ | Clearance of erythrocytes and blood pathogens; iron metabolism; mediators of immunological tolerance | ↑Iron metabolism (Spi-C, NRF2, HO-1), lipid and cholesterol handling (PPARγ, LXRα); ↓Glycolysis (upregulated upon stimulation) | [3, 4, 7, 70, 91, 94–104, 106, 107, 109] |
| Erythroid island macrophages | Bone marrow | Adult bone marrow and fetal liver (likely) | F4/80+ VCAM1+ CD169+ | Support of erythropoiesis, iron handing | ↑Iron metabolism (Spi-C, HO-1) and fatty acid metabolism signatures | [106, 107] |
| Small peritoneal macrophages | Peritoneum | Adult bone marrow/blood monocytes | F4/80-low CD11b-low MHCII-hi | Immune sentinel functions and inflammatory regulation | ↑Glycolysis and OXPHOS upon activation compared with large peritoneal macrophages | [110, 111] |
| Large peritoneal macrophages | Peritoneum | Yolk sac progenitors | F4/80hi CD11bhi MHCIIlo | Clearance of dead cells/bacteria, inflammatory regulation, antimicrobial defense |
Naïve: ↑ETC/CII, ROS, lipid and cholesterol handling (GATA6, C/EBPβ, RXRα/β) Stimulated: context-dependent OXPHOS; ↓Lipid metabolism/FAO; ↑Glycolysis |
[70, 78–80, 86, 110–120] |
| Microglia | Central nervous system | Yolk sac progenitors | F4/80+ CX3CR1+ CD11b+ | Immune sentinel functions; clearance of apoptotic cells; regulation of brain homeostasis, neurogenesis and synaptic activity |
Naïve: ↑OXPHOS, context-dependent fuel use (mainly glucose) and metabolic pathway activation Stimulated: ↑Glycolysis; ↓OXPHOS |
[121–126] |
| Osteoclasts | Bone marrow, spleen, blood | Adult bone marrow/blood monocytes | TRAP+ (tartrate- resistant acid phosphatase) | Bone resorption (dissolution of collagen and mineralized bone) |
Naïve: ↑OXPHOS/CI activity, FAO, glutaminolysis Bone-exposed: ↑Glycolysis, HIF-1α, lactate production |
[128–135] |
| Intestinal lamina propria macrophages | Intestine | Adult bone marrow/blood monocytes | CD64+ MHCIIhi CD206+ | Clearance of dead cells, maintenance of epithelial homeostasis, immune sentinel functions, antimicrobial activity |
Butyrate-exposed: ↑ROS production; Unaltered OXPHOS; ↓Glycolysis and mTOR signaling |
[136–138] |
| Kidney-resident macrophages | Kidney | Yolk sac and/or fetal liver progenitors | CD64+ F4/80+ CD11c+ | Clearance of dead cells, likely regulation of ureteric bud branching and vascular development | ↑Fatty acid metabolism-, ↓OXPHOS- and glycolysis-related gene expression (healthy compared with lupus-like disease) | [116, 139, 140] |
| White adipose tissue macrophages | Lean white adipose tissue | Yolk sac progenitors (predominantly) | F4/80+ CD11b+ CD206+ | Efferocytosis and apoptotic cell clearance | Metabolically quiescent (↓Glycolysis and ↓OXPHOS compared with macrophages from obese fat) | [189, 191, 196] |
| Embryonic cardiac macrophages | Heart | Yolk sac and fetal liver progenitors |
CD64+ CX3CR1+ |
Efferocytosis and immune sentinel functions | Metabolically quiescent (↓Glycolysis and ↓OXPHOS compared with macrophages upon MI) | [141, 146] |
| Monocyte-derived cardiac macrophages | Heart | Adult bone marrow/blood monocytes | CCR2+ MerTK+ CD64+ CD11chi CD206+ | Immune surveillance | ↑Glycolysis upon MI; ↑OXPHOS and ↓Glycolysis from Day 3 after MI. | [67, 141, 146] |
| Skeletal muscle macrophages | Skeletal muscle | Embryonic and bone marrow precursors | CD11b+ F4/80+ CD64+ | Maintenance of tissue homeostasis, muscle growth and regeneration | To be investigated | [145] |
Fig. 2.
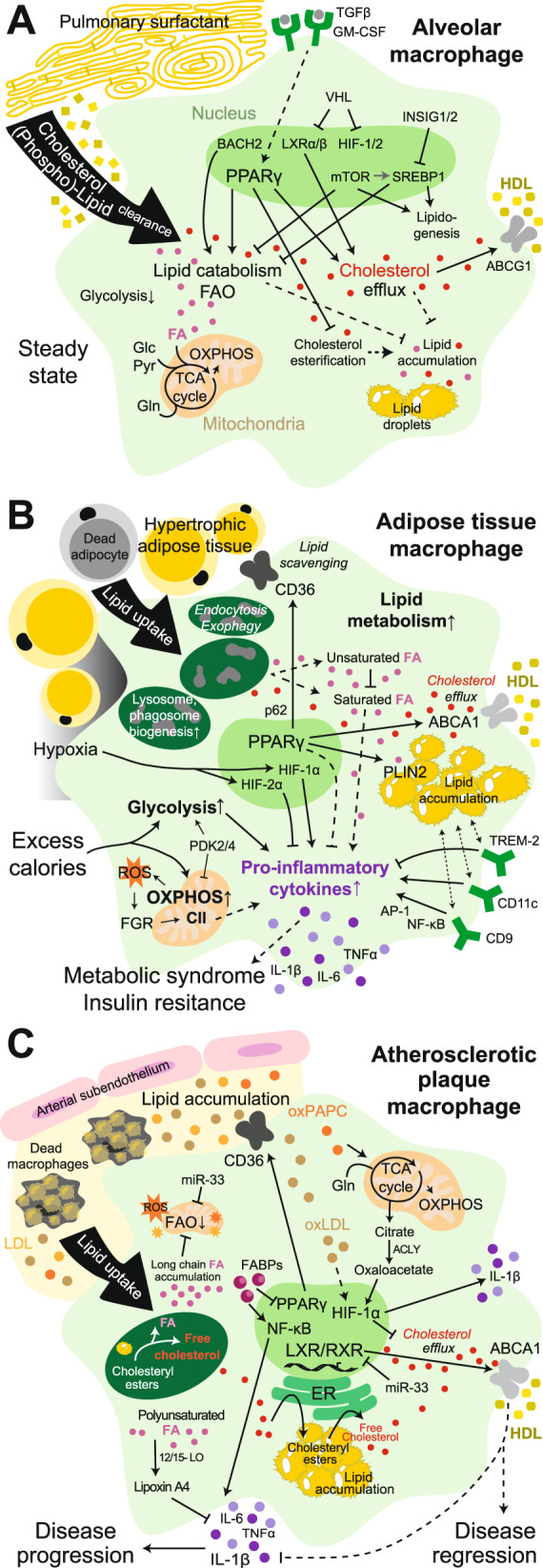
Lipid handling by tissue macrophages. A The metabolism of alveolar macrophages present in lung tissue is specialized for lipid catabolism and trafficking for effective clearance of pulmonary surfactant. B Excess calorie intake causes adipose tissue hypertrophy, hypoxia and adipocyte death. In response, adipose tissue macrophages become bioenergetically activated, scavenge resulting lipids and elevate their lipid metabolism. Ultimately, they become lipid-laden and proinflammatory and contribute to systemic metabolic syndrome and insulin resistance. C In atherosclerotic lesions, macrophages are exposed to a variety of lipids (i.e., oxLDL, LDL, oxPAPC, long-chain fatty acids, and cholesterol crystals) that either promote or attenuate the proatherogenic environment. Excessive free cholesterol and fatty acids, which are generated in endolysosomes upon lipid uptake, alter the metabolism of macrophages, leading to the production of proinflammatory cytokines. Conversely, effective cholesterol efflux restores macrophage functions, promoting atherosclerosis resolution. CII, complex II; FA, fatty acid; Glc, glucose; Gln, glutamine; LOX1, oxidized low-density lipoprotein receptor 1; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; Pyr, pyruvate; SRA1, steroid receptor RNA activator 1. Solid lines: direct relationships; dashed lines: indirect relationships. Purple circles: proinflammatory cytokines; gray circles: growth factors; brown and orange circles: bound cholesterol/LDL/oxLDL or oxPARC; yellow and ochre circles: bound cholesterol/HDL; red circles: free cholesterol; pink circles: fatty acids; orange stars: ROS
Interference with AM presence or AM lipid metabolism causes pathological surfactant accumulation in the lung, a rare disease termed pulmonary alveolar proteinosis [66, 71, 75], of which the inability of AMs to clear cholesterol has been suggested to be the primary underlying factor [81]. Indeed, lipid-laden macrophage-like cells are found in the alveoli of mice deficient in liver X receptor (LXR)α and β transcription factors and cholesterol/oxysterol sensors regulating reverse cholesterol transport [82] and upon loss of the LXRα target gene ATP-binding cassette (ABC) transporter ABCG1 that mediates cellular cholesterol and phospholipid efflux to high-density lipoprotein (HDL) [83]. In line with this, treatment with PPARγ agonists, LXR agonists or statins that lower systemic cholesterol levels reduces proteinosis pathology in mice [81, 84], which further supports the importance of active lipid and cholesterol metabolism in AMs (Fig. 2A).
In addition, oxygen-sensing pathways, such as von Hippel-Lindau (VHL)/HIF, regulate lipid metabolism and the identity of AMs [85]. VHL-deficient murine AMs with forced HIF-1/2-target gene expression exhibit an immature phenotype and a reduced ability to clear lung surfactant. In detail, LXR/retinoid X receptor (RXR) activation is increased in AMs upon VHL loss, resulting in increased expression of cholesterol efflux genes [85], which confirms the relevance of appropriate lipid handling for AM identity (Fig. 2A). Consistently, the homeostatic bioenergetics of AMs seem tuned toward lipid catabolism. AMs appear impaired in glycolytic metabolism, and the transcriptomic glycolysis signature is downregulated during their maturation in the lung after birth. In general, AMs display high basal respiration using glucose, glutamine, and pyruvate or fatty acid fuels, which is diminished upon VHL loss. This metabolic feature appears to be imposed upon AMs by the lung tissue microenvironment [85, 86] (Fig. 2A).
Interstitial macrophages (IntMs, CXC3R1+ CD11b+ SiglecF–, Table 1) are derived from blood monocytes. They are a rather scarce population mainly located between the epithelium and capillaries in the steady state, but their numbers can increase notably upon immune challenges [65]. Their predominant function is immune surveillance in the lung [5]. In agreement, in proinflammatory settings, the cellular metabolism of IntMs is reminiscent of that of M1-like BMDMs [87] (see Section 1). Analyses of Mycobacterium tuberculosis (Mtb)-infected murine AMs and IntMs have shown enriched OXPHOS, fatty acid metabolism and cholesterol homeostasis gene signatures as well as a higher lipid content and FAO capacity in AMs, while IntMs secrete more lactate and express higher levels of glycolysis-related genes, including iNOS [87], which inhibits OXPHOS [27, 45, 46]. These observations confirm the commitment of AMs to FAO and mitochondrial respiration, while IntMs display more glycolytic metabolism upon proinflammatory Mtb infection [87]. Nevertheless, lung macrophage populations undergo specific changes in the contexts of distinct lung diseases that also differentially affect their metabolism; this has recently been reviewed in detail [88].
Splenic and liver macrophage populations
To date, four different macrophage types have been distinguished in the spleen in the steady state based on their locations and surface marker expression [1] (Table 1). The monocyte-derived splenic macrophage subsets comprise SIGNR1+ marginal zone macrophages (MZMs) and CD169+ sialoadhesin+ marginal metallophilic macrophages (MMMs) in the marginal zone as well as F4/80− CD68+ tingible body macrophages (TBMs) in the white pulp. However, the most abundant splenic macrophage population is F4/80+ VCAM1+ CD11blo red pulp macrophages (RPMs), which are derived from the yolk sac and fetal liver progenitors and self-maintain in the splenic red pulp [89, 90]. A population of reticular fibroblasts secrete M-CSF to provide a niche for RPMs in the red pulp [9]. RPMs act as immune sentinels; however, their predominant homeostatic functions are erythrocyte and platelet phagocytosis and iron recycling [89, 90]. TBMs functionally specialize in phagocytosis of B cells that undergo apoptosis during germinal center reactions, while MZMs and MMMs harbor a predominant immunological function, capturing and clearing blood-borne pathogens such as bacteria, parasites and viruses [89]. While little is known about the metabolic features of TBMs, the development of MZMs and MMMs is controlled by LXRα/β [91]. Interestingly, rather than involving regulation of reverse cholesterol transport, the function of LXRs in macrophages of the marginal zone is connected with their immune function and clearance of phagocytosed cargo, as LXRα/β-deficient mice exhibit increased susceptibility to infection due to defective microbe control. Indeed, intracellular bacteria and apoptotic cells activate LXRs in cultured macrophages, which is required for their optimal clearance [92, 93].
In the homeostatic liver, F4/80+ Clec4F+ Tim4+ Kupffer cells (KCs), which form the largest tissue-resident macrophage population in the body, are found alongside F4/80+ CX3CR1+ MHCII+ liver capsular macrophages (LCMs, Table 1) and occasionally recruited peritoneal or other monocyte-derived macrophages. KCs originate from fetal liver monocytic precursors that colonize and self-maintain in the sinusoidal lumen, while blood monocyte-derived LCMs are present in the hepatic capsule [94, 95]. KCs can mediate immunological tolerance against blood-borne antigens from nutrients or dead cells but also clear damaged erythrocytes and circulating pathogens [95]. Upon immunogenic activation, KCs upregulate glucose uptake and pyruvate dehydrogenase kinase (PDK)-dependent glycolytic metabolism, which diminishes their tolerogenic function of IL-10 production [96, 97]. LCMs were only recently described to participate in immune surveillance and neutrophil recruitment, and apart from analysis of the enrichment of metabolic pathways in KCs compared with LCMs, their metabolism remains to be investigated [98].
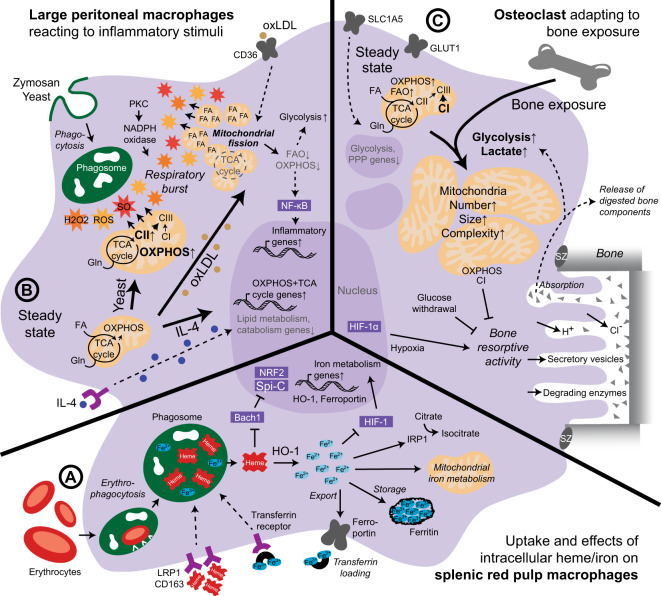
The bona fide macrophage populations resident in the spleen and liver, RPMs and KCs, respectively, are metabolically very dynamic cells during homeostasis and share many metabolic traits. Both populations constantly phagocytose defective erythrocytes and engage actively in the recycling, storage and metabolism of iron [89, 95, 99]. Recently, iron metabolism in macrophages was the subject of several reviews and is summarized here [100–103]. Molecularly, most genes involved in iron handling are induced by Spi-C and nuclear factor erythroid 2–related factor (NRF)2 transcription factors, both of which are expressed by murine RPMs and KCs [99, 104]. During in vivo differentiation of new resident macrophage populations from monocytes after their loss, Spi-C expression is induced in RPMs by the metabolite heme [105]. In differentiating KCs, Spi-C is mediated by Notch ligands derived from liver sinusoidal endothelial cells within a KC niche that also comprises stellate cells and hepatocytes [7, 8]. Spi-C and NRF2 are, however, repressed by BACH1 (Fig. 3A). Intracellular iron or heme, which is recycled from erythrocytes or taken up by RPMs and KCs via various receptors, such as the hemoglobin scavenger receptor CD163 or LDLR-related protein 1 (LRP1), sequesters BACH1, resulting in the expression of genes required for iron metabolism, including heme oxygenase 1 (HO-1), ferroportin and ferritin. HO-1 degrades heme using oxygen and NADPH to release iron into the cytoplasm, where it is stored by ferritin or regulates cellular metabolic processes such as HIF signaling, steps of the TCA cycle via iron-responsive element-binding protein 1 or mitochondrial iron metabolism. Iron is exported from macrophages by ferroportin located in the plasma membrane and, once extracellular, loaded onto transferrin [100–103] (Fig. 3A). In the bone marrow, erythroblasts take up transferrin-bound iron, which is aided by F4/80+ VCAM1+ CD169+ erythroid island macrophages (EIMs) that specialize in iron handling [106]. Indeed, HO-1-deficient mice display a notable loss of macrophages in the spleen, liver and bone marrow [107]. Spi-C has long been known to be fundamental for RPM and EIM development, and Spi-C loss reduces the numbers of these cells but, interestingly, does not affect KCs. The remaining Spi-C-deficient RPMs exhibit an impaired ability to phagocytose and clear erythrocytes [104, 105]. KCs, however, also regulate systemic iron metabolism via their ability to suppress hepatocyte-expressed hepcidin, which causes degradation of ferroportin upon high plasma iron levels and limits iron export from macrophages [99, 102].
Fig. 3.
Microenvironmental influence on tissue macrophage metabolism. A Splenic red pulp macrophages scavenge defective erythrocytes for iron recycling. B Large peritoneal macrophages adapt their bioenergetics after detection of different microenvironmental factors, such as yeast, oxLDL or IL-4, to facilitate the respiratory burst. C Osteoclasts shift their cellular metabolism when exposed to bone, promoting bone resorptive activity. CI-III, complex I-III; FA, fatty acid; Gln, glutamine; IRP1, iron-responsive element-binding protein; PKC, protein kinase C; SO, superoxide; SZ, sealing zone. Solid lines: direct relationships; dashed lines: indirect relationships. Purple circles: cytokines; brown circles: bound cholesterol/LDL/oxLDL; red and orange stars: ROS, SO and H2O2
In addition, evidence points toward active lipid and cholesterol handling of murine hepatic KCs and splenic RPMs, primarily their strong lipid metabolism signature compared with that of other macrophage populations. In detail, both cell types express notable levels of PPARγ and LXRα as well as many of their target genes involved in lipid metabolism and cholesterol trafficking [3, 4, 70, 99, 108]. SREBP1 and SREBP2, which are LXR target genes that control fatty acid and cholesterol synthesis, are also expressed by KCs [109]. Similar to Spi-C expression, LXRα expression is also induced in differentiating KCs by liver sinusoidal endothelial cells of the KC niche via Notch ligands. Notably, LXRα appears to be the transcription factor with the highest expression in KCs and has been described to be KC lineage determining [7, 8]. Indeed, whereas LXRα/β deficiency does not affect RPM presence [91], a lack of LXRα results in a mixed population of embryonic KCs and monocyte-derived cells, stressing the importance of LXRα for KC identity [7, 108]. On the other hand, KCs appear unaltered by PPARγ deletion, while the differentiation of RPMs is notably compromised upon PPARγ loss [66]. Nevertheless, apart from comparison of the transcriptomic signatures, the actual metabolic activity of KCs or RPMs handling lipids and cholesterol or its relevance for the functions of these cells in homeostasis remains largely unclear. Intriguingly, there may be a connection between iron and lipid/cholesterol metabolism in KCs and RPMs, as the transcriptome of bone marrow EIMs, which shows their specialization in iron handling, also reveals a strong fatty acid metabolism signature [106].
