Abstract
Objectives
Monitoring is essential to safe anticoagulation prescribing and requires close collaboration among pathologists, clinicians, and pharmacists.
Methods
We describe our experience in the evolving strategy for laboratory testing of unfractionated heparin (UFH).
Results
An intrainstitutional investigation revealed significant discordance between activated partial thromboplastin time (aPTT) and antifactor Xa (anti-Xa) assays, prompting a transition from the former to the latter in 2013. With the increasing use of oral factor Xa inhibitors (eg, apixaban, rivaroxaban, edoxaban, betrixaban), which interfere with the anti-Xa assay, we adapted our protocol again to incorporate aPTT in patients admitted on oral Xa inhibitors who require transition to UFH.
Conclusions
Our experience demonstrates key challenges in anticoagulation and highlights the importance of clinical pathologists in helping health systems adapt to the changing anticoagulation landscape.
Keywords: Anticoagulation, Heparin, Direct oral anticoagulants
Key Points.
Two assays are commonly used for monitoring and dose adjustment of unfractionated heparin (UFH): activated partial thromboplastin time (aPTT) and antifactor Xa (anti-Xa).
Because aPTT is influenced by multiple preanalytic and biologic factors, the anti-Xa assay has increasingly been used because of its ability to reflect heparin concentration more directly.
Because oral factor Xa inhibitors interfere with the heparin anti-Xa assay, patients who require transition to UFH need an alternative test. The aPTT assay is the most widely available alternative.
INTRODUCTION
Collaboration among pathologists, clinicians, and pharmacists has long been an essential part of safe and effective anticoagulation because commonly used oral drugs (eg, warfarin) and parenteral anticoagulants (eg, heparin) have required close laboratory monitoring to ensure appropriate dosing. With the advent of direct oral anticoagulants (DOACs), clinical pathologists have had to adapt to meet the needs these new agents present. Two classes of DOACs are currently available: (1) direct thrombin inhibitors (dabigatran) and (2) factor Xa inhibitors (apixaban, rivaroxaban, edoxaban, and betrixaban). Although DOACs have predictable pharmacokinetics and pharmacodynamics, which enables clinicians to prescribe them at a fixed dose without laboratory monitoring, new issues have arisen from their interference with existing assays, including activated partial thromboplastin time (aPTT) and the antifactor Xa (anti-Xa) assay.1,2
We describe the evolution of anticoagulation monitoring at our institution, starting with a historical description of the transition from aPTT to anti-Xa assay and concluding with a discussion of how oral Xa inhibitors have prompted modification of this protocol. Our experience highlights the importance of close collaboration between clinical team members (including the pharmacist), who are aware of the patient’s medications, and laboratory specialists, who understand the roles and limitations of the available assays. This multidisciplinary team is necessary to ensure the safe and effective use of anticoagulation.
MONITORING UNFRACTIONATED HEPARIN
Discovered in 1916,3 unfractionated heparin (UFH) has been the foundation for anticoagulation in medical practice. The majority of its anticoagulant effect is mediated by binding to and accelerating the action of antithrombin (AT), which inactivates thrombin (factor IIa), factor Xa, factor IXa, and factor XIIa.4 UFH includes branched glycosaminoglycans of various sizes, with the inhibition of factor Xa mediated by a specific pentasaccharide sequence. UFH can be administered as a continuous intravenous (IV) infusion or as a subcutaneous injection; the former is more commonly used in hospitalized patients who have venous or arterial thrombosis because of its ability to achieve rapid therapeutic effect. The pharmacokinetics of IV UFH is unpredictable, because it binds to plasma proteins, endothelial cells, and macrophages and has variable clearance.4 Therefore, laboratory monitoring is required to avoid under- and overanticoagulation. Two assays are widely used to monitor IV UFH: aPTT and the anti-Xa assay.
Heparin Monitoring With aPTT
The aPTT has been the standard of UFH monitoring, although the optimal therapeutic range continues to be debated. It was originally defined as 1.5 to 2.5 times the aPTT of normal plasma,5 but significant variation in aPTT results based on reagents and coagulometers hinders interlaboratory consistency, even when attempts are made to correlate with heparin activity assays (including anti-Xa and protamine titration assays).6,7 Current recommendations acknowledge these limitations and expect that individual institutions will define a laboratory-specific therapeutic aPTT range based on the anti-Xa assay.7,8 An additional limitation of using aPTT is that it is affected by a variety of factors common in medical practice, such as increased coagulation factor levels, as seen in acute-phase reaction Table 1 . Thus, prolongation or shortening of aPTT by such factors introduces the potential for under- or overdosing of UFH as well as the risk of thrombosis progression or bleeding complications.
