Abstract
Pediatric sepsis is a common problem worldwide and is associated with significant morbidity and mortality. Best practice recommendations have been published by both the American College of Critical Care Medicine and the Surviving Sepsis Campaign to guide the recognition and treatment of pediatric sepsis. However, implementation of these recommendations can be challenging due to the complexity of the care required and intensity of resources needed to successfully implement programs. This paper outlines the experience with implementation of a pediatric sepsis quality improvement program at Primary Children’s Hospital, a free-standing, quaternary care children’s hospital in Salt Lake City. The hospital has implemented sepsis projects across multiple care settings. Challenges, lessons learned, and suggestions for implementation are described.
Keywords: pediatrics, sepsis, septic shock, quality improvement, guideline adherence, pediatric emergency medicine
Plain Language Summary
Sepsis is a life-threatening condition that results from an inappropriate response to an infection by the body’s immune system. All children are potentially susceptible to sepsis, with nearly 8,000 children dying from the disease in the US each year. Sepsis is a complicated disease, and several international groups have published guidelines to help hospital teams treat children with sepsis appropriately. However, because recognizing and treating sepsis in children is challenging and takes a coordinated effort from many different types of healthcare team members, following the international sepsis guidelines effectively can be difficult and resource intensive. This paper describes how one children’s hospital (Primary Children’s Hospital in Salt Lake City, Utah) approached the challenge of implementing pediatric sepsis guidelines, some lessons learned from their experience, and suggestions for others interested in implementing sepsis guidelines for children.
Introduction
Pediatric sepsis is common problem worldwide, and is a major contributor to global morbidity, mortality, and healthcare utilization in children [1–4]. Based on a large meta-analysis of pediatric sepsis in twelve middle and high-income countries, it is estimated that per 100,000 population, 48 children develop sepsis and 22 children develop severe sepsis annually [2]. Extrapolated to a global scale, the predicted incidence is 1.2 million cases of pediatric sepsis annually [2]. An international study of 128 Pediatric Intensive Care Units (PICUs) showed high point prevalence of pediatric severe sepsis, with approximately 8% of critically ill children having severe sepsis at any given time [3]. Mortality rates in pediatric sepsis range widely from 2–25% overall, and are influenced by multiple factors, including the presence of underlying chronic disease, geographic location, location within the hospital (e.g., emergency department [ED], intensive care units, general medical floor), and reporting and diagnostic coding practices [1–6]. Notably, there is limited epidemiologic data from low-income countries making accurate quantification of overall global burden challenging, but it is clear that pediatric sepsis remains a critical public health problem.
Pediatric Sepsis Definitions
Despite the high prevalence and healthcare impacts, the definition of pediatric sepsis lacks standardization and broad consensus agreement, with varying approaches used to identify children in real-time at the bedside, versus for retrospective study or quality improvement (QI) efforts, versus for enrollment in clinical trials. The first pediatric sepsis definitions were published in 2002 by the American College of Critical Care Medicine (ACCM) and provided a framework for identifying children with septic shock at the bedside [7]. An International Pediatric Sepsis Consensus Conference was held in 2005 with the goal of modifying adult Systemic Inflammatory Response Syndrome (SIRS) criteria for children and developing pediatric definitions for organ dysfunction, sepsis, severe sepsis, and septic shock specifically for identifying participants for pediatric sepsis clinical trials [8]. While there have been recent updates to international consensus definitions in adult sepsis (Sepsis-3) [9], standardized definitions in the pediatric population reflecting the current understanding of pediatric sepsis pathophysiology are needed. In response to this need, the Society of Critical Care Medicine (SCCM) has recently convened the Pediatric Sepsis Definition Taskforce to provide clarity on this important challenge and to update the pediatric sepsis definition based on more recent investigations [10].
International Guideline Development
The 2002 ACCM pediatric sepsis definitions publication also included the first pediatric sepsis management guidelines [7] which were widely disseminated and sanctioned by the SCCM. The pediatric septic shock management algorithm was incorporated into Pediatric Advance Life Support (PALS), and many children’s hospitals actively implemented these guidelines [11]. The recommendations were found to be generally useful and effective without evidence of harm in resource rich settings [11]. In resource poor settings lacking mechanical ventilation, intravenous (IV) infusion pumps, inotropic and vasoactive medications, and intensive care monitoring, study outcomes were mixed [12]. Following publication of the updated ACCM guidelines in 2007, there has been increasing focus in the United States on septic shock recognition and treatment, particularly in regards to resuscitation within the first hour of presentation [12]. The latest update, released in 2017, recommended that each institution develop tools including a recognition bundle to optimize identification of patients with suspected septic shock, resuscitation and stabilization bundles to promote adherence to best practice, and a performance bundle to monitor, improve and sustain adherence to best practice [12].
In parallel to the ACCM pediatric hemodynamic support guidelines, the Surviving Sepsis Campaign (SSC) developed evidence-based best practice recommendations to guide identification, resuscitation and management of (primarily adult) sepsis [13]. The SSC guidelines were first published in 2004 and have been updated every four years, with subsequent iterations including more pediatric specific recommendations [13–17]. In 2020, the first pediatric specific SSC guideline was published, providing best practice recommendations for clinicians caring for children with septic shock and sepsis-associated organ dysfunction [4]. The recommendations are applicable to children ranging in age from infancy to late adolescence presenting in acute care, emergency, and inpatient settings [4].
In addition to the publication of the ACCM and SSC guidelines, there have been numerous efforts in the United States to improve prompt recognition and treatment of sepsis. In 2013, New York issued a statewide sepsis mandate titled “Rory’s Regulations” in the wake of the death of Rory Staunton, a previously healthy 12-year-old boy who died of unrecognized septic shock. The law requires implementation of a 1-hour sepsis bundle in EDs, inpatient units, and PICUs, which include timely completion of blood cultures, broad-spectrum antibiotics, and IV fluid bolus in patients with suspected sepsis [18]. Following implementation of the New York state mandate, completion of the 1-hour sepsis bundle has been associated with lower risk-adjusted in-hospital mortality among patients with pediatric sepsis and septic shock [18,19]. Illinois (2016) and New Jersey (2018) have also mandated implementation of in-hospital sepsis protocols [20].
