Abstract
Objective:
To evaluate the discriminatory ability of different repetition increments of saccades and gaze stability testing for diagnosing concussion in adolescents.
Design:
Cross-sectional
Setting:
Suburban high school and academic pediatric tertiary care center
Participants:
69 adolescent athletes within 28 days of a sports- or recreation-related concussion and 69 adolescent athletes without recent concussion
Assessment of Independent Variables:
Symptom provocation with horizontal and vertical saccades and gaze stability testing performed up to 30 repetitions
Main Outcome Measures:
Sensitivity and specificity at 10-repetition increments (≤10, ≤20, ≤30) and area under the receiver operating characteristic curves (AUC) of a visio-vestibular examination (VVE) subscore, scored 0-4 based on the number of assessments with symptom provocation, at each repetition increment.
Results:
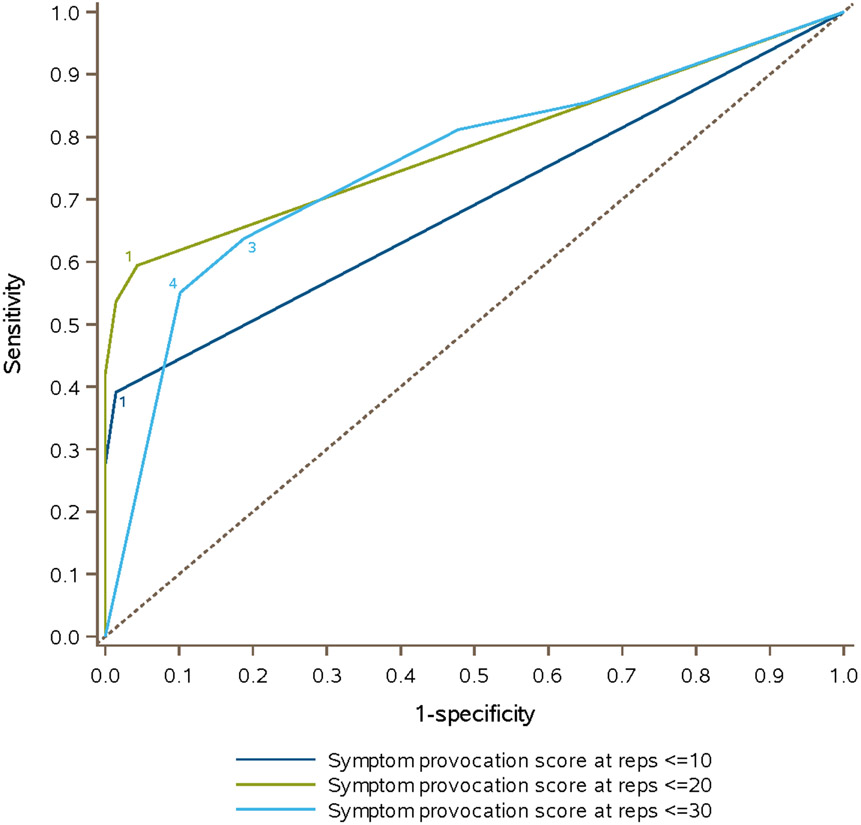
Sensitivity improved when increasing from ≤10 to ≤20 to ≤30 repetitions for horizontal (25% to 50% to 69%) and vertical (32% to 52% to 74%) saccades and horizontal (19% to 45% to 71%) and vertical (23% to 45% to 72%) gaze stability. Specificity was comparable at ≤10 and ≤20 repetitions, but decreased at ≤30 repetitions across assessments. For a VVE subscore (0-4) based on the number of symptomatic assessments, the discriminatory ability of the test was highest at ≤20 repetitions (AUC of 0.79) with an optimal subscore of one (sensitivity 59%, specificity 96%).
Conclusions:
A VVE including a higher threshold level of repetitions for saccades and gaze stability has improved discriminatory ability for concussion, with an optimized AUC of 0.79 at ≤20 repetitions.
