Abstract
Effective strategies to support PrEP adherence among adolescent girls and young women (AGYW) are needed. We examined PrEP use disclosure and its effect on adherence among 200 AGYW ages 16–25 initiating PrEP in South Africa to help inform these strategies. We estimated the relative prevalence of high adherence (intracellular tenofovir-diphosphate concentration ≥ 700 fmol/punch) 3- and 6-months after PrEP initiation among those who disclosed vs. did not disclose their PrEP use, both overall and by age. Most AGYW disclosed to a parent (58%), partner (58%), or friend (81%) by month 6. We did not observe a strong effect of disclosure on adherence overall; however, among younger AGYW (≤ 18 years), those who disclosed to a parent were 6.8 times as likely to have high adherence at month 6 than those who did not (95% CI 1.02, 45.56). More work is needed to understand parents’ roles as allies and identify ways peers and partners can motivate PrEP use for AGYW.
Keywords: Adolescent girls and young women, Pre-exposure prophylaxis, PrEP, HIV prevention, Disclosure
Introduction
Adolescent girls and young women (AGYW) represent a population at high risk of acquiring HIV infection [1]. In sub-Saharan Africa, where the vast majority of HIV infections occur globally, nearly 25% of all new infections occur among AGYW, and AGYW are more than twice as likely to become infected with HIV compared to young men [1,2]. AGYW are at a decisive age in their developmental trajectories, often facing milestones such as sexual debut and increased engagement in sexual partnerships. Thus, AGYW critically need access to a range of HIV prevention options, including biomedical HIV prevention strategies [3], along with information and skills to facilitate sexual decision-making [4, 5].
Oral antiretroviral pre-exposure prophylaxis (PrEP) is an effective biomedical HIV prevention strategy that holds enormous potential to substantially reduce HIV acquisition in diverse populations globally [6]. Evolving data from randomized trials and demonstration projects among adult men have shown a reduction in HIV acquisition of greater than 90% with oral PrEP when adherence is high, with the majority of breakthrough infections occurring among those who discontinue PrEP [7–9]. Among young African women, however, adherence has been challenging, compromising PrEP’s efficacy in two trials [10, 11]. Further, adolescents under 18 years of age have only recently been included in biomedical HIV prevention studies, and thus there is a limited understanding of factors impacting adherence in this young age group [12–15].
Adolescent girls and young women are likely to need support for effective PrEP use. As described among young people living with HIV, disclosure can be an important means of eliciting social support for adherence to antiretroviral therapy [16, 17]. Research examining the importance of disclosure and social support to PrEP adherence among African women has focused on their ability to harness support from male partners [18–20]. In the transition to young adulthood, adolescent development is guided by a diverse network of social influences that are likely to impact health-related behavioral decisions in different ways than in adults [21–23]. To better support AGYW initiating PrEP, information is needed on the effect of disclosure to specific social groups, including parents, partners, and peers, on PrEP adherence. In this study, we sought to examine: (1) PrEP use disclosure by AGYW to specific social groups, and (2) the effect of PrEP use disclosure on PrEP adherence, both overall and according to age.
Methods
Parent Trial Design
We used longitudinal data from the 3Ps for Prevention study (3P), a randomized trial evaluating PrEP acceptability and adherence among South African AGYW [24]. Between March 2017 and March 2018, 200 AGYW aged 16–25 years who were enrolled into 3P initiated daily oral PrEP and were followed monthly for 3 months and then quarterly through month 12. At enrollment, participants were randomized 1:1 to receive (or not receive) a short-term incentive conditional on tenofovir-diphosphate (TFV-DP) concentrations in dried blood spots (DBS) to promote adherence. Additional detail on the study is provided elsewhere [24].
Study Population and Procedures
3P was conducted at a youth-friendly research center located in a peri-urban township of approximately 30,000 people 40 km outside Cape Town in the Southern Peninsula district of South Africa’s Western Cape province. Participants were recruited through a social marketing campaign to raise PrEP awareness, educate AGYW about PrEP, and enumerate PrEP demand [25]. Sexually active, HIV-uninfected AGYW aged 16–25 years who were not currently enrolled in any other research studies, had not previously participated in an oral PrEP study, and were intending to take PrEP for up to 12 months were eligible to enroll into the 3P study.
AGYW who met the inclusion criteria and consented to study participation were offered once-daily oral emtricitabine 200 mg/tenofovir disoproxil fumarate 300 mg (FTC/TDF). PrEP was dispensed at months 1, 2, 3, 6, and 9. At each visit, participants received counseling on the importance of high PrEP adherence, information on potential side effects, and strategies for confidential storage of pills and anticipated barriers to adherence. All participants were compensated with a 100 ZAR shopping voucher (approximately 7 USD) at each visit, with those in the incentive arm having conditional opportunities to receive up to three additional vouchers.
3P study procedures were approved by the University of Cape Town’s Human Research Ethics Committee and this analysis was approved by the University of North Carolina at Chapel Hill’s Institutional Review Board. All participants provided written informed consent in English or isiXhosa. A parental consent waiver was granted for participants aged 16–17 years.