Building a Sepsis Quality Improvement Program: The Primary Children’s Hospital Experience
Primary Children’s Hospital (PCH) is a quaternary, 289-bed, free standing children’s hospital in Salt Lake City, Utah. The sepsis QI work started at PCH in 2006 in the ED following two sentinel events within months of each other: deaths secondary to septic shock following delayed recognition and inadequate resuscitation. These events sparked a deep investigation of septic shock recognition and treatment, and set the stage for what would be a dedicated, long-term commitment to improving pediatric sepsis care. Our ED clinical team acknowledged that we lacked a systematic approach to the recognition and treatment of septic shock, and that a systematic approach would be critical to providing high quality care for this patient population. The initial ED program started in 2007 (Figure 1), and has continued to evolve. Early analysis of the project two years after implementation revealed significant improvements in timely sepsis treatment, and hospital length of stay [21], and more recent analysis demonstrates sustained improvements in timely interventions and mortality for ED sepsis patients.[22] The PCH sepsis teams now have active projects in the ED, PICU, Cardiac Intensive Care Unit (CICU), Medical/Surgical Units, and are developing a workflow for the Cancer/Transplant Unit.
Figure 1.
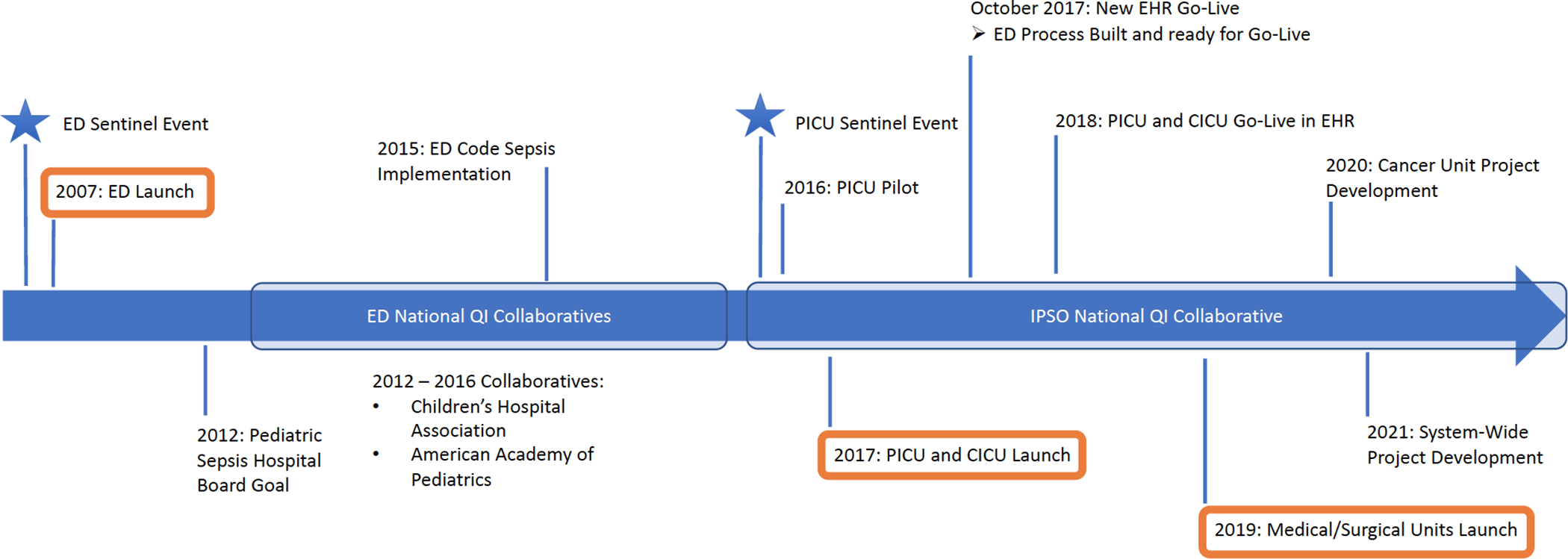
Timeline of pediatric sepsis quality improvement program implementation at Primary Children’s Hospital. Pediatric sepsis initiatives began in the Emergency Department in 2007, followed by the Pediatric Intensive Care Unit and Cardiac Intensive Care Unit in 2017, and the Medical/Surgical Units in 2019. Ongoing and future areas for implementation include the Cancer Unit and other hospitals within the Intermountain Healthcare System.
Data and Infrastructure
A central component to any improvement work is the ability to obtain and trust the data that drive the improvement process. At PCH, our team worked through several iterations of an operational definition of pediatric septic shock, ultimately landing on a two-step process to identify patients for inclusion in our sepsis database and improvement work. Step one consists of generating a “candidate list” of patients to be reviewed for inclusion. This list is generated from data that can be extracted from the Enterprise Data Warehouse (EDW) in an automated process. The current algorithm for generating the candidate list is taken from the Improving Pediatric Sepsis Outcomes (IPSO) national sepsis collaborative definition [23] and is based on a combination of diagnostic codes and treatments provided. Charts from patients on the candidate list are then manually reviewed by trained members of the team to determine inclusion in the cohort based on documented evidence of shock, as outlined by the ACCM [11,12]. While this process is labor intensive, it has been valuable in our experience, as we are confident that our data represents true cases of septic shock, allowing us to focus our improvement efforts where most needed.
For adult sepsis improvement work, many hospitals choose to define their sepsis cohorts using the definitions from the Centers for Medicare & Medicaid Services (CMS), as these are tied to the “Sep-1” measures that drive CMS reimbursement [24]. However, Sep-3 is the most current clinical definition and has some advantages, including the use of organ dysfunction as a key component of sepsis and the removal of SIRS criteria and “severe sepsis” from the definition framework [24]. Each program will need to decide what makes the most sense, weighing the pros and cons of each approach with respect to ease of data extraction and operationalization, time intensity, accuracy of the definition in identifying the cohort, and need for meeting CMS requirements.
