Abstract
Introduction:
Laboratory studies have demonstrated that antibiotic use in conjunction with bowel purgatives causes alterations to the gut microbiota. Because gut microbiota changes may be a trigger for the development of irritable bowel syndrome (IBS), we sought to assess whether individuals who undergo bowel cleansing for colonoscopy and have concurrent antibiotic exposure develop IBS at higher rates than individuals who undergo colonoscopy without antibiotic exposure.
Methods:
We used data from Optum’s de-identified Clinformatics® Data Mart Database in the United States to study a cohort of 50 – 55 year-olds who underwent screening colonoscopy. Individuals exposed to antibiotics within 14 days of colonoscopy were propensity-score matched to individuals who were not exposed to antibiotics around colonoscopy. The primary outcome was a new IBS diagnosis and the composite outcome was a new claim for IBS, IBS medications, or IBS symptoms. The association of antibiotic exposure and the outcomes was calculated using Cox proportional hazards regression.
Results:
408,714 individuals met criteria for the screening colonoscopy cohort. Of these, 24,617 (6.0%) were exposed to antibiotics around the time of colonoscopy, and they were propensity-score matched to 24,617 individuals not exposed to antibiotics. There was no statistically significant association between antibiotic use and IBS (HR 1.11, 95% CI 0.89 – 1.39), but there was a weak association between antibiotic use and the composite outcome (HR 1.12, 95% CI 1.02 – 1.24, number needed to harm 94).
Conclusion:
Individuals concurrently exposed to antibiotics and bowel purgative had slightly higher rates of surrogate IBS outcomes compared to matched controls who did not receive antibiotics concurrently with bowel purgative.
Keywords: Irritable bowel syndrome, antibiotics, colonoscopy, health services research
Introduction
Irritable bowel syndrome (IBS) is a chronic disorder of brain-gut interaction characterized by recurrent abdominal pain and changes in bowel habits.1, 2 Although the etiology of IBS is incompletely understood, it is thought to be multifactorial from host factors, such as genetic susceptibility and visceral hypersensitivity,3 and environmental triggers, such as diet, psychosocial stressors, and enteric infections.1, 4, 5 As IBS has been associated with dysbiosis,6–8 alteration to the composition of gut microbiota may also be an environmental trigger. Antibiotic exposure and bowel purgative use have each been demonstrated to induce short-term, reversible changes to the composition of the gut microbiota.9–13 Furthermore, concurrent treatment with bowel purgative and antibiotics alters the composition and quantity of gut microbiota in humans, and these changes may be prolonged depending on host factors.14–16 The clinical effects of these gut microbiota alterations in humans are unknown.
As part of clinical care, there are individuals who are incidentally exposed to the combination of antibiotics and bowel purgatives, such as individuals undergoing bowel cleanse prior to colonoscopy who also receive antibiotics for prophylaxis or treatment of infections. The availability of administrative health claims data for clinical research provides an opportunity to study such individuals to determine if they are at increased risk of subsequently developing IBS or IBS symptoms. As such, we used longitudinal patient-level health claims data from the United States to conduct a retrospective cohort study of individuals undergoing screening colonoscopy. We compared the relative rates of IBS and IBS symptoms in those with and without coincident antibiotic exposure to test the hypothesis that combined exposure to antibiotics and bowel purgatives is associated with development of IBS.
Methods
Data source
This retrospective cohort study was performed using data from Optum’s de-identified Clinformatics® Data Mart Database (Optum). Optum is a patient-level database consisting of the inpatient, outpatient, pharmacy, and procedure claims of 88 million unique enrollees of large commercial and Medicare Advantage health plans in the United States from May 1, 2000 – June 30, 2020. We used data from January 1, 2005 – December 31, 2019 to derive the study results. Additional details regarding the data source are presented in Supplemental methods. The Institutional Review Board of the University of Pennsylvania has classified research using Optum as exempt.
Screening colonoscopy cohort inclusion and exclusion criteria
Among all individuals in Optum, we identified those with at least 365 days of continuous follow-up to allow for adequate time to assess covariates.17 For those with multiple discontinuous enrollments in Optum, only the first enrollment was considered to avoid misclassification of exposures, outcomes, and covariates that may have occurred during gaps in enrollment. From these individuals, we identified a cohort of 50 – 55 year-olds who underwent outpatient screening colonoscopy. We restricted the cohort to those undergoing screening colonoscopy because these individuals should not have pre-existing gastrointestinal symptoms that could represent underlying IBS, which would introduce protopathic bias to the study conclusions. The cohort was limited to 50 – 55 year-olds because this was the recommended colorectal cancer screening initiation age during most of the study period by society guidelines in the United States.18, 19 Because procedure codes for screening colonoscopy are often misclassified,20 we determined whether a colonoscopy was a screening colonoscopy using a validated algorithm incorporating International Classification of Diseases (ICD) medical claims, Current Procedural Terminology codes, and Healthcare Common Procedure Coding System codes in the 365 days prior to colonoscopy.21 This approach has been demonstrated to have 89% sensitivity, 91% specificity, and area under the curve of 0.96 for distinguishing screening colonoscopy from non-screening colonoscopy. For individuals who underwent multiple colonoscopies during enrollment, only the first was considered to maintain independence of observations. Cohort exclusion criteria were designed to reduce the possibility of including individuals with prevalent IBS in the cohort and to avoid confounding by indication. These exclusion criteria are listed in Figure 1 and detailed in Supplemental methods.