Keywords: Visio-vestibular examination, saccades, gaze stability, vestibulo-ocular reflex, pediatric concussion
INTRODUCTION
Sports-related concussions (SRC) are common among youth athletes, with a recent epidemiological study estimating 1.1 to 1.9 million sports- and recreation-related concussions occurring annually in the US among children 18 years of age or younger.1 Since youth concussion has grown as a public health concern, more research has focused on improving identification and management. Amidst this body of knowledge, there is emerging evidence that visual- and vestibular-related impairments are common after concussion2-4 and are an important part of concussion evaluation,5-7 as they have been linked to worse outcomes, including delayed return to school and sport.2,8-11
In order to identify vestibular and ocular motor deficits in the clinical setting, the Vestibular/Ocular Motor Screening (VOMS) tool was developed and validated as a brief examination, assessing smooth pursuits, saccades, gaze stability (the vestibulo-ocular reflex), visual motion sensitivity (VMS), and near point of convergence (NPC).12 This tool measures self-reported headache, dizziness, nausea, and fogginess (each rated on a 0-10 scale), assessing severity of symptom provocation with each assessment by comparing pre- and post-assessment symptoms. Previous studies have investigated the validity and reliability of VOMS13-17, risk factors for visio-vestibular dysfunction after concussion,13,18-20 and the association of visio-vestibular dysfunction with prolonged recovery and negative outcomes after concussion.4,8-11,21
Since concussions often manifest with only mild physical examination deficits, concern for ceiling effects has emerged for many concussion tests, including VOMS.22 As a result, there has been interest in adding difficulty or complexity to standard concussion assessments to improve sensitivity, as exemplified by dual-task gait, in which a concurrent cognitive task is performed during tandem gait testing.23,24 It is increasingly recognized that symptom provocation with physical, cognitive, and visio-vestibular testing is more closely related to functional deficits in real-world settings, such as school and sport, compared to symptoms at rest,5,8,25 and testing that is not challenging enough may fail to detect mild deficits seen in concussion. We hypothesized that 10 repetitions of saccades and gaze stability on VOMS may have limited sensitivity, and thus increasing testing beyond 10 repetitions may better differentiate between concussed and non-concussed youth. However, another study of this visio-vestibular exam in neurologically normal children suggested that increasing to 30 repetitions may increase false positives. In this study, 19% of the non-concussed cohort developed symptoms with saccadic testing, and nearly all of them (97%) developed symptoms between 10 and 30 repetitions.26
No study has determined the ideal number of repetitions of saccades and gaze stability to maximize differentiation between concussed and non-concussed youth. To address this gap, the primary objective of this study was to evaluate the discriminatory ability of saccades and gaze stability testing at different repetition increments to determine the optimal threshold level of repetitions for differentiating adolescent athletes with and without concussion.
METHODS
Study Design and Population
Concussed athletes and healthy controls underwent a comprehensive visio-vestibular examination (VVE) between August 2017 and May 2018, including saccades and gaze stability, as well as smooth pursuits, VMS, and NPC, as part of a large, prospective observational study.27 Healthy controls were recruited from a private suburban high school prior to their respective soccer, basketball, or lacrosse seasons. Concussed athletes were recruited from the same high school, as well as a specialty referral concussion program at a large pediatric academic center. Concussions were diagnosed by board-certified sports medicine physicians according to the definition outlined in the 5th Consensus Statement on Concussion in Sport.28 Inclusion criteria for athletes with concussion included sports- or recreation-related injury with initial assessment within 28 days of injury. Subjects within one month of clearance from a previous concussion were excluded from both the concussion and healthy control groups. Healthy controls who sustained a concussion during the study period were included only in the concussion group. The study was approved by our institution’s Institutional Review Board (IRB) with subjects providing adult consent and/or child assent via electronic consent forms in the Research Electronic Data Capture (REDCap) tool hosted at the same hospital network.29
Data Collection
Demographic data, including sex, age, and race/ethnicity, were abstracted from the electronic health record for concussed athletes and collected via self-reported questionnaire for healthy controls. We collected self-reported data from both concussed athletes and healthy controls regarding past medical history, including previous concussion(s) and diagnosis of mood disorder (anxiety or depression), vision abnormality (strabismus or amblyopia), motion sickness, or ADHD, as these medical co-morbidities may affect symptom reporting or performance on visio-vestibular assessments. The VVE was conducted by sports medicine physicians or research staff, all of whom underwent standardized training by a single sports medicine physician. Healthy controls completed testing in a dedicated space at the participating high school while concussed athletes completed testing in the same space of the high school or in a sports medicine office at our institution. The VVE was performed as part of a larger battery of clinical and objective measures, as described previously.27 All study data were entered and stored electronically in REDCap.
Assessments
The VVE used in this study is derived but distinct from VOMS as it includes assessments of smooth pursuits, saccades (horizontal and vertical), gaze stability (horizontal and vertical), visual motion sensitivity, near point of convergence, accommodation, and gait/balance testing.8 The examination is always completed in the sequence listed above.