In addition to determining the definition, building the data infrastructure is critical to any sepsis QI initiative. When we first began our sepsis work at PCH, we started with Microsoft Excel (2003) for data collection. As our program grew and our process for chart review, data collection, and data analysis became more refined, we found it worked best to use REDCap for data entry and management [25,26]. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies. We have also included a data analyst in our workgroup to improve communication and understanding of the work between clinically-oriented team members and those working with the data. Building data infrastructure and including a data analyst on the team will ensure that the data collected are available, informative, and usable to drive change.
The sepsis initiatives at PCH have now been growing and evolving for almost 15 years. During that time, we have met many successes, and even more challenges, as we have worked to figure out how best to implement care pathways in different areas of the hospital with unique patient populations, care team composition, hospital coverage, and stakeholders.
Challenges to Implementation
Emergency Department Challenge #1: Bundle Compliance
The pediatric emergency department (PED) sepsis program was launched in January 2007, accompanied by a recognition and management guideline to facilitate and standardize key care elements such as fluid resuscitation, antibiotic administration and obtaining a blood culture. The primary process measure we monitored was compliance with the 2-element bundle: administration of both intravenous fluids (IVF) and broad-spectrum antibiotics within one hour of recognition. Wide variation in process measure compliance occurred despite multiple education campaigns and stake holder program updates. As order sets with pre-selected items can help improve care compliance in a variety of clinical situations [27–29], including sepsis [30–33], we chose this intervention to try to improve our 2-elemenent compliance.
We introduced a standardized order set in 2011 that included pre-selected key processes such as intravenous (IV) access, IVF bolus, ceftriaxone administration and blood tests including lactate, complete blood count and blood culture. Within four months, 2-element bundle compliance increased from approximately 58% to > 95% and variation decreased appreciably in a sustained fashion. Order set implementation represents the most substantial iterative change effecting 2-element bundle compliance to date (Figure 2). Improvement in 2-element bundle compliance has also been associated with a significant decrease in sepsis mortality; the odds of survival are 5 times as high for children who receive bundle-compliant care compared to those who do not [22], and mortality decreased from 8% to 2%. In a subsequent analysis of individual care elements, we found that both IV fluid administration within 1 hour and recognition of sepsis at triage were both significantly associated with decreased mortality, though antibiotic timing was not.[34]
Figure 2.
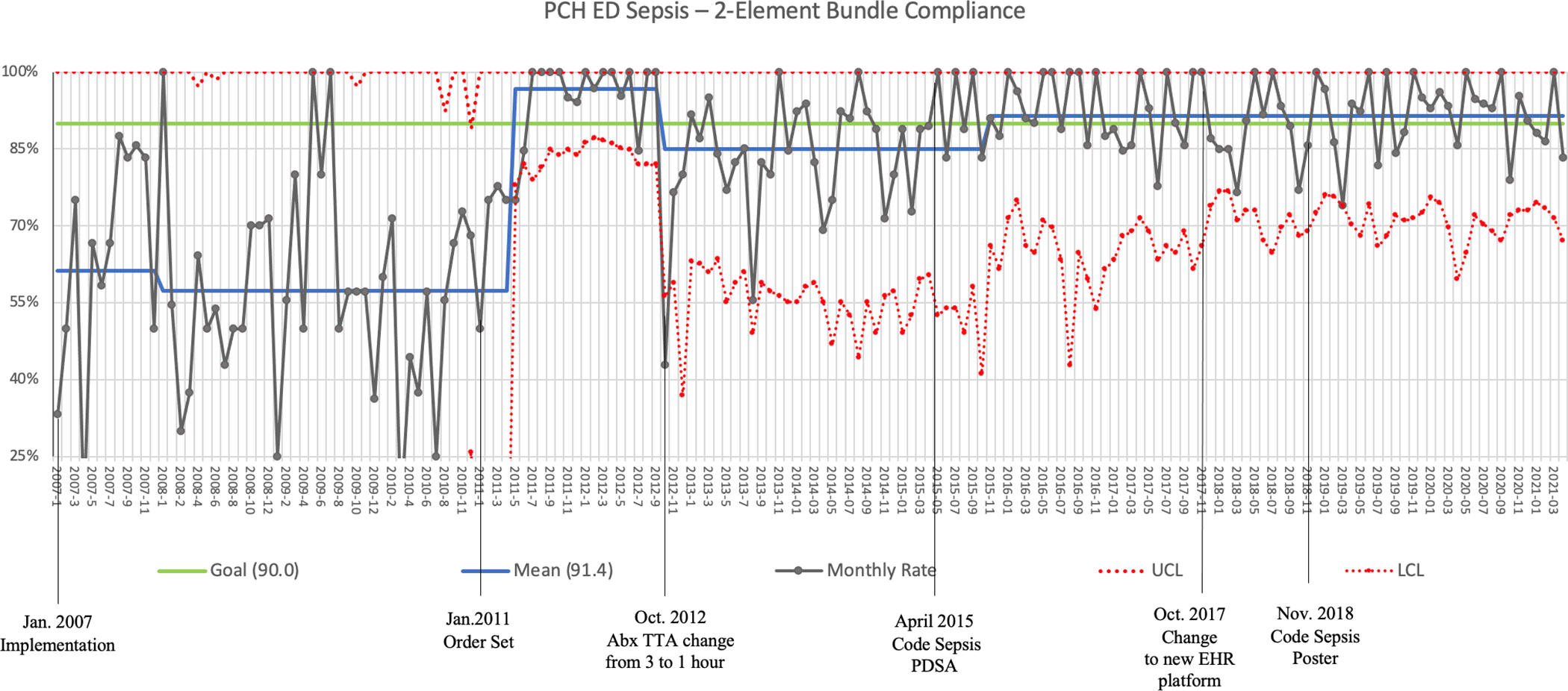
Annotated statistical process control (SPC) p-chart of 2-Element Bundle Compliance in the Primary Children’s Hospital Emergency Department from January, 2007 through May, 2021. The 2-Element Bundle consists of delivery of intravenous fluids to restore perfusion and/or hypotension and administration of broad-spectrum intravenous antibiotics. Compliance is defined as receipt of both bundle elements within 60 minutes of sepsis recognition. Order set implementation had the greatest impact on compliance and Code Sepsis PDSA had the most influence on decreasing variability.