Data Collection
Socio-demographic data were collected from all participants prior to initiating PrEP. At all follow-up visits, participants completed a behavioral survey that assessed partnership characteristics, disclosure of PrEP use, and reactions participants received following disclosure events. Disclosure of PrEP use was assessed by asking participants if they had told anyone about their PrEP use since their last visit (yes/no). Participants who responded affirmatively were then asked if they had disclosed their PrEP use separately to at least one person in each of several specific social groups: a sex partner, a parent, a sister or brother, an “other family member”, a friend, a neighbor, a nurse or doctor, and “other” persons. For each specific social group to which a participant reported disclosing her PrEP use to at least one person, she received an additional question inquiring if she received a supportive reaction in that group. DBS were collected at follow-up months 1, 2, 3, 6, and 12 for storage and retrospective drug-level testing for the main study, further described under “Outcome assessment” below.
Exposure Assessment
We assessed our exposure, PrEP use disclosure, at two timepoints: month 3 and month 6. At month 3, participants who reported disclosing their PrEP use at follow-up months 1, 2, or 3 were considered exposed. At month 6, those who reported disclosing their PrEP use at follow-up months 1, 2, 3, or 6 were considered exposed. We created several exposure variables to capture different dimensions of disclosure that could potentially affect PrEP adherence. First, to assess disclosure to at least one person, we created a binary ‘disclosure to anyone’ variable (yes/no). Second, to assess the total number of different social groups to which a participant reported disclosing to at least one member, we created a continuous ‘sum of disclosure groups’ variable (range 0–8). Third, to assess disclosure to at least one parent, partner, and friend, we created three separate binary disclosure variables (yes/no) for each social group.
Outcome Assessment
Intracellular TFV-DP concentrations in DBS were measured at the University of Cape Town Division of Clinical Pharmacology Laboratory, which developed and validated an indirect method for the quantification of TFV-DP in 50 μl human DBS, in collaboration with the University of Colorado Pharmacology Laboratory, which developed the assay and determined thresholds with directly observed dosing [26]. DBS provide a reliable measure of cumulative PrEP adherence in the month prior to a sample being collected [26].
We assessed our outcome, high adherence, at months 3 and 6 for all participants. High adherence was defined as TFV-DP ≥ 700 fmol/punch at a given visit. This threshold correlated with an average of ≥ 4 doses/week in the prior month and was associated with 100% protection against HIV acquisition among men who have sex with men (MSM) in iPrEX OLE [7, 26]. In primary analyses, participants with a missing DBS result (either due to missing the study visit or not providing a DBS) were imputed as non-adherent under the assumption that they were likely to have stopped taking PrEP. We probed the impact of this assumption in a sensitivity analysis (described below).
Statistical Analysis
We conducted descriptive analyses of demographic and behavioral characteristics assessed prior to PrEP initiation. We then determined the proportion of participants who reported disclosing to anyone and to at least one member of a given social group during the first 3 and 6 months of follow-up, as well as the proportion of participants who received at least one supportive reaction following disclosure to at least one member of a given social group. For each social group, we also calculated at each visit the number of participants with a new group-level disclosure, defined as a reported disclosure since the last visit to at least one person in a given social group. We then assessed the cumulative number of group-level disclosures over time for each group. To determine if PrEP use disclosure varied by age, we used log-binomial regression to estimate a prevalence ratio (PR) for disclosure of PrEP use to each social group in the first 6 months of follow-up for younger (≤ 18 years) vs. older (> 18 years) AGYW.
Modified Poisson regression with a robust error variance [27] was used to estimate the relative prevalence of high adherence among those who disclosed their PrEP use compared to those who did not. With these PR estimates, we examined associations between (1) disclosure of PrEP use over the first 3 months of follow-up and high adherence at month 3, and (2) disclosure of PrEP use over the first 6 months of follow-up and high adherence at month 6. For both the 3-month and 6-month outcomes, we examined the overall impact on adherence of PrEP use disclosure to anyone and to a greater number of different social groups. To reduce bias, we identified potential confounders of the disclosure-adherence relationship using a directed acyclic graph (supplemental Appendix A) [28], and we included available covariates in multivariable models to calculate adjusted PR (aPR) estimates. All full-sample adjusted models controlled for age, trial arm, and disclosure of intentions to use PrEP reported at enrollment.
Then, because we hypothesized that the effects of disclosure to certain groups might vary by age (i.e., younger AGYW who are more likely to be living at home and/or be in school may obtain support from different social groups than older AGYW), we calculated overall and age-stratified PR estimates separately for disclosure to partners, parents, and friends, and we assessed for evidence of effect measure modification by age by including an interaction term (disclosure × dichotomous age group) in adjusted regression models. A Wald test with an alpha value of 0.15 was used to assess the statistical significance of the interaction. The age categorization of ≤ 18 and > 18 years maximized balance between groups given our limited sample size. Age-stratified models adjusted for trial arm and enrollment disclosure. In models examining disclosure to partners, we also adjusted for participants having a primary sex partner and a history of any intimate partner violence (IPV) in the past year, both of which were assessed at enrollment to ensure they preceded assessment of the exposure. For all full-sample and age-stratified PR and aPR estimates, an effect was considered statistically significant if the 95% confidence interval (CI) did not contain the null value of 1.0.