Figure 1. Screening colonoscopy cohort inclusion and exclusion criteria.
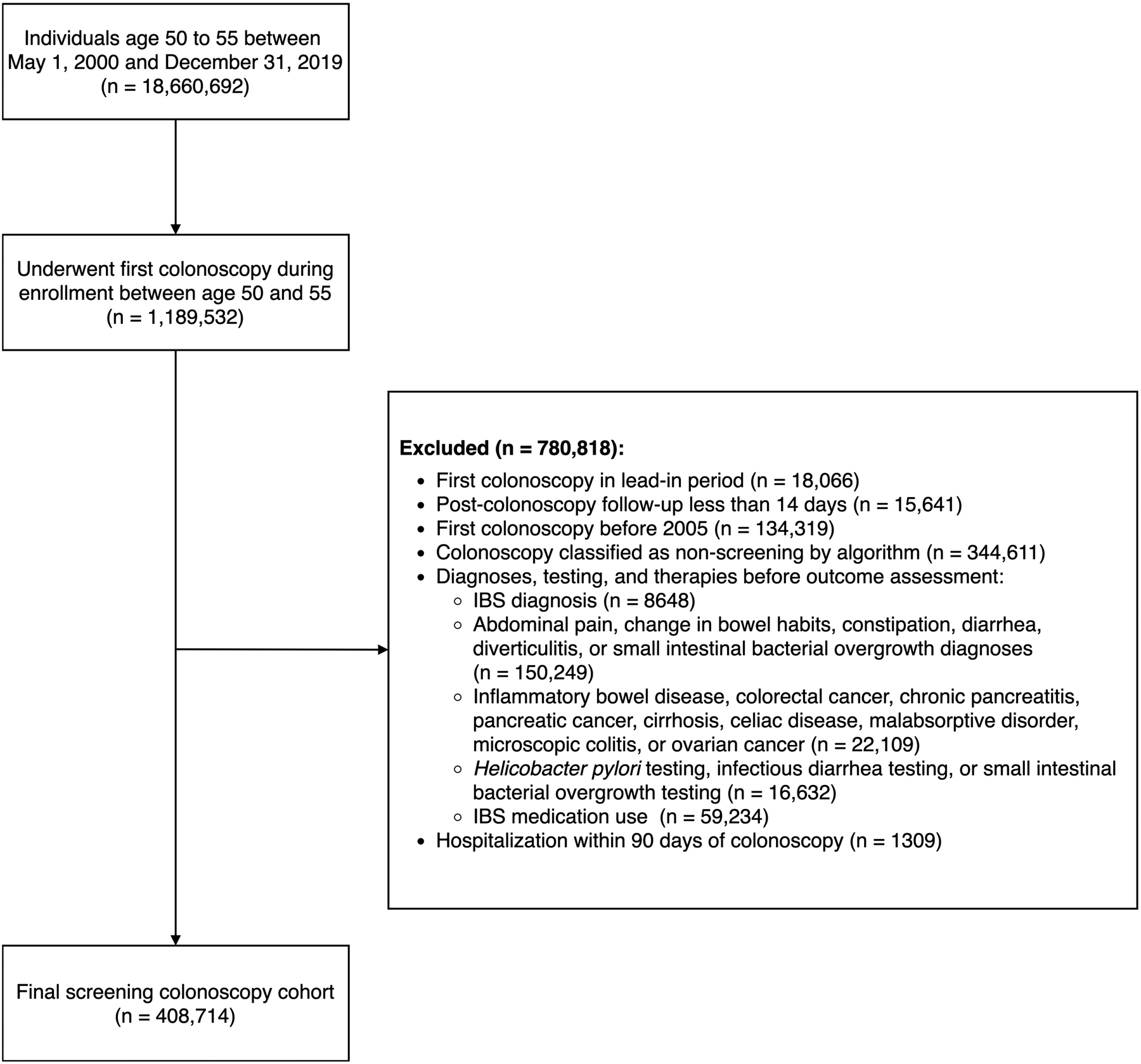
Exclusions applied in listed order
Antibiotic use
We identified individuals who underwent screening colonoscopy and also received antibiotics within 14 days of the colonoscopy through filled prescription claims with an American Hospital Formulary Service code starting with “0812.” Only oral antibiotics were considered as subcutaneous and intravenous antibiotics are less common in the outpatient setting and ophthalmic, otic, and topical antibiotics have negligible interaction with the gut microbiota. Individuals were considered exposed to the antibiotic if there was at least one dose that would be administered within the exposure window of 14 days before colonoscopy to 14 days after colonoscopy based on the fill date and days supplied. We described the possible clinical indications for antibiotic prescriptions by tabulating the frequency of all medical claims in the 14 days prior to the antibiotic fill date.
Outcomes
The outcome of interest was a new diagnosis of IBS from 195 – 1095 days after index colonoscopy. Day 195 was chosen as the start date of the outcome assessment window to allow for 14 days of potential exposure to antibiotics after the colonoscopy and 180 days for development of IBS symptoms per the Rome IV criteria.2 Day 1095 (approximately 3 years from colonoscopy) was selected as the end of the outcome assessment period to allow for sufficient time for formal recording of IBS within the expected window of effect of antibiotics on the gut microbiota.22 Diagnoses of IBS were determined using a validated ICD-claims based algorithm.23 This algorithm classifies individuals as having IBS if they have an ICD code for IBS in any diagnostic position and no ICD codes for Crohn’s disease, colorectal cancer, ulcerative colitis, chronic pancreatitis, pancreatic cancer, cirrhosis, celiac disease, malabsorptive syndromes, microscopic colitis, or ovarian cancer (codes in Supplemental table 1). Individuals with any of these diagnoses prior to day 15 after colonoscopy were excluded from the cohort. The algorithm is estimated to have 99% sensitivity and 91% positive predictive value relative to 120 medical records. Individuals with a diagnosis of IBS prior to day 195 after colonoscopy were censored from the analysis to be consistent with the Rome IV criteria.