Emergency Department Challenge #2: Intravenous Access Delay
In 2014, we noted decreased compliance in both IVF administration and antibiotic delivery. A Pareto analysis of reasons for delays identified IV access as a major contributor (Figure 3a). Using QI methodologies (e.g., Fishbone diagram, Key Driver diagram, multiple brainstorming sessions, Gemba walk), a multi-part Code Sepsis PDSA was implemented in April 2015. Components of the PDSA included: 1) creation of “Code Sepsis” designation; 2) addition of PED overhead announcement to include “patient arrival to room” with re-broadcast if no response within 5 minutes; 3) standardization of bedside huddle and handoff from triage; 4) expanding response group to include IV team and pharmacist; 5) addition of trauma charge nurse response to code sepsis activations; 6) removal of “pump” administration option for IVF bolus from order set; 7) tasking a PED technician to bring an intraosseous (IO) drill to bedside for all alerts; 8) addition of IO placement to skills day. Within one month, we noted sustained improvements in IV access time and decreased variation with 2-element bundle compliance. A follow-up Pareto analysis identified IV access as being an insignificant contributor to process delays (Figure 3b). Seven months after PDSA implementation a center line shift from 85% to 92% occurred for 2-element bundle compliance (Figure 2).
Figure 3.
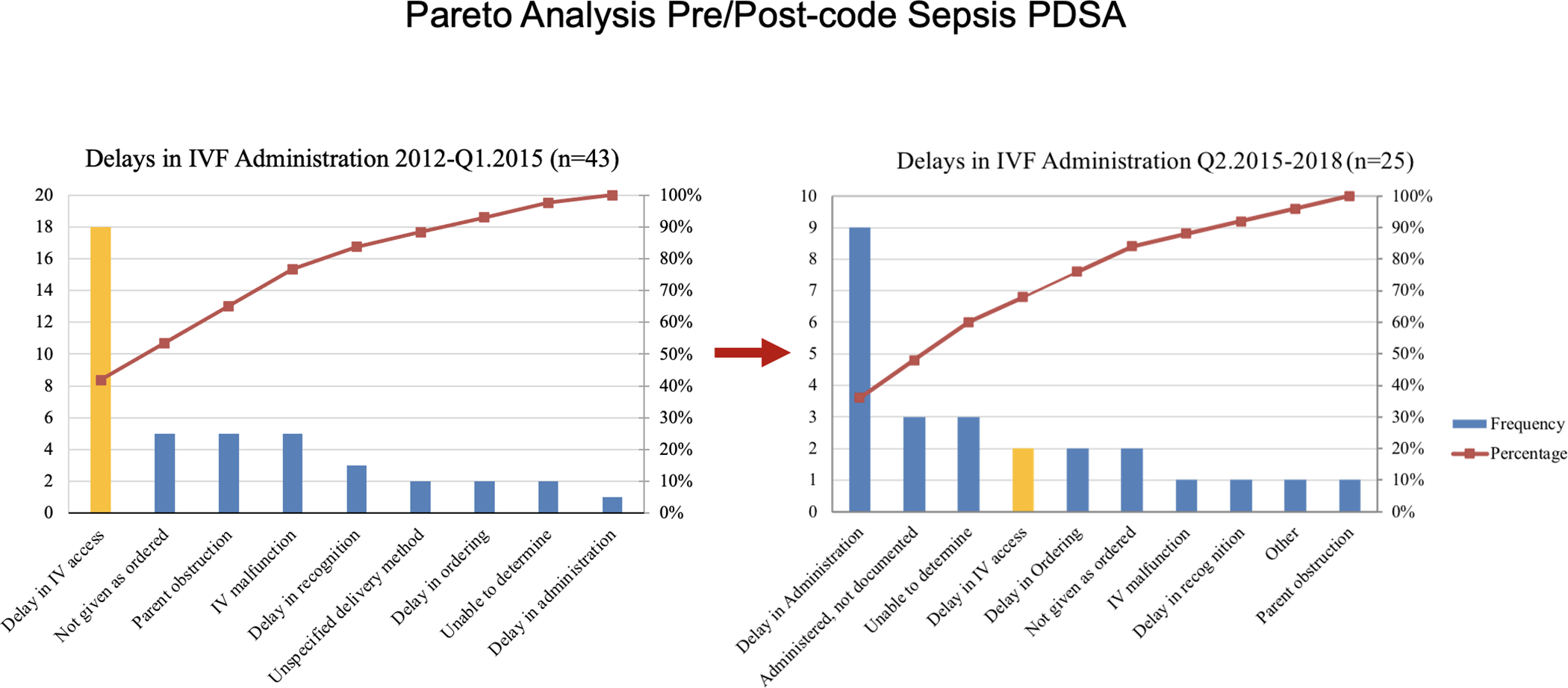
Pareto analysis of time to intravenous (IV) fluid administration delays. Panel A. Baseline analysis of 2012 through quarter 1 of 2015 demonstrated that IV access was the largest single contributor to IV fluid administration delays. Panel B. From quarter 2, 2015 through 2018, following the Code Sepsis Plan-Do-Study-Act (PDSA) cycle, IV access was no longer a significant contributor to IV fluid administration delays.