To assess the sensitivity of results to our primary analysis assumption that missing DBS values represented non-adherence, we conducted a complete-case sensitivity analysis in which we restricted our analytic sample to participants with an available DBS result at a given time point. All statistical analyses were conducted in SAS software, version 9.4 (Cary, NC).
Results
Sample Characteristics and Patterns of PrEP Use Disclosure
The 200 3P participants had a median age of 19 years (IQR 17–21) (Table 1). Most (86%) had a primary sex partner at screening, of whom 42% did not know their partner’s HIV status. Over half (55%) of participants were worried “a lot” about getting HIV. During the first 3 months of follow-up, 90% of participants reported disclosing their PrEP use to at least one person, with 82% reporting disclosure to at least one friend, 56–59% reporting disclosure to at least one parent, partner, sibling or other family member (separately), 27% reporting disclosure to at least one neighbor, and 8% reporting disclosure to at least one nurse or doctor (Table 2). Among participants who disclosed to at least one member of a given social group during the first 3 months of follow-up, almost all (> 96% for each group) reported that at least one disclosure was followed by a supportive reaction within a given group.
Table I.
Participant characteristics prior to PrEP initiation
| N (%) or median (IQR) |
|
|---|---|
| Total Participants | 200 |
| Age (median, IQR) | 19 (17–21) |
| Age | |
| ≤18 years | 89 (44.5%) |
| >18 years | 111 (55.5%) |
| Education | |
| Primary or some secondary school | 113 (61.8%) |
| Completed secondary school or higher | 60 (30.2%) |
| Has a primary sexual partner at enrollment | |
| Yes | 172 (86.0%) |
| No | 28 (14.0%) |
| HIV status of primary sexual partner | |
| HIV negative | 98 (57.0%) |
| HIV positive | 1 (0.6%) |
| Participant doesn’t know | 73 (42.4%) |
| IPV in the past year at enrollment | |
| Any | 37 (18.6%) |
| None | 162 (81.4%) |
| “How worried are you about getting HIV?” | |
| Not worried | 43 (21.6%) |
| Worried some | 46 (23.1%) |
| Worried a lot | 110 (55.3%) |
Table II.
Disclosure and social support by social group and follow-up period
| Study period (Total non-missing observations) |
First 3 months of follow-up (n=191) |
First 6 months of follow-up (n=193) |
|---|---|---|
| Disclosed to anyone, n (%) a | 172 (90%) | 172 (89%) |
| Disclosed to a parent, n (%) b | 109 (57%) | 111 (58%) |
| Supportive reaction, n (%)c | 107 (98%) | 109 (98%) |
| Disclosed to a partner, n (%) | 107 (56%) | 111 (58%) |
| Supportive reaction, n (%) | 107 (100%) | 111 (100%) |
| Disclosed to a sibling, n (%) | 110 (58%) | 113 (59%) |
| Supportive reaction, n (%) | 109 (99%) | 112 (99%) |
| Disclosed to other family, n (%) | 113 (59%) | 114 (59%) |
| Supportive reaction, n (%) | 113 (100%) | 114 (100%) |
| Disclosed to a friend, n (%) | 156 (82%) | 156 (81%) |
| Supportive reaction, n (%) | 152 (97%) | 152 (97%) |
| Disclosed to a neighbor, n (%) | 51 (27%) | 54 (28%) |
| Supportive reaction, n (%) | 49 (96%) | 53 (98%) |
| Disclosed to nurse/doctor n (%) | 15 (8%) | 17 (9%) |
| Supportive reaction, n (%) | 15 (100%) | 17 (100%) |
% = number who disclosed to anyone / total non-missing observations
% = number who disclosed to at least one member of a given group / total non-missing observations
% = number who reported at least one supportive reaction / number who disclosed to at least one member of a given group
