Abstract
Irrigation schemes provide an ideal habitat for Anopheles mosquitoes particularly during the dry season. Reliable estimates of outdoor host-seeking behaviour are needed to assess the impact of vector control options and this is particularly the case for Anopheles arabiensis which displays a wide range of behaviours that circumvent traditional indoor-insecticide based control. In this study we compared the sampling efficiency of the host decoy trap (HDT) with the human landing catch (HLC) and Suna trap in a repeated Latin square design in two villages (Lengwe and Mwanza) on an irrigated sugar estate in southern Malawi. Over the course of 18 trapping nights, we caught 379 female Anopheles, the majority of which were identified as An. arabiensis. Across both villages, there was no detectable difference in Anopheles catch between the HDT compared with the HLC (RR = 0.85, P = 0.508). The overall sensitivity of the HLC was greater than the Suna trap regardless of mosquito density (Lengwe, α = 2.75, 95% credible interval: 2.03–3.73; Mwanza, α = 3.38, 95% credible interval: 1.50–9.30) whereas the sensitivity of the HDT was only greater than the Suna trap when mosquito numbers were high (Lengwe, α = 2.63, 95% credible interval: 2.00–3.85).We conclude that the HDT is an effective sampling device for outdoor host seeking An. arabiensis in southern Malawi. The presence of An. arabiensis in irrigated lands during the dry season poses a challenge for ongoing indoor vector control efforts.
Subject terms: Malaria, Entomology
Introduction
Irrigation provides a refuge for malaria mosquito vectors (in the genus Anopheles) during periods of the year when environmental conditions would otherwise be too hot or too dry to sustain local mosquito populations. The flooding, seepage or spill over of water from irrigation and drainage channels creates the ideal microhabitat for the aquatic life stages of anophelines1. Depending on the agro-ecosystem, the cultivation of land can permanently shift local mosquito population dynamics and living in proximity to agricultural schemes is a risk factor for exposure to potentially infectious mosquito bites2,3.
One of the main species to benefit from irrigated agriculture is Anopheles arabiensis (a member of the Anopheles gambiae species complex), particularly in eastern and southern parts of Africa. This species is generally associated with dry-savannah habitats and adults oviposit in temporary, small, sunlit pools4, meaning that irrigated cropland provides ideal conditions all year-round. In Kenya, for example, the pattern of larval and adult population densities of An. arabiensis reflect the rice cropping cycle with peak abundances occurring when rice stalks are immature after transplantation independent of seasonal rains5. The shallow periphery of microdams—small water harvesting structures—support dominant An. arabiensis populations in Western Kenya6 and Ethiopia7, and several studies have observed increases in both indoor and outdoor densities of An. arabiensis, entomological inoculation rates and malaria incidence as a function of distance from small or large agricultural schemes7–10.
Controlling An. arabiensis via contemporary vector control methods such as insecticide treated nets (ITNs) and indoor residual spraying (IRS) is compromised by the large amount of behavioural plasticity exhibited by this species11. Anopheles arabiensis displays a continuum of feeding (zoophagy and exophagy) and resting (exophily) patterns which can circumvent indoor insecticide-based control. In many areas of East Africa, including Malawi, An. arabiensis has superseded the more anthropophilic An. gambiae s.s. as the dominant vector of the Anopheles gambiae species complex following the upscale of ITNs12–14 although little is currently known concerning the magnitude of outdoor biting by An. arabiensis and its contribution to residual transmission in Malawi. Complementary interventions which target the full spectrum of An. arabiensis behaviour are needed to reduce residual transmission. This requires the simultaneous development of reliable and practical means of quantifying the host-seeking behaviour of outdoor biting vectors to determine the efficacy of these interventions.
Unlike other crops, which are fallow for part of the year, sugarcane is a perennial crop which can be irrigated all year round15. This provides an ideal environment for malaria vectors to flourish and extend the transmission season beyond the typical window of the rainy season. Large commercial sugar estates process sugarcane at source, reducing transport and production costs. This encourages the movement of employees and families into the area increasing the local population density. The combination of year-round mosquito breeding and human settlements within and adjacent to the sugar fields creates a ‘malaria microcosm’ leading many private sugar-processing firms to invest in their own bespoke malaria control programmes as part of healthcare initiatives for the workforce16.
The Illovo Sugar company operates two estates in Malawi with the larger of these estates (Nchalo) located next to the Shire River in the southern district of Chikwawa. Malaria prevalence is historically high and the current Plasmodium falciparum prevalence in children aged 2–10 years old is estimated at 12.2%17. Illovo has conducted an IRS programme since 1990 to reduce malaria incidence18 and currently target all households with a single-application of the organophosphate, pirimiphos-methyl.
The ability to reliably monitor mosquito host-seeking behavior is an essential component of evaluating vector control. While most infectious bites continue to occur indoors19, quantifying the level of outdoor transmission has become increasingly important, particularly in parts of East Africa where there is now extensive evidence for shifts in biting behaviour12 and where the highly adaptable An. arabiensis has become the dominant vector20. Trapping methods are required that capture representative samples of the outdoor host-seeking Anopheles population, but entomological monitoring remains highly reliant on the gold-standard but ethically flawed human landing catch (HLC)21. The HLC requires volunteers to manually aspirate mosquitoes as they land to take a blood-feed. This exposes volunteers to potentially infectious mosquito bites which presents ethical concerns21. Aside from the safety issues, the data collected by HLC volunteers varies by individual, imposing a layer of experimental bias22.