Composite outcome.
Since there is likely a lag between symptom onset and a medically coded diagnosis of IBS,22 we performed an analysis in which individuals who newly started IBS medications or were newly diagnosed with IBS symptoms were also considered to have IBS. In this analysis, the outcome was the composite of:
A new IBS claim 195 – 1095 days after colonoscopy
New prescriptions for IBS-associated treatments 195 – 1095 days after colonoscopy. These treatments were alosetron, dicyclomine, diphenoxylate-atropine, eluxadoline, hyoscyamine, linaclotide, loperamide, lubiprostone, plecanatide, or rifaximin (codes in Supplemental table 2).
- New claims for potential IBS symptoms 195 – 1095 days after colonoscopy. Based on Rome IV criteria, these symptoms were:
- At least 1 claim for abdominal pain AND
- At least 1 claim for a defecatory symptom (change in bowel habits, constipation, or diarrhea) AND
- No more than 90 days between abdominal pain and defecatory symptoms to ensure concurrence
For individuals meeting composite outcome criterion 3, the composite outcome date was considered the later of the date of the first abdominal pain claim or date of the first defecatory symptom claim. Individuals meeting the composite outcome criteria prior to day 195 after colonoscopy were censored from the analysis to be consistent with the Rome IV criteria. To avoid recording gastrointestinal symptoms related to non-IBS etiologies, individuals with diagnoses of Crohn’s disease, colorectal cancer, ulcerative colitis, chronic pancreatitis, pancreatic cancer, cirrhosis, celiac disease, malabsorptive syndromes, microscopic colitis, or ovarian cancer were censored from the analysis.
Other covariates
In order to control for potential confounders of the association between antibiotic use and IBS, we assessed for the presence of several patient factors prior to day 15 after colonoscopy. These included:
Patient demographics: Age, sex, colonoscopy year, race/ethnicity, and U.S. census division
Charlson-Deyo score for comorbid diagnoses24
Comorbidities associated with antibiotic prophylaxis for colonoscopy: Heart-valve replacement, orthopedic joint replacement25
Bowel preparation type (Types of bowel preparation regimens in Supplemental table 3. Bowel preparation was extracted from prescription data no more than 30 days before colonoscopy. Individuals without a prescription for a bowel cleanse were assumed to have taken over-the-counter polyethylene glycol electrolyte solution with sports drink, as it is most common over-the-counter bowel purgative.26)
Health care utilization: Number of hospitalizations, number of total outpatient visits in the 365 days prior to colonoscopy, number of office visits between 14 days before colonoscopy and 14 days after colonoscopy, number of office visits 0 – 14 days after colonoscopy, and number of antibiotic prescriptions from enrollment to 14 days before colonoscopy.
Based on preliminary analyses, we suspected that there was potential confounding between health care utilization, antibiotic use, and a formal diagnosis of IBS.27 For example, individuals who have frequent office visits with healthcare providers may be more likely to receive antibiotics for potentially self-limited viral upper respiratory tract infections and may be more likely to receive formal diagnoses of IBS for chronic abdominal symptoms. To adjust for this confounding, we utilized high-dimensional propensity scores from all diagnoses, procedures, and prescriptions recorded in Optum.28 Among individuals in the screening colonoscopy cohort, potential high-dimensional propensity score covariates were selected from the 200 most prevalent ICD-9 diagnoses, the 200 most prevalent ICD-10 diagnoses, the 50 most prevalent procedures, and the 200 most prevalent prescriptions. From these 650 codes, the 200 codes with the strongest associations with IBS were selected for the final high-dimensional propensity score model.
Statistical analyses
The overall study design is graphically depicted in Supplemental figure 1. Stata version 16 (College Station, TX, USA) was used for data extraction and all statistical analyses. Continuous variables are described as means with 95% confidence intervals (CIs) and categorical variables are described as proportions. To control for confounding, we used propensity score described above to match individuals exposed to antibiotics to similar individuals not exposed to antibiotics via propensity score matching with a caliper width of 0.10. The propensity score was generated from a logistic regression model with dependent variable of antibiotic use and independent variables of age, sex, race/ethnicity, U.S. census division, year of colonoscopy, history of prosthetic heart valve or orthopedic prosthesis, Charlson-Deyo score, bowel preparation type, utilization of antibiotics, office visits, and hospitalizations prior to colonoscopy, and the 200 high-dimensional propensity score covariates. Propensity score balance was assessed using standardized mean differences (SMD), and SMD < 0.10 was considered balanced.
