Abstract
The accumulation and propagation of misfolded proteins in the brain is a pathological hallmark shared by many neurodegenerative diseases, such as the depositions of β-amyloid and hyperphosphorylated tau proteins in Alzheimer's disease. Initial evidence shows the role of nitric oxide synthases in the development of neurodegenerative diseases. A recent, in an exciting paper (Bourgognon et al. in Proc Natl Acad Sci USA 118, 1–11, 2021. 10.1073/pnas.2009579118) it was shown that the inducible nitric oxide synthase plays an important role in promoting oxidative and nitrergic stress leading to neuroinflammation and consequently neuronal function impairments and decline in synaptic strength in mouse prion disease. In this context, we reviewed the possible mechanisms of nitric oxide synthase in the generation of neurodegenerative diseases.
Keywords: iNOS, Neurodegeneration, Prion model, Oxidative stress, Nitrergic stress
Introduction
Alzheimer's Disease (AD) is a progressive neurodegenerative disease that affects around 50 million people worldwide and this number is estimated to double every 20 years (Prince et al. 2013). Cumulative evidence showed that AD etiology is characterized by the depositions of the β-amyloid and hyperphosphorylated tau proteins. The proposed prion mechanism in AD is marked by misshapen proteins that spread through the brain like an infection leading normal proteins to adopt a misfolded shape contributing to the neural dysfunction (Watts and Prusiner 2018; Condello et al. 2020). AD and other neurodegenerative diseases share the common characteristic of steroretyped protein accumulation in the brain.
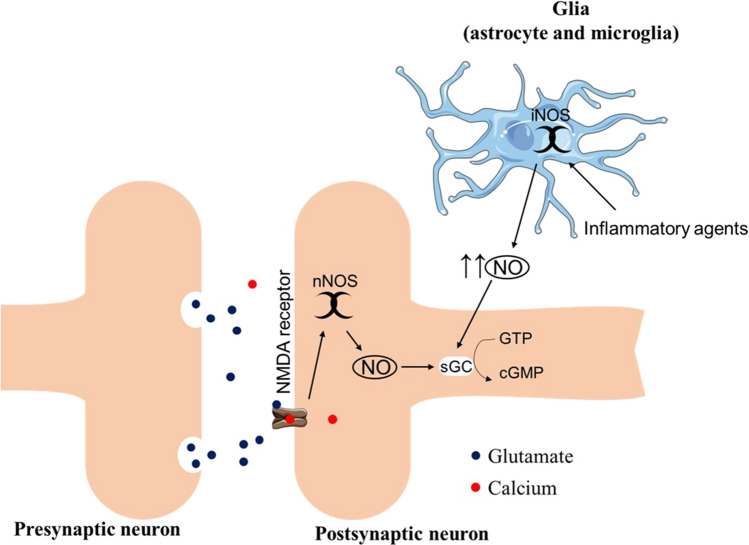
Although nitric oxide (NO) plays an essential role in synaptic transmission and brain plasticity, especially in the cortex and hippocampus, the cellular stress mediated by high levels of NO and nitrergic/oxidative stress can lead to the impairment of synaptic plasticity and premature neurodegeneration (Steinert et al. 2010; Balez and Ooi 2016). In the brain, the major source of NO production is the neural nitric oxide synthase (nNOS), which is activated mainly when the N-methyl-d-aspartate (NMDA) receptor binds to glutamate in the postsynaptic neuron. However, the inducible nitric oxide synthase (iNOS) is expressed by the action of inflammatory agents, such as interleukin-1 (IL-1), interferon-γ, and NK-κB in astrocytes and microglia (Lüth et al. 2001; Guix et al. 2005; Harooni et al. 2009) (Fig. 1).
Fig. 1.
