Abstract
BACKGROUND
Several breast cancer studies have reported the use of adjuvant opioids with the paravertebral block (PVB) to improve outcomes. However, there is no level-1 evidence justifying its use.
AIM
To elucidate if the addition of opioids to PVB improves pain control in breast cancer surgery patients.
METHODS
We conducted an electronic literature search across PubMed, Embase, Scopus, and Google Scholar databases up to October 20, 2020. Only randomized controlled trials (RCTs) comparing the addition of opioids to PVB with placebo for breast cancer surgery patients were included.
RESULTS
Six RCTs were included. Our meta-analysis indicated significantly reduced 24-h total analgesic consumption with the addition of opioids to PVB as compared to placebo [standardized mean difference (SMD) -1.57, 95% confidence interval (CI): -2.93, -0.21, I2 = 94%]. However, on subgroup analysis, the results were non-significant for studies using single PVB (SMD: -1.76, 95%CI: -3.65, 0.13 I2 = 95.09%) and studies using PVB infusion (SMD: -1.30, 95%CI: -4.26, 1.65, I2 = 95.49%). Analysis of single PVB studies indicated no significant difference in the time to first analgesic request between opioid and placebo groups (mean difference -11.28, 95%CI: -42.00, 19.43, I2 = 99.39%). Pain scores at 24 h were marginally lower in the opioid group (mean difference -1.10, 95%CI: -2.20, 0.00, I2 = 0%). There was no difference in the incidence of postoperative nausea and vomiting between the two groups.
CONCLUSION
Current evidence suggests a limited role of adjuvant opioids with PVB for breast cancer surgery patients. Further homogenous RCTs with a large sample size are needed to clarify the beneficial role of opioids with PVB.
Keywords: Opioids, Pain, Surgery, Breast cancer, Nerve block, Paravertebral block
Core Tip: The use of opioids as adjuvants for nerve blocks has been increasing. However, it is not clear if the addition of opioids to paravertebral block (PVB) improves outcomes for breast cancer surgery patients. In our first systematic review and meta-analysis, we pooled data from six randomized controlled trials assessing the role of adjuvant opioids for PVB in patients undergoing breast cancer surgery. Our results indicate that the addition of opioids to PVB has a limited role in reducing 24-h analgesic consumption, time to first analgesic request, and pain scores as compared to placebo. Further high-quality studies are required to strengthen the evidence.
INTRODUCTION
Breast cancer is the most common malignancy in females worldwide, and surgical intervention is the primary mode of management even in advanced cases (basically palliative in selected populations)[1]. Studies have shown improved survival with mastectomy in patients with breast cancer[1,2]. While general anesthesia is the standard-setting used for surgical interventions for these patients, a substantial number of individuals encounter significant postoperative pain[3,4]. Inadequate analgesia in the immediate postoperative period can lead to a prolonged hospital stay, increased healthcare cost, and reduced patient satisfaction[5]. Optimal management of acute pain in breast cancer survivors can also reduce the development of chronic post-surgical pain[6].
Over the last decade, several quality-improvement protocols have been described to improve peri-operative management and optimize pain control in breast cancer patients[7]. One such method is the use of locoregional anesthetic techniques like the paravertebral block (PVB), intercostal nerve block, erector spinae plane block, and pectoral block[5,8,9]. Of these, the PVB has been widely used to provide better analgesia after surgery in breast cancer patients. The clinical efficacy of PVB has also been demonstrated by several studies[10,11]. Terkawi et al[12] in a meta-analysis of 24 studies demonstrated that the use of PVB decreased opioid consumption and postoperative pain scores at 2, 24, 48, and 72 h after surgery. However, the effects of PVB were found to be modest with a limited beneficial effect on postoperative recovery. In this context, several researchers have evaluated the addition of adjuvants to PVB to improve its efficacy. It is hypothesized that the addition of drugs like opioids, clonidine, and dexmedetomidine would lead to better analgesic efficacy of PVB[13,14]. Opioids have been used in combination with local anesthetics for several locoregional anesthetic techniques leading to better pain control in the immediate postoperative period[15,16]. However, it is not known if the addition of opioids to PVB would lead to better outcomes in breast cancer patients. Despite several studies reporting the use of adjuvant opioids with PVB, there is a lack of pooled evidence to guide clinical practice. Thus, this systematic review and meta-analysis aimed to answer the following clinical question: Does the addition of opioids to PVB lead to improved pain control in the immediate post-operative period in patients undergoing surgery for breast cancer?