Table 1.
Variables That Influence Activated Partial Thromboplastin Time and the Antifactor Xa Assay and Interfere With the Accuracy of Unfractionated Heparin Dose Determination9
| Variables that prolong aPPT with no effect on the anti-Xa assay |
|---|
| Underfilled sodium citrate collection tube10,11 |
| Decreased clotting factor production from liver disease12,13 |
| Decreased clotting factor production from congenital deficiencies12,13 |
| Decreased production of factors not associated with increased bleeding risk (ie, factor XII, prekallikrein, HMW kininogen)12,13 |
| Increased clotting factor consumption (ie, DIC)12,13 |
| Presence of lupus anticoagulant or acquired factor inhibitors12,13 |
| Variables that shorten aPPT with no effect on the anti-Xa assay |
| Increased acute-phase reactants (factor VIII, fibrinogen)13,14 |
| Variable that overestimates UFH in the anti-Xa assay with no effect on aPPT |
| Hypertriglyceridemia (>690 mg/dL)15,a |
| Variables that underestimate UFH in the anti-Xa assay with no effect on aPPT |
| Hyperbilirubinemia (conjugated >29 mg/dL; unconjugated >14 mg/dL)15,a |
| Gross hemolysis (>1.5 g/dL)15,a |
Anti-Xa, antifactor Xa; aPPT, activated partial thromboplastin time; DIC, disseminated intravascular coagulation; HMW, high molecular weight; UFH, unfractionated heparin.
aValues for STA-Liquid Anti-Xa reagent (Diagnostica Stago) as cited. Other reagents may have different thresholds for interference.
Introduction of the Anti-Xa Assay
The anti-Xa assay is a direct measure of UFH activity determined by mixing the patient’s plasma with a known amount of purified factor Xa, and then measuring residual Xa activity colorimetrically. In this assay, the amount of color generated inversely correlates with the concentration of UFH-AT complex in the plasma, which inhibits the enzymatic conversion of a Xa-specific chromogenic substrate to colored product by factor Xa. The College of American Pathologists16 and the American College of Chest Physicians8 define a therapeutic anti-Xa assay range as 0.3 to 0.7 U/mL for UFH,17 but similar to aPTT, optimal therapeutic range is also debated. The anti-Xa assay has become increasingly popular for UFH monitoring because it is not influenced by most biological and preanalytical factors that affect aPTT Table 1 . Importantly, aPTT and the anti-Xa assay can remain persistently low in patients with congenital or acquired AT deficiency, representing in vivo failure of heparin anticoagulation effect. Therefore, AT levels should be evaluated in patients who do not achieve therapeutic levels despite high UFH doses (>35,000 U/day).
Large randomized comparisons between the anti-Xa assay and aPTT have not been performed, so a definitive benefit of one monitoring strategy over another remains unknown. Nevertheless, given the theoretical advantage of the specificity of the anti-Xa assay to the amount of UFH in circulation, many institutions have opted to transition monitoring from aPTT to the anti-Xa assay.18-21
TRANSITION FROM APTT TO THE ANTI-XA ASSAY: A SINGLE-INSTITUTION EXPERIENCE
To determine how the aPTT result in patients receiving IV UFH in our institution correlated with the anti-Xa assay, we performed a prospective investigation. Approval was obtained from the local institutional review board, and the study was conducted under international human research ethics standards. Although dose adjustments of UFH were performed using aPTT, an anti-Xa assay was collected simultaneously 18 to 24 hours after UFH initiation. We used the STA-Liquid Anti-Xa reagent on the STA-R analyzer (Diagnostica Stago) without exogenous dextran sulfate or AT, calibrated with STA-Multi Hep Calibrator (Diagnostica Stago), and defined the therapeutic range as 0.3 to 0.7 U/mL. Following the College of American Pathologists recommendations,16 we determined our therapeutic aPTT range by comparing the results of the aPTT with the anti-Xa assay using approximately 100 samples from inpatients on UFH. We performed this comparison annually, and the aPTT range has been consistently 71 to 105 seconds (normal range, 25-35 seconds). For the prospective evaluation, we included all 342 patients consecutively tested with both assays (56% men and 44% women; mean [SD] age, 59 [15] years; 58% were White, 41% were Black, and less than 1% were Asian or Hispanic. Most patients (58%) had arterial thrombosis, 41% had venous thrombosis, and 1% had both.