To assess the association of antibiotic use and IBS among the propensity-matched screening colonoscopy cohort, we used Cox proportional hazards regression to calculate hazard ratios (HR) of developing IBS or the composite outcome based on antibiotic exposure. Follow-up ended on the earliest of 1095 days from colonoscopy or IBS diagnosis. Individuals were censored from the analysis upon disenrollment from the database or if they were diagnosed with the outcome before the start of the outcome assessment period 195 days after colonoscopy. We calculated the number of antibiotic exposures that would cause one additional IBS outcome (number needed to harm).29 We also performed secondary analyses individually assessing exposure to antibiotics commonly prescribed in the outpatient setting. These antibiotic classes were aminopenicillins, first-generation cephalosporins, macrolides, quinolones, and sulfonamides. To control for multiple comparisons, these secondary analyses were performed with a Bonferroni-corrected type I error rate of 0.005.30
Results
Screening colonoscopy cohort description
From 2000 – 2019, 18,660,692 unique individuals in Optum were age 50 – 55 and met eligibility criteria. Of these, 1,189,532 underwent their first colonoscopy in Optum between ages 50 – 55. After exclusions, the final cohort consisted of 408,714 unique individuals. The most common exclusions were post-colonoscopy follow-up less than 365 days, colonoscopy before 2005, and non-screening colonoscopy (Figure 1). 24,617 individuals were exposed to antibiotics within the 14 days before or after colonoscopy and 384,097 were not exposed. Characteristics of patients exposed and not exposed to antibiotics are presented in Table 1. Notable differences between exposed versus unexposed individuals included higher proportion female, higher proportion with a heart valve replacement or orthopedic prosthetic, higher Charlson-Deyo comorbidity score, higher antibiotic usage prior to colonoscopy, and higher office visit utilization prior to colonoscopy. All antibiotic-exposed individuals were matched to individuals without antibiotic exposure in the propensity-score matched analysis, and all SMDs were < 0.10, indicating covariate balance. The most common claims in the 14 days prior to antibiotic fill date are presented in Table 2. Among potential indications for antibiotics, upper respiratory tract infections, sinusitis, pharyngitis, and urinary tract infections were most common.
Table 1.
Cohort characteristics stratified by antibiotic use
| Antibiotic exposed | Not exposed to antibiotics | Not exposed to antibiotics (propensity-score matched) | Unadjusted SMD | Adjusted SMD | |
|---|---|---|---|---|---|
| (n = 24,617) | (n = 384,097) | (n = 24,617) | |||
| Age at colonoscopy | 51.56 | 51.55 | 51.55 | 0.01 | 0.00 |
| Female | 0.50 | 0.45 | 0.50 | 0.10 | −0.00 |
| Year of colonoscopy | 2011.44 | 2011.83 | 2011.35 | −0.10 | 0.03 |
| Years from enrollment to colonoscopy | 5.45 | 5.61 | 5.64 | −0.04 | −0.05 |
| Total years of follow-up from enrollment | 9.36 | 9.43 | 9.60 | −0.01 | −0.05 |
| Prosthetic heart valve or orthopedic prosthetic | 0.02 | 0.01 | 0.02 | 0.10 | 0.01 |
| Charlson-Deyo score | 1.44 | 1.21 | 1.47 | 0.23 | −0.02 |
| Bowel preparation | -- | -- | |||
| PEG | 0.86 | 0.86 | 0.85 | -- | -- |
| Magnesium sulfate | 0.00 | 0.00 | 0.00 | -- | -- |
| Sodium phosphate | 0.03 | 0.02 | 0.03 | -- | -- |
| Sodium sulfate | 0.11 | 0.11 | 0.12 | -- | -- |
| PEG and sodium sulfate | 0.00 | 0.00 | 0.00 | -- | -- |
| Days of antibiotics from enrollment to 14 days before colonoscopy | 125.63 | 79.35 | 125.42 | 0.21 | 0.00 |
| Hospitalizations 90 to 365 days before colonoscopy | 0.02 | 0.01 | 0.02 | 0.07 | 0.00 |
| Office visits 1 to 365 days before colonoscopy | 0.85 | 0.57 | 0.87 | 0.12 | −0.01 |
| Office visits 1 to 14 days before colonoscopy | 0.07 | 0.04 | 0.07 | 0.11 | 0.00 |
| Office visits 1 to 14 days after colonoscopy | 0.06 | 0.03 | 0.05 | 0.12 | 0.01 |
Notes: Continues variables presented as means, categorical variables presented as proportions.
Abbreviations: SMD—standardized mean difference (< 0.10 indicates covariate balance), PEG—polyethylene glycol
Table 2.