Emergency Department Challenge # 3: Transition from Paper to Electronic Sepsis Tools
In October 2017, Intermountain Healthcare adopted the Cerner® electronic health record (EHR) platform. Prior to the new EHR launch, the paper-based sepsis screening tool previously utilized was adapted for the EHR. We incorporated sepsis screening into the EHR-based PED triage form, ensuring that every patient presenting to the PCH ED would be screened for sepsis. Expectations of the new system included improvements in screen performance as well as data acquisition and accuracy. We envisioned that increased accessibility of electronic data would allow for new areas of investigation and improvement. Despite the benefits of implementing the new EHR, the unanticipated challenges were numerous.
We found several issues with data entry and data accuracy. For example, front line provider feedback indicated charting was often completed after interventions (e.g., IV placement) instead of in real time. Timestamps associated with interventions were thus recorded based on memory or estimates, resulting in inaccuracies and apparent decreases in measure compliance. To address this, a PDSA cycle was implemented involving a laminated “Code Sepsis” poster which focused on key time-based interventions (Appendix A). A laminated poster was placed in every PED room and hung on a prominent cabinet for code sepsis activations along with a dry erase marker, providing an easy, just-in-time method for recording the time an intervention was performed. When the patient’s clinical status allowed, the nurse transferred the data to the EHR, improving data entry accuracy.
Transition to the EHR also impacted the apparent performance of our pediatric sepsis screening tool, with an increase in the positive predictive value from 14% to 26% but a decrease in the sensitivity from 98% to 56%, despite no change in the elements of the screening tool. The two biggest contributing factors were (1) missing data on the ED triage form, and (2) seasoned nurses appropriately identifying a suspected sepsis patient and initiating a sepsis huddle even if the patient did not meet exact screening criteria. Because the sepsis screen is reliant on triage data, inaccuracies in triage charting were significantly impacting the performance of the screening tool. To improve triage data entry, numerous interventions have been implemented including just-in-time feedback in person or via email, educational updates, incorporating triage documentation into regular required training, and most recently mandatory triage audits by every nurse. Improvements have been noted in documentation and screen performance since the interventions.
Another unanticipated data challenge with transition from paper to electronic sepsis screening was the negative consequence of inflexibility of the electronic alert. The PED nurses had gained remarkable, sustained expertise in sepsis recognition after 5 years of utilizing the paper tool. With transition to the EHR, triage nurses often appropriately identified a child with possible sepsis, even when the electronic algorithm did not. For example, the electronic screen might fail to identify a patient with possible sepsis if a vital sign missed the threshold by a few points (e.g., a child with heart rate of 89 and screening tool cut off of 90). However, an experienced nurse, able to assess the nuance and not beholden to the ‘hard-stops’ of the EHR-based tool, could appropriately recognize this child’s risk and call for a sepsis huddle. Because the electronic tool cannot perform this type of clinical judgement, there were more patients now falling into the “false negative” category. To accommodate for excellent nursing judgement, an additional triage radio button has been added to the electronic triage form that allows the nurse to select “concern for sepsis,” generating an electronic sepsis alert even if vital sign thresholds or other criteria are not fully met.
Pediatric Intensive Care Unit and Cardiac Intensive Care Unit Challenges
The ED sepsis program was well established prior to spreading the sepsis QI work to other areas of the hospital, allowing other units to capitalize on the ED experience. A critical challenge identified with implementation in the PICU and CICU setting was identifying true early warning signs of potential sepsis among all the other diagnostic possibilities for critically ill children. While many patients in the ICU have evidence of SIRS or shock at the time of their admission, or underlying physiology that mimics sepsis, the development of septic shock among PICU and CICU patients is a rare event. Identifying a new episode of septic shock among all the patients with vital sign and laboratory derangements is difficult, and false positive sepsis alerts have been frequent. In response to this issue, we are continuing to evaluate our process.
Some potential solutions to decreasing the false positive rate include (1) excluding certain patients from sepsis screening, such as those in the first 24 hours following cardiac surgery, (2) building in a nurse driven perfusion check in response to an initial alert, prior to initiating a bedside huddle, and (3) developing a machine learning model to more accurately identify patients. Although we must keep the concern for alert fatigue in mind, some PICU team members have commented that the false positives are often just as helpful as the true positives, as they alert the team to a potential change in patient condition (even if not sepsis-related), providing an opportunity to reassess the patient trajectory and intervene with other treatment modalities.
Medical/Surgical Units Challenges
The Primary Children’s Hospital Medical/Surgical units were the third group to implement the sepsis evaluation and treatment bundle. These units are comprised of five inpatient areas and one observation unit, over three hospital floors. Each unit provides specialized care based on the patient’s needs and admitting diagnosis. The units are staffed by a wide variety of medicine and surgical teams including pediatric hospital medicine, general surgery and several other medical and surgical subspecialties.
Implementation in the Medical/Surgical units posed two unique challenges. Over the previous decade, several different electronic alert systems were attempted. Each of these systems failed due to the high number of false positive alerts. This experience led to initial resistance from providers, heightening the importance of “getting it right” when rolling out a new process. We addressed this issue by running the algorithm in the background for several months prior to implementation of the screening process. This allowed the sepsis team to modify the screening criteria to reduce the number of false positives while not missing patients who may be showing signs of sepsis. Following implementation of the sepsis screening process, there were approximately seven alerts per week across all the Medical/Surgical units.
The second major challenge was variability in provider coverage across different services. While the pediatric hospitalist and general surgery teams have in-house coverage 24/7, many of the other inpatient teams do not, particularly at night. Our protocol requires a patient to be evaluated by a provider within 10 minutes of an alert activation, which includes the attending of record. For the teams who are not able to respond in this time frame, the protocol recommends a Rapid Response activation. Utilizing the rapid response system facilitates timely evaluation and treatment of potential sepsis patients. After a year of utilizing the sepsis screening and treatment guidelines, we have gained significant sepsis awareness among the Medical/Surgical caregivers, driving earlier sepsis recognition.
Suggestions for Building a Sepsis Improvement Program
Based on our experience building, growing, and sustaining our pediatric sepsis QI program over the past 14 years, we have gained considerable insight. Table 1 summarizes suggested Core Pediatric Sepsis QI Program Best Practices and the next sections provide suggestions for the implementation of a successful, sustainable pediatric sepsis QI program.