Peritoneal macrophages
During homeostasis, exudates of the peritoneum contain two main macrophage populations, F4/80hi CD11bhi MHCIIlo large peritoneal macrophages (LPMs) and F4/80lo CD11blo MHCIIhi small peritoneal macrophages (SPMs, Table 1). LPMs originate from yolk sac progenitors, self-maintain, and are involved in dead cell/bacteria phagocytosis and inflammatory responses. SPMs, which are constantly differentiated from blood monocytes, act as potent immune sentinels and inflammatory regulators [110]. LPMs are more numerous than SPMs in the steady state; however, the presence of SPMs increases profoundly upon stimulation or inflammation, when SPMs out-populate LPMs in a process termed the “macrophage disappearance reaction” [110]. In a comparative study, thioglycolate-elicited SPMs exhibited not only an elevated activation status and activity but also a much higher extracellular acidification rate (ECAR) and OCR than resident LPMs [111]. This fact has to be taken into consideration when examining features of different peritoneal macrophage populations, including their metabolism.
The metabolic state of murine LPMs supports the induction of immune responses and antimicrobial defense. This state involves elevated mitochondrial and ETC activity linked to mtROS production upon inflammatory stimulation, pointing toward a role of LPMs in controlling infection [112, 113] (Fig. 3B). LPMs display a high oxidative respiration rate fueled by glutamine and fatty acids in the steady state compared with that of BMDMs or AMs [86, 114, 115] but not with that of elicited SPMs [111]. In fact, the high levels of the metabolite glutamate in the peritoneum have been proposed to be a “tissue-niche fuel” for LPMs [114]. Upon stimulation of LPMs with zymosan or yeast, mitochondria are recruited to phagosomes contributing to an antimicrobial respiratory burst, specifically via glutaminolysis-mediated induction of ETC complex II [114] (Fig. 3B). LPMs also appear very responsive to in vivo stimulation with the canonical type 2 cytokine IL-4 and, notably, further upregulate genes involved in OXPHOS and the TCA cycle, while genes involved in lipid metabolism, such as those in the PPARγ signature, are downregulated [86] (Fig. 3B).
In contrast, exposure of thioglycolate-elicited murine SPMs and LPMs to a distinct class of stimulants such as CD36-binding oxidized low-density lipoprotein (oxLDL) results in reductions in FAO and OXPHOS and upregulation of glycolysis in concert with increased nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation and inflammatory cytokine production [115]. Stimulation of LPMs with ovalbumin opsonized with IgG has a similar effect decreasing the OCR [116]. However, oxLDL-induced inflammatory activation is in fact dependent on a primary repurposing of the ETC toward ROS and superoxide production, with oxLDL promoting structural changes in the mitochondria which suppress OXPHOS. The timing suggests the oxLDL-mediated glycolytic switch in peritoneal macrophages to be a secondary effect following the metabolic adaption of mitochondrial structure and functionality to facilitate ROS generation [115] (Fig. 3B).
In addition, studies on the LPM-expressed signature transcription factors GATA6, C/EBPβ and RXRα/β suggest a relevance of lipid handling for LPM identity that is in line with the functions of LPMs as phagocytes [80, 117–119]. LPMs express higher levels of proteins involved in lipid handling and cholesterol transport than RPMs [120]. GATA6-deficient LPMs display an even further increased OCR, in line with their observed alternative activation, and diminished aspartoacylase expression. Aspartoacylase can regulate acetyl-CoA formation as well as lipid synthesis, and aspartoacylase-mutant LPMs are more prone to death than their wild-type counterparts [117]. In accordance, C/EBPβ controls lipid metabolism-related genes in LPMs, and C/EBPβ-deficient LPMs exhibit striking upregulation of LXRα and PPARγ [70, 78, 79]. Moreover, loss of RXRs in LPMs also results in an enhanced lipid metabolism signature and lipid accumulation, especially via lipid-containing lysosome-like vesicles [119]. Notably, all three transcription factors have been reported to be vital for murine LPM differentiation, survival, maturation and polarization [80, 117–119]. In line with this, mixed thioglycolate-elicited SPMs and LPMs show a strong TCA cycle, FAO and FAS transcriptional profile [115].
Microglia in the central nervous system
F4/80+ CX3CR1+ CD11b+ microglia originate from embryonic yolk sac macrophages and reside in the central nervous system, where they self-maintain (Table 1). They function as immune sentinels to protect the brain from pathogens but are also required to maintain brain homeostasis by regulating neurogenesis, synaptic activity and apoptotic cell clearance [121]. Their intrinsic metabolism has been reviewed recently [122–124] and is summarized here.
Microglia engage in active mitochondrial OXPHOS metabolism to meet their energetic demands in the steady state and undergo a glycolytic switch upon activation, similar to cultured BMDMs. In the mouse brain, compared to astrocytes and neurons, microglia express similar levels of TCA cycle and glycolytic genes but higher levels of OXPHOS-related genes. Microglia also express factors involved in glucose uptake, which appears to be required for ROS production [122–124]. Ex vivo, untreated and anti-inflammatory (IL-13+IL-4)-stimulated microglia show high basal and maximal respiratory rates, while proinflammatory (LPS + IFNγ) stimulation collapses the OCR of microglia and reduces the OCR/ECAR ratio, suggesting a glycolytic switch [125].
Murine microglia have striking metabolic plasticity, adapting their OXPHOS activity to their microenvironment, for example, the presence of serum in culture medium [125]. Furthermore, using in vivo and in situ imaging of intracellular NADPH, microglia have been shown to adjust to hypoglycemia/aglycemia and switch from glucose as their main fuel to glutamine to support their OXPHOS metabolism, a process requiring mTOR [126]. As microglia are in constant communication with their microenvironment, numerous metabolic pathways are involved in their function, such as uptake and metabolism of free fatty acids/FAO, lactate and ketone bodies [122–124, 126].
Finally, an energy-deficient state of microglia in the aging mouse brain fosters inflammation. During aging, the prostaglandin E2/EP2 signaling axis causes reduced glycolytic flux and OXPHOS in microglia via glucose sequestration into glycogen. Blocking the EP2 cascade rescues bioenergetics in microglia, ameliorates aging-associated neural inflammation and improves memory [127]. These findings highlight the importance of functional energy metabolism in a tissue macrophage population for the maintenance of neural homeostasis.
Osteoclasts in mineralized bone
Osteoclasts, which are present in the bone marrow, spleen and blood, are multinucleated and terminally differentiated from monocytes (Table 1). They specialize in bone resorption and dissolve collagen and mineral bone matrix by releasing proteolytic enzymes and acids. As this activity comes with high bioenergetic demands, mature human and mouse osteoclasts contain more mitochondria than other cell types, and these mitochondria are of greater size and complexity [128] (Fig. 3C).
Murine osteoclastogenesis is driven by osteoblasts producing receptor activator of nuclear factor kappa-Β ligand (RANKL) and osteoprotegerin (OPG), which is termed the RANK-RANKL-OPG system [6]. In vivo and in vitro, this system seems to rely on mitochondrial biogenesis and OXPHOS, especially complex I of the ETC, which is regulated by iron uptake, PGC-1β and alternative NF-κB (RelB and NIK) signaling [129–131]. In addition, human and mouse osteoclast differentiation with RANKL and M-CSF induces an ECAR decrease and increases the gene expression of ETC components and the mitochondrial OCR [131–133]. In line with this, hypoxic conditions limit human and murine osteoclastogenesis in vitro [128], and in human bone sections, osteoclasts are associated with high FAO activity and low expression of glycolytic or PPP enzymes [134]. OXPHOS is likely fueled by glutaminolysis (Fig. 3C) and controlled by c-Myc. This is evidenced by the upregulation of glutamine importer solute carrier family 1 member 5 (Slc1a5) and glutaminase-1 during osteoclastogenesis and the fact that glutamine withdrawal as well as inhibition of Slc1a5 or c-Myc reduces osteoclast differentiation [135]. Upregulation of glycolytic genes/glucose transporters and suppression of osteoclast differentiation by mTOR inhibition or 5ʹ AMP-activated protein kinase (AMPK) activation have also been observed [135]. However, osteoclastogenesis has been reported to be unaffected by 2-deoxy-d-glucose [132] and reduced by lactate [129]. This discrepancy is likely explained by the metabolic switch of osteoclasts toward glycolysis upon bone absorption [128] (Fig. 3C). Activation of murine osteoclasts with bone powder does induce enhanced glycolytic activity compared to that in unstimulated cells, and the bone resorption activity of osteoclasts is driven by enhanced glycolysis, HIF-1α and lactate production [132, 135]. In addition, the collagen degradation activity of human osteoclasts is diminished when the cells are cultured in the absence of glucose, which enforces an OXPHOS-driven metabolism. In turn, complex I inhibition by rotenone augments the resorptive function of osteoclasts [133].
Other macrophage populations
Intestinal macrophages residing in the lamina propria are exposed to numerous nutrients and metabolites during homeostasis that influence their metabolism and activities, primarily microbiota-derived factors [136]. The functions of monocyte-derived CD64+ MHCIIhi CD206+ lamina propria macrophages include apoptotic cell removal, promotion of epithelial integrity, immunoregulation and antimicrobial activity [137]. For example, microbiota-derived butyrate triggers enhanced ROS production in murine macrophages, while OCR is unaltered and glycolysis and mTOR signaling are inhibited, resulting in increased bactericidal functions [138] (Table 1).
The predominant macrophage type in the kidneys appears to be embryo-derived CD64+ F4/80+ CD11c+ kidney resident macrophages (KRMs). Functionally, they contribute to dead cell clearance, ureteric bud branching and likely vascular development [139, 140]. Murine KRMs appear metabolically quiescent in the steady state. In comparison, KRMs from mice with lupus erythematosis-like disease display stronger OXPHOS and glycolysis-related, but weaker fatty acid metabolism, gene signatures compared with disease-free mice (Table 1). Inhibition of this glycolytic switch by KRMs upon disease may represent a therapeutic approach controlling kidney inflammation [116]. Interestingly, human synovial macrophages from patients with rheumatoid arthritis, which is also an immune complex-associated disease, show higher glycolytic gene expression but weaker OXPHOS and fatty acid metabolism signatures than those from healthy donors [116].
Overall, various populations of macrophages (co-)exist throughout the body and specialize in distinct, mostly tissue-specific activities. While these populations undoubtedly have to adapt to changing environments, the diverse metabolic programs of macrophages in tissues emerge to be perfectly optimized to facilitate the homeostatic functions of these cells and contribute to their identities (Table 1).
Macrophage metabolism in tissue regeneration
Upon tissue damage, macrophages play an active role in the early initiation of acute inflammation and later in the anti-inflammatory phase of cell proliferation and remodeling associated with the resolution of inflammation (Fig. 1B). After the initial injury, embryo-derived tissue-resident macrophages are rapidly replaced by monocytes [141, 142], which show a switch toward an alternatively activated anti-inflammatory state within the first days after damage. These monocyte-derived macrophages infiltrating tissues upon sterile injury drive tissue regeneration, which is impaired in mice lacking CCR2 [143]. Upon resolution of inflammation, steady-state self-maintenance of macrophages is recovered [144].
Most studies addressing the importance of macrophage metabolism in tissue regeneration use skeletal muscle injury or myocardial infarction (MI) models. CD11b+ F4/80+ CD64+ macrophages are present in skeletal muscle during homeostasis and stem from embryonic and adult bone marrow precursors, showing remarkable diversity, with at least 4 different subsets identified using single-cell RNA sequencing [145]. CD64+ CX3CR1+ resident cardiac macrophages originate from the yolk sac and fetal liver progenitors, but monocyte-derived macrophages are also present in the heart (Table 1). Functionally, they clear dead cells and act as immune sentinels with pro- and anti-inflammatory functions in the steady state; however, little is known about their cellular metabolism and how it affects their functions in homeostasis and upon injury [146].
Metabolic changes in macrophages after tissue injury
Damage-associated molecular patterns released from dead cells upon sterile tissue injury activate macrophages via pattern recognition receptors. This activation is linked to a rewiring of cellular metabolism and happens along with changes in nutrient availability that may both precede and be the consequence of the metabolic changes taking place. An integral view of how metabolism regulates injury and repair has been provided elsewhere [147]; here, we will focus on the intrinsic changes in macrophage metabolism during tissue regeneration.
Within 24 h upon proinflammatory activation, macrophages enhance their glycolytic metabolism [21] (Fig. 1B). This change has been confirmed in an indirect manner via transcriptomic analysis in models of MI or skeletal muscle repair, in which upregulated glycolysis and hypoxia response signatures have been found in macrophages one day after tissue injury [141, 142]. The hypoxic environment caused by skeletal muscle injury is maintained for several days with a peak on Day 4 [148]. The infiltration of proinflammatory macrophages in the tissue is HIF-dependent, as shown in mice with specific deletion of HIF-1α and HIF-2α in lysozyme M (LysM)-expressing cells [148, 149]. Although HIF is reported to be an essential transcription factor for M2 polarization upon hypoxia/lactic acid/IL-4 sensing [150], the deletion of HIF does not affect the shift of macrophages toward an anti-inflammatory phenotype in the context of skeletal muscle regeneration, as indicated by typical M2 marker expression in vitro and in vivo [148]. In line with this, tissue regeneration was not affected by HIF deletion in LysM-expressing cells in two models of muscle injury [148] and was only slightly affected in a third model of mild tissue trauma [149]. These observations imply that although the macrophage transition from a proinflammatory to a resolutive state takes place in a hypoxic environment, it is independent of HIFs. This finding correlates with the rapid decline in HIF expression by macrophages 3 days after MI [141]. Consistent with HIF expression, following an initial glycolytic burst on Day 1, glycolytic genes are promptly downregulated at Days 2-3 (Fig. 1B), as shown by two studies that longitudinally analyzed the transcriptomes of macrophages after MI [141] and muscle injury [142].
Switch of macrophage metabolism for tissue repair and remodeling
As early as 24–72 h upon tissue injury, macrophage function changes toward an anti-inflammatory phenotype that promotes cell proliferation and tissue remodeling. At this time, glycolysis-related genes are promptly downregulated [141, 142], while mitochondrial metabolism-related genes (including TCA cycle and ETC genes) are upregulated, an effect that is sustained during the tissue regeneration phase [142]. In line with the in vitro findings, additional evidence supports the role of mitochondrial metabolism in the anti-inflammatory function of macrophages during tissue repair (Fig. 1B).
First, AMPK1α, a key metabolic enzyme that can enhance OXPHOS, increases its activity in macrophages shortly upon tissue injury and is essential for the anti-inflammatory phenotype of macrophages and appropriate muscle regeneration [151]. Furthermore, a key function of macrophages in terminating the production of inflammatory mediators and promoting inflammation resolution is efferocytosis, which is required for the production of anti-inflammatory cytokines [152]. A growing body of literature shows that this process depends on metabolic rewiring, which, after an early phase that is dependent on increased glucose uptake and aerobic glycolysis [153], focuses on mitochondrial and fatty acid metabolism (Fig. 1B). In an in vivo model of apoptotic cell clearance in the thymus upon dexamethasone treatment, mitochondrial uncoupling protein 2 [154] and dynamin-related protein 1 [155], which reduce the mitochondrial membrane potential and mitochondrial fission, respectively, were found to be required for effective and continuous efferocytosis by macrophages. The catabolism of phagocytosed apoptotic cells by macrophages leads to an increase in the OCR fueled by FAO. In this context, ETC dysfunction by depletion of Rieske iron-sulfur protein (RISP), an essential subunit of mitochondrial complex III, in LysM-expressing cells in mice leads to a decrease in IL-10 expression that translates into a loss of ventricular systolic function upon MI [156]. IL-10 expression upon ETC interference in myeloid cells can be rescued with the NAD+ precursor nicotinamide mononucleotide [156], which has been found to be cardioprotective in an ischemia and reperfusion model [157]. For instance, hydrogels loaded with glutamine may increase mitochondrial spare respiratory capacity, and the administration of nicotinamide mononucleotide preclinically improves recovery upon sterile tissue injury [156–158]. In addition, IL-10 production is increased in macrophages from nonhypoxic regions of the heart, suggesting that the progressive recovery of oxygen supply in the tissue may work as an environmental cue that promotes tissue regeneration [156].
The significant external lipid substrate provided by apoptotic cells also increases the expression of PPARδ in macrophages, which increases opsonin expression and activates a transcriptional program required for apoptotic cell clearance and anti-inflammatory gene expression [159]. Ly6C− macrophages, which are anti-inflammatory, also show high expression of PPARγ upon tissue injury [160] (Fig. 1B). While alternative macrophage polarization in vitro is regulated by PPARγ [57], macrophages lacking PPARγ can transition toward an anti-inflammatory phenotype in vivo, as Ly6C+ and Ly6C− macrophages have been found to appear over time in a model of skeletal muscle damage [160]. However, PPARγ mediates the transcriptional control of growth factors produced by macrophages that can regulate skeletal muscle regeneration, which is subsequently impaired in mouse models with PPARγ-deficient macrophages [160]. Consistent with this, longitudinal mass spectrometry-based lipidomics during the transition from inflammation to resolution and regeneration in skeletal muscle injury have indicated that macrophages are both sources and sensors of lipid mediators. These factors play a role in the temporal transition from Ly6Chi pro-inflammatory to Ly6Clo pro-resolution macrophages. In particular, macrophage production of polyunsaturated fatty acid derivatives such as resolvins, which are specialized pro-resolving mediators, increases over time during tissue repair [161] (Fig. 1B). Resolvin D2 is first expressed in Ly6Clo macrophages at Day 2 upon tissue injury; at the same time, its receptor G protein-coupled receptor 18 is highly expressed on Ly6Chi macrophages, suggesting directional anti-inflammatory signaling cues. In line with this, the expression of lipoxygenases, the key enzymes for polyunsaturated fatty acid metabolism, is also increased in macrophages in the resolution phase [161].
Regarding amino acid metabolism, transcriptomic analyses have shown increases in glutamine metabolism genes in macrophages at the late stages of tissue recovery [142]. Muscle injuries are characterized by low glutamine levels; however, macrophages synthesize and secrete glutamine to promote the growth of satellite cells and improve muscle regeneration. Furthermore, macrophage-targeted inhibition of glutamine oxidation by glutamine dehydrogenase-1 improves muscle regeneration in muscle injury and ischemia models [162]. In addition, while proinflammatory macrophages use arginine for the generation of NO via iNOS [163], a diverging pathway competes for arginine during tissue remodeling. Arginase-1 catalyzes the production of ornithine by BMDMs, which is used as a substrate for the synthesis of the collagen precursor proline and polyamines, which support mitochondrial metabolism in alternatively activated macrophage models [52]. Hence, arginase-1 activity in macrophages may provide the extracellular matrix and promote the proliferation of stromal and satellite cells during wound healing [164, 165] (Fig. 1B).