MATERIALS AND METHODS
Search strategy
The authors planned and executed this study conforming to the recommendations of the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses)[17] and the Cochrane Handbook for Systematic Reviews of Intervention[18]. Protocol registration was, however, not carried out. We conducted an electronic literature search across PubMed, Embase, Scopus, and Google Scholar databases. Two reviewers independently carried out the literature search. Search limits were from the inception of databases to October 20, 2020. The search terms included “breast surgery”, “mastectomy”, “paravertebral block”, “opioid”, “morphine”, “fentanyl”, “buprenorphine”, and “tramadol”. Supplementary Table 1 presents the search strategy and the result of the PubMed database. At first, the search records were reviewed by their titles and abstracts. Relevant articles to the review were identified, and full texts of the articles were extracted. Both the reviewers assessed individual articles based on the inclusion and exclusion criteria. Any disagreements were resolved by discussion. The bibliography of studies meeting the inclusion criteria was also hand-searched for any missed references.
Inclusion criteria
We defined the inclusion and exclusion criteria of the review based on the PICOS (Population, Intervention, Comparison, Outcome, Study type) framework a priori. Population: Studies conducted on patients undergoing breast cancer surgery and receiving PVB before general anesthesia. The Intervention was to be the addition of an opioid to the PVB. The comparison was the addition of placebo or no drug to the PVB. Studies were to report at least one of the following outcomes: 24 h total analgesic consumption, pain scores, time to the first analgesic, and/or incidence of Postoperative nausea and vomiting (PONV). Only randomized controlled trials (RCTs) were eligible to be included in the review. No language restriction was placed. Studies comparing opioids with any other active drugs were excluded. We also excluded studies using opioids not as an addition to PVB but via other routes like intravenous, subcutaneous, etc. Furthermore, non-RCTs, retrospective studies, single-arm studies, and studies not reporting relevant data were also excluded.
Data extraction and quality assessment
Data were extracted using a data extraction form by two reviewers independently. Name of the first author, publication year, study type, study location, age of patients, surgery type, sample size, intervention drug and dose, PVB protocol, use of other analgesics, and study outcomes were extracted. The primary outcome of the interest of our analysis was 24-h total analgesic consumption. The secondary outcomes were time to first analgesic demand, pain scores, and incidence of PONV. Furthermore, a descriptive analysis of other outcomes reported by the included studies was also performed.
Two reviewers assessed the quality of each RCT using the Cochrane Collaboration risk assessment tool[18]. Each study was assessed for bias in random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The study was judged to have a "high", "unclear", or "low" risk of bias for each domain. Any disagreements were resolved by discussion.
Statistical analysis
The software “Open MetaAnalyst” was used for the meta-analysis[19]. Meta-analysis was conducted only if at least three trials reported similar outcomes. Owing to the methodological heterogeneity of the included studies, we used a random-effects model to calculate the pooled effect size for all analyses. Continuous data not reported on the same scale were summarized using standardized mean difference (SMD) with 95% confidence interval (CI) or else mean difference (MD) was used. Specifically, different analgesics were used by the individual studies for the outcome of ‘total analgesic consumption’, hence we used SMD to pool this variable. For studies not reporting mean and standard deviation (SD) scores of continuous variables, the method described by Wan et al[20] was used to calculate data from the median and interquartile range. For the incidence of PONV, we calculated odds ratios (OR) with 95%CI. Sub-group analysis was conducted for single and continuous PVB. Heterogeneity was assessed using the I2 statistic. I2 values of 25%-50% represented low, values of 50%-75% represented medium, and more than 75% represented substantial heterogeneity. As < 10 studies were included per meta-analysis, funnel plots were not used to assess publication bias.
RESULTS
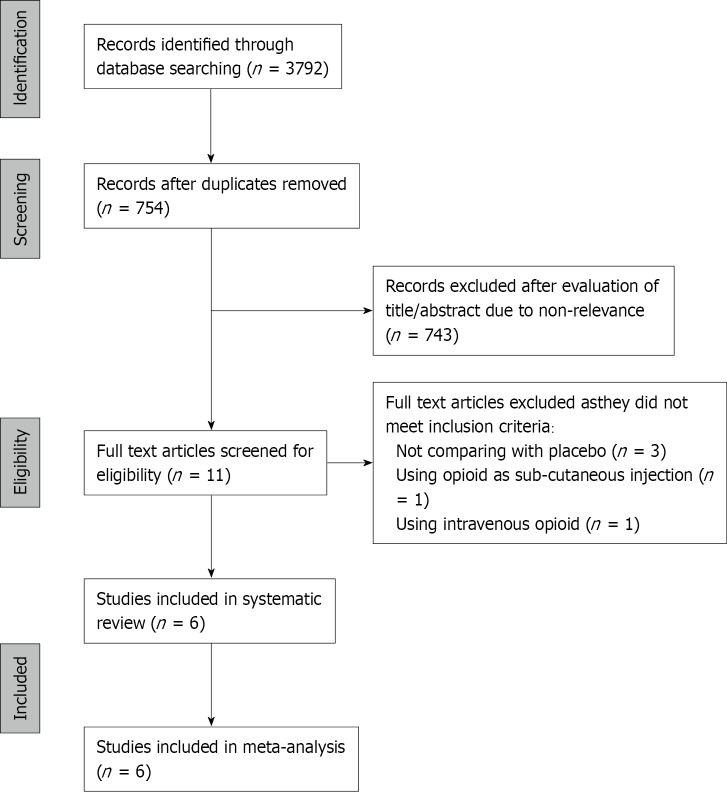
The PRISMA flowchart of the review is presented in Figure 1. Of the 11 studies assessed by the full-texts, five were excluded with reasons, and a total of six RCTs fulfilled the inclusion criteria[21-26]. Characteristics of studies included in the review are presented in Table 1. Three trials[22,23,25] were conducted exclusively on modified radical mastectomy patients, while the remaining three included other breast cancer surgeries as well. The sample size of the included studies was small, ranging from 12-20 patients per group. The opioids used as an adjunct to PVB were fentanyl in three trials, while morphine, tramadol, and buprenorphine were used in one study each. Two trials[22,26] used continuous PVB infusions, while the remaining used single PVB.
