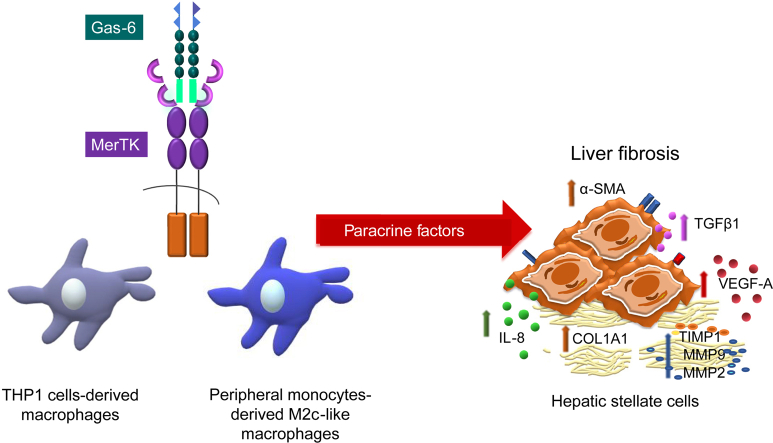
Abstract
Background & Aims
Activation of Kupffer cells and recruitment of monocytes are key events in fibrogenesis. These cells release soluble mediators which induce the activation of hepatic stellate cells (HSCs), the main fibrogenic cell type within the liver. Mer tyrosine kinase (MerTK) signaling regulates multiple processes in macrophages and has been implicated in the pathogenesis of non-alcoholic steatohepatitis-related fibrosis. In this study, we explored if MerTK activation in macrophages influences the profibrogenic phenotype of HSCs.
Methods
Macrophages were derived from THP-1 cells or differentiated from peripheral blood monocytes towards MerTK+/CD206+/CD163+/CD209- macrophages. The role of MerTK was assessed by pharmacologic and genetic inhibition. HSC migration was determined in Boyden chambers, viability was measured by the MTT assay, and proliferation was evaluated by the BrdU incorporation assay.
Results
Gas-6 induced MerTK phosphorylation and Akt activation in macrophages, and these effects were inhibited by UNC569. During polarization, MerTK+/CD206+/CD163+/CD209- macrophages exhibited activation of STAT3, ERK1/2, p38 and increased expression of VEGF-A. Activation of MerTK in THP-1 macrophages induced a secretome which promoted a significant increase in migration, proliferation, viability and expression of profibrogenic factors in HSCs. Similarly, conditioned medium from MerTK+ macrophages induced a significant increase in cell migration, proliferation, STAT3 and p38 phosphorylation and upregulation of IL-8 expression in HSCs. Moreover, conditioned medium from Gas-6-stimulated Kupffer cells induced a significant increase in HSC proliferation. These effects were specifically related to MerTK expression and activity in macrophages, as indicated by pharmacologic inhibition and knockdown experiments.
Conclusions
MerTK activation in macrophages modifies the secretome to promote profibrogenic features in HSCs, implicating this receptor in the pathogenesis of hepatic fibrosis.
Lay summary
Fibrosis represents the process of scarring occurring in patients with chronic liver diseases. This process depends on production of scar tissue components by a specific cell type, named hepatic stellate cells, and is regulated by interaction with other cells. Herein, we show that activation of MerTK, a receptor present in a population of macrophages, causes the production of factors that act on hepatic stellate cells, increasing their ability to produce scar tissue.
Keywords: Gas-6, THP-1, M2c-like macrophages, NASH, liver fibrosis
Abbreviations: CM, conditioned medium; ECM, extracellular matrix; Gas-6, growth arrest-specific gene 6; HSC(s), hepatic stellate cells; KC(s), Kupffer cell(s); M-CSF, macrophage colony-stimulating factor; MerTK, Myeloid-epithelial-reproductive tyrosine kinase; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PMA, phorbol 12-myristate 13-acetate; siRNA, small-interfering RNA; TGFβ1, transforming growth factor-β1; TIMP1, tissue inhibitor of metalloproteinase 1; VEGF-A, vascular endothelial growth factor-A
Graphical abstract
Highlights
-
•
MerTK, a member of the TAM family of proteins, is highly expressed in MerTK+/CD206+/CD163+/CD209- macrophages.
-
•
In these macrophages, activation of MerTK induces phosphorylation of Akt, STAT3, ERK1/2, p38 and increased expression of VEGF-A.
-
•
MerTK activation in macrophages modulates the secretome to promote the profibrogenic phenotype of human HSCs.
-
•
Profibrogenic effects of macrophages expressing high levels of MerTK were blocked by knockdown or inhibition of MerTK.
Introduction
Liver fibrosis is a wound-healing response that occurs in the context of chronic liver injury, and is characterized by excessive extracellular matrix (ECM) accumulation and fibrous scar formation, leading to liver dysfunction.1 Hepatic stellate cells (HSCs) are the main cellular effectors of hepatic fibrogenesis. Through a process termed ‘activation’, HSCs acquire a myofibroblast-like phenotype, characterized by increased proliferation, contractility, chemotaxis and ECM production.2 The development of fibrosis is the result of a complex cross-talk involving diverse cell types, either resident in the liver, or infiltrating the tissue as a response to injury. Hepatic macrophages comprise Kupffer cells (KCs) and infiltrating, bone marrow-derived monocytes/macrophages.3 Various populations of hepatic macrophages have been identified, expressing specific markers and exhibiting specialized functions.4 Environmental stimuli define macrophage polarization into classic M1, and alternative M2, which can be further differentiated into diverse subtypes, each induced by different molecules and eliciting different signals.5 During the early stages of liver injury, bone marrow-derived monocytes are recruited to the liver and differentiate into M1 macrophages.6 M1 macrophages can rapidly switch to an M2 phenotype and mediate tissue repair in case of cessation of liver injury.7 However, when the lesion is persistent, M2 macrophages mediate an important pro-fibrotic function.8
Myeloid-epithelial-reproductive tyrosine kinase (MerTK) is a member of the Tyro-3, Axl, and Mer (TAM) receptor tyrosine kinase family9 and is expressed at high levels in macrophages displaying the M2c-like phenotype, characterized by a marked involvement in tissue repair, matrix remodeling and promotion of angiogenesis through secretion of pro-angiogenic factors.10,11 Growth arrest-specific gene 6 (Gas-6), a glycoprotein considered a new potential fibrosis biomarker,[12], [13], [14] is one of the best characterized ligands for MerTK. A single nucleotide polymorphism (SNP) associated with lower MerTK expression has been linked to the reduced progression of liver fibrosis in patients with non-alcoholic fatty liver disease (NAFLD) or HCV.[15], [16], [17] In hepatic tissue from patients with NAFLD, MerTK was found to be mostly expressed in macrophages and partially in HSCs, and was loosely aggregated within inflammatory foci.16 Moreover, activation of MerTK in HSCs resulted in the induction of profibrogenic actions.
Cross-talk between HSCs and macrophages is crucial for fibrosis development,18 but the biological role played by macrophage-derived MerTK is still unclear. Herein, we show that macrophages expressing high levels of MerTK promote a profibrogenic phenotype in HSCs (via paracrine signals), inducing a significant increase in cell migration, viability, proliferation and the expression of profibrogenic factors.