In this study, we quantify outdoor host-seeking malaria vectors in two villages situated inside the sugar estate boundary in Nchalo. Our primary goal was to test the efficacy of the host decoy trap (HDT), a relatively new device for sampling mosquito vectors outdoors23,24, with the HLC and the odour-based Suna trap25, a device used in other nearby studies to measure outdoor catches in the lower Shire Valley14. Our aim was to compare the performance of the HDT as an outdoor Anopheles sampling device in an area where the risk of exposure to Anopheles bites is perceptibly higher due to the irrigated habitat provided by the sugarcane fields. We measure outdoor host-seeking in the face of the high IRS coverage in Illovo and during the middle of the dry season as part of our efforts to understand the ecology of the main vectors in the area for improved control.
Results
We collected a total of 2,096 mosquitoes over the course of 18 nights during the middle of the dry season (June–July 2019) on the Nchalo sugar estate using three outdoor sampling methods. Altogether, we identified 379 Anopheles and 1717 culicines. We primarily focused our analyses on the 379 Anopheles, all of which were female.
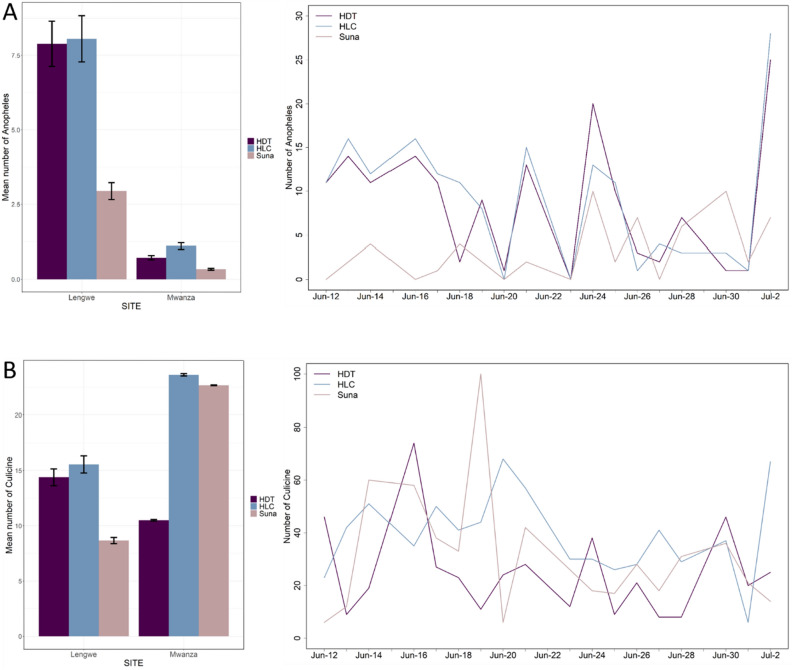
There was a stark difference in the abundance of Anopheles caught between the two study villages despite their proximity (~ 8 km apart) with a 90% reduction in Mwanza (RR = 0.10, P = 2e−16). Across both villages the HDT and HLC caught comparable numbers of Anopheles per night (RR = 0.85, P = 0.508) whereas significantly fewer Anopheles were caught by the Suna trap compared to the HLC (RR = 0.36, P = 0.0005). In Lengwe, the HDT sampled a nightly mean of 7.9 Anopheles (95% CI: 4.6–11.1) compared with 8.0 by HLC (95% CI: 4.3–11.8) while the Suna trap caught 2.8 Anopheles (Fig. 1A). In Mwanza, where the overall number of Anopheles was considerably lower, the HDT caught a nightly average of 0.7 (95% CI: 0.002–1.44) compared with 1.2 by the HLC (95% CI: 0.037–0.63) whereas only 0.3 (CI: 0.056–2.28) were caught using the Suna trap (Fig. 1A). In contrast to the Anopheles catch data, more culicines were caught in Mwanza compared to Lengwe (RR = 1.43, P = 0.041). When culicine data were pooled across villages there was no evidence for differences in the relative capture efficacy of the Suna trap (R = 0.76, P = 0.197) or HDT (R = 0.67, P = 0.060) against the HLC, however, performance was clearly dependent on the village of collection (Fig. 1B).
Figure 1.
The mean number of mosquitoes caught per trap per night for each trapping method in Mwanza and Lengwe (± s.e.m) and a breakdown of the total number of mosquitoes caught per night in both villages. Data are presented for Anopheles (panel A) and culicines (panel B).
At the species level most Anopheles specimens were identified as An. gambiae s.l. (87%, n = 330) followed by An. coustani (11%, n = 40) and An. funestus (2%, n = 9) (Table 1). All An. gambiae s.l. underwent PCR to determine sibling species although we were unable to clarify 43 individual specimens due to non-amplification of PCR products. The proportion of unidentified An. gambiae s.l. specimens caught per trap type was similar (HDT = 13.9%, HLC = 9.0%, Suna = 10.9%). Out of the remaining 287 An. gambiae s.l. samples, the predominant species was An. arabiensis (94.4%, n = 271) followed by An. quadriannulatus (4.2%, n = 12), with very few An. gambiae s.s. (1.4%, n = 4).