Participant exposure status was coded as missing if they had missing data for all visits during a given study period (i.e., month 1, 2, and 3 visits during the first 3 months of follow-up and month 1, 2, 3, and 6 visits during the first 6 months of follow-up)
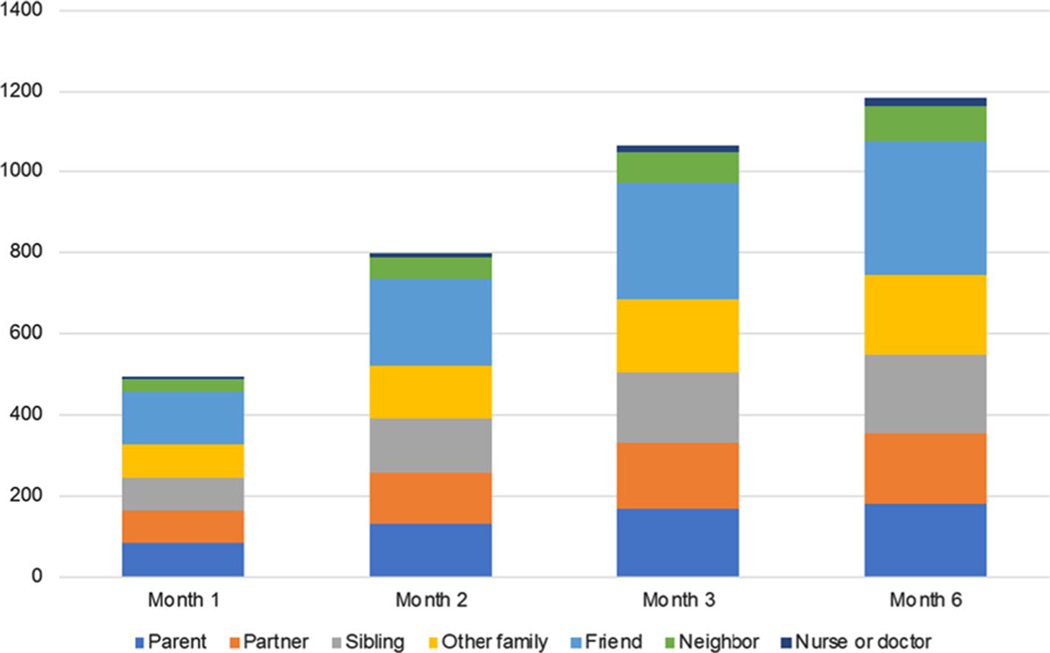
During the first 6 months of follow-up, a similar proportion of participants had reported disclosing their PrEP use to at least one member of a given social group as had been reported during the first 3 month of follow-up (Table 2). Further, similar to month 3, within a given social group, most participants (> 97%) reported that at least one disclosure was followed by a supportive reaction. Participants reported disclosing to a median of 4 different social groups by both month 3 and 6 of follow-up (IQR 2–5 and 2–6, respectively). The cumulative number of new group-level disclosures increased most substantially over the first 3 months of follow-up, and participants reported a total of 181 group-level disclosures to parents, 176 to partners, 192 to siblings, 194 to other family members, 334 to friends, 87 to neighbors, and 20 to nurses or doctors through month 6 (Fig. 1). Younger AGYW (≤ 18 years) were significantly more likely than older AGYW (> 18 years) to disclose to a parent (PR = 1.31, 95% CI 1.03, 1.67) (Table 3). There were no other statistically significant associations between age and disclosure to other groups.
Fig. 1.
Cumulative number of new group-level disclosures reported over follow-up
Table III.
The relative prevalence of PrEP use disclosure in the first 6 months of follow-up among those ≤ 18 years vs. > 18 years (N = 193)
| Disclosure prevalence | ||||
|---|---|---|---|---|
| ≤18 years | >18 years | PRa | 95% CI | |
|
|
||||
| Total, n | 86 | 107 | ||
| Disclosed to anyone, n (%) | 76 (88%) | 96 (90%) | 0.99 | 0.89, 1.09 |
| Disclosed to a parent, n (%) | 57 (66%) | 54 (50%) | 1.31 | 1.03, 1.67 |
| Disclosed to a partner, n (%) | 50 (58%) | 61 (57%) | 1.02 | 0.80, 1.30 |
| Disclosed to a sibling, n (%) | 49 (57%) | 64 (60%) | 0.95 | 0.75, 1.21 |
| Disclosed to other family, n (%) | 55 (64%) | 59 (55%) | 1.16 | 0.92, 1.46 |
| Disclosed to a friend, n (%) | 72 (84%) | 84 (79%) | 1.07 | 0.93, 1.22 |
| Disclosed to a neighbor, n (%) | 23 (27%) | 31 (29%) | 0.92 | 0.58, 1.46 |
| Disclosed to nurse/doctor n (%) | 10 (12%) | 7 (7%) | 1.78 | 0.71, 4.47 |
>18 years = referent group
PR=prevalence ratio; CI=confidence interval
PR and aPR estimates and 95% CIs were calculated using log-binomial regression
95% CI overlap with the null value of 1.0 was used to assess statistical significance of PR estimates
The Effect of PrEP Use Disclosure on Adherence at Months 3 and 6
At months 3 and 6, 40% (80/200) and 10% (20/200) of participants were classified as having high adherence, respectively. Overall, we did not find a strong effect of PrEP use disclosure on PrEP adherence in the full study population. For example, the prevalence of high adherence was only about 1.3 times as high among those reporting vs. not reporting disclosure of PrEP use to anyone at both month 3 (aPR = 1.31, 95% CI 0.66, 2.58) and month 6 (aPR = 1.33, 95% CI 0.41, 4.26) (Table 4). Similarly, disclosure to a greater number of different social groups did not appear to meaningfully affect adherence at month 3. At month 6, only a small effect with moderate precision was observed for a one-unit increase in the number of different social groups to which a participant had reported disclosing (aPR = 1.21, 95% CI 0.98, 1.50).
Table IV.