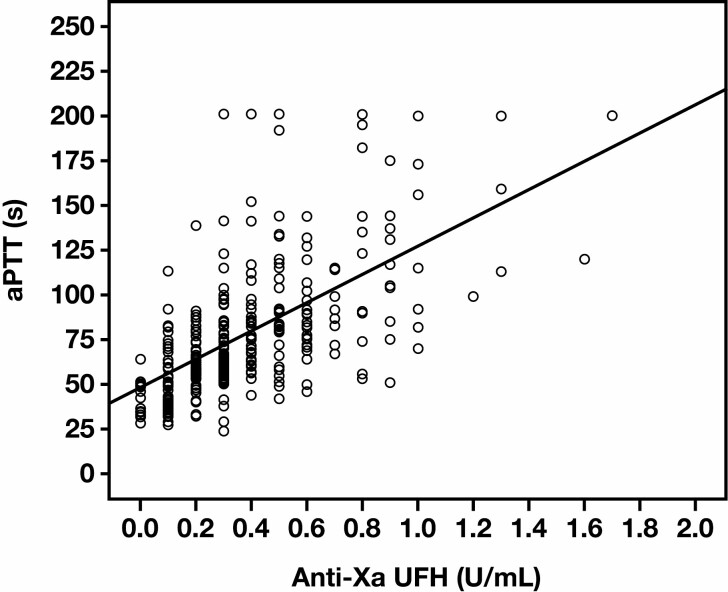
Figure 1 shows that the aPTT and anti-Xa assay results were concordant in only 203 of 342 (59%) patients, with low correlation based on linear regression analysis (r = 0.65, R2 = 0.372), a κ value of 0.22 (P < .001),22 and a Pearson χ 2 analysis (P < .001). The greatest concordance was noted when the anti-Xa assay value was above or below the therapeutic range (>0.7 U/mL or <0.3 U/mL, respectively). Overall, the results showed that 19% of patients were likely underdosed and 22% overdosed based on the aPTT result Table 2 , assuming that the anti-Xa assay is more accurate, while recognizing its limitations.
Figure 1.
Scatterplot of paired measurements of antifactor Xa (anti-Xa) and activated partial thromboplastin time (aPTT) assays. In our institutional evaluation (n = 342), aPTT and anti-Xa results demonstrated low correlation based on linear regression analysis (r = 0.65, R2 = 0.372).
Table 2.
Paired Measurements of the Activated Partial Thromboplastin Time and Antifactor Xa Assay (n = 342)
Anti-Xa, antifactory Xa; aPTT, activated partial thromboplastin time; UFH, unfractionated heparin.
aConcordance between anti-Xa and aPTT.
bDiscordance between anti-Xa and aPTT, potential for UFH underdosing.
cDiscordance between anti-Xa and aPTT, potential for UFH overdosing.
Similar discrepancies have been reported in the literature, and fluctuations in aPTT have been attributed to prothrombin deficiency or high factor VIII.23 It has also been shown that compared with aPTT, anti-Xa assay monitoring of UFH results in faster achievement of therapeutic concentration and more time in the therapeutic range.18-21 We presume that this occurs because the anti-Xa assay provides a more predictable dose-response curve, so fewer lab draws are required. Therefore, providers need to make fewer dose adjustments. In patients who require high doses of UFH to achieve therapeutic aPTT, similar bleeding and thrombosis rates were observed when using the anti-Xa assay and the aPTT.24 Single-institution observational studies have described conflicting results, with some reporting no difference in bleeding rates between monitoring strategies21 and others reporting increased bleeding with aPTT elevation and therapeutic anti-Xa assay. Of note, the latter are based on a small number of bleeding events.19,25 Discordance between anti-Xa and aPTT is associated with worse clinical outcomes. Price et al26 demonstrated that patients with at least 2 consecutive values at increased aPTT compared with the anti-Xa assay had a higher risk of major bleeding and 30-day mortality. Although the increased cost of anti-Xa assay reagents has been a hindrance at some institutions, the total reagent cost may be lower because the more predictable dose response allows for fewer lab draws per patient.9
Given our findings and those reported in the literature, our institution opted to transition to anti-Xa assay monitoring of UFH in 2013. The transition required multidisciplinary team meetings over several months to adequately prepare for the change. Efforts included education for nursing and pharmacy on specimen collection and dose adjustments, training of laboratory personnel to perform the anti-Xa assay around the clock, modification of satellite laboratory operations, creation of electronic health record (EHR) ordering protocols, budgeting for the initial cost increase of the anti-Xa assay reagents, and generation of physician awareness and buy-in. These efforts ultimately resulted in the development of an Anticoagulation Subcommittee of our Pharmacy and Therapeutics Committee, which continues to function in a supervisory capacity to promote appropriate and safe anticoagulation. Since that time, additional resources have been published to assist institutions with the aPTT to anti-Xa assay transition.27 Our institutional heparin dosing protocol using the anti-Xa assay is presented in Table 3 .
