Abstract
Retinal inflammation underlies multiple prevalent ocular and neurological diseases. Similar inflammatory processes are observed in glaucomatous optic neuropathy, age-related macular degeneration, retinitis pigmentosa, posterior uveitis, Alzheimer’s disease, and Parkinson’s disease. In particular, human and animal studies have demonstrated the important role microglia/macrophages play in initiating and maintaining a pro-inflammatory environment in degenerative processes impacting vision. On the other hand, microglia have also been shown to have a protective role in multiple central nervous system diseases. Identifying the mechanisms underlying cell dysfunction and death is the first step towards developing novel therapeutics for these diseases impacting the central nervous system. In addition to reviewing recent key studies defining important mediators of retinal inflammation, with an emphasis on translational studies that bridge this research from bench to bedside, we also highlight a promising therapeutic class of medications, the glucagon-like peptide-1 receptor agonists. Finally, we propose areas where additional research is necessary to identify mechanisms that can be modulated to shift the balance from a neurotoxic to a neuroprotective retinal environment.
Keywords: retina, glaucoma, age-related macular degeneration, retinitis pigmentosa, Alzheimer’s disease, Parkinson’s disease, inflammation, microglia, monocytes, macrophages
Introduction
A number of recent publications have illuminated the relationship between retinal inflammation and diseases impacting the visual pathway. The central nervous system (CNS) is a site of immune privilege that is relatively shielded from the systemic circulation and is where microglia function as the resident myeloid cells. Like macrophages in the periphery, microglia play many important roles in both health and disease, and they may be either neuroprotective or neurotoxic.
Studies in both humans and animal models have highlighted the roles macrophage infiltration and microglia activation play in ophthalmic diseases. In particular, the contribution of myeloid cells to pathogenesis in glaucoma, retinal degenerative processes such as age-related macular degeneration (AMD) and retinitis pigmentosa (RP), and inflammatory conditions like posterior uveitis, are increasingly well-characterized. As a direct extension of the CNS, the retina provides a window through which neurodegenerative diseases impacting the brain, such as Parkinson’s and Alzheimer’s diseases, may be imaged and studied in vivo.
This review provides an updated perspective on the roles myeloid cells play in retinal inflammation, both in ocular diseases and brain neurodegenerations with ocular extensions. An abbreviated overview of ocular anatomy provides the introductory background upon which these diseases are then discussed in detail. Particular attention will be given to the posterior segment including the retina and the optic nerve. We highlight conditions where myeloid cells have been suggested to be a significant driver in the disease process. Finally, we propose that a class of medications, glucagon-like peptide-1 receptor (GLP-1R) agonists, demonstrates potential as a novel treatment modality for neurodegenerative processes impacting the eye and the brain.
Anatomy of the Eye
The eye is most often conceptualized as two separate compartments (Figure 1). The posterior segment denotes the area lying behind the crystalline lens, while the anterior segment consists of structures in front of, and including, the lens. The anterior segment can be further divided into an anterior and a posterior chamber by the iris, which has a central pupillary opening that connects the two chambers, allowing aqueous humor to flow into the anterior chamber from behind the iris [1].
Figure 1.
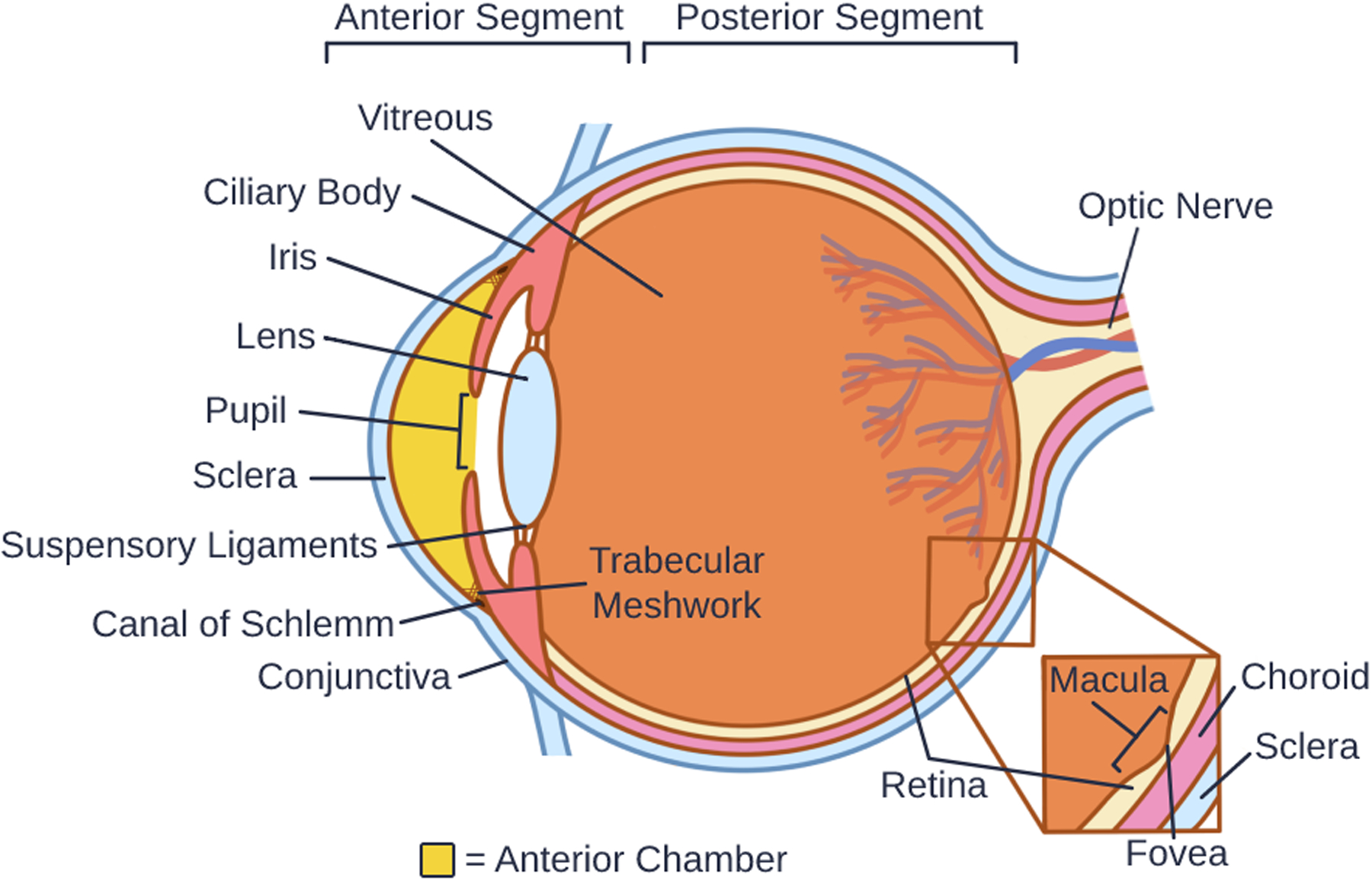
Anatomy of the Eye
Externally, the sclera is the white-appearing collagenous shell that maintains the rounded shape of the eye. Covering the sclera and continuous with the inner surface of the eyelids is the conjunctiva, a mucous membrane that provides immune protection from the outside environment and lubricates the eye. The cornea is the clear, avascular window of the eye that provides approximately 80% of its refractive power. It consists of a collagenous stroma sandwiched between an epithelial and an endothelial layer and their respective basement membranes [2]. Posterior to the cornea lies the iris, a heavily pigmented structure that contains both longitudinal and circular smooth muscles that adjust the size of the pupil in response to light intensity. The crystalline lens sits behind the pupil and is suspended from the ciliary body by zonular ligaments [3]. These zonular suspensory ligaments allow the muscular contractions within the ciliary body to modify the shape of the lens, and the eye to focus on images at various distances [4]. Behind the lens, the gel-like vitreous humor fills the posterior chamber [5, 6]. The retina surrounds this vitreous cavity and is the site of the transduction of light to neural impulses [7]. Lying beneath the retina and within the sclera is the choroid, a layer of blood vessels that nourishes the outer layers of the retina [8].
In addition to adjusting the shape of the lens, the ciliary body also secretes aqueous humor [9]. The aqueous fluid flows through the pupillary opening to circulate within the anterior chamber, where it provides nutrients to ocular structures, after which it drains through the trabecular meshwork in the iridocorneal angle to remove metabolic waste [10]. From the trabecular meshwork, aqueous humor continues into the Canal of Schlemm, which is a modified capillary that drains into the episcleral venous system surrounding the eye [1]. Should aqueous drainage become obstructed as during angle closure, intraocular pressure (IOP) can increase dramatically within the eye. Elevated IOP, which can happen even with open iridocorneal angles, is a major risk factor for the development of glaucomatous neurodegeneration. All currently available glaucoma treatments focus on IOP lowering [11, 12].
Retinal Anatomy and Visual Signal Transduction
The retina and optic nerve are extensions of the CNS that develop from the optic cup, a protrusion of the forebrain [7]. As the retina is an extension of the CNS, the blood-retina barrier (BRB) functions like the blood-brain barrier to offer the retina a degree of immune privilege [7, 13, 14]. In primates, the retina contains an oval-shaped macula lutea, and within the macula the fovea provides for the highest degree of visual acuity [15]. The human retina itself is a complex structure with 10 distinct layers (Figures 1 and 2). The outermost layer is the retinal pigment epithelium (RPE), situated between the choroid and the photoreceptors of the retina. The RPE contains melanin that absorbs light to minimize light reflections off of the sclera, and also forms the BRB [7]. Internal to the RPE lies the photoreceptor layer, containing extensions of rods and cones that respond to incoming light. Internal to this, the external limiting membrane is formed by a layer of cellular connections between Müller cells and the photoreceptors [16]. Moving even closer to the vitreous cavity, the outer nuclear layer (ONL) contains the nuclei of the photoreceptors, followed by the outer plexiform layer (OPL), where the photoreceptors synapse with the dendrites of bipolar and horizontal cells. The inner nuclear layer (INL) contains the nuclei of the bipolar cells, horizontal cells, amacrine cells, and Müller glia. Adjacent to the INL is the inner plexiform layer (IPL), where bipolar and amacrine cells synapse with retinal ganglion cells (RGCs). Ganglion cell bodies reside in the adjacent ganglion cell layer (GCL). Finally, RGC axons traverse the retinal nerve fiber layer (RNFL) and coalesce to form the optic nerve, which primarily transmits to the visual cortex in the occipital lobe of the brain [7]. To form the optic nerve, RGC axons exit the eye through openings in the lamina cribrosa, a multilayered connective tissue comprised of plates of collagen that are a part of the optic nerve head (ONH) [17]. The internal limiting membrane sits adjacent to the vitreous and is formed by the footplates of Müller cells and segregates the vitreous humor from the retina [16].
Figure 2. Resting and Activated Myeloid Cells in the Retina and Optic Nerve.
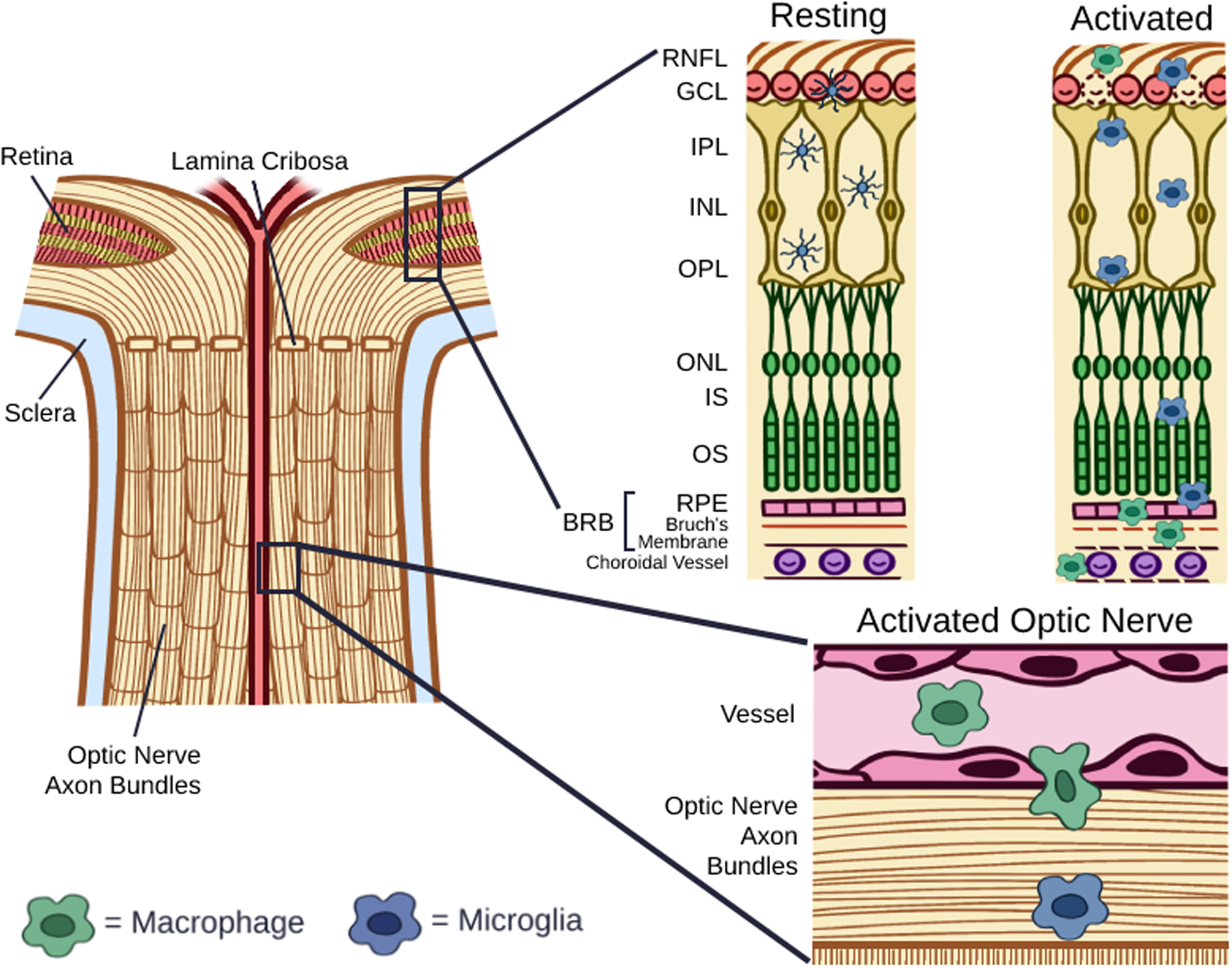
In the resting state, microglia have a dendritic morphology and localize to the ganglion cell layer (GCL), inner plexiform layer (IPL), and outer plexiform layer (OPL), and macrophages are largely absent in the retina. Following activation, microglia take on an enlarged, amoeboid shape and migrate to the subretinal space above the RPE. Within the retina, activated microglia release pro-inflammatory cytokines, including interleukin-1α (IL-1α), tumor necrosis factor α (TNF-α), complement component 1q (C1q), reactive oxygen species (ROS), and nitric oxide (NO), and are associated with the death of both retinal ganglion cells and photoreceptors. Recruited macrophages have been shown to infiltrate the retina via the optic nerve.
Within the photoreceptor layer, relative to cones, rods are more abundant in the peripheral retina and are highly sensitive to changes in light and dark. Cones are most densely packed in the fovea and are responsible for color vision in bright light [7]. Mouse and human retinas differ in that mouse retinas lack a macula lutea, and mouse retinas have two cone opsins, one for UV light and one for green light [18, 19]. In contrast, there are three types of cones in the human retina, with each maximally responsive to different wavelengths of light: red, green and blue [20]. Rods and cones convert light to electrical signals in neurons in a process called phototransduction. While multiple bipolar cells synapse with a single cone, many rods converge onto a single bipolar cell to increase light sensitivity at the cost of acuity. Amacrine cells modulate RGC firing by sending inhibitory signals to the axon terminals of bipolar cells synapsing with RGCs. Horizontal cells receive input from photoreceptors and send inhibitory signals as feedback to rods and cones, thereby inhibiting adjacent photoreceptors to enhance contrast [21]. The retina contains 3 types of glial cells: Müller cells, microglia, and astrocytes. Müller cells modulate the exchange of nutrients from the circulation to neurons in the retina [22]. Like Müller cells, astrocytes associate with blood vessels in the GCL and contribute to the BRB, while also sharing synaptic pruning responsibilities with microglia [13].