Table 1.
Core Pediatric Sepsis QI Program Best Practices-critical elements in italics
| Pediatric sepsis improvement team | 1. Workgroup should include key stake holders and front-line providers (e.g., nurses, technicians, advanced practice providers, pharmacists, respiratory therapists, IV team), other key members (e.g., project manager, improvement specialist, unit specific educator, family advisory board member, data analysist, EHR specialist, administrative leadership representative). 2. Meet regularly to review program and analytics/metrics. 3. Establish evidence-based metrics for interventions. 4. Consider creating an evidence-based pediatric sepsis guideline. 5. Have a project manager. |
| Data and analytics | 1. Team should include a dedicated analyst familiar with QI statistics/methodologies. 2. Though not required, a consistent data abstractor is highly recommended as part of the team. 3. Develop a case definition for cohort inclusion based on published, evidence-based recommendations [4,9,12,13]. 4. Build an algorithm for cohort identification leveraging elements extractable from the EHR. Utilize previously published methods [22], and modify for site-specific requirements. 5. If feasible, perform manual chart review of possible cohort candidates, particularly in the beginning, and for those who received non-compliant care. 6. Develop a case review algorithm that allows for consistent determination of cohort inclusion. Discuss ambiguous cases during regular team meetings. 7. Depending on patient volumes, perform EHR query for cohort candidates on a weekly or bi-monthly basis. 8. Establish data elements for analysis (leverage EHR) including process, outcome and balancing metrics, clinical parameters, laboratory results (e.g., lactate), demographics, disposition. |
| Recognition strategy | 1. Pediatric specific early recognition screen should include age adjusted vital signs, incorporate high risk conditions, be actionable and forward facing to providers. 2. Consider adding a hard-stop to the screen alert that is actionable (e.g., requires providers to acknowledge the alert and select a management option). 3. Structure the screen to maximize sensitivity and positive predictive value (will likely require multiple rapid improvement cycles). |
| Escalation and management strategy | 1. The notification system should alert key personnel to the bedside for patients with suspected sepsis (e.g., overhead broadcast, communication system alert such as Vocera®) 2. Following an alert, initiate a multi-disciplinary bedside huddle (minimum of nurse and attending physician) for all patients with a positive screen. 3. Standardized order set should be easily accessible and include key components of recommended sepsis care [4]. |
| De-escalation strategy | 1. Discontinue vasoactive medications as soon as allowable. 2. Discontinue lines and tubes as soon as allowable. 3. Tailor antibiotics utilizing available data including culture and/or susceptibility results. 4. Transfer from a critical care unit as soon as is feasible. |
Pre-implementation Phase
Strategic work prior to implementation is imperative. A critical first step at the beginning of program development is to engage the hospital’s administrative leadership for support, oversight, and guidance. Additionally, identifying and engaging key stake holders at both the hospital or system level, as well as at the individual unit level is essential for developing processes that will be effective, be supported by frontline teams, and help drive change.
Developing Clinical Decision Support
During the pre-implementation phase, if an EHR is available but resources to build electronic tools are scarce, consider starting with a paper-based order set, screen and other tools. Iterative changes are frequent during the implementation phase, and this strategy allows for rapid cycle improvements utilizing fewer resources. When utilizing an EHR-based screening tool, run the screen silently in the background for at least a few months. This exercise can provide extremely helpful contextual tool performance feedback and data. For example, a screening algorithm may identify fewer cases than anticipated, or alternatively may have an unacceptably high false positive rate. These findings can give the implementation team an opportunity to fine-tune the tools and improve performance prior to going live, which may allow for a smoother roll-out.
Establishing Case Definition, Identification Methods, and Data Storage Plan
As discussed above, sepsis definitions for both adult and pediatric populations have changed substantially over the last 30 years and continue to be a source of debate, consternation and discussion [8,9,35–40]. We recommend utilizing evidence based or consensus derived published definitions that are clinically pragmatic [4,12] as opposed to research focused. Anticipate being nimble as definitions are likely to evolve over time. Case identification can be challenging and will inherently differ from site-to-site depending on resources, EHR, data analytic support, and location of implementation. Leverage the EHR for data extraction if possible, and utilize methods previously published [23]. We recommend using a combination of criteria including clinical variables, process variables, laboratory data, and sepsis specific International Classification of Disease (ICD) codes. Using specific ICD codes for pediatric severe sepsis and septic shock in isolation is not sufficient and will likely miss a significant percentage of cases [41]. Build the data collection and storage system prior to implementation, and determine in advance the process, outcome, and balancing metrics that will allow the team to use the data to drive change.
Educational Efforts
Finally, prior to implementation, perform extensive and overlapping education for all front-line providers, utilizing a variety of educational methods including formal presentations, newsletter announcements, unit bulletin boards, email, reminders during unit safety huddles, and simulation. Simulation allows for the detection of unanticipated nuances in the system, barriers to use, and EHR coding errors, and team members can provide insight into what does and does not work for their team and care setting. Be open to and elicit feedback, engage frontline providers, enable champions, and encourage continued input.
Implementation
When considering the implementation strategy, we recommend implementing the program one unit at a time. This allows for greater learning as each unit has nuances and unique challenges that may require specific adaptations of the process and/or tools. For example, general care inpatient units may involve multiple services (e.g., general surgery, hospitalist, neurosurgery, pulmonology) and some may not have in-house coverage 24 hours per day. A rapid response system may be helpful for patients found to have sepsis in this setting; however, this would not be applicable to most ED settings. To allow for robust data capture, consider first implementing in a unit that has a higher frequency of pediatric sepsis, such as the ED or critical care unit. Identify unit champions to improve program success and promote ongoing communication with program leads.
Post-implementation
Following implementation, elicit front line provider feedback, and share data and results often and widely with providers, stake holders, and executive sponsors. Celebrate successes and anticipate multiple rapid improvement cycles to improve metrics and boost compliance. Providing individual provider-specific compliance compared with colleagues can be very impactful and result in improved metric adherence.