Table 3.
Institutional Heparin Dosing Protocol Based on the Antifactor Xa Assay
| Indication | |||
|---|---|---|---|
| Venous Thrombosis | Cardiac | CNS or Postpartum Thrombosis | |
| Starting dose | |||
| Bolus | 80 U/kg | 70 U/kg | None |
| Starting rate | 18 U/kg/h | 15 U/kg/h | 12 U/kg/h |
| Monitoring | Check anti-Xa assay 6 h after UFH initiation | ||
| Dose adjustments based on the anti-Xa assay result, U/mL | |||
| <0.2 | Bolus: 30 U/kg | Bolus: None | |
| Infusion: Increase rate by 3 U/kg/h | Infusion: Increase rate by 3 U/kg/h | ||
| Monitoring: Repeat anti-Xa in 6 h | Monitoring: Repeat anti-Xa in 6 h | ||
| 0.2-0.29 | Bolus: 30 U/kg | Bolus: None | |
| Infusion: Increase rate by 3 U/kg/h | Infusion: Increase rate by 2 U/kg/h | ||
| Monitoring: Repeat anti-Xa in 6 h | Monitoring: Repeat anti-Xa in 6 h | ||
| 0.3-0.7 | No change | ||
| Monitoring: Repeat anti-Xa with next day’s labs | |||
| 0.71-0.8 | Infusion: Decrease rate by 1 U/kg/h | ||
| Monitoring: Repeat anti-Xa in 6 h | |||
| 0.81-0.99 | Infusion: Decrease rate by 2 U/kg/h | ||
| Monitoring: Repeat anti-Xa in 6 h | |||
| ≥1 | Infusion: Hold for 1 h; at restart, decrease rate by 2 U/kg/h | ||
| Monitoring: Repeat anti-Xa in 6 h |
Anti-Xa, antifactory Xa; CNS, central nervous system; UFH, unfractionated heparin.
NEW CHALLENGES IN UFH MONITORING: DOACS THAT INHIBIT FACTOR XA
The anticoagulation landscape was revolutionized with the approval of the first oral factor Xa inhibitor, rivaroxaban, by the US Food and Drug Administration (FDA) in 2011,28 followed by apixaban,29 edoxaban,30 and betrixaban31 in the years that followed. With their ease of use, use of oral Xa inhibitors to manage venous thrombosis and atrial fibrillation has outpaced warfarin in ambulatory patients.32,33 Furthermore, the use of oral Xa inhibitors continues to expand, with new approvals from the FDA and the European Medicines Agency for venous thrombosis prophylaxis (eg, orthopedic surgery, patients with cancer) and arterial thrombotic disorders (eg, coronary artery disease, peripheral artery disease).
As would be expected from their mechanism of action, oral Xa inhibitors affect anti-Xa assays used for UFH monitoring. Although commercially available assays are not calibrated to assess oral Xa inhibitor concentration, studies have suggested a linear relationship between drug concentration and anti-Xa assay result.34,35 A challenge arises when ambulatory patients taking factor Xa inhibitors are admitted to the hospital and require transition to UFH.
In this scenario, many facilities have opted to return to using the aPTT until the oral Xa inhibitor is cleared.34,35 Importantly, the aPTT is also prolonged by oral Xa inhibitors, but the degree of influence varies significantly based on the reagent used and the drug present (ie, less effect with apixaban vs rivaroxaban).36,37 Furthermore, the degree of prolongation does not accurately reflect the oral Xa inhibitor concentration, so readers should be cautioned that a normal aPTT does not indicate the absence of medication.38 Our institution uses the STA-PTT-A reagent (Diagnostica Stago) on the STA-R analyzer. This reagent has lower sensitivity to oral Xa inhibitors: The concentration needed to double the clotting time with rivaroxaban37 is above the expected range for peak therapeutic concentration.39 Given these considerations, we rely on the same therapeutic range for aPTT (71-105 seconds), as determined by the method previously described. Although additional strategies for heparin monitoring in the presence of oral Xa inhibitors have been evaluated (eg, drug absorption,40 anti-IIa assay,41 a modified anti-Xa assay that can account for oral Xa inhibitor interference42), the feasibility of these approaches is limited because they are not commercially available or approved by the FDA for this indication.