Microglia and Macrophages
Microglia serve as important mediators of immune response within the CNS [23]. In healthy eyes, microglia have a highly branched morphology, with cell bodies located in several layers of the inner retina: the RNFL/GCL, IPL, and OPL [24]. Microglia have multiple functions, including immune surveillance, synaptic pruning, modulation of neuronal activity, and regulation of neuronal soma and axon growth [25]. Activated microglia, which exhibit an amoeboid appearance, may serve either neuroprotective or neurotoxic roles depending on their functional states. They have been shown to migrate to the subretinal space and adhere to the RPE in response to photoreceptor damage and aging [26, 27].
In their activated states, microglia remodel neural synapses to facilitate plasticity during development, phagocytose cellular debris, and produce neurotrophic factors to regulate neuron survival and apoptosis [28–36]. Activated microglia can also produce superoxide ions, release pro-inflammatory cytokines, and present self-antigens in autoimmune responses [24, 37, 38]. Neurotoxic microglia may phagocytose stressed but viable neurons during development, inflammation, and in pathological states such as CNS ischemia [39, 40].
Although microglia and macrophages have similar functions, microglia are developmentally and functionally distinct from monocyte-derived macrophages. Microglia arise from myeloid progenitors in the yolk sac early in development whereas peripheral macrophages derive not only from the yolk sac, but from both fetal monocytes and postnatal hematopoietic stem cells [41–44]. Further, microglia are distinct from prenatal-derived tissue-resident macrophages located throughout other organs including the eye [26, 45, 46]. Monocytes travel through the systemic circulation before extravasating into end tissues in response to specific chemoattractant signals, after which they differentiate into tissue-specific macrophages [47, 48]. The population of microglia cells is maintained via self-renewal, largely separate from the influence of blood-derived monocytes [28, 49].
Microglia can occupy the IPL, OPL and subretinal spaces, and monocyte-derived macrophages expectedly show an association with perivascular spaces prior to migrating into, and residing in, multiple layers of the retina [26, 46, 50]. After infiltrating the CNS in response to injury, peripheral macrophages adopt morphology and surface markers similar to those of resident microglia [49, 51]. If the microglia population is depleted, macrophages can replace microglia in the central nervous system where they take on similar characteristics, distributions, and gene-expression profiles as resident microglia [52, 53]. In particular, yolk sac-derived macrophages express many signature microglia genes including Tmem119, Fcrls, Hexb, and Olfml3 when injected into the brains of microglia-deficient mice [51]. The similarities between macrophages and microglia complicate efforts to differentiate resident microglia from infiltrating macrophages. Genetic fate mapping approaches have mitigated without entirely resolving this limitation [44, 54–56].
The M1/M2 paradigm is an imperfect, and arguably outdated, way to conceptualize cytotoxic and homeostatic macrophages, respectively, and holds less relevance for microglia [57–59]. M1 macrophages were often described as performing the role of phagocytosing dead or dying tissue and releasing pro-inflammatory cytokines. Thus, they were seen to serve a neurotoxic role in the nervous system [60, 61]. Stimulation by lipopolysaccharides (LPS) and interferon-gamma (IFN-γ) from Th1 helper T cells were shown to induce M1 macrophages to produce pro-inflammatory cytokines tumor necrosis factor-alpha (TNF-α), interleukin 1 (IL-1), and nitric oxide synthase (NOS) [60]. M2 macrophages in vitro, in contrast, were shown to produce ornithine following stimulation by interleukin 4 (IL-4) from Th2 helper T-cells, and stimulate cell division [58]. So-called M2 macrophages may also inhibit inflammation via cellular signaling, and along with increased expression of neurotrophic factors like brain-derived neurotrophic factor (BDNF), act in a neuroprotective manner in the nervous system [61, 62]. The M1/M2 classification framework represents a simplified approach to macrophage characterization, when in reality these 2 phenotypes may represent 2 functional states among many [58, 60, 61].
Within the retina, as is the case within other parts of the CNS, microglia constantly survey for noxious stimuli. Under sustained toxic stimulation, exaggerated microglial inflammatory responses can lead to the release of pro-inflammatory mediators [40]. A sustained, chronic pro-inflammatory environment is common to many retinal degenerative diseases and neurodegenerative disorders affecting vision, as discussed in detail below [63].
Glaucoma
Glaucoma is the leading global cause of irreversible blindness. The number of people with glaucoma worldwide is expected to increase from 64.3 million in 2013 to 111.8 million in 2040 [64]. Glaucoma encompasses a group of optic neuropathies that share a common phenotype of RGC death and axon degeneration with characteristic optic nerve cupping and visual field loss [65]. The specific etiologies behind glaucoma are being continuously elucidated, but elevated IOP, along with age, sex, and ethnicity, are well-known risk factors in glaucomatous neurodegeneration [66, 67].
More recently, both microglia activation and macrophage recruitment have been implicated in glaucoma pathogenesis. In the eyes of patients with glaucoma, activated microglia have been found clustered at the lamina cribrosa of the ONH and surrounding blood vessels [68]. Margeta and colleagues identified myeloid cells positive for CD163, a marker of macrophages involved in tissue repair, in the optic nerves of eyes with both early and advanced glaucoma [69]. Macrophage chemoattractant protein-1 (MCP-1), also known as CCL2 (C-C motif ligand 2), is a key chemotactic factor in monocyte recruitment and often implicated in initiating inflammation [70, 71]. A recent prospective, longitudinal study in patients with normal-tension glaucoma demonstrated that elevated systemic levels of MCP-1 are associated with progressive loss on visual field testing [72]. These studies on human patients with glaucoma highlight an emerging role for monocytes in the pathogenesis of glaucoma.
Extravasation of myeloid cells from the ONH has also been implicated in glaucoma pathogenesis and following optic nerve injury. In the DBA/2J mouse model of chronic glaucoma, pharmacological and genetic inhibition of monocyte entry into the eye prevented loss of RGC soma and axons [73]. Crush injury of the optic nerve has also been shown to attract myeloid cells to injured RGCs [74]. Through parabiosis and fate mapping, the responding myeloid cells were demonstrated to be retinal in origin as opposed to derived from circulating macrophages. Interestingly, total optic nerve transection prevented this myeloid cellular response, suggesting that the optic nerve serves as either a reservoir or a conduit for reactive myeloid cells following optic nerve injury [74]. Recently, our group showed that a macrophage-enriched population of retinal cells was responsible for pro-inflammatory cytokine release early on after microbead-induced IOP elevation, whereas a microglia-enriched population did not contribute to the inflammatory response until weeks later, further suggesting monocyte infiltration may be a driver of astrocyte activation [75].
Recent experimental evidence suggests that elevated IOP in one eye was sufficient to elicit early and widespread microglial activation that precedes RGC death. Following laser-induced ocular hypertension in one eye, activation of microglia extended to all retinal layers in both eyes [76]. In a rat model of intermittent ocular hypertension, unilateral IOP elevation also led to bilateral microglial activation and a significant decrease in RGC and axon density [77]. Unilateral microbead-induced IOP elevation has also been found to enhance microglial reactivity in the contralateral, normotensive optic nerve and its central projections in rats [78]. Interestingly, however, RGC death in the contralateral eye did not occur without IOP elevation. Taken together, results suggest that a focal, unilateral insult of IOP elevation is sufficient to induce a global inflammatory response throughout the retina and the central visual system.
IOP elevation also causes transcriptomic changes impacting macrophage/microglial function that vary depending on the timeframe of glaucomatous injury. Early on, after IOP elevation but prior to RGC axon loss, Oikawa and colleagues demonstrated transcriptomic evidence of inflammatory pathway activation and microglial proliferation in the eyes of domestic cats [17]. In the DBA/2J model of chronic IOP elevation and glaucoma, transcriptomic profiling of microglial mRNA from the ONH showed disruptions of homeostatic functions in microglia the authors characterized as neither definitively pro- or anti-inflammatory [79]. Transcriptomic signatures also appeared to differ between infiltrating macrophages and resident microglia in these animals, suggesting a way to distinguish between the two cellular populations.
Several mediators of RGC death in the setting of ocular hypertension have been elucidated. Multiple studies have demonstrated elevated levels of TNF-α in the aqueous humor, retina, and optic nerves of patients with glaucoma [80–83]. Cueva Vargas and colleagues showed that TNF-α is released by microglia and Müller cells following elevated IOP in a rat model of glaucoma, and that pharmacological inhibition of TNF-α enhanced RGC soma and axon survival [84]. Nakazawa and colleagues demonstrated that inducing ocular hypertension in mice resulted in a sequence of events including upregulation of TNF-α, microglial activation, optic nerve oligodendrocyte death, and RGC death [85]. Genetic deletion of TNF-α or its receptor, and neutralizing antibodies to TNF-α both prevented the damaging effects of ocular hypertension [85]. Inhibition of TNF-α in a mouse model of corneal chemical injury was also shown to prevent monocyte infiltration and microglial activation as well as reduce susceptibility to secondary glaucoma and RGC death [86]. Etanercept is a TNF-α blocking decoy receptor that is approved for the treatment of conditions where pro-inflammatory signaling is central to disease pathophysiology, such as rheumatoid arthritis and psoriatic arthritis [87, 88]. In a rat model of hypertensive glaucoma, etanercept inhibited the activation of microglia around the ONH and promoted RGC survival [89]. In short, existing evidence suggests that TNF-α plays an important role in both IOP-dependent and independent RGC death, while TNF-α antagonism may represent a potential treatment pathway in glaucoma.
Fas is a member of the TNF receptor superfamily that signals apoptotic cell death after binding the Fas ligand. Fas is constitutively expressed in murine microglia and significantly upregulated following TNF-α stimulation [90]. A small peptide antagonist of the Fas receptor inhibited microglia activation and expression of TNF-α, C1q, and C3, and reduced RGC death following microbead-induced IOP elevation [91]. In a separate publication by the same group, inhibition of Fas ligand signaling by its cleavage product was shown to protect against RGC and axon degeneration in both the DBA/2J mouse model of glaucoma and following microbead-induced IOP elevation [92]. These results provide additional evidence that TNF-α-associated pro-inflammatory pathways contribute to glaucomatous neurodegeneration.
The complement cascade actively regulates the inflammatory response and has been implicated in glaucoma [93–98]. C1q, in particular, is a major complement component with upregulated expression in the human glaucomatous retina and animal models of the disease [99]. In a microbead-induced primate model of glaucoma, C1q upregulation was shown in conjunction with decreased RGC axon density after 28 weeks of sustained IOP elevation [100]. Inhibition of the complement cascade prevented RGC dendritic pruning and synaptic loss, which occurs early in the disease process prior to axon and soma degeneration [94]. Genetic and pharmacological inhibition of C1 prevented these early changes in both genetic and induced models of glaucoma [94, 98]. These findings elucidate the role of complement in retinal degenerative processes including glaucoma and highlight the potential for complement-modulating therapeutics in glaucoma treatment.
The IL-1 family containing both pro- and anti-inflammatory cytokines contributes directly to a number of retinal diseases as well as glaucoma [101–105]. In a study of 50 patients with primary open-angle glaucoma, circulating levels of IL-1β mRNA in blood lymphocytes as well as IL-1β protein in the aqueous fluid were elevated compared to age- and sex-matched controls [106]. The expression of IL-1α, IL-1β, and IL-6 were also shown to be increased in the trabecular meshwork cells of glaucomatous eyes compared to normal eyes [107]. Further, IL-1 was shown to stimulate the activation of the pro-inflammatory transcription factor nuclear factor-kappa B (NF-κB), which upon release from I-κB inhibition, translocates to the nucleus and mediates the expression of a battery of pro-inflammatory cytokines including IL-1α, IL-1β, IL-6, and TNF-α [107, 108].
Recently, our group demonstrated that induced ocular hypertension stimulates macrophage/microglia to upregulate IL-1α, TNF-α, and C1q, a trio of cytokines shown to be necessary and sufficient to induce astrocyte conversion to a neurotoxic (so-called A1) phenotype, causing RGC death [109]. Genetic knockout mice deficient in IL-1α, TNF-α, and C1qa (triple knockout) exhibited reduced astrocyte transformation and RGC death in ocular hypertension [75]. These findings are corroborated by the work of Guttenplan and colleagues, who demonstrated increased survival of electrophysiologically-viable RGCs in triple knockout animals following both optic nerve crush and microbead injections, and showed that focal injury is a prerequisite to astrocyte-induced RGC death [110]. Following optic nerve crush-induced synaptic degeneration in the dorsal lateral geniculate nucleus, microglia, in a process mediated in part by C1q, have been shown to be the responding post-injury phagocytes [111]. It remains to be determined whether this process promotes neuroprotection by removing toxic debris or exacerbates neurodegeneration by further compromising synaptic integrity.
In summary, evidence increasingly suggests that neuroinflammatory pathways play in a role in glaucoma pathogenesis at all stages of the disease process impacting both the outflow pathway in the iridocorneal angle and the retina/optic nerve. In particular, following focal injury or stress, macrophage/microglia activation may directly contribute to RGC death and optic nerve degeneration in glaucoma. Targeting retinal inflammatory pathways and identifying ways to switch microglial activation toward neuroprotection may function in a synergistic fashion with IOP lowering to improve RGC survival.
Age-Related Macular Degeneration
Age-related macular degeneration (AMD) is a progressive, common, and heritable deterioration of the macula impacting central vision [112]. The global prevalence in 2020 is 8.69% (196 million people), with a projected increase to 288 million people by 2040 [113]. Clinical features of AMD include the presence of drusen, consisting of extracellular deposits of lipids, proteins, and inflammation-related cytokines, between the RPE and the underlying Bruch’s basement membrane [114, 115]. AMD is categorized by 2 clinical forms: 1) Neovascular or exudative AMD, characterized by the growth of immature, leaky choroidal vessels either beneath or through the RPE into the avascular outer retina, and 2) Nonexudative AMD, in the advanced geographic atrophy form, characterized by slowly expanding areas of degeneration of the RPE and photoreceptors. [112, 116, 117].
An abundance of evidence suggests that subretinal macrophages/microglia are part of a pro-inflammatory milieu contributing to photoreceptor death in retinal degeneration. Under physiologic conditions, the photoreceptor layer and the subretinal space are inherently immunosuppressive and lack monocytes [118]. In AMD, however, monocytic infiltration and microglial activation have been observed in both regions in all stages of the disease process [118, 119]. Microglia and recruited macrophages produce high levels of pro-inflammatory cytokines including IL-1β, TNF-α, and IL-6, resulting in increased vasodilation and vascular permeability/exudation [120]. A similar pro-inflammatory environment created by subretinal macrophages/microglia has been shown to directly induce the death of nearby photoreceptors in a mouse model of acute retinal degeneration [121]. Conditioned media from reactive human and murine microglial cells have been shown to trigger caspase-mediated photoreceptor cell death in vitro [122, 123]. In addition to MCP-1, other chemokines implicated in retinal macrophage recruitment include the Cx3cr1 receptor ligand fractalkine and the complement receptor C5aR [124, 125]. In summary, accumulation of retinal macrophages/microglia and their pro-inflammatory cytokine production may play a mechanistic role in worsening photoreceptor degeneration and disease progression in AMD.
Macrophages are the primary infiltrating immune cells in choroidal neovascularization (CNV), a hallmark of exudative AMD [126]. Higher levels of MCP-1 have been identified in the serum of patients with exudative AMD compared to unaffected age- and gender-matched controls [127]. Blocking Müller MCP-1 expression reduced monocyte/microglial infiltration and activation in a rat model of light-induced retinal degeneration [128]. The monocyte marker CD163, along with other cytokines implicated in inflammation, may be an indicator of disease status in AMD. The levels of multiple cytokines including CD163 in the peripheral blood of human patients differed between exudative AMD, nonexudative AMD, and age-matched controls, depending on the presence of active leakage in exudative cases [126]. Higher peripheral blood levels of C-reactive protein and IL-6, for example, have been associated with AMD progression [129, 130]. Recurrent CNV leads to subretinal scarring and fibrosis, an important contributor to vision loss [131]. Infiltrating macrophages may also contribute directly to macular fibrosis by transdifferentiating into myofibroblasts in experimental CNV and in human eyes with exudative AMD [132]. Targeting specific populations of proangiogenic and pro-fibrotic macrophages represents a potential therapeutic strategy for AMD.