Most common medical claims in the 14 days prior to antibiotic fill date stratified by before/after colonoscopy and ICD era
| BEFORE COLONOSCOPY | AFTER COLONOSCOPY | ||||||
|---|---|---|---|---|---|---|---|
| Rank | Code | Cat. | Code | Cat. | |||
| ICD-9 | 1 | V76.51 | Screening for malignant neoplasm of the colon | C | 461.9 | Acute sinusitis NOS | HT |
| 2 | V70.0 | Routine medical exam | 466.0 | Acute bronchitis | R | ||
| 3 | 211.3 | Benign neoplasm of the large bowel | C | 465.9 | Acute upper respiratory tract infection NOS | R | |
| 4 | 272.4 | Hyperlipidemia NOS | 599.0 | Urinary tract infection NOS | U | ||
| 5 | 461.9 | Acute sinusitis NOS | HT | 786.2 | Cough | R | |
| 6 | 401.9 | Hypertension NOS | 401.9 | Hypertension NOS | |||
| 7 | V72.31 | Routine gynecologic examination | V70.0 | Routine medical exam | |||
| 8 | 401.1 | Benign hypertension | 462 | Acute pharyngitis | HT | ||
| 9 | 599.0 | Urinary tract infection NOS | U | 272.4 | Hyperlipidemia NOS | ||
| 10 | V76.12 | Screening mammogram | 211.3 | Benign neoplasm of the large bowel | C | ||
| Rank | Code | Cat. | Code | ||||
| ICD-10 | 1 | Z12.11 | Encounter for screening for malignant neoplasm of colon | C | I10 | Essential hypertension | |
| 2 | Z00.00 | Encounter for general adult medical exam | R05 | Cough | R | ||
| 3 | I10 | Essential (primary) hypertension | J01.90 | Acute sinusitis, unspecified | HT | ||
| 4 | Z23 | Need for immunization against single bacterial diseases | Z23 | Need for immunization against single bacterial diseases | |||
| 5 | R05 | Cough | R | Z00.00 | Encounter for general adult medical examination | ||
| 6 | E78.5 | Hyperlipidemia, unspecified | E11.9 | Type 2 diabetes without complications | |||
| 7 | Z12.31 | Screening mammogram for malignant neoplasm of breast | J06.9 | Acute upper respiratory tract infection, unspecified | R | ||
| 8 | J01.90 | Acute sinusitis, unspecified | HT | J02.9 | Acute pharyngitis, unspecified | HT | |
| 9 | J06.9 | Acute upper respiratory tract infection, unspecified | R | N39.0 | Urinary tract infection, site not specified | U | |
| 10 | R53.83 | Other fatigue | J20.9 | Acute bronchitis, unspecified | R | ||
Categories: C—Related to colonoscopy; HT—Head and throat infections R—Upper respiratory tract infections; U—Urinary tract infections
IBS outcomes
Between 15 – 1095 days after colonoscopy, 166 antibiotic-exposed individuals and 151 propensity-matched individuals without antibiotic exposure were diagnosed with IBS (incidence rates 3.3 versus 3.0 per 1000 person-years respectively). The HR of antibiotic exposure was 1.11 (95% CI 0.89 – 1.39). Between 15 – 1095 days after colonoscopy, 834 antibiotic-exposed individuals and 754 propensity-matched individuals without antibiotic exposure were diagnosed with the composite outcome of IBS, new prescriptions for IBS medications, or IBS symptoms of abdominal pain and a new defecatory symptom (incidence rates 17.0 versus 15.2 per 1000 person-years respectively). The HR of antibiotic exposure for the composite outcome was 1.12 (95% CI 1.02 – 1.24). Kaplan-Meier failure curves of the IBS and composite outcomes are depicted in Figure 2. For the composite outcome, the number needed to harm at 1, 2, and 3 years was 243 (95% CI 175 – 361), 130 (85 – 229), and 94 (95% CI 60 – 176), respectively. Of those with the composite outcome, 78% with antibiotic exposure and 76% propensity-matched individuals without antibiotic exposure had follow-up claims for IBS or IBS symptoms. Abdominal pain and diarrhea were the most frequent symptom claims (Supplemental table 4).
Figure 2.
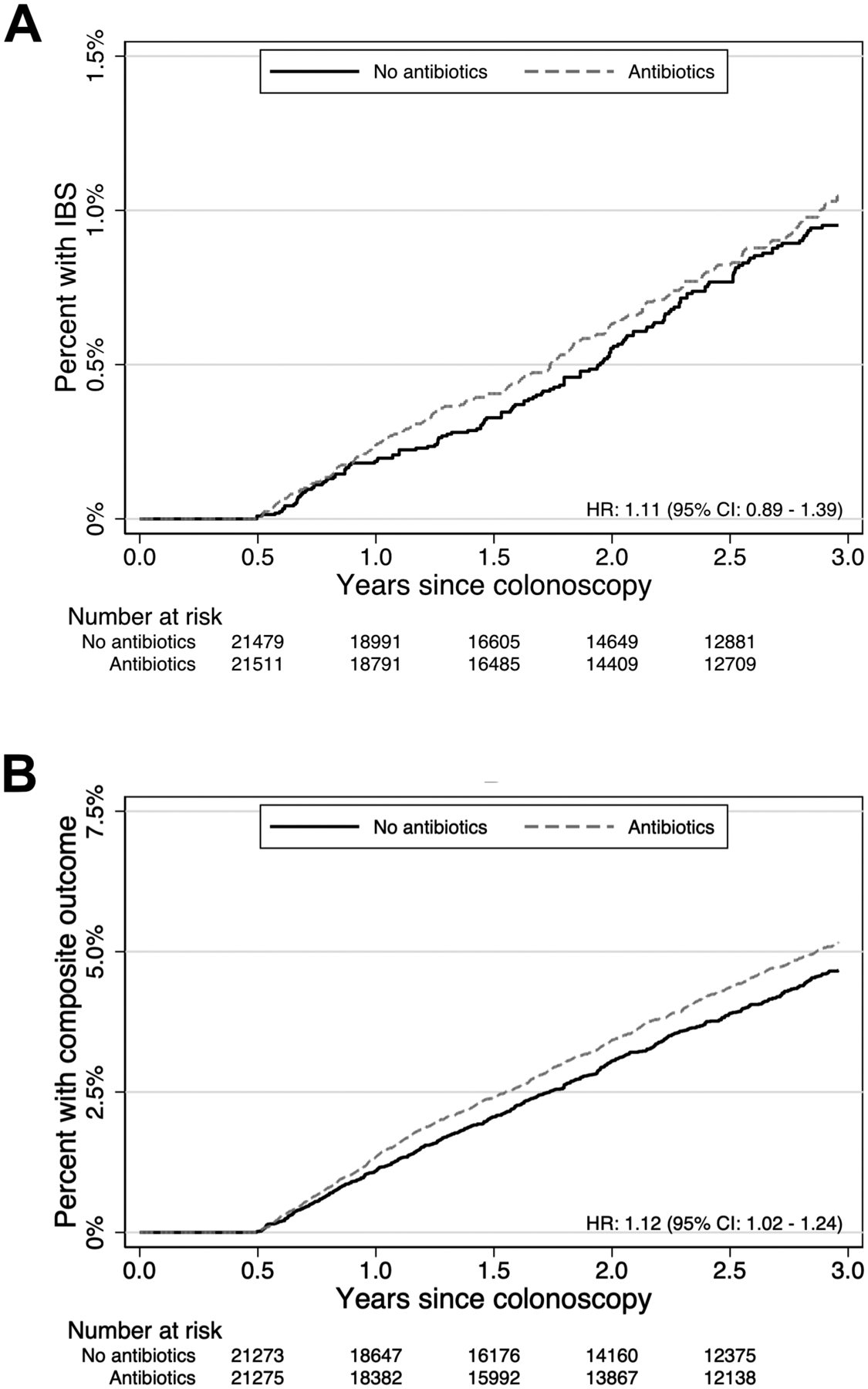
Kaplan-Meier failure curves of IBS diagnoses (panel A) and composite outcome of IBS diagnoses, IBS medications, or IBS symptoms (panel B)
Secondary analyses of specific antibiotic classes
For the outcome of a new IBS diagnosis, none of the five tested antibiotic classes were associated with antibiotic use using the Bonferroni-corrected 99.5% CIs. For the composite outcome of IBS, new prescriptions for IBS medications, or IBS symptoms of abdominal pain and a new defecatory symptom, quinolone antibiotics were associated with aHR 1.36 (99.5% CI 1.08 – 1.72, Table 3).
Table 3.
Association of specific antibiotic classes with IBS and the composite outcome among 24,617 individuals exposed to antibiotics around colonoscopy and 24,617 propensity-matched individuals not exposed to antibiotics around colonoscopy
| Primary outcome: IBS | Composite outcome: IBS, IBS medications, or IBS symptoms | |||