Acknowledging the limitations of aPTT, many institutions have adopted a hybrid approach using limited-duration aPTT–based monitoring for the expected time required for the oral Xa inhibitor to clear followed by an anti-Xa assay–based protocol as soon as the oral drug is expected to be eliminated.27,43 This solution is admittedly imperfect because of the influence of oral Xa inhibitors on aPTT and the limitations of aPTT in the presence of other variables, as previously described Table 1 , but it remains the most readily available and feasible option. Furthermore, the decision of when to transition can be complex because the timing of the last oral Xa inhibitor dose may be unknown and its clearance may be prolonged because of decreased kidney function or concomitant use of medications that inhibit CYP3A4 or P-glycoprotein (P-gp). For these reasons, a hybrid approach is particularly dependent on close coordination among the laboratory, pharmacy, and clinical teams. Our approach, which is similar to what was published by Faust et al,43 arose after multidisciplinary discussions and is described below:
When a patient on a Xa-inhibitor is admitted to the hospital and requires IV anticoagulation, begin UFH infusion without a bolus at the time of the next scheduled dose of the oral agent. If the time of the last dose and/or next scheduled dose is unknown, initiate UFH infusion on admission. Consider collecting a baseline anti-Xa assay before UFH initiation if there is uncertainty as to whether the patient has recently taken an oral Xa inhibitor. Importantly, use the baseline anti-Xa assay to screen for the presence of an oral Xa inhibitor, not to quantify its anticoagulant effect. We consider an anti-Xa assay result less than 0.1 U/mL to indicate the absence of an oral Xa inhibitor and an indication that the patient can be started on UFH with the standard anti-Xa assay monitoring protocol.
Monitor and titrate UFH infusion by using the aPTT protocol Table 4 , aiming for a range of 75 to 105 seconds.
Continue the aPTT protocol for 48 to 72 hours based on estimated time for oral Xa inhibitor clearance, taking into account kidney function and concurrent medications, such as the P-gp inhibitors or inducers and CYP3A4 inhibitors or inducers.
When the estimated time for clearance has been reached, initiate the anti-Xa assay protocol for continued UFH monitoring.
Table 4.
Institutional Heparin Dosing Protocol Based on Activated Partial Thromboplastin Time Values
| Indication | |||
|---|---|---|---|
| Venous Thrombosis | Cardiac | CNS or Postpartum Thrombosis | |
| Starting dose | |||
| Bolus | 80 U/kg | 70 U/kg | None |
| Starting rate | 18 U/kg/h | 15 U/kg/h | 12 U/kg/h |
| Monitoring | Check aPTT 6 h after UFH initiation | ||
| Dose adjustments by aPTT, s | |||
| <64 | Bolus: 30 U/kg | Bolus: None | |
| Infusion: Increase rate by 3 U/kg/h | Infusion: Increase rate by 3 U/kg/h | ||
| Monitoring: Repeat aPTT in 6 h | Monitoring: Repeat aPTT in 6 h | ||
| 64-70 | Bolus: 30 U/kg | Bolus: None | |
| Infusion: Increase rate by 3 U/kg/h | Infusion: Increase rate by 2 U/kg/h | ||
| Monitoring: Repeat aPTT in 6 h | Monitoring: Repeat aPTT in 6 h | ||
| 71-105 | No change | ||
| Monitoring: Repeat aPTT with next day’s labs | |||
| 106-116 | Infusion: Decrease rate by 1 U/kg/h | ||
| Monitoring: Repeat aPTT in 6 h | |||
| 117-132 | Infusion: Decrease rate by 2 U/kg/h | ||
| Monitoring: Repeat aPTT in 6 h | |||
| ≥133 | Infusion: Hold for 1 h; at restart, decrease rate by 2 U/kg/h | ||
| Monitoring: Repeat aPTT in 6 h |
aPTT, activated partial thromboplastin time; CNS, central nervous system; UFH, unfractionated heparin.