Table 1.
Number of mosquitoes caught per trap per village which were identified to species.
| Trap | An. arabiensis | An. coustani | An. funestus | An. gambiae s.s | An. tenebrosus | An. quadriannulatus | Total |
|---|---|---|---|---|---|---|---|
| Lengwe | |||||||
| HDT | 107 | 5 | 3 | 1 | 2 | 6 | 124 |
| HLC | 98 | 15 | 3 | 0 | 12 | 4 | 132 |
| Suna | 38 | 2 | 2 | 0 | 2 | 0 | 44 |
| Total | 243 | 22 | 8 | 1 | 16 | 10 | 300 |
| Mwanza | |||||||
| HDT | 10 | 0 | 0 | 1 | 0 | 1 | 12 |
| HLC | 13 | 1 | 1 | 2 | 1 | 1 | 19 |
| Suna | 5 | 0 | 0 | 0 | 0 | 0 | 5 |
| Total | 28 | 1 | 1 | 3 | 1 | 2 | 36 |
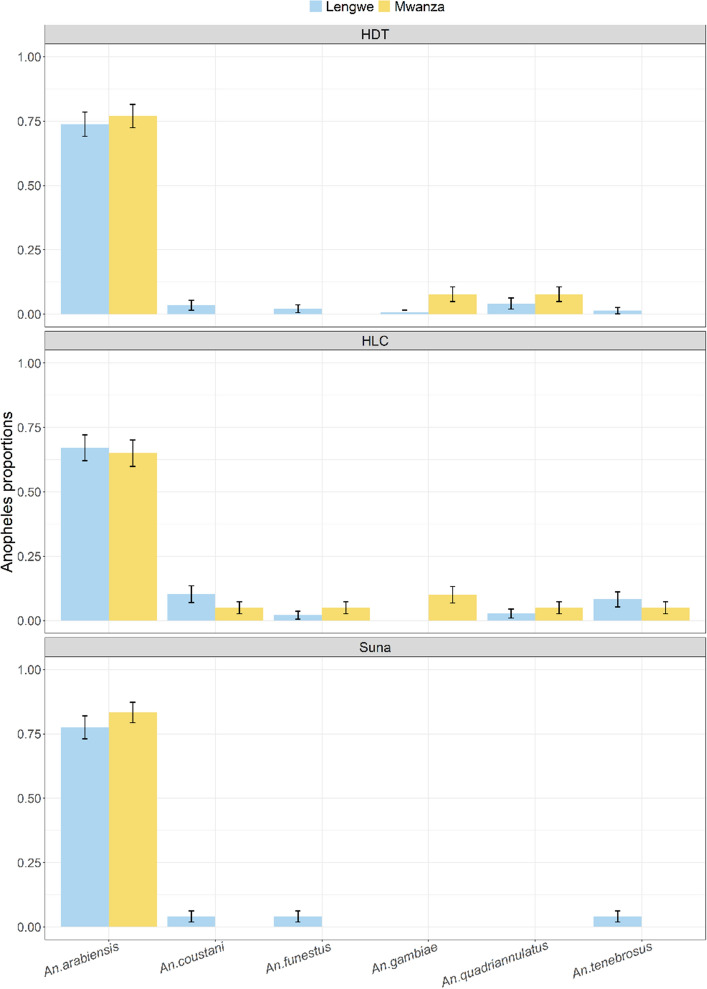
The relative proportions of Anopheles species did not substantially vary by trap and were consistent across Lengwe and Mwanza. An. arabiensis was the predominant species in all trap types in both villages although the relative proportions were slightly higher in the HDT (Lengwe = 0.74; Mwanza = 0.77) and Suna trap (Lengwe = 0.77; Mwanza = 0.83) compared with the HLC (Lengwe = 0.67; Mwanza = 0.65). This reflects the greater diversity of species caught using the HLC (Fig. 2). In Lengwe, where Anopheles numbers were greater, the secondary vectors, An. coustani and An. tenebrosus, comprised 0.10 and 0.08 of HLC collections respectively; relatively higher than the proportion caught by either the HDT (0.03 and 0.01) or Suna trap (0.04 and 0.04). The very low numbers of other anopheline vectors caught in this study (An. gambiae s.s., An. funestus and An. quadriannulatus) make comparisons for these species trivial, although it is noteworthy that only the HDT and HLC caught An. quadriannulatus.
Figure 2.
Relative Anopheles species composition (proportions ± s.e.m) for the HDT, HLC and Suna trap in the Nchalo sugar estate, Malawi. Data are presented for each study village.
All the Anopheles mosquitoes collected were females and the abdominal status were classified as either fed, gravid or unfed, although only six gravid mosquitoes were caught in the entire study. Over one-fifth of female Anopheles caught by HLC had taken a feed prior to collection (21.4%), much higher than the HDT (9.6%) and Suna trap (3.6%).
We investigated whether the sampling efficiency of each trap varied with Anopheles density using a modified test for linearity26–28. The density dependence (ß) and the sampling efficiency (α) parameters for the linear and non-linear models are provided in Table 2.