|---|---|---|---|---|
| HR | 99.5% CI | HR | 99.5% CI | |
| Aminopenicillins (n = 6998) | 0.93 | 0.59 – 1.48 | 0.98 | 0.80 – 1.20 |
| First-generation cephalosporins (n = 2082) | 1.37 | 0.69 – 2.71 | 1.09 | 0.77 – 1.52 |
| Macrolides (n = 5368) | 1.22 | 0.76 – 1.94 | 1.19 | 0.96 – 1.47 |
| Quinolones (n = 3856) | 1.16 | 0.66 – 2.02 | 1.36 | 1.08 – 1.72 |
| Sulfonamides (n = 1895) | 1.16 | 0.54 – 2.51 | 0.95 | 0.66 – 1.39 |
Discussion
In this cohort study of 50 – 55 year-olds in the United States who underwent screening colonoscopy, individuals who received antibiotics in the 14 days before or after colonoscopy were diagnosed with IBS at similar rates to propensity-score matched individuals who did not receive antibiotics around the time of colonoscopy (HR 1.11, 95% CI 0.89 – 1.39). In an analysis accounting for possible under recording of IBS diagnoses in medical claims data, there was a weak association between concurrent antibiotic use around the time of colonoscopy and the composite outcome of IBS, IBS medications, or IBS symptoms (HR 1.12, 95% CI 1.02 – 1.24). While laboratory studies have demonstrated marked changes to the diversity and quantity of the gut microbiota of individuals concurrently exposed to antibiotics and bowel, this study utilizing real-world clinical data found only a small association between antibiotic use around the time of colonoscopy and subsequent IBS symptoms. A possible reason for the weak clinical association could be that the gut microbiota begins to recover to baseline features as soon as nine days after exposure.14, 16 Furthermore, the experimental studies used oral vancomycin and neomycin, which in combination have broad-spectrum antibiotic activity. In the outpatient clinical setting, very few patients have indications to receive such a broad-spectrum antibiotic regimen. It is also possible that by not accounting for interactions between gut microbiota changes and dietary habits, our study underappreciated the effects of excess intestinal gas production, immune-modulating metabolites, and visceral hypersensitivity.31. In our secondary analysis assessing the association of individual antibiotic classes with IBS outcomes, there was a moderate association between quinolones, which have Gram-positive and Gram-negative coverage, and the composite outcome of IBS, IBS medications, or IBS symptoms (HR 1.36, 99.5% CI 1.08 – 1.72). This supports the concept that the antibiotic spectrum of activity may be a key risk factor for subsequent gastrointestinal side effects and underscores the importance of antibiotic stewardship that limits antibiotic use to conditions proven to be secondary to bacterial infections. Indeed, 30% of antibiotics prescribed in the United States are considered inappropriate,32 and in this study, upper respiratory tract infection, sinusitis, and pharyngitis—all of which are most commonly secondary to viral infections—were among the most common medical claims in the 14 days preceding antibiotic fill date.33–35
This study has several strengths. First, the cohort was constructed from a validated algorithm to identify individuals undergoing screening colonoscopy. This was critical to the study design because including individuals undergoing diagnostic colonoscopy for assessment of abdominal symptoms may have resulted in protopathic bias, in which prodromal symptoms of undiagnosed IBS lead to the exposure of interest. For example, without exclusion of individuals undergoing diagnostic colonoscopy, it is possible that a patient with undiagnosed IBS-diarrhea subtype could receive empiric antibiotics to treat presumed infectious diarrhea or small intestinal bacterial overgrowth and then undergo colonoscopy for further evaluation. If such a patient was included in the study, it would appear as if the antibiotics were associated with the subsequent diagnosis of IBS when in reality the IBS lead to the antibiotic prescription. A second strength of the study was high statistical power from the large sample size that included nearly 25,000 individuals exposed to antibiotics around the time of colonoscopy. A third strength of this study is the incorporation of high-dimensional propensity scores and propensity-score matching to adjust for healthcare utilization confounding the relationship between antibiotic use and IBS. This technique also controls for other sources of confounding between antibiotic use and IBS that were not directly accounted for in the exclusion criteria or regression models.