Schematic illustration of the mechanism of nNOS and iNOS production of NO. The presynaptic release of glutamate activates the ionotropic receptor NMDA which leads to the entrance of calcium into the postsynaptic neuron. The increased amount of calcium in the postsynaptic neuron induces the activation of nNOS and consequently the release of low levels of NO by the nNOS. The iNOS expression and activation are mediated by the action of inflammatory agents (e.g., interleukin-1, INF-γ, and NK-κB) leading to the production of high levels of NO. The bioavailability of NO stimulates the enzyme soluble guanylyl cyclase catalyzing the conversion of guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP) which acts in the maintenance of several neural physiological processes, such as neurotransmission, synaptic plasticity, and cognition
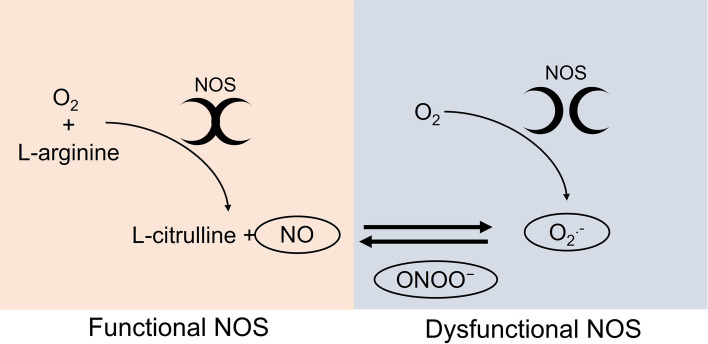
However, in dysfunctional states (e.g., inflammation, hypoxia, brain ischemia), the overstimulation of the iNOS enzyme leads to the uncoupling of the NOS dimers, causing a massive generation of peroxynitrite (Fig. 2). As consequence, the nitration of the protein-bound and free tyrosine residues by peroxynitrite forms nitrotyrosine that leads to structural disruption and dysfunction of the proteins (Bandookwala and Sengupta 2020; Bourgognon et al. 2021). The high levels of peroxynitrite seem to be an important mechanism in AD pathology. A previous study showed that peroxynitrite leads to the nitration of β-amyloid protein and is highly toxic to neurons inducing synaptic transmission impairment and accelerating the aggregation of the β-amyloid peptide (Kummer et al. 2011; Guivernau et al. 2016).
Fig. 2.
Schematic illustration of the mechanism of formation of NO, superoxide anion (O2.-), and peroxynitrite (ONOO-) by NOS. The functional dimer of NOS has the coupled form that uses oxygen (O2) and L-arginine as cofactors for the formation of NO and L-citrulline. In its dysfunctional state due to inflammation, hypoxia, brain ischemia, and NOS overstimulation, the NOS presented in it is uncoupled form. In this state, the enzyme uses O2 to produce O2.-. Due to the high affinity of O2.- for NO, these molecules interact and form another reactive species, peroxynitrite (ONOO-)
During aging, there is an increase in oxidative stress in the brain independent of clinical neurological manifestations. Interestingly, the CA1 region of the hippocampus is the brain region most susceptible to oxidative damage and also one of the most sensitive areas to neurodegeneration associated with AD (Wang and Michaelis 2010; Salim 2017). Importantly, beta-amyloid protein deposition can promote oxidative stress generation, which consequently leads to the formation of neuritic plaques, forming a vicious cycle of brain impairment (Reynolds et al. 2005; Butterfield and Halliwell 2019).