Materials and methods
Cell culture and treatments
The THP-1 human monocytic leukemic cell line, kindly provided by Dr. Elisabetta Rovida (University of Florence, Italy), was differentiated into macrophages by 48 h or 144 h of incubation with phorbol 12-myristate 13-acetate (PMA) (10 ng/ml) at 37°C in 5% CO2, as described by Kurihara et al.19,20 Human monocytes were isolated from buffy coats of healthy blood donors who provided written informed consent. A two-step procedure was employed, as described by De Almeida et al.,21 using Lympholyte-H (Cedarlane, Burlington, CA, USA) followed by slight hyperosmolar Percoll gradient (GE Healthcare Life Sciences, RD, St. Louis, MO, USA). Primary human HSCs were isolated from normal liver tissue unsuitable for transplantation as previously described in detail.22 All patients provided written, informed consent for cell isolation, and the study was approved by the local institutional review board. KCs were purchased from Thermo Scientific (Waltham, MA USA).
Studies with conditioned medium
THP-1 monocytes were differentiated into macrophages by 144 h of incubation with 10 ng/ml PMA at 37°C in 5% CO2, hereafter referred to as “THP-1 macrophages”. siRNA-transfected THP-1 macrophages were incubated for 24 h in serum-free medium and then stimulated with Gas-6 (200 ng/ml) for 8 h. When the MerTK inhibitor UNC56923 was used, both THP-1-derived and circulating monocyte-derived MerTK+/CD206+/CD163+/CD209- macrophages were incubated in serum-free medium for 24 h and then exposed to 5 μM UNC569 or to DMSO for 90 min before stimulation with Gas-6 (200 ng/ml) for 8 h. All macrophages were then rinsed 3 times with PBS and further incubated overnight in serum-free medium. Conditioned medium (CM) was collected, centrifuged at 2,000 x g for 10 min and stored at -80°C for further use. Although the traditional classification (M1, M2a, M2b, M2c), which is based on induction of in vitro polarization, does not accurately describe the phenotypic heterogeneity of in vivo macrophages, for ease of reading, the CM of MerTK+/CD206+/CD163+/CD209- macrophages is defined as ‘M2c CM’.
Statistical analysis
Data in bar graphs represent mean ± SD from at least 3 independent experiments. Luminograms are representative of at least 3 experiments with similar results.
Statistical analysis was performed using Student’s t test.
For further details regarding the materials and methods used, please refer to the CTAT table and supplementary information.
Results
MerTK expression and activation in THP-1 macrophages
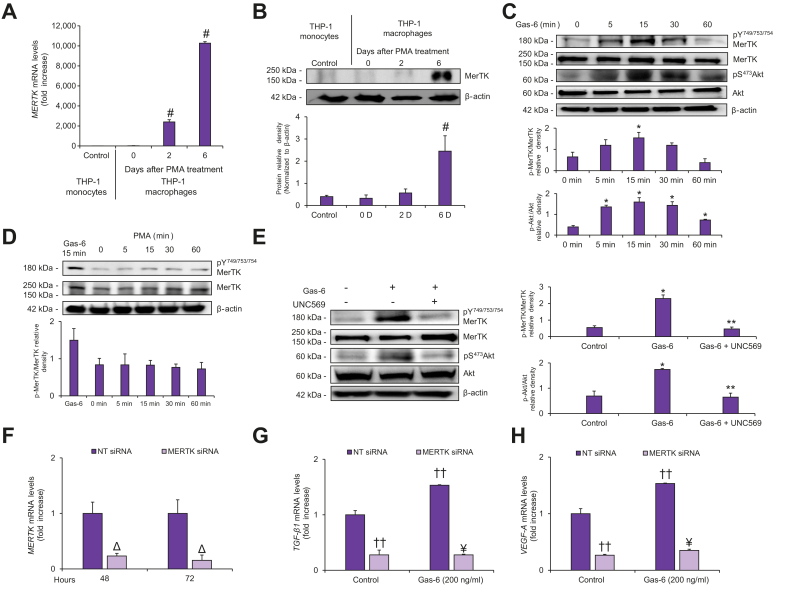
To elucidate the involvement of MerTK in the interaction between macrophages and HSCs, we initially studied macrophages derived from THP-1 cells, an immortalized monocytic line which differentiates into macrophages when exposed to PMA.19 In PMA-differentiated THP-1 macrophages, the expression of MerTK, but not of Axl and Tyro3, was substantially increased (data not shown).20 Accordingly, MERTK mRNA levels were several thousand-fold higher in THP-1 macrophages after 6 days of treatment with PMA (Fig. 1A). This increase in MerTK expression was confirmed by western blot analysis (Fig. 1B). Consistent with previous findings,24,25 exposure of THP-1 macrophages to Gas-6, a ligand of MerTK, increased MerTK phosphorylation and downstream activation of Akt, in a time-dependent manner (Fig. 1C). In contrast, exposure to PMA did not result in any increase in MerTK phosphorylation (Fig. 1D).
Fig. 1.
MerTK inhibition or silencing affect fibrogenic pathways in THP-1 macrophages.
(A) Total RNA was isolated from serum-deprived THP-1 cells in unstimulated conditions (Control) (THP-1 monocytes) or after treatment with PMA (10 ng/ml) (THP-1 macrophages). MerTK expression was evaluated by qRT-PCR and normalized to β-actin. (B) 30 μg of cell lysate were analyzed by western blotting, using antibodies against MerTK; β-Actin was used as a control for equal loading. PMA-treated serum-deprived THP-1 macrophages for 6 days were used for following experiments. (C) Serum-deprived THP-1 macrophages were exposed to Gas-6 for the indicated time points. 30 μg of cell lysates were analyzed by western blotting with the indicated antibodies. (D) Serum-deprived THP-1 macrophages were exposed to Gas-6 (200 ng/ml) or to PMA (100 ng/ml) for the indicated time points. 30 μg of cell lysates were analyzed by western blotting with the indicated antibodies. (E) Serum-deprived THP-1 macrophages were exposed to UNC569 or DMSO before stimulation with Gas-6 for 15 min. 30 μg of cell lysates were analyzed by western blotting, using the indicated antibodies. (F-H) THP-1 macrophages were transfected with MERTK-specific siRNA (light purple columns) or with NT siRNA (purple columns), (F) Total RNA was isolated 48 h and 72 h after transfection and MERTK expression was determined by qRT-PCR and normalized to β-actin. (G-H) 72 h after transfection, THP-1 macrophages were exposed to Gas-6 for 8 h. Total RNA was isolated and the expression of (G) VEGF-A and (H) TGFβ1 was evaluated by qRT-PCR and normalized to β-actin. Densitometries of p-MerTK/MerTK and p-Akt/Akt expression (n = 3) were shown in the graphs. Data are mean ± SEM. Statistical significance was assessed by Student's t test. #p <0.05 vs. THP-1 monocytes; ∗p <0.05 vs. Control; ∗∗p <0.05 vs. THP-1 macrophage stimulated with Gas-6 without inhibition for MerTK; Δp <0.05 vs. NT siRNA; ††p <0.05 vs. NT siRNA unstimulated; ¥p <0.05 vs. NT siRNA stimulated with Gas-6. NT, non-targeting; PMA, phorbol 12-myristate 13-acetate; qRT-PCR, quantitative real-time PCR; siRNA, small-interfering RNA.
Pre-incubation with UNC569, an inhibitor specific to MerTK, reduced MerTK phosphorylation and downstream Akt activation23 (Fig. 1E). To rule out that the effects of UNC569 were due to non-specific inhibition, THP-1 macrophages were transfected with MERTK-specific small-interfering RNA (siRNA) and knockdown efficiency was evaluated by real-time PCR (Fig. 1F). Exposure of THP-1 macrophages to Gas-6 induced a significant increase in gene expression of vascular endothelial growth factor-A (VEGF-A) and transforming growth factor-β1 (TGFβ1) and these effects were completely abolished by MerTK silencing (Fig. 1G and H). Taken together, these data indicate that, in macrophages, MerTK regulates the expression of factors implicated in liver repair, in a MerTK-dependent fashion.