Recently, the AMD-associated minor haplotype of the chromosome 10q26 was shown to account for a large proportion of the genetic risk of AMD [133]. Disease risk increased up to tenfold in homozygous carriers [134, 135]. The disease-associated haplotype and the common non-risk haplotype only differ by several single nucleotide polymorphisms, located in a non-coding DNA sequence within the promoter of the high-temperature requirement A serine peptidase 1 (HRTA1) [136, 137]. In both in vitro and in vivo experiments, HTRA1 reduced the ability of thrombospondin-1 to form CD47 complexes in mice, a step that mediates monocyte elimination from the subretinal space [137, 138]. Injection of HTRA1 into the subretinal space increased the survival of monocytes in a CD47-dependent manner, and monocytes from homozygous carriers of the minor haplotype were shown to express high levels of HTRA1 [137]. Conversely, HTRA1-induced monocyte survival is dependent on the induction of osteopontin, and genetic ablation of osteopontin reduced subretinal inflammation and monocyte survival [137]. These studies suggest that the mechanism behind 10q26 haplotype-associated AMD risk is the promotion of monocyte survival and inflammation within the retina.
In summary, retinal inflammation and macrophage/microglial activation clearly contribute to retinal degeneration in AMD. Developing effective therapies for AMD necessitates a clear understanding of the inflammatory responses at all stages of the disease process.
Retinitis Pigmentosa
Retinitis pigmentosa (RP) is a heterogeneous group of inherited retinal degenerative disorders characterized by the progressive loss of photoreceptors resulting in severe vision loss [139]. In human retinas with RP, activated, amoeboid myeloid cells are found in the ONL containing debris from phagocytosed rod cells in regions of photoreceptor degeneration [119]. A retrospective, observational study identified increased density of inflammatory cells and higher levels of pro-inflammatory cytokines including IL-1α, TNF-α, and MCP-1 in the aqueous humor and anterior vitreous cavity of RP patients compared to controls [140]. Using the rd10 mouse model of RP, the same group also found microglial activation and elevated expression of pro-inflammatory cytokines IL-1β, MCP-1, and TNF-α preceding photoreceptor apoptosis [141]. They further demonstrated anti-inflammatory effects as well as photoreceptor rescue following treatment with the antioxidant N-acetylcysteine (NAC). Topical and oral NAC were also shown to reduce superoxide radicals, as well as rescue and maintain cone function in the rd1 and rd10 mouse models of RP [142]. More recently, a phase I clinical trial evaluated the effect of oral NAC administration in RP patients, and demonstrated improvements in best-corrected visual acuity after 24 weeks of treatment [143].
Inhibition of a specific pro-inflammatory pathway demonstrated the treatment potential of strategies that mitigate exaggerated immune activation in an animal model of RP. Myeloid differentiation factor 88 (MyD88) is an adaptor protein for the IL-1 receptor and Toll-like receptor (TLR) families of innate immunity receptors, and mediates inflammatory responses to cellular injury by activating NF-κB [144]. Using a MyD88-blocking peptide in a mouse model of RP, Garces and Hackam demonstrated increased rod photoreceptor function and reduced apoptosis compared to controls [145]. MyD88 inhibition also resulted in fewer microglia/macrophage cells in the photoreceptor layer, while the total number of microglia/macrophages within the retina was unchanged, suggesting that MyD88 promotes the migration of microglia/macrophages within the retina to site of injury.
Activated microglia, rather than recruited monocytes, have been shown to phagocytose stressed, but viable photoreceptors in models of retinal degeneration [40]. In the rd10 mouse model of RP, activated microglia in the ONL contributed to retinal degeneration by phagocytosing non-apoptotic rods in early rod degeneration [40]. Further, both genetic microglia ablation induced by tamoxifen pulse and inhibition of the phagocytosis receptor vitronectin ameliorated photoreceptor death in these animals. Compared to microglia, recruited macrophages appeared to play a lesser role in photoreceptor clearance. Recruited monocytes were found to localize to the subretinal space more than the ONL and morphologically showed little evidence of phagocytosis [40]. In a follow up publication by the same group, longitudinal tamoxifen treatment was unexpectedly found to reduce microglial activation in vivo independent of depletion, and this modulation was linked to improved photoreceptor survival in vitro [146]. Taken together, these results raise the possibility that tamoxifen administration to ablate CX3CR1-expressing microglia may have also rescued photoreceptors by blunting microglia activation [40].
Consistent with the finding that microglia play a larger role than recruited monocytes in models of retinal degeneration, rd10 mice with genetic deletion of CCR2, the receptor to the major macrophage chemokine MCP-1, demonstrated only moderate, if significant, decrease in the numbers of F4/80 positive myeloid cells in the retina. CCR2 deletion also delayed, but did not ultimately reduce, photoreceptor degeneration relative to rd10 mice with functional CCR2 [147]. These results suggest that while macrophage recruitment may contribute to pathogenesis early on in RP retinal degeneration, microglia instead of recruited macrophages are primarily responsible for photoreceptor death [147]. Findings highlight myeloid activation as both a cause and a response to photoreceptor loss, and support targeting myeloid cell infiltration and activation in therapeutic strategies in RP treatment.
In the RhoP23H/wt mouse model of autosomal dominant RP, O’Koren and Saban found age-related accumulation of IBA1-positive myeloid cells in the subretinal space [26]. Cx3cr1-positive cells in the subretinal space adherent to the RPE were shown to be microglia using lineage tracing. Further, microglia depletion resulted in decreased number of IBA1-positive cells in the subretinal space [26]. Following photoreceptor damage, macrophages did not occupy the subretinal space even after microglia depletion, suggesting that recruited monocytes and resident microglia occupy different niches in response to disease. Interestingly, the authors also showed that different sub-populations of microglia contributed to different steps of visual transduction, with ablation of all retinal microglia resulting in reduction of both photoreceptor and bipolar signaling on ERG testing [148]. In addition, subretinal microglia was shown to improve photoreceptor-RPE interdigitation, thereby functioning in a protective capacity in this model of RP [26]. These findings provide evidence that activated microglia can also function in neuroprotective in addition to the traditionally-recognized cyto-destructive roles. Selective immunomodulation will be an important consideration in the development of therapies to prevent and treat RP-like retinal degeneration.
Posterior Uveitis
Inflammation of the posterior uveal tract can also involve the retina and the optic nerve, and are otherwise referred to as choroiditis or chorioretinitis depending on the level of affected tissues [149]. Posterior uveitis has both infectious and non-infectious/autoimmune causes. Autoimmune uveitis is a vision-threatening ocular inflammatory condition in which the retina and uveal tissues are targeted by autoreactive immune activation, and is often associated with systemic manifestations [150]. Autoimmune uveitis include some of the most common causes of posterior inflammation, including Behçet’s disease, sarcoidosis, and Vogt-Koyanagi-Harada disease [151, 152]. Animal models of posterior uveitis have recently highlighted the dynamic function of myeloid cells in this disease.
Microglia have been shown to be essential for inducing the retinal inflammatory response in experimental autoimmune uveitis (EAU) [150]. EAU is a T cell-mediated mouse model of chorioretinitis whereby inflammation is induced by immunization with retinal antigens [153]. Microglia ablation prior to the development of EAU was sufficient to block the inflammatory response [150]. When microglia were depleted after inflammation had already begun, however, disease timeline and severity remained unchanged. EAU mice with locally inducible microglia ablation had significantly fewer adherent leukocytes at retinal vessels compared to control mice, suggesting that microglia recruited leukocytes by promoting vascular cell adherence and extravasation at the BRB, and mediate the entry of circulating leukocytes into the retina [150]. Following microglia depletion, circulating immune cells were therefore unable to penetrate the BRB.
In vivo fundus imaging in an animal model of posterior uveitis has revealed the relative contribution and migration pattern of macrophages in this disease, further elucidating their function in inflammatory retinal conditions. Cx3cr1+ and CD11c+ myeloid cells have been shown to accumulate around the ONH and retinal vessels 2 weeks after immunization in EAU [154]. Bremer and colleagues used in vivo two-photon microscopy to track the motility pattern of immune cells, and found that CD4+ T cells and LysM+ peripheral phagocytes radially migrated within the retina away from the ONH following immunization [155]. CD4+ T cells were shown to arrive in the retina prior to phagocytes, with migration away from the ONH. Taken together, these results suggest that peripheral macrophages may be attracted by T cells and subsequently use the optic nerve as a conduit for entering the retina.
Breakdown of the BRB is strongly implicated in the pathogenesis of posterior uveitis [150, 155, 156]. In addition to leukocyte extravasation, BRB breakdown also allows fluid and protein leakage into the retina to cause uveitic macular edema, an often refractory condition impacting visual acuity in more than 40% of patients [157–159]. In EAU, CD4+ T cells and macrophage/microglia have been shown in the retina both perivascularly and in tertiary lymphoid clusters [154, 160]. When EAU mice were given an inhibitor to integrin VLA-4, an adhesion molecule implicated in CD4+ T cell recruitment, fewer Th17+ subset of T cells and Ly6C+ monocyte/macrophages crossed the BRB, resulting in reduced clinical disease severity [153]. Repeated exposure to systemic LPS also led to BRB breakdown, retinal microglia proliferation and activation, and macrophage infiltration [161]. Treatment with the colony-stimulating factor 1 receptor inhibitor PLX5622 resulted in microglia depletion and normalization of cytokine levels following LPS challenge, preserving BRB integrity [161]. Results suggest that microglia activation may lead to increased vascular permeability to facilitate infiltration of immune cells into the retina and exacerbate retinal inflammation in posterior uveitis.
Ocular Manifestations of Neurodegenerative Processes
The retina and brain share the same embryological origin as extensions from the neural tube [162]. The retina responds similarly to the brain following many forms of cellular injury, and certain central neurodegenerative processes can be detected, and more importantly, directly visualized in the retina. We will review recent findings describing the ocular manifestations of Alzheimer’s and Parkinson’s diseases and highlight the role myeloid cells play in disease pathogenesis.
Alzheimer’s Disease
Alzheimer’s disease (AD) is the leading cause of dementia worldwide [163]. It is associated with a cumulative economic burden of $305 billion in 2020, and predicted to surpass $1 trillion by 2050, making it the most costly disease in the United States [164]. AD is a group of heterogeneous neurodegenerative processes characterized by early hippocampal involvement impacting memory and executive function [165–167]. Definitive diagnosis of AD occurs postmortem following demonstration of extracellular amyloid-β (Aβ) plaques cleaved from amyloid precursor protein and intracellular accumulation of neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau [168]. Few diagnostic biomarkers of AD currently exist, and there is a critical need to identify reliable biomarkers earlier in the disease process [169].
Visual perception and abnormalities of the retina have been investigated to provide diagnostic clues in AD. AD pathology may manifest in visual field defects, changes in contrast sensitivity and visual acuity, and impaired color recognition [170–177]. RGC degeneration, Aβ deposition with local myeloid and astrocyte infiltration, hyperphosphorylated tau, and optic nerve axon loss have all been described in AD eyes [178–183]. Retinal structural alterations reported in patients with AD include thinning of the inner retina on optical coherence tomography (OCT), specifically the RNFL and the ganglion cell-inner plexiform layer [184–187]. Thinning of the RNFL in AD is associated with decreased retinal function, as evidenced by reduced amplitude on pattern ERG [188]. Circadian dysfunction is a hallmark of AD, and loss of melanopsin RGCs, a retinal cell type associated with circadian rhythm regulation, has been associated with Aβ deposition in postmortem AD retinas [181]. While these findings suggest RNFL and GCL degeneration are potential ocular biomarkers for AD, still other studies failed to demonstrate a strong association between AD and RNFL thinning, challenging the utility of inner retinal degeneration as a reliable biomarker for AD [189–191]. In contrast, another related neurodegenerative disease, frontotemporal dementia, has been associated with thinning of the photoreceptor layer, which may prove helpful as a diagnostic discriminator between AD and frontotemporal dementia [192].
Studies in human retina and mouse models of AD demonstrate microglia/macrophage activation. Retinal microglia/macrophages in a mouse model of AD showed a ramified, resting phenotype before symptom onset [193]. Following symptom onset, however, myeloid cells transitioned to an activated, amoeboid morphology in the retina. The same group also demonstrated histological evidence of neurotoxic astrocytes and microglia activation in the retinas of AD patients [194]. Co-localization of microglia/macrophages with tau oligomers has been demonstrated in the retinas of a mouse model of AD and in the brains of human AD patients, suggesting that tau oligomers are pro-inflammatory in AD [195]. Aβ deposits were also shown to accumulate with age in the retinas of a mouse model of AD, and correlated with increased expression of the macrophage chemokine MCP-1 and microglia proliferation in the GCL [196]. Microglial recognition and phagocytosis of Aβ plaques, and subsequent formation of dense-core Aβ plaques possibly as a way to confine toxic Aβ oligomers, has been shown to be mediated by the TAM receptor tyrosine kinases Axl and Mer [35]. Aβ deposition disrupted retinal function in a mouse model of AD and was associated with increased retinal microglia activity [197].
Evidence of microglia-mediated neuroprotection is plentiful in the AD brain if comparatively lacking in the AD retina. Mutations affecting the microglia gene triggering receptor expressed on myeloid cells 2 (TREM2) have been associated with decreased sensing, compaction, and phagocytosis of Aβ plaques by microglia in the brains of mice and humans with AD [198]. The protective barrier effect of microglia is further demonstrated by the finding that Aβ plaque regions not enveloped by microglia are associated with more severe axonal damage [199]. A subtype of microglia associated with neurodegenerative diseases (DAM) may be activated for plaque clearance to possibly restrict AD progression through both TREM2-dependent and independent mechanisms [200]. These findings link plaque depositions characteristic of AD with an increase in focal, specific microglia/macrophage reactivity, potentially as an inducible mechanism to mitigate the accumulation of toxic protein aggregates.
Parkinson’s Disease
Parkinson’s disease (PD) is the second most common neurodegenerative disorder following AD [201]. PD is characterized by the progressive loss of dopaminergic neurons in the substantia nigra and nerve terminals of the striatum [202]. Ocular disturbances associated with PD include blepharospasm, blepharitis/dry eye, visual hallucinations, as well as impaired acuity and decreased contrast sensitivity, motion perception and color vision [203–205]. The depletion of retinal dopamine may play a causative role in many of the visual processing impairments observed in PD [206, 207]. Structural changes in PD eyes include decreased small vessel and perfusion density in both the retina and the choroid [208]. Retinal and specifically, RGC, dysfunction have been demonstrated using ERG early in the disease process, and was found to correlate with disease severity [209, 210].
Lewy bodies are intracytoplasmic aggregates found in the neurons of patients with PD, and are comprised mainly of α-synuclein [211, 212]. In the normal retina, α-synuclein is found in photoreceptor outer segments and axons, bipolar, and amacrine cells [213]. In patients with PD, aggregates of α-synuclein localized to the inner retina, including the GCL, IPL, and INL [214]. α-synuclein has been shown to induce the activation of microglia [215], initiating a pro-inflammatory cascade that leads to accumulation of activated glial cells in the substantia nigra [215, 216]. Accordingly, increased reactivity of microglia/macrophages, astrocytes, and Müller cells were shown to first occur in the inner retina of a mouse model of MPTP-induced PD [217]. A transgenic mouse model overexpressing human α-synuclein also demonstrated an association between α-synuclein deposition, Müller and microglia/macrophage activation, and photoreceptor death within the retina [218]. Human postmortem tissue from AD and PD patients demonstrated the presence of reactive, neurotoxic astrocytes, which were shown to be induced by activated microglia [109].
Several mechanisms for myeloid cell activation in PD have been described. In one, α-synuclein deposits are identified by cell surface TLRs, resulting in microglia activation and secretion of pro-inflammatory cytokines IL-1β, IL-6 and IL-18 by astrocytes [219–222]. Specifically, α-synuclein has been shown to stimulate the NF-κB pathway downstream of microglia activation [223]. In another, an in vitro model of PD showed that α-synuclein phagocytosis is mediated by Fcγ receptors on microglia, and when these receptors are genetically ablated, NF-κB mediated pro-inflammatory signaling was reduced [215].
The increasing characterization of myeloid cell-mediated pro-inflammatory pathways in the pathogenesis of neurodegenerative conditions like AD and PD has led to the development novel therapeutics, a class of which will be discussed in the following section.