Table 2.
Summary of the model parameter estimates for each trapping comparison.
| Model comparison | Location | Model 1 | Model 2 | |
|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | ||
| HDT versus Suna | Lengwe | 0.38 (0.26, 0.50) | 8.56 (0.38, 13.40) | 0.27 (0.12,0.98) |
| Mwanza | 0.43 (0.15,1.17) | 0.44 (0.00, 430) | 0.05 (0.00,0.87) | |
| HDT versus HLC | Lengwe | 1.03 (0.80, 1.30) | 0.65 (0.34,1.68) | 1.23 (0.83,1.82) |
| Mwanza | 1.52 (0.83, 3.17) | 1.76 (0.67,14.41) | 0.88 (0.32,195) | |
| Suna versus HLC | Lengwe | 2.75 (2.03, 3.73) | 0.41 (0.29,0.64) | 8.84 (3.01,31.47) |
| Mwanza | 3.38 (1.50, 9.30) | 2.88 (1.01,41.63) | 1.25 (0.21,10.48) | |
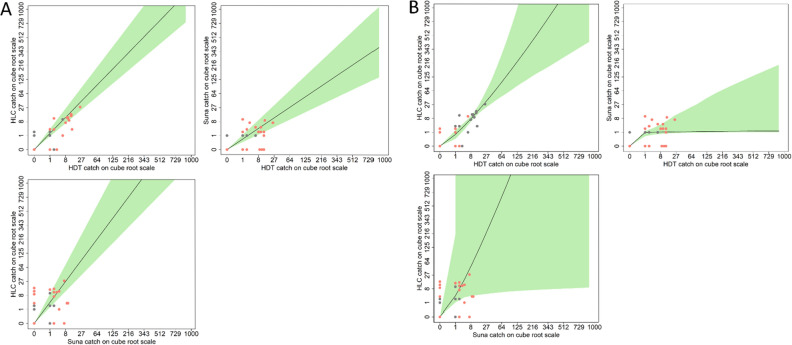
Figure 3 shows the fitted linear (model 1) and non-linear (model 2) relative sampling efficiencies for each trap comparison. When considering the density effects in model 2, the only curve which remains close to a straight line is for the HLC and HDT (Fig. 3b). The mean catch per night for the HLC and HDT was closely correlated (Fig. 1a) and for both Lengwe and Mwanza the 95% credible intervals for ß include the value for density independence (ß = 1) (Table 2). We note, however, that the fewer number of mosquitoes caught in Mwanza (n = 39) lead to extremely wide credibility intervals (particularly in the power model) and demonstrate the limit of this approach when mosquito catches are so few in individual sites. Nevertheless, across the two study villages, the fitted curves and density dependence estimates show a broad agreement between the sampling efficiencies of the HDT and HLC compared with the relationship between these two methods and the Suna trap.
Figure 3.
The fitted relative sampling efficiencies for each trap comparison. For each panel the number of mosquitoes caught per night are plotted against each other for the HDT versus HLC (top left), HDT versus Suna (top right) and HLC versus Suna (bottom left). Sampling efficiencies are shown for the linear model in panel A and for the non-linear (power) model in panel B. The shaded green area corresponds to 95% credible intervals for the best fitting curve for each model comparison.
Discussion
In this study, we add further evidence that the human baited HDT can be an efficient method for catching outdoor Anopheles vectors. We discuss the results in the context of sampling mosquitoes during the dry season in the lower Shire Valley of southern Malawi and within an extensive irrigated landscape.
Based on Anopheles catches per night, and within both villages on the Nchalo sugar estate, the HDT caught similar numbers of Anopheles as the HLC and outperformed the Suna trap (Fig. 1). The predominance of An. arabiensis as the main vector (82% of identified anophelines and 94% of all An. gambiae s.l.) caught during our study means that few species-specific conclusions can be made concerning the diversity of vectors caught by each trap, although we note that the HLC did catch small numbers of a greater variety of anophelines including An. gambiae s.s., An. coustani and An. tenebrosus. As we did not perform simultaneous indoor collections, it is not known whether the lack of An. funestus or An. gambiae s.s. caught in any trap was due to their endophagic behaviour, their relatively low density at this time of year or whether the continued IRS campaign within Illovo has greatly reduced their populations. Over a 38-month sampling period in Majete (approximately 20 kms away from Nchalo) between 2015 and 2018, over twice as many An. arabiensis were caught compared to An. funestus using the Suna trap both indoors and outdoors29. It is likely, therefore, that An. arabiensis is the major vector in this area.