In THP-1 macrophages, MerTK activation induces the release of soluble factors which modulate the biology of HSCs
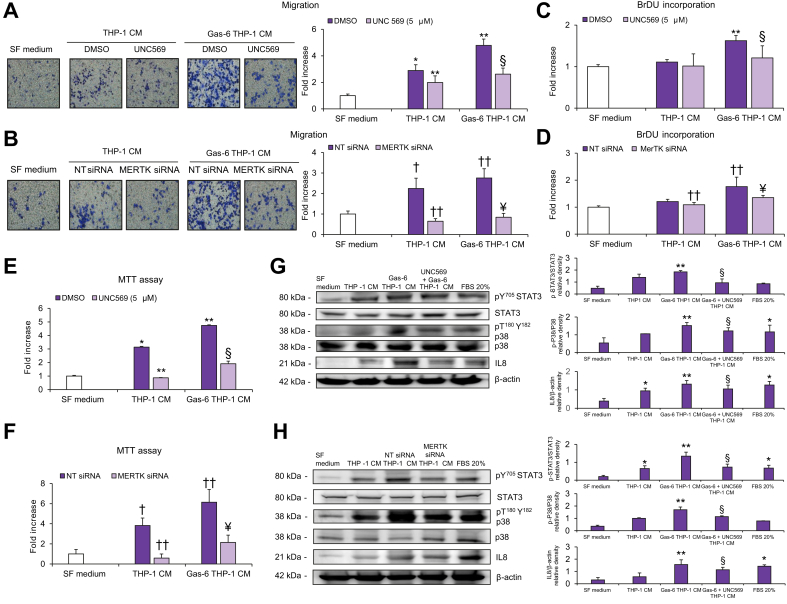
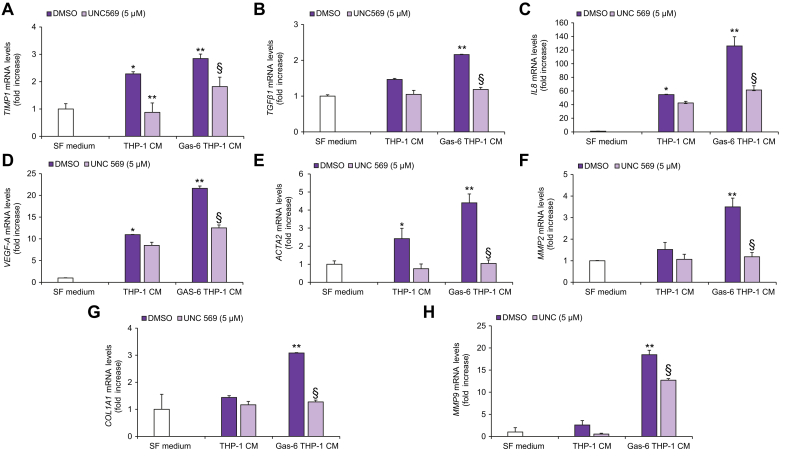
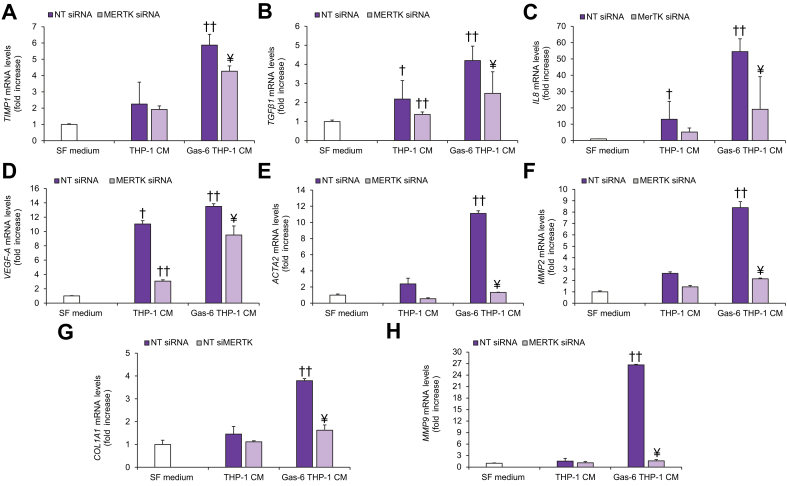
To examine the involvement of MerTK signaling in the cross-talk between macrophages and HSCs, we studied the effects of CM obtained from THP-1 macrophages on the biological features of HSCs. HSCs exposed to THP-1-CM showed a significant increase in migration, that was more evident when macrophages were treated with Gas-6 (Fig. 2A). To evaluate whether these effects were mediated by MerTK activation in macrophages, we used CM from THP-1 macrophages with pharmacologic or genetic inhibition of MerTK. Using both these strategies, increased migration of HSCs in response to CM from Gas-6-stimulated THP-1 macrophages was significantly reduced (Fig. 2A,B). Similar results were obtained when we explored the effects of THP-1-CM on HSC proliferation (Fig. 2C,D) and viability (Fig. 2E,F). In HSCs exposed to CM from Gas-6-stimulated THP-1 macrophages, increased activation of STAT3 and p38 was observed, together with an increase in IL-8 protein expression. These effects were inhibited by pre-treatment of THP-1 macrophages with UNC569 or after MerTK silencing (Fig. 2G-H). To better elucidate the role of THP-1 macrophage MerTK in modulating the profibrogenic properties of HSCs, we analyzed the expression of genes upregulated upon HSC activation. In HSCs treated with CM from Gas-6-stimulated THP-1 macrophages, mRNA expression of tissue inhibitor of metalloproteinase 1 (TIMP1), TGFβ1, interleukin 8 (IL8), VEGF-A, actin alpha 2, smooth muscle (ACTA2), matrix metalloproteinase 2 (MMP2), collagen type I alpha 1 chain (COL1A1) and matrix metalloproteinase 9 (MMP9) was significantly increased compared to that in HSCs treated with CM from unstimulated macrophages (Fig. 3). These effects were blunted by macrophage pre-treatment with UNC569, indicating that the actions of Gas-6 are mediated by MerTK (Fig. 3). Next, we stimulated HSCs with CM from THP-1 macrophages after MERTK depletion, with or without exposure to Gas-6. Upon MerTK knockdown, CM from macrophages showed a reduced capability to induce expression of fibrogenic genes in HSCs (Fig. 4). Taken together, these data indicate that MerTK expressed by differentiated THP-1 cells mediates profibrogenic actions in HSCs via soluble mediators.
Fig. 2.
Migration, proliferation, viability, and activated signaling pathways in HSCs exposed to CM from MerTK-inhibited THP-1 macrophages.
(A-C-E) Serum-starved HSCs were exposed to SF medium (Control, white columns) or to CM from THP-1 macrophages left untreated (DMSO, purple columns), treated with UNC569 (light purple columns) or stimulated with Gas-6 in the presence (light purple columns) or absence (purple columns) of UNC569. (B-D-F) Serum-starved HSCs were exposed to SF medium (Control, white columns) or to CM of THP-1 macrophages transfected with NT siRNA (purple columns) or MERTK-siRNA (light purple columns) and left untreated or treated with Gas-6. (A-B) HSC migration was measured in modified Boyden chambers. (C-D) HSC proliferation was assayed by BrdU Cell Proliferation ELISA Kit. (E-F) HSC viability was measured by MTT assay. (G) Serum-starved HSCs were exposed to CM from THP-1 macrophages unstimulated or stimulated Gas-6 in the presence or absence of UNC569 or (H) to CM from THP-1 macrophages transfected with NT siRNA or MERTK-siRNA stimulated with or without Gas-6 for 24 h. 30 μg of total cell lysates were subjected to immunoblot analysis to detect different proteins or phosphoproteins. Densitometries of p-STAT3/STAT3, p-P38/P38 and IL8/β-Actin expression (n = 3) were shown in the graphs. Data are mean ± SEM. Statistical significance was assessed by Student's t test. ∗p <0.05 vs. SF medium; ∗∗p <0.05 vs. CM of untreated THP-1 macrophages; §p <0.05 vs. CM of THP-1 macrophage stimulated with Gas-6 without inhibition for MerTK; †p <0.05 vs. SF medium; ††p <0.05 vs. CM of NT siRNA unstimulated THP-1 macrophages; ¥p <0.05 vs. CM of NT siRNA THP-1 macrophage stimulated with Gas-6. CM, conditioned media; HSC(s), hepatic stellate cells; NT, non-targeting; SF, serum free; siRNA, small-interfering RNA.