Metabolic defects in macrophages in unresolved tissue regeneration
Macrophages play key roles in models of unresolved tissue injury, such as idiopathic pulmonary fibrosis [166]. AMs from fibrosis murine models first increase glycolysis and then switch metabolically to FAO, as tested by extracellular flux analysis and as indicated by the increased expression of key enzymes [167, 168]. The FAO increase is dependent on the mitochondrial calcium uniporter (MCU) and mtROS increase-driven expression of PGC-1α. Indeed, blockade of this mechanism in AMs causes metabolic reversal to glycolysis and protects mice from fibrosis [168]. Itaconate and its synthesizing enzyme immune-responsive gene 1 (IRG1) are reduced in AMs from idiopathic pulmonary fibrosis patients. Consistently, AMs lacking itaconate are more profibrotic than those with itaconate and lead to exaggerated persistent fibrosis. In addition, itaconate administration is preclinically used for the treatment of fibrosis, providing further evidence of its immunoregulatory role [169].
Iron concentrations are increased in bronchoalveolar lavage fluid and AMs from idiopathic pulmonary fibrosis patients, and these increases are associated with a proinflammatory phenotype and ROS production [170, 171]. In addition, an increased frequency of transferrin receptor 1 (CD71)− macrophages, which are characterized by the expression of profibrotic genes, is inversely correlated with survival in these patients [172]. Strategies to improve lung fibrosis using drugs that affect metabolism have been tested in the clinic. However, reduction of ROS via N-acetylcysteine (clinical trial identifier NCT 00650091) or the use of metformin [173], which was efficient in preclinical research due to AMPK activation [174], fails to impact clinical outcomes. Notably, none of these strategies are specifically directed toward macrophages or based on macrophage-related immune-metabolic evidence.
Altogether, these data suggest that the targeted manipulation of cellular metabolism in macrophages is a promising target to increase the speed of wound healing and prevent inadequate tissue regeneration like that in fibrosis [88].
Metabolic adaptations of adipose tissue macrophages upon overnutrition
Nutritional challenges, such as refeeding after starvation or excess calorie intake, systemically affect the activities and metabolism of macrophages in tissues, including white adipose tissue (WAT), pancreas, liver, peritoneum and brain. Prolonged diet-driven perturbations of tissue macrophages are associated with pathologies such as type II diabetes, nonalcoholic fatty liver disease (NAFLD) and cancer [99, 175–179]. During obesity, persistent overnutrition causes lipid accumulation and hypertrophy of WAT. The imposed mechanical stress, as well as oxygen shortage, results in adipocyte death. In conjunction, this activates adipose tissue macrophages (ATMs) to secrete proinflammatory mediators such as tumor necrosis factor (TNF)α or IL-1β that, in turn, stimulate inflammatory pathways such as the c-Jun N-terminal kinase (JNK) or inhibitor of nuclear factor kappa-B kinase subunit (IKK)β pathways in adipocytes. These mechanisms interfere with insulin signaling, culminating in insulin resistance, lipid accumulation in the liver or NAFLD and metabolic syndrome [175–177]. Liver-resident macrophages, such as KCs or monocyte-derived macrophages, react to systemic inflammation, gut-derived metabolites and other factors during NAFLD and can foster its progression to nonalcoholic steatohepatitis (NASH), fibrosis and liver cirrhosis. In livers with NASH, causative remodeling of macrophage subpopulations and a shift toward a proinflammatory phenotype have been reported, which makes macrophages a promising target in NAFLD/NASH. This phenomenon and the underlying metabolic alterations in liver macrophages have been the subjects of several recent reviews [70, 95, 99, 109, 175, 180–182]. Hence, here, we focus on the interplay of metabolic and functional adaptations of ATMs upon overnutrition that promote obesity and metabolic syndrome (Fig. 2B).
Overall, in lean or obese WAT, there are distinct ATM populations associated with proinflammatory functions (lipid-laden CD9+ ATMs, CD11c+ ATMs), anti-inflammatory functions (TREM-2+ lipid-associated macrophages [LAMs], vasculature-associated macrophages, Txnrd1+ HO1+ Mox-like macrophages) or dual functions (glucose+insulin+palmitate-treated BMDMs [MMe-like ATMs]) [183–189]. Moreover, sympathetic neuron-associated macrophages that accumulate in hypertrophic WAT and produce TNFα and IL-1α [190] as well as homeostatic Ly6C+ ATMs with an anti-inflammatory profile [183] have been described.
Bioenergetic activation of adipose tissue macrophages upon excess calorie intake
The key role of ATMs in obesity-associated low-grade inflammation has long been known to be driven by notable changes in the cellular metabolism of ATMs. ATMs from obese WAT display a glycolytic and proinflammatory state. However, comparisons of lean vs. obese ATMs and unstimulated vs. LPS-activated BMDMs have revealed that ATMs have different transcriptomic and proteomic profiles than M1-polarized macrophages, both in mice and human [183, 187, 191, 192]. This highlights the uniqueness and complexity of the metabolic and functional states of distinct ATM populations upon overnutrition.
Healthy WAT predominantly contains F4/80+ CD11b+ CD206+ anti-inflammatory or redox-regulatory (Txnrd1+ HO1+ Mox) macrophages derived from the embryonic yolk sac, with some contribution of monocyte precursors [193, 194] (Table 1). In addition, homeostatic WAT contains embryo-derived vasculature-associated ATMs, which associate with blood vessels, endocytose blood-borne macromolecules and are filled with lipid droplets [186]. Lean ATMs have been proposed to engage in OXPHOS/FAO-driven metabolism [195]. Yet, ATMs in lean mice appear metabolically quiescent with low OXPHOS and glycolytic gene expression and a low OCR and ECAR. Rapid bioenergetic/metabolic activation of murine ATMs is observed upon high-fat diet (HFD) feeding, as evidenced by increases in OCR, ECAR and lactate release as well as glycolysis and OXPHOS gene expression signatures [189, 191, 196]. CD14+ myeloid cells from visceral WAT of obese patients with diabetes display a comparable metabolic activation, in contrast to myeloid cells from nondiabetic obese individuals [191]. Moreover, co-culture of BMDMs with lean or obese WAT has corroborated the bioenergetic metabolic activation of ATMs upon HFD feeding [191]. WAT expression of FGR kinase (a tyrosine kinase from the Src family), mainly by macrophages, is also linked to obesity, liver steatosis and insulin resistance [197]. MtROS generated in stressed ATMs activate FGR, which mediates mitochondrial complex II activation and is associated with proinflammatory cytokine production [37, 41, 197] (Fig. 2B). Thus, FGR deletion in bone marrow-derived cells prevents insulin resistance and liver steatosis upon HFD feeding in mice [197]. In one study, obesity-associated pathologies were also ameliorated in mice treated with the near-infrared fluorophore IR-61, which preferentially targets macrophages, increasing OXPHOS and ameliorating WAT inflammation [198]. However, the effect of IR-61 on mitochondrial metabolism of adipocytes, whose impairment is a hallmark of obesity in mice [199], was unfortunately not investigated in that study.
The unique metabolic activation of ATMs during diet-induced obesity can largely be ascribed to characteristics of the dramatically expanded WAT microenvironment, the creation of hypoxia due to inadequate angiogenesis, the release of danger signals by dying adipocytes and the abundance of (adipocyte-derived) lipids and fatty acids [175, 176, 193]. However, changes in the bioenergetics of ATMs may also be influenced by the almost exclusive monocytic origin of ATMs in obese mice [189, 193, 194].
Hypoxia, HIF, glycolysis and the PPP in adipose tissue macrophages
Upon refeeding after fasting, murine ATMs in lean WAT, in contrast to several other types of tissue macrophages, induce a proinflammatory IL-1 pathway response and a transcriptional lipid metabolism signature (PPAR signaling, glycerolipid metabolism, fatty acid degradation) [178]. Inhibition of glycolysis, glutaminolysis and FAO reduces proinflammatory cytokine secretion by cultured lean ATMs, while only glycolysis drives the enhanced release of IL-6 and CXCL1 by ATMs from obese WAT [191]. Hypoxia sensing and proinflammatory stimulation associate with the glycolytic metabolism of ATMs during overnutrition (Fig. 2B). Murine ATMs in obese compared with lean WAT display enhanced activation of proinflammatory HIF-1α, resulting in the expression of IL-1β and glycolytic genes [196]. Consistent with HIF-1α driving a glycolytic metabolism and proinflammatory state, ATM accumulation and transcription of IL-1β appear to be reduced in WAT of HFD-fed LysM-Cre HIF-1αf/f mice which also exhibit mildly improved glucose tolerance and increased expression of angiogenic factors. However, the effects of HIF-1α loss in ATMs on general adipose tissue inflammation, angiogenesis, adiposity or insulin sensitivity are context-dependent [191, 196, 200]. Supporting the dependence of proinflammatory functions of ATMs on their glycolytic metabolism, HFD-fed mice with PDK2/4-deficient bone marrow display lower ATM numbers and inflammation (TNFα, IL-6 and CCL2 expression) in WAT as well as ameliorated insulin resistance [201].
In contrast, HIF-2α expression, also observed in lean and obese ATMs, is rather associated with an inflammation-resolving (M2-like) ATM state, reduced expression of TNFα or IL-12 [202] and ameliorated inflammasome activation. In BMDMs and peritoneal macrophages, HIF-2α deficiency enhances the OCR and FAO via carnitine palmitoyltransferase 1 A upregulation during inflammasome activation, which drives IL-1β and IL-18 secretion [203]. Adipocyte and peritoneal macrophage co-cultures have confirmed the anti-inflammatory role of HIF-2α in macrophages, which includes the induction of arginase-1 and limitation of NO and proinflammatory gene expression in adipocytes [202]. HIF-2α+/– mice indeed display ATM accumulation, insulin resistance and susceptibility to adipose tissue inflammation (TNFα and IL-6) upon overnutrition [202]. HFD-fed LysM-Cre HIF-2αf/f mice also exhibit signs of metabolic syndrome and elevated IL-1β and IL-18 levels in plasma. Notably, stabilization of HIF-2α by treatment with its agonist FG-4592 alleviates overnutrition-induced inflammasome activation and insulin resistance [203].
Finally, activation of the PPP is also associated with proinflammatory features of macrophages during excess calorie intake. First, glucose-6-phosphate dehydrogenase (G6PD), the initial enzyme of the oxidative branch of the PPP, is highly expressed in ATMs from obese compared with lean mice, and its levels in human adipose tissue correlate with several parameters of obesity [204]. G6PD-deficient ATMs from obese mice exhibit decreased TNFα and CCL2 expression, and G6PD-deficient peritoneal macrophages are less responsive to LPS stimulation ex vivo than their wild-type counterparts. Indeed, glucose intolerance; crown-like structure (CLS) number; and TNFα, IL-6 and CCL2 levels in adipose tissue are improved in HFD-fed mice grafted with G6PD-deficient bone marrow compared with controls [205]. Second, the potential involvement of sedoheptulokinase, which is part of the nonoxidative branch and negatively regulates the flux through the PPP by producing sedoheptulose 7-phosphate, in overnutrition-induced pathologies has also been proposed. Sedoheptulokinase is expressed by M2-like macrophages in vitro and downregulated to allow proinflammatory M1-like macrophage features [206].
Lipid handling by adipose tissue macrophages
The metabolic state of ATMs in expanded WAT is affected by dietary or adipocyte-derived fatty acids [207]. ATMs accumulate in CLS around dying adipocytes in obese WAT and clear released lipids via endocytosis or larger particles via exophagy, causing foam cell formation [208]. Enhanced lipid uptake and lipid droplet formation are linked to lysosomal biogenesis in murine ATMs from obese WAT, and ATMs accumulate more lipids upon inhibition of lysosome function [192]. Herein, we outline the key concepts of lipid metabolism in ATMs investigated in vivo (Fig. 2B); while adaptations of macrophages after fatty acid/lipid exposure have been recently reviewed [207, 209].
Full-length oxidized phospholipids, which are predominantly found in WAT of HFD-fed mice, augment BMDM bioenergetics and proinflammatory gene expression [189]. In contrast, truncated oxidized phospholipids diminish the OCR and ECAR of cultured BMDMs while increasing the expression of Mox-like antioxidant genes. Moreover, (long-chain) saturated fatty acids stimulate not only glycolysis, lipid metabolism and OXPHOS in macrophages but also a switch to a proinflammatory phenotype [209]. Indeed, murine BMDMs increase glycolysis and HIF-1α expression as well as the basal OCR when exposed to the saturated fatty acid palmitate, which culminates in IL-1β induction [196]. Conversely, unsaturated fatty acids show anti-inflammatory effects, even opposing saturated fatty acids [209]. TLR4-dependent priming of BMDMs that causes alterations in cellular metabolism, lipid handling and membrane composition is required for the proinflammatory effects of palmitate [210].
However, palmitate treatment of BMDMs does not entirely reproduce the bioenergetic activation of obese ATMs, as the maximal OCR is actually reduced [196]. This observation suggests that additional mechanisms regulate ATM metabolism upon overnutrition. In addition to bioenergetic adaptations and proinflammatory activation, ATMs in obese WAT also trigger anti-inflammatory lipid metabolism programs. Those programs are driven by PPARγ (the master regulator of lipid catabolism) and p62 (a signaling-regulatory scaffold protein) and/or triggering receptor expressed on myeloid cells (TREM)-2 [185–187]. First, the PPARγ-target genes ATP binding cassette subfamily A member 1 (ABCA1, crucial for cholesterol export), CD36 (a scavenger receptor for lipid uptake) and/or perilipin 2 (PLIN2, a lipid droplet protein) are upregulated in omental and subcutaneous human ATMs from obese vs. nonobese individuals and in murine ATMs from obese WAT in concert with TNFα and IL-1β. Palmitate exposure also elevates PLIN2 and p62 expression in BMDMs. Blocking PPARγ and p62 in MMe-like activated BMDMs (treated with high levels of glucose, insulin, and palmitate) enhances IL-1β and/or TNFα expression. This observation illustrates the anti-inflammatory features of PPARγ/p62 and MMe-like activation, which is nevertheless distinct from M2-like activation [187]. Furthermore, a protective population of lipid-associated ATMs (LAMs) accumulated in CLS in mice and humans upon obesity. LAMs express TREM-2 and are characterized by transcriptional signatures of lysosomes/phagosomes, endocytosis, lipid metabolism (PPARγ) and OXPHOS. Although their functions have not been directly investigated, TREM-2 loss remodels ATM populations in obesity and enhances weight gain and adiposity [185]. In line with this, WAT ATMs are reduced in HFD-fed LysM-Cre PPARγf/f mice, but their adiposity and insulin and glucose tolerance are significantly enhanced [57].
In contrast, HFD-fed chimeric mice bearing bone marrow deficient in C/EBPβ, another regulator of lipid metabolism, also harbor reduced ATMs in WAT, but WAT inflammation (TNFα, IL-6, CCL2 and NLRP3) is also reduced. Despite the regulatory role of C/EBPβ in induced cytokine production [211], these results may suggest a context-dependent proinflammatory role of lipid accumulation in WAT ATMs [79]. Supporting this, proinflammatory CD11c+ ATMs in obese mice are known to express higher TNFα and IL-1β levels and accumulate more lipid droplets than anti-inflammatory CD11c− ATMs [184, 192]. CD9+ proinflammatory ATMs are also lipid laden, accumulate in obese WAT and reside within CLS [183]. They display enhanced AP-1 and NF-κB activity and express proinflammatory gene signatures (TNFα, IL-1α, CCL2, IL-18) and lipid metabolism/lysosome gene signatures (PLIN2, CD63, LAMP2, LPL, LIPA). In addition, lipid-accumulating CD9+ ATMs have been found in the CLS of human WAT and are correlated with body mass. Notably, adoptive peritoneal transfer of obese fat-derived CD9+ ATMs into lean mice induces an inflammatory transcriptomic response in lean WAT highly reminiscent of the response in WAT upon overnutrition [183]. However puzzlingly, the described ATM populations appear to overlap; for example, TREM-2+ LAMs highly express CD9 [185], and the presence of ATMs is dynamic during obesity progression, with some populations increasing or decreasing over time.
However, the proposed functions of ATMs do not correlate with gross metabolic features, such as intracellular lipid accumulation. One explanation would be the nature/type of internalized lipids in ATMs, as full-length vs. truncated oxidized phospholipids or saturated vs. unsaturated fatty acids can induce opposing functions [189, 209].
Moreover, in addition to being diverse, ATMs may play different roles depending on the extent of obesity. Adipose tissue remodeling upon overnutrition is progressive and reversible upon HFD withdrawal. Despite increasing over time, the presence of (CD11c+) ATMs expressing proinflammatory genes (TNFα, IL-1β, IL-6) and lipid metabolism genes (CD36, PLIN2, ABCA1) and an inflammatory state in the WAT of HFD-fed mice actually precedes adipocyte death and CLS formation [184, 188]. The TLR2-myeloid differentiation factor (MYD)88-NADPH oxidase (NOX)2 axis controls both inflammation and lysosomal exocytosis, which is necessary for dead adipocyte clearance [208], in cultured MMe-like BMDMs. Consistently, NOX2−/− mice display improved glucose tolerance correlated with decreased TNFα, IL-1β and IL-6 expression by WAT ATMs upon short-term HFD feeding. Conversely, at later time points, NOX2−/− mice develop hepatosteatosis, insulin resistance and lipoatrophy associated with dead adipocyte accumulation and defective expression of lysosomal exocytosis genes by ATMs [188]. Moreover, upon switching of mice from a HFD to a normal chow diet, the numbers of proinflammatory CD11c+ ATMs are maintained in WAT, but TNFα and IL-1β expression in these cells is reduced, and insulin sensitivity is improved [184]. Hence, depending on the extent of WAT hypertrophy, the activities of ATMs may be beneficial or unfavorable, and lipid-laden ATMs can adapt their proinflammatory properties accordingly.
These factors should be considered when designing future studies to improve our understanding of how metabolism dictates ATM function, thus defining potential targets to combat overnutrition-associated pathologies.
Macrophage metabolism in atherosclerosis
Atherosclerosis is a chronic inflammatory disease in which lipids build up in arteries, causing local inflammation and the development of atheroma plaques. These plaques, which are infiltrated by immune cells, can impede blood flow and eventually cause blood clots due to their rupture. In humans, macrophage plaque infiltration is related to altered plaque metabolism (increased glycolysis and hypoxia [212, 213]), necrotic core formation, enhanced plaque rupture and acute clinical cardiovascular events [214, 215]. Indeed, macrophages play a prominent role in atherosclerosis, influencing disease initiation, progression and regression [216].
At early stages, macrophages take up (modified) LDL that is retained in the arterial subendothelium, which leads to intracellular lipid accumulation and macrophage foam cell formation [217]. Macrophage foam cells have a diminished migratory capacity, which reduces their ability to egress plaques [218, 219], an important contributor to potential atherosclerosis regression, and show increased expression of lipid handling genes [220]. In plaques, macrophages can also display a proinflammatory phenotype that promotes the secretion of cytokines and chemokines and amplifies the immune response by recruiting monocytes, T cells and neutrophils [216].
As the lesion progresses, macrophages proliferate, release cytokines and proteases, and ultimately become apoptotic upon unresolved lipid and endoplasmic reticulum (ER) stress [216]. In advanced plaques, efferocytosis (phagocytosis of dead cells) is impaired, contributing to necrotic core formation. If dyslipidemia is resolved by transplanting the atherosclerotic aortic arches from apolipoprotein E (ApoE)−/− mice into healthy recipients or via dietary changes in regression models, CD68+ plaque macrophage numbers decrease. In part, this is achieved by CC-chemokine receptor (CCR)7-mediated egress of macrophages from the plaques. The remaining plaque macrophages display transcriptional changes, including increased expression of the anti-inflammatory markers arginase-1 and CD163 [221, 222].