Figure 1.
Study flow chart.
Table 1.
Details of included studies
|
Ref.
|
Yr
|
Study location
|
Surgery type
|
Sample size
|
Age (yr)
|
Common protocol for PVB
|
Opioid added to PVB in intervention group)
|
Drug added to PVB in control group
|
Post-operative analgesics used
|
|
|
Study
|
Control
|
|||||||||
| Mostafa et al[21] | 2018 | Egypt | MRM or breast conservation surgery with axillary node dissection | 20 | 20 | 18-78 | Nalbuphine 10 mg | PVB at the level of T4 with bupivacaine 0.5% 0.3 mL/kg | No drug | Tramadol as PCA |
| Pushparajan et al[22] | 2017 | India | MRM | 20 | 20 | 18-60 | Fentanyl 2 μg/mL at 0.1 mL/kg/h for 24 h | Continuous PVB at the level of T4 with 0.2% ropivacaine for 24 h | No drug | Fentanyl as PCA. Paracetamol or tramadol or fentanyl for breakthrough pain |
| Morsy et al[23] | 2017 | Egypt | MRM | 15 | 15 | NR | Morphine 2 mg | PVB at the level of T3 with 20 mL of bupivacaine 0.25% | No drug | Meperidine for breakthrough pain |
| Bhuvaneshwari et al[24] | 2012 | India | Total mastectomy and axillary lymph node dissection | 12 | 12 | Study: 49.1 ± 7.1; Control: 50.7 ± 11 | Fentanyl 2 μg/mL | PVB at the level of T3 with bupivacaine 0.25% and epinephrine 5 μg/mL | No drug | Morphine for breakthrough pain |
| Omar et al[25] | 2011 | Egypt | MRM | 19 | 20 | Study: 47.5 ± 9.3; Control: 49.3 ± 10.5 | Tramadol 1.5 mg/kg (maximum of 150 mg) | PVB at the level of T1 (1/3rd of the dose) and T4 (2/3rd of the dose) with bupivacaine 0.5% 2 mg/kg | No drug | Fentanyl as PCA. Paracetamol 1 g thrice daily and ibuprofen 400-600 mg thrice daily |
| Burlacu et al[26] | 2006 | Ireland | Wide local excisions (at least one breast quadrant), mastectomies, and mastectomies with reconstruction | 13 | 13 | Study: 54 ± NR; Control: 51 ± NR | Fentanyl 50 μg with bolus followed by 4 μg/mL infusion | Continuous PVB at the level of T3 with initial bolus of 19 mL levobupivacaine 0.25% followed by continuous infusion of 0.1% solution for 24 h | Saline | Morphine as PCA |
PVB: Paravertebral block; MRM: Modified radical mastectomy; PCA: Patient controlled analgesia; T: Thoracic vertebral level.
Outcomes
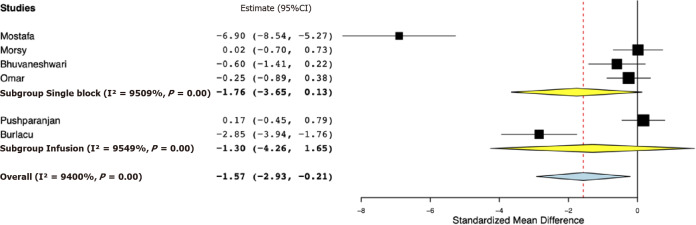
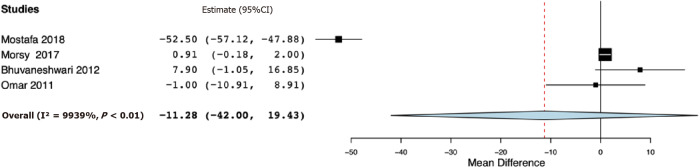
Table 2 presents the results of the outcomes reported by the included studies. For the primary outcome, data were reported by all six studies. Our meta-analysis indicated significantly reduced 24-h total analgesic consumption with the addition of opioids to PVB as compared to placebo (SMD: -1.57, 95%CI: -2.93, -0.21, I2 = 94%) (Figure 2). However, on subgroup analysis, the results were non-significant for studies using single PVB (SMD: -1.76, 95%CI: -3.65, 0.13, I2 = 95.09%) and studies using PVB infusion (SMD: -1.30, 95%CI: -4.26, 1.65, I2 = 95.49%). Data on time to first analgesic request were reported by four studies using a single PVB. Our analysis demonstrated no significant difference in the time to first analgesic request between opioid and placebo groups in hours (MD -11.28, 95%CI: -42.00, 19.43, I2 = 99.39%) (Figure 3).