Fig. 3.
HSC expression of proinflammatory and profibrogenic genes in response to CM from MerTK-inhibited THP-1 macrophages.
Serum-starved HSCs were exposed to SF medium (Control, white columns) or to CM from THP-1 macrophages left untreated (DMSO, purple columns), treated with UNC569 (light purple columns) or stimulated with Gas-6 in the presence (light purple columns) or absence (purple columns) of UNC569 for 48 h. (A-H) Total RNA was isolated and mRNA expression of different genes was evaluated by qRT-PCR and normalized for the expression of β-actin. Data are mean ± SEM. Statistical significance was assessed by Student's t test. ∗p <0.05 vs. SF medium; ∗∗p <0.05 vs. CM of untreated THP-1 macrophages; §p <0.05 vs. CM of THP-1 macrophage stimulated with Gas-6 without inhibition for MerTK. CM, conditioned media; HSC(s), hepatic stellate cell(s); qRT-PCR, quantitative real-time PCR; SF, serum free.
Fig. 4.
HSC expression of proinflammatory and profibrogenic markers in response to CM from MERTK-silenced THP-1 macrophages.
Serum-starved HSCs were exposed to SF medium (Control, white columns) or to CM from THP-1 macrophages transfected with NT siRNA (purple columns) or MERTK-siRNA (light purple columns) and left untreated or treated with Gas-6 for 48 h. (A-H) Total RNA was isolated and mRNA expression of different genes was evaluated by qRT-PCR and normalized to β-actin. Data are mean ± SEM. Statistical significance was assessed by Student's t test. †p <0.05 vs. SF medium; ††p <0.05 vs. CM of NT siRNA unstimulated THP-1 macrophages; ¥p <0.05 vs. CM of NT siRNA THP-1 macrophage stimulated with Gas-6. CM, conditioned media; HSCs, hepatic stellate cells; NT, non-targeting; qRT-PCR, quantitative real-time PCR; SF, serum free; siRNA, small-interfering RNA.
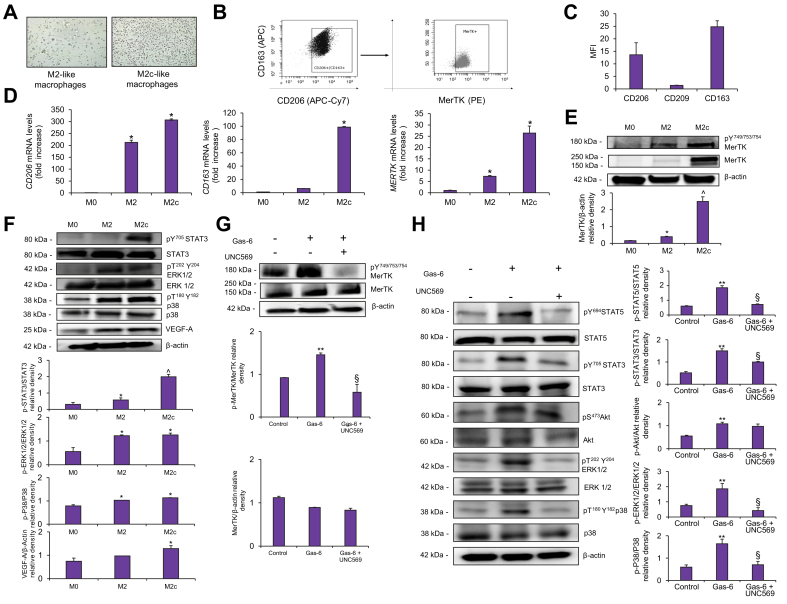
Profibrogenic effects of MerTK activation in MerTK+/CD206+/CD163+/CD209- macrophages via cross-talk with HSCs
Macrophage polarization towards a M2c-like phenotype is associated with a marked increase in MerTK expression.11 Because THP-1 cells only partially recapitulate the biologic effects of tissue-infiltrating macrophages, we next investigated whether the effects of MerTK activation could be reproduced in a population of monocyte-derived MerTK+/CD206+/CD163+/CD209- macrophages (Fig. 5A). Exposure to a culture medium rich in macrophage colony-stimulating factor (M-CSF) and IL-10 induced high expression of CD206 (a marker for alternatively activated macrophages26), CD163 (a specific marker for M2c cells27), and low expression of CD209 (a specific marker for M2a phenotype11) (Fig. 5C). Flow cytometry analyses confirmed a high expression of MerTK in the CD206+/CD163+/CD209- phenotype (Fig. 5B,C). Furthermore, MERTK expression was gradually increased along MerTK+/CD206+/CD163+/CD209- macrophage differentiation. CD206 expression significantly increased on the fifth day of exposure to M-CSF, while a significant increase in CD163 expression was observed after the last 3 days of treatment (Fig. 5D).28 These findings were associated with marked MerTK upregulation at the protein level and increased tyrosine phosphorylation on activation-specific residues (Fig. 5E). Moreover, acquisition of a M2c-like-polarization was associated with activation of several signaling pathways, including STAT3, ERK 1/2, and p38, compared to M0-like macrophages. In addition, increased VEGF-A expression was observed in MerTK+/CD206+/CD163+/CD209- macrophages (Fig. 5F). Exposure of MerTK+/CD206+/CD163+/CD209- macrophages to Gas-6 further increased phosphorylation of MerTK (Fig. 5G) and led to an increase in phosphorylation of STAT5, STAT3, Akt, ERK1/2 and p38, that was reverted by pre-treatment with UNC569 (Fig. 5H).
Fig. 5.
Expression and activation of MerTK and downstream signaling in monocytes differentiated towards M2c-like macrophages.
(A) Morphologic analysis of culture of naïve (M0) macrophages derived from peripheral blood monocytes and of peripheral blood monocytes that were treated with M-CSF (50 ng/ml) for 5 days (M2-like macrophages), followed by cultivation with M-CSF (50 ng/ml) and IL-10 (50 ng/ml) for additional 3 days (M2c-like macrophages). (B-C) Cells were stained for CD206, CD163, CD209 or MerTK and analyzed by flow cytometry. (B) Representative Dot plots depicting the gating strategy for analyzing MerTK expression in (CD206+ CD163+ CD209-) macrophages. (C) Histograms show MFI. (D) CD206 CD163 and MERTK mRNA expression was analyzed by qRT-PCR and normalized to β-actin. (E-F) Macrophages were deprived of serum and lysed. (G-H) Serum-deprived M2c macrophages were exposed to UNC569 or DMSO for 90 min before stimulation with Gas-6 for 15 min. 30 μg of cell lysates were analyzed by western blotting to detect different proteins or phosphoproteins. Densitometries of MerTK/ β-Actin, p-MerTK/MerTK, p-STAT3/STAT3, p-STAT5/STAT5, p-Akt/Akt p-ERK1/2/ERK1/2, p-P38/P38 and VEGF-A/ β-Actin expression (n = 3) were shown in the graphs. Data are mean ± SEM. Statistical significance was assessed by Student's t test. ∗p <0.05 vs. M0 macrophages; ˆp <0.05 vs. M2 macrophages; ∗∗p <0.05 vs. Control M2c; §p <0.05 vs. M2c macrophage stimulated with Gas-6 without inhibition for MerTK. M-CSF, macrophage colony-stimulating factor; MFI, mean fluorescence intensity; qRT-PCR, quantitative real-time PCR.