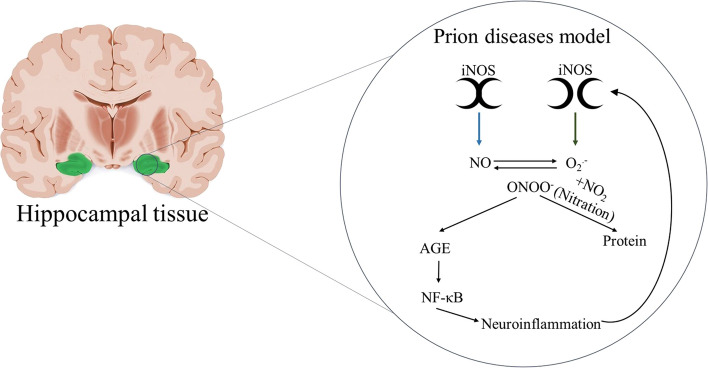
A recent manuscript aimed to understand the role of nitrergic stress in the development of neurodegenerative diseases used prion-mediated degeneration as the disease model system (Bourgognon et al. 2021).This model was characterized by the mRNA overexpression of iNOS and it was associated with synaptic and neural dysfunction measured by the electrophysiology recordings of the evoked postsynaptic currents trains and current-evoked action potential, respectively. Moreover, in the neurodegeneration model, the iNOS produced higher amounts of peroxynitrite, which leads to the nitration of protein-bound and free tyrosine residues by peroxynitrite forms of nitrotyrosine promoting structural disruption and dysfunction of the protein. The peroxynitrite promoted the accumulation of advanced glycation end-products (AGE), which activate the NF-κB signaling and consequently neuroinflammation. It is important to highlight that neuroinflammation induced the iNOS activation generating a progressive cycle of the neuroinflammation, as illustrated in Fig. 3. To prove that iNOS promotes neuroinflammation, Bourgognon and colleagues treated the prion mice with the NOS inhibitor, L-NAME. Interestingly, the synaptic and neural activities were restored and the AGE and nitrergic/oxidative signaling were preserved. This stimulating study suggests the iNOS role on neurodegenerative disease and its effects on neuroinflammation and nitrergic/oxidative. It also suggests exciting new horizons for potential treatments for these diseases, including AD.
Fig. 3.
The role of iNOS on neuroinflammation. The iNOS produces NO and O2.−. The reaction of these two molecules generates the ONOO- which leads to the nitration of protein and activation of advanced glycation end-products (AGE) signaling cascade and activation of NF- κB promoting neuroinflammation. The neuroinflammation induces the iNOS expression in a continuous cycle
It is important to highlight that NO molecule plays a vital role in several physiologic processes, and the inhibition of the NOS enzymes may lead to bladder underactivation and endothelial dysfunction (De Oliveira et al. 2019; Siragusa et al. 2020; Justo and Afonso 2021). Although Bourgognon and colleagues showed important evidence on the iNOS role for neurodegenerative diseases, the pharmacological inhibitor for iNOS (L-NAME) is very unspecific, acting in all isoforms. For example, L-NAME acts with 10- to 30-fold selectivity for the eNOS and nNOS isoforms (Boer et al. 2000). In Bourgognon’s study, the animals were treated with 20 mg/kg of L-NAME for three weeks, which can be used to inhibit the endothelial nitric oxide synthase (eNOS), as a well-characterized rodent model for hypertension (Kumar et al. 2012). The genomic structure of the NOS isoforms shares 50–60% homology, and there is no available specific iNOS inhibitor. Although the latest clinical trials with “specific” iNOS inhibitors have great inhibitory effects on the inflammation mediated by iNOS, they also exhibited severe toxicities in human subjects, such as elevated blood pressure that were attributed to their non-selectivity effect on all forms of NOS (Minhas et al. 2020). Although this recent study opens the discussion of the possible role of the nNOS, iNOS, and eNOS isoforms on the neurodegenerative diseases and their role on oxidative/nitrergic stress and neuroinflammation, additional studies will be crucial to confirm this hypothesis and test new therapeutic targets to inhibit specific NOS forms.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest related to this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Balez R, Ooi L. Getting to NO Alzheimer’s disease: Neuroprotection versus neurotoxicity mediated by nitric oxide. Oxid Med Cell Longev. 2016 doi: 10.1155/2016/3806157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandookwala M, Sengupta P. 3-Nitrotyrosine: a versatile oxidative stress biomarker for major neurodegenerative diseases. Int J Neurosci. 2020;130:1047–1062. doi: 10.1080/00207454.2020.1713776. [DOI] [PubMed] [Google Scholar]