Lastly, sepsis improvement programs should incorporate the learning healthcare system concept of utilizing patient level data and analytics to inform best practices, which are then translated back to the clinicians, patients, and stakeholders in a timely fashion. This information can be used to inform subsequent improvement cycles [42]. Incorporating patient-reported outcomes may result in identification of important metrics for future investigation (e.g., days out of school following an in-hospital sepsis episode, caregiver days of work lost).
Future Directions
While there have been many outstanding advancements in pediatric sepsis care, there is much work to be done. This work falls into three broad categories: (1) advancing QI initiatives beyond dedicated children’s hospitals, (2) understanding sepsis phenotypes and biomarker profiles, and potentially incorporating these into diagnostic and treatment algorithms, and (3) understanding and addressing health disparities.
Implementation of pediatric sepsis guidelines has focused on tertiary care children’s hospitals; however, over 70% of children seeking emergency care are first seen in a general ED, many of which are under-prepared to care for children [43]. Greenwald et al. found that children presenting to general EDs with septic shock are less likely to be recognized [44], and only 24% receive care that is compliant with published guidelines [45]. As children’s hospitals become more adept with sepsis care across care settings, researchers, implementation scientists, and QI experts are beginning to turn their efforts to improving recognition and treatment of pediatric sepsis in community hospitals, before children arrive at the tertiary or quaternary center. Our team is now laying the ground work for implementation of a pediatric sepsis care pathway throughout the Intermountain Healthcare system, with the goal of providing high quality pediatric sepsis care at every point of access with the healthcare system.
Several groups are exploring sepsis phenotypes, how best to identify them and categorize them, and if there are treatment strategies that can be tailored to different phenotypes [46–48]. There are multiple pediatric and adult focused randomized clinical trials ongoing to evaluate these questions, paving the way for the entry of personalized medicine into sepsis care. This is a truly exciting area of research that has potential to significantly improve mortality and long-term complications from pediatric sepsis.
Literature identifying racial and ethnic disparities in pediatric sepsis care and outcomes is beginning to emerge. A single-center study indicated that non-Hispanic Black children were less likely to be treated with the hospital’s sepsis pathway than non-Hispanic White children [49]. In an investigation of pediatric severe sepsis in the Kids’ Inpatient Database (KID), compared to White children, Black children had higher odds of mortality, and both Black and Hispanic children had longer lengths of hospital stay [50]. Further investigations are needed to determine why these disparities exist, and what the underlying mechanisms are that drive differences in care and outcomes among racial and ethnic minorities.
Conclusion
Pediatric sepsis is a common problem associated with significant worldwide morbidity and mortality. Best-practice guidelines are available for the recognition and treatment of pediatric sepsis [4], but can be difficult to implement given the challenge of identifying patients, complexity and timeliness of care required, nuances of clinical settings where care is provided, and intensity of resources required for successful implementation. Here we have discussed our hospital’s approach to improving care for this patient population, outlining challenges, lessons learned, and suggestions for implementation.
Supplementary Material
Declaration of funding
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002538. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of financial/other relationships
JKW is supported by the Intermountain Foundation through the University of Utah CTSI Partner Career Development Program.
Footnotes
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- [1.].Balamuth F, Weiss SL, Neuman MI, et al. Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med. 2014;15(9):798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2.].Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, et al. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223–230. [DOI] [PubMed] [Google Scholar]
- [3.].Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191(10):1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4.].Weiss SL, Peters MJ, Alhazzani W, et al. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr Crit Care Med. 2020;21(2):e52–e106. [DOI] [PubMed] [Google Scholar]
- [5.].Ames SG, Davis BS, Angus DC, et al. Hospital Variation in Risk-Adjusted Pediatric Sepsis Mortality. Pediatr Crit Care Med. 2018;19(5):390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6.].Prout AJ, Talisa VB, Carcillo JA, et al. Children with Chronic Disease Bear the Highest Burden of Pediatric Sepsis. J Pediatr. 2018;199:194–199.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7.].Carcillo JA, Fields AI, American College of Critical Care Medicine Task Force Committee M. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002;30(6):1365–1378. [DOI] [PubMed] [Google Scholar]
- [8.].Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. [DOI] [PubMed] [Google Scholar]
- [9.].Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10.].Menon K, Schlapbach LJ, Akech S, et al. Pediatric Sepsis Definition-A Systematic Review Protocol by the Pediatric Sepsis Definition Taskforce. Crit Care Explor. 2020;2(6):e0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11.].Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37(2):666–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12.].Davis AL, Carcillo JA, Aneja RK, et al. American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock. Crit Care Med. 2017;45(6):1061–1093. [DOI] [PubMed] [Google Scholar]
- [13.].Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004. Mar;32(3):858–873. [DOI] [PubMed] [Google Scholar]
- [14.].Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45(3):486–552. [DOI] [PubMed] [Google Scholar]
- [15.].Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. [DOI] [PubMed] [Google Scholar]
- [16.].Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. [DOI] [PubMed] [Google Scholar]
- [17.].Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 Update. Crit Care Med. 2018;46(6):997–1000. [DOI] [PubMed] [Google Scholar]