Table 2.
Outcomes reported by included studies
|
Ref.
|
Outcome
|
Results
|
| Mostafa et al[21] | Time to first analgesic request | Significantly longer in the opioid group |
| Post-operative analgesic time | Significantly longer in the opioid group | |
| 24 h total analgesic consumption | Significantly lower in the opioid group | |
| Pain scores up to 24 h | Significantly lower in the opioid group | |
| HR, SBP, DBP | No difference between the two groups | |
| Ramsay sedation scores | Patients in the control group were more agitated then opioid group in the first four hours after the operation. No difference between the two groups after four hours | |
| Pushparajan et al[22] | Pain scores up to discharge | Significantly lower scores in the opioid group only at 24 h and not at other time periods |
| 24 h total analgesic consumption | No difference between the two groups | |
| PONV | No difference between the two groups | |
| Urinary retention, pruritis | No difference between the two groups | |
| Patient satisfaction | No difference between the two groups | |
| Morsy et al[23] | 24 h total analgesic consumption | No difference between the two groups |
| Time to first analgesic request | No difference between the two groups | |
| Ramsay sedation scores | No difference between the two groups | |
| PONV | No difference between the two groups | |
| HR, SBP, DBP | No difference between the two groups | |
| Bhuvaneshwari et al[24] | Time to first analgesic request | Significantly longer in the opioid group |
| 24 h total analgesic consumption | Significantly lower in the opioid group | |
| Cumulative pain scores at 24 h | Significantly lower in the opioid group | |
| PONV | No difference between the two groups | |
| Patient satisfaction | Significantly higher in the opioid group | |
| Omar et al[25] | Time to first analgesic request | No difference between the two groups |
| 24 h total analgesic consumption | No difference between the two groups | |
| Pain scores up to 24 h | No difference between the two groups | |
| PONV | No difference between the two groups | |
| Burlacu et al[26] | Total analgesic consumption | Significantly lower in the opioid group |
| Pain scores up to 24 h | No difference between the two groups | |
| Nausea scores | Significantly higher in the opioid group | |
| SBP | No difference between the two groups | |
| Patient satisfaction | Significantly higher in the opioid group |
PONV: Postoperative nausea and vomiting; HR: Heart rate; SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
Figure 2.
Forest plot of 24-h total analgesic consumption with sub-group analysis based on type of paravertebral block.
Figure 3.
Forest plot of time to first analgesic request in hours.
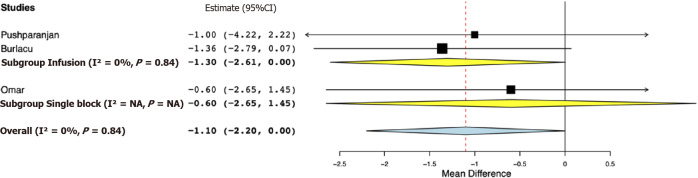
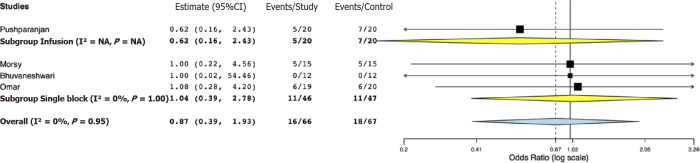
Data for a meta-analysis on pain scores on the Visual Analog Scale were available only from three studies. The study of Bhuvaneshwari et al[24] reported cumulative 24-h pain scores, while SD values of pain scores were not reported by Mostafa et al[21]. Both these studies reported significantly lower pain scores in the opioid group. For the remaining studies, our pooled analysis indicated pain scores at 24 h were marginally lower in the opioid group (MD -1.10, 95%CI: -2.20, 0.00, I2 = 0%) (Figure 4). Sub-group analysis demonstrated marginal difference favoring the opioid group in the studies using PVB infusion (MD -1.30, 95%CI: -2.61, 0.00, I2 = 0%); however, for the study using single PVB no difference was noted (MD -0.60, 95%CI: -2.65, 1.45). Data on the incidence of PONV were reported by four studies. Our analysis indicated no statistically significant difference between opioid and placebo groups (OR 0.87, 95%CI: 0.39, 1.93, I2 = 0%) (Figure 5). Results were non-significant on subgroup analysis as well.
Figure 4.
Forest plot of 24-h visual analog scale pain score with sub-group analysis based on type of paravertebral block.
Figure 5.
Forest plot of incidence of postoperative nausea and vomiting with sub-group analysis based on type of paravertebral block.