- Boer R, Ulrich WR, Klein T, et al. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol. 2000;58:1026–1034. doi: 10.1124/mol.58.5.1026. [DOI] [PubMed] [Google Scholar]
- Bourgognon JM, Spiers JG, Robinson SW, et al. Inhibition of neuroinflammatory nitric oxide signaling suppresses glycation and prevents neuronal dysfunction in mouse prion disease. Proc Natl Acad Sci USA. 2021;118:1–11. doi: 10.1073/pnas.2009579118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20:148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condello C, DeGrado WF, Prusiner SB. Prion biology: implications for Alzheimer’s disease therapeutics. Lancet Neurol. 2020;19:802–803. doi: 10.1016/S1474-4422(20)30274-X. [DOI] [PubMed] [Google Scholar]
- De Oliveira MG, Alexandre EC, Bonilla-Becerra SM, et al. Autonomic dysregulation at multiple sites is implicated in age associated underactive bladder in female mice. Neurourol Urodyn. 2019;38:1212–1221. doi: 10.1002/nau.23990. [DOI] [PubMed] [Google Scholar]
- Guivernau XB, Bonet XJ, Godoy JA, et al. Amyloid-β peptide nitrotyrosination stabilizes oligomers and enhances NMDAR-mediated toxicity. J Neurosci. 2016;36:11693–11703. doi: 10.1523/JNEUROSCI.1081-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guix FX, Uribesalgo I, Coma M, Muñoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Harooni HE, Naghdi N, Sepehri H, Rohani AH. The role of hippocampal nitric oxide (NO) on learning and immediate, short- and long-term memory retrieval in inhibitory avoidance task in male adult rats. Behav Brain Res. 2009;201:166–172. doi: 10.1016/j.bbr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Justo AFO, Afonso PPL. The role of vascular endothelial protein tyrosine phosphatase on nitric oxide synthase function in diabetes: from molecular biology to the clinic. J Cell Commun Signal. 2021 doi: 10.1007/s12079-021-00611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Prahalathan P, Raja B. Syringic acid ameliorates L-NAME-induced hypertension by reducing oxidative stress. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:1175–1184. doi: 10.1007/s00210-012-0802-7. [DOI] [PubMed] [Google Scholar]
- Kummer MP, Hermes M, Delekarte A, et al. Nitration of tyrosine 10 critically enhances amyloid b aggregation and plaque formation. Cell Press. 2011;71:833–844. doi: 10.1016/j.neuron.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Lüth HJ, Holzer M, Gärtner U, et al. Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer’s disease, in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology. Brain Res. 2001;913:57–67. doi: 10.1016/S0006-8993(01)02758-5. [DOI] [PubMed] [Google Scholar]
- Minhas R, Bansal Y, Bansal G. Inducible nitric oxide synthase inhibitors: a comprehensive update. Med Res Rev. 2020;40:823–855. doi: 10.1002/med.21636. [DOI] [PubMed] [Google Scholar]
- Prince M, Bryce R, Albanese E, et al. The global prevalence of conduct disorder: a systematic review and meta-analysis. Alzheimer’s Dement. 2013;9:63–75. doi: 10.18502/ijps.v16i2.5822. [DOI] [PubMed] [Google Scholar]
- Reynolds MR, Berry RW, Binder LI. Site-specific nitration and oxidative dityrosine bridging of the τ protein by peroxynitrite: Implications for Alzheimer’s disease. Biochemistry. 2005;44:1690–1700. doi: 10.1021/bi047982v. [DOI] [PubMed] [Google Scholar]
- Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. 2017;360:201–205. doi: 10.1124/jpet.116.237503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siragusa M, Justo AFO, Malacarne PF, et al. VE-PTP inhibition elicits eNOS phosphorylation to blunt endothelial dysfunction and hypertension in diabetes. Cardiovasc Res. 2020;117:1546–1556. doi: 10.1093/cvr/cvaa213. [DOI] [PubMed] [Google Scholar]
- Steinert JR, Chernova T, Forsythe ID. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist. 2010;16:435–452. doi: 10.1177/1073858410366481. [DOI] [PubMed] [Google Scholar]
- Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:1–13. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Prusiner SB. b-Amyloid prions and the pathobiology of Alzheimer’s disease Joel. Cold Spring Harb Perspect Med. 2018;8:1–14. doi: 10.1101/cshperspect.a023507. [DOI] [PMC free article] [PubMed] [Google Scholar]