For the purposes of this analysis, results from saccades and gaze stability testing were examined since these two assessments were performed up to 30 repetitions, rather than 10 repetitions as previously published in VOMS.12 Saccadic testing evaluates a subject’s ability to rapidly move their eyes between the examiner’s two fingers, held shoulder-width apart to test horizontally and forehead-to-chest to test vertically. Gaze stability testing, which assesses the vestibulo-ocular reflex (VOR), was performed with the subject fixing their gaze on the examiner’s thumb while nodding “yes” (vertical) and then shaking their head “no” (horizontal). Prior to each of these assessments, subjects were instructed to inform the examiner if symptoms were provoked during testing, at which point the individual test was terminated and the number of repetitions recorded. If subjects completed all 30 repetitions without reporting symptoms, they were asked about the presence or worsening of symptoms at the end of 30 repetitions. Common symptoms among patients with concussion were recorded, including headache, dizziness, and nausea, as well as specific vision complaints, including eye fatigue and eye pain, since visual disturbances are prevalent and often undetected after concussion and are known to have a significant effect on successful return to school.3 Unlike VOMS, which quantifies the severity of symptoms provoked while performing each assessment, we evaluated symptom provocation as a binary element based on the addition of worsening of any symptom. An assessment was considered abnormal if a subject reported symptom provocation at any number of repetitions, up to and including 30.
Since our data were multi-dimensional with both a binary (presence or absence of symptom provocation) and continuous component (number of repetitions), we assessed symptom provocation at ≤10, ≤20, and ≤30 repetitions for each task (horizontal and vertical saccades; horizontal and vertical gaze stability). Additionally, we calculated a VVE subscore of 0-4 by summing the number of assessments with symptom provocation (horizontal and vertical saccades; horizontal and vertical gaze stability).
Statistical Analysis
Demographic and clinical characteristics were compared between athletes with concussion and healthy controls using F-tests for continuous variables and chi-square statistics for categorical variables. We calculated sensitivity and specificity for symptom provocation at 10-repetition increments for each assessment. Receiver operating characteristic (ROC) curves were generated based on the VVE subscores. For each of the 10-repetition increments, we identified the optimal subscore as the point on the ROC curve furthest vertically from the chance line (positive diagonal) that maximizes sensitivity and specificity for a continuous test (the Youden index). The area under the receiver operating characteristic curve (AUC) was then calculated using the optimal subscore at ≤10, ≤20, and ≤30 repetitions to identify the repetition threshold level that maximizes the discriminatory ability of the test.
We also conducted a sensitivity analysis comparing healthy controls to concussed athletes who were seen ≤7 days from injury to examine more acutely injured subjects. Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
The cohort consisted of 69 concussed athletes and 69 healthy controls. These two groups did not differ significantly across sociodemographic characteristics, including sex, age, race/ethnicity, and past medical history (Table 1). Concussed athletes were seen a median of 10 days (IQR 5,15) post-injury. Of the 69 concussed athletes, 11 (16%) completed testing at the high school and the remaining 58 (84%) at the sports medicine center.
Table 1.
Demographic and clinical characteristics of concussed athletes and healthy controls.
| Concussed athletes (n=69) |
Healthy controls (n=69) |
p-value | |
|---|---|---|---|
| Age in years, mean (SD) | 16.0 (1.3) | 15.8 (1.2) | 0.33 |
| Male, n (%) | 34 (49) | 37 (54) | 0.61 |
| Race/ethnicity, n (%) | 0.97 | ||
| Non-Hispanic white | 52 (75) | 51 (74) | |
| Non-Hispanic black | 8 (12) | 8 (12) | |
| Other/unknown | 9 (13) | 10 (14) | |
| History of prior concussions, n (%)* | 32 (46) | 24 (35) | 0.14 |
| Past medical history, n (%) | |||
| Mood disorder (anxiety or depression) | 11 (16) | 4 (6) | 0.06 |
| ADHD | 8 (12) | 10 (14) | 0.61 |
| Motion sickness | 5 (7) | 4 (6) | 0.73 |
| Vision abnormality (strabismus or amblyopia) | 3 (4) | 1 (1) | 0.31 |
| Days from injury to assessment, median (IQR) | 10 (5,15) | n/a |
One concussed athlete had missing data on history of prior concussion.