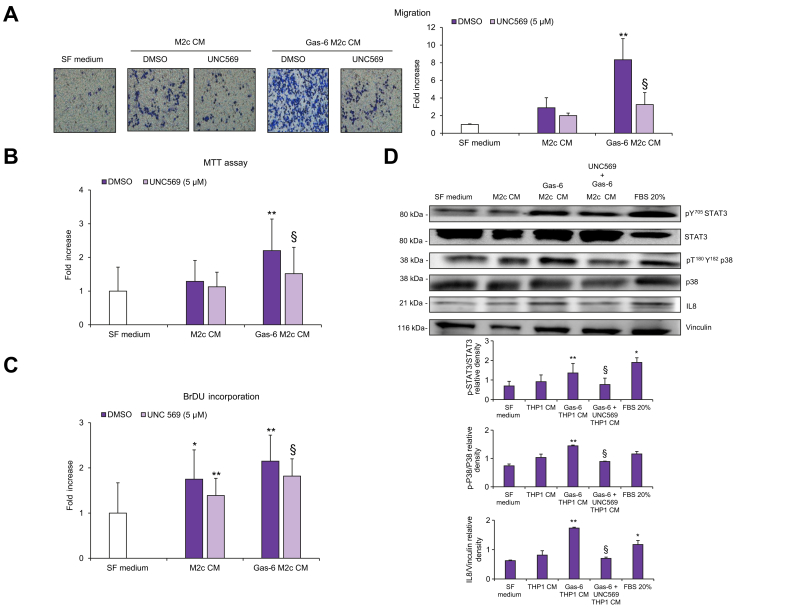
Having confirmed the high expression levels of MerTK in M2c-like macrophages, we next evaluated the involvement of this kinase in the interplay between macrophages and HSCs, analyzing the effects of CM from MerTK+/CD206+/CD163+/CD209- macrophages on the fibrogenic features of HSCs. Incubation of HSCs with CM from MerTK+/CD206+/CD163+/CD209- macrophages caused a significant increase in HSC chemotaxis. This effect was further increased by exposure to Gas-6 and markedly reduced by macrophage pre-treatment with UNC569 (Fig. 6A). Similarly, exposure of HSCs to CM from Gas-6-stimulated MerTK+/CD206+/CD163+/CD209- macrophages led to a significant increase in HSC viability and proliferation, and these effects were also reduced by pre-treatment with the MerTK inhibitor (Fig. 6B,C). To better understand the role of MerTK in the induction of a profibrogenic phenotype, we examined the activation of different signaling pathways. In HSCs exposed to CM from Gas-6-stimulated MerTK+/CD206+/CD163+/CD209- macrophages, we observed increased activation of STAT3 and p38, together with an increase in IL-8 expression. These effects were inhibited by pre-treatment of MerTK+/CD206+/CD163+/CD209- macrophages with UNC569 (Fig. 6D).
Fig. 6.
Migration, viability, proliferation, and activated signaling pathways in HSCs exposed to CM from MerTK-inhibited M2c-like macrophages.
(A-C) Serum-starved HSCs were exposed to SF medium (Control, white columns) or to CM from M2c macrophages left untreated (DMSO, purple columns), treated with UNC569 (light purple columns) or stimulated with Gas-6 in the presence (light purple columns) or absence (purple columns) of UNC569. (A) HSC migration was measured using modified Boyden chambers. (B) HSC viability was measured by MTT assay. (C) HSC proliferation was assayed by BrdU Cell Proliferation ELISA Kit. (D) Serum-starved HSCs were exposed to CM from M2c macrophages unstimulated or stimulated with Gas-6 in the presence or absence of UNC569 for 24 h. 30 μg of total cell lysates were subjected to immunoblot analysis to detect different proteins or phosphoproteins. Densitometries of p-STAT3/STAT3, p-P38/P38 and IL8/ Vinculin expression (n = 3) were shown in the graphs. Data are mean ± SEM. Statistical significance was assessed by Student's t test. ∗p <0.05 vs. SF medium; ∗∗p <0.05 vs. CM of untreated M2c macrophages; §p <0.05 vs. CM of M2c macrophage stimulated with Gas-6 without inhibition for MerTK. CM, conditioned media; HSC(s), hepatic stellate cell(s); SF, serum-free.
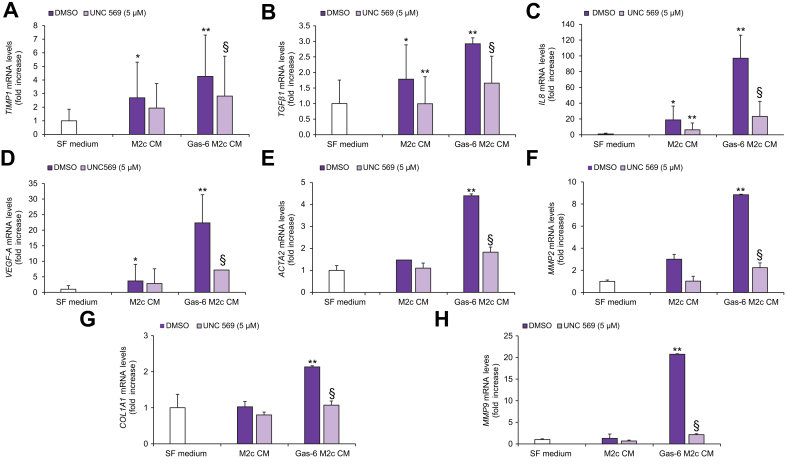
We next evaluated the regulation of profibrogenic genes in HSCs treated with CM from Gas-6-stimulated MerTK+/CD206+/CD163+/CD209- macrophages. Expression of TIMP1, TGFβ1, IL8, VEGF-A, ACTA2, MMP2, COL1A1 and MMP9 was increased compared to that in HSCs treated with CM from unstimulated macrophages. These actions were significantly reduced when macrophages were pre-treated with UNC569, indicating that MerTK activation was required (Fig. 7). Taken together, these data indicate that activation of MerTK in alternatively activated macrophages results in the upregulation of profibrogenic signals in HSCs.
Fig. 7.
HSC expression of proinflammatory and profibrogenic genes in response to CM from MerTK-inhibited M2c macrophages.
Serum-starved HSCs were exposed to SF medium (Control, white columns) or to CM of M2c macrophages left untreated (DMSO, purple columns), treated with UNC569 (light purple columns) or stimulated with Gas-6 in the presence (light purple columns) or absence (purple columns) of UNC569 for 48 h. (A-H) Total RNA was isolated and levels of different genes were determined by qRT-PCR and normalized to β-actin. Data are mean ± SEM. Statistical significance was assessed by Student's t test. ∗p <0.05 vs. SF medium; ∗∗p <0.05 vs. CM of untreated M2c macrophages; §p <0.05 vs. CM of M2c macrophage stimulated with Gas-6 without inhibition for MerTK. CM, conditioned media; HSC(s), hepatic stellate cell(s); qRT-PCR, quantitative real-time PCR; SF, serum-free.