Modified Poisson regression analysis estimating the relative prevalence of high adherence at months 3 and 6 among AGYW who disclosed to anyone and to a greater number of different social groups
| Follow-up month | Events/N or median | % or IQR | PR (95% CI) | aPR (95% CI) |
|---|---|---|---|---|
|
High Adherence, TFV-DP ≥700 fmol/punch (n=80)
|
||||
| Month 3 (N=191) | ||||
| Disclosed to anyone | 74/172 | 43.0 | 1.36 (0.69, 2.70) | 1.31 (0.66, 2.58) |
| Sum of disclosure groups (range=0–8) | 4 | 2–5 | 0.98 (0.91, 1.06) | 0.97 (0.90, 1.05) |
|
| ||||
|
High Adherence, TFV-DP ≥700 fmol/punch (n=20)
|
||||
| Month 6 (N=193) | ||||
| Disclosed to anyone | 18/172 | 10.5 | 1.10 (0.27, 4.41) | 1.33 (0.41, 4.26) |
| Sum of disclosure groups (range=0–8) | 4 | 2–6 | 1.20 (0.95, 1.53) | 1.21 (0.98, 1.50) |
% = prevalence; PR= prevalence ratio; CI=confidence interval; aPR = adjusted prevalence ratio
Adjusted models controlled for age, trial arm, and disclosure at enrollment
PR and aPR estimates and 95% CIs were calculated using modified Poisson regression with a robust error variance
95% CI overlap with the null value of 1.0 was used to assess statistical significance of PR and aPR estimates
Social groups included partners, parents, siblings, other family, friends, neighbors, nurses/doctors, or other PR and aPR for the “sum of disclosure groups” exposure represent the ratio corresponding to a one-unit increase in the number of different social groups to which disclosure occurred prior to a given time point
There were no statistically significant associations, either in the full study population or in the separate age strata, between high adherence and disclosure of PrEP use to a partner or a friend. Disclosure to a parent, however, was associated with high adherence at month 6 in the younger age stratum (Table 5). Specifically, among younger AGYW (≤ 18 years), disclosure of PrEP use to a parent was asssociated with high month-6 adherence (aPR = 6.83, 95% CI 1.02, 45.56). In contrast, among older AGYW (> 18 years), those who disclosed their PrEP use to a parent were slightly less likely than those who did not disclose to a parent to have high month-6 adherence (aPR = 0.69, 95% CI 0.17, 2.90) (Wald test statistic for interaction (z) = −1.87, p = 0.062). At month 3, no meaningful associations between parent disclosure and adherence were found.
Table V.
Overall and age-stratified modified Poisson regression analysis estimating the relative prevalence of high adherence at months 3 and 6 among AGYW who disclosed their PrEP use to a partner, parent, and friend (separately) compared to those who did not
| Younger AGYW (≤18 years) | Older AGYW (>18 years) | Age × Disclosure | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| High Adherence | High Adherence | High Adherence | Wald test | ||||||||
|
|
|||||||||||
| Social group | Month | Events/N | % | aPR (95% CI) | Events/N | % | aPR (95% CI) | Events/N | % | aPR (95% CI) | |
| Parent | |||||||||||
| Did not disclose | 3 | 34/82 | 41.5 | 1.00 (ref) | 13/29 | 44.8 | 1.00 (ref) | 21/53 | 39.6 | 1.00 (ref) | z=0.38, p=.707 |
| Disclosed | 3 | 46/109 | 42.2 | 1.00 (0.71, 1.40) | 23/56 | 41.1 | 0.93 (0.59, 1.54) | 23/53 | 43.4 | 1.05 (0.67, 1.66) | |
| Did not disclose | 6 | 6/82 | 7.3 | 1.00 (ref) | 1/29 | 3.5 | 1.00 (ref) | 5/53 | 9.4 | 1.00 (ref) | z=−1.87, p=.062 |
| Disclosed | 6 | 14/111 | 12.6 | 2.03 (0.82, 5.00) | 11/57 | 19.3 | 6.83 (1.02, 45.56) | 3/54 | 5.6 | 0.69 (0.17, 2.90) | |
|
| |||||||||||
| Partner | |||||||||||
| Did not disclose | 3 | 37/84 | 44.1 | 1.00 (ref) | 17/37 | 46.0 | 1.00 (ref) | 20/47 | 42.6 | 1.00 (ref) | z=0.41, p=.683 |
| Disclosed | 3 | 43/107 | 40.2 | 0.94 (0.67, 1.33) | 19/48 | 39.6 | 0.87 (0.52, 1.45) | 24/59 | 40.7 | 1.00 (0.64, 1.57) | |
| Did not disclose | 6 | 8/82 | 9.8 | 1.00 (ref) | 5/36 | 13.9 | 1.00 (ref) | 3/46 | 6.5 | 1.00 (ref) | z=0.22, p=.823 |
| Disclosed | 6 | 12/111 | 10.8 | 1.19 (0.49, 2.89) | 7/50 | 14.0 | 1.09 (0.36, 3.29) | 5/61 | 8.2 | 1.34 (0.32, 5.62) | |
|
| |||||||||||
| Friend | |||||||||||
| Did not disclose | 3 | 15/35 | 42.9 | 1.00 (ref) | 7/13 | 53.9 | 1.00 (ref) | 8/22 | 36.4 | 1.00 (ref) | z=0.89, p=.374 |
| Disclosed | 3 | 65/156 | 41.7 | 0.92 (0.60, 1.42) | 29/72 | 40.3 | 0.75 (0.42, 1.31) | 36/84 | 42.9 | 1.09 (0.58, 2.03) | |
| Did not disclose | 6 | 3/37 | 8.1 | 1.00 (ref) | 1/14 | 7.1 | 1.00 (ref) | 2/23 | 8.7 | 1.00 (ref) | z=−0.87, p=.387 |
| Disclosed | 6 | 17/156 | 10.9 | 1.31 (0.43, 3.96) | 11/72 | 15.3 | 2.30 (0.36, 14.83) | 6/84 | 7.1 | 0.75 (0.17, 3.35) | |
% = prevalence; CI=confidence interval; aPR = adjusted prevalence ratio; z = Wald test statistic; p = Wald p-value (α=.15)
High adherence was defined as TFV-DP ≥700 fmol/punch
Adjusted models controlled for age, trial arm, and disclosure at enrollment; partner models also adjusted for having a primary partner and history of IPV in the past year
Prevalence ratio estimates and 95% CIs were calculated using modified Poisson regression with a robust error variance
95% CI overlap with the null value of 1.0 was used to assess statistical significance of aPR estimates
In the complete-case sensitivity analysis, 48% (80/166) and 14% (20/138) of participants were classified as having high adherence at month 3 and month 6, respectively. Associations of PrEP use disclosure with high adherence in the sensitivity analysis remained largely consistent with the primary analysis in direction and magnitude of effect (supplemental Appendix B).