Analysis of the metabolic adaptations of atherosclerotic plaque macrophages is challenging due to the scarcity of these cells, and the existing knowledge is largely based on transcriptomics and the use of animal models with genetic deficiencies. Here, we outline current models of the metabolism of atheroma macrophages, which appear to be highly disease stage- or context-dependent and are often even opposing. This issue can additionally be explained by the fact that current knowledge on atherosclerotic macrophages is largely obtained by studies using BMDMs or peritoneal macrophages as surrogates. Given the functional and metabolic diversity of tissue macrophage types (Table 1) and BMDMs (outlined above), their resemblance may be incomplete.
Lipid handling impacts macrophage function during hypercholesterolemia/atherosclerosis
Efferocytosis, macropinocytosis and phagocytosis together with diverse scavenger receptors (including CD36, SRA1/2, SRB1, SR-PSOX, LOX and LRP1) are involved in lipid uptake by macrophages [217]. In plaques, macrophages internalize environmental lipids such as LDLs, modified LDLs (mainly oxidized), oxidized phospholipids, fatty acids, and apoptotic cell-derived lipids (Fig. 2C).
Oxidized phospholipids are phosphocholine-containing phospholipids with polyunsaturated fatty acid moieties (mainly arachidonic acid) that are oxidized (e.g., by free radicals from the inflammatory environment) [223]. Oxidized phospholipids are recognized by CD36 and induce proatherogenic immune activation in peritoneal macrophages [224, 225]. In BMDMs, stimulation with oxidized phospholipid 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (oxPAPC) and LPS drives simultaneous glycolysis and OXPHOS as well as glutamine catabolism and oxaloacetate accumulation. This metabolic reprogramming potentiates HIF-1 stabilization, which, in turn, increases hyperinflammation by promoting IL-1β production [226] (Fig. 2C). The oxPAPC-dependent genetic signature in mice is also upregulated in human individuals with pro-atherosclerotic lipid profiles [226].
As detailed earlier, the oxLDL/CD36 axis switches fatty acid metabolism and mitochondrial OXPHOS toward glycolysis, superoxide production and proinflammatory activity in peritoneal macrophages ex vivo [115] (Fig. 3B). Under atherogenic conditions in HFD-fed ApoE−/− mice, increased GLUT1 expression and mitochondrial ROS levels are detectable in blood Ly6Chi monocytes; the latter are also found in aortic lesional CD36-expressing F4/80+ macrophages [115]. Cholesterol crystals formed by CD36-mediated uptake of oxLDL by peritoneal macrophages activate the NLRP3 inflammasome [227], which is linked to Western diet-induced inflammation and aortic plaque formation in LDL receptor (LDLR)−/− mice [228]. In this line, ApoE−/− IL-1β−/− mice show reduced aortic lesion sizes [227, 229], and anti-IL1β therapy has been found to lead to a lower rate of recurrent cardiovascular events in a human cohort independent of lipid-level lowering [230].
Cholesteryl esters of LDL are metabolized in the endolysosomal compartment into free cholesterol and fatty acids. Free cholesterol is either effluxed from cells to HDL (reverse cholesterol transport) via the lipid transporters ABCA1 and ABCG1, key players in atherosclerosis, or trafficked to the ER, where it is reconverted into cholesteryl esters and stored in lipid droplets [231] (Fig. 2C). As the lesion progresses, impairment of cholesterol trafficking in macrophages and the resulting accumulation of cytotoxic free cholesterol have been hypothesized to occur. In cultured peritoneal macrophages, cholesterol exposure activates the unfolded protein response, leading to ER stress and increased C/EBP homologous protein (CHOP)-mediated apoptosis [232]. Furthermore, cultured ABCA1−/− and/or ABCG1−/− macrophages increase their cellular cholesterol content (in lipid rafts), which activates TLR4 and NF‐κB signaling, resulting in the secretion of proinflammatory cytokines [233, 234] (Fig. 2C). LDLR+/− mice that received ABCA1−/− ABCG1−/− bone marrow display enhanced foam cell presence and aortic lesion sizes [234], supporting the hypothesis that foamy macrophages are proinflammatory and drive atherosclerosis pathology.
In contrast, peritoneal macrophages of LDLR−/− mice fed a high cholesterol/high fat (HCHF) diet increase their cholesterol content, leading to a subsequent decrease in the cholesterol biosynthesis pathway that leads to the accumulation of desmosterol, a cholesterol precursor that acts as a LXR ligand [235]. Desmosterol promotes LXR-mediated cholesterol efflux in peritoneal macrophages via LXR target genes such as ABC transporters, which is linked with attenuation of TLR signaling and decreased inflammatory signatures [233, 235, 236]. Decreased inflammation in conditions of hypercholesterolemia has also been shown to occur in peritoneal macrophages from LDLR−/− mice fed an HCHF diet via suppression of the PPP and NRF2 oxidative stress pathways [22].
These discrepancies could be explained by the characterization of foamy and non-foamy macrophages that coexist in atherosclerotic plaques and exhibit different features [220]. Foamy macrophages in lesions show increased expression of OXPHOS-related genes as well as cholesterol metabolism, lysosome activity and PPAR signaling signatures. Conversely, non-foamy macrophages are enriched with genes involved in inflammatory processes, suggesting that these newly recruited macrophages promote local inflammation and plaque progression [220]. Hence, the differences between pro- and anti-inflammatory features exhibited by atherosclerotic plaque macrophages could be related to the coexistence of distinct macrophage types or states at different stages of disease.
Moreover, even the roles of individual factors involved in cholesterol handling by aortic plaque macrophages appear to be context dependent, such as in the case of LRP1, a marker usually linked with plaque progression under proatherogenic conditions [237]. First, peritoneal macrophages deficient in LRP1 display increased reverse cholesterol efflux, and ApoE−/− mice grafted with LRP1−/− bone marrow exhibit accelerated plaque regression [222]. These mice show reduced plaque CD68+ macrophage numbers and enhanced macrophage CCR7 expression, which contributes to atherosclerosis resolution in addition to the increased reverse cholesterol transport induced by LRP1 deficiency [222]. These two mechanisms may be intricately linked, as macrophage LXR signaling, a key pathway in cholesterol homeostasis, is required for maximal CCR7 expression and macrophage egress from plaques [238]. In contrast, a study using a LRP1Y63F knock-in mouse model that carries a point mutation that impedes LRP1 phosphorylation and subsequent signal transduction has highlighted a role for LRP1 in promoting atherosclerosis [239]. Peritoneal macrophages from LDLR−/− mice carrying LRP1Y63F bone marrow show enhanced intracellular lipid accumulation and decreased clearance of apoptotic cells during disease progression due to a reduction in LXR- and ABCA1-mediated cholesterol efflux. Overall, the contradictory roles of the same pathway/receptor in atherosclerosis are possibly related to the different stages of atherosclerosis (regression vs. progression) [240].
Finally, in vitro studies in BMDMs, peritoneal macrophages and cell lines have shown cholesterol efflux to be controlled by microRNA (miR)-33, which affects several aspects of cellular metabolism, including FAO, mitochondrial respiration, gluconeogenesis and autophagy [241–244] (Fig. 2C). High miR-33a/b and low mitochondrial gene expression have been reported in human atherosclerotic plaques compared with healthy arteries, while other metabolic processes have not been assessed. Antagonism of miR-33 in ApoE−/− or LDLR−/− mice reduces aortic lesion size, lipid content, CD68+ plaque macrophage presence and ABCA1 expression, as determined after laser-capture microdissection [241, 245]. However, mitochondrially impaired PGC-1α−/− peritoneal macrophages do not intrinsically alter their ATP levels but exhibit blunted miR-33 efficacy, suggesting additional mechanisms by which miR-33 controls ATP and cholesterol efflux [241]. Indeed, a steroidogenic acute regulatory protein (StAR)/mitochondrial sterol 27-hydroxylase (CYP27A1) axis also activates LXR-dependent cholesterol efflux and inflammatory gene expression in cultured macrophages and cell lines [246, 247]. In line with this, ApoE−/− mice systemically overexpressing StAR due to infection with a recombinant CMV-StAR adenovirus exhibit decreased cholesterol and triglyceride accumulation in the liver as well as decreased aortic neutral lipid levels, although the relevance of these findings for macrophages have not been investigated [246].
Fatty acid accumulation can also promote a proinflammatory macrophage phenotype by inducing the production of fatty acid binding proteins (FABPs) in peritoneal macrophages [248, 249] (Fig. 2C). FABP4 deficiency attenuates inflammatory cytokine production in macrophage cultures [248], and FABP5 limits PPARγ activation and promotes Akt- and NF-κB-linked inflammation in peritoneal macrophages [249]. This results in reduced inflammation and decreased atherosclerosis in ApoE−/− or LDLR−/− mice transplanted with FABP4−/− and FABP5−/− bone marrow, respectively [248, 249]. In contrast, polyunsaturated fatty acids, mainly omega-3 fatty acids and arachidonic acid, can be converted through lipoxygenase pathways into pro-resolving lipid mediators such as lipoxins or resolvins that induce macrophage efferocytosis and anti-inflammatory gene expression [250] (Fig. 2C). Macrophages overexpressing 12/15-lipoxygenase (12/15-LO) show increased levels of lipoxin A4, which downregulates several proinflammatory cytokines, resulting in atheroprotection in ApoE−/− mice overexpressing 12/15-LO in macrophages and increased atherosclerosis in ApoE−/− 12/15-LO−/− mice [251].
Changes in glucose and amino acid metabolism in macrophages as atherosclerosis evolves
The atherosclerotic plaque microenvironment is characterized by hypoxic regions [252, 253] that drive metabolic reprogramming of cultured BMDMs and human blood monocytes toward increased glycolysis via the activation of HIF-1α, which in turn stimulates IL-1β production [254, 255]. In murine and human plaques, HIF-1α has been found to colocalize with CD68+ macrophages and highly expressed proteins involved in glucose metabolism, including GLUT1 and Hexokinase-2. High levels of IL-1β are also correlated with macrophage-rich regions [254, 255]. Cultured macrophages with stabilized HIF-1α in hypoxia increase triglyceride and sterol biosynthesis and halt cholesterol efflux via ABCA1 [254] (Fig. 2C), a potential mechanism by which plaque macrophages accumulate cholesterol. The inflammatory stimuli oxLDL and oxPAPC can also activate HIF-1α, stimulating glucose uptake and enhancing IL-1β production, respectively [115, 226]. HIF-1α−/− bone marrow transplantation into LDLR−/− mice results in decreased aortic lesion sizes, indicating the importance of HIF-1α in plaque macrophages [256].
In vitro studies have suggested that the effects of HIF-1α are due to its regulation of glucose uptake and glycolysis [256]; however, the role of glycolysis in plaque macrophages remains unclear. Bone marrow transplants overexpressing Glut1 increase macrophage glycolysis and PPP activation but do not alter inflammation or atheroma plaques in LDLR−/− mice [257]. Similarly, LDLR−/− mice with macrophage-specific deficiency of Glut1 show no difference in aortic lesion size but show an increased proportion of plaques with necrotic cores and decreased plaque stability [153, 258]. Moreover, an enhanced 18F-FDG signal, an indication of glucose uptake, corresponds to macrophage abundance and/or inflammatory activation in atherosclerotic plaques in both humans [212] and rabbits [259]. Of note, compared with healthy control monocytes, ex vivo LPS/IFNγ-stimulated circulating monocytes from atherosclerosis patients show increased glycolysis and PPP gene expression as well as enhanced IL-1β and IL-6 production via the ROS/pyruvate kinase M2/STAT3 pathway [260].
Amino acids in the microenvironment can also play roles in macrophage metabolism and function in atherosclerosis. For instance, the increased systemic availability of leucine upon high-protein diet feeding is associated with increased atherosclerotic plaque complexity via alteration of the mTORC1/autophagy axis in peritoneal macrophages [261]. Macrophage culture experiments have shown that leucine first synergizes with proatherogenic lipids (i.e., 7-ketocholesterol and cholesterol crystals) to induce mitochondrial uncoupling and increase macrophage ROS production. Then, mTORC1 is activated, which inhibits the mitophagy of dysfunctional mitochondria. These leucine-induced mechanisms trigger macrophage apoptosis in plaques, which in turn contributes to necrotic core formation and increases plaque instability upon high-protein diet feeding [261].
Moreover, oxPAPC-triggered hyperinflammation in BMDMs is dependent on glutamine and ATP citrate lyase (ACLY)-dependent conversion of citrate into oxaloacetate. Indeed, the reductions in atherosclerotic plaques in LDLR−/− mice upon treatment with glutaminase or ACLY inhibitors as well as in LDLR−/− mice harboring LysM-Cre ACLYf/f bone marrow support the importance of glutamine catabolism and oxaloacetate accumulation in atherosclerosis [226, 262].
In summary, different types of metabolites (lipids, glucose, and amino acids), lipid handling processes and the involved signaling factors have been shown to foster diverse features in atheroma macrophages during disease progression or regression. This controversy highlights the need for accurate evaluation of the specific microenvironmental conditions (i.e., lipid type/source, stage of the disease, hypoxic regions) [263] as well as of the model system used (i.e., ex vivo vs. in vivo, peritoneal and/or aortic macrophages) [179] when investigating complex diseases such as atherosclerosis.
The metabolic challenges of macrophages in cancer
Overview of the tropic functions of tumor-associated macrophages and underlying metabolic features
Solid tumors form complex structures with requirements similar to those of developing organs [264]. Tumors recruit circulating monocytes into the tumor microenvironment (TME), where they become tumor-associated macrophages (TAMs) [265]. However, TAMs are not only of monocytic origin but can also be derived from tissue-resident embryo-derived macrophages [266, 267]. Depending on the tumor type, the pro- or anti-tumorigenic roles of monocytes or embryo-derived macrophages vary [268, 269], and the field of tumor macrophage ontogeny is in its infancy.
Generally, a high TAM content correlates with a poor prognosis [270, 271], and TAMs are associated with anti-inflammatory properties as well as a broad spectrum of metabolic features [272]. Notably, anti-inflammatory TAMs strongly rely on glycolysis, in contrast to cultured BMDMs (Fig. 1A), demonstrating the complexity of in vivo macrophage polarization [273, 274]. TAM heterogeneity also extends to different tumor nodules of the same animal [275], as the TAM phenotype changes over time depending on tumor progression. The ways in which TAMs support tumor development are also diverse, such as generation of an immunosuppressive TME by anti-inflammatory cytokines and programmed death-ligand 1 (PD-L1) expression [276, 277], extracellular matrix remodeling [278] and facilitation of the angiogenic switch for large tumor nodules [279]. However, macrophages are not always tumorigenic [280]. Increased macrophage infiltration prolongs survival in cervical carcinoma patients and is associated with mature CD163- proinflammatory macrophages that promote cytotoxic CD8+ T cell responses [281–283]. In colorectal cancer, an improved prognosis also correlates with macrophage infiltration, regardless of phenotype (based on nitric oxide synthase 2 [NOS2] or CD163 expression) [284, 285].
One of the main metabolic pathways in macrophages shown to influence tumor growth is amino acid metabolism, and protumoral TAMs frequently highly express arginase-1 [14, 286] (Fig. 4.1). Tumor-associated myeloid cells express cationic amino acid transporters 1 and 2B at higher levels than nontumor-associated myeloid cells, leading to higher uptake of arginine and its depletion in the TME [287, 288]. The result of this is three-fold: First, arginase-1 converts arginine into ornithine and urea, inhibiting tumoricidal NO synthesis. Second, arginine is metabolized into ornithine and polyamines, which support tumor growth. Myeloid-specific deletion of ornithine decarboxylase, a rate-limiting factor in the polyamine biosynthesis pathway, also leads to increased production of M1-associated cytokines, including TNFα, IL-1β, IFNγ, and NOS2, resulting in decreased tumor burden and improved survival in a model of colitis-associated carcinogenesis [289]. Third, the depletion of arginine from the TME suppresses the anti-tumoral activity of T cells [286–288, 290].
Fig. 4.
Tumor-associated macrophage and cancer cell cross-talk. Cancer cells and tumor-associated macrophages engage in metabolic cross-talk, which has been shown primarily to support tumor cell growth and survival. This includes increased arginase expression by macrophages promoting arginine metabolism (1); tryptophan metabolism in cancer cells via IDO (2); glutamine metabolism in cancer cells to promote their proliferation and in macrophages to drive anti-inflammatory gene expression (3); glucose metabolism-mediated increases in extracellular lactate, which modulates macrophage function (4); the induction of and response to a hypoxic microenvironment (5); cholesterol export from macrophages (via ABC transporters) to cancer cells (via LDLR) (6); heme metabolism and iron efflux from macrophages (7); and mitochondrial ROS production (8). More details of these pathways can be found in the main text. CO, carbon monoxide. Solid lines: direct relationships; dashed lines: indirect relationships. Brown circles: bound cholesterol/LDL/oxLDL; dark green circles: lactate; light green circles: hyaluronic acid; orange stars: ROS
Tryptophan metabolism is also implicated in modulating anti-tumor immune responses (Fig. 4.2). The enzyme indoleamine-2,3-dioxygenase (IDO) performs the first step of the kynurenine pathway, which converts tryptophan to N-formylkynurenine. In the context of tumors, this has been linked to T cell suppression via tryptophan depletion from the TME and promotion of regulatory T cell responses [291]. However, IDO-overexpressing tumors also recruit more TAMs into the TME, where they express CD206 and high levels of TGFβ but low levels of NOS2, CD86, and IL-12—characteristics of protumorigenic macrophages [291]. This macrophage modulation appears to be dependent on IDO-induced macrophage expression of the arylhydrocarbon receptor [291, 292]. Arylhydrocarbon receptor signaling also induces the expression of cytochrome p450 enzymes, such as CYP1A1, which are involved in steroid and lipid metabolism [293], yet their roles in cell-intrinsic TAM metabolism require further study.
Glutamine metabolism is known to fuel biosynthesis pathways in cancer cells, contributing to cell survival; however, this pathway may also directly contribute to TAM function [294] (Fig. 4.3). In cultured BMDMs, glutamine drives M2 macrophage polarization via epigenetic modifications such as Jmjd3 demethylation that enhance the expression of key M2 genes, such as arginase-1, and UDP-GlcNAc synthesis [25, 56]. Supporting these findings, genetic deletion of glutamine synthase in macrophages leads to more M1-like features, as defined by MHCIIhi expression, and reduces the presence of CD206+ M2 macrophages. This strategy does not affect the primary tumor burden; however, it does inhibit metastasis and increase T cell infiltration [295].
How are TAMs metabolically repurposed to be protumorigenic?
TAMs and tumor cells continuously interact, similar to macrophages and their surrounding parenchyma in homeostasis [296]. For example, tumor-produced M-CSF stimulates TAM production of epidermal and vascular endothelial growth factor (EGF and VEGF), which activate tumor cells in a paracrine loop to promote extravasation and enhance metastasis [279, 297, 298]. Tumor cells and TAMs also engage in metabolic crosstalk. Tumor cells are typically highly glycolytic due to exclusive expression of the M2 isoform of pyruvate kinase (PKM2) [39, 299], which can lead to competition for nutrients with other glycolytic cells in the TME [300]. TAMs have the highest level of glucose uptake, followed by T cells and then tumor cells, all of which are dependent on mTORC1 activity [301]. In contrast, tumor cells have the highest uptake of glutamine, indicating that glycolytic tumors may not be immunosuppressive through direct nutrient competition but via more complex intercellular regulation [301]. The end-product of tumor cell glycolysis is lactate, which promotes the production of VEGF and arginase-1 in TAMs to support tumor vascularization and proliferation (Fig. 4.4). Indeed, tumor cells genetically modified to produce less lactic acid show reduced growth in vivo. This effect is dependent on lactic acid uptake via monocarboxylate transporters (MCT1-4) and macrophage expression of HIF-1α [150]. This finding is supported in vitro, where lactate induces anti-inflammatory gene expression programs, e.g., an increase in arginase-1 and a decrease in iNOS, in BMDMs through histone lactylation [302]. Alongside lactate, the hypoxic TME also induces HIF-1α stabilization in TAMs, which again triggers VEGF and arginase-1 expression [150] (Fig. 4.5). In line with this, analysis of TAM heterogeneity has shown denser localization of anti-inflammatory macrophages in more hypoxic areas of a mammary adenocarcinoma model [303].