Overall, 86% of concussed athletes and 65% of healthy controls had at least one symptomatic assessment across the testing paradigm, with most symptomatic assessments for healthy controls occurring at 30 repetitions. The proportions of concussed athletes and healthy controls with symptomatic assessments at ≤10, ≤20, and ≤30 repetitions are described in Table 2. At every increment of ≤10, ≤20, and ≤30 repetitions, significantly more concussed athletes reported symptoms than healthy controls across all four tests. When comparing symptom provocation based on number of repetitions, healthy controls who reported symptoms did so at a higher number of repetitions compared to concussed athletes. Only one healthy control (1%) reported symptoms within the first 10 repetitions of any assessment.
Table 2.
Proportion of subjects with symptoms provoked and associated sensitivities and specificities at different repetition increments of horizontal and vertical saccades and gaze stability testing.
| Concussed athletes (n=69) |
Healthy controls (n=69) |
p-value | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| Symptoms provoked at ≤10 repetitions, n (%) | |||||
| Horizontal saccades* | 17 (25) | 0 (0) | <0.001 | 0.25 | 1.00 |
| Vertical saccades | 22 (32) | 1 (1) | <0.001 | 0.32 | 0.99 |
| Horizontal gaze stability | 13 (19) | 0 (0) | <0.001 | 0.19 | 1.00 |
| Vertical gaze stability** | 16 (23) | 0 (0) | <0.001 | 0.23 | 1.00 |
| Symptoms provoked at ≤20 repetitions, n (%) | |||||
| Horizontal saccades* | 34 (49) | 1 (1) | <0.001 | 0.50 | 0.99 |
| Vertical saccades | 36 (52) | 3 (4) | <0.001 | 0.52 | 0.96 |
| Horizontal gaze stability | 31 (45) | 0 (0) | <0.001 | 0.45 | 1.00 |
| Vertical gaze stability** | 31 (45) | 0 (0) | <0.001 | 0.45 | 1.00 |
| Symptoms provoked at ≤30 repetitions, n (%) | |||||
| Horizontal saccades* | 47 (68) | 32 (46) | 0.007 | 0.69 | 0.54 |
| Vertical saccades | 51 (74) | 32 (46) | <0.001 | 0.74 | 0.54 |
| Horizontal gaze stability | 49 (71) | 18 (26) | <0.001 | 0.71 | 0.74 |
| Vertical gaze stability** | 50 (72) | 16 (23) | <0.001 | 0.72 | 0.76 |
One concussed athlete had missing data for symptoms provoked by horizontal saccades.
One healthy control had missing data for symptoms provoked by vertical gaze stability.
There was an improvement in sensitivity when increasing from ≤10 to ≤20 repetitions for all four assessments: horizontal (25% to 50%) and vertical (32% to 52%) saccades and horizontal (19% to 45%) and vertical (23% to 45%) gaze stability (Table 2). Increasing to ≤30 repetitions further improved sensitivity for horizontal and vertical saccades (69% and 74%, respectively), as well as horizontal and vertical gaze stability (71% and 72%, respectively). Specificities ranged from 96% to 100% at ≤10 and ≤20 repetitions for all 4 assessments. However, increasing to ≤30 repetitions decreased specificity for all 4 assessments (54% and 54% for horizontal and vertical saccades; 74% and 76% for horizontal and vertical gaze stability).
The proportions of concussed athletes and healthy controls with VVE subscores of 0-4 at increments of ≤10, ≤20, and ≤30 repetitions are described in Table 3. Receiver operating characteristic (ROC) curves for the VVE subscores at each repetition increment are shown in Figure 1. The optimal subscore, or number of assessments with symptom provocation, was one at both ≤10 repetitions (sensitivity 39%, specificity 99%) and ≤20 repetitions (sensitivity 59%, specificity 96%), and equivalent with a subscore of three or four at ≤30 repetitions (sensitivity 64%, specificity 81% with subscore of three; sensitivity 55%, specificity 90% with subscore of four). The AUC was 0.69 (95% CI 0.63, 0.75) at ≤10 repetitions, 0.79 (95% CI 0.72, 0.85) at ≤20 repetitions, and 0.76 (95% CI 0.68, 0.84) at ≤30 repetitions. In the sensitivity analysis comparing healthy controls to concussed athletes assessed ≤7 days from injury (n = 27), we found no difference in sensitivity or specificity for the four assessments at ≤10, ≤20, or ≤30 repetition increments compared to the entire cohort of concussed athletes.