Discussion
In this study of 200 AGYW, most participants disclosed their PrEP use to at least one other person. During 6 months of follow-up, just over half of participants reported disclosing their PrEP use to at least one parent, partner, sibling, or other family member; a greater proportion reported disclosing to friends and fewer reported disclosing to neighbors and nurses or doctors. In response to their disclosure, the majority of participants reported at least one supportive reaction within a given group. Disclosure to certain groups varied by age, with younger AGYW more likely to disclose to a parent than older AGYW. This finding is important given that parents can have a strong influence on health-related motivations, decisions, and behaviors [22], particularly among younger AGYW who may be more likely to be living at home.
Recent recommendations for improving PrEP adherence suggest that tailored PrEP delivery programs are necessary to meet the needs of African AGYW [29]. In this investigation aimed at informing these programs, we found that disclosure of PrEP use to anyone and to a greater number of different social groups did not affect adherence. While qualitative research has highlighted the potential association between disclosure and adherence among AGYW, the perceived impact of PrEP disclosure reported in qualitative studies seems to hinge upon reactions and subsequent levels of support or stigma [30–32]. In the current study, we were not able to adequately assess support following disclosure, as the survey did not allow participants to report multiple reactions if they disclosed to more than one person within a given social group during a given time period. Further, binary (yes/no) answer options for supportive reactions did not capture any nuance in support. From previous research, we know disclosure is not a binary construct [33]. Although the vast majority of participants reported supportive reactions following disclosure experiences, qualitative data from 3P shows more variability in support [31]. Therefore, it is possible that we did not observe a strong overall effect of disclosure in our study because disclosure did not yield support for PrEP adherence. Research is needed to understand the conditions under which disclosure elicits support for PrEP use among AGYW.
In our stratified analysis, effects of disclosure to a parent on adherence varied significantly by age. Among younger AGYW, disclosure of PrEP use to a parent was associated with high adherence at month 6. Among older AGYW, the reverse effect was observed. These findings suggest that parents may play an important role in facilitating PrEP use among younger AGYW. Qualitative research from the 3P study also found that disclosure to parents, and particularly mothers, was common, and often perceived as having either a strong positive or negative impact on PrEP use. Support for PrEP use from mothers was often related to whether they understood participant explanations of PrEP, their normative beliefs about sexual and reproductive health matters and pill-taking behavior more broadly, and their concerns surrounding the resemblance of PrEP to HIV treatment [31]. More work is needed to develop strategies for engaging parents to support their child’s PrEP use and to understand how fears surrounding disclosure to parents may be impacting PrEP uptake and adherence.
Similar to parent disclosure, just over half of AGYW reported disclosing to a partner in our study population, but disclosure to a partner did not appear to affect adherence. In PrEP and microbicide placebo-controlled trials, male partners have been shown to have a strong influence on African women’s product uptake and use [18–20]. Among AGYW and in open-label studies such as 3P, however, the impact of partner disclosure may differ. Narratives from qualitative research suggest that disclosure to partners can alter perceptions of trust and expose young women to violence and stigma [18, 30, 31]. Therefore, disclosure to partners should be carefully considered, and AGYW initiating PrEP should be provided with strategies for discreet use and non-disclosure to partners who may impede their successful PrEP use.
The majority of AGYW disclosed to at least one friend, and disclosure to friends did not have a clear effect on PrEP adherence. The absence of a clear effect in our study is somewhat surprising, since peer support interventions have been shown to improve treatment outcomes among adolescents on antiretroviral therapy [34]. In EMPOWER, however, adherence clubs did benefit young women’s PrEP use [35]. Adherence clubs and other peer support interventions for African AGYW should be evaluated to determine how peers can help motivate PrEP use. Further, outside of PrEP programs, providers should consider the potentially important role of peers given AGYW are frequent disclosers to this group [30], and research should explore which peer relationships (close friends vs. acquaintances) are most beneficial. For example, qualitative findings from POWER suggest that close friends within an AGYW’s inner circle may positively influence PrEP use, whereas broader friend groups may be negative influencers [36].