At our institution, the treating provider and collaborating pharmacist make the decision to use the aPTT-based protocol and when to transition to the anti-Xa assay protocol for an individual patient. To facilitate appropriate use, EHR order sets for both protocols include indications and instructions. Despite these efforts, our institution has discovered that certain clinical teams continue to order the aPTT-based protocol in situations that are not recommended. Furthermore, they may concurrently order aPTT and anti-Xa assays, leading to confusion when discordance occurs and about which value to follow. Multidisciplinary efforts continue to reinforce the role of each protocol.
An important limitation of our experience is that we have not prospectively evaluated the outcomes of patients on oral Xa inhibitors monitored using the hybrid approach. Our Anticoagulation Subcommittee, however, continually audits anticoagulation-related safety events throughout the health system, and no major adverse events associated with this protocol have been identified. Given that each nursing unit also has designated pharmacists who work closely with the clinical teams, it is unlikely that such events would go unnoticed. To expand institutional efforts to promote anticoagulation safety as well as appropriate use and monitoring of such medications, we are also in the process of developing an Anticoagulation Stewardship Program (as proposed by the Anticoagulation Forum44). This program will provide additional oversight of patients receiving UFH infusions using the aPTT monitoring protocol as well as any other anticoagulant and protocol. This program will also require collaboration with clinical pathologists and pharmacists to be effective.
REVERSAL AGENT FOR ORAL FACTOR XA INHIBITORS AND THE ANTI-XA ASSAY
Our institution encountered another challenge regarding laboratory monitoring of UFH with the introduction of andexanet alfa,45 the antidote for apixaban and rivaroxaban. Although not recommended on the package insert, providers interested in confirming the reversal of the Xa inhibitor drug started ordering the anti-Xa assay to assess effect. With this practice, we learned that andexanet alfa paradoxically causes falsely increased functional anti-Xa levels because of the dissociation of the oral Xa inhibitor from andexanet alfa in highly diluted in vitro conditions.45 A modified anti-Xa assay has been developed to overcome this limitation, but it is not widely available.46 The duration of this effect has not been evaluated because clinical trials have used a modified anti-Xa assay that is not commercially available.47 In addition, if andexanet alfa is used immediately before an urgent cardiac bypass in patients taking apixaban or rivaroxaban, another IV anticoagulant must be employed because andexanet alfa not only interferes with the anti-Xa assays but also causes UFH unresponsiveness.45,48 Clinical pathologist awareness of these potential pitfalls of andexanet alfa use and influence on monitoring is essential, as use of this agent becomes more pervasive and clinicians rely on expert consultation to interpret results of these assays.
CONCLUSION
Monitoring of UFH is complex, with guidance based on limited data, and decisions often heavily depend on institution-specific considerations. Furthermore, just as many facilities were attempting to leave aPTT monitoring, increasing use of oral Xa inhibitors introduced a new challenge to anti-Xa assay–based protocols. Our institution has adopted a hybrid approach, favoring the anti-Xa assay in the majority of patients but returning to aPTT for patients transitioning to UFH from oral Xa inhibitors. It is likely that health systems will continue to adapt approaches to UFH monitoring as new assays are developed and new anticoagulants are introduced, all in an effort to reach the common goal: a UFH monitoring strategy that consistently prevents thrombosis while minimizing bleeding risk.
Funding: Dr Gangaraju received funding from the National Center for Advancing Translational Sciences (NCATS) (grant No. KL2TR003097).
Disclosure: Dr Gangaraju is a consultant for Sanofi Genzyme and Alexion.