Risk of bias analysis
Table 3 presents the risk of bias assessment of included studies. The majority of studies (5/6) were of good quality with a low risk of bias across six of the seven domains. Only the trial of Morsy et al[23] did not provide adequate information on randomization, allocation concealment, and blinding.
Table 3.
Risk of bias in included studies
|
Ref.
|
Random sequence generation
|
Allocation concealment
|
Blinding of participants and personnel
|
Blinding of outcome assessment
|
Incomplete outcome data
|
Selective reporting
|
Other bias
|
| Mostafa et al[21] | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | Low risk |
| Pushparajan et al[22] | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Morsy et al[23] | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| Bhuvaneshwari et al[24] | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Omar et al[25] | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Burlacu et al[26] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
DISCUSSION
The results of our review assessing the role of adjuvant opioids to PVB for breast cancer surgery patients indicate that: (1) Total analgesic consumption in the immediate postoperative period may not be reduced with the addition of opioids to a single PVB or PVB infusion; (2) Time to first analgesic request is not increased with the addition of opioids to a single PVB; (3) There is only a marginal difference in pain score at 24 h with the addition of opioids; and (4) Adjuvant opioids do not increase the incidence of PONV.
PVB as a technique of regional anesthesia has gained popularity for pain control in patients undergoing breast surgery. The procedure involves the deposition of local anesthetic just lateral to the spinous process of the vertebrae in the area where spinal nerves emerge out of the intervertebral foramina. The local anesthetic blocks the somatic and sympathetic nerve supply of the dermatome of interest thereby providing postsurgical analgesia[27]. Its efficacy has been tested in a randomized setting by numerous authors not only for breast surgery but also for thoracic surgeries[12,28]. However, one important limitation of nerve blocks, in general, is the short duration of action of the local anesthetic. Even with agents with a longer duration of action, like bupivacaine and ropivacaine, the duration of analgesia is often inadequately sustained in the postoperative period[29]. Prolonging the duration of analgesia by increasing the dose of local anesthetic entails the risk of adverse events involving the cardiovascular and central nervous systems[30]. To overcome this issue, several adjuvants have been used with local anesthetics to prolong the analgesic effect while maintaining the safety of regional anesthesia.
One of the earliest adjuvants used with local anesthetics was opioids. Their use with local anesthetics has accelerated since studies reported the presence of peripheral opioid receptors in the primary afferent neurons and peripheral sensory nerves[31]. It is thought that opioids exhibit a local anesthetic-like action causing hyperpolarization of the afferent sensory neuron through G protein‐coupled receptor mechanism[32]. Nishikawa et al[33] have suggested that adjuvant opioids with local anesthetics can lead to the increased duration and improved quality of local anesthetic blockade. However, the results of our review indicate that this mechanism may have a limited role especially for PVB in breast cancer surgery patients. For the primary outcome of 24-h total analgesic consumption, our analysis demonstrated a statistically significant difference in favor of the opioid group, but no such difference was noted on sub-group analysis of single PVB and PVB infusions studies. It is important to note that the lower end of 95%CI of the meta-analysis was on the higher side (-3.65 for single PVB sub-group and -4.26 for PVB infusion group) and the upper end of the 95%CI close to zero (for single PVB sub-group) in the meta-analysis. Similar was the case in the analysis of 24-h pain scores with the upper end of 95%CI at 0, albeit with a very limited number of studies. The addition of opioids was found to be safe with no increase in the incidence of PONV. Therefore, while our results do not demonstrate a clear advantage of adding opioids to PVB they are suggestive of a probable role of adjuvant opioids in PVB for breast cancer surgery patients, which needs further research.
Such inconclusive result was also evident on descriptive analysis of the included studies with three RCTs[22,23,25] reporting no benefit of adding opioids to PVB, while the remaining three[21,24,26] reporting significant advantage in favor of adjuvant opioids. Such varied results with local anesthetic adjuvants have been reported with other nerve blocks as well. Soulioti et al[34] in an RCT reported improved outcomes with the addition of 100 mg of tramadol to brachial plexus block with ropivacaine. In contrast, no such benefit was noted by Kesimci et al[35] using a similar dose of tramadol with ropivacaine. Several other RCTs have reported the limited benefit of adding opioids to peripheral nerve blocks. In a recently published trial, Kim et al[36] failed to prove any beneficial effect of adding fentanyl to continuous femoral nerve blocks for knee arthroplasty patients. Two RCTs have shown that the addition of fentanyl to transverse abdominus plane block did not improve postoperative outcomes in patients undergoing cesarean section and gynecological surgeries[37,38]. The contrasting results on the role of adjuvant opioids in literature as well as in our review can be attributed to several factors, like the type of surgery, type of nerve block, type and dosage of local anesthetic, type and dose of opioid, pain threshold of patients, the post-operative pain control protocol, etc. In our review, while the type of surgery and nerve block were the same, four different opioids were used with varying doses in the included studies. Postoperative pain control protocol was different across studies, which could also have contributed to the heterogeneous results. However, such heterogeneity is expected in clinical trials conducted in different geographical settings. Several other meta-analyses in literature have also pooled outcomes of different opioids in a single analysis[39-41].