GLP-1R Agonists as Disease Modifying Agents in Neurodegenerative Diseases
In light of the prominent role neuroinflammation plays in AD and PD pathogenesis, treatments that target inflammatory pathways are increasingly sought after to curtail neurodegenerative processes. The incretin hormone glucagon-like peptide 1 (GLP-1) is best known for its role in regulating glucose homeostasis, weight, and satiety by acting through the GLP-1R. Agents that activate the GLP-1R, such as GLP-1R agonists and dipeptidyl peptidase 4 (DPP-IV) inhibitors, constitute popular classes of therapy for type 2 diabetes, which can cause retinopathy and is a risk factor for glaucoma [224–226]. In mice with diet-induced obesity and insulin resistance, treatment with the GLP-1R agonist liraglutide improved cognitive function and hippocampal synaptic plasticity [227]. In human patients, long-term treatment with the GLP-1R agonist dulaglutide was associated with moderate improvements on cognitive testing in patients with type 2 diabetes [228]. The most common side effect of GLP-1R agonists is gastrointestinal symptoms, which are often self-limiting and rarely results in discontinuation of therapy [229]. Additional benefits include weight-loss and long-term cardiovascular protection associated with decreased mortality in patients with diabetes [230–233].
GLP-1R activation enhances protein synthesis, cellular proliferation, and mitochondrial biogenesis, while inhibiting apoptosis, inflammation, and protein aggregation, jointly leading to improved cellular survival [234]. GLP-1R agonists readily cross the blood-brain barrier, and GLP-1R is expressed in neurons, microglia, and astrocytes of the CNS [235–240]. In the eye, GLP-1R expression has been localized to the GCL, INL, and ONL of the mouse retina [241, 242]. The specific retinal cell types mediating the effects of GLP-1R agonists in the eye, however, are not known.
Over a decade of preclinical studies have demonstrated the potential of GLP-1R agonists for improving neuronal survival and function in neurodegenerative diseases. GLP-1 reduced the level of Aβ in the brain of diabetic mice and decreased the level of amyloid precursor protein in cultured neuronal cells, while GLP-1 and the GLP-1R agonist exendin-4 protected cultured hippocampal neurons from Aβ or iron-induced death [243]. Exendin-4 has also been shown to reduced brain levels of Aβ and amyloid precursor proteins in mouse models of AD [244]. GLP-1R agonists Val(8)GLP-1 and liraglutide effectively prevented Aβ-induced deficits in spatial learning and memory in rats [245, 246]. Val(8)GLP-1 was also shown to improve both pathological and age-related synaptic degeneration in a mouse model of AD, and to enhance dentate gyrus neurogenesis in wild type mice [247, 248]. Liraglutide similarly improved memory, synaptogenesis, and neurogenesis in a mouse model of AD [249].
Randomized clinical trials assessing GLP-1R agonists in patients with AD demonstrated mixed results. In a double-blind pilot, 38 patients with AD were randomized to either liraglutide treatment or placebo [250]. While Aβ load was no different between groups after 6 months, liraglutide was shown to halt the decline of glucose metabolism on PET imaging [250]. The follow-up randomized, double-blinded phase IIb clinical trial of liraglutide versus placebo (the ELAD study), failed to show a treatment effect in its primary endpoint of cerebral glucose metabolism after 12 months [251, 252]. The study did find a positive difference in the secondary endpoint of a measure of cognitive function. A longer-duration clinical trial with a greater number of subjects per treatment group may be necessary to definitively assess for protective effects of GLP-1R agonists in AD.
In preclinical models of PD, exendin-4 increased the number of neural stem/progenitor cells isolated from the subventricular zone in vitro, normalized dopamine balance and increased the number of cells expressing markers of dopaminergic neurons following 6-hydroxydopamine injury in vivo [253]. Exendin-4 also arrested the progression of nigral lesions following 6-hydroxydopamine and LPS injections in rats [254]. Liraglutide and lixisenatide, both GLP-1R agonists with longer half-lives than exendin-4, improved measures of motor impairment following MPTP-induced neuro-injury [255]. In human PD, a small 45-patient single-blind trial evaluated the effect of exenatide, a synthetic version of exendin-4, versus controls and found improvements in off-medication motor and cognitive measures after 12 months of treatment [256]. Subsequently, a double-blind trial with 62 patients evaluated the effect of exenatide versus placebo in PD [257]. Following 12 months of treatment and a wash-out period of 3 months, patients given exenatide at a dose approved for diabetes treatment scored higher on the motor subscale of the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) compared to placebo. This study also showed that exenatide readily crossed the blood-brain barrier and was detectable in human cerebral spinal fluid at a concentration comparable to those reported in preclinical animal models.
Mechanistically speaking, evidence suggests that GLP-1R agonists slow the progression of neurodegeneration in AD and PD by inhibiting the release of pro-inflammatory cytokines, macrophage/microglia activation, and the formation of neurotoxic astrocytes [234]. Exenatide was shown to inhibit the streptozocin-induced increase in pro-inflammatory cytokine TNF-α in a rat model of AD [258]. In the MPTP-mouse model of PD, exendin-4 prevented microglia activation and expression of pro-inflammatory cytokines MMP-3, IL-1β and TNF-α in the substantia nigra and striatum, rescued dopaminergic neurons while maintaining dopamine levels, and improved motor function [259, 260]. In cultured dopaminergic neurons, GLP-1 and exendin-4 conferred neuroprotection following 6-hydroxydopamine administration in a GLP-1R-dependent manner [260]. Exendin-4 has also been shown to inhibit monocyte/macrophage adhesion to arterial walls prior to extravasation, mediated by a reduction in TNF-α expression [261]. Following exendin-4 treatment, expression of NF-κB, a key mediator of activated myeloid cells, was shown to be decreased in rats with high-fat diet-induced obesity [262]. Finally, liraglutide has been shown to reduce TNF-α production by monocytes, and halved microglia activation in a mouse model of AD [249, 263]
NLY01 is a pegylated form of exendin-4 with a long half-life in both non-human primates (88 hours) and mice (38 hours), where it penetrates the blood-brain barrier resulting in elevated concentrations in the CNS [264]. NLY01 has been shown to act on microglial GLP-1R to dampen neuroinflammatory signaling, thereby reducing dopaminergic cell death in a mouse model of PD [264]. Recently, NLY01 was also shown to block microglial-induced neurotoxic astrocyte transformation, resulting in preservation of spatial learning and memory in mouse models of AD [265]. Ongoing phase IIb trials are evaluating whether NLY01 treatment can manifest meaningful clinical benefits in patients with early, untreated PD and AD [266].
Following microbead-induced IOP elevation, our group showed that treatment with NLY01 reduced expression of IL-1α, TNF-α, and C1q by microglia/macrophages in the retina, decreased neurotoxic astrocyte activation, and rescued RGCs [75] (Figure 3). Experimentation by our group is underway to examine the mechanisms driving GLP-1R agonist-induced RGC rescue. In a separate retrospective database study, we showed that treatment with GLP-1R agonists halved the risk for a new diagnosis of glaucoma among diabetic patients [267]. A larger scale study is necessary to examine whether GLP-1R agonists alter the risk of glaucoma progression.
Figure 3. Glucagon-Like Peptide-1 Receptor (GLP-1R) Agonists Decrease Retinal Inflammation.
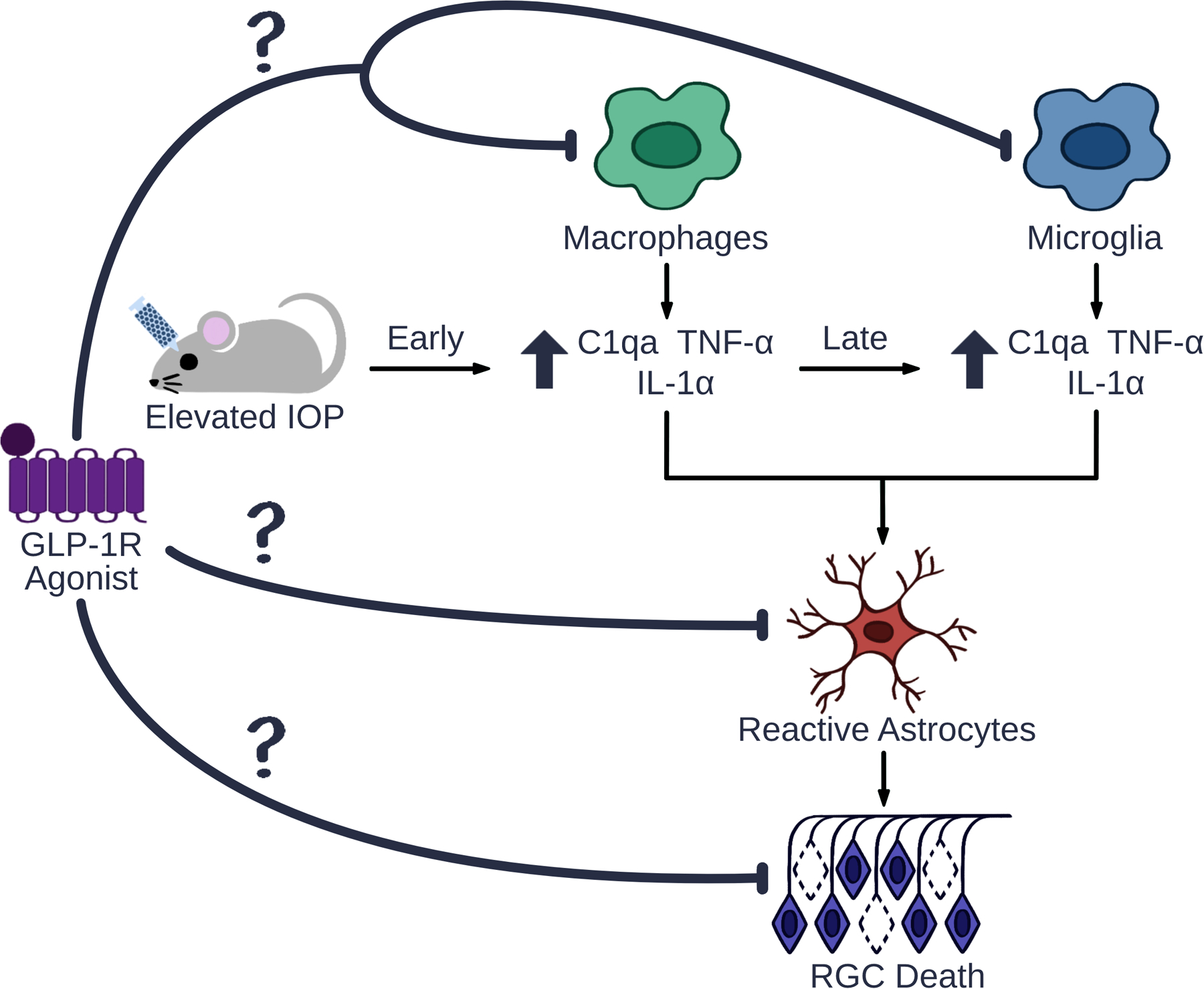
Elevated intraocular pressure (IOP) increases production of the pro-inflammatory cytokines interleukin-1α (IL-1α), tumor necrosis factor α (TNF-α), and complement component 1q (C1q) by activated microglia and recruited macrophages. These cytokines activate neurotoxic astrocytes, and results in retinal ganglion cell (RGC) death. Our lab has shown that the GLP-1R agonist NLY01 inhibits activation of microglia, macrophages, and astrocytes, and prevents RGC death in eyes with microbead-induced IOP elevation. However, the mechanism behind NLY01-induced RGC rescue, including the cell types mediating its protective effects as well as the relative contribution of macrophages versus microglia, has not been examined.
Conclusions
Underlying the dissection of ocular and neurological diseases in this review is the role of inflammation in chronic diseases affecting vision. Glaucoma, AMD, RP, posterior uveitis, AD, and PD exhibit similar inflammatory changes, marked by microglia and macrophage activation and migration. The leading cause of irreversible blindness worldwide, glaucoma, is associated with activated microglia and recruited macrophages [68, 69]. In AMD, pro-inflammatory cytokines produced by macrophages and subretinal microglia may worsen subretinal fibrosis [118, 132]. Inflammation in RP is mediated by microglia as opposed to macrophages; these microglia phagocytose both stressed and dying photoreceptors [119]. Experimental models of posterior uveitis have implicated microglia in the induction of an autoimmune response and the breakdown of the BRB [150, 161]. In AD, the presence of hypertrophic retinal microglia and perivascular macrophages implicate increased myeloid reactivity in pathogenesis [193, 194]. Activated microglia have also been shown to induce neurotoxic reactive astrocytes in AD and PD [109, 265]. Further work is needed to identify the mechanisms that can be pharmacologically modulated to tip the macrophage and microglia balance from a neurotoxic to a neuroprotective milieu in the retina. Recent findings suggest that one such novel strategy may involve GLP-1R agonists.
Interestingly, several common themes arise from the comparison of these diseases. Macrophage infiltration, either from a compromised BRB or from the ONH, which serves as a reservoir for myeloid cells, plays an important inciting role in ocular diseases such as glaucoma, AMD, and posterior uveitis [73, 74, 119, 120, 128, 132, 153–155, 160, 268]. Corroboratively, elevated levels of MCP-1, a major chemokine in macrophage recruitment, is associated with pathogenesis and disease progression in animal models of glaucoma, AMD, RP, and AD [72, 118, 127, 128, 140, 141, 147, 161, 196]. Further, upregulation of pro-inflammatory cytokines including TNF-α, the IL-1 family, and complement constitute a common pathway through which activated myeloid cells exert their downstream effects, possibly by promoting transformation of astrocytes into neurotoxic states.
The process of unpacking risk factors in these diseases has identified several pathways that can be targeted by selective therapeutics. In AMD, the 10q26 minor haplotype induced the overexpression of the serine peptidase HTRA1, which prevented the clearance of retinal monocytes in an osteopontin-dependent fashion. Inhibition of osteopontin reversed this effect and reduced retinal inflammation, suggesting that inhibition of monocyte activation and cytokine release may also provide a therapeutic benefit in AMD [137]. In AD, microglia exert a neuroprotective effect through Aβ plaque clearance, mediated by both TREM2-dependent and independent pathways [198–200]. In glaucoma, PD, and possibly AD, the pro-inflammatory milieu appears to be incited, in part, through the Fas receptor, TNF-α, the IL-1 family of cytokines, and complement activation [84–86, 89, 91, 92, 94, 98–100, 107, 215, 216, 219–223]. Inhibition of some of these pathways with GLP-1R agonists decreased the activation of neurotoxic astrocytes by microglia/macrophages, and rescues neurons in animal models of glaucoma, PD, and AD [75, 249, 258–265]. The efficacy of GLP-1R agonists as a disease modifying agent in these neurodegenerative diseases require validation in human studies, which are largely ongoing [75, 243–251, 253–259, 264, 266]. These clinical trials may help to identify effective treatments for multiple progressive neurodegenerative diseases and lessen the significant burden placed on patients, caregivers and health system resources.
Acknowledgements
We wish to thank Amy Chen for assistance with figure design and F. Chris Bennett and Jacob K. Sterling for fruitful discussions and support. The authors are supported by the Jeffrey W. Berger Memorial Medical Student Research Award (MG); National Institutes of Health/National Eye Institute grants K08EY029765 and K12EY015398, Glaucoma Research Foundation, and Neuraly, Inc. (QNC); National Institutes of Health/National Eye Institute grants R01EY015240 and R01EY028916 (JLD); Research to Prevent Blindness; the F. M. Kirby Foundation; and the Paul and Evanina Bell Mackall Foundation Trust, a gift in memory of Dr. Lee F. Mauger.
Abbreviations:
- AD
Alzheimer’s disease
- AMD
age-related macular degeneration
- BRB
blood-retina barrier
- C1q
complement component 1q
- CNS
central nervous system
- ERG
electroretinography
- GLP-1R
glucagon-like peptide-1 receptor
- IL
interleukin
- IOP
intraocular pressure
- MCP-1
macrophage chemoattractant protein-1
- NF-κB
nuclear factor-kappa B
- ONH
optic nerve head
- PD
Parkinson’s disease
- RGC
retinal ganglion cell
- RP
retinitis pigmentosa
- RPE
retinal pigment epithelium
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/FEBS.16177
Conflicts of Interest:
Michelle Guo – no disclosures related to this work
Turner D. Schwartz – no disclosures related to this work
Joshua L. Dunaief – no disclosures related to this work
Qi N. Cui – sponsored research agreement (Neuraly, Inc.)