REFERENCES
- 1. Gosselin RC, Adcock DM, Douxfils J. An update on laboratory assessment for direct oral anticoagulants (DOACs). Int J Lab Hematol. 2019;41:33-39. [DOI] [PubMed] [Google Scholar]
- 2. Conway SE, Hwang AY, Ponte CD, et al. Laboratory and clinical monitoring of direct acting oral anticoagulants: what clinicians need to know. Pharmacotherapy. 2017;37:236-248. [DOI] [PubMed] [Google Scholar]
- 3. McLean J. The thromboplastic action of cephalin. Am J Physiol. 1916;41:250-257. [Google Scholar]
- 4. Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):188S-203S. [DOI] [PubMed] [Google Scholar]
- 5. Basu D, Gallus A, Hirsh J, et al. A prospective study of the value of monitoring heparin treatment with the activated partial thromboplastin time. N Engl J Med. 1972;287:324-327. [DOI] [PubMed] [Google Scholar]
- 6. Cuker A, Ptashkin B, Konkle BA, et al. Interlaboratory agreement in the monitoring of unfractionated heparin using the anti-factor Xa-correlated activated partial thromboplastin time. J Thromb Haemost. 2009;7:80-86. [DOI] [PubMed] [Google Scholar]
- 7. Marlar RA, Clement B, Gausman J. Activated partial thromboplastin time monitoring of unfractionated heparin therapy: issues and recommendations. Semin Thromb Hemost. 2016;43:253-260. [DOI] [PubMed] [Google Scholar]
- 8. Garcia DA, Baglin TP, Weitz JI, et al. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl):e24S-e43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vandiver JW, Vondracek TG. Antifactor Xa levels versus activated partial thromboplastin time for monitoring unfractionated heparin. Pharmacotherapy. 2012;32:546-558. [DOI] [PubMed] [Google Scholar]
- 10. Payne S, MacKinnon K, Keeney M, et al. Effect of 3.2 vs. 3.8% sodium citrate concentration on anti-Xa levels for patients on therapeutic low molecular weight heparin. Clin Lab Haematol. 2003;25:317-319. [DOI] [PubMed] [Google Scholar]
- 11. Adcock DM, Kressin DC, Marlar RA. Minimum specimen volume requirements for routine coagulation testing: dependence on citrate concentration. Am J Clin Pathol. 1998;109:595-599. [DOI] [PubMed] [Google Scholar]
- 12. Kitchens CS. To bleed or not to bleed? Is that the question for the PTT? J Thromb Haemost. 2005;3:2607-2611. [DOI] [PubMed] [Google Scholar]
- 13. Eikelboom JW, Hirsh J. Monitoring unfractionated heparin with the aPTT: time for a fresh look. Thromb Haemost. 2006;96:547-552. [PubMed] [Google Scholar]
- 14. Lehman CM, Frank EL. Laboratory monitoring of heparin therapy: partial thromboplastin time or anti-Xa assay? Lab Med. 2009;40:47-51. [Google Scholar]
- 15. STA-liquid anti-Xa colorimetric assay of heparins (UFH and LMWH) [package insert]. Parsippany, NJ:Diagnostica Stago Inc; 2014. [Google Scholar]
- 16. Olson JD, Arkin CF, Brandt JT, et al. College of American Pathologists Conference XXXI on Laboratory Monitoring of Anticoagulant Therapy: laboratory monitoring of unfractionated heparin therapy. Arch Pathol Lab Med. 1998;122:782-798. [PubMed] [Google Scholar]
- 17. Smythe MA, Mattson JC, Koerber JM. The heparin anti-Xa therapeutic range: are we there yet? Chest. 2002;121:303-304. [DOI] [PubMed] [Google Scholar]
- 18. Guervil DJ, Rosenberg AF, Winterstein AG, et al. Activated partial thromboplastin time versus antifactor Xa heparin assay in monitoring unfractionated heparin by continuous intravenous infusion. Ann Pharmacother. 2011;45:861-868. [DOI] [PubMed] [Google Scholar]
- 19. Samuel S, Allison TA, Sharaf S, et al. Antifactor Xa levels vs. activated partial thromboplastin time for monitoring unfractionated heparin: a pilot study. J Clin Pharm Ther. 2016;41:499-502. [DOI] [PubMed] [Google Scholar]
- 20. Whitman-Purves E, Coons JC, Miller T, et al. Performance of anti-factor Xa versus activated partial thromboplastin time for heparin monitoring using multiple nomograms. Clin Appl Thromb Hemost. 2018;24:310-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frugé KS, Lee YR. Comparison of unfractionated heparin protocols using antifactor Xa monitoring or activated partial thrombin time monitoring. Am J Health Syst Pharm. 2015;72:S90-S97. [DOI] [PubMed] [Google Scholar]
- 22. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [PubMed] [Google Scholar]
- 23. Takemoto CM, Streiff MB, Shermock KM, et al. Activated partial thromboplastin time and anti-Xa measurements in heparin monitoring: biochemical basis for discordance. Am J Clin Pathol. 2013;139:450-456. [DOI] [PubMed] [Google Scholar]
- 24. Levine MN, Hirsh J, Gent M, et al. A randomized trial comparing activated thromboplastin time with heparin assay in patients with acute venous thromboembolism requiring large daily doses of heparin. Arch Intern Med. 1994;154:49-56. [PubMed] [Google Scholar]
- 25. McLaughlin K, Rimsans J, Sylvester KW, et al. Evaluation of antifactor-Xa heparin assay and activated partial thromboplastin time values in patients on therapeutic continuous infusion unfractionated heparin therapy. Clin Appl Thromb Hemost. 2019;25:1076029619876030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Price EA, Jin J, Nguyen HM, et al. Discordant aPTT and anti-Xa values and outcomes in hospitalized patients treated with intravenous unfractionated heparin. Ann Pharmacother. 2013;47:151-158. [DOI] [PubMed] [Google Scholar]
- 27. Dager W; AC Forum Board of Directors. Guide to transitioning from the aPTT to anti-Xa assay to manage heparin infusions; from The Anticoagulation Forum.https://acforum-excellence.org/Resource-Center/resource_files/1524-2020-04-29-101004.pdf. Updated April 28, 2020. Accessed July 24, 2021.