Table 3.
Proportion of subjects with a VVE subscore of 0-4 at increments of ≤10, ≤20, and ≤30 repetitions with associated sensitivities/specificities.
| p-value | Subscore | Concussed athletes (n=69) |
Healthy controls (n=69) |
Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Symptoms provoked at ≤10 repetitions, n (%) | <0.001 | 0 | 42 (61) | 68 (99) | 1 | 0 |
| 1 | 8 (12) | 1 (1) | 0.39 | 0.99 | ||
| 2 | 5 (7) | 0 (0) | 0.28 | 1 | ||
| 3 | 6 (9) | 0 (0) | 0.2 | 1 | ||
| 4 | 8 (12) | 0 (0) | 0.12 | 1 | ||
| Symptoms provoked at ≤20 repetitions, n (%) | <0.001 | 0 | 28 (41) | 66 (96) | 1 | 0 |
| 1 | 4 (6) | 2 (3) | 0.59 | 0.96 | ||
| 2 | 8 (12) | 1 (1) | 0.54 | 0.99 | ||
| 3 | 4 (6) | 0 (0) | 0.42 | 1 | ||
| 4 | 25 (36) | 0 (0) | 0.36 | 1 | ||
| Symptoms provoked at ≤30 repetitions, n (%) | <0.001 | 0 | 10 (14) | 24 (35) | 1 | 0 |
| 1 | 3 (4) | 12 (17) | 0.86 | 0.35 | ||
| 2 | 12 (17) | 20 (29) | 0.81 | 0.52 | ||
| 3 | 6 (9) | 6 (9) | 0.64 | 0.81 | ||
| 4 | 38 (55) | 7 (10) | 0.55 | 0.9 |
Figure 1.
Receiver operating characteristic (ROC) curves depicting the area under the curve (AUC) for differentiating concussed athletes and healthy controls using a VVE subscore (number of abnormal assessments of horizontal saccades, vertical saccades, horizontal gaze stability, vertical gaze stability) at increments of ≤10, ≤20, and ≤30 repetitions.
DISCUSSION
This study evaluated symptom provocation patterns with horizontal and vertical saccades and gaze stability completed up to 30 repetitions, specifically examining sensitivity and specificity beyond the standard VOMS repetition threshold level of 10. We found that overall sensitivity across saccades and gaze stability assessments in our VVE was poor at 10 repetitions, increased at 20 repetitions, and increased further at 30 repetitions. Conversely, specificity across all assessments was outstanding with 10 repetitions, remained high with 20 repetitions, and then dropped considerably with 30 repetitions. When looking at all four assessments in combination with a VVE subscore of 0-4 based on number of symptomatic assessments, we found that the discriminatory ability of the test was highest at ≤20 repetitions (AUC of 0.79) with an optimal subscore of one (sensitivity 59%, specificity 96%).
Characteristics of the VOMS screening tool and its utility in the clinical assessment of vestibular and ocular motor deficits after concussion were first described in 2014.12 The horizontal saccades, vertical saccades, and horizontal gaze stability domains of this screening tool were found to differentiate concussed participants from healthy controls with AUC of 0.68, 0.65, and 0.78, respectively.12 Several additional research studies have demonstrated that VOMS has high internal consistency and reliability13,16,17 and may be superior to other assessments of vestibular and ocular motor function, such as the King-Devick (KD) test and Balance Error Scoring System (BESS).14,15,30 VOMS has been shown to have low rates of false positives among uninjured collegiate and youth athletes;13,16,17 however, a more recent study highlighted a lack of sensitivity with VOMS testing.7 This is especially salient, as false negatives may result in an athlete’s premature return to play.
Our study demonstrates that increasing test difficulty of two of the visio-vestibular functions assessed within VOMS, saccades and gaze stability, improves sensitivity, indicating that some concussed athletes may have a higher provocation threshold before deficits become apparent. Using the traditional assessment, these athletes represent potential false negatives, at risk for a missed diagnosis of concussion if only 10 repetitions are performed. However, our study also highlighted that there is a drawback to increasing the number of repetitions too high. We noted a drop-off in specificity with an increase to 30 repetitions, which is consistent with our previous study demonstrating a relatively high incidence of provoked symptoms among non-concussed controls completing this exam,26 suggesting there is an upper limit to test difficulty beyond which the risk of false positives increases. In light of these data, a threshold level of 20 repetitions optimizes sensitivity while preserving specificity among the repetition increments we examined.