References
- 1.Tamm ER, Braunger BM & Fuchshofer R (2015) Intraocular Pressure and the Mechanisms Involved in Resistance of the Aqueous Humor Flow in the Trabecular Meshwork Outflow Pathways, Prog Mol Biol Transl Sci. 134, 301–14. [DOI] [PubMed] [Google Scholar]
- 2.Eghrari AO, Riazuddin SA & Gottsch JD (2015) Overview of the Cornea: Structure, Function, and Development, Prog Mol Biol Transl Sci. 134, 7–23. [DOI] [PubMed] [Google Scholar]
- 3.Delaye M & Tardieu A (1983) Short-range order of crystallin proteins accounts for eye lens transparency, Nature. 302, 415–7. [DOI] [PubMed] [Google Scholar]
- 4.Koretz JF & Handelman GH (1988) How the human eye focuses, Sci Am. 259, 92–9. [DOI] [PubMed] [Google Scholar]
- 5.Grus FH, Joachim SC & Pfeiffer N (2007) Proteomics in ocular fluids, Proteomics Clin Appl. 1, 876–88. [DOI] [PubMed] [Google Scholar]
- 6.Berman E (1991) Vitreous in Biochemistry of the Eye pp. 291–307, Perspectives in Vision Research, Boston, MA. [Google Scholar]
- 7.Grossniklaus HE, Geisert EE & Nickerson JM (2015) Introduction to the Retina, Prog Mol Biol Transl Sci. 134, 383–96. [DOI] [PubMed] [Google Scholar]
- 8.Willoughby CE, Ponzin D, Ferrari S, Lobo A, Landau K & Omidi Y (2010) Anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function - a review, Clinical and Experimental Ophthalmology. 38, 2–11.20447093 [Google Scholar]
- 9.Gupta D & Chen PP (2016) Glaucoma, Am Fam Physician. 93, 668–74. [PubMed] [Google Scholar]
- 10.Weinreb RN, Aung T & Medeiros FA (2014) The pathophysiology and treatment of glaucoma: a review, JAMA. 311, 1901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez M, Rodriguez FD, Sharma SC & Vecino E (2009) Immunohistochemical changes in rat retinas at various time periods of elevated intraocular pressure, Mol Vis. 15, 2696–709. [PMC free article] [PubMed] [Google Scholar]
- 12.Von Thun Und Hohenstein-Blaul N, Kunst S, Pfeiffer N & Grus FH (2017) Biomarkers for glaucoma: from the lab to the clinic, Eye (Lond). 31, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vecino E, Rodriguez FD, Ruzafa N, Pereiro X & Sharma SC (2016) Glia-neuron interactions in the mammalian retina, Prog Retin Eye Res. 51, 1–40. [DOI] [PubMed] [Google Scholar]
- 14.Hosoya K & Tomi M (2005) Advances in the cell biology of transport via the inner blood-retinal barrier: establishment of cell lines and transport functions, Biol Pharm Bull. 28, 1–8. [DOI] [PubMed] [Google Scholar]
- 15.R G & Balasubramanian L (2018) Macula segmentation and fovea localization employing image processing and heuristic based clustering for automated retinal screening, Comput Methods Programs Biomed. 160, 153–163. [DOI] [PubMed] [Google Scholar]
- 16.Awh C (2020) Basic histology of the eye and accessory structures in EyeWiki [Google Scholar]
- 17.Oikawa K, Ver Hoeve JN, Teixeira LBC, Snyder KC, Kiland JA, Ellinwood NM & McLellan GJ (2020) Sub-region-Specific Optic Nerve Head Glial Activation in Glaucoma, Mol Neurobiol. 57, 2620–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volland S, Esteve-Rudd J, Hoo J, Yee C & Williams DS (2015) A comparison of some organizational characteristics of the mouse central retina and the human macula, PLoS One. 10, e0125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadal-Nicolas FM, Kunze VP, Ball JM, Peng BT, Krishnan A, Zhou G, Dong L & Li W (2020) True S-cones are concentrated in the ventral mouse retina and wired for color detection in the upper visual field, Elife. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathans J, Thomas D & Hogness DS (1986) Molecular genetics of human color vision: the genes encoding blue, green, and red pigments, Science. 232, 193–202. [DOI] [PubMed] [Google Scholar]
- 21.Masland RH (2001) The fundamental plan of the retina, Nat Neurosci. 4, 877–86. [DOI] [PubMed] [Google Scholar]
- 22.Newman E & Reichenbach A (1996) The Muller cell: a functional element of the retina, Trends Neurosci. 19, 307–12. [DOI] [PubMed] [Google Scholar]
- 23.Zeng HL & Shi JM (2018) The role of microglia in the progression of glaucomatous neurodegeneration- a review, Int J Ophthalmol. 11, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okunuki Y, Mukai R, Pearsall EA, Klokman G, Husain D, Park DH, Korobkina E, Weiner HL, Butovsky O, Ksander BR, Miller JW & Connor KM (2018) Microglia inhibit photoreceptor cell death and regulate immune cell infiltration in response to retinal detachment, Proc Natl Acad Sci U S A. 115, E6264–E6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rathnasamy G, Foulds WS, Ling EA & Kaur C (2019) Retinal microglia - A key player in healthy and diseased retina, Prog Neurobiol. 173, 18–40. [DOI] [PubMed] [Google Scholar]
- 26.O’Koren EG, Yu C, Klingeborn M, Wong AYW, Prigge CL, Mathew R, Kalnitsky J, Msallam RA, Silvin A, Kay JN, Bowes Rickman C, Arshavsky VY, Ginhoux F, Merad M & Saban DR (2019) Microglial Function Is Distinct in Different Anatomical Locations during Retinal Homeostasis and Degeneration, Immunity. 50, 723–737 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris DR, Bounds SE, Liu H, Ding WQ, Chen Y, Liu Y & Cai J (2020) Exosomal MiRNA Transfer between Retinal Microglia and RPE, Int J Mol Sci. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, Sierra A, Molnar Z, Cragg MS, Garaschuk O, Perry VH & Gomez-Nicola D (2017) Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain, Cell Rep. 18, 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman SM & Wong WT (2018) Microglia in the Retina: Roles in Development, Maturity, and Disease, Annu Rev Vis Sci. 4, 45–77. [DOI] [PubMed] [Google Scholar]
- 30.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW & Barres BA (2007) The classical complement cascade mediates CNS synapse elimination, Cell. 131, 1164–78. [DOI] [PubMed] [Google Scholar]
- 31.Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, Schwab Y & Gross CT (2018) Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction, Nat Commun. 9, 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomes C, Ferreira R, George J, Sanches R, Rodrigues DI, Goncalves N & Cunha RA (2013) Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia, J Neuroinflammation. 10, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, Hempstead BL, Littman DR & Gan WB (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor, Cell. 155, 1596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz-Aparicio I, Paris I, Sierra-Torre V, Plaza-Zabala A, Rodriguez-Iglesias N, Marquez-Ropero M, Beccari S, Huguet P, Abiega O, Alberdi E, Matute C, Bernales I, Schulz A, Otrokocsi L, Sperlagh B, Happonen KE, Lemke G, Maletic-Savatic M, Valero J & Sierra A (2020) Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome, J Neurosci. 40, 1453–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, Happonen KE, Burrola PG, O’Connor C, Hah N, Huang L, Nimmerjahn A & Lemke G (2021) Microglia use TAM receptors to detect and engulf amyloid beta plaques, Nat Immunol. 22, 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nimmerjahn A, Kirchhoff F & Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo, Science. 308, 1314–8. [DOI] [PubMed] [Google Scholar]
- 37.Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM & Langmann T (2015) Retinal microglia: just bystander or target for therapy?, Prog Retin Eye Res. 45, 30–57. [DOI] [PubMed] [Google Scholar]
- 38.Wakselman S, Bechade C, Roumier A, Bernard D, Triller A & Bessis A (2008) Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor, J Neurosci. 28, 8138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown GC & Neher JJ (2014) Microglial phagocytosis of live neurons, Nat Rev Neurosci. 15, 209–16. [DOI] [PubMed] [Google Scholar]
- 40.Zhao L, Zabel MK, Wang X, Ma W, Shah P, Fariss RN, Qian H, Parkhurst CN, Gan WB & Wong WT (2015) Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration, EMBO Mol Med. 7, 1179–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ & Mann DL (2014) Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation, Immunity. 40, 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M & Ginhoux F (2015) C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages, Immunity. 42, 665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheng J, Ruedl C & Karjalainen K (2015) Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells, Immunity. 43, 382–93. [DOI] [PubMed] [Google Scholar]
- 44.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM & Merad M (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages, Science. 330, 841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guilliams M & Svedberg FR (2021) Does tissue imprinting restrict macrophage plasticity?, Nat Immunol. 22, 118–127. [DOI] [PubMed] [Google Scholar]
- 46.Wieghofer P, Hagemeyer N, Sankowski R, Schlecht A, Staszewski O, Amann L, Gruber M, Koch J, Hausmann A, Zhang P, Boneva S, Masuda T, Hilgendorf I, Goldmann T, Bottcher C, Priller J, Rossi FM, Lange C & Prinz M (2021) Mapping the origin and fate of myeloid cells in distinct compartments of the eye by single-cell profiling, EMBO J. 40, e105123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hume DA, Irvine KM & Pridans C (2019) The Mononuclear Phagocyte System: The Relationship between Monocytes and Macrophages, Trends Immunol. 40, 98–112. [DOI] [PubMed] [Google Scholar]
- 48.Guilliams M, Mildner A & Yona S (2018) Developmental and Functional Heterogeneity of Monocytes, Immunity. 49, 595–613. [DOI] [PubMed] [Google Scholar]
- 49.Ajami B, Bennett JL, Krieger C, Tetzlaff W & Rossi FM (2007) Local self-renewal can sustain CNS microglia maintenance and function throughout adult life, Nat Neurosci. 10, 1538–43. [DOI] [PubMed] [Google Scholar]
- 50.Lapenna A, De Palma M & Lewis CE (2018) Perivascular macrophages in health and disease, Nat Rev Immunol. 18, 689–702. [DOI] [PubMed] [Google Scholar]
- 51.Bennett FC, Bennett ML, Yaqoob F, Mulinyawe SB, Grant GA, Hayden Gephart M, Plowey ED & Barres BA (2018) A Combination of Ontogeny and CNS Environment Establishes Microglial Identity, Neuron. 98, 1170–1183 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruttger J, Karram K, Wortge S, Regen T, Marini F, Hoppmann N, Klein M, Blank T, Yona S, Wolf Y, Mack M, Pinteaux E, Muller W, Zipp F, Binder H, Bopp T, Prinz M, Jung S & Waisman A (2015) Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System, Immunity. 43, 92–106. [DOI] [PubMed] [Google Scholar]
- 53.Lund H, Pieber M, Parsa R, Han J, Grommisch D, Ewing E, Kular L, Needhamsen M, Espinosa A, Nilsson E, Overby AK, Butovsky O, Jagodic M, Zhang XM & Harris RA (2018) Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells, Nat Commun. 9, 4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Z, Gu Y, Chakarov S, Bleriot C, Kwok I, Chen X, Shin A, Huang W, Dress RJ, Dutertre CA, Schlitzer A, Chen J, Ng LG, Wang H, Liu Z, Su B & Ginhoux F (2019) Fate Mapping via Ms4a3-Expression History Traces Monocyte-Derived Cells, Cell. 178, 1509–1525 e19. [DOI] [PubMed] [Google Scholar]
- 55.Werner Y, Mass E, Ashok Kumar P, Ulas T, Handler K, Horne A, Klee K, Lupp A, Schutz D, Saaber F, Redecker C, Schultze JL, Geissmann F & Stumm R (2020) Cxcr4 distinguishes HSC-derived monocytes from microglia and reveals monocyte immune responses to experimental stroke, Nat Neurosci. 23, 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F & Rodewald HR (2015) Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors, Nature. 518, 547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Italiani P & Boraschi D (2014) From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation, Front Immunol. 5, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mills CD, Kincaid K, Alt JM, Heilman MJ & Hill AM (2000) M-1/M-2 macrophages and the Th1/Th2 paradigm, J Immunol. 164, 6166–73. [DOI] [PubMed] [Google Scholar]
- 59.Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist?, Nat Neurosci. 19, 987–91. [DOI] [PubMed] [Google Scholar]
- 60.Nahrendorf M & Swirski FK (2016) Abandoning M1/M2 for a Network Model of Macrophage Function, Circ Res. 119, 414–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinez FO & Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment, F1000Prime Rep. 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miao H, Li R, Han C, Lu X & Zhang H (2018) Minocycline promotes posthemorrhagic neurogenesis via M2 microglia polarization via upregulation of the TrkB/BDNF pathway in rats, J Neurophysiol. 120, 1307–1317. [DOI] [PubMed] [Google Scholar]
- 63.Chen M & Xu H (2015) Parainflammation, chronic inflammation, and age-related macular degeneration, J Leukoc Biol. 98, 713–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tham YC, Li X, Wong TY, Quigley HA, Aung T & Cheng CY (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis, Ophthalmology. 121, 2081–90. [DOI] [PubMed] [Google Scholar]
- 65.Quigley HA (2011) Glaucoma, Lancet. 377, 1367–77. [DOI] [PubMed] [Google Scholar]
- 66.Boland MV & Quigley HA (2007) Risk factors and open-angle glaucoma: classification and application, J Glaucoma. 16, 406–18. [DOI] [PubMed] [Google Scholar]
- 67.Coleman AL & Miglior S (2008) Risk factors for glaucoma onset and progression, Surv Ophthalmol. 53 Suppl1, S3–10. [DOI] [PubMed] [Google Scholar]
- 68.Neufeld AH (1999) Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma, Arch Ophthalmol. 117, 1050–6. [DOI] [PubMed] [Google Scholar]
- 69.Margeta MA, Lad EM & Proia AD (2018) CD163+ macrophages infiltrate axon bundles of postmortem optic nerves with glaucoma, Graefes Arch Clin Exp Ophthalmol. 256, 2449–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee NY, Park HY, Park CK & Ahn MD (2012) Analysis of systemic endothelin-1, matrix metalloproteinase-9, macrophage chemoattractant protein-1, and high-sensitivity C-reactive protein in normal-tension glaucoma, Curr Eye Res. 37, 1121–6. [DOI] [PubMed] [Google Scholar]
- 71.Gschwandtner M, Derler R & Midwood KS (2019) More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis, Front Immunol. 10, 2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee NY, Kim MH & Park CK (2017) Visual Field Progression is Associated with Systemic Concentration of Macrophage Chemoattractant Protein-1 in Normal-Tension Glaucoma, Curr Eye Res. 42, 1002–1006. [DOI] [PubMed] [Google Scholar]
- 73.Williams PA, Braine CE, Kizhatil K, Foxworth NE, Tolman NG, Harder JM, Scott RA, Sousa GL, Panitch A, Howell GR & John SWM (2019) Inhibition of monocyte-like cell extravasation protects from neurodegeneration in DBA/2J glaucoma, Mol Neurodegener. 14, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heuss ND, Pierson MJ, Roehrich H, McPherson SW, Gram AL, Li L & Gregerson DS (2018) Optic nerve as a source of activated retinal microglia post-injury, Acta Neuropathol Commun. 6, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sterling JK, Adetunji MO, Guttha S, Bargoud AR, Uyhazi KE, Ross AG, Dunaief JL & Cui QN (2020) GLP-1 Receptor Agonist NLY01 Reduces Retinal Inflammation and Neuron Death Secondary to Ocular Hypertension, Cell Rep. 33, 108271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rojas B, Gallego BI, Ramirez AI, Salazar JJ, de Hoz R, Valiente-Soriano FJ, Aviles-Trigueros M, Villegas-Perez MP, Vidal-Sanz M, Trivino A & Ramirez JM (2014) Microglia in mouse retina contralateral to experimental glaucoma exhibit multiple signs of activation in all retinal layers, J Neuroinflammation. 11, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gramlich OW, Teister J, Neumann M, Tao X, Beck S, von Pein HD, Pfeiffer N & Grus FH (2016) Immune response after intermittent minimally invasive intraocular pressure elevations in an experimental animal model of glaucoma, J Neuroinflammation. 13, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tribble JR, Kokkali E, Otmani A, Plastino F, Lardner E, Vohra R, Kolko M, Andre H, Morgan JE & Williams PA (2021) When Is a Control Not a Control? Reactive Microglia Occur Throughout the Control Contralateral Pathway of Retinal Ganglion Cell Projections in Experimental Glaucoma, Transl Vis Sci Technol. 10, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tribble JR, Harder JM, Williams PA & John SWM (2020) Ocular hypertension suppresses homeostatic gene expression in optic nerve head microglia of DBA/2 J mice, Mol Brain. 13, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan X, Tezel G, Wax MB & Edward DP (2000) Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head, Arch Ophthalmol. 118, 666–73. [DOI] [PubMed] [Google Scholar]