- 28. Xarelto [package insert]. Beerse, Belgium:Janssen Pharmaceuticals Inc; 2016. [Google Scholar]
- 29. Eliquis [package insert]. New York, NY:Bristol Myers Squibb; 2012. [Google Scholar]
- 30. Savaya [package insert]. Chuo City, Tokyo, Japan:Daiichi Sankyo Inc; 2015. [Google Scholar]
- 31. Bevyxxa [package insert]. San Francisco, CA:Portola Pharmaceuticals Inc; 2017. [Google Scholar]
- 32. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300-5.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lutsey PL, Walker RF, MacLehose RF, et al. Direct oral anticoagulants and warfarin for venous thromboembolism treatment: trends from 2012 to 2017. Res Pract Thromb Haemost. 2019;3:668-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cuker A, Husseinzadeh H. Laboratory measurement of the anticoagulant activity of edoxaban: a systematic review. J Thromb Thrombolysis. 2015;39:288-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cuker A, Siegal DM, Crowther MA, et al. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014;64:1128-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Douxfils J, Chatelain C, Chatelain B, et al. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost. 2013;110:283-294. [DOI] [PubMed] [Google Scholar]
- 37. Douxfils J, Mullier F, Loosen C, et al. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res. 2012;130:956-966. [DOI] [PubMed] [Google Scholar]
- 38. Adcock DM, Gosselin RC. The danger of relying on the APTT and PT in patients on DOAC therapy, a potential patient safety issue. Int J Lab Hematol. 2017;39:37-40. [DOI] [PubMed] [Google Scholar]
- 39. Gosselin RC, Adcock DM, Bates SM, et al. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018;118:437-450. [DOI] [PubMed] [Google Scholar]
- 40. Exner T, Rigano J, Favaloro EJ. The effect of DOACs on laboratory tests and their removal by activated carbon to limit interference in functional assays. Int J Lab Hematol. 2020;42(suppl 1):41-48. [DOI] [PubMed] [Google Scholar]
- 41. Hanslik A, Kitzmüller E, Tran US, et al. Anti-activated factor II assay for monitoring unfractionated heparin in children: results of the HEARTCAT study. J Thromb Haemost. 2017;15:38-46. [DOI] [PubMed] [Google Scholar]
- 42. Strickland SW, Palkimas S, Acker M, et al. A novel laboratory assay to monitor unfractionated heparin dosing in patients taking apixaban prior to hospital admission. J Appl Lab Med. 2020;6:378-386. [DOI] [PubMed] [Google Scholar]
- 43. Faust AC, Kanyer D, Wittkowsky AK. Managing transitions from oral factor Xa inhibitors to unfractionated heparin infusions. Am J Health Syst Pharm. 2016;73:2037-2041. [DOI] [PubMed] [Google Scholar]
- 44. Anticoagulation Forum. Core elements of anticoagulation stewardship programs. https://acforum.org/web/downloads/ACF%20Anticoagulation%20Stewardship%20Guide.pdf. 2019.. Accessed May 1, 2021.
- 45. Andexxa [package insert]. San Francisco, CA:Portola Pharmaceuticals Inc; 2020. [Google Scholar]
- 46. Cardenas J, Kotha J, Lu G, et al. Validation of a modified anti-FXa assay on STA-compact analyzer for measuring FXa inhibitor levels in the presence of andexanet alfa. Blood. 2020;136(suppl 1):37-38. [Google Scholar]
- 47. Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19:446-451. [DOI] [PubMed] [Google Scholar]
- 48. Levy JH, Connors JM. Andexanet alfa use in cardiac surgical patients: a Xa inhibitor and heparin reversal agent. J Cardiothorac Vasc Anesth. 2021;35:265-266. [DOI] [PubMed] [Google Scholar]