Gas-6 expression in differentiated macrophages and in KCs
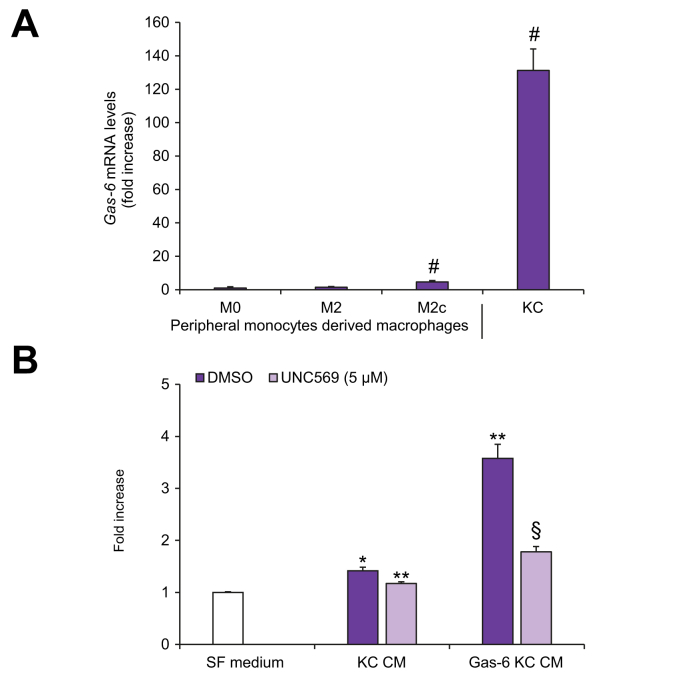
Gas-6 was produced by THP-1, M2c-like macrophages and KCs, with higher levels in MerTK+/CD206+/CD163+/CD209- macrophages and in KCs (Fig. S1A-D). However, pharmacologic inhibition or MERTK gene silencing did not result in significant changes in the levels of Gas-6 (Fig. S1A-D). Gas-6 mRNA levels were significantly increased in THP-1 macrophages after 2 and 6 days of treatment with PMA (Fig. S1E), as well as during macrophage polarization towards a M2c-like phenotype (Fig. 8A). Of note, Gas-6 expression was markedly elevated in KCs compared to naïve (M0) or M2c-like-polarized macrophages (Fig. 8A). KCs expressed transcripts for MERTK at levels comparable to those measured in differentiated macrophages (data not shown).
Fig. 8.
Expression of Gas-6 in KCs and effect of MerTK activation in KCs on HSC proliferation.
Total RNA was isolated from serum-deprived peripheral monocyte-derived macrophages and from KCs. (A) Gas-6 mRNA expression was analyzed by qRT-PCR and normalized to β-actin. (B) Serum-starved HSCs were exposed to SF medium (Control, white column) or to CM from KCs left untreated (DMSO, purple columns), treated with UNC569 (light purple column) or stimulated with Gas-6 in the presence (light purple column) or absence (purple column) of UNC569. HSC proliferation was assayed by BrdU Cell Proliferation ELISA Kit. Data are mean ± SEM. Statistical significance was assessed by Student's t test. #p <0.05 vs. M0 macrophages. ∗p <0.05 vs. SF medium; ∗∗p <0.05 vs. CM of untreated KCs; §p <0.05 vs. CM of KCs stimulated with Gas-6 without inhibition for MerTK. CM, conditioned media; HSC(s), hepatic stellate cell(s); KCs, Kupffer cells; qRT-PCR, quantitative real-time PCR; SF, serum-free.
MerTK activation in KCs induces HSC proliferation
To investigate whether MerTK expression in KCs contributes to the fibrogenic cross-talk with HSCs, we obtained CM from cultured human KCs in different experimental conditions.
HSCs exposed to KC-CM showed a significant increase in cell proliferation (Fig. 8B). This effect was more evident when KCs were treated with Gas-6 and significantly reduced upon pre-treatment of KCs with UNC569.
Discussion
Hepatic fibrogenesis is the result of a complex cross-talk among different resident and infiltrating cell types.29 Accumulating evidence points to the pivotal role of macrophages in the generation of profibrogenic signals as well as in the process of fibrosis resolution.30 Evidence for different macrophage phenotypes adds complexity to the system31 and the relative role of differentially polarized cells has not been completely unraveled. In this study, we provide evidence that activation of the tyrosine kinase receptor MerTK on the surface of M2c-like-polarized macrophages modifies the secretome to induce profibrogenic actions in HSCs.
MerTK has been under intense investigation over the last few years for its involvement in immune regulatory activities.32 A first indication of a possible link between MerTK and hepatic fibrosis has been provided by a genome-wide association study in patients with hepatitis C, where a SNP in MerTK was more prevalent in patients with advanced fibrosis.15 We recently reported a higher hepatic expression of MERTK in patients with NAFLD and in murine models of fibrogenesis.16 Moreover, 2 intronic SNPs in MERTK rs6726639 A33 and rs4374383 A[15], [16], [17] were associated with decreased hepatic MerTK expression and a reduced risk of liver fibrosis. We also previously provided evidence for a direct role of MerTK in the regulation of the profibrogenic actions of HSCs,16 because exposure of cultured human HSCs to Gas-6 resulted in induction of a profibrogenic phenotype. Moreover circulating levels of Gas-6 were increased in patients with cirrhosis (concentrations around 60-80 ng/ml13,14) and in animal models of chronic liver damage (concentrations around 40 ng/ml14). Taken together, these data suggested a relevant role of MerTK activation in the fibrogenic process, especially in the context of non-alcoholic steatohepatitis (NASH).16
Immunohistochemical analysis of livers with NASH fibrosis demonstrated that macrophages are major contributors to the expression of MerTK.16 Macrophages localize in close proximity to activated HSCs, and have been shown to play a key role in the progression of liver injury from inflammation to fibrosis, and eventually cirrhosis and hepatocellular carcinoma.34 Based on this background, we differentiated cultured macrophages to an M2c-like phenotype, which is known to express MerTK at high levels, and evaluated the contribution of activation of this kinase in the regulation of the HSC phenotype. Specifically, we employed CM obtained from macrophage cultures, which is known to contain soluble factors, exosomes and microparticles that may modulate the phenotype of HSCs.35 We first used THP-1 macrophages, which express high levels of MerTK, Gas-6, VEGF-A and TGFβ1, factors characterizing a wound-healing macrophagic phenotype which promotes cellular proliferation and blood vessel development,[36], [37], [38] as a cellular model.20,38 Our findings indicate that Gas-6 induces MerTK phosphorylation and activation of Akt, a well-recognized downstream effector of MerTK,23 confirming the functionality of this pathway.
More important, CM from Gas-6-treated THP-1 cells induced the increase in migration, proliferation and viability of HSCs, and these actions were blocked by exposure of macrophages to UNC569, a small-molecule inhibitor specific for MerTK.23 The role of MerTK-expressing macrophages in a profibrogenic cross-talk with HSCs was further supported by experiments using siRNAs to downregulate MerTK expression, which provided similar results to those obtained with the chemical inhibitor. Interestingly, exposure of HSCs to medium conditioned by MerTK-expressing THP-1 cells also resulted in a significant upregulation of the expression of several genes known to be involved in fibrogenesis, such as TIMP1, TGFβ1, ACTA2, MMP2, COL1A1 and MMP9. In addition, upregulated expression of VEGF-A, which participates in the regulation of angiogenesis during tissue repair,39 and of IL8, a proinflammatory chemokine,40 at the gene and protein levels, was observed. Also in this case, stimulation of macrophages with Gas-6 increased the effects, while pharmacologic inhibition or genetic knockdown of MerTK blocked these actions. Interestingly, Gas-6 also upregulated the expression of TGFβ1 and VEGF-A in macrophages, indicating that paracrine and autocrine loops involving these factors are regulated by MerTK.