Tumor-derived succinate also promotes succinate receptor 1 (SUCNR1)/phosphoinositide 3-kinase (PI3K)/HIF-1α-dependent polarization of peritoneal macrophages in vitro, enhancing macrophage expression of arginase-1 [304]. Apart from modulating the TME, tumors can actively export exosomes that alter TAM functions. The microRNA let-7a, found in tumor exosomes, inhibits mTOR signaling in TAMs, leading to increased OXPHOS and expression of the M2-like markers CD206 and arginase-1 [305]. It is not clear whether protumoral macrophage polarization occurs in newly recruited macrophages or upon repolarization of the proinflammatory phenotype of existing TAMs, as reversion toward an anti-tumoral TAM phenotype appears to be limited [46].
Lipid handling by macrophages in the TME is also key to determining cancer outcomes. ABCG1-deficient macrophages, with reduce cholesterol efflux, improve outcomes in bladder carcinoma and melanoma models due to a shift to M1-like gene expression—increasing iNOS, MHCII, and CD86 expression while decreasing arginase-1 and CCL22 [306]. Similarly, in vitro, ABCG1-deficient macrophages show defective lipid efflux, accumulate cholesterol in lipid rafts and enhance proinflammatory cytokine production [307, 308]. The secretion of hyaluronic acid by ovarian cancer cells, an occurrence observed in many cancers, promotes plasma membrane cholesterol efflux in TAMs via binding of hyaluronic acid to CD44 [309] (Fig. 4.6). This reduction in intracellular cholesterol enhances IL-4 receptor (IL-4R)/STAT6/PI3K signaling in vitro and in vivo, promoting the expression of the typical M2 marker arginase-1 while inhibiting NOS2 and proinflammatory IL-12 expression, thereby promoting tumor progression [309, 310]. This cholesterol efflux is dependent on PI3K and Akt activation, which can upregulate ABCA1 expression in macrophages [309, 311]. Moreover, cholesterol efflux leads to decreases in lipid rafts in macrophages, which act as signaling platforms for various receptors, including IFNγR, decreasing IFNγ-dependent expression of the proinflammatory genes IL-12 and iNOS [309]. Indeed, myeloid-specific deletion of cholesterol efflux genes ABCA1 and ABCG1 or inhibition of IL-4R signaling, STAT6, or PI3K has been found to significantly impair tumor progression in an ovarian cancer model [309]. Moreover, cancer cells upregulate cholesterol receptors to utilize the cholesterol lost from TAMs [312].
In addition to promoting cholesterol efflux, PI3K signaling regulates many key genes for immune activation or suppression in the TME [313, 314]. However, lipid handling requires a delicate balance, as lipid accumulation in RXRα-deficient macrophages can lead to apoptosis in the cancer setting. Peritoneal LPMs are the main infiltrating immune cells in ovarian cancer, where they support tumor growth, and their lipid-induced apoptosis decreases tumor growth [119]. In another context, compared with healthy control cells, TAMs from the bone marrow of patients with myeloma display increased CD36-mediated lipid uptake and storage [315]. This finding correlates with an increased FAO transcriptional signature and mitochondrial respiration in TAMs and supports the protumoral functions of these cells via the expression of arginase-1, VEGF, HIF-1α, and CCL2 [315].
Increased pyrimidine synthesis and release by protumoral macrophages also nurtures tumor cells [316]. Similarly, while proinflammatory macrophages typically sequester iron to limit pathogen growth, protumoral TAMs increase iron export, which is hijacked by tumors to promote cell proliferation and tissue repair [317]. A distinct set of bone marrow-derived F4/80hi TAMs with high expression of the hemoglobin scavenger receptor CD163 and the heme catabolism enzyme HO-1 accumulate at the invasive margins of tumors [318] (Fig. 4.7). These TAMs rely on an NF-κB1/M-CSF receptor/C3a anaphylatoxin receptor signaling axis to drive HO-1 expression. The effect of heme catabolism is to remove cytotoxic labile heme from the TME and release carbon monoxide, which enhances STAT3/STAT6 activation in TAMs to induce anti-inflammatory cytokines such as IL-10 (Fig. 4.7). In a myeloid-specific HO-1-deficient mouse model, TAM heme catabolism was found to have no effect on primary tumors but to support metastasis by promoting angiogenesis and epithelial-to-mesenchymal transition [318].
ROS and reactive nitrogen species (RNS) are produced by macrophages to kill pathogens, but their range of other functions can be both pro- and anti-tumorigenic [319]. Macrophage-derived NO can directly induce tumor cell apoptosis [320]. However, TAM-derived ROS can also activate mitogen-activated protein kinase (MAPK)/PPARy signaling by tumors, promoting their survival (Fig. 4.8). TAMs are also enriched with antioxidant transcriptional signatures that are correlated with anti-tumorigenic responses [321]. In the context of peritoneal grafted cancer models, increases in FAO, OXPHOS, and glycolysis are observed in peritoneal resident macrophages [322]. IRG1 and itaconate are highly upregulated in these macrophages, resulting in increased OCR and mtROS production, which activates MAPK in nearby tumor cells to enhance their survival [322] (Fig. 4.8). Certain tumors can also induce ROS accumulation to support their own growth and influence other cells in their microenvironment, including TAMs [323]. Indeed, in a carcinogen-induced lung cancer model, administration of the ROS inhibitor butylated hydroxyanisole (BHA) prevented TAM recruitment into the TME and subsequently decreased tumor burden [324].
Finally, while TAMs are promising therapeutic targets, the current knowledge suggests that their targeting should be context dependent. Apart from clinical strategies reducing TAM presence, repurposing of TAMs from an anti-inflammatory to an anti-tumor phenotype is being pursued [179, 325]. For instance, CpG oligodeoxynucleotides, which are TLR9 agonists, induce a unique type of metabolic rewiring in macrophages whereby they do not utilize exogenous fatty acids but incorporate glucose into acetyl-CoA to enhance de novo lipid biosynthesis for FAO and membrane expansion [326]. This metabolic change increases membrane fluidity and subsequently phagocytic activity, enhancing TAM anti-tumor functions in a model of pancreatic ductal adenocarcinoma [326]. Similarly, macrophages that utilize FAO appear to increase efferocytosis via PPAR activation [327]. However, a better understanding of the unique metabolism of TAMs will improve future therapeutic approaches.
Concluding remarks
Early in vitro studies associated the metabolic states of macrophages with functional features adopted upon activation (Fig. 1A). Indeed, parallels of this metabolic and functional polarization are observed during tissue injury, wound healing and regeneration. Macrophages initially adopt a proinflammatory and glycolytic state before switching to a resolving state and predominantly mitochondria-driven metabolism (Fig. 1B).
However, the homeostatic metabolic activities of tissue macrophage populations appear to be as diverse as their identities, the variety of microenvironments they reside in and, subsequently, the functions they execute to maintain homeostasis (Table 1). For example, osteoclasts specialize in bone matrix degradation, which is driven by glycolysis, while splenic RPMs engage in iron recycling when phagocytosing defective erythrocytes, and peritoneal LPMs require active mitochondria to generate a respiratory burst upon encountering yeast (Fig. 3). In addition, numerous tissue macrophage populations, such as pulmonary AMs, splenic RPMs, hepatic KCs, and peritoneal LPMs, express the metabolic machinery for lipid and cholesterol handling to clear environmental factors (Fig. 2A). Anatomical tissue niches for resident macrophages thus direct tissue macrophage identity and contribute to the metabolic features the macrophages adopt. For example, epithelial or stromal cell-derived signaling factors such as GM-CSF or Notch ligands induce PPARγ in AMs or Spi-C and LXRα expression in KCs, respectively. Additionally, metabolites present in “soluble” tissue niches foster metabolic traits of tissue macrophages, such as splenic heme for RPMs and glutamate for LPMs.
In line with this, environmental changes accompanying or underlying certain pathologies can overwhelm or hijack tissue macrophages that try to maintain their homeostatic activity. The resulting metabolic adaptations of tissue macrophages not only alter macrophage functionality but can also contribute to or drive disease severity. Indeed, adipose tissue macrophages during overnutrition-mediated WAT hypertrophy or plaque macrophages during atherosclerosis can be overpowered in their attempts to clear the accumulating extracellular lipid load, which culminates in proinflammatory responses (Fig. 2B, C). Tumor-associated macrophages also react functionally with underlying metabolic changes to cues present in cancer microenvironments and actively engage in metabolic crosstalk with cancer (and other) cells (Fig. 4), which often fosters cancer progression. Despite these emerging profound changes in tissue macrophage metabolism that underlie their functionality in pathologic settings, this avenue has been explored surprisingly little as a therapeutic option in the clinic [179]. Currently, efforts predominantly aim to target macrophages [180, 328, 329] or metabolic features locally/systemically [156–158, 173, 330, 331] in distinct pathophysiological settings. A joint effort of these approaches to affect metabolism specifically in macrophages is needed and may hold great therapeutic potential and limit side effects.
To facilitate translational application, we have to improve our knowledge of tissue macrophage metabolism in homeostasis and disease, since related reports often extrapolate observations from cultured surrogates, which themselves represent cells under metabolic challenge, to in vivo tissue macrophages. While AMs and LPMs can be harvested without tissue dissociation, the purification of macrophages located within the tissue stroma is more complex and can alter the metabolic profiles of the macrophages. Nevertheless, for some mainly murine macrophage populations, very informative techniques such as bulk transcriptomics and metabolomics have been performed, and single-cell-based analyses of murine and human tissues are emerging [332]. Met-Flow and SCENITH, flow cytometry-based methods using specific inhibitors to dissect functional (energetic) metabolic profiles at a single-cell level, represent feasible alternatives for complex tissues/samples [333, 334]. CyTOF panels with large numbers of key metabolic enzymes have been successfully used in recent years to study metabolic changes [335]. These cytometry-based techniques have also been applied to human myeloid cells in blood or TAMs. Spectroscopy and fluorescence imaging techniques using ratiometric fluorescence probes that detect changes in metabolic features [126, 336] are emerging as valuable tools to study tissue macrophage metabolism as well as enzyme histochemistry and histocytometry for measurement of enzyme activity. Finally, whole-body imaging systems such as positron emission tomography, single-photon emission computed tomography or nuclear magnetic resonance have also been successfully applied [12].
In addition, genetically modified animal models represent valuable tools for the investigation of tissue macrophage metabolism. Cre recombinase-based driver lines, such as LysM-Cre lines, and/or bone marrow replacement strategies have been successfully used to dissect the effects of metabolic alterations in macrophages in vivo. However, apart from a few exceptions [108, 337], these approaches are mostly not specific for particular tissue macrophage subsets or even macrophages, which might limit the interpretation of the results. The fact that bona fide macrophages are often rapidly out-populated by bone marrow-derived macrophages in tissues in many disease settings (the macrophage disappearance reaction) [110] can further influence the complexity of studying tissue macrophage metabolism. Moreover, some features related to cellular metabolism have been found to be distinctly regulated in murine and human macrophages, such as iNOS and arginase expression [338]. In addition, the dependence of the bioenergetics of monocytes on glucose or mitochondrial metabolism differs between humans and mice [333]. These observations clearly highlight the need for the validation of findings from mouse model systems in the human setting to be clinically relevant.
Overall, in light of the emerging importance of the microenvironment for cellular metabolism (Figs. 2–4), a complementary combination of the currently available techniques applied to tissue macrophages in vivo appears most suitable for reliable investigation of the metabolic traits, dependencies and adaptations of these cells. Understanding the metabolic features of tissue macrophages will provide valuable information for the maintenance of tissue homeostasis and likely help to control pathologies that are driven by metabolic changes in macrophages.
Acknowledgements
We thank all members of the DS laboratory at CNIC, the Iborra laboratory at the Universidad Complutense de Madrid, Veronica Miguel and Santiago Lamas from the Centro de Biología Molecular “Severo Ochoa” for scientific discussions and comments on the manuscript.
Author contributions
SKW, GD, IHM, and AM prepared tables and figures and wrote parts of the manuscript. SKW and DS conceptualized and wrote the manuscript. GD and IHM helped with conceptualization of the manuscript. All authors contributed to manuscript editing and read and approved the final version.
Funding
SKW and the project that gave rise to these results received support in the form of a fellowship from the La Caixa Foundation (ID 100010434). The fellowship code is LCF/BQ/PR20/11770008. GD is supported by a European Molecular Biology Organization Long-term Fellowship (ALTF 379-2019). This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 892965. IHM is supported by a La Caixa INPhINIT fellowship (ID 100010434, fellowship code: LCF/BQ/IN17/11620074). Work in the DS laboratory is funded by the CNIC, by the European Research Council (ERC-2016-Consolidator Grant 725091), by the Agencia Estatal de Investigación (PID2019-108157RB), by the Comunidad de Madrid (B2017/BMD-3733 Immunothercan-CM), by Atresmedia (Constantes y Vitales prize), by the Fondo Solidario Juntos (Banco Santander), and by the Fundació La Marató de TV3 (201723). The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the MICINN and the Pro CNIC Foundation.
Competing interests
The authors declare no competing interests.
Contributor Information
Stefanie K. Wculek, Email: stefanie.wculek@cnic.es
David Sancho, Email: dsancho@cnic.es.