The comparative evaluation of mosquito trapping methods is context specific and depends on the diversity of local vector species, the environment and season. Rarely, if ever, will mosquito traps demonstrate an equivalent efficiency across all environmental contexts, and this is certainly the case for the HDT. In Burkina Faso, the HDT consistently outperformed the HLC regardless of season or mosquito genera with HDT catches of the main anthropophilic and endophagic vector, An. coluzzi, ten-fold higher than with the HLC24. In western Kenya, however, where An. arabiensis and An. gambiae s.s. are the dominant vector species, trap catches were dependent on the type of bait used23 with cattle-baited HDT catching sevenfold more Anopheles (mainly An. arabiensis) than the HLC. In contrast, when the HDT was baited with human odour, the HLC caught approximately sixfold more Anopheles. The efficacy of the HDT may therefore be linked to species-specific differences in behavior; a conclusion supported by collections in the island of Sulawesi, Indonesia where a greater diversity of Anopheles species exists30. The HDT showed comparable efficacy to the HLC when pooling catch data over both villages within the sugar estate (RR = 0.85), however, it is important to recognise that the few mosquitoes caught in Mwanza, means that robust generalizations comparing the performance of outdoor HDT versus the HLC remain limited and further work will be needed in different ecological settings within the country.
The HLC and HDT caught between 2- and fourfold more Anopheles than the Suna trap depending on the village. The Suna trap has shown promise as a relatively inexpensive device for both indoor and outdoor mosquito collections in laboratory and semi-field trials25. In recent field evaluations as part of the Majete Malaria Project—located approximately 30 km away from Nchalo—the sampling efficiency of the Suna trap was similar to the HLC for both indoor and outdoor anophelines14. We note, however, that the relative proportion of the vectors in Majete (ratio of An. gambiae s.l.: An. funestus = 60:40) differs to those from Nchalo, providing a possible explanation for the discrepancy in trap performance.
We caught 4.5-fold more culicines than anophelines over the course of the 18 days of the study. The capture of relatively greater numbers of outdoor culicines compared to Anopheles is not unusual in field evaluations of mosquito traps. Indeed, similar findings were observed with the HLC and Suna trap in nearby Majete14. In contrast to the anopheline data, the overall pattern of culicines caught by each trapping method contrasted between the two villages (Fig. 1). As we did not distinguish culicine specimens to species level we cannot make any robust conclusions about the relative efficacy of each trap to sample culicines, but we hypothesize that the differential catches in Lengwe and Mwanza are due to the species composition.
Alternatives to the HLC must be able to collect enough mosquitoes to justify their use and demonstrate the same functionality in terms of indoor/outdoor biting, have stable capture rates across the night and comparable efficiency irrespective of local mosquito densities31,32. Due to the nature of our study design, we did not address all these criteria, but we were able to investigate density dependence. Two traps are said to be density dependent if the relative sampling sensitivity varies with mosquito density. For example, in the case of the mosquito electrocuting trap (MET), which shows promise as an outdoor sampling tool, the relative sensitivity of the MET and HLC to catch Anopheles is largely unaffected by mosquito density and the traps can be considered density independent26,27. Here, we show that the HDT and HLC have broadly comparable sensitivities for catching an outdoor vector population consisting largely of An. arabiensis, irrespective of density although the small number of mosquitoes caught in Mwanza and the single seasonal period of study (dry-season) means we did not capture the full cycle of annual variation needed to provide more robust conclusions on density dependence.
Irrigated sugar fields are a perennial source of habitat for anopheline vectors. In cultivated sugar farms across eastern and southern Africa, An. arabiensis is one of the main beneficiary species, thriving in the transient and dynamic surface waters provided by irrigation channels and drainage systems. Plenty of reports exist documenting higher mosquito densities and infection rates in localities in proximity to irrigated agricultural land dominated by An. arabiensis from Kenya, Ethiopia and Tanzania6,7,9. The finding that An. arabiensis is the main outdoor host-seeking vector within Nchalo during the dry season is significant as this will impact current vector control efforts. Since 1990, Illovo has conducted a private IRS programme to protect its employees and their families, and now routinely conducts an annual spray of all houses within the estate with a long-lasting application of pirimiphos-methyl (Actellic 300CS). An analysis of monthly malaria health facility data over a five-year period shows that IRS is having a positive impact on reducing malaria cases, but it is almost certain that residual outdoor transmission is occurring and is most likely driven by An. arabiensis. Year-round monitoring of indoor and outdoor mosquito behaviour in the area is needed to determine how to effectively tailor vector control efforts in such a landscape. A recent cluster randomized trial in the Majete Wildlife Reserve showed that the mean nightly entomological inoculation rate (EIR) was very low, with an annual EIR of 1.3 ib/person/year for An. arabiensis29. Our low sample size precluded any meaningful need to investigate sporozoite rates, but based on other surveys in the area, we hypothesise that outdoor rates are similarly low although larger future entomological studies are needed to determine relative EIR in indoor versus outdoor An. arabiensis and whether EIR continues to be a useful metric going forward for measuring vector control impact.
We propose that the HDT is an efficient method for sampling outdoor host-seeking anophelines during the dry-season in southern Malawi. Further investigations are needed to make comparisons with the HLC in other environmental contexts within Malawi to ensure that samples caught by the HDT are representative of local populations and catches remain stable throughout the night. While this study was conducted during just a single dry season, the predominance of An. arabiensis suggests that the irrigated sugar farms provide a year-round refuge for this species and efforts should be directed towards understanding the behavior of An. arabiensis particularly in the context of the local IRS programme.