To provide additional data supporting our findings, we used MerTK+/CD206+/CD163+/CD209- macrophages derived from peripheral blood monocytes. This subpopulation expresses high levels of MerTK and, consistently, we observed a marked increase in activation of signaling pathways such as STAT3, ERK1/2, p38,41 and in the expression of VEGF-A,42 all downstream targets of MerTK. Of note, no differences between M2-like and MerTK+/CD206+/CD163+/CD209- macrophages were observed for ERK1/2 and p38 activation, possibly reflecting the fact that several agonists converge on these pathways. Also, in these differentiated and well-characterized cells, Gas-6 induced a substantial increase in phosphorylation of MerTK and Akt, and downstream targets of MerTK such as STAT5, STAT3, ERK1/2 and p38, which was reverted by pre-treatment with UNC569. Using these freshly prepared cells, we corroborated the findings indicating that CM from Gas-6-stimulated MerTK+/CD206+/CD163+/CD209- macrophages promotes the profibrogenic actions of HSCs, inducing a significant increase in HSC migration, cell viability, cell proliferation and expression of profibrotic and proinflammatory genes. Lastly, we found that CM from Gas-6-stimulated MerTK+/CD206+/CD163+/CD209- macrophages induced, in HSCs, the phosphorylation of STAT3 and p38, which are fundamental in the profibrogenic phenotype of HSCs,43 and increased the expression of IL8, an inflammatory chemokine that is closely associated with angiogenesis.40 Importantly, the results obtained in THP-1 cells and in peripheral monocytes derived M2c-like macrophages could be extended to KCs, where transcripts for MerTK were detected, and Gas-6 was expressed at the gene and protein levels.
Functionally, CM from Gas-6-treated KCs increased HSC proliferation, and this effect was blocked by exposure of KCs to UNC569. Taken together, these data strongly suggest that macrophage MerTK could play an important role in the liver fibrogenic process by upregulating several functions associated with the activated phenotype of HSCs. These studies provide an additional mechanism to support previously reported data indicating that holo- or myeloid-specific MerTK targeting in NASH mice decreases liver fibrosis.38
Data reported herein are in clear agreement with previous studies highlighting the reduced risk of liver fibrosis in carriers of the (rs4374383 G>A) variant of MerTK, both in hepatitis C and in NASH.[15], [16], [17] Moreover, in a recent study, myeloid-specific targeting of MerTK reduced fibrosis development in NASH mice. Interestingly, shedding of MerTK from the surface of macrophages limited profibrogenic activities, which were dependent, at least in part, on TGFβ1-mediated signals.38 These findings are consistent with recent data reporting single-cell RNA sequence analysis of cirrhotic human livers, showing that MerTK is primarily expressed in liver macrophages.44
These data should be put in the context of the growing interest in other members of the TAM family, such as Axl, in the pathophysiology of liver fibrosis.45 Blocking activation of this receptor in different cell types was reported to blunt fibrogenesis in experimental models.13,14 Because tyrosine kinase receptors are generally considered druggable targets, different lines of evidence, including those reported in the present study, indicate the TAM family should be the topic of further investigation as a new target for pharmacologic treatment of hepatic fibrosis.12,13
The identification of a role for MerTK in fibrogenesis expands the number of hepatic conditions where this receptor is implicated. In patients with acute-on-chronic liver failure, monocytes and macrophages expressing MerTK were increased, and MerTK expression was associated with a reduced ability to produce proinflammatory cytokines, resulting in suppression of the innate immune response to microbes.46 On the other hand, in mice with acute liver failure, MerTK-deficient animals exhibited persistent liver injury and inflammation, identifying a protective role of MerTK+ macrophages in this setting.47
In conclusion, MerTK expressed on macrophages induces the secretion of factors which modify the phenotype of stellate cells in a profibrogenic fashion, highlighting a novel cross-talk between intrahepatic cells. Together with other data, our findings warrant additional investigation to substantiate the role of this receptor as a therapeutic target for antifibrotic treatments.
Financial support
This work was supported by the Horizon 2020 project ‘Elucidating Pathways of Steatohepatitis’ (Epos) under Grant Agreement no. 634413 and the University of Florence. MP was supported in part by a “Mario Coppo” Hepatology Scholarship from the Italian Association for the Study of the Liver (AISF).
Authors’ contributions
MP, FM designed the study and wrote the manuscript. MP, AC, CR, NN, BP, GD, ER, MPP, LL, FL, KB and SP provided materials, performed the experiments, collected the data, and analyzed the results. MP, AC, FM supervised the project and critically revised the manuscript.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of interest
The authors declare no conflicts of interest to disclose related to this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100444.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Cordero-Espinoza L., Huch M. The balancing act of the liver: tissue regeneration versus fibrosis. J Clin Invest. 2018;128:85–96. doi: 10.1172/JCI93562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuchida T., Friedman S.L. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 3.Guillot A., Tacke F. Liver macrophages: old dogmas and new insights. Hepatol Commun. 2019;3:730–743. doi: 10.1002/hep4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sica A., Invernizzi P., Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 5.Spiller K.L., Wrona E.A., Romero-Torres S., Pallotta I., Graney P.L., Witherel C.E., et al. Differential gene expression in human, murine, and cell line-derived macrophages upon polarization. Exp Cell Res. 2016;347:1–13. doi: 10.1016/j.yexcr.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Schuster S., Cabrera D., Arrese M., Feldstein A.E. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15:349–364. doi: 10.1038/s41575-018-0009-6. [DOI] [PubMed] [Google Scholar]
- 7.Pradere J.P., Kluwe J., De Minicis S., Jiao J.J., Gwak G.Y., Dapito D.H., et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–1473. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braga T.T., Agudelo J.S., Camara N.O. Macrophages during the fibrotic process: M2 as friend and foe. Front Immunol. 2015;6:602. doi: 10.3389/fimmu.2015.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastore M., Grimaudo S., Pipitone R.M., Lori G., Raggi C., Petta S., et al. Role of myeloid-epithelial-reproductive tyrosine kinase and macrophage polarization in the progression of atherosclerotic lesions associated with nonalcoholic fatty liver disease. Front Pharmacol. 2019;10:604. doi: 10.3389/fphar.2019.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S.Y., Lim E.J., Yoon Y.S., Ahn Y.H., Park E.M., Kim H.S., et al. Liver X receptor and STAT1 cooperate downstream of Gas6/Mer to induce anti-inflammatory arginase 2 expression in macrophages. Sci Rep. 2016;6:29673. doi: 10.1038/srep29673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zizzo G., Hilliard B.A., Monestier M., Cohen P.L. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189:3508–3520. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smirne C., Rigamonti C., De Benedittis C., Sainaghi P.P., Bellan M., Burlone M.E., et al. Gas6/TAM signaling components as novel biomarkers of liver fibrosis. Dis Markers. 2019;2019:2304931. doi: 10.1155/2019/2304931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tutusaus A., de Gregorio E., Cucarull B., Cristóbal H., Aresté C., Graupera I., et al. A functional role of GAS6/TAM in nonalcoholic steatohepatitis progression implicates AXL as therapeutic target. Cell Mol Gastroenterol Hepatol. 2020;9:349–368. doi: 10.1016/j.jcmgh.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bárcena C., Stefanovic M., Tutusaus A., Joannas L., Menéndez A., García-Ruiz C., et al. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. J Hepatol. 2015;63:670–678. doi: 10.1016/j.jhep.