We note that our sample size was small, and the proportion of participants with high adherence at month 6 was minimal, limiting the precision of estimates and our ability to detect meaningful associations. Additionally, missing DBS data was common, requiring imputation of adherence values for analysis. Although our sensitivity analysis produced similar results, it is possible that missing outcome data biased our findings to some extent. There is also potential for exposure misclassification, as self-reported disclosure is subject to recall error and social desirability bias. Further, there is likely unmeasured confounding and variation in disclosure experience and opportunity, which may dampen the validity of causal inferences. For example, the survey did not ask participants who they were living with, a potentially important covariate that may have impacted both disclosure/non-disclosure opportunity and PrEP adherence. Moreover, when examining disclosure to specific social groups, we were not able to control for variability in disclosure to other specific group types. Finally, we based our threshold for high adherence on research conducted among MSM and adults [7, 26]. To facilitate future investigations of PrEP adherence among South African AGYW, research is needed to identify the most relevant adherence thresholds for women and AGYW specifically.
This investigation informs several recommendations for future research. First, larger, open-label studies are needed to assess the effects we examined here, with the inclusion of additional covariates and expanded measures of disclosure and social support. A larger sample would also provide more power to determine if the effects of disclosure vary by age, as we observed for disclosure to parents despite limited precision. Next, the effects of disclosure on adherence to daily oral PrEP should be compared to the effects of disclosure on adherence to alternative dosing regimens (e.g., intermittent, on-demand) and formulations (e.g., topical, injectable, insertable) to determine if disclosure is more important for daily pill-taking compared to more discreet or longer-acting formulations. Last, more research is needed to understand barriers and facilitators to both disclosure and PrEP adherence. For example, qualitative research has identified anticipated stigma as a barrier to disclosure among AGYW and highlights the importance of multi-level programs (e.g., media campaigns, community outreach) in changing norms around sexuality and increasing community knowledge around PrEP [32].
Conclusions
We quantitatively investigated PrEP use disclosure and adherence in a population that continues to experience high HIV incidence. Despite limited power, our results suggest that more work is needed to understand parents’ roles as allies, and to develop strategies for engaging parents in their child’s PrEP use. Further, adherence support programs for AGYW should find ways that peers and partners can help motivate effective PrEP use, and additional research should be conducted to identify other drivers of PrEP adherence. Examination of the effect of PrEP use disclosure to various social groups on adherence in larger cohorts with more nuanced measures of disclosure and social support could provide additional evidence for tailored PrEP delivery programs and future intervention efforts.
Supplementary Material
Acknowledgments
Funding This research was supported by Grants R01MH107251 (PIs: Bekker and Celum) and F31MH119965 (PI: Giovenco) from the National Institutes of Health.
Footnotes
Data Availability Data may contain identifying or sensitive patient information. To preserve participant confidentiality, these data cannot be shared publicly. The Principal Investigators of this study, Connie Celum (ccelum@uw.edu) and Linda-Gail Bekker (linda-gail.bekker@hivresearch.org.za), may be contacted with requests to access these data. Access may be granted to those wishing to use the data for research purposes.
Code Availability Analysis code may be shared upon request to Danielle Giovenco (danielle.giovenco1@gmail.com).
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10461-021-03455-xx.
Declarations
Conflict of interest The authors have declared that no competing interests exist.
Ethical Approval All study procedures were approved by the University of Cape Town’s Human Research Ethics Committee and this analysis was approved by the University of North Carolina at Chapel Hill’s Institutional Review Board. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to Participate All participants provided written informed consent in English or isiXhosa. A parental consent waiver was granted for participants aged 16–17 years.