Methods
Study site
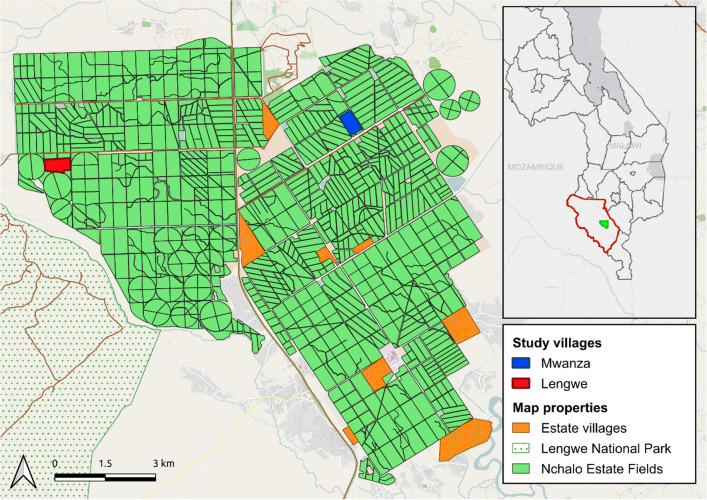
Illovo Sugar is the major producer of sugarcane in the Southern African Development Community (SADC) region. In Malawi, Illovo has two cane growing sites producing 2 million tonnes of sugarcane combined per year. Our study was conducted in two villages, Mwanza (− 16.1995; 34.8009) and Lengwe (− 16.1869; 34.8805), within the Nchalo estate which sits within the low-lying Shire Valley of the southern district of Chikwawa (70 m.a.s.l.) (Fig. 4). Temperatures in the valley can rise up to 40 °C during the dry season which lasts from May to November while peak rainfall occurs sometime between December and February33. The two major malaria vectors found in the regions are An. arabiensis and An. funestus, their relative proportions varying seasonally34. The Nchalo estate incorporates ~ 13,000 ha of sugarcane plots irrigated by a range of methods supplied by water from the Shire River. Illovo has conducted an IRS programme since 1990 using a variety of pyrethroid-based compounds until 2014 before a switch to a single annual application of Actellic 300 CS (pirimiphos-methyl) prior to the onset of the rainy season18. Households within Nchalo receive bednets as part of the National Malaria Control Programme distribution campaigns.
Figure 4.
The location of the Illovo sugar estate within Malawi (inset) and study villages Mwanza and Lengwe. Map created using QGIS version 2.18 (https://qgis.org/en/site/).
Study design and mosquito sample collection
We selected three houses within each of Mwanza and Lengwe for mosquito sampling. All houses were roofed with iron sheets and had fully closed eaves. Sampling houses were approximately 100 m apart to reduce the influence of nearby traps on mosquito collections at other houses. Mosquito sampling was performed using three different methods: the HDT, HLC and Suna trap. The HLC was considered the gold-standard to which catches from the HDT and Suna trap were compared against. All mosquito collections were conducted in the outdoor environment and traps were set-up approximately three metres within the compound of selected houses. For our study we used a repeated 3 × 3 Latin square experimental design (trap x house x night). Two concurrent Latin square rotations were conducted within each village (i.e., six houses containing either HDT, Suna trap or HLC during any single night). The two Latin squares were repeated six nights a week for three weeks (18 nights of data collection per village and 36 trap nights) between the months of June and July 2019. All experiments began at 1800 and ran until 0600 the following morning.
A standardized version of the HDT (developed by the University of Greenwich and Biogents) with some minor modifications was used in all experiments. In brief, the HDT uses a protected host as bait from which odour is funnelled down a 6 m PVC pipe towards a visually contrasting black and thermally heated trap. A transparent adhesive plastic sheet (Barrettine Environmental Health, Bristol, UK) covers the circumference of the trap to catch mosquitoes as they land. During each collection night a volunteer slept in a tent, positioned approximately five meters from the house, as a source of odour. At the end of the collection period, the adhesive sheets were transported to the laboratory and mosquitoes removed using forceps and non-toxic Mobe-Moat solvent (Barrettine Environmental Health, Bristol, UK). Suna traps were suspended outside the house approximately 30 cm above ground level25. Suna traps were baited with the MB5 attractant blend, suspended outside the house approximately 30 cm above ground level and supplied with CO2 produced from yeast and molasses (from the Illovo estate) for fermentation. Mosquitoes were collected from the HDT and Suna traps between 0700 and 0900.
Four HLC collectors were recruited from Mwanza and Lengwe and trained prior to the study. All collectors were given malaria prophylaxis (doxycycline) at a daily dose of 100 mg for the duration of collections and for five days before and after the study. Two volunteers were assigned per house with each working one shift per night (1800-0000, 0000-0600) and taking a 15-min break per hour. Collectors were sitting approximately 3 m from the house with their legs exposed from their knees down. Mosquitoes landing on their legs were collected using a mouth aspirator. To minimize individual host-attraction bias, after six hours (mid-point) during a single collection night, HLC and HDT volunteers swapped places.
Mosquitoes were stored individually, separated by sex and morphologically identified to genus level35 to differentiate anopheline and culicine catches. All anophelines were identified by species complex and sub-species level using PCR36 and classified as either fed or unfed. Ethical approval for this study was obtained from the College of Medicine Research Ethics Committee (COM-REC) (No. 2605).