One potential explanation for the improved sensitivity with increasing number of repetitions is that concussion leads to cognitive fatigability, or the inability to maintain performance over time during a task requiring sustained attention.31 Möller et al. were the first to describe and apply the concept of cognitive fatigability to mild traumatic brain injury (mTBI), suggesting that it could be quantified by calculating decreased performance on a task over time.32,33 In a recent study, Möller et al. compared fatigability with saccadic eye movements in patients with mTBI and orthopedic controls. They found that acquired fatigue correlated to prosaccade latency for the mTBI group, with slower eye movements toward a target detected by an objective eye tracker.34 Their finding that decrements in quantitative saccadic performance may relate to cognitive fatigability after concussion lends support to our findings that increasing repetitions and extending the duration of the task may be a useful means to detect subtle underlying deficits in concussion and improve diagnostic utility.
There are several limitations to this study. Foremost, the findings of this study were dependent on subjective symptom report with testing. Adolescent athletes may underreport symptoms to avoid detection of concussion and removal from athletic play,35,36 yielding a greater number of false negatives with testing and contributing to the sensitivity ceiling observed in our results. An additional symptom-related limitation is that we recorded symptom provocation on a binary scale, with the onset or worsening of any concussion-related symptom indicating provocation, while VOMS quantifies changes in symptoms on a 10-point scale and defines a change in symptom severity of ≥2 as clinically meaningful. However, using a binary threshold for defining clinically meaningful symptom provocation did not significantly change our false positive rate at 10 repetitions compared to that previously reported for VOMS, as both approximated zero (the mean symptom score for controls was 0.1 out of 10 on the comparable VOMS domains).12 This study examined adolescent athletes with concussion who were primarily patients recruited from a tertiary sports medicine referral center, which may represent concussions with more severe symptoms and impairments. As a result, the findings may not be generalizable to concussions presenting to other health care settings, other age groups, and non-athletes; however, the school from which our athletes were recruited has mandatory sports participation, and these athletes may be more representative of the general adolescent population than collegiate athletes. Finally, our concussed athletes were most commonly enrolled in the subacute post-injury period; consequently, we cannot determine the ability of the tests to distinguish concussed adolescents in the very acute post-injury phase, as vestibulo-ocular performance has been shown to change over the course of recovery. However, our sensitivity analysis showed similar results among the concussed athletes assessed within 7 days of injury indicating the robustness of our results.37 Future studies should compare performance on saccades and gaze stability with higher repetitions before and after concussion, as well as longitudinally over the course of recovery, to determine its utility as a prognostic measure.
In conclusion, the findings of this study yield important considerations for health care professionals evaluating and managing sports-related concussion. By increasing the number of repetitions for saccades and gaze stability testing, clinicians can improve the sensitivity of their clinical examination and better identify concussions among youth and adolescents while preserving adequate specificity. Although increasing beyond 10 repetitions inevitably adds some time to the examination, the minimally added time likely provides the clinician with valuable additional diagnostic capability by identifying a deficit that might otherwise go undetected. We have previously demonstrated that this VVE is feasible for clinicians to use across multiple settings, including both primary care and acute care, and thus is potentially generalizable to many practice settings.38,39 The results of this study suggest that increasing task difficulty of two clinical assessments may be an inexpensive, feasible, and readily available means by which clinicians can improve their detection of visio-vestibular deficits in concussed youth and more accurately diagnose concussion across any clinical setting.
Clinical Relevance:
The findings in this study suggest that a higher threshold level of repetitions of two commonly used visio-vestibular assessments enables clinicians to more accurately diagnose youth concussion.
Acknowledgements:
We would like to acknowledge Ronni Kessler, Fairuz Mohammed, Olivia Podolak, Ari Fish, Julia Vanni, Shelly Sharma, Alexis Brzuchalski, Taylor Valerio, and Kate Rownd for their contributions to data collection. We are grateful to the students and parents from the Shipley School and families at Children’s Hospital of Philadelphia for their participation in this research study. We would also like to thank the Shipley School administration and athletic training staff, in particular Mark Duncan, Director of Athletics, and Drs. Steve Piltch and Michael G. Turner, former and current Head of School, for their support.