There are potential limitations to consider when interpreting these study results. First, the algorithm for identifying IBS using claims data is not exact, and there are frequently delays between IBS onset and recorded diagnosis.22 To address this limitation, we assessed a composite outcome of IBS, IBS medications, or IBS symptoms. Second, this study did not assess the association of bowel preparation alone or antibiotics alone with subsequent IBS. In preliminary analyses, we found few new IBS diagnoses in 50 – 55 year-olds who did not have colonoscopy, thereby preventing the formation of a non-colonoscopy control group. Similarly, because of high prevalence of lifetime antibiotic exposure,36 it was difficult to identify an appropriate index date for non-antibiotic-exposed controls. Third, we could not directly confirm adherence to antibiotic prescriptions. However, this study using claims data has an advantage over studies ascertaining medication use through prescription data because individuals who fill and pay for medications at pharmacies are more likely to adhere to medications than those who simply received a prescription. Fourth, dietary and behavioral IBS therapies were not considered as part of the outcome definitions. While these are important treatments for IBS, we expect that individuals who have medical claims for these therapies also have associated IBS claims in order to justify the service. Fifth, this study was restricted to individuals 50 – 55 in order to limit protopathic bias by excluding individuals undergoing diagnostic colonoscopy. While this potentially limits the generalizability of the study results to 50 – 55 year-olds with medical insurance, the association between bowel purgative, antibiotics, and IBS in the general population may be stronger because the majority of individuals with IBS are younger than age 45.22 Finally, although we included several safeguards to eliminate confounding, we cannot exclude that the weak association between bowel preparation, antibiotics, and the composite IBS outcome is not due to unmeasured confounding or type I error.
In summary, this study did not demonstrate increased rates of IBS for individuals concurrently exposed to antibiotics and bowel purgative around the time of screening colonoscopy, but did demonstrate a small, 12% higher rate for the composite outcome of IBS, IBS medications, or IBS symptoms. Additionally, a secondary analysis identified that quinolone antibiotics were moderately associated with the composite outcome. Together, these results indicate that while the population-level impact of antibiotic use around the time of colonoscopy is small, there are individuals who may be at risk for IBS-related morbidity based on patient and treatment factors. These results may inform future studies that assess potential IBS risk factors and investigate the pathophysiology of IBS through direct assessment of gut microbiota perturbations.
Supplementary Material
WHAT YOU NEED TO KNOW.
Background:
Concurrent antibiotic and bowel purgative use induces robust alterations to the composition of gut microbiota in laboratory studies. The clinical significance of these alterations is unclear.
Findings:
In this retrospective cohort study of enrollees of large commercial and Medicare Advantage health plans in the United States, individuals exposed to bowel purgative for colonoscopy and antibiotics were diagnosed with IBS at similar rates compared to those exposed to bowel purgative for colonoscopy without antibiotics. Secondary analyses demonstrated that individuals exposed to quinolone antibiotics had higher rates of IBS symptoms after colonoscopy.
Implications for patient care:
The population-level impact of antibiotic use around the time of colonoscopy is small, but there may be individuals who are at risk for IBS based on patient and treatment factors.
Grant support:
Hashem B. El-Serag: NIH/NIDDK—P30-DK056338
Josephine Ni: NIH/NIDDK—K08-DK123316, Burroughs Wellcome Fund Career Awards for Medical Scientists
Shivani U. Thanawala: NIH/NIDDK—T32-DK007740
Ravy K. Vajravelu: NIH/NIDDK—K08-DK119475
Role of the funding source:
This research was funded by the National Institutes of Health in the United States (NIH/NIDDK—K08-DK119475 to RKV). The funders of this study had no role in the study design, data collection, data analysis, data interpretation, or presentation of the results.
Disclosures:
Dr. El-Serag has no conflicts of interest to disclose.
Dr. Lewis has served as a consultant to AbbVie, Arena Pharmaceuticals, Bristol-Myers Squibb, Bridge Biotherapeutics, Celgene, Gilead, Janssen, Johnson & Johnson Consumer Inc, Lilly, Merck, Nestle Health Science, Pfizer, Samsung Bioepis, Takeda, and UCB. The scope of work for each of these was unrelated to this research. He has received research funding from Nestle Health Science, Janssen and Takeda, also unrelated to this research.