References
- 1.UNAIDS. AIDS by the numbers [Internet]. 2016. https://www.unaids.org/sites/default/files/media_asset/AIDS-by-the-numbers-2016_en.pdf
- 2.Karim SS, Baxter C. HIV incidence rates in adolescent girls and young women in sub-Saharan Africa. Lancet Glob Health. 2019;7(11):e1470–1. [DOI] [PubMed] [Google Scholar]
- 3.Pettifor A, Bekker LG, Hosek S, et al. Preventing HIV among young people: research priorities for the future. J Acquir Immune Defic Syndr. 2013;63(2):S155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celum CL, Delany-Moretlwe S, McConnell M, et al. Rethinking HIV prevention to prepare for oral PrEP implementation for young African women. J Int AIDS Soc. 2015;18:20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velloza J, Delany-Moretlwe S, Baeten JM. Comprehensive HIV risk reduction interventions for 2020 and beyond: product choices and effective service-delivery platforms for individual needs and population-level impact. Curr Opin HIV AIDS. 2019;14(5):423–32. [DOI] [PubMed] [Google Scholar]
- 6.Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molina JM, Charreau I, Spire B, et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV. 2017;4(9):e402–10. [DOI] [PubMed] [Google Scholar]
- 9.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill K, Johnson L, Dietrich J, et al. Acceptability, safety, and patterns of use of oral tenofovir disoproxil fumarate and emtricitabine for HIV pre-exposure prophylaxis in South African adolescents: an open-label single-arm phase 2 trial. Lancet Child Adolesc Health. 2020;4:875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosek SG, Landovitz RJ, Kapogiannis B, et al. Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatr. 2017;171:1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celum C, Hosek S, Tsholwana M, et al. PrEP uptake, persistence, adherence, and effect of retrospective drug level feedback on PrEP adherence among young women in southern Africa: Results from HPTN 082, a randomized controlled trial. PLoS Med. 2021;18(6):e1003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosek S, Celum C, Wilson CM, et al. Preventing HIV among adolescents with oral PrEP: observations and challenges in the United States and South Africa. J Int AIDS Soc. 2016;19:21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S, Yamazaki M, Harris DR, Harper GW, Ellen J. Social support and Human Immunodeficiency Virus-Status disclosure to friends and family: implications for human immunodeficiency virus-positive youth. J Adolesc Health. 2015;57(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith R, Rossetto K, Peterson BL. A meta-analysis of disclosure of one’s HIV-positive status, stigma and social support. AIDS Care. 2008;20(10):1266–75. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery ET, van der Straten A, Stadler J, et al. Male partner influence on women’s HIV prevention trial participation and use of pre-exposure prophylaxis: the importance of “understanding.” AIDS Behav. 2015;19(5):784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corneli A, Perry B, Agot K, et al. Facilitators of adherence to the study pill in the FEM-PrEP clinical trial. PLoS ONE. 2015;10(4):e0125458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanham M, Wilcher R, Montgomery ET, et al. Engaging male partners in women’s microbicide use: Evidence from clinical trials and implications for future research and microbicide introduction. J Int AIDS Soc. 2014;17(3):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viner RM, Ozer EM, Denny S, et al. Adolescence and the social determinants of health. Lancet. 2012;379:1641–52. [DOI] [PubMed] [Google Scholar]
- 22.Grotevant HD, Cooper CR. Patterns of interaction in family relationships and the development of identity exploration in adolescence. Child Dev. 1985;56:415–28. [PubMed] [Google Scholar]
- 23.Patton GC, Sawyer SM, Santelli JS, et al. Our future: a Lancet commission on adolescent health and wellbeing. Lancet. 2016;387(10036):2423–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celum CL, Gill K, Morton JF, et al. Incentives conditioned on tenofovir levels to support PrEP adherence among young South African women: a randomized trial. J Int AIDS Soc. 2020;23(11):e25636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morton JF, Myers L, Gill K, Bekker L-G, et al. Evaluation of a behavior-centered design strategy for creating demand for oral PrEP among young women in Cape Town, South Africa. Gates Open Res. 2020;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother. 2018;62(1):e0171017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 28.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haberer JE, Mugo N, Baeten JM, Pyra M, Bukusi E, Bekker LG. PrEP as a lifestyle and investment for adolescent girls and young women in Sub-Saharan Africa. J Int Assoc Provid AIDS Care. 2019;18:2325958219831011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scorgie F, Khoza N, Baron D, et al. Disclosure of PrEP use by young women in South Africa and Tanzania: qualitative findings from a demonstration project. Cult Health Sex. 2019;23:1–6. [DOI] [PubMed] [Google Scholar]
- 31.Giovenco D, Gill K, Fynn L, et al. Experiences of oral pre-exposure prophylaxis (PrEP) use disclosure among South African adolescent girls and young women and its perceived impact on adherence. PLoS ONE. 2021;16(3):e0248307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velloza J, Khoza N, Scorgie F, et al. The influence of HIV-related stigma on PrEP disclosure and adherence among adolescent girls and young women in HPTN 082: a qualitative study. J Int AIDS Soc. 2020;23(3):e25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahin-Hodoglugil NN, van der Straten A, Cheng H, et al. Degrees of disclosure: a study of women’s covert use of the diaphragm in an HIV prevention trial in sub-Saharan Africa. Soc Sci Med. 2009;69(10):1547–55. [DOI] [PubMed] [Google Scholar]
- 34.Tsondai PR, Wilkinson LS, Grimsrud A, Mdlalo PT, Ullauri A, Boulle A. High rates of retention and viral suppression in the scale-up of antiretroviral therapy adherence clubs in Cape Town, South Africa. J Int AIDS Soc. 2017;20:21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramskin L, Baron D, Makgamathe K, et al. EMPOWER clubs for young women using oral PrEP as part of an HIV prevention package: what we’ve learned so far. Adherence to HIV prevention and treatment. Adherence to HIV Prevention and Treatment; April 26–27; Harare, Zimbabwe; 2018. [Google Scholar]
- 36.Katz AW, Rousseau E, Khoza N, Mogaka F, Bukusi E, Delany-Moretlwe S, Bekker LG, Morton J, Johnson R, Celum C, Baeten J. Using social maps to explore young women’s experiences with social support of their oral PrEP use in Kenya and South Africa. J Int AIDS Soc. 2021;24:78–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.