- 81.Tezel G, Li LY, Patil RV & Wax MB (2001) TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes, Invest Ophthalmol Vis Sci. 42, 1787–94. [PubMed] [Google Scholar]
- 82.Sawada H, Fukuchi T, Tanaka T & Abe H (2010) Tumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucoma, Invest Ophthalmol Vis Sci. 51, 903–6. [DOI] [PubMed] [Google Scholar]
- 83.Peng C, Wu Y, Ding X, Chen D, Zeng C, Xu L & Guo W (2020) Characteristic Cytokine Profiles of Aqueous Humor in Glaucoma Secondary to Sturge-Weber Syndrome, Front Immunol. 11, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cueva Vargas JL, Osswald IK, Unsain N, Aurousseau MR, Barker PA, Bowie D & Di Polo A (2015) Soluble Tumor Necrosis Factor Alpha Promotes Retinal Ganglion Cell Death in Glaucoma via Calcium-Permeable AMPA Receptor Activation, J Neurosci. 35, 12088–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW & Benowitz LI (2006) Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma, J Neurosci. 26, 12633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paschalis EI, Lei F, Zhou C, Kapoulea V, Thanos A, Dana R, Vavvas DG, Chodosh J & Dohlman CH (2018) The Role of Microglia and Peripheral Monocytes in Retinal Damage after Corneal Chemical Injury, Am J Pathol. 188, 1580–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nanda S & Bathon JM (2004) Etanercept: a clinical review of current and emerging indications, Expert Opin Pharmacother. 5, 1175–86. [DOI] [PubMed] [Google Scholar]
- 88.Ramiro S, Radner H, van der Heijde D, van Tubergen A, Buchbinder R, Aletaha D & Landewe RB (2011) Combination therapy for pain management in inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, other spondyloarthritis), Cochrane Database Syst Rev, CD008886. [DOI] [PubMed] [Google Scholar]
- 89.Roh M, Zhang Y, Murakami Y, Thanos A, Lee SC, Vavvas DG, Benowitz LI & Miller JW (2012) Etanercept, a widely used inhibitor of tumor necrosis factor-alpha (TNF-alpha), prevents retinal ganglion cell loss in a rat model of glaucoma, PLoS One. 7, e40065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee SJ, Zhou T, Choi C, Wang Z & Benveniste EN (2000) Differential regulation and function of Fas expression on glial cells, J Immunol. 164, 1277–85. [DOI] [PubMed] [Google Scholar]
- 91.Krishnan A, Kocab AJ, Zacks DN, Marshak-Rothstein A & Gregory-Ksander M (2019) A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma, J Neuroinflammation. 16, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krishnan A, Fei F, Jones A, Busto P, Marshak-Rothstein A, Ksander BR & Gregory-Ksander M (2016) Overexpression of Soluble Fas Ligand following Adeno-Associated Virus Gene Therapy Prevents Retinal Ganglion Cell Death in Chronic and Acute Murine Models of Glaucoma, J Immunol. 197, 4626–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garcia-Bermudez MY, Freude KK, Mouhammad ZA, van Wijngaarden P, Martin KK & Kolko M (2021) Glial Cells in Glaucoma: Friends, Foes, and Potential Therapeutic Targets, Front Neurol. 12, 624983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams PA, Tribble JR, Pepper KW, Cross SD, Morgan BP, Morgan JE, John SW & Howell GR (2016) Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma, Mol Neurodegener. 11, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reinehr S, Gomes SC, Gassel CJ, Asaad MA, Stute G, Schargus M, Dick HB & Joachim SC (2019) Intravitreal Therapy Against the Complement Factor C5 Prevents Retinal Degeneration in an Experimental Autoimmune Glaucoma Model, Front Pharmacol. 10, 1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borucki DM, Toutonji A, Couch C, Mallah K, Rohrer B & Tomlinson S (2020) Complement-Mediated Microglial Phagocytosis and Pathological Changes in the Development and Degeneration of the Visual System, Front Immunol. 11, 566892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harder JM, Williams PA, Braine CE, Yang HS, Thomas JM, Foxworth NE, John SWM & Howell GR (2020) Complement peptide C3a receptor 1 promotes optic nerve degeneration in DBA/2J mice, J Neuroinflammation. 17, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Howell GR, MacNicoll KH, Braine CE, Soto I, Macalinao DG, Sousa GL & John SW (2014) Combinatorial targeting of early pathways profoundly inhibits neurodegeneration in a mouse model of glaucoma, Neurobiol Dis. 71, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stasi K, Nagel D, Yang X, Wang RF, Ren L, Podos SM, Mittag T & Danias J (2006) Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes, Invest Ophthalmol Vis Sci. 47, 1024–9. [DOI] [PubMed] [Google Scholar]
- 100.Lambert WS, Carlson BJ, Ghose P, Vest VD, Yao V & Calkins DJ (2019) Towards A Microbead Occlusion Model of Glaucoma for a Non-Human Primate, Sci Rep. 9, 11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wooff Y, Man SM, Aggio-Bruce R, Natoli R & Fernando N (2019) IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases, Front Immunol. 10, 1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eandi CM, Charles Messance H, Augustin S, Dominguez E, Lavalette S, Forster V, Hu SJ, Siquieros L, Craft CM, Sahel JA, Tadayoni R, Paques M, Guillonneau X & Sennlaub F (2016) Subretinal mononuclear phagocytes induce cone segment loss via IL-1beta, Elife. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao M, Bai Y, Xie W, Shi X, Li F, Yang F, Sun Y, Huang L & Li X (2015) Interleukin-1beta Level Is Increased in Vitreous of Patients with Neovascular Age-Related Macular Degeneration (nAMD) and Polypoidal Choroidal Vasculopathy (PCV), PLoS One. 10, e0125150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu SJ, Calippe B, Lavalette S, Roubeix C, Montassar F, Housset M, Levy O, Delarasse C, Paques M, Sahel JA, Sennlaub F & Guillonneau X (2015) Upregulation of P2RX7 in Cx3cr1-Deficient Mononuclear Phagocytes Leads to Increased Interleukin-1beta Secretion and Photoreceptor Neurodegeneration, J Neurosci. 35, 6987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chi W, Li F, Chen H, Wang Y, Zhu Y, Yang X, Zhu J, Wu F, Ouyang H, Ge J, Weinreb RN, Zhang K & Zhuo Y (2014) Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1beta production in acute glaucoma, Proc Natl Acad Sci U S A. 111, 11181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Markiewicz L, Pytel D, Mucha B, Szymanek K, Szaflik J, Szaflik JP & Majsterek I (2015) Altered Expression Levels of MMP1, MMP9, MMP12, TIMP1, and IL-1beta as a Risk Factor for the Elevated IOP and Optic Nerve Head Damage in the Primary Open-Angle Glaucoma Patients, Biomed Res Int. 2015, 812503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang N, Chintala SK, Fini ME & Schuman JS (2001) Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype, Nat Med. 7, 304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang X, Zeng Q, Baris M & Tezel G (2020) Transgenic inhibition of astroglial NF-kappaB restrains the neuroinflammatory and neurodegenerative outcomes of experimental mouse glaucoma, J Neuroinflammation. 17, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B & Barres BA (2017) Neurotoxic reactive astrocytes are induced by activated microglia, Nature. 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guttenplan KA, Stafford BK, El-Danaf RN, Adler DI, Munch AE, Weigel MK, Huberman AD & Liddelow SA (2020) Neurotoxic Reactive Astrocytes Drive Neuronal Death after Retinal Injury, Cell Rep. 31, 107776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Norris GT, Smirnov I, Filiano AJ, Shadowen HM, Cody KR, Thompson JA, Harris TH, Gaultier A, Overall CC & Kipnis J (2018) Neuronal integrity and complement control synaptic material clearance by microglia after CNS injury, J Exp Med. 215, 1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ambati J & Fowler BJ (2012) Mechanisms of age-related macular degeneration, Neuron. 75, 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY & Wong TY (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis, Lancet Glob Health. 2, e106–16. [DOI] [PubMed] [Google Scholar]
- 114.Spaide RF & Curcio CA (2010) Drusen characterization with multimodal imaging, Retina. 30, 1441–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen L, Messinger JD, Sloan KR, Wong J, Roorda A, Duncan JL & Curcio CA (2020) ABUNDANCE AND MULTIMODAL VISIBILITY OF SOFT DRUSEN IN EARLY AGE-RELATED MACULAR DEGENERATION: A Clinicopathologic Correlation, Retina. 40, 1644–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakamura R, Sene A, Santeford A, Gdoura A, Kubota S, Zapata N & Apte RS (2015) IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis, Nat Commun. 6, 7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chichagova V, Hallam D, Collin J, Zerti D, Dorgau B, Felemban M, Lako M & Steel DH (2018) Cellular regeneration strategies for macular degeneration: past, present and future, Eye (Lond). 32, 946–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guillonneau X, Eandi CM, Paques M, Sahel JA, Sapieha P & Sennlaub F (2017) On phagocytes and macular degeneration, Prog Retin Eye Res. 61, 98–128. [DOI] [PubMed] [Google Scholar]
- 119.Gupta N, Brown KE & Milam AH (2003) Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration, Exp Eye Res. 76, 463–71. [DOI] [PubMed] [Google Scholar]
- 120.Wynn TA, Chawla A & Pollard JW (2013) Macrophage biology in development, homeostasis and disease, Nature. 496, 445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scholz R, Sobotka M, Caramoy A, Stempfl T, Moehle C & Langmann T (2015) Minocycline counter-regulates pro-inflammatory microglia responses in the retina and protects from degeneration, J Neuroinflammation. 12, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Madeira MH, Rashid K, Ambrosio AF, Santiago AR & Langmann T (2018) Blockade of microglial adenosine A2A receptor impacts inflammatory mechanisms, reduces ARPE-19 cell dysfunction and prevents photoreceptor loss in vitro, Sci Rep. 8, 2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wiedemann J, Rashid K & Langmann T (2018) Resveratrol induces dynamic changes to the microglia transcriptome, inhibiting inflammatory pathways and protecting against microglia-mediated photoreceptor apoptosis, Biochem Biophys Res Commun. 501, 239–245. [DOI] [PubMed] [Google Scholar]
- 124.Zhang M, Xu G, Liu W, Ni Y & Zhou W (2012) Role of fractalkine/CX3CR1 interaction in light-induced photoreceptor degeneration through regulating retinal microglial activation and migration, PLoS One. 7, e35446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Song D, Sulewski ME Jr., Wang C, Song J, Bhuyan R, Sterling J, Clark E, Song WC & Dunaief JL (2017) Complement C5a receptor knockout has diminished light-induced microglia/macrophage retinal migration, Mol Vis. 23, 210–218. [PMC free article] [PubMed] [Google Scholar]
- 126.Daftarian N, Zandi S, Piryaie G, Nikougoftar Zarif M, Ranaei Pirmardan E, Yamaguchi M, Behzadian Nejad Q, Hasanpour H, Samiei S, Pfister IB, Soheili ZS, Nakao S, Barakat A, Garweg JG, Ahmadieh H & Hafezi-Moghadam A (2020) Peripheral blood CD163(+) monocytes and soluble CD163 in dry and neovascular age-related macular degeneration, FASEB J. 34, 8001–8011. [DOI] [PubMed] [Google Scholar]
- 127.Zor RK, Ersan S, Kucuk E, Yildirim G & Sari I (2020) Serum malondialdehyde, monocyte chemoattractant protein-1, and vitamin C levels in wet type age-related macular degeneration patients, Ther Adv Ophthalmol. 12, 2515841420951682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rutar M, Natoli R & Provis JM (2012) Small interfering RNA-mediated suppression of Ccl2 in Muller cells attenuates microglial recruitment and photoreceptor death following retinal degeneration, J Neuroinflammation. 9, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Seddon JM, Gensler G, Milton RC, Klein ML & Rifai N (2004) Association between C-reactive protein and age-related macular degeneration, JAMA. 291, 704–10. [DOI] [PubMed] [Google Scholar]
- 130.Seddon JM, George S, Rosner B & Rifai N (2005) Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers, Arch Ophthalmol. 123, 774–82. [DOI] [PubMed] [Google Scholar]
- 131.Daniel E, Toth CA, Grunwald JE, Jaffe GJ, Martin DF, Fine SL, Huang J, Ying GS, Hagstrom SA, Winter K, Maguire MG & Comparison of Age-related Macular Degeneration Treatments Trials Research, G. (2014) Risk of scar in the comparison of age-related macular degeneration treatments trials, Ophthalmology. 121, 656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Little K, Llorian-Salvador M, Tang M, Du X, Marry S, Chen M & Xu H (2020) Macrophage to myofibroblast transition contributes to subretinal fibrosis secondary to neovascular age-related macular degeneration, J Neuroinflammation. 17, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fritsche LG, Igl W, Bailey JN, Grassmann F, Sengupta S, Bragg-Gresham JL, Burdon KP, Hebbring SJ, Wen C, Gorski M, Kim IK, Cho D, Zack D, Souied E, Scholl HP, Bala E, Lee KE, Hunter DJ, Sardell RJ, Mitchell P, Merriam JE, Cipriani V, Hoffman JD, Schick T, Lechanteur YT, Guymer RH, Johnson MP, Jiang Y, Stanton CM, Buitendijk GH, Zhan X, Kwong AM, Boleda A, Brooks M, Gieser L, Ratnapriya R, Branham KE, Foerster JR, Heckenlively JR, Othman MI, Vote BJ, Liang HH, Souzeau E, McAllister IL, Isaacs T, Hall J, Lake S, Mackey DA, Constable IJ, Craig JE, Kitchner TE, Yang Z, Su Z, Luo H, Chen D, Ouyang H, Flagg K, Lin D, Mao G, Ferreyra H, Stark K, von Strachwitz CN, Wolf A, Brandl C, Rudolph G, Olden M, Morrison MA, Morgan DJ, Schu M, Ahn J, Silvestri G, Tsironi EE, Park KH, Farrer LA, Orlin A, Brucker A, Li M, Curcio CA, Mohand-Said S, Sahel JA, Audo I, Benchaboune M, Cree AJ, Rennie CA, Goverdhan SV, Grunin M, Hagbi-Levi S, Campochiaro P, Katsanis N, Holz FG, Blond F, Blanche H, Deleuze JF, Igo RP Jr., Truitt B, Peachey NS, Meuer SM, Myers CE, Moore EL, Klein R, et al. (2016) A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants, Nat Genet. 48, 134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen W, Xu W, Tao Q, Liu J, Li X, Gan X, Hu H & Lu Y (2009) Meta-analysis of the association of the HTRA1 polymorphisms with the risk of age-related macular degeneration, Exp Eye Res. 89, 292–300. [DOI] [PubMed] [Google Scholar]
- 135.Rivera A, Fisher SA, Fritsche LG, Keilhauer CN, Lichtner P, Meitinger T & Weber BH (2005) Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk, Hum Mol Genet. 14, 3227–36. [DOI] [PubMed] [Google Scholar]
- 136.Liao SM, Zheng W, Zhu J, Lewis CA, Delgado O, Crowley MA, Buchanan NM, Jaffee BD & Dryja TP (2017) Specific correlation between the major chromosome 10q26 haplotype conferring risk for age-related macular degeneration and the expression of HTRA1, Mol Vis. 23, 318–333. [PMC free article] [PubMed] [Google Scholar]
- 137.Beguier F, Housset M, Roubeix C, Augustin S, Zagar Y, Nous C, Mathis T, Eandi C, Benchaboune M, Drame-Maigne A, Carpentier W, Chardonnet S, Touhami S, Blot G, Conart JB, Charles-Messance H, Potey A, Girmens JF, Paques M, Blond F, Leveillard T, Koertvely E, Roger JE, Sahel JA, Sapieha P, Delarasse C, Guillonneau X & Sennlaub F (2020) The 10q26 Risk Haplotype of Age-Related Macular Degeneration Aggravates Subretinal Inflammation by Impairing Monocyte Elimination, Immunity. 53, 429–441 e8. [DOI] [PubMed] [Google Scholar]