In addition to the inter-study heterogeneity, other limitations of our review should also be taken into account while interpreting the results. Firstly, only six RCTs were available for analysis in this review, all with limited sample size. Thus, our review may not have been statistically powered to detect significant differences between the groups. Secondly, data on pain scores at different periods were not available from included studies for a meta-analysis. Furthermore, data as mean and SD were not presented by all studies, further restricting our analysis. Thirdly, pain score and analgesic consumption after surgery can depend on several surgical and patient-dependent factors, like duration and complexity of the surgical procedure, the pain threshold of the patient, etc. Such uncontrolled factors could have also influenced the outcomes of the included trials. Lastly, we were unable to register the review protocol on any online database, and this is a significant limitation of our review. Nevertheless, our study has certain novelties. We have presented the first systematic review and meta-analysis assessing the role of adjuvant opioids in PVB for breast cancer surgery patients. The overall quality of the included RCTs was high, and this lends credibility to the overall review.
CONCLUSION
To conclude, there is a limited role of adjuvant opioids with PVB for breast cancer surgery patients. The addition of opioids had no significant effect on 24-h total analgesic consumption, time to first analgesic, and 24-h pain score. Further homogenous RCTs with a large sample size are needed to clarify the beneficial role of opioids with PVB.
ARTICLE HIGHLIGHTS
Research background
Opioids have been used in combination with local anesthetics for several locoregional anesthetic techniques, leading to better pain control in the immediate postoperative period. However, it is not known if the addition of opioids to paravertebral block (PVB) would lead to better outcomes in breast cancer patients.
Research motivation
No meta-analysis has summarized evidence to assess the value of adding opioids to PVB in breast cancer patients undergoing surgical intervention.
Research objectives
To compare total analgesic consumption, time to first analgesic request, and pain scores with and without the addition of opioids to PVB in breast cancer surgery patients.
Research methods
We conducted an electronic literature search across PubMed, Embase, Scopus, and Google Scholar databases up to October 20, 2020 for randomized controlled trials (RCTs) comparing the addition of opioids to PVB with placebo for breast cancer surgery patients.
Research results
Analysis of six RCTs demonstrated that the addition of opioids to PVB significantly reduced 24-h total analgesic consumption but had no impact on the time to first analgesic request. Pain scores at 24 h were marginally lower with the addition of opioids.
Research conclusions
Current evidence suggests a limited role of adjuvant opioids with PVB for breast cancer surgery patients.
Research perspectives
Further homogenous RCTs with a large sample size are needed to clarify the beneficial role of opioids with PVB.
Footnotes
Conflict-of-interest statement: The authors deny any conflict of interest.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: July 19, 2021
First decision: October 16, 2021
Article in press: January 19, 2022
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lepot A, Valencia GA S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
Contributor Information
Meng-Hua Chen, Lanzhou University Medical College, Lanzhou 730000, Gansu Province, China.
Zheng Chen, Department of Breast, Shandong Second Provincial General Hospital, Jinan 250021, Shandong Province, China.
Da Zhao, Department of Oncology, The First Hospital of Lanzhou University, Lanzhou 730000, Gansu Province, China. zhaoda_dr@163.com.
References
- 1.Harris E, Barry M, Kell MR. Meta-analysis to determine if surgical resection of the primary tumour in the setting of stage IV breast cancer impacts on survival. Ann Surg Oncol. 2013;20:2828–2834. doi: 10.1245/s10434-013-2998-2. [DOI] [PubMed] [Google Scholar]
- 2.Arciero C, Liu Y, Gillespie T, Subhedar P. Surgery and survival in patients with stage IV breast cancer. Breast J. 2019;25:644–653. doi: 10.1111/tbj.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sada A, Thiels CA, Britain MK, Dudakovic A, Bergquist WJ, Nickel SR, Moran MJ, Martinez-Jorge J, Jakub JW. Optimizing Discharge Opioid Prescribing Practices After Mastectomy With Immediate Reconstruction. Mayo Clin Proc Innov Qual Outcomes. 2019;3:183–188. doi: 10.1016/j.mayocpiqo.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron RH. Surgical management of breast cancer. Semin Oncol Nurs. 2007;23:10–19. doi: 10.1016/j.soncn.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Calì Cassi L, Biffoli F, Francesconi D, Petrella G, Buonomo O. Anesthesia and analgesia in breast surgery: the benefits of peripheral nerve block. Eur Rev Med Pharmacol Sci. 2017;21:1341–1345. [PubMed] [Google Scholar]