References
- 1.Nobs SP, Kopf M. Tissue-resident macrophages: guardians of organ homeostasis. Trends Immunol. 2021;42:495–507. doi: 10.1016/j.it.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–44. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–26. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–28. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mass E. Delineating the origins, developmental programs and homeostatic functions of tissue-resident macrophages. Int Immunol. 2018;30:493–501. doi: 10.1093/intimm/dxy044. [DOI] [PubMed] [Google Scholar]
- 6.T’Jonck W, Guilliams M, Bonnardel J. Niche signals and transcription factors involved in tissue-resident macrophage development. Cell Immunol. 2018;330:43–53. doi: 10.1016/j.cellimm.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakai M, Troutman TD, Seidman JS, Ouyang Z, Spann NJ, Abe Y, et al. Liver-derived signals sequentially reprogram myeloid enhancers to initiate and maintain kupffer cell identity. Immunity. 2019;51:655–.e8. doi: 10.1016/j.immuni.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnardel J, T'Jonck W, Gaublomme D, Browaeys R, Scott CL, Martens L, et al. Stellate cells, hepatocytes, and endothelial cells imprint the kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity. 2019;51:638–.e9. doi: 10.1016/j.immuni.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellomo A, Mondor I, Spinelli L, Lagueyrie M, Stewart BJ, Brouilly N, et al. Reticular fibroblasts expressing the transcription factor WT1 define a stromal niche that maintains and replenishes splenic red pulp macrophages. Immunity. 2020;53:127–.e7. doi: 10.1016/j.immuni.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Van den Bossche J, O’Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38:395–406. doi: 10.1016/j.it.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Ryan DG, O’Neill LAJ. Krebs cycle reborn in macrophage immunometabolism. Annu Rev Immunol. 2020;38:289–313. doi: 10.1146/annurev-immunol-081619-104850. [DOI] [PubMed] [Google Scholar]
- 12.Zago G, Saavedra PHV, Keshari KR, Perry JSA. Immunometabolism of tissue-resident macrophages—an appraisal of the current knowledge and cutting-edge methods and technologies. Front Immunol. 2021;12:1–20. doi: 10.3389/fimmu.2021.665782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newsholme P, Curi R, Gordon S, Newsholme EA. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem J. 1986;239:121–5. doi: 10.1042/bj2390121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–88. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Prados J-C, Través PG, Cuenca J, Rico D, Aragonés J, Martín-Sanz P, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–14. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 17.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–89. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 19.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–42. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289:7884–96. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palsson-Mcdermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, et al. Pyruvate kinase M2 regulates hif-1α activity and il-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;21:65–80. doi: 10.1016/j.cmet.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baardman J, Verberk S, Prange K, van Weeghel M, van der Velden S, Ryan DG, et al. A defective pentose phosphate pathway reduces inflammatory macrophage responses during hypercholesterolemia. Cell Rep. 2018;25:2044–.e5. doi: 10.1016/j.celrep.2018.10.092. [DOI] [PubMed] [Google Scholar]
- 23.Meiser J, Krämer L, Sapcariu SC, Battello N, Ghelfi J, D'Herouel AF, et al. Pro-inflammatory macrophages sustain pyruvate oxidation through pyruvate dehydrogenase for the synthesis of itaconate and to enable cytokine expression. J Biol Chem. 2016;291:3932–46. doi: 10.1074/jbc.M115.676817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauterbach MA, Hanke JE, Serefidou M, Mangan M, Kolbe CC, Hess T, et al. Toll-like receptor signaling rewires macrophage metabolism and promotes histone acetylation via ATP-citrate lyase. Immunity. 2019;51:997–1011.e7. doi: 10.1016/j.immuni.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–30. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Koenis DS, Medzikovic L, van Loenen PB, van Weeghel M, Huveneers S, Vos M, et al. Nuclear receptor Nur77 limits the macrophage inflammatory response through transcriptional reprogramming of mitochondrial metabolism. Cell Rep. 2018;24:2127–.e7. doi: 10.1016/j.celrep.2018.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey JD, Diotallevi M, Nicol T, McNeill E, Shaw A, Chuaiphichai S, et al. Nitric oxide modulates metabolic remodeling in inflammatory macrophages through TCA cycle regulation and itaconate accumulation. Cell Rep. 2019;28:218–.e7. doi: 10.1016/j.celrep.2019.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–80. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan J, Krautkramer KA, Feldman JL, Denu JM. Metabolic regulation of histone post-translational modifications. ACS Chem Biol. 2015;10:95–108. doi: 10.1021/cb500846u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei X, Song H, Yin L, Rizzo MG, Sidhu R, Covey DF, et al. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature. 2016;539:294–8. doi: 10.1038/nature20117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dennis EA, Deems RA, Harkewicz R, Quehenberger O, Brown HA, Milne SB, et al. A mouse macrophage lipidome*. J Biol Chem. 2010;285:39976–85. doi: 10.1074/jbc.M110.182915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CGL, Jenkins NA, Gilbert DJ, Copeland NG, O’Brien WE. Cloning and analysis of gene regulation of a novel LPS-inducible cDNA. Immunogenetics. 1995;41:263–70. doi: 10.1007/BF00172150. [DOI] [PubMed] [Google Scholar]
- 33.Strelko CL, Lu W, Dufort FJ, Seyfried TN, Chiles TC, Rabinowitz JD, et al. Itaconic acid is a mammalian metabolite induced during macrophage activation. J Am Chem Soc. 2011;133:16386–9. doi: 10.1021/ja2070889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci USA. 2013;110:7820–5. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordes T, Wallace M, Michelucci A, Divakaruni AS, Sapcariu SC, Sousa C, et al. Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J Biol Chem. 2016;291:14274–84. doi: 10.1074/jbc.M115.685792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016;24:158–66. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills EL, Kelly B, Logan A, Costa A, Varma M, Bryant CE, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–.e13. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 39.Warburg O. Injuring of respiration the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 40.Swain A, Bambouskova M, Kim H, Andhey PS, Duncan D, Auclair K, et al. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nat Metab. 2020;2:594–602. doi: 10.1038/s42255-020-0210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garaude J, Acín-Pérez R, Martínez-Cano S, Enamorado M, Ugolini M, Nistal-Villán E, et al. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat Immunol. 2016;17:1037–45. doi: 10.1038/ni.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halvorsen B, Espeland MZ, Andersen GØ, Yndestad A, Sagen EL, Rashidi A, et al. Increased expression of NAMPT in PBMC from patients with acute coronary syndrome and in inflammatory M1 macrophages. Atherosclerosis. 2015;243:204–10. doi: 10.1016/j.atherosclerosis.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Cameron AM, Castoldi A, Sanin DE, Flachsmann LJ, Field CS, Puleston DJ, et al. Inflammatory macrophage dependence on NAD+salvage is a consequence of reactive oxygen species–mediated DNA damage. Nat Immunol. 2019;20:420–32. doi: 10.1038/s41590-019-0336-y. [DOI] [PubMed] [Google Scholar]
- 44.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–80. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cleeter MWJ, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AHV. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–4. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 46.Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17:684–96. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Postat J, Olekhnovitch R, Lemaître F, Bousso P. A metabolism-based quorum sensing mechanism contributes to termination of inflammatory responses. Immunity. 2018;49:654–.e5. doi: 10.1016/j.immuni.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 48.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–16. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 49.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corraliza IM, Soler G, Eichmann K, Modolell M. Arginase induction by suppressors of nitric oxide synthesis (IL-4, IL-10 and PGE2) in murine bone-marrow-derived macrophages. Biochem Biophys Res Commun. 1995;206:667–73. doi: 10.1006/bbrc.1995.1094. [DOI] [PubMed] [Google Scholar]
- 51.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, et al. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:255–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puleston DJ, Buck MD, Klein Geltink RI, Kyle RL, Caputa G, O'Sullivan D, et al. Polyamines and eIF5A hypusination modulate mitochondrial respiration and macrophage activation. Cell Metab. 2019;30:352–.e8. doi: 10.1016/j.cmet.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang F, Zhang S, Vuckovic I, Jeon R, Lerman A, Folmes CD, et al. Glycolytic stimulation is not a requirement for M2 macrophage differentiation. Cell Metab. 2018;28:463–.e4. doi: 10.1016/j.cmet.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang SC, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD, Pearce EJ. Metabolic reprogramming mediated by the mTORC2-IRF4 signaling axis is essential for macrophage alternative activation. Immunity. 2016;45:817–30. doi: 10.1016/j.immuni.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang SC, Everts B, Ivanova Y, O'Sullivan D, Nascimento M, Smith AM, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–55. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu PS, Wang H, Li X, Chao T, Teav T, Christen S, et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 2017;18:985–94. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- 57.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feng J, Han J, Pearce SF, Silverstein RL, Gotto AM, Jr, Hajjar DP, et al. Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-γ. J Lipid Res. 2000;41:688–96. [PubMed] [Google Scholar]
- 59.Nomura M, Liu J, Rovira II, Gonzalez-Hurtado E, Lee J, Wolfgang MJ, et al. Fatty acid oxidation in macrophage polarization. Nat Immunol. 2016;17:216–7. doi: 10.1038/ni.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Namgaladze D, Brüne B. Fatty acid oxidation is dispensable for human macrophage IL-4-induced polarization. Biochim Biophys Acta—Mol Cell Biol Lipids. 2014;1841:1329–35. doi: 10.1016/j.bbalip.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 61.Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114:3244–54. doi: 10.1182/blood-2009-04-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–44. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 63.Covarrubias, AJ, Aksoylar HI, Yu J, Snyder NW, Worth AJ, Iyer SS, et al. Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife. 2016:5:e11612. [DOI] [PMC free article] [PubMed]
- 64.Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, et al. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 2013;4:2834. doi: 10.1038/ncomms3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evren E, Ringqvist E, Willinger T. Origin and ontogeny of lung macrophages: from mice to humans. Immunology. 2020;160:126–38. doi: 10.1111/imm.13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider C, Nobs SP, Kurrer M, Rehrauer H, Thiele C, Kopf M. Induction of the nuclear receptor PPAR-γ 3 by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol. 2014;15:1026–37. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- 67.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krafft MP. Overcoming inactivation of the lung surfactant by serum proteins: a potential role for fluorocarbons? Soft Matter. 2015;11:5982–94. doi: 10.1039/c5sm00926j. [DOI] [PubMed] [Google Scholar]
- 69.Fessler MB, Summer RS. Surfactant lipids at the host-environment interface metabolic sensors, suppressors, and effectors of inflammatory lung disease. Am J Respir Cell Mol Biol. 2016;54:624–35. doi: 10.1165/rcmb.2016-0011PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Remmerie A, Scott CL. Macrophages and lipid metabolism. Cell Immunol. 2018;330:27–42. doi: 10.1016/j.cellimm.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baker AD, Malur A, Barna BP, Ghosh S, Kavuru MS, Malur AG, et al. Targeted PPARγ deficiency in alveolar macrophages disrupts surfactant catabolism. J Lipid Res. 2010;51:1325–31. doi: 10.1194/jlr.M001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu X, Buttgereit A, Lelios I, Utz SG, Cansever D, Becher B, et al. The cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity. 2017;47:903–.e4. doi: 10.1016/j.immuni.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 73.Park MD, Merad M. Cooperation between the alveolar epithelium and lung-resident basophils shapes alveolar macrophages. Nat Rev Immunol. 2021;21:344–344. doi: 10.1038/s41577-021-00561-8. [DOI] [PubMed] [Google Scholar]
- 74.Ebina-Shibuya R, Matsumoto M, Kuwahara M, Jang KJ, Sugai M, Ito Y, et al. Inflammatory responses induce an identity crisis of alveolar macrophages, leading to pulmonary alveolar proteinosis. J Biol Chem. 2017;292:18098–112. doi: 10.1074/jbc.M117.808535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakamura A, Ebina-Shibuya R, Itoh-Nakadai A, Muto A, Shima H, Saigusa D, et al. Transcription repressor Bach2 is required for pulmonary surfactant homeostasis and alveolar macrophage function. J Exp Med. 2013;210:2191–204. doi: 10.1084/jem.20130028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Plantier L, Besnard V, Xu Y, Ikegami M, Wert SE, Hunt AN, et al. Activation of sterol-response element-binding proteins (SREBP) in alveolar type II cells enhances lipogenesis causing pulmonary lipotoxicity. J Biol Chem. 2012;287:10099–114. doi: 10.1074/jbc.M111.303669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sinclair C, Bommakanti G, Gardinassi L, Loebbermann J, Johnson MJ, Hakimpour P, et al. MTOR regulates metabolic adaptation of APCs in the lung and controls the outcome of allergic inflammation. Science. 2017;357:1014–21. doi: 10.1126/science.aaj2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Millward CA, Heaney JD, Sinasac DS, Chu EC, Bederman IR, Gilge DA, et al. Mice with a deletion in the gene for CCAAT/enhancer-binding protein β are protected against diet-induced obesity. Diabetes. 2007;56:161–7. doi: 10.2337/db06-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rahman SM, Janssen RC, Choudhury M, Baquero KC, Aikens RM, de la Houssaye BA, et al. CCAAT/Enhancer-binding Protein β (C/EBPβ) expression regulates dietary-induced inflammation in macrophages and adipose tissue in mice. J Biol Chem. 2012;287:34349–60. doi: 10.1074/jbc.M112.410613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cain DW, O'Koren EG, Kan MJ, Womble M, Sempowski GD, Hopper K, et al. Identification of a tissue-specific, C/EBPβ-dependent pathway of differentiation for murine peritoneal macrophages. J Immunol. 2013;191:4665–75. doi: 10.4049/jimmunol.1300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sallese A, Suzuki T, McCarthy C, Bridges J, Filuta A, Arumugam P, et al. Targeting cholesterol homeostasis in lung diseases. Sci Rep. 2017;7:10211. doi: 10.1038/s41598-017-10879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schuster GU, Parini P, Wang L, Alberti S, Steffensen KR, Hansson GK, et al. Accumulation of foam cells in liver X receptor-deficient mice. Circulation. 2002;106:1147–53. doi: 10.1161/01.cir.0000026802.79202.96. [DOI] [PubMed] [Google Scholar]
- 83.Out R, Hoekstra M, Hildebrand RB, Kruit JK, Meurs I, Li Z, et al. Macrophage ABCG1 deletion disrupts lipid homeostasis in alveolar macrophages and moderately influences atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2295–2300. doi: 10.1161/01.ATV.0000237629.29842.4c. [DOI] [PubMed] [Google Scholar]
- 84.McCarthy C, Lee E, Bridges JP, Sallese A, Suzuki T, Woods JC, et al. Statin as a novel pharmacotherapy of pulmonary alveolar proteinosis. Nat Commun. 2018;9:1–9. doi: 10.1038/s41467-018-05491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Izquierdo HM, Brandi P, Gómez MJ, Conde-Garrosa R, Priego E, Enamorado M, et al. Von Hippel-Lindau protein is required for optimal alveolar macrophage terminal differentiation, self-renewal, and function. Cell Rep. 2018;24:1738–46. doi: 10.1016/j.celrep.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Svedberg FR, Brown SL, Krauss MZ, Campbell L, Sharpe C, Clausen M, et al. The lung environment controls alveolar macrophage metabolism and responsiveness in type 2 inflammation. Nat Immunol. 2019;20:571–80. doi: 10.1038/s41590-019-0352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang L, Nazarova EV, Tan S, Liu Y, Russell DG. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J Exp Med. 2018;215:1135–52. doi: 10.1084/jem.20172020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ogger PP, Byrne AJ. Macrophage metabolic reprogramming during chronic lung disease. Mucosal Immunol. 2021;14:282–95. doi: 10.1038/s41385-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.A-Gonzalez N, Castrillo A. Origin and specialization of splenic macrophages. Cell Immunol. 2018;330:151–8. doi: 10.1016/j.cellimm.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 90.Kurotaki D, Uede T, Tamura T. Functions and development of red pulp macrophages. Microbiol Immunol. 2015;59:55–62. doi: 10.1111/1348-0421.12228. [DOI] [PubMed] [Google Scholar]
- 91.A-Gonzalez N, Guillen JA, Gallardo G, Diaz M, de la Rosa JV, Hernandez IH, et al. The nuclear receptor LXRα controls the functional specialization of splenic macrophages. Nat Immunol. 2013;14:831–9. doi: 10.1038/ni.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–58. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 94.Blériot C, Ginhoux F. Understanding the heterogeneity of resident liver macrophages. Front Immunol. 2019;10:1–6. doi: 10.3389/fimmu.2019.02694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nguyen-Lefebvre AT, Horuzsko A. Kupffer cell metabolism and function. J Enzymol Metab. 2016;1:1–26. [PMC free article] [PubMed] [Google Scholar]
- 96.Meszaros K, Bojta J, Bautista AP, Lang CH, Spitzer JJ. Glucose utilization by Kupffer cells, endothelial cells, and granulocytes in endotoxemic rat liver. Am J Physiol—Gastrointest Liver Physiol. 1991;260:G7–G12. doi: 10.1152/ajpgi.1991.260.1.G7. [DOI] [PubMed] [Google Scholar]
- 97.Na YR, Jung D, Song J, Park JW, Hong JJ, Seok SH. Pyruvate dehydrogenase kinase is a negative regulator of interleukin-10 production in macrophages. J Mol Cell Biol. 2020;12:543–55. doi: 10.1093/jmcb/mjz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sierro F, Evrard M, Rizzetto S, Melino M, Mitchell AJ, Florido M, et al. A liver capsular network of monocyte-derived macrophages restricts hepatic dissemination of intraperitoneal bacteria by neutrophil recruitment. Immunity. 2017;47:374–.e6. doi: 10.1016/j.immuni.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 99.Scott CL, Guilliams M. The role of Kupffer cells in hepatic iron and lipid metabolism. J Hepatol. 2018;69:1197–9. doi: 10.1016/j.jhep.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Winn, NC, Volk, KM & Hasty, AH. Regulation of tissue iron homeostasis: The macrophage “ferrostat”. JCI Insight. 2020:5:e132964. [DOI] [PMC free article] [PubMed]
- 101.Ganz, T. Macrophages and iron metabolism. Myeloid Cells Heal. Dis. A Synth. 217:203–12.
- 102.Nairz M, Theurl I, Swirski FK, Weiss G. “Pumping iron”—how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflug Arch Eur J Physiol. 2017;469:397–418. doi: 10.1007/s00424-017-1944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sukhbaatar N, Weichhart T. Iron regulation: macrophages in control. Pharmaceuticals. 2018;11:137. doi: 10.3390/ph11040137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kohyama M, Ise W, Edelson BT, Wilker PR, Hildner K, Mejia C, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457:318–21. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haldar M, Kohyama M, So AY, Kc W, Wu X, Briseño CG, et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell. 2014;156:1223–34. doi: 10.1016/j.cell.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li W, Wang Y, Zhao H, Zhang H, Xu Y, Wang S, et al. Identification and transcriptome analysis of erythroblastic island macrophages. Blood. 2019;134:480–91. doi: 10.1182/blood.2019000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kovtunovych G, Eckhaus MA, Ghosh MC, Ollivierre-Wilson H, Rouault TA. Dysfunction of the heme recycling system in heme oxygenase 1-deficient mice: effects on macrophage viability and tissue iron distribution. Blood. 2010;116:6054–62. doi: 10.1182/blood-2010-03-272138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scott CL, T'Jonck W, Martens L, Todorov H, Sichien D, Soen B, et al. The transcription factor ZEB2 is required to maintain the tissue-specific identities of macrophages. Immunity. 2018;49:312–.e5. doi: 10.1016/j.immuni.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bennett H, Troutman TD, Sakai M, Glass CK. Epigenetic regulation of Kupffer cell function in health and disease. Front Immunol. 2021;11:1–17. doi: 10.3389/fimmu.2020.609618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cassado AA, D’Império Lima MR, Bortoluci KR. Revisiting mouse peritoneal macrophages: Heterogeneity, development, and function. Front Immunol. 2015;6:1–9. doi: 10.3389/fimmu.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pavlou S, Wang L, Xu H, Chen M. Higher phagocytic activity of thioglycollate-elicited peritoneal macrophages is related to metabolic status of the cells. J Inflamm. 2017;14:12–7. doi: 10.1186/s12950-017-0151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rouzer CA, Scott WA, Griffith OW, Hamill AL, Cohn ZA. Glutathione metabolism in resting and phagocytizing peritoneal macrophages. J Biol Chem. 1982;257:2002–8. [PubMed] [Google Scholar]