- 138.Calippe B, Augustin S, Beguier F, Charles-Messance H, Poupel L, Conart JB, Hu SJ, Lavalette S, Fauvet A, Rayes J, Levy O, Raoul W, Fitting C, Denefle T, Pickering MC, Harris C, Jorieux S, Sullivan PM, Sahel JA, Karoyan P, Sapieha P, Guillonneau X, Gautier EL & Sennlaub F (2017) Complement Factor H Inhibits CD47-Mediated Resolution of Inflammation, Immunity. 46, 261–272. [DOI] [PubMed] [Google Scholar]
- 139.Cuenca N, Fernandez-Sanchez L, Campello L, Maneu V, De la Villa P, Lax P & Pinilla I (2014) Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases, Prog Retin Eye Res. 43, 17–75. [DOI] [PubMed] [Google Scholar]
- 140.Yoshida N, Ikeda Y, Notomi S, Ishikawa K, Murakami Y, Hisatomi T, Enaida H & Ishibashi T (2013) Clinical evidence of sustained chronic inflammatory reaction in retinitis pigmentosa, Ophthalmology. 120, 100–5. [DOI] [PubMed] [Google Scholar]
- 141.Yoshida N, Ikeda Y, Notomi S, Ishikawa K, Murakami Y, Hisatomi T, Enaida H & Ishibashi T (2013) Laboratory evidence of sustained chronic inflammatory reaction in retinitis pigmentosa, Ophthalmology. 120, e5–12. [DOI] [PubMed] [Google Scholar]
- 142.Lee SY, Usui S, Zafar AB, Oveson BC, Jo YJ, Lu L, Masoudi S & Campochiaro PA (2011) N-Acetylcysteine promotes long-term survival of cones in a model of retinitis pigmentosa, J Cell Physiol. 226, 1843–9. [DOI] [PubMed] [Google Scholar]
- 143.Campochiaro PA, Iftikhar M, Hafiz G, Akhlaq A, Tsai G, Wehling D, Lu L, Wall GM, Singh MS & Kong X (2020) Oral N-acetylcysteine improves cone function in retinitis pigmentosa patients in phase I trial, J Clin Invest. 130, 1527–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang S, Li CZ, Yan H, Qiu W, Chen YG, Wang PH, Weng SP & He JG (2012) Identification and function of myeloid differentiation factor 88 (MyD88) in Litopenaeus vannamei, PLoS One. 7, e47038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Garces K, Carmy T, Illiano P, Brambilla R & Hackam AS (2020) Increased Neuroprotective Microglia and Photoreceptor Survival in the Retina from a Peptide Inhibitor of Myeloid Differentiation Factor 88 (MyD88), J Mol Neurosci. 70, 968–980. [DOI] [PubMed] [Google Scholar]
- 146.Wang X, Zhao L, Zhang Y, Ma W, Gonzalez SR, Fan J, Kretschmer F, Badea TC, Qian HH & Wong WT (2017) Tamoxifen Provides Structural and Functional Rescue in Murine Models of Photoreceptor Degeneration, J Neurosci. 37, 3294–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guo C, Otani A, Oishi A, Kojima H, Makiyama Y, Nakagawa S & Yoshimura N (2012) Knockout of ccr2 alleviates photoreceptor cell death in a model of retinitis pigmentosa, Exp Eye Res. 104, 39–47. [DOI] [PubMed] [Google Scholar]
- 148.Wang X, Zhao L, Zhang J, Fariss RN, Ma W, Kretschmer F, Wang M, Qian HH, Badea TC, Diamond JS, Gan WB, Roger JE & Wong WT (2016) Requirement for Microglia for the Maintenance of Synaptic Function and Integrity in the Mature Retina, J Neurosci. 36, 2827–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jabs DA, Nussenblatt RB, Rosenbaum JT & Standardization of Uveitis Nomenclature Working, G. (2005) Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop, Am J Ophthalmol. 140, 509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Okunuki Y, Mukai R, Nakao T, Tabor SJ, Butovsky O, Dana R, Ksander BR & Connor KM (2019) Retinal microglia initiate neuroinflammation in ocular autoimmunity, Proc Natl Acad Sci U S A. 116, 9989–9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Goto H, Mochizuki M, Yamaki K, Kotake S, Usui M & Ohno S (2007) Epidemiological survey of intraocular inflammation in Japan, Jpn J Ophthalmol. 51, 41–4. [DOI] [PubMed] [Google Scholar]
- 152.Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P & Murray PI (2004) Degree, duration, and causes of visual loss in uveitis, Br J Ophthalmol. 88, 1159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen YH, Eskandarpour M, Zhang X, Galatowicz G, Greenwood J, Lightman S & Calder V (2021) Small-molecule antagonist of VLA-4 (GW559090) attenuated neuro-inflammation by targeting Th17 cell trafficking across the blood-retinal barrier in experimental autoimmune uveitis, J Neuroinflammation. 18, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen X, Kezic JM, Forrester JV, Goldberg GL, Wicks IP, Bernard CC & McMenamin PG (2015) In vivo multi-modal imaging of experimental autoimmune uveoretinitis in transgenic reporter mice reveals the dynamic nature of inflammatory changes during disease progression, J Neuroinflammation. 12, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bremer D, Pache F, Gunther R, Hornow J, Andresen V, Leben R, Mothes R, Zimmermann H, Brandt AU, Paul F, Hauser AE, Radbruch H & Niesner R (2016) Longitudinal Intravital Imaging of the Retina Reveals Long-term Dynamics of Immune Infiltration and Its Effects on the Glial Network in Experimental Autoimmune Uveoretinitis, without Evident Signs of Neuronal Dysfunction in the Ganglion Cell Layer, Front Immunol. 7, 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Forrester JV, Kuffova L & Dick AD (2018) Autoimmunity, Autoinflammation, and Infection in Uveitis, Am J Ophthalmol. 189, 77–85. [DOI] [PubMed] [Google Scholar]
- 157.Lardenoye CW, van Kooij B & Rothova A (2006) Impact of macular edema on visual acuity in uveitis, Ophthalmology. 113, 1446–9. [DOI] [PubMed] [Google Scholar]
- 158.Rothova A, Suttorp-van Schulten MS, Frits Treffers W & Kijlstra A (1996) Causes and frequency of blindness in patients with intraocular inflammatory disease, Br J Ophthalmol. 80, 332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu H, Forrester JV, Liversidge J & Crane IJ (2003) Leukocyte trafficking in experimental autoimmune uveitis: breakdown of blood-retinal barrier and upregulation of cellular adhesion molecules, Invest Ophthalmol Vis Sci. 44, 226–34. [DOI] [PubMed] [Google Scholar]
- 160.Kielczewski JL, Horai R, Jittayasothorn Y, Chan CC & Caspi RR (2016) Tertiary Lymphoid Tissue Forms in Retinas of Mice with Spontaneous Autoimmune Uveitis and Has Consequences on Visual Function, J Immunol. 196, 1013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kokona D, Ebneter A, Escher P & Zinkernagel MS (2018) Colony-stimulating factor 1 receptor inhibition prevents disruption of the blood-retina barrier during chronic inflammation, J Neuroinflammation. 15, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.London A, Benhar I & Schwartz M (2013) The retina as a window to the brain-from eye research to CNS disorders, Nat Rev Neurol. 9, 44–53. [DOI] [PubMed] [Google Scholar]
- 163.Fani Maleki A & Rivest S (2019) Innate Immune Cells: Monocytes, Monocyte-Derived Macrophages and Microglia as Therapeutic Targets for Alzheimer’s Disease and Multiple Sclerosis, Front Cell Neurosci. 13, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wong W (2020) Economic burden of Alzheimer disease and managed care considerations, Am J Manag Care. 26, S177–S183. [DOI] [PubMed] [Google Scholar]
- 165.Morris JC, Blennow K, Froelich L, Nordberg A, Soininen H, Waldemar G, Wahlund LO & Dubois B (2014) Harmonized diagnostic criteria for Alzheimer’s disease: recommendations, J Intern Med. 275, 204–13. [DOI] [PubMed] [Google Scholar]
- 166.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S & Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease, Alzheimers Dement. 7, 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ferreira D, Verhagen C, Hernandez-Cabrera JA, Cavallin L, Guo CJ, Ekman U, Muehlboeck JS, Simmons A, Barroso J, Wahlund LO & Westman E (2017) Distinct subtypes of Alzheimer’s disease based on patterns of brain atrophy: longitudinal trajectories and clinical applications, Sci Rep. 7, 46263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Huang HC & Jiang ZF (2009) Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: relationship and links in Alzheimer’s disease, J Alzheimers Dis. 16, 15–27. [DOI] [PubMed] [Google Scholar]
- 169.Paraskevaidi M, Allsop D, Karim S, Martin FL & Crean S (2020) Diagnostic Biomarkers for Alzheimer’s Disease Using Non-Invasive Specimens, J Clin Med. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Frost S, Martins RN & Kanagasingam Y (2010) Ocular biomarkers for early detection of Alzheimer’s disease, J Alzheimers Dis. 22, 1–16. [DOI] [PubMed] [Google Scholar]
- 171.Valenti DA (2010) Alzheimer’s disease: visual system review, Optometry. 81, 12–21. [DOI] [PubMed] [Google Scholar]
- 172.Cronin-Golomb A, Corkin S, Rizzo JF, Cohen J, Growdon JH & Banks KS (1991) Visual dysfunction in Alzheimer’s disease: relation to normal aging, Ann Neurol. 29, 41–52. [DOI] [PubMed] [Google Scholar]
- 173.Lakshminarayanan V, Lagrave J, Kean ML, Dick M & Shankle R (1996) Vision in dementia: contrast effects, Neurol Res. 18, 9–15. [DOI] [PubMed] [Google Scholar]
- 174.Sadun AA, Borchert M, DeVita E, Hinton DR & Bassi CJ (1987) Assessment of visual impairment in patients with Alzheimer’s disease, Am J Ophthalmol. 104, 113–20. [DOI] [PubMed] [Google Scholar]
- 175.Bassi CJ, Solomon K & Young D (1993) Vision in aging and dementia, Optom Vis Sci. 70, 809–13. [DOI] [PubMed] [Google Scholar]
- 176.Polo V, Rodrigo MJ, Garcia-Martin E, Otin S, Larrosa JM, Fuertes MI, Bambo MP, Pablo LE & Satue M (2017) Visual dysfunction and its correlation with retinal changes in patients with Alzheimer’s disease, Eye (Lond). 31, 1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pablo Pinero D, Monllor B, Moncho V & de Fez D (2016) Visual function alterations in Alzheimer Disease: A case report, Can J Ophthalmol. 51, e16–8. [DOI] [PubMed] [Google Scholar]
- 178.Blanks JC, Hinton DR, Sadun AA & Miller CA (1989) Retinal ganglion cell degeneration in Alzheimer’s disease, Brain Res. 501, 364–72. [DOI] [PubMed] [Google Scholar]
- 179.Koronyo Y, Biggs D, Barron E, Boyer DS, Pearlman JA, Au WJ, Kile SJ, Blanco A, Fuchs DT, Ashfaq A, Frautschy S, Cole GM, Miller CA, Hinton DR, Verdooner SR, Black KL & Koronyo-Hamaoui M (2017) Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease, JCI Insight. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schon C, Hoffmann NA, Ochs SM, Burgold S, Filser S, Steinbach S, Seeliger MW, Arzberger T, Goedert M, Kretzschmar HA, Schmidt B & Herms J (2012) Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice, PLoS One. 7, e53547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.La Morgia C, Ross-Cisneros FN, Koronyo Y, Hannibal J, Gallassi R, Cantalupo G, Sambati L, Pan BX, Tozer KR, Barboni P, Provini F, Avanzini P, Carbonelli M, Pelosi A, Chui H, Liguori R, Baruzzi A, Koronyo-Hamaoui M, Sadun AA & Carelli V (2016) Melanopsin retinal ganglion cell loss in Alzheimer disease, Ann Neurol. 79, 90–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Danesh-Meyer HV, Birch H, Ku JY, Carroll S & Gamble G (2006) Reduction of optic nerve fibers in patients with Alzheimer disease identified by laser imaging, Neurology. 67, 1852–4. [DOI] [PubMed] [Google Scholar]
- 183.Liu B, Rasool S, Yang Z, Glabe CG, Schreiber SS, Ge J & Tan Z (2009) Amyloid-peptide vaccinations reduce {beta}-amyloid plaques but exacerbate vascular deposition and inflammation in the retina of Alzheimer’s transgenic mice, Am J Pathol. 175, 2099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Polo V, Garcia-Martin E, Bambo MP, Pinilla J, Larrosa JM, Satue M, Otin S & Pablo LE (2014) Reliability and validity of Cirrus and Spectralis optical coherence tomography for detecting retinal atrophy in Alzheimer’s disease, Eye (Lond). 28, 680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kromer R, Serbecic N, Hausner L, Froelich L, Aboul-Enein F & Beutelspacher SC (2014) Detection of Retinal Nerve Fiber Layer Defects in Alzheimer’s Disease Using SD-OCT, Front Psychiatry. 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cheung CY, Ong YT, Hilal S, Ikram MK, Low S, Ong YL, Venketasubramanian N, Yap P, Seow D, Chen CL & Wong TY (2015) Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease, J Alzheimers Dis. 45, 45–56. [DOI] [PubMed] [Google Scholar]
- 187.Marziani E, Pomati S, Ramolfo P, Cigada M, Giani A, Mariani C & Staurenghi G (2013) Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography, Invest Ophthalmol Vis Sci. 54, 5953–8. [DOI] [PubMed] [Google Scholar]
- 188.Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG & Pierelli F (2001) Morphological and functional retinal impairment in Alzheimer’s disease patients, Clin Neurophysiol. 112, 1860–7. [DOI] [PubMed] [Google Scholar]
- 189.Sanchez D, Castilla-Marti M, Rodriguez-Gomez O, Valero S, Piferrer A, Martinez G, Martinez J, Serra J, Moreno-Grau S, Hernandez-Olasagarre B, De Rojas I, Hernandez I, Abdelnour C, Rosende-Roca M, Vargas L, Mauleon A, Santos-Santos MA, Alegret M, Ortega G, Espinosa A, Perez-Cordon A, Sanabria A, Ciudin A, Simo R, Hernandez C, Villoslada P, Ruiz A, Tarraga L & Boada M (2018) Usefulness of peripapillary nerve fiber layer thickness assessed by optical coherence tomography as a biomarker for Alzheimer’s disease, Sci Rep. 8, 16345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Garcia-Martin ES, Rojas B, Ramirez AI, de Hoz R, Salazar JJ, Yubero R, Gil P, Trivino A & Ramirez JM (2014) Macular thickness as a potential biomarker of mild Alzheimer’s disease, Ophthalmology. 121, 1149–1151 e3. [DOI] [PubMed] [Google Scholar]
- 191.Gharbiya M, Trebbastoni A, Parisi F, Manganiello S, Cruciani F, D’Antonio F, De Vico U, Imbriano L, Campanelli A & De Lena C (2014) Choroidal thinning as a new finding in Alzheimer’s disease: evidence from enhanced depth imaging spectral domain optical coherence tomography, J Alzheimers Dis. 40, 907–17. [DOI] [PubMed] [Google Scholar]
- 192.Kim BJ, Irwin DJ, Song D, Daniel E, Leveque JD, Raquib AR, Pan W, Ying GS, Aleman TS, Dunaief JL & Grossman M (2017) Optical coherence tomography identifies outer retina thinning in frontotemporal degeneration, Neurology. 89, 1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Grimaldi A, Brighi C, Peruzzi G, Ragozzino D, Bonanni V, Limatola C, Ruocco G & Di Angelantonio S (2018) Inflammation, neurodegeneration and protein aggregation in the retina as ocular biomarkers for Alzheimer’s disease in the 3xTg-AD mouse model, Cell Death Dis. 9, 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Grimaldi A, Pediconi N, Oieni F, Pizzarelli R, Rosito M, Giubettini M, Santini T, Limatola C, Ruocco G, Ragozzino D & Di Angelantonio S (2019) Neuroinflammatory Processes, A1 Astrocyte Activation and Protein Aggregation in the Retina of Alzheimer’s Disease Patients, Possible Biomarkers for Early Diagnosis, Front Neurosci. 13, 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nilson AN, English KC, Gerson JE, Barton Whittle T, Nicolas Crain C, Xue J, Sengupta U, Castillo-Carranza DL, Zhang W, Gupta P & Kayed R (2017) Tau Oligomers Associate with Inflammation in the Brain and Retina of Tauopathy Mice and in Neurodegenerative Diseases, J Alzheimers Dis. 55, 1083–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ning A, Cui J, To E, Ashe KH & Matsubara J (2008) Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease, Invest Ophthalmol Vis Sci. 49, 5136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Perez SE, Lumayag S, Kovacs B, Mufson EJ & Xu S (2009) Beta-amyloid deposition and functional impairment in the retina of the APPswe/PS1DeltaE9 transgenic mouse model of Alzheimer’s disease, Invest Ophthalmol Vis Sci. 50, 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR & Colonna M (2015) TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model, Cell. 160, 1061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Condello C, Yuan P, Schain A & Grutzendler J (2015) Microglia constitute a barrier that prevents neurotoxic protofibrillar Abeta42 hotspots around plaques, Nat Commun. 6, 6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M & Amit I (2017) A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease, Cell. 169, 1276–1290 e17. [DOI] [PubMed] [Google Scholar]