Data analysis
The primary outcome was the mean number of mosquitoes collected per trap per night. Data was analyzed in R version 3.5.137. Generalized linear mixed models (GLMM) were used to compare the mean number of mosquitoes collected per trap per night with a negative binomial distribution to account for the over-dispersion observed in the initial Poisson model. Models were formally compared using Akaike information criteria (AIC) and log-likelihood ratio tests (LRT). Trap type and village were considered independent fixed effects with the night of collection fit as a random effect. Separate models were fit for nightly collections of Anopheles and Culicine mosquitoes.
The number of mosquitoes caught by two trapping methods targeting the same population should be roughly proportional across the range of mosquito densities. To estimate the comparative sampling efficiencies of each trap we followed the approach by Briet et al.21 and fitted the linear model below:
In this model, is the expected number of mosquitoes caught by a trap Y during the night; is the expected number of mosquitoes caught by trap X on the same night; is the relative sampling efficacy corresponding to site (Lengwe or Mwanza). It is assumed that the mosquito counts in each trap follows a Poisson distribution such that and . Mosquito densities were assumed to follow a log-normal distribution, i.e. . Three models were fitted for each pair of traps (i.e. HDT with HLC, HDT with Suna and HLC with Suna) and only Anopheles data were assessed.
For the density dependent analysis, the linear model was modified as follows:
The parameter is the site-specific exponent governing the relationship between the two traps. Density independence (or proportionality) is achieved when approximates 1 and density-dependent effects are considered if is different from 1.
Model fitting was done in a Bayesian framework using Markov chain Monte Carlo algorithm in WinBUGS 1.4 through the R2WinBUGS R interface. We assigned non-informative uniform distribution priors to all the model parameters and constrained them to be positive. For each model, we ran 40,000 iterations, a burnin of 20,000 and a thinning parameter equal to 20 thus giving 1000 posterior samples for inference.
Ethical approval
The study was conducted with ethical approval from the College of Medicine Research and Ethics Committee (COMREC) (P.02/19/2605) in Malawi. All study procedures were performed in accordance with the relevant national guidelines. Prior to the commencement of the study, we obtained support from the District Health Officer of Chikwawa and from village leaders in Mwanza and Lengwe. Written informed consent was obtained for all volunteers and householders recruited for trap operations. Study information including the purposes, benefits and risks was provided to all participants in both English and Chichewa.
Acknowledgements
We thank the volunteers and households who participated in the mosquito sampling in Lengwe and Mwanza. We are grateful to Dr Albert Mkumbwa and Illovo Sugar Ltd for permission to conduct research on the Nchalo estate. Wisck Agagi provided the shape files for the map of the Illovo sugar estate. KZ was supported by an institutional training grant awarded as part of the Wellcome Strategic award number 206545/Z/17/Z to the Malawi-Liverpool-Wellcome Trust Clinical Research Programme (MLW), administered under the joint MLW/Kamuzu University of Health Sciences Training Committee. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Author contributions
K.Z. and C.J. conceived the study; K.Z.., C.J. and F.M.H. designed the study; K.Z., L.D. and P.N. performed field and lab experiments; J.C., K.Z., D.M. and C.J. analysed the data; K.Z. and C.J. wrote the manuscript; all authors read, commented on, and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ijumba JN, Lindsay SW. Impact of irrigation on malaria in Africa: Paddies paradox. Med. Vet. Entomol. 2001;15:1–11. doi: 10.1046/j.1365-2915.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- 2.Kibret S, Wilson GG, Ryder D, Tekie H, Petros B. The influence of dams on malaria transmission in Sub-Saharan Africa. EcoHealth. 2017;14:408–419. doi: 10.1007/s10393-015-1029-0. [DOI] [PubMed] [Google Scholar]
- 3.Djègbè I, et al. Minimal tillage and intermittent flooding farming systems show a potential reduction in the proliferation of Anopheles mosquito larvae in a rice field in Malanville, Northern Benin. Malar. J. 2020;19:1–10. doi: 10.1186/s12936-020-03406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinka ME, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic précis. Parasites Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muturi EJ, et al. Mosquito species diversity and abundance in relation to land use in a riceland agroecosystem in Mwea, Kenya. JVEC. 2006;31:129–137. doi: 10.3376/1081-1710(2006)31[129:msdaai]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.McCann RS, Gimnig JE, Bayoh MN, Ombok M, Walker ED. Microdam impoundments provide suitable habitat for larvae of malaria vectors: An observational study in Western Kenya. J. Med. Entomol. 2018;55:723–730. doi: 10.1093/jme/tjy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kibret S, Wilson GG, Ryder D, Tekie H, Petros B. Malaria impact of large dams at different eco-epidemiological settings in Ethiopia. Trop. Med. Health. 2017;45:4. doi: 10.1186/s41182-017-0044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghebreyesus TA, et al. Incidence of malaria among children living near dams in northern Ethiopia: Community based incidence survey. BMJ. 1999;319:663–666. doi: 10.1136/bmj.319.7211.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demissew A, et al. Impact of sugarcane irrigation on malaria vector Anopheles mosquito fauna, abundance and seasonality in Arjo-Didessa, Ethiopia. Malar. J. 2020;19:344. doi: 10.1186/s12936-020-03416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonio-Nkondjio C, et al. Malaria transmission and rice cultivation in Lagdo, northern Cameroon. Trans. R. Soc. Trop. Med. Hyg. 2008;102:352–359. doi: 10.1016/j.trstmh.