- 113.Buchmüller-Rouiller Y, Mauël J. Impairment of the oxidative metabolism of mouse peritoneal macrophages by intracellular Leishmania spp. Infect Immun. 1987;55:587–93. doi: 10.1128/iai.55.3.587-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davies LC, Rice CM, Palmieri EM, Taylor PR, Kuhns DB, McVicar DW. Peritoneal tissue-resident macrophages are metabolically poised to engage microbes using tissue-niche fuels. Nat Commun. 2017;8:2074. doi: 10.1038/s41467-017-02092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Y, Yang M, Huang W, Chen W, Zhao Y, Schulte ML, et al. Mitochondrial metabolic reprogramming by CD36 signaling drives macrophage inflammatory responses. Circ Res. 2019;125:1087–102. doi: 10.1161/CIRCRESAHA.119.315833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jing C, Castro-Dopico T, Richoz N, Tuong ZK, Ferdinand JR, Lok LSC, et al. Macrophage metabolic reprogramming presents a therapeutic target in lupus nephritis. Proc Natl Acad Sci USA. 2020;117:15160–71. doi: 10.1073/pnas.2000943117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gautier EL, Ivanov S, Williams JW, Huang SC, Marcelin G, Fairfax K, et al. Gata6 regulates aspartoacylase expression in resident peritoneal macrophages and controls their survival. J Exp Med. 2014;211:1525–31. doi: 10.1084/jem.20140570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rosas M, Davies LC, Giles PJ, Liao CT, Kharfan B, Stone TC, et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344:645–8. doi: 10.1126/science.1251414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Casanova-Acebes M, Menéndez-Gutiérrez MP, Porcuna J, Álvarez-Errico D, Lavin Y, García A, et al. RXRs control serous macrophage neonatal expansion and identity and contribute to ovarian cancer progression. Nat Commun. 2020;11:1655. doi: 10.1038/s41467-020-15371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nagala M, Crocker PR. Towards understanding the cell surface phenotype, metabolic properties and immune functions of resident macrophages of the peritoneal cavity and splenic red pulp using high resolution quantitative proteomics. Wellcome Open Res. 2020;5:165. [Google Scholar]
- 121.Ginhoux F, Prinz M. Origin of microglia: current concepts and past controversies. Cold Spring Harb Perspect Biol. 2015;7:1–15. doi: 10.1101/cshperspect.a020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ghosh S, Castillo E, Frias ES, Swanson RA. Bioenergetic regulation of microglia. Glia. 2018;66:1200–12. doi: 10.1002/glia.23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aldana BI. Microglia-specific metabolic changes in neurodegeneration. J Mol Biol. 2019;431:1830–42. doi: 10.1016/j.jmb.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 124.Lauro C, Limatola C. Metabolic reprograming of microglia in the regulation of the innate inflammatory response. Front Immunol. 2020;11:1–8. doi: 10.3389/fimmu.2020.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Montilla A, Zabala A, Matute C, Domercq M. Functional and metabolic characterization of microglia culture in a defined medium. Front Cell Neurosci. 2020;14:1–11. doi: 10.3389/fncel.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bernier LP, York EM, Kamyabi A, Choi HB, Weilinger NL, MacVicar BA. Microglial metabolic flexibility supports immune surveillance of the brain parenchyma. Nat Commun. 2020;11:1559. doi: 10.1038/s41467-020-15267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Minhas PS, Latif-Hernandez A, McReynolds MR, Durairaj AS, Wang Q, Rubin A, et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature. 2021;590:122–8. doi: 10.1038/s41586-020-03160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arnett TR, Orriss IR. Metabolic properties of the osteoclast. Bone. 2018;115:25–30. doi: 10.1016/j.bone.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 129.Jin Z, Wei W, Yang M, Du Y, Wan Y. Mitochondrial complex i activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab. 2014;20:483–98. doi: 10.1016/j.cmet.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zeng R, Faccio R, Novack DV. Alternative NF-κB regulates RANKL-induced osteoclast differentiation and mitochondrial biogenesis via independent mechanisms. J Bone Miner Res. 2015;30:2287–99. doi: 10.1002/jbmr.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, et al. Coordination of PGC-1β and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med. 2009;15:259–66. doi: 10.1038/nm.1910. [DOI] [PubMed] [Google Scholar]
- 132.Taubmann J, Krishnacoumar B, Böhm C, Faas M, Müller D, Adam S, et al. Metabolic reprogramming of osteoclasts represents a therapeutic target during the treatment of osteoporosis. Sci Rep. 2020;10:1–9. doi: 10.1038/s41598-020-77892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lemma S, Sboarina M, Porporato PE, Zini N, Sonveaux P, Di Pompo G, et al. Energy metabolism in osteoclast formation and activity. Int J Biochem Cell Biol. 2016;79:168–80. doi: 10.1016/j.biocel.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 134.Dodds RA, Gowen M, Bradbeer JN. Microcytophotometric analysis of human osteoclast metabolism: Lack of activity in certain oxidative pathways indicates inability to sustain biosynthesis during resorption. J Histochem Cytochem. 1994;42:599–606. doi: 10.1177/42.5.8157931. [DOI] [PubMed] [Google Scholar]
- 135.Indo Y, Takeshita S, Ishii KA, Hoshii T, Aburatani H, Hirao A, et al. Metabolic regulation of osteoclast differentiation and function. J Bone Miner Res. 2013;28:2392–9. doi: 10.1002/jbmr.1976. [DOI] [PubMed] [Google Scholar]
- 136.Zhang Z, Tang H, Chen P, Xie H, Tao Y. Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct Target Ther. 2019;4:41. doi: 10.1038/s41392-019-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bain CC, Schridde A. Origin, differentiation, and function of intestinal macrophages. Front Immunol. 2018;9:1–15. doi: 10.3389/fimmu.2018.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50:432–.e7. doi: 10.1016/j.immuni.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Munro DAD, Hughes J. The origins and functions of tissue-resident macrophages in kidney development. Front Physiol. 2017;8:1–13. doi: 10.3389/fphys.2017.00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Puranik AS, Leaf IA, Jensen MA, Hedayat AF, Saad A, Kim KW, et al. Kidney-resident macrophages promote a proangiogenic environment in the normal and chronically ischemic mouse kidney. Sci Rep. 2018;8:1–15. doi: 10.1038/s41598-018-31887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mouton AJ, DeLeon-Pennell KY, Rivera Gonzalez OJ, Flynn ER, Freeman TC, Saucerman JJ, et al. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res Cardiol. 2018;113:1–18. doi: 10.1007/s00395-018-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Varga T, Mounier R, Horvath A, Cuvellier S, Dumont F, Poliska S, et al. Highly dynamic transcriptional signature of distinct macrophage subsets during sterile inflammation, resolution, and tissue repair. J Immunol. 2016;196:4771–82. doi: 10.4049/jimmunol.1502490. [DOI] [PubMed] [Google Scholar]
- 143.Martinez CO, McHale MJ, Wells JT, Ochoa O, Michalek JE, McManus LM, et al. Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am J Physiol - Regul Integr Comp Physiol. 2010;299:832–42. doi: 10.1152/ajpregu.00797.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–69. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang X, Sathe AA, Smith GR, Ruf-Zamojski F, Nair V, Lavine KJ, et al. Heterogeneous origins and functions of mouse skeletal muscle-resident macrophages. Proc Natl Acad Sci USA. 2020;117:20729–40. doi: 10.1073/pnas.1915950117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lafuse WP, Wozniak DJ, Rajaram MVS. Role of cardiac macrophages on cardiac inflammation, fibrosis and tissue repair. Cells. 2020;10:51. doi: 10.3390/cells10010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Eming SA, Murray PJ, Pearce EJ. Metabolic orchestration of the wound healing response. Cell Metab. 2021;33:1726–43. doi: 10.1016/j.cmet.2021.07.017. [DOI] [PubMed] [Google Scholar]
- 148.Gondin J, Théret M, Duhamel G, Pegan K, Mathieu JR, Peyssonnaux C, et al. Myeloid HIFs are dispensable for resolution of inflammation during skeletal muscle regeneration. J Immunol. 2015;194:3389–99. doi: 10.4049/jimmunol.1401420. [DOI] [PubMed] [Google Scholar]
- 149.Scheerer N, Dehne N, Stockmann C, Swoboda S, Baba HA, Neugebauer A, et al. Myeloid hypoxia-inducible factor-1α is essential for skeletal muscle regeneration in mice. J Immunol. 2013;191:407–14. doi: 10.4049/jimmunol.1103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mounier R, Théret M, Arnold L, Cuvellier S, Bultot L, Göransson O, et al. AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18:251–64. doi: 10.1016/j.cmet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 152.Poon IKH, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–80. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Morioka S, Perry J, Raymond MH, Medina CB, Zhu Y, Zhao L, et al. Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature. 2018;563:714–8. doi: 10.1038/s41586-018-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Park D, Han CZ, Elliott MR, Kinchen JM, Trampont PC, Das S, et al. Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature. 2011;477:220–4. doi: 10.1038/nature10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Y, Subramanian M, Yurdagul A, Jr, Barbosa-Lorenzi VC, Cai B, de Juan-Sanz J, et al. Mitochondrial fission promotes the continued clearance of apoptotic cells by macrophages. Cell. 2017;171:331–.e22. doi: 10.1016/j.cell.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang S, Weinberg S, DeBerge M, Gainullina A, Schipma M, Kinchen JM, et al. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab. 2019;29:443–.e5. doi: 10.1016/j.cmet.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One. 2014;9:e98972. doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Thangavel P, Ramachandran B, Chakraborty S, Kannan R, Lonchin S, Muthuvijayan V. Accelerated healing of diabetic wounds treated with l-glutamic acid loaded hydrogels through enhanced collagen deposition and angiogenesis: an in vivo study. Sci Rep. 2017;7:10701. doi: 10.1038/s41598-017-10882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, et al. PPAR-δ senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009;15:1266–72. doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Varga T, Mounier R, Patsalos A, Gogolák P, Peloquin M, Horvath A, et al. Macrophage PPARγ, a lipid activated transcription factor controls the growth factor gdf3 and skeletal muscle regeneration. Immunity. 2016;45:1038–51. doi: 10.1016/j.immuni.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Giannakis N, Sansbury BE, Patsalos A, Hays TT, Riley CO, Han X, et al. Dynamic changes to lipid mediators support transitions among macrophage subtypes during muscle regeneration. Nat Immunol. 2019;20:626–36. doi: 10.1038/s41590-019-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shang M, Cappellesso F, Amorim R, Serneels J, Virga F, Eelen G, et al. Macrophage-derived glutamine boosts satellite cells and muscle regeneration. Nature. 2020;587:626–31. doi: 10.1038/s41586-020-2857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kingery JR, Hamid T, Lewis RK, Ismahil MA, Bansal SS, Rokosh G, et al. Leukocyte iNOS is required for inflammation and pathological remodeling in ischemic heart failure. Basic Res Cardiol. 2017;112:1–25. doi: 10.1007/s00395-017-0609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Caputa G, Flachsmann LJ, Cameron AM. Macrophage metabolism: a wound-healing perspective. Immunol Cell Biol. 2019;97:268–78. doi: 10.1111/imcb.12237. [DOI] [PubMed] [Google Scholar]
- 165.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l-arginine metabolism. J Immunol. 2001;167:6533–44. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 166.Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214:2387–404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xie N, Cui H, Ge J, Banerjee S, Guo S, Dubey S, et al. Metabolic characterization and RNA profiling reveal glycolytic dependence of profibrotic phenotype of alveolar macrophages in lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2017;313:L834–L844. doi: 10.1152/ajplung.00235.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gu L, Larson Casey JL, Andrabi SA, Lee JH, Meza-Perez S, Randall TD, et al. Mitochondrial calcium uniporter regulates PGC-1α expression to mediate metabolic reprogramming in pulmonary fibrosis. Redox Biol. 2019;26:101307. doi: 10.1016/j.redox.2019.101307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ogger, PP, Albers GJ, Hewitt RJ, O’Sullivan BJ, Powell JE, Calamita E, et al. Itaconate controls the severity of pulmonary fibrosis. Sci Immunol. 2020:5:eabc1884. [DOI] [PMC free article] [PubMed]
- 170.Puxeddu E, Comandini A, Cavalli F, Pezzuto G, D'Ambrosio C, Senis L, et al. Iron laden macrophages in idiopathic pulmonary fibrosis: the telltale of occult alveolar hemorrhage? Pulm Pharmacol Ther. 2014;28:35–40. doi: 10.1016/j.pupt.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 171.Lee J, Arisi I, Puxeddu E, Mramba LK, Amicosante M, Swaisgood CM, et al. Bronchoalveolar lavage (BAL) cells in idiopathic pulmonary fibrosis express a complex pro-inflammatory, pro-repair, angiogenic activation pattern, likely associated with macrophage iron accumulation. PLoS One. 2018;13:1–15. doi: 10.1371/journal.pone.0194803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Allden SJ, Ogger PP, Ghai P, McErlean P, Hewitt R, Toshner R, et al. The transferrin receptor CD71 delineates functionally distinct airway macrophage subsets during idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;200:209–19. doi: 10.1164/rccm.201809-1775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Spagnolo P, Kreuter M, Maher TM, Wuyts W, Bonella F, Corte TJ, et al. Metformin does not affect clinically relevant outcomes in patients with idiopathic pulmonary fibrosis. Respiration. 2018;96:314–22. doi: 10.1159/000489668. [DOI] [PubMed] [Google Scholar]
- 174.Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, et al. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med. 2018;24:1121–7. doi: 10.1038/s41591-018-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lefere S, Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: Crosstalk with metabolism. JHEP Rep. 2019;1:30–43. doi: 10.1016/j.jhepr.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang S, Van Den Bossche J, Ramalho T. Macrophage metabolism at the crossroad of metabolic diseases and cancer. Immunometabolism. 2020;2:1–26. [Google Scholar]
- 177.Orliaguet L, Dalmas E, Drareni K, Venteclef N, Alzaid F. Mechanisms of macrophage polarization in insulin signaling and sensitivity. Front Endocrinol (Lausanne) 2020;11:1–23. doi: 10.3389/fendo.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brykczynska U, Geigges M, Wiedemann SJ, Dror E, Böni-Schnetzler M, Hess C, et al. Distinct transcriptional responses across tissue-resident macrophages to short-term and long-term metabolic challenge. Cell Rep. 2020;30:1627–.e7. doi: 10.1016/j.celrep.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 179.Geeraerts X, Bolli E, Fendt SM, Van Ginderachter JA. Macrophage metabolism as therapeutic target for cancer, atherosclerosis, and obesity. Front Immunol. 2017;8:289. doi: 10.3389/fimmu.2017.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kazankov K, Jørgensen S, Thomsen KL, Møller HJ, Vilstrup H, George J, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145–59. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 181.Oates JR, McKell MC, Moreno-Fernandez ME, Damen MSMA, Deepe GS, Qualls JE, et al. Macrophage function in the pathogenesis of non-alcoholic fatty liver disease: the Mac attack. Front Immunol. 2019;10:1–16. doi: 10.3389/fimmu.2019.02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Moreno-Fernandez ME, Miraldi ER, Divanovic S. Not chopped liver—a careful, fate-mapping study of Macrophages in NASH. Cell Metab. 2020;32:328–30. doi: 10.1016/j.cmet.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 183.Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci USA. 2018;115:E5096–E5105. doi: 10.1073/pnas.1802611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li P, Lu M, Nguyen M, Bae EJ, Chapman J, Feng D, et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010;285:15333–45. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell. 2019;178:686–.e14. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Silva HM, Báfica A, Rodrigues-Luiz GF, Chi J, Santos PEA, Reis BS, et al. Vasculature-associated fat macrophages readily adapt to inflammatory and metabolic challenges. J Exp Med. 2019;216:786–806. doi: 10.1084/jem.20181049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–25. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Coats BR, Schoenfelt KQ, Barbosa-Lorenzi VC, Peris E, Cui C, Hoffman A, et al. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep. 2017;20:3149–61. doi: 10.1016/j.celrep.2017.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Serbulea V, Upchurch CM, Schappe MS, Voigt P, DeWeese DE, Desai BN, et al. Macrophage phenotype and bioenergetics are controlled by oxidized phospholipids identified in lean and obese adipose tissue. Proc Natl Acad Sci USA. 2018;115:E6254–E6263. doi: 10.1073/pnas.1800544115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sánchez NM, Mahú I, et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med. 2017;23:1309–18. doi: 10.1038/nm.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Boutens L, Hooiveld GJ, Dhingra S, Cramer RA, Netea MG, Stienstra R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia. 2018;61:942–53. doi: 10.1007/s00125-017-4526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW., Jr Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–30. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li Y, Yun K, Mu R. A review on the biology and properties of adipose tissue macrophages involved in adipose tissue physiological and pathophysiological processes. Lipids Health Dis. 2020;19:1–9. doi: 10.1186/s12944-020-01342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Daemen S, Schilling JD. The interplay between tissue niche and macrophage cellular metabolism in obesity. Front Immunol. 2020;10:1–16. doi: 10.3389/fimmu.2019.03133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Caputa G, Castoldi A, Pearce EJ. Metabolic adaptations of tissue-resident immune cells. Nat Immunol. 2019;20:793–801. doi: 10.1038/s41590-019-0407-0. [DOI] [PubMed] [Google Scholar]
- 196.Sharma M, Boytard L, Hadi T, Koelwyn G, Simon R, Ouimet M, et al. Enhanced glycolysis and HIF-1α activation in adipose tissue macrophages sustains local and systemic interleukin-1β production in obesity. Sci Rep. 2020;10:1–12. doi: 10.1038/s41598-020-62272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Acín-Pérez R, Iborra S, Martí-Mateos Y, Cook E, Conde-Garrosa R, Petcherski A, et al. Fgr kinase is required for proinflammatory macrophage activation during diet-induced obesity. Nat Metab. 2020;2:974–88. doi: 10.1038/s42255-020-00273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang Y, Tang B, Long L, Luo P, Xiang W, Li X, et al. Improvement of obesity-associated disorders by a small-molecule drug targeting mitochondria of adipose tissue macrophages. Nat Commun. 2021;12:102. doi: 10.1038/s41467-020-20315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schöttl T, Kappler L, Fromme T, Klingenspor M. Limited OXPHOS capacity in white adipocytes is a hallmark of obesity in laboratory mice irrespective of the glucose tolerance status. Mol Metab. 2015;4:631–42. doi: 10.1016/j.molmet.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Takikawa A, Mahmood A, Nawaz A, Kado T, Okabe K, Yamamoto S, et al. HIF-1α in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes. 2016;65:3649–59. doi: 10.2337/db16-0012. [DOI] [PubMed] [Google Scholar]
- 201.Min BK, Park S, Kang HJ, Kim DW, Ham HJ, Ha CM, et al. Pyruvate dehydrogenase kinase is a metabolic checkpoint for polarization of macrophages to the M1 phenotype. Front Immunol. 2019;10:1–14. doi: 10.3389/fimmu.2019.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Choe SS, Shin KC, Ka S, Lee YK, Chun JS, Kim JB. Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes. 2014;63:3359–71. doi: 10.2337/db13-1965. [DOI] [PubMed] [Google Scholar]
- 203.Li X, Zhang X, Xia J, Zhang L, Chen B, Lian G, et al. Macrophage HIF-2α suppresses NLRP3 inflammasome activation and alleviates insulin resistance. Cell Rep. 2021;36:109607. doi: 10.1016/j.celrep.2021.109607. [DOI] [PubMed] [Google Scholar]
- 204.Ham M, Lee JW, Choi AH, Jang H, Choi G, Park J, et al. Macrophage glucose-6-phosphate dehydrogenase stimulates proinflammatory responses with oxidative stress. Mol Cell Biol. 2013;33:2425–35. doi: 10.1128/MCB.01260-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ham M, Choe SS, Shin KC, Choi G, Kim JW, Noh JR, et al. Glucose-6-phosphate dehydrogenase deficiency improves insulin resistance with reduced adipose tissue inflammation in obesity. Diabetes. 2016;65:2624–38. doi: 10.2337/db16-0060. [DOI] [PubMed] [Google Scholar]
- 206.Ge T, Yang J, Zhou S, Wang Y, Li Y, Tong X. The role of the pentose phosphate pathway in diabetes and cancer. Front Endocrinol (Lausanne) 2020;11:1–11. doi: 10.3389/fendo.2020.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dahik VD, Frisdal E, Goff WL. Rewiring of lipid metabolism in adipose tissue macrophages in obesity: Impact on insulin resistance and type 2 diabetes. Int J Mol Sci. 2020;21:1–30. doi: 10.3390/ijms21155505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Haka AS, Barbosa-Lorenzi VC, Lee HJ, Falcone DJ, Hudis CA, Dannenberg AJ, et al. Exocytosis of macrophage lysosomes leads to digestion of apoptotic adipocytes and foam cell formation. J Lipid Res. 2016;57:980–92. doi: 10.1194/jlr.M064089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Davanso MR, Crisma AR, Murata G, Newsholme P, Curi R. Impact of dietary fatty acids on macrophage lipid metabolism. Signal Funct Immunometabolism. 2020;2:1–41. [Google Scholar]
- 210.Lancaster GI, Langley KG, Berglund NA, Kammoun HL, Reibe S, Estevez E, et al. Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab. 2018;27:1096–.e5. doi: 10.1016/j.cmet.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 211.Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, et al. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990;9:1897–906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Verweij SL, Duivenvoorden R, Stiekema L, Nurmohamed NS, van der Valk FM, Versloot M, et al. CCR2 expression on circulating monocytes is associated with arterial wall inflammation assessed by 18F-FDG PET/CT in patients at risk for cardiovascular disease. Cardiovasc Res. 2018;114:468–75. doi: 10.1093/cvr/cvx224. [DOI] [PubMed] [Google Scholar]