- [18.].Gigli KH, Davis BS, Yabes JG, et al. Pediatric Outcomes After Regulatory Mandates for Sepsis Care. Pediatrics. 2020;146(1):e20193353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19.].Evans IVR, Phillips GS, Alpern ER, et al. Association Between the New York Sepsis Care Mandate and In-Hospital Mortality for Pediatric Sepsis. Jama. 2018;320(4):358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20.].Sepsis Alliance [Internet]. San Diego (CA): Sepsis Alliance. New Jersey Now One of Three States to Mandate Sepsis Protocols; 2018. Jan 18 [cited 2021 May 26]. Available from: https://www.sepsis.org/news/new-jersey-now-one-three-states-mandate-sepsis-protocols/ [Google Scholar]
- [21.].Larsen GY, Mecham N, Greenberg R. An emgergency department septic shock protocol and care guideline for children initiated at triage. Pediatrics. 2011;127(6):e1585–e1592. [DOI] [PubMed] [Google Scholar]
- [22.].Lane RD, Funai T, Reeder R, et al. High reliability pediatric septic shock quality improvement initiative and decreasing mortality. Pediatrics. 2016;138(4):e20154153. [DOI] [PubMed] [Google Scholar]
- [23.].Scott HF, Brilli RJ, Paul R, et al. Evaluating Pediatric Sepsis Definitions Designed for Electronic Health Record Extraction and Multicenter Quality Improvement. Crit Care Med. 2020;48(10):e916–e926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24.].Kalantari A, Mallemat H, Weingart SD. Sepsis Definitions: The Search for Gold and What CMS Got Wrong. West J Emerg Med. 2017;18(5):951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25.].Harris PA, Taylor R, Theilke R, et al. Research electronic data capture (REDCap) — A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26.].Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an International community of software partners. Journal of Biomedical Informatics. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27.].Ballard DJ, Ogola G, Fleming NS, et al. The Impact of Standardized Order Sets on Quality and Financial Outcomes. In: Henriksen K, Battles JB, Keyes MA, et al. , editors. Advances in Patient Safety: New Directions and Alternative Approaches (Vol. 2: Culture and Redesign). Rockville (MD): Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- [28.].Jacobs BR, Hart KW, Rucker DW. Reduction in clinical variance using targeted design changes in computerized provider order entry (CPOE) order sets: Impact on hospitalized children with acute asthma exacerbation. Appl Clin Inform. 2012;3(1):52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29.].Zeidan AM, Streiff MB, Lau BD, et al. Impact of a venous thromboembolism prophylaxis “smart order set”: Improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549. [DOI] [PubMed] [Google Scholar]
- [30.].Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2006;34(11):2707–2713. [DOI] [PubMed] [Google Scholar]
- [31.].Thiel SW, Asghar MF, Micek ST, et al. Hospital-wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med. 2009;37(3):819–824. [DOI] [PubMed] [Google Scholar]
- [32.].Probst CA, Shaffer VA, Chan YR. The effect of defaults in an electronic health record on laboratory test ordering practices for pediatric patients. Health Psychol. 2013;32(9):995–1002. [DOI] [PubMed] [Google Scholar]
- [33.].Balamuth F, Weiss SL, Fitzgerald JC, et al. Protocolized treatment is associated with decreased organ dysfunction in pediatric severe sepsis. Pediatr Crit Care Med. 2016;17(9):817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34.].Lane RD, Olson J, Reeder R, et al. Antibiotic timing in pediatric septic shock. Hosp Pediatr. 2020;10(4):311–317. [DOI] [PubMed] [Google Scholar]
- [35.].Angus DC, Seymour CW, Coopersmith CM, et al. A framework for the development and interpretation of different sepsis definitions and clinical criteria. Crit Care Med. 2016;44(3):e113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36.].Weiss SL, Parker B, Bullock ME, et al. Defining pediatric sepsis by different criteria: discrepancies in populations and implications for clinical practice. Pediatr Crit Care Med. 2012;13(4):e219–226. [DOI] [PubMed] [Google Scholar]
- [37.].Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017;171(10):e172352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38.].Brilli RJ, Goldstein B. Pediatric sepsis definitions: past, present, and future. Pediatr Crit Care Med. 2005;6(3 Suppl):S6–8. [DOI] [PubMed] [Google Scholar]
- [39.].Schlapbach LJ, Kissoon N. Defining pediatric sepsis. JAMA Pediatr. 2018;172(4):312–314. [DOI] [PubMed] [Google Scholar]
- [40.].Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016; 315(8):775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41.].Balamuth F, Weiss SL, Hall M, et al. Identifying pediatric severe sepsis and septic shock: accuracy of diagnosis codes. J Pediatr. 2015;167(6):1295–1300e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42.].Friedman C, Rubin J, Brown J, et al. Toward a science of learning systems: a research agenda for the high-functioning learning health system. J Am Med Inform Assoc. 2015;22(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43.].Schappert S, Bhuiya F. Availability of pediatric services and equipment in emergency departments: United States 2006. National Health Statistics Reports. Hyattsville, MD: National Center for Health Statistics; 2012. [PubMed] [Google Scholar]
- [44.].Scott HF, Greenwald EE, Bajaj L, et al. The sensitivity of clinician diagnosis of sepsis in tertiary and community-based emergency settings. J Pediatr. 2018;195:220–227 e1. [DOI] [PubMed] [Google Scholar]
- [45.].Greenwald E, Olds E, Leonard J, et al. Pediatric sepsis in community emergency care settings: Guideline concordance and outcomes. Pediatr Emerg Care. 2020. [cited 2021 Jun 17]. doi: 10.1097/PEC.0000000000002120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46.].Carcillo JA, Halstead ES, Hall MW, et al. Three hypothetical inflammation pathobiology phenotypes and pediatric sepsis-induced multiple organ failure outcome. Pediatr Crit Care Med. 2017;18(6):513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47.].Wong HR, Cvijanovich NZ, Anas N, et al. Pediatric sepsis biomarker risk model-ii: redefining the pediatric sepsis biomarker risk model with septic shock phenotype. Crit Care Med. 2016;44(11):2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48.].DeMerle KM, Angus DC, Baillie JK, et al. Sepsis subclasses: A framework for development and interpretation. Crit Care Med. 2021;49(5):748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49.].Raman J, Johnson TJ, Hayes K, et al. Racial differences in sepsis recognition in the emergency department. Pediatrics. 2019;144(4):e20190348. [DOI] [PubMed] [Google Scholar]
- [50.].Mitchell HK, Reddy A, Montoya-Williams D, et al. Hospital outcomes for children with severe sepsis in the USA by race or ethnicity and insurance status: a population-based, retrospective cohort study. Lancet Child Adolesc Health. 2021;5(2):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