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Killeen GF, et al. Measuring, manipulating and exploiting behaviours of adult mosquitoes to optimise malaria vector control impact. BMJ Glob Health. 2017;2:e000212. doi: 10.1136/bmjgh-2016-000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell TL, et al. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCann RS, et al. The effect of community-driven larval source management and house improvement on malaria transmission when added to the standard malaria control strategies in Malawi: A cluster-randomized controlled trial. Malar. J. 2021;20:232. doi: 10.1186/s12936-021-03769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mburu MM, et al. Assessment of the Suna trap for sampling mosquitoes indoors and outdoors. Malar. J. 2019;18:51. doi: 10.1186/s12936-019-2680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess TM, et al. A sweet deal? Sugarcane, water and agricultural transformation in Sub-Saharan Africa. Glob. Environ. Change. 2016;39:181–194. doi: 10.1016/j.gloenvcha.2016.05.003. [DOI] [Google Scholar]
- 16.Jones RT, et al. The role of the private sector in supporting malaria control in resource development settings. J. Infect. Dis. 2020;222:S701–S708. doi: 10.1093/infdis/jiaa488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chipeta MG, et al. Geostatistical analysis of Malawi’s changing malaria transmission from 2010 to 2017. Wellcome Open Res. 2019;4:57. doi: 10.12688/wellcomeopenres.15193.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roll Back Malaria. PROGRESS & IMPACT SERIES Focus on Malawi Country Reports Number 6 April 2013 Ministry of Health. (2013).
- 19.Sherrard-Smith E, et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc. Natl. Acad. Sci. USA. 2019;116:15086–15095. doi: 10.1073/pnas.1820646116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitau J, et al. Species shifts in the anopheles gambiae complex: Do LLINs successfully control Anopheles arabiensis? PLoS ONE. 2012;7:e31481. doi: 10.1371/journal.pone.0031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achee NL, Youngblood L, Bangs MJ, Lavery JV, James S. Considerations for the use of human participants in vector biology research: A tool for investigators and regulators. Vector-Borne Zoonotic Dis. 2015;15:89–102. doi: 10.1089/vbz.2014.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Service M. Critical-review of procedures for sampling populations of adult mosquitos. Bull. Entomol. Res. 1977;67:343–382. doi: 10.1017/S0007485300011184. [DOI] [Google Scholar]
- 23.Abong’o B, et al. Host decoy trap (HDT) with cattle odour is highly effective for collection of exophagic malaria vectors. Parasites Vect. 2018;11:533. doi: 10.1186/s13071-018-3099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkes FM, Dabiré RK, Sawadogo SP, Torr SJ, Gibson G. Exploiting Anopheles responses to thermal, odour and visual stimuli to improve surveillance and control of malaria. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-17632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiscox A, et al. Development and optimization of the Suna trap as a tool for mosquito monitoring and control. Malar. J. 2014;13:1–14. doi: 10.1186/1475-2875-13-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanou A, et al. Evaluation of mosquito electrocuting traps as a safe alternative to the human landing catch for measuring human exposure to malaria vectors in Burkina Faso. Malar. J. 2019;18:386. doi: 10.1186/s12936-019-3030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Govella NJ, et al. An improved mosquito electrocuting trap that safely reproduces epidemiologically relevant metrics of mosquito human-feeding behaviours as determined by human landing catch. Malar. J. 2016;15:465. doi: 10.1186/s12936-016-1513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Briët OJT, et al. Applications and limitations of Centers for Disease Control and Prevention miniature light traps for measuring biting densities of African malaria vector populations: A pooled-analysis of 13 comparisons with human landing catches. Malar. J. 2015;14:247. doi: 10.1186/s12936-015-0761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amoah B, et al. Identifying plasmodium falciparum transmission patterns through parasite prevalence and entomological inoculation rate. Elife. 2021;10:e65682. doi: 10.7554/eLife.65682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson JR, et al. Characterization of vector communities and biting behavior in South Sulawesi with host decoy traps and human landing catches. Parasites Vect. 2020;13:329. doi: 10.1186/s13071-020-04205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monroe A, et al. Methods and indicators for measuring patterns of human exposure to malaria vectors. Malar. J. 2020;19:207. doi: 10.1186/s12936-020-03271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farlow R, Russell TL, Burkot TR. Nextgen vector surveillance tools: Sensitive, specific, cost-effective and epidemiologically relevant. Malar. J. 2020;19:432. doi: 10.1186/s12936-020-03494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chirombo J, et al. Childhood malaria case incidence in Malawi between 2004 and 2017: Spatio-temporal modelling of climate and non-climate factors. Malar. J. 2020;19:1–13. doi: 10.1186/s12936-019-3097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mzilahowa T, Hastings IM, Molyneux ME, McCall PJ. Entomological indices of malaria transmission in Chikhwawa district, Southern Malawi. Malar. J. 2012;11:380. doi: 10.1186/1475-2875-11-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae) Malar. J. 2020;19:70. doi: 10.1186/s12936-020-3144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles Gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 37.R Core Team. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, 2020).