- 6.Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, Dworkin RH. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006;7:626–634. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temple-Oberle C, Shea-Budgell MA, Tan M, Semple JL, Schrag C, Barreto M, Blondeel P, Hamming J, Dayan J, Ljungqvist O ERAS Society. Consensus Review of Optimal Perioperative Care in Breast Reconstruction: Enhanced Recovery after Surgery (ERAS) Society Recommendations. Plast Reconstr Surg. 2017;139:1056e–1071e. doi: 10.1097/PRS.0000000000003242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Z, Durrands T, Li R, Gan TJ, Lin J. Pectoral block vs paravertebral block: a systematic review, meta-analysis and trial sequential analysis. Reg Anesth Pain Med. 2020;45:727–732. doi: 10.1136/rapm-2020-101512. [DOI] [PubMed] [Google Scholar]
- 9.Leong RW, Tan ESJ, Wong SN, Tan KH, Liu CW. Efficacy of erector spinae plane block for analgesia in breast surgery: a systematic review and meta-analysis. Anaesthesia. 2021;76:404–413. doi: 10.1111/anae.15164. [DOI] [PubMed] [Google Scholar]
- 10.Kasimahanti R, Arora S, Bhatia N, Singh G. Ultrasound-guided single- vs double-level thoracic paravertebral block for postoperative analgesia in total mastectomy with axillary clearance. J Clin Anesth. 2016;33:414–421. doi: 10.1016/j.jclinane.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Salviz EA, Sivrikoz N, Ozonur A, Orhan-Sungur M, Savran-Karadeniz M, Altun D, Hocaoglu E, Celet-Ozden B, Tugrul KM. Ultrasound-Guided Bilateral Thoracic Paravertebral Blocks as an Adjunct to General Anesthesia in Patients Undergoing Reduction Mammaplasty: A Historical Cohort Study. Plast Reconstr Surg. 2017;139:20e–28e. doi: 10.1097/PRS.0000000000002842. [DOI] [PubMed] [Google Scholar]
- 12.Terkawi AS, Tsang S, Sessler DI, Terkawi RS, Nunemaker MS, Durieux ME, Shilling A. Improving Analgesic Efficacy and Safety of Thoracic Paravertebral Block for Breast Surgery: A Mixed-Effects Meta-Analysis. Pain Physician. 2015;18:E757–E780. [PubMed] [Google Scholar]
- 13.Priya S, Bamba C. Comparison of Morphine and Clonidine as Adjuvants in Paravertebral Block. Anesth Essays Res. 2018;12:459–463. doi: 10.4103/aer.AER_27_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohta M, Kalra B, Sethi AK, Kaur N. Efficacy of dexmedetomidine as an adjuvant in paravertebral block in breast cancer surgery. J Anesth. 2016;30:252–260. doi: 10.1007/s00540-015-2123-8. [DOI] [PubMed] [Google Scholar]
- 15.Hamed MA, Ghaber S, Reda A. Dexmedetomidine and Fentanyl as an Adjunct to Bupivacaine 0.5% in Supraclavicular Nerve Block: A Randomized Controlled Study. Anesth Essays Res. 2018;12:475–479. doi: 10.4103/aer.AER_50_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akhondzadeh R, Rashidi M, Gousheh M, Olapour A, Tasbihi B. Comparison of the Ketamine-Lidocaine and Fentanyl-Lidocaine in Postoperative Analgesia in Axillary Block in Upper Limb Fractures By Ultrasound Guidance. Anesth Pain Med. 2019;9:e92695. doi: 10.5812/aapm.92695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. Version 6. Cochrane; 2019. [Google Scholar]
- 19.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omar Mostafa M, Makram Botros J, Sayed Khaleel AM. Effect of Dexmedetomidine Versus Nalbuphine as an Adjuvant on Paravertebral Block to Manage Postoperative Pain After Mastectomies. Anesth Pain Med. 2018;8:e13308. doi: 10.5812/aapm.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pushparajan HK, Punj J, Pandey R, Darlong V, Srivastava A, Batra RK, Bhan A. Addition of Fentanyl to Ropivacaine Infusion in Continuous Thoracic Paravertebral Infusion Does Not Improve Its Analgesic Effect Following Modified Radical Mastectomy: A Randomized Controlled Trial. AANA J. 2017;85:352–356. [PubMed] [Google Scholar]
- 23.Morsy A, Abd-elmaksoud M, Abdel Aziz R, Metwally M. Comparison between dexmedetomidine vs morphine added to bupivacaine in paravertebral block in patients scheduled for modified radical mastectomy. Res Opin Anesth Intensive Care. 2017;4:59–64. [Google Scholar]