- 213.Tomas L, Edsfeldt A, Mollet IG, Perisic Matic L, Prehn C, Adamski J, et al. Altered metabolism distinguishes high-risk from stable carotid atherosclerotic plaques. Eur Heart J. 2018;39:2301–10. doi: 10.1093/eurheartj/ehy124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fleg JL, Stone GW, Fayad ZA, Granada JF, Hatsukami TS, Kolodgie FD, et al. Detection of high-risk atherosclerotic plaque: report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc Imaging. 2012;5:941–55. doi: 10.1016/j.jcmg.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–66. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 216.Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118:653–67. doi: 10.1161/CIRCRESAHA.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tabas I, Bornfeldt KE. Intracellular and intercellular aspects of macrophage immunometabolism in atherosclerosis. Circ Res. 2020;126:1209–27. doi: 10.1161/CIRCRESAHA.119.315939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–45. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–43. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kim K, Shim D, Lee JS, Zaitsev K, Williams JW, Kim KW, et al. Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models. Circ Res. 2018;123:1127–42. doi: 10.1161/CIRCRESAHA.118.312804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Feig JE, Vengrenyuk Y, Reiser V, Wu C, Statnikov A, Aliferis CF, et al. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS One. 2012;7:e39790. doi: 10.1371/journal.pone.0039790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mueller PA, Zhu L, Tavori H, Huynh K, Giunzioni I, Stafford JM, et al. Deletion of macrophage low-density lipoprotein receptor-related protein 1 (LRP1) accelerates atherosclerosis regression and increases C-C chemokine receptor type 7 (CCR7) expression in plaque macrophages. Circulation. 2018;138:1850–63. doi: 10.1161/CIRCULATIONAHA.117.031702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lee S, Birukov KG, Romanoski CE, Springstead JR, Lusis AJ, Berliner JA. Role of phospholipid oxidation products in atherosclerosis. Circ Res. 2012;111:778–99. doi: 10.1161/CIRCRESAHA.111.256859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Que X, Hung MY, Yeang C, Gonen A, Prohaska TA, Sun X, et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature. 2018;558:301–6. doi: 10.1038/s41586-018-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kamstrup PR, Hung M-Y, Witztum JL, Tsimikas S, Nordestgaard BG. Oxidized phospholipids and risk of calcific aortic valve disease: the copenhagen general population study. Arterioscler Thromb Vasc Biol. 2017;37:1570–8. doi: 10.1161/ATVBAHA.116.308761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Di Gioia M, Spreafico R, Springstead JR, Mendelson MM, Joehanes R, Levy D, et al. Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat Immunol. 2020;21:42–53. doi: 10.1038/s41590-019-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–20. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Christ A, Günther P, Lauterbach M, Duewell P, Biswas D, Pelka K, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell. 2018;172:162–.e14. doi: 10.1016/j.cell.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–60. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 230.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 231.Kellner-Weibel G, Jerome WG, Small DM, Warner GJ, Stoltenborg JK, Kearney MA, et al. Effects of intracellular free cholesterol accumulation on macrophage viability: a model for foam cell death. Arterioscler Thromb Vasc Biol. 1998;18:423–31. doi: 10.1161/01.atv.18.3.423. [DOI] [PubMed] [Google Scholar]
- 232.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–92. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 233.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, et al. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–47. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–8. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–52. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, et al. Macrophage ABCA1 reduces MyD88-dependent toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51:3196–206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Overton CD, Yancey PG, Major AS, Linton MF, Fazio S. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ Res. 2007;100:670–7. doi: 10.1161/01.RES.0000260204.40510.aa. [DOI] [PubMed] [Google Scholar]
- 238.Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J Clin Invest. 2010;120:4415–24. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Xian X, Ding Y, Dieckmann M, Zhou L, Plattner F, Liu M, et al. LRP1 integrates murine macrophage cholesterol homeostasis and inflammatory responses in atherosclerosis. Elife. 2017;6:1–31. doi: 10.7554/eLife.29292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen J, Su Y, Pi S, Hu B, Mao L. The dual role of low-density lipoprotein receptor-related protein 1 in atherosclerosis. Front Cardiovasc Med. 2021;8:1–20. doi: 10.3389/fcvm.2021.682389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Karunakaran D, Thrush AB, Nguyen MA, Richards L, Geoffrion M, Singaravelu R, et al. Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by anti-miR33 in atherosclerosis. Circ Res. 2015;117:266–78. doi: 10.1161/CIRCRESAHA.117.305624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dávalos A, Goedeke L, Smibert P, Ramírez CM, Warrier NP, Andreo U, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci USA. 2011;108:9232–7. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ramírez CM, Goedeke L, Rotllan N, Yoon JH, Cirera-Salinas D, Mattison JA, et al. MicroRNA 33 regulates glucose metabolism. Mol Cell Biol. 2013;33:2891–902. doi: 10.1128/MCB.00016-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ouimet M, Koster S, Sakowski E, Ramkhelawon B, van Solingen C, Oldebeken S, et al. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat Immunol. 2016;17:677–86. doi: 10.1038/ni.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–31. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ning Y, Xu L, Ren S, Pandak WM, Chen S, Yin L. StAR overexpression decreases serum and tissue lipids in apolipoprotein E-deficient mice. Lipids. 2009;44:511–9. doi: 10.1007/s11745-009-3299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Taylor JMW, Borthwick F, Bartholomew C, Graham A. Overexpression of steroidogenic acute regulatory protein increases macrophage cholesterol efflux to apolipoprotein AI. Cardiovasc Res. 2010;86:526–34. doi: 10.1093/cvr/cvq015. [DOI] [PubMed] [Google Scholar]
- 248.Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Babaev VR, Runner RP, Fan D, Ding L, Zhang Y, Tao H, et al. Macrophage Mal1 deficiency suppresses atherosclerosis in low-density lipoprotein receptor-null mice by activating peroxisome proliferator-activated receptor-γ-regulated genes. Arterioscler Thromb Vasc Biol. 2011;31:1283–90. doi: 10.1161/ATVBAHA.111.225839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J Publ Fed Am Soc Exp Biol. 2008;22:3595–606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Marsch E, Theelen TL, Demandt JA, Jeurissen M, van Gink M, Verjans R, et al. Reversal of hypoxia in murine atherosclerosis prevents necrotic core expansion by enhancing efferocytosis. Arterioscler Thromb Vasc Biol. 2014;34:2545–53. doi: 10.1161/ATVBAHA.114.304023. [DOI] [PubMed] [Google Scholar]
- 253.Vink A, Schoneveld AH, Lamers D, Houben AJ, van der Groep P, van Diest PJ, et al. HIF-1 alpha expression is associated with an atheromatous inflammatory plaque phenotype and upregulated in activated macrophages. Atherosclerosis. 2007;195:e69–75. doi: 10.1016/j.atherosclerosis.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 254.Parathath S, Mick SL, Feig JE, Joaquin V, Grauer L, Habiel DM, et al. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ Res. 2011;109:1141–52. doi: 10.1161/CIRCRESAHA.111.246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Folco EJ, Sukhova GK, Quillard T, Libby P. Moderate hypoxia potentiates interleukin-1β production in activated human macrophages. Circ Res. 2014;115:875–83. doi: 10.1161/CIRCRESAHA.115.304437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Aarup A, Pedersen TX, Junker N, Christoffersen C, Bartels ED, Madsen M, et al. Hypoxia-inducible factor-1α expression in macrophages promotes development of atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:1782–90. doi: 10.1161/ATVBAHA.116.307830. [DOI] [PubMed] [Google Scholar]
- 257.Nishizawa T, Kanter JE, Kramer F, Barnhart S, Shen X, Vivekanandan-Giri A, et al. Testing the role of myeloid cell glucose flux in inflammation and atherosclerosis. Cell Rep. 2014;7:356–65. doi: 10.1016/j.celrep.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Freemerman AJ, Zhao L, Pingili AK, Teng B, Cozzo AJ, Fuller AM, et al. Myeloid Slc2a1-deficient murine model revealed macrophage activation and metabolic phenotype are fueled by GLUT1. J Immunol. 2019;202:1265–86. doi: 10.4049/jimmunol.1800002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yamashita A, Zhao Y, Matsuura Y, Yamasaki K, Moriguchi-Goto S, Sugita C, et al. Increased metabolite levels of glycolysis and pentose phosphate pathway in rabbit atherosclerotic arteries and hypoxic macrophage. PLoS One. 2014;9:e86426. doi: 10.1371/journal.pone.0086426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213:337–54. doi: 10.1084/jem.20150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhang X, Sergin I, Evans TD, Jeong SJ, Rodriguez-Velez A, Kapoor D, et al. High-protein diets increase cardiovascular risk by activating macrophage mTOR to suppress mitophagy. Nat Metab. 2020;2:110–25. doi: 10.1038/s42255-019-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Baardman J, Verberk S, van der Velden S, Gijbels M, van Roomen C, Sluimer JC, et al. Macrophage ATP citrate lyase deficiency stabilizes atherosclerotic plaques. Nat Commun. 2020;11:6296. doi: 10.1038/s41467-020-20141-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Almeida L, Everts B. Fa(c)t checking: how fatty acids shape metabolism and function of macrophages and dendritic cells. Eur J Immunol. 2021;51:1628–40. doi: 10.1002/eji.202048944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Developmental Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559–72. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, et al. Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity. 2017;47:597. doi: 10.1016/j.immuni.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Loyher PL, Hamon P, Laviron M, Meghraoui-Kheddar A, Goncalves E, Deng Z, et al. Macrophages of distinct origins contribute to tumor development in the lung. J Exp Med. 2018;215:2536–53. doi: 10.1084/jem.20180534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kalbasi A, Komar C, Tooker GM, Liu M, Lee JW, Gladney WL, et al. Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23:137–48. doi: 10.1158/1078-0432.CCR-16-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, et al. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep. 2016;17:2445–59. doi: 10.1016/j.celrep.2016.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4:155. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ojalvo LS, King W, Cox D, Pollard JW. High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol. 2009;174:1048–64. doi: 10.2353/ajpath.2009.080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.M. de-Brito N, Duncan-Moretti J, C. da-Costa H, Saldanha-Gama R, Paula-Neto HA, G. Dorighello G, et al. Aerobic glycolysis is a metabolic requirement to maintain the M2-like polarization of tumor-associated macrophages. Biochim Biophys Acta—Mol Cell Res. 2020;1867:118604. doi: 10.1016/j.bbamcr.2019.118604. [DOI] [PubMed] [Google Scholar]
- 274.Liu D, Chang C, Lu N, Wang X, Lu Q, Ren X, et al. Comprehensive proteomics analysis reveals metabolic reprogramming of tumor-associated macrophages stimulated by the tumor microenvironment. J Proteome Res. 2017;16:288–97. doi: 10.1021/acs.jproteome.6b00604. [DOI] [PubMed] [Google Scholar]
- 275.Cuccarese MF, Dubach JM, Pfirschke C, Engblom C, Garris C, Miller MA, et al. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat Commun. 2017;8:14293. doi: 10.1038/ncomms14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, et al. Autocrine production of IL-10 mediates defective IL-12 production and NF-κB activation in tumor-associated macrophages. J Immunol. 2000;164:762–7. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 277.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, et al. Macrophages induce invasiveness of epithelial cancer cells Via NF-κB and JNK. J Immunol. 2005;175:1197–205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 279.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–46. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 280.Q wen Zhang, L Liu, C yang Gong, H shan Shi, Y hui Zeng, X ze Wang, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2021:7:e50946. [DOI] [PMC free article] [PubMed]
- 281.De Vos Van Steenwijk PJ, Ramwadhdoebe TH, Goedemans R, Doorduijn EM, van Ham JJ, Gorter A, et al. Tumor-infiltrating CD14-positive myeloid cells and CD8-positive T-cells prolong survival in patients with cervical carcinoma. Int J Cancer. 2013;133:2884–94. doi: 10.1002/ijc.28309. [DOI] [PubMed] [Google Scholar]
- 282.Skytthe MK, Graversen JH, Moestrup SK. Targeting of CD163+ macrophages in inflammatory and malignant diseases. Int J Mol Sci. 2020;21:5497. doi: 10.3390/ijms21155497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Etzerodt A, Tsalkitzi K, Maniecki M, Damsky W, Delfini M, Baudoin E, et al. Specific targeting of CD163+ TAMs mobilizes inflammatory monocytes and promotes T cell–mediated tumor regression. J Exp Med. 2019;216:2394–411. doi: 10.1084/jem.20182124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Edin S, Wikberg ML, Dahlin AM, Rutegård J, Öberg Å, Oldenborg PA, et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH, Ding Y, et al. The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med. 2010;8:13. doi: 10.1186/1479-5876-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Mills CD, Shearer J, Evans R, Caldwell MD. Macrophage arginine metabolism and the inhibition or stimulation of cancer. J Immunol. 1992;149:2709–14. [PubMed] [Google Scholar]
- 287.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167:829–.e13. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–49. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 289.Singh K, Coburn LA, Asim M, Barry DP, Allaman MM, Shi C, et al. Ornithine decarboxylase in macrophages exacerbates colitis and promotes colitis-associated colon carcinogenesis by impairing M1 immune responses. Cancer Res. 2018;78:4303–15. doi: 10.1158/0008-5472.CAN-18-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chang CI, Liao JC, Kuo L. Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer Res. 2001;61:1100–6. [PubMed] [Google Scholar]
- 291.Campesato LF, Budhu S, Tchaicha J, Weng CH, Gigoux M, Cohen IJ, et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat Commun. 2020;11:4011. doi: 10.1038/s41467-020-17750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Takenaka MC, Gabriely G, Rothhammer V, Mascanfroni ID, Wheeler MA, Chao CC, et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci. 2019;22:729–40. doi: 10.1038/s41593-019-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hankinson O. The role of AHR-inducible cytochrome P450s in metabolism of polyunsaturated fatty acids. Drug Metab Rev. 2016;48:342–50. doi: 10.1080/03602532.2016.1197240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:749–749. doi: 10.1038/nrc.2016.114. [DOI] [PubMed] [Google Scholar]
- 295.Palmieri EM, Menga A, Martín-Pérez R, Quinto A, Riera-Domingo C, De Tullio G, et al. Pharmacologic or genetic targeting of glutamine synthetase skews macrophages toward an M1-like phenotype and inhibits tumor metastasis. Cell Rep. 2017;20:1654–66. doi: 10.1016/j.celrep.2017.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2016;17:9–17. doi: 10.1038/ni.3320. [DOI] [PubMed] [Google Scholar]
- 297.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–56. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 298.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 299.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 300.Chang C-H, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–41. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593:282–8. doi: 10.1038/s41586-021-03442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–80. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 304.Wu J-Y, Huang TW, Hsieh YT, Wang YF, Yen CC, Lee GL, et al. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol Cell. 2020;77:213–.e5. doi: 10.1016/j.molcel.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 305.Park JE, Dutta B, Tse SW, Gupta N, Tan CF, Low JK, et al. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift. Oncogene. 2019;38:5158–73. doi: 10.1038/s41388-019-0782-x. [DOI] [PubMed] [Google Scholar]
- 306.Sag D, Cekic C, Wu R, Linden J, Hedrick CC. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat Commun. 2015;6:6354. doi: 10.1038/ncomms7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–41. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Pradel LC, Mitchell AJ, Zarubica A, Dufort L, Chasson L, Naquet P, et al. ATP-binding cassette transporter hallmarks tissue macrophages and modulates cytokine-triggered polarization programs. Eur J Immunol. 2009;39:2270–80. doi: 10.1002/eji.200838867. [DOI] [PubMed] [Google Scholar]
- 309.Goossens P, Rodriguez-Vita J, Etzerodt A, Masse M, Rastoin O, Gouirand V, et al. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab. 2019;29:1376–.e4. doi: 10.1016/j.cmet.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 310.Lenart M, Rutkowska-Zapala M, Baj-Krzyworzeka M, Szatanek R, Węglarczyk K, Smallie T, et al. Hyaluronan carried by tumor-derived microvesicles induces IL-10 production in classical (CD14++CD16−) monocytes via PI3K/Akt/mTOR-dependent signalling pathway. Immunobiology. 2017;222:1–10. doi: 10.1016/j.imbio.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 311.Okoro EU, Guo Z, Yang H. Akt isoform-dependent regulation of ATP-binding cassette A1 expression by apolipoprotein E. Biochem Biophys Res Commun. 2016;477:123–8. doi: 10.1016/j.bbrc.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Guillaumond F, Bidaut G, Ouaissi M, Servais S, Gouirand V, Olivares O, et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 2015;112:2473–8. doi: 10.1073/pnas.1421601112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kaneda MM, Cappello P, Nguyen AV, Ralainirina N, Hardamon CR, Foubert P, et al. Macrophage PI3Kγ drives pancreatic ductal adenocarcinoma progression. Cancer Disco. 2016;6:870–85. doi: 10.1158/2159-8290.CD-15-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, et al. PI3Kγ 3 is a molecular switch that controls immune suppression. Nature. 2016;539:437–42. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Su P, Wang Q, Bi E, Ma X, Liu L, Yang M, et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res. 2020;80:1438–50. doi: 10.1158/0008-5472.CAN-19-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Halbrook CJ, Pontious C, Kovalenko I, Lapienyte L, Dreyer S, Lee HJ, et al. Macrophage-released pyrimidines inhibit gemcitabine therapy in pancreatic cancer. Cell Metab. 2019;29:1390–.e6. doi: 10.1016/j.cmet.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Mertens C, Akam EA, Rehwald C, Brüne B, Tomat E, Jung M. Intracellular iron chelation modulates the macrophage iron phenotype with consequences on tumor progression. PLoS One. 2016;11:e0166164. doi: 10.1371/journal.pone.0166164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Consonni FM, Bleve A, Totaro MG, Storto M, Kunderfranco P, Termanini A, et al. Heme catabolism by tumor-associated macrophages controls metastasis formation. Nat Immunol. 2021;22:595–606. doi: 10.1038/s41590-021-00921-5. [DOI] [PubMed] [Google Scholar]
- 319.Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB. Upsides and downsides of reactive oxygen species for Cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal. 2012;16:1295–322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Cui S, Reichner JS, Mateo RB, Albina JE. Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or -independent mechanisms. Cancer Res. 1994;54:2462–7. [PubMed] [Google Scholar]
- 321.Lin X, Zheng W, Liu J, Zhang Y, Qin H, Wu H, et al. Oxidative stress in malignant melanoma enhances tumor necrosis factor-α secretion of tumor-associated macrophages that promote cancer cell invasion. Antioxid Redox Signal. 2013;19:1337–55. doi: 10.1089/ars.2012.4617. [DOI] [PubMed] [Google Scholar]
- 322.Weiss JM, Davies LC, Karwan M, Ileva L, Ozaki MK, Cheng RY, et al. Itaconic acid mediates crosstalk between macrophage metabolism and peritoneal tumors. J Clin Invest. 2018;128:3794–805. doi: 10.1172/JCI99169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA. 2010;107:8788–93. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu ZG. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23:898–914. doi: 10.1038/cr.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Anfray C, Ummarino A, Andón FT, Allavena P. Current strategies to target tumor-associated-macrophages to improve anti-tumor immune responses. Cells. 2019;9:46. doi: 10.3390/cells9010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Liu M, O'Connor RS, Trefely S, Graham K, Snyder NW, Beatty GL. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47−mediated ‘don’t-eat-me’ signal. Nat Immunol. 2019;20:265–75. doi: 10.1038/s41590-018-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARγ and PPARδ negatively regulate specific subsets of lipopolysaccharide and IFN-γ target genes in macrophages. Proc Natl Acad Sci USA. 2003;100:6712–7. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Hu, D, Wang, Z, Wang, Y & Liang, C. Targeting macrophages in atherosclerosis. Curr Pharm Biotechnol. 2021:22. [DOI] [PubMed]
- 329.Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther. 2021;6:1–21. doi: 10.1038/s41392-021-00506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Poznyak AV, Zhang D, Orekhova V, Grechko AV, Wetzker R, Orekhov AN. A brief overview of currently used atherosclerosis treatment approaches targeting lipid metabolism alterations. Am J Cardiovasc Dis. 2020;10:62–71. [PMC free article] [PubMed] [Google Scholar]
- 331.Doerstling SS, O’Flanagan CH, Hursting SD. Obesity and cancer metabolism: a perspective on interacting tumor-intrinsic and extrinsic factors. Front Oncol. 2017;7:1–11. doi: 10.3389/fonc.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Artyomov MN, Van Den Bossche J. Immunometabolism perspective Immunometabolism in the single-cell era. Cell Metab. 2020;32:710–25. doi: 10.1016/j.cmet.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Argüello RJ, Combes AJ, Char R, Gigan JP, Baaziz AI, Bousiquot E, et al. SCENITH: a flow cytometry-based method to functionally profile energy metabolism with single-cell resolution. Cell Metab. 2020;32:1063–.e7. doi: 10.1016/j.cmet.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Ahl PJ, Hopkins RA, Xiang WW, Au B, Kaliaperumal N, Fairhurst AM, et al. Met-flow, a strategy for single-cell metabolic analysis highlights dynamic changes in immune subpopulations. Commun Biol. 2020;3:1–15. doi: 10.1038/s42003-020-1027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Hartmann FJ, Bendall SC. Immune monitoring using mass cytometry and related high-dimensional imaging approaches. Nat Rev Rheumatol. 2020;16:87–99. doi: 10.1038/s41584-019-0338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhang Z, Cheng X, Zhao Y, Yang Y. Lighting up live-cell and in vivo central carbon metabolism with genetically encoded fluorescent sensors. Annu Rev Anal Chem. 2020;13:293–314. doi: 10.1146/annurev-anchem-091619-091306. [DOI] [PubMed] [Google Scholar]
- 337.Shi J, Hua L, Harmer D, Li P, Ren G. Cre driver mice targeting macrophages. Methods Mol Biol. 2018;1784:263–75. doi: 10.1007/978-1-4939-7837-3_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Schneemann M, Schoeden G. Macrophage biology and immunology: man is not a mouse. J Leukoc Biol. 2007;81:579–579. doi: 10.1189/jlb.1106702. [DOI] [PubMed] [Google Scholar]