- 201.Nussbaum RL & Ellis CE (2003) Alzheimer’s disease and Parkinson’s disease, N Engl J Med. 348, 1356–64. [DOI] [PubMed] [Google Scholar]
- 202.Dauer W & Przedborski S (2003) Parkinson’s disease: mechanisms and models, Neuron. 39, 889–909. [DOI] [PubMed] [Google Scholar]
- 203.Biousse V, Skibell BC, Watts RL, Loupe DN, Drews-Botsch C & Newman NJ (2004) Ophthalmologic features of Parkinson’s disease, Neurology. 62, 177–80. [DOI] [PubMed] [Google Scholar]
- 204.Archibald NK, Clarke MP, Mosimann UP & Burn DJ (2009) The retina in Parkinson’s disease, Brain. 132, 1128–45. [DOI] [PubMed] [Google Scholar]
- 205.Guo L, Normando EM, Shah PA, De Groef L & Cordeiro MF (2018) Oculo-visual abnormalities in Parkinson’s disease: Possible value as biomarkers, Mov Disord. 33, 1390–1406. [DOI] [PubMed] [Google Scholar]
- 206.Djamgoz MB, Hankins MW, Hirano J & Archer SN (1997) Neurobiology of retinal dopamine in relation to degenerative states of the tissue, Vision Res. 37, 3509–29. [DOI] [PubMed] [Google Scholar]
- 207.Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM & McMahon DG (2012) Retinal dopamine mediates multiple dimensions of light-adapted vision, J Neurosci. 32, 9359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Robbins CB, Thompson AC, Bhullar PK, Koo HY, Agrawal R, Soundararajan S, Yoon SP, Polascik BW, Scott BL, Grewal DS & Fekrat S (2021) Characterization of Retinal Microvascular and Choroidal Structural Changes in Parkinson Disease, JAMA Ophthalmol. 139, 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Moschos MM, Tagaris G, Markopoulos I, Margetis I, Tsapakis S, Kanakis M & Koutsandrea C (2011) Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss, Eur J Ophthalmol. 21, 24–9. [DOI] [PubMed] [Google Scholar]
- 210.Garcia-Martin E, Rodriguez-Mena D, Satue M, Almarcegui C, Dolz I, Alarcia R, Seral M, Polo V, Larrosa JM & Pablo LE (2014) Electrophysiology and optical coherence tomography to evaluate Parkinson disease severity, Invest Ophthalmol Vis Sci. 55, 696–705. [DOI] [PubMed] [Google Scholar]
- 211.Orr CF, Rowe DB & Halliday GM (2002) An inflammatory review of Parkinson’s disease, Prog Neurobiol. 68, 325–40. [DOI] [PubMed] [Google Scholar]
- 212.George S & Brundin P (2015) Immunotherapy in Parkinson’s Disease: Micromanaging Alpha-Synuclein Aggregation, J Parkinsons Dis. 5, 413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Martinez-Navarrete GC, Martin-Nieto J, Esteve-Rudd J, Angulo A & Cuenca N (2007) Alpha synuclein gene expression profile in the retina of vertebrates, Mol Vis. 13, 949–61. [PMC free article] [PubMed] [Google Scholar]
- 214.Bodis-Wollner I, Kozlowski PB, Glazman S & Miri S (2014) alpha-synuclein in the inner retina in parkinson disease, Ann Neurol. 75, 964–6. [DOI] [PubMed] [Google Scholar]
- 215.Cao S, Standaert DG & Harms AS (2012) The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia, J Neuroinflammation. 9, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Croisier E, Moran LB, Dexter DT, Pearce RK & Graeber MB (2005) Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition, J Neuroinflammation. 2, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen ST, Hsu JR, Hsu PC & Chuang JI (2003) The retina as a novel in vivo model for studying the role of molecules of the Bcl-2 family in relation to MPTP neurotoxicity, Neurochem Res. 28, 805–14. [DOI] [PubMed] [Google Scholar]
- 218.Mammadova N, Summers CM, Kokemuller RD, He Q, Ding S, Baron T, Yu C, Valentine RJ, Sakaguchi DS, Kanthasamy AG, Greenlee JJ & Heather West Greenlee M (2019) Accelerated accumulation of retinal alpha-synuclein (pSer129) and tau, neuroinflammation, and autophagic dysregulation in a seeded mouse model of Parkinson’s disease, Neurobiol Dis. 121, 1–16. [DOI] [PubMed] [Google Scholar]
- 219.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E & Lee SJ (2010) Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies, J Biol Chem. 285, 9262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gonzalez H, Elgueta D, Montoya A & Pacheco R (2014) Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases, J Neuroimmunol. 274, 1–13. [DOI] [PubMed] [Google Scholar]
- 221.Doorn KJ, Moors T, Drukarch B, van de Berg W, Lucassen PJ & van Dam AM (2014) Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients, Acta Neuropathol Commun. 2, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK & Stefanova N (2013) Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia, Glia. 61, 349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang QS, Heng Y, Yuan YH & Chen NH (2017) Pathological alpha-synuclein exacerbates the progression of Parkinson’s disease through microglial activation, Toxicol Lett. 265, 30–37. [DOI] [PubMed] [Google Scholar]
- 224.Hayes MR, Mietlicki-Baase EG, Kanoski SE & De Jonghe BC (2014) Incretins and amylin: neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose, Annu Rev Nutr. 34, 237–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Duh EJ, Sun JK & Stitt AW (2017) Diabetic retinopathy: current understanding, mechanisms, and treatment strategies, JCI Insight. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhou M, Wang W, Huang W & Zhang X (2014) Diabetes mellitus as a risk factor for open-angle glaucoma: a systematic review and meta-analysis, PLoS One. 9, e102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Porter DW, Kerr BD, Flatt PR, Holscher C & Gault VA (2010) Four weeks administration of Liraglutide improves memory and learning as well as glycaemic control in mice with high fat dietary-induced obesity and insulin resistance, Diabetes Obes Metab. 12, 891–9. [DOI] [PubMed] [Google Scholar]
- 228.Cukierman-Yaffe T, Gerstein HC, Colhoun HM, Diaz R, Garcia-Perez LE, Lakshmanan M, Bethel A, Xavier D, Probstfield J, Riddle MC, Ryden L, Atisso CM, Hall S, Rao-Melacini P, Basile J, Cushman WC, Franek E, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH & Temelkova-Kurktschiev T (2020) Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial, Lancet Neurol. 19, 582–590. [DOI] [PubMed] [Google Scholar]
- 229.Filippatos TD, Panagiotopoulou TV & Elisaf MS (2014) Adverse Effects of GLP-1 Receptor Agonists, Rev Diabet Stud. 11, 202–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Kober L, Petrie MC & McMurray JJV (2019) Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials, Lancet Diabetes Endocrinol. 7, 776–785. [DOI] [PubMed] [Google Scholar]
- 231.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, Committee LS & Investigators LT (2016) Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes, N Engl J Med. 375, 311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Potts JE, Gray LJ, Brady EM, Khunti K, Davies MJ & Bodicoat DH (2015) The Effect of Glucagon-Like Peptide 1 Receptor Agonists on Weight Loss in Type 2 Diabetes: A Systematic Review and Mixed Treatment Comparison Meta-Analysis, PLoS One. 10, e0126769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, Jeppesen OK, Christiansen E, Hertz CL, Haluzik M & Investigators P (2019) PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison With Placebo in Patients With Type 2 Diabetes, Diabetes Care. 42, 1724–1732. [DOI] [PubMed] [Google Scholar]
- 234.Athauda D & Foltynie T (2016) The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action, Drug Discov Today. 21, 802–18. [DOI] [PubMed] [Google Scholar]
- 235.Gong N, Xiao Q, Zhu B, Zhang CY, Wang YC, Fan H, Ma AN & Wang YX (2014) Activation of spinal glucagon-like peptide-1 receptors specifically suppresses pain hypersensitivity, J Neurosci. 34, 5322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Timper K, Del Rio-Martin A, Cremer AL, Bremser S, Alber J, Giavalisco P, Varela L, Heilinger C, Nolte H, Trifunovic A, Horvath TL, Kloppenburg P, Backes H & Bruning JC (2020) GLP-1 Receptor Signaling in Astrocytes Regulates Fatty Acid Oxidation, Mitochondrial Integrity, and Function, Cell Metab. 31, 1189–1205 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Reiner DJ, Mietlicki-Baase EG, McGrath LE, Zimmer DJ, Bence KK, Sousa GL, Konanur VR, Krawczyk J, Burk DH, Kanoski SE, Hermann GE, Rogers RC & Hayes MR (2016) Astrocytes Regulate GLP-1 Receptor-Mediated Effects on Energy Balance, J Neurosci. 36, 3531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shan Y, Tan S, Lin Y, Liao S, Zhang B, Chen X, Wang J, Deng Z, Zeng Q, Zhang L, Wang Y, Hu X, Qiu W, Peng L & Lu Z (2019) The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke, J Neuroinflammation. 16, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Marina N, Turovsky E, Christie IN, Hosford PS, Hadjihambi A, Korsak A, Ang R, Mastitskaya S, Sheikhbahaei S, Theparambil SM & Gourine AV (2018) Brain metabolic sensing and metabolic signaling at the level of an astrocyte, Glia. 66, 1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kastin AJ, Akerstrom V & Pan W (2002) Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier, J Mol Neurosci. 18, 7–14. [DOI] [PubMed] [Google Scholar]
- 241.Hebsgaard JB, Pyke C, Yildirim E, Knudsen LB, Heegaard S & Kvist PH (2018) Glucagon-like peptide-1 receptor expression in the human eye, Diabetes Obes Metab. 20, 2304–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hernandez C, Bogdanov P, Corraliza L, Garcia-Ramirez M, Sola-Adell C, Arranz JA, Arroba AI, Valverde AM & Simo R (2016) Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes, Diabetes. 65, 172–87. [DOI] [PubMed] [Google Scholar]
- 243.Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM & Greig NH (2003) Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron, J Neurosci Res. 72, 603–12. [DOI] [PubMed] [Google Scholar]
- 244.Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, Tweedie D, Perry T, Mattson MP, Kapogiannis D, Sambamurti K, Lahiri DK & Greig NH (2010) GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease, J Alzheimers Dis. 19, 1205–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wang XH, Li L, Holscher C, Pan YF, Chen XR & Qi JS (2010) Val8-glucagon-like peptide-1 protects against Abeta1-40-induced impairment of hippocampal late-phase long-term potentiation and spatial learning in rats, Neuroscience. 170, 1239–48. [DOI] [PubMed] [Google Scholar]
- 246.Han WN, Holscher C, Yuan L, Yang W, Wang XH, Wu MN & Qi JS (2013) Liraglutide protects against amyloid-beta protein-induced impairment of spatial learning and memory in rats, Neurobiol Aging. 34, 576–88. [DOI] [PubMed] [Google Scholar]
- 247.Gengler S, McClean PL, McCurtin R, Gault VA & Holscher C (2012) Val(8)GLP-1 rescues synaptic plasticity and reduces dense core plaques in APP/PS1 mice, Neurobiol Aging. 33, 265–76. [DOI] [PubMed] [Google Scholar]
- 248.McGovern SF, Hunter K & Holscher C (2012) Effects of the glucagon-like polypeptide-1 analogue (Val8)GLP-1 on learning, progenitor cell proliferation and neurogenesis in the C57B/16 mouse brain, Brain Res. 1473, 204–13. [DOI] [PubMed] [Google Scholar]
- 249.McClean PL, Parthsarathy V, Faivre E & Holscher C (2011) The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease, J Neurosci. 31, 6587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gejl M, Gjedde A, Egefjord L, Moller A, Hansen SB, Vang K, Rodell A, Braendgaard H, Gottrup H, Schacht A, Moller N, Brock B & Rungby J (2016) In Alzheimer’s Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial, Front Aging Neurosci. 8, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Femminella GD, Frangou E, Love SB, Busza G, Holmes C, Ritchie C, Lawrence R, McFarlane B, Tadros G, Ridha BH, Bannister C, Walker Z, Archer H, Coulthard E, Underwood BR, Prasanna A, Koranteng P, Karim S, Junaid K, McGuinness B, Nilforooshan R, Macharouthu A, Donaldson A, Thacker S, Russell G, Malik N, Mate V, Knight L, Kshemendran S, Harrison J, Holscher C, Brooks DJ, Passmore AP, Ballard C & Edison P (2019) Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: study protocol for a randomised controlled trial (ELAD study), Trials. 20, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Cairns E (2020) CTAD 2020 – Elad fails, but GLP-1s could still have a future in Alzheimer’s in Evaluate Vantage
- 253.Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Ronnholm H & Wikstrom L (2008) Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease, J Neurosci Res. 86, 326–38. [DOI] [PubMed] [Google Scholar]
- 254.Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS & Whitton PS (2008) Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease, J Neuroinflammation. 5, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Liu W, Jalewa J, Sharma M, Li G, Li L & Holscher C (2015) Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease, Neuroscience. 303, 42–50. [DOI] [PubMed] [Google Scholar]
- 256.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P & Foltynie T (2013) Exenatide and the treatment of patients with Parkinson’s disease, J Clin Invest. 123, 2730–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S & Foltynie T (2017) Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial, Lancet. 390, 1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Solmaz V, Cinar BP, Yigitturk G, Cavusoglu T, Taskiran D & Erbas O (2015) Exenatide reduces TNF-alpha expression and improves hippocampal neuron numbers and memory in streptozotocin treated rats, Eur J Pharmacol. 765, 482–7. [DOI] [PubMed] [Google Scholar]
- 259.Kim S, Moon M & Park S (2009) Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease, J Endocrinol. 202, 431–9. [DOI] [PubMed] [Google Scholar]
- 260.Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y & Greig NH (2009) GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism, Proc Natl Acad Sci U S A. 106, 1285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R & Watada H (2010) Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4, Diabetes. 59, 1030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Han L, Yu Y, Sun X & Wang B (2012) Exendin-4 directly improves endothelial dysfunction in isolated aortas from obese rats through the cAMP or AMPK-eNOS pathways, Diabetes Res Clin Pract. 97, 453–60. [DOI] [PubMed] [Google Scholar]
- 263.Ahern T, Tobin AM, Corrigan M, Hogan A, Sweeney C, Kirby B & O’Shea D (2013) Glucagon-like peptide-1 analogue therapy for psoriasis patients with obesity and type 2 diabetes: a prospective cohort study, J Eur Acad Dermatol Venereol. 27, 1440–3. [DOI] [PubMed] [Google Scholar]
- 264.Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, Kwon SH, Park YJ, Karuppagounder SS, Park H, Kim S, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, Kang SU, Lee Y, Lee KC, Na DH, Kim D, Lee SH, Roschke VV, Liddelow SA, Mari Z, Barres BA, Dawson VL, Lee S, Dawson TM & Ko HS (2018) Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease, Nat Med. 24, 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Park JS, Kam TI, Lee S, Park H, Oh Y, Kwon SH, Song JJ, Kim D, Kim H, Jhaldiyal A, Na DH, Lee KC, Park EJ, Pomper MG, Pletnikova O, Troncoso JC, Ko HS, Dawson VL, Dawson TM & Lee S (2021) Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer’s disease, Acta Neuropathol Commun. 9, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.(2021) A clinical study of NLY01 in patients with early Parkinson’s disease in ClinicalTrials.gov.
- 267.Sterling JK, Hua P, Dunaief JL, Cui QN & VanderBeek BL (2021) Exposure to glucagon-like peptide 1 receptor (GLP-1R) agonists reduces glaucoma risk, medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ebneter A, Kokona D, Schneider N & Zinkernagel MS (2017) Microglia Activation and Recruitment of Circulating Macrophages During Ischemic Experimental Branch Retinal Vein Occlusion, Invest Ophthalmol Vis Sci. 58, 944–953. [DOI] [PubMed] [Google Scholar]