Funding:
Funding for this research has been provided by the Pennsylvania Department of Health. Research reported in this publication was also supported by National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R01NS097549. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and Recreation-Related Concussions in US Youth. Pediatrics. 2016;138(1). [DOI] [PubMed] [Google Scholar]
- 2.Corwin DJ, Wiebe DJ, Zonfrillo MR, et al. Vestibular Deficits following Youth Concussion. J Pediatr. 2015;166(5):1221–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Master CL, Scheiman M, Gallaway M, et al. Vision Diagnoses Are Common After Concussion in Adolescents. Clin Pediatr (Phila). 2016;55(3):260–267. [DOI] [PubMed] [Google Scholar]
- 4.Ellis MJ, Cordingley D, Vis S, et al. Vestibulo-ocular dysfunction in pediatric sports-related concussion. J Neurosurg Pediatr. 2015;16(3):248–255. [DOI] [PubMed] [Google Scholar]
- 5.Mayer AR, Wertz C, Ryman SG, et al. Neurosensory Deficits Vary as a Function of Point of Care in Pediatric Mild Traumatic Brain Injury. J Neurotrauma. 2018;35(10):1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elbin RJ, Sufrinko A, Anderson MN, et al. Prospective Changes in Vestibular and Ocular Motor Impairment After Concussion. J Neurol Phys Ther. 2018;42(3):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherry NS, Fazio-Sumrok V, Sufrinko A, et al. Multimodal Assessment of Sport-Related Concussion. Clin J Sport Med. [published online ahead of print March 18, 2019]. doi: 10.1097/JSM.0000000000000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Master CL, Master SR, Wiebe DJ, et al. Vision and Vestibular System Dysfunction Predicts Prolonged Concussion Recovery in Children. Clin J Sport Med. 2018;28(2):139–145. [DOI] [PubMed] [Google Scholar]
- 9.Anzalone AJ, Blueitt D, Case T, et al. A Positive Vestibular/Ocular Motor Screening (VOMS) Is Associated With Increased Recovery Time After Sports-Related Concussion in Youth and Adolescent Athletes. Am J Sports Med. 2017;45(2):474–479. [DOI] [PubMed] [Google Scholar]
- 10.Sinnott AM, Elbin RJ, Collins MW, et al. Persistent vestibular-ocular impairment following concussion in adolescents. J Sci Med Sport. 2019;22(12):1292–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis MJ, Cordingley DM, Vis S, et al. Clinical predictors of vestibulo-ocular dysfunction in pediatric sports-related concussion. J Neurosurg Pediatr. 2017;19(1):38–45. [DOI] [PubMed] [Google Scholar]
- 12.Mucha A, Collins MW, Elbin RJ, et al. A Brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42(10):2479–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kontos AP, Sufrinko A, Elbin RJ, et al. Reliability and Associated Risk Factors for Performance on the Vestibular/Ocular Motor Screening (VOMS) Tool in Healthy Collegiate Athletes. Am J Sports Med. 2016;44(6):1400–1406. [DOI] [PubMed] [Google Scholar]
- 14.Worts PR, Schatz P, Burkhart SO. Test Performance and Test-Retest Reliability of the Vestibular/Ocular Motor Screening and King-Devick Test in Adolescent Athletes During a Competitive Sport Season. Am J Sports Med. 2018;46(8):2004–2010. [DOI] [PubMed] [Google Scholar]
- 15.Yorke AM, Smith L, Babcock M, et al. Validity and Reliability of the Vestibular/Ocular Motor Screening and Associations With Common Concussion Screening Tools. Sports Health. 2017;9(2):174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iverson GL, Cook NE, Howell DR, et al. Preseason Vestibular Ocular Motor Screening in Children and Adolescents. Clin J Sport Med. [published online ahead of print June 19, 2019]. doi: 10.1097/JSM.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 17.Moran RN, Covassin T, Elbin RJ, et al. Reliability and Normative Reference Values for the Vestibular/Ocular Motor Screening (VOMS) Tool in Youth Athletes. Am J Sports Med. 2018;46(6):1475–1480. [DOI] [PubMed] [Google Scholar]
- 18.Womble MN, McAllister-Deitrick J, Marchetti GF, et al. Risk Factors for Vestibular and Oculomotor Outcomes After Sport-Related Concussion. Clin J Sport Med. [published online ahead of print June 11, 2019]. doi: 10.1097/JSM.0000000000000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sufrinko AM, Kegel NE, Mucha A, et al. History of High Motion Sickness Susceptibility Predicts Vestibular Dysfunction Following Sport/Recreation-Related Concussion. Clin J Sport Med. 2019;29(4):318–323. [DOI] [PubMed] [Google Scholar]