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patin E., Kutalik Z., Guergnon J., Bibert S., Nalpas B., Jouanguy E., et al. Genome-wide association study identifies variants associated with progression of liver fibrosis from HCV infection. Gastroenterology. 2012;143:1244–1252.e1212. doi: 10.1053/j.gastro.2012.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petta S., Valenti L., Marra F., Grimaudo S., Tripodo C., Bugianesi E., et al. MERTK rs4374383 polymorphism affects the severity of fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2016;64:682–690. doi: 10.1016/j.jhep.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Jiménez-Sousa M., Gómez-Moreno A.Z., Pineda-Tenor D., Brochado-Kith O., Sánchez-Ruano J.J., Artaza-Varasa T., et al. The myeloid-epithelial-reproductive tyrosine kinase (MERTK) rs4374383 polymorphism predicts progression of liver fibrosis in hepatitis C virus-infected patients: a longitudinal study. J Clin Med. 2018;7 doi: 10.3390/jcm7120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pakshir P., Hinz B. The big five in fibrosis: macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018;68-69:81–93. doi: 10.1016/j.matbio.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Kurihara Y., Furue M. Interferon-γ enhances phorbol myristate acetate-induced cell attachment and tumor necrosis factor production via the NF-κB pathway in THP-1 human monocytic cells. Mol Med Rep. 2013;7:1739–1744. doi: 10.3892/mmr.2013.1419. [DOI] [PubMed] [Google Scholar]
- 20.Liao D., Wang X., Li M., Lin P.H., Yao Q., Chen C. Human protein S inhibits the uptake of AcLDL and expression of SR-A through Mer receptor tyrosine kinase in human macrophages. Blood. 2009;113:165–174. doi: 10.1182/blood-2008-05-158048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Almeida M.C., Silva A.C., Barral A., Barral Netto M. A simple method for human peripheral blood monocyte isolation. Mem Inst Oswaldo Cruz. 2000;95:221–223. doi: 10.1590/s0074-02762000000200014. [DOI] [PubMed] [Google Scholar]
- 22.Casini A., Pinzani M., Milani S., Grappone C., Galli G., Jezequel A.M., et al. Regulation of extracellular matrix synthesis by transforming growth factor beta 1 in human fat-storing cells. Gastroenterology. 1993;105:245–253. doi: 10.1016/0016-5085(93)90033-9. [DOI] [PubMed] [Google Scholar]
- 23.Christoph S., Deryckere D., Schlegel J., Frazer J.K., Batchelor L.A., Trakhimets A.Y., et al. UNC569, a novel small-molecule mer inhibitor with efficacy against acute lymphoblastic leukemia in vitro and in vivo. Mol Cancer Ther. 2013;12:2367–2377. doi: 10.1158/1535-7163.MCT-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai B., Kasikara C., Doran A.C., Ramakrishnan R., Birge R.B., Tabas I. MerTK signaling in macrophages promotes the synthesis of inflammation resolution mediators by suppressing CaMKII activity. Sci Signal. 2018;11 doi: 10.1126/scisignal.aar3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alciato F., Sainaghi P.P., Sola D., Castello L., Avanzi G.C. TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J Leukoc Biol. 2010;87:869–875. doi: 10.1189/jlb.0909610. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y., Shirai M., Asada K., Yasui H., Karayama M., Hozumi H., et al. Macrophage mannose receptor, CD206, predict prognosis in patients with pulmonary tuberculosis. Sci Rep. 2018;8:13129. doi: 10.1038/s41598-018-31565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambarus C.A., Krausz S., van Eijk M., Hamann J., Radstake T.R., Reedquist K.A., et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012;375:196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Zizzo G., Cohen P.L. Antibody cross-linking of CD14 activates MerTK and promotes human macrophage clearance of apoptotic neutrophils: the dual role of CD14 at the crossroads between M1 and M2c polarization. Inflammation. 2018;41:2206–2221. doi: 10.1007/s10753-018-0864-x. [DOI] [PubMed] [Google Scholar]
- 29.Li H., Zhou Y., Wang H., Zhang M., Qiu P., Zhang R., et al. Crosstalk between liver macrophages and surrounding cells in nonalcoholic steatohepatitis. Front Immunol. 2020;11:1169. doi: 10.3389/fimmu.2020.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tacke F., Zimmermann H.W. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–1096. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Zigmond E., Samia-Grinberg S., Pasmanik-Chor M., Brazowski E., Shibolet O., Halpern Z., et al. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J Immunol. 2014;193:344–353. doi: 10.4049/jimmunol.1400574. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L., DeBerge M., Wang J., Dangi A., Zhang X., Schroth S., et al. Receptor tyrosine kinase MerTK suppresses an allogenic type I IFN response to promote transplant tolerance. Am J Transpl. 2019;19:674–685. doi: 10.1111/ajt.15087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavalli M., Pan G., Nord H., Wallén Arzt E., Wallerman O., Wadelius C. Genetic prevention of hepatitis C virus-induced liver fibrosis by allele-specific downregulation of MERTK. Hepatol Res. 2017;47:826–830. doi: 10.1111/hepr.12810. [DOI] [PubMed] [Google Scholar]
- 34.Ambade A., Satishchandran A., Saha B., Gyongyosi B., Lowe P., Kodys K., et al. Hepatocellular carcinoma is accelerated by NASH involving M2 macrophage polarization mediated by hif-1. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2016.1221557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman S.L., Arthur M.J. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989;84:1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka M., Siemann D.W. Gas6/Axl signaling pathway in the tumor immune microenvironment. Cancers (Basel) 2020;12 doi: 10.3390/cancers12071850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai B., Dongiovanni P., Corey K.E., Wang X., Shmarakov I.O., Zheng Z., et al. Macrophage MerTK promotes liver fibrosis in nonalcoholic steatohepatitis. Cell Metab. 2020;31:406–421.e407. doi: 10.1016/j.cmet.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrari G., Cook B.D., Terushkin V., Pintucci G., Mignatti P. Transforming growth factor-beta 1 (TGF-beta1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J Cell Physiol. 2009;219:449–458. doi: 10.1002/jcp.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu B., Lin N., Zhang M., Zhu Y., Cheng H., Chen S., et al. Activated hepatic stellate cells promote angiogenesis via interleukin-8 in hepatocellular carcinoma. J Transl Med. 2015;13:365. doi: 10.1186/s12967-015-0730-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cummings C.T., Deryckere D., Earp H.S., Graham D.K. Molecular pathways: MERTK signaling in cancer. Clin Cancer Res. 2013;19:5275–5280. doi: 10.1158/1078-0432.CCR-12-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howangyin K.Y., Zlatanova I., Pinto C., Ngkelo A., Cochain C., Rouanet M., et al. Myeloid-epithelial-reproductive receptor tyrosine kinase and milk fat globule epidermal growth factor 8 coordinately improve remodeling after myocardial infarction via local delivery of vascular endothelial growth factor. Circulation. 2016;133:826–839. doi: 10.1161/CIRCULATIONAHA.115.020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kagan P., Sultan M., Tachlytski I., Safran M., Ben-Ari Z. Both MAPK and STAT3 signal transduction pathways are necessary for IL-6-dependent hepatic stellate cells activation. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramachandran P., Dobie R., Wilson-Kanamori J.R., Dora E.F., Henderson B.E.P., Luu N.T., et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575:512–518. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linger R.M., Keating A.K., Earp H.S., Graham D.K. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernsmeier C., Pop O.T., Singanayagam A., Triantafyllou E., Patel V.C., Weston C.J., et al. Patients with acute-on-chronic liver failure have increased numbers of regulatory immune cells expressing the receptor tyrosine kinase MERTK. Gastroenterology. 2015;148:603–615.e614. doi: 10.1053/j.gastro.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 47.Triantafyllou E., Pop O.T., Possamai L.A., Wilhelm A., Liaskou E., Singanayagam A., et al. MerTK expressing hepatic macrophages promote the resolution of inflammation in acute liver failure. Gut. 2018;67:333–347. doi: 10.1136/gutjnl-2016-313615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.