Dr. Ni has no conflicts of interest to disclose.
Dr. Shapiro has no conflicts of interest to disclose.
Dr. Thanawala has no conflicts of interest to disclose.
Dr. Vajravelu has no conflicts of interest to disclose.
Abbreviations:
- CI
Confidence interval
- HR
Hazard ratio
- IBS
Irritable bowel syndrome
- ICD
International Classification of Diseases
- SMD
Standardized mean difference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology 2016. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016;150:1257–61. [DOI] [PubMed] [Google Scholar]
- 3.Ek WE, Reznichenko A, Ripke S, et al. Exploring the genetics of irritable bowel syndrome: a GWA study in the general population and replication in multinational case-control cohorts. Gut 2015;64:1774–82. [DOI] [PubMed] [Google Scholar]
- 4.Mayer EA, Labus JS, Tillisch K, et al. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol 2015;12:592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015;313:949–58. [DOI] [PubMed] [Google Scholar]
- 6.Kim G, Deepinder F, Morales W, et al. Methanobrevibacter smithii is the predominant methanogen in patients with constipation-predominant IBS and methane on breath. Dig Dis Sci 2012;57:3213–8. [DOI] [PubMed] [Google Scholar]
- 7.Tap J, Derrien M, Tornblom H, et al. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology 2017;152:111–123 e8. [DOI] [PubMed] [Google Scholar]
- 8.Jeffery IB, O’Toole PW, Ohman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012;61:997–1006. [DOI] [PubMed] [Google Scholar]
- 9.Rodino-Janeiro BK, Vicario M, Alonso-Cotoner C, et al. A Review of Microbiota and Irritable Bowel Syndrome: Future in Therapies. Adv Ther 2018;35:289–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rashid MU, Zaura E, Buijs MJ, et al. Determining the Long-term Effect of Antibiotic Administration on the Human Normal Intestinal Microbiota Using Culture and Pyrosequencing Methods. Clin Infect Dis 2015;60 Suppl 2:S77–84. [DOI] [PubMed] [Google Scholar]
- 11.Stewardson AJ, Gaia N, Francois P, et al. Collateral damage from oral ciprofloxacin versus nitrofurantoin in outpatients with urinary tract infections: a culture-free analysis of gut microbiota. Clin Microbiol Infect 2015;21:344 e1–11. [DOI] [PubMed] [Google Scholar]
- 12.Nagata N, Tohya M, Fukuda S, et al. Effects of bowel preparation on the human gut microbiome and metabolome. Sci Rep 2019;9:4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature 2012;489:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni J, Shen TD, Chen EZ, et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen TC, Albenberg L, Bittinger K, et al. Engineering the gut microbiota to treat hyperammonemia. J Clin Invest 2015;125:2841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanes C, Bittinger K, Gao Y, et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis JD, Bilker WB, Weinstein RB, et al. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf 2005;14:443–51. [DOI] [PubMed] [Google Scholar]
- 18.Rex DK, Boland CR, Dominitz JA, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2017;112:1016–1030. [DOI] [PubMed] [Google Scholar]
- 19.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 20.Sheffield KM, Han Y, Kuo YF, et al. Potentially inappropriate screening colonoscopy in Medicare patients: variation by physician and geographic region. JAMA Intern Med 2013;173:542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams KF, Johnson EA, Chubak J, et al. Development of an Algorithm to Classify Colonoscopy Indication from Coded Health Care Data. EGEMS (Wash DC) 2015;3:1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hungin AP, Chang L, Locke GR, et al. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther 2005;21:1365–75. [DOI] [PubMed] [Google Scholar]
- 23.Sands BE, Duh MS, Cali C, et al. Algorithms to identify colonic ischemia, complications of constipation and irritable bowel syndrome in medical claims data: development and validation. Pharmacoepidemiol Drug Saf 2006;15:47–56. [DOI] [PubMed] [Google Scholar]
- 24.Deyo R, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 25.Khashab MA, Chithadi KV, Acosta RD, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2015;81:81–9. [DOI] [PubMed] [Google Scholar]
- 26.Saltzman JR, Cash BD, Pasha SF, et al. Bowel preparation before colonoscopy. Gastrointest Endosc 2015;81:781–94. [DOI] [PubMed] [Google Scholar]
- 27.Jaramillo M Antibiotics after colonoscopy linked with higher risk of subsequent IBS. Healio.com, 2020. [Google Scholar]
- 28.Schneeweiss S, Rassen JA, Glynn RJ, et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009;20:512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319:1492–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn OJ. Multiple Comparisons among Means. Journal of the American Statistical Association 1961;56:52–64. [Google Scholar]
- 31.Rajilic-Stojanovic M, Jonkers DM, Salonen A, et al. Intestinal microbiota and diet in IBS: causes, consequences, or epiphenomena? Am J Gastroenterol 2015;110:278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA 2016;315:1864–73. [DOI] [PubMed] [Google Scholar]
- 33.Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis 2012;54:e72–e112. [DOI] [PubMed] [Google Scholar]
- 34.Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med 2001;134:521–9. [DOI] [PubMed] [Google Scholar]
- 35.Cooper RJ, Hoffman JR, Bartlett JG, et al. Principles of appropriate antibiotic use for acute pharyngitis in adults: background. Ann Intern Med 2001;134:509–17. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother 2014;69:234–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