- 20.Moran RN, Covassin T, Wallace J. Premorbid migraine history as a risk factor for vestibular and oculomotor baseline concussion assessment in pediatric athletes. J Neurosurg Pediatr. [published online ahead of print Jan 11, 2019]. doi: 10.3171/2018.10.PEDS18425.1-6. [DOI] [PubMed] [Google Scholar]
- 21.Sufrinko AM, Marchetti GF, Cohen PE, et al. Using Acute Performance on a Comprehensive Neurocognitive, Vestibular, and Ocular Motor Assessment Battery to Predict Recovery Duration After Sport-Related Concussions. Am J Sports Med. 2017;45(5):1187–1194. [DOI] [PubMed] [Google Scholar]
- 22.Echemendia RJ, Broglio SP, Davis GA, et al. What tests and measures should be added to the SCAT3 and related tests to improve their reliability, sensitivity and/or specificity in sideline concussion diagnosis? A systematic review. Br J Sports Med. 2017;51(11):895–901. [DOI] [PubMed] [Google Scholar]
- 23.Howell DR, Myer GD, Grooms D, et al. Examining Motor Tasks of Differing Complexity After Concussion in Adolescents. Arch Phys Med Rehabil. 2019;100(4):613–619. [DOI] [PubMed] [Google Scholar]
- 24.Howell DR, Wilson JC, Brilliant AN, et al. Objective clinical tests of dual-task dynamic postural control in youth athletes with concussion. J Sci Med Sport. 2019;22(5):521–525. [DOI] [PubMed] [Google Scholar]
- 25.Ellis MJ, Leddy J, Cordingley D, et al. A Physiological Approach to Assessment and Rehabilitation of Acute Concussion in Collegiate and Professional Athletes. Front Neurol. 2018;9:1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corwin DJ, Zonfrillo MR, Wiebe DJ, et al. Vestibular and oculomotor findings in neurologically-normal, non-concussed children. Brain Inj. 2018;32(6):794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corwin DJ, McDonald CC, Arbogast KB, et al. Clinical and Device-based Metrics of Gait and Balance in Diagnosing Youth Concussion. Med Sci Sports Exerc. 2020;52(3):542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–847. [DOI] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alkathiry AA, Kontos AP, Furman JM, et al. Vestibulo-Ocular Reflex Function in Adolescents With Sport-Related Concussion: Preliminary Results. Sports Health. 2019;11(6):479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwid SR, Tyler CM, Scheid EA, et al. Cognitive fatigue during a test requiring sustained attention: a pilot study. Mult Scler. 2003;9(5):503–508. [DOI] [PubMed] [Google Scholar]
- 32.Moller MC, Nygren de Boussard C, Oldenburg C, et al. An investigation of attention, executive, and psychomotor aspects of cognitive fatigability. J Clin Exp Neuropsychol. 2014;36(7):716–729. [DOI] [PubMed] [Google Scholar]
- 33.Moller MC, Nordin LE, Bartfai A, et al. Fatigue and Cognitive Fatigability in Mild Traumatic Brain Injury are Correlated with Altered Neural Activity during Vigilance Test Performance. Front Neurol. 2017;8:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moller MC, Johansson J, Matuseviciene G, et al. An observational study of trait and state fatigue, and their relation to cognitive fatigability and saccade performance. Concussion. 2019;4(2):CNC62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chrisman SP, Quitiquit C, Rivara FP. Qualitative study of barriers to concussive symptom reporting in high school athletics. J Adolesc Health. 2013;52(3):330–335 e333. [DOI] [PubMed] [Google Scholar]
- 36.Wallace J, Covassin T, Nogle S, et al. Knowledge of Concussion and Reporting Behaviors in High School Athletes With or Without Access to an Athletic Trainer. J Athl Train. 2017;52(3):228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheever KM, McDevitt J, Tierney R, et al. Concussion Recovery Phase Affects Vestibular and Oculomotor Symptom Provocation. Int J Sports Med. 2018;39(2):141–147. [DOI] [PubMed] [Google Scholar]
- 38.Corwin DJ, Propert KJ, Zorc JJ, et al. Use of the vestibular and oculomotor examination for concussion in a pediatric emergency department. Am J Emerg Med. 2019;37(7):1219–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Master CL, Grady MF. Office-based management of pediatric and adolescent concussion. Pediatr Ann. 2012;41(9):1–6. [DOI] [PubMed] [Google Scholar]