- 24.Bhuvaneswari V, Wig J, Mathew PJ, Singh G. Post-operative pain and analgesic requirements after paravertebral block for mastectomy: A randomized controlled trial of different concentrations of bupivacaine and fentanyl. Indian J Anaesth. 2012;56:34–39. doi: 10.4103/0019-5049.93341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omar AM, Mansour MA, Abdelwahab HH, Aboushanab OH. Role of ketamine and tramadol as adjuncts to bupivacaine 0.5% in paravertebral block for breast surgery: A randomized double-blind study. Egypt J Anaesth. 2011;27:101–105. [Google Scholar]
- 26.Burlacu CL, Frizelle HP, Moriarty DC, Buggy DJ. Fentanyl and clonidine as adjunctive analgesics with levobupivacaine in paravertebral analgesia for breast surgery. Anaesthesia. 2006;61:932–937. doi: 10.1111/j.1365-2044.2006.04793.x. [DOI] [PubMed] [Google Scholar]
- 27.Vila H Jr, Liu J, Kavasmaneck D. Paravertebral block: new benefits from an old procedure. Curr Opin Anaesthesiol. 2007;20:316–318. doi: 10.1097/ACO.0b013e328166780e. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Hao Z, Li W, Duan C, Fan X, Xin J, Ren C. The Efficacy and Safety of Paravertebral Block Combined with Parecoxib During Video-Assisted Thoracic Surgery: A Randomized Controlled Trial. J Pain Res. 2020;13:355–366. doi: 10.2147/JPR.S244787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansour NA. Ropivacaine vs Bupivacaine in Postoperative Pain Control. J Biotechnol Biomater. 2012;02:1–8. [Google Scholar]
- 30.Krishna Prasad GV, Khanna S, Jaishree SV. Review of adjuvants to local anesthetics in peripheral nerve blocks: Current and future trends. Saudi J Anaesth. 2020;14:77–84. doi: 10.4103/sja.SJA_423_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- 32.Gissen AJ, Gugino LD, Datta S, Miller J, Covino BG. Effects of fentanyl and sufentanil on peripheral mammalian nerves. Anesth Analg. 1987;66:1272–1276. [PubMed] [Google Scholar]
- 33.Nishikawa K, Kanaya N, Nakayama M, Igarashi M, Tsunoda K, Namiki A. Fentanyl improves analgesia but prolongs the onset of axillary brachial plexus block by peripheral mechanism. Anesth Analg. 2000;91:384–387. doi: 10.1097/00000539-200008000-00028. [DOI] [PubMed] [Google Scholar]
- 34.Soulioti E, Tsaroucha A, Makris A, Koutsaki M, Sklika E, Mela A, Megaloikonomos PD, Mavrogenis AF, Fassoulaki A. Addition of 100 mg of Tramadol to 40 mL of 0.5% Ropivacaine for Interscalene Brachial Plexus Block Improves Postoperative Analgesia in Patients Undergoing Shoulder Surgeries as Compared to Ropivacaine Alone-A Randomized Controlled Study. Medicina (Kaunas) 2019;55 doi: 10.3390/medicina55070399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kesimci E, Izdes S, Gozdemir M, Kanbak O. Tramadol does not prolong the effect of ropivacaine 7.5 mg/mL for axillary brachial plexus block. Acta Anaesthesiol Scand. 2007;51:736–741. doi: 10.1111/j.1399-6576.2007.01308.x. [DOI] [PubMed] [Google Scholar]
- 36.Kim GH, Lee JW, Kim GE, Lee SS, Son SL, Kim BU, Cho HN, Kwon MY, Koo MS, Kim JE, Yun MJ. Analgesic effect of ropivacaine with fentanyl in comparison with ropivacaine alone for continuous femoral nerve block after knee replacement arthroplasty: a prospective, randomized, double-blinded study. Anesth Pain Med (Seoul) 2020;15:209–216. doi: 10.17085/apm.2020.15.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Q, Liu X, Zhong X, Yang B. Addition of dexmedetomidine or fentanyl to ropivacaine for transversus abdominis plane block: evaluation of effect on postoperative pain and quality of recovery in gynecological surgery. J Pain Res. 2018;11:2897–2903. doi: 10.2147/JPR.S178516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang LZ, Liu X, Zhang YF, Hu XX, Zhang XM. Addition of fentanyl to the ultrasound-guided transversus abdominis plane block does not improve analgesia following cesarean delivery. Exp Ther Med. 2016;11:1441–1446. doi: 10.3892/etm.2016.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frauenknecht J, Kirkham KR, Jacot-Guillarmod A, Albrecht E. Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: a systematic review and meta-analysis. Anaesthesia. 2019;74:651–662. doi: 10.1111/anae.14582. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Li QB, Wu YY, Wang BN, Kang JL, Xu XW. Efficacy and Safety of Opioids for the Prevention of Etomidate-Induced Myoclonus: A Meta-Analysis. Am J Ther. 2018;25:e517–e523. doi: 10.1097/MJT.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 41.Luo N, Tan S, Li X, Singh S, Liu S, Chen C, Huang Z, Feng S, Lin Y, Cen H, Liang M, Chen M. Efficacy and Safety of Opioids in Treating Cancer-Related Dyspnea: A Systematic Review and Meta-Analysis Based on Randomized Controlled Trials. J Pain Symptom Manage. 2021;61:198–210.e1. doi: 10.1016/j.jpainsymman.2020.07.021. [DOI] [PubMed